Abstract
Previous studies provided inconsistent results on the effects of antioxidant nutrient intake on lung cancer prevention. We aimed to evaluate the association between antioxidant consumption from food and supplemental sources and lung cancer incidence. Data were obtained from the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial. A total of 98,451 participants were included in the data analysis. We used a multivariable Cox proportional hazards regression model to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between antioxidant intake and lung cancer risk. Dose-response assessments for individual nutrients were conducted. We also selected the model for the best combination of antioxidants for reducing lung cancer risk using machine learning methods. After the median follow-up of 12.2 years, 1642 new cases were identified. Intake of the calculated HRs indicated a trend for a higher quartile of food-based Composite Dietary Antioxidant Index (fCDAI) associated with a lower lung cancer risk after adjusting for covariates (HRQ4vs.Q1 = 0.64, 95% CI: 0.52, 0.79; P for trend < 0.001). Protective effects of dietary antioxidant intake were observed across all individual antioxidant micronutrients except magnesium. Random forests model suggested the dietary intake group of α-carotene, magnesium, vitamin C, vitamin E, lycopene, selenium, lutein, and zeaxanthin, and β-carotene had the most favorable effects on lung cancer prevention. Higher consumption of antioxidants from food sources has a protective effect against lung cancer, while no effects were shown in the supplemental group. It is recommended to consume a combination of various antioxidants due to the potential benefits from the interaction, while more research should be performed to investigate the underlying mechanisms of antioxidant synergic effects on lung cancer risk reduction.
Keywords: antioxidant micronutrients, vitamins, minerals, lung cancer incidence, machine learning
1. Introduction
Lung cancer is one of the most diagnosed malignancies worldwide, with high incidence and mortality rates each year [1]. In 2020, there were 22.4 cases per 100,000 persons across all ages and both sexes, with an estimation of 2,206,771 new cases and 1,796,144 deaths worldwide [2]. Several risk factors for lung cancer development have been identified in previous studies, such as cigarette smoking, exposure to second-hand smoke, asbestos, and air pollution [1,3].
Evidence has also suggested that diet, such as antioxidant intake, is associated with lung cancer incidence [4,5]. Antioxidants, such as vitamins C, vitamin E, β-carotene, and other phytochemicals, have abilities to combat free radicals and inhibit oxidation [6]. Previous epidemiological studies have shown that higher consumption of antioxidant-rich fruits and vegetables is linked to certain cancer risk reduction, including lung cancer [6,7,8]. According to the current updates from World Cancer Research Fund (WCRF), there is moderate evidence indicating that the consumption of dietary sources of antioxidants, vegetables, and fruits may be a protective factor against lung cancer occurrence, as well as retinol- or carotenoids-rich foods [9]. However, the individual roles of antioxidant nutrients on lung cancer risk remain inconclusive. A well-documented antioxidant bioactive compound, β-carotene, has demonstrated an inverse association with lung cancer incidence in several studies [8,10]. Strong evidence has shown that high-dose β-carotene supplemental intake increases lung cancer risk among current and former smokers [9]. In addition, a systematic review and meta-analysis highlighted that the favorable effects of selenium supplementation have only been shown in populations with a lower baseline serum selenium status, while another meta-analysis noted an inverse association between selenium exposure and lung cancer risk without identifying a threshold effect in the dose-response analyses [11,12]. These studies yield mixed results on cancer development, which are sophisticated and require further research on the individual roles of antioxidants.
Many studies have examined the individual effects of certain antioxidant intake, while there are inadequate studies investigating the combined effects of antioxidant consumption from both dietary and supplemental sources. Due to the potential interactions between different antioxidants, separately looking at individual micronutrients could not fully account for the synergic effects of antioxidants intake on lung cancer risk. The food-based Composite Dietary Antioxidant Index (fCDAI) has been developed and used in multiple longitudinal studies to capture the overall antioxidant intake from various food groups and evaluate the associations with cancer risk [13,14,15]. We utilized this index to predict lung cancer risk in a generally healthy population. Meanwhile, we also employed machine learning methods to investigate the optimal antioxidant combination in lung cancer prevention.
Therefore, in this study, we aimed to examine the effects of the independent and combined intake of antioxidant nutrients on lung cancer risk, differentiate the effects based on dietary and supplemental sources, and investigate the optimal combination of individual antioxidants on lung cancer prevention in different subgroups in a large population-based cancer screening trial.
2. Materials and Methods
2.1. Study Population and Study Design
The data used in this study were from the Prostate, Lung, Colorectal, and Ovarian (PLCO) study, a cancer screening trial, which included a total of 155,000 participants recruited from 10 centers across the US. The trial aimed to evaluate the effects of screening exams on decreasing cancer mortality rates. Participants were enrolled first in 1993, randomized into the control arm (usual care) or the intervention arm (screening exams). Eligible participants were aged between 55 and 74 years old at the enrollment and free of prostate, lung, colorectal, or ovarian cancer history. Baseline information of participants was collected through a baseline questionnaire (BQ). Dietary history questionnaire (DHQ) has been provided to both arms since 1998. Around 77% or 113,000 participants completed DHQ with a 3-year median time into study collected. Subjects were followed for approximately 12 years to collect data on cancer diagnoses.
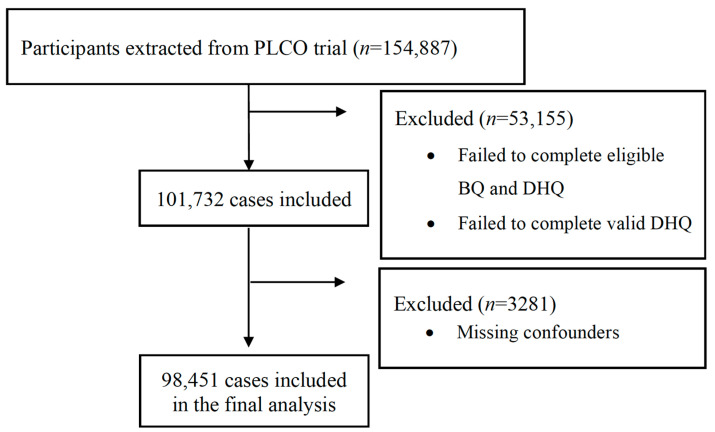
In total, there were 154,887 participants extracted from the PLCO trial. After excluding 53,155 individuals who failed to complete valid BQ and DHQ, 101,732 subjects remained in the study. Eligible participants who completed a valid BQ had no history of lung cancer prior to the trial but had time at risk for developing their first cancer. A valid DHQ was defined as the completion of the questionnaire with a completion date before date of death, missing no more than 7 frequency responses, and not having extreme energy intake (top and bottom 1% of each sex group). We further excluded 3281 participants due to the missing of important covariates (study arm, sex, education, body mass index, marital status, family history of lung cancer or any other cancer, smoking status, pack-years of cigarettes, and alcohol drinking status). Finally, there were 98,451 cases included in the analytical dataset. A flow diagram is presented in Figure 1.
Figure 1.
Flow diagram of selecting individuals from the PLCO trial.
The PLCO screening trial was approved by the Institutional Review Board of the National Cancer Institute (NCI), and written consent forms were obtained from subjects prior to and after the randomization for agreements of participating trial activities and screening. This Cancer Data Access System (CDAS) project was approved by NCI, and the project ID is PLCO-974.
2.2. Data Collection
Baseline data on demographics, medical history, and other risk factor information, such as smoking status, were self-reported by participants. In our study, we obtained age, sex, race, study arm, education, body mass index (BMI), marital status, family history of any cancer, family history of lung cancer, smoking status, pack-years of cigarettes, and alcohol drinking status. Dietary data were collected through DHQ, which included 156 questions to assess alcohol use, nutrient intake, supplement intake, total energy intake, and daily consumption of foods and beverages in grams and frequencies in the past year. Specifically, we obtained dietary intake of vitamin A, vitamin C, vitamin E, α-carotene, β-carotene, magnesium, selenium, zinc, lycopene, lutein, and zeaxanthin. Nutrition Data System for Research (NDS-R), along with U.S. Department of Agriculture (USDA)’s Continuing Survey of Food Intakes by Individuals (CSFII), were used to estimate the amount of nutrients intake. Additionally, daily supplemental intake of several vitamins and minerals, which was available, was also retrieved.
2.3. fCDAI Score Calculation
The food-based Composite Dietary Antioxidant Index (fCDAI) was used to calculate a summary score of individual’s dietary intake of antioxidants in respect of the mean intake of the entire cohort. The index has been validated in other studies, which found it to be inversely associated with several pro-inflammatory biomarkers and helpful in evaluating the effects of antioxidant intake on health outcomes [13,14,15]. An fCDAI score was calculated for each participant by estimating the dietary consumption of six antioxidants, including vitamins A, C, E, magnesium, selenium, and zinc, using the following formula:
where xi is the daily consumption of antioxidant i. stands for the mean intake of antioxidant i across entire study population, and Si is the SD for for antioxidant i.
2.4. Ascertment of Lung Cancer Cases
The outcome of interest was the incidence of lung cancer. Study participants self-reported lung cancer diagnoses through annual questionnaires. Abnormal chest x-ray screening, death certificates, and relative informed cases were followed up. The lung cancer diagnoses were all confirmed through medical record abstraction (MRA) later. The lung cancer histopathologic types derived from International Classification of Diseases for Oncology, 2nd Edition (ICD-O-2) morphology, including non-small cell lung cancer and small cell lung cancer. Carcinoid tumors were not considered during screening in the trial and were not included in this study as confirmed cases.
2.5. Statistical Analysis
We used Cox proportional hazards regression model to estimate the hazard ratio (HR) and its 95% confidence intervals (CIs) for the association between antioxidants intake and lung cancer risk. Time of follow-up time was defined as cohort entry date till the date of cancer diagnoses, death, drop-out, or the end of study through 2009. The daily antioxidant intake and fCDAI score of study population were divided into quartiles. Both dietary and supplemental intake were assessed for their associations with lung cancer incidence. Models were age-adjusted and additionally adjusted for other covariates, including study arm (usual care, screening), sex (male, female), education (<8 years, 8–11 years, high school, college, postgraduate), BMI (continuous), marital status (married, divorced, separated, widowed), family history of lung cancer (yes, no) or any other cancer (yes, no), smoking status (never, current, former), pack-years of cigarettes (continuous), and alcohol drinking status (yes, no). Tests for linear trend were conducted, and median intakes were used to denote corresponding quartiles in regression models. Dose-response analyses were further performed to examine the relationship between daily intake (dietary and supplemental) of antioxidants and lung cancer incidence using the restricted cubic splines. The 20th percentile of intake was set as the reference value. Lastly, we used random forest to select models of best combination of antioxidants for lung cancer prevention, grouped by sources of intake (food, supplements, all sources). All models adopted 80% of data as a train set and 20% of data as a test set. Average decrease in accuracy and mean-reduced Gini coefficient were two sorting methods used for random forests. All statistical analyses were performed by R Studio (4.2.1, Boston, MA, USA) and STATA (16.0, College Station, TX, USA).
3. Results
3.1. Baseline Characteristics
After the median follow-up of 12.2 years, there were 98,451 subjects, including 1642 cases of lung cancer being diagnosed. Table 1 shows the baseline characteristics of included participants by the quartile distribution of fCDAI scores. At the baseline, the mean (SD) age of subjects was 62.4 (5.28) years old. Males had a higher dietary antioxidant consumption than the female population (p < 0.001). Other significant differences were exhibited regarding age (p < 0.001), race (p < 0.001), study arm (p = 0.0023), education (p < 0.001), BMI (p < 0.001), marital status (p < 0.001), family history of any cancer (p = 0.0158), smoking status (p < 0.001), alcohol drinking status (p < 0.001), and total energy intake (p < 0.001). In addition, participants who consumed higher amounts of dietary antioxidants were more educated and more likely to be former cigarette smokers.
Table 1.
Baseline Characteristics of 98,451 participants from PLCO Cancer Screening Trial by fCDAI Quartiles.
| Title 1 | 1 (n = 24,613) |
2 (n = 24,613) |
3 (n = 24,613) |
4 (n = 24,612) |
Overall (n = 98,451) |
p-Value |
|---|---|---|---|---|---|---|
| Age | ||||||
| Mean (SD) | 62.7 (5.34) | 62.5 (5.28) | 62.4 (5.27) | 62.0 (5.20) | 62.4 (5.28) | <0.001 |
| Median [Min, Max] | 62.0 [53.0, 75.0] | 62.0 [54.0, 74.0] | 62.0 [54.0, 78.0] | 61.0 [54.0, 75.0] | 62.0 [53.0, 78.0] | |
| Sex | ||||||
| Male | 8861 (36.0%) | 10,742 (43.6%) | 12,585 (51.1%) | 15,474 (62.9%) | 47,662 (48.4%) | <0.001 |
| Female | 15,752 (64.0%) | 13,871 (56.4%) | 12,028 (48.9%) | 9138 (37.1%) | 50,789 (51.6%) | |
| Race | ||||||
| White, Non-Hispanic | 21,868 (88.8%) | 22,605 (91.8%) | 22,806 (92.7%) | 22,299 (90.6%) | 89,578 (91.0%) | <0.001 |
| Black, Non-Hispanic | 992 (4.0%) | 730 (3.0%) | 645 (2.6%) | 844 (3.4%) | 3211 (3.3%) | |
| Hispanic | 394 (1.6%) | 316 (1.3%) | 344 (1.4%) | 386 (1.6%) | 1440 (1.5%) | |
| Asian | 1182 (4.8%) | 817 (3.3%) | 664 (2.7%) | 874 (3.6%) | 3537 (3.6%) | |
| Pacific Islander | 111 (0.5%) | 103 (0.4%) | 102 (0.4%) | 139 (0.6%) | 455 (0.5%) | |
| American Indian | 58 (0.2%) | 31 (0.1%) | 45 (0.2%) | 62 (0.3%) | 196 (0.2%) | |
| Missing | 8 (0.0%) | 11 (0.0%) | 7 (0.0%) | 8 (0.0%) | 34 (0.0%) | |
| Study arm | ||||||
| Intervention | 12,422 (50.5%) | 12,468 (50.7%) | 12,722 (51.7%) | 12,795 (52.0%) | 50,407 (51.2%) | 0.00229 |
| Control | 12,191 (49.5%) | 12,145 (49.3%) | 11,891 (48.3%) | 11,817 (48.0%) | 48,044 (48.8%) | |
| Education | ||||||
| Less Than 8 Years | 177 (0.7%) | 115 (0.5%) | 138 (0.6%) | 167 (0.7%) | 597 (0.6%) | <0.001 |
| 8–11 Years | 1539 (6.3%) | 1307 (5.3%) | 1203 (4.9%) | 1216 (4.9%) | 5265 (5.3%) | |
| 12 Years or Completed High School | 6938 (28.2%) | 5883 (23.9%) | 5284 (21.5%) | 4815 (19.6%) | 22,920 (23.3%) | |
| Post High School Training Other than College | 3251 (13.2%) | 3204 (13.0%) | 3238 (13.2%) | 3036 (12.3%) | 12,729 (12.9%) | |
| Some College | 5515 (22.4%) | 5218 (21.2%) | 5213 (21.2%) | 5210 (21.2%) | 21,156 (21.5%) | |
| College Graduate | 3686 (15.0%) | 4440 (18.0%) | 4605 (18.7%) | 4607 (18.7%) | 17,338 (17.6%) | |
| Postgraduate | 3507 (14.2%) | 4446 (18.1%) | 4932 (20.0%) | 5561 (22.6%) | 18,446 (18.7%) | |
| BMI | ||||||
| 0–18.5 | 186 (0.8%) | 186 (0.8%) | 156 (0.6%) | 136 (0.6%) | 664 (0.7%) | <0.001 |
| 18.5–25 | 8780 (35.7%) | 8610 (35.0%) | 8338 (33.9%) | 7449 (30.3%) | 33,177 (33.7%) | |
| 25–30 | 10,041 (40.8%) | 10,373 (42.1%) | 10,595 (43.0%) | 10,940 (44.4%) | 41,949 (42.6%) | |
| 30+ | 5606 (22.8%) | 5444 (22.1%) | 5524 (22.4%) | 6087 (24.7%) | 22,661 (23.0%) | |
| Marital status | ||||||
| Married Or Living As Married | 18,461 (75.0%) | 19,464 (79.1%) | 19,755 (80.3%) | 19,575 (79.5%) | 77,255 (78.5%) | <0.001 |
| Widowed | 2545 (10.3%) | 2024 (8.2%) | 1812 (7.4%) | 1571 (6.4%) | 7952 (8.1%) | |
| Divorced | 2647 (10.8%) | 2199 (8.9%) | 2150 (8.7%) | 2385 (9.7%) | 9381 (9.5%) | |
| Separated | 196 (0.8%) | 162 (0.7%) | 172 (0.7%) | 228 (0.9%) | 758 (0.8%) | |
| Never Married | 764 (3.1%) | 764 (3.1%) | 724 (2.9%) | 853 (3.5%) | 3105 (3.2%) | |
| Family history of any cancer | ||||||
| No | 10,701 (43.5%) | 10,811 (43.9%) | 10,854 (44.1%) | 11,075 (45.0%) | 43,441 (44.1%) | 0.0158 |
| Yes, Immediate Family Member | 2665 (10.8%) | 2573 (10.5%) | 2556 (10.4%) | 2504 (10.2%) | 10,298 (10.5%) | |
| Possibly Relative or Cancer Type Not Clear | 632 (2.6%) | 559 (2.3%) | 581 (2.4%) | 591 (2.4%) | 2363 (2.4%) | |
| Smoking status | ||||||
| Never Smoked Cigarettes | 12,318 (50.0%) | 12,152 (49.4%) | 11,757 (47.8%) | 11,310 (46.0%) | 47,537 (48.3%) | <0.001 |
| Current Cigarette Smoker | 2579 (10.5%) | 2204 (9.0%) | 2135 (8.7%) | 2189 (8.9%) | 9107 (9.3%) | |
| Former Cigarette Smoker | 9716 (39.5%) | 10,257 (41.7%) | 10,721 (43.6%) | 11,113 (45.2%) | 41,807 (42.5%) | |
| Alcohol drinking status | ||||||
| Never | 3008 (12.2%) | 2593 (10.5%) | 2236 (9.1%) | 2103 (8.5%) | 9940 (10.1%) | <0.001 |
| Former | 3759 (15.3%) | 3494 (14.2%) | 3446 (14.0%) | 3570 (14.5%) | 14,269 (14.5%) | |
| Current | 17,001 (69.1%) | 17,838 (72.5%) | 18,323 (74.4%) | 18,308 (74.4%) | 71,470 (72.6%) | |
| Unknown | 845 (3.4%) | 688 (2.8%) | 608 (2.5%) | 631 (2.6%) | 2772 (2.8%) | |
| Total energy | ||||||
| Mean (SD) | 1050 (300) | 1480 (325) | 1850 (411) | 2570 (740) | 1740 (735) | <0.001 |
| Median [Min, Max] | 1030 [466, 4970] | 1450 [550, 5390] | 1810 [622, 5540] | 2450 [644, 5620] | 1610 [466, 5620] | |
| fCADI | ||||||
| Mean (SD) | −5.07 (1.23) | −2.07 (0.727) | 0.682 (0.928) | 6.46 (3.93) | −0.0000000675 (4.76) | <0.001 |
| Median [Min, Max] | −4.85 [−10.1, −3.34] | −2.08 [−3.34, −0.805] | 0.618 [−0.805, 2.43] | 5.29 [2.43, 42.0] | −0.805 [−10.1, 42.0] |
3.2. Association between Individual Antioxidants and Risk of Lung Cancer
Table 2 shows the associations between individual antioxidant nutrient intake from diets and supplements and lung cancer incidents among all subjects, stratified by quartiles of intake. α-carotene, β-carotene, vitamin A, vitamin C, vitamin E, magnesium, selenium, zinc, lycopene, lutein, and zeaxanthin were major antioxidants with high bioactivities we investigated. As shown in Table 2, both the age-adjusted and multi-adjusted models indicated that the higher dietary and total intake of β-carotene was associated with a decreased lung cancer risk. Compared with the lowest quartile, the highest quartile of total β-carotene intake, with a mean of 9775 mcg/day, appeared to be linked to a reduced lung cancer incidence (HR = 0.69, 95% CI: 0.59–0.80). A significant trend across quartiles was observed (p for trend < 0.001). A nonsignificant trend towards potentially harmful effects was shown among those who took daily β-carotene supplements (HR: 1.04, 95% CI: 0.94–1.15, p = 0.453) in the multi-adjusted model.
Table 2.
Hazard Ratios (HRs) of the association between total, dietary, and supplemental antioxidants intake and lung cancer incidence by quartiles of intake.
| Nutrients | Range | Mean | Cohort | Cases | Incidence Rate (95% CI) a | Age-Adjusted HR (95% CI), p-Value | Multi-Adjusted HR (95% CI), p-Value | |
|---|---|---|---|---|---|---|---|---|
| Total β-carotene (mcg/day) | ||||||||
| Q1 | <2278.77 | 1556.37 | 24,614 | 516 | 0.052 (0.047–0.056) | Reference group | Reference group | |
| Q2 | ≥2278.77 to <3504.26 | 2864.17 | 24,616 | 455 | 0.045 (0.041–0.050) | 0.86 (0.76–0.97), p = 0.017 | 0.98 (0.86–1.11), p = 0.761 | |
| Q3 | ≥3504.26 to <5665.90 | 4422.85 | 24,609 | 374 | 0.037 (0.034–0.041) | 0.70 (0.61–0.80), p = 1.04 × 10−7 | 0.86 (0.75–0.99), p = 0.039 | |
| Q4 | ≥5665.90 | 9775.35 | 24,612 | 297 | 0.030 (0.027–0.033) | 0.55 (0.48–0.63), p < 2 × 10−16 | 0.69 (0.59–0.80), p = 1.36 × 10−6 | |
| P for linear trend | P for trend < 2 × 10−16 | P for trend = 1.75 × 10−7 | ||||||
| Dietary β-carotene | ||||||||
| Q1 | <1634.06 | 1137.74 | 24,613 | 547 | 0.055 (0.050–0.059) | Reference group | Reference group | |
| Q2 | ≥1634.06 to <2654.56 | 2119.15 | 24,613 | 426 | 0.043 (0.039–0.047) | 0.76 (0.67–0.86), p = 2.30 × 10−5 | 0.84 (0.74–0.95), p = 0.007 | |
| Q3 | ≥2654.56 to <4428.63 | 3423.88 | 24,612 | 379 | 0.038 (0.034–0.042) | 0.67 (0.58- 0.76), p = 1.17 × 10−9 | 0.79 (0.69–0.91), p = 0.001 | |
| Q4 | ≥4428.63 | 7527.37 | 24,613 | 290 | 0.029 (0.026–0.033) | 0.51 (0.44–0.59), p ≤ 2 × 10−16 | 0.62 (0.53–0.72), p = 1.03 × 10−9 | |
| P for linear trend | P for trend < 2 × 10−16 | P for trend = 3.79 × 10−9 | ||||||
| β-carotene supplements | ||||||||
| No | 0 | 0 | 37,113 | 651 | 0.065 (0.060–0.070) | Reference group | Reference group | |
| Yes | ≤2700 | 294.93 | 61,338 | 991 | 0.099 (0.093–0.105) | 0.93 (0.84–1.02), p = 0.125 | 1.04 (0.94–1.15), p = 0.453 | |
| Total vitamin A (IU/day) | ||||||||
| Q1 | <7422.5 | 4991.567 | 24,613 | 490 | 0.049 (0.045–0.054) | Reference group | Reference group | |
| Q2 | ≥7422.5 to <10,810.0 | 9139.481 | 24,616 | 475 | 0.048 (0.043–0.052) | 0.94 (0.83–1.07), p = 0.378 | 1.05 (0.92–1.19), p = 0.450 | |
| Q3 | ≥10,810.0 to <15,489.5 | 12,873.09 | 24,609 | 375 | 0.037 (0.034–0.041) | 0.73 (0.64–0.84), p = 4.58 × 10−6 | 0.86 (0.74–0.98), p = 0.029 | |
| Q4 | ≥15,489.5 | 22,986.43 | 24,613 | 302 | 0.030 (0.027–0.034) | 0.58 (0.51–0.67), p = 2.23 × 10−13 | 0.72 (0.61–0.84), p = 2.28 × 10−5 | |
| P for linear trend | P for trend = 9.16 × 10−16 | P for trend = 1.60 × 10−6 | ||||||
| Dietary vitamin A | ||||||||
| Q1 | <4993.5 | 3652.943 | 24,615 | 518 | 0.052 (0.048–0.056) | Reference group | Reference group | |
| Q2 | ≥4993.5 to <7513.7 | 6212.687 | 24,612 | 446 | 0.045 (0.041–0.049) | 0.83 (0.73–0.94), p = 0.004 | 0.85 (0.75–0.97), p = 0.0155 | |
| Q3 | ≥7513.7 to <11,610.5 | 9303.387 | 24,612 | 375 | 0.038 (0.034–0.041) | 0.68 (0.60–0.78), p = 2.21 × 10−8 | 0.75 (0.65–0.87), p = 0.0001 | |
| Q4 | ≥11,610.5 | 18,619.62 | 24,612 | 303 | 0.030 (0.027–0.034) | 0.55 (0.48–0.64), p < 2 × 10−16 | 0.63 (0.54–0.74), p = 1.73 × 10−8 | |
| P for linear trend | P for trend <2 × 10−16 | P for trend = 2.88 × 10−8 | ||||||
| Vitamin A supplements | ||||||||
| No | 0 | 0 | 37,183 | 653 | 0.065 (0.060–0.071) | Reference group | Reference group | |
| Yes | ≤30,000 | 4901.844 | 61,268 | 989 | 0.099 (0.093–0.105) | 0.93 (0.84–1.02), p = 0.124 | 1.03 (0.93–1.14), p = 0.578 | |
| Total vitamin C (mg/day) | ||||||||
| Q1 | <127.85 | 85.368 | 24,613 | 536 | 0.054 (0.049–0.058) | Reference group | Reference group | |
| Q2 | ≥127.85 to <210.22 | 165.918 | 24,615 | 364 | 0.036 (0.033–0.040) | 0.65 (0.57–0.74), p = 1.40 × 10−10 | 0.82 (0.71–0.93), p = 0.003 | |
| Q3 | ≥210.22 to <563.31 | 328.331 | 24,610 | 358 | 0.036 (0.032–0.040) | 0.64 (0.56–0.73), p = 3.44 × 10−11 | 0.81 (0.70–0.93), p = 0.003 | |
| Q4 | ≥563.31 | 925.296 | 24,613 | 384 | 0.038 (0.035–0.042) | 0.67 (0.59–0.76), p = 2.03 × 10−9 | 0.82 (0.72–0.94), p = 0.005 | |
| P for linear trend | P for trend = 0.0001 | P for trend = 0.0625 | ||||||
| Dietary vitamin C | ||||||||
| Q1 | <80.03 | 56.195 | 24,616 | 558 | 0.056 (0.051–0.061) | Reference group | Reference group | |
| Q2 | ≥80.03 to <120.77 | 100.178 | 24,614 | 393 | 0.039 (0.036–0.043) | 0.67 (0.59–0.76), p = 8.22 × 10−10 | 0.81 (0.71–0.92), p = 0.001 | |
| Q3 | ≥120.77 to <171.92 | 144.357 | 24,614 | 342 | 0.034 (0.031–0.038) | 0.56 (0.49–0.64), p < 2 × 10−16 | 0.72 (0.62–0.82), p = 2.98 × 10−6 | |
| Q4 | ≥171.92 | 251.426 | 24,607 | 349 | 0.035 (0.031–0.039) | 0.58 (0.50–0.66), p = 6.34 × 10−16 | 0.73 (0.63–0.85), p = 4.43 × 10−5 | |
| P for linear trend | P for trend = 2.84 × 10−15 | P for trend = 4.04 × 10−5 | ||||||
| Vitamin C supplements | ||||||||
| No | 0 | 0 | 26,670 | 506 | 0.051 (0.046–0.055) | Reference group | Reference group | |
| Yes | ≤2102.86 | 326.698 | 71,781 | 1136 | 0.114 (0.107–0.120) | 0.83 (0.75–0.93), p = 0.0007 | 0.98 (0.88–1.09), p = 0.656 | |
| Total vitamin E (mg/day) | ||||||||
| Q1 | <13.23 | 7.452 | 24,614 | 465 | 0.046 (0.042–0.051) | Reference group | Reference group | |
| Q2 | ≥13.23 to <34.42 | 24.914 | 24,613 | 452 | 0.045 (0.041–0.050) | 0.97 (0.86–1.11), p = 0.685 | 1.07 (0.93–1.21), p = 0.348 | |
| Q3 | ≥34.42 to <291.94 | 189.001 | 24,612 | 342 | 0.034 (0.031–0.038) | 0.71 (0.62–0.81), p = 1.23 × 10−6 | 0.83 (0.72–0.96), p = 0.012 | |
| Q4 | ≥291.94 | 387.723 | 24,612 | 383 | 0.038 (0.035–0.042) | 0.79 (0.69–0.91), p = 0.0007 | 0.89 (0.78–1.02), p = 0.103 | |
| P for linear trend | P for trend = 5.81 × 10−7 | P for trend = 0.0016 | ||||||
| Dietary vitamin E | ||||||||
| Q1 | <4.971 | 3.768 | 24,644 | 477 | 0.048 (0.044–0.052) | Reference group | Reference group | |
| Q2 | ≥4.971 to <6.901 | 5.924 | 24,613 | 391 | 0.039 (0.035–0.043) | 0.83 (0.73–0.95), p = 0.007 | 0.82 (0.71–0.92), p = 0.006 | |
| Q3 | ≥6.901 to <9.591 | 8.119 | 24,582 | 399 | 0.040 (0.036–0.044) | 0.86 (0.75–0.98), p = 0.028 | 0.82 (0.62–0.82), p = 0.008 | |
| Q4 | ≥9.591 | 13.531 | 24,612 | 375 | 0.038 (0.034–0.041) | 0.84 (0.73–0.96), p = 0.009 | 0.72 (0.63–0.85), p = 0.0005 | |
| P for linear trend | P for trend = 0.031 | P for trend = 0.0017 | ||||||
| Vitamin E supplements | ||||||||
| No | 0 | 0 | 25,133 | 490 | 0.049 (0.045–0.054) | Reference group | Reference group | |
| Yes | ≤ 690.1 | 192.445 | 73,318 | 1152 | 0.115 (0.109–0.122) | 0.79 (0.71–0.88), p = 1.17 × 10−5 | 0.91 (0.82–1.02), p = 0.097 | |
| Total magnesium (mg/day) | ||||||||
| Q1 | <273.73 | 213.779 | 24,615 | 397 | 0.040 (0.036–0.044) | Reference group | Reference group | |
| Q2 | ≥273.73 to <354.16 | 314.646 | 24,612 | 392 | 0.039 (0.036–0.043) | 0.98 (0.86–1.13), p = 0.824 | 0.95 (0.82–1.10), p = 0.477 | |
| Q3 | ≥354.16 to <446.29 | 397.172 | 24,612 | 418 | 0.042 (0.038–0.046) | 1.04 (0.91–1.20), p = 0.547 | 0.97 (0.83–1.12), p = 0.657 | |
| Q4 | ≥446.29 | 554.459 | 24,612 | 435 | 0.044 (0.040–0.048) | 1.12 (0.97–1.28), p = 0.112 | 0.92 (0.77–1.10), p = 0.369 | |
| P for linear trend | P for trend = 0.0684 | P for trend = 0.4266 | ||||||
| Dietary magnesium | ||||||||
| Q1 | <233.03 | 183.725 | 24,616 | 389 | 0.039 (0.035–0.043) | Reference group | Reference group | |
| Q2 | ≥233.03 to <303.16 | 268.121 | 24,610 | 388 | 0.039 (0.035–0.043) | 0.98 (0.85–1.13), p = 0.822 | 0.94 (0.81–1.08), p = 0.371 | |
| Q3 | ≥303.16 to <389.11 | 342.821 | 24,613 | 404 | 0.040 (0.037–0.045) | 1.03 (0.89–1.18), p = 0.724 | 0.90 (0.77–1.05), p = 0.187 | |
| Q4 | ≥389.11 | 495.427 | 24,612 | 461 | 0.046 (0.042–0.051) | 1.21 (1.05–1.38), p = 0.007 | 0.94 (0.77–1.14), p = 0.546 | |
| P for linear trend | P for trend = 0.0025 | P for trend = 0.5619 | ||||||
| Magnesium supplements | ||||||||
| No | 0 | 0 | 45,192 | 806 | 0.081 (0.075–0.086) | Reference group | Reference group | |
| Yes | ≤100 | 87.787 | 53,259 | 836 | 0.084 (0.078–0.089) | 0.89 (0.81–0.98), p = 0.021 | 1.00 (0.90–1.10), p = 0.933 | |
| Total selenium (mcg/day) | ||||||||
| Q1 | <59.57 | 45.411 | 24,621 | 416 | 0.042 (0.038–0.046) | Reference group | Reference group | |
| Q2 | ≥59.57 to <81.65 | 70.528 | 24,612 | 383 | 0.038 (0.035–0.042) | 0.93 (0.81–1.07), p = 0.336 | 0.88 (0.76–1.02), p = 0.079 | |
| Q3 | ≥81.65 to <110.78 | 94.956 | 24,613 | 401 | 0.040 (0.036–0.044) | 1.00 (0.87–1.15), p = 0.972 | 0.86 (0.73–1.00), p = 0.054 | |
| Q4 | ≥110.78 | 147.879 | 24,605 | 442 | 0.044 (0.040–0.049) | 1.16 (1.01–1.33), p = 0.031 | 0.84 (0.68–1.03), p = 0.093 | |
| P for linear trend | P for trend = 0.0086 | P for trend = 0.1306 | ||||||
| Dietary selenium | ||||||||
| Q1 | <58.33 | 44.648 | 24,624 | 416 | 0.042 (0.038–0.046) | Reference group | Reference group | |
| Q2 | ≥58.33 to <79.27 | 68.767 | 24,611 | 379 | 0.038 (0.034–0.042) | 0.92 (0.80–1.06), p = 0.263 | 0.86 (0.74–0.99), p = 0.037 | |
| Q3 | ≥79.27 to <106.76 | 91.811 | 24,605 | 414 | 0.041 (0.038–0.046) | 1.04 (0.91–1.19), p = 0.588 | 0.88 (0.75–1.03), p = 0.105 | |
| Q4 | ≥106.76 | 142.739 | 24,611 | 433 | 0.043 (0.039–0.048) | 1.14 (1.00–1.30), p = 0.059 | 0.75 (0.61–0.93), p = 0.010 | |
| P for linear trend | P for trend = 0.0139 | P for trend = 0.0187 | ||||||
| Selenium supplements | ||||||||
| No | 0 | 0 | 92,249 | 1550 | 0.155 (0.147–0.163) | Reference group | Reference group | |
| Yes | ≤42.86 | 42.86 | 6202 | 92 | 0.009 (0.008–0.011) | 0.88 (0.71–1.08), p = 0.219 | 0.89 (0.72–1.09), p = 0.263 | |
| Total zinc (mg/day) | ||||||||
| Q1 | <9.95 | 7.012 | 24,616 | 446 | 0.045 (0.041–0.049) | Reference group | Reference group | |
| Q2 | ≥9.95 to <19.84 | 14.354 | 24,632 | 397 | 0.040 (0.036–0.044) | 0.93 (0.81–1.06), p = 0.265 | 0.89 (0.77–1.02), p = 0.098 | |
| Q3 | ≥19.84 to <25.77 | 22.811 | 24,602 | 395 | 0.040 (0.036–0.044) | 0.90 (0.78–1.03), p = 0.113 | 0.94 (0.82–1.08), p = 0.399 | |
| Q4 | ≥25.77 | 34.401 | 24,601 | 404 | 0.040 (0.037–0.045) | 0.92 (0.81–1.06), p = 0.243 | 0.86 (0.74–1.00), p = 0.053 | |
| P for linear trend | P for trend = 0.219 | P for trend = 0.1519 | ||||||
| Dietary zinc | ||||||||
| Q1 | <7.00 | 5.381 | 24,626 | 413 | 0.041 (0.038–0.045) | Reference group | Reference group | |
| Q2 | ≥7.00 to <9.48 | 8.234 | 24,688 | 398 | 0.040 (0.036–0.044) | 0.97 (0.84–1.11), p = 0.650 | 0.94 (0.81–1.08), p = 0.367 | |
| Q3 | ≥9.48 to <12.90 | 11.016 | 24,528 | 406 | 0.041 (0.037–0.045) | 1.02 (0.89–1.17), p = 0.812 | 0.93 (0.79–1.09), p = 0.351 | |
| Q4 | ≥12.90 | 17.816 | 24,609 | 425 | 0.042 (0.039–0.047) | 1.09 (0.95–1.25), p = 0.204 | 0.82 (0.67–0.99), p = 0.041 | |
| P for linear trend | P for trend = 0.119 | P for trend = 0.0430 | ||||||
| Zinc supplements | ||||||||
| No | 0 | 0 | 42,257 | 765 | 0.076 (0.071–0.082) | Reference group | Reference group | |
| Yes | ≤ 36.43 | 15.825 | 56,194 | 877 | 0.088 (0.082–0.094) | 0.87 (0.79–0.96), p = 0.004 | 0.96 (0.87–1.06), p = 0.460 | |
| Dietary lycopene (mg/day) | ||||||||
| Q1 | <3108.61 | 2170.982 | 24,613 | 469 | 0.047 (0.043–0.051) | Reference group | Reference group | |
| Q2 | ≥3108.61 to <4761.09 | 3908.915 | 24,613 | 390 | 0.039 (0.035–0.043) | 0.85 (0.75–0.98), p = 0.0220 | 0.87 (0.76–0.99), p = 0.0408 | |
| Q3 | ≥4761.09 to <7491.66 | 5948.377 | 24,612 | 373 | 0.037 (0.034–0.041) | 0.85 (0.74–0.97), p = 0.0175 | 0.84 (0.73–0.97), p = 0.0157 | |
| Q4 | ≥7491.66 | 13,823.78 | 24,613 | 410 | 0.041 (0.037–0.045) | 0.96 (0.84–1.10), p = 0.5347 | 0.83 (0.71–0.96), p = 0.0144 | |
| P for linear trend | P for trend = 0.977 | P for trend = 0.0437 | ||||||
| Dietary lutein and zeaxanthin (mg/day) | ||||||||
| Q1 | <1246.48 | 897.2885 | 24,613 | 511 | 0.051 (0.047–0.056) | Reference group | Reference group | |
| Q2 | ≥1246.48 to <1906.74 | 1563.793 | 24,613 | 428 | 0.043 (0.039–0.047) | 0.83 (0.73–0.94), p = 0.0042 | 0.95 (0.83–1.08), p = 0.4021 | |
| Q3 | ≥1906.74 to <3002.04 | 2379.873 | 24,612 | 364 | 0.036 (0.033–0.040) | 0.71 (0.62–0.81), p = 4.47 × 10−7 | 0.83 (0.72–0.96), p = 0.0112 | |
| Q4 | ≥3002.04 | 5674.224 | 24,613 | 339 | 0.034 (0.030–0.038) | 0.66 (0.57–0.76), p = 2.84 × 10−9 | 0.78 (0.67–0.91), p = 0.0015 | |
| P for linear trend | P for trend = 1.02 × 10−8 | P for trend = 0.0011 | ||||||
| Dietary α-carotene (mg/day) | ||||||||
| Q1 | <295.22 | 193.6037 | 24,615 | 532 | 0.053 (0.049–0.058) | Reference group | Reference group | |
| Q2 | ≥295.22 to <545.47 | 412.4326 | 24,612 | 460 | 0.046 (0.042–0.050) | 0.84 (0.74–0.95), p = 0.0059 | 0.91 (0.80–1.03), p = 0.1321 | |
| Q3 | ≥545.47 to <995.90 | 745.5172 | 24,611 | 365 | 0.037 (0.033–0.040) | 0.64 (0.56–0.74), p = 9.46 × 10−11 | 0.76 (0.66–0.87), p = 9.23 × 10−5 | |
| Q4 | ≥995.90 | 2010.004 | 24,613 | 285 | 0.029 (0.025–0.032) | 0.50 (0.44–0.58), p < 2 × 10−16 | 0.64 (0.55–0.74), p = 1.12 × 10−8 | |
| P for linear trend | P for trend < 2 × 10−16 | P for trend = 4.55 × 10−9 | ||||||
a. Per 10,000 person-years.
After adjusting multiple covariates, protective effects were also found in total and dietary intake of vitamin A, dietary vitamin C, total and dietary vitamin E, dietary selenium, dietary zinc, dietary lycopene, dietary lutein and zeaxanthin, and dietary α-carotene, where significant trends across quartiles were observed in each antioxidant nutrient. The lowest HR was observed in the third quartile of total and dietary vitamin C consumption, compared to HRs of other quartiles. When total vitamin C intake was treated as a continuous variable, a decreased and then slightly increased risk of lung cancer was observed using the restricted cubic spline model (reference value = 113.31 mg/day, p for nonlinear = 0.005) (Figure S1). A similar trend was observed in dietary lycopene (Figure S2). Significant nonlinear dose-response curves were also shown in total β-carotene, dietary α-carotene, dietary lutein, and zeaxanthin intake with a sharp decrease of risk at a lower dosage level followed by a continuous but slower decreased risk at higher dosage levels (Figures S3–S5). Noted, there were no significant associations observed among supplemental β-carotene, vitamin A, vitamin C, vitamin E, magnesium, and zinc intake with lung cancer risk (Figures S6–S11).
3.3. Association between fCADI Score and Risk of Lung Cancer
Table 3 demonstrates the associations between fCADI scores and lung cancer incidence by quartiles. The overall HRs showed that a higher quartile indicated a lower risk of lung cancer in the multi-adjusted model. Compared to the 1st quartile, the highest quartile of fCADI scores was linked to a 36% risk reduction in lung cancer (HR = 0.64, 95% CI: 0.52, 0.79). Lung cancer incidence decreased as fCADI scores increased (P for trend < 0.001).
Table 3.
Hazard Ratios (HRs) of the associations between fCADI score and lung cancer incidence by quartiles of intake.
| fCADI in Quartile | Range | Cohort | Cases | Incidence Rate (95% CI) a |
Age-Adjusted HR (95%CI), p-Value | Multi-Adjusted HR (95% CI), p-Value |
|---|---|---|---|---|---|---|
| Overall | ||||||
| Q1 | <−3.3440 | 24,613 | 459 | 0.046 (0.042–0.050) | Reference group | Reference group |
| Q2 | ≥−3.3440 to <−0.8054 | 24,613 | 417 | 0.042 (0.038–0.046) | 0.90 (0.79–1.03), p = 0.127 | 0.85 (0.74–0.97), p = 0.019 |
| Q3 | ≥−0.8054 to <2.4260 | 24,613 | 369 | 0.037 (0.033–0.041) | 0.80 (0.70–0.92), p = 0.002 | 0.70 (0.60–0.82), p = 1.32 × 10−5 |
| Q4 | ≥2.4260 | 24,612 | 397 | 0.040 (0.036–0.044) | 0.89 (0.78–1.02), p = 0.084 | 0.64 (0.52–0.79), p = 2.78 × 10−5 |
| P for linear trend | P for trend = 0.062 | P for trend < 0.001 |
a. Per 10,000 person-years.
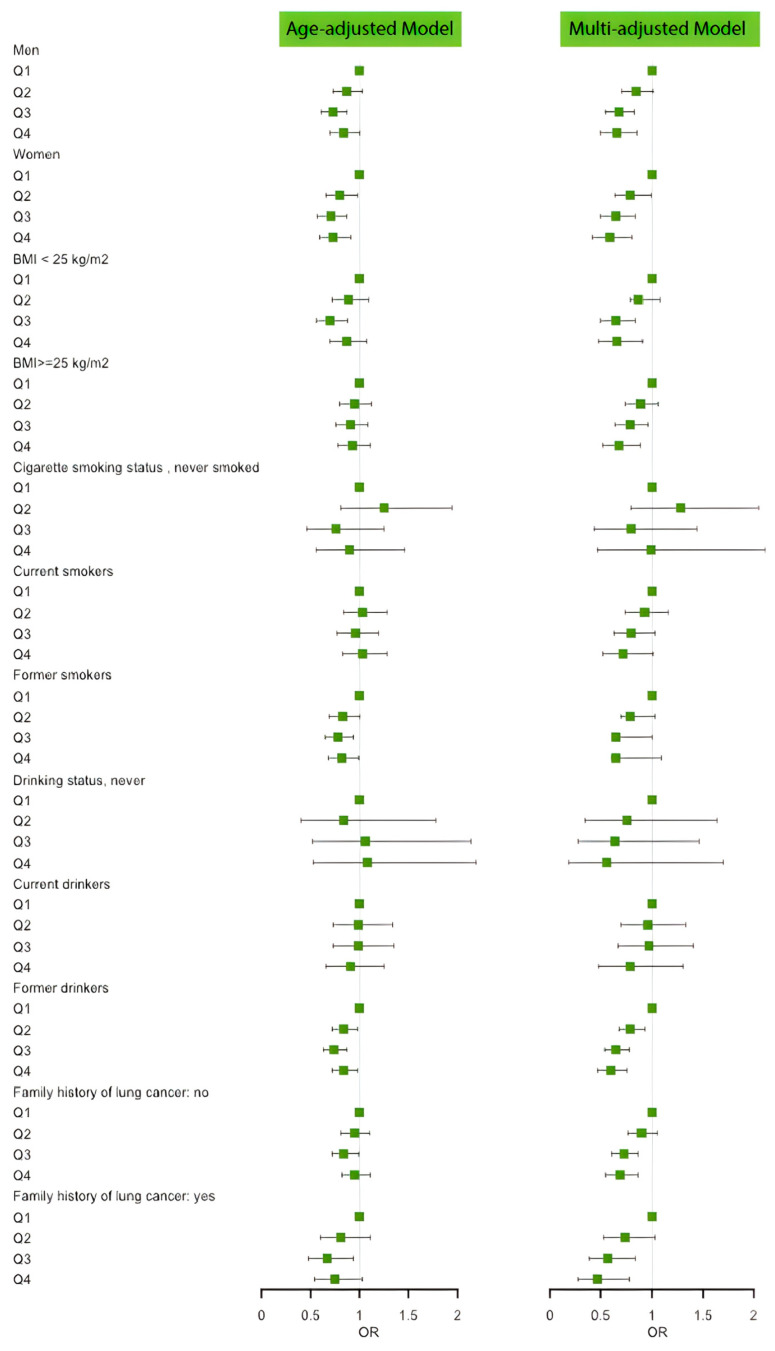
Figure 2 and Table A1 show the results of the subgroup analyses in different populations. Pronounced protective linear trends were also observed among male, female, normal weight and overweight individuals, current cigarette smokers, current alcohol drinkers, and individuals with and without a family history of lung cancer. However, no effects were noted among individuals who have never smoked or formerly smoked and individuals who have never drunk or formerly drank.
Figure 2.
Forest plot of the associations between fCADI Score and lung cancer incidence by subgroups.
3.4. Best Combination of Antioxidants Model Selection and Random Forest
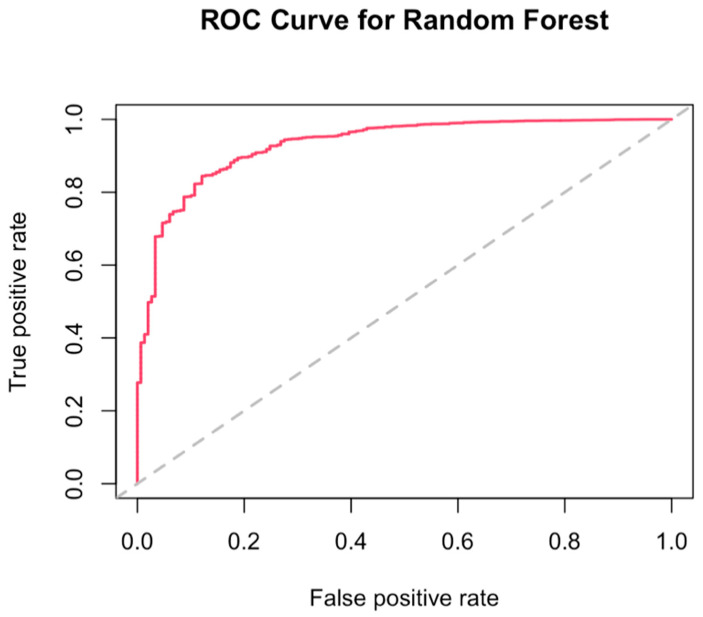
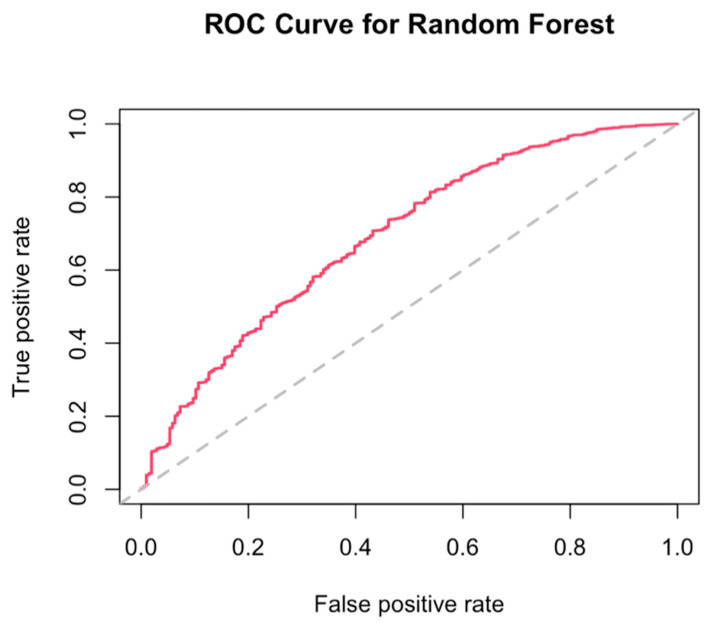
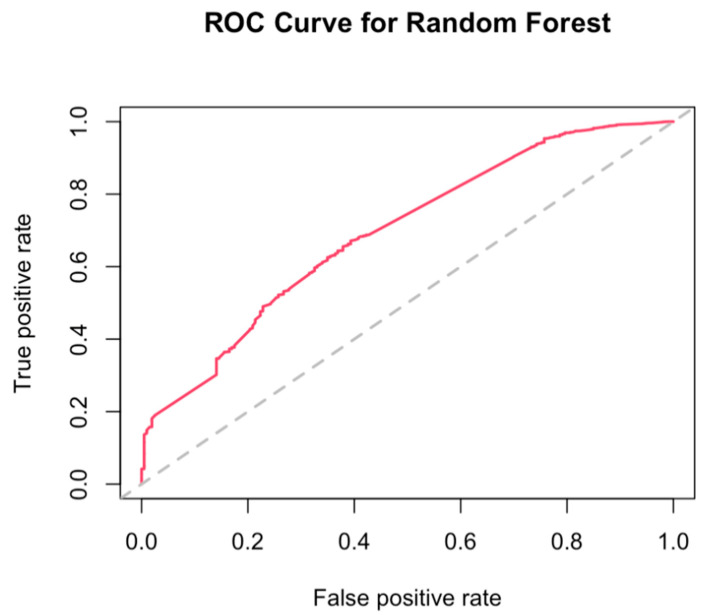
Figure 3 shows the receiver operating characteristic (ROC) curve to illustrate the possibly optimal models of antioxidants combination on lung cancer incidence reduction, grouped by sources of intake. For the dietary intake group, the variable importance ranking was α-carotene, followed by magnesium, vitamin C, vitamin E, lycopene, selenium, lutein, and zeaxanthin, and β-carotene. When the mtry value (the random number used by each tree) equaled three, it had the lowest out-of-bag data error rate. The prediction accuracy was 0.88, and the area under the ROC curve (AUC) was 0.89, which indicated a good fit. The models for the supplemental group and total intake group were less acceptable than the dietary group, with AUC equaling 0.69 for both (Figure A1 and Figure A2).
Figure 3.
A receiver operating characteristic (ROC) curve for the dietary group.
4. Discussion
This study investigated the association between antioxidant micronutrient intake and the risk of lung cancer in a large US population, with the majority being Caucasians. After adjusting for age, sex, BMI, smoking, and other risk factors for lung cancer based on previous literature, we found an inverse association between overall dietary antioxidants intake and incidence of lung cancer, as illustrated by the fCADI score. Higher total individual antioxidant intakes, including β-carotene, vitamin A, and vitamin E, were also associated with a decreased risk of lung cancer. Protective effects of dietary antioxidant intake were observed across all individual antioxidant micronutrients except magnesium. However, dietary magnesium played the second most important role in the best dietary model selected by random forest for lung cancer prevention, and the antioxidant combination was α-carotene, magnesium, vitamin C, vitamin E, lycopene, selenium, lutein and zeaxanthin, and β-carotene. Moreover, we did not notice any significant effects of supplemental antioxidant intake on lung cancer risk. In subgroup analyses, protective effects were not modified by sex, BMI, or family history of lung cancer. Effect modifications were shown among never and former smokers and drinkers, and the advantages of total diet-derived antioxidants were only observed among current smokers and current alcohol drinkers.
Previous evidence suggested an inverse association between dietary antioxidant intake and several cancer risks, including prostate cancer, digestive cancer, and lung cancer [16,17,18]. In the present study, we confirmed previous results on lung cancer risk and moved the field forward by individually examining each micronutrient from both dietary and supplemental sources. We found that total β-carotene, vitamin A, and vitamin E intake was associated with a decreased risk of lung cancer, while we only observed protective effects from dietary vitamin C, selenium, and zinc. Moreover, we only obtained data on lycopene, lutein and zeaxanthin, and α-carotene from dietary sources, and protective effects were found in all of them. Magnesium was the only micronutrient not linked to lung cancer risk in both dietary and supplemental forms. The results from the present study were in line with another prospective population-based cohort study in the Netherlands, which demonstrated dietary zinc intake decreased the risk of lung cancer by 42%, while no association was observed with dietary magnesium [19].
Although the protective effects of the above nutrients were recorded in many previous studies and confirmed in the current study, one randomized controlled trial and meta-analyses did not advocate for the high-dose use of β-carotene, vitamin C, and vitamin E supplementation for cancer prevention purposes in the generally healthy population, as no beneficial effects on total cancer incidence and mortality were shown [20,21]. A recent Swedish study illustrated the potential biological basis behind the scenes [22]. Given that lung cancer cells require lots of energy, such as sugar, to grow and multiply rapidly, this faster energy-making process would cause tremendous oxidative stress on cells by generating free oxygen radicals [22,23]. The antioxidant supplements would support lung cancer cells to withstand the stress and thrive [22]. In line with the result of this present study that the daily use of β-carotene supplementation, with a mean of 295 mcg/day, might potentially have a 4% incremental risk of developing lung cancer. The effect was not significant, and this might be due to the dosage being relatively low compared to the dosage in clinical interventions, that we were unable to observe the potential harmful effects at a high dosage. In addition, since the baseline population was generally healthy, we deemed participants who reported the daily consumption of β-carotene supplementation did not take it separately but as part of the multivitamin formula, which might minimize the harmful effects of sole supplemental β-carotene. When β-carotene supplement is consumed individually, a high risk of cardiovascular outcomes is associated, and the harmful effects are more pronounced among current smokers [24]. This is probably due to the fact that under high oxygen concentrations, β-carotene undergoes a pro-oxidant mechanism, which may indirectly induce negative health consequences [24]. However, when β-carotene is obtained from food sources or consumed with other micronutrient supplements together, the interactions between different antioxidants may produce synergic effects on attenuating the pro-oxidant property of β-carotene under certain circumstances [24]. Besides β-carotene, the dose-response analyses in current study also revealed a decreased and slightly increased trend for total vitamin C intake, showing that moderate consumption (500 mg/day) was optimal for lung cancer prevention and the high intake of vitamin C should be treated with caution. Hence, the sources where the antioxidants being obtained from and the dosage level of antioxidants consumption yield mixed results on lung cancer incidence prevention, which requires more evidence to make firm conclusions in future studies.
It should be noticed that lifestyle factors also play an important role in effect modification. Previous large clinical trials have demonstrated that among smoking populations, β-carotene supplementation increases the risk of lung cancer, independent of tar or nicotine level of cigarettes, and this is due to the property of β-carotene as a pro-oxidant under the free-radical-rich condition of smokers [25,26,27,28]. In the present observational study, we noted a 4% nonsignificantly elevated risk among people who took β-carotene supplementation daily, while we did not observe a dose-response relationship between the supplementation and lung cancer events since a very small portion of participants consumed a high-dose basis (>30 mg/d). Additionally, in the current study, we noted that among current smokers and drinkers, a higher fCADI score was associated with a significant risk reduction of lung cancer. In the subgroup of current smokers, we detected a significant linear trend of protective effects of total diet-derived antioxidants intake, while the effects were not shown among never or former users. The results were consistent with a recent case-control study conducted in Canada that among heavy and moderate smokers, an inverse association was found between elevated dietary intakes of β-carotene, α-carotene, and lycopene in male and vitamin C in females and lung cancer risk [29]. However, current smokers only took up 9.3% of the total population in our study, and the finding should be treated with caution. Given the above, the results of the present study suggested that individuals with different lifestyles might have varied responses to the effects of antioxidants on lung cancer risk. Therefore, when conducting future dietary interventions, additional considerations should be made in assessing different lifestyle factors. More research is also needed to explore the interactions between antioxidant intake and lifestyle factors.
We applied the fCADI score to examine the combined effects of individual antioxidants and proved that jointly consumed antioxidants together could significantly reduce lung cancer risk. Furthermore, to the best of our knowledge, this is the first study using the machine learning method to select the optimal model of a random forest, rank the importance of antioxidants, and find the best combination by groups. Since previous studies yielded mixed results on the effects of diet-derived antioxidants in different cohort settings, we applied this method to incorporate more input parameters to fit the prediction model with a potentially higher accuracy level. We noticed that the dietary group model with antioxidants intake from food sources had the highest prediction value on lung cancer prevention, with α-carotene intake being the most important factor, while models for the other two groups did not present sufficient prediction value. The strong protective effects of α-carotene were also demonstrated in a pooled analysis of two large U.S. cohorts that increased α-carotene intake was associated with a 63% risk reduction of lung cancer among non-smokers [30]. Another population-based study using data from the Third Nutrition and Health Examination Survey (NHANES III) also showed that a high serum level of α-carotene at baseline was linked to a lower risk of lung cancer and a 46% risk reduction of death among current smokers [31]. Moreover, although we did not observe the protective effect of individual dietary intake of magnesium in this cohort, it was the second most important predictor in the model, after α-carotene. This might be due to the potential synergic effect which altered the association, while the underlying biological mechanisms are still unclear, which require further research in future studies.
Of note, the present study has several unique strengths. First, this study has a relatively long follow-up period, approximately 12 years, to collect lung cancer diagnoses. Second, the study cohort is very large and has a wide representativeness of the US population. Study participants were recruited across 10 screening centers in the US. Third, due to the nature of this prospective cohort study, the reverse causation bias could be reduced. Forth, in the subgroup analyses, we interestingly noted the inverse association between fCADI score and lung cancer incidence is more pronounced among current smokers and drinkers.
Nevertheless, several limitations need to be addressed. First, the dietary information was only collected once at the baseline, while the diets might change during the long follow-up period, which could lead to misclassifications. However, this could also help minimize the bias of reverse causation. Second, because DHQ was self-reported, the information might not be accurate enough, and recall bias could exist. Third, 36.4% of study participants were excluded due to failure to complete valid BQ or DHQ and missing important covariates. We could not evaluate the difference between the selected group and excluded group, leading to possible selection bias.
5. Conclusions
Our results suggested that a higher overall dietary antioxidant consumption from various food sources was associated with a lower risk of lung cancer. No protective effects were shown in antioxidant supplements. Lifestyle factors, including smoking and alcohol drinking, might modify the observed associations. In addition, future studies should investigate the potential interactions between vitamins and minerals in lung cancer prevention.
Acknowledgments
We gratefully thank the NCI for providing access to the PLCO cancer screening trial dataset and acknowledge all participants for their commitments to the study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12020338/s1, Figure S1: Dose-response curve for total vitamin C intake and lung cancer incidence; Figure S2: Dose-response curve for dietary lycopene intake and lung cancer incidence; Figure S3: Dose-response curve for total β-carotene intake and lung cancer incidence; Figure S4: Dose-response curve for dietary α-carotene intake and lung cancer incidence; Figure S5: Dose-response curve for dietary lutein and zeaxanthin intake and lung cancer incidence; Figure S6: Dose-response curve for supplemental β-carotene intake and lung cancer incidence; Figure S7: Dose-response curve for supplemental vitamin A intake and lung cancer incidence; Figure S8: Dose-response curve for supplemental vitamin C intake and lung cancer incidence; Figure S9: Dose-response curve for supplemental vitamin E intake and lung cancer incidence; Figure S10: Dose-response curve for supplemental magnesium intake and lung cancer incidence; Figure S11: Dose-response curve for supplemental zinc intake and lung cancer incidence.
Appendix A
Table A1.
Hazard Ratios (HRs) of the associations between fCADI Score and lung cancer incidence by quartiles of intake and subgroups.
| fCADI in Quartile | Range | Cohort | Cases | Incidence Rate (95% CI) a | Age-Adjusted HR (95% CI), p-Value | Multi-Adjusted HR (95% CI), p-Value |
|---|---|---|---|---|---|---|
| Men | ||||||
| Q1 | <−2.5551 | 11,916 | 285 | 0.029 (0.025–0.032) | Reference group | Reference group |
| Q2 | ≥−2.5551 to <−0.1780 | 11,915 | 250 | 0.025 (0.022–0.028) | 0.87 (0.73–1.03), p = 0.1012 | 0.85 (0.71–1.01), p = 0.0672 |
| Q3 | ≥0.1780 to <3.6439 | 11,915 | 207 | 0.021 (0.018–0.024) | 0.73 (0.61- 0.87), p = 0.0007 | 0.68 (0.55–0.83), p = 0.0003 |
| Q4 | ≥3.6439 | 11,916 | 228 | 0.023 (0.020–0.026) | 0.84 (0.70–1.00), p = 0.0473 | 0.66 (0.50–0.86), p = 0.0024 |
| P for linear trend | P for trend = 0.0344 | P for trend = 0.00284 | ||||
| Women | ||||||
| Q1 | <−3.9276 | 12,697 | 208 | 0.021 (0.018–0.024) | Reference group | Reference group |
| Q2 | ≥−3.9276 to <−1.6222 | 12,697 | 168 | 0.017 (0.014–0.020) | 0.80 (0.66–0.98), p = 0.0350 | 0.79 (0.64–0.99), p = 0.0378 |
| Q3 | ≥1.6222 to <1.2269 | 12,698 | 147 | 0.015 (0.013–0.017) | 0.71 (0.57- 0.87), p = 0.0012 | 0.65 (0.50–0.84), p = 0.0009 |
| Q4 | ≥1.2269 | 12,697 | 149 | 0.015 (0.013–0.017) | 0.73 (0.59–0.91), p = 0.0039 | 0.59 (0.42–0.81), p = 0.0015 |
| P for linear trend | P for trend = 0.003 | P for trend < 0.001 | ||||
| BMI < 25 kg/m2 | ||||||
| Q1 | <−3.4911 | 8460 | 185 | 0.018 (0.016–0.021) | Reference group | Reference group |
| Q2 | ≥−3.4911 to <−1.0459 | 8460 | 165 | 0.017 (0.014–0.019) | 0.89 (0.72–1.09), p = 0.258 | 0.87 (0.79–1.08), p = 0.208 |
| Q3 | ≥−1.0459 to <2.0027 | 8461 | 132 | 0.013 (0.011–0.016) | 0.70 (0.56–0.88), p = 0.002 | 0.65 (0.50–0.84), p = 0.001 |
| Q4 | ≥2.0027 | 8460 | 160 | 0.016 (0.014–0.019) | 0.87 (0.70–1.07), p = 0.184 | 0.66 (0.48–0.91), p = 0.011 |
| P for linear trend | P for trend = 0.077 | P for trend = 0.002 | ||||
| BMI ≥ 25 kg/m2 | ||||||
| Q1 | <−3.2569 | 16,153 | 266 | 0.027 (0.024–0.030) | Reference group | Reference group |
| Q2 | ≥−3.2569 to <−0.6718 | 16,152 | 254 | 0.025 (0.022–0.029) | 0.95 (0.80–1.12), p = 0.531 | 0.89 (0.74–1.06), p = 0.185 |
| Q3 | ≥−0.6718 to <2.6493 | 16,152 | 240 | 0.024 (0.021–0.027) | 0.91 (0.76–1.08), p = 0.263 | 0.79 (0.64–0.96), p = 0.020 |
| Q4 | ≥2.6493 | 16,153 | 240 | 0.024 (0.021–0.027) | 0.93 (0.78–1.11), p = 0.431 | 0.68 (0.52–0.89), p = 0.005 |
| P for linear trend | P for trend = 0.389 | P for trend = 0.002 | ||||
| Cigarette smoking status: never smoked | ||||||
| Q1 | <−3.4377 | 11,884 | 36 | 0.004 (0.003–0.005) | Reference group | Reference group |
| Q2 | ≥−3.4377 to <−0.9573 | 11,884 | 45 | 0.004 (0.003–0.006) | 1.25 (0.81–1.94), p = 0.319 | 1.28 (0.80–2.05), p = 0.304 |
| Q3 | ≥0.9573 to <2.2189 | 11,885 | 27 | 0.003 (0.002–0.004) | 0.76 (0.46- 1.25), p = 0.285 | 0.80 (0.44–1.44), p = 0.448 |
| Q4 | ≥2.2189 | 11,884 | 31 | 0.003 (0.002–0.004) | 0.90 (0.56–1.46), p = 0.670 | 0.99 (0.47–2.11), p = 0.987 |
| P for linear trend | P for trend = 0.17 | P for trend = 0.306 | ||||
| Current smokers | ||||||
| Q1 | <−3.7053 | 2277 | 165 | 0.017 (0.014–0.019) | Reference group | Reference group |
| Q2 | ≥−3.7053 to <−1.0678 | 2276 | 175 | 0.018 (0.015–0.020) | 1.03 (0.84–1.28), p = 0.757 | 0.93 (0.74–1.16), p = 0.5094 |
| Q3 | ≥−1.0678 to <2.2474 | 2277 | 159 | 0.016 (0.014–0.019) | 0.96 (0.77–1.19), p = 0.681 | 0.80 (0.63–1.03), p = 0.0890 |
| Q4 | ≥2.2474 | 2277 | 165 | 0.016 (0.014–0.019) | 1.03 (0.83–1.28), p = 0.800 | 0.72 (0.52–1.01), p = 0.0554 |
| P for linear trend | P for trend = 0.901 | P for trend = 0.004 | ||||
| Former smokers | ||||||
| Q1 | <−3.1491 | 10,452 | 248 | 0.025 (0.022–0.028) | Reference group | Reference group |
| Q2 | ≥−3.1491 to <−0.5835 | 10,451 | 204 | 0.020 (0.018–0.023) | 0.83 (0.69–1.00), p = 0.0464 | 0.79 (0.70–1.03), p = 0.104 |
| Q3 | ≥−0.5835 to <2.6865 | 10,452 | 193 | 0.019 (0.017–0.022) | 0.78 (0.65–0.94), p = 0.0106 | 0.65 (0.64–1.00), p = 0.053 |
| Q4 | ≥2.6865 | 10,452 | 194 | 0.019 (0.017–0.022) | 0.82 (0.68–0.99), p = 0.0403 | 0.65 (0.61–1.09), p = 0.162 |
| P for linear trend | P for trend = 0.107 | P for trend = 0.133 | ||||
| Drinking status: Never drinkers | ||||||
| Q1 | <−3.8669 | 2485 | 15 | 0.002 (0.001–0.002) | Reference group | Reference group |
| Q2 | ≥−3.8669 to <−1.4275 | 2485 | 13 | 0.001 (0.001–0.002) | 0.84 (0.40–1.78), p = 0.6566 | 0.76 (0.35–1.64), p = 0.4765 |
| Q3 | ≥−1.4275 to <1.7642 | 2485 | 16 | 0.002 (0.001–0.003) | 1.06 (0.52–2.14), p = 0.8784 | 0.64 (0.28–1.46), p = 0.2877 |
| Q4 | ≥1.7642 | 2485 | 16 | 0.002 (0.001–0.003) | 1.08 (0.53–2.19), p = 0.8296 | 0.56 (0.19–1.70), p = 0.3056 |
| P for linear trend | P for trend = 0.433 | P for trend = 0.513 | ||||
| Former drinkers | ||||||
| Q1 | <−3.4802 | 3567 | 81 | 0.008 (0.007–0.010) | Reference group | Reference group |
| Q2 | ≥−3.4802 to <−0.8979 | 3567 | 81 | 0.008 (0.007–0.010) | 0.99 (0.73–1.34), p = 0.935 | 0.96 (0.70–1.33), p = 0.8097 |
| Q3 | ≥−0.8979 to <2.4313 | 3568 | 81 | 0.008 (0.007–0.010) | 0.99 (0.73–1.35), p = 0.971 | 0.97 (0.67–1.40), p = 0.8734 |
| Q4 | ≥2.4313 | 3567 | 72 | 0.007 (0.006–0.009) | 0.91 (0.66–1.25), p = 0.565 | 0.79 (0.48–1.30), p = 0.3625 |
| P for linear trend | P for trend = 0.488 | P for trend = 0.271 | ||||
| Current drinkers | ||||||
| Q1 | <−3.2122 | 17,868 | 359 | 0.036 (0.032–0.040) | Reference group | Reference group |
| Q2 | ≥−3.2122 to <−0.6797 | 17,867 | 303 | 0.030 (0.027–0.034) | 0.84 (0.72–0.98), p = 0.0224 | 0.79 (0.68–0.93), p = 0.0047 |
| Q3 | ≥−0.6797 to <2.5210 | 17,867 | 268 | 0.027 (0.024–0.030) | 0.74 (0.63–0.87), p = 0.0002 | 0.65 (0.54–0.78), p = 3.75 × 10−6 |
| Q4 | ≥2.5210 | 17,868 | 294 | 0.029 (0.026–0.033) | 0.84 (0.72–0.98), p = 0.0264 | 0.60 (0.47–0.76), p = 1.75 × 10−5 |
| P for linear trend | P for trend = 0.025 | P for trend < 0.001 | ||||
| Family history of lung cancer: No | ||||||
| Q1 | <−3.3261 | 21,448 | 347 | 0.009 (0.007–0.011) | Reference group | Reference group |
| Q2 | ≥−3.3261 to <−0.7930 | 21,447 | 331 | 0.007 (0.006–0.009) | 0.95 (0.81–1.10), p = 0.4799 | 0.90 (0.77–1.05), p = 0.1762 |
| Q3 | ≥−0.7930 to <2.4407 | 21,447 | 294 | 0.006 (0.005–0.008) | 0.84 (0.72–0.99), p = 0.0324 | 0.73 (0.61–0.87), p = 0.0006 |
| Q4 | ≥2.4407 | 21,448 | 321 | 0.006 (0.005–0.008) | 0.95 (0.82–1.11), p = 0.5181 | 0.69 (0.55–0.87), p = 0.0019 |
| P for linear trend | P for trend = 0.413 | P for trend < 0.001 | ||||
| Family history of lung cancer: Yes | ||||||
| Q1 | <−3.4296 | 2575 | 87 | 0.035 (0.031–0.039) | Reference group | Reference group |
| Q2 | ≥−3.4296 to <−0.9070 | 2574 | 72 | 0.033 (0.030–0.037) | 0.81 (0.60–1.11), p = 0.1981 | 0.74 (0.53–1.03), p = 0.0727 |
| Q3 | ≥0.9070 to <2.3121 | 2574 | 59 | 0.029 (0.026–0.033) | 0.67 (0.48–0.94), p = 0.0197 | 0.57 (0.39–0.84), p = 0.0047 |
| Q4 | ≥2.3121 | 2575 | 64 | 0.032 (0.029–0.036) | 0.75 (0.54–1.03), p = 0.0770 | 0.47 (0.28–0.78), p = 0.0036 |
| P for linear trend | P for trend = 0.061 | P for trend = 0.002 |
a. Per 10,000 person-years.
Figure A1.
A receiver operating characteristic (ROC) curve for the total intake group.
Figure A2.
A receiver operating characteristic (ROC) curve for the supplemental group.
Author Contributions
A.Z. provided support for conceptualization and methodology; X.N. supported methodology and software; J.Y. performed data curation, investigation, and validation; J.Y. and S.Q. conducted formal analysis and visualization; J.Y. prepared the writing of the original draft; A.Z. and J.Y. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of United States NCI (CDAS project “PLCO-974”, 9 May 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data are contained within this article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Thai A.A., Solomon B.J., Sequist L.V., Gainor J.F., Heist R.S. Lung cancer. Lancet. 2021;398:535–554. doi: 10.1016/S0140-6736(21)00312-3. [DOI] [PubMed] [Google Scholar]
- 2.Huang J., Deng Y., Tin M.S., Lok V., Ngai C.H., Zhang L., Lucero-Prisno D.E., III, Xu W., Zheng Z.-J., Elcarte E. Distribution, risk factors, and temporal trends for lung cancer incidence and mortality: A global analysis. Chest. 2022;161:1101–1111. doi: 10.1016/j.chest.2021.12.655. [DOI] [PubMed] [Google Scholar]
- 3.Uguen M., Dewitte J.D., Marcorelles P., Loddé B., Pougnet R., Saliou P., De Braekeleer M., Uguen A. Asbestos-related lung cancers: A retrospective clinical and pathological study. Mol. Clin. Oncol. 2017;7:135–139. doi: 10.3892/mco.2017.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu N., Su X., Wang Z., Dai B., Kang J. Association of dietary vitamin A and β-carotene intake with the risk of lung cancer: A meta-analysis of 19 publications. Nutrients. 2015;7:9309–9324. doi: 10.3390/nu7115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo J., Shen L., Zheng D. Association between vitamin C intake and lung cancer: A dose-response meta-analysis. Sci. Rep. 2014;4:6161. doi: 10.1038/srep06161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borek C. Dietary antioxidants and human cancer. Integr. Cancer Ther. 2004;3:333–341. doi: 10.1177/1534735404270578. [DOI] [PubMed] [Google Scholar]
- 8.Roswall N., Olsen A., Christensen J., Dragsted L.O., Overvad K., Tjønneland A. Source-specific effects of micronutrients in lung cancer prevention. Lung Cancer. 2010;67:275–281. doi: 10.1016/j.lungcan.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Clinton S.K., Giovannucci E.L., Hursting S.D. The world cancer research fund/American institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: Impact and future directions. J. Nutr. 2020;150:663–671. doi: 10.1093/jn/nxz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y., Wu J., Yoon H.-S., Buchowski M.S., Cai H., Deppen S.A., Steinwandel M.D., Zheng W., Shu X.-O., Blot W.J. Associations of Dietary Intakes of Carotenoids and Vitamin A with Lung Cancer Risk in a Low-Income Population in the Southeastern United States. Cancers. 2022;14:5159. doi: 10.3390/cancers14205159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz H., Kennedy D., Fergusson D., Fernandes R., Cooley K., Seely A., Sagar S., Wong R., Seely D. Selenium and lung cancer: A systematic review and meta analysis. PLoS ONE. 2011;6:e26259. doi: 10.1371/journal.pone.0026259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai X., Wang C., Yu W., Fan W., Wang S., Shen N., Wu P., Li X., Wang F. Selenium Exposure and Cancer Risk: An Updated Meta-analysis and Meta-regression. Sci. Rep. 2016;6:19213. doi: 10.1038/srep19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luu H.N., Wen W., Li H., Dai Q., Yang G., Cai Q., Xiang Y.B., Gao Y.T., Zheng W., Shu X.O. Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels? Antioxid. Redox Signal. 2015;22:951–959. doi: 10.1089/ars.2014.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright M.E., Mayne S.T., Stolzenberg-Solomon R.Z., Li Z., Pietinen P., Taylor P.R., Virtamo J., Albanes D. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am. J. Epidemiol. 2004;160:68–76. doi: 10.1093/aje/kwh173. [DOI] [PubMed] [Google Scholar]
- 15.Yu Y.C., Paragomi P., Wang R., Jin A., Schoen R.E., Sheng L.T., Pan A., Koh W.P., Yuan J.M., Luu H.N. Composite dietary antioxidant index and the risk of colorectal cancer: Findings from the Singapore Chinese Health Study. Int. J. Cancer. 2022;150:1599–1608. doi: 10.1002/ijc.33925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egnell M., Fassier P., Lécuyer L., Gonzalez R., Zelek L., Vasson M.P., Hercberg S., Latino-Martel P., Galan P., Druesne-Pecollo N., et al. Antioxidant intake from diet and supplements and risk of digestive cancers in middle-aged adults: Results from the prospective NutriNet-Santé cohort. Br. J. Nutr. 2017;118:541–549. doi: 10.1017/S0007114517002392. [DOI] [PubMed] [Google Scholar]
- 17.Huang J., Weinstein S.J., Yu K., Männistö S., Albanes D. A Prospective Study of Serum Vitamin E and 28-Year Risk of Lung Cancer. J. Natl. Cancer Instig. 2020;112:191–199. doi: 10.1093/jnci/djz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin P.-H., Aronson W., Freedland S.J. Nutrition, dietary interventions and prostate cancer: The latest evidence. BMC Med. 2015;13:3. doi: 10.1186/s12916-014-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muka T., Kraja B., Ruiter R., Lahousse L., de Keyser C.E., Hofman A., Franco O.H., Brusselle G., Stricker B.H., Kiefte-de Jong J.C. Dietary mineral intake and lung cancer risk: The Rotterdam Study. Eur. J. Nutr. 2017;56:1637–1646. doi: 10.1007/s00394-016-1210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J., Cook N.R., Albert C., Zaharris E., Gaziano J.M., Van Denburgh M., Buring J.E., Manson J.E. Vitamins C and E and beta carotene supplementation and cancer risk: A randomized controlled trial. J. Natl. Cancer Instig. 2009;101:14–23. doi: 10.1093/jnci/djn438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortés-Jofré M., Rueda J.R., Asenjo-Lobos C., Madrid E., Bonfill Cosp X. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst. Rev. 2020;3:Cd002141. doi: 10.1002/14651858.CD002141.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiel C., Le Gal K., Ibrahim M.X., Jahangir C.A., Kashif M., Yao H., Ziegler D.V., Xu X., Ghosh T., Mondal T., et al. BACH1 Stabilization by Antioxidants Stimulates Lung Cancer Metastasis. Cell. 2019;178:330–345.e322. doi: 10.1016/j.cell.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Lignitto L., LeBoeuf S.E., Homer H., Jiang S., Askenazi M., Karakousi T.R., Pass H.I., Bhutkar A.J., Tsirigos A., Ueberheide B., et al. Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1. Cell. 2019;178:316–329.e318. doi: 10.1016/j.cell.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J., Zhang Y., Na X., Zhao A. β-Carotene Supplementation and Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2022;14:1284. doi: 10.3390/nu14061284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middha P., Weinstein S.J., Männistö S., Albanes D., Mondul A.M. β-carotene supplementation and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: The role of tar and nicotine. Nicotine Tob. Res. 2019;21:1045–1050. doi: 10.1093/ntr/nty115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Group A.-T.B.C.C.P.S. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 27.Albanes D., Heinonen O.P., Huttunen J.K., Taylor P.R., Virtamo J., Edwards B., Haapakoski J., Rautalahti M., Hartman A., Palmgren J. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am. J. Clin. Nutr. 1995;62:1427S–1430S. doi: 10.1093/ajcn/62.6.1427S. [DOI] [PubMed] [Google Scholar]
- 28.Omenn G.S., Goodman G.E., Thornquist M.D., Balmes J., Cullen M.R., Glass A., Keogh J.P., Meyskens Jr F.L., Valanis B., Williams Jr J.H. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. JNCI J. Natl. Cancer Instig. 1996;88:1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 29.Shareck M., Rousseau M.C., Koushik A., Siemiatycki J., Parent M.E. Inverse Association between Dietary Intake of Selected Carotenoids and Vitamin C and Risk of Lung Cancer. Front. Oncol. 2017;7:23. doi: 10.3389/fonc.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michaud D.S., Feskanich D., Rimm E.B., Colditz G.A., Speizer F.E., Willett W.C., Giovannucci E. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am. J. Clin. Nutr. 2000;72:990–997. doi: 10.1093/ajcn/72.4.990. [DOI] [PubMed] [Google Scholar]
- 31.Min K.B., Min J.Y. Serum carotenoid levels and risk of lung cancer death in US adults. Cancer Sci. 2014;105:736–743. doi: 10.1111/cas.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are contained within this article and supplementary material.