ABSTRACT
Aims/Introduction
Little is known about the relationship between cardiovascular health (CVH) metrics and the risk of developing prediabetes or diabetes. We examined the association of CVH metrics with the annual risk of developing prediabetes or diabetes.
Materials and Methods
We carried out this study including 403,857 participants aged 18–71 years with available data on fasting plasma glucose (FPG) data for five consecutive years and with normal FPG (<100 mg/dL) at the initial health checkup. We identified the following ideal CVH metrics: non‐smoking, body mass index of <25 kg/m2, maintaining physical activity, taking breakfast, untreated blood pressure of <120/80 mmHg and untreated total cholesterol of <200 mg/dL. We defined the primary end‐point as prediabetes (FPG 100–125 mg/dL) or diabetes (FPG ≥126 mg/dL or use of antihyperglycemic medications). We examined the relationship of CVH metrics with the annual incidence of prediabetes or diabetes. Additionally, we examined the association of 1‐year changes in CVH metrics with the risk for prediabetes or diabetes.
Results
The median age was 44 years, and 65.6% were men. An increasing number of non‐ideal CVH metrics was associated with an elevated risk of prediabetes or diabetes. A non‐ideal body mass index was most strongly associated with the risk of prediabetes or diabetes. The risk of developing prediabetes or diabetes rose as the number of non‐ideal CVH metrics increased over 1 year.
Conclusions
CVH metrics could stratify the risk of the annual development of prediabetes or diabetes. The risk of developing prediabetes or diabetes might be reduced by improving CVH metrics.
Keywords: Cardiovascular health metrics, Diabetes, Prediabetes
CVH metrics can be used to stratify the risk of the annual development of prediabetes or diabetes. Improving CVH metrics may reduce the risk of prediabetes or diabetes.

INTRODUCION
Diabetes and prediabetes are common metabolic disorders that are associated with adverse clinical outcomes. The prevalence of prediabetes or diabetes is 34.5 and 13.0% among adults aged ≥18 years in the USA (National Diabetes Statistics Report 2020. Estimates of diabetes and its burden in the United States [cdc.gov]). Diabetes is the leading etiology for renal failure and blindness. Furthermore, diabetes is also associated with a greater risk of cardiovascular disease (CVD), non‐alcoholic fatty liver disease and cancer 1 , 2 , 3 , 4 , 5 , 6 . Prediabetes is also associated with a higher risk of CVD and cancer 5 , 6 . Given the high prevalence and poor prognosis of complications of prediabetes or diabetes, risk stratification and preventive strategies are required. Due to the fact that the majority of individuals with prediabetes or diabetes have no symptoms, hyperglycemia is frequently detected through screenings, such as health checkups. The latest statement from the U.S. Preventive Services Task Force suggests screening people (aged 35–70 years) with overweight or obesity for prediabetes and diabetes every 3 years 7 . However, there have been limited epidemiological data identifying the optimal screening intervals for prediabetes or diabetes. To validate the updated statement, additional data are necessary on the annual incidence and determinants of prediabetes or diabetes in persons with normal fasting plasma glucose (FPG). Comorbid modifiable risk factors; for example, unhealthy lifestyles and obesity, are linked with a greater risk of diabetes 8 , 9 . Cardiovascular health (CVH) metrics consisting of seven modifiable risk and lifestyle factors, such as smoking, body mass index (BMI), physical activity, eating habits, blood pressure, FPG level and total cholesterol level, could be a concise risk stratification approach for predicting future CVD risk. Furthermore, a previous study including Native Americans without diabetes presented a potential link between CVH metrics and incident diabetes 10 . However, the association of these modifiable risk factors with the risk of developing prediabetes is unclear. Furthermore, there are no available data regarding the association between CVH metrics and the annual occurrence of prediabetes or diabetes, for which it is necessary to determine the most appropriate risk stratification and screening interval. Furthermore, whether modifying CVH metrics could prevent the development of prediabetes or diabetes requires elucidation. Thus, the present study analyzed a nationwide epidemiological dataset including 403,857 individuals with FPG data available for five consecutive years and with normal FPG (<100 mg/dL) at the initial health checkup, and analyzed the link between CVH metrics and the annual incidence of prediabetes or diabetes. Additionally, we carried out sex‐ and age‐stratified subgroup analyses, and evaluated the association between 1‐year changes in CVH metrics over the initial year of follow up and future risk of prediabetes or diabetes development.
MATERIALS AND METHODS
Study design and data source
We used data from the JMDC Claims Database (JMDC; Tokyo, Japan) for the present observational cohort study between 2005 and 2021 11 , 12 , 13 . The JMDC has contracts with >60 insurers, and includes data on health checkup and insurance claim records of insured individuals. The JMDC Claims Database is a collection of the health checkup records including data on blood examinations (e.g., FPG), current medications and the individuals' responses to a standard questionnaire administered during such periodic health checkups 14 . We extracted FPG data from 416,355 individuals with available FPG checkup data for five consecutive years who were normoglycemic (<100 mg/dL) and without any missing data on CVH metrics components at the initial checkup. To obtain information, we set a lookback period of 4 months, and necessitated the population be covered by insurance for at least 4 months before the first checkup. After excluding individuals with prescriptions for diabetes within 3 months before (n = 1,101) and after (n = 59) the baseline checkup, those with a history of CVD (n = 11,233), and those undergoing renal replacement therapy (n = 105), 403,857 participants were included in the analysis in the present study (Figure S1).
Checkups carried out 1, 2, 3, 4 and 5 years after the initial checkup were defined as the oldest checkups among those carried out between 1, 2, 3, 4 and 5 years ± a half year after the date of the initial checkup, respectively.
Primary end‐point
The primary end‐point was defined as prediabetes or diabetes. We defined prediabetes as an FPG 100–125 mg/dL and diabetes as an FPG ≥126 mg/dL or the use of antidiabetic medications. Antidiabetic drugs were defined as drugs with World Health Organization Anatomical Therapeutic Chemical Classification System codes starting with “A10”.
Individuals meeting the definition of diabetes were also included in the prediabetes category. Diagnoses of prediabetes or diabetes were fixed. For instance, if an individual was diagnosed with prediabetes 1 year after the initial health checkup, this diagnosis was utilized at 2, 3, 4 and 5 years after the original health checkup, regardless of the follow‐up FPG levels.
Ethics
We carried out the present study in compliance with the ethical guidelines of the University of Tokyo (approval number: 2018–10,862) and the Declaration of Helsinki's principles. Informed consent was not required for this study, because all data in the JMDC Claims Database were anonymized. The International Conference on Harmonization guidelines were followed by all data 15 .
CVH metrics
The original American Heart Association definitions of CVH metrics were modified to fit the JMDC Claims Database according to our previous studies 12 , 16 . The original and modified definitions used in the present study of CVH metrics are shown in Table S1. The ideal CVH metrics were as follows: BMI of <25 kg/m2; non‐smoking; 30 min of physical activity ≥twice weekly or ≥1 h of walking per day; eating habits: skipping breakfast <3 times per week; systolic untreated blood pressure/diastolic blood pressure of <120/80 mmHg; and total cholesterol of <200 mg/dL. Using measurements of low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol and triglycerides, the untreated total cholesterol levels were calculated.
Statistical procedure
Descriptive analysis
We presented continuous and categorical data as medians (interquartile range) and numbers (percentage), respectively. Using one‐way analysis of variance and χ2‐tests, we compared continuous and categorical data between the seven groups based on the number of non‐ideal CVH metrics (0–6).
Main analysis
We calculated the odds ratios (ORs) of the number of non‐ideal CVH metrics for developing prediabetes or diabetes at 1, 2, 3, 4, and 5 years following the initial health check‐up, adjusting for age, sex, and FPG at the initial health check‐up. In the multivariable logistic regression analysis, we included the six CVH metric components in addition to age, sex and FPG at the initial health check‐up to evaluate the associations between each CVH metric component and the risk of prediabetes or diabetes. We also calculated the number needed to screen for diabetes or prediabetes, as previously described 17 .
Subgroup analysis
We carried out subgroup analyses stratified by sex and age (<45 vs ≥45 years). This cut‐off was derived from the American Diabetes Association statement for screening for diabetes 7 .
Secondary analysis
After excluding participants who developed prediabetes or diabetes (n = 40,303) or with missing data on CVH metrics 1 year after the baseline year (n = 9,537) from the original cohort (n = 403,857), we analyzed 354,017 participants who maintained FPG <100 mg/dL and with data for the repeated assessment of CVH metrics 1 year after the initial health checkup to evaluate the relationship between the change in CVH metrics and the risk of subsequently developing diabetes or prediabetes.
Additional analysis
Considering that the cut‐off age for screening might be controversial 17 , we assessed the effect of age on the association between CVH metrics and the risk of prediabetes or diabetes, and applied a non‐linear model using a restricted cubic spline with 3 knots (10th, 50th and 90th percentiles) and an age of 45 years set as the reference, adjusting for sex, FPG at the initial health check‐up and CVH metrics. To confirm the BMI cut‐off, we also applied a non‐linear model for BMI using a BMI of 22 kg/m2 as the reference, adjusting for age, sex, FPG at the initial health checkup and CVH metrics other than BMI.
Sensitivity analysis
Considering that glycated hemoglobin (HbA1c) is a stable assay to assess the presence of diabetes or prediabetes, and the diagnostic criteria for diabetes include FPG ≥126 mg/dL and HbA1c ≥6.5%, among those whose HbA1c values were available for five consecutive years (n = 301,676) out of the included population, we analyzed the development of prediabetes or diabetes after exclusion of those with HbA1c ≥5.7% at the baseline checkup (n = 44,900) using the following definitions: prediabetes as FPG ≥100 mg/dL and HbA1c ≥5.7%, and diabetes as FPG ≥126 mg/dL and HbA1c ≥6.5%.
P‐values of <0.05 were considered statistically significant. We carried out all statistical analyses using Stata (version 17; StataCorp LLC, College Station, TX, USA).
RESULTS
Characteristics of the study population
Baseline clinical characteristics of the study participants are shown in Table 1. The median age was 44 years (interquartile range 40–50 years), and male participants accounted for 265,113 (65.6%). The median FPG level was 89 mg/dL (interquartile range 84–94 mg/dL) and the median number of non‐ideal CVH metrics was two (interquartile range 1–3). As the number of non‐ideal CVH measurements increased, the proportion of men and FPG values rose.
Table 1.
Characteristics of study population
| No. non‐ideal CVH components | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 (n = 26,952) | 1 (n = 92,490) | 2 (n = 121,014) | 3 (n = 95,182) | 4 (n = 49,993) | 5 (n = 15,814) | 6 (n = 2,412) | P‐value | |
| Fasting plasma glucose (mg/dL) | 87 (83–92) | 88 (83–92) | 89 (84–93) | 90 (85–94) | 91 (86–95) | 91 (86–95) | 91 (87–95) | <0.001 |
| Non‐ideal body mass index | 0 (0.0) | 2,113 (2.3) | 11,771 (9.7) | 25,826 (27.1) | 26,692 (53.4) | 11,653 (73.7) | 2,412 (100.0) | <0.001 |
| Non‐ideal smoking | 0 (0.0) | 6,475 (7.0) | 24,403 (20.2) | 35,448 (37.2) | 28,310 (56.6) | 13,050 (82.5) | 2,412 (100.0) | <0.001 |
| Non‐ideal physical activity | 0 (0.0) | 41,351 (44.7) | 73,972 (61.1) | 71,066 (74.7) | 42,611 (85.2) | 14,636 (92.6) | 2,412 (100.0) | <0.001 |
| Non‐ideal eating habit | 0 (0.0) | 3,197 (3.5) | 13,915 (11.5) | 22,261 (23.4) | 19,636 (39.3) | 10,175 (64.3) | 2,412 (100.0) | <0.001 |
| Non‐ideal blood pressure | 0 (0.0) | 14,150 (15.3) | 51,167 (42.3) | 64,006 (67.2) | 41,173 (82.4) | 14,774 (93.4) | 2,412 (100.0) | <0.001 |
| Non‐ideal total cholesterol | 0 (0.0) | 25,204 (27.3) | 66,800 (55.2) | 66,939 (70.3) | 41,550 (83.1) | 14,782 (93.5) | 2,412 (100.0) | <0.001 |
| Age (years) | 42 (37–47) | 43 (38–48) | 44 (40–50) | 45 (40–51) | 44 (40–50) | 44 (40–49) | 42 (39–48) | <0.001 |
| Men, n (%) | 12,016 (44.6) | 45,309 (49.0) | 75,668 (62.5) | 72,322 (76.0) | 42,860 (85.7) | 14,648 (92.6) | 2,290 (94.9) | <0.001 |
| Body mass index (kg/m2) | 20.6 (19.2–22.1) | 20.9 (19.4–22.6) | 21.8 (20.1–23.5) | 23 (21.1–25.2) | 25.1 (22.4–27) | 26.1 (24.7–28) | 27.1 (25.9–29.1) | <0.001 |
| Systolic blood pressure (mmHg) | 106 (100–112) | 109 (102–116) | 116 (106–125) | 122 (113–130) | 126 (120–134) | 128 (122–136) | 129 (124–137) | <0.001 |
| Diastolic blood pressure (mmHg) | 65 (60–70) | 67 (61–73) | 72 (65–79) | 77 (70–83) | 80 (73–86) | 82 (76–88) | 82 (77.5–88) | <0.001 |
| Total cholesterol (mg/dL) | 177.6 (163.6–189) | 186.4 (169.8–202.2) | 202.6 (181–224.6) | 212 (191.6–233.2) | 219 (203–239.6) | 224.8 (209–245.6) | 231.1 (214.4–253.6) | <0.001 |
Data are presented as the median (interquartile range) for continuous variables or numbers (percentages) for categorical variables. Continuous and categorical variables were compared using one‐way analysis of variance and χ2 between seven groups according to the number of non‐ideal cardiovascular health (CVH) metrics components.
CVH metrics and the annual incidence of prediabetes or diabetes
Figure 1 shows the annual incidence of prediabetes or diabetes for 5 years after the initial health checkup according to the number of non‐ideal CVH metrics. Overall, the incidence of prediabetes and diabetes increased from 10.0% and 0.2% at 1 year after the initial checkup to 32.9% and 1.1%, respectively, 5 years after the initial health check‐up. Accordingly, 10 and 651 participants were required to screen for prediabetes and diabetes at 1 year after the initial checkup, whereas three and 95 participants, respectively, were required at 5 years after the initial health checkup. At 1–5 years after the initial checkup, the incidence of prediabetes or diabetes increased with an increasing number of non‐ideal CVH metrics. The number of non‐ideal CVH metrics was significantly associated with the risk for prediabetes or diabetes development at all time points after the initial health checkups in multivariable logistic regression analyses. The odds ratios per 1‐point increase in the number of non‐ideal CVH metrics for prediabetes or diabetes at 5 years after the initial health checkup were 1.21 (95% confidence interval [CI] 1.20–1.21) and 1.61 (95% CI 1.57–1.65), respectively (Table 2).
Figure 1.
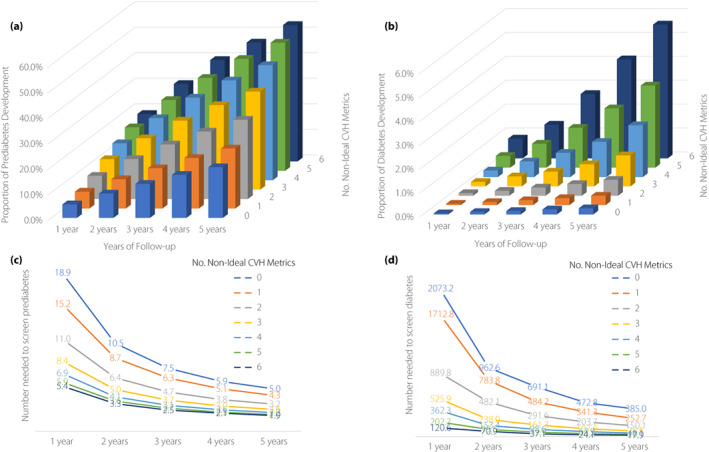
Cardiovascular health metrics and incidence of prediabetes or diabetes. (a, b) Proportions for (a) incident prediabetes or (b) diabetes. (c, d) Number required to screen (c) incident prediabetes or (d) diabetes.
Table 2.
Association between the number of non‐ideal cardiovascular health metrics and the risk of prediabetes or diabetes
| Years of follow up | 1 Year | 2 Years | 3 Years | 4 Years | 5 Years | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Prediabetes | Diabetes | Prediabetes | Diabetes | Prediabetes | Diabetes | Prediabetes | Diabetes | Prediabetes | Diabetes | |
| No. events | 40,303 | 620 | 68,549 | 1,316 | 92,683 | 2,071 | 113,956 | 3,036 | 133,022 | 4,245 | |
| No. non‐Ideal CVH metrics | 0 | Reference | Reference | Reference | Reference | Reference | |||||
| 1 | 1.10 | 1.13 | 1.07 | 1.15 | 1.07 | 1.34 | 1.07 | 1.30 | 1.11 | 1.43 | |
| [1.03, 1.17] | [0.62, 2.08] | [1.02, 1.12] | [0.76, 1.74] | [1.03, 1.12] | [0.95, 1.89] | [1.03, 1.12] | [0.98, 1.73] | [1.07, 1.15] | [1.11, 1.85] | ||
| 2 | 1.26 | 1.94 | 1.25 | 1.66 | 1.28 | 1.97 | 1.30 | 1.94 | 1.35 | 2.15 | |
| [1.18, 1.33] | [1.10, 3.43] | [1.20, 1.31] | [1.12, 2.46] | [1.23, 1.33] | [1.42, 2.74] | [1.25, 1.35] | [1.48, 2.55] | [1.30, 1.40] | [1.68, 2.75] | ||
| 3 | 1.47 | 2.99 | 1.47 | 3.06 | 1.52 | 3.24 | 1.55 | 3.34 | 1.63 | 3.84 | |
| [1.39, 1.56] | [1.70, 5.28] | [1.41, 1.55] | [2.08, 4.51] | [1.45, 1.58] | [2.34, 4.49] | [1.50, 1.62] | [2.55, 4.38] | [1.57, 1.69] | [3.01, 4.89] | ||
| 4 | 1.70 | 4.11 | 1.75 | 4.48 | 1.82 | 4.99 | 1.88 | 4.98 | 2.00 | 6.04 | |
| [1.59, 1.80] | [2.32, 7.30] | [1.66, 1.84] | [3.04, 6.62] | [1.74, 1.90] | [3.59, 6.94] | [1.80, 1.96] | [3.79, 6.54] | [1.93, 2.08] | [4.74, 7.72] | ||
| 5 | 2.00 | 7.15 | 2.03 | 6.71 | 2.13 | 7.97 | 2.24 | 8.18 | 2.41 | 9.35 | |
| [1.86, 2.15] | [3.95, 12.96] | [1.92, 2.16] | [4.47, 10.08] | [2.02, 2.24] | [5.67, 11.20] | [2.13, 2.35] | [6.17, 10.84] | [2.30, 2.53] | [7.26, 12.03] | ||
| 6 | 2.18 | 11.93 | 2.28 | 9.28 | 2.42 | 12.69 | 2.45 | 13.55 | 2.71 | 15.16 | |
| [1.93, 2.47] | [5.89, 24.15] | [2.05, 2.53] | [5.59, 15.39] | [2.20, 2.68] | [8.48, 18.98] | [2.22, 2.69] | [9.73, 18.87] | [2.46, 2.98] | [11.29, 20.34] | ||
| Odds ratio for 1‐point increase in the number of non‐ideal CVH metrics | |||||||||||
| 1.16 | 1.54 | 1.17 | 1.55 | 1.18 | 1.56 | 1.19 | 1.58 | 1.21 | 1.61 | ||
| [1.15, 1.17] | [1.44, 1.64] | [1.16, 1.18] | [1.48, 1.62] | [1.17, 1.19] | [1.51, 1.62] | [1.18, 1.20] | [1.54, 1.63] | [1.20, 1.21] | [1.57, 1.65] | ||
Multivariable logistic regression analysis included the number of ideal cardiovascular health (CVH) metrics, age, sex and fasting plasma glucose at the initial health check‐up. OR, odds ratio.
Individual CVH metric components and the annual incidence of prediabetes or diabetes
The relationships of each CVH metric with the risk of the development of prediabetes or diabetes are shown in Table S2. Non‐ideal status of BMI, smoking and blood pressure were related to increased risks of prediabetes or diabetes at all time points; however, it was not clear whether non‐ideal physical activity was associated with a higher incidence of prediabetes or diabetes at all time points after the initial health checkup. Non‐ideal total cholesterol and non‐ideal eating habits were associated with a higher incidence of prediabetes at all time points and diabetes at all time points except 1 year after the initial health checkup (Table S2).
Subgroup analyses
We stratified the study participants by sex (265,113 men and 138,744 women). The association between the number of non‐ideal CVH metrics and the increasing incidence of prediabetes or diabetes was observed in both sexes. The number of non‐ideal CVH metrics was associated with the increasing incidence of prediabetes or diabetes in both men and women (Figure S2). Logistic regression analyses showed that a higher number of non‐ideal CVH metrics was related to a higher incidence of prediabetes or diabetes in both men and women (Table S3). Non‐ideal BMI, non‐ideal smoking and non‐ideal blood pressure were associated with increased risks of prediabetes or diabetes in both men and women; however, the coefficients of non‐ideal BMI for prediabetes or diabetes development were augmented in women (Table S4). The study population was also stratified by age (213,691 individuals aged <45 years and 190,166 individuals aged ≥45 years). The risk of developing prediabetes or diabetes was linked with the number of non‐ideal CVH metrics in both age groups (Figure S3). In logistic regression analyses, an increasing number of non‐ideal CVH metrics was linked with an increasing risk of prediabetes or diabetes development in both age groups (Table S5). Non‐ideal BMI, non‐ideal smoking and non‐ideal blood pressure were associated with increased risks of prediabetes or diabetes in both age groups. The coefficients of BMI for the risk of diabetes development were augmented in participants aged <45 years as compared with those aged ≥45 years (Table S6).
Changes in the number of non‐ideal CVH metric components and the risk for prediabetes or diabetes
We analyzed 354,017 participants with FPG <100 mg/dL and available data for the repeated assessment of CVH metrics at 1 year after the initial health checkup. After adjusting for covariates and baseline CVH metrics, a 1‐point increase (at 1 year) in the number of non‐ideal CVH metric components was related to an increasing risk for developing prediabetes or diabetes annually 2–5 years after the initial health checkup (Table 3). The odds ratios of a 1‐point increase (at 1 year) in the number of non‐ideal CVH metric components for prediabetes and diabetes at 5 years after the initial health checkup were 1.15 (95% CI 1.14–1.16) and 1.38 (95% CI 1.31–1.45), respectively. This relationship was consistent across subgroups stratified by sex or age.
Table 3.
Baseline risks for cardiovascular health metrics and the association between the change in the number of non‐ideal cardiovascular health metrics components over 1 year, and the subsequent risk of transition to prediabetes or diabetes
| Population | Years of follow up | 2 Years | 3 Years | 4 Years | 5 Years | ||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Prediabetes | Diabetes | Prediabetes | Diabetes | Prediabetes | Diabetes | Prediabetes | Diabetes | |
| Whole | 1‐point increase of baseline CVH metrics | 1.19 | 1.52 | 1.21 | 1.59 | 1.22 | 1.67 | 1.24 | 1.77 |
| [1.17, 1.20] | [1.40, 1.64] | [1.20, 1.22] | [1.50, 1.69] | [1.21, 1.23] | [1.59, 1.75] | [1.23, 1.25] | [1.70, 1.83] | ||
| 1‐point increase of 1‐year CVH metrics change | 1.13 | 1.14 | 1.14 | 1.22 | 1.14 | 1.32 | 1.15 | 1.38 | |
| [1.11, 1.15] | [1.02, 1.27] | [1.13, 1.15] | [1.12, 1.32] | [1.13, 1.15] | [1.23, 1.40] | [1.14, 1.16] | [1.31, 1.45] | ||
| Female | 1‐point increase of baseline CVH metrics | 1.24 | 1.38 | 1.26 | 1.56 | 1.27 | 1.51 | 1.29 | 1.73 |
| [1.21, 1.28] | [1.13, 1.67] | [1.24, 1.29] | [1.36, 1.80] | [1.25, 1.30] | [1.35, 1.70] | [1.27, 1.32] | [1.57, 1.90] | ||
| 1‐point increase of 1‐year CVH metrics change | 1.17 | 1.07 | 1.17 | 1.19 | 1.17 | 1.28 | 1.18 | 1.26 | |
| [1.13, 1.21] | [0.83, 1.38] | [1.14, 1.20] | [0.98, 1.44] | [1.14, 1.20] | [1.09, 1.49] | [1.15, 1.20] | [1.11, 1.44] | ||
| Male | 1‐point increase of baseline CVH metrics | 1.17 | 1.56 | 1.19 | 1.59 | 1.20 | 1.70 | 1.22 | 1.77 |
| [1.16, 1.18] | [1.43, 1.70] | [1.18, 1.20] | [1.49, 1.70] | [1.19, 1.21] | [1.61, 1.78] | [1.21, 1.23] | [1.70, 1.85] | ||
| 1‐point increase of 1‐year CVH metrics change | 1.12 | 1.16 | 1.13 | 1.22 | 1.13 | 1.32 | 1.14 | 1.40 | |
| [1.10, 1.14] | [1.02, 1.31] | [1.12, 1.15] | [1.12, 1.34] | [1.12, 1.15] | [1.23, 1.42] | [1.13, 1.16] | [1.32, 1.48] | ||
| Aged <45 years | 1‐point increase of baseline CVH metrics | 1.20 | 1.56 | 1.22 | 1.63 | 1.23 | 1.75 | 1.26 | 1.87 |
| [1.18, 1.22] | [1.39, 1.74] | [1.20, 1.24] | [1.51, 1.77] | [1.22, 1.24] | [1.65, 1.87] | [1.24, 1.27] | [1.77, 1.96] | ||
| 1‐point increase of 1‐year CVH metrics change | 1.13 | 1.1 | 1.14 | 1.17 | 1.15 | 1.31 | 1.17 | 1.39 | |
| [1.10, 1.15] | [0.95, 1.29] | [1.12, 1.16] | [1.05, 1.30] | [1.13, 1.17] | [1.20, 1.43] | [1.15, 1.18] | [1.30, 1.49] | ||
| Aged ≥45 years | 1‐point increase of baseline CVH metrics | 1.17 | 1.53 | 1.19 | 1.56 | 1.20 | 1.59 | 1.22 | 1.67 |
| [1.15, 1.19] | [1.37, 1.72] | [1.17, 1.20] | [1.43, 1.70] | [1.19, 1.21] | [1.48, 1.70] | [1.20, 1.23] | [1.58, 1.76] | ||
| 1‐point increase of 1‐year CVH metrics change | 1.13 | 1.19 | 1.13 | 1.29 | 1.13 | 1.32 | 1.13 | 1.36 | |
| [1.11, 1.15] | [1.02, 1.40] | [1.11, 1.15] | [1.14, 1.46] | [1.11, 1.15] | [1.20, 1.46] | [1.12, 1.15] | [1.26, 1.47] | ||
Odds ratios of 1‐point increase (at 1 year) in the number of non‐ideal cardiovascular health (CVH) metrics components were adjusted for age, sex, fasting plasma glucose at the initial health checkup and the number of non‐ideal CVH metrics components at the initial health checkup. We calculated odds ratios of 1‐point increase (at 1 year) in the number of non‐ideal CVH metrics components in the sex‐stratified analysis, adjusted similarly except for sex.
Age modification effect on the associations between CVH metrics and the development of prediabetes or diabetes with age as a non‐linear continuous variable
When we treated age as a non‐linear continuous variable and used a cubic spline model with 45 years of age as the reference, the relationship between age and prediabetes development was nearly linear, whereas that between age and diabetes development was not (Figure S4). Age >45 years was related to a steep increase in the risk of diabetes development.
Modification effect of BMI on the associations between other CVH metrics and the development of prediabetes or diabetes
When we treated BMI as a non‐linear continuous variable and used a cubic spline model with 22 kg/m2 as the reference, the relationship between BMI and prediabetes development was almost linear, whereas that between BMI and diabetes development was not (Figure S5). Regarding the association between BMI and diabetes development, for BMI >23 kg/m2, an increase in BMI affected the risk of diabetes development at an accelerated rate.
Sensitivity analysis
Analysis of 256,776 people having FPG <100 mg/dL and HbA1c 5.7% at the initial‐year checkup showed similar results. The number of non‐ideal CVH metrics was positively linked with a higher risk of developing prediabetes or diabetes, as in the primary analysis (Figure S6). This result remained consistent, even after adjusting for covariates (Table S7). Among the components, a non‐ideal status of BMI was most strongly associated with the risk for prediabetes and diabetes (Table S8).
DISCUSSION
Using a nationwide administrative claims database, we found that the number of non‐ideal CVH metrics was correlated with the risk of the subsequent development of prediabetes or diabetes among individuals with FPG <100 mg/dL at baseline. Among participants with all non‐ideal CVH metrics, >30% and >50% developed prediabetes in 3 and 5 years, respectively. The present study is the first to describe the natural history of FPG trends in a middle‐aged population, as well as the importance of CVH metrics in the development of prediabetes or diabetes.
We reported the annual incidence of prediabetes and diabetes among individuals with blood glucose levels within the normal range at baseline. Although previous epidemiological studies have reported the incidence of diabetes 18 , 19 , data on the annual incidence of prediabetes are scarce. As diabetes is one of the most essential healthcare issues with adverse clinical outcomes and increasing prevalence worldwide 20 , 21 , we also pay attention to prediabetes, which is the most striking predisposing factor for the development of diabetes. Furthermore, prediabetes itself increases the risk of various subsequent complications 5 , 22 . Therefore, it is important to take appropriate preventive measures for both prediabetes and diabetes, based on concrete risk stratification. For this purpose, determining the detailed incidence of prediabetes and diabetes, and identifying the risk factors for both conditions are important. Furthermore, to our knowledge, there are no data on the relationship between modifiable risk factors and the risk of the annual development of prediabetes or diabetes.
The present results showed that BMI had the greatest impact on the development of prediabetes and diabetes among the components of CVH metrics, particularly diabetes. This finding was supported by our additional analysis showing an association between BMI and prediabetes or diabetes (Figure S5). Although the association between BMI and prediabetes was nearly linear, that between BMI and diabetes increased exponentially with increased BMI. These findings were consistent with those of previous studies that showed the contribution of BMI to subsequent hyperglycemic development 23 , 24 . The exponential risk augmentation in diabetes risk along with BMI increase was also reported in other previous studies 25 , 26 . Interestingly, this exponential association between BMI and risk of diabetes was found to be independent of genetic or environmental factors, as shown using Mendelian randomization 26 . A possible background behind this fact was suggested by the fact that those with prediabetes were more likely to develop diabetes when they had higher BMI and experienced further weight gain, and that this association was augmented among people with higher BMI 27 . Another interesting finding in the present study is that the risk of prediabetes increased almost linearly, whereas that of diabetes increased non‐linearly along with age increase from normal glycemic ranges. An association of age with prediabetes and diabetes risk was reported in a previous article 28 showing that the risk of diabetes increased more steeply than that of prediabetes with the age advance.
The optimal interval for carrying out checkups remains controversial 7 . As approximately 25–30% of participants with four or more non‐ideal CVH metrics developed prediabetes, FPG in this population should be carefully observed at an interval of at least 2–3 years, if not annually. Because 2.7 and 5.6% of individuals with non‐ideal CVH metrics developed diabetes at 3 years and 5 years, respectively, biennial or triennial checkups might be sufficient to detect diabetes in this population. However, as hyperglycemia below the diabetes level is reportedly associated with an increased risk for the development of diabetes, cardiovascular disease, proteinuria and cancer 5 , 6 , the community should take measures against such “hyperglycemic sequelae”.
The present study had several strengths. First, we described the annual changes in FPG among those with FPG <100 mg/dL; that is, individuals without prediabetes or diabetes at baseline. This was enabled by the requirement for annual checkups by the Japanese government for companies. Furthermore, we confirmed the robustness of our results by defining prediabetes and diabetes using both FPG and HbA1c levels. Second, we revisited, among a normoglycemic population, the importance of CVH metrics previously shown to be associated with the development of atherosclerotic cardiovascular events 12 . Most CVH metrics were modifiable factors; therefore, the present findings might influence policy‐making in public health. We showed that changes in CVH metrics might improve the risk of developing hyperglycemia.
Despite the novelty of the present study, it had several limitations. First, our findings might not be generalizable to other populations. East Asian populations develop diabetes at a relatively low BMI compared with Western populations 29 . However, as the risk factors for hyperglycemia are similar between these populations 30 , the present results could be useful in other countries. Second, although we adjusted for common confounders, such as age, sex and modifiable physical or lifestyle factors, we could not adjust for social factors, such as socioeconomic status, family history of diabetes or other metabolic diseases, which might have affected our results. Further studies are required to consider such social, lifestyle and genetic factors. Dietary components (e.g., Dietary Approaches to Stop Hypertension diet) are used to determine eating behaviors in the original formulation of CVH metrics. Unfortunately, our dataset did not provide any information on dietary components. Because skipping breakfast is known to increase the risk of lifestyle‐related disease or cardiovascular disease, we determined that skipping breakfast was a non‐ideal eating habit in the present study. We obtained the information on lifestyles from the self‐reported questionnaires at health checkups. As the present results were partly derived from a subjective questionnaire, we have to interpret the results with caution. Finally, we failed to show the cost‐effectiveness of health checkups, which is also important in preparing for decision‐making in public health policy.
In conclusion, a higher number of non‐ideal CVH metrics was related to a higher annual incidence of prediabetes or diabetes in normoglycemic adults aged 18–71 years. Obesity, high blood pressure and cigarette smoking were associated with a higher annual incidence of prediabetes or diabetes at almost all time points. These results confirmed the important role of modifiable risk factors in the pathogenesis of prediabetes or diabetes, highlighting the potential of CVH metrics as indicators for the optimal screening of prediabetes or diabetes and implementing preventive measures. Furthermore, a 1‐year change in CVH metrics was related to a change in the risk for developing prediabetes or diabetes, suggesting the potential benefit of lifestyle interventions for preventing prediabetes or diabetes. The present results can be used to motivate people to optimize their lifestyle and risk factors to prevent the development of prediabetes or diabetes.
DISCLOSURES
Akira Okada and Satoko Yamaguchi are members of the Department of Prevention of Diabetes and Lifestyle‐related Diseases, which is a cooperative program between The University of Tokyo and Asahi Mutual Life Insurance Company. Research funding and scholarship funds (Hidehiro Kaneko and Katsuhito Fujiu) from Medtronic Japan Co., Ltd, Abbott Medical Japan Co., Ltd, Boston Scientific Japan Co., Ltd, and Fukuda Denshi, Central Tokyo Co., Ltd. The other authors declare no conflict of interest.
Approval of the research protocol: This study was carried out in accordance with the ethical guidelines of the University of Tokyo (approval by the Ethical Committee of the University of Tokyo: 2018–10,862) and the principles of the Declaration of Helsinki.
Informed consent: As all data in the JMDC Claims Database were de‐identified, the requirement for informed consent was waived.
Registry and the registration no. of the study/trial: N/A.
Animal study: N/A.
Supporting information
Figure S1 | Flowchart.
Figure S2 | Cardiovascular health metrics and incidence of prediabetes or diabetes stratified by sex.
Figure S3 | Cardiovascular health metrics and incidence of prediabetes or diabetes stratified by age category.
Figure S4 | Association between age and development of prediabetes or diabetes in 5 years.
Figure S5 | Association between body mass index and development of prediabetes or diabetes in 5 years.
Figure S6 | Cardiovascular health metrics and incidence of prediabetes or diabetes when using both fasting plasma glucose and glycated hemoglobin levels.
Table S1 | Definitions of ideal cardiovascular health metrics.
Table S2 | Odds ratio of each component of cardiovascular health metrics for the risk of transition to prediabetes or diabetes.
Table S3 | Odds ratio per 1‐point increase in the number of non‐ideal cardiovascular health metrics.
Table S4 | Odds ratio of each component of cardiovascular health metrics for the risk of transition to prediabetes or diabetes stratified by sex.
Table S5 | Odds ratio per 1‐point increase in the number of non‐ideal cardiovascular health metrics stratified by age category.
Table S6 | Odds ratio of each component of cardiovascular health metrics for the risk of transition to prediabetes or diabetes stratified by age category.
Table S7 | Association between the number of non‐ideal cardiovascular health metrics and the risk of prediabetes or diabetes when using both fasting plasma glucose and glycated hemoglobin levels as the definition of prediabetes or diabetes.
Table S8 | Odds ratio of each component of cardiovascular health metrics for the risk of transition to prediabetes or diabetes when using both fasting plasma glucose and glycated hemoglobin levels as the definition of prediabetes or diabetes.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Health, Labor and Welfare, Japan (21AA2007), and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907, 21H03159 and 21 K08123). The funding sources had no role in the design, analysis, interpretation, manuscript preparation, or decision to publish.
REFERENCES
- 1. Emerging Risk Factors C, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta‐analysis of 102 prospective studies. Lancet 2010; 375: 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Almdal T, Scharling H, Jensen JS, et al. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: A population‐based study of 13,000 men and women with 20 years of follow‐up. Arch Intern Med 2004; 164: 1422–1426. [DOI] [PubMed] [Google Scholar]
- 3. Lee YH, Cho Y, Lee BW, et al. Nonalcoholic fatty liver disease in diabetes. Part I: Epidemiology and diagnosis. Diabetes Metab J 2019; 43: 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: A consensus report. Diabetes Care 2010; 33: 1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaneko H, Itoh H, Kiriyama H, et al. Fasting plasma glucose and subsequent cardiovascular disease among young adults: Analysis of a nationwide epidemiological database. Atherosclerosis 2021; 319: 35–41. [DOI] [PubMed] [Google Scholar]
- 6. Itoh H, Kaneko H, Okada A, et al. Fasting plasma glucose and incident colorectal cancer: Analysis of a Nationwide epidemiological database fasting plasma glucose and colorectal cancer. J Clin Endocrinol Metab 2021; 106: e4448–e4458. [DOI] [PubMed] [Google Scholar]
- 7. Force USPST , Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US preventive services task Force recommendation statement. JAMA 2021; 326: 736–743. [DOI] [PubMed] [Google Scholar]
- 8. Mozaffarian D, Kamineni A, Carnethon M, et al. Lifestyle risk factors and new‐onset diabetes mellitus in older adults: The cardiovascular health study. Arch Intern Med 2009; 169: 798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001; 345: 790–797. [DOI] [PubMed] [Google Scholar]
- 10. Fretts AM, Howard BV, McKnight B, et al. Life's simple 7 and incidence of diabetes among American Indians: The strong heart family study. Diabetes Care 2014; 37: 2240–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaneko H, Yano Y, Itoh H, et al. Association of blood pressure classification using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with risk of heart failure and atrial fibrillation. Circulation 2021; 143: 2244–2253. [DOI] [PubMed] [Google Scholar]
- 12. Kaneko H, Itoh H, Kamon T, et al. Association of cardiovascular health metrics with subsequent cardiovascular disease in young adults. J Am Coll Cardiol 2020; 76: 2414–2416. [DOI] [PubMed] [Google Scholar]
- 13. Ohbe H, Goto T, Miyamoto Y, et al. Risk of cardiovascular events after Spouse's ICU admission. Circulation 2020; 142: 1691–1693. [DOI] [PubMed] [Google Scholar]
- 14. Fukasawa T, Tanemura N, Kimura S, et al. Utility of a specific health checkup database containing lifestyle behaviors and lifestyle diseases for employee health Insurance in Japan. J Epidemiol 2020; 30: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dixon JR Jr. The international conference on harmonization good clinical practice guideline. Qual Assur 1998; 6: 65–74. [DOI] [PubMed] [Google Scholar]
- 16. Kaneko H, Itoh H, Yotsumoto H, et al. Cardiovascular health metrics of 87,160 couples: Analysis of a Nationwide epidemiological database. J Atheroscler Thromb 2021; 28: 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chung S, Azar KM, Baek M, et al. Reconsidering the age thresholds for type II diabetes screening in the U.S. Am J Prev Med 2014; 47: 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gonzalez EL, Johansson S, Wallander MA, et al. Trends in the prevalence and incidence of diabetes in the UK: 1996‐2005. J Epidemiol Community Health 2009; 63: 332–336. [DOI] [PubMed] [Google Scholar]
- 19. Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980‐2012. JAMA 2014; 312: 1218–1226. [DOI] [PubMed] [Google Scholar]
- 20. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res Clin Pract 2019; 157: 107843. [DOI] [PubMed] [Google Scholar]
- 21. Xu G, Liu B, Sun Y, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: Population based study. BMJ 2018; 362: k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Itoh H, Kaneko H, Okada A, et al. Fasting plasma glucose and incident colorectal cancer: Analysis of a Nationwide epidemiological database. J Clin Endocrinol Metab 2021; 106: e4448–e4458. [DOI] [PubMed] [Google Scholar]
- 23. Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity‐related health risk factors, 2001. JAMA 2003; 289: 76–79. [DOI] [PubMed] [Google Scholar]
- 24. Teufel F, Seiglie JA, Geldsetzer P, et al. Body‐mass index and diabetes risk in 57 low‐income and middle‐income countries: A cross‐sectional study of nationally representative, individual‐level data in 685 616 adults. Lancet 2021; 398: 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leong A, Rahme E, Dasgupta K. Spousal diabetes as a diabetes risk factor: A systematic review and meta‐analysis. BMC Med 2014; 12: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wainberg M, Mahajan A, Kundaje A, et al. Homogeneity in the association of body mass index with type 2 diabetes across the UK biobank: A Mendelian randomization study. PLoS Med 2019; 16: e1002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu H, Kawasaki Y, Kuwahara K, et al. Trajectories of body mass index and waist circumference before the onset of diabetes among people with prediabetes. Clin Nutr 2020; 39: 2881–2888. [DOI] [PubMed] [Google Scholar]
- 28. Broz J, Malinovska J, Nunes MA, et al. Prevalence of diabetes and prediabetes and its risk factors in adults aged 25‐64 in The Czech Republic: A cross‐sectional study. Diabetes Res Clin Pract 2020; 170: 108470. [DOI] [PubMed] [Google Scholar]
- 29. Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006; 368: 1681–1688. [DOI] [PubMed] [Google Scholar]
- 30. Bellou V, Belbasis L, Tzoulaki I, et al. Risk factors for type 2 diabetes mellitus: An exposure‐wide umbrella review of meta‐analyses. PLoS One 2018; 13: e0194127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Flowchart.
Figure S2 | Cardiovascular health metrics and incidence of prediabetes or diabetes stratified by sex.
Figure S3 | Cardiovascular health metrics and incidence of prediabetes or diabetes stratified by age category.
Figure S4 | Association between age and development of prediabetes or diabetes in 5 years.
Figure S5 | Association between body mass index and development of prediabetes or diabetes in 5 years.
Figure S6 | Cardiovascular health metrics and incidence of prediabetes or diabetes when using both fasting plasma glucose and glycated hemoglobin levels.
Table S1 | Definitions of ideal cardiovascular health metrics.
Table S2 | Odds ratio of each component of cardiovascular health metrics for the risk of transition to prediabetes or diabetes.
Table S3 | Odds ratio per 1‐point increase in the number of non‐ideal cardiovascular health metrics.
Table S4 | Odds ratio of each component of cardiovascular health metrics for the risk of transition to prediabetes or diabetes stratified by sex.
Table S5 | Odds ratio per 1‐point increase in the number of non‐ideal cardiovascular health metrics stratified by age category.
Table S6 | Odds ratio of each component of cardiovascular health metrics for the risk of transition to prediabetes or diabetes stratified by age category.
Table S7 | Association between the number of non‐ideal cardiovascular health metrics and the risk of prediabetes or diabetes when using both fasting plasma glucose and glycated hemoglobin levels as the definition of prediabetes or diabetes.
Table S8 | Odds ratio of each component of cardiovascular health metrics for the risk of transition to prediabetes or diabetes when using both fasting plasma glucose and glycated hemoglobin levels as the definition of prediabetes or diabetes.


