Abstract
Susceptibility analysis is an intelligent technique that not only assists decision makers in assessing the suspected severity of any sort of brain tumour in a patient but also helps them diagnose and cure these tumours. This technique has been proven more useful in those developing countries where the available health-based and funding-based resources are limited. By employing set-based operations of an arithmetical model, namely fuzzy parameterised complex intuitionistic fuzzy hypersoft set (FPCIFHSS), this study seeks to develop a robust multi-attribute decision support mechanism for appraising patients’ susceptibility to brain tumours. The FPCIFHSS is regarded as more reliable and generalised for handling information-based uncertainties because its complex components and fuzzy parameterisation are designed to deal with the periodic nature of the data and dubious parameters (sub-parameters), respectively. In the proposed FPCIFHSS-susceptibility model, some suitable types of brain tumours are approximated with respect to the most relevant symptoms (parameters) based on the expert opinions of decision makers in terms of complex intuitionistic fuzzy numbers (CIFNs). After determining the fuzzy parameterised values of multi-argument-based tuples and converting the CIFNs into fuzzy values, the scores for such types of tumours are computed based on a core matrix which relates them with fuzzy parameterised multi-argument-based tuples. The sub-intervals within [0, 1] denote the susceptibility degrees of patients corresponding to these types of brain tumours. The susceptibility of patients is examined by observing the membership of score values in the sub-intervals.
Keywords: brain tumour, complex intuitionistic fuzzy set, core matrix, fuzzy parameterisation, hypersoft set, susceptibility analysis
1. Introduction
Over the course of time, human intelligence coupled with advancements in biomedical systems have allowed for the development of methodologies and procedures that allow medical professionals to access cancerous masses in a more in-depth manner and more concisely. However, these advancements still fall short when considering curing methods of cancerous tumours [1]. Multiple avenues of research can be generated with a single research question while following different methodologies for solving that problem with unique analytical pipelines. So, when considering the field of functional neuroimaging coupled with the development of diagnostic systems in its fledgling nature, analytical exploration is an inescapable process of the scientific method and can lead to significant discoveries over time.
The brain is the most complex organ in the human body and even a slight abnormality can have catastrophic effects on the whole body. The statistics indicate that about 23,000 people fell prey to cancerous tumours in the brain in the USA alone, indicating it as one of the major emerging ailments [2]. The cancer indicator reports indicate the probability of development of these tumours is the same for adults and children [3]. Another report indicated that a total of 80,000 new cases of brain tumours were reported in 2018, which were divided into four classes based on their location in the brain: meningioma represented 36.3% (29,320), gliomas 26.5% (21,200), pituitary tumours represented nearly 16.2% (13,210) and the rest of the cases belonged to other types of brain tumour such as malignant, medulloblastoma and lymphomas [4]. The majority of the cases of reported meningiomas start as benign lesions from a histological perspective [5]. Based on these figures, the timely diagnosis and effective handling of a tumour are essential for the patient. Advancements in the field of medical and neural imaging had allowed for timely detection of these lesions in the pre-symptomatic stages, unlike a couple of decades ago when these tumours were only detected when they became large and severely symptomatic [6]. When studying these tumours, some of them never became symptomatic while others progressed to cause symptoms, raising the question of which patient to select for treatment and what treatment methods to opt for for optimal results [7,8,9]. In the case of most tumours, surgery is the very first option for tumours with large size, while for those masses that are close to radiosensitive structures like the optic apparatus, SRS is a viable option for the treatment of small meningiomas [10,11,12].
Now, the mode of therapy to choose for effective treatment of the tumour relies on the pathological nature of the tumour, the stage at which it is diagnosed and the tumour category. When diagnosing a patient with a particular disease, medical imaging techniques and the intuition of medical professionals go hand in hand. The diagnostic process is highly reliant on how the medical images are perceived by the medical professionals while considering other symptomatic conditions simultaneously. Medical professionals use computer-aided diagnosis (CAD) to efficiently analyse and classify pathological and imaging data obtained for diagnosing brain tumours [13]. With recent advances in machine learning, data mining and artificial intelligence, these CAD models have come a long way. In the case of brain tumour diagnosis, the accuracy of these models is still sub-par for regular medical use. Researchers are trying to improve the accuracy of these models by processing huge data sets of images and diagnostic test data of brain tumours using deep learning models for improvement in diagnostic accuracy [14,15]. Another approach that is on par with these models is applying fuzzy set theory concepts to design diagnostic support systems. When diagnosing a patient with a particular disease, medical imaging techniques and the intuition of medical professionals go hand in hand. The diagnostic process is highly reliant on how the medical images are perceived by the medical professionals while considering other symptomatic conditions at the same time. So, this diagnostic process presents itself as an MADM (a particular type of MCDM) problem where the medical professional decides, based on the numerous factors of different natures, to come to a tentative decision in the form of a diagnosis. Fuzzy set theory has been extensively used in designing decision support systems as it is considered to handle human intuition using mathematical syntax. The idea was introduced by Zadeh [16]. This theory became the basis of numerous other decision-making studies and led to the development of complex hybrid mathematical structures such as the concept of rough set and period mathematics.
As an extension of existing structures [17,18,19,20], Smarandache put forward the concept of hypersoft set (HSS) [21] which is an emerging field of research to tackle data-based vagueness and uncertainties. Recently, Saeed et al. [22] presented various set-based operations of HSS. The HSS has been a subject of great interest for many researchers since its introduction in 2018. Its hybridised models have been applied in many multidisciplinary areas while dealing with MADM problems. Saeed et al. [23,24,25,26] studied the diagnoses of diseases such as COVID-19, tuberculosis, allergy-based diseases, and hepatitis, respectively, by using the hybrids of HSS. Similarly, Rahman et al. [27,28] studied the diagnosis of heart disease by proposing robust algorithmic MADM techniques based on hybrids of HSS.
Analysis of susceptibility to brain tumours has attracted many researchers but the most significant contributions are the following: Romano et al. [29] discussed clinical applications of dynamic susceptibility contrast perfusion-weighted MR imaging in brain tumours. Järnum et al. [30] investigated the perfusion MRI of brain tumours by providing a comparative study of pseudo-continuous arterial spin labeling and dynamic susceptibility contrast imaging. Fayed et al. [31] proposed a robust technique for malignancy assessment of brain tumours with magnetic resonance spectroscopy and dynamic susceptibility contrast MRI. Lehmann et al. [32] presented the comparative study of perfusion measurement in brain tumours at 3 Tesla MR on the basis of arterial spin labeling versus dynamic susceptibility contrast-enhanced MRI. Park et al. [33] combined the high-resolution susceptibility-weighted imaging and the apparent diffusion coefficient by assigning value to brain tumour imaging and clinical feasibility of non-contrast MRI at 3T. The majority of the studies involve the application of fuzzy hybrid structures with reference to brain tumours that employ fuzzy logic to classify brain tumours based on data obtained from neuroimaging techniques. Some examples include the use of a picture fuzzy clustering method for segmentations of brain MRI images [34], tumour symmetry analysis using spatially constrained deformable models based on fuzzy classification of 3D MRI images [35], classification of brain tumour type using fuzzy cognitive maps [36] and studies involving the concept of hypersoft mapping for the suggestion of an appropriate treatment method based on the type of tumour [37].
Although the research work conducted by Saeed et al. [37] regarding the diagnosis of brain tumours is convincing, it is not sufficient for real-world MADM situations such as a medical diagnosis in which the regular data-based periodicity is observed. Similarly, they employed the concept hypersoft mapping with an indeterminate setting with many computation-based complexities. As the work of Saeed et al. is the research item most relevant to this study, the scarcity of such research motivates the authors to initiate this paper. The proposed structure FPCIFHSS is more flexible and easily understandable as compared to the mentioned studies. It has a lesser degree of computational complexities, which enables readers from multidisciplinary fields of study to understand its findings and computations with great ease.
The prominent contributions of the paper can be outlined as follows:
The ambiguous nature of parameters (symptoms) and their related sub-parameters are managed by applying the concept of fuzzy parameterisation, which assigns them a fuzzy membership grade. An innovative algebraic method, namely the FPCIFHSS-Ranking method, is used to find out the fuzzy parameterised values of parametric valued tuples based on their complex intuitionistic fuzzy numbers-based approximation. The data-based periodicity is treated with the use of amplitude and phase values (complex plane setting). The amplitude value is meant for the membership magnitude and the other is for its periodic value.
A novel robust MADM-based algorithm i.e., Pythagorean Means-based Scoring Algorithm (PMBSA) is proposed by using set-based operations of FPCIFHSS to assess analyses of the susceptibility of patients to brain tumours. These steps of the proposed algorithms are easily understandable and free of computational complexities.
The expert opinions of decision makers (medical experts with expertise in dealing with brain tumours such as neurologists, neuropathologists, internists, oncologists, radiation oncologists, neuro-oncologists and neurosurgeons) are gathered in terms of CIFNs, which are not only easily computable but also easily transformable to fuzzy values. The patients are first assigned a susceptibility degree in subintervals within [0, 1] then scores of brain tumours, computed from the proposed algorithm, are matched on their respective
As far as the organisation of the remainder of this paper is concerned, the next section (Section 2) consists of some preliminary knowledge which is provided to understand the main methodology and the presented mathematical structure with great ease. Section 3 describes the various stages of the proposed methodology, including the characterisation of the basic notions of FPCIFHSS, the role of fuzzy parameterisation for the symptoms and their related subcategories, arithmetic criteria for the transformation of CIFNs to CFNs and then CFNs to fuzzy values, the process for the selection of parameters and sub-parameters and the profiles of decision makers for this evaluation. It also includes an MADM system based on the proposal of a robust algorithm for assessing the susceptibility of patients to brain tumours using aggregation-based operations of FPCIFHSS. Section 4 presents the discussion, sensitivity analysis and comparison of the proposed study. Finally, Section 5 concludes the paper with limitations and future scope.
Abbreviations and Acronyms
All the abbreviations and acronyms (that are used in the paper) along with their full names are presented in Table 1.
Table 1.
Abbreviations and their full names.
| Symbol | Stands for |
|---|---|
| MCDM | Multi-criteria decision making |
| MRI | Magnetic resonance imaging |
| DMP | Decision making problem |
| FS | Fuzzy set |
| CFS | Complex fuzzy set |
| IFS | Intuitionistic fuzzy set |
| CIFS | Complex intuitionistic fuzzy set |
| SS | Soft set |
| HSS | Hypersoft set |
| MADM | Muti-attribute decision making |
| FPFHSS | Fuzzy parameterised fuzzy hypersoft set |
| FPIFHSS | Fuzzy parameterised intuitionistic fuzzy hypersoft set |
| CFHSS | Complex fuzzy hypersoft set |
| CIFHSS | Complex intuitionistic fuzzy hypersoft set |
| CIFN | Complex intuitionistic fuzzy number |
| CFN | Complex fuzzy number |
| MAGAF | Multi-argument approximate function |
| CVF | Complex valued function |
| AT | Amplitude term |
| PT | Phase term |
| FPCIFHSS | Fuzzy parameterised complex intuitionistic fuzzy hypersoft set |
2. Preliminary Knowledge
This section reviews some preliminary definitions to assist the main results. The following applies throughout the remainder of the paper.
Definition 1
(Atanassov, [17]). An intuitionistic fuzzy set over is stated as
(1) here such that and the values and are true and false belonging grades of , respectively. The grade of hesitancy is .
Example 1.
Let be a collection of objects with , , , , , , and ; then an IFS is constructed as
with hesitancy grades , , and .
Definition 2
(Alkouri, [19]). A complex intuitionistic fuzzy set over is stated as
(2) where , are complex valued true and false belonging mappings such that and . The values and are the amplitude terms for true and false belonging grades of , respectively. Similarly, the values and are the phase terms for true and false belonging grades of , respectively. The grades of hesitancy for amplitude and phase terms are and .
Example 2.
Let be a collection of objects with , , , , , , and ; then a CIFS is constructed as
with hesitancy grades , , , , , , and .
Definition 3
(Molodtsov, [20]). Let and be the sets consisting of evaluating features (attributes) and alternatives, respectively. An SS over is stated as
(3) in which is an approximate mapping and the value is an -approximate entity of .
Definition 4
(Smarandache, [21], Rahman et al. [27]). Let be the sets containing the sub-parametric values of the parameters belonging to such that for any two , the corresponding sets and are non-overlapping. The HSS over is stated as
(4) n which is a multi argument approximate mapping, and the value is a -multi-approximate entity of .
3. Salient Features of Proposed Methodology
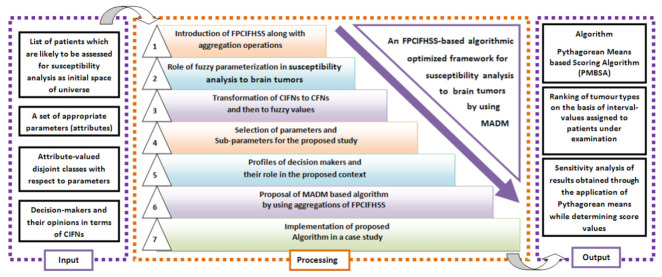
This section discusses the various aspects of stages involved in the proposed methodology. In the first stage, a novel algebraic model, i.e., fuzzy parameterised complex intuitionistic fuzzy hypersoft set (FPCIFHSS), is introduced, which best suits the analysis of susceptibility to brain tumours by considering its expected uncertainties. The stages of the adopted methodology are presented briefly in Figure 1.
Figure 1.
The brief pictorial description of proposed decision support framework.
3.1. The FPCIFHSS and Its Set-Theoretic Operations
In this part, the definition of FPCIFHSS and its aggregation operations are presented with examples.
Definition 5.
Let be the sets containing the sub-parametric values of the parameters belonging to such that for any two , the corresponding sets and are non-overlapping and for where k is the total number of elements in , is a fuzzy set over , then an FPCIFHSS over can be stated as
(5) where with
(6)
(7) are complex valued true and false belonging mappings such that and . The values and are the amplitude terms for true and false belonging grades of , respectively. Similarly, the values and are the phase terms for true and false belonging grades of , respectively. The grades of hesitancy for amplitude and phase terms are and .
Example 3.
Let be a collection of objects, be the set of parameters and the respective parametric valued non-overlapping sets be , and , respectively, such that , then the fuzzy set over is , then FPCIFHSS over can be constructed as
with hesitancy grades , , , , , , and .
In Example 3, the approximate element of sub-parametric tuple (with 10% fuzzy parameterised grade) is
in which means that truth- and falsity-based amplitude values of are 0.2 and 0.3, respectively, similarly truth- and falsity-based phase values of are and , respectively, in the approximation of sub-parametric tuple . Similarly, all other terms can also be interpreted.
Definition 6.
Let and be FPCIFHSSs over such that
(8) where with
(9)
(10) and
(11) where with
(12)
(13) then
(1) is another FPCIFHSS over such that
where with
and
(2) is another FPCIFHSS over such that
with
and
Example 4.
Reassuming Example 3, we have
and
then
and
3.2. Role of Fuzzy Parameterisation in Analysis of Susceptibility to Brain Tumours
The idea of fuzzy parameterisation is meant to tackle uncertainties attached to the procedure of selecting parameters for the analysis and ranking of objects under observation. Let be a space of objects under consideration and be an FS over the set of attribute-valued tuples . Let and be amplitude values of in true-belonging and false-belonging components of CIFNs with respect to . Similarly, let and be phase values of in true-belonging and false-belonging components of CIFNs with respect to . Then, the fuzzy parameterised value of can be computed as
| (14) |
3.3. Algebraic Criterion for the Transformation of CIFNs
If is a CIFN for corresponding to fuzzy parameterised tuples , then CIFN can be converted to CFN by using the following arithmetical formula
| (15) |
If is a CFN, then fuzzy values can be obtained from it by using the following formula
| (16) |
3.4. Selection Criterion for Parameters and Sub-Parameters
The characteristics (criteria) and sub-attributes (sub-criteria) are the main elements that directly connect to the MADM problem and potentially have a significant impact on decisions. Because of this, it is advised to choose parameters and sub-parameters with intelligence. Interviewing people and conducting questionnaire-based surveys are seen as appropriate methods for gathering information that will help select parameters and sub-parameters. However, only the parameters and sub-parameters compatible with the chosen algebraic model will likely be taken into account. Only those parameters (criteria) that are likely to be divided into disjoint sub-classes with sub-parametric values (sub-criteria) are accepted after reviewing the pertinent literature and utilising the suggested model.
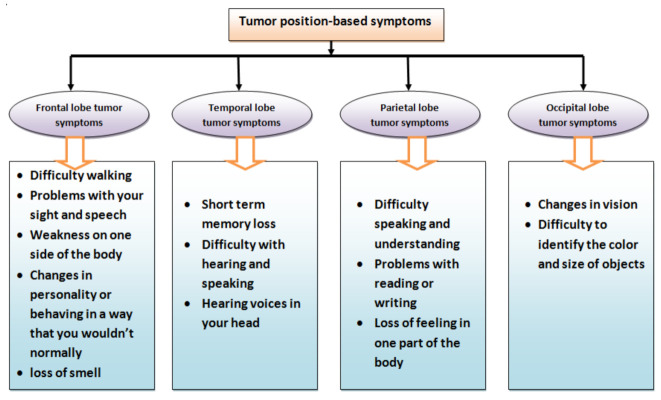
According to the American Association of Neurological Surgeons [38], many types of tumours may appear in the body for several uncertain reasons, but this study considered only those tumours which relate to the brain based on their positions in the cerebrum. A brief description of them is provided in Figure 2. Since there are many symptoms which may lead to the suffering of brain tumours [39,40], the most relevant symptoms and their sub-categories are considered as parameters (criteria) and sub-parameters (sub-criteria) for the evaluation of susceptibility to brain tumours. The purpose of choosing them is based on their relevance and suitability with respect to brain tumours. Figure 3 presents a brief description of adopted modified parameters and sub-parameters. To study their roles in detail, one can visit the web pages [39,40].
Figure 2.
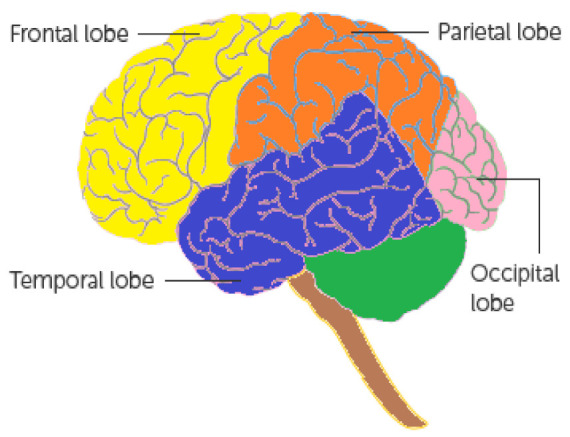
Classification of cerebrum (source: [39]).
Figure 3.
Classification of tumour location-based symptoms (source: [39]).
3.5. Profile of Decision Makers and Their Roles
The key players in MADM practice are decision makers (experts) who make the decisions necessary for the evaluation process to be completed successfully. Their shared conflicts of interest or other relevant disagreements might result in biased judgments. Therefore, it is common to employ professionals with multi-disciplinary competence areas from many sources (departments). A few of the main duties of decision makers in MADM include the following:
Analysing raw data (information) gathered from various sources.
Processing the analysed data statistically.
Examination of options and constraints.
Analysing processed data using various parameters.
A concise set of appropriate parameters for assessing alternatives.
Using an appropriate algebraic model to offer recommendations for approximating options based on parameters.
Sorting of objects under observation.
In the present study, the decision makers are qualified doctors such as neurologists and neuropathologists who specialise in issues concerning the brain and central nervous system. Although they can manage a major part of the evaluation easily, due to the involvement of many other factors, they can be assisted by other doctors such as internists, oncologists, radiation oncologists, neuro-oncologists and neurosurgeons [40].
3.6. Designing of Decision Support System
In this section, a decision support mechanism is presented, which assists the decision makers in assessing the susceptibility of patients to brain tumours by following the easy steps of the proposed robust algorithm.
Patients with brain tumours are typically directed to the oncology department at urban hospitals in some developing Asian countries such as Pakistan. There, the tumours’ conditions are first assessed before an appropriate course of therapy is suggested. Lack of resources makes it nearly impossible to diagnose a particular type of brain tumour and then treat it locally. Therefore, it is preferable to just evaluate a patient’s susceptibility to brain tumours at this local level before referring them to the appropriate facilities for treatment.
Now, a robust algorithm is being proposed using the aggregations of the proposed algebraic model FPCIFHSS and other arithmetical cum decision-making techniques.
A brief step-wise description of Algorithm 1 is presented in Figure 4. Now, Algorithm 1 is explained by the following case study-based numerical example. In this case, the opinions of decision makers are hypothetical.
| Algorithm 1 Pythagorean Means based Scoring Algorithm (PMBSA): The algorithm is divided into following four major stages |
|
Input:
Construction:
Computation:
Output:
|
Figure 4.
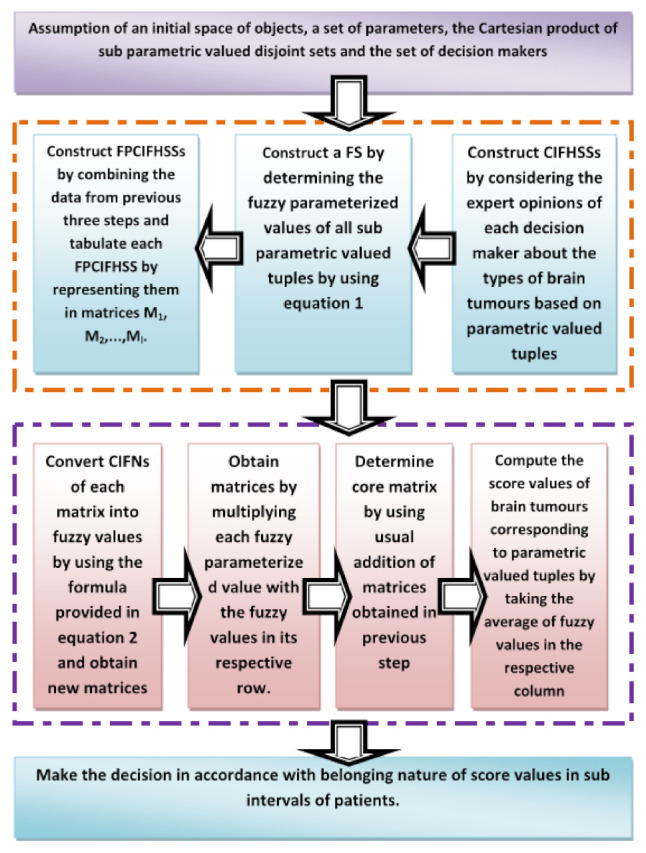
Flowchart of proposed PMBSA algorithm.
Example 5.
The administration of a cancer hospital, “MEDICARE" (a hypothetical name), is very much concerned with the increasing ratio of suspected brain tumour patients being referred by various hospitals from all over the country. In order to evaluate the susceptibility level of patients to the specific type of brain tumours, a departmental committee is constituted consisting of two neurologists and one neuropathologist, which is considered as a set of decision makers . According to the terms and conditions provided to the committee, the following are the tasks of the committee:
1. Shortlist the patients for analysis of susceptibility to brain tumours and assign susceptibility degrees as subintervals of [0, 1].
2. List the expected types of brain tumours and their related symptoms after a close analysis of the literature and other related sources.
3. Provide opinions for the approximations of brain tumour types separately based on multi-argument-based symptoms by considering fuzzy parameterisation, complex intuitionistic fuzzy setting and hypersoft setting.
After mutual consultation, three types of brain tumours are shortlisted which are enclosed as a set of initial spaces of objects . Figure 5, Figure 6 and Figure 7 present the pictorial display of these types of tumours, respectively. Six patients, , , , , and , are shortlisted and their susceptibility degrees are supposed to be contained in subintervals [0, 0.3), [0.2, 0.4), [0.2, 0.6), [0.3, 0.7), [0.3, 0.85) and [0.85, 1], respectively. The committee has selected some appropriate symptoms in accordance with the location of brain tumours and considered them as evaluating parameters for this case. These symptoms form a set of parameters . For the sake of having reliable evaluations, the chosen parameters are then classified on a preferential basis into their respective sub-parametric valued non-overlapping sets:
,
,
,
.
For the sake of including multiple arguments simultaneously, the Cartesian product of is calculated, that is
.
Now, all the members provide their expert opinions separately for the approximations of three types of brain tumours based on sub-parametric valued tuples , , and in terms of CIFNs, that is
For :
For :
For :
Now, for sake of assessing the ambiguous nature of sub-parametric valued tuples, their respective fuzzy parameterised values computed by using Equation (14) are presented in Table 2.
In this stage, the opinions and fuzzy parameterised degrees of each decision maker are compiled as FPCIFHSSs and are presented in Table 3, Table 4 and Table 5.
Now, all the CIFN-based entries of FPCIFHSSs , and are converted to CFNs by using the arithmetical criterion given in Equation (15). The new matrices thus obtained are presented in Table 6, Table 7 and Table 8.
In this step, CFN-based entries of matrices , and are transformed to fuzzy values first by employing the criterion provided in Equation (16) and then each fuzzy parameterised value corresponding to sub-parametric valued tuples , , and is multiplied with the fuzzy valued-based entries in their respective rows. The matrices , and thus obtained are presented in Table 9, Table 10 and Table 11.
Now, in this phase, the core matrix is obtained by the ordinary addition of matrices , and . Its tabulation formation is provided in Table 12.
The score values for the types of brain tumours are computed by taking the average of the entries in their corresponding rows of core matrix . The score values are presented in Table 13.
From Table 13, it is clear that , and ; therefore, it is concluded that patients , and are suspected to have brain tumours “craniopharyngioma" and “brain-metastases", whereas patients and are suspected to have “medulloblastomas". Patient is outside the scope of the analysis of susceptibility to these three types of brain tumours. This can also be seen in Figure 8.
Figure 5.
Craniopharyngioma: brain tumour of type 1 (source: https://en.wikipedia.org/wiki/Craniopharyngioma) (accessed on 16 October 2022).
Figure 6.
Brain-metastases: brain tumour of type 2 (source: https://www.mayoclinic.org/diseases-conditions/brain-metastases/symptoms-causes/syc-20350136) (accessed on 18 September 2022).
Figure 7.
Medulloblastomas: brain tumour of type 3 (source: https://www.mayoclinic.org/diseases-conditions/medulloblastoma/cdc-20363524) (accessed on 18 September 2022).
Table 2.
Fuzzy parameterised values corresponding to .
| DMs | ||||
|---|---|---|---|---|
| 0.15 | 0.25 | 0.35 | 0.45 | |
| 0.51 | 0.52 | 0.53 | 0.54 | |
| 0.54 | 0.53 | 0.52 | 0.295 |
Table 3.
Matrix formation of FPCIFHSS constructed with .
Table 4.
Matrix formation of FPCIFHSS constructed with .
Table 5.
Matrix formation of FPCIFHSS constructed with .
Table 6.
Matrix formation of with entries in terms of CFNs.
Table 7.
Matrix formation of with entries in terms of CFNs.
Table 8.
Matrix formation of with entries in terms of CFNs.
Table 9.
Tabular formation of with entries in terms of fuzzy values.
| 0.0098 | 0.0113 | 0.0120 | |
| 0.0288 | 0.0313 | 0.0325 | |
| 0.0578 | 0.0613 | 0.0630 | |
| 0.0968 | 0.1013 | 0.1035 |
Table 10.
Tabular formation of with entries in terms of fuzzy values.
| 0.0791 | 0.1301 | 0.1556 | |
| 0.0832 | 0.1352 | 0.3224 | |
| 0.0875 | 0.1405 | 0.1670 | |
| 0.0918 | 0.1458 | 0.1728 |
Table 11.
Tabular formation of with entries in terms of fuzzy values.
| 0.0918 | 0.1458 | 0.1728 | |
| 0.0875 | 0.1405 | 0.1670 | |
| 0.0832 | 0.1352 | 0.1612 | |
| 0.0266 | 0.0163 | 0.0605 |
Table 12.
Tabular formation of core matrix .
| 0.1807 | 0.2872 | 0.3404 | |
| 0.1995 | 0.3070 | 0.5219 | |
| 0.2285 | 0.3370 | 0.3912 | |
| 0.2152 | 0.2634 | 0.3368 |
Table 13.
Scores of alternatives corresponding to .
| Brain Tumour Types | : Score Values of |
|---|---|
| 0.2060 | |
| 0.2987 | |
| 0.3976 |
Figure 8.
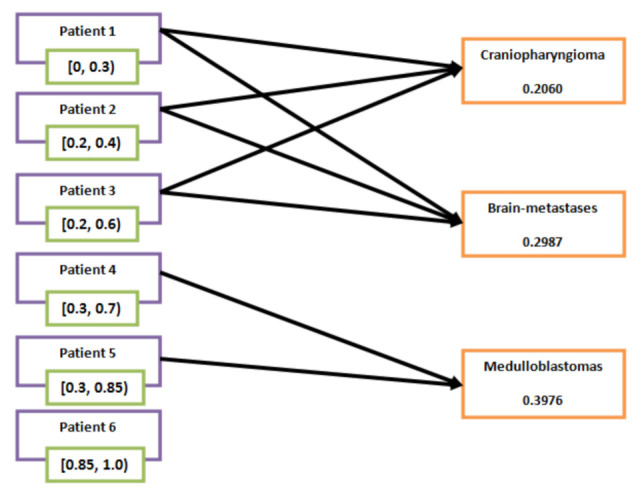
Relation between the patients and brain tumours.
4. Discussion, Sensitivity Analysis and Comparison
The proposed mathematical model is new and has not been used by any scholar in the literature for the proposed study. Moreover, no relevant literature exists regarding the assessment of analyses of susceptibility of patients to brain tumours by using fuzzy set-like or soft set-like structures. Therefore, the proposed study is not comparable with any existing literature studies based on computational results. However, a comparison is made with its own results by employing different statistical techniques for the determination of scoring values of brain tumours.
As in Example 5, the susceptibility level of patients is assessed by applying the concept of arithmetic mean for determining the score values of brain tumours based on sub-parametric valued tuples and thus five overlapping results and one neutral result are observed. However, the computed scores may vary if other means are applied to find score values that definitely alter the final findings. In this regard, the following cases can be considered:
Case 1.
The score values for the types of brain tumours are computed by taking the geometric means of the entries in their corresponding rows of the core matrix . The score values are presented in Table 14.
Table 14.
Scores of alternatives corresponding to computed through geometric mean.
| Brain Tumour Types | : Score Values of |
|---|---|
| 0.2052 | |
| 0.2974 | |
| 0.3911 |
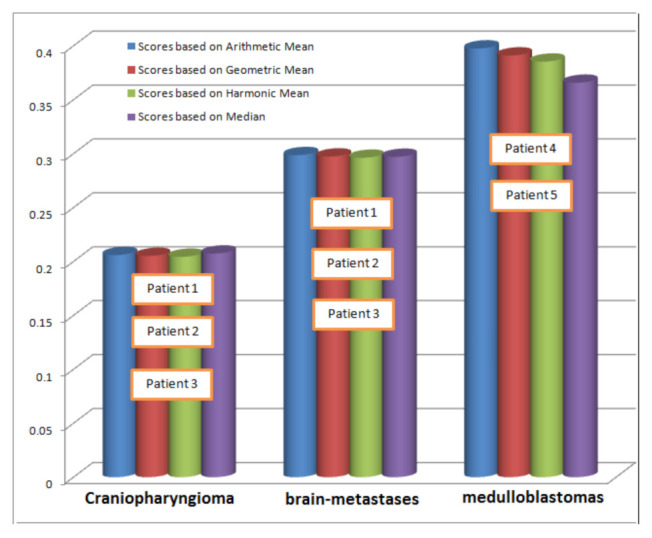
From Table 14, it is clear that and ; therefore, it is concluded that patients , and are suspected to have brain tumours “craniopharyngioma” and “brain-metastases”, whereas patients and are suspected to have “medulloblastomas”. Patient is outside the scope of the analysis of susceptibility to these three types of brain tumours.
Case 2.
The score values for the types of brain tumours are computed by taking the harmonic means of the entries in their corresponding rows of core matrix . The score values are presented in Table 15.
Table 15.
Scores of alternatives corresponding to computed through harmonic mean.
| Brain Tumour Types | : Score Values of |
|---|---|
| 0.2044 | |
| 0.2962 | |
| 0.3854 |
From Table 15, it is clear that and , ; therefore, it is concluded that patients , and are suspected to have brain tumours “craniopharyngioma” and “brain-metastases”, whereas patients and are suspected to have “medulloblastomas”. Patient is outside the scope of the analysis of susceptibility to these three types of brain tumours.
Case 3.
The score values for the types of brain tumours are computed by taking the harmonic means of the entries in their corresponding rows of core matrix . The score values are presented in Table 16.
Table 16.
Scores of alternatives corresponding to computed through median.
| Brain Tumour Types | : Score Values of |
|---|---|
| 0.2074 | |
| 0.2971 | |
| 0.3658 |
From Table 16, it is clear that and , ; therefore, it is concluded that patients , and are suspected to have brain tumours “craniopharyngioma” and “brain-metastases”, whereas patients and are suspected to have “medulloblastomas”. Patient is outside the scope of the analysis of susceptibility to these three types of brain tumours.
In all the above cases, the same results are received by applying different Pythagorean means. The combined comparison of scores computed through these cases are presented in Figure 9.
Figure 9.
Comparison of scores computed through different statistical techniques.
Now, we compare our proposed study with some the most relevant already developed models such as Kumar et al. [34], Papageorgiou et al. [36] and Saeed et al. [37]. This comparison is based on some significant evaluating indicators and is presented in Table 17.
Table 17.
Structural comparison of proposed study with the most relevant existing model.
| References | Entitlement of Fuzzy Parameterisation | Handling of Information-Based Periodicity | Provision of Susceptibility-Based Ranking for Brain Tumours | Pythagorean Means-Based Sensitivity Analysis of Results |
|---|---|---|---|---|
| Kumar et al. [34] | Inadequate | Inadequate | Inadequate | Inadequate |
| Papageorgiou et al. [36] | Inadequate | Inadequate | Inadequate | Inadequate |
| Saeed et al. [37] | Inadequate | Inadequate | Inadequate | Inadequate |
| Proposed model | Parameters and sub-parameter-based uncertainties are managed by using the concept of fuzzy parameterisation | Information-based periodicity is tackled by using amplitude and phase values in complex plane settings | Patients and types of brain tumours are matched and ranked in accordance with the susceptibility scores | Sensitivity of computed scores is observed through employing the formulations of Pythagorean means (i.e., arithmetic mean, geometric mean and harmonic mean) |
5. Conclusions
In the present research, the set-based operations of a flexible arithmetic model FPCIFHSS are used to design an MADM system based on the proposal of a robust algorithm for the assessment of analyses of patients’ susceptibility to brain tumours. This task is accomplished by characterising the basic notions of FPCIFHSS, studying the role of fuzzy parameterisation for the symptoms and their related subcategories, studying arithmetic criteria for the transformation of CIFNs to CFNs and then CFNs to fuzzy values, understanding the process for the selection of parameters and sub-parameters and studying the profiles of decision makers for this evaluation. The suggested method is reliable and flexible, although it has some restrictions on the decision makers’ right to neutral or indefinite membership grades in exchange for their input. The decision makers in the current approach are required to approximatively weigh the alternatives based on their opinions of dependent belonging and non-belonging grades within [0, 1]. When used in a neutrosophic environment, however, where participants can autonomously express their ideas regarding belonging, non-belonging and indeterminate grades within [0, 3], this approach can produce more trustworthy results. Additionally, the information utilised in the form of decision makers’ judgments is fictitious. So it is simple to use this method to discuss a case study that includes actual data from the concerned department. A broad range of research fields, including artificial intelligence, soft computing (fuzzy logic), image processing and classification, pattern recognition and data clustering may fall under the purview of this study. By utilising suitable pseudo-codes through machine learning tools, the proposed algorithm (i.e., the decision support system) can be used further in the classification of brain tumours based on MRI.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: 22UQU4400257DSR88.
Author Contributions
Conceptualization, A.U.R., M.H.S., M.S., D.A.Z., M.A.M. and A.S.A.-W.; Methodology, A.U.R., M.H.S., M.S., A.S.A.-W. and M.A.; Software, A.U.R., D.A.Z., M.A. and K.H.A.; Validation, D.A.Z. and K.H.A.; Formal analysis, A.U.R., M.H.S., M.S. and K.H.A.; Investigation, A.U.R., M.H.S., M.S., D.A.Z. and M.A.; Data curation, D.A.Z., M.A.M. and K.H.A.; Writing of the original draft, A.U.R., M.H.S., M.S. and M.A.M.; Writing—review and editing, A.U.R., M.A.M. and A.S.A.-W.; Visualization, A.U.R., M.S. and M.A.; Supervision, M.S., A.S.A.-W. and M.A.; Project administration, M.A.M. and K.H.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Noreen N., Palaniappan S., Qayyum A., Ahmad I., Imran M., Shoaib M. A deep learning model based on concatenation approach for the diagnosis of brain tumour. IEEE Access. 2020;8:55135–55144. doi: 10.1109/ACCESS.2020.2978629. [DOI] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2020;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.American Brain Tumor Association. [(accessed on 13 January 2023)]. Available online: https://www.abta.org/about-brain-tumors/
- 5.Pollock B.E. Stereotactic radiosurgery of benign intracranialtumours. J. Neuro-Oncol. 2009;92:337–343. doi: 10.1007/s11060-009-9831-6. [DOI] [PubMed] [Google Scholar]
- 6.Vernooij M.W., Ikram M.A., Tanghe H.L., Vincent A.J., Hofman A., Krestin G.P., van der Lugt A. Incidental findings on brain MRI in thegeneral population. N. Engl. J. Med. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 7.Braunstein J.B., Vick N.A. Meningiomas: The decision not to operate. Neurology. 1997;48:1459–1462. doi: 10.1212/WNL.48.5.1459. [DOI] [PubMed] [Google Scholar]
- 8.Firsching R.P., Fischer A., Peters R., Thun F., Klug N. Growth rate of incidental meningiomas. J. Neurosurg. 1990;73:545–547. doi: 10.3171/jns.1990.73.4.0545. [DOI] [PubMed] [Google Scholar]
- 9.Herscovici Z., Rappaport Z., Sulkes J., Danaila L., Rubin G. Natural history of conservatively treated meningiomas. Neurology. 2004;63:1133–1134. doi: 10.1212/01.WNL.0000138569.45818.50. [DOI] [PubMed] [Google Scholar]
- 10.Flickinger J.C., Kondziolka D., Maitz H., Lunsford L.D. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int. J. Radiat. Oncol. Biol. Phys. 2003;56:801–806. doi: 10.1016/S0360-3016(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 11.Iwai Y., Yamanaka K., Ikeda H. Gamma Knife radiosurgery forskull basemeningioma: Long-term results of low-dose treatment, Clinical article. J. Neurosurg. 2008;109:804–810. doi: 10.3171/JNS/2008/109/11/0804. [DOI] [PubMed] [Google Scholar]
- 12.Kondziolka D., Levy E.L., Niranjan A., Flickinger J.C., Lunsford L.D. Long term outcomes after meningioma radiosurgery: Physician and patient perspectives. J. Neurosurg. 1999;91:44–50. doi: 10.3171/jns.1999.91.1.0044. [DOI] [PubMed] [Google Scholar]
- 13.Deepak S., Ameer P.M. Brain tumour classification using deep CNN features via transfer learning. Comput. Biol. Med. 2019;111:103345. doi: 10.1016/j.compbiomed.2019.103345. [DOI] [PubMed] [Google Scholar]
- 14.Naseer A., Rani M., Naz S., Razzak M.I., Imran M., Xu G. Refining Parkinson’s neurological disorder identification through deep transfer learning. Neural Comput. Appl. 2019;32:839–854. doi: 10.1007/s00521-019-04069-0. [DOI] [Google Scholar]
- 15.Zuo H., Fan H., Blasch E., Ling H. Combining convolutional and recurrent neural networks for human skin detection. IEEE Signal Process. Lett. 2017;24:289–293. doi: 10.1109/LSP.2017.2654803. [DOI] [Google Scholar]
- 16.Zadeh L.A. Fuzzy sets. Inf. Control. 1965;8:338–353. doi: 10.1016/S0019-9958(65)90241-X. [DOI] [Google Scholar]
- 17.Atanassov K.T. Intuitionistic fuzzy sets. Fuzzy Sets Syst. 1986;20:87–96. doi: 10.1016/S0165-0114(86)80034-3. [DOI] [Google Scholar]
- 18.Ramot D., Milo R., Friedman M., Kandel A. Complex fuzzy sets. IEEE Trans. Fuzzy Syst. 2002;10:171–186. doi: 10.1109/91.995119. [DOI] [Google Scholar]
- 19.Alkouri A.M.D.J.S., Salleh A.R. Complex intuitionistic fuzzy sets. AIP Conf. Proc. 2012;1482:464–470. doi: 10.1063/1.4757515. [DOI] [Google Scholar]
- 20.Molodtsov D. Soft set theory—First results. Comput. Math. Appl. 1999;37:19–31. doi: 10.1016/S0898-1221(99)00056-5. [DOI] [Google Scholar]
- 21.Smarandache F. Extension of soft set of hypersoft set and then to plithogenic hypersoft set. Neutrosophic Sets Syst. 2018;22:168–170. doi: 10.5281/zenodo.2838716. [DOI] [Google Scholar]
- 22.Saeed M., Rahman A.U., Ahsan M., Smarandache F. Theory of Hypersoft Sets: Axiomatic Properties, Aggregation Operations, Relations, Functions and Matrices. Neutrosophic Sets Syst. 2022;51:744–765. doi: 10.5281/zenodo.7135413. [DOI] [Google Scholar]
- 23.Saeed M., Ahsan M., Saeed M.H., Rahman A.U., Mehmood A., Mohammed M.A., Jaber M.M., Damaševičius R. An optimized decision support model for COVID-19 diagnostics based on complex fuzzy hypersoft mapping. Mathematics. 2022;10:2472. doi: 10.3390/math10142472. [DOI] [Google Scholar]
- 24.Saeed M., Ahsan M., Saeed M.H., Rahman A.U., Mohammed M.A., Nedoma J., Martinek R. An algebraic modeling for tuberculosis disease prognosis and proposed potential treatment methods using fuzzy hypersoft mappings. Biomed. Signal Process. Control. 2023;80:104267. doi: 10.1016/j.bspc.2022.104267. [DOI] [Google Scholar]
- 25.Saeed M., Ahsan M., Saeed M.H., Mehmood A., Khalifa H.A.E.W., Mekawy I. The Prognosis of Allergy-Based Diseases Using Pythagorean Fuzzy Hypersoft Mapping Structures and Recommending Medication. IEEE Access. 2022;10:5681–5696. doi: 10.1109/ACCESS.2022.3141092. [DOI] [Google Scholar]
- 26.Saeed M., Ahsan M., Saeed M.H., Mehmood A., Abdeljawad T. An application of neutrosophic hypersoft mapping to diagnose hepatitis and propose appropriate treatment. IEEE Access. 2021;9:70455–70471. doi: 10.1109/ACCESS.2021.3077867. [DOI] [Google Scholar]
- 27.Rahman A.U., Saeed M., Mohammed M.A., Krishnamoorthy S., Kadry S., Eid F. An Integrated Algorithmic MADM Approach for Heart Diseases’ Diagnosis Based on Neutrosophic Hypersoft Set with Possibility Degree-Based Setting. Life. 2022;12:729. doi: 10.3390/life12050729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman A.U., Saeed M., Mohammed M.A., Jaber M.M., Garcia-Zapirain B. A novel fuzzy parameterised fuzzy hypersoft set and riesz summability approach based decision support system for diagnosis of heart diseases. Diagnostics. 2022;12:1546. doi: 10.3390/diagnostics12071546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romano A., Espagnet R., Calabria L.F., Coppola V., Talamanca L.F., Cipriani V., Minniti G., Pierallini A., Fantozzi L.M., Bozzao A. Clinical applications of dynamic susceptibility contrast perfusion-weighted MR imaging in brain tumours. Radiol. Medica. 2012;117:445–460. doi: 10.1007/s11547-011-0715-4. [DOI] [PubMed] [Google Scholar]
- 30.Järnum H., Steffensen E.G., Knutsson L., Fründ E.T., Simonsen C.W., Lundbye-Christensen S., Shankaranarayanan A., Alsop D.C., Jensen F.T., Larsson E.M. Perfusion MRI of brain tumours: A comparative study of pseudo-continuous arterial spin labeling and dynamic susceptibility contrast imaging. Neuroradiology. 2010;52:307–317. doi: 10.1007/s00234-009-0616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fayed N., Dávila J., Medrano J., Olmos S. Malignancy assessment of brain tumours with magnetic resonance spectroscopy and dynamic susceptibility contrast MRI. Eur. J. Radiol. 2008;67:427–433. doi: 10.1016/j.ejrad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 32.Lehmann P., Monet P., de Marco G., Saliou G., Perrin M., Stoquart-Elsankari S., Bruniau A., Vallée J.N. A comparative study of perfusion measurement in brain tumours at 3 Tesla MR: Arterial spin labeling versus dynamic susceptibility contrast-enhanced MRI. Eur. Neurol. 2010;64:21–26. doi: 10.1159/000311520. [DOI] [PubMed] [Google Scholar]
- 33.Park S.M., Kim H.S., Jahng G.H., Ryu C.W., Kim S.Y. Combination of high-resolution susceptibility-weighted imaging and the apparent diffusion coefficient: Added value to brain tumour imaging and clinical feasibility of non-contrast MRI at 3T. Br. J. Radiol. 2010;83:466–475. doi: 10.1259/bjr/34304111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S.A., Harish B.S., Aradhya V.M. A picture fuzzy clustering approach for brain tumour segmentation; Proceedings of the 2016 Second International Conference on Cognitive Computing and Information Processing (CCIP); Mysuru, India. 12–13 August 2016; pp. 1–6. [DOI] [Google Scholar]
- 35.Khotanlou H., Colliot O., Atif J., Bloch I. 3D brain tumour segmentation in MRI using fuzzy classification, symmetry analysis and spatially constrained deformable models. Fuzzy Sets Syst. 2009;160:1457–1473. doi: 10.1016/j.fss.2008.11.016. [DOI] [Google Scholar]
- 36.Papageorgiou E.I., Spyridonos P.P., Glotsos D.T., Stylios C.D., Ravazoula P., Nikiforidis G.N., Groumpos P.P. Brain tumour characterization using the soft computing technique of fuzzy cognitive maps. Appl. Soft Comput. 2008;8:820–828. doi: 10.1016/j.asoc.2007.06.006. [DOI] [Google Scholar]
- 37.Saeed M., Ahsan M., Rahman A.U., Saeed M.H., Mehmood A. An application of neutrosophic hypersoft mapping to diagnose brain tumour and propose appropriate treatment. J. Intell. Fuzzy Syst. 2021;41:1677–1699. doi: 10.3233/JIFS-210482. [DOI] [Google Scholar]
- 38.American Association of Neurological Surgeons. [(accessed on 16 October 2022)]. Available online: https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Brain-tumours.
- 39.Cancer Research UK [(accessed on 25 October 2022)]. Available online: https://www.cancerresearchuk.org/about-cancer/brain-tumours/symptoms.
- 40.Mayo Clinic. [(accessed on 18 September 2022)]. Available online: https://www.mayoclinic.org/diseases-conditions/brain-tumour/symptoms-causes/syc-20350084.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.