Abstract
Background: Anthracyclines such as doxorubicin remain a primary treatment for hematological malignancies and breast cancers. However, cardiotoxicity induced by anthracyclines, possibly leading to heart failure, severely limits their application. The pathological mechanisms of anthracycline-induced cardiac injury are believed to involve iron-overload-mediated formation of reactive oxygen species (ROS), mitochondrial dysfunction, and inflammation. The dietary thione, ergothioneine (ET), is avidly absorbed and accumulated in tissues, including the heart. Amongst other cytoprotective properties, ET was shown to scavenge ROS, decrease proinflammatory mediators, and chelate metal cations, including Fe2+, preventing them from partaking in redox activities, and may protect against mitochondrial damage and dysfunction. Plasma ET levels are also strongly correlated to a decreased risk of cardiovascular events in humans, suggesting a cardioprotective role. This evidence highlights ET’s potential to counteract anthracycline cardiotoxicity. Methods and Findings: We investigated whether ET supplementation can protect against cardiac dysfunction in mice models of doxorubicin-induced cardiotoxicity and revealed that it had significant protective effects. Moreover, ET administration in a mouse breast cancer model did not exacerbate the growth of the tumor or interfere with the chemotherapeutic efficacy of doxorubicin. Conclusion: These results suggest that ET could be a viable co-therapy to alleviate the cardiotoxic effects of anthracyclines in the treatment of cancers.
Keywords: ergothioneine, doxorubicin, cardiotoxicity, anthracycline, chemotherapy
1. Introduction
Anthracyclines such as doxorubicin are widely used chemotherapy drugs in both adults and children for hematologic malignancies, soft-tissue sarcomas, and solid tumors of the breast, lung, stomach, bladder, and ovaries. Despite their use as a standard for effective curative chemotherapy for decades, their clinical application can be severely limited due to dose-specific cardiotoxic side effects, which range from asymptomatic echocardiographic changes to severe cardiomyopathy and heart failure [1]. These cardiotoxic effects can persist long after cessation of chemotherapy, and in some cases myocardial damage may not be apparent until many years after completion of chemotherapy and can be exacerbated by other cardiometabolic co-morbidities, such as hypertension, coronary artery disease, and diabetes [2]. Doxorubicin, one of the most effective anthracycline drugs, is frequently prescribed; however, it also poses a greater risk of cardiac injury. It is estimated that over one quarter of patients receiving a cumulative dose of doxorubicin exceeding 550 mg/m2 will develop heart failure [2]. Although such doses are rarely applied in current clinical practice, the problem of cardiotoxicity has not gone away. Despite decades of research and increased understanding of anthracycline cardiotoxicity, maintaining chemotherapeutic efficacy while preventing cardiotoxicity remains a challenge, especially in children [1].
While the exact pathological mechanisms of anthracycline cardiotoxicity are not fully understood, it is known to involve multiple pathways, including excessive oxidative stress [3], activation of pro-inflammatory pathways [4], mitochondrial dysfunction [5], mitochondrial iron-overload-induced lipid peroxidation [6], and activation of apoptotic pathways [7]. Studies suggest a strong affinity of anthracyclines to cardiolipin, a phospholipid found abundantly on the inner mitochondrial membrane, explaining the tendency for doxorubicin and its complexes with iron to accumulate in the mitochondria [8]. Since cardiomyocytes are rich in mitochondria to meet their high energy demands, this may explain the predisposition of the heart to damage by anthracyclines. Redox cycling by doxorubicin involving mitochondrial complex I [3], together with mitochondrial iron overload [9], leads to the formation of reactive oxygen species (ROS), such as superoxide and hydroxyl radials, and hydrogen peroxide. Together with anthracycline-induced downregulation of glutathione peroxidase 4, this contributes to excessive lipid peroxidation and ultimately cardiomyocyte ferroptosis; a process that has been shown to be mitigated by iron chelators [6].
The maintenance of redox homeostasis is critical to the preservation of normal cardiomyocyte function [10], and the disruption of this by anthracyclines through the pro-oxidative mechanisms mentioned earlier can disrupt redox-sensitive signaling pathways and damage important cellular components, ultimately causing cardiomyocyte death [6,11]. As oxidative stress has been hypothesized to be one of the primary drivers of anthracycline cardiotoxicity, some studies have evaluated the application of antioxidant compounds, such as vitamins C [12] and E [13], and N-acetylcysteine [14], to prevent cardiac injury. While outcomes are somewhat mixed, a few studies have yielded positive outcomes in vitro and in acute toxicity in vivo models; however, none have proven successful in a clinical setting [15,16]. Moreover, there is concern as to whether these compounds may interfere with chemotherapy [17,18], exacerbate tumor growth, or promote metastasis, as has been suggested by a few animal studies (e.g., [19,20,21]). At present, the iron chelator, dexrazoxane, is the only drug approved by the US FDA to counteract anthracycline cardiotoxicity. It appears to work by binding iron from the anthracycline–iron complexes and inhibiting the formation of ROS [22]. However, it does not eliminate cardiotoxic risk, and there are concerns of secondary hematological malignancies and reduced response to chemotherapy, which is possibly why dexrazoxane is only prescribed to a small percentage of patients undergoing doxorubicin chemotherapy [23].
The effectiveness and widespread application of anthracyclines in cancer therapy mean their removal is not a viable solution; hence, the absence of an effective prophylactic cardioprotectant against anthracyclines highlights an important therapeutic gap. Prior studies have shown that a low-molecular-weight dietary thione, ergothioneine (ET), may act as a physiological cytoprotectant (for detailed reviews on this compound, please see [24,25,26,27]). ET is not known to be produced in animals and humans but is abundant in diets, especially mushrooms, and is avidly taken up and accumulated in tissues, including the heart [25,28], by the cation transporter, OCTN1, which is also known as the ET transporter due to its specificity for ET [29,30]. Many studies have highlighted the cytoprotective properties of ET, including its ability to scavenge free radicals [31]; decrease pro-inflammatory mediators [32]; chelate divalent metal cations (such as Fe2+), preventing their redox activities [31,33,34]; and prevent mitochondrial damage and dysfunction [35,36], suggesting that it could be a cardioprotective agent against anthracyclines. Indeed, higher levels of ET in human plasma were identified as an independent marker of a lower risk of cardiometabolic disease and associated mortality in a longitudinal study involving 3236 participants [37]. Other studies have also demonstrated that ET is able to protect the vascular system against diabetes-induced cardiovascular injury in rats [38], protect the endothelium against a range of toxins to prevent endothelial dysfunction [32,39,40], and protect tissues against ischemia–reperfusion injury [41,42,43]. Moreover, ET decreased levels of proinflammatory cytokines [43] and the expression of vascular adhesion molecules (VCAM-1, ICAM-1 and E-selectin) [44], and was also shown to reduce the pro-oxidant ferrylmyoglobin to metmyoglobin [45], highlighting the possible ways that ET could protect the heart from inflammatory and oxidative injury induced by anthracyclines. Untargeted metabolomic studies revealed that levels of ET were elevated in the heart of mice subjected to either transverse aortic constriction (pressure overload) or myocardial infarction [46], supporting our prior hypothesis [47] that tissues may upregulate expression of the ET transporter to increase ET uptake in injured tissue as a cytoprotective mechanism [48].
This evidence suggests that ET could be an excellent candidate to counteract multiple pathological mechanisms of anthracycline cardiotoxicity; however, to date, this has not been investigated. The present study demonstrates that ET is able to improve cardiac function in a mouse model of doxorubicin-induced cardiotoxicity. Moreover, in a breast cancer model, supplementation of ET in the presence or absence of doxorubicin administration demonstrated that ET neither exacerbated tumor growth nor interfered with the chemotherapeutic efficacy of doxorubicin. This study presents a strong case for ET to be used in conjunction with anthracycline chemotherapy to counter cardiac injury and lays the foundations for a future clinical evaluation of ET against anthracycline cardiotoxicity.
2. Materials and Methods
2.1. Chemicals and Reagents
L-ergothioneine (>98%), L-ergothioneine-d9 (ET-d9), hercynine, and hercynine-d9, were provided by Tetrahedron (Paris, France). Doxorubicin and hydrochloride salt (>99%) was purchased from LC Laboratories (Woburn, MA, USA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise stated.
2.2. Animal Studies
Mice (C57BL-6J, BALB-C) were randomly assigned to cages in groups of 4–5 animals and provided with water and standard mice chow (Teklad, Envigo, IN, USA) ad libitum. The animals were kept in a barrier animal facility with a 12 h/12 h light–dark cycle. Animals were cared for in accordance with the protocols and guidelines stipulated in the Institutional Animal Care and Use Committee (IACUC), National University of Singapore. All the procedures were performed with prior approval by the IACUC, NUS, protocol No. R17-1242.
2.3. Doxorubicin Cardiotoxicity Model
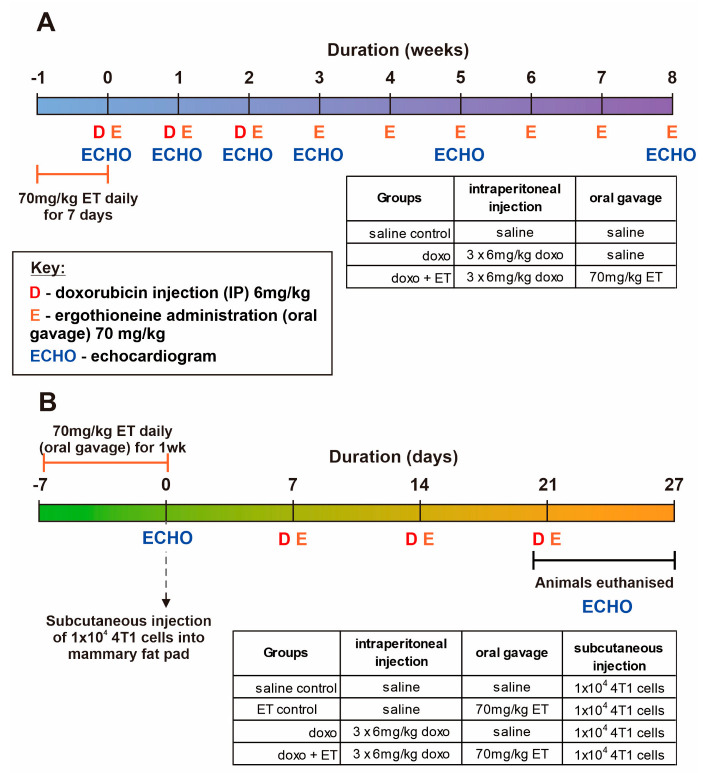
To induce anthracycline cardiotoxicity, C57BL-6J mice (Jackson Laboratories; ~10 weeks old) were administered with 6 mg/kg doxorubicin (in sterile saline solution; 0.9% w/v NaCl) intraperitoneally once weekly for 3 weeks (cumulative dose of 18 mg/kg). Animals were monitored during the administration and for a further 5 weeks with transthoracic echocardiographic measurements (Vevo® 2100 system, as detailed below) taken at baseline and weeks 1, 2, 3, 5, and 8. For ET-treated animals, 70 mg/kg ET (dissolved in sterile saline; dose was established previously in [28] to significantly elevate levels in the heart) was administered by oral gavage daily for 1 week prior to administration of doxorubicin, then once weekly for the 8-week duration of the study (Figure 1A). Animals were assigned to 3 groups comprising saline control (no doxorubicin; n = 7), doxorubicin alone (cardiotoxicity model; n = 10), and doxorubicin with ET administration (evaluation of ET treatment; n = 10). Mice were euthanized within 48 h after the final echocardiogram, and blood and tissues (heart, liver, kidney, and bone marrow) were collected for ET and biomarker analysis. In addition to measurements of cardiac function, the levels of ET and related metabolites, OCTN1 expression, and oxidative biomarkers were also measured in the blood and tissues of the animals.
Figure 1.
Timeline of animal studies and groups. (A) Doxorubicin cardiotoxicity animal study in C57BL6 mice with or without ET administration. Mice (except for saline controls) were given 6 mg/kg doxorubicin weekly for 3 weeks and monitored periodically for a further 5 weeks. (B) A breast cancer model using injection of 4T1 cells into the mammary fat pad of BALB-C mice was used to evaluate the direct effect of ET on tumor growth and doxorubicin chemotherapy. Animals administered with doxorubicin were given 6 mg/kg doxorubicin weekly for 3 weeks. In both studies, animals supplemented with ET were given 70 mg/kg ET daily for 7 days prior to the commencement of the study, then once weekly thereafter until the completion of the study.
2.4. Mouse Breast Cancer Model
A mouse breast cancer model was used to evaluate if ET interferes with the chemotherapeutic effect of doxorubicin and/or stimulates the growth of the cancer. BALB-C mice at 8 weeks of age were subcutaneously injected with 50 µL of a 4T1 cell (murine mammary gland carcinoma) suspension (containing 1 × 104 cells) into the female mouse mammary fat pad. All mice were given the breast tumor injections and randomly assigned to groups (n = 6/group), before being given either saline (vehicle control) alone, doxorubicin alone, or doxorubicin with ET administration (Figure 1B). For ET administration, 70 mg/kg were given to animals daily for 7 days prior to commencement of the study and once weekly until animals were euthanized. Animals administered doxorubicin were given 3 doses of 6 mg/kg intraperitoneally once weekly for 3 weeks, with the first dose commencing 1 week following injection of the breast cancer (Figure 1B). Only baseline and endpoint echocardiographic measurements were taken in this study due to the premature euthanasia of animals to prevent undue stress to animals due to high dose of doxorubicin (required to suppress the aggressive tumor growth) or overgrown or ulcerated tumor in saline controls. However, these were sufficient to establish the cardiotoxic effects of doxorubicin with and without ET. Mice were euthanized within 24 h after the final echocardiogram, and blood and tissues (heart, liver, kidney, and bone marrow) were collected for ET and biomarker analysis. The tumor size was measured with a vernier caliper after excision and subsequently weighed.
2.5. Echo Measurement and Assessment
Animal cardiac function was assessed using a high-frequency ultrasound system Vevo® 2100 (VisualSonics, Toronto, Canada) and analyzed with Vevo® 2100 software, version 1.7.0, as established previously [49,50]. In brief, echocardiography was performed on mice under general anesthesia (1–1.5% v/v isoflurane, Baxter, Singapore), B-mode recordings were obtained from a long-axis view of the LV to analyze cardiac left ventricular volumes and left ventricular ejection fraction (LVEF). To assess global cardiac dysfunction (both systolic and diastolic) in doxorubicin-induced cardiomyopathy, myocardial performance index (MPI), a parameter defined as the sum of the isovolumic contraction time and relaxation time divided by the ejection time, was determined by Doppler echocardiography of left ventricular inflow and outflow [51,52]. Echocardiography was performed and analyzed by a researcher blinded for the treatment of animals.
2.6. Quantitative PCR of Mouse Heart RNA
RNA was isolated from heart samples using the NucleoSpin TriPrep kit with on-column DNase digestion (Macherey-Nagel, Dueren, Germany). Extracted RNAs were quantified and checked by 260/280 nm measurement using the Synergy H1 spectrophotometer with Take 3 microvolume plate (BioTek; Agilent Technologies, Santa Clara, CA, USA). Reverse-transcription PCR (RT-PCR) was performed using QuantiTect RT kit (Qiagen, Hilden, Germany), and quantitative PCR (qPCR) was performed using BlitzAmp Hotstart qPCR mix (MiRXES, Singapore) with an Applied Biosystem 7500 Real-Time PCR system (Thermo Fisher Scientific, MA, USA). Primers sequences are listed in Appendix A (Table A1), with gene expression normalized to β-actin.
2.7. Extraction of Blood and Tissues ET and its Metabolites
We mixed 10 µL of plasma or ~10 mg of heart tissue (accurately weighed) with ET-d9 and hercynine-d9 internal standards and ultrapure water (Arium; Sartorius, Gottingen, Germany) to 100 µL final volume. For plasma ET, samples were precipitated with 50 µL of methanol at −20 °C overnight. For heart, tissue was homogenized with a motorized pellet pestle in 150 µL of ultrapure water containing internal standards. After homogenization, 750 µL of ice-cold methanol was added and samples were vortexed for 30 s before incubating at −20 °C overnight. Precipitated plasma and heart tissue samples were then centrifuged (20,000× g, 15 min, 4 °C) and supernatants were evaporated under a stream of N2 gas before reconstituting in 100 µL ultrapure water. Any debris was removed by centrifugation before transferring into silanized glass inserts with vials (Agilent CrossLab, Santa Clara, CA, USA) for analysis by liquid chromatography–mass spectrometry (LC-MS/MS). ET or hercynine levels were normalized against the accurate mass of tissue and expressed in mass per wet weight of tissue ± SEM.
2.8. Quantifying Biomarkers of Oxidative Damage to RNA and DNA
Levels of 8-hydroxyguanosine (8OHG) and 8-hydroxydeoxyguanosine (8OHdG) were measured in the heart tissue as biomarkers of oxidative damage to RNA and DNA, respectively. Approximately 20 mg of heart tissue was used to extract RNA and DNA using TRIzol reagent (Invitrogen, MA, USA) as per the manufacturer’s protocol. All procedures were performed on ice to reduce artefactual oxidation. Isolated RNA was dissolved in RNase-free water, while DNA was dissolved in 160 µL of 10 mM Tris pH 8.0 buffer at 4 °C overnight. We enzymatically hydrolyzed 50 µg of RNA at 37 °C using 20 µg RNase A, 1 mU phosphodiesterase (P3242; Sigma-Aldrich, St. Louis, MO, USA), and 2 U alkaline phosphatase (A2356; Sigma-Aldrich, St. Louis, MO, USA) in buffer (10 mM Tris pH 8.0, 5 mM MgCl2) in a volume of 100 µL for 1 h. Isolated DNA was hydrolyzed at 37 °C with 1 U of benzonase (Merck, NJ, USA), 3 mU phosphodiesterase, and 4 U alkaline phosphatase in 200µL of buffer for 6 h. Internal standards were added before hydrolysis. The reaction was quenched with 5 volumes of ice-cold methanol, vortexed, and stored at −20 °C overnight. All samples were centrifuged (20,000× g, 15 min, 4 °C) and supernatants were evaporated under a stream of N2 gas before reconstituting in 60 µL of ultrapure water. Any precipitates were removed by centrifugation before transferring into silanized glass inserts with vials for LC-MS/MS analysis.
2.9. Protein Carbonylation ELISA
Diluted plasma (25x in PBS) and TRIzol-extracted heart proteins were derivatized with 4 mM DNPH (in 2 M HCl) as per manufacturer’s instructions using Oxyblot Protein Oxidation Detection Kit (Merck Millipore, MA, USA). We left 2 µg of proteins in 200 µL 100 mM pH 9.6 bicarbonate buffer to bind to the ELISA plate overnight at 4 °C. The plate was washed 5 times with PBS and blocked with 100 µL Pierce Protein-Free T20 buffer for 1 h. Plate was washed with PBS-T (0.1% v/v Tween-20) and incubated with 100 µL 1:200 primary antibody (Oxyblot kit) for 2 h with shaking. Plate was then washed and incubated with 100 µL 1:400 secondary antibody (Oxyblot kit) for 1 h. Plate was washed and 100 µL of TMB (Abcam, Cambridge, UK) was added. The reaction was stopped with 100 µL of 2 M H2SO4 and absorbance was determined at 450 nm using the Synergy H1 spectrophotometer (BioTek; Agilent Technologies, Santa Clara, CA, USA). Carbonylation levels were quantified against a standard curve.
2.10. Liquid Chromatography–Mass Spectrometry Analysis
LC-MS/MS was carried out using an Agilent 1290 UPLC system coupled to an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). Samples were kept at 10 °C in the autosampler during analysis. For ET and hercynine analysis, 2 µL of the processed samples were injected into a Cogent Diamond-Hydride column (4 µm, 150 × 2.1 mm, 100 Å; MicroSolv Technology Corporation, Leland, NC, USA) maintained at 40 °C. Solvent A was acetonitrile in 0.1% v/v formic acid, and Solvent B was 0.1% v/v formic acid in ultrapure water. Chromatography was carried out at a flow rate of 0.5 mL/min using the following gradient elution: 1 min of 20% solvent B, followed by a gradual increase to 40% solvent B over 3 min to elute ET. Solvent B was further increased to 90% over 1 min to elute hercynine, and this was maintained for 3.5 min before returning to 20% for 3.5 min to re-equilibrate the column. The total run time was 12 min. The retention times for ET and hercynine were 4.2 and 6.8 min, respectively.
For the 8OHG and 8OHdG analysis, 10 µL of the processed samples were injected into an Accucore PFP column (150 × 3.0 mm, 2.6 µm; Thermo Fisher Scientific, Waltham, MA, USA) maintained at 30 °C. Solvent A was acetonitrile; Solvent B was 0.1% v/v formic acid in ultrapure water. Chromatography was carried out at a flow rate of 0.5 mL/min using the following gradient elution: 1.5 min of 98% solvent B, followed by a 4 min gradual decrease in solvent B to 95%. The column was washed with 5% solvent B for 2.5 min and re-equilibrated with 98% solvent B for 4 min. The total run time was 12 min. The retention times for guanosine, 8OHG, dG, and 8OHdG were 3.7, 4.3, 4.8, and 6.1 min, respectively.
For all targets, MS was carried out under positive ion ESI and a multiple reaction monitoring mode. Capillary voltage was set at 3200 V, and gas temperature was kept at 350 °C. Nitrogen sheath gas pressure for nebulizing sample was at 50 psi, and gas flow was set at 12 L/min. Ultra-high purity nitrogen was used as collision gas. The optimized precursor to product ion transitions and their respective fragmentor voltages and collision energies are listed in Appendix A (Table A2).
2.11. Statistical Analysis
Data were tabulated using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). Statistical analyses (1-way analysis of variance (ANOVA) with Tukey’s multiple comparison test or 2-way ANOVA, with multiple column comparison) were performed, and graphs were generated using GraphPad Prism version 9.2.0 (GraphPad Software, California, CA, USA). Unless otherwise stated, all data are expressed as mean ± standard error, with p < 0.05 considered statistically significant.
3. Results
3.1. Animal Weights in Doxorubicin Treated Mice
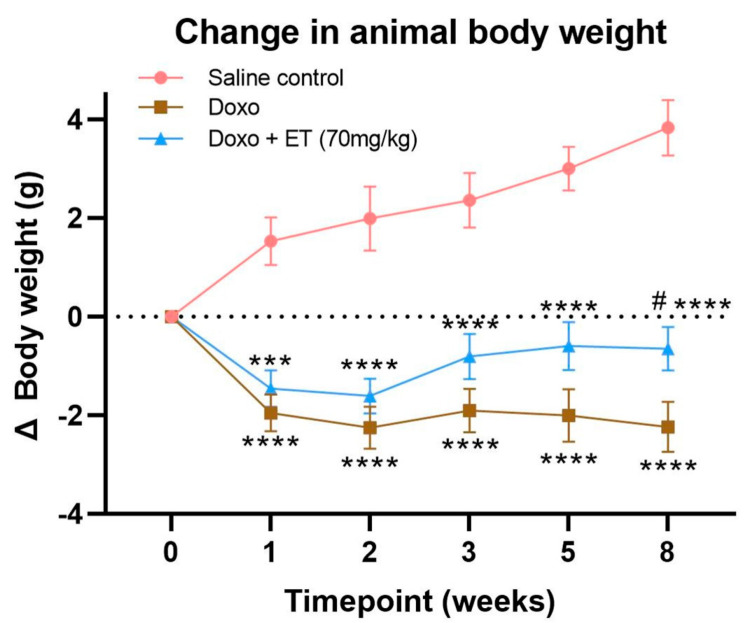
Administration of 6 mg/kg doxorubicin weekly for 3 weeks (total cumulative dose of 18 mg/kg) in C57BL-6J mice led to significant decreases in animal weights (Figure 2) compared with saline-treated controls, whose weights continued to increase over time. Animal weights in doxorubicin-treated animals remained low for the entire duration of the 8-week study, even after doxorubicin administration had ceased. Doxorubicin-treated animals supplemented with 70 mg/kg ET also declined in weight; however, their weights were able to gradually recover after doxorubicin administration had ceased, which were significantly greater than doxorubicin alone by week 8.
Figure 2.
Animal weights. Animal weights were taken at each timepoint to compare weights of animals with doxorubicin administration with (doxo + ET) and without (doxo) ET supplementation with untreated (saline control) animals. Mice administered with doxorubicin had a significant decline in mean body weight compared with saline controls. Doxorubicin-treated animals supplemented with ET also had significant declines in body weight; however, their body weights were able to recover, being significantly greater than doxorubicin-treated animals alone by week 8. A 2-way analysis of variance (ANOVA) using multiple column comparison was performed with significances of **** p < 0.0001, *** p < 0.001 vs. saline control, and # p < 0.05 vs. doxorubicin alone.
3.2. Echocardiographic Assessment of Cardiac Function in Doxorubicin-Treated Mice
Left ventricular systolic function, as assessed by an echocardiographic assessment of the change in LVEF, is a primary measure of cardiac function [1,49]. As expected, the LVEF in saline-treated controls remained constant relative to the baseline over the 8-week study period (Figure 3A). In contrast, administration of doxorubicin significantly decreased LVEF from baseline (ΔEF) from the second week onward (corresponding to second dose of doxorubicin; Figure 3A). To comprehensively evaluate the impact of doxorubicin on cardiac function, we also performed cardiac Doppler echocardiography to determine the MPI that reflects myocardial performance in both systolic and diastolic aspects [51,52]. As shown in Figure 3B, a significant increase in MPI was observed in doxorubicin-treated mice relative to saline-treated controls, indicating impaired cardiac function by administration of doxorubicin in mice. Remarkably, the oral supplementation of mice with 70 mg/kg ET prior to doxorubicin administration, as illustrated in Figure 1A, prevented doxorubicin-induced cardiac dysfunction, as evidenced by preservation of LVEF and a significantly lower increase in MPI (Figure 3A,B).
Figure 3.
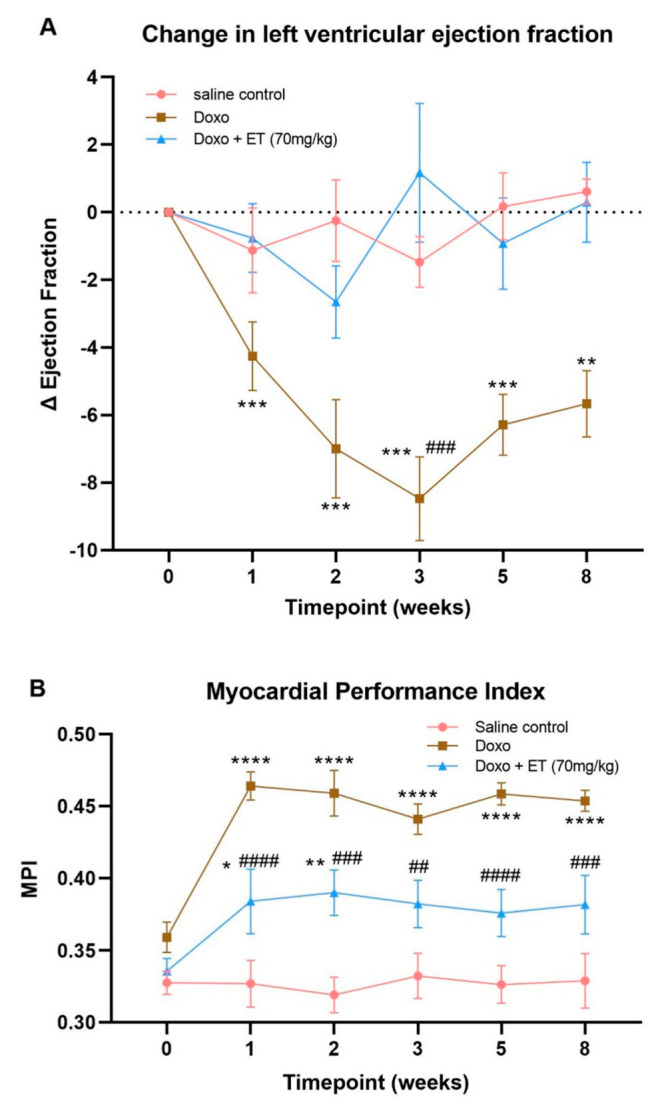
Assessment of cardiac function. (A) The change in LVEF was measured as an indicator of cardiac (systolic) function. Significant decreases in LVEF were seen with doxorubicin-alone-administered animals, which was significant versus control from 2 weeks. Supplementation of doxorubicin-treated animals with ET prevented this decline in LVEF, which was significantly different to doxorubicin-alone-treated animals at the 3-week time point. (B) Similarly, myocardial performance index was significantly increased in doxorubicin-treated animals (indicating declining cardiac function). ET administration decreased MPI but not to baseline/saline control levels. A 2-way ANOVA using multiple column comparison was performed, with indicated significance levels; **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05 vs. saline control, and #### p < 0.0001, ### p < 0.001, ## p < 0.01 vs. doxorubicin alone.
3.3. Expression of ET Transporter mRNA in Heart
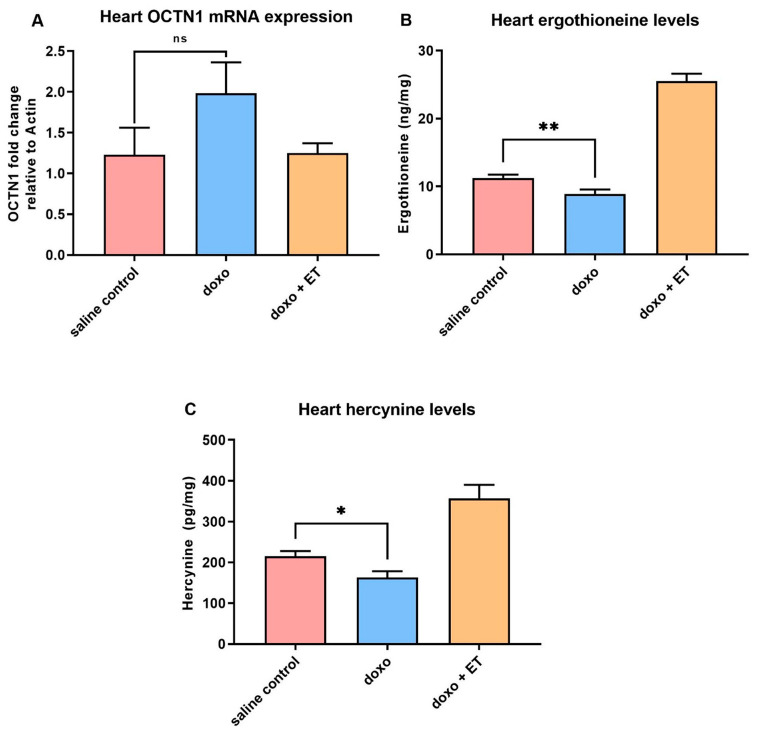
A trend towards an increase in the mRNA expression of OCTN1 was observed in the heart following administration of doxorubicin compared with saline controls; however, this was not significant (Figure 4A). No change was observed in OCTN1 expression in animals supplemented with ET prior to administration of doxorubicin when compared with saline controls.
Figure 4.
Levels of ergothioneine and hercynine in the heart. (A) Real-time PCR analysis of OCTN1 (the ET transporter) mRNA expression in heart tissue. Although a slight increase in OCTN1 mRNA was seen with doxorubicin administration, this was not significant. No changes were seen in doxorubicin-treated animals with ET supplementation. (B) Significantly lower levels of ET were seen in the heart with doxorubicin treatment. This may be due to increased consumption of ET as it scavenges ROS. The heart levels of hercynine (C) also decreased significantly relative to saline control. As expected, supplementation with ET increased levels of hercynine (prior studies show that levels of hercynine correlated closely with ET [54]). A 1-way ANOVA with Tukey’s multiple comparison test was performed; ** p < 0.01, * p < 0.05 vs. saline control; p < 0.0001 saline control or doxorubicin alone vs. doxorubicin + ET groups in (B,C); ns—not significant.
3.4. Levels of Ergothioneine and Hercynine in Heart Tissues
It should be noted that as with prior studies (Tang et al. [28]), detectable basal levels of ET are present in both blood and heart due to the avid accumulation and retention from the trace levels present in the standard mouse diet. Doxorubicin administration significantly decreased levels of ET in the heart (Figure 4B), which is presumably through utilization (i.e., oxidation). Levels of the ET metabolite hercynine also decreased with doxorubicin administration alone (Figure 4C). As expected, oral supplementation of ET significantly increased the levels of ET (~2.5 fold higher than baseline; Figure 4B), as well as levels of hercynine (Figure 4C).
3.5. Levels of Ergothioneine and Hercynine in Plasma
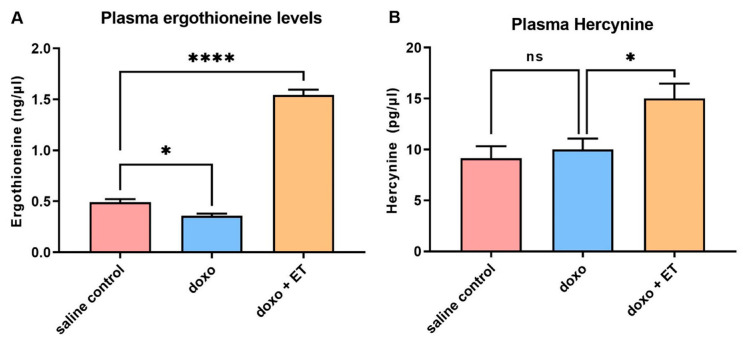
The levels of ET in the plasma also followed a similar trend to the heart tissues, with a significant drop in ET levels following doxorubicin administration (Figure 5A). However, levels of hercynine (a metabolite of ET) had an increasing trend, although this was not significant, which could be due to efflux from tissues (Figure 5B). Again, supplementation with ET significantly increased the levels of ET (~3-fold increase), as well as hercynine in the plasma.
Figure 5.
Plasma ergothioneine and hercynine. A significant decrease was seen in plasma (A) ET levels with administration of doxorubicin relative to saline controls, similar to what was observed in the heart. A non-significant increasing trend for plasma hercynine was observed following doxorubicin administration (B). As expected, supplementation increased plasma levels of ET and hercynine. A 1-way ANOVA with Tukey’s multiple comparison test was performed; (A) * p < 0.05, **** p < 0.0001 vs. saline controls; (B) * p < 0.05 vs. doxorubicin alone.
3.6. Oxidative Damage Biomarkers
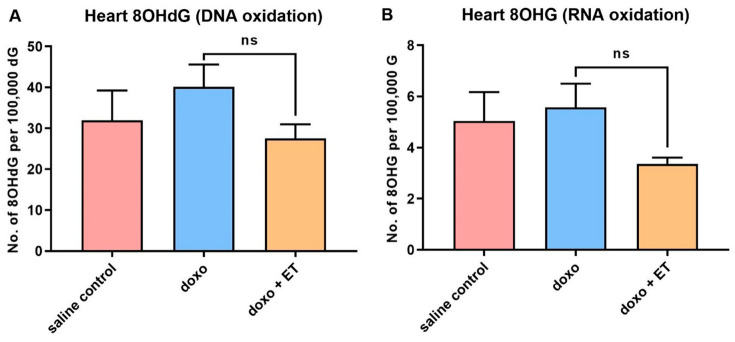
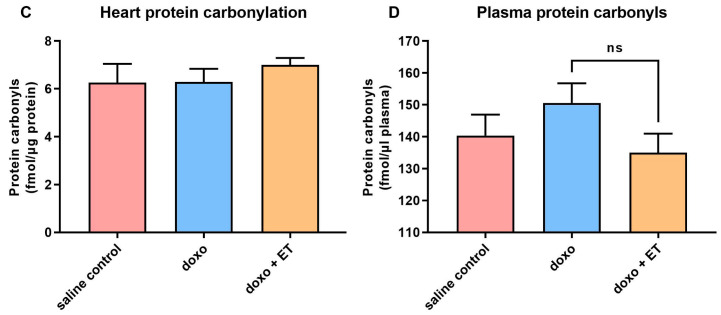
The levels of 8OHdG, 8OHG, and protein carbonyls were measured in the heart tissues as established biomarkers of oxidative damage to DNA, RNA, and protein, respectively [11,53]. A slight but non-significant increase in DNA and RNA oxidation was observed with doxorubicin administration (Figure 6A,B), while supplementing ET appeared to decrease DNA and RNA oxidation markers; however, again, this was not significant relative to animals treated with doxorubicin alone. No apparent trends were observed for protein carbonyls in the heart tissue (Figure 6C); however, plasma protein carbonyls appeared to follow a similar non-significant trend compared to DNA and RNA oxidation markers in the heart (Figure 6D).
Figure 6.
Oxidative damage biomarkers. Although slight increases were observed in the levels of 8OHdG, a biomarker of oxidative DNA damage (A), and 8OHG, a biomarker of oxidative RNA damage (B), with doxorubicin administration relative to saline controls, this was not significant (ns; 1-way ANOVA, Tukey’s multiple comparison test). Supplementation with ET also appeared to decrease oxidative markers of both DNA and RNA, but this was not significant (A,B). No differences were seen in heart protein carbonyls (C) between any of the groups. A similar trend in plasma protein carbonyls (D) was seen as with nucleic acid oxidation markers in heart, but again this was not significantly different.
3.7. Breast Cancer Model
In the BALB-C breast cancer model, the body weights of animals between the saline and ET control groups (without doxorubicin) were not different. However, doxorubicin administration caused a significant loss of body weight and premature death in these animals, regardless of ET supplementation (Figure 7A). As with the C57BL-6J doxorubicin cardiotoxicity model, the administration of doxorubicin to the BALB-C mice led to a significant decrease in LVEF relative to the baseline (Figure 7B). Supplementation with ET almost completely abolished this doxorubicin-induced decrease in LVEF, and animals fared significantly better than animals with doxorubicin administration alone. These results indicate that ET protects against doxorubicin-induced cardiac dysfunction, even in the presence of breast cancer.
Figure 7.
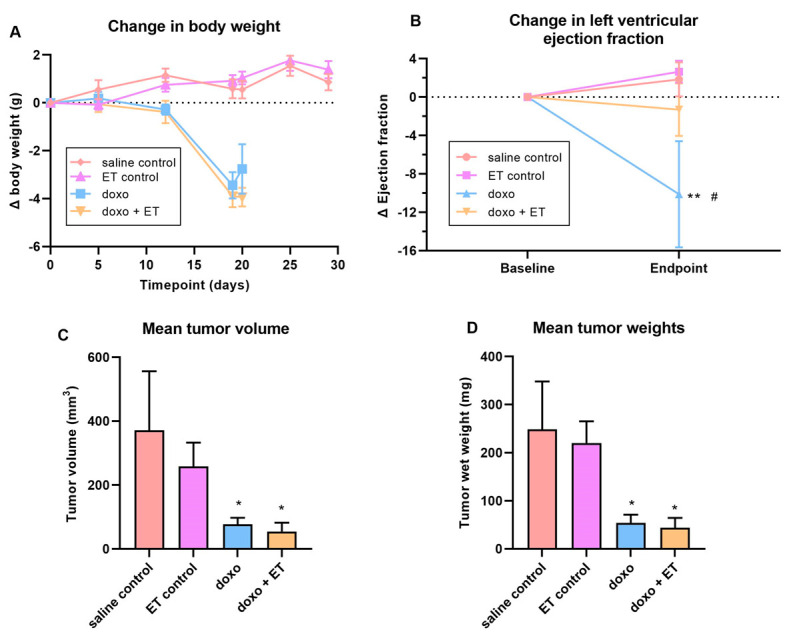
Breast cancer model. (A) Administration of doxorubicin resulted in a significant (p < 0.0001; 1-way ANOVA multiple comparison test) decline in body weights with or without ET supplementation relative to saline controls. ET-supplemented-animal weights remained similar to saline controls. (B) Cardiac function (LVEF) was measured at baseline and endpoint in the breast cancer model. As with the C57BL-6J doxorubicin model, a significant decrease in LVEF was seen with administration of doxorubicin relative to saline control animals. However, supplementation with ET significantly prevented this decline in cardiac function (2-way ANOVA with multiple comparison test; ** p < 0.01 vs. saline control # p < 0.05 vs. doxo + ET). The average volume (C) (estimated based on caliper measurements; Vol(mm3) = [width(mm)]2 x [length(mm)/2]) and weight (D) of the excised tumors are shown. Saline control animals had the largest tumor size, but administration of doxorubicin significantly decreased volume/weight of the tumor (* p < 0.05; 1-way ANOVA with multiple comparison test). Supplementation of animals with ET alone had a slight, but not significant, decrease in tumor size compared with saline controls. Likewise, ET supplementation in doxorubicin-treated animals had a slight but non-significant decrease in tumor size relative to doxorubicin alone. This indicates that ET does not aggravate tumor growth nor interfere with the chemotherapeutic effect of doxorubicin.
Following euthanasia, the excised breast tumors were measured with a caliper to estimate the volume and were subsequently weighed (images are shown in Appendix A; Figure A1). The estimated volume (Figure 7C) and mass of the tumors (Figure 7D) were closely correlated. The tumor size and mass in saline control animals were the highest; however, doxorubicin administration significantly reduced the volume and mass of the tumor. Interestingly a slight decline in tumor size was observed in animals supplemented with ET alone or with doxorubicin when compared with saline controls or doxorubicin alone, respectively, however this was not significant (Figure 7C,D). Supplementation of doxorubicin-treated animals with ET thus did not appear to affect the chemotherapeutic efficacy of doxorubicin.
4. Discussion
Cardiotoxicity is one of the leading causes of non-cancer related death in patients undergoing or having undergone cancer treatment [55]. Indeed, anthracyclines, which are commonly applied as chemotherapy drugs against leukemia and breast cancer (although they also find application in many soft tissue sarcomas and tumors of the stomach, lung, ovaries, and other organs), are known to be associated with cumulative dose-related cardiotoxicity (i.e., risk of cardiotoxicity increases with total cumulative dose). Anthracycline-induced cardiomyopathy is mostly irreversible and may lead to congestive heart failure. In breast cancer survivors, cardiovascular disease accounts for 35% of non-cancer-related deaths in survivors aged ≥50 years [56]. Although some cases of acute cardiotoxicity occur during or soon after anthracycline administration, most cases are chronic cardiotoxicity occurring within the first year (early onset) or decades following completion of chemotherapy (late-onset) [57]. Up to 65% of adult survivors of late-onset cardiotoxicity, particularly relevant to treatment of childhood malignancies with doxorubicin, present evidence of left ventricular contractile abnormalities [58]. Furthermore, concomitant application of certain other anti-cancer drugs (despite absence of cardiotoxicity from those drugs alone) may further exacerbate anthracycline cardiotoxicity and increase the risk of cardiac dysfunction [59].
We established that an optimized cumulative dose of 18 mg/kg doxorubicin over 3 weeks was able to induce cardiotoxicity and significantly decrease LVEF in the C57BL-6J mice (Figure 3A). Although the EF partially recovered over the 5-week follow-up period, this remained significantly lower than the control mice for the entire study. Supplementing this doxorubicin cardiotoxic mouse model with ET (70 mg/kg; daily for 7 days prior to doxorubicin administration and subsequently once weekly via oral gavage) abolished the doxorubicin-induced decrease in LVEF. This dose was previously established in mice [28] to raise the tissue ET levels of the animal, including the heart. By the endpoint, a ~2.5-fold increase in ET levels was observed in the heart tissue compared with saline controls with ET supplementation (Figure 5A). This is a critical point, as many antioxidants or therapeutics may not possess a specific transporter or may be rapidly metabolized and/or excreted, leading to poor bioavailability. We have similarly observed the accumulation and strong retention of ET in human subjects following oral supplementation [54], with transcriptomics revealing the presence of slc22a4 (gene-encoding OCTN1) mRNA in human heart [25], suggesting a similar accumulation of ET as with mice. Indeed, the uptake of ET is dictated by the expression of OCTN1, and knocking out this transporter in mice leads to tissues devoid of ET [60,61], which seems to predispose tissues to oxidative stress and inflammation [61,62,63]. Conversely, we hypothesized that tissue stress and injury may feedback to upregulate OCTN1 expression and increase ET uptake at the site of tissue injury [47,48]. Supporting this, a metabolomic study revealed a significant increase in ET levels in the heart following pressure overload (by aortic constriction) or myocardial infarction [46]. As seen in our cardiotoxicity model, doxorubicin administration alone tended to increase the expression of OCTN1 mRNA, although this was not significant (Figure 4A). However, when high levels of ET were present (due to oral supplementation), the induction of OCTN1 expression was not observed. As the tissues were collected at the endpoint, it is possible that the expression levels of OCTN1 had already declined, since this was more than 5 weeks following the final doxorubicin administration. This stabilization is evident from observing the animal weights, which significantly declined during doxorubicin administration with or without ET (Figure 2); however, they somewhat recovered following the cessation of doxorubicin administration. Animals supplemented with ET appeared to recover faster (from 3-week timepoint onwards), and their mean body weights were significantly higher compared to animals treated with doxorubicin alone.
Doxorubicin administration also significantly increased MPI scores, an assessment of global cardiac dysfunction that combines both systolic and diastolic performance [64] relative to saline controls (Figure 3B). The supplementation with ET in doxorubicin-treated animals significantly lowered this doxorubicin-induced increase in MPI score. However, ET supplementation did not reduce this to the levels observed in the saline controls, possibly indicating a subclinical left ventricular cardiotoxicity [65]. Perhaps prolonged ET administration or a longer observation period is required to demonstrate the efficacy of ET against late-onset cardiac dysfunction (which can occur decades after completion of anthracycline chemotherapy in humans). Since LVEF and MPI derived from 2D echocardiography were sufficient to evaluate the cardioprotective effects of ET, we did not perform other cardiac function measurements, such as 3D echocardiography or global longitudinal strain analysis, which requires special instrumentation and software [66,67]. The measurement of some conventional biomarkers of myocardial damage, such as troponins or myocardial histology, may provide further insights into the extent of cardioprotection by ET.
A significant decrease in the basal heart levels of ET (compared with saline controls) was observed with doxorubicin administration alone, despite the observed trend toward an increase in OCTN1 expression (Figure 4A). This may be indicative of ET utilization/oxidation in the heart. Indeed, doxorubicin-mediated iron overload in mitochondria and mitochondrial redox cycling have been shown to produce excessive ROS [5,11]. ET has been previously shown to scavenge a range of ROS, including singlet oxygen [68], hydroxyl radicals [31], peroxynitrite [69], or ferryl complexes [45,70], and has also been shown to protect against damage by hydrogen peroxide [71,72] and superoxide [73,74]. Hercynine is the purported stable end-product of ET oxidation (via ergothioneine sulphonate [75]). Indeed, we have shown that animal tissues devoid of ET in OCTN1-knockout mice also lack hercynine, indicating it is a metabolite of ET [60]. Levels of hercynine in the heart tissues were seen to decline significantly following doxorubicin administration alone, while they significantly increased following ET administration (Figure 4C) in a similar trend to ET levels in the heart. This is not surprising given that we have previously shown that hercynine levels closely correlate to levels of ET [54]. In contrast to ET, hercynine is unlikely to be retained in the cardiomyocytes, and any transient increase in hercynine due to ET oxidation may not be detectable by the endpoint (5-weeks post-doxorubicin), when the tissues were collected. Supporting this, the levels in plasma appear to be slightly elevated (Figure 5B), although this is not significant, possibly due to excretion from the body. Another possible explanation is that hercynine may not be the final oxidation product and may react with oxidants, such as hypochlorite, as was suggested recently [76].
A trend of increasing oxidative damage to DNA and RNA (Figure 6A,B) was observed with the administration of doxorubicin, while the supplementation of doxorubicin-treated animals with ET appeared to decrease levels of DNA and RNA oxidative markers; however, in both cases, this was not significant. Unfortunately, as the tissues were only collected at the endpoint (8 weeks) to allow for the observation of cardiac function following the cessation of doxorubicin administration, this meant that earlier increases in oxidative damage biomarkers may have normalized. With highly active cellular DNA repair mechanisms [11] and high RNA and protein turnover, it is possible that the peak of oxidative injury was not captured. As such, it is difficult to ascertain if ET is protecting the heart due to a decrease in oxidative damage to DNA, RNA, or protein, mediated through either chelation of Fe2+ or the prevention of mitochondrial dysfunction in cardiomyocytes. Iron plays a key role in doxorubicin cardiotoxicity through the formation of doxorubicin–Fe complexes [77] and iron-overload in mitochondria. Animal studies found that excessive iron exacerbates doxorubicin cardiotoxicity [78,79]. Conversely, iron chelators, such as dexrazoxane and deferoxamine, have been shown to decrease cardiotoxicity in animal models and some clinical studies [78]. ET can also bind divalent metal ions, such as Fe2+and Cu2+ [33], forming redox-inactive complexes and preventing damage to critical cellular components, such as DNA and mitochondria [34]. Indeed, the protective role of ET during ischemia–reperfusion is suggested to be in part mediated by iron chelation [80]. Similarly, the chelation of iron could prevent the association with doxorubicin and formation of complexes and/or inhibit its ability to partake in redox activities. Elevations in γ-glutamyl transpeptidase (GGT) are observed with ischemia–reperfusion cardiac injury [81] and cardiovascular diseases (serving as an independent risk factor and marker to predict mortality due to cardiovascular events in coronary heart disease patients) [82,83]. However, recent studies have reported that ET may inhibit GGT activity [84,85], indicating a possible mechanism of cardioprotection. Another possibility is that ET may activate Nrf2, as shown previously [84,86], thereby upregulating expression of downstream antioxidant and anti-inflammatory response genes to protect cardiac tissues. However, further studies are needed to evaluate the exact cardioprotective mechanism(s) of ET.
In our breast cancer model, supplementation with ET did not have any significant effect on tumor size or weight, both alone or with doxorubicin, indicating that ET does not exacerbate tumor growth, nor does it interfere with the chemotherapeutic efficacy of doxorubicin, unlike other antioxidants [19,20,21]. This is especially important to establish. It is interesting to note that while not significant, ET administration itself slightly decreased the size of the tumor, both in the absence and presence of doxorubicin. This suggests that ET alone may have a slight anti-tumor effect and may warrant further studies. Indeed, studies by D’Onofrio et al. [87] noted a dose-dependent anti-cancer effect of ET in colorectal cancer cells by inducing necroptosis through SIRT3/MLKL pathways.
While further studies are needed to elucidate the mechanisms of protection by ET and to compare its protective actions with that of dexrazoxane [22,23], this study paves the foundations for future clinical evaluations of ET as a cardioprotectant against anthracyclines in chemotherapy. Furthermore, ET is thought to be safe for clinical use, being bestowed with GRAS (generally recognized as safe) status by the US FDA, and has received the European Food Safety Authority (EFSA) approval for use as a food supplement, even in infants and children [88,89].
5. Conclusions
Anthracyclines such as doxorubicin remain a primary chemotherapeutic against hematological malignancies and tumors, such as breast cancer and sarcomas, due to their effectiveness. However, counteracting their cardiotoxic effects remains a major challenge. We demonstrated that the unique cytoprotective thione, ET, which can accumulate in heart tissues, decreases the cardiotoxic effects of doxorubicin in our mouse model. Moreover, using a murine breast cancer model, we established that ET did not interfere with the chemotherapeutic efficacy of doxorubicin nor exacerbate the growth of the breast tumor. Although further work is still needed to elucidate the protective mechanisms against doxorubicin, this study highlights the potential for ET to be used as an agent for alleviating anthracycline cardiotoxicity in cancer patients, which certainly warrants further attention.
Acknowledgments
The authors wish to thank Tetrahedron (Paris, France) for kindly providing the L-ergothioneine, L-ergothioneine-d9, L-hercynine, and hercynine-d9 used in our studies.
Appendix A
Table A1.
Forward and reverse primer sequences used for qPCR.
| Genes | Forward Primer 5’→3’ | Reverse Primer 5’→3’ |
|---|---|---|
| OCTN1 | AAGTCCTCTTTGCAACCATGGC | CTGTGTTGTTTGCCATTGTGGG |
| β-ACTIN | TGACAGGATGCAGAAGGAGA | CGCTCAGGAGGAGCAATG |
Table A2.
Mass spectrometry–multiple reaction monitoring (MRM) transitions, as well as the optimized fragmentor voltages and collision energies for those transitions.
| Targets | MRM Transition | Fragmentor Voltage (V) | Collision Energy (eV) |
|---|---|---|---|
| ET | 230 → 186 | 103 | 9 |
| ET-d9 | 239 → 195 | 98 | 9 |
| Hercynine | 198 → 95 | 94 | 21 |
| Hercynine-d9 | 207 → 95 | 103 | 9 |
| 8OHdG | 284 → 168 | 80 | 10 |
| 8OHdG-13C2,15N1 | 287 → 171 | 80 | 10 |
| 8OHG | 300 → 168 | 85 | 10 |
| 8OHG-13C1,15N2 | 303 → 171 | 85 | 10 |
| dG | 268 → 152 | 70 | 6 |
| dG-13C1,15N2 | 271 → 155 | 65 | 5 |
| Guanosine | 284 → 152 | 70 | 10 |
| Guanosine-15N5 | 289 → 157 | 85 | 10 |
Figure A1.
Image of excised tumours from each animal (animals euthanized at differing points corresponding to images (A–C)). Allocation of groups can be found in the table.
Author Contributions
Conceptualization—I.K.C., J.-W.W. and B.H.; methodology—I.K.C., R.M.Y.T., X.W., K.S., J.-W.W. and B.H.; validation—I.K.C., R.M.Y.T., X.W. and J.-W.W.; formal analysis—I.K.C., R.M.Y.T., X.W., S.Y.C., J.-W.W. and B.H.; experimental investigation—R.M.Y.T., X.W., I.K.C., K.S. and S.Y.C.; writing—original draft preparation—I.K.C., R.M.Y.T. and B.H.; writing—review and editing—I.K.C., R.M.Y.T., B.H. and J.-W.W.; supervision—J.-W.W., B.H. and L.H.K.L.; project administration—I.K.C., J.-W.W. and B.H.; funding acquisition—I.K.C., B.H. and J.-W.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Animals were cared for in accordance with the protocols and guidelines stipulated in the Institutional Animal Care and Use Committee (IACUC) of the National University of Singapore. The animal study protocol was approved by IACUC, National University of Singapore, protocol R17-1242 on the 19th of January 2018.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this study are available in the appendices, or available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Ministry of Education AcRF Tier 1, grant number NUHSRO/2017/055/T1; the Tan Chin Tuan Centennial Foundation, the Ministry of Health—National Academy of Medicine Healthy Longevity Catalyst Award (HLCA20Jan-0057); and the National Medical Research Council (individual research grants NMRC/1264/2010/082/12 and NMRC/OFYIRG/0081/2018). S.Y. Chong was supported by the ESR/TENG GL PhD scholarship program.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Raj S., Franco V.I., Lipshultz S.E. Anthracycline-induced cardiotoxicity: A review of pathophysiology, diagnosis, and treatment. Curr. Treat Options Cardiovasc. Med. 2014;16:315. doi: 10.1007/s11936-014-0315-4. [DOI] [PubMed] [Google Scholar]
- 2.Swain S.M., Whaley F.S., Ewer M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 3.Doroshow J.H., Davies K.J. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J. Biol. Chem. 1986;261:3068–3074. doi: 10.1016/S0021-9258(17)35747-2. [DOI] [PubMed] [Google Scholar]
- 4.Fabiani I., Aimo A., Grigoratos C., Castiglione V., Gentile F., Saccaro L., Arzilli C., Cardinale D., Passino C., Emdin M. Oxidative stress and inflammation: Determinants of anthracycline cardiotoxicity and possible therapeutic targets. Heart Fail Rev. 2021;26:881–890. doi: 10.1007/s10741-020-10063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J., Wu R., Chen L., Yang Z., Yan D., Li M. Understanding Anthracycline Cardiotoxicity from Mitochondrial Aspect. Front. Pharmacol. 2022;13:811406. doi: 10.3389/fphar.2022.811406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tadokoro T., Ikeda M., Ide T., Deguchi H., Ikeda S., Okabe K., Ishikita A., Matsushima S., Koumura T., Yamada K., et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5:e132747. doi: 10.1172/jci.insight.132747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller I., Jenner A., Bruchelt G., Niethammer D., Halliwell B. Effect of concentration on the cytotoxic mechanism of doxorubicin--apoptosis and oxidative DNA damage. Biochem. Biophys. Res. Commun. 1997;230:254–257. doi: 10.1006/bbrc.1996.5898. [DOI] [PubMed] [Google Scholar]
- 8.Goormaghtigh E., Chatelain P., Caspers J., Ruysschaert J.M. Evidence of a specific complex between adriamycin and negatively-charged phospholipids. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1980;597:1–14. doi: 10.1016/0005-2736(80)90145-5. [DOI] [PubMed] [Google Scholar]
- 9.Ichikawa Y., Ghanefar M., Bayeva M., Wu R., Khechaduri A., Prasad S.V.N., Mutharasan R.K., Naik T.J., Ardehali H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014;124:617–630. doi: 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z., Dalan R., Hu Z., Wang J., Chew N., Poh K., Tan R., Soong T., Dai Y., Ye L., et al. Reactive Oxygen Species Scavenging Nanomedicine for the Treatment of Ischemic Heart Disease. Adv. Mater. 2022;34:e2202169. doi: 10.1002/adma.202202169. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B., Gutteridge J.M. Free Radicals in Biology and Medicine. 5th ed. Clarendon Press; Oxford, UK: 2015. [Google Scholar]
- 12.Akolkar G., da Silva Dias D., Ayyappan P., Bagchi A.K., Jassal D.S., Salemi V.M.C., Irigoyen M.C., De Angelis K., Singal P. Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2017;313:H795–H809. doi: 10.1152/ajpheart.00253.2017. [DOI] [PubMed] [Google Scholar]
- 13.Bjelogrlic S.K., Radic J., Jovic V., Radulovic S. Activity of d,l-alpha-tocopherol (vitamin E) against cardiotoxicity induced by doxorubicin and doxorubicin with cyclophosphamide in mice. Basic Clin. Pharmacol. Toxicol. 2005;97:311–319. doi: 10.1111/j.1742-7843.2005.pto_166.x. [DOI] [PubMed] [Google Scholar]
- 14.Arica V., Demir I.H., Tutanc M., Basarslan F., Arica S., Karcoiglu M., Ozturk H., Nacar A. N-acetylcysteine prevents doxorubucin-induced cardiotoxicity in rats. Hum. Exp. Toxicol. 2013;32:655–661. doi: 10.1177/0960327112467043. [DOI] [PubMed] [Google Scholar]
- 15.Vincent D.T., Ibrahim Y.F., Espey M.G., Suzuki Y.J. The role of antioxidants in the era of cardio-oncology. Cancer Chemother. Pharmacol. 2013;72:1157–1168. doi: 10.1007/s00280-013-2260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavalcanti I.D.L., Soares J.C.S., Medeiros S.M.d.F.R.d.S., Cavalcanti I., Lira Nogueira M.C.d.B. Can antioxidant vitamins avoid the cardiotoxicity of doxorubicin in treating breast cancer? PharmaNutrition. 2021;16:100259. doi: 10.1016/j.phanu.2021.100259. [DOI] [Google Scholar]
- 17.Ambrosone C.B., Zirpoli G.R., Hutson A.D., McCann W.E., McCann S.E., Barlow W.E., Kelly K.M., Cannioto R., Sucheston-Campbell L., Hershman D., et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients with Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221) J. Clin. Oncol. 2020;38:804–814. doi: 10.1200/JCO.19.01203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawenda B.D., Kelly K.M., Ladas E.J., Sagar S.M., Vickers A., Blumberg J.B. Should Supplemental Antioxidant Administration Be Avoided During Chemotherapy and Radiation Therapy? JNCI J. Natl. Cancer Inst. 2008;100:773–783. doi: 10.1093/jnci/djn148. [DOI] [PubMed] [Google Scholar]
- 19.Zou Z.V., Le Gal K., El Zowalaty A.E., Pehlivanoglu L.E., Garellick V., Gul N., Ibrahim M., Bergh P., Henricsson M., Wiel C., et al. Antioxidants Promote Intestinal Tumor Progression in Mice. Antioxidants. 2021;10:241. doi: 10.3390/antiox10020241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sayin V.I., Ibrahim M.X., Larsson E., Nilsson J.A., Lindahl P., Bergo M. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014;6:221ra15. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 21.Breau M., Houssaini A., Lipskaia L., Abid S., Born E., Marcos E., Czibik G., Attwe A., Beaulieu D., Palazzo A., et al. The antioxidant N-acetylcysteine protects from lung emphysema but induces lung adenocarcinoma in mice. JCI Insight. 2019;4:e127647. doi: 10.1172/jci.insight.127647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buss J.L., Hasinoff B. The one-ring open hydrolysis product intermediates of the cardioprotective agent ICRF-187 (dexrazoxane) displace iron from iron-anthracycline complexes. Agents Actions. 1993;40:86–95. doi: 10.1007/BF01976756. [DOI] [PubMed] [Google Scholar]
- 23.Shaikh F., Dupuis L., Alexander S., Gupta A., Mertens L., Nathan P. Cardioprotection and Second Malignant Neoplasms Associated with Dexrazoxane in Children Receiving Anthracycline Chemotherapy: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2016;108:djv357. doi: 10.1093/jnci/djv357. [DOI] [PubMed] [Google Scholar]
- 24.Cheah I.K., Halliwell B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta. 2012;1822:784–793. doi: 10.1016/j.bbadis.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Cheah I.K., Halliwell B. Ergothioneine, recent developments. Redox Biol. 2021;42:101868. doi: 10.1016/j.redox.2021.101868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melville D.B. Ergothioneine. Vitam. Horm. 1959;17:155–204. [Google Scholar]
- 27.Halliwell B., Cheah I., Tang R.M.Y. Ergothioneine—A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018;592:3357–3366. doi: 10.1002/1873-3468.13123. [DOI] [PubMed] [Google Scholar]
- 28.Tang R.M.Y., Cheah I., Yew T., Halliwell B. Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci. Rep. 2018;8:1601. doi: 10.1038/s41598-018-20021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucker R.A.J., Cheah I., Halliwell B. Specificity of the ergothioneine transporter natively expressed in HeLa cells. Biochem. Biophys. Res. Commun. 2019;513:22–27. doi: 10.1016/j.bbrc.2019.02.122. [DOI] [PubMed] [Google Scholar]
- 30.Grundemann D., Hartmann L., Flogel S. The ergothioneine transporter (ETT): Substrates and locations, an inventory. FEBS Lett. 2022;596:1252–1269. doi: 10.1002/1873-3468.14269. [DOI] [PubMed] [Google Scholar]
- 31.Akanmu D., Cecchini R., Aruoma O.I., Halliwell B. The antioxidant action of ergothioneine. Arch. Biochem. Biophys. 1991;288:10–16. doi: 10.1016/0003-9861(91)90158-F. [DOI] [PubMed] [Google Scholar]
- 32.Koh S.S., Ooi S., Lui N., Qiong C., Ho L., Cheah I., Halliwell B., Herr D., Ong W. Effect of Ergothioneine on 7-Ketocholesterol-Induced Endothelial Injury. Neuromolecular Med. 2021;23:184–198. doi: 10.1007/s12017-020-08620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motohash N., Mori I., Sugiura Y., Tanaka H. Metal-complexes of ergothioneine. Chem. Pharm. Bull. 1974;22:654–657. doi: 10.1248/cpb.22.654. [DOI] [Google Scholar]
- 34.Zhu B.Z., Mao L., Fan R., Zhu J., Zhang Y., Wang J., Kalyanaraman B., Frei B. Ergothioneine prevents copper-induced oxidative damage to DNA and protein by forming a redox-inactive ergothioneine-copper complex. Chem. Res. Toxicol. 2011;24:30–34. doi: 10.1021/tx100214t. [DOI] [PubMed] [Google Scholar]
- 35.Paul B.D., Snyder S. The unusual amino acid L-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 2010;17:1134–1140. doi: 10.1038/cdd.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williamson R.D., McCarthy F., Manna S., Groarke E., Kell D., Kenny L., McCarthy C. L-(+)-Ergothioneine Significantly Improves the Clinical Characteristics of Preeclampsia in the Reduced Uterine Perfusion Pressure Rat Model. Hypertension. 2020;75:561–568. doi: 10.1161/HYPERTENSIONAHA.119.13929. [DOI] [PubMed] [Google Scholar]
- 37.Smith E., Ottosson F., Hellstrand S., Ericson U., Orho-Melander M., Fernandez C., Melander O. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart. 2020;106:691–697. doi: 10.1136/heartjnl-2019-315485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dare A., Elrashedy A., Channa M., Nadar A. Cardioprotective Effects and in-silico Antioxidant Mechanism of L-Ergothioneine in Experimental Type-2 Diabetic Rats. Cardiovasc. Hematol. Agents Med. Chem. 2022;20:133–147. doi: 10.2174/1871525719666210809122541. [DOI] [PubMed] [Google Scholar]
- 39.Gökçe G., Arun M., Ertuna E. Ergothioneine prevents endothelial dysfunction induced by mercury chloride. Exp. Ther. Med. 2018;15:4697–4702. doi: 10.3892/etm.2018.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li R.W., Yang C., Sit A., Kwan Y., Lee S., Hoi M., Chan S., Hausman M., Vanhoutte P., Leung G. Uptake and protective effects of ergothioneine in human endothelial cells. J. Pharmacol. Exp. Ther. 2014;350:691–700. doi: 10.1124/jpet.114.214049. [DOI] [PubMed] [Google Scholar]
- 41.Sakrak Ö., Kerem M., Bedirli A., Pasaoğlu H., Alper M., Ofluoğlu E., Yılmaz T. Ergothioneine prevents acute lung injury in mesenteric ischemia and reperfusion injury in rats. J. Crit. Care. 2008;23:268–269. doi: 10.1016/j.jcrc.2008.03.021. [DOI] [Google Scholar]
- 42.Bedirli A., Sakrak O., Muhtaroglu S., Soyuer I., Guler I., Erdogan A.R., Sozuer E. Ergothioneine pretreatment protects the liver from ischemia-reperfusion injury caused by increasing hepatic heat shock protein 70. J. Surg Res. 2004;122:96–102. doi: 10.1016/j.jss.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Sakrak O., Kerem M., Bedirli A., Pasaoglu H., Akyurek N., Ofluoglu E., Gultekin F. Ergothioneine modulates proinflammatory cytokines and heat shock protein 70 in mesenteric ischemia and reperfusion injury. J. Surg Res. 2008;144:36–42. doi: 10.1016/j.jss.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Martin K.R. The bioactive agent ergothioneine, a key component of dietary mushrooms, inhibits monocyte binding to endothelial cells characteristic of early cardiovascular disease. J. Med. Food. 2010;13:1340–1346. doi: 10.1089/jmf.2009.0194. [DOI] [PubMed] [Google Scholar]
- 45.Arduini A., Eddy L., Hochstein P. The reduction of ferryl myoglobin by ergothioneine: A novel function for ergothioneine. Arch. Biochem. Biophys. 1990;281:41–43. doi: 10.1016/0003-9861(90)90410-Z. [DOI] [PubMed] [Google Scholar]
- 46.Sansbury B.E., DeMartino A., Xie Z., Brooks A., Brainard R., Watson L., DeFilippis A., Cummins T., Harbeson M., Brittian K., et al. Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ. Heart Fail. 2014;7:634–642. doi: 10.1161/CIRCHEARTFAILURE.114.001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halliwell B., Cheah I., Drum C. Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem. Biophys. Res. Commun. 2016;470:245–250. doi: 10.1016/j.bbrc.2015.12.124. [DOI] [PubMed] [Google Scholar]
- 48.Cheah I.K., Tang R., Ye P., Yew T., Lim K., Halliwell B. Liver ergothioneine accumulation in a guinea pig model of non-alcoholic fatty liver disease. A possible mechanism of defence? Free Radic. Res. 2016;50:14–25. doi: 10.3109/10715762.2015.1099642. [DOI] [PubMed] [Google Scholar]
- 49.Wang J.W., Fontes M., Wang X., Chong S., Kessler E., Zhang Y., de Haan J., Arslan F., de Jager S., Timmers L., et al. Leukocytic Toll-Like Receptor 2 Deficiency Preserves Cardiac Function and Reduces Fibrosis in Sustained Pressure Overload. Sci. Rep. 2017;7:9193. doi: 10.1038/s41598-017-09451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Kleijn D.P.V., Chong S., Wang X., Yatim S., Fairhurst A., Vernooij F., Zharkova O., Chan M., Foo R., Timmers L., et al. Toll-like receptor 7 deficiency promotes survival and reduces adverse left ventricular remodelling after myocardial infarction. Cardiovasc. Res. 2019;115:1791–1803. doi: 10.1093/cvr/cvz057. [DOI] [PubMed] [Google Scholar]
- 51.Tei C. New non-invasive index for combined systolic and diastolic ventricular function. J. Cardiol. 1995;26:135–136. [PubMed] [Google Scholar]
- 52.Salemi V.M., Pires M., Cestari I., Cestari I., Picard M., Leirner A., Mady C. Echocardiographic assessment of global ventricular function using the myocardial performance index in rats with hypertrophy. Artif. Organs. 2004;28:332–337. doi: 10.1111/j.1525-1594.2004.47350.x. [DOI] [PubMed] [Google Scholar]
- 53.Murphy M.P., Bayir H., Belousov V., Chang C., Davies K., Davies M., Dick T., Finkel T., Forman H., Janssen-Heininger Y., et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022;4:651–662. doi: 10.1038/s42255-022-00591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheah I.K., Tang R., Yew T., Lim K., Halliwell B. Administration of pure ergothioneine to healthy human subjects; Uptake, metabolism and effects on biomarkers of oxidative damage and inflammation. Antioxid. Redox Signal. 2017;26:193–206. doi: 10.1089/ars.2016.6778. [DOI] [PubMed] [Google Scholar]
- 55.Zaorsky N.G., Churilla T., Egleston B., Fisher S., Ridge J., Horwitz E., Meyer J. Causes of death among cancer patients. Ann. Oncol. 2017;28:400–407. doi: 10.1093/annonc/mdw604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schairer C., Mink P., Carroll L., Devesa S. Probabilities of death from breast cancer and other causes among female breast cancer patients. J. Natl. Cancer Inst. 2004;96:1311–1321. doi: 10.1093/jnci/djh253. [DOI] [PubMed] [Google Scholar]
- 57.Florescu M., Cinteza M., Vinereanu D. Chemotherapy-induced Cardiotoxicity. Maedica (Bucur) 2013;8:59–67. [PMC free article] [PubMed] [Google Scholar]
- 58.Grenier M.A., Lipshultz S. Epidemiology of anthracycline cardiotoxicity in children and adults. Semin. Oncol. 1998;25((Suppl. 10)):72–85. [PubMed] [Google Scholar]
- 59.Gianni L., Salvatorelli E., Minotti G. Anthracycline cardiotoxicity in breast cancer patients: Synergism with trastuzumab and taxanes. Cardiovasc. Toxicol. 2007;7:67–71. doi: 10.1007/s12012-007-0013-5. [DOI] [PubMed] [Google Scholar]
- 60.Halliwell B., Tang R., Cheah I. Diet-Derived Antioxidants: The Special Case of Ergothioneine. Annu. Rev. Food Sci. Technol. 2023;14 doi: 10.1146/annurev-food-060822-122236. [DOI] [PubMed] [Google Scholar]
- 61.Kato Y., Kubo Y., Iwata D., Kato S., Sudo T., Sugiura T., Kagaya T., Wakayama T., Hirayama A., Sugimoto M., et al. Gene knockout and metabolome analysis of carnitine/organic cation transporter OCTN1. Pharm. Res. 2010;27:832–840. doi: 10.1007/s11095-010-0076-z. [DOI] [PubMed] [Google Scholar]
- 62.Pfeiffer C., Bach M., Bauer T., da Ponte J.C., Schomig E., Grundemann D. Knockout of the ergothioneine transporter ETT in zebrafish results in increased 8-oxoguanine levels. Free Radic. Biol. Med. 2015;83:178–185. doi: 10.1016/j.freeradbiomed.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 63.Cheah I.K., Ong R., Gruber J., Yew T., Ng L., Chen C., Halliwell B. Knockout of a putative ergothioneine transporter in Caenorhabditis elegans decreases lifespan and increases susceptibility to oxidative damage. Free Radic. Res. 2013;47:1036–1045. doi: 10.3109/10715762.2013.848354. [DOI] [PubMed] [Google Scholar]
- 64.Tei C., Ling L., Hodge D., Bailey K., Oh J., Rodeheffer R., Tajik A., Seward J. New index of combined systolic and diastolic myocardial performance: A simple and reproducible measure of cardiac function—A study in normals and dilated cardiomyopathy. J. Cardiol. 1995;26:357–366. [PubMed] [Google Scholar]
- 65.Sofia R., Melita V., De Vita A., Ruggiero A., Romano A., Attina G., Birritella L., Lamendola P., Lombardo A., Lanza G., et al. Cardiac Surveillance for Early Detection of Late Subclinical Cardiac Dysfunction in Childhood Cancer Survivors after Anthracycline Therapy. Front. Oncol. 2021;11:624057. doi: 10.3389/fonc.2021.624057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dawson D., Lygate C., Saunders J., Schneider J., Ye X., Hulbert K., Noble J., Neubauer S. Quantitative 3-dimensional echocardiography for accurate and rapid cardiac phenotype characterization in mice. Circulation. 2004;110:1632–1637. doi: 10.1161/01.CIR.0000142049.14227.AD. [DOI] [PubMed] [Google Scholar]
- 67.Chong S.Y., Zharkova O., Yatim S., Wang X., Lim X., Huang C., Tan C., Jiang J., Ye L., Tan M., et al. Tissue factor cytoplasmic domain exacerbates post-infarct left ventricular remodeling via orchestrating cardiac inflammation and angiogenesis. Theranostics. 2021;11:9243–9261. doi: 10.7150/thno.63354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Obayashi K., Kurihara K., Okano Y., Masaki H., Yarosh D. L-Ergothioneine scavenges superoxide and singlet oxygen and suppresses TNF-alpha and MMP-1 expression in UV-irradiated human dermal fibroblasts. J. Cosmet. Sci. 2005;56:17–27. doi: 10.1111/j.0142-5463.2005.00265_2.x. [DOI] [PubMed] [Google Scholar]
- 69.Aruoma O.I., Whiteman M., England T., Halliwell B. Antioxidant action of ergothioneine: Assessment of its ability to scavenge peroxynitrite. Biochem. Biophys. Res. Commun. 1997;231:389–391. doi: 10.1006/bbrc.1997.6109. [DOI] [PubMed] [Google Scholar]
- 70.Chaudiere J., Ferrari-Iliou R. Intracellular antioxidants: From chemical to biochemical mechanisms. Food Chem. Toxicol. 1999;37:949–962. doi: 10.1016/S0278-6915(99)00090-3. [DOI] [PubMed] [Google Scholar]
- 71.Colognato R., Laurenza I., Fontana I., Coppede F., Siciliano G., Coecke S., Aruoma O., Benzi L., Migliore L. Modulation of hydrogen peroxide-induced DNA damage, MAPKs activation and cell death in PC12 by ergothioneine. Clin. Nutr. 2006;25:135–145. doi: 10.1016/j.clnu.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 72.Aruoma O.I., Spencer J., Mahmood N. Protection against oxidative damage and cell death by the natural antioxidant ergothioneine. Food Chem. Toxicol. 1999;37:1043–1053. doi: 10.1016/S0278-6915(99)00098-8. [DOI] [PubMed] [Google Scholar]
- 73.Gokce G., Arun M. Ergothioneine produces relaxation in isolated rat aorta by inactivating superoxide anion. Eur. Rev. Med. Pharmacol. Sci. 2014;18:3339–3345. [PubMed] [Google Scholar]
- 74.Servillo L., D’Onofrio N., Casale R., Cautela D., Giovane A., Castaldo D., Balestrieri M. Ergothioneine products derived by superoxide oxidation in endothelial cells exposed to high-glucose. Free Radic. Biol. Med. 2017;108:8–18. doi: 10.1016/j.freeradbiomed.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 75.Servillo L., Castaldo D., Casale R., D’Onofrio N., Giovane A., Cautela D., Balestrieri M. An uncommon redox behavior sheds light on the cellular antioxidant properties of ergothioneine. Free Radic. Biol. Med. 2015;79:228–236. doi: 10.1016/j.freeradbiomed.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 76.Ando C., Morimitsu Y. A proposed antioxidation mechanism of ergothioneine based on the chemically derived oxidation product hercynine and further decomposition products. Biosci. Biotechnol. Biochem. 2021;85:1175–1182. doi: 10.1093/bbb/zbab006. [DOI] [PubMed] [Google Scholar]
- 77.Myers C.E., Gianni L., Simone C., Klecker R., Greene R. Oxidative destruction of erythrocyte ghost membranes catalyzed by the doxorubicin-iron complex. Biochemistry. 1982;21:1707–1712. doi: 10.1021/bi00537a001. [DOI] [PubMed] [Google Scholar]
- 78.Hershko C., Link G., Tzahor M., Kaltwasser J., Athias P., Grynberg A., Pinson A. Anthracycline toxicity is potentiated by iron and inhibited by deferoxamine: Studies in rat heart cells in culture. J. Lab. Clin. Med. 1993;122:245–251. [PubMed] [Google Scholar]
- 79.Panjrath G.S., Patel V., Valdiviezo C., Narula N., Narula J., Jain D. Potentiation of Doxorubicin cardiotoxicity by iron loading in a rodent model. J. Am. Coll Cardiol. 2007;49:2457–2464. doi: 10.1016/j.jacc.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 80.Arkadopoulos N., Nastos C., Kalimeris K., Economou E., Theodoraki K., Kouskouni E., Pafiti A., Kostopanagiotou G., Smyrniotis V. Iron chelation for amelioration of liver ischemia-reperfusion injury. Hemoglobin. 2010;34:265–277. doi: 10.3109/03630269.2010.484766. [DOI] [PubMed] [Google Scholar]
- 81.Koyama T., Tsubota A., Sawano T., Tawa M., Watanabe B., Hiratake J., Nakagawa K., Matsumura Y., Ohkita M. Involvement of gamma-Glutamyl Transpeptidase in Ischemia/Reperfusion-Induced Cardiac Dysfunction in Isolated Rat Hearts. Biol. Pharm. Bull. 2019;42:1947–1952. doi: 10.1248/bpb.b19-00434. [DOI] [PubMed] [Google Scholar]
- 82.Lee D.S., Evans J., Robins S., Wilson P., Albano I., Fox C., Wang T., Benjamin E., D’Agostino R.B., Vasan R. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2007;27:127–133. doi: 10.1161/01.ATV.0000251993.20372.40. [DOI] [PubMed] [Google Scholar]
- 83.Ndrepepa G., Colleran R., Kastrati A. Gamma-glutamyl transferase and the risk of atherosclerosis and coronary heart disease. Clin. Chim. Acta. 2018;476:130–138. doi: 10.1016/j.cca.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 84.Salama S.A., Abd-Allah G., Mohamadin A., Elshafey M., Gad H. Ergothioneine mitigates cisplatin-evoked nephrotoxicity via targeting Nrf2, NF-kappaB, and apoptotic signaling and inhibiting gamma-glutamyl transpeptidase. Life Sci. 2021;278:119572. doi: 10.1016/j.lfs.2021.119572. [DOI] [PubMed] [Google Scholar]
- 85.Brancaccio M., Russo M., Masullo M., Palumbo A., Russo G., Castellano I. Sulfur-containing histidine compounds inhibit gamma-glutamyl transpeptidase activity in human cancer cells. J. Biol. Chem. 2019;294:14603–14614. doi: 10.1074/jbc.RA119.009304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hseu Y.C., Lo H., Korivi M., Tsai Y., Tang M., Yang H. Dermato-protective properties of ergothioneine through induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated Human keratinocytes. Free Radic. Biol. Med. 2015;86:102–117. doi: 10.1016/j.freeradbiomed.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 87.D’Onofrio N., Martino E., Balestrieri A., Mele L., Cautela D., Castaldo D., Balestrieri M. Diet-derived ergothioneine induces necroptosis in colorectal cancer cells by activating the SIRT3/MLKL pathway. FEBS Lett. 2022;596:1313–1329. doi: 10.1002/1873-3468.14310. [DOI] [PubMed] [Google Scholar]
- 88.Turck D., Bresson J., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., Hirsch-Ernst K., Mangelsdorf I., McArdle H., Naska A., et al. Safety of synthetic l-ergothioneine (Ergoneine®) as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2016;14:4629. doi: 10.2903/j.efsa.2016.4629. [DOI] [Google Scholar]
- 89.Turck D., Bresson J., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., Hirsch-Ernst K., Mangelsdorf I., McArdle H., Naska A., et al. Statement on the safety of synthetic l-ergothioneine as a novel food—Supplementary dietary exposure and safety assessment for infants and young children, pregnant and breastfeeding women. EFSA J. 2017;15:5060. doi: 10.2903/j.efsa.2017.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data presented in this study are available in the appendices, or available on request from the corresponding authors.