“Every way makes my gain … ”Othello, Act V, Scene I
Sensing various chemicals in the environment and responding to changes in their concentrations is a fundamental property of a living cell. It is especially important for unicellular organisms that constantly interact with the environment. Microorganisms possess simple yet effective systems that allow them to regulate numerous cellular functions in response to changes in their surroundings (for recent reviews, see references (34 and 54). Active motility of microbial cells along chemical gradients, usually referred to as chemotaxis, is controlled by probably the best-studied signal transduction system. Taxis responses allow motile microorganisms to rapidly move toward a microenvironment optimal for their growth and survival. The mechanism of flagellar motility and its control via chemotaxis have been studied in great detail in Escherichia coli and Salmonella enterica serovar Typhimurium (for reviews, see references (8, 15, 55, and 56). Enteric bacteria measure concentrations of chemicals outside the cell using transmembrane receptors (chemotaxis transducers) that transmit information into the cell interior. Transmembrane chemoreceptors are arranged into arrays, resulting in the sensor system, which is extremely sensitive to subtle conformational changes (14, 55). The transmembrane signaling in E. coli is a current paradigm for chemical sensing (for a recent review, see reference (21). However, there is a growing evidence that transmembrane signaling is not the only way to sense chemicals. This minireview focuses on alternative strategies used by microorganisms in order to monitor the chemical composition of the environment.
METABOLISM-INDEPENDENT CHEMICAL SENSING
Transmembrane chemoreceptors.
In 1969, Julius Adler provided for the first time extensive biochemical and genetic evidence that E. coli chemotaxis is independent of uptake or metabolism of the chemical stimulus (1). For example, chemotaxis to galactose was normal in mutants deficient in galactose transport and metabolism. From that time on, research focused on the metabolism-independent information flow from membrane receptors to flagellar motors. E. coli possess five chemoreceptors, and four of them are involved in transmembrane signaling: Tar for aspartate, Tsr for serine, Trg for ribose and galactose, and Tap for dipeptides (for reviews, see references (21 and 56). These chemoreceptors have essentially the same membrane topology: two transmembrane helices anchor the receptor in the membrane and demarcate a periplasmic ligand-binding domain and a cytoplasmic signaling module. Binding of a chemoeffector (attractant or repellent) or chemoeffector-occupied binding protein to the periplasmic domain of the chemoreceptor is necessary and sufficient to cause changes in the cell behavior. Conformational changes in the receptor are transmitted to the histidine autokinase CheA, which serves as the phosphodonor for the cognate response regulator CheY. Phosphorylated CheY controls swimming behavior by binding the flagellar motor and changing its rotational direction from counterclockwise to clockwise (for a recent review, see reference (14). Transmembrane chemoreceptors Tar, Tsr, Trg, and Tap, which recognize chemical stimuli extracellularly, account for 90% of the total number of chemotaxis transducer molecules in E. coli (32; F. Roy, M. S. Johnson, and B. L. Taylor, unpublished data). The dominance of the ligand-binding mechanism of chemoreception explains the classical postulates of Adler: (i) essentially nonmetabolizable analogues of metabolizable attractants are still attractants, (ii) mutation in the metabolism of a chemical attractant does not affect chemotaxis, and (iii) chemicals attract bacteria even in the presence of another metabolizable chemical.
PTS taxis: transport-dependent behavior.
In addition to chemotactic responses mediated by transmembrane receptors, E. coli also exhibits chemotaxis to substrates that are transported by the phosphoenolpyruvate (PEP)-dependent carbohydrate phosphotransferase system (PTS). Transport, but not metabolism, of the PTS substrates is required for a behavioral response (2). During transport, the sugar is phosphorylated by a membrane-bound sugar-specific enzyme II (EII) transport protein. EII accepts phosphate from a nonspecific donor, a PEP-dependent histidine kinase enzyme I (EI). EI and a phosphohistidine carrier protein (Hpr) constitute the phosphorelay to EII. EI interacts directly with CheA, and the phosphorylation state of EI regulates the autophosphorylation of CheA (40) by a transport-induced dephosphorylation of the PTS (41). An unusual variation on a theme in PTS chemotaxis was reported for Bacillus subtilis, where a chemoreceptor was directly involved in mediating the response (23).
Metabolism-independent chemotaxis was found in several bacterial species, including chemotaxis to amino acids in B. subtilis (25, 44) and to aromatic acids in Pseudomonas putida (20).
CHEMICAL SENSING VIA ENERGY TAXIS
Metabolism-dependent behavior.
In contrast to the metabolism-independent behavior described above, some chemotactic responses in bacteria require metabolism of a chemical attractant. These include chemotaxis of E. coli to proline (17), glycerol (71), and succinate (13). The presence of an alternative substrate or a mutation in glycerol metabolism prevents chemotaxis to glycerol (71), a clear disagreement with Adler's postulates for metabolism-independent chemotaxis.
Metabolism-dependent chemotaxis to most chemical attractants was demonstrated in an α-proteobacterium, Azospirillum brasilense, a diazotrophic soil organism capable of colonizing the rhizosphere of many important cereals and grasses (4, 69). In A. brasilense, (i) nonmetabolizable analogues of metabolizable attractants are not attractants, (ii) inhibition of the metabolism of a chemical attractant completely abolishes chemotaxis to and only to this attractant, and (iii) the presence of another metabolizable chemical (exogenous or endogenous) prevents chemotaxis to all attractants studied. In addition, in A. brasilense, there is a direct correlation between the efficiency of a chemical as a growth substrate and as a chemoeffector (4).
Experimental evidence suggests that chemotaxis to certain attractants is metabolism dependent in another α-proteobacterium, Rhodobacter sphaeroides (8–10). First, all attractants are metabolites, the strongest being organic acids, such as succinate, similar to what occurs in A. brasilense. Other chemoattractants include a wide range of sugars, polyols, and amino acids. No repellents have been identified so far in R. sphaeroides. Involvement of metabolism in chemotaxis of R. sphaeroides to sugars was shown (36). Stronger chemotactic responses are observed when cells are grown on the sugar under test as the growth substrate, and a period of starvation enhances the responses. Furthermore, a mutant impaired in the metabolism of a sugar does not show chemotaxis to this sugar.
Similarly, chemotaxis to some attractants, such as proline, was suggested to be metabolism dependent in Sinorhizobium meliloti, a nitrogen-fixing symbiont of leguminous plants (27). Chemotaxis to root exudates is a prerequisite for the establishment of the symbiotic association between the plant and the bacterium. S. meliloti cells are attracted by organic acids, amino acids, carbohydrates, and flavones, all of which are present in root exudates (19, 50). The chemotactic responses in S. meliloti are stronger if cells have been starved before being chemotactically stimulated, suggesting that metabolism is required.
Spirochaeta aurantia is a free-living spirochete found in aquatic environments. A relationship between energy metabolism and chemotaxis in this bacterium was suggested by the observation that chemotactic responses to low concentrations of d-ribose and d-xylose were stronger if the cells were grown under carbon-limited conditions (62). Moreover, addition of d-xylose to the cells caused changes in membrane potential (28, 29), suggesting that monitoring intracellular energy status is the origin of the chemotactic signal toward d-xylose.
Metabolism-dependent chemotaxis to pyruvate, a rapidly metabolizable substrate, has also been recently reported for Campylobacter jejuni, a microaerophilic ɛ-proteobacterium, which is a common commensal organism of the gastrointestinal tract in animals (33).
Energy taxis.
A correlation was reported between temporal changes in behavior and in electron transport/membrane potential during taxis to proline (17) and glycerol (71) in E. coli and during taxis to all strong attractants in A. brasilense (4). These studies suggested that metabolism-dependent chemotaxis shares the signaling pathway with other behavioral responses collectively known as energy taxis.
Energy taxis is broadly defined as a behavioral response to stimuli that affect cellular energy levels (60). It encompasses responses that are directly linked to electron transport/energy generation, such as aerotaxis, phototaxis, redox taxis, and taxis to alternative electron acceptors (for recent reviews, see references 8, 59, and 61). The signal for this type of behavior originates within the electron transport system, where changes in the rate of electron transport (or a related parameter) are detected by a signal transduction system. This bacterial behavior was analyzed extensively with respect to electron acceptors (8, 11, 24, 30, 58) and light, which initiates electron transport (8, 31). Rapidly metabolizable substrates that input reducing equivalents into the electron transport system have rarely been studied as chemoeffectors. However, the donors of reducing equivalents directly determine the rate of electron transport and thus are being sensed as attractants via the energy taxis mechanism. Therefore, metabolizable substrates can be chemical stimuli for bacteria as long as a receptor measuring changes in the electron transport system is present.
Electron transport system is required for chemical sensing in A. brasilense.
In energy taxis, a functional electron transport system is required for the behavioral response (Fig. 1.). For example, electron transport to the terminal oxidases is essential for aerotaxis in E. coli (52) and for taxis to light, oxygen, and dimethyl sulfoxide in R. sphaeroides (24, 31). A. brasilense became the first microorganism for which direct evidence was obtained that a functional electron transport system is required for chemotaxis to all major chemical attractants and repellents (4).
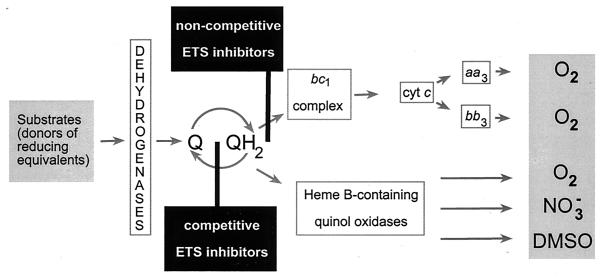
FIG. 1.
Attractants and repellents in energy taxis. Metabolizable substrates and electron acceptors are attractants (gray boxes) because they maintain the flow of reducing equivalents through the electron transport system (ETS). Chemicals that inhibit the flow are repellents (black boxes). Adapted from reference 4 with modifications.
First, only chemicals that interact directly with the electron transport system were found to be repellents for A. brasilense. Substituted quinones that are strong competitive inhibitors of the respiratory system in azospirilla (3) were found to cause a repellent response (4), which correlated with their reduction potential, similar to negative redox taxis in E. coli (11). Noncompetitive inhibitors of the cytochrome c oxidase-terminated respiratory branch, such as myxothiazol, were also found to cause a repellent response. The cytochrome c oxidase-terminated branches in bacteria are the most efficient in energy generation (63). Inhibition of this respiratory branch by myxothiazol caused a strong repellent response in A. brasilense, which lasted several minutes in a temporal gradient assay. Under these conditions, supplying reducing equivalents beyond the inhibition site by using artificial electron donors caused an attractant response (4).
Second, there was a correlation between the magnitude of the chemotactic response, rate of electron transport, and membrane potential upon addition of a chemical attractant to starved cells (4).
Third and most important, a mutant lacking the cytochrome cbb3-type terminal oxidase had significantly diminished chemotaxis to all major attractants, but only under microaerobic conditions (4). When assayed under fully aerobic conditions, where this respiratory branch is not functional (42), chemotactic responses in the mutant and the wild-type strain were identical (4). Altogether, the results showed that the signal for chemotaxis toward major attractants and repellents originated within a functional electron transport system in A. brasilense.
The cytochrome c-containing branch may play a specific role in sensing and signaling to the tactic machinery when changes in the electron transport system occur. This respiratory branch is dominant under microaerophilic conditions (42), where A. brasilense generates maximal energy (70). Therefore, a mutation or inhibition of this electron transport branch will have the strongest effect on the energy status of the cell. It is not known whether a change in the redox state of components of the electron transport system or an ion motive force is the signal for chemotaxis in A. brasilense and in other species where chemicals are sensed via energy taxis. Characterization of chemoreceptors capable of measuring changes within the electron transport system is required in order to conclusively establish sensing mechanisms.
A GROWING FAMILY OF RECEPTORS FOR ENERGY TAXIS
A general scheme showing a variety of energy taxis receptors discussed below is shown on Fig. 2.
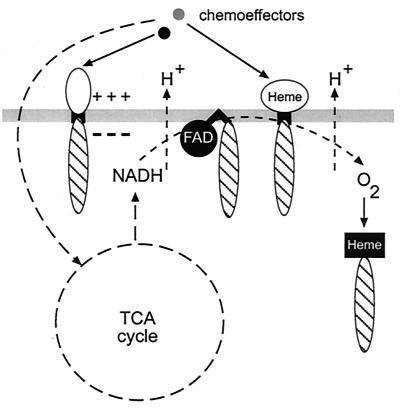
FIG. 2.
Microbial chemotaxis transducers. Metabolism-independent chemotaxis is governed by transmembrane receptors that have a periplasmic ligand-binding domain (shown in white). Energy taxis is governed by all membrane-associated receptors shown: either redox-responsive cofactors (FAD, heme) or charge-sensitive amino acid residues in a close proximity to the membrane might serve as the sensors (see the text for details). A specialized receptor binding oxygen directly is shown on the right. Hatched ovals represent conserved signaling modules. Cytoplasmic sensing domains are shown in black.
Aer.
Although a receptor for energy taxis, which would measure proton motive force or a similar parameter, was theoretically predicted 20 years ago (26, 57), the first such receptor, Aer of E. coli, was described only in 1997 (13, 47). As all members of the chemotaxis transducer superfamily (68), the Aer protein has a conserved C-terminal signaling module. Almost the entire N-terminal region of Aer consists of the PAS domain (named PAS for Per, ARNT, Sim) (72). By using computerized database searches, this domain was detected in hundreds of signal transduction proteins from all three kingdoms of cellular life that are implicated in sensing of oxygen, light, and redox potential (46, 60, 72). The PAS domain of Aer contains the flavin adonine dinucleotide (FAD) cofactor (12, 13, 49), and presumably the redox status of FAD within Aer reflects that of an interacting component of the electron transport system. Aer was conclusively shown to mediate the response to succinate (13) and glycerol (47), chemicals that were proposed to signal via energy taxis (71). The importance of the redox-responsive FAD for Aer-mediated responses was well documented by using site-specific mutagenesis of the PAS domain and the HAMP domain (named HAMP for histidine kinases, adenylylcyclases, methyl-accepting chemotaxis proteins) (12, 49); Q. Ma and B. L. Taylor, unpublished data), which is a signaling module found in many microbial sensor proteins (7). In Aer, the HAMP domain is thought to transmit the signal from the PAS domain to the C-terminal signaling module. Aer is the best-studied receptor that governs chemotaxis to rapidly metabolizable chemical substrates by using the energy-sensing mechanism.
Tsr.
The serine chemoreceptor of E. coli, Tsr, has also been identified as an energy taxis transducer (47), but the sensing mechanism employed by Tsr remains unknown. Tsr responds to all stimuli that are recognized by Aer (47), including artificial stimulation or inhibition of the electron transport by redox-active compounds (11). However, no redox-responsive (or any other) prosthetic group is found in the receptor. Tsr was implicated in sensing both intracellular and extracellular pH (38, 48, 53). Thus, it was logical to suggest that the mechanism of pH sensing and energy sensing might be similar, because the pH gradient is a component of the electrochemical proton gradient generated by the electron transport system (39, 59). Positively charged residues on the cytoplasmic side of the membrane that are adjacent to the transmembrane helices might be the actual sensor elements. These residues are found in most membrane-spanning proteins and are subject to direct control by the electrochemical proton gradient (51).
In addition to Aer and Tsr of E. coli, several chemoreceptors were identified in other bacterial species that may mediate chemical sensing via energy taxis.
DcrA in Desufovibrio vulgaris.
The DcrA chemoreceptor of D. vulgaris is the most intriguing sensor and potential energy taxis transducer. Experimental evidence suggests that DcrA contains a C-type heme in its periplasmic region (22); therefore, it may be responsive to redox changes in the electron transport system or/and may bind oxygen directly. Moreover, the second putative sensing module in DcrA is the PAS domain, which is located intracellularly between the second transmembrane region and the C-terminal signaling module (60). Unfortunately, lack of behavioral studies renders the term “putative” for this unique sensor.
Ptr in Rhodospirillum centenum.
Another putative energy taxis transducer has been recently described for a photosynthetic α-proteobacterium, R. centenum. This transducer, termed Ptr, was shown to mediate photosensory behavior in R. centenum, where disruption of the ptr gene eliminated responses to light (37). Based on the presence in the predicted periplasmic sensing region of a short motif resembling known heme-binding sites, Jiang and Bauer (37) suggested that Ptr might sense the redox state of a periplasmic component of the photosynthetic electron transport system, such as cytochrome c. Although this is an attractive model, significant experimental evidence is still required to prove it. Chemotaxis to rapidly metabolizable substrates was not analyzed in the ptr mutant, and Ptr homologs are present in bacteria that lack a photosynthetic respiratory system, for example the McpB protein in Rhizobium leguminosarum (66). Until further evidence is obtained, it is reasonable to assume that Ptr is a general energy taxis transducer. Ptr has the same membrane topology as Tsr of E. coli, and no prosthetic group was experimentally proven to be associated with either transducer. Therefore, the sensing mechanism for Tsr and Ptr may in fact be the same and requires further characterization.
Receptors in R. sphaeroides.
R. sphaeroides responds to a variety of electron acceptors, and at least some chemoattractants, especially sugars (36), appear to signal via energy taxis. Several chemoreceptors have been identified in R. sphaeroides. Two of them, TlpA and TlpB, are cytoplasmic and were proposed to sense cytoplasmic metabolic intermediates (9). Deletion of TlpA results in a reduction of chemotaxis toward all compounds, but only under aerobic conditions, supporting the idea that TlpA senses a change in a metabolite intermediate (64). Cytoplasmic receptors may not necessarily be metabolism dependent or energy taxis sensors, but they certainly represent a strategy where chemical sensing occurs intracellularly.
Rhizobial chemoreceptors.
Two cytoplasmic chemoreceptors in S. meliloti, TlpA and TlpB, were proposed to be the sensors monitoring internal stimuli that reflect the metabolic or energy state of a cell (9), similar to the cytoplasmic receptors in R. sphaeroides. Several chemoreceptors were also identified in R. leguminosarum (66). However, mutating most chemoreceptor genes did not result in a clear nonchemotactic phenotype. Only deficiency in the McpB chemoreceptor resulted in a reduction of chemotaxis toward all attractants tested and in a reduced ability to infect the host plant, illustrating the importance of chemotaxis in the plant-microbe symbiosis (66). However, as in most other species, the nature of the signal sensed by the chemoreceptor remains to be elucidated.
CetA and CetB in C. jejuni.
Two proteins, CetA and CetB (Campylobacter energy taxis), show significant homology with the PAS domain and conserved signaling chemoreceptor domain of the E. coli energy-taxis receptor Aer, respectively. cetA and cetB mutants of C. jejuni are impaired in chemotaxis toward pyruvate and fumarate. Pyruvate is rapidly metabolized by C. jejuni in the presence of oxygen as a terminal electron acceptor, and fumarate is the preferred terminal electron acceptor even in the presence of oxygen (33). Thus, C. jejuni is able to sense its intracellular energy status by using a bipartite system consisting of CetA and CetB in a manner analogous to Aer-mediated energy taxis in E. coli and P. putida (13, 33, 43).
Specialized receptors that sense energy-related parameters.
Some chemoreceptors are apparent sensors of energy-related parameters, such as oxygen and light. However, they employ mechanisms of direct sensing and in this sense resemble classical ligand-binding transmembrane chemoreceptors in E. coli. A novel chemoreceptor that senses an energy-related parameter, the intracellular concentration of oxygen, has been identified recently in Halobacterium salinarum and B. subtilis (35). The HemAT transducer governs the aerotactic response in both species and contains heme B, which directly binds oxygen similarly to hemoglobin and some heme-based sensors and differently from sensors interacting with the electron transport system. Similarly, a receptor that responds directly to light, and not to light-mediated electron transport, has been identified in the cyanobacterium Synechocystis sp. (65, 67). In its N-terminal region, the Slr0041 receptor contains two photosensory GAF domains (named GAF for cGMP-phosphodiesterases, adenylate cyclases, and FhlA) (67) that are known phototransducing elements in microorganisms, plants, and animals (6). Conserved attachment sites for a light-responsive tetrapyrrole chromophore are present in both GAF domains of Slr0041 (67), and a mutation in the corresponding gene abolishes phototaxis to red/far red light that excites tetrapyrrole (65). Thus, in aerotaxis and phototaxis there is also more than one way to sense the stimuli.
CONCLUSIONS
Measuring changes in the electron transport system is the most effective way to monitor energy metabolism in general and concentrations of available metabolizable substrates in particular. In energy taxis, the strength of the response depends on the presence of both a substrate and an appropriate electron acceptor (Fig. 1). Should the optimal concentration of a substrate decrease, the cells will execute “chemotaxis,” moving up the chemical gradient. Should the optimal concentration of an electron acceptor (for example, oxygen) decrease, the cells will execute “aerotaxis,” moving up the oxygen gradient. Thus, via energy taxis the cells seek optimal balance between the oxidizable substrate and oxygen in order to generate maximal energy.
In E. coli, two receptors, Aer and Tsr, monitor intracellular energy levels, whereas receptors utilizing the ligand-binding mechanism are not redundant. The presence of two receptors monitoring different parameters of energy status provides the cell with a more flexible energy-sensing mechanism and therefore an obvious advantage. The lack of a readily measurable phenotype upon mutation of receptor genes in some bacteria may be due to the presence of redundant energy taxis receptors. Indeed, if such receptors measure an energy-related parameter, then a diminished response to all attractants sensed via energy taxis or no phenotype at all is expected, depending on whether the energy-related parameter measured by the receptor is strong enough under the conditions tested. For example, the reduction in chemotaxis in the cbb3 mutant of A. brasilense can only be observed under microaerobic conditions where the oxidase is active (4). Revealing such subtle differences requires sensitive methods, such as the temporal gradient assay.
Three major classes of chemical sensors are widespread in microorganisms: chemotaxis transducers (68), histidine kinases (34, 54), and adenylate cyclases/phosphodiesterases (16, 18, 45). All three classes share several known sensor domains, such as PAS (46, 60, 72), Cache/ESENS (5, 68), and GAF (6). All of these sensor elements are also present in eukaryotic signal transduction networks. Therefore, fundamental principals of chemical sensing appear to be well conserved throughout the tree of life. Sequences of thousands of various sensor proteins are now available in public databases. Data mining and, most importantly, careful experiments will soon show us even more ways microorganisms can sense chemicals in the environment that are crucial for their growth and survival.
ACKNOWLEDGMENTS
We thank Quinhong Ma, Mark Johnson, and Barry Taylor for sharing unpublished data and Carrie Harwood for providing reprints and preprints related to this review.
The work from the authors' laboratory was supported in part by grant 96-35305-3795 from the U.S. Department of Agriculture and by startup funds from Georgia Institute of Technology (to I.B.Z.).
REFERENCES
- 1.Adler J. Chemoreceptors in bacteria. Science. 1969;166:1588–1597. doi: 10.1126/science.166.3913.1588. [DOI] [PubMed] [Google Scholar]
- 2.Adler J, Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci USA. 1974;71:2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandre G, Bally R, Taylor B L, Zhulin I B. Loss of cytochrome c oxidase and acquisition of resistance to extracellular quinones in laccase-positive Azospirillum lipoferum. J Bacteriol. 1999;181:6730–6738. doi: 10.1128/jb.181.21.6730-6738.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexandre G, Greer S E, Zhulin I B. Energy taxis is the dominant behavior in Azospirillum brasilense. J Bacteriol. 2000;182:6042–6048. doi: 10.1128/jb.182.21.6042-6048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anantharaman V, Aravind L. Cache—a signaling domain common to animal Ca(2+)-channel subunits and a class of prokaryotic chemotaxis receptors. Trends Biochem Sci. 2000;25:535–537. doi: 10.1016/s0968-0004(00)01672-8. [DOI] [PubMed] [Google Scholar]
- 6.Aravind L, Ponting C P. The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem Sci. 1997;22:458–459. doi: 10.1016/s0968-0004(97)01148-1. [DOI] [PubMed] [Google Scholar]
- 7.Aravind L, Ponting C P. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol Lett. 1999;176:111–116. doi: 10.1111/j.1574-6968.1999.tb13650.x. [DOI] [PubMed] [Google Scholar]
- 8.Armitage J P. Behavioural responses of bacteria to light and oxygen. Arch Microbiol. 1997;168:249–261. doi: 10.1007/s002030050496. [DOI] [PubMed] [Google Scholar]
- 9.Armitage J P, Schmitt R. Bacterial chemotaxis: Rhodobacter sphaeroides and Sinorhizobium meliloti—variations on a theme? Microbiology. 1997;143:3671–3682. doi: 10.1099/00221287-143-12-3671. [DOI] [PubMed] [Google Scholar]
- 10.Armitage J P. Bacterial tactic responses. Adv Microb Physiol. 1999;41:229–289. doi: 10.1016/s0065-2911(08)60168-x. [DOI] [PubMed] [Google Scholar]
- 11.Bespalov V A, Zhulin I B, Taylor B L. Behavioral responses of Escherichia coli to changes in redox potential. Proc Natl Acad Sci USA. 1996;93:10084–10089. doi: 10.1073/pnas.93.19.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bibikov S I, Barnes L A, Gitin Y, Parkinson J S. Domain organization and flavin adenine dinucleotide-binding determinants in the aerotaxis signal transducer Aer of Escherichia coli. Proc Natl Acad Sci USA. 2000;97:5830–5835. doi: 10.1073/pnas.100118697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bibikov S I, Biran R, Rudd K E, Parkinson S J. A signal transducer for aerotaxis in Escherichia coli. J Bacteriol. 1997;179:4075–4079. doi: 10.1128/jb.179.12.4075-4079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bray D, Levin M D, Morton-Firth C J. Receptor clustering as a cellular mechanism to control sensitivity. Nature. 1998;393:85–88. doi: 10.1038/30018. [DOI] [PubMed] [Google Scholar]
- 15.Bren A, Eisenbach M. How signals are heard during bacterial chemotaxis: protein-protein interactions in sensory signal propagation. J Bacteriol. 2000;182:6865–6873. doi: 10.1128/jb.182.24.6865-6873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang A L, Tuckerman J R, Gonzalez G, Mayer R, Weinhouse H, Volman G, Amikam D, Benziman M, Gilles-Gonzalez M-A. Phosphodiesterase A1, a regulator of cellulose synthesis in Acetobacter xylinum, is a heme-based sensor. Biochemistry. 2001;40:3420–3426. doi: 10.1021/bi0100236. [DOI] [PubMed] [Google Scholar]
- 17.Clancy M, Madill K A, Wood J M. Genetic and biochemical requirements for chemotaxis to l-proline in Escherichia coli. J Bacteriol. 1981;146:902–906. doi: 10.1128/jb.146.3.902-906.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delgado-Nixon V M, Gonzalez G, Gilles-Gonzalez M A. Dos, a heme-binding PAS protein from Escherichia coli, is a direct oxygen sensor. Biochemistry. 2000;39:2685–2691. doi: 10.1021/bi991911s. [DOI] [PubMed] [Google Scholar]
- 19.Dharmatilake A J, Bauer W D. Chemotaxis of Rhizobium meliloti towards nodulation gene-inducible compounds from alfalfa roots. Appl Enviorn Microbiol. 1992;58:1153–1158. doi: 10.1128/aem.58.4.1153-1158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ditty J L, Harwood C S. Conserved cytoplasmic loops are important for both the transport and chemotaxis functions of PcaK, a protein from Pseudomonas putida with 12 membrane-spanning regions. J Bacteriol. 1999;181:5068–5074. doi: 10.1128/jb.181.16.5068-5074.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falke J J, Hazelbauer G L. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem Sci. 2001;26:257–265. doi: 10.1016/s0968-0004(00)01770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu R, Wall J D, Voordouw G. DcrA, a c-type heme-containing methyl-accepting protein from Desulfovibrio vulgaris Hildenborough, senses the oxygen concentration or redox potential of the environment. J Bacteriol. 1994;176:344–350. doi: 10.1128/jb.176.2.344-350.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrity L F, Schiel S L, Merrill R, Reizer J, Saier M H, Jr, Ordal G W. Unique regulation of carbohydrate chemotaxis in Bacillus subtilis by the phosphoenolpyruvate-dependent phosphotransferase system and the methyl-accepting chemotaxis protein McpC. J Bacteriol. 1998;180:4475–4480. doi: 10.1128/jb.180.17.4475-4480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauden D E, Armitage J P. Electron-transport dependent taxis in Rhodobacter sphaeroides. J Bacteriol. 1995;177:5853–5859. doi: 10.1128/jb.177.20.5853-5859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gestwicki J E, Strong L E, Kiessling L L. Tuning chemotactic responses with synthetic multivalent ligands. Chem Biol. 2000;7:583–591. doi: 10.1016/s1074-5521(00)00002-8. [DOI] [PubMed] [Google Scholar]
- 26.Glagolev A N. Reception of the energy level in bacterial taxis. J Theor Biol. 1980;82:171–185. doi: 10.1016/0022-5193(80)90097-1. [DOI] [PubMed] [Google Scholar]
- 27.Götz R, Limmer N, Ober K, Schmitt R. Motility and chemotaxis in two strains of Rhizobium with complex flagella. J Gen Microbiol. 1982;128:789–798. [Google Scholar]
- 28.Goulbourne E A, Jr, Greenberg E P. Chemotaxis of Spirochaeta aurantia: involvement of membrane potential in chemosensory signal transduction. J Bacteriol. 1981;148:837–844. doi: 10.1128/jb.148.3.837-844.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goulbourne E A, Jr, Greenberg E P. A voltage clamp inhibits chemotaxis of Spirochaeta aurantia. J Bacteriol. 1983;153:916–920. doi: 10.1128/jb.153.2.916-920.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grishanin R N, Chalmina I I, Zhulin I B. Behaviour of Azospirillum brasilense in a spatial gradient of oxygen and in a “redox” gradient of artificial electron acceptor. J Gen Microbiol. 1991;137:2781–2785. [Google Scholar]
- 31.Grishanin R N, Gauden D E, Armitage J P. Photoresponses in Rhodobacter sphaeroides: role of photosynthetic electron transport. J Bacteriol. 1997;179:24–30. doi: 10.1128/jb.179.1.24-30.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hazelbauer G L, Harayama S. Sensory transduction in bacterial chemotaxis. Int Rev Cytol. 1983;81:33–70. doi: 10.1016/s0074-7696(08)62334-7. [DOI] [PubMed] [Google Scholar]
- 33.Hendrixson D R, Akerley B J, DiRita V J. Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility. Mol Microbiol. 2001;40:214–224. doi: 10.1046/j.1365-2958.2001.02376.x. [DOI] [PubMed] [Google Scholar]
- 34.Hoch J A. Two-component and phosphorelay signal transduction. Curr Opin Microbiol. 2000;3:165–170. doi: 10.1016/s1369-5274(00)00070-9. [DOI] [PubMed] [Google Scholar]
- 35.Hou S, Larsen R W, Boudko D, Riley C W, Karatan E, Zimmer M, Ordal G W, Alam M. Myoglobin-like aerotaxis transducers in Archaea and Bacteria. Nature. 2000;403:540–544. doi: 10.1038/35000570. [DOI] [PubMed] [Google Scholar]
- 36.Jeziore-Sassoon Y, Hamblin P A, Bootle-Wilbraham C A, Poole P S, Armitage J P. Metabolism is required for chemotaxis to sugars in Rhodobacter sphaeroides. Microbiology. 1998;144:229–239. doi: 10.1099/00221287-144-1-229. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Z Y, Bauer C E. Component of the Rhodospirillum centenum photosensory apparatus with structural and functional similarity to methyl-accepting chemotaxis protein chemoreceptors. J Bacteriol. 2001;183:171–177. doi: 10.1128/JB.183.1.171-177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kihara M, Macnab R M. Cytoplasmic pH mediates pH taxis and weak-acid repellent taxis of bacteria. J Bacteriol. 1981;145:1209–1221. doi: 10.1128/jb.145.3.1209-1221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levit M N, Stock J B. pH sensing in bacterial chemotaxis. Novartis Found Symp. 1999;221:38–50. doi: 10.1002/9780470515631.ch4. [DOI] [PubMed] [Google Scholar]
- 40.Lux R, Jahreis K, Bettenbrock K, Parkinson J S, Lengeler J W. Coupling the phosphotransferase system and the methyl-accepting chemotaxis protein-dependent chemotaxis signaling pathways of Escherichia coli. Proc Natl Acad Sci USA. 1995;92:11583–11587. doi: 10.1073/pnas.92.25.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lux R, Munasinghe V R, Castellano F, Lengeler J W, Corrie J E, Khan S. Elucidation of a PTS-carbohydrate chemotactic signal pathway in Escherichia coli using a time-resolved behavioral assay. Mol Biol Cell. 1999;10:1133–1146. doi: 10.1091/mbc.10.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchal K, Sun J, Keijers V, Haaker H, Vanderleyden J. A cytochrome cbb3 (cytochrome c) terminal oxidase in Azospirillum brasilense Sp7 supports microaerobic growth. J Bacteriol. 1998;180:5689–5696. doi: 10.1128/jb.180.21.5689-5696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nichols N N, Harwood C S. An aerotaxis transducer gene from Pseudomonas putida. FEMS Microbiol Lett. 2000;182:177–183. doi: 10.1111/j.1574-6968.2000.tb08893.x. [DOI] [PubMed] [Google Scholar]
- 44.Ordal G W, Villani D P, Nicholas R A, Hamel F G. Independence of proline chemotaxis and transport in Bacillus subtilis. J Biol Chem. 1978;253:4916–4919. [PubMed] [Google Scholar]
- 45.Pei J, Grishin N V. GGDEF domain is homologous to adenylyl cyclase. Proteins. 2001;42:210–216. doi: 10.1002/1097-0134(20010201)42:2<210::aid-prot80>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 46.Ponting C P, Aravind L. PAS: a multifunctional domain family comes to light. Curr Biol. 1997;7:R674–R677. doi: 10.1016/s0960-9822(06)00352-6. [DOI] [PubMed] [Google Scholar]
- 47.Rebbapragada A, Johnson M S, Harding G P, Zuccarelli A J, Fletcher H M, Zhulin I B, Taylor B L. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and tranduce oxygen, redox, and energy signals for Escherichia coli behavior. Proc Natl Acad Sci USA. 1997;94:10541–10546. doi: 10.1073/pnas.94.20.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Repaske D R, Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol. 1981;145:1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Repik A V, Rebbapragada A, Johnson M S, Haznedar J O, Zhulin I B, Taylor B L. PAS domain residues involved in signal transduction by the Aer redox sensor of Escherichia coli. Mol Microbiol. 2000;36:806–816. doi: 10.1046/j.1365-2958.2000.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson J B, Bauer W D. Relationships between C4 dicarboxylic acid transport and chemotaxis in Rhizobium meliloti. J Bacteriol. 1993;175:2284–2291. doi: 10.1128/jb.175.8.2284-2291.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuenemann T A, Delgado-Nixon V M, Dalbey R E. Direct evidence that the proton motive force inhibits membrane translocation of positively charged residues within membrane proteins. J Biol Chem. 1999;274:6855–6864. doi: 10.1074/jbc.274.11.6855. [DOI] [PubMed] [Google Scholar]
- 52.Shioi J, Tribhuwan R C, Berg S T, Taylor B L. Signal transduction in chemotaxis to oxygen in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1988;170:5507–5511. doi: 10.1128/jb.170.12.5507-5511.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slonczewski J L, Macnab R M, Alger J R, Castle A M. Effects of pH and repellent tactic stimuli on protein methylation levels in Escherichia coli. J Bacteriol. 1982;152:384–399. doi: 10.1128/jb.152.1.384-399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stock A M, Robinson V L, Goudreau P N. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 55.Stock J, Levit M. Signal transduction: hair brains in bacterial chemotaxis. Curr Biol. 2000;10:R11–R14. doi: 10.1016/s0960-9822(99)00248-1. [DOI] [PubMed] [Google Scholar]
- 56.Stock J B, Surette M G. Chemotaxis. In: Neidhardt F C, Curtiss III R, Ingraham J I, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1103–1129. [Google Scholar]
- 57.Taylor B L. Role of proton motive force in sensory transduction in bacteria. Annu Rev Microbiol. 1983;37:551–573. doi: 10.1146/annurev.mi.37.100183.003003. [DOI] [PubMed] [Google Scholar]
- 58.Taylor B L, Miller J B, Warrick H M, Koshland D E., Jr Electron acceptor taxis and blue light effect on bacterial chemotaxis. J Bacteriol. 1979;140:567–573. doi: 10.1128/jb.140.2.567-573.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor B L, Zhulin I B. In search of higher energy: metabolism-dependent behaviour in bacteria. Mol Microbiol. 1998;28:683–690. doi: 10.1046/j.1365-2958.1998.00835.x. [DOI] [PubMed] [Google Scholar]
- 60.Taylor B L, Zhulin I B. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor B L, Zhulin I B, Johnson M S. Aerotaxis and other energy-sensing behavior in bacteria. Annu Rev Microbiol. 1999;53:103–128. doi: 10.1146/annurev.micro.53.1.103. [DOI] [PubMed] [Google Scholar]
- 62.Terracciano J S, Canale-Parola E. Enhancement of chemotaxis in Spirochaeta aurantia grown under conditions of nutrient limitation. J Bacteriol. 1984;159:173–178. doi: 10.1128/jb.159.1.173-178.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trumpower B L, Gennis R B. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu Rev Biochem. 1994;63:675–716. doi: 10.1146/annurev.bi.63.070194.003331. [DOI] [PubMed] [Google Scholar]
- 64.Ward M J, Harrison D M, Ebner M J, Armitage J P. Identification of a methyl-accepting chemotaxis protein in Rhodobacter sphaeroides. Mol Microbiol. 1995;18:115–121. doi: 10.1111/j.1365-2958.1995.mmi_18010115.x. [DOI] [PubMed] [Google Scholar]
- 65.Yoshihara S, Suzuki F, Fujita H, Geng X X, Ikeuchi M. Novel putative photoreceptor and regulatory genes required for the positive phototactic movement of the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2000;41:1299–1304. doi: 10.1093/pcp/pce010. [DOI] [PubMed] [Google Scholar]
- 66.Yost C K, Rochepeau P, Hynes M F. Rhizobium leguminosarum contains a group of genes that appear to code for methyl-accepting chemotaxis proteins. Microbiology. 1998;144:1945–1956. doi: 10.1099/00221287-144-7-1945. [DOI] [PubMed] [Google Scholar]
- 67.Zhulin I B. A novel phototaxis receptor hidden in the cyanobacterial genome. J Mol Microbiol Biotechnol. 2000;2:491–493. [PubMed] [Google Scholar]
- 68.Zhulin I B. The superfamily of chemotaxis transducers: from physiology to genomics and back. Adv Microb Physiol. 2001;45:157–198. doi: 10.1016/s0065-2911(01)45004-1. [DOI] [PubMed] [Google Scholar]
- 69.Zhulin I B, Armitage J P. Motility, chemokinesis, and methylation-independent chemotaxis in Azospirillum brasilense. J Bacteriol. 1993;175:952–958. doi: 10.1128/jb.175.4.952-958.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhulin I B, Bespalov V A, Johnson M S, Taylor B L. Oxygen taxis and proton motive force in Azospirillum brasilense. J Bacteriol. 1996;178:5199–5204. doi: 10.1128/jb.178.17.5199-5204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhulin I B, Rowsell E H, Johnson M S, Taylor B L. Glycerol elicits energy taxis of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1997;179:3196–3201. doi: 10.1128/jb.179.10.3196-3201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhulin I B, Taylor B L, Dixon R. PAS domain S-boxes in Archaea, Bacteria and sensors for oxygen and redox. Trends Biochem Sci. 1997;22:331–333. doi: 10.1016/s0968-0004(97)01110-9. [DOI] [PubMed] [Google Scholar]