Abstract
Aims/Introduction
There has been an increase in research on diabetes‐related stigma and its association with glycated hemoglobin (HbA1c) over the past years. However, little is known about the association of self‐stigma with HbA1c in persons with type 1 diabetes. This study aims to examine the association between self‐stigma and HbA1c in Japanese people with type 1 diabetes.
Materials and Methods
This cross‐sectional study was conducted at a clinic in Tokyo. Questionnaires using nine items from the Japanese version of the Self‐Stigma Scale was distributed to outpatients with type 1 diabetes, aged ≥18 years. We excluded outpatients with serious mental disorder, those who required urgent medical treatment or received hemodialysis. Adjusted linear regression analyses tested the association between the score of the 9‐item Self‐Stigma Scale and HbA1c.
Results
Questionnaires were distributed to 166 eligible participants. A total of 109 participants were included in the final analysis after excluding participants with incomplete answers and laboratory data. After adjusting for age, sex, employment status, body mass index, duration of diabetes and insulin secretion, there was a significant positive association between self‐stigma and HbA1c (β = 0.05, 95% confidence interval 0.01 to 0.08).
Conclusions
This cross‐sectional study showed a significant association between self‐stigma and HbA1c in persons with type 1 diabetes. Addressing self‐stigma might be as equally essential as measuring HbA1c in evaluating glycemic outcome among individuals with type 1 diabetes.
Keywords: Glycated hemoglobin, Self‐stigma, Type 1 diabetes
A significant association was observed between self‐stigma and glycated hemoglobin in persons with type 1 diabetes.

INTRODUCTION
The term ‘stigma’ was first defined by Goffman in 1963, as ‘the situation of the individual who is disqualified from full social acceptance’. 1 In recent years, stigma is defined in three main concepts: experienced stigma, perceived stigma and self‐stigma. 1 Experienced stigma refers to the actual episodes of social discrimination and/or participation restrictions due to the lack of social acceptance toward their illness. 2 , 3 , 4 , 5 Perceived stigma is caused by the fear of experiencing stigma in public and a feeling of shame associated with the illness. 3 , 4 , 5
In contrast to experienced or perceived stigma, which is associated with negative reactions of the general public toward individuals, self‐stigma represents their own negative beliefs, emotional reactions and behaviors toward themselves as a result of their illness. Self‐stigma occurs when individuals internalize societal stereotypes, prejudicial attitude and discrimination associated with their illness by experiencing or perceiving stigma. 6 , 7 , 8
Stigma in people with diabetes has gained focus over the past years, and a certain number of scales have been developed to assess diabetes‐related stigma. 7 , 8 , 9 , 10 , 11 Using validated scales, there has been an increase in quantitative researches on diabetes‐related stigma among people with type 1 diabetes. 12 , 13 , 14
Previous studies have reported the association between experienced/perceived stigma with psychological and clinical outcomes in people with type 1 diabetes over the past few years. For example, two studies showed negative associations of experienced/perceived stigma with diabetes distress and depressive symptoms among persons with type 1 diabetes. 13 , 14 Notably, recent cross‐sectional studies have also found that experienced/perceived stigma negatively associates with glycemic outcome among persons with type 1 diabetes. 12 , 13 Although numerous studies regarding experienced/perceived stigma in type 1 diabetes have been reported, there are very few studies of self‐stigma in people with type 1 diabetes.
In 2014, the first self‐stigma scale in Japanese language, rigorously adapted and translated from the original Self‐Stigma Scale 8 was published by Kato et al. 7 The Japanese version of the Self‐Stigma Scale enabled further research into the association of self‐stigma with clinical, psychological and behavioral factors in Japanese persons with type 2 diabetes. Recent studies have shown that self‐stigma was associated with lower patient activation for self‐management in persons with type 2 diabetes. 15 , 16 This result suggested the importance of assessing and reducing self‐stigma in persons with diabetes to increase attainment of behavioral goals, so as to optimize treatment outcomes.
Thus, self‐stigma in people with diabetes is an essential issue to be assessed. However, to the best of our knowledge, self‐stigma in people with type 1 diabetes remains under‐researched. The aim of the present study was to investigate self‐stigma and its associations with glycated hemoglobin (HbA1c) in persons with type 1 diabetes in Japan.
MATERIALS AND METHODS
Study design
The present study was a single‐center, cross‐sectional study carried out at the Institute of Medical Science, Asahi Life Foundation, Tokyo, between August 2021 and November 2021.
Participants and procedure
Eligible participants were Japanese adults aged ≥18 years with doctor‐reported diagnosis of type 1 diabetes, treated with insulin injection.
The following were excluded: those with serious mental disorder, such as dementia, depression and schizophrenia, that would affect their cognition on stigmatization, those who required urgent medical treatment due to acute infection or malignant tumor and those who received hemodialysis.
The purpose of the study was explained by the doctor‐in‐charge at the clinical visit, and informed consent was obtained from those who agreed to the terms of this study during enrollment. Participants were able to choose either to answer the questionnaire alone during the clinic visit, or to answer at home and mail it back within a week from the visit. Participants who had not submitted the questionnaire within a month were reminded once by the doctor‐in‐charge at the next clinical visit.
Ethics approval
This research was approved by the Human Subjects Review Committee at the Institute for Medical Science (approval number 12605). Written informed consent was obtained from each participant.
Measurements
Self‐stigma
Self‐stigma was assessed using nine items from the Japanese version of the Self‐Stigma Scale. 7 The Japanese version of the Self‐Stigma Scale, which consists of 39 items, was translated and adapted from the original Self‐Stigma Scale created by Mak and Cheung. 8 For further clinical use of the Self‐Stigma Scale, Mak and Cheung reported the reliability and validity of the nine‐item Self‐Stigma Scale‐Short with retained adequate psychometric properties among concealable minority groups. 8 Later, the Self‐Stigma Scale‐Short was also validated among people with mental illness 17 , 18 and type 2 diabetes. 19 The strengths of the short version compared with the original scale are being feasible for persons with diabetes to complete within limited space or time at clinical visits, a higher acceptability of the questionnaire in larger population, better response rates and lower rates of missing data. 8 , 17 , 20 Also, as items in the Self‐Stigma Scale include questions that could cause psychological distress, the short form enables persons with diabetes to answer with less distress. Regarding the published evidence on the reliability and validity of the Self‐Stigma Scale‐Short and the strengths of the use of the short form, we chose nine items from the Japanese version of Self‐Stigma Scale as a preliminary questionnaire, according to the original Self‐Stigma Scale‐Short in three dimensions (Cognitive, Affective and Behavioral), each with three items (Table S1). Using 4‐point Likert scale of agreement, participants were asked to answer each statement with the following choices: 0 (strongly disagree), 1 (disagree), 2 (agree) and 3 (strongly disagree). The total score ranged from 0 to 27, where higher scores indicated greater self‐stigma.
Glycemic outcome
Average HbA1c value was calculated to evaluate glycemic outcome. HbA1c data were obtained from electronic health records. Average HbA1c was calculated by HbA1c values measured in the last three clinical visits within 6 months, starting from the day questionnaire was distributed.
Clinical characteristics
Self‐reported clinical characteristics, such as duration of diabetes, were collected from the survey. Data on body mass index (BMI) and serum C‐peptide immunoreactivity (CPR) were obtained from electronic health records.
Insulin secretion level was divided into two categories: fasting or random serum CPR <0.1 ng/mL as ‘depletion’ 21 , and fasting serum CPR ≥0.1 ng/mL as ‘non‐depletion’. The time of blood collection for the measurement of CPR levels was defined within the past 12 months. Individuals who lacked data of CPR in the past 12 month were categorized as ‘depletion’ if a random serum CPR <0.1 ng/mL was confirmed since the first visit. Participants were excluded if none of the CPR data since the first visit were <0.1 ng/mL and fasting CPR was not measured within a year.
Demographic characteristics
Data on employment status (full‐time/part‐time/unemployed or retired) were self‐reported and extracted from the survey.
Statistical analysis
Descriptive statistics
Data were summarized using counts and percentages for categorical or binary data, or mean and standard deviation for continuous data. Median and interquartile range were provided where continuous data were non‐normally distributed.
Statistical methods
HbA1c value was considered a dependent variable. First, univariate linear regression analysis was carried out to assess the relationship between dependent variable and each independent variable. After assessing independent variables that had a significant association with HbA1c, multivariate linear regression analyses were carried out to estimate the mean difference in HbA1c (%) for every 1‐point increase in the nine‐item Self‐Stigma Scale score. Covariates were selected based on the result of univariate analysis and literature reviews, and we adjusted the model for age, sex, BMI, duration of diabetes and employment status. 13 , 14 , 16 Serum CPR was also included as a covariate, in consideration of the significant association between insulin depletion and higher HbA1c in type 1 diabetes reported previously. 21 , 22 Only participants with complete data were included in the linear regression models. Variance inflation factor was calculated for each variable to evaluate multicollinearity of the model.
The regression results were interpreted in 95% confidence intervals of the effect estimates and P‐values. P < 0.05 were considered statistically significant. All the statistical analyses were carried out using JMP 16 (SAS Institute, Cary, NC, USA).
RESULTS
The process for selecting participants for inclusion is shown in Figure 1. Questionnaires were distributed to 166 outpatients with doctor‐reported diagnosis of type 1 diabetes. Of these participants, 140 participants responded, making the collection rate 84.3%. Three participants had missing answers in the nine‐item Self‐Stigma Scale, and 30 participants lacked data on insulin secretion. One participant had a falsely low value of HbA1c due to anemia and was excluded. After excluding these participants, the present study included a total of 109 participants in the analytic cohort.
Figure 1.
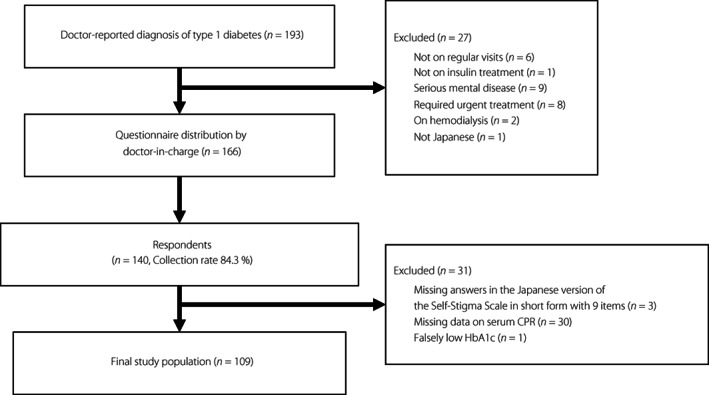
Flow chart showing the process for selecting participants for inclusion in the study. There were 193 outpatients with doctor‐reported diagnosis of type 1 diabetes. Among them, 27 outpatients were excluded due to the following reasons; not on regular visits, not on insulin treatment, had serious mental disease, required urgent treatment, on hemodialysis and not Japanese. Questionnaires were distributed by the doctor‐in‐charge to 166 eligible participants. Of these participants, 140 responded, corresponding to a collection rate of 84.3%. Three participants with missing answers in the Japanese version of the Self‐Stigma Scale in short form with nine items and 30 participants with missing data on serum CPR were excluded. One participant showed a falsely low value of HbA1c due to anemia and was excluded. Finally, 109 participants were included in the final study population. CPR, C‐peptide immunoreactivity; HbA1c, glycated hemoglobin.
Table 1 shows the demographic and clinical characteristics of the final analytic cohort. In the cohort, 43.1% were women, and the mean age was 58.3 years. A total of 50.4% were employed full‐time, and 13.7% were employed part‐time. The mean duration of diabetes was 25.5 years. Insulin secretion in the majority of participants was categorized as depleted (86.2%). The mean BMI was 22.9. The median of the total scores of the nine‐item Self‐Stigma Scale was 11. The median scores of Cognitive, Affective and Behavioral subscales were 5, 3 and 1, respectively. The mean HbA1c was 7.6%.
Table 1.
Demographics and clinical characteristics of participants
| Missing | ||
|---|---|---|
| Demographics | ||
| Age (years) | 58.3 (13.5) | ― |
| Sex | ― | |
| Male | 62 (56.8) | |
| Female | 47 (43.1) | |
| Employment | ― | |
| Full‐time | 55 (50.4) | |
| Part‐time | 15 (13.7) | |
| Retired/unemployed | 39 (35.7) | |
| Clinical characteristics | ||
| Duration (years) | 25.5 (13.8) | ― |
| Insulin secretion | ― | |
| Depletion † (fasting or random CPR <0.1 ng/mL) | 94 (86.2) | |
| Non‐depletion ‡ (fasting CPR ≥0.1 ng/mL) | 15 (13.7) | |
| BMI (kg/m2) | 22.9 (4.0) | ― |
| Self‐stigma | ||
| SSS‐J in short form with 9 items (total score) | 11 (7, 14) | ― |
| Cognitive (subscale) | 5 (4, 6) | |
| Affective (subscale) | 3 (2, 6) | |
| Behavioral (subscale) | 1 (0, 3) | |
| Glycemic outcome | ||
| Average HbA1c (%) of the last three clinical visits | 7.6 (0.9) | ― |
Total n = 109. Data are n (%) or mean (standard deviation). Where continuous variables are nonnormally distributed, median (interquartile range) are reported.
Depletion was defined as fasting or random serum C‐peptide immunoreactivity (CPR) <0.1 ng/mL ever confirmed since first visit.
Non‐depletion was defined as fasting serum CPR ≥0.1 ng/mL in the last 12 months.
BMI, body mass index; HbA1c, glycated hemoglobin; SSS‐J, The Japanese version of Self‐Stigma Scale.
Unadjusted and adjusted linear regression models assessing the association between self‐stigma scores and HbA1c are shown in Table 2. The unadjusted model showed significant associations between HbA1c and self‐stigma, age, employment status, BMI and duration of diabetes, respectively. The association of insulin depletion with higher HbA1c was not significant. After adjusting for age, sex, employment status, BMI, duration of diabetes and insulin secretion, the positive association between self‐stigma and HbA1c remained significant (β = 0.05, 95% confidence interval 0.01 to 0.08).
Table 2.
Unadjusted and adjusted linear regression exploring association between self‐stigma and glycated hemoglobin
| Independent variables | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|
| β (95% CI) | P‐value | β (95% CI) | β′ | P‐value | |
| Self‐stigma † | 0.04 (0.005 to 0.08) | 0.026 | 0.05 (0.01 to 0.08) | 0.23 | 0.009 |
| Age (years) | −0.01 (−0.02 to −0.0007) | 0.039 | −0.005 (−0.02 to 0.01) | −0.06 | 0.59 |
| Sex | |||||
| Male | Reference | Reference | |||
| Female | 0.12 (−0.06 to 0.31) | 0.18 | 0.09 (−0.10 to 0.29) | 0.09 | 0.33 |
| Employment status | |||||
| Full‐time | −0.67 (−1.23 to −0.11) | 0.019 | −0.88 (−1.42 to −0.34) | −0.44 | 0.001 |
| Part‐time | Reference | Reference | |||
| Retired/unemployed | −0.75 (−1.33 to −0.17) | 0.011 | −0.73 (−1.28 to −0.17) | −0.35 | 0.010 |
| BMI (kg/m2) | 0.07 (0.03 to 0.12) | <0.001 | 0.08 (0.04 to 0.13) | 0.36 | <0.001 |
| Duration (years) | −0.01 (−0.02 to −0.0008) | 0.037 | −0.009 (−0.02 to 0.005) | −0.12 | 0.22 |
| Insulin secretion | |||||
| Depletion ‡ | 0.11 (−0.38 to 0.61) | 0.65 | 0.39 (−0.06 to 0.86) | 0.15 | 0.092 |
| Non‐depletion § | Reference | Reference | |||
Total n = 109. Model adjusted for age, sex, duration of diabetes, body mass index (body mass index) and insulin secretion.
Self‐stigma was evaluated by the total score of the Japanese version of the Self‐Stigma Scale in short form with nine items.
Depletion was defined as fasting or random serum C‐peptide immunoreactivity (CPR) <0.1 ng/mL ever confirmed since first visit.
Non‐depletion was defined as fasting serum CPR ≥0.1 ng/mL in the last 12 months.
β, regression coefficient; β′, standardized regression coefficient; CI, confidence interval; HbA1c, glycated hemoglobin.
Variance inflation factors for each independent variable were lower than 2.7. Therefore, no multicollinearity problems were considered to exist. 23
DISCUSSION
The present single‐center, cross‐sectional study showed a significant association between self‐stigma and HbA1c in Japanese adults with type 1 diabetes. To the best of our knowledge, this is the first study to report an association between self‐stigma and HbA1c in persons with type 1 diabetes.
The result of the present study differed from the previous study of self‐stigma in Japanese adults with type 2 diabetes, which reported that the association between self‐stigma and HbA1c was statistically insignificant. 24 Comparing stigma depending on the types of diabetes, it has been previously reported that persons with type 1 diabetes experience and perceive more stigma than those with type 2 diabetes. 25 Increased stigma in persons with type 1 diabetes might have affected the association between self‐stigma and HbA1c differently from the stigma in persons with type 2 diabetes.
The difference in how stigma develops in each type of diabetes might be one of the reasons for the increased stigma in persons with type 1 diabetes. It is reported that the greatest difference of stigma between type 1 and type 2 diabetes is related to less public awareness of type 1 diabetes, and misunderstandings about diabetes that all types arise the same way. 25 Although it is evident that persons with type 2 diabetes also perceive stigma that ‘they brought the condition on themselves’ 26 , persons with type 1 diabetes have a tendency to struggle with the diagnosis of ‘diabetes the lifestyle disease’, despite the different onset mechanisms, and think that type 1 diabetes ruined their lives. 27 These emotions might even relate to strong feelings of annoyance, anger and hatred against diabetes 28 , and possibly interfere with development of self‐stigma for diabetes.
In addition to the difference of public awareness, the pathophysiology is different between type 1 and type 2 diabetes. Type 1 diabetes is characterized by autoimmune β‐cell destruction usually leading to absolute lack of insulin, and requires lifelong daily insulin use. 29 The need for multiple daily insulin injection or pump therapy among type 1 diabetes patients might also be the reason for increased stigma 25 , as stigma increases with the intensity of treatment (i.e., from non‐insulin to non‐intensive insulin to intensive insulin therapy). 14 , 25 Insulin use is highly visible, and concerns about how insulin therapy is perceived in public are common among individuals with diabetes, leading to delayed or omitting insulin administration. 30 It is known that inappropriate timing of insulin administration is associated with poorer glycemic outcome, as measured by HbA1c. 31 , 32
Collectively, it is possible that increased experienced/perceived stigma and its internalization in type 1 diabetes due to misunderstandings about the general causes of diabetes, and the high intensity treatment of multiple daily insulin use might have had an impact on the association between self‐stigma and HbA1c in a different way from type 2 diabetes. Further research including both people with type 1 and type 2 diabetes should be carried out on how different aspects of self‐stigma regarding treatment intensity and types of diabetes relate to glycemic outcome over time.
The present result was consistent with prior reports on experienced/perceived stigma in persons with type 1 diabetes. Previous cross‐sectional studies have reported the association between experienced/perceived stigma and HbA1c among persons with type 1 diabetes. 12 , 13 Although there are studies that highlight the importance of addressing experienced/perceived stigma in persons with type 1 diabetes, self‐stigma among type 1 diabetes patients is relatively underexamined. As far as we know, this is the first study to report an association of self‐stigma with HbA1c in persons with type 1 diabetes.
The current study provides a possible association between self‐stigma and HbA1c in persons with type 1 diabetes. Our result supports the framework from a qualitative study in type 1 diabetes, which proposed that suboptimal HbA1c was one of the consequences of diabetes‐related stigma, aligned with impaired psychological well‐being, social interaction and behavioral management of type 1 diabetes. 28 It has also been proposed previously that diabetes‐related stigma might possibly lead to suboptimal HbA1c, and in the very long‐term, the development of diabetes complications. 28 , 33 However, we were unable to elucidate the causal relationship between self‐stigma and HbA1c due to the cross‐sectional nature of the study, and clarifying the causality was beyond the scope of our study. Further longitudinal study is required to examine the causal relationship between self‐stigma and HbA1c in persons with type 1 diabetes.
A clinical implication from the present result is that addressing the levels of self‐stigma might be important for optimizing glycemic outcome in persons with type 1 diabetes. The Japanese Diabetes Society states that objectives of diabetes management are maintaining quality of life and life expectancy, through preventing the development or progression of diabetes complications and conditions associated with diabetes, and eliminating stigma. 34 , 35 The importance of eliminating stigma in the cycle of person‐centered glycemic management is also supported by the American Diabetes Association. 36 Although efforts are still necessary to reduce stigma, the present study provides a statistically significant result that self‐stigma is associated with HbA1c in persons with type 1 diabetes, thereby supporting further interventions to promote diabetes advocacy. 37
Although the present study showed an association between self‐stigma and HbA1c, we should be aware that every person with diabetes, regardless of their present HbA1c values, might suffer from stigma in many aspects. Several participants commented in the questionnaire additionally that if they had answered the questionnaire soon after diagnosis of type 1 diabetes, they would have answered the self‐stigma scale worse. These answers may suggest that the degree of self‐stigma changes over time. A recent study also found the association between self‐stigma and duration of type 2 diabetes. 38 The levels of self‐stigma should be regularly assessed for all persons with diabetes, regardless of the clinical data.
The present study has several limitations. First, due to the cross‐sectional nature of this study, the causality between self‐stigma and HbA1c was not proven. Also, we did not prove the clinical mechanism underlying the association between self‐stigma and HbA1c. Multiyear prospective research is required in the future to verify the causality between stigmatic status and glycemic outcome over time. Second, as this study was carried out at a single center, the external validity was limited. Major characteristics of persons with type 1 diabetes at our institution included long duration of diabetes and high percentages of affluent persons. These characteristics might have influenced the scores of self‐stigma. Furthermore, missing data and limited number of participants might have affected the statistical analysis. Therefore, multicenter studies are required to confirm the result in a larger scale. Third, the Japanese version of the Self‐Stigma Scale has only been validated among people with type 2 diabetes, which limits its generalizability to type 1 diabetes. Further research is necessary to validate a scale in Japanese language that assesses diabetes‐related stigma among Japanese people with type 1 diabetes. Additionally, we used nine items from the Japanese version of the Self‐Stigma Scale. Even though the original Self‐Stigma Scale‐Short has been validated among several samples of people. 8 , 17 , 18 , 19 , the short version of the Japanese version of the Self‐Stigma Scale was yet to be confirmed. The use of the short‐form measure in the present study might have decreased the reliability and validity compared with the original long form. 39 Fourth, we were unable to assess experienced or perceived stigma in the present study, for there are no scales in publish that assess experienced or perceived stigma in Japanese at present. Fifth, we conducted the study during the COVID‐19 pandemic, and the spread of COVID‐19 might have had an impact on glycemic values. 40 , 41 , 42 In addition, the present study did not include assessments of total daily insulin dose and experiences of diabetes education with a certified diabetes educator, which might relate to self‐stigma or glycemic outcome.
In conclusion, the present cross‐sectional study showed an important finding that self‐stigma is associated with HbA1c among adults with type 1 diabetes. Our study suggests that treatment of diabetes should be provided to persons with type 1 diabetes with considerations that HbA1c is associated with self‐stigma.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: This research was approved by the Human Subjects Review Committee at the Institute for Medical Science (approval number 12605).
Informed consent: Written informed consent was obtained from all participants.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Table S1 | The Japanese version of the Self‐Stigma Scale in short form with nine items.
ACKNOWLEDGMENTS
The authors thank all the study participants for attending this study. The authors also acknowledge great appreciation to Rieko Ichihashi, Nobuhiro Tachibana and all the other staff members for their skilled assistance.
REFERENCES
- 1. Goffman E. Stigma: Notes on the Management of Spoiled Identity. Harmondsworth: Penguin, 1963. [Google Scholar]
- 2. Brohan E, Slade M, Clement S, et al. Experiences of mental illness stigma, prejudice and discrimination: A review of measures. BMC Health Serv Res 2010; 10: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacoby A. Felt versus enacted stigma: A concept revisited. Evidence from a study of people with epilepsy in remission. Soc Sci Med 1994; 38: 269–274. [DOI] [PubMed] [Google Scholar]
- 4. van Brakel WH, Anderson AM, Mutatkar RK, et al. The participation scale: Measuring a key concept in public health. Disabil Rehabil 2006; 28: 193–203. [DOI] [PubMed] [Google Scholar]
- 5. Scrambler G, Hopkins A. Being epileptic: Coming to the terms with stigma. Sociol Health Illn 1986; 8: 26–43. [Google Scholar]
- 6. Corrigan PW, Watson AC. The paradox of self‐stigma and mental illness. Clin Psychol Sci Pract 2002; 9: 35–53. [Google Scholar]
- 7. Kato A, Takada M, Hashimoto H. Reliability and validity of the Japanese version of the self‐stigma scale in patients with type 2 diabetes. Health Qual Life Outcomes 2014; 12: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mak WW, Cheung RY. Self‐stigma among concealable minorities in Hong Kong: Conceptualization and unified measurement. Am J Orthopsychiatry 2010; 80: 267–281. [DOI] [PubMed] [Google Scholar]
- 9. Browne JL, Ventura AD, Mosely K, et al. Measuring type 1 diabetes stigma: Development and validation of the type 1 diabetes stigma assessment scale (DSAS‐1). Diabet Med 2017; 34: 1773–1782. [DOI] [PubMed] [Google Scholar]
- 10. Browne JL, Ventura AD, Mosely K, et al. Measuring the stigma surrounding type 2 diabetes: Development and validation of the type 2 diabetes stigma assessment scale (DSAS‐2). Diabetes Care 2016; 39: 2141–2148. [DOI] [PubMed] [Google Scholar]
- 11. Mulvaney SA, Hood KK, Schlundt DG, et al. Development and initial validation of the barriers to diabetes adherence measure for adolescents. Diabetes Res Clin Pract 2011; 94: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brazeau AS, Nakhla M, Wright M, et al. Stigma and its association with glycemic control and hypoglycemia in adolescents and young adults with type 1 diabetes: Cross‐sectional study. J Med Internet Res 2018; 20: e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hansen UM, Olesen K, Willaing I. Diabetes stigma and its association with diabetes outcomes: A cross‐sectional study of adults with type 1 diabetes. Scand J Public Health 2020; 48: 855–861. [DOI] [PubMed] [Google Scholar]
- 14. Holmes‐Truscott E, Ventura AD, Thuraisingam S, et al. Psychosocial moderators of the impact of diabetes stigma: Results from the second diabetes MILES ‐ Australia (MILES‐2) study. Diabetes Care 2020; 43: 2651–2659. [DOI] [PubMed] [Google Scholar]
- 15. Kato A, Fujimaki Y, Fujimori S, et al. How self‐stigma affects patient activation in persons with type 2 diabetes: A cross‐sectional study. BMJ Open 2020; 10: e034757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kato A, Fujimaki Y, Fujimori S, et al. Association between self‐stigma and self‐care behaviors in patients with type 2 diabetes: A cross‐sectional study. BMJ Open Diabetes Res Care 2016; 4: e000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu TH, Chang CC, Chen CY, et al. Further psychometric evaluation of the self‐stigma scale‐short: Measurement invariance across mental illness and gender. PLoS One 2015; 10: e0117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang CC, Lin CY, Gronholm PC, et al. Cross‐validation of two commonly used self‐stigma measures, Taiwan versions of the internalized stigma mental illness scale and self‐stigma scale‐short, for people with mental illness. Assessment 2018; 25: 777–792. [DOI] [PubMed] [Google Scholar]
- 19. Ouedraogo J, Pana M, Dipankui MT, et al. French stigma scale: French translation and validation of a stigma scale to measure diabetes‐related stigma. Res Sq 2020. doi: 10.21203/rs.3.rs-16906/v1 [DOI] [Google Scholar]
- 20. Grossi E, Groth N, Mosconi P, et al. Development and validation of the short version of the psychological general well‐being index (PGWB‐S). Health Qual Life Outcomes 2006; 4: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Survey on diagnostic criteria and the development of severity assessment of type 1 diabetes in Japan focused on daily life and socialty, funded by the Ministry of Health, Labour and Welfare [article online], 2018. Available from: https://mhlw‐grants.niph.go.jp/project/26490 Accessed June 15, 2022 (Japanese).
- 22. Sugihara S, Kikuchi T, Urakami T, et al. Residual endogenous insulin secretion in Japanese children with type 1A diabetes. Clin Pediatr Endocrinol 2021; 30: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glantz SA, Slinker BK. Primer of Applied Regression and Analysis of Variance. New York, NY: McGraw‐Hill, 1990. [Google Scholar]
- 24. Kato A, Fujimaki Y, Fujimori S, et al. Psychological and behavioural patterns of stigma among patients with type 2 diabetes: A cross‐sectional study. BMJ Open 2017; 7: e013425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu NF, Brown AS, Folias AE, et al. Stigma in people with type 1 or type 2 diabetes. Clin Diabetes 2017; 35: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Browne JL, Ventura A, Mosely K, et al. ‘I call it the blame and shame disease’: A qualitative study about perceptions of social stigma surrounding type 2 diabetes. BMJ Open 2013; 3: e003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nishio I, Chujo M. Self‐stigma of patients with type 1 diabetes and their coping strategies. Yonago Acta Med 2017; 60: 167–173. [PMC free article] [PubMed] [Google Scholar]
- 28. Browne JL, Ventura A, Mosely K, et al. ‘I'm not a druggie, I'm just a diabetic’: A qualitative study of stigma from the perspective of adults with type 1 diabetes. BMJ Open 2014; 4: e005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes‐2021. Diabetes Care 2021; 44: S15–S33. [DOI] [PubMed] [Google Scholar]
- 30. Bermeo‐Cabrera J, Almeda‐Valdes P, Riofrios‐Palacios J, et al. Insulin adherence in type 2 diabetes in Mexico: Behaviors and barriers. J Diabetes Res 2018; 2018: 3190849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schaper NC, Nikolajsen A, Sandberg A, et al. Timing of insulin injections, adherence, and glycemic control in a multinational sample of people with type 2 diabetes: A cross‐sectional analysis. Diabetes Ther 2017; 8: 1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gomes MB, Negrato CA. Adherence to insulin therapeutic regimens in patients with type 1 diabetes. A nationwide survey in Brazil. Diabetes Res Clin Pract 2016; 120: 47–55. [DOI] [PubMed] [Google Scholar]
- 33. Schabert J, Browne JL, Mosely K, et al. Social stigma in diabetes: A framework to understand a growing problem for an increasing epidemic. Patient 2013; 6: 1–10. [DOI] [PubMed] [Google Scholar]
- 34. The Japanese Diabetes Society . Treatment Guide for Diabetes 2022–2023. Tokyo: Bunkoudou, 2022. [Google Scholar]
- 35. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 2020; 11: 165–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. American Diabetes Association . Standards of medical care in diabetes—2022 abridged for primary care providers 2022. Clin Diabetes 2022; 40: 10–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hilliard ME, Oser SM, Close KL, et al. From individuals to international policy: Achievements and ongoing needs in diabetes advocacy. Curr Diab Rep 2015; 15: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kato A, Fujimaki Y, Fujimori S, et al. Associations between diabetes duration and self‐stigma development in Japanese people with type 2 diabetes: A secondary analysis of cross‐sectional data. BMJ Open 2021; 11: e055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Widaman K, Little T, Preacher K, et al. On creating and using short forms of scales in secondary research. In: Trzesniewski KH, Donnellan MB, Lucas R (eds). Secondary Data Analysis. Washington, DC: American Psychological Association, 2011; 39–61. [Google Scholar]
- 40. Onishi Y, Ichihashi R, Yoshida Y, et al. Substitution of telemedicine for clinic visit during the COVID‐19 pandemic of 2020: Comparison of telemedicine and clinic visit. J Diabetes Investig 2022; 13: 1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duarte V, Mota B, Ferreira S, et al. Impact of COVID‐19 lockdown on glycemic control in type 1 diabetes. Arch Pediatr 2022; 29: 27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shic C, Zhu H, Liu J, et al. Barriers to self‐management of type 2 diabetes during COVID‐19 medical isolation: A qualitative study. Diabetes Metab Syndr Obes 2020; 13: 3713–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | The Japanese version of the Self‐Stigma Scale in short form with nine items.


