Abstract
In Streptococcus pneumoniae oxygen availability is a major determinant for competence development in exponentially growing cultures. NADH oxidase activity is required for optimal competence in cultures grown aerobically. The implication of oxidative metabolism and more specifically of Nox on central metabolism has been examined. Glycolytic flux throughout exponential growth revealed homolactic fermentation with a lactate production/glucose utilization ratio close to 2, whatever the aerobiosis level of the culture. Loss-of-function mutations in nox, which encodes NADH oxidase, did not change this trait. Consistently, mRNA levels of glyceraldehyde-3-phosphate dehydrogenase, l-lactate dehydrogenase, pyruvate oxidase, and NADH oxidase remained comparable to wild-type levels, as did the specific activities of key enzymes which control central metabolism. Competence regulation by oxygen involving the NADH oxidase activity is not due to significant modification of carbon flux through glycolysis. Failure to obtain loss-of-function mutation in L-ldh, which encodes the l-lactate dehydrogenase, indicates its essential role in pneumococci whatever their growth status.
The aerotolerant anaerobe Streptococcus pneumoniae is a human pathogen showing high transformability by soluble DNA. Central metabolism in these bacteria is classically described as homolactic and genome analysis revealed the absence of most of the genes involved in aerobic respiration (29 genes searched for), the tricarboxylic acid (TCA) cycle, and gluconeogenesis (2), suggesting that fermentative metabolism is the major catabolic pathway. However, mutational analysis revealed the role of oxidases having O2 as the substrate in both virulence and competence. Notably, insertion mutations in spxB (GenBank accession number L39074), which encodes a putative pyruvate oxidase, and in nox (GenBank accession number AF014458), which encodes the H2O-producing NADH oxidase (Nox), severely reduce experimental virulence and bacterial persistence in tissues of infected mice (1, 15). Interestingly, the NADH oxidase activity is also required for optimal competence in laboratory strains (1). Oxidative metabolism is thus involved in the physiological specialization of S. pneumoniae in animals and in fermentative conditions in the laboratory. Indeed, O2 availability and Nox activity are major determinants for transcriptional regulation of the early competence genes comCDE (5). The operon comCDE encodes the procompetence activator and its dedicated two-component signaling system ComDE. Maturation and export of ComC to the competence-stimulating peptide CSP requires the ComAB ATP-binding cassette (ABC) transporter. CSP activates the autokinase ComD, with subsequent activation of the response regulator ComE (9). This signaling pathway is involved in the transduction of putative signals produced when bacteria are grown aerobically (5).
In lactic acid bacteria, the presence of oxygen in strains possessing oxidase activities has a distinct effect on carbon metabolism. Pyruvate metabolism can shift towards mixed-acid fermentation, thereby increasing ATP production via acetate generation under various environmental conditions. Induction of alternative pyruvate metabolic pathways has been attributed to the antagonistic control of the l-lactate dehydrogenase (l-Ldh) (GenBank accession number AJ000336) and of the other pyruvate-consuming reactions, catalyzed either by pyruvate formate lyase or by pyruvate dehydrogenase, by the NADH/NAD+ ratio (7, 13). Constraints imposed on glyceraldehyde-3-phosphate dehydrogenase (Gap) activity (6) by the NADH/NAD+ ratio trigger this metabolic shift, leading to significant reorientation of pyruvate metabolism toward acetate synthesis with the associated gain in energy generation, plus deviation of carbon flux toward alternative pathways of pyruvate metabolism, notably the acetoin pathway. Metabolic reorientation is accentuated in the presence of oxygen (14), and metabolic engineering strategies have exploited this, by overexpression of the nox gene, which encodes NADH oxidase (11). However, the effects on central metabolism of a loss-of-function mutation in nox have not been addressed. In view of the correlation between Nox activity and functional characteristics of S. pneumoniae such as competence and virulence, the possibility that these traits can be related to changes in energy metabolism due to modified carbon flux through glycolysis was investigated. The data obtained did not reveal a shift in central metabolism when the oxidative status of the cultures was changed or when the NADH oxidase activity of the bacteria was abolished by mutation. This suggests that the implication of Nox in competence involves functions other than the direct oxidation of NADH associated with the central metabolic pathways.
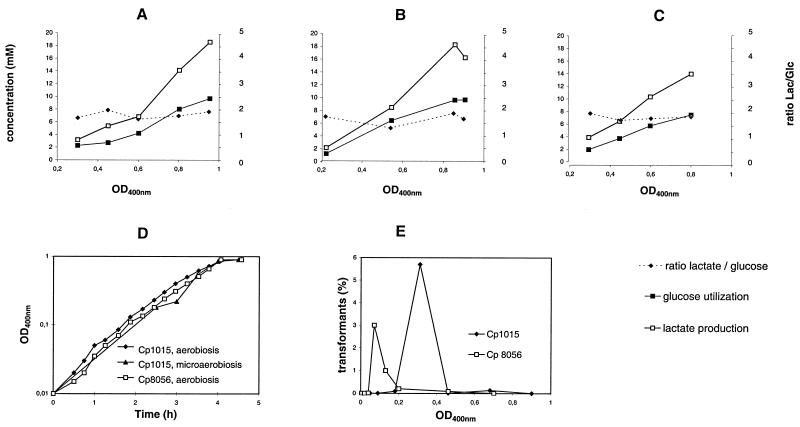
Influence of O2 availability on competence and glucose fermentation in the nox+ and nox mutant genetic backgrounds.
Metabolic flux in bacteria was measured by quantifying the fermentative metabolites produced throughout exponential growth using high-performance liquid chromatography (HPLC). Competence development was determined in parallel in the same cultures by the measurement of transformant recovery throughout growth (Fig. 1D and E) in a medium where O2 availability is the single parameter determining competence development in the wild-type strain Cp1015 (5). Strain Cp1015 showed a similar pattern of glucose utilization and lactate production during growth whatever the oxygen status of the culture (Fig. 1A and B), and this trait was comparable to that of the nox mutant strain Cp8056 growing in the presence of oxygen (Fig. 1C). In an OD400 (optical density at 400 nm) window of 0.2 to 0.8, lactate production/glucose utilization ratios were close to 2, reflecting an essentially homolactic metabolism, despite minor trace amounts of both acetate and ethanol in the medium. In order to investigate the mechanism of such a strong regulation, the transcript levels of genes identified as having an important role in controlling carbon flux through energy metabolism, i.e., genes encoding l-Ldh, Nox, SpxB, and putative Gap (see Table 1), were compared by Northern blotting of total RNA with specific probes and also by measurements of the specific activities of the corresponding enzymes in crude extracts of nox+ and nox mutant strains.
FIG. 1.
Kinetics of glucose utilization and lactate production during growth in CTM medium (1) under aerobiosis (A) and microaerobiosis (B) of the wild-type strain Cp1015, compared to that of the nox mutant strain Cp8056, grown under aerobiosis (C). Growth curves (D) were obtained by OD400 measurements. Profiles of competence development throughout aerobic growth of Cp1015 and Cp8056 are also presented (E). For transformation tests, chromosomal DNA (1 μg/ml) carrying the rif23 allele allowing resistance to rifampin (2 μg/ml) was used. Rifr transformants were selected on plates, and their frequency in the population was calculated as described previously (1). For HPLC analysis, supernatants of cultures were filtered through 0.2-μm-pore-size Millipore filters.
TABLE 1.
Bacterial strains, plasmids, and oligonucleotides used in this work
| Strain, plasmid, or oligonucleotide | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| Cp1015 | Wild type; str1 hexA | 12 |
| Cp8056 | str1 hexA nox K71−→stop codon | 1 |
| Plasmids | ||
| pAM239 | PBR322 derivative; ColE1 origin; lacZα selection | 8 |
| pLDH1 | PstI/XbaI fragment of pTSS7 (GenBank accession no. AF000336, positions 434 to 1103) cloned into pAM239 | This work |
| pLDH2 | LDH11-LDH12a PCR fragment of ldh cloned into pAM239 (PstI/XbaI) | This work |
| pTSS7 | Mutated PCR fragment (241G→T and 243T→A) of the ldh gene (GenBank accession no. AF000336, positions 233 to 1103) cloned into pBluescript (12a) | This work |
| pTSS13 | PCR fragment of the spxB gene (GenBank accession no. L39074, positions 23 to 1983) cloned into pBluescript (12a) | This work |
| Probes | ||
| nox | A 1,124-bp BamHI-EcoRI nox fragment from pNOX2 (1) (GenBank accession no. AF014458, positions 10 to 1133) | This work |
| ldh | An 847-bp HpaI-SpeI ldh fragment from pTSS7 (GenBank accession no. AF000336, positions 267 to 1103) | This work |
| spxB | A 1,206-bp HincII-HincII spxB fragment from pTSS13 (GenBank accession no. L39074, positions 475 to 1680) | This work |
| S16 | A 650-bp fragment from pP16 (GenBank accession no. X58312, positions 166 to 816) | 5 |
| gap | A 699-bp GAPD1-GAPD2 PCR fragmentb | |
| Oligonucleotide primers | ||
| GAPD1 | 5′-CCTGAGAAGCTTGCGTCAAACAATGAACCGTA | This work |
| HindIII | ||
| GAPD2 | 5′-CCACTGCTGCAGGGTCGTCTTGCTTTCCGTCG | This work |
| PstI | ||
| FPAM | 5′-CGAGCTCGGTACCCGGGG | This work |
| LDH11 | 5′-CCACACCTGCAGGGGTGAACACGGTGACTCTG | This work |
| PstI | ||
| LDH12 | 5′-CCACACTCTAGATGTGGGAAGATGTATAATGG | This work |
| XbaI |
The LDH11 primer was designed from the GenBank (accession number AF000336) sequence (positions 720 to 739), whereas the LDH12 sequence was retrieved from the 3836 contig sequence of the TIGR unfinished genome sequences database (positions 149 to 168 upstream of the stop codon of the ldh gene).
The gap gene was identified on the basis of its homology to the gapC gene of Streptococcus equisimilis. The gapC gene sequence from S. equisimilis (GenBank accession number Y12602) was used to search for homolog sequences into the TIGR unfinished genome sequences database. A 1,005-nucleotide sequence that showed 87% identity with the Streptococcus equisimilis gapC gene was retrieved in contig 3836.
Expression of key enzymes controlling the carbohydrate flux.
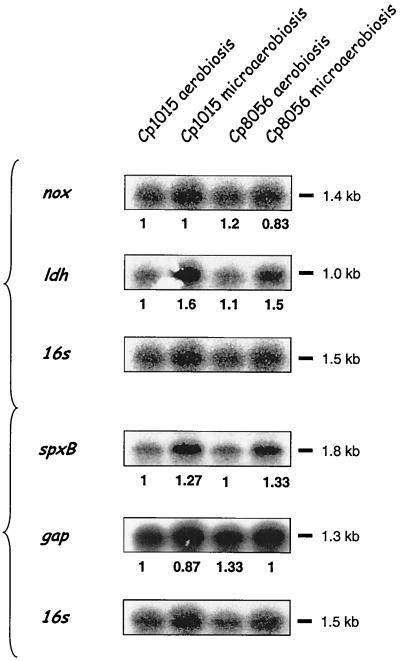
Northern blot analysis of total-RNA preparations from cultures (OD400, 0.1) of the wild-type strain Cp1015 and the Nox0 mutant Cp8056 was performed using as specific probes a 1,124-bp nox fragment from pNOX2 (GenBank accession number AF014458, positions 10 to 1133), an 847-bp L-ldh fragment from pTSS7 (GenBank accession number AF000336, positions 267 to 1103), a 1,206-bp spxB fragment from pTSS13 (GenBank accession number L39074, positions 475 to 1680), a 650-bp fragment from pP16 (GenBank accession number X58312, positions 166 to 816), and a 699-bp GAPD1–GAPD2 PCR fragment (Table 1). Although it has been extensively shown that in nox mutants competence peaks at an OD400 of 0.1, in contrast to the wild-type strain, which becomes competent at an OD400 of 0.3, both strains showed essentially identical levels for the 1-kb L-ldh, 1.4-kb nox, 1.8-kb spxB, and 1.3-kb gap mRNAs in bacteria from cultures at an OD400 of 0.1 (Fig. 2). It should be noted that the calculated sizes of the mRNAs are close to the sizes of their corresponding open reading frames, indicating that monocistronic operons encode these different proteins.
FIG. 2.
Effect of nox loss-of-function mutation and oxygen limitation on nox, L-ldh, spxB, and gap mRNA levels in cultures at an OD400 of 0.1. Total-RNA preparations from competent cultures of the nox strain Cp8056 were subjected to Northern blot analysis with probes specific for ldh, nox, gap, and spxB. 16S rRNA was taken as a qualitative and quantitative internal control (see Table 1). A parallel analysis was performed on noncompetent cultures of the wild-type strain Cp1015 grown aerobically or microaerobically to an OD400 of 0.1. Signals were quantified by densitometry, and ratios of specific mRNA to 16S rRNA are given. The experiments were repeated with independent cultures to test reproducibility.
At this point in growth, no measurable difference was found among the specific activities of the encoded Gap, l-Ldh, and Nox. In order to assess other checkpoints in the carbon flux, the specific activities of the fructose diphosphate aldolase and phosphoglycerate isomerase were also measured, confirming that the cellular glycolytic potential was not changed by the nox loss-of-function mutation (Table 2).
TABLE 2.
Specific activities of key glycolytic enzymes in the wild-type and nox strains
| Enzyme | Mean sp acta (SD) in cultures of:
|
|
|---|---|---|
| Cp1015 | Cp8056 | |
| Glyceraldehyde 3-phosphate dehydrogenase | 1.79 (0.11) | 1.93 (0.17) |
| NADH oxidase | 0.87 (0.07) | 0 |
| Lactate dehydrogenase | 3.23 (0.18) | 3.61 (0.26) |
| Fructose diphosphate aldolase | 1.93 (0.12) | 2.08 (0.05) |
| Phosphoglycerate isomerase | 1.49 (0.01) | 1.33 (0.07) |
Expressed in micromoles per milligram of protein per minute; measured from cultures at an OD400 of 0.1. Values are means of at least five measurements with two different extracts. Cp1015, wild type; Cp8056, nox mutant.
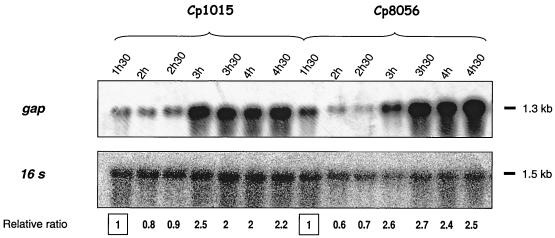
Another trait that characterized the Nox0 strains was the rapid loss of competence when cultures grew to an OD400 greater than 0.1 (Fig. 1E). To assess whether such a trait involved specific regulation of carbohydrate metabolism, the levels of the previously mentioned mRNAs in cells were compared throughout growth up to an OD400 of 0.8. For both the wild-type and nox mutant strains, cellular mRNA levels were constant for L-ldh, spxB, and nox (data not shown); cellular gap mRNA levels increased slightly (relative value, 1 to 2) when cultures reached the late-exponential-growth phase (Fig. 3). The physiological importance of growth phase-related variation in gap transcript levels is not yet known but is probably related to medium acidification phenomena. The data obtained indicate that expression of the enzymes considered in this study did not vary during competence development and, furthermore, was not influenced by Nox activity.
FIG. 3.
Evolution of gap mRNA levels throughout growth in cultures from the wild-type strain Cp1015 and the Nox0 mutant Cp8056. Aliquots of cultures of Cp1015 (A) and Cp8056 (nox) (B) growing under aerobiosis in CTM medium (1) were withdrawn at 30-min intervals. Total-RNA preparations from these cultures were subjected to Northern blotting with a gap-specific probe. 16S rRNA was used as a qualitative and quantitative internal control. Signals were quantified by densitometry, and the 16S rRNA/gap mRNA ratio was calculated. For each strain, results were expressed relative to data obtained for cultures grown for 1 h 30 min, taken as 1. The experiments were repeated with independent cultures to test reproducibility.
Although it has been clearly demonstrated that competence is dependent upon both the presence of a functional Nox (1) and adequate aeration (5), no significant reorientation of pyruvate metabolism from normal homolactic characteristics could be demonstrated under competence conditions and in nox strains. The consequence of a forced reorientation of the glycolytic flux on competence expression was assessed further by a mutational strategy.
Mutagenesis of L-ldh.
The enzymes providing an alternative pathway of pyruvate metabolism are present in the cell, as seen by the presence of trace amounts of mixed-acid fermentation products. Since this potential is not exploited to increase ATP generation in the presence of oxygen, at least in competence medium (1), an attempt was made to force the metabolic flux through this pathway, thereby revealing a putative “oxidative phenotype,” by mutational blocking of l-Ldh activity.
Attempts to introduce a stop codon mutation by mutating the nucleotides at positions 241 and 243 (GenBank accession number AJ000336) in the 5′ region of the L-ldh gene (10) using mutational strategies routinely used in the laboratory (1, 5) were unsuccessful (Table 1 and data not shown). Furthermore, insertional mutation by single-crossover integration of the nonreplicative plasmid pAM239 carrying an L-ldh fragment in the resident chromosome was tried. Both an internal L-ldh fragment (positions 434 to 1103) and a fragment containing the 3′ end of L-ldh (positions 720 to 1173) were cloned into pAM239, and the recombinant plasmids pLDH1 and pLDH2 were used to transform Cp1015. Recombinant colonies carrying the inserted plasmid and expressing the spectinomycin resistance gene present on the plasmid were screened for their growth in spectinomycin-containing medium. Recombinant clones were obtained only when plasmid pLDH2 was used as donor DNA (10 independent experiments). Insertion in L-ldh in these clones was verified by PCR amplification of a 650-nucleotide fragment with primers LDH11 and FPAM (Fig. 4). Transformation of Cp1015 with the mutagenic plasmids under microaerobic conditions in GasPak jars (5) gave identical results (data not shown). Plasmid insertion was tolerated only with plasmid pLDH2, since it probably allowed the reconstitution of a wild-type copy of L-ldh (Fig. 4). This suggests that disruption of L-ldh cannot be obtained and therefore the l-Ldh pathway is essential in S. pneumoniae, despite the presence of pyruvate oxidase/NADH oxidase and the theoretically better energetic yield of the mixed-acid pathway compared to homolactic fermentation (4). This finding is reminiscent of work with Streptococcus mutans in which a loss-of-function mutation in L-ldh was not obtained (3).
FIG. 4.
L-ldh mutagenesis by plasmid insertion duplication. The wild-type strain Cp1015 was transformed with the nonreplicative plasmids pLDH1, containing an internal fragment of L-ldh (A) and pLDH2, containing a fragment corresponding to the part of L-ldh encoding the C terminus of the enzyme (B). (C) Recombinant colonies were selected for resistance to spectinomycin (spcR) and verified by PCR amplification of the chromosome with oligonucleotides LDH11 and FPAM (lane 1, Eurogentec SmartLadder molecular weight marker, lane 2, PCR product of the pLDH2 plasmid, taken as a control; lanes 3 to 8, PCR products of transformed colonies).
In S. pneumoniae expression of the key enzymes Gap and l-Ldh, and also of SpxB, is strictly regulated, as shown by constant levels of the corresponding cellular mRNAs, regardless of the oxygen status of the cultures and the presence or absence of functional Nox. Furthermore, Nox has no impact on the specific activity of Gap, l-Ldh, fructose diphosphate aldolase, or phosphoglycerate isomerase. Neither variations in O2 availability nor in NADH oxidase activity influence the carbon flux under the conditions tested, which remained essentially homolactic. In agreement with these data, attempts to obtain mutants devoid of l-Ldh activity by plasmid insertion mutagenesis were unsuccessful. Despite the requirements of oxygen and Nox for optimal competence expression under the control of the two-component signal transducing systems CiaRH and ComDE (5), no change in the glycolytic flux was related to the pattern of competence expression in response to oxygen availability. The present work shows that these regulations, culminating in competence, are not related to stimulation or reorientation of central metabolism, resulting in increased production of phosphodonors and subsequent activation of signal transduction by phosphotransfer through two-component signalling systems. The role of oxidative metabolism in competence regulation remains under investigation.
Acknowledgments
This work was supported by Université Paul Sabatier, EA 3036, and Rhône-Poulenc Rorer (RPR), Vitry, France. L.M.-M. was supported by an RPR postdoctoral fellowship.
We thank Delphine Dos Santos and Ghislain Fournous for preparing the bacterial cultures for different analyses and Suzanne Eychenne for technical assistance.
REFERENCES
- 1.Auzat I, Chapuy-Regaud S, Le Bras G, Dos Santos D, Ogunniyi A D, Le Thomas I, Garel J-R, Paton J C, Trombe M-C. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol Microbiol. 1999;34:1018–1028. doi: 10.1046/j.1365-2958.1999.01663.x. [DOI] [PubMed] [Google Scholar]
- 2.Baltz R H, Norris F H, Matsushima P, Dehoff B S, Rockey P, Porter G, Burgett S, Peery R, Hoskins J, Braverman L, Jenkins I, Solenberg P, Young M, McHenney M A, Rosteck P R, Skatrud P L. DNA sequence sampling and gene disruption for identification of new antibacterial targets in Streptococcus pneumoniae. In: Tomasz A, editor. Streptococcus pneumoniae: molecular biology and mechanisms of disease. Larchmont, N.Y: Mary Ann Liebert, Inc.; 2000. pp. 33–44. [Google Scholar]
- 3.Chen A, Hillman J D, Duncan M. l-(+)-Lactate dehydrogenase deficiency is lethal in Streptococcus mutans. J Bacteriol. 1994;176:1542–1545. doi: 10.1128/jb.176.5.1542-1545.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Condon S. Responses of lactic acid bacteria to oxygen. FEMS Microbiol Rev. 1987;46:269–280. [Google Scholar]
- 5.Echenique J R, Chapuy-Regaud S, Trombe M-C. Competence regulation by oxygen in Streptococcus pneumoniae: involvement of ciaRH and comCDE. Mol Microbiol. 2000;36:688–696. doi: 10.1046/j.1365-2958.2000.01891.x. [DOI] [PubMed] [Google Scholar]
- 6.Even S, Garrigues C, Loubière P, Lindley N D, Cocaign-Bousquet M. Pyruvate metabolism in Lactococcus lactis is dependent upon glyceraldehyde-3-phosphate dehydrogenase activity. Metab Eng. 1999;1:198–205. doi: 10.1006/mben.1999.0120. [DOI] [PubMed] [Google Scholar]
- 7.Garrigues C, Loubière P, Lindley N D, Cocaign-Bousquet M. Control of the shift from homolactic-acid to mixed-acid fermentation in Lactococcus lactis: predominent role of NADH/NAD+ ratio. J Bacteriol. 1997;179:5282–5287. doi: 10.1128/jb.179.17.5282-5287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gil D. Elaboration et caractérisation d'un nouveau type de vecteur de clonage à nombre de copies régulable. Thèse de l'Université. Toulouse, France: Université Paul Sabatier; 1990. [Google Scholar]
- 9.Håvarstein L S. Identification of a competence regulon in Streptococcus pneumoniae by genomic analysis. Trends Microbiol. 1998;6:297–299. doi: 10.1016/s0966-842x(98)01328-6. [DOI] [PubMed] [Google Scholar]
- 10.Jado I, Fenoll A, Casal J, Perez A. Nucleotide sequence and chromosomal location of l-lactate dehydrogenase gene from Streptococcus pneumoniae. Curr Microbiol. 1998;37:77–79. doi: 10.1007/s002849900342. [DOI] [PubMed] [Google Scholar]
- 11.Lopez de Felipe F, Kleerebezem M, de Vos W M, Hugenholtz J. Cofactor engineering: a novel approach to metabolic engineering in Lactococcus lactis by controlled expression of NADH oxidase. J Bacteriol. 1998;180:3804–3808. doi: 10.1128/jb.180.15.3804-3808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison D A, Trombe M-C, Hayden M K, Waszak G A, Chen J D. Isolation of transformation-deficient Streptococcus pneumoniae mutants defective in control of competence, using insertion-duplication mutagenesis with the erythromycin resistance determinant of pAMβ1. J Bacteriol. 1984;159:870–876. doi: 10.1128/jb.159.3.870-876.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Short J H, Fernandez J H, Sorge J A, Huge WD. Lambda ZAP: a bacteriophage lambda expression vector with in vitro excision properties. Nucleic Acids Res. 1988;16:7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snoep J L, Teixeira de Mattos M J, Neijssel O M. Effect of the energy source on the NADH/NAD+ ratio and on pyruvate catabolism in anaerobic chemostat cultures of Enterococcus faecalis NCTC 775. FEMS Microbiol Lett. 1991;81:63–66. [Google Scholar]
- 14.Snoep J L, de Graef M R, Teixeira de Mattos M J, Neijssel O M. Pyruvate catabolism during transient state conditions in chemostat cultures of Enterococcus faecalis NCTC775: importance of internal pyruvate concentration and NADH/NAD+ ratio. J Gen Microbiol. 1992;138:2015–2020. doi: 10.1099/00221287-138-10-2015. [DOI] [PubMed] [Google Scholar]
- 15.Spellerberg B, Cundell D R, Sandros J, Pearce B J, Idänpäan-Heikkilä I, Rosenow C, Masure H R. Pyruvate oxidase as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol. 1996;19:803–813. doi: 10.1046/j.1365-2958.1996.425954.x. [DOI] [PubMed] [Google Scholar]