Abstract
Transapical transcatheter aortic valve implantation (TA-TAVI) is generally considered to be associated with increased morbidity and mortality compared with transfemoral transcatheter aortic valve implantation TAVI (TF-TAVI). We aimed to compare different patient risk profiles, access-related complications, and long-term survival using inverse probability treatment weighting. This is a retrospective, single-center analysis of 925 consecutive patients with aortic valve stenosis undergoing TF-TAVI (n = 802) or TA-TAVI (n = 123) at the University Hospital Basel, Switzerland, as a single procedure between September 2011 and August 2020. Baseline characteristics revealed a higher perioperative risk as reflected in the EuroSCORE II (geometric mean 2.3 (95% confidence interval (CI) 2.2 to 2.4) vs. 3.7 (CI 3.1 to 4.5); before inverse probability of treatment weighting (IPTW) p < 0.001) in the transfemoral than in the transapical group, respectively. After 30 days, TF-TAVI patients had a higher incidence of any bleeding than TA-TAVI patients (TF-TAVI n = 146 vs. TA-TAVI n = 15; weighted hazard ratio (HR) 0.52 (0.29 to 0.95); p = 0.032). After 5 years, all-cause mortality did not differ between the two groups (TF-TAVI n = 162 vs. TA-TAVI n = 45; weighted HR 1.31, (0.92 to 1.88); p = 0.138). With regard to our data, we could demonstrate, despite a higher perioperative risk, the short- and long-term safety and efficacy of the transapical approach for TAVI therapies. Though at higher perioperative risk, transapically treated patients suffered from less bleeding or vascular complications than transfemorally treated patients. It is of utmost interest that 5-year mortality did not differ between the groups.
Keywords: aortic valve replacement, transcatheter, mortality
1. Introduction
Aortic valve stenosis is the most common valvular heart disease in Europe and North America and its prevalence is steadily increasing due to ageing of the population. Most patients with symptomatic aortic valve stenosis require intervention [1,2]. At an advanced stage of the disease, an intervention is inevitable to ensure survival and improve quality of life. In the past, surgical aortic valve replacement (SAVR) was the gold standard. However, since the first transcatheter aortic valve implantation (TAVI) via an anterograde transseptal approach in 2002 [3], TAVI has since become an established therapy and is now the default therapy in high-risk patients and serves as an alternative to SAVR in intermediate and low-risk patients [4]. In parallel, continuous development in the management and techniques of SAVR has constantly improved the outcome of patients with severe aortic valve stenosis. Nevertheless, the PARTNER trials have proven the efficacy and safety in high- and moderate-risk patients receiving TAVI without the need for cardiopulmonary bypass [5,6,7,8].
Due to continuous improvements in the size and flexibility of delivery systems, the small skin incision and vascular closure devices, as well as increased experience of the interventional cardiologist, transfemoral (TF) access has become the default access route for TAVI [7,9,10]. However, some patients cannot be managed by TF access due to severe calcifications, small vessel caliber, or tortuosity of the peripheral arteries or the aorta. Therefore, several access alternatives have been developed to enable minimally invasive transcatheter treatment for aortic stenosis whenever TF is unfavorable. In 2005, the first case of transapical (TA) TAVI without cardiopulmonary bypass was performed. [9] Since then, the TA approach rapidly developed as the most frequently used alternative access route for patients with unsuitable vascular anatomy [10,11,12].
Patients who underwent TA-TAVIs have been reported to have high rates of 30-day and long-term mortality [13,14]. This observation is aggravated by the high-risk profile of TA-TAVI patients, because they suffer more commonly from coronary artery disease (CAD), peripheral arterial disease (PAD), renal dysfunction, prior cardiac surgery or coronary artery bypass surgery (CABG), and a porcelain aorta [13,14]. Therefore, it is unclear to what extent this increased mortality merely reflects the sicker patient population with an intrinsic higher TA periprocedural risk, or the more invasive TA procedure itself.
Although alternative access routes have been explored, TA currently is the most frequent alternative access route at the University Hospital Basel, a tertiary care center in cardiovascular medicine. Before each intervention, the patient’s profile is evaluated depending on the risk factors in an interdisciplinary heart team meeting involving cardiac surgeons, interventional cardiologists, anesthesiologists, as well as radiologists. Together, we seek agreement on the best approach based on international guidelines and the patients’ needs and profiles. Even though a thorough evaluation of each patient is done, little is known about the long-term survival rate between TF- and TA-TAVI approaches as well as the long-term complications. With this analysis, we aim to investigate risk factors for outcomes and compare the 5-year survival rate between the TF-TAVI and TA-TAVI approaches using propensity modeling to adjust for baseline variables.
2. Material and Methods
All data for this analysis were assessed for the SwissTAVI Registry (date of access 19 October 2020), a national, prospective, multi-center registry in Switzerland focusing on improving the management of patients with aortic valve disease (clinicaltrials.gov NCT01368250). The study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees (ethics committee in Basel (reference number 305/11; 2011; since then permission through the ethics committee Bern, project ID: 2021-01738). The SwissTAVI Registry uses for data collection a web-based database (www.swisstavi.ch) with standardized case report forms at all centers performing TAVI in Switzerland. The first patient receiving the TAVI procedure was recruited in September 2011. The data of all participating centers are collected at baseline and during follow up, which is performed according to a prespecified protocol. All clinical events are prospectively collected and adjudicated by a dedicated clinical event committee according to the standardized criteria of the Valve Academic Research Consortium 2 (VARC-2) [15]. The study protocol of the SwissTAVI registry was approved by the institutional review board of all participating sites and by the local cantonal ethics committee. Written informed consent was obtained from all patients. The authors designed the study, gathered, and analyzed the data.
2.1. Study Population
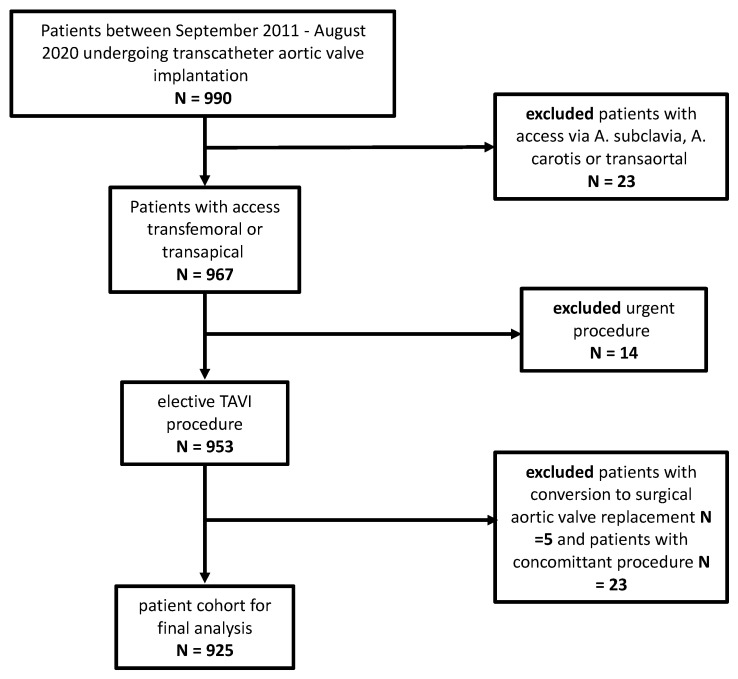
Data of 990 consecutive patients with aortic valve stenosis undergoing TAVI procedures at the University Hospital of Basel, Switzerland, between September 2011 and August 2020, were extracted from the SwissTAVI Registry. Patients with concomitant procedures or other vascular access (via subclavian artery or transaortic) were excluded, leaving a study sample of 925 patients. (Figure 1) The most common indication in both TAVI groups was a severe stenosis of the native aortic valve, while 22 patients (3%) in the TF-TAVI group suffered from severe stenosis of a biological aortic prosthesis, and one patient from a combined severe regurgitation and stenosis of a biological aortic valve prosthesis. Among the TA-TAVI patients, one patient each had a severe stenosis of a biological valve, a severe regurgitation of a biological or a degenerative previous TAVI prosthesis.
Figure 1.
Flowchart.
2.2. Pre-Interventional Diagnostics
All patients with severe aortic stenosis considered for an intervention underwent coronary angiography, transthoracic echocardiography, and three-dimensional cardiovascular computer tomography (CT). After evaluation, the patient’s profile was evaluated depending on the risk factors in an interdisciplinary heart team meeting involving cardiac surgeons, interventional cardiologists, anesthesiologists, as well as radiologists. The CT scan was carefully analyzed using the 3-mensio planning software (3mensio Medical Imaging, Bilthoven, The Netherlands) to determine valvular specifications, such as annular size, degree of valve calcification, distance to coronary ostia, and implantation angle. Vascular entities were analyzed with regard to diameter and characteristics of the iliofemoral and aortic axis. In case of a small peripheral vessel access, severe annular calcification, abdominal aortic aneurysm or vessel tortuosity, TA was considered to be the best option. The TAVI procedure was performed in the cardiac catheterization lab (TF) or hybrid operating room (TA) with an interdisciplinary team of cardiac surgeons, interventional cardiologists, anesthesiologists supported by scrub nurses and radiological technologists.
2.3. TAVI Devices
Over the years, several different devices with different generations were implanted in our center. All percentage distributions of implanted TAVIs are listed in Table S1 in Supplementary Materials. The most common devices for TF-TAVI were St. Jude Medical PorticoTM (22%) (St. Jude Medical, Saint Paul, MN, USA), Edwards SapienTM 3 (19%), SapienTM XT (8.6%), (Edwards Lifesciences, Irvine, CA, USA), Medtronic CoreValve (8.6%), Boston Scientific LotusTM (8.5%), Medtronic Evolut RTM (7.5%), Medtronic Evolut ProTM (4.7%) (Medtronic Inc., Minneapolis, MN, USA), and Lotus EdgeTM (2.4%) (Boston Scientific, Marlborough, MA, USA)
In case of TA-TAVI, the most commonly used valves were the JenaValve (46%) (JenaValve Technology, Inc., Munich, Germany) and the Edwards SapienTM 3 (43%) (Edwards Lifesciences, Irvine, CA, USA).
2.4. Study End Points
The primary endpoint of this study was all-cause mortality at 5 years in patients undergoing either TF- or TA-TAVI. The secondary endpoints included outcomes at 30 days according to the VARC-2 definitions, which include major adverse cardiac and cerebrovascular events such as death, myocardial infarction, stroke, bleeding, and aortic valve re-intervention [15].
2.5. Statistical Analysis
To investigate to what degree the outcome might be associated with the treatment, we used propensity modeling to achieve balanced treatment groups with respect to risk factors, given the observational origin of the data. We used inverse probability of treatment weighting (IPTW) to calculate average treatment effects. The propensity score with the kernel density (Figure S1 in Supplementary Materials) and the standardized differences before and after IPTW (Figure S2 in Supplementary Materials) are mentioned in the Supplementary Materials. We included age, sex, Society of Thoracic Surgeons (STS) score (after log transformation), chronic obstructive pulmonary disease (COPD), CAD, PAD, atrial fibrillation, hypertension, body surface area, and diabetes as covariates into the propensity model. We censored treatment weights exceeding the 1st and 99th percentiles [16] and calculated standardized differences for each variable to assess residual imbalances between the groups, using the formulae proposed in Austin et al. [17]. Standardized differences of ±0.1 standard deviation units or less are considered to indicate irrelevant difference, ±0.2 might still be considered acceptable. We analyzed our primary outcome survival in a time-to-event manner after IPTW, including a 30-day landmark to balance risk differences related to the procedure. We plotted Kaplan–Meier curves for visualization and calculated hazard ratios using Cox regression, with results are referred to as “weighted hazard ratio”. Proportional hazard assumptions were checked using Schoenfeld residuals.
Continuous variables were presented as mean ± standard deviation if normally distributed, or as geometric mean if distribution was skewed, with standard deviations back transformed from the log scale. Corresponding p-values were calculated using linear regression on the variable or on the log transformed variable, respectively. Categories were presented as numbers and percentages, p-values were calculated using logistic regression for binary variables or multinomial regression else. After IPTW, all p values and confidence intervals were based on robust standard deviations. Statistical analyses were performed using Stata 16 (StataCorp LLC, College Station, TX, USA).
3. Results
3.1. Patient’s Characteristics
From September 2011 to August 2020, a total of 990 documented patients were eligible for this analysis. In addition, 71% of the patients were octogenarians (mean 82 ± 7 years). A total of 925 patients received either a TF-TAVI or TA-TAVI (TF-TAVI: n = 802 (86.7%) vs. TA-TAVI: n = 123 (13.3%)) as a single procedure. Patients with a TA approach had a higher prevalence of COPD, stroke, history of cardiac surgery, previous CABG, PAD, and suffered more often from arterial hypertension, syncope, as well as diabetes mellitus (Table 1), whereas patients with TF-TAVI more often had previous valve surgery and atrial fibrillation. Overall, TA-TAVI patients had a higher operative risk as reflected in the EuroSCORE II value (TF-TAVI: geometric mean 2.3 (95% confidence interval (CI) 2.2 to 2.4) vs. TA-TAVI: 3.7 (CI 3.1 to 4.5); before inverse probability of treatment weighting (IPTW) p < 0.001) and STS score (TF-TAVI: 4.0 (CI 3.8 to 4.1) vs. TA-TAVI 5.4 (CI 4.8 to 6.0); before IPTW p < 0.001). Overall, no significant difference in preoperative anti-platelet and anti-coagulation therapy was seen, except for Aspirin, with a higher medication in the TA-TAVI group TF_TAVI: n = 433 (54%) vs. TA-TAVI n = 90 (73%); p < 0.001). (Table S3 in Supplementary Materials).
Table 1.
Baseline characteristics before and after IPTW.
| Before IPTW | After IPTW | |||||||
|---|---|---|---|---|---|---|---|---|
| TF-TAVI n = 802 |
TA-TAVI n = 123 |
SD | p | TF-TAVI n = 802 |
TA-TAVI n = 123 |
SD | p | |
| Age (years) | 82 ± 6 | 82 ± 7 | −0.110 | 0.235 | 82 ± 6 | 82 ± 6 | 0.008 | 0.933 |
| Body mass index (kg/m2) | 27 ± 5 | 26 ± 5 | −0.242 | 0.014 | 27 ± 5 | 27 ± 7 | 0.012 | 0.908 |
| Euro SCORE II Value | 2.3 (2.2 to 2.4) | 3.7 (3.1 to 4.5) | 0.641 | <0.001 | 2.4 (2.2 to 2.5) | 3.3 (2.7 to 4.1) | 0.487 | 0.047 |
| STS risk score | 4.0 (3.8 to 4.1) | 5.4 (4.8 to 6.0) | 0.689 | <0.001 | 4.1 (3.9 to 4.3) | 4.6 (4.0 to 5.3) | 0.243 | 0.243 |
| LVEF (%) | 52 (51 to 53) | 53 (50 to 55) | 0.764 | 0.712 | 51 (50 to 53) | 52 (49 to 56) | 0.735 | 0.678 |
| Female sex – no. (%) | 432 (54%) | 50 (41%) | 0.267 | 0.007 | 416 (52%) | 55 (44%) | 0.150 | 0.178 |
| Diabetes mellitus – no. (%) | 236 (29%) | 34 (28%) | 0.040 | 0.685 | 236 (29%) | 35 (29%) | 0.015 | 0.892 |
| Arterial hypertension – no. (%) | 649 (81%) | 106 (86%) | −0.142 | 0.163 | 656 (82%) | 107 (87%) | −0.152 | 0.172 |
| Dyslipidemia – no. (%) | 467 (58%) | 84 (68%) | −0.210 | 0.035 | 473 (59%) | 80 (65%) | −0.132 | 0.243 |
| COPD – no. (%) | 71 (9%) | 32 (26%) | −0.464 | <0.001 | 88 (11%) | 20 (16%) | −0.150 | 0.117 |
| History of Stroke – no. (%) | 102 (13%) | 17 (14%) | −0.033 | 0.734 | 105 (13%) | 18 (15%) | −0.054 | 0.620 |
| Previous aortic valvuloplasty – no. (%) | 29 (4%) | 3 (2%) | 0.069 | 0.509 | 30 (4%) | 3 (2%) | 0.073 | 0.547 |
| Coronary artery disease – no. (%) | 440 (55%) | 91 (74%) | −0.408 | <0.001 | 461 (57%) | 83 (67%) | −0.204 | 0.081 |
| History of PCI – no. (%) | 276 (34%) | 60 (49%) | −0.295 | 0.002 | 291 (36%) | 52 (42%) | −0.115 | 0.288 |
| History of MI – no. (%) | 123 (15%) | 26 (21%) | −0.151 | 0.105 | 131 (16%) | 22 (18%) | −0.037 | 0.723 |
| Atrial fibrillation – no. (%) | 166 (21%) | 13 (11%) | 0.282 | 0.010 | 155 (19%) | 14 (12%) | 0.211 | 0.082 |
| Peripheral artery disease | 103 (13%) | 52 (42%) | −0.698 | <0.001 | 133 (17%) | 29 (23%) | −0.165 | 0.072 |
| Previous cardiac surgery | 84 (10%) | 23 (19%) | −0.235 | 0.009 | 91 (11%) | 19 (15%) | −0.113 | 0.257 |
| Previous CABG – no. (%) | 64 (8%) | 23 (19%) | −0.319 | <0.001 | 71 (9%) | 19 (15%) | −0.196 | 0.043 |
| Previous valve surgery – no. (%) | 31 (4%) | 3 (2%) | 0.082 | 0.438 | 32 (4%) | 1 (1%) | 0.178 | 0.078 |
| Syncope – no. (%) | 63 (8%) | 17 (14%) | −0.195 | 0.029 | 63 (8%) | 20 (16%) | −0.256 | 0.012 |
| NYHA (III or IV) – no. (%) | 429 (54%) | 75 (61%) | −0.154 | 0.118 | 430 (54%) | 72 (59%) | −0.101 | 0.369 |
| Indication | 0.365 | <0.001 | ||||||
| severe stenosis of native valve | 779 (97%) | 120 (98%) | 0.027 | 778 (97%) | 122 (99%) | 0.128 | ||
| severe stenosis of bioprosthesis | 22 (3%) | 1 (1%) | −0.146 | 23 (3%) | 0 (0%) | −0.207 | ||
| severe regurgitation of an aortic bioprosthesis | 1 (0%) | 1 (1%) | 0.101 | 1 (0%) | 0 (0%) | 0.013 | ||
| degenerative transcatheter heart valve prosthesis | 0 (0%) | 1 (1%) | 0.128 | 0 (0%) | 1 (1%) | 0.120 | ||
IPTW: inverse probability of treatment weighting; TF-TAVI: transfemoral transcatheter aortic valve implantation; TA-TAVI: transapical transcatheter aortic valve implantation; SD: standard deviation; STS: Society of Thoracic Surgeons; LVEF: left ventricular ejection fraction; COPD: chronic obstructive lung disease; PCI: percutaneous coronary intervention; MI: myocardial infarction; CABG: coronary artery bypass graft; NYHA: New York Heart Association.3.2. Procedural Details.
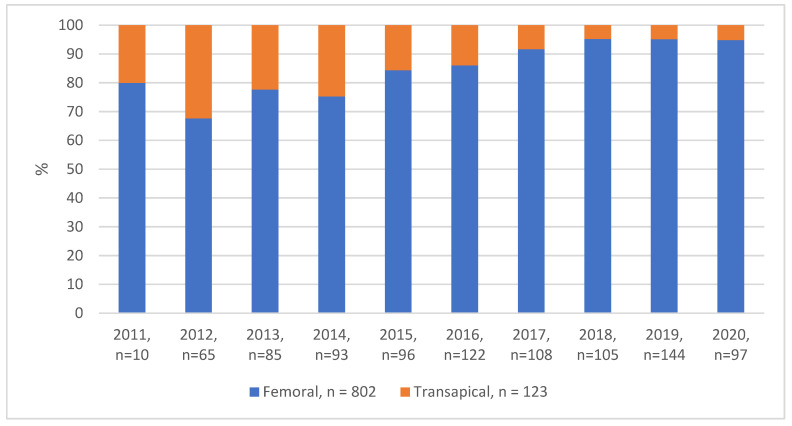
Over the last decade, there has been a noted shift in the choice of access route—while TF as an access route increased over time, there was a decrease in TA-TAVIs (Figure 2). In case of TF-TAVI, 90% (n = 723) of the patients had local anesthesia and 10% (n = 78) had general anesthesia. All TA-TAVI patients (n = 123 (100%)) received general anesthesia. In the TF-TAVI group, procedural time (68 ± 26 min vs. 86 ± 43 min; before IPTW p < 0.001) was shorter and less radiocontrast agent was used as compared to TA-TAVI (190 ± 73 mL vs. 154 ± 78 mL, p < 0.001). The main access site in the TF-TAVI group was predominantly treated with preclosure devices (n = 787, 98%). In the TA-TAVI group, the main access site (apex) was closed with purse-string sutures (n = 123 (100%)) (Table 2). The detailed numerical distribution per year is in Table S2 in the Supplementary Materials.
Figure 2.
Percentage distribution of transfemoral and transapical TAVI per year: Shown is the percentage distribution for both procedures in each year. At the beginning of 2015, there is a trend in favor of using transfemoral access and a decrease of using transapical access for TAVI each year. Since 2018, around 5% of patients per year undergo transapical approach.
Table 2.
Procedural details.
| Before IPTW | After IPTW | |||||||
|---|---|---|---|---|---|---|---|---|
| Procedural Details | TF-TAVI n = 802 |
TA-TAVI n = 123 |
SD | p | TF-TAVI n = 802 |
TA-TAVI n = 123 |
SD | p |
| Procedure time – min | 68 ± 26 | 86 ± 43 | 0.532 | <0.001 | 67 ± 26 | 85 ± 50 | 0.434 | <0.001 |
| Total contrast administered – ml | 190 ± 73 | 154 ± 78 | −0.473 | <0.001 | 190 ± 76 | 156 ± 91 | −0.400 | <0.001 |
| Valve size – mm | 27 (27 to 27) | 25 (25 to 26) | 0.862 | <0.001 | 27 (27 to 27) | 25 (25 to 26) | 0.858 | <0.001 |
| Balloon valvuloplasty – no. (%) | 680 (85%) | 113 (92%) | −0.222 | 0.040 | 682 (85%) | 114 (93%) | −0.253 | 0.042 |
| Device closure of femoral artery | 788 (98%) | 41 (34%) | 1.864 | <0.001 | 787 (98%) | 37 (30%) | 2.021 | <0.001 |
| Number of valves used | 0.672 | <0.001 | ||||||
| 1 | 777 (97%) | 118 (96%) | −0.052 | 776 (97%) | 118 (96%) | −0.029 | ||
| 2 | 21 (3%) | 5 (4%) | 0.081 | 22 (3%) | 5 (4%) | 0.057 | ||
| 3 | 4 (1%) | 0 (0%) | −0.100 | 4 (0%) | 0 (0%) | −0.099 | ||
IPTW: inverse probability of treatment weighting; TF-TAVI: transfemoral transcatheter aortic valve implantation; TA-TAVI: transapical transcatheter aortic valve implantation; SD: standard deviation.
3.2. Procedural Complication and Early Procedural Outcomes
Overall, both groups had a low incidence of procedural complications (Table 3). The two groups (TF-TAVI n = 24, (3%) vs. TA-TAVI n = 2, (2%); after IPTW p = 0.454) showed a low incidence of aortic valve dislocation. Only three cases were reported within the group of TF-TAVI that required conversion to another access route, either due to massive, unexpected calcification, tortuosity, or undersized diameter of the femoral artery. In 6% of the TF-TAVI patients (n = 46), an access vessel complication was reported and in 2% (n = 17), a vascular surgeon had to intervene for an open vascular surgical repair. None of these complications occurred in the TA-TAVI group. At discharge, aortic regurgitation grades were measured via trans esophageal echocardiography and were similar in both groups (p = 0.646). In both groups, more than 50% did not have aortic regurgitation at all (TF-TAVI n = 454 (57%), TA-TAVI n = 64 (52%)) and approximately 40% had mild aortic regurgitation (TF-TAVI n = 325 (41%), TA-TAVI n = 57 (46%)). The mean gradient above the prosthetic aortic valve was comparably low at discharge in both groups (TF-TAVI 8mmHg (CI 8 to 9 mmHg) vs. TA-TAVI 11 mmHg (CI 10 to 12 mmHg); after IPTW p = 0.007). Patients who underwent the transapical approach stayed slightly longer in the intensive care unit (TF-TAVI 2 days (CI 2 to 2 days) vs. TA-TAVI 2 days (CI 2 to 3 days); after IPTW p = 0.019), however longer on the general ward (TF-TAVI 5 days (CI 5 to 6 days) vs. TA-TAVI 9 days (CI 8 to 10 days); after IPTW p < 0.001).
Table 3.
Procedural complication and early outcome.
| Before IPTW | After IPTW | |||||||
|---|---|---|---|---|---|---|---|---|
| Procedural Complication and Early Outcome | TF-TAVI n = 802 |
TA-TAVI n = 123 |
SD | p | TF-TAVI n = 802 |
TA-TAVI n = 123 |
SD | p |
| Reposition with snare | 6 (1%) | 0 (0%) | 0.123 | 1.000 | 6 (1%) | 0 (0%) | 0.123 | 1.000 |
| Valve in valve | 18 (2%) | 4 (3%) | −0.06 | 0.497 | 18 (2%) | 3 (3%) | −0.029 | 0.753 |
| Valve retrieval | 12 (1%) | 0 (0%) | 0.174 | 0.385 | 13 (2%) | 0 (0%) | 0.182 | 0.385 |
| Valve dislocation | 23 (3%) | 2 (2%) | 0.084 | 0.435 | 24 (3%) | 2 (2%) | 0.086 | 0.454 |
| Conversion to transapical approach | 3 (0%) | 0 (0%) | 0.087 | 1.000 | 3 (0%) | 0 (0%) | 0.084 | 1.000 |
| Cardiac tamponade or rupture | 7 (1%) | 1 (1%) | 0.007 | 0.947 | 8 (1%) | 1 (1%) | 0.041 | 0.659 |
| Hemodynamic instability requiring treatment | 29 (4%) | 3 (2%) | 0.069 | 0.509 | 30 (4%) | 2 (1%) | 0.150 | 0.104 |
| Resuscitation | 14 (2%) | 1 (1%) | 0.065 | 0.584 | 14 (2%) | 1 (0%) | 0.121 | 0.210 |
| Femoral artery complication | 44 (5%) | 0 (0%) | 0.341 | 0.002 | 46 (6%) | 0 (0%) | 0.347 | 0.002 |
| - Stenosis | 6 (1%) | 0 (0%) | 0.123 | 1.000 | 7 (1%) | 0 (0%) | 0.128 | 1.000 |
| - Rupture | 2 (0%) | 0 (0%) | 0.071 | 1.000 | 3 (0%) | 0 (0%) | 0.085 | 1.000 |
| - Hematoma (>2 units transfusion) | 4 (0%) | 0 (0%) | 0.100 | 1.000 | 4 (1%) | 0 (0%) | 0.101 | 1.000 |
| - Stent placed | 11 (1%) | 0 (0%) | 0.167 | 0.377 | 11 (1%) | 0 (0%) | 0.168 | 0.377 |
| - Need for vascular surgery | 17 (2%) | 0 (0%) | 0.208 | 0.150 | 17 (2%) | 0 (0%) | 0.211 | 0.150 |
| Coronary artery occlusion | 1 (0%) | 0 (0%) | 0.050 | 1.000 | 1 (0%) | 0 (0%) | 0.049 | 1.000 |
| Annulus rupture/aortic dissection | 4 (0%) | 0 (0%) | 0.100 | 1.000 | 5 (1%) | 0 (0%) | 0.113 | 1.000 |
| Type of femoral artery complication | 1.000 | <0.001 | ||||||
| - dissection | 15 (2%) | 0 (0%) | −0.19 | 16 (2%) | 0 (0%) | −0.203 | ||
| - perforation | 13 (2%) | 0 (0%) | −0.18 | 12 (2%) | 0 (0%) | −0.176 | ||
| - occlusion | 13 (2%) | 0 (0%) | −0.18 | 14 (2%) | 0 (0%) | −0.184 | ||
| Aortic valve peak gradient (mmHg)Peak gradient (mmHg) | 15 (15 to 16) | 19 (17 to 21) | 0.705 | 0.003 | 15 (15 to 16) | 19 (17 to 21) | 0.691 | 0.003 |
| Aortic valve mean gradient (mmHg) | 8 (8 to 9) | 11 (9 to 12) | 0.690 | 0.015 | 8 (8 to 9) | 11 (10 to 12) | 0.698 | 0.007 |
| Aortic regurgitation grade postmeasure/discharge | 0.667 | 0.646 | ||||||
| - none | 453 (57%) | 63 (51%) | −0.106 | 454 (57%) | 64 (52%) | −0.089 | ||
| - mild | 327 (41%) | 56 (46%) | 0.096 | 325 (41%) | 57 (46%) | 0.113 | ||
| - moderate | 19 (2%) | 3 (2%) | 0.004 | 19 (2%) | 2 (1%) | −0.084 | ||
| - severe | 3 (0%) | 1 (1%) | 0.057 | 4 (0%) | 0 (0%) | −0.010 | ||
| Intensive care unit – days | 2 (2 to 2) | 2 (2 to 3) | 0.678 | 0.005 | 2 (2 to 2) | 2 (2 to 3) | 0.653 | 0.019 |
| General ward - days | 5 (5 to 6) | 9 (8 to 10) | 0.860 | <0.001 | 5 (5 to 6) | 9 (8 to 10) | 0.877 | <0.001 |
IPTW: inverse probability of treatment weighting; TF-TAVI: transfemoral transcatheter aortic valve implantation; TA-TAVI: transapical transcatheter aortic valve implantation; SD: standard deviation.3.4. 30-Day Follow-Up.
According to the VARC-2 criteria, bleeding and vascular complications within 30 days after intervention were seen more often after TF-TAVI than after TA-TAVI, corresponding to an IPT weighted hazard ratio of 0.52, 95% CI 0.29 to 0.95, p = 0.032, and 0.18, CI 0.07 to 0.42, p < 0.001, respectively. (Table 4) We did not see a difference regarding 30-day mortality, weighted hazard ratio (HR) 1.11, CI 0.43 to 2.87, p = 0.82, nor regarding any other pre-specified outcome.
Table 4.
Short and long-term follow up (landmark 30 days).
| Before IPTW | After IPTW | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | Time Interval | TF-TAVI n = 802 |
TA-TAVI n = 123 |
HR | p | HR | p |
| Death | ≤30 d | 17 | 7 | 2.67 (1.11 to 6.45) | 0.028 | 1.11 (0.43 to 2.87) | 0.834 |
| 30 d-5 y | 145 | 38 | 1.18 (0.83 to 1.69) | 0.354 | 1.33 (0.91 to 1.95) | 0.141 | |
| 0-5 y | 162 | 45 | 1.31 (0.94 to 1.82) | 0.115 | 1.31 (0.92 to 1.88) | 0.138 | |
| Overall | 164 (20%) | 47 (38%) | |||||
| Stroke | ≤30 d | 30 | 7 | 1.52 (0.67 to 3.47) | 0.315 | 1.11 (0.42 to 2.92) | 0.832 |
| 30 d-5 y | 17 | 2 | 0.59 (0.14 to 2.58) | 0.488 | 1.01 (0.21 to 4.81) | 0.987 | |
| 0-5 y | 47 | 9 | 1.14 (0.56 to 2.33) | 0.715 | 1.07 (0.47 to 2.47) | 0.867 | |
| Overall | 47 (6%) | 10 (8%) | |||||
| MI | ≤30 d | 10 | 2 | 1.30 (0.29 to 5.94) | 0.733 | 0.43 (0.09 to 2.06) | 0.294 |
| 30 d-5 y | 11 | 3 | 1.25 (0.35 to 4.49) | 0.735 | 1.09 (0.27 to 4.41) | 0.900 | |
| 0-5 y | 21 | 5 | 1.27 (0.48 to 3.38) | 0.633 | 0.82 (0.27 to 2.42) | 0.713 | |
| Overall | 21 (3%) | 6 (5%) | |||||
| AKI | ≤30 d | 15 | 4 | 1.73 (0.57 to 5.20) | 0.332 | 1.14 (0.35 to 3.69) | 0.828 |
| 30 d-5 y | 1 | 1 | 5.77 (0.36 to 92.3) | 0.215 | 3.78 (0.24 to 60.2) | 0.347 | |
| 0-5 y | 16 | 5 | 2.00 (0.73 to 5.46) | 0.176 | 1.55 (0.51 to 4.71) | 0.439 | |
| Overall | 16 (2%) | 5 (4%) | |||||
| Any bleeding | ≤30 d | 146 | 15 | 0.65 (0.38 to 1.11) | 0.114 | 0.52 (0.29 to 0.95) | 0.032 |
| 30 d-5 y | 26 | 5 | 0.88 (0.34 to 2.29) | 0.787 | 0.62 (0.20 to 1.93) | 0.407 | |
| 0-5 y | 172 | 20 | 0.69 (0.44 to 1.10) | 0.124 | 0.54 (0.32 to 0.92) | 0.022 | |
| Overall | 172 (21%) | 20 (16%) | |||||
| Life threatening bleeding | ≤30 d | 15 | 5 | 2.17 (0.79 to 5.96) | 0.134 | 1.75 (0.56 to 5.50) | 0.339 |
| 30 d-5 y | 13 | 4 | 1.49 (0.48 to 4.58) | 0.489 | 1.22 (0.34 to 4.35) | 0.761 | |
| 0-5 y | 28 | 9 | 1.81 (0.85 to 3.86) | 0.121 | 1.47 (0.62 to 3.48) | 0.377 | |
| Overall | 28 (3%) | 9 (7%) | |||||
| Major bleeding | ≤30 d | 54 | 7 | 0.84 (0.38 to 1.84) | 0.659 | 0.77 (0.32 to 1.85) | 0.562 |
| 30 d-5 y | 4 | 0 | no event | 1.00 | no event | 1.00 | |
| 0-5 y | 58 | 7 | 0.77 (0.35 to 1.70) | 0.523 | 0.71 (0.30 to 1.70) | 0.444 | |
| Overall | 58 (7%) | 7 (6%) | |||||
| Minor bleeding | ≤30 d | 77 | 3 | 0.25 (0.08 to 0.79) | 0.018 | 0.13 (0.04 to 0.48) | 0.002 |
| 30 d-5 y | 9 | 1 | 0.50 (0.06 to 3.95) | 0.511 | 0.14 (0.02 to 1.07) | 0.058 | |
| 0-5 y | 86 | 4 | 0.29 (0.10 to 0.78) | 0.014 | 0.13 (0.04 to 0.41) | <0.001 | |
| Overall | 86 (11%) | 4 (3%) | |||||
| Vascular bleeding | ≤30 d | 137 | 6 | 0.28 (0.12 to 0.64) | 0.002 | 0.18 (0.07 to 0.42) | <0.001 |
| 30 d-5 y | 2 | 0 | no event | 1.000 | no event | ||
| 0-5 y | 139 | 6 | 0.28 (0.12 to 0.63) | 0.002 | 0.17 (0.07 to 0.41) | <0.001 | |
| Overall | 139 (17%) | 6 (5%) | |||||
| SVD | ≤30 d | 3 | 1 | 2.17 (0.23 to 20.87) | 0.502 | 4.23 (0.45 to 39.8) | 0.207 |
| 30 d-5 y | 20 | 3 | 0.72 (0.21 to 2.42) | 0.590 | 0.62 (0.16 to 2.39) | 0.485 | |
| 0-5 y | 23 | 4 | 0.87 (0.30 to 2.51) | 0.792 | 0.99 (0.29 to 3.34) | 0.986 | |
| Overall | 24 (3%) | 4 (3%) | |||||
| Reintervention | ≤30 d | 13 | 2 | 1.00 (0.23 to 4.44) | 0.998 | 0.85 (0.19 to 3.80) | 0.831 |
| 30 d-5 y | 9 | 2 | 1.09 (0.24 to 5.09) | 0.908 | 1.49 (0.25 to 8.93) | 0.661 | |
| 0-5 y | 22 | 4 | 1.04 (0.36 to 3.04) | 0.936 | 1.13 (0.35 to 3.67) | 0.841 | |
| Overall | 22 (3%) | 5 (4%) | |||||
| PM | ≤30 d | 169 | 7 | 0.25 (0.12 to 0.54) | <0.001 | 0.22 (0.09 to 0.53) | 0.001 |
| 30 d-5 y | 12 | 3 | 1.05 (0.30 to 3.73) | 0.941 | 1.17 (0.33 to 4.12) | 0.803 | |
| 0-5 y | 181 | 10 | 0.33 (0.17 to 0.62) | 0.001 | 0.31 (0.15 to 0.62) | 0.001 | |
| Overall | 181 (23%) | 10 (8%) | |||||
IPTW: inverse probability of treatment weighting; TF-TAVI: transfemoral transcatheter aortic valve implantation; TA-TAVI: transapical transcatheter aortic valve implantation; HR: hazard ratio; MI: myocardial infarction; AKI: acute kidney injury; SVD: structural valve deterioration; PM: pacemaker.
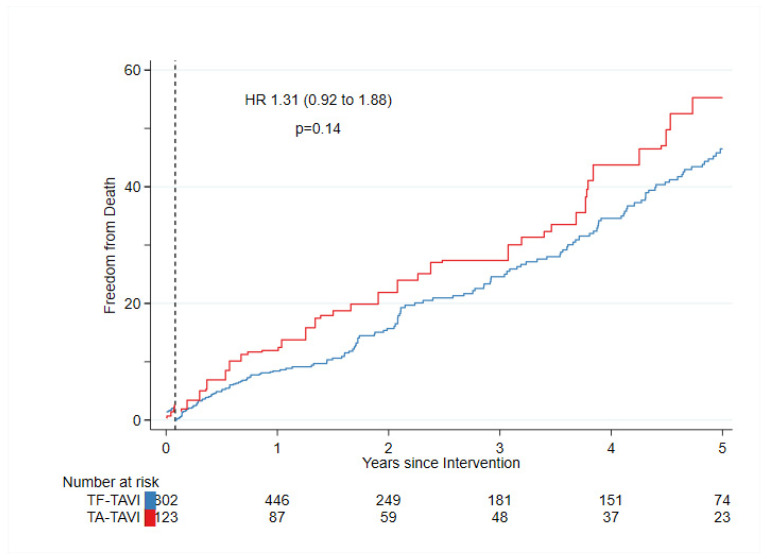
More patients needed a permanent pacemaker after TF-TAVI than after TA-TAVI, (weighted HR 0.22, CI 0.09 to 0.53, p = 0.001) due to a difference in valve selection and a substantially higher incidence of a high-grade atrioventricular blockage. Figure 3 shows IPT-weighted 30 days’ landmark estimates for freedom from death. A crude (before IPTW) Kaplan-Meier curve with confidence bands which overlap is in Figure S3 in Supplementary Materials.
Figure 3.
Mortality in patients who underwent TA-TAVI was similar to patients who underwent TF-TAVI after IPTW with a landmark inserted at 30 days after the procedure.
3.3. 5-Year Follow-Up
The results of the long-term follow-up of patients after TF- and TA-TAVI are shown in Table 4. We did not find an association of treatment and death (weighted HR TF/TA TAVI: 0–5 years after IPTW; 1.31 (0.92 to 1.88); p = 0.138). Similarly, upon calculating the HR between both intervention groups, no favor for one or the other intervention in outcomes, such as stroke, myocardial infarction (MI), acute kidney injury or life-threatening bleeding, major bleeding, structural valve deterioration, or reintervention, was observed (Table 4). Minor bleeding was slightly lower after TA TAVI (weighted HR TF/TA TAVI: 0–5 years after IPTW; 0.13 (0.04 to 0.48); p ≤ 0.001).
4. Discussion
Ever since the introduction of transcatheter aortic valve implantation, the two main access routes are transfemoral and transapical. As reported from other centers [18], a constant shift to the TF-TAVI was also visible at our institute (Figure 2). The improved accuracy of computed tomography and planning software, the decreased sheath size and increased flexibility of the TF-TAVI systems, no need for general anesthesia or post-interventional pleural drainage, are beneficial. As a result, the majority of patients qualify for a TF-TAVI. This stimulates a widespread controversial discussion of TA-TAVI being a high-risk intervention. Nevertheless, due to ongoing ageing of the population and increasing numbers of severe PAD, the TF-TAVI might not be the favorable route of access due to the lack of sufficient vessel diameter in the ilio-femoral artery or aorta, and the risk for dissection, rupture, or thrombosis. Regarding our single center study, we can report seven major findings.
First, patients in the TA-TAVI group had a higher perioperative risk due to differences in baseline characteristics. However, even though the group of TA-TAVI reflected higher risk patients, the primary and secondary outcomes did not differ between TF- and TA-TAVI. Therefore, TA-TAVI should be always considered as a safe alternative access with a similar survival rate. Similar results were reported by a prospective single center study with 1000 patients by Schymik, G et al. [19], which supports our findings. Second, the scarce need for intraoperative conversion to another access route reflects a concise treatment selection for each patient within the interdisciplinary heart team. This is even lower compared to rates reported by the SENTINAL and SOURCE registries (TF 4.7% and 1.7%; TA 3.2% and 3.5%, respectively) [20,21]; Third, even though the incidence was low, the TF-TAVI cohort had a higher incidence of bleeding complications during the procedure. Major bleeding and vascular complications occur significantly more often in TF-TAVI as already reported by previous studies [21,22,23,24]. However, the low incidence of access bleeding in the current analysis might be due to the use of an ultrasound-driven puncture of the main access (the femoral artery) to reduce the risk of puncturing the false vessel or calcified area of the access vessel. Fourth, permanent pacemaker implantation rates were low (15%) in the overall group compared to data from a large multicenter collaborative study [22]. However, they were four times higher in TF-TAVI than TA-TAVI patients. Most of the patients received a dual chamber pacemaker due to a higher grade of atrioventricular block. In contrast to these findings, the propensity matched analysis in the PARTNER TRIAL I showed no such differences in both groups [10]. We assume the use of prosthesis with low pacemaker rates for TA-TAVI procedures, such as the JenaValve (JenaValve Technology GmbH, Munich, Germany) causative for this [25]. Fifth, 30-day mortality after propensity modeling was similar for both groups. In our center, we were able to achieve lower mortality rates in comparison to other trials, such as the SOURCE, UK TAVI, PRAGMATIC, and complete SwissTAVI, which range from 3.6% to 6.4% for TF-TAVI and 9.5% to 15.7% for TA-TAVI [21,22,23,24]. Considering the baseline characteristics, the patients’ factors are unlikely to be responsible for this difference. We consider the improvement of the delivery and valve systems as well as the increasing operator experience causal [26,27]. Sixth, TA-TAVI were all performed in the modern Hybrid operating room with the newest imaging solution (ARTIS pheno®, Siemens Healthineers) and TA-TAVI patients were as well under a cardiac anesthesiologist’s care during the implantation with the broad spectrum of treatment options. Seventh, upon evaluation of the long-term outcomes, we did not find an association of treatment and death and there were no significant differences in the occurrence of stroke, acute renal injury, or MI.
Our findings validate and broaden previous studies regarding the understanding and importance of meticulous evaluation of the heart to establish the best possible treatment. With this, we can ensure a safe access route and comparable long term survival rate for both patient’s groups. Despite findings from previous literature, we could show convincing results indicating that TA is an appropriate therapy and can be used successfully as a bail-out strategy for TF in an unfavorable or even hostile environment.
Some limitations should be considered when interpreting these findings. First, it is a single-center study and there is a lack of random assignment to treatment groups due to the heart team´s decisions. Second, from the beginning of the study until this retrospective analysis, there has been major technical development, especially in the sense of transfemoral delivery systems. With the latest delivery systems, smaller and more precise vascular access has become feasible and procedure-related bleedings may be more preventable. Third, as can be seen from the standardized differences, propensity modeling did not achieve fully balanced treatment groups with respect to the risk of outcomes, which indicates that there might be some residual confounding factors.
5. Conclusions
In this single-center observational study involving 925 consecutive patients with aortic valve stenosis undergoing TF- or TA-TAVI, we demonstrated compared short- and long-term safety of both treatment groups. Major vascular complications and indications for permanent pacemaker implantation were higher in the TF-TAVI group. The present study shows the importance of the interdisciplinary heart team to talk over the best individual patient treatment option. In contrast to previous literature and despite the ongoing shift to TF-TAVI procedures, the present study shows that TA-TAVI is a safe and efficacious treatment and is an adequate alternative to TF-TAVI.
Acknowledgments
We thank Ayse Mettler for improving language and style.
Abbreviations
| CABG | Coronary Artery Bypass Grafting |
| CAD | Coronary Artery Disease |
| CI | Confidence Interval |
| CT | Computer Tomography |
| COPD | Chronic Obstructive Pulmonary Disease |
| HR | Hazard Ratio |
| IPTW | Inverse probability of treatment weighting |
| MI | Myocardial Infarction |
| PAD | Peripheral Artery Disease |
| PM | Pacemaker |
| SAVR | Surgical Aortic Valve Replacement |
| STS | Society of Thoracic Surgeons |
| TA | Transapical |
| TAVI | Transcatheter Aortic Valve Implantation |
| TF | Transfemoral |
| VARC | Valve Aortic Research Consortium |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bioengineering10020156/s1, Figure S1: Propensity Score, kernel density; Figure S2: Standardized Differences before and after IPTW; Figure S3: Kaplan–Meier curve before IPTW; Table S1: Numbers of Implants during the observation period; Table S2: Numerical distribution of TF-TAVI and TA-TAVI per year; Table S3: Preoperative medications.
Author Contributions
Conceptualization, C.M., R.T., B.G. and O.R.; methodology, C.M., R.T., B.G. and O.R.; software, C.M., R.T., B.G. and O.R.; validation, C.M., R.T., B.G. and O.R.; formal analysis, B.G.; investigation, C.M., R.T., B.G. and O.R.; resources, C.M., R.T., B.G., F.E., R.J., C.K. and O.R.; data curation, C.M., R.T., B.G. and O.R.; writing—original draft preparation, C.M., R.T., B.G. and O.R.; writing—review and editing, C.M., R.T., B.G., F.E., R.J., C.K.and O.R.; visualization, C.M., R.T., B.G. and O.R.; supervision, C.M., R.T. and O.R.; project administration, O.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees (ethics committee beider Basel (reference number 305/11; 2011; since then permission through the ethics committee Bern, project ID: 2021-01738).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
All data generated and analyzed during this study are included in this study published article (and its Supplementary Materials).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Vahanian A., Beyersdorf F., Praz F., Milojevic M., Baldus S., Bauersachs J., Capodanno D., Conradi L., de Bonis M., de Paulis R., et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 2.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., Gentile F., Jneid H., Krieger E.V., Mack M., McLeod C., et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:E72–E227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 3.Cribier A., Eltchaninoff H., Bash A., Borenstein N., Tron C., Bauer F., Derumeaux G., Anselme F., Laborde F., Leon M.B. Percutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis: First Human Case Description. Circulation. 2002;106:3006–3008. doi: 10.1161/01.CIR.0000047200.36165.B8. [DOI] [PubMed] [Google Scholar]
- 4.Booth K., Beattie R., McBride M., Manoharan G., Spence M., Jones J.M. High Risk Aortic Valve Replacement – the Challenges of Multiple Treatment Strategies with an Evolving Technology. Ulster Med. J. 2016;85:18–22. [PMC free article] [PubMed] [Google Scholar]
- 5.Cribier A., Eltchaninoff H., Tron C., Bauer F., Agatiello C., Sebagh L., Bash A., Nusimovici D., Litzler P.Y., Bessou J.P., et al. Early Experience with Percutaneous Transcatheter Implantation of Heart Valve Prosthesis for the Treatment of End-Stage Inoperable Patients with Calcific Aortic Stenosis. J. Am. Coll. Cardiol. 2004;43:698–703. doi: 10.1016/j.jacc.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Popma J.J., Deeb G.M., Yakubov S.J., Mumtaz M., Gada H., O’Hair D., Bajwa T., Heiser J.C., Merhi W., Kleiman N.S., et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 7.Baumgartner H., Falk V., Bax J.J., De Bonis M., Hamm C., Holm P.J., Iung B., Lancellotti P., Lansac E., Rodriguez Muñoz D., et al. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 8.Mack M.J., Leon M.B., Thourani V.H., Makkar R., Kodali S.K., Russo M., Kapadia S.R., Malaisrie S.C., Cohen D.J., Pibarot P., et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 9.Ye J., Cheung A., Lichtenstein S.V., Carere R.G., Thompson C.R., Pasupati S., Webb J.G. Transapical Aortic Valve Implantation in Humans. J. Thorac. Cardiovasc. Surg. 2006;131:1194–1196. doi: 10.1016/j.jtcvs.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Blackstone E.H., Suri R.M., Rajeswaran J., Babaliaros V., Douglas P.S., Fearon W.F., Miller D.C., Hahn R.T., Kapadia S., Kirtane A.J., et al. Propensity-Matched Comparisons of Clinical Outcomes after Transapical or Transfemoral Transcatheter Aortic Valve Replacement a Placement of Aortic Transcatheter Valves (PARTNER)-I Trial Substudy. Circulation. 2015;131:1989–1999. doi: 10.1161/CIRCULATIONAHA.114.012525. [DOI] [PubMed] [Google Scholar]
- 11.Ludman P.F., Moat N., De Belder M.A., Blackman D.J., Duncan A., Banya W., MacCarthy P.A., Cunningham D., Wendler O., Marlee D., et al. Transcatheter Aortic Valve Implantation in the United Kingdom: Temporal Trends, Predictors of Outcome, and 6-Year Follow-up: A Report from the UK Transcatheter Aortic Valve Implantation (TAVI) Registry, 2007 to 2012. Circulation. 2015;131:1181–1190. doi: 10.1161/CIRCULATIONAHA.114.013947. [DOI] [PubMed] [Google Scholar]
- 12.Gilard M., Eltchaninoff H., Iung B., Donzeau-Gouge P., Chevreul K., Fajadet J., Leprince P., Leguerrier A., Lievre M., Prat A., et al. Registry of Transcatheter Aortic-Valve Implantation in High-Risk Patients. N. Engl. J. Med. 2012;366:1705–1715. doi: 10.1056/nejmoa1114705. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa N., Kuss O., Emmel E., Scholtz S., Scholtz W., Fujita B., Ensminger S., Gummert J.F., Börgermann J. Minimally Invasive versus Transapical versus Transfemoral Aortic Valve Implantation: A One-to-One-to-One Propensity Score–Matched Analysis. J. Thorac. Cardiovasc. Surg. 2018;156:1825–1834. doi: 10.1016/j.jtcvs.2018.04.104. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari E., Eeckhout E., Keller S., Muller O., Tozzi P., Berdajs D., von Segesser L.K. Transfemoral versus Transapical Approach for Transcatheter Aortic Valve Implantation: Hospital Outcome and Risk Factor Analysis. J. Cardiothorac. Surg. 2017;12 doi: 10.1186/s13019-017-0638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kappetein A.P., Head S.J., Généreux P., Piazza N., Van Mieghem N.M., Blackstone E.H., Brott T.G., Cohen D.J., Cutlip D.E., Van Es G.A., et al. Updated Standardized Endpoint Definitions for Transcatheter Aortic Valve Implantation: The Valve Academic Research Consortium-2 Consensus Document. J. Thorac. Cardiovasc. Surg. 2013;145:6–23. doi: 10.1016/j.jtcvs.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Austin P.C., Stuart E.A. Moving towards Best Practice When Using Inverse Probability of Treatment Weighting (IPTW) Using the Propensity Score to Estimate Causal Treatment Effects in Observational Studies. Stat. Med. 2015;34:3661. doi: 10.1002/SIM.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011;46:399. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reents W., Barth S., Griese D.P., Winkler S., Babin-Ebell J., Kerber S., Diegeler A., Zacher M., Hamm K. Transfemoral versus Transapical Transcatheter Aortic Valve Implantation: A Single-Centre Experience. Eur. J. Cardio-Thorac. Surg. 2019;55:744–750. doi: 10.1093/ejcts/ezy363. [DOI] [PubMed] [Google Scholar]
- 19.Schymik G., Würth A., Bramlage P., Herbinger T., Heimeshoff M., Pilz L., Schymik J.S., Wondraschek R., Süselbeck T., Gerhardus J., et al. Long-Term Results of Transapical versus Transfemoral TAVI in a Real World Population of 1000 Patients with Severe Symptomatic Aortic Stenosis. Circ. Cardiovasc. Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.113.000761. [DOI] [PubMed] [Google Scholar]
- 20.Di Mario C., Eltchaninoff H., Moat N., Goicolea J., Ussia G.P., Kala P., Wenaweser P., Zembala M., Nickenig G., Barrero E.A., et al. The 2011-12 Pilot European Sentinel Registry of Transcatheter Aortic Valve Implantation: In-Hospital Results in 4,571 Patients. EuroIntervention. 2013;8:1362–1371. doi: 10.4244/EIJV8I12A209. [DOI] [PubMed] [Google Scholar]
- 21.Thomas M., Schymik G., Walther T., Himbert D., Lefèvre T., Treede H., Eggebrecht H., Rubino P., Michev I., Lange R., et al. Thirty-Day Results of the SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) Registry: A European Registry of Transcatheter Aortic Valve Implantation Using the Edwards SAPIEN Valve. Circulation. 2010;122:62–69. doi: 10.1161/CIRCULATIONAHA.109.907402. [DOI] [PubMed] [Google Scholar]
- 22.van der Boon R.M.A., Marcheix B., Tchetche D., Chieffo A., van Mieghem N.M., Dumonteil N., Vahdat O., Maisano F., Serruys P.W., Kappetein A.P., et al. Transapical versus Transfemoral Aortic Valve Implantation: A Multicenter Collaborative Study. Ann. Thorac. Surg. 2014;97:22–28. doi: 10.1016/j.athoracsur.2013.09.088. [DOI] [PubMed] [Google Scholar]
- 23.Blackman D.J., Baxter P.D., Gale C.P., Moat N.E., Maccarthy P.A., Hildick-Smith D., Trivedi U., Cunningham D., de Belder M.A., Ludman P.F. Do Outcomes from Transcatheter Aortic Valve Implantation Vary According to Access Route and Valve Type? The UK TAVI Registry. J. Interv. Cardiol. 2014;27:86–95. doi: 10.1111/joic.12084. [DOI] [PubMed] [Google Scholar]
- 24.Wenaweser P., Stortecky S., Heg D., Tueller D., Nietlispach F., Falk V., Pedrazzini G., Jeger R., Reuthebuch O., Carrel T., et al. Short-Term Clinical Outcomes among Patients Undergoing Transcatheter Aortic Valve Implantation in Switzerland: The Swiss TAVI Registry. EuroIntervention. 2014;10:982–989. doi: 10.4244/EIJV10I8A166. [DOI] [PubMed] [Google Scholar]
- 25.Reuthebuch O., Koechlin L., Kaufmann B.A., Kessel-Schaefer A., Gahl B., Eckstein F.S. Transapical Transcatheter Aortic Valve Implantation Using the JenaValve: A One-Year Follow-Up. Thorac. Cardiovasc. Surg. 2015;63:493–500. doi: 10.1055/S-0035-1552980. [DOI] [PubMed] [Google Scholar]
- 26.Hayashida K., Lefvre T., Chevalier B., Hovasse T., Romano M., Garot P., Mylotte D., Uribe J., Farge A., Donzeau-Gouge P., et al. Transfemoral Aortic Valve Implantation: New Criteria to Predict Vascular Complications. JACC Cardiovasc. Interv. 2011;4:851–858. doi: 10.1016/j.jcin.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Bourantas C.V., Modolo R., Baumbach A., Søndergaard L., Prendergast B.D., Ozkor M., Kennon S., Mathur A., Mullen M.J., Serruys P.W. The Evolution of Device Technology in Transcatheter Aortic Valve Implantation. EuroIntervention. 2019;14:e1826–e1833. doi: 10.4244/EIJ-D-18-01048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during this study are included in this study published article (and its Supplementary Materials).