Background
Metabolic-associated fatty liver disease (MAFLD) is a new term of nonalcoholic fatty liver disease (NAFLD), with newly proposed diagnostic criteria. The applicability of common noninvasive testing for screening NAFLD is unclear for the detection of MAFLD and requires reevaluation. We aimed to validate the effectiveness of traditional NAFLD-related steatosis indices for diagnosing MAFLD and to determine the optimal cutoff values as well as compare their accuracy between NAFLD and MAFLD diagnosis.
Methods
This study enrolled 1866 participants from the National Health and Nutrition Examination Survey (NHANES) database (2017–2018). The diagnostic performances of fatty liver index (FLI), Framingham Steatosis Index (FSI), Zhejiang University index (ZJU), lipid accumulation product (LAP), hepatitis steatosis index (HSI) and visceral adiposity index (VAI) were evaluated using the area under the receiver operator characteristic (AUROC) curve and the optimal cutoff points were calculated according to maximum Youden’s index.
Results
FLI had the highest AUROC (0.840) for predicting MAFLD in the whole population, with a cutoff value of 56.93. The AUROCs of FLI, FSI, ZJU, LAP, HSI and VAI for predicting MAFLD/NAFLD were 0.840/0.812, 0.833/0.811, 0.826/0.811, 0.826/0.799, 0.814/0.803 and 0.747/0.729, respectively. The AUROC values of all indices decreased in the subgroup of the population with overweight or diabetes.
Conclusion
The NAFLD-related scores would be equally useful to screen MAFLD and seemed to be more compatible with MAFLD. The FLI was optimal in both MAFLD and NAFLD diagnoses. However, a new predictive indicator suitable for various characteristics of the population is worth further development in the future.
Keywords: metabolic-associated fatty liver disease, National Health and Nutrition Examination Survey, noninvasive diagnosis, steatosis indices
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a global public health issue, which shows an increasing prevalence with the growing epidemic of diabetes and obesity [1]. In 2020, an international expert consensus redefined NAFLD as metabolic-associated fatty liver disease (MAFLD), and updated diagnostic criteria were proposed [2]. MAFLD was diagnosed if there is evidence of hepatic steatosis by imaging, blood biomarkers/scores or histology, plus one of the three conditions: overweight/obesity or type 2 diabetes (T2DM) or the presence of metabolic risk abnormalities [2]. With changes in diet, lifestyle and health, MAFLD is expected to become the leading cause of chronic liver disease worldwide in the coming decades. In addition, MAFLD may progress and develop into cirrhosis and hepatocellular carcinoma. Therefore, the detection of MAFLD is crucial for public health research and the prevention of progress into advanced diseases for the individual.
Liver biopsy is the gold standard to diagnose fatty liver disease but invasiveness and unavoidable complications limit its clinical application. Abdominal ultrasonography is the recommended primary diagnostic tool for detecting hepatic steatosis of MAFLD [3]. However, for large-scale epidemiological investigations, it is difficult to perform ultrasonography on each participant due to its cost and the need for specialized equipment and staff. Steatosis indices, such as the fatty liver index (FLI) [4], Framingham Steatosis index (FSI) [5], Zhejiang University index (ZJU) [6], lipid accumulation product (LAP) [7], hepatitis steatosis index (HSI) [8] and visceral adiposity index (VAI) [9] have been tested and generally used in diagnosing NAFLD [10]. MAFLD is the new term of NAFLD, with new diagnostic criteria that do not rule out significant alcohol consumption or any other liver diseases. Given the significant changes in diagnostic criteria between MAFLD and NAFLD, the utility of the common steatosis indices for identifying MAFLD is unclear and needs to be re-evaluated. The controlled attenuation parameter (CAP) is an accurate parameter to reflect liver steatosis detected by vibration-controlled transient elastography (VCTE) [11] and MAFLD has not been studied using VCTE and hepatic steatosis index. Because the National Health and Nutritional Examination Survey (NHANES) of the 2017–2018 cycle first performed VCTE in the US nationally representative sample, this study aimed to select the VCTE-diagnosed MAFLD group to examine the diagnostic capabilities of widely used NAFLD-related indices for diagnosing MAFLD and calculate the cutoff values as well as compare the diagnostic reliability and the cutoff values of the indices studied for MAFLD with those for NAFLD.
Materials and methods
Data source and study population
The NHANES is a continuous cross-sectional survey conducted by the National Center for Health Statistics (NCHS) for the assessment of health and nutritional status among the general public of the USA, which included unbiased demographics, dietary, examination, laboratory and questionnaire data. The NHANES has become a frequent database used to study MAFLD or NAFLD because of recently available data on FibroScan liver steatosis assessment [12–15]. The study cohort was obtained from the NHANES database (2017–2018). There were 9254 participants in NHANES 2017–2018 and the individuals were included in our study if he/she met the below criteria: (1) The fasting time before the venipuncture was at least 8 h; (2) Elastography examination status was described as complete [fasting >3 h; at least 10 valid measures; liver stiffness interquartile range/median E (IQRe) <30%]; (3) Complete records for the diagnosis of MAFLD and calculations of FLI, FSI, ZJU, LAP, HIS and VAI. The NHANES 2017–2018 survey was approved by the NCHS Ethics Review Board and informed consent was obtained from all participants. All NHANES datasets are anonymous and can be accessed online for free (https://www.cdc.gov/nchs/nhanes/index.htm).
Data collection and definitions
Data from interviews and physical examinations were collected for analysis, involving age, gender, ethnicity, waist circumference, BMI, SBP, DBP (SBP/DBP measured three times and the mean of the three measurements was adopted), alanine aminotransferase (ALT), aspartate aminotransferase (AST), high-density lipoprotein (HDL), triglyceride, gamma-glutamyl-transferase (GGT), fasting glucose, fasting insulin, hemoglobin A1c (HbA1c), high-sensitivity C-reactive protein (hs-CRP), markers of infection with hepatitis viruses, alcohol intake and the results of the liver ultrasound transient elastography. Detailed descriptions of the procedures and methodologies for measurements of the above data are reported elsewhere [16]. Homeostasis model assessment-estimated insulin resistance (HOMA-IR) was calculated as [fasting insulin (mU/ml) × fasting glucose (mmol/l)/22.5]. The markers of liver steatosis (FLI, FSI, ZJU, LAP, HIS and VAI) were calculated as Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/EJGH/A810 and cutoff values of FLI, ZJU, LAP, HIS and VAI for detecting NAFLD were determined according to previous literature, with the cutoff points of 60 [4], 38 [6], 38 [17], 36 [8] and 1.78 [18], respectively.
Hypertension was identified if: (1) SBP value ≥140 mmHg and/or a DBP value ≥90 mmHg; (2) told by a doctor or other health professional and (3) current use of antihypertensive medications [19]. Diabetes was defined if: (1) told by a doctor or other health professional; (2) Use of antidiabetic medications; (3) HbA1c (%) >6.5; (4) fasting glucose (mmol/L) ≥7.0 mmol/L and (5) random blood glucose (mmol/L) ≥11.1 mmol/L [20]. MAFLD was diagnosed by the evidence of hepatic steatosis and at least one among the following: overweight/obesity, presence of T2DM and metabolic dysregulation [2]. Steatosis was defined as the median CAP ≥288 dB/m according to a recent study that compared CAP with MRI proton density fat fraction (MRI-PDFF) [21] and significant fibrosis was defined as the liver stiffness measurements ≥8 kPa [13]. We also used CAP scores of 288 with an IQR of CAP <30 dB/m to define steatosis for sensitivity analysis considering that the diagnostic accuracy of CAP for the detection of hepatic steatosis is more reliable when an IQR of CAP is <30 dB/m [21]. Overweight/obesity was defined as BMI ≥25 kg/m2. Metabolic dysregulation was defined as the occurrence of two or more of the following conditions: (1) waist circumference ≥102 cm in males and 88 cm in females, (2) blood pressure ≥130/85 mmHg or treatment of specific drug, (3) triglyceride ≥1.70 mmol/L or treatment of specific drug, (4) HDL <1.0 mmol/L in men and <1.3 mmol/l in women, (5) prediabetes (i.e. fasting glucose: 5.6–6.9 mmol/l or 2-h post-load glucose: 7.8–11.0 mmol/l or HbA1c: 5.7–6.4%), (6) HOMA-IR ≥2.5 and (7) hs-CRP >2 mg/L.
We defined NAFLD as the presence of liver steatosis in the absence of other causes for steatosis (e.g. viral hepatitis and overdose of alcohol) [22]. Viral hepatitis was defined by the presence of HCV-RNA and/or confirmed hepatitis C antibodies (hepatitis C virus) or the hepatitis B surface antigen (hepatitis B virus) [15]. Overdose alcohol was indicated if >30 g/day for men and >20 g/day for women [15].
Statistical analysis
Continuous variables were expressed as median (interquartile range) and categorical variables were presented as counts (percentages, %). Mann-Whitney U-test was used for continuous variables and the χ2 test for categorical variables. The area under the receiver operating characteristic (AUROC) curve was used to determine diagnostic performance and calculate the optimal cutoffs, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). The highest value of Youden’s index was used to determine optimal cutoffs. Pairwise comparisons between AUROC values of different steatosis indices were conducted by the DeLong method. Statistical analyses were performed using SPSS version 22.0 and MedCalc version 19.0.4 software. A two-tailed P value <0.05 was considered statistically significant.
Results
Characteristics of participants
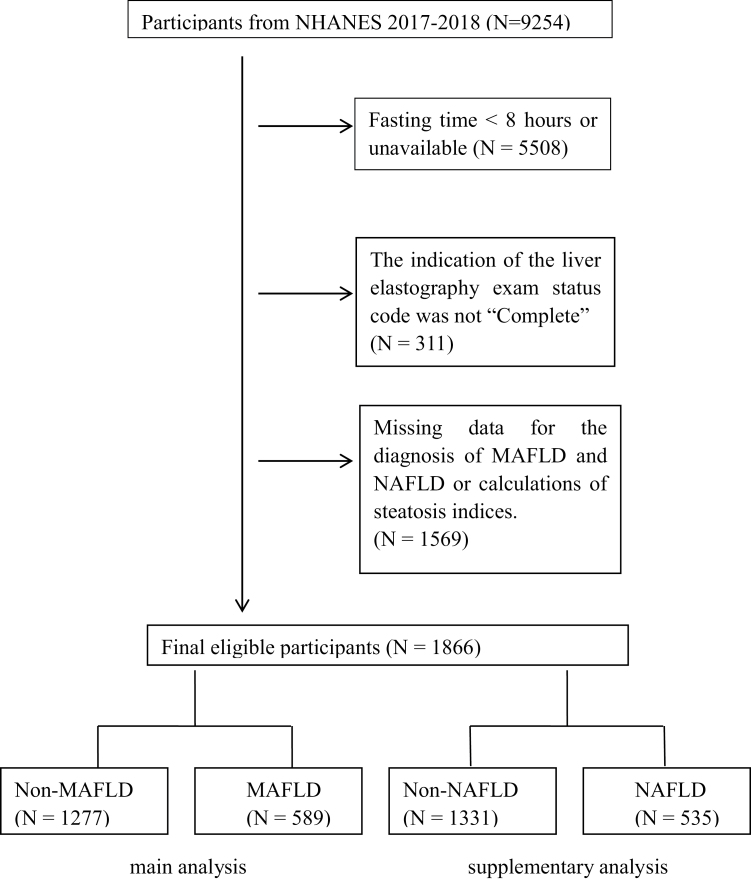
After excluding unreliable elastographic examinations as well as missing data for diagnosis of MAFLD or NAFLD and calculations of steatosis indices, a total of 1866 participants from NHANES in the 2017–2018 cycle were included in our study (Fig. 1). Of these, 589 participants (280 female and 309 male) complied with diagnostic criteria of MAFLD and 535 participants (266 female and 269 male) was identified as NAFLD. As presented in Table 1, the prevalence of diabetes, hypertension and significant fibrosis in the MAFLD/NAFLD group were significantly higher than non-MAFLD/non-NAFLD group. The participants with MAFLD or NAFLD have higher waist circumference, BMI, SBP, DBP, ALT, AST, triglyceride, GGT and lower HDL than those of the control group. The values of all steatosis indices were significantly higher in people with MAFLD/NAFLD than without MAFLD/NAFLD. The characteristics of the 705 subjects with IQR of CAP <30 dB/m for analysis of sensitivity were shown in Supplementary Table 2, Supplemental digital content 2, http://links.lww.com/EJGH/A811.
Fig. 1.
Flowchart of the enrolled participants in the study.
Table 1.
Characteristics of the subjects according to MAFLD or NAFLD
| Characteristics | Non-MAFLD (n = 1277) | MAFLD (n = 589) | P value | Non-NAFLD (n = 1331) | NAFLD (n = 535) | P value |
|---|---|---|---|---|---|---|
| Age (year) | 40.00 | 54.00 | <0.001 | 41.00 | 54.00 | <0.001 |
| (23.00–60.00) | (39.00–64.00) | (23.00–60.00) | (38.00–64.00) | |||
| Female (N, %) | 692, 54.2% | 280, 47.5% | 0.008 | 706, 53.0% | 266, 49.7% | 0.194 |
| Race (N, %) | <0.001 | <0.001 | ||||
| Mexican American | 148, 11.6% | 126, 21.4% | 161, 12.1% | 113, 21.1% | ||
| Other Hispanic | 114, 8.9% | 54, 9.2% | 121, 9.1% | 47, 8.8% | ||
| Non-Hispanic | 759, 59.4% | 326, 55.3% | 789, 59.3% | 296, 55.3% | ||
| Other Race | 256, 20.0% | 83, 14.1% | 260, 19.5% | 79, 14.8% | ||
| Diabetes (N, %) | 139, 10.9% | 210, 35.7% | <0.001 | 156, 11.7% | 193, 36.1% | <0.001 |
| Hypertension (N, %) | 394, 30.9% | 324, 55.0% | <0.001 | 427, 32.1% | 291, 54.4% | <0.001 |
| Significant fibrosis (N, %) | 62, 4.9% | 99, 16.8% | <0.001 | 71, 5.3% | 90, 16.8% | <0.001 |
| Waist circumference (cm) | 90.30 | 109.80 | <0.001 | 91.70 | 109.80 | <0.001 |
| (80.40–101.50) | (99.80–119.80) | (80.80–102.50) | (99.80–120.10) | |||
| BMI (kg/m2) | 25.80 | 32.50 | <0.001 | 26.00 | 32.40 | <0.001 |
| (22.40–29.90) | (28.80–37.00) | (22.60–30.20) | (28.85–37.25) | |||
| SBP (mmHg) | 115.0 | 126.0 | <0.001 | 116.0 | 125.0 | <0.001 |
| (107.0–129.0) | (116.0–139.0) | (108.0–130.0) | (115.0–138.0) | |||
| DBP (mmHg) | 69.00 | 74.00 | <0.001 | 70.00 | 74.00 | <0.001 |
| (63.00–77.00) | (66.00–81.00) | (63.00–77.00) | (65.00–81.00) | |||
| ALT (U/L) | 15.00 | 22.00 | <0.001 | 16.00 | 21.00 | <0.001 |
| (11.00–22.00) | (16.00–33.00) | (11.00–22.00) | (15.00–32.00) | |||
| AST (U/L) | 18.00 | 20.00 | <0.001 | 19.00 | 20.00 | <0.001 |
| (15.00–22.00) | (16.00–27.00) | (15.00–23.00) | (16.00–26.00) | |||
| HDL (mmol/L) | 1.40 | 1.19 | <0.001 | 1.40 | 1.19 | <0.001 |
| (1.16–1.66) | (1.03–1.40) | (1.16–1.66) | (1.03–1.40) | |||
| TG (mmol/L) | 0.82 | 1.32 | <0.001 | 0.85 | 1.30 | <0.001 |
| (0.58–1.23) | (0.96–1.86) | (0.59–1.29) | (0.93–1.85) | |||
| GGT (U/L) | 17.00 | 25.00 | <0.001 | 18.00 | 24.00 | <0.001 |
| (12.00–25.00) | (18.00–40.00) | (12.00–27.00) | (17.00–38.00) | |||
| Fasting glucose (mmol/l) | 5.55 | 6.11 | <0.001 | 5.55 | 6.11 | <0.001 |
| (5.27–5.94) | (5.61–7.11) | (5.27–6.00) | (5.61–7.11) | |||
| Fasting insulin (mU/ml) | 8.25 | 15.74 | <0.001 | 8.40 | 15.74 | <0.001 |
| (5.63–12.32) | (10.09–24.12) | (5.79–12.74) | (10.16–24.52) | |||
| HbA1c | 5.40 | 5.80 | <0.001 | 5.40 | 5.80 | <0.001 |
| (5.20–5.70) | (5.40–6.40) | (5.20–5.70) | (5.40–6.40) | |||
| hs-CRP (mg/L) | 1.27 | 2.94 | <0.001 | 1.29 | 2.99 | <0.001 |
| (0.59–2.96) | (1.33–5.86) | (0.60–2.99) | (1.35–6.00) | |||
| HOMA-IR | 2.07 | 4.63 | <0.001 | 2.12 | 4.68 | <0.001 |
| (1.39–3.27) | (2.81–7.46) | (1.42–3.38) | (2.82–7.55) | |||
| FLI | 26.20 | 83.88 | <0.001 | 29.49 | 83.43 | <0.001 |
| (7.41–59.93) | (59.57–94.84) | (8.19–63.64) | (58.43–94.82) | |||
| FSI | 10.10 | 44.72 | <0.001 | 11.09 | 44.37 | <0.001 |
| (3.88–24.69) | (23.86–70.44) | (4.08–26.57) | (23.37–70.42) | |||
| ZJU | 36.10 | 45.35 | <0.001 | 36.45 | 45.47 | <0.001 |
| (32.15–41.31) | (40.97–50.87) | (32.35–41.75) | (40.92–50.94) | |||
| LAP | 24.04 | 63.71 | <0.001 | 25.46 | 62.05 | <0.001 |
| (12.11–43.69) | (42.30–94.95) | (12.66–46.15) | (40.32–94.82) | |||
| HSI | 34.00 | 43.61 | <0.001 | 34.47 | 43.71 | <0.001 |
| (30.00–39.47) | (38.74–48.87) | (30.20–39.85) | (38.79–49.02) | |||
| VAI | 0.97 | 1.92 | <0.001 | 0.99 | 1.88 | <0.001 |
| (0.60–1.54) | (1.21–2.86) | (0.62–1.60) | (1.16–2.86) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; FLI, fatty liver index; FSI, Framingham Steatosis Index; GGT, gamma-glutamyl-transferase; HbA1c, Hemoglobin A1c; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein; HSI, hepatitis steatosis index; LAP, lipid accumulation product; MAFLD, metabolic dysfunction-associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease; TG, triglyceride; VAI, visceral adiposity index; ZJU, Zhejiang University index.
Comparisons between index systems for MAFLD and NAFLD prediction
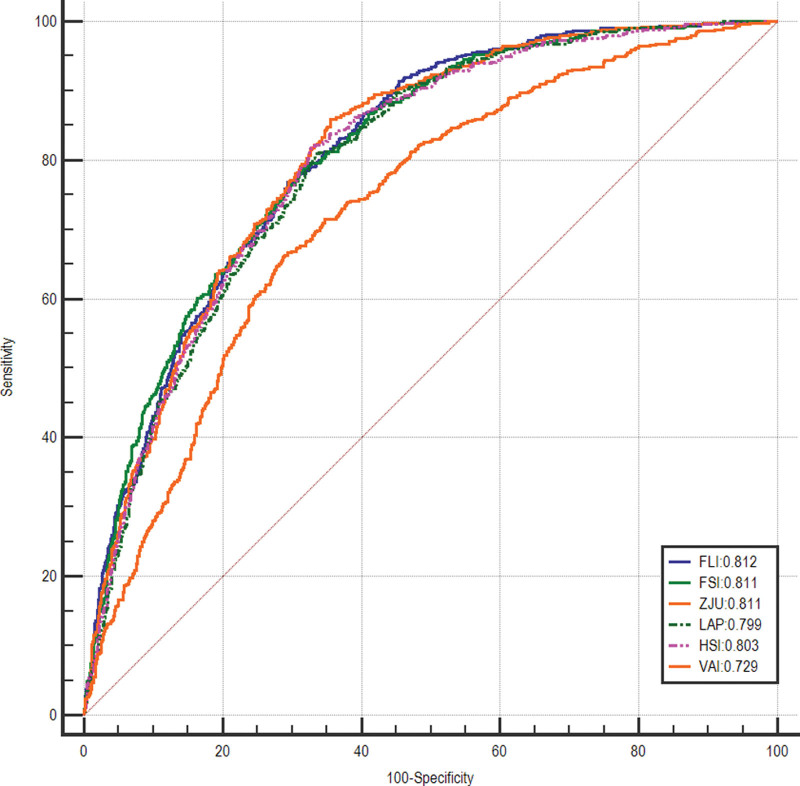
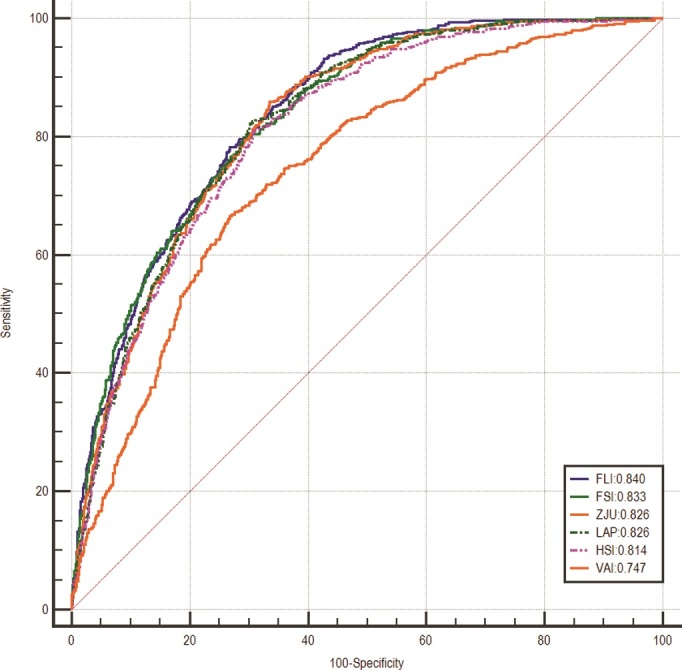
The AUROC values of steatosis indices for the prediction of MAFLD and NAFLD were calculated and pairwise comparisons between indices were performed. The predicted indices showed adequate diagnostic performance in detecting MAFLD, with AUROC values over 0.800 except for VAI had an acceptable performance with AUROC of 0.747. The results of pairwise comparisons of different indices were listed in Table 2. The FLI had the highest AUROC of 0.840 [95% confidence interval (CI), 0.822–0.858], followed by the FSI (0.833; 95% CI, 0.815–0.852), ZJU (0.826; 95% CI, 0.808–0.845), LAP (0.826; 95% CI, 0.807–0.844), HSI (0.814; 95% CI, 0.795–0.834) and VAI (0.747; 95% CI, 0.723–0.770) (Fig. 2 and Table 3). The cut-off value of FLI was 56.93 for the diagnosis of MAFLD, with a sensitivity of 0.783, a specificity of 0.732, a PPV of 0.574, and an NPV of 0.880 (Table 3). The AUROCs of studied tools in detecting NAFLD were above 0.800 besides VAI but slightly lower than those of MAFLD, with the values of 0.812 (95% CI, 0.792–0.832) for FLI, 0.811 (95% CI, 0.791–0.832) for FSI, 0.811 (95% CI, 0.791–0.831) for ZJU, 0.799 (95% CI, 0.778–0.820) for LAP, 0.803 (95% CI, 0.782–0.823) for HSI and 0.729 (95% CI, 0.705–0.754) for VAI, respectively (Fig. 3 and Table 3). The FLI still had the highest AUROC value of 0.812 for screening NAFLD, with a sensitivity of 0.727, a specificity of 0.724 and an NPV of 0.868 when applying the cut-off point of 60 (Table 3). The sensitivity analysis showed similar results (Supplementary Table 3, Supplemental digital content 3, http://links.lww.com/EJGH/A812 and Supplementary Table 4, Supplemental digital content 4, http://links.lww.com/EJGH/A813).
Table 2.
Pairwise comparisons of receiver operatingcharacteristic curves of different steatosis indices
| Indices | FLI | FSI | ZJU | LAP | HSI | VAI |
|---|---|---|---|---|---|---|
| Accuracy for MAFLD diagnosis | ||||||
| AUROC | 0.840 | 0.833 | 0.826 | 0.826 | 0.814 | 0.747 |
| (95% CI) | (0.822–0.858) | (0.815–0.852) | (0.808–0.845) | (0.807–0.844) | (0.795–0.834) | (0.723–0.770) |
| FLI | – | – | – | – | – | – |
| FSI | P = 0.122 | – | – | – | – | – |
| ZJU | P = 0.008 | P = 0.196 | – | – | – | – |
| LAP | P = 0.026 | P = 0.272 | P = 0.937 | – | – | – |
| HSI | P < 0.001 | P < 0.112 | P < 0.001 | P = 0.253 | – | – |
| VAI | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | – |
| Accuracy for NAFLD diagnosis | ||||||
| AUROC | 0.812 | 0.811 | 0.811 | 0.799 | 0.803 | 0.729 |
| (95% CI) | (0.792–0.832) | (0.791–0.832) | (0.791–0.831) | (0.778–0.820) | (0.782–0.823) | (0.705–0.754) |
| FLI | – | – | – | – | – | – |
| FSI | P = 0.889 | – | – | – | – | – |
| ZJU | P = 0.836 | P = 0.935 | – | – | – | – |
| LAP | P = 0.052 | P = 0.087 | P = 0.179 | – | – | – |
| HSI | P = 0.129 | P = 0.165 | P = 0.014 | P = 0.714 | – | – |
| VAI | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | – |
Endash indicates repeated comparisons.
AUROC, area under the receiver operating characteristic; CI, confidence interval; FLI, fatty liver index; FSI, Framingham Steatosis Index; HSI, hepatitis steatosis index; LAP, lipid accumulation product; MAFLD, metabolic dysfunction-associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease; VAI, visceral adiposity index; ZJU, Zhejiang University index.
Fig. 2.
Receiver operating characteristic curves of FLI, FSI, ZJU, LAP, HSI, and VAI for screening MAFLD. FLI, fatty liver index; FSI, Framingham Steatosis Index; HSI, hepatitis steatosis index; LAP, lipid accumulation product; MAFLD, metabolic dysfunction-associated fatty liver disease; VAI, visceral adiposity index; ZJU, Zhejiang University index.
Table 3.
Diagnostic performances of different steatosis indices
| Index systems | AUROC | SEN | SPE | PPV | NPV | Cut-off value |
|---|---|---|---|---|---|---|
| Diagnostic ability for MAFLD | ||||||
| FLI | 0.840 (0.822–0.858) | 0.783 | 0.732 | 0.574 | 0.880 | 56.93 |
| FSI | 0.833 (0.815–0.852) | 0.793 | 0.710 | 0.558 | 0.881 | 21.12 |
| ZJU | 0.826 (0.808–0.845) | 0.859 | 0.665 | 0.542 | 0.911 | 39.16 |
| LAP | 0.826 (0.807–0.844) | 0.827 | 0.694 | 0.555 | 0.897 | 36.74 |
| HSI | 0.814 (0.795–0.834) | 0.810 | 0.691 | 0.547 | 0.887 | 37.85 |
| VAI | 0.747 (0.723–0.770) | 0.666 | 0.731 | 0.533 | 0.826 | 1.47 |
| Diagnostic ability for NAFLD | ||||||
| FLI | 0.812 (0.792–0.832) | 0.727 | 0.724 | 0.515 | 0.868 | 60.00 |
| FSI | 0.811 (0.791–0.832) | 0.753 | 0.708 | 0.509 | 0.877 | 23.00 |
| ZJU | 0.811 (0.791–0.831) | 0.892 | 0.583 | 0.462 | 0.930 | 38.00 |
| LAP | 0.799 (0.778–0.820) | 0.787 | 0.678 | 0.495 | 0.888 | 38.00 |
| HSI | 0.803 (0.782–0.823) | 0.877 | 0.571 | 0.451 | 0.920 | 36.00 |
| VAI | 0.729 (0.705–0.754) | 0.533 | 0.787 | 0.501 | 0.807 | 1.78 |
AUROC, area under the receiver operating characteristic; FLI, fatty liver index; FSI, Framingham Steatosis Index; HSI, hepatitis steatosis index; LAP, lipid accumulation product; NAFLD, nonalcoholic fatty liver disease; NPV, negative predictive value; PPV, positive predictive value; ROC, receiver operating characteristic; SEN, sensitivity; SPE, specificity; VAI, visceral adiposity index; ZJU, Zhejiang University index.
Fig. 3.
Receiver operating characteristic curves of FLI, FSI, ZJU, LAP, HSI, and VAI for screening NAFLD. FLI, fatty liver index; FSI, Framingham Steatosis Index; HSI, hepatitis steatosis index; LAP, lipid accumulation product; NAFLD, nonalcoholic fatty liver disease; VAI, visceral adiposity index; ZJU, Zhejiang University index.
Diagnostic accuracy of indices for diagnosing MAFLD in different subgroups.
Subgroup analysis was conducted by dividing the participants into different groups according to age, gender, BMI and the presence of diabetes. The FLI showed better diagnostic ability in different subgroups than other steatosis indices except for the male group. In the male group, the AUROC of FLI was 0.845, which was slightly lower than FSI (AUROC, 0.849) and ZJU (AUROC, 0.846). The AUROC values of all indices for diagnosing MAFLD tended to decrease in those having diabetes and BMI over 25 compared with those in the other subgroups. Table 4 showed the diagnostic performance and population-specific cut-off values of these indices in different subgroups.
Table 4.
Diagnostic performance of different indices for diagnosing MAFLD in different subgroups
| Indices | ROC (95% CI) | SEN | SPE | PPV | NPV | Cut-off value |
|---|---|---|---|---|---|---|
| Age <60 | ||||||
| FLI | 0.858 (0.837–0.878) | 0.892 | 0.672 | 0.510 | 0.942 | 41.50 |
| FSI | 0.853 (0.832–0.874) | 0.775 | 0.770 | 0.564 | 0.899 | 21.25 |
| ZJU | 0.843 (0.822–0.865) | 0.917 | 0.636 | 0.492 | 0.952 | 37.97 |
| LAP | 0.845 (0.823–0.867) | 0.806 | 0.754 | 0.557 | 0.910 | 36.72 |
| HSI | 0.832 (0.810–0.855) | 0.836 | 0.691 | 0.509 | 0.917 | 37.74 |
| VAI | 0.767 (0.739–0.795) | 0.669 | 0.768 | 0.525 | 0.858 | 1.47 |
| Age ≥60 | ||||||
| FLI | 0.791 (0.754–0.827) | 0.777 | 0.658 | 0.605 | 0.814 | 58.04 |
| FSI | 0.777 (0.739–0.815) | 0.677 | 0.770 | 0.665 | 0.779 | 32.47 |
| ZJU | 0.783 (0.746–0.821) | 0.856 | 0.593 | 0.587 | 0.859 | 39.14 |
| LAP | 0.768 (0.730–0.807) | 0.677 | 0.729 | 0.628 | 0.769 | 51.00 |
| HSI | 0.778 (0.740–0.817) | 0.782 | 0.676 | 0.620 | 0.821 | 37.88 |
| VAI | 0.690 (0.646–0.733) | 0.616 | 0.699 | 0.580 | 0.729 | 1.61 |
| Male | ||||||
| FLI | 0.845 (0.820–0.870) | 0.812 | 0.721 | 0.606 | 0.879 | 56.93 |
| FSI | 0.849 (0.824–0.875) | 0.783 | 0.754 | 0.627 | 0.868 | 24.18 |
| ZJU | 0.846 (0.820–0.872) | 0.812 | 0.744 | 0.626 | 0.882 | 39.16 |
| LAP | 0.832 (0.805–0.858) | 0.806 | 0.718 | 0.601 | 0.875 | 37.31 |
| HSI | 0.828 (0.800–0.855) | 0.793 | 0.718 | 0.598 | 0.868 | 37.19 |
| VAI | 0.753 (0.721–0.786) | 0.641 | 0.769 | 0.595 | 0.802 | 1.45 |
| Female | ||||||
| FLI | 0.835 (0.810–0.860) | 0.925 | 0.604 | 0.486 | 0.952 | 35.74 |
| FSI | 0.818 (0.791–0.845) | 0.771 | 0.710 | 0.518 | 0.885 | 21.12 |
| ZJU | 0.824 (0.798–0.850) | 0.904 | 0.607 | 0.482 | 0.940 | 39.36 |
| LAP | 0.822 (0.796–0.848) | 0.843 | 0.682 | 0.518 | 0.915 | 36.72 |
| HSI | 0.814 (0.788–0.841) | 0.868 | 0.640 | 0.494 | 0.923 | 37.84 |
| VAI | 0.749 (0.716–0.782) | 0.768 | 0.632 | 0.457 | 0.871 | 1.29 |
| BMI <25 | ||||||
| FLI | 0.870 (0.810–0.931) | 0.781 | 0.840 | 0.216 | 0.986 | 19.82 |
| FSI | 0.837 (0.768–0.905) | 0.844 | 0.714 | 0.142 | 0.988 | 5.69 |
| ZJU | 0.827 (0.743–0.911) | 0.656 | 0.911 | 0.292 | 0.979 | 35.33 |
| LAP | 0.861 (0.788–0.934) | 0.875 | 0.781 | 0.183 | 0.991 | 22.68 |
| HSI | 0.757 (0.667–0.846) | 0.750 | 0.730 | 0.135 | 0.981 | 31.37 |
| VAI | 0.824 (0.735–0.912) | 0.844 | 0.761 | 0.166 | 0.989 | 1.19 |
| BMI ≥25 | ||||||
| FLI | 0.752 (0.725–0.778) | 0.702 | 0.675 | 0.630 | 0.742 | 69.12 |
| FSI | 0.743 (0.716–0.770) | 0.632 | 0.741 | 0.658 | 0.719 | 35.75 |
| ZJU | 0.728 (0.700–0.756) | 0.664 | 0.683 | 0.623 | 0.721 | 43.24 |
| LAP | 0.738 (0.710–0.765) | 0.724 | 0.638 | 0.612 | 0.745 | 46.12 |
| HSI | 0.716 (0.688–0.744) | 0.671 | 0.656 | 0.606 | 0.717 | 41.06 |
| VAI | 0.679 (0.649–0.708) | 0.670 | 0.634 | 0.590 | 0.709 | 1.45 |
| Diabetes | ||||||
| FLI | 0.736 (0.683–0.790) | 0.524 | 0.827 | 0.821 | 0.535 | 88.23 |
| FSI | 0.736 (0.682–0.789) | 0.719 | 0.655 | 0.759 | 0.607 | 36.48 |
| ZJU | 0.739 (0.686–0.792) | 0.690 | 0.676 | 0.763 | 0.591 | 44.55 |
| LAP | 0.724 (0.669–0.778) | 0.600 | 0.748 | 0.783 | 0.553 | 69.57 |
| HSI | 0.719 (0.663–0.774) | 0.881 | 0.460 | 0.712 | 0.719 | 38.08 |
| VAI | 0.658 (0.600–0.717) | 0.729 | 0.561 | 0.715 | 0.578 | 1.63 |
| No diabetes | ||||||
| FLI | 0.841 (0.820–0.862) | 0.908 | 0.609 | 0.436 | 0.952 | 35.19 |
| FSI | 0.829 (0.807–0.851) | 0.747 | 0.748 | 0.496 | 0.897 | 21.12 |
| ZJU | 0.820 (0.797–0.842) | 0.807 | 0.708 | 0.480 | 0.917 | 39.17 |
| LAP | 0.824 (0.802–0.846) | 0.792 | 0.731 | 0.495 | 0.913 | 36.74 |
| HSI | 0.812 (0.788–0.835) | 0.768 | 0.721 | 0.479 | 0.903 | 37.85 |
| VAI | 0.727 (0.709–0.765) | 0.704 | 0.670 | 0.416 | 0.872 | 1.22 |
FLI, fatty liver index; FSI, Framingham Steatosis Index; HSI, hepatitis steatosis index; LAP, lipid accumulation product; NAFLD, nonalcoholic fatty liver disease; NPV, negative predictive value; PPV, positive predictive value; ROC, receiver operating characteristic; SEN, sensitivity; SPE, specificity; VAI, visceral adiposity index; ZJU, Zhejiang University index.
Discussion
Liver biopsy or imaging used to diagnose MAFLD is burdensome and hard to implement on a large scale, so applying noninvasive algorithms is a valuable alternative method. This study for the first time validated the NAFLD-related steatosis indices for screening VCTE-diagnosed MAFLD and compared the diagnostic validity of noninvasive clinical scores between NAFLD and MAFLD. FLI, FSI, ZJU, LAP and HSI showed satisfactory performance in the whole and subgroups in detecting MAFLD whereas the AUROC of VAI varied greatly in the different subgroups. The FLI had the highest AUROC in the general US population with a sensitivity of 0.783 and a specificity of 0.732. The cut-off value of FLI was 56.93. In subgroup analysis, the diagnostic accuracy of all indices declined in people with high BMI (BMI ≥25) or diabetes. Furthermore, our findings suggested the diagnostic capacity of NAFLD-related indices had not been compromised by MAFLD nomenclature whereas all the scores appear to be more compatible with MAFLD with few differences between the cutoff points. The FLI outperformed other scores in both diagnoses of MAFLD and NAFLD in the general population.
NAFLD has been redefined as MAFLD, which is no longer an exclusion diagnosis. In addition, the accuracy of indices is influenced by the age, sex, ethnicity and region of the sample populations. Therefore, further validation of the indices widely used in NAFLD diagnosis is required for different cohorts to distinguish between people with MAFLD and those without. So far, only a few studies examined the validity of the steatosis index in discriminating MAFLD. A comparison of the performance of hepatic steatosis indices in identifying MAFLD was conducted by Han and Lee [23] based on the Korean population. Although ethnicity and environment are different from Europe, their findings were similar to those of our study showing FLI performs better at diagnosing MAFLD. It is worth noting that FLI has been recommended for diagnosing fatty liver in large population studies by guidelines [3]. The predictive ability of the FSI for MAFLD has not been validated and the prediction power of ZJU, an algorithm developed in China, is unclear in western populations although it showed clinically acceptable capability for the detection of MAFLD in eastern populations [23]. Our study observed that FSI and ZJU had similar diagnostic performances as FLI and their AUROC values in the male group were slightly higher than FLI. Unfortunately, they contain more parameters and their calculations are more complex than FLI [5,6]. LAP, a cost-effective index only based on waist circumference and triglyceride [7], is a good choice for screening MAFLD when FLI is unavailable, especially in the normal BMI US population (BMI <25) as its AUROC value is second only to FLI in the normal-weight subgroup. HSI was developed based on ALT/AST ratio and BMI [8] and is simpler than FLI but it is not as well as FLI. Some studies have validated VAI for the prediction of MAFLD while its predictive role requires further determination [24]. The VAI had a variable accuracy among subgroups and relatively unsatisfactory diagnostic value in the whole population compared to other scores according to our findings.
These noninvasive clinical scores have shown useful diagnostic value in NAFLD. In the original studies, the AUROC values of FLI, FSI, ZJU and HSI for identifying steatosis were 0.840, 0.845, 0.822 and 0.812, respectively, with cutoff values of 60, 23, 38 and 36, respectively [4–6,8]. Although LAP and VAI were developed for predicting cardiovascular disease, previous studies have demonstrated that the discriminative capacity of these steatosis scores for NAFLD could reach 75%, with the cutoff points of 38 for LAP and 1.78 for VAI based on biopsy-proven NAFLD [17,18]. One study in Italy reported FLI (0.77), ZJU (0.76), HSI (0.75) and LAP (0.74) in the diagnosis of NAFLD [25], another study from China showed promising results of FLI (0.880), FSI (0.864), ZJU (0.861), LAP (0.853) and HSI (0.833) for identifying NAFLD [26]. To compare the diagnostic reliability and the cutoff values of the indices between NAFLD and MAFLD, we validated these indices again in our cross-sectional cohort, and our results showing they are reliable predictors for NAFLD were consistent with the available evidence. Moreover, the performances of the studied noninvasive scores in MAFLD are as good as previously reported for NAFLD and the optimal cutoff points for predicting MAFLD were 56.93 (FLI), 21.12 (FSI), 39.16 (ZJU), 36.74 (LAP), 37.85 (HSI) and 1.47 (LAP), respectively, which was similar with those previously reported cutoff points of 60 (FLI), 23 (FSI), ZJU (38) HSI (36) and VAI (1.78) for NAFLD. FLI performs best both in diagnosing MAFLD and NAFLD. These data support the notion that a name change from NAFLD to MAFLD seems not to influence the diagnostic accuracy for NAFLD-related steatosis indices. Furthermore, according to the results showing AUROC values for all indices were somewhat higher in MAFLD than in NAFLD of this study, NAFLD-related steatosis indices seem to be more compatible with MAFLD. The MAFLD diagnostic criteria include waist circumference, BMI, triglyceride, HDL and fasting glucose, which are parameters in steatosis indices, so this may explain why indices predict MAFLD well.
Recently, another study investigated the diagnostic accuracy of common hepatic steatosis formulas for MAFLD based on the NHANES database from 1988 to 1994 [27]. However, as the authors admitted in their original literature, the diagnosis of hepatic steatosis was based on ultrasonography rather than CAP measurement by VCTE, a more sensitive method for assessing steatosis. On the other hand, considering the prevalence of MAFLD has gradually risen with the growing incidence of obesity and T2DM in these years according to a recent study based on the NHANES database [13], it seems reasonable to reconsider the accuracy of traditional steatosis indices in differentiating MAFLD among current US population. Interestingly, in this study, all fatty liver formulae had declined AUROC values in the subgroup with high BMI (≥25 kg/m2) rather than low BMI (<25 kg/m2). Our findings were contrary, which is probably explained by the rising prevalence of obesity as the result of socioeconomic changes and the rapid shift from malnutrition to over-calorie eating habits. Besides overweight people, the performance of these scores was poor in people with diabetes. Moreover, the cutoff values varied significantly in different subgroups. Thus, a novel efficient index that is suitable for all populations is required for the detection of MAFLD patients, with population-specific cutoff values.
We have to acknowledge some limitations in our study. On the one hand, the evidence of hepatic steatosis was assessed by VCTE rather than a liver biopsy. However, CAP using transient elastography is regarded as a reliable method to diagnose liver steatosis according to previous literature [28,29] and biopsy is not easy to perform in clinical settings. On the other hand, the data used in the study consisted mainly of Caucasians in the USA and the performance of steatosis indices in other cohorts remains unclear. More studies in more regions and races are required to explore the accuracy of indices.
In conclusion, the FLI, FSI, ZJU, LAP, HIS and VAI as frequently used in NAFLD were still useful in identifying VCTE-diagnosed MAFLD and even seem to improve MAFLD. The FLI performed best in the whole population, calculated by serum biomarkers, and may serve as a practical and alternative tool to screen MAFLD when imaging modalities, such as in large-scale epidemiological studies, are not available or feasible. A new prediction model considering all kinds of characteristics of the population is hoped to be developed in the future.
Acknowledgements
The authors thank all researchers who are dedicated to the study.
M.D. and G.L. conceived and designed the study. J.C. and X.M. performed the analysis. J.C., X.M. and G.L. wrote and reviewed the article.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Dr. Jie Chen and Dr. Xueying Mao contributed equally to the writing of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.eurojgh.com.
References
- 1.Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology 2020; 158:1851–1864. [DOI] [PubMed] [Google Scholar]
- 2.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020; 73:202–209. [DOI] [PubMed] [Google Scholar]
- 3.Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian Pacific Association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int 2020; 14:889–919. [DOI] [PubMed] [Google Scholar]
- 4.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006; 6:33. Published 2006 Nov 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long MT, Pedley A, Colantonio LD, Massaro JM, Hoffmann U, Muntner P, et al. Development and validation of the Framingham steatosis index to identify persons with hepatic steatosis. Clin Gastroenterol Hepatol 2016; 14:1172–1180.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Xu C, Xun Y, Lu Z, Shi J, Yu C, et al. ZJU index: a novel model for predicting nonalcoholic fatty liver disease in a Chinese population. Sci Rep 2015; 5:16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn HS. The ‘lipid accumulation product’ performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison [published correction appears in BMC Cardiovasc Disord. 2006;6:5]. BMC Cardiovasc Disord 2005; 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010; 42:503–508. [DOI] [PubMed] [Google Scholar]
- 9.Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al.; AlkaMeSy Study Group. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010; 33:920–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Zhang M, Wang M, Wang M, Zhang R, Wang H, et al. External validation and comparison of simple tools to screen for nonalcoholic fatty liver disease in Chinese community population. Eur J Gastroenterol Hepatol 2022; 34:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao YT, Xiang LL, Qi F, Zhang YJ, Chen Y, Zhou XQ. Accuracy of controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) for assessing steatosis and fibrosis in non-alcoholic fatty liver disease: a systematic review and meta-analysis. EClinicalMedicine 2022; 51:101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Goh GB, Huang J, Wu Y, Wang M, Kumar R, et al. Validation of non-invasive fibrosis scores for predicting advanced fibrosis in metabolic-associated fatty liver disease. J Clin Transl Hepatol 2022; 10:589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younossi ZM, Paik JM, Al Shabeeb R, Golabi P, Younossi I, Henry L. Are there outcome differences between NAFLD and metabolic-associated fatty liver disease? Hepatology 2022; 76:1423–1437. [Epub ahead of print, 2022 Apr 1]. [DOI] [PubMed] [Google Scholar]
- 14.Weng Z, Ou W, Huang J, Singh M, Wang M, Zhu Y, et al. Circadian misalignment rather than sleep duration is associated with MAFLD: a population-based propensity score-matched study. Nat Sci Sleep 2021; 13:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Tan L, Liu Z, Shi R. The association between non-alcoholic fatty liver disease (NAFLD) and advanced fibrosis with blood selenium level based on the NHANES 2017-2018. Ann Med 2022; 54:2259–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for disease control and prevention. 2017: National Health and Nutrition Examination Survey (NHANES). U.S. Department of health and human services; 2017. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2017. Volume 2020. [Google Scholar]
- 17.Siddiqui MS, Patidar KR, Boyett S, Smith PG, Sanyal AJ, Sterling RK. Validation of noninvasive methods for detecting hepatic steatosis in patients with human immunodeficiency virus infection. Clin Gastroenterol Hepatol 2015; 13:402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vural Keskinler M, Mutlu HH, Sirin A, Erkalma Senates B, Colak Y, Tuncer I, et al. Visceral adiposity index as a practical tool in patients with biopsy-proven nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Metab Syndr Relat Disord 2021; 19:26–31. [DOI] [PubMed] [Google Scholar]
- 19.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al.; Authors/Task Force Members. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018; 36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 2020; 43:S14–S31. [DOI] [PubMed] [Google Scholar]
- 21.Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2018; 67:1348–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paik JM, Deshpande R, Golabi P, Younossi I, Henry L, Younossi ZM. The impact of modifiable risk factors on the long-term outcomes of non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2020; 51:291–304. [DOI] [PubMed] [Google Scholar]
- 23.Han AL, Lee HK. Comparison of the diagnostic performance of steatosis indices for discrimination of CT-diagnosed metabolic dysfunction-associated fatty liver disease. Metabolites 2022; 12:664. Published 2022 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi X, Zhu S, Zhu L. Diagnostic accuracy of the visceral adiposity index in patients with metabolic-associated fatty liver disease: a meta-analysis. Lipids Health Dis 2022; 21:28. Published 2022 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foschi FG, Conti F, Domenicali M, Giacomoni P, Borghi A, Bevilacqua V, et al.; Group Bagnacavallo Study. External validation of surrogate indices of fatty liver in the general population: the Bagnacavallo study. J Clin Med 2021; 10:520. Published 2021 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, He M, Zhang Y, Li T, Liu Y, Xu Z, et al. Validation of simple indexes for nonalcoholic fatty liver disease in western China: a retrospective cross-sectional study. Endocr J 2018; 65:373–381. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Liu S, Huang J, Zhu Y, Lin S. Validation of five hepatic steatosis algorithms in metabolic-associated fatty liver disease: a population based study. J Gastroenterol Hepatol 2022; 37:938–945. [DOI] [PubMed] [Google Scholar]
- 28.Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019; 156:1717–1730. [DOI] [PubMed] [Google Scholar]
- 29.Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 2017; 66:1022–1030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.