Abstract
Cannabis sativa products have historically been used for healing purposes; now their biological properties are supported with scientific evidence, but modern research has not yet fully developed its therapeutic potential. This study focuses on the cultivar of C. sativa called strawberry to understand the biological and medical potentials of hydroalcoholic extracts from two different parts of the plant: leaves and inflorescences. Two biological assets were investigated including antioxidant and antimicrobial potential. Additionally, quantitative determination of phenolic and terpenophenol compounds was conducted. The antimicrobial action was highlighted for the hydroalcoholic extract from inflorescences, especially against Escherichia coli and Bacillus subtilis. Among the dermatophytes’ strains, the most sensitive was Arthroderma currey. These effects could be related albeit partially to the pattern of the phenolics detected, among which the most prominent one was benzoic acid. On the other hand, antioxidant and antimicrobial effects of the extracts could be also mediated by the main terpenophenolics identified and quantified, namely cannabidiolic acid and cannabidiol. Collectively, the present data point to the potential use of the inflorescences from the C. sativa cultivar strawberry as a valuable plant material for the development of bioactive extracts with antioxidant and antimicrobial effects
Keywords: Cannabis sativa, antimicrobial, antioxidant, phenolic compounds, benzoic acid, cannabidiol, cannabidiolic acid
1. Introduction
Plants have long been used for health-promoting effects, due to the bioactivity of specialized metabolites that may work as medicines, flavorings, and recreational substances in humans [1,2,3,4].
In this context, a great deal of attention has been paid to Cannabis sativa L. [5], an annual, robust, fast-growing, and generally dioecious plant that produces male and female flowers on separate individuals, although it has the tendency to be monoecious [6,7].
Industrial hemp has been long cultivated as a valuable source of fibers and nutrients [5], and numerous European countries, including Italy, have promoted the valorization of the hemp productive chain, thus driving the registration of new cultivars, with more than 60, throughout Europe. This market is currently ruled by the EU (Regulation (EC) N° 1251/99 and subsequent amendments), according to which only hemp cultivars registered can be cultivated and after the verification of Δ9-tetrahydrocannabinol content, which has to be lower than 0.2% w/w [8].
Non-psychotropic terpenophenols, cannabidiol and cannabigerol, are the main phytochemicals responsible for hemp’s pharmacological effects, especially in the brain [9,10,11,12,13]. On the other hand, specialized metabolites present in trichomes, including terpenes and phenolic compounds, may influence the biological properties of inflorescence extracts [14,15,16,17,18].
Previous studies have suggested the potential pharmacological applications of polar extracts from inflorescences, with antioxidant/anti-inflammatory and antimicrobial properties related, albeit partially, to the pattern of phenolic compounds [19,20]. In addition, the aerial parts of the plant such as the leaves are rich in trichomes thus suggesting that leaves also contain active ingredients and their biological activities could be investigated.
Actually, limited data are available about the potential applicability of hemp leaves as a source of bioactive extracts with health-promoting effects [21]. Therefore, the objective of this study was to investigate the antimicrobial and antioxidant activity of hydroalcoholic extracts from inflorescences and leaves of the C. sativa strawberry cultivar. The extracts were prepared via ultrasound-assisted extraction (UAE) and analyzed through liquid chromatography for the quantitative determination of phenolic and terpenophenolic compounds.
2. Materials and Methods
2.1. Hemp Material
Hemp dry leaves and inflorescences of Cannabis sativa L. cultivar “strawberry” were cultivated in the Umbria region, Italy. All of the samples were kindly supplied by J.j. Farm Società Agricola Semplice (Castiglione Del Lago (PG), Italy) during the cultivation season of 2021. Morphological identification was made by Prof. Paola Angelini, Associate Professor at the Department of Chemistry, Biology and Biotechnology, Università degli Studi di Perugia, Perugia (Italy).
2.2. Chemicals and Reagents
1,1-Diphenyl-2-picryl-hydrazyl-hydrate (DPPH), Trolox, 2,4,6-tri(2-pyridyl)-1,5,5-triazine ligand (TPTZ), 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) or ABTS, ferric chloride, acetate buffer, Mueller–Hinton broth (MHB), Rose bengal chloramphenicol agar (RBCA), malt extract agar (MEA), Sabouraud dextrose agar (SDA), RPMI (Roswell Park Memorial Institute) 1640 medium, Morpholinepropanesulphonic acid (MOPS), fluconazole, and purity grade organic solvent ethanol absolute were purchased from Sigma (Sigma-Aldrich GmbH, Taufkirchen, Germany).
2.3. Molecular Identification
To test our identification based on the morphological characters of C. sativa, total genomic DNA was extracted using a ZR plant/seed DNA kit (Euroclone S.p.A., Milan, Italy). The genomic DNA’s quality and quantity were evaluated according to the literature [22].
2.4. Preparation of Plant Extracts
Female Cannabis flowers and leaves were weighed; after, they were gently crushed into small-sized pieces and they were placed into different flasks. To extract the active ingredients, maceration of the plant’s parts in aqueous and hydroalcoholic solutions is required [23], according to Salhi et al. [24] The optimal ratio is 1:10 w/v. Briefly, hydroalcoholic extracts (70% ethanol) were obtained by soaking the flowers and leaves in different bottles with distilled water. The samples were taken into a dark place at room temperature (21 2 °C) for 72 h. According to Abubakar and Haque [25] the extraction was carried out by placing flasks in an ultrasonic bath (Bransonic, Dietzenbach, Germany) for 2 h for each sample. The solutions were filtered using filter paper into clean flasks. Each extract was filtered using a syringe filter with a 0.45 μm pore Ø, bought by Corning (Wiesbaden, Germany). The sterilized extracts were stored at −20 °C. Ten mL of each extract was brought to dryness in the oven at 60 °C until a constant mass was obtained; the dry weight was found and used to calculate the concentration (Table 1).
Table 1.
Cannabis sativa L. cultivar strawberry extracts.
| Sample ID | Plant Material | Type of Extract | Concentration (g/10 mL) |
|---|---|---|---|
| E1 | Inflorescences | Hydroalcoholic | 0.2125 |
| E2 | Leaves | Hydroalcoholic | 0.1699 |
2.5. Antimicrobial Tests
Fungal and Bacterial Strains
Extracts E1–E2 were tested for in vitro antifungal activity against different yeasts and dermatophyte species: Candida albicans (YEPGA 6183), C. tropicalis (YEPGA 6184), C. albicans (YEPGA 6379), C. parapsilopsis (YEPGA 6551), Arthroderma curreyi (CCF 5207), A. gypseum (CCF 6261), A. insingulare (CCF 5417), A. quadrifidum (CCF 5792), Trichophyton mentagrophytes (CCF 4823), T. mentagrophytes (CCF 5930), T. rubrum (CCF 4933), and T. tonsurans (CCF 4834).
Furthermore, the same samples were assayed for the antimicrobial test against Gram-negative bacterial strains: Escherichia coli (ATCC 10536), E. coli (PeruMycA 2), E. coli (PeruMycA 3), Pseudomonas aeruginosa (ATCC 15442), and Salmonella typhy (PeruMyc 7), and also against Gram-positive strains: Bacillus cereus (PeruMycA 4), B. subtilis (PeruMyc 6), and Staphylococcus aureus (ATCC 6538). The microbial cultures were maintained in the culture collection of the Department of Chemistry, Biology and Biotechnology (DCBB University of Perugia, Perugia, Italy) named “PeruMyc” and are available upon request.
Antifungal and antibacterial activities were assessed as previously reported [19,26,27,28].
2.6. Antioxidant Tests and Determination of Phenolic Compounds
The scavenging/reducing effects of the extracts were evaluated through DPPH, ABTS, and FRAP assays. The detailed protocols are reported in the literature [29,30,31,32].
The total amount of phenolic compounds content in C. sativa strawberry extracts was determined according to the Folin–Ciocalteu assay [33].
2.7. High-Performance Liquid Chromatography (HPLC) Analysis of Phenolic Compounds
The extracts were analyzed for phenol and terpenophenol quantitative determination using a reversed phase HPLC-DAD in gradient elution mode [16]. The separation is fully detailed in the Supplementary Materials.
3. Results and Discussion
3.1. Plant Identification
Recently, there has been renewed global interest in the therapeutic potential of Cannabis, given its unique chemical components. The characterization and identification of this medicinal plant is fundamental for the knowledge of all of its phytochemistry, in such a way as to express its full potential for pharmaceutical applications [34]. The morphological characteristics of C. sativa correspond to those reported by Small [11]. The taxonomic affiliation of the plant was performed via targeting the trnL-F region of the chloroplast genome. Additionally, BLAST research (Table 2) has highlighted that the highest omology was observed with the strains cv. Dagestani, Cheungsam, Yoruba, and Carmagnola.
Table 2.
Comparison between the sample of C. sativa cv. strawberry and the sequences present on GenBank.
| Correspondence with Genbank seq. | % Identity | Base Pair | Accession no. |
|---|---|---|---|
| Cannabis sativa | 100 | 153,945 | KY084475.1 |
| Cannabis sativa cultivar Dagestani | 100 | 153,867 | KR779995.1 |
| Cannabis sativa subsp. sativa cultivar Cheungsam | 100 | 153,848 | KR184827.1 |
| Cannabis sativa cultivar Yoruba | 100 | 153,854 | NC_027223.1 |
| Cannabis sativa cultivar Carmagnola | 100 | 153,871 | NC_026562.1 |
| Cannabis sativa | 100 | 153,849 | OM479429.1 |
| Cannabis sativa | 100 | 780 | AF501598.1 |
| Cannabis sativa | 100 | 750 | AY958392.1 |
| Cannabis sativa | 100 | 687 | KF250352.1 |
| Cannabis sativa | 100 | 681 | AY958387.1 |
| Cannabis sativa | 99.86% | 153,910 | MH118118.1 |
| Cannabis sativa | 99.86% | 127,897 | KY419963.1 |
| Cannabis sativa | 99.86% | 153,927 | OK523376.1 |
| Cannabis sativa | 99.86% | 153,873 | MT721158.1 |
| Cannabis sativa cultivar Yunma 7 | 99.86% | 153,899 | MW013540.1 |
| Cannabis sativa | 99.86% | 749 | AY958393.1 |
| Cannabis sativa | 99.86% | 716 | JN040359.1 |
| Cannabis sativa | 99.85% | 680 | AY958388.1 |
| Cannabis sativa | 99.58% | 750 | AJ390367.1 |
3.2. Antimicrobial Activity
Table 3, Table 4 and Table 5 shows the MIC range and geometric means of cv strawberry extracts and synthetic drugs (ciprofloxacin, fluconazole, and griseofulvin) against the tested bacterial, yeasts and dermatophytes strains. All of them showed antimicrobial activity in the concentration range of <1.95–200 μg/mL, but with a wide variability in terms of potency and selectivity.
Table 3.
Minimal inhibitory concentrations (MICs) of Cannabis sativa L. cv. strawberry extracts against bacterial strains.
| MIC (µg mL−1) * | ||||||||
|---|---|---|---|---|---|---|---|---|
| Escherichia | Escherichia | Escherichia | Bacillus | Pseudomonas | Bacillus | Salmonella | Staphylococcus | |
| coli | coli | coli | cereus | aeruginosa | subtilis | typhy | aureus | |
| (ATCC 10536) | (PeruMycA 2) | (PeruMycA 3) | (ATCC 12826) | (ATCC 15442) | (PeruMycA 6) | (PeruMycA 7) | (ATCC 6538) | |
| E1 | 4.96 (3.13–6.25) | 15.74 (12.5–25) | >200 | >200 | 39.68(25–50) | 1.56 < - 1.56 | >200 | 15.74 (12.5–25) |
| E2 | 7.87 (6.25–12.5) | 39.68 (25–50) | >200 | >200 | 62.99 (50–100) | 19.84 (12.5–25) | >200 | 62.99 (50–100) |
| Ciprofloxacin (µg/mL) | 31.49 (25–50) | 9.92 (6.25–12.5) | 79.37 (50–100) | 125.99 (100–200) | 125.99 (100–200) | 125.99 (100–200) | 79.37 (50–100) | 200 - > 200 |
* The MIC values are reported as geometric means of three independent replicates (n = 3). The MIC range concentrations are reported within brackets.
Table 4.
Minimal inhibitory concentrations (MICs) of Cannabis sativa L. cv. strawberry extracts against yeast strains.
| MIC (µg mL−1) * | ||||
|---|---|---|---|---|
| Candida | Candida | Candida | Candida | |
| tropicalis | albicans | parapsilosis | albicans | |
| (YEPGA 6184) | (YEPGA 6379) | (YEPGA 6551) | (YEPGA 6183) | |
| E1 | >200 | >200 | <6.25 | 15.75 (12.5–25) |
| E2 | 15.75 (12.5–25) | >200 | <6.25 | 15.75 (12.5–25) |
| Fluconazole (µg/mL) | 2 | 1 | 4 | 2 |
* The MIC values are reported as geometric means of three independent replicates (n = 3). The MIC range concentrations are reported within brackets.
Table 5.
Minimal inhibitory concentrations of Cannabis sativa L. cv. strawberry extracts against dermatophyte strains.
| MIC (µg mL−1) * | ||||||||
|---|---|---|---|---|---|---|---|---|
| Trichophyton | Trichophyton | Trichophyton | Arthroderma | Trichophyton | Arthroderma | Arthroderma | Arthroderma | |
| mentagrophytes | tonsurans | rubrum | quadrifidum | erinacei | gypseum | currey | insingulare | |
| (CCF 4823) | (CCF 4834) | (CCF 4933) | (CCF 5792) | (CCF 5930) | (CCF 6261) | (CCF 5207) | (CCF 5417) | |
| E1 | 39.68 (25–50) | 62.99 (50–100) | 62.99 (50–100) | 31.49 (25–50) | 39.68 (25–50) | 125.99 (100–200) | <6.25 | 125.99 (100–200) |
| E2 | 125.99 (100–200) | 79.37 (50–100) | 79.37 (50–100) | 125.99 (100–200) | 125.99 (100–200) | 158.74 (100–200) | <6.25 | 125.99 (100–200) |
| Griseofulvin (µg/mL) | 2.52 (2–4) | 0.198 (0.125–0.25) | 1.26 (1–2) | >8 | 3.174 (2–4) | 1.587 (1–2) | >8 | >8 |
* The MIC values are reported as geometric means of three independent replicates (n = 3). The MIC range concentrations are reported within brackets.
Regarding bacteria, the strongest inhibition was observed for E1 extract [MIC < 1.56 µg/mL against Bacillus subtilis (PeruMycA 6)]. Almost all of the bacterial strains were sensitive to both hydro-alcoholic extracts with MIC values lower than 62.99 μg/mL.
The extracts were also effective in inhibiting dermatophyte growth. A. currey (CCF 5207) was the most sensitive fungal species to the extracts with MIC values < 6.25 μg/mL. Based on our knowledge, reports on the screening of the antimicrobial activity of C. sativa cv. strawberry extracts against different bacterial, yeast, and dermatophyte strains have not been published, yet. On the other hand, the present results are consistent with our previous investigations about the antimycotic effects of the water extract from the inflorescences of the industrial hemp cultivar Futura 75 [20].
3.3. Phytochemical Analysis
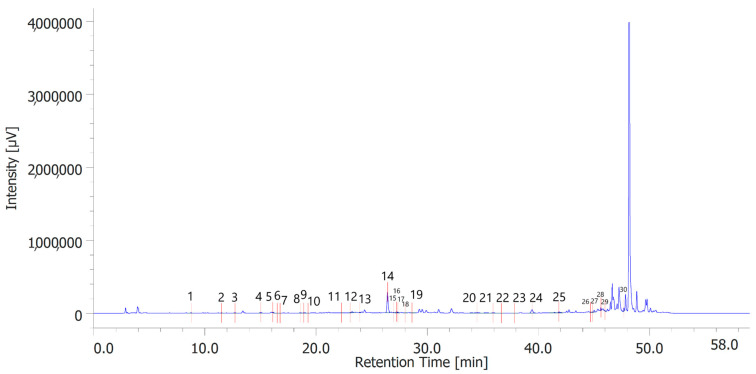
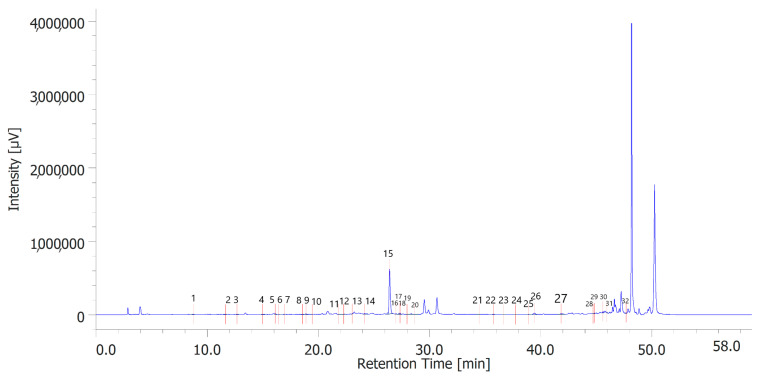
Table 6 shows the concentration of the total phenols contained (TPC) in the hydroalcoholic extracts of the leaves and inflorescences of C. sativa cv. strawberry. Total phenols data are expressed as % means ± SD referred to gallic acid equivalents. Phenolic compounds are an important class of active biological compounds synthesized by hemp. Their biological function is related to their antioxidant activity for the protection of important cellular structures such as membranes, structural proteins, enzymes, and cell membrane lipids. Different phenolic compounds have been identified in polar extracts from hemp inflorescences, including gallic acid, catechin, vanillic acid and rutin, and a close relationship between antioxidant activity and the content of phenolic matter [20]. In the present study, antiradical effects were measured via ABTS, DPPH, and FRAP assays (Table 7), as well. The EC50 values related to these assays reflected the MIC values observed against bacterial and fungal strains, thus further indicating the content of phenolics in the extracts as responsible, albeit in part, for the observed antioxidant and antimicrobial effects [35,36]. In order to improve our knowledge on the phytochemical composition of the extracts, liquid chromatography analyses were conducted (Figure 1 and Figure 2). A total of 30 compounds were identified and quantified by comparison with a pure standard. The list of the identified compounds is included in Table 3. Among the identified phytochemicals, benzoic acid was the prominent compound, in both the inflorescence (peak #14: 766.75 µg/mL) and leaf (peak # 15: 1683.43 µg/mL) extracts. This is also consistent with our recent study indicating benzoic acid as one of the prominent phytochemicals in the polar extracts from the pollen collected by male hemp inflorescences [16].
Table 6.
Total phenolics compounds contained in C. sativa cv. strawberry extracts.
| Sample | GAE | ±SD |
|---|---|---|
| E1 | 14.97 | 1.51 |
| E2 | 13.79 | 1.39 |
Table 7.
Antiradical activity of the tested C. sativa extracts.
| DPPH Test | ABTS Test | FRAP Test | |||
|---|---|---|---|---|---|
| Sample | EC50 μg/mL |
Trolox Equivalents | EC50 μg/mL |
Trolox Equivalents | Trolox Equivalents |
| E1 | 73 ± 2 | 11.45 ± 0.39 | 2 ± 0.04 | 1.13 ± 0.02 | 95 ± 50 |
| E2 | 67 ± 5 | 10.31 ± 0.85 | 2 ± 0.1 | 1.29 ± 0.06 | 76 ± 12 |
Figure 1.
Phenolic compounds identified in the hydroalcoholic extract of hemp inflorescences. Among the compounds quantitavely determined, benzoic acid (peak #14) was the prominent.
Figure 2.
Phenolic compounds identified in the hydroalcoholic extract of hemp leaves. Among the compounds quantitatively determined, benzoic acid (peak #15) was the most prominent.
The presence of significant amounts of benzoic acid in the extracts can also explain the observed antimicrobial properties against bacteria and fungi.
Indeed, benzoic acid and its derivatives have long been used as food preservatives [37]. They are effective as antimicrobials against a wide number of microbial strains, and this can be related to their capability to penetrate the phospholipid bilayer, and disrupt phospholipids’ interactions, thus leading to membrane disintegration.
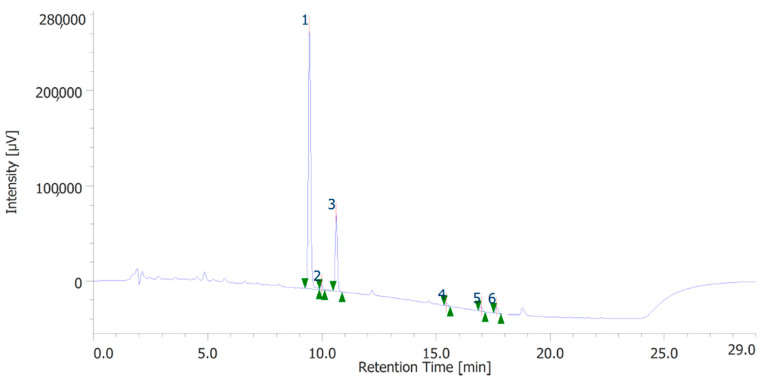
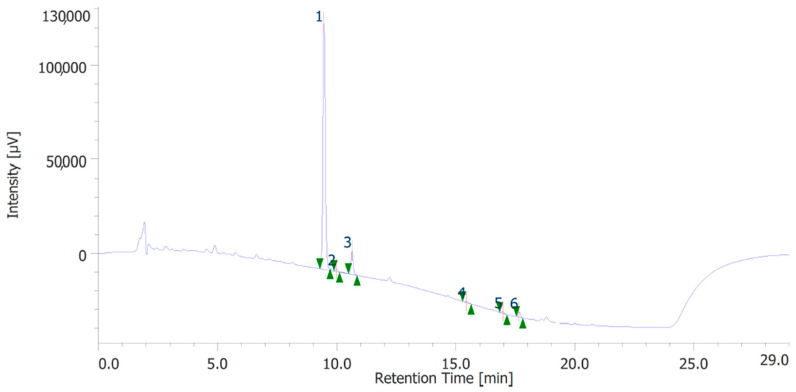
The extracts were also quantitatively analyzed for the content of terpenophenols, as reported in Figure 3 and Figure 4. Both the leaves and inflorescences showed a higher content of cannabidiolic acid (CBDA, peak #1) and cannabidiol (CBD, peak # 3) compared to the other terpenophenols, whose list is reported in Table S4. Specifically, the inflorescences were richer in CBDA and CBD than the leaves. The respective concentrations of terpenophenols are indicated in the legends of Figure 3 and Figure 4.
Figure 3.
Terphenolic compounds identified in the hydroalcoholic extract of hemp inflorescences. Among the compounds quantitatively determined, cannabidiolic acid (peak #1: 45.99 µg/mL) and cannabidiol (peak #3: 23.55 µg/mL) were the most prominent.
Figure 4.
Terphenolic compounds identified in the hydroalcoholic extract of hemp leaves. Among the compounds quantitatively determined, cannabidiolic acid (peak #1: 21.71 µg/mL) and cannabidiol (peak #3: 3.52 µg/mL) were the most prominent.
Although these phytochemicals are present at concentrations quite lower compared with benzoic acid, we cannot exclude that CBDA and CBD may contribute to the intrinsic scavenging/reducing [10] and antimicrobial properties [38] demonstrated by the extracts.
Additionally, their presence may also suggest the extracts’ putative protective effects [16] which deserve further investigation.
4. Conclusions
As widely reported in the literature, C. sativa is a valuable crop from which multiple extracts and bioactive compounds can be obtained for innovative applications of this traditional botany resource [17]. The present study focused on the properties of different types of extracts from the strawberry hemp cultivar, with regards to phenolic composition, antioxidant and antimicrobial effects. From the outcomes highlighted above, the extracts derived from inflorescences seem to have greater biological activity than those derived from leaves, probably due to the fact that a greater number of different trichomes are present in the inflorescences and therefore there is greater and more differentiated production of active metabolites. This aspect, however, needs more in-depth studies.
The results show that this crop has antimicrobial and antioxidant activities so there are promising translational potentials in different fields. These effects could be related, albeit partially, to the total content of phenolic compounds. With specific regard to the antimicrobial effects, the higher concentrations of benzoic acid in the extracts, compared with the other phenolics, could play a pivotal role in the observed antimicrobial effects [39]. The antioxidant and antimicrobial effects of hemp extracts could be also mediated, at least in part, by the main terpenophenolics identified and quantified, namely cannabidiolic acid and cannabidiol [10,38]. Although further studies still need to increase our knowledge on the chemical composition, extracts prepared from this cultivar might be considered for the development of innovative antioxidant and antimicrobial products in the food and pharmaceutical industries. This would further improve the whole productive chain of industrial hemp.
Acknowledgments
The work is also part of the third mission activity of the botanic garden “Giardino dei Semplici”, “G. d’Annunzio” University Chieti-Pescara.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12020219/s1. High-performance liquid chromatography (HPLC) analysis of phenolic and terpenophenolic compounds; Table S1: Gradient elution condition; Table S2: Retention times and wavelength of quantification of the standards used to identify and quantify phenolics in the extracts.; Table S3: Gradient elution of HPLC-DAD; Table S4: Retention times and wavelength of the quantification of the standards used to identify and quantify the terpenophenolics in the extracts.
Author Contributions
Conceptualization, P.A., L.M. and C.F.; methodology, P.A., L.M. and C.F.; software, P.A., L.M. and C.F.; validation, G.A.F.; formal analysis, P.A., L.M. and C.F.; investigation, G.A.F., B.T., G.C., L.S., A.A., D.B. and S.C.D.S.; resources, P.A., L.M. and C.F.; data curation, P.A., L.M. and C.F.; writing—original draft preparation, P.A. and G.A.F.; writing—review and editing, P.A., L.M., C.F., G.Z. and G.O.; visualization, R.V., G.O. and G.Z.; supervision, R.V.; project administration, P.A., L.M. and C.F.; funding acquisition, P.A., L.M. and C.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Fitzgerald M., Heinrich M., Booker A. Medicinal plant analysis: A historical and regional discussion of emergent complex techniques. Front. Pharmacol. 2020;10:1480. doi: 10.3389/fphar.2019.01480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sofowora A., Ogunbodede E., Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complement. Altern. Med. 2013;10:210–229. doi: 10.4314/ajtcam.v10i5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandar H., Hijazi A., Rammal H., Hachem A., Saad Z., Badran B. Techniques for the extraction of bioactive compounds from Lebanese Urtica Dioica. Am. J. Phytomedicine Clin. Ther. 2013;1:507–513. [Google Scholar]
- 4.Choudhary N., Siddiqui M., Bi S., Khatoon S. Variation in preliminary phytochemicals screening of Cannabis sativa L. leaf, stem and root. Int. J. Pharm. 2014;1:516–519. [Google Scholar]
- 5.Montserrat-de la Paz S., Marín-Aguilar F., García-Gimenez M.D., Fernández-Arche M. Hemp (Cannabis sativa L.) seed oil: Analytical and phytochemical characterization of the unsaponifiable fraction. J. Agric. Food Chem. 2014;62:1105–1110. doi: 10.1021/jf404278q. [DOI] [PubMed] [Google Scholar]
- 6.Amaducci S., Scordia D., Liu F.-H., Zhang Q., Guo H., Testa G., Cosentino S.L. Key cultivation techniques for hemp in Europe and China. Ind. Crop. Prod. 2015;68:2–16. doi: 10.1016/j.indcrop.2014.06.041. [DOI] [Google Scholar]
- 7.Clarke R.C. The Botany and Ecology of Cannabis. Pods Press; Ben Lomond, CA, USA: 1977. [Google Scholar]
- 8.Da Porto C., Decorti D., Natolino A. Separation of aroma compounds from industrial hemp inflorescences (Cannabis sativa L.) by supercritical CO2 extraction and on-line fractionation. Ind. Crop. Prod. 2014;58:99. doi: 10.1016/j.indcrop.2014.03.042. [DOI] [Google Scholar]
- 9.di Giacomo V., Chiavaroli A., Orlando G., Cataldi A., Rapino M., Di Valerio V., Leone S., Brunetti L., Menghini L., Recinella L., et al. Neuroprotective and Neuromodulatory Effects Induced by Cannabidiol and Cannabigerol in Rat Hypo-E22 cells and Isolated Hypothalamus. Antioxidants. 2020;9:71. doi: 10.3390/antiox9010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.di Giacomo V., Chiavaroli A., Recinella L., Orlando G., Cataldi A., Rapino M., Di Valerio V., Ronci M., Leone S., Brunetti L., et al. Antioxidant and Neuroprotective Effects Induced by Cannabidiol and Cannabigerol in Rat CTX-TNA2 Astrocytes and Isolated Cortexes. Int. J. Mol. Sci. 2020;21:3575. doi: 10.3390/ijms21103575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calapai F., Cardia L., Esposito E., Ammendolia I., Mondello C., Lo Giudice R., Gangemi S., Calapai G., Mannucci C. Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives. Evid. -Based Complement. Altern. Med. Ecam. 2022;2022:3336516. doi: 10.1155/2022/3336516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landucci E., Mazzantini C., Lana D., Calvani M., Magni G., Giovannini M.G., Pellegrini-Giampietro D.E. Cannabidiol inhibits microglia activation and mitigates neuronal damage induced by kainate in an in-vitro seizure model. Neurobiol. Dis. 2022;174:105895. doi: 10.1016/j.nbd.2022.105895. [DOI] [PubMed] [Google Scholar]
- 13.Jastrząb A., Jarocka-Karpowicz I., Skrzydlewska E. The Origin and Biomedical Relevance of Cannabigerol. Int. J. Mol. Sci. 2022;23:7929. doi: 10.3390/ijms23147929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores-Sanchez I.J., Verpoorte R. Secondary metabolism in cannabis. Phytochem. Rev. 2008;7:615–639. doi: 10.1007/s11101-008-9094-4. [DOI] [Google Scholar]
- 15.Pellati F., Borgonetti V., Brighenti V., Biagi M., Benvenuti S., Corsi L. Cannabis sativa L. and nonpsychoactive cannabinoids: Their chemistry and role against oxidative stress, inflammation, and cancer. BioMed Res. Int. 2018;2018:1691428. doi: 10.1155/2018/1691428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acquaviva A., Di Simone S.C., Canini A., Braglia R., Di Marco G., Campana C., Angelini P., Flores G.A., Venanzoni R., Libero M.L. Phytochemical and biological investigations on the pollen from industrial hemp male inflorescences. Food Res. Int. 2022;161:111883. doi: 10.1016/j.foodres.2022.111883. [DOI] [PubMed] [Google Scholar]
- 17.Di Sotto A., Gullì M., Acquaviva A., Tacchini M., Di Simone S.C., Chiavaroli A., Recinella L., Leone S., Brunetti L., Orlando G. Phytochemical and pharmacological profiles of the essential oil from the inflorescences of the Cannabis sativa L. Ind. Crops Prod. 2022;183:114980. doi: 10.1016/j.indcrop.2022.114980. [DOI] [Google Scholar]
- 18.Shamsudin N.F., Ahmed Q.U., Mahmood S., Ali Shah S.A., Khatib A., Mukhtar S., Alsharif M.A., Parveen H., Zakaria Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules. 2022;27:1149. doi: 10.3390/molecules27041149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrante C., Recinella L., Ronci M., Menghini L., Brunetti L., Chiavaroli A., Leone S., Di Iorio L., Carradori S., Tirillini B. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019;125:452–461. doi: 10.1016/j.fct.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 20.Orlando G., Adorisio S., Delfino D., Chiavaroli A., Brunetti L., Recinella L., Leone S., D’Antonio M., Zengin G., Acquaviva A. Comparative investigation of composition, antifungal, and anti-inflammatory effects of the essential oil from three industrial hemp varieties from Italian cultivation. Antibiotics. 2021;10:334. doi: 10.3390/antibiotics10030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mastellone G., Marengo A., Sgorbini B., Scaglia F., Capetti F., Gai F., Peiretti P.G., Rubiolo P., Cagliero C. Characterization and Biological Activity of Fiber-Type Cannabis sativa L. Aerial Parts at Different Growth Stages. Plants. 2022;11:419. doi: 10.3390/plants11030419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taberlet P., Gielly L., Pautou G., Bouvet J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991;17:1105–1109. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- 23.Azwanida N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants. 2015;4:196. [Google Scholar]
- 24.Salhi N., Mohammed Saghir S.A., Terzi V., Brahmi I., Ghedairi N., Bissati S. Antifungal activity of aqueous extracts of some dominant Algerian medicinal plants. BioMed Res. Int. 2017;2017:7526291. doi: 10.1155/2017/7526291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abubakar A.R., Haque M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020;12:1. doi: 10.4103/jpbs.JPBS_175_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CLSI . CLSI standard M38. 3rd ed. Clinical Laboratory Standards Institute; Wayne, PA, USA: 2017. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. [Google Scholar]
- 27.CLSI . Approved Standard; Document M38. Clinical Laboratory Standards Institute; Wayne, PA, USA: 2018. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. [Google Scholar]
- 28.CLSI . CLSI document M07-A10. 10th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2015. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard. [Google Scholar]
- 29.Öztürk M., Duru M.E., Kivrak Ş., Mercan-Doğan N., Türkoglu A., Özler M.A. In vitro antioxidant, anticholinesterase and antimicrobial activity studies on three Agaricus species with fatty acid compositions and iron contents: A comparative study on the three most edible mushrooms. Food Chem. Toxicol. 2011;49:1353–1360. doi: 10.1016/j.fct.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 31.Wołosiak R., Drużyńska B., Derewiaka D., Piecyk M., Majewska E., Ciecierska M., Worobiej E., Pakosz P. Verification of the Conditions for Determination of Antioxidant Activity by Abts and Dpph Assays—A Practical Approach. Molecules. 2021;27:50. doi: 10.3390/molecules27010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svečnjak L., Marijanović Z., Okińczyc P., Marek Kuś P., Jerković I. Mediterranean propolis from the Adriatic Sea islands as a source of natural antioxidants: Comprehensive chemical biodiversity determined by GC-MS, FTIR-ATR, UHPLC-DAD-QqTOF-MS, DPPH and FRAP assay. Antioxidants. 2020;9:337. doi: 10.3390/antiox9040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 34.Ren G., Zhang X., Li Y., Ridout K., Serrano-Serrano M.L., Yang Y., Liu A., Ravikanth G., Nawaz M.A., Mumtaz A.S. Large-scale whole-genome resequencing unravels the domestication history of Cannabis sativa. Sci. Adv. 2021;7:eabg2286. doi: 10.1126/sciadv.abg2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bottari N.B., Lopes L.Q.S., Pizzuti K., dos Santos Alves C.F., Corrêa M.S., Bolzan L.P., Zago A., de Almeida Vaucher R., Boligon A.A., Giongo J.L. Antimicrobial activity and phytochemical characterization of Carya illinoensis. Microb. Pathog. 2017;104:190–195. doi: 10.1016/j.micpath.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 36.de Camargo A.C., Regitano-d’Arce M.A.B., Rasera G.B., Canniatti-Brazaca S.G., do Prado-Silva L., Alvarenga V.O., Sant’Ana A.S., Shahidi F. Phenolic acids and flavonoids of peanut by-products: Antioxidant capacity and antimicrobial effects. Food Chem. 2017;237:538–544. doi: 10.1016/j.foodchem.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Díaz I.M., Medina E., Page C.A., Johanningsmeier S.D., Daughtry K.V., Moeller L. Prevention of microbes-induced spoilage in sodium chloride–free cucumber fermentations employing preservatives. J. Food Sci. 2022;87:5054–5069. doi: 10.1111/1750-3841.16345. [DOI] [PubMed] [Google Scholar]
- 38.Martinenghi L.D., Jønsson R., Lund T., Jenssen H. Isolation, Purification, and Antimicrobial Characterization of Cannabidiolic Acid and Cannabidiol from Cannabis sativa L. Biomolecules. 2020;10:900. doi: 10.3390/biom10060900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Synowiec A., Żyła K., Gniewosz M., Kieliszek M. An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli. Open Life Sci. 2021;16:594–601. doi: 10.1515/biol-2021-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data are available from the corresponding author.