Abstract
Background: The immune system (innate and adaptive) is influenced by vitamin D3, which affects gene expression and inflammatory pathways. An umbrella review was conducted to evaluate the power and accuracy of data connecting vitamin D3 to the outcomes of COVID-19 infection and to appraise the proof provided by published meta-analyses. Methods: MEDLINE, Embase, and the Cochrane Library were searched from database inception to 31 May 2022. Meta-analyses of prospective or retrospective observational studies and randomized trials were included. Evidence of association was graded according to the established criteria: strong, highly suggestive, suggestive, weak, or not significant. Results: From 74 publications, 27 meta-analyses described five associations between vitamin D3 levels and supplementation and COVID-19 outcomes. Low levels of vitamin D3 were significantly associated with severity (highly suggestive evidence; OR = 1.97 [95% CI, 1.55–2.51], p < 0.01; I2 = 77%, p < 0.01) and mortality risk due to COVID-19 disease (OR = 1.83 [95% CI, 1.55–2.16], p < 0.01; I2 = 50%, p < 0.01). Vitamin D3 supplementation, after a diagnosis of COVID-19 infection, was associated with significantly reduced infection severity (e.g., ICU admission) and mortality. Conclusions: This umbrella review of the available evidence suggests that insufficient vitamin D3 may increase COVID-19 infection risk, severity, and mortality, in addition to showing a highly suggestive association between vitamin D3 supplementation and reduced severity and mortality among infected patients.
Keywords: vitamin D3, COVID-19, infection, meta-analysis, umbrella review
1. Introduction
Vitamin D modulates the systemic inflammatory response and may actively protect against respiratory tract infections and other diseases [1]. A recent meta-analysis showed that vitamin D supplementation reduces all-cause mortality [2]. In addition, vitamin D supplementation reduces the risk of acute respiratory infections, despite having a small effect size [3]. The outbreak of coronavirus during the last quarter of 2019 resulted in a global pandemic. The virus responsible for the illness was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease it caused was called coronavirus disease 2019 (COVID-19). COVID-19 infection can range from asymptomatic to mild respiratory tract symptoms to severe pneumonia requiring mechanical ventilation, and may require supportive measures, such as admission to an intensive care unit. The disease has no specific treatment, with options ranging from no intervention to administration of oxygen, steroids, and antivirals.
Multiple systematic reviews and meta-analyses have investigated the correlation between vitamin D3 levels and COVID-19 outcomes. However, the reported associations may not be valid because of inherent biases, such as selective bias, publication bias, or other factors. Additionally, the evidence has not been systematically evaluated; therefore, we conducted a meta-analysis of observational and randomized studies to confirm and grade the burden of data. The purpose of this study was to provide an up-to-date assessment of the evidence, evaluating the strength of the published evidence regarding the association between vitamin D3 levels and supplementation with various COVID-19 outcomes.
2. Material and Methods
This study adhered to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline [4].
2.1. Literature Search
We searched PubMed, Embase, and the Cochrane Database of Systematic Reviews from the beginning of May 2022. The search terms used were (“meta-analysis”) AND (“vitamin D3” OR “25-hydroxyvitamin D”) AND (“COVID-19”). In addition, we manually screened references to identify eligible articles. A literature search was independently conducted using FP and AG. The differences between the FP and AG were discussed and resolved using AL.
2.2. Selection Criteria
Two authors (FP and AG) independently reviewed the full text of potentially eligible articles. Only meta-analyses of epidemiological studies examining the relationship between vitamin D3 and COVID-19 outcomes in adults have been considered. In addition, narrative or systematic reviews without meta-analysis and analyses of the effect of vitamin D3 on other respiratory diseases, conference abstracts, and letters to the editors were excluded. When several meta-analyses from the same authors reported the same health outcomes, we included the meta-analysis with the largest number of studies. In summary, meta-analyses of prospective (observational or randomized) or retrospective studies evaluating the correlation between vitamin levels, according to the cutoff chosen by each author, were analyzed to address three main outcomes: COVID-19 infection risk, infection severity (ICU admissions), and mortality. In addition, meta-analyses with the same criteria as above, exploring the association between vitamin D3 supplementation at infection diagnosis and severe illness (ICU admission or death) in adult patients, were included.
2.3. Data Extraction
Data from the included studies were extracted separately by two authors (FP and AG). For each eligible meta-analysis, we extracted the following information: first author, publication year, study design, number of participants and trials, crude or multivariable-adjusted summary risk estimates and corresponding 95% CIs (odds ratios [ORs], risk ratios [RRs], or hazard ratios [HRs]), p values of pooled effects, Egger’s test measurement, and I2 with p for significance. Discrepancies were resolved through discussion. We re-examined the estimated risk of an event for each relationship using random effects models. The three measures of association (OR, RR, and HR) were expected to produce comparable results, as the associated risks of the outcomes we studied were likely to be low. To keep things simple, all risk estimates were explained in terms of odds ratios (ORs).
2.4. Assessment of Methodological Quality
Two authors carefully assessed the quality of each meta-analysis using the reliable AMSTAR 2 [5] tool. Based on the scores, four grades were assigned to describe methodological quality: high, moderate, low, and critically low. A high score was assigned when there was no or only one minor defect present, and a moderate score was assigned when there was more than one defect.
2.5. Grading of the Evidence
Meta-analyses showing nominally significant associations were categorized into 4 evidence groups: strong, highly suggestive, suggestive, and weak [6]. Associations were considered to be supported by strong evidence if all the following criteria were met: the meta-analysis included >1000 cases, a threshold that provides 80% power for hazard ratios ≥ 1.20 (α = 0.05); the random-effects model had a p value ≤10−6 (under the assumption that the tests of statistical significance of the effect in each meta-analysis were valid); absence of high heterogeneity (I2 < 50%); 95% prediction intervals excluded the null value; and no evidence of small-study effects and excess significance bias. There was strong evidence associated with meta-analyses containing more than one thousand cases, with a random-effects p-value of 10−6 or lower. The largest study in the meta-analysis was nominally statistically significant. Meta-analyses with more than 1000 cases and a random-effects p-value of 10−3 or lower yielded suggestive evidence. All other nominally significant associations were considered weak.
2.6. Data Analysis
Sensitivity analysis was performed after excluding studies with a significant risk of bias and low-quality levels. When the p-value was <0.05, the total effects of the pooled meta-analyses were considered significant. I2 and Q tests were used to evaluate heterogeneity between studies and Egger’s test was used to calculate publication bias. p < 0.1 for both heterogeneity and publication bias was considered significant.
3. Results
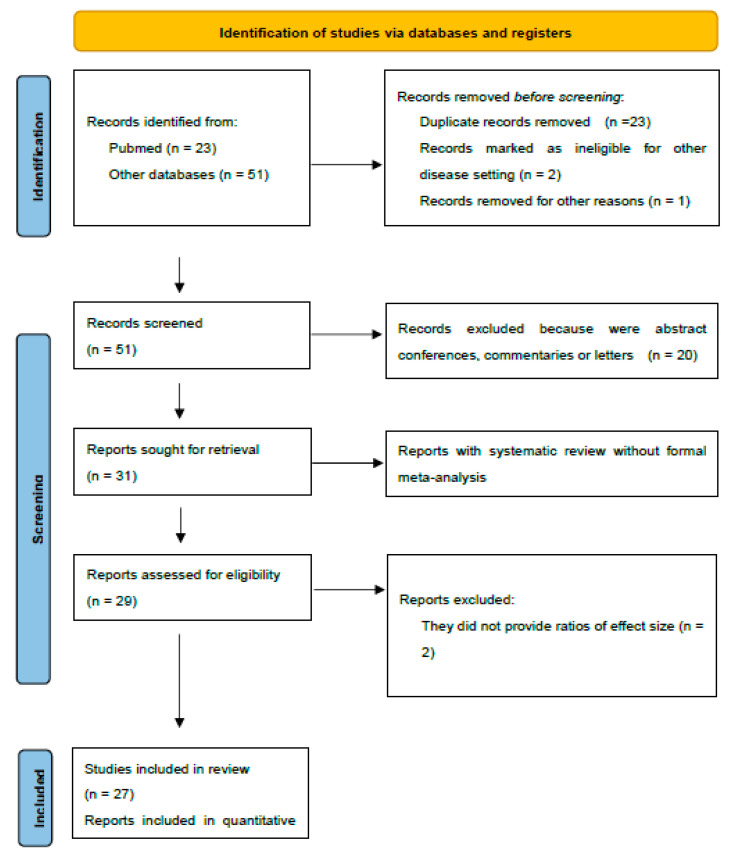
This umbrella review identified 74 publications and included 27 meta-analyses (36%) describing five associations. Figure 1 in the Supplement lists the 47 articles (65%) that were excluded and the reasons for their exclusion.
Figure 1.
Flow diagram of the included studies.
Table 1 describes the characteristics of the 27 eligible meta-analyses [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33], which were published between 2021 and 2022.
Table 1.
Characteristics of the included studies.
| Author/ Year |
N° Studies/ N° pts |
Type of Studies | Time of Search | Vitamina D3 Cutoff (ng/mL) | Vitamin D3 Dose/Timing | Outcome Evaluated | Severity Definition |
Type of Metric/ Type of Analysis |
Small Study Effect (p Egger Test) |
Overall Quality Assessment (AMSTAR2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Akbar/2021 | 14/999,179 | 9 Retrosp observational | Up to 9 December 2020 | 20–30 | - | - Risk of infection and VitD levels | Criteria for severe CAP (ICU, MV) | OR/- | Severity (p 0.047) Mortality (p 0.046) | Moderate |
| 2 Prosp Observational | - Risk of severity and VitD levels | |||||||||
| 2 Cross-Sect | - Risk of death and VitD levels | |||||||||
| 1 Observational study | ||||||||||
| Al Kiyumi/2021 | 43/254,963 | 18 Retrosp | Until 20 December 2020 | 20 | - | - Prevalence of VDD/VDI in patients with COVID-19 | ARDS, severe radiology, ICU admission, O2 therapy, ventilator support | OR/MVA | Severity (p 0.071) Mortality (p 0.023) | Moderate |
| 11 Cross-Sect | - Severity of COVID-19 | |||||||||
| 8 Prosp | - Case-fatality rate | |||||||||
| 3 Case-control | ||||||||||
| 1 Observational study | ||||||||||
| 1 pilot study | ||||||||||
| 1 Case series | ||||||||||
| Bassatne/2021 | 31/8209 | 15 Cross-Sect | Until 20 January 2021 | 20 | 357 to 60,000 IU die/from one week to 12 months | Association of low serum 25(OH)D with COVID-19 related health outcomes: | - | RR/- | Not performed | High |
| 11 Cohort | - Mortality | |||||||||
| 4 Case-control | - ICU admission | |||||||||
| - Invasive ventilation | ||||||||||
| - Non-invasive ventilation | ||||||||||
| - Hospitalization | ||||||||||
| - Length of hospital stay | ||||||||||
| - Disease severity | ||||||||||
| - ARDS | ||||||||||
| Serum 25(OH)D levels in COVID-19 infected patients compared to those not infected | ||||||||||
| Ben-Eltriki/ 2021 |
24/3637 | 7 Retrosp observational | Up to 30 March 2021 | 30 | - | - All-cause mortality | Total number of severe cases, hospital duration, MV | RR/- | Not reported | Critically low |
| 6 Prosp cohort | - COVID-19 severity | |||||||||
| 3 Cross-Sect | - Difference in biological markers and disease severity | |||||||||
| 2 Retrosp | ||||||||||
| 2 Prosp observational | ||||||||||
| 2 Cohort | ||||||||||
| 2 Case-control | ||||||||||
| Beran/2022 | 14/3497 | 4 Retrosp cohort | Through 5 December 2021 | - | Various | -Risk of mortality | - | RR/- | (p 0.047) | High |
| 4 RCT | - Intubation rate | |||||||||
| 3 Non-RCT | ||||||||||
| 2 Cross-Sect | ||||||||||
| 1 Case-control | ||||||||||
| Chen/2021 | 13/536,338 | 10 Retrosp cohort | Up to 5 June 2021 | 20–30 | Various | - Effect of low VitD level on COVID-19 | ICU admission | OR/MVA | Not performed | Moderate |
| 2 RCT | - Effect of VitD supplements on ICU admission or death | |||||||||
| 1 Prosp C | ||||||||||
| Chiodini/ 2021 |
54/ 1,403,715 |
37 Retrosp | Until 31 March 2021 | 25 | - | - VitD status as a predictor of in-hospital COVID-19 severity | ICU admission | OR/- | Severity (p 0.816) Mortality (p 0.110) | Moderate |
| 17 Prosp | - VitD and SARS-CoV-2 infection or COVID-19 related hospitalization | |||||||||
| Crafa/2021 | 29/380,172 | 11 Retrosp | Up to January 2021 | 20 | - | - 25(OH)D levels in patients SARS-CoV-2 pos or neg | Severe pneumonia, ICU, severe radiology or combo | OR/- | Not reported | Moderate |
| 6 Prosp | - 25(OH)D levels in patients with severe or non-severe COVID-19 | |||||||||
| 5 Case-control | - 25(OH)D levels in COVID-19 patients who died compared to those discharged | |||||||||
| 3 Observational study | - Risk of severe COVID-19 in patients with VitD deficiency | |||||||||
| 1 pilot study | - Mortality risk in patients with VitD deficiency | |||||||||
| 1 Cohort | ||||||||||
| 1 Cross-Sect | ||||||||||
| 1 Popul-based | ||||||||||
| Dissanayake/ 2022 |
72/ 1,975,551 |
28 Retrosp cohort | - | Various | - | - Susceptibility to infection | Hospitalization, hypoxia (O2, non/invasive ventilation, ARDS or combo), death or composite | OR/- | (p 0.0001) | Moderate |
| 26 Case-control | - Risk of developing severe COVID-19 | |||||||||
| 17 Prosp cohort | - Mortality | |||||||||
| - VitD in the treatment of COVID-19 | ||||||||||
| Ghasemian/ 2021 |
23/11,901 | 9 Retrosp cohort | Up to 18 December 2020 | 20–30 | - | - Frequency of VitD status in | - | OR/- | Severity (p 0.14) Mortality (p 0.62) | Critically low |
| 5 Case-control | COVID-19 patients | |||||||||
| 4 Cross-Sect | - Mean serum 25(OH)D concentration | |||||||||
| 4 Prosp cohort | - VitD deficiency and SARS-CoV-2 infection | |||||||||
| 1 RCT | - VitD deficiency and COVID-19 severity | |||||||||
| - VitD deficiency and | ||||||||||
| COVID-19 mortality | ||||||||||
| Halim/2022 | 11/1424 | 4 Retrosp cohort | Up to the year 2021 | 20 | - | - VitD and COVID-19 severity | Various | OR/- | Not reported | Critically low |
| 4 Cohort | - VitD and COVID-19 mortality | |||||||||
| 3 Cross-Sect | ||||||||||
| Hosseini/2022 | 23/ 5,870,189 |
9 RCT | Up until January 2022 | 25 | Various | - Risk of COVID-19 infection (primary prevention | - | RR/- | Risk of bias detected in some studies | High |
| 6 Retrosp cohort | studies on uninfected individuals) | |||||||||
| 4 Prosp cohort | - Hospital admission (secondary prevention studies on mild COVID-19 cases) | |||||||||
| 3 Retrosp | - ICU admission and mortality rate (tertiary prevention studies on hospitalized | |||||||||
| 1 Case-control | COVID-19 patients) | |||||||||
| Hu/2022 | 20/12,806 | 6 Retrosp | On 1 May 2021 | 25 | - | - Effect of VitD serum concentration on mortality | ICU admission, ventilator support, length of hospital stay | RR/- | High risk | Low |
| 4 Retrosp observational | - Effect of VitD serum concentration on ICU admission | |||||||||
| 4 Prosp observational | - Effect of VitD serum concentration on ventilator support requirement | |||||||||
| 2 Retrosp cohort | - Length of hospital stay | |||||||||
| 2 Cross-Sect | ||||||||||
| 1 Prosp | ||||||||||
| 1 Prosp cohort | ||||||||||
| Kaya/2021 | 21/205,869 | 11 Case-control | Between 1 January and 15 December 2020 | 20 | - | - VitD and COVID-19 infection | - | OR/- | Severity (p 0.064) Mortality (p 0.911) | Low |
| 9 Cohort | - VitD and COVID-19 severity | |||||||||
| 6 Cross-Sect | - VitD and COVID-19 mortality | |||||||||
| Kazemi/2021 | 39/13,333 | 11 Case-control | Up to 26 November 2020 | Various | - | - Association of 25(OH)D status with SARS-CoV-2 infection | Various | OR/- | (p 0.002) | High |
| 9 Cross-Sect | - Association of VitD status with COVID-19 severity | |||||||||
| 6 Retrosp cohort | - Composite severity | |||||||||
| 4 Retrosp observational | - ICU admission or stay | |||||||||
| 3 Retrosp | - Pulmonary complications | |||||||||
| 3 Prosp | - Hospitalization | |||||||||
| 3 Descriptive | - Concentration of 25(OH)D between severe and less | |||||||||
| 3 Cohort | - severe status of disease | |||||||||
| 2 Prosp cohort | - Inflammatory markers | |||||||||
| 2 Quasi-exp | - Mortality | |||||||||
| 2 RCT | ||||||||||
| Liu/2021 | 10/361,934 | 10 Case-control | To 25 September 2020 | Various | - | - Association between | - | OR/- | (p 0.001; p 0.009) | Moderate |
| VitD deficiency or insufficiency and COVID-19 infection | ||||||||||
| - VitD levels in COVID-19-positive and-negative participants | ||||||||||
| Oscanoa/2021 | 23/2692 | 11 Case-control | Between December 2019 and December 2020 | Various | - | - Mortality and severity | ICU admission, ARDS and/or need for MV | RR/- | Not reported | Critically low |
| 5 Cross-Sect | - proportions in COVID-19 patients with 25(OH)D deficiency | |||||||||
| 5 Cohort | ||||||||||
| 2 Observational study | ||||||||||
| Pai/2022 | 13/2933 | 10 Observational study | Until 8 June 2021 | Various | Various | - ICU admission and/or mortality in COVID-19 patients receiving VitD supplementation | - | OR/- | Risk of bias detected in some studies | High |
| 3 RCT | ||||||||||
| Pereira/2022 | 25/8176 | 9 Cohort | Up to 9 October 2020 | 50 | - | - Prevalence of VitD deficiency in severe cases of COVID-19 | - | OR/- | Risk of bias detected in some studies | Critically low |
| 7 Retrosp | ||||||||||
| 6 Cross-Sect | ||||||||||
| 1 Prosp cohort | ||||||||||
| 1 Popul-based | ||||||||||
| 1 Case series | ||||||||||
| Petrelli/2021 | 43/612,601 | 24 Retrosp | Until 31 January 2021 | 15–30 | Various | - Association between VitD and risk, severity, and mortality for COVID-19 infection | Various | OR/MVA | (p 0.04) | Critically low |
| 7 Prosp | ||||||||||
| 4 Case-control | ||||||||||
| 2 Prosp cohort | ||||||||||
| 2 Cross-Sect | ||||||||||
| 1 Observational | ||||||||||
| 1 Registry data | ||||||||||
| 1 Popul-based | ||||||||||
| 1 RCT | ||||||||||
| Rawat/2021 | 5/467 | 3 RCT | Until 18 May 2021 | - | Various | - Mortality | ICU admission, MV | RR/- | Risk of bias detected in some studies | Critically low |
| 2 Quasi-Exp | - Mechanical ventilation | |||||||||
| - Admission to ICU | ||||||||||
| - Acute markers | ||||||||||
| Shah/2021 | 3/532 | 2 RCT | Between December 2019 and 17 December 2020 | - | Various | - ICU admission | ICU admission, need for MV, mortality | OR/MVA | Severity (p 0.253) Mortality (p 0.138) | Critically low |
| 1 Retrosp case-control | - Death | |||||||||
| - Hospital length of stay | ||||||||||
| - Mechanical ventilation requirement | ||||||||||
| - Serum level of VitD and | ||||||||||
| - biomarkers | ||||||||||
| Szarpak/2022 | 8/2322 | 3 Observational | Until 10 July 2021 | - | Various | - Primary end points were 14-day and in-hospital mortality. | - | OR/- | Risk of bias detected in some studies | Critically low |
| 1 Retrosp cohort | - Secondary end points were ICU admission, need of mechanical ventilation, | |||||||||
| 1 Retrosp case-control | - radiological improvement and secondary infection incidence | |||||||||
| 1 Quasi-exp | ||||||||||
| 1 Pilot study random | ||||||||||
| 1 RCT | ||||||||||
| Tentolouris/ 2021 |
9/278 | 2 Cohort | On 26 March 2021 | - | Various | - Mortality | Various | OR/- | Severity (p 0.011) Mortality (p 0.676) | Moderate |
| 1 RCT | - ICU admissions | |||||||||
| 1 pilot study random | ||||||||||
| 1 Quasi-exp | ||||||||||
| 1 Prosp observ. study | ||||||||||
| 1 Prosp cross-Sect | ||||||||||
| 1 Case-control | ||||||||||
| 1 Retrosp cross-Sect | ||||||||||
| 1 Retrosp | ||||||||||
| Teshome/ 2021 |
14/91,120 | 5 Cohort | From 15 May 2020 to 20 December 2020 | - | - | - Risk of developing COVID-19 infection among VDD and normal VitD levels | - | OR/- | (p 0.764) * | Critically low |
| 5 Case-control | ||||||||||
| 3 Cross-Sect | ||||||||||
| 1 Interim audit | ||||||||||
| Varikasuvu/ 2022 |
6/551 | 6 RCT | To 5 August 2021 | - | Various | - Severity | - | RR/- | (p 0.14) | Moderate |
| - ICU care | ||||||||||
| - Mortality | ||||||||||
| - Seropositivity | ||||||||||
| - RT-PCR positivity | ||||||||||
| Wang/2021 | 17/2756 | 17 Observational | From 1 January 2019 to 3 December 2020 | 12–25 | - | - Mortality | Mortality, hospital admission, lenght of hospital stay | OR/- | High risk | Critically low |
| - Hospital admission | ||||||||||
| - Length of hospital stay | ||||||||||
| - ICU admission |
ICU, intensive care unit; CAP, community-acquired pneumonia; ARDS, acute respiratory distress syndrome; MV, mechanical ventilation; VDD/VDI, vitamin D deficiency/insufficiency; OR, odds ratio; RR, risk ratio; MVA, multivariate analysis; RCT, randomized controlled trial; Case-control, case-control study; Cross-Sect, cross-sectional study; Quasi-Exp, quasi-experimental study; Popul-based, population-based study; Retrosp, retrospective; Prosp, prospective; *, Begg’s test.
The median number of studies included in the meta-analysis was 20. The median sample size in the meta-analysis was 11,901. Overall, 12,767,045 patients were included in these 27 meta-analyses.
Using the AMSTAR 2 method (Table 1), we evaluated the methodological quality of five meta-analyses as high (18.5%) and found that nine (33.3%) were of moderate quality. Two meta-analyses (7.4%) were rated as low-quality, and 11 (40.7%) were rated as critically low-quality.
A description and summary of the association between vitamin D3 levels and supplementation with endpoints are presented in Table 2.
Table 2.
The strength of epidemiologic evidence of the 5 unique health outcomes.
| Endpoint | Author/Year | N° Studies | Effect Size (95% CI) | Heterogenity (I2, p) | Overall Effect |
|---|---|---|---|---|---|
| COVID-19 infection | Akbar/2021 | 6 | OR = 2.71 [1.72, 4.29] | 92.6%, 0.001 | OR = 1.72 (95% CI 1.51–1.97) p < 0.01; I2 = 76%, p for heterogeneity < 0.01 Highly suggestive association |
| Bassatne/2021 | 3 | RR = 1.35 (0.93–1.96) | 76%, 0.11 | ||
| Chen/2021 | 3 | RR = 1.61 (0.92–1.8) | 92%, 0.09 | ||
| Chiodini/2021 | 7 | OR = 1.49 (1.16–1.91) | 79%, 0.1 | ||
| Dissanayake/2022 | 19 | OR = 1.46 (1.28–1.65) | 92%, <0.001 | ||
| Gasemian/2021 | 3 | OR = 3.36 (2.58–4.37) | NR | ||
| Kaya/2021 | 8 | OR = 1.64 (1.32–2.04) | 85%, <0.01 | ||
| Kazemi/2021 | 3 | OR = 1.77 (1.24–2.53) | 44%, 0.16 | ||
| Liu/2021 | 4 | OR = 1.43 (1–2.05) | 64%, 0.03 | ||
| Pereira/2022 | 4 | OR = 1.21 (0.83–1.6) | 78%, 0.003 | ||
| Petrelli/2021 | 7 | OR = 1.5 (1.08–2.08) | 95%, <0.001 | ||
| Teshome/2021 | 8 | OR = 1.8 (1.72–1.88) | 71%, <0.001 | ||
| Severe COVID-19 infection | Akbar/2021 | 5 | OR = 1.90 [1.24, 2.93] | 64.2%, 0.02 | OR = 1.97 (95% CI 1.55–2.51) p < 0.01; I2 = 77%, p for heterogeneity < 0.01 Highly suggestive association |
| Al Kiyumi/2021 | 5 | OR = 3.38 (1.94–5.87) | 67%, <0.01 | ||
| Bassatne/2021 | 7 | RR = 1.34 (0.64–2.79) | 0, 0.43 | ||
| Ben Eltriki/2021 | 12 | RR = 1.5 (1.1–2.05) | 92%, 0.01 | ||
| Chiodini/2021 | 10 | OR = 2.83 (1.74–4.6) | 84%, 0.94 | ||
| Crafa/2021 | 8 | OR = 4.58 (2.24–9.36) | 81%, 0.001 | ||
| Dissanayake/2022 | 36 | OR = 1.9 (1.52–2.38) | 81%, <0.001 | ||
| Gasemian/2021 | 13 | OR = 5.18 (2.61–10.31) | NR | ||
| Hu/2022 | 3 | RR = 0.87 (0.67–1.14) | 0, 0.31 | ||
| Kaya/2021 | 9 | OR = 1.24 (0.71–2.17) | 92%, <0.01 | ||
| Kazemi/2021 | 6 | OR = 10.61 (2.07–54.38) | 90, <0.01 | ||
| Oscanoa/2021 | 4 | RR = 1.79 (1.3–2.46) | 81%, <0.001 | ||
| Pereira/2022 | 10 | OR = 1.65 (1.3–2.09) | 35%, 0.12 | ||
| Petrelli/2021 | 17 | OR = 2.6 (1.84–3.67) | 87%, <0.001 | ||
| Wang/2021 | 4 | OR = 5.44 (0.38–78.42) | 83%, NR | ||
| COVID-19 mortality | Akbar/2021 | 8 | OR = 3.08 [1.35, 7.00] | 80.3%, 0.001 | OR = 1.83 (95% CI 1.55–2.16) p < 0.01; I2 = 50%, p for heterogeneity = 0.01 Highly suggestive association |
| Al Kiyumi/2021 | 4 | OR = 2.3 [1.47–3.6] | 0, 0.07 | ||
| Bassatne/2021 | 7 | RR = 2.09 (0.92–4.77) | 76%, 0.08 | ||
| Ben Eltriki/2021 | 18 | RR = 1.6 (1.1–2.32) | 68%, 0.01 | ||
| Chen/2021 | 4 | RR = 2.36 (1.1–5.06) | 0%, 0.71 | ||
| Chiodini/2021 | 19 | OR = 4.15 (1.76–9.79) | 44%, 0.33 | ||
| Crafa/2021 | 9 | OR = 4.92 (0.83–29.31) | 94%, <0.001 | ||
| Dissanayake/2022 | 20 | OR = 2.07 (1.28–3.35) | 73%, 0.003 | ||
| Gasemian/2021 | 7 | OR = 1.6 (0.57–4.45) | NR | ||
| Halim/2022 | 5 | OR = 1.15 (0.52–2.49) | 66%, 0.34 | ||
| Hu/2022 | 20 | RR = 1.49 (1.34–1.66) | 83%, 0.88 | ||
| Kaya/2021 | 5 | OR = 1.58 (0.76–3.28) | 83%, <0.01 | ||
| Kazemi/2021 | 8 | OR = 2.62 (1.13–6.08) | 83%, <0.001 | ||
| Oscanoa/2021 | 4 | RR = 2.67 (1.2–5.94) | 83%, <0.001 | ||
| Pereira/2022 | 5 | OR = 1.82 (1.06–2.58) | 59%, 0.04 | ||
| Petrelli/2021 | 19 | OR = 1.22 (1.04–1.43) | 74%, 0.01 | ||
| Wang/2021 | 12 | OR = 2.47 (1.5–4.05) | 30%, NR | ||
| Effect of supplementation of vitamin D3 on COVID-19 infection severity | Beran/2022 | 6 | RR = 0.55 (0.32–0.97) | 48%, 0.04 | OR = 0.38 (95% CI 0.28–0.5) p < 0.01; I2 = 0%, p for heterogeneity = 0.71 Highly suggestive association |
| Chen/2021 | 2 | OR = 0.14 (0–4.9) | 90%, 0.28 | ||
| Hosseini/2022 | 7 | RR = 0.35 (0.20–0.62) | 75%, <0.001 | ||
| Petrelli/2021 | 6 | OR = 0.27 (0.11–0.66) | 49%, 0.004 | ||
| Rawat/2021 | 2 | RR= 0.2 (0.01–4.26) | 89%, 0.3 | ||
| Shah/2021 | 3 | OR = 0.15 (0.01–1.45) | 82%, NR | ||
| Szarpak/2021 | 5 | OR = 0.19 (0.06–0.54) | 77%, 0.002 | ||
| Tentolouris/2021 | 6 | OR = 0.32 (0.14–0.71) | 60%, 0.028 | ||
| Varikasuvu/2022 | 6 | RR = 0.46 (0.23–0.93) | 52%, 0.06 | ||
| Effect of supplementation of vitamin D3 on COVID-19 infection mortality | Beran/2022 | 9 | RR = 0.64 (0.33–1.27) | 77%, 0.25 | OR = 0.53 (95% CI 0.42–0.67) p < 0.01: I2 = 0%, p for heterogeneity = 0.88 Highly suggestive association |
| Chen/2021 | 2 | OR = 0.57 (0.04–7.78) | 64%, 0.67 | ||
| Hosseini/2022 | 11 | RR = 0.46 (0.30–0.7) | 54%, <0.001 | ||
| Pai/2022 | 7 * | OR = 0.35 (0.14–0.85) | 67%, 0.03 | ||
| Petrelli/2021 | 7 | OR = 0.41 (0.21–0.81) | 72%, 0.01 | ||
| Rawat/2021 | 4 | RR= 0.55 (0.22–1.39) | 58%, 0.21 | ||
| Shah/2021 | 3 | OR = 0.93 (0.41–2.11) | 21%, 0.27 | ||
| Szarpak/2021 | 7 | OR = 0.56 (0.23–1.37) | 74%, 0.2 | ||
| Tentolouris/2021 | 9 | OR = 0.59 (0.31–1.12) | 62%, 0.006 | ||
| Varikasuvu/2022 | 4 | RR = 0.78 (0.25–2.4) | 48%, 0.03 |
*, 2 studies reported ICU admission and ICU admission or mortality as outcomes.
There was considerable heterogeneity among the three associations (those that correlated levels with outcomes: I2 = 76, 77, and 50%). Neither of the other two associations (vitamin D3 supplementation and outcome) were heterogeneous (I2 = 0%). There were small-study effects in 6, 7, 10, 4, and 4 studies for each association (50, 50, 58, 44, and 40%, respectively), so grade levels were downgraded for each association. In the largest study, all associations were statistically significant at p < 0.05 for all effect sizes.
Each of the five associations reached statistical significance at p < 0.01, suggesting vitamin D3 supplementation or adequate vitamin D3 levels may protect against COVID-19 risk and unfavorable outcomes.
4. Grading of Evidence
4.1. Susceptibility to Infection
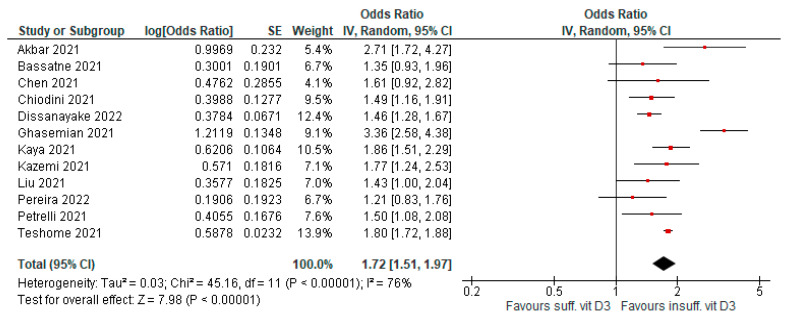
Low levels of vitamin D3 were associated with a significant risk of COVID-19 infection compared to sufficient levels. Twelve meta-analyses evaluated this outcome (AMSTAR 2 quality level was moderate to high in seven papers). Evidence was graded as highly suggestive (OR = 1.72 [95% CI, 1.51–1.97], p < 0.01; I2 = 76%, p < 0.01; Figure 2).
Figure 2.
Forest plot showing results for the association of vitamin D3 levels and COVID-19 infection risk.
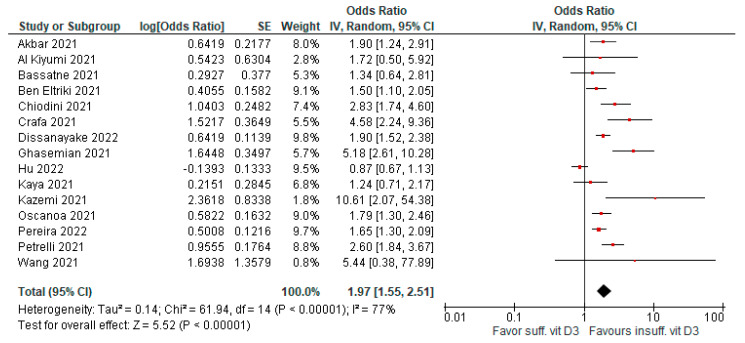
4.2. Risk of Severe Infection (ICU Admission)
Low levels of vitamin D3 were associated with significant risk of severity compared to patients with sufficient levels. Fifteen meta-analyses evaluated this outcome (AMSTAR 2 quality level was moderate to high in seven papers). Evidence was graded as highly suggestive (OR = 1.97 [95% CI, 1.55–2.51], p < 0.01; I2 = 77%, p < 0.01; Figure 3).
Figure 3.
Forest plot showing results for the association of vitamin D3 levels and COVID-19 severity.
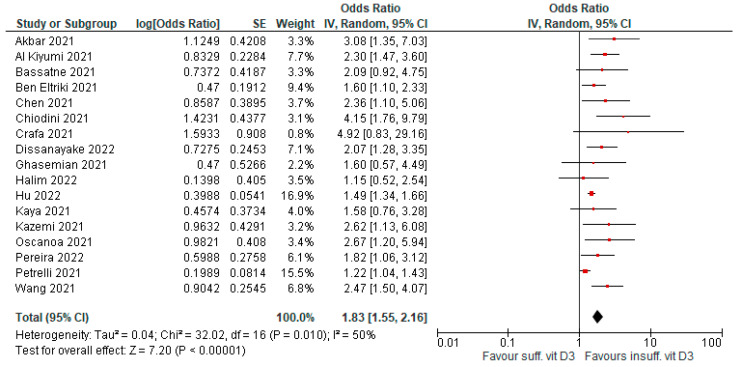
4.3. Risk of Death
Low levels of vitamin D3 were associated with significant mortality risk compared to patients with sufficient levels. Seventeen meta-analyses evaluated this outcome (AMSTAR 2 quality level was moderate to high in eight papers). Evidence was graded as highly suggestive (OR = 1.83 [95% CI, 1.55–2.16], p < 0.01; I2 = 50%, p = 0.01; Figure 4).
Figure 4.
Forest plot showing results for the association of vitamin D3 levels and COVID-19 mortality.
4.4. Supplementation to Improve Infection Severity
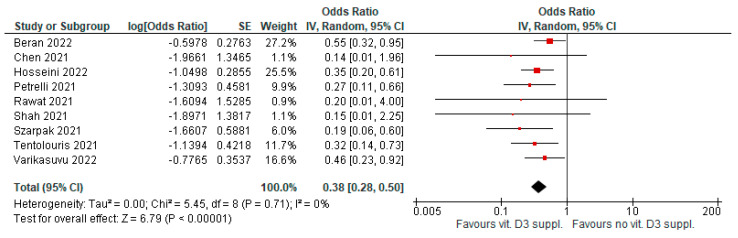
Vitamin D3 supplementation, after a diagnosis of COVID-19 infection, was associated with significantly reduced infection severity (e.g., ICU admission). Nine meta-analyses evaluated this outcome (AMSTAR2 had a moderate-high level quality in five papers). Evidence was graded as highly suggestive for evidence of publication bias in two meta-analyses (OR = 0.38 [95% CI, 0.28–0.5], p < 0.01; I2 = 0%, p = 0.71; Figure 5).
Figure 5.
Forest plot showing results for the association of vitamin D3 supplementation and COVID-19 severity.
4.5. Supplementation to Improve Infection Mortality
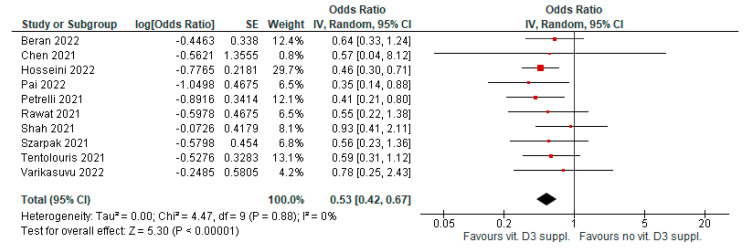
Vitamin D3 supplementation, after a diagnosis of COVID-19 infection, was associated with significantly reduced infection mortality. Ten meta-analyses evaluated this outcome (AMSTAR 2 quality level was moderate to high in six papers). Evidence was graded as highly suggestive for evidence of publication bias in three meta-analyses (OR = 0.53 [95% CI, 0.42–0.67], p < 0.01; I2 = 0%, p = 0.88; Figure 6).
Figure 6.
Forest plot showing results for the association of vitamin D3 supplementation and COVID-19 mortality.
4.6. Sensitivity Analysis
In studies where the risk of infection and disease severity were correlated with vitamin D3 levels, the strength of association remained unchanged after the exclusion of biased meta-analyses (I2 85 and 51%). After exclusion of low levels, the meta-analysis association became strong for risk of infection and remained highly suggestive of disease severity (I2 41 and 75%). For correlation with mortality, the association became strong after excluding papers that retained significant bias and low-quality levels (I2 0 and 46%). Both outcomes explored in supplementation studies maintained an unchanged grade of association (highly suggestive) after excluding studies with significant bias and low-quality levels (I2 0 and 0%). After considering papers that only calculated adjusted ORs (seven papers), the association between vitamin D3 levels and infection and mortality become strong.
5. Discussion
The most common forms of vitamin D supplementation are cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2), which are the precursors of 1,25(OH)2D3 (the active form of vitamin D). Besides its essential biological functions (classical), such as bone metabolism and calcium and phosphorus homeostasis, vitamin D is also involved in other roles (non-classical) involving immune modulation, lung and muscle function, cardiovascular health, and infectious disease prevention.
Many reports made in Europe, not among those included in these meta-analyses, based on observations made during the first pandemic wave, have suggested an association between vitamin D deficiency, risk of SARS-CoV-2 infection, incidence and severity of COVID-19, and mortality. Speculative observations related to the higher prevalence of hypovitaminosis D among European countries and the very high prevalence of SARS-CoV-2 and COVID-19 infections, especially in the northern regions, have defined the association between the two events without verifying the causal link or excluding causality. Vitamin D status, risk of infection, and development of severe forms of the disease are complex phenomena dependent on innumerable variables whose complex interdependent relationship cannot be described by their mere sum. Therefore, only studies conducted in large cohorts and/or pooled/umbrella analyses can assume epidemiological relevance.
Here, we present the first umbrella review of published meta-analyses on the association between vitamin D3 and COVID-19. We included 27 published meta-analyses, which comprised five summary risk estimates for the association vitamin D3 insufficiency (<30 ng/mL) and supplementation have with COVID-19 outcomes. We found a significant effect size that supported all the statistically significant associations in the primary and sensitivity analyses. There is highly suggestive evidence for an association between low vitamin D3 levels and COVID-19 incidence and disease severity (ICU admission). Furthermore, suggestive evidence exists for positive associations between insufficient vitamin D3 levels and mortality in infected patients (83% increased risk compared with normally replete patients). However, we did not analyze continuous vitamin D3 level/effect associations due to data limitations. Furthermore, after exclusion of biased or low-quality meta-analyses, or those that included unadjusted (crude) ORs, the association with mortality became strong. Finally, we found positive, highly suggestive evidence of an association between vitamin D administration and risk of death reduction.
Vitamin D regulates other cellular functions, in addition to calcium and bone homeostasis. The vitamin D receptor is universally expressed in nucleated cells. A large amount of epidemiological data indicate that the risks of cancer and infectious, autoimmune, and vascular diseases are higher when 25-hydroxyvitamin D (25[OH]D) levels are <20 ng/mL, and that risks decrease with higher 25(OH)D concentrations [34]. However, no convincing randomized trial data has shown that vitamin D supplements can decrease cancer risk or prognosis, decrease the risk or severity of infections or autoimmune diseases, or decrease cardiovascular risks or metabolic diseases. Subjects with normal vitamin D3 levels may be healthier and less prone to many diseases, as well as to COVID-19 infection and potential consequences (e.g., intubation). In particular, in COVID-19 patients, a meta-analysis showed that patients with sufficient vitamin D had significantly lower levels of interleukin-6, C-reactive protein, ferritin, lactate dehydrogenase, fibrinogen, and D-dimer compared to those in the vitamin D deficient group [35].
It is highly plausible that the proposed biological mechanisms explain the observed associations. Several genes involved in the immune system (innate and adaptive immunity) and the downstream inflammatory cascade are influenced by vitamin D, which affects the susceptibility to and severity of infectious diseases. Patients with COVID-19 may benefit from vitamin D supplementation as a preventive and therapeutic agent. Vitamin D binds to its receptor and affects two main pathways: (a) it inhibits pro-inflammatory cytokines, interfering with the TNF-induced NFkB1 pathway, and (b) it activates the Jak-Stat pathway by inducing the expression of interferon-stimulating genes [36,37,38].
More specifically, 1,25(OH)2D has antimicrobial activity, as it can induce the expression of cathelicidin and β-defensin 2 proteins with both direct and indirect antimicrobial efficacy (through stimulation of chemotaxis of cells of the immune system, inducing the expression of pro-inflammatory cytokines, resulting in the removal of infected cells in the respiratory tract). The expression of β-defensin [39] is stimulated by vitamin D through the induction of nucleotide-binding oligomerization domain-containing protein 2 (NOD2) 2. Furthermore, 1,25(OH)2D inhibits the expression of hepcidin and, therefore, determines the elimination of the hepcidin-mediated block of iron export dependent on ferroportin, resulting in increased efflux of iron from the infected cell and, consequently, the reduction in the availability of this element for microbial growth [40]. Indeed, vitamin D has multiple antimicrobial effects, including stimulation of the barrier function of the intestine [41] and alveolar epithelia [42], production of reactive oxygen species (ROS) [43], neutrophil function [44], and phagocytic and autophagocytic activities (through the induction of the key effectors of autophagy, LC3, beclin 1, and PI3Kγ3) of macrophages [45]. Both the induction of cathelicidin and defensins and stimulation of one of the pro-autophagic pathways in antigen-presenting cells have an important antiviral effect, inhibit virus replication [46], and aid in the clearance of virus particles [47]. In the context of adaptive immunity, calcitriol limits the activation of T lymphocytes [48], induces the expression of regulatory phenotypes (Tregs) that mediate immune tolerance, and limits abnormal immune responses and phenotypic shifts from T helper Th1/Th17 to Th2 (from pro-inflammatory to regulatory) [49]. The effectiveness of vitamin D is a function of the activity of its receptor, VDR. Single nucleotide polymorphisms (SNPs) in the VDR gene affect protein responsiveness and have been associated with numerous immune dysfunctions. Compared to the CT and CC genotypes, the TT genotype of the FokI polymorphism has been associated with a greater risk of respiratory syncytial virus (SCV) infections [50].
The attack/replication sequence into human cells explains another possible therapeutic role of vitamin D. Through its spike protein, SARS-CoV-2 enters the human body via its cognate angiotensin, converting enzyme-2 (ACE-2) receptor on the cells of the lungs and other organs [51]. As soon as the viral RNA enters the host cell, it replicates continuously, using the host’s polymerase system. This leads to severe pathological changes and, finally, cell death. The virus uses multiple mechanisms to evade the host immune system, including excessive stimulation of the renin-angiotensin system (RAS) [52]. SARS-CoV-2 interferes with and modulates the RAS system, resulting in an excess of angiotensin II being generated, activating the cytokine storm, and downregulating the host’s immune system. Dysfunctional immune responses in some COVID-19 cases lead to overproduction of pro-inflammatory cytokines in circulation that trigger acute respiratory distress syndrome (ARDS) and eventually death. Vitamin D has been hypothesized to play a role in acute respiratory distress syndrome (ARDS). ACE2, which, as previously reported, serves as the docking site for viral protein S, converts angiotensin II (Ang-II) to angiotensin 1–7 [Ang(1–7)]. The latter has vasodilator, anti-inflammatory, and protective effects against organ damage actions [53]. Following binding to protein S, the ACE2-viral particle complex is internalized and, therefore, the enzymatic activity of ACE2 is downregulated. Downregulation of ACE2 is associated with an abnormal inflammatory response that can cause tissue damage, leading to further downregulation of ACE2. This process can result in acute respiratory distress syndrome (ARDS) [54,55]. Vitamin D has a protective role against ARDS, given the ability to inhibit the expression of renin and the activity of the ACE/Ang-II/AT1R axis and to stimulate, instead, the ACE2/Ang-(1–7)/MasG (G protein-associated Mas receptor). Thus, vitamin D acts as a negative endocrine modulator of the renin-angiotensin-aldosterone system (RAAS) [56,57,58].
An abnormal inflammatory response resulting from SARS-CoV-2 infection is responsible for the development of COVID-19 and, in some cases, for manifestations of increasing severity. The so-called “cytokine storm”, characterized by the massive and sustained release of pro-inflammatory cytokines (IL-1, IL-6, TNFα, and IFNγ), is responsible for the symptoms and organ damage (particularly affecting the lungs and heart). In severe COVID-19 cases, IL-6 levels were 2.9 times higher than in cases with less severe disease. This evidence supports the role of vitamin D in reducing cytokine storms by inducing anti-inflammatory mediators (IL-10, IL-4, and TGFβ). Furthermore, 1,25(OH)2D can reduce the hyperinflammatory response and, therefore, the manifestations of COVID-19 by enhancing the expression of anti-inflammatory and regulatory phenotypes Th2 and T-reg at the expense of pro-inflammatory Th1/Th17, which is also more prominently involved in the cytokine storm [59].
The results of this study suggest a positive association between vitamin D3 supplementation and the severity and mortality of COVID-19 (a reduction in events by approximately 60% and 50%, respectively), as described by Albergamo et al. [60]. However, its dosage and timing are yet to be determined. A recent randomized trial found that the administration of vitamin D did not affect the severity of COVID-19 [61]. In this trial, a single dose of 200 000 IU vitamin D was administered to hospitalized patients with moderate or severe disease. Whether high-dose bolus or continuous low-dose administration is the preferred regimen remains unclear. In addition, whether patients with only true deficiency (<20 ng/mL) or the whole COVID-19 population should be treated with cholecalciferol is still unknown. Current recommendations suggest treating patients with vitamin D3 insufficiency with daily doses of 400–800 units for up to 3 months [62]. Only those with severe vitamin D3 deficiency (<10 ng/mL) may be offered higher doses; however, in general, higher monthly doses can increase the risk of falls in older adults [63]. NICE has proposed specific guidelines related to vitamin D3 administration during the COVID-19 pandemic. In this document, all adults (including women who are pregnant or breastfeeding), young people, and children older than 4 years should consider taking a daily supplement containing 400 units of vitamin D between October and early March because people do not produce enough vitamin D from sunlight in these months [64].
Other micronutrients or vitamin D agents may also play a role in this disease. In particular, compounds such as vitamin C and zinc may play a role; however, Beran et al. [27] found that vitamin C and zinc might not reduce the mortality or severity of COVID-19.
This study has several limitations. First, we must acknowledge that most of the studies examined were retrospective in nature, and a few made adjustments for common variables (e.g., age and pre-existing chronic diseases). In addition, few randomized controlled trials that are capable of managing confounding factors due to their design are available for supplementation. Additionally, many of the included studies showed publication bias based on Egger’s test and a moderate to low quality according to the AMSTAR 2 checklist, leading to the recommendation that researchers follow methodology guidelines to ensure the methodological quality of future meta-analyses. This resulted in the evidence for two associations being downgraded from strong to highly suggestive. When studies with intrinsic bias or low quality were excluded, vitamin D3 levels strongly correlated with COVID-19 mortality. Furthermore, we were unable to perform subgroup analyses due to the absence of data (e.g., sex, age, previous use of vitamin D3, comorbidities, and country of origin).
The following considerations can be made based on the results of these studies. Even if further controlled studies are needed, vitamin D appears to be more effective against COVID-19 (both due to the speed of negativization and the benign evolution of the disease in case of infection) if administered prior to the manifested infection, especially in elderly, frail, and institutionalized subjects. The minimum optimal plasma target of 25(OH)D to be reached in the preventive setting would be ≥30 ng/mL, for which it is necessary to administer high doses of cholecalciferol, also in relation to the basal levels of the patient, up to 4000 IU/day [37,65,66]. With the preventive administration of oral cholecalciferol (up to 4000 IU/day), the use of vitamin D, which, even at high doses, does not present substantial side effects, is useful for correcting a situation of specific general deficiency in the population, especially in winter, regardless of SARS-CoV-2 infection [67]. Additionally, higher parenteral doses of vitamin D3 could have an effect on the final outcome, as bypassing liver vitamin D3 will also be available to CYP11A1 for metabolism in organs expressing this enzyme [68]. In addition to targeting COVID-19 through both nuclear receptor-dependent and-independent mechanisms, new CYP11A1-derived active forms of vitamin D and lumisterol can be used as antiviral drugs and supplements to prevent and attenuate it [69].
6. Conclusions
In conclusion, the results of the present study support and reaffirm the recommendation for vitamin D3 supplementation as helpful in preventing COVID-19 (particularly for elderly or frail people, housebound individuals, or individuals living in nursing homes and who spend a great deal of their time indoors due to COVID-19), while emphasizing its potential anti-inflammatory and immunomodulatory properties to lessen the severity and mortality of infected individuals. Additionally, this evidence supports the possibility of monitoring vitamin D3 levels, especially in high-risk populations. However, additional research is necessary to determine the optimal dose, timing, and duration of vitamin D3 therapy for infected patients.
Author Contributions
Conceptualization, F.P. and A.G.; methodology, F.P.; software, F.P.; validation, F.P. and A.G.; formal analysis, F.P.; data curation, A.G. and S.O.; writing—original draft preparation, F.P.; writing—review and editing, K.B., M.C., M.C.P., D.P., V.L., L.D., C.R., G.D., M.G., S.O., F.P. and A.G.; visualization, A.L.; supervision, F.P.; project administration, F.P. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Heath A.K., Kim I.Y., Hodge A.M., English D.R., Muller D.C. Vitamin D Status and Mortality: A Systematic Review of Observational Studies. Int. J. Environ. Res. Public Health. 2019;16:383. doi: 10.3390/ijerph16030383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y., Fang F., Tang J., Jia L., Feng Y., Xu P., Faramand A. Association between vitamin D supplementation and mortality: Systematic review and meta-analysis. BMJ. 2019;366:l4673. doi: 10.1136/bmj.l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martineau A.R., Jolliffe D.A., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., Goodall E.C., et al. Vitamin D supplementation to prevent acute respiratory infections: Individual participant data meta-analysis. Health Technol. Assess. 2019;23:1–44. doi: 10.3310/hta23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 5.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theodoratou E., Tzoulaki I., Zgaga L., Ioannidis J.P.A. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbar M.R., Wibowo A., Pranata R., Setiabudiawan B. Low Serum 25-hydroxyvitamin D (Vitamin D) Level Is Associated with Susceptibility to COVID-19, Severity, and Mortality: A Systematic Review and Meta-Analysis. Front. Nutr. 2021;8:660420. doi: 10.3389/fnut.2021.660420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Kiyumi M., Kalra S., Davies J., Kalhan A. The impact of vitamin d deficiency on the severity of symptoms and mortality rate among adult patients with Covid-19: A systematic review and meta-analysis. Indian J. Endocrinol. Metab. 2021;25:261. doi: 10.4103/ijem.ijem_115_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaya M.O., Pamukçu E., Yakar B. The role of vitamin D deficiency on COVID-19: A systematic review and meta-analysis of observational studies. Epidemiology Health. 2021;43:e2021074. doi: 10.4178/epih.e2021074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazemi A., Mohammadi V., Aghababaee S.K., Golzarand M., Clark C.C.T., Babajafari S. Association of Vitamin D Status with SARS-CoV-2 Infection or COVID-19 Severity: A Systematic Review and Meta-analysis. Adv. Nutr. Int. Rev. J. 2021;12:1636–1658. doi: 10.1093/advances/nmab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu N., Sun J., Wang X., Zhang T., Zhao M., Li H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021;104:58–64. doi: 10.1016/j.ijid.2020.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oscanoa T.J., Amado J., Vidal X., Laird E., Ghashut A.R., Romero-Ortuno R. The Relationship Between the Severity and Mortality of SARS-CoV-2 Infection and 25-hydroxyvitamin D Concentration—A Metaanalysis. Adv. Respir. Med. 2021;89:145–157. doi: 10.5603/ARM.a2021.0037. [DOI] [PubMed] [Google Scholar]
- 13.Pereira M., Dantas Damascena A.D., Galvão Azevedo L.M.G., de Almeida Oliveira T.D.A., da Mota Santana J.D.M. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022;62:1308–1316. doi: 10.1080/10408398.2020.1841090. [DOI] [PubMed] [Google Scholar]
- 14.Petrelli F., Luciani A., Perego G., Dognini G., Colombelli P.L., Ghidini A. Therapeutic and prognostic role of vitamin D for COVID-19 infection: A systematic review and meta-analysis of 43 observational studies. J. Steroid Biochem. Mol. Biol. 2021;211:105883. doi: 10.1016/j.jsbmb.2021.105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah K., Saxena D., Mavalankar D. Vitamin D supplementation, COVID-19 and disease severity: A meta-analysis. QJM Int. J. Med. 2021;114:175–181. doi: 10.1093/qjmed/hcab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szarpak L., Filipiak K.J., Gasecka A., Gawel W., Koziel D., Jaguszewski M.J., Chmielewski J., Gozhenko A., Bielski K., Wroblewski P., et al. Vitamin D supplementation to treat SARS-CoV-2 positive patients. Evidence from meta-analysis. Cardiol. J. 2022;29:188–196. doi: 10.5603/CJ.a2021.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tentolouris N., Samakidou G., Eleftheriadou I., Tentolouris A., Jude E.B. The effect of vitamin D supplementation on mortality and intensive care unit admission of COVID-19 patients. A systematic review, meta-analysis and meta-regression. Diabetes/Metab. Res. Rev. 2022;38:e3517. doi: 10.1002/dmrr.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teshome A., Adane A., Girma B., Mekonnen Z.A. The Impact of Vitamin D Level on COVID-19 Infection: Systematic Review and Meta-Analysis. Front. Public Health. 2021;9:624559. doi: 10.3389/fpubh.2021.624559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben-Eltriki M., Hopefl R., Wright J.M., Deb S. Association between Vitamin D Status and Risk of Developing Severe COVID-19 Infection: A Meta-Analysis of Observational Studies. J. Am. Coll. Nutr. 2022;41:679–689. doi: 10.1080/07315724.2021.1951891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varikasuvu S.R., Thangappazham B., Vykunta A., Duggina P., Manne M., Raj H., Aloori S. COVID-19 and vitamin D (Co-VIVID study): A systematic review and meta-analysis of randomized controlled trials. Expert Rev. Anti-infective Ther. 2022;20:907–913. doi: 10.1080/14787210.2022.2035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., Joshi A., Leopold K., Jackson S., Christensen S., Nayfeh T., Mohammed K., Creo A., Tebben P., Kumar S. Association of vitamin D deficiency with COVID-19 infection severity: Systematic review and meta-analysis. Clin. Endocrinol. 2021;96:281–287. doi: 10.1111/cen.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal R., Banerjee M., Bhadada S.K., Shetty A.J., Singh B., Vyas A. Vitamin D supplementation and clinical outcomes in COVID-19: A systematic review and meta-analysis. J. Endocrinol. Investig. 2022;45:53–68. doi: 10.1007/s40618-021-01614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassatne A., Basbous M., Chakhtoura M., El Zein O., Rahme M., Fuleihan G.E.-H. The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis. Metabolism. 2021;119:154753. doi: 10.1016/j.metabol.2021.154753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dissanayake H.A., de Silva N.L., Sumanatilleke M., de Silva S.D.N., Gamage K.K.K., Dematapitiya C., Kuruppu D.C., Ranasinghe P., Pathmanathan S., Katulanda P. Prognostic and Therapeutic Role of Vitamin D in COVID-19: Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2021;107:1484–1502. doi: 10.1210/clinem/dgab892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rawat D., Roy A., Maitra S., Shankar V., Khanna P., Baidya D.K. “Vitamin D supplementation and COVID-19 treatment: A systematic review and meta-analysis”. Diabetes Metab. Syndr. Clin. Res. Rev. 2021;15:102189. doi: 10.1016/j.dsx.2021.102189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosseini B., El Abd A., Ducharme F.M. Effects of Vitamin D Supplementation on COVID-19 Related Outcomes: A Systematic Review and Meta-Analysis. Nutrients. 2022;14:2134. doi: 10.3390/nu14102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beran A., Mhanna M., Srour O., Ayesh H., Stewart J.M., Hjouj M., Khokher W., Mhanna A.S., Ghazaleh D., Khader Y., et al. Clinical significance of micronutrient supplements in patients with coronavirus disease 2019: A comprehensive systematic review and meta-analysis. Clin. Nutr. ESPEN. 2022;48:167–177. doi: 10.1016/j.clnesp.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J., Mei K., Xie L., Yuan P., Ma J., Yu P., Zhu W., Zheng C., Liu X. Low vitamin D levels do not aggravate COVID-19 risk or death, and vitamin D supplementation does not improve outcomes in hospitalized patients with COVID-19: A meta-analysis and GRADE assessment of cohort studies and RCTs. Nutr. J. 2021;20:89. doi: 10.1186/s12937-021-00744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiodini I., Gatti D., Soranna D., Merlotti D., Mingiano C., Fassio A., Adami G., Falchetti A., Eller-Vainicher C., Rossini M., et al. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front. Public Health. 2021;9:736665. doi: 10.3389/fpubh.2021.736665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crafa A., Cannarella R., Condorelli R.A., Mongioì L.M., Barbagallo F., Aversa A., La Vignera S., Calogero A.E. Influence of 25-hydroxy-cholecalciferol levels on SARS-CoV-2 infection and COVID-19 severity: A systematic review and meta-analysis. Eclinicalmedicine. 2021;37:100967. doi: 10.1016/j.eclinm.2021.100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghasemian R., Shamshirian A., Heydari K., Malekan M., Alizadeh-Navaei R., Ebrahimzadeh M.A., Warkiani M.E., Jafarpour H., Bazaz S.R., Shahmirzadi A.R., et al. The role of vitamin D in the age of COVID-19: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021;75:e14675. doi: 10.1111/ijcp.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halim C., Mirza A.F., Sari M.I. The Association between TNF-α, IL-6, and Vitamin D Levels and COVID-19 Severity and Mortality: A Systematic Review and Meta-Analysis. Pathogens. 2022;11:195. doi: 10.3390/pathogens11020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Y., Kung J., Cave A., Banh H.L. Effects of Vitamin D Serum Level on Morbidity and Mortality in Patients with COVID-19: A Systematic Review and Meta-Analysis. J. Pharm. Pharm. Sci. 2022;25:84–92. doi: 10.18433/jpps32590. [DOI] [PubMed] [Google Scholar]
- 34.Bouillon R., Marcocci C., Carmeliet G., Bikle D., White J.H., Dawson-Hughes B., Lips P., Munns C.F., Lazaretti-Castro M., Giustina A., et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2018;40:1109–1151. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hopefl R., Ben-Eltriki M., Deb S. Association Between Vitamin D Levels and Inflammatory Markers in COVID-19 Patients: A Meta-Analysis of Observational Studies. J. Pharm. Pharm. Sci. 2022;25:124–136. doi: 10.18433/jpps32518. [DOI] [PubMed] [Google Scholar]
- 36.Zdrenghea M.T., Makrinioti H., Bagacean C., Bush A., Johnston S.L., Stanciu L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017;27:e1909. doi: 10.1002/rmv.1909. [DOI] [PubMed] [Google Scholar]
- 37.Arboleda J.F., Urcuqui-Inchima S. Vitamin D Supplementation: A Potential Approach for Coronavirus/COVID-19 Therapeutics? Front. Immunol. 2020;11:1523. doi: 10.3389/fimmu.2020.01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed F. A Network-Based Analysis Reveals the Mechanism Underlying Vitamin D in Suppressing Cytokine Storm and Virus in SARS-CoV-2 Infection. Front. Immunol. 2020;11:590459. doi: 10.3389/fimmu.2020.590459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T.-T., Dabbas B., Laperriere D., Bitton A.J., Soualhine H., Tavera-Mendoza L.E., Dionne S., Servant M.J., Bitton A., Seidman E.G., et al. Direct and Indirect Induction by 1,25-Dihydroxyvitamin D3 of the NOD2/CARD15-Defensin β2 Innate Immune Pathway Defective in Crohn Disease. J. Biol. Chem. 2010;285:2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bacchetta J., Zaritsky J.J., Sea J.L., Chun R., Lisse T.S., Zavala K., Nayak A., Wesseling-Perry K., Westerman M., Hollis B.W., et al. Suppression of Iron-Regulatory Hepcidin by Vitamin D. J. Am. Soc. Nephrol. 2014;25:564–572. doi: 10.1681/ASN.2013040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong J., Zhang Z., Musch M.W., Ning G., Sun J., Hart J., Bissonnette M., Li Y.C. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G208–G216. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y.-Y., Liu T.-J., Fu J.-H., Xu W., Wu L.-L., Hou A.-N., Xue X.-D. Vitamin D/VDR signaling attenuates lipopolysaccharide-induced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol. Med. Rep. 2016;13:1186–1194. doi: 10.3892/mmr.2015.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gough M.E., Graviss E.A., May E.E. The dynamic immunomodulatory effects of vitamin D3 during Mycobacterium infection. Innate Immun. 2017;23:506–523. doi: 10.1177/1753425917719143. [DOI] [PubMed] [Google Scholar]
- 44.Subramanian K., Bergman P., Henriques-Normark B. Vitamin D Promotes Pneumococcal Killing and Modulates Inflammatory Responses in Primary Human Neutrophils. J. Innate Immun. 2017;9:375–386. doi: 10.1159/000455969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mushegian A.A. Autophagy and vitamin D. Sci. Signal. 2017;10:eaan2526. doi: 10.1126/scisignal.aan2526. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed A., Siman-Tov G., Hall G., Bhalla N., Narayanan A. Human Antimicrobial Peptides as Therapeutics for Viral Infections. Viruses. 2019;11:704. doi: 10.3390/v11080704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mao J., Lin E., He L., Yu J., Tan P., Zhou Y. Autophagy and Viral Infection. Adv. Exp. Med. Biol. 2019;1209:55–78. doi: 10.1007/978-981-15-0606-2_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Etten E., Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J. Steroid Biochem. Mol. Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Daniel C., Sartory N.A., Zahn N., Radeke H.H., Stein J.M. Immune Modulatory Treatment of Trinitrobenzene Sulfonic Acid Colitis with Calcitriol Is Associated with a Change of a T Helper (Th) 1/Th17 to a Th2 and Regulatory T Cell Profile. J. Pharmacol. Exp. Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 50.Laplana M., Royo J.L., Fibla J. Vitamin D Receptor polymorphisms and risk of enveloped virus infection: A meta-analysis. Gene. 2018;678:384–394. doi: 10.1016/j.gene.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 51.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asperen R.M.W.-V., Lutter R., Specht P.A., Moll G.N., Van Woensel J.B., Van Der Loos C.M., Van Goor H., Kamilic J., Florquin S., Bos A.P. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J. Pathol. 2011;225:618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 55.Grace J.A., Klein S., Herath C.B., Granzow M., Schierwagen R., Masing N., Walther T., Sauerbruch T., Burrell L.M., Angus P.W., et al. Activation of the Mas Receptor by Angiotensin-(1–7) in the Renin–Angiotensin System Mediates Mesenteric Vasodilatation in Cirrhosis. Gastroenterology. 2013;145:874–884.e5. doi: 10.1053/j.gastro.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 56.Xu J., Yang J., Chen J., Luo Q., Zhang Q., Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017;16:7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bilezikian J.P., Bikle D., Hewison M., Lazaretti-Castro M., Formenti A.M., Gupta A., Madhavan M.V., Nair N., Babalyan V., Hutchings N., et al. Mechanisms in Endocrinology: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020;183:R133–R147. doi: 10.1530/eje-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song Y., Qayyum S., Greer R.A., Slominski R.M., Raman C., Slominski A.T., Song Y. Vitamin D3 and its hydroxyderivatives as promising drugs against COVID-19: A computational study. J. Biomol. Struct. Dyn. 2022;40:11594–11610. doi: 10.1080/07391102.2021.1964601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulivieri F.M., Banfi G., Camozzi V., Colao A., Formenti A.M., Frara S., Lombardi G., Napoli N., Giustina A. Vitamin D in the Covid-19 era: A review with recommendations from a G.I.O.S.E.G. expert panel. Endocrine. 2021;72:597–603. doi: 10.1007/s12020-021-02749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Albergamo A., Apprato G., Silvagno F. The Role of Vitamin D in Supporting Health in the COVID-19 Era. Int. J. Mol. Sci. 2022;23:3621. doi: 10.3390/ijms23073621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murai I.H., Fernandes A.L., Sales L.P., Pinto A.J., Goessler K.F., Duran C.S.C., Silva C.B.R., Franco A.S., Macedo M.B., Dalmolin H.H.H., et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. Med. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 63.Ginde A.A., Blatchford P., Breese K., Zarrabi L., Linnebur S.A., Wallace J.I., Schwartz R.S. High-Dose Monthly Vitamin D for Prevention of Acute Respiratory Infection in Older Long-Term Care Residents: A Randomized Clinical Trial. J. Am. Geriatr. Soc. 2017;65:496–503. doi: 10.1111/jgs.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guidelines. [(accessed on 15 June 2022)]. Available online: https://www.nice.org.uk/guidance/ng187.
- 65.Balla M., Merugu G.P., Konala V.M., Sangani V., Kondakindi H., Pokal M., Gayam V., Adapa S., Naramala S., Malayala S.V. Back to basics: Review on vitamin D and respiratory viral infections including COVID-19. J. Community Hosp. Intern. Med. Perspect. 2020;10:529–536. doi: 10.1080/20009666.2020.1811074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maghbooli Z., Sahraian M.A., Ebrahimi M., Pazoki M., Kafan S., Tabriz H.M., Hadadi A., Montazeri M., Nasiri M., Shirvani A., et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE. 2020;15:e0239799. doi: 10.1371/journal.pone.0239799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Murai I.H., Fernandes A.L., Antonangelo L., Gualano B., Pereira R.M.R. Effect of a Single High-Dose Vitamin D3 on the Length of Hospital Stay of Severely 25-Hydroxyvitamin D-Deficient Patients with COVID-19. Clinics. 2021;76:e3549. doi: 10.6061/clinics/2021/e3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slominski R.M., Stefan J., Athar M., Holick M.F., Jetten A.M., Raman C., Slominski A.T. COVID-19 and Vitamin D: A lesson from the skin. Exp. Dermatol. 2020;29:885–890. doi: 10.1111/exd.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qayyum S., Slominski R.M., Raman C., Slominski A.T. Novel CYP11A1-Derived Vitamin D and Lumisterol Biometabolites for the Management of COVID-19. Nutrients. 2022;14:4779. doi: 10.3390/nu14224779. [DOI] [PMC free article] [PubMed] [Google Scholar]