Abstract
Several genetic disorders have variable degree of central nervous system white matter abnormalities. We retrieved and reviewed 422 genetic conditions with prominent and consistent involvement of white matter from the literature. We herein describe the current definitions, classification systems, clinical spectrum, neuroimaging findings, genomics and molecular mechanisms of these conditions. Though diagnosis for most of these disorders relies mainly on genomic tests, specifically exome sequencing, we collate several clinical and neuroimaging findings still relevant in diagnosis of clinically recognizable disorders. We also review the current understanding of pathophysiology and therapeutics of these disorders.
Keywords: Leukodystrophies, genetic leukoencephalopathies, inherited white matter disorders, hypomyelination, demyelination, leukoencephalopathy, delayed myelination, myelination
Introduction
Genetic disorders with involvement of central nervous system (CNS) white matter are heterogeneous entities. Over the years, classifications based on neuropathology, imaging, genetic and molecular mechanisms have been devised.1-3 Essentially, these disorders have been put under a single umbrella term in order to provide a diagnostic framework and the term leukodystrophies (LD) has been used interchangeably to describe most genetic white matter disorders.1, 2, 4-7 However, the rapid increase in delineation of novel phenotypes and underlying genetic mechanisms has made it challenging to accommodate these disorders into the current definitions and classification systems. The diagnostic modalities for these disorders have transformed from pathology to pattern recognition on the magnetic resonance imaging (MRI) of the brain and more recently to direct genomic testing.4 Several, but not all these disorders, share molecular pathways and patho-mechanisms necessary for devising therapeutic modalities.
These disorders appear to represent a continuum ranging from isolated and primary myelin defects to those with other structural white matter components involvement and finally to those where extensive white matter involvement is secondary to metabolic defects or neuronal pathology. Acknowledging the limitations of current insight into underlying pathomechanisms, all disorders with significant and high penetrance of CNS white matter abnormalities (CNS WMAs) evident on neuroimaging have been included in this review. We attempt to devise a pragmatic approach for diagnosis of these disorders in the genomic era. We describe the current clinical spectrum, classification and its limitations, diagnostic modalities, role of genomic testing in rapid diagnosis, pathophysiology and therapeutic modalities for these disorders.
Definitions and Classifications
Traditionally, all genetic disorders with CNS WMAs were referred to as leukodystrophies. Recently, an attempt was made by the Global Leukodystrophy Initiative 1 to define and classify white matter disorders into three categories i.e leukoencephalopathies, genetic leukoencephalopathies and leukodystrophies based on consensus of a panel of experts in the field. Leukoencephalopathies was defined as all disorders with white matter abnormalities of the central nervous system, both acquired and genetic. Leukoencephalopathies with an underlying genetic defect were termed as genetic leukoencephalopathies (gLE). The term leukodystrophies (LD) was used for a subclass of genetic leukoencephalopathies characterized by primary glial cell and myelin sheath pathology of variable etiology where secondary axonal pathology can emerge as the disease progresses. There were several limitations to the classification. For example, L-2 hydroxyglutaric aciduria in which there is neuropathology evidence for primary WM involvement, was classified as genetic leukoencephalopathy and not as leukodystrophy. Cerebrotendinous xanthomatosis, classified as a true primary disorder of WM, has involvement of grey matter structures as well on MRI as well as systemic involvement.8 Several disorders for which a neuropathology evidence was lacking were designated as leukodystrophy based on the brain imaging data.
Classification of leukodystrophies based on pathological changes and pathogenetic mechanisms that takes into account the primary involvement of any white matter component has also been proposed recently.2 Categories in this classification are the myelin disorders due to a primary defect in oligodendrocytes or myelin (hypomyelinating and demyelinating leukodystrophies, leukodystrophies with myelin vacuolization); astrocytopathies; leuko-axonopathies; microgliopathies; and leuko-vasculopathies.
Currently, the myelin-focused concept has been abandoned and all genetic disorders with involvement of any component within CNS white matter i.e myelin, oligodendrocytes, astrocytes, microglia, axons, and blood vessels are referred to as LD.3, 6 All genetic disorders irrespective of the structural white matter component involved, the molecular process affected and the disease course, are referred to as LD. Hence, several disorders, which were designated as gLE earlier, have now been reclassified as LD.4 The exhaustive list of all disorders including metabolic, mitochondrial and those designated as leukoencephalopathies have been brought under this term. Hence, the concept of only white matter involvement, primary white matter involvement or true white matter involvement stands blurred. The experts agree that the precise meaning of this term is lost and retains the popular concept of selective, primary, predominant involvement of white matter with a progressive disease course.
Though LD is used practically for all genetic white matter disorders, this definition, which is largely based on neuropathology, does not contribute to categorizing the newly recognized phenotypes diagnosed by neuroimaging and genomic testing. The list of these disorders is bound to grow, and the diagnostic approach is likely to evolve into a combination of deep phenotyping complemented by genomic testing. Classifications based on brain imaging findings has been discussed in the respective section of the review.
Epidemiology
There is limited information on cumulative incidence and prevalence of these disorders with CNS WMAs owing to immense heterogeneity. The incidence in a pediatric cohort of genetic white matter disorders with molecular diagnosis was noted to be 1.2 in 100,000 live births9 which was comparable to the incidence of acquired white matter disorders in this age group. However, in a decade-long study of cohort of MRI diagnosed cases, the incidence of white matter disorders was estimated to be 1 in 7,663 live births.10 An earlier study based on either MRI and/or biochemically confirmed cases reported the incidence of 2 per 100,000 live births.11 More robust epidemiological data is available for common and well characterized disorders. A recent study based on genetic diagnosis by targeted and exome sequencing revealed relatively high frequency of Aicardi-Goutières syndrome, TUBB4A-related leukodystrophy, peroxisomal biogenesis disorders, POLR3-related leukodystrophy, vanishing white matter, and Pelizaeus-Merzbacher disease.12 The prevalence is noted to be 1 in 4,845 to 50,000 for adrenoleukodystrophy (to cite PMID 32003821) 13, 1 in 40,000 to 1,60,000 for metachromatic leukodystrophy 14 and 1 in 2,50,000 for Krabbe disease 15 across different populations. The mortality rates of Krabbe disease, Pelizaeus-Merzbacher disease, Canavan disease, Alexander disease and metachromatic leukodystrophy are reported by Barczykowski et al to be 0.089, 0.031, 0.012, 0.031 and 0.140 per 10,00,000 individuals of all ages respectively. 15 The mortality rates in children below 5 years of age were noted to be three to nine folds more than those above 5 years of age.
Etiology
CNS WMAs are known to occur due to genetic as well as acquired causes such as autoimmune, toxins, hypoxic ischemia, infections and several unknown factors.15 This review is focused mainly on disorders with an underlying genetic etiology. Literature search followed by manual curation revealed 422 genetic conditions with predominant CNS WMAs. The details of search methodology are provided in the supplemental data.
Monogenic disorders
Most of the well described disorders with CNS WMAs are of monogenic etiology. A total of 406 monogenic disorders caused by pathogenic variants in 410 genes were retrieved (Table S1). One-hundred-nineteen conditions were designated as LD and one-hundred-nine as gLE earlier.1, 4, 5 Thirty-one conditions have been referred to as both LD and gLE in the literature. We retrieved an additional 147 disorders with variable but consistent CNS WMAs on neuroimaging.
Subclassification of monogenic disorders based on appropriate and common principles is not achievable at present due to limited understanding of patho-mechanisms involved. Based on the current knowledge, we categorize monogenic disorders affecting a known cellular or molecular process. However, these categories are not exclusive and often a disorder can be placed in more than one of these. There are very few disorders known to be caused by defects in myelin specific proteins (3 disorders, 3 genes). The largest subgroup is that of nuclear mitochondrial disorders (91 disorders, 102 genes). Other common categories are organelle dysfunctions such as lysosomal (30 disorders, 28 genes) and peroxisomal (23 disorders, 17 genes). Defects in several enzymes involved in metabolic pathways of amino acids and organic acids (29 disorders, 32 genes), fatty acids (4 disorders, 4 genes), carbohydrate (4 disorders, 4 genes) and glycolipids (4 disorders, 4 genes) also result in marked CNS WMAs. Disorders affecting the membrane transport due to disturbed intravesicular transport (21 disorders, 20 genes) and iron and water homeostasis (39 disorders, 39 genes) are increasingly being recognized. Other prominent groups include disorders of DNA replication, transcription and their regulation (17 disorders, 17 genes), disorders of DNA repair mechanism (10 disorders, 9 genes), disorders of mRNA translation (29 disorders, 33 genes), translation modification and editing (7 disorders, 7 genes), disorders of cell-cell adhesion (4 disorders, 4 genes), cell cycle and differentiation (13 disorders, 11 genes) and apoptosis (4 disorders, 4 genes). Remaining disorders (74 disorders, 72 genes) have been listed in the miscellaneous category. All disorders of monogenic etiology and their subclassifications have been listed in the supplementary table S1.
Twenty-two of all monogenic disorders listed above have neuropathological findings of vascular defects and are known as genetic vasculopathies.2, 4 Vascular defects involve small vessels of the brain, small veins, capillaries, small arteries and arterioles.16 Regardless of similar pathology, the underlying cellular and processes involved are variable, mainly DNA replication and transcription regulation (6 disorders), cellular growth, differentiation and apoptosis (6 disorders) and one disorder each in the categories of DNA repair mechanisms and subcellular dysfunction (lysosomal).
Mitochondrial disorders
Four disorders due to variants in mitochondrial genome have CNS WMAs (Table S1).17, 18 Three of these, mitochondrial encephalopathy with lactic acidosis and stroke-like episodes, Leigh disease and mitochondrial respiratory chain complex deficiency are caused due to defects in either protein coding (10 genes) or mitochondrial encoded transfer RNA (12 genes). The protein coding genes span subunits of all respiratory chain complexes, namely complex I (5 genes), III (2 genes), IV (2 genes) and V (1 gene), except complex II, which is entirely nuclear encoded. One disorder, Kearns-Sayre syndrome, is known to be caused by rearrangements in the mitochondrial genome.
Chromosomal abnormalities and microdeletion/microduplication syndromes
CNS WMAs are known to occur in very few chromosomal abnormalities and microdeletion/microduplications syndromes consistently. The chromosomal causes include tetrasomy 12p,19, 20 ring chromosome 1821, 22 and 49, XXXXY syndrome.23, 24 Chromosome 18q deletion syndrome (MIM#601808) is the most common and is consistently associated with WMAs.25, 26 Other rare microdeletion/duplication syndromes with WMAs include 6p25 microdeletion,27-29 3p21.31 deletion,30-32 14q12-q13.1 triplication,33 5q14.3 deletion,34 11q14.3 deletion, 35, 36 11q24 deletion,37 17p13.3 deletion,38-41 and 22q11.2q13 duplication.42 Supplementary Table S2 provides the clinical and radiological findings associated with these disorders.
Diagnosis
The clinical heterogeneity and ever-increasing number of disorders with CNS WMAs pose a significant challenge in clinical diagnosis. Though genomic testing is increasingly being applied as a first line diagnostic test, a combination of inheritance pattern, age of onset, characteristic neurological or non-neurological clinical findings and MRI brain pattern is helpful in accomplishing a clinical diagnosis and more often a set of differential diagnosis for these disorders.
Inheritance pattern
Most disorders with CNS WMAs follow an autosomal recessive inheritance pattern (322 conditions). Sixty-four disorders are inherited exclusively in an autosomal dominant pattern and 4 disorders can be inherited both as autosomal recessive and dominant. Sixteen disorders show X-linked inheritance patterns. Four disorders follow mitochondrial inheritance. Hence, pedigrees with autosomal dominant or X-linked patterns provide a good handle for clinical diagnosis. The sporadic cases must be carefully evaluated to rule out the acquired conditions. The inheritance patterns of all disorders are provided in supplementary table S1.
Age of onset
The age of onset of most disorders is in the pediatric age group. However, several disorders have onset ranging from pediatric to adulthood (Table S3). Very few disorders with CNS WMAs are known to be exclusively adult onset conditions and are listed in table 1.43 These disorders would need dissection from the late onset forms of other disorders and acquired conditions.
Table 1.
Disorders with exclusive adulthood onset
| S. No. | Condition | MIM# | Gene |
|---|---|---|---|
| 1 | Hereditary diffuse leukoencephalopathy with spheroids | 221820 | CSF1R |
| 2 | Autosomal dominant adult onset demyelinating leukodystrophy | 169500 | LMNB1 |
| 3 | Adult polyglucosan body disease | 263570 | GBE1 |
| 4 | Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy | 125310 | NOTCH3 |
| 5 | Cerebral autosomal recessive cerebral arteriopathy with subcortical infarcts and leukoencephalopathy | 600142 | HTRA1 |
| 6 | Cathepsin A-related arteriopathy with strokes and leukoencephalopathy | - | CTSA |
| 7 | Cerebral leukodystrophy with retinal vasculopathy | 192315 | TREX1 |
| 8 | Small vessel disease with ocular abnormalities | 175180 | COL4A1 |
| 9 | Gordon Holmes syndrome | 212840 | RNF216 |
| 10 | Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy 1 | 221770 | TYROBP |
| 11 | Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy 2 | 618193 | TREM2 |
Clinical features
Several but not all disorders with CNS WMAs are progressive disorders.6 Those with a progressive course present with regression of milestones after a period of normalcy and the others as delayed development. Often hypotonia is the presenting feature that progresses to hypertonia and spasticity, ataxia, nystagmus, swallowing and speech difficulties later in the course.5 Those disorders which begin with motor manifestations alone usually manifest cognitive and behavioral changes as the disease advances. The neurological findings alone are seldom useful for establishing a clinical diagnosis owing to the immense heterogeneity of these disorders. However, extra-neurological features involving endocrine, ophthalmologic, auditory, musculoskeletal, skin, gastrointestinal and cardiovascular systems in presence of significant white matter involvement often aids clinical diagnosis (Table 2).
Table 2.
Clinical findings as a diagnostic clue for disorders with CNS WMAs
| Clinical feature | Disorders |
|---|---|
| Macrocephaly | Alexander disease, Canavan disease, Megalencephalic leukoencephalopathy with subcortical cysts, 1,2-hydroxy glutaric aciduria, GM2 gangliosidosis |
| Coarse facies | Sialic acid storage disease, Fucosidosis, Multiple sulfatase deficiency, Mucopolysaccharidosis |
| Progeroid appearance | Cockayne syndrome |
| Enamel hypoplasia and other enamel defects | Oculodentodigital dysplasia, Peroxisomal disorders |
| Oligodontia, hypodontia, delayed eruption, altered sequence of eruption, abnormal colour /shape | POLR3 related disorders (not universal and highly variable) Oculo-dentodigital dysplasia |
| Propensity for cavities | Cockayne syndrome |
| Cataract |
At birth Hypomyelination with congenital cataract, Childhood ataxia with central nervous system hypomyelination (only connatal cases), Peroxisomal disorders Childhood onset Cerebrotendinous xanthomatosis, POLR3 related disorders |
| Cherry red spot | Sialidosis, GM2 gangliosidosis, Metachromatic leukodystrophy (some cases) |
| Glaucoma | Aicardi–Goutières Syndrome, Oculodentodigital dysplasia |
| Optic atrophy | Metachromatic leukodystrophy, Canavan disease Childhood ataxia with central nervous system hypomyelination, Cerebrotendinous xanthomatosis, Peroxisomal disorders (+/−), POLR3 related disorders (+/−), Hypomyelinating leukodystrophies, Mitochondrial disorders, Oculodentodigital dysplasia |
| Retinitis pigmentosa | Refsum disease (adolescent and adult onset), Peroxisomal disorders |
| Vascular retinal defects | Cerebroretinal microangiopathy with calcifications and cysts (Coats plus syndrome) |
| Angiokeratoma corporis diffusum | Fucosidosis |
| Ichthyosis |
Congenital Sjogren-Larsson syndrome, Ichthyotic keratoderma, spasticity, hypomyelination, and dysmorphic facies Childhood onset Multiple sulfatase deficiency, Sialic acid storage disorder, Peroxisome biogenesis disorders including neonatal Zelleweger syndrome Adrenoleukodystrophy and Infantile Refsum disease Adulthood onset Refsum disease |
| Hyperpigmentation | X-Adrenoleukodystrophy, Mitochondrial neurogastrointestinal encephalopathy |
| Xanthomas | Cerebrotendinous xanthomatosis |
| Photosensitivity | Cockayne syndrome, Tay syndrome |
| Adrenal insufficiency | X-linked Adrenoleukodystrophy, Peroxisome biogenesis disorders |
| Hypothyroidism | POLR3 related disorders, Aicardi–Goutières Syndrome, Cerebrotendinous xanthomatosis, Peroxisomal biogenesis disorders |
| Hypogonadotropic hypogonadism | POLR3 related disorders |
| Growth Hormone deficiency | POLR3 related disorders, Aicardi–Goutières Syndrome |
| Ovarian dysgenesis (Premature ovarian failure) | Ovarioleukodystrophy, AARS2-related leukoencephalopathy Peroxisome biogenesis disorders |
| Hepatosplenomegaly | Multiple sulfatase deficiency, Galactosialidosis, Sialic acid storage disorders |
| Hepatic dysfunction | Peroxisomal disorders, Aicardi–Goutières Syndrome, Mitochondriopathies Fucosidosis, Sialic acid storage disorders |
| Chondrodysplasia punctata | Peroxisomal disorders |
| Dysostosis multiplex | Multiple sulfatase deficiency, Sialidosis |
Acquired disorders may present with acute or subacute onset following an episode of infection, toxicity or hypoxia. They may have monophasic illness of acute onset followed by partial or complete recovery as in acute disseminated encephalomyelitis44 or a chronic illness with recurrent episodes of relapsing signs and symptoms as seen in multiple sclerosis.45, 46 Often, they may mimic disorders of genetic etiology in clinical presentation, especially mitochondrial disorders precipitated by an intercurrent illness.5
Neuroimaging
MRI has a vital role in the diagnosis of disorders with CNS WMAs. The minimum requirements for a standard MRI investigation are T1-weighted, T2-weighted and fluid-attenuated inversion-recovery (FLAIR) images. The radiological diagnostic algorithm devised by van der Knaap et al remains a useful aid for clinical diagnosis of common and recognizable disorders.3 The pattern recognition on neuroimaging involves differentiation into hypomyelination or other white matter pathologies, the confluency and predominant area of localization of WMAs and certain specific MRI characteristics. Additionally, other magnetic resonance sequences including contrast enhanced MRI, susceptibility weighted imaging and diffusion-weighted sequences are useful diagnostic tools for disorders with inflammatory component, calcifications and/or vascular lesions.5 MR spectroscopy serves as a sensitive method for diagnoses of metabolic and mitochondrial disorders among these disorders (Table 3).
Table 3.
Neuroimaging characteristics of common disorders with de/dysmyelination
| Confluency and area of predominance | ||
|---|---|---|
| Diffuse and symmetric | Area of predominance | Disorders |
| Subcortical | Glutaric aciduria (Fig 1B) Canavan disease (Fig 1B) Urea cycle defects |
|
| Frontal lobe | Alexander disease Metachromatic leukodystrophy (Fig 1B) Neuroaxonal leukodystrophy with axonal spheroids |
|
| Periventricular | Metachromatic leukodystrophy (Fig 1B) Krabbe disease Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation Periventricular leukomalacia (Fig S1) |
|
| Occipital | Krabbe disease (Fig 1B) X-linked adrenoleukodystrophy Peroxisomal disorders |
|
| Diffuse cerebral | Vanishing white matter disease (Fig 1B) Megalencephalic leukoencephalopathy with subcortical cysts (Fig 1B) Merosin deficient congenital muscular dystrophy (Fig 1B) Mitochondrial disorders Most leukodystrophies at the advanced stages HIV encephalopathy (Fig S1) Toxic leukoencephalopathy (Fig S1) |
|
| Cerebellar | Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation Leigh disease (Fig 1B) Alexander disease Maple syrup urine disease (Fig 1B) Adult onset autosomal dominant leukodystrophy |
|
| Brainstem | Leigh disease (Fig 1B) Wilson disease Alexander disease Krabbe disease (Fig 1B) Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation |
|
| Spinal Cord | Alexander disease Mitochondrial disorders (Fig 1B) Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation |
|
| Focal and asymmetric | Genetic vasculopathies | Brain small vessel disease Proliferative vasculopathy and hydranencephaly-hydrocephaly syndrome Microangiopathy and leukoencephalopathy, pontine, autosomal dominant Aicardi-Goutières syndrome (Fig 1B) Cerebral amyloid angiopathy Leukoencephalopathy with calcifications and cysts Cerebral AD arteriopathy with subcortical infarcts and leukoencephalopathy Cerebral AR arteriopathy with subcortical infarcts and leukoencephalopathy (Fig 1B) RNASET2-related leukodystrophy NGLY1 related congenital disorder of deglycosylation Cathepsin A-related arteriopathy with strokes and leukodystrophy |
| Acquired disorders with white matter abnormalities | Optic neuritis Transverse myelitis Acute disseminated encephalomyelitis (Fig S1) Multiple sclerosis (Fig S1) |
|
| Special MRI characteristics | ||
| Cystic changes | Megalencephalic leukoencephalopathy with subcortical cysts (Fig 1B) Mitochondrial disorders (Fig 1B) Vanishing white matter disease (Fig 1B) Glutaric aciduria (basal ganglia) |
|
| Calcifications | Aicardi-Goutieres syndrome (Fig 1B) Galactosialidosis Brain small vessel disease with or without ocular anomalies Labrune's Syndrome or Leukoencephalopathy with Calcifications and Cysts Cerebro Retinal Microangiopathy with Calcifications and Cysts (Coats Plus Syndrome) Pseudo-TORCH syndrome 1 AARS2 related disease KARS2 related disease Adult onset leukoencephalopathy with axonal spheroids and pigmented glia COL4A1-related disorders Retinal vasculopathy with cerebral leukoencephalopathy Cerebral amyloid angiopathy Primary familial brain calcification Pseudohypoparathyroidism Leukoencephalopathy with calcifications and cysts |
|
| Magnetic Resonance Spectroscopy | Elevated lactate | Mitochondrial disorders (Fig 1B) Some metabolic disorders (mild elevation) |
| Altered metabolites | Ribose 5-phosphate isomerase deficiency (Elevated levels of arabitol and ribitol) Canavan disease (elevated N-acetylaspartic acid) (Fig 1B) |
|
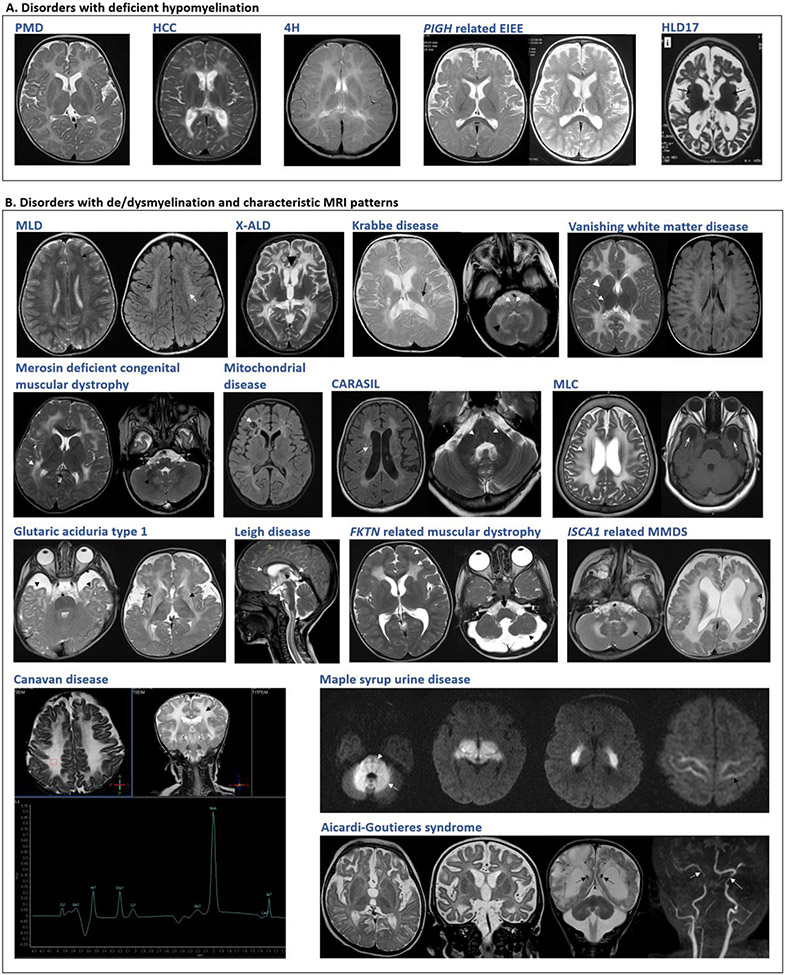
Deficient myelination, either permanent hypomyelination or delayed myelination (Fig. 1A), is seen as less marked T2-weighted hyperintensities and T1-weighted hypointensities, isointensities or mild hyperintensities of white matter relative to grey matter.3 Seventy-six disorders with deficient CNS myelination were retrieved from the literature (Supplementary table 4). Of these, forty-six conditions are reported with hypomyelination and twenty-seven with delayed myelination consistently. Hypomyelination and delayed myelination have been used interchangeably in three conditions. The number of disorders with deficient myelination, particularly permanent hypomyelination is limited. Hence, hypomyelination on MRI in conjunction with other specific findings is a good clinical handle for diagnosis of these disorders. Two major group of disorders with deficient myelination are hypomyelinating leukodystrophies and early infantile onset epileptic encephalopathies (EIEE). Nineteen disorders with permanent hypomyelination have been catalogued as hypomyelinating leukodystrophies (HLDs, PS312080) in OMIM. WMAs, mainly delayed myelination, has been observed in thirty-five of the eighty-four (EIEE) reported till date (OMIM PS308350). This could be attributed to seizure activity arresting the normal process of myelination which progresses and usually normalizes after seizure control.47 Clinically identifiable disorders with hypomyelination are listed in table 4 and other disorders with deficient myelination are provided in table S4
Figure 1. Neuroimaging findings in selected disorders.
A. Disorders with deficient myelination. Variable degrees of hypomyelination in Pelizaeus-Merzbacher disease (PMD) at 11 months, hypomyelination with congenital cataract (HCC) at 5 years and 4H leukodystrophy (4H) at 5 years. Delayed myelination at 1 year in PIGH related early infantile epileptic encephalopathy which improved by 2 years. Advanced stage in hypomyelinating leukodystrophy type 17 showing cerebral, cerebellar atrophy. B. Disorders with de/dysmyelination and characteristic MRI patterns. Metachromatic leukodystrophy (MLD): T2W and FLAIR symmetrical white matter hyperintensity, sparing of subcortical U-fibers with tigroid pattern. X-linked adrenoleukodystrophy (X-ALD): T2W hyperintensities involving the white matter of bilateral parieto-temporal and frontal lobes. Krabbe disease: T2W hyperintense signals in the periventricular white matter, posterior limb of the internal capsule, brainstem along the pyramidal tracts and cerebellar white matter. Vanishing white matter disease: T2W hyperintensities and FLAIR hypointensities in deep and subcortical white matter, sparing basal ganglia and internal capsule. Merosin deficient congenital muscular dystrophy: T2W bilateral diffuse hyperintensities in deep cerebral white matter. Mitochondrial: Necrotizing leukoencephalopathy, FLAIR hyperintensity of bilateral white matter with cystic changes. Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL): FLAIR and T2W hyperintensities with lacunar infarcts and T2W hyperintensities indicating pons-arc sign. Megalencephalic leukoencephalopathy with subcortical cysts (MLC): T2W hyperintensities with involvement of subcortical U-fibers. Subcortical cysts in anterior temporal lobes. Glutaric aciduria type 1: Temporal lobe hypoplasia, widened sylvian fissures and hyperintense basal ganglia with periventricular deep and subcortical white matter involvement. Leigh disease: Hyperintensities in corpus callosum, brain stem, spinal cord and thalamus. FKTN related dystroglycanopathy: Symmetric frontal lobar polymicrogyria-pachygyria complex, symmetrical changes in frontal lobe white matter. Brainstem and cerebellar hypoplasia seen. ISCA1 related multiple mitochondrial dysfunctions syndrome (MMDS) 5: Diffuse cerebellar and cerebral white matter hyperintensities, ventriculomegaly and pachygyria. Canavan disease: Diffuse bilateral symmetrical T2W white matter hyperintensity with subcortical U-fiber involvemen, Elevated NAA on MRS. Maple syrup urine disease (MSUD): Diffusion-weighted images show symmetrical diffusion restriction in cerebellar white matter, brain stem, cerebral peduncles, posterior limb of internal capsule, thalami, and perirolandic cerebral white matter. Aicardi-Goutieres syndrome: Delayed myelination, high signal in frontotemporal white matter with atrophy. SWI sequence shows calcifications, paucity of white matter, cerebellar atrophy and arteriopathy in advanced stage.
Table 4.
Clinically identifiable disorders with deficient myelination
| Disease | Gene | Clinical clues | Neuroimaging findings |
|---|---|---|---|
| Leukodystrophy, hypomyelinating, 1 (Pelizaeus-Merzbacher disease) | PLP1 | Developmental delay, hypotonia, rotatory nystagmus | Hypomyelination (FIG 1A) Thinning of corpus callosum |
| Leukodystrophy, hypomyelinating, 2 (Pelizaeus-Merzbacher like disease 1) | GJC2 | Developmental delay, hypotonia, rotatory nystagmus | Hypomyelination Involvement of the corticospinal tracts with abnormal T2-weighted signal extending into the brain stem resulting in extensive brain stem involvement not seen in Pelizaeus-Merzbacher disease Cerebral atrophy at advanced stages |
| Leukodystrophy, hypomyelinating 5 (Hypomyelination with congenital cataract) | FAM126A | Sudden motor regression Cataract |
Hypomyelination (FIG 1A) Cerebral atrophy at advanced stages |
| Pol III related leukodystrophiesLeukodystrophy, hypomyelinating, 8, with or without oligodontia and/or hypogonadotropic hypogonadism | POLR3B | Hypodontia Hypogonadotropic hypogonadism |
Hypomyelination (FIG 1A) Thinning of corpus callosum |
| Leukodystrophy, hypomyelinating, 7, with or without oligodontia and/or hypogonadotropic hypogonadism | POLR3A | Hypodontia Hypogonadotropic hypogonadism |
Hypomyelination (FIG 1A) Thinning of corpus callosum |
| Ichthyosis, acanthosis nigricans, hypomyelination, spastic paraplegia, high frequency deafness and optic atrophy | ELOVL1 | Ichthyosis, acanthosis nigricans, deafness |
Hypomyelination |
| Hypomyelinating neuropathy, congenital, 3 | CNTNAP1 | Arthrogryposis multiplex congenita | Hypomyelination Thin corpus callosum Cerebellar atrophy Pontine atrophy |
| Hypermethioninemia with deficiency of S-adenosylhomocysteine hydrolase | AHCY | Facial dysmorphism Abnormal hair and teeth Myocardiopathy Hypermethioninemia |
Severely delayed myelination |
De/dysmyelination presents with T2-weighted hyperintensities and T1-weighted hypointensities of affected white matter relative to the grey matter.3 Confluent, bilateral and symmetric signal abnormalities are predominantly observed in genetic disorders while focal asymmetric involvement of white matter may be indicative of a non-genetic cause. However, there are several exceptions to this phenomenon. Specific pattern recognition of WMAs including predominant areas of white matter dysmyelination in MRI remains vital for diagnosis of several common disorders like Krabbe disease, metachromatic leukoencephalopathy, megalencephalic leukoencephalopathy (Fig 1B), Leigh disease etc (Fig 1B). Seldom, it also aids in diagnosis of rare disorders with very characteristic and unique combination of brain imaging findings such as multiple mitochondrial dysfunction syndrome 5 (MIM# 617613) (Fig 1B) with extensive diffuse cerebral WMAs, ventriculomegaly, pachygyria and cerebellar atrophy.48-50 Characteristic neuroimaging findings for common disorders is provided in table 3 and Figure 1. Of the 147 disorders not categorized as LD/gLE earlier, twenty-four (21 with dysmyelination, 2 with hypomyelination along with dysmyelination and 1 with hypomyelination) present with confluent and recognizable pattern of WMAs (Table S5).
Acquired white matter disorders are more likely to have multifocal asymmetric white matter abnormalities.3 However, this imaging appearance may also be noted in several genetic disorders such as neuroaxonal leukodystrophy with spheroids, CADASIL, vascular leukoencephalopathies (Table 3) and some disorders of mitochondrial etiology (Figure 1B). Conversely, some acquired causes such as hypoxic ischemia, periventricular leukomalacia, HIV encephalopathy and toxic leukoencephalopathies lead to symmetrical and confluent white matter abnormalities thus mimicking genetic disorders. Large and ill-defined lesions which involve grey matter structures as well suggest the possibility of acquired demyelinating disorders.3 MRI may reveal a single or multiple lesions in both white matter (periventricular and subcortical) and grey matter (basal ganglia, thalamus, cortex) in individuals affected with acute disseminated encephalomyelitis.44, 51 Multiple sclerosis is diagnosed by neuroimaging evidence of two or more brain and/or spinal cord lesions disseminated by space and time (McDonald criteria, 2005).45, 46 Neuroimaging findings of common acquired white matter disorders is provided in Figure S1.
Genetic testing
Exome sequencing (ES) has emerged as a highly efficient diagnostic modality for genetic disorders with CNS WMAs as most of these disorders are monogenic and predominantly recessive. The earlier diagnostic rate of 50% has now increased to more than 70–80% with the use of ES.6, 7 The widespread availability of ES relative to several other specialized tests has also led to a decrease in the discrepancy in patients receiving a definitive diagnosis worldwide. In the past decade, approximately 72 novel gene-disease associations for these disorders have been identified with the application of ES.
However, ES has limitations in terms of diagnosis of genomic variants beyond exonic single nucleotide variants and small indels owing to technical difficulties.52 Variants in regions with repetitive sequence, CNVs, intronic and variants in regulatory regions cannot be identified by exome sequencing. The most common genomic variants for disorders like Pelizaeus-Merzbacher disease and Krabbe disease are large deletions/duplications of PLP1 and GALC respectively, which may not be detected in ES, thus emphasizing the role of clinical diagnosis in the era of genomics. A good clinical diagnosis or a set of narrow differential diagnoses is more likely to facilitate definitive molecular diagnosis and overcome the uncertainties of broad-spectrum genomic tests like exome and genome sequencing.
Literature on the utility of gene panels for diagnosis of these disorders is very limited. Yield of a custom panel was noted to be 13.3% in a cohort of individuals with adult onset leukodystrophies.53 In an Argentinian cohort of individuals with genetic disorder with WMAs, a virtual panel analysis from exome data rendered a diagnostic yield of 46.1%.54 Though whole genome sequencing (WGS) outweighs the diagnostic yield of ES, there is limited data on use of this technique as well for investigation of these disorders.55 48 A recent study reported trio genome sequencing in 41 families who were undiagnosed with trio exome sequencing.56 This resulted in a diagnosis in 14 (34%) additional families. Further decrease in sequencing cost and increase in the ease of data analysis is likely to result in WGS as a test of choice for these disorders.
Biochemical tests
The role of biochemical testing for genetic disorders including those with CNS WMAs is getting redefined in the era of broad-spectrum genomic testing. In scenarios with a diagnostic MRI pattern, biochemical analysis aids in validating the clinical diagnosis (Table 5). This can be followed by often inexpensive targeted genetic testing. Also, easily available biochemical tests like enzyme assays may often be used to resolve the variants of uncertain significance, a major concern with the broad-spectrum genomic tests. In these cases, biochemical testing adds to the evidence of pathogenicity while interpreting the observed variants.
Table 5.
Biochemical testing in common disorders
| Testing parameter | Disorders |
|---|---|
| Creatine phosphokinase (Serum) |
Muscular dystrophies Merosin deficient congenital muscular dystrophy Dystroglycanopathies |
| Lactate, pyruvate (Plasma, Cerebrospinal fluid) | Mitochondrial disorders |
| Glycosaminoglycans (Urine) | Metachromatic leukodystrophy Multiple sulfatase deficiency |
| Organic acids (Urine) | L-2-hydroxyglutarate N-acetylaspartic acid for Canavan disease Metabolic disorders of Krebs cycle Other mitochondrial disorders |
| Amino acids (Urine) | Aminoacidopathies Maple syrup urine disease Homocystinuria Hyperornithinemia-hyperammonemia-homocitrullinemia syndrome Pyruvate dehydrogenase complex deficiency |
| Very long chain fatty acids (Plasma) | Peroxisomal disorders X-linked adrenoleukodystrophy |
| Lysosomal enzymes (leukocytes/fibroblasts) | Krabbe (galactosyl cerebrosidase) Metachromatic leukodystrophy (arylsulfatase A) Multiple sulfatase deȴciency (arylsulfatase A,B,C,D) GM1 gangliosidosis (beta galactosidase) GM2 gangliosidosis (hexosaminidase A & B) Sialidosis (neuraminidase) Galactosialidosis (neuraminidase and beta galactosidase) |
| 1,4-alpha-glucan-branching enzyme activity | Adult polyglucosan body disease |
| Cholestanol | Cerebrotendinous xanthomatosis |
| Fatty aldehyde dehydrogenase enzyme | Sjogren-Larsson syndrome |
Pathophysiology
Genetic disorders with CNS WMAs result from molecular defects which lead to abnormalities in myelin production and maintenance or myelin destruction.2 The myelin sheath is a modified plasma membrane of the oligodendrocytes and is wrapped around neuronal axons. It consists of lipids (70%–85%) and proteins (15%– 30%).57 The production of myelin in developmental stages and its maintenance through adult life requires tightly regulated cues within the oligodendrocytes, glial cells and the neuronal axons.58 Most of the oligodendrocytic organelles including nucleus, rough endoplasmic reticulum, Golgi apparatus, microtubules, lysosomes and peroxisomes play critical and unique roles in formation and maintenance of CNS white matter.
There are three main mechanisms of white matter abnormalities based on cellular pathology. First, is a primary defect in oligodendrocytic activity of myelin production and maintenance. The illustrative example for defect in myelin production is duplication variants in PLP1 which cause misfolding of myelin followed by mislocalization. This leads to accumulation of PLP1 in the late endosome resulting in oligodendrocytic death. Second, is a defect on astrocytic regulation of myelination process, as seen in Alexander disease, where mutant glial fibrillary acidic protein accumulation activates multiple stress pathways inside the astrocytes. An astrocytic defect may also result in an ionic imbalance and fluid accumulation in myelin (vacuolation) as observed in several mitochondrial disorders and megalencephalic leukoencephalopathy with subcortical cysts. Third, there is no formation or there is degeneration of previously formed neuronal axons. Variants in neuronal axon specific genes may lead to improper formation of axons (FAM126A related hypomyelinating leukodystrophy) or degeneration of previously formed neuronal axons (GM1 gangliosidosis).
In addition to this, WMAs can occur as a consequence of genetic defects which lead to vascular pathology.2 The classical example is cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, caused by defects in NOTCH3. Variants and deletions of NOTCH3 result in accumulation of the aberrant protein which ultimately causes a reduced cerebral blood flow in brain white matter leading to white matter abnormalities.59 Several external factors like metabolic, mitochondrial, and non-genetic factors (toxins, hypoxic insults) can also lead to WMAs. Defects in several genes which are not yet known to have a direct role in oligodendrocyte or glial cell function are increasingly being identified to cause these disorders.
Treatment
A substantial insight into the patho-mechanisms has enabled advances in specific therapeutic strategies for these disorders. The timing of initiating therapy is of utmost importance as these therapies are effective only if initiated before or immediately after the onset of symptoms. Hence, an early diagnosis is warranted for optimal management and outcome for these disorders. However, supportive care remains the mainstay of treatment for most and involves management of spasticity, adequate nutrition including supplementation of vitamin D and calcium, treatment of neuropathic pain, epilepsy, drugs for sialorrhea and insomnia and monitoring for orthopedic complications like hip dislocation and scoliosis. One of the most common symptoms, spasticity, can be managed effectively by oral spasmolytic drugs, intramuscular botulinum toxin, intrathecal baclofen,60 or selective dorsal rhizotomy.61
The effective and curative therapies developed till date for these disorders include hematopoietic stem cell transplant and ex vivo gene therapy. Other forms of therapies like antisense oligonucleotides, targeted drug therapy, enzyme replacement therapy and stem cell-based therapy need further evidence before they are proved efficacious in the clinic. Table 6 summarizes the currently recommended therapeutic measures and table S6 lists the emerging treatment modalities for common and selected disorders.4, 62-95
Table 6.
Therapeutic strategies
| S. No. | Disorder | Therapy | References | |
|---|---|---|---|---|
| Recommended therapies | ||||
| ALD | Asymptomatic males | Lorenzo oil and dietary restriction of very long chain fatty acids | Moser et al., 2005; Sassa et al., 2014 | |
| Males with adrenal insufficiency | Oral corticosteroid replacement | Raymond, Moser, & Fatemi, 1993 | ||
| Cerebral ALD | HSCT effectively arrests progression of demyelination if initiated early in course of disease | Shapiro et al., 2000; Peters et al., 2004; Miller et al., 2011 | ||
| Adrenomyeloneuropathy | Corticosteroid replacement for adrenocortical insufficiency Supportive treatment |
Raymond, Moser, & Fatemi, 1993 | ||
| 2 | MLD | Early onset forms | Cholecystectomy for preventing complications like gall bladder dysfunction, polyps and carcinoma Variable outcomes and several complications in patients treated with allogenic HSCT |
Peters et al., 2004 |
| Juvenile or adult forms | Allogenic HSCT halts demyelination in minimally symptomatic Individuals | Peters et al., 2004; Weinberg 2005 | ||
| 3 | Krabbe disease | Infantile onset | HSCT prevents progression of disease in presymptomatic individuals | Escolar et al., 2005 |
| Late onset | HSCT may be beneficial | Krivit et al 1999; Krivit 2004 | ||
| 4 | Aicardi-Goutieres syndrome | Systemic corticosteroids are effective for treatment of autoimmune manifestations but not for neurologic symptoms | Chahwan and Chahwan 2012 | |
| 5 | Vanishing white matter disease | Preventive care to avoid trauma and infections Treatment of ovarian failure |
van der Knaap et al., 2019 | |
| 6 | Canavan disease | Oral lithium citrate showed improvement in myelination in 6 patients but minimal clinically effectivity in tolerable lithium dosage | Assadi et al.,2010; Pleasure et al., 2020 | |
| 7 | Cerebrotendinous xanthomatosis | Oral chenodeoxycholic acid Cholic acid | Berginer et al 1984; Mandia et a., 2019 | |
| 8 | Adult onset polyglucosan body disease | Triheptanoin, a 7-carbon triglyceride has been found to be beneficial in slowing the clinical course | Roe et al 2010; (NCT00947960) | |
| 9 | Hypomyelination with brain stem and spinal cord abnormalities and leg spasticity | Steroids may be beneficial in patients with subacute disease onset | Wolf et al., 2014 | |
| 12 | Pol-III related leukodystrophies | Supplemental hormonal therapies including growth hormone, thyroid hormone and hormone replacement therapy for hypogonadism | Bernard and Vanderver, 1993 | |
X-ALD – X-linked adrenoleukodystrophy; HSCT- Hematopoietic stem cell transplantation; MLD- Metachromatic leukodystrophy
Conclusion
The last few decades have added several insights for disorders with CNS WMAs. A combination of clinical, radiographic, biochemical and genomic expertise has helped us to overcome the challenges of a definitive diagnosis in disorders with CNS WMAs. The improvement in understanding of etiopathogenesis in future is likely to result in sustained advancement in therapeutics for several of these disorders.
Supplementary Material
Acknowledgements
We thank Department of Health Research, Ministry of Health and Family Welfare, Government of India for funding the project titled “Clinical and Molecular Characterization of Leukodystrophies in Indian Children” (V.25011/379/2015-GIA/HR) which led to our understanding of disorders with central nervous system white matter abnormalities and assisted in this review.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest
Data availability:
Data sharing is not applicable to this article as it is a review of pre-existing information.
References
- 1.Vanderver A, Prust M, Tonduti D, et al. Case definition and classification of leukodystrophies and leukoencephalopathies. Molecular genetics and metabolism. Apr 2015;114(4):494–500. doi: 10.1016/j.ymgme.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Knaap MS, Bugiani M. Leukodystrophies: a proposed classification system based on pathological changes and pathogenetic mechanisms. Sep 2017;134(3):351–382. doi: 10.1007/s00401-017-1739-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiffmann R, van der Knaap MS. Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology. Feb 24 2009;72(8):750–9. doi: 10.1212/01.wnl.0000343049.00540.c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Knaap MS, Schiffmann R, Mochel F, Wolf NI. Diagnosis, prognosis, and treatment of leukodystrophies. The Lancet Neurology. Oct 2019;18(10):962–972. doi: 10.1016/s1474-4422(19)30143-7 [DOI] [PubMed] [Google Scholar]
- 5.Parikh S, Bernard G, Leventer RJ, et al. A clinical approach to the diagnosis of patients with leukodystrophies and genetic leukoencephelopathies. Molecular genetics and metabolism. Apr 2015;114(4):501–515. doi: 10.1016/j.ymgme.2014.12.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kevelam SH, Steenweg ME, Srivastava S, et al. Update on Leukodystrophies: A Historical Perspective and Adapted Definition. Neuropediatrics. Dec 2016;47(6):349–354. doi: 10.1055/s-0036-1588020 [DOI] [PubMed] [Google Scholar]
- 7.Vanderver A, Simons C, Helman G. Whole exome sequencing in patients with white matter abnormalities. Jun 2016;79(6):1031–1037. doi: 10.1002/ana.24650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salsano E. Leukodystrophy or genetic leukoencephalopathy? Nature does not make leaps. Molecular genetics and metabolism. Apr 2015;114(4):491–3. doi: 10.1016/j.ymgme.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 9.Vanderver A, Hussey H, Schmidt JL, Pastor W, Hoffman HJ. Relative incidence of inherited white matter disorders in childhood to acquired pediatric demyelinating disorders. Seminars in pediatric neurology. Dec 2012;19(4):219–23. doi: 10.1016/j.spen.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonkowsky JL, Nelson C, Kingston JL, Filloux FM, Mundorff MB, Srivastava R. The burden of inherited leukodystrophies in children. Neurology. Aug 24 2010;75(8):718–25. doi: 10.1212/WNL.0b013e3181eee46b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heim P, Claussen M, Hoffmann B, et al. Leukodystrophy incidence in Germany. American journal of medical genetics. Sep 5 1997;71(4):475–8. [PubMed] [Google Scholar]
- 12.Schmidt JL, Pizzino A. Estimating the relative frequency of leukodystrophies and recommendations for carrier screening in the era of next-generation sequencing. Jun 23 2020;doi: 10.1002/ajmg.a.61641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bezman L, Moser AB, Raymond GV, et al. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Annals of neurology. Apr 2001;49(4):512–7. [PubMed] [Google Scholar]
- 14.Gomez-Ospina N. Arylsulfatase A Deficiency. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews(®). University of Washington, Seattle: Copyright © 1993-2020, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.; 1993. [Google Scholar]
- 15.Barczykowski AL, Foss AH, Duffner PK, Yan L, Carter RL. Death rates in the U.S. due to Krabbe disease and related leukodystrophy and lysosomal storage diseases. American journal of medical genetics Part A. Nov 2012;158a(11):2835–42. doi: 10.1002/ajmg.a.35624 [DOI] [PubMed] [Google Scholar]
- 16.Pantoni L Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. The Lancet Neurology. Jul 2010;9(7):689–701. doi: 10.1016/s1474-4422(10)70104-6 [DOI] [PubMed] [Google Scholar]
- 17.Thorburn DR, Rahman J, Rahman S. Mitochondrial DNA-Associated Leigh Syndrome and NARP. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews(®). University of Washington, Seattle: Copyright © 1993-2020, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.; 1993. [Google Scholar]
- 18.Chinnery PF. Mitochondrial Disorders Overview. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews(®). University of Washington, Seattle: Copyright © 1993-2020, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.; 1993. [Google Scholar]
- 19.Barkovich EJ, Lateef TM, Whitehead MT. Neuroimaging findings in Pallister-Killian syndrome. The neuroradiology journal. Aug 2018;31(4):403–411. doi: 10.1177/1971400917744798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito Y, Masuko K, Kaneko K, et al. Brain MRI findings of older patients with Pallister-Killian syndrome. Brain & development. Jan 2006;28(1):34–8. doi: 10.1016/j.braindev.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 21.Benini R, Saint-Martin C, Shevell MI, Bernard G. Abnormal myelination in ring chromosome 18 syndrome. Journal of child neurology. Aug 2012;27(8):1042–7. doi: 10.1177/0883073811430268 [DOI] [PubMed] [Google Scholar]
- 22.Anzai M, Arai-Ichinoi N, Takezawa Y, et al. Patchy white matter hyperintensity in ring chromosome 18 syndrome. Pediatrics international : official journal of the Japan Pediatric Society. Sep 2016;58(9):919–22. doi: 10.1111/ped.13043 [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Cazorla A, Sans A, Baquero M, et al. White matter alterations associated with chromosomal disorders. Developmental medicine and child neurology. Mar 2004;46(3):148–53. [DOI] [PubMed] [Google Scholar]
- 24.Buller MA, Pfeifer CM. Severe myelinopathy in 49,XXXXY syndrome. The neuroradiology journal. Oct 2018;31(5):523–525. doi: 10.1177/1971400917703989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feenstra I, Vissers LE, Orsel M, et al. Genotype-phenotype mapping of chromosome 18q deletions by high-resolution array CGH: an update of the phenotypic map. American journal of medical genetics Part A. Aug 15 2007;143a(16):1858–67. doi: 10.1002/ajmg.a.31850 [DOI] [PubMed] [Google Scholar]
- 26.Tanaka R, Iwasaki N, Hayashi M, et al. Abnormal brain MRI signal in 18q-syndrome not due to dysmyelination. Brain & development. Mar 2012;34(3):234–7. doi: 10.1016/j.braindev.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 27.Vernon HJ, Bytyci Telegrafi A, Batista D, Owegi M, Leigh R. 6p25 microdeletion: white matter abnormalities in an adult patient. American journal of medical genetics Part A. Jul 2013;161a(7):1686–9. doi: 10.1002/ajmg.a.35937 [DOI] [PubMed] [Google Scholar]
- 28.Cellini E, Disciglio V, Novara F, et al. Periventricular heterotopia with white matter abnormalities associated with 6p25 deletion. American journal of medical genetics Part A. Jul 2012;158a(7):1793–7. doi: 10.1002/ajmg.a.35416 [DOI] [PubMed] [Google Scholar]
- 29.DeScipio C. The 6p subtelomere deletion syndrome. American journal of medical genetics Part C, Seminars in medical genetics. Nov 15 2007;145c(4):377–82. doi: 10.1002/ajmg.c.30156 [DOI] [PubMed] [Google Scholar]
- 30.Lovrecic L, Bertok S, Zerjav Tansek M. A New Case of an Extremely Rare 3p21.31 Interstitial Deletion. Molecular syndromology. May 2016;7(2):93–8. doi: 10.1159/000445227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eto K, Sakai N, Shimada S, et al. Microdeletions of 3p21.31 characterized by developmental delay, distinctive features, elevated serum creatine kinase levels, and white matter involvement. American journal of medical genetics Part A. Dec 2013;161a(12):3049–56. doi: 10.1002/ajmg.a.36156 [DOI] [PubMed] [Google Scholar]
- 32.Haldeman-Englert CR, Gai X, Perin JC, et al. A 3.1-Mb microdeletion of 3p21.31 associated with cortical blindness, cleft lip, CNS abnormalities, and developmental delay. European journal of medical genetics. Jul-Aug 2009;52(4):265–8. doi: 10.1016/j.ejmg.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rea G, Stallings RL, Mullarkey M, McKinstry CS, McManus D, Morrison PJ. Subcortical white matter abnormalities because of previously undescribed de-novo 14q12-q13.1 triplication. Clinical dysmorphology. Apr 2013;22(2):71–2. doi: 10.1097/MCD.0b013e32835f7465 [DOI] [PubMed] [Google Scholar]
- 34.Hotz A, Hellenbroich Y, Sperner J, et al. Microdeletion 5q14.3 and anomalies of brain development. American journal of medical genetics Part A. Sep 2013;161a(9):2124–33. doi: 10.1002/ajmg.a.36020 [DOI] [PubMed] [Google Scholar]
- 35.Goizet C, Coupry I, Rooryck C, et al. Molecular characterization of an 11q14.3 microdeletion associated with leukodystrophy. European journal of human genetics : EJHG. Mar 2004;12(3):245–50. doi: 10.1038/sj.ejhg.5201128 [DOI] [PubMed] [Google Scholar]
- 36.Coupry I, Taine L, Goizet C, et al. Leucodystrophy and oculocutaneous albinism in a child with an 11q14 deletion. Journal of medical genetics. Jan 2001;38(1):35–8. doi: 10.1136/jmg.38.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto T, Shimada S, Shimojima K, Sangu N, Ninomiya S, Kubota M. Leukoencephalopathy associated with 11q24 deletion involving the gene encoding hepatic and glial cell adhesion molecule in two patients. European journal of medical genetics. Sep 2015;58(9):492–6. doi: 10.1016/j.ejmg.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 38.Hirasawa-Inoue A, Takeshita E, Shimizu-Motohashi Y, et al. Static Leukoencephalopathy Associated with 17p13.3 Microdeletion Syndrome: A Case Report. Neuropediatrics. Dec 2019;50(6):387–390. doi: 10.1055/s-0039-1693972 [DOI] [PubMed] [Google Scholar]
- 39.Emrick LT, Rosenfeld JA, Lalani SR, et al. Microdeletions excluding YWHAE and PAFAH1B1 cause a unique leukoencephalopathy: further delineation of the 17p13.3 microdeletion spectrum. Genetics in medicine : official journal of the American College of Medical Genetics. Jul 2019;21(7):1652–1656. doi: 10.1038/s41436-018-0358-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiff M, Delahaye A, Andrieux J, et al. Further delineation of the 17p13.3 microdeletion involving YWHAE but distal to PAFAH1B1: four additional patients. European journal of medical genetics. Sep-Oct 2010;53(5):303–8. doi: 10.1016/j.ejmg.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 41.Heide S, Keren B, Billette de Villemeur T, et al. Copy Number Variations Found in Patients with a Corpus Callosum Abnormality and Intellectual Disability. The Journal of pediatrics. Jun 2017;185:160–166.e1. doi: 10.1016/j.jpeds.2017.02.023 [DOI] [PubMed] [Google Scholar]
- 42.Falah N, Posey JE, Thorson W, et al. 22q11.2q13 duplication including SOX10 causes sex-reversal and peripheral demyelinating neuropathy, central dysmyelinating leukodystrophy, Waardenburg syndrome, and Hirschsprung disease. American journal of medical genetics Part A. Apr 2017;173(4):1066–1070. doi: 10.1002/ajmg.a.38109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Köhler W, Curiel J, Vanderver A. Adulthood leukodystrophies. Nature reviews Neurology. Feb 2018;14(2):94–105. doi: 10.1038/nrneurol.2017.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee YJ. Acute disseminated encephalomyelitis in children: differential diagnosis from multiple sclerosis on the basis of clinical course. Korean journal of pediatrics. Jun 2011;54(6):234–40. doi: 10.3345/kjp.2011.54.6.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filippi M, Rocca MA, Ciccarelli O, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. The Lancet Neurology. Mar 2016;15(3):292–303. doi: 10.1016/s1474-4422(15)00393-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurwitz BJ. The diagnosis of multiple sclerosis and the clinical subtypes. Annals of Indian Academy of Neurology. Oct 2009;12(4):226–30. doi: 10.4103/0972-2327.58276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holmes GL, Milh MD, Dulac O. Maturation of the human brain and epilepsy. Handbook of clinical neurology. 2012;107:135–43. doi: 10.1016/b978-0-444-52898-8.00007-0 [DOI] [PubMed] [Google Scholar]
- 48.Shukla A, Kaur P, Girisha KM. Report of the Third Family with Multiple Mitochondrial Dysfunctions Syndrome 5 Caused by the Founder Variant p.(Glu87Lys) in ISCA1. Journal of pediatric genetics. Sep 2018;7(3):130–133. doi: 10.1055/s-0038-1641177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shukla A, Narayanan DL, Kaur P, Girisha KM. ISCA1-Related Multiple Mitochondrial Dysfunctions Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews(®). University of Washington, Seattle: Copyright © 1993-2020, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.; 1993. [PubMed] [Google Scholar]
- 50.Shukla A, Hebbar M, Srivastava A, et al. Homozygous p.(Glu87Lys) variant in ISCA1 is associated with a multiple mitochondrial dysfunctions syndrome. Journal of human genetics. Jul 2017;62(7):723–727. doi: 10.1038/jhg.2017.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarbu N, Shih RY, Jones RV, Horkayne-Szakaly I, Oleaga L, Smirniotopoulos JG. White Matter Diseases with Radiologic-Pathologic Correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. Sep-Oct 2016;36(5):1426–47. doi: 10.1148/rg.2016160031 [DOI] [PubMed] [Google Scholar]
- 52.Biesecker LG, Green RC. Diagnostic clinical genome and exome sequencing. The New England journal of medicine. Jun 19 2014;370(25):2418–25. doi: 10.1056/NEJMra1312543 [DOI] [PubMed] [Google Scholar]
- 53.Kunii M, Doi H, Ishii Y, et al. Genetic analysis of adult leukoencephalopathy patients using a custom-designed gene panel. Aug 2018;94(2):232–238. doi: 10.1111/cge.13371 [DOI] [PubMed] [Google Scholar]
- 54.Cohen L, Manín A, Medina N, et al. Argentinian clinical genomics in a leukodystrophies and genetic leukoencephalopathies cohort: Diagnostic yield in our first 9 years. Annals of human genetics. Jan 2020;84(1):11–28. doi: 10.1111/ahg.12345 [DOI] [PubMed] [Google Scholar]
- 55.Bick D, Jones M, Taylor SL. Case for genome sequencing in infants and children with rare, undiagnosed or genetic diseases. Dec 2019;56(12):783–791. doi: 10.1136/jmedgenet-2019-106111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helman G, Lajoie BR, Crawford J, et al. Genome sequencing in persistently unsolved white matter disorders. Jan 2020;7(1):144–152. doi: 10.1002/acn3.50957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nave KA. Myelination and support of axonal integrity by glia. Nature. Nov 11 2010;468(7321):244–52. doi: 10.1038/nature09614 [DOI] [PubMed] [Google Scholar]
- 58.Gaesser JM, Fyffe-Maricich SL. Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Experimental neurology. Sep 2016;283(Pt B):501–11. doi: 10.1016/j.expneurol.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.André C. CADASIL: pathogenesis, clinical and radiological findings and treatment. Arquivos de neuro-psiquiatria. Apr 2010;68(2):287–99. doi: 10.1590/s0004-282x2010000200026 [DOI] [PubMed] [Google Scholar]
- 60.van der Veldt N, van Rappard DF, van de Pol LA, et al. Intrathecal baclofen in metachromatic leukodystrophy. Feb 2019;61(2):232–235. doi: 10.1111/dmcn.13919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gump WC, Mutchnick IS, Moriarty TM. Selective dorsal rhizotomy for spasticity not associated with cerebral palsy: reconsideration of surgical inclusion criteria. Neurosurgical focus. Nov 2013;35(5):E6. doi: 10.3171/2013.8.focus13294 [DOI] [PubMed] [Google Scholar]
- 62.Eichler F, Duncan C, Musolino PL, et al. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. The New England journal of medicine. Oct 26 2017;377(17):1630–1638. doi: 10.1056/NEJMoa1700554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hironaka K, Yamazaki Y, Hirai Y, et al. Enzyme replacement in the CSF to treat metachromatic leukodystrophy in mouse model using single intracerebroventricular injection of self-complementary AAV1 vector. Scientific reports. Aug 18 2015;5:13104. doi: 10.1038/srep13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sessa M, Lorioli L, Fumagalli F, et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet (London, England). Jul 30 2016;388(10043):476–87. doi: 10.1016/s0140-6736(16)30374-9 [DOI] [PubMed] [Google Scholar]
- 65.Berardi AS, Pannuzzo G, Graziano A, Costantino-Ceccarini E, Piomboni P, Luddi A. Pharmacological chaperones increase residual β-galactocerebrosidase activity in fibroblasts from Krabbe patients. Molecular genetics and metabolism. Aug 2014;112(4):294–301. doi: 10.1016/j.ymgme.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 66.LeVine SM, Pedchenko TV, Bronshteyn IG, Pinson DM. L-cycloserine slows the clinical and pathological course in mice with globoid cell leukodystrophy (twitcher mice). Journal of neuroscience research. Apr 15 2000;60(2):231–6. doi: [DOI] [PubMed] [Google Scholar]
- 67.Hawkins-Salsbury JA, Shea L, Jiang X. Mechanism-based combination treatment dramatically increases therapeutic efficacy in murine globoid cell leukodystrophy. Apr 22 2015;35(16):6495–505. doi: 10.1523/jneurosci.4199-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rice GI, Meyzer C, Bouazza N, et al. Reverse-Transcriptase Inhibitors in the Aicardi–Goutières Syndrome. The New England journal of medicine. Dec 6 2018;379(23):2275–7. doi: 10.1056/NEJMc1810983 [DOI] [PubMed] [Google Scholar]
- 69.Kothur K, Bandodkar S, Chu S, et al. An open-label trial of JAK 1/2 blockade in progressive IFIH1-associated neuroinflammation. Neurology. Feb 6 2018;90(6):289–291. doi: 10.1212/wnl.0000000000004921 [DOI] [PubMed] [Google Scholar]
- 70.Dooves S, Leferink PS, Krabbenborg S, et al. Cell Replacement Therapy Improves Pathological Hallmarks in a Mouse Model of Leukodystrophy Vanishing White Matter. Stem cell reports. Mar 5 2019;12(3):441–450. doi: 10.1016/j.stemcr.2019.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dooves S, Bugiani M, Wisse LE, Abbink TEM, van der Knaap MS, Heine VM. Bergmann glia translocation: a new disease marker for vanishing white matter identifies therapeutic effects of Guanabenz treatment. Neuropathology and applied neurobiology. Jun 2018;44(4):391–403. doi: 10.1111/nan.12411 [DOI] [PubMed] [Google Scholar]
- 72.Hagemann TL, Powers B, Mazur C, et al. Antisense suppression of glial fibrillary acidic protein as a treatment for Alexander disease. Annals of neurology. Jan 2018;83(1):27–39. doi: 10.1002/ana.25118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leone P, Shera D, McPhee SW, et al. Long-term follow-up after gene therapy for canavan disease. Science translational medicine. Dec 19 2012;4(165):165ra163. doi: 10.1126/scitranslmed.3003454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hull V, Wang Y, Burns T, Zhang S, Sternbach S, McDonough J. Antisense Oligonucleotide Reverses Leukodystrophy in Canavan Disease Mice. Mar 2020;87(3):480–485. doi: 10.1002/ana.25674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Osorio MJ, Rowitch DH, Tesar P, Wernig M, Windrem MS, Goldman SA. Concise Review: Stem Cell-Based Treatment of Pelizaeus-Merzbacher Disease. Stem cells (Dayton, Ohio). Feb 2017;35(2):311–315. doi: 10.1002/stem.2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gupta N, Henry RG, Strober J, et al. Neural stem cell engraftment and myelination in the human brain. Science translational medicine. Oct 10 2012;4(155):155ra137. doi: 10.1126/scitranslmed.3004373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raymond GV, Moser AB, Fatemi A. X-Linked Adrenoleukodystrophy. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews(®). University of Washington, Seattle: Copyright © 1993-2020, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.; 1993. [Google Scholar]
- 78.Helman G, Van Haren K, Escolar ML, Vanderver A. Emerging treatments for pediatric leukodystrophies. Pediatric clinics of North America. Jun 2015;62(3):649–66. doi: 10.1016/j.pcl.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Helman G, Van Haren K, Bonkowsky JL, et al. Disease specific therapies in leukodystrophies and leukoencephalopathies. Molecular genetics and metabolism. Apr 2015;114(4):527–36. doi: 10.1016/j.ymgme.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Assadi M, Janson C, Wang DJ, et al. Lithium citrate reduces excessive intra-cerebral N-acetyl aspartate in Canavan disease. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. Jul 2010;14(4):354–9. doi: 10.1016/j.ejpn.2009.11.006 [DOI] [PubMed] [Google Scholar]
- 81.Pleasure D, Guo F, Chechneva O, et al. Pathophysiology and Treatment of Canavan Disease. Neurochemical research. Mar 2020;45(3):561–565. doi: 10.1007/s11064-018-2693-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berginer VM, Salen G, Shefer S. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. The New England journal of medicine. Dec 27 1984;311(26):1649–52. doi: 10.1056/nejm198412273112601 [DOI] [PubMed] [Google Scholar]
- 83.Mandia D, Chaussenot A, Besson G, et al. Cholic acid as a treatment for cerebrotendinous xanthomatosis in adults. Aug 2019;266(8):2043–2050. doi: 10.1007/s00415-019-09377-y [DOI] [PubMed] [Google Scholar]
- 84.Roe CR, Bottiglieri T, Wallace M, Arning E, Martin A. Adult Polyglucosan Body Disease (APBD): Anaplerotic diet therapy (Triheptanoin) and demonstration of defective methylation pathways. Molecular genetics and metabolism. Oct-Nov 2010;101(2-3):246–52. doi: 10.1016/j.ymgme.2010.06.017 [DOI] [PubMed] [Google Scholar]
- 85.Bernard G, Vanderver A. POLR3-Related Leukodystrophy. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews(®). University of Washington, Seattle: Copyright © 1993-2020, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.; 1993. [Google Scholar]
- 86.Wolf NI, Toro C, Kister I, et al. DARS-associated leukoencephalopathy can mimic a steroid-responsive neuroinflammatory disorder. Neurology. Jan 20 2015;84(3):226–30. doi: 10.1212/wnl.0000000000001157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moser HW, Raymond GV, Lu SE, et al. Follow-up of 89 asymptomatic patients with adrenoleukodystrophy treated with Lorenzo's oil. Archives of neurology. Jul 2005;62(7):1073–80. doi: 10.1001/archneur.62.7.1073 [DOI] [PubMed] [Google Scholar]
- 88.Shapiro E, Krivit W, Lockman L, et al. Long-term effect of bone-marrow transplantation for childhood-onset cerebral X-linked adrenoleukodystrophy. Lancet (London, England). Aug 26 2000;356(9231):713–8. doi: 10.1016/s0140-6736(00)02629-5 [DOI] [PubMed] [Google Scholar]
- 89.Peters C, Charnas LR, Tan Y, et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. Aug 1 2004;104(3):881–8. doi: 10.1182/blood-2003-10-3402 [DOI] [PubMed] [Google Scholar]
- 90.Miller WP, Rothman SM, Nascene D, et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood. Aug 18 2011;118(7):1971–8. doi: 10.1182/blood-2011-01-329235 [DOI] [PubMed] [Google Scholar]
- 91.Escolar ML, Poe MD, Provenzale JM, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. The New England journal of medicine. May 19 2005;352(20):2069–81. doi: 10.1056/NEJMoa042604 [DOI] [PubMed] [Google Scholar]
- 92.Krivit W, Peters C, Shapiro EG. Bone marrow transplantation as effective treatment of central nervous system disease in globoid cell leukodystrophy, metachromatic leukodystrophy, adrenoleukodystrophy, mannosidosis, fucosidosis, aspartylglucosaminuria, Hurler, Maroteaux-Lamy, and Sly syndromes, and Gaucher disease type III. Current opinion in neurology. Apr 1999;12(2):167–76. doi: 10.1097/00019052-199904000-00007 [DOI] [PubMed] [Google Scholar]
- 93.Krivit W. Allogeneic stem cell transplantation for the treatment of lysosomal and peroxisomal metabolic diseases. Springer seminars in immunopathology. Nov 2004;26(1-2):119–32. doi: 10.1007/s00281-004-0166-2 [DOI] [PubMed] [Google Scholar]
- 94.Chahwan C, Chahwan R. Aicardi-Goutieres syndrome: from patients to genes and beyond. Clinical genetics. May 2012;81(5):413–20. doi: 10.1111/j.1399-0004.2011.01825.x [DOI] [PubMed] [Google Scholar]
- 95.Weinberg KI. Early use of drastic therapy. The New England journal of medicine. May 19 2005;352(20):2124–6. doi: 10.1056/NEJMe058071 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as it is a review of pre-existing information.