Abstract
Ventilator-associated pneumonia (VAP) remains a common risk in mechanically ventilated patients. Different care bundles have been proposed to succeed VAP reduction. We aimed to identify the combined interventions that have been used to by ICUs worldwide from the implementation of “Institute for Healthcare Improvement Ventilator Bundle”, i.e., from December 2004. A search was performed on the PubMed, Scopus and Science Direct databases. Finally, 38 studies met our inclusion criteria. The most common interventions monitored in the care bundles were sedation and weaning protocols, semi-recumbent positioning, oral and hand hygiene, peptic ulcer disease and deep venus thrombosis prophylaxis, subglottic suctioning, and cuff pressure control. Head-of-bed elevation was implemented by almost all studies, followed by oral hygiene, which was the second extensively used intervention. Four studies indicated a low VAP reduction, while 22 studies found an over 36% VAP decline, and in ten of them, the decrease was over 65%. Four of these studies indicated zero or nearly zero after intervention VAP rates. The studies with the highest VAP reduction adopted the “IHI Ventilator Bundle” combined with adequate endotracheal tube cuff pressure and subglottic suctioning. Multifaced techniques can lead to VAP reduction at a great extent. Multidisciplinary measures combined with long-lasting education programs and measurement of bundle’s compliance should be the gold standard combination.
Keywords: ventilator-associated pneumonia, care bundles, intensive care units, prevention
1. Introduction
Ventilator-associated pneumonia (VAP) is one of the main types of infection in critically ill mechanically ventilated patients, leading to increased mortality, morbidity, hospital stay, economic and psychological costs for patients and their families [1,2,3,4]. During the last two decades, many guidelines have been proposed to reduce the incidence of VAP. It has been scientifically proven that interventions must be combined in order to be useful [5,6,7]. Bundle is a set of individual components, combined to make a set of quality indicators for a specific system, procedure, or treatment [8]. These interventions must be all implemented together to achieve significantly better results [9].
In 1983, the CDC published the guidelines for the prevention of nosocomial pneumonia, which were specialized for VAP in 2003 [9]. In December 2004, the Institute for Healthcare Improvement (IHI), during the promotion of the “100,000 Lives Campaign”, inserted the “IHI Ventilator Bundle”, consisting of four elements: (1) elevation of the head of bed (HOB) to 30°–45°; (2) daily “sedation vacation” and assessment of readiness to extubate; (3) peptic ulcer disease (PUD) prophylaxis and (4) deep venus thrombosis (DVT) prophylaxis [9]. In 2010, IHI added a fifth intervention: (5) daily oral care with chlorhexidine. In 2016, the Intensive Care Society proposed a bundle called “Recommended bundle of Interventions for the prevention of VAP”, including elevation of head of bed, daily sedation vacation and assessment of readiness to extubate, use of subglottic secretion drainage, avoidance of scheduled ventilator circuit changes, oral hygiene without chlorhexidine and PUD prophylaxis (only for high-risk patients), without mentioning DVT prophylaxis. Next year, the European Respiratory Society, in collaboration with the European Society of Intensive Care Medicine, the European Society of Clinical Microbiology and Infectious Diseases and the American Latin Thoracic Association published the “International guidelines for the management of VAP”, introducing the use of Selective Digestive and Oropharyngeal Decontamination and proposing Oral Decontamination without chlorhexidine. Most of the ICUs worldwide adopted the “IHI Ventilator Bundle”, adapting it to their own needs. As a result, there has been a variation in the included interventions of VAP bundles among ICUs and until now there is no common bundle which can be agreed to be implemented by the communities worldwide [10].
In the last two decades, a major problem has been the increasing rates of occurrence of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) and hospital-associated MRSA (HA-MRSA). Apart from that, the communities have faced several viral outbreaks, such as SARS-1, SARS-2, and MERS. All the above severe respiratory syndromes and population aging have led to increased rates of ventilated patients [11]. As VAP is one of the most common preventable lung infections in critically ill intubated patients, it is imperative to determine the most efficient preventive measures for VAP reduction. When there is such heterogeneity in ventilator bundles, the question is which ventilator bundle may be more effective?
A great number of studies examined the effectiveness of the different combinations of interventions for VAP prevention. Several reviews have summarized the findings of those studies [11,12,13,14]. A previous systematic review [11] synthesized all the VAP bundles; however, it was published seven years ago, and several studies have been published since then. Moreover, the most recent review was published in 2022, but its main purpose was to summarize the strategies for improvement of care bundle compliance [14]. Therefore, the aim of our systematic review was to present all the multidimensional interventions used in ICUs, from the introduction of the “IHI Ventilator Bundle”, i.e., from December 2004, which is chronologically a point of intersection, as most of the ICUs started to adopt the “IHI Ventilator Bundle”, either alone or combined with other interventions in order to prevent VAP.
2. Materials and Methods
2.1. Data Sources and Strategies
A systematic literature review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [15]. A comprehensive search was performed on the PubMed, Scopus, and Science Direct databases. We used the following strategy: “ventilator-associated pneumonia” AND “care bundles” AND ”intensive care units” AND ”prevention”. Databases were searched from January 2005 to 23 October 2022. The selected period was intended because in December 2004, the IHI introduced the “IHI Ventilator Bundle”. This systematic review has been registered on PROSPERO registry (ID: CRD42022384828).
2.2. Selection and Eligibility Criteria
Three independent authors were responsible for removing the duplicates, screening the title and abstract, and analyzing the full content of the studies in accordance with the inclusion criteria. Two independent authors selected the included studies and another independent author resolved possible disagreements. To be included in our systematic review, studies had to (a) be published in the English language; (b) be pre–post observational studies; (c) include adult critically ill patients, intubated at least 48 h, admitted to all kinds of adult ICUs; (d) evaluate the implementation of care bundles in VAP prevention by thoroughly presenting all the combined interventions and calculating the pre and post intervention VAP rate; (e) compare with the individual intervention’s implementation for VAP prevention; and (f) be published after the implementation of “IHI Ventilator Bundle”. Additionally, we excluded protocols, conference papers, abstracts, posters, and letters to editors and editorials.
2.3. Data Extraction and Risk of Bias Assessment
For each study, the main characteristics were extracted: authors, country, data collection period, study setting, sample size, age of patients, measures, implementation of an educational program, and main findings. The appraisal of quality of the included studies was performed by using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) [16]. The checklist consisted of seven domains of bias: confounding, participants’ selection, interventions’ classification, deviation from intended interventions, missing data, outcomes’ measurement, and reporting biases. After completing the checklist, all studies were classified as low risk, moderate risk, serious risk, critical risk, or “no information”.
3. Results
3.1. Identification and Selection of Studies
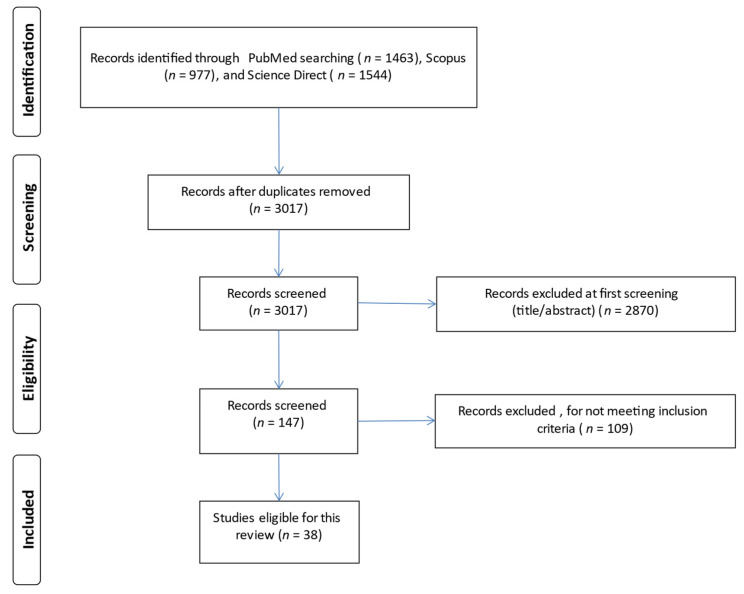
The study selection process is demonstrated in Figure 1. After an initial database search, a total of 3984 studies were identified. After title, abstract, and full content screening, 38 studies were finally included in the review.
Figure 1.
Flow diagram according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
3.2. Characteristics of the Studies Included in This Review
Finally, 38 studies were included in our review. The main characteristics of the studies are presented in Table 1. Fifteen studies took place in Asia, ten in Europe, six in South America, five in North America, one in Australia, and one in Africa. The data collection period ranged from October 2003 [17] to February 2021 [18]. Two studies [5,19] were conducted in Saudi Arabia, at the period of MERS outburst and one study [18] was carried out in Egypt during the coronavirus pandemic. The total sample size ranged from 43 intubated patients [20] to 171,237 intubated patients [21]. All the studies, due to the inclusion criteria, had a pre- and post-intervention observational study design. The studied populations were critically ill ventilated patients admitted to general, medical, surgical, neurosurgical, trauma, and cardiovascular ICUs. Most of the included studies were performed in general ICUs and fifteen of them were multicenter.
Table 1.
Main characteristics of the reviewed studies (n = 38).
| ID | Reference | Country | Data Collection Period | Study Setting |
Sample Size | Mean Age of Patients (Standard Deviation) | Educational Program (Yes/No/NA) |
|---|---|---|---|---|---|---|---|
| 1. | Al-Tawfiq et al. (2010) | Saudi Arabia | 1 January 2006–31 December 2008 | One 18-bed ICU | NA | NA | yes |
| 2. | Bouadma et al. (2010) | France | 2-year period (Months and year NA) |
One 20-bed MICU | 1649 ventilator days | NA | yes |
| 3. | Bird et al. (2010) | USA | 1 March 2006–31 May 2009 | Two SICUs | NA | NA | NA |
| 4. | Ban et al. (2011) | Korea | 31 October 2005–28 February 2006 | One ICU | 155 patients | NA | yes |
| 5. | Berenholtz et al. (2011) | USA | 1 October 2003–30 September 2005 | 81 ICUs | 550,800 ventilator days | NA | yes |
| 6. | Morris et al. (2011) | Scotland | NA | One 18-bed ICU | pre: 1460 patients post: 501 patients |
pre: 60 (47–72) * post: 59 (48–70) * |
yes |
| 7. | Gallagher et al. (2012) | USA | 31 August 2010–30 September 2011 | One ICU | 83 patients | 63 (NA) | yes |
| 8. | Moore et al. (2012) | USA | 1 January 2011–30 June 2012 | One 16-bed combined Neurosurgical & Trauma ICU | 1987 patients | NA | NA |
| 9. | Gatell et al. (2012) | Spain | 1 January 2008–31 May 2009 | One 16-bed GICU | NA | NA | yes |
| 10. | Guanche-Garcell et al. (2013) | Cuba | 31 January 2007–30 November 2010 | One ICU | pre: 67 patients post: 1008 patients |
pre: 60 (17.6) post: 61.4 (17.6) |
yes |
| 11. | Leblebicioglu et al. (2013) | Turkey | 31 August 2003–31 January 2009 | 11 ICUs | pre: 448 patients post: 3864 patients |
pre: 52.4 (22.5) post: 49 (21.6) |
yes |
| 12. | Mehta et al. (2013) | India | 31 July 2004–31 October 2011 | 21 ICUs | pre: 3979 patients post: 42,966 patients |
pre: 54.8 (17.8) post: 54.5 (18.3) |
yes |
| 13. | Micik et al. (2013) | Australia | 1 April 2011–31 August 2012 | One 8-bed cardiothoracic ICU | NA | NA | yes |
| 14. | Viana et al. (2013) | Brazil | 1 January 2014–30 June 2008 | One 14-bed ICU | pre: 294 patients post: 224 patients |
pre: 77 (65–85) * post: 76 (61–83) * |
NA |
| 15. | Chen et al. (2014) | Taiwan | 1 January 2010–31 December 2012 | One MICU & one SICU |
NA | NA | yes |
| 16. | Docher et al. (2014) | USA | 1 April 2009–30 September 2012 | One 18-bed MICU | 713 patients | 58.8 (17.5) | yes |
| 17. | Eom et al. (2014) | Korea | 31 July 2010–30 June 2011 | 6 ICUs | NA | NA | yes |
| 18. | Righi et al. (2014) | Italy | 31 January 2004–31 December 2010 | One 10-bed ICU | 1372 patients | 61.1 (17.1) | NA |
| 19. | Ismail et al. (2015) | Lebanon | NA | One CCU | pre: 15 patients post: 28 patients |
pre: 67.1 (16.5) post: 56.2 (25.7) |
yes |
| 20. | Lim et al. (2015) | Taiwan | 1 January 2006–31 March 2013 | 5 SICUs | 27,125 patients pre: 12,913 patients post: 14,212 patients |
pre: 63.2 (NA) post: 62.8 (NA) |
yes |
| 21. | Zeng et al. (2015) | China | 1 December 2011–31 May 2014 | One MICU | 375 patients | NA | NA |
| 22. | Alcan et al. (2016) | Turkey | 7 April 2014–31 October 2014 | One GICU | 128 patients | NA | yes |
| 23. | Khan et al. (2016) | Saudi Arabia | 2008–2013 | One GICU | 3665 patients | 53.2 (21) | yes |
| 24. | Mogyorodi et al. (2016) | Hungary | 1 January 2015–31 December 2015 | One 12-bed ICU | 535 patients pre: 275 patients post: 260 patients |
pre: 69.8 (14.3) post: 68.7 (14.0) |
yes |
| 25. | Marini et al. (2016) | Saudi Arabia | 31 October 2012–30 June 2014 | One GICU | NA | NA | yes |
| 26. | Parisi et al. (2016) | Greece | 2–year study period | One 30-bed ICU | pre: 226 patients post: 136 patients |
pre: 59 (41–73) * post: 58 (42–72) * |
yes |
| 27. | Alvarez–Lerma et al. (2018) | Spain | 1 April 2011–31 December 2012 | One hundred eighty-one ICUs | 171,237 patients | NA | yes |
| 28. | Burja et al. (2018) | Slovenia | 1 September 2014–30 April 2015 | One 12-bed MICU | pre: 55 patients post: 74 patients |
pre: 67.8 (14.5) post: 64.8 (13.7) |
yes |
| 29. | Landelle et al. (2018) | Switzerland | 31 August 2014–31 July 2016 | One 34-bed ICU | pre: 291 patients post: 356 patients |
pre: 61.9 (48.6–73.4) * post: 60.5 (49.4–71.2) * |
yes |
| 30. | Cengiz et al. (2019) | Turkey | 1 January 2015–30 January 2016 | 9 ICUs | NA | NA | yes |
| 31. | Kao et al. (2019) | Taiwan | 1 January 2012–31 October 2014 | 7 SICUs, one CV/SICU, two MICUs | NA | NA | yes |
| 32. | Sousa et al. (2019) | Portugal | 31 October 2015–31 March 2017 | 3 ICUs | 828 patients | NA | yes |
| 33. | Branco et al. (2020) | Brazil | 30 June 2017–30 June 2018 | One GICU | 302 patients | 62.4 (17.1) | yes |
| 34. | Fortaleza et al. (2020) | Brazil | 1 January 2007–30 June 2019 | Two ICUs | NA | NA | yes |
| 35. | Liu et al. (2020) | China | 1 June 2017–31 May 2019 | 6 ICUs | 4716 patients | NA | NA |
| 36. | Michelangelo et al. (2020) | Argentina | 31 January 2016–31 December 2018 | 3 ICUs | NA | NA | yes |
| 37. | Ochoa-Hein et al. (2020) | Mexico | 2015–2018 | One 14-bed ICU | NA | NA | yes |
| 38. | Shaban et al. (2021) | Egypt | 31 March 2020–28 February 2021 | Two ICUs | pre: 52 patients post: 52 patients |
pre: 58.4 (4.4) post: 57.8 (2.9) |
NA |
Abbreviations: CCU: Critical Care Unit; CV: Cardiovascular; GICU: General Intensive Care Unit; ICU: Intensive Care Unit; IP: Intervention Phase; MICU: Medical Intensive Care Unit; NA: Not Applicable; SICU: Surgical Intensive Care Unit. * median (interquartile range).
3.3. Risk of Bias Assessment
The risk of bias assessment of the reviewed studies is demonstrated in Supplementary Table S1. The risk of bias was low in seven studies [2,5,22,23,24,25,26], serious and critical in 11 studies [3,6,7,17,19,27,28,29,30,31] and moderate in the remaining studies. The most common bias in the pre-post observational studies was the bias due to deviations from intended interventions.
3.4. Ventilator-Associated Care Bundles—Patients’ Outcomes
All of the studies used multifaceted strategies to prevent VAP, as it was one of the inclusion criteria. The number of the included ventilator bundle elements varied from four to thirteen and is shown in Table 2. Head-of-Bed elevation, with a range of 30° to 45°, was implemented by all the reviewed studies, except one [32]. The second most widely used intervention was oral hygiene using chlorhexidine 0.12%. Only one study [3] used sodium bicarbonate and another one [33] sponges and mouthwashes, albeit without a particular change in VAP reduction. Six studies did not adopt the measure of oral care [3,17,18,29,30,32]. Nine studies adopted the IHI Ventilator Bundle [5,6,19,20,23,33,34,35,36]. In two studies, one additional preventive measure was selective oropharyngeal decontamination by using colistin, tobramycin, and nystatin, three times daily, with remarkable VAP reduction rates [25,37].
Table 2.
Preventive VAP measures monitored in revised studies.
| ID | Reference | Physicians’ Interventions | Nurses’ Interventions | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IHI Ventilator | Bundle | |||||||||||||||||||||
| Avoid Nasogastric Tube | Avoid Nasotracheal Intubation | PUD Prophylaxis | DVT Prophylaxis | Daily Sedation Vacation | Daily Assessment of Readiness for Extubating | HOB Elevation 30°–45° | Oral Care/Chlorhexidine 0.12% | Adequate ETT Cuff Pressure (20–30 cm H2O) | Subglottic Suctioning | Hand Hygiene | Aseptic Suctioning Technique | Avoid Accidental Extubation | Avoid Gastric Overdistension | Adherence to Recommended Frequency of Equipment Change ** | Suction When Necessary | Suitable Use & Replacement of HME Filters | Closed Suction System | Change Soiled/Damaged VC | Condensate Removal | Other | ||
| 1. | Al-Tawfiq et al. (2010) | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||
| 2. | Bird et al. (2010) | √ | √ | √ | √ | √ | ||||||||||||||||
| 3. | Bouadma et al. (2010) | √ | √ | √ | √ | √ | √ | √ | gloves gowns |
|||||||||||||
| 4. | Ban et al. (2011) | √ | √ | √ | √ | √ | √ | gloves | ||||||||||||||
| 5. | Berenholtz et al. (2011) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||
| 6. | Gallagher et al. (2012) | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||
| 7. | Morris et al. (2011) | √ | √ | √ | √ | |||||||||||||||||
| 8. | Moore et al. (2012) | √ | √ | √ | √ | √ | ||||||||||||||||
| 9. | Gatell et al. (2012) | √ | √ | √ | √ | √ | √ | √ | √ | (b) | ||||||||||||
| 10. | Guanche-Garcell et al. (2013) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | (c) | ||||||||
| 11. | Leblebicioglu et al. (2013) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | (c) (j) |
||||||||||
| 12. | Mehta et al. (2013) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | (c) (j) |
||||||||||
| 13. | Micik et al. (2013) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||
| 14. | Viana et al. (2013) | √ | √ | √ | √ | √ | √ **** | |||||||||||||||
| 15. | Chen et al. (2014) | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||
| 16. | Docher et al. (2014) | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||
| 17. | Eom et al. (2014) | √ | √ | √ | √ | |||||||||||||||||
| 18. | Righi et al. (2014) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | SDD | ||||||||||
| 19. | Ismail et al. (2015) | √ | √ | √ | √ | √ | √ | √ | √ | gloves | ||||||||||||
| 20. | Lim et al. (2015) | √ | √ | √ | √ | √ | √ | √ | √ | (a) | ||||||||||||
| 21. | Zeng et al. (2015) | √ | √ | √ | √ | √ | √ | √ | √ | (l) | ||||||||||||
| 22. | Alcan et al. (2016) | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||
| 23. | Khan et al. (2016) | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||
| 24. | Mogyorodi et al. (2016) | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||||||
| 25. | Marini et al. (2016) | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||
| 26. | Parisi et al. (2016) | √ | √ | √ | √ | √ | √ *** | |||||||||||||||
| 27. | Alvarez-Lerma et al. (2018) | √ | √ | √ | √ | √ | √ | √ | (e) | |||||||||||||
| 28. | Burja et al. (2018) | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||
| 29. | Landelle et al. (2018) | √ | √ | √ | √ | √ | √ | √ | (i) SOD |
|||||||||||||
| 30. | Cengiz et al. (2019) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | (f) | ||||||||||
| 31. | Kao et al. (2019) | √ | √ | √ | √ | √ | √ | (h) | ||||||||||||||
| 32. | Sousa et al. (2019) | √ | √ | √ | √ | √ | √ | √ | (c) (i) |
|||||||||||||
| 33. | Branco et al. (2020) | √ | √ | √ | √ | √ | √ | |||||||||||||||
| 34. | Fortaleza et al. (2020) | √ | √ | √ | √ | (h) | ||||||||||||||||
| 35. | Liu et al. (2020) | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||
| 36. | Michelangelo et al. (2020) | √ | √ | √ | √ | |||||||||||||||||
| 37. | Ochoa-Hein et al. (2020) | √ * | √ * | √ | √ | √ | √ | √ | √ | (k) | ||||||||||||
| 38. | Shaban et al. (2021) | √ | √ | √ | √ | √ | ||||||||||||||||
Abbreviations: HOB: Head-of-bed; PUD: Peptic Ulcer Disease; DVT: Deep Vein Thrombosis; ETT: Endotracheal Tube; VC: Ventilator Circuit; SOD: Selective Oropharyngeal Decontamination (with colistin, tobramycin, nystatin, 3 times/day); IHI: Institute of Healthcare Improvement; SDD: Selective Digestive Track Decontamination; ** Adherence to recommended frequency of equipment change: HME filter 48 h, breathing circuit only solid, closed suction system 72 h; *** With sodium bicarbonate;**** With sponges & mouthwashes/no chlorhexidine; (a): high-level sterilization andstorage of the ventilator tubing; moisten the devices with sterile water; (b): smallest possible calibre nasogastric tube; (c): non-invasive positive pressure ventilator; (e): procedures andprotocols to reduce duration of MV; selective decontamination of the oropharyngeal and the digestive tract; (f): not using routine saline solution in aspiration; confirm feeding tube placement; (h): emptying water from the respirator tube; (i): active mobilization; (j): avoidance of histamine receptor 2 (H2)-blocking agents & proton pump inhibitors; use of sterile water to rinse reusable respiratory equipment; (k): tooth brushing; patient position changes; non-invasive ventilation in selected patients (acute cardiogenic pulmonary edema andtype 2 respiratory insufficiency in patients with chronic obstructive pulmonary disease (introduced in January 2016); * and use of non-invasive ventilatory assistance or high—flow nasal cannula in extubated patients (introduced in January 2018); (l): personal protective equipment for suctioning, daily cleaning of the ventilator andsuction bottle with sterile distilled water; sterilization of the circuit by pasteurization; use of an independent care room.
Patients’ outcomes are shown in Table 3. Thirty-six of the included studies indicated a reduction in VAP incidence. VAP reduction rates ranged from 13% [2] to 100% [23,36]. Only four studies showed low VAP reduction [2,38,39,40]. The majority of the studies showed a reduction of 36–64% and twelve of them a reduction over 65% [5,6,19,20,23,24,25,27,29,30,36,41]. None of the studies that found low rates of VAP reduction, used the “IHI Ventilator Bundle” and more specifically, all of them did not adopt the PUD and DVT prophylaxis, with three of them adopting the rest measures of the “IHI Ventilator Bundle” (daily sedation vacation, daily assessment of readiness for extubation, head-of-bed elevation and oral care with chlorhexidine). Fourteen studies [2,5,17,18,19,20,21,22,25,35,38,40,42,43] included subglottic suctioning in their bundles, with four of them [5,19,20,25] to achieve more than 65% VAP reduction.
Table 3.
Patient outcomes in each study.
| ID | Reference | Pre-Intervention VAP | Post-Intervention VAP | p-Value | Comments |
|---|---|---|---|---|---|
| 1. | Al-Tawfiq et al. (2010) | 9.3 | 1-year after: 2.3 2-years after: 2.2 |
p < 0.001 | |
| 2. | Bouadma et al. (2010) | 23.5 (26.7%) | 1-year after: 14.9 (15.3%) 2-years after: 11.5(11.1%) |
p < 0.0001 | |
| 3. | Bird et al. (2010) | 10.2 | 3.4 | NA | |
| 4. | Ban et al. (2011) | 17.4 | 11.04 | p = 0.074 | |
| 5. | Berenholz et al. (2011) | 6.9 | 16-months after: 3.4 28–30 months after: 2.4 |
NA | |
| 6. | Morris et al. (2011) | 32.0 | 12.0 | p < 0.001 | |
| 7. | Gallagher et al. (2012) | 25.5 | 0.0 | p = 0.003 | |
| 8. | Moore et al. (2012) | 4.5 | Ranged per quarter | NA | The VAP rate per quarter (total 6 quarters) ranged from 1.94 to 6.55 (M = 4.33, SD: 1.65) |
| 9. | Gatell et al. (2012) | 9.9 | 9.3 | p = 0.36 | VAP incidence (>4 days after intubation): 4.6 vs. 3.1 |
| 10. | Guanche-Garcell et al. (2013) | 52.6 | 15.3 | p = 0.003 | 70% reduction |
| 11. | Leblebicioglu et al. (2013) | 31.1 | 16.8 | p = 0.0001 | |
| 12. | Mehta et al. (2013) | 17.4 | 10.8 | p = 0.0001 | 38% reduction |
| 13. | Micik et al. (2013) | 13.4 | 7.7 | NA | |
| 14. | Viana et al. (2013) | 18.6 | 11.8 | p = 0.002 | |
| 15. | Chen et al. (2014) | 1.5 | 0.0 | NA | |
| 16. | Docher et al. (2014) | 9.3 | Ranged per month | p < 0.001 | ●Mean after IVR: 3.2 (SD: 5.71). ●Average VAP reduction/month: 0.27 |
| 17. | Eom et al. (2014) | 4.08 | 1.16 | NA | |
| 18. | Righi et al. (2014) | 15.9% | 6.7% | p < 0.001 | * VAP bundle period: 2004–2007 * VAP bundle & SOD period: 2008–2010 ●EVAP (6.6% to 1.9%) ●LVAP (9.3% to 4.7%) |
| 19. | Ismail et al. (2015) | 66.7% | 21.4% | p = 0.003 | |
| 20. | Lim et al. (2015) | 13.63 | 3.9 | p < 0.001 | ●Ventilator utilization ratio decreased by 9.9% & VAP density reduced by 1.9 cases/1000 ventilator days (up to a 57.6% reduction) |
| 21. | Zeng et al. (2015) | 0.495 | 0.281 | p = 0.001 | |
| 22. | Alcan et al. (2016) | 15.91 | 8.50 | p = 0.0001 | |
| 23. | Khan et al. (2016) | 8.6 | 2.0 | p < 0.001 | |
| 24. | Mogyorodi et al. (2016) | 21.5 (95% CI: 14.17–31.10) | 12.0 (95% CI: 7.2–19.49) | NA | Relative risk reduction: 44% (95% CI: −0.5 to 0.98) |
| 25. | Marini et al. (2016) | 4.0 | 0.8 | NA | |
| 26. | Parisi et al. (2016) | 21.6 | 11.6 | p = 0.01 | |
| 27. | Alvarez-Lerma et al. (2018) | 9.83 (95% CI: 8.42–11.48) | 4.34 (95% CI: 3.22–5.84) | NA | |
| 28. | Burja et al. (2018) | Total: 41.8% EVAP: 10.9% LVAP: 30.9% |
Total: 25.7% EVAP: 12.2% LVAP: 13.5% |
Total: p = 0.061 EVAP: p > 0.99 LVAP: 0.027 |
|
| 29. | Landelle et al. (2018) | 24.0 | 3.9 | p < 0.001 | ●IVR without SOD: reduction 42% ●IVR with SOD: reduction 70% |
| 30. | Cengiz et al. (2019) | 12.856 | 6.866 | p = 0.036 | |
| 31. | Kao et al. (2019) | Total: 1.9 CV/SICU: 4.5 SICUs: 2.1 MICUs: 0.5 |
Total: 1.5 CV/SICU: 4.5 SICUs: 1.4 MICUs: 1.0 |
Total: p = 0.005 CV/SICU: p = 0.5391 SICUs: p < 0.001 MICUs: p = 0.0489 |
|
| 32. | Sousa et al. (2019) | Total: 7.89 ICU A: 4.0% ICU B: 2.4% ICU C: 7.1% |
Total: 6.81 ICU A: 4.7% ICU B: 2.1% ICU C: 3.5% |
Total: p = 0.552 ICU A: p = 0.539 ICU B: p = 0.001 ICU C: p = 0.02 |
|
| 33. | Branco et al. (2020) | 7.99 | 4.28 | p < 0.001 | |
| 34. | Fortaleza et al. (2020) | 36.58 | 12.04 | p < 0.001 | |
| 35. | Liu et al. (2020) | 18.85 | 13.70 | p = 0.019 | |
| 36. | Michelangelo et al. (2020) | 6.11 | 3.55 | p < 0.01 | |
| 37. | Ochoa et al. (2020) | 8.2 | 3.1 | p = 0.019 | |
| 38. | Shaban et al. (2021) | 62.20% | 26.90% | p < 0.001 |
Abbreviations: CV: Cardiovascular; EVAP (onset ≤ 7 days after intubation): early-onset ventilator associated pneumonia; ICU: Intensive Care Unit; IVR: intervention VAP rate; LVAP (onset > 8 days after intubation): late-onset ventilator associated pneumonia; SICU: surgical intensive care unit; VAP: ventilator-associated pneumonia; MICU: Medical Intensive Care Unit; SOD: Selective Oropharyngeal Decontamination. All VAP rates are expressed per 1000 ventilator-days.
In nine studies [7,17,19,21,23,24,26,30,42], statistical significance was not mentioned, and in four studies [2,32,38,43], the results were not statistically significant, while 24 studies reported a statistically significant reduction. One single center study [43] showed that there were no significant differences in after-intervention VAP and early onset VAP rates but only in late onset VAP rates. In our systematic review, p-values of less than 0.05 were considered significant.
The studies with the highest rate of reduction [5,19,25] implemented the “IHI Ventilator Bundle” combined with adequate ETT cuff pressure at 20–30 cm H2O and subglottic suctioning. Moreover, the study of Gallagher et al. (2012) [36] indicated zero after intervention VAP rate, giving great importance not only to the adoption of “IHI Ventilator Bundle” but also to hand washing and condensate removal. Another study with zero after intervention VAP rate was the study of Chen et al. (2014) [23], where “IHI Ventilator Bundle” with adequate ETT cuff pressure were used as preventive measures.
3.5. Educational Program
We identified thirty-three studies that adopted an education program, as demonstrated in Supplementary Table S2. Education program included self-learning packets, presentations, discussions, knowledge questionnaires, posters, checklists, videos, reminder signs in patients’ rooms, nursing and medical champions, stimulation scenarios and feedback meetings. Thirty-two studies measured the compliance to ventilator bundles and/or education program, and in all of them there was a significant increase in VAP bundle adherence after the education program.
4. Discussion
Our systematic review demonstrated the variety of interventions included in ventilator care bundles and their implementation in VAP decrease in adult ICUs. Thirty-eight studies met our inclusion criteria and we found that the combined measures can prevent VAP toa greater extent. Moreover, our review showed that the number of intervention strategies varied widely in the included studies. Most of the studies examined whether ventilator bundle’s compliance could be increased through health workers’ training.
VAP is one of the main healthcare-associated infections in intubated critically ill patients. VAP is associated with an exceeded duration of mechanical ventilation. The most common microorganisms responsible for VAP are pseudomonas aeruginosa, escherichiacoli, klebsiella pneumoniae and the Acinetobacter species from Gram-negative microorganisms and staphylococcus aureus from Gram-positive microorganisms. VAP diagnostic criteria differ among ICUs but usually require factors such as fever, leukocytosis, progressive infiltrate on chest X-ray, positive cultures from respiratory secretions and reduction in gas exchange [1,9].
During the last decades, there has been a great scientific concern about finding the best strategies to prevent VAP and ventilator-associated events [1]. Worldwide, scientists’ aim to decrease VAP incidence in order: first, to improve ventilated patients’ outcomes, and thereafter to decrease mortality, hospital length of stay and healthcare expenditures. VAP diagnostic criteria, methods of respiratory sampling, interventions in VAP bundles and study designs varies, at a great extent, among the ICUs worldwide [24]. Nevertheless, VAP care bundles combined with a focused education program, which could lead to increased compliance, have proved their efficacy in VAP reduction [6].
A multimodal approach for VAP prevention includes functional, mechanical, and pharmacological measures [21]. The most important intervention for preventing VAP, is avoiding intubation, by using noninvasive positive pressure ventilation whenever possible [1]. By the review of the existing studies, the most frequent proposed interventions, when the patient should be intubated, were sedation and weaning protocols, semi-recumbent positioning, oral and hand hygiene, PUD and DVT prophylaxis, subglottic suctioning, and cuff pressure control. However, interventions as, avoidance of nasogastric tubes, nasotracheal intubation, accidental extubation and gastric overdistension, aseptic suctioning technique and adherence to recommended frequency of equipment change were monitored in the minority of the included studies. Two studies used as an additional preventive measure Selective Oropharyngeal Decontamination (SOD) [25,37]. The care bundle without SOD was associated with a decrease of 42% in VAP rates, while with the implementation of SOD there was a further decrease of 70% in VAP rates [25].
A semi-recumbent position was established with the introduction of “IHI Ventilator Bundle”, though it was proposed much earlier in studies conducted from 1992 to 1999 to test its contribution in aspiration prevention [9]. The implementation of this measure by almost all studies shows the acceptance and the recognition of its importance in VAP treatment. In our systematic review, most of the studies involved oral hygiene in their bundles. Oral care remains an important tool for dental plaque removal and the promotion of a normal microbial community inside the oral cavity, thus preventing the growth of microorganisms in the trachea and the creation of VAP [44,45,46,47]. Fourteen studies aimed to figure out the benefits of subglottic secretion drainage as the secretions concentrated between the vocal cords and the ETT may cause VAP because of the potential growth of pathogens. These studies showed that subglottic suctioning seem to be a useful preventive measure.
A notable result of our study is that ten studies did not control the daily readiness for extubating, when the presence of the endotracheal tube is one of the major predisposing factors for VAP development, as pathogens enter the trachea either by micro aspiration around the ETT cuff or by the biofilm formed in the inner side of the ETT. Moreover, only nine of the included studies followed the “IHI VAP preventive Bundle”. All of them, achieved a VAP reduction at least 36%, five of them showed a reduction of over 65% and two of them managed a zero after intervention VAP rate. These results may indicate the important contribution of the IHI VAP preventive Bundle, alone or in combination with other measures, in VAP reduction. It is noteworthy that the revised studies, carried out the last six years, did not use the “IHI Ventilator Bundle” but adopted some of its interventions.
Another thing that was remarkable was that in many studies, hand hygiene was not mentioned to be monitored as an element in the VAP care bundles. We assume that measures such as hand hygiene and aseptic suctioning technique are of the most basic techniques and that they were taken for granted, along with all the other interventions for VAP prevention in the included studies. Another interesting conclusion in some studies [19,23,28,36] was the after intervention zero-VAP rate, which could be due to many factors, such as country health care policy makers, diversity in VAP diagnostic criteria and methods of BAL sampling [48].
Over the past two decades, humanity has faced several viral outbreaks, causing severe respiratory syndromes, such as SARS-1, SARS-2 and MERS, which may have increased the percentage of intubated patients at a local level. According to the inclusion criteria, all the included studies were conducted after December 2004. Taking into consideration the data collection periods of the revised studies, in two studies [5,19] carried out the period of MERS outbreak in Saudi Arabia, the viral load of the study population and the patient outcomes might have been influenced. Furthermore, only one of the included studies [18] has been performed since the beginning of the coronavirus pandemic. In all three studies, the authors did not mention the possibility of any influence in patients’ outcomes. The data collection periods of the remaining studies did not coincide with the above viral outbreaks’ periods.
The study of Alvarez-Lerma et al. (2018) [21] was the largest study, including 181 ICUs and 171,237 patients, demonstrating the effectiveness of implementing a VAP prevention bundle at a national level. The study of Kao et al. (2019) [39] indicated that compliance rates of VAP bundle care and VAP reduction rates differ between the different types of ICUs (medical, surgical, cardiovascular). Three studies [41,49,50] adopted the INICC multidimensional approach, including the following practices: (1) bundle for VAP prevention (2) education (3) outcome surveillance (4) process surveillance (5) feedback on VAP rates and (6) performance feedback of infection control practices.
Finally, most of the included studies, apart from the implementation of care bundles, adopted at the same time a multifaced educational program. Presentations, posters, videos, discussions, stimulating scenarios and feedback meetings were some of the ways that ICU staff was trained. Our systematic review underlined the wide range in VAP reduction. This wide fluctuation may have been affected, to some extent, by either the country’s healthcare system or the extent variety of educational programs and different degree of personnel’s adherence, but at the same time the studies’ results highlighted that multidisciplinary health professionals’ education and frequent monitoring of adherence can lead to better patients’ outcomes.
5. Limitations
There are several limitations in this systematic review. At first, as we searched for relevant studies only in English language, other potential studies written in different languages were not included in our review. Also, we included three databases in our research, and thus additional studies could be found in other databases. In addition, application of vigorous inclusion and exclusion criteria may preclude some studies to be included in our review. Moreover, among the reviewed studies, there was a heterogeneity, to a greater extent, of the study settings. In particular, differences in study quality are indicative of great differences in studies design, since seven studies had a high level of quality, 20 had a moderate level, and 11 a low level. However, we should notice that heterogeneity among studies was not a limitation of our review but an unavoidable effect of the studies design. Therefore, we should consider our results with caution since it is difficult to establish solid conclusions.
6. Conclusions
From our systematic review, it emerged that there is a considerable variation between the VAP care bundles and education programs in ICUs worldwide. This variation leads to discrepancies and does not allow comparability, to a greater extent, between the studies to find the gold standard VAP care bundle. The IHI VAP preventive Bundle seems to be a very useful tool in VAP reduction, combined with adequate ETT cuff pressure and subglottic suctioning, without forgetting the hand hygiene and aseptic suctioning technique. ICUs should adopt basic practices that prevent or decrease VAP rates, and as a result, mortality, duration of mechanical ventilation, length of stay, and healthcare costs. Moreover, the strategies should be multifaceted and supported by a long-term education program by ensuring compliance in the care bundle. These multidisciplinary strategies and education programs should be common in all ICUs. At least in the same country, national or cross-sectional randomized controlled studies need to be carried out in order to be able to compare the measures’ efficacy and find the best combination of preventive interventions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12020227/s1, Supplementary Table S1: Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I); Supplementary Table S2: Impact of multidisciplinary educational program in the reviewed studies.
Author Contributions
Conceptualization, M.M., T.K., P.G., A.K. and P.M.; methodology, M.M., T.K., P.G. and P.M.; validation, M.M., T.K. and P.G.; investigation, M.M., T.K., P.G., A.K. and P.M.; resources: P.M.; data curation, M.M., T.K. and P.G.; writing-original draft preparation: M.M., T.K., P.G., A.K. and P.M.; writing-review and editing: M.M., T.K., P.G. and P.M.; supervision: P.M.; funding acquisition: P.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding Statement
Special Account for Research Grants of the National and Kapodistrian University of Athens.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Klompas M., Branson R., Eichenwald E.C., Greene L.R., Howell M.D., Lee G., Magill S., Maragakis L.L., Priebe G.P., Speck K., et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 Update. Infect. Control. Hosp. Epidemiol. 2014;35:133–154. doi: 10.1017/S0899823X00193894. [DOI] [PubMed] [Google Scholar]
- 2.Sousa A.S., Ferriso C., Paira J.A. Application of a ventilator associated pneumonia prevention guideline and outcomes: A quasi-experimental study. Intensive Crit. Care Nurs. 2019;51:50–56. doi: 10.1016/j.iccn.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Parisi M., Gerovasili V., Dimopoulos D., Kampisiouli E., Goga C., Perivolioti E., Argyropoulou A., Routsi C., Tsiodras S., Nanas S. Use of Ventilator Bundle and Staff Education to Decrease Ventilator Associated Pneumonia in Intensive Care Patients. Crit. Care Nurse. 2016;36:e1–e7. doi: 10.4037/ccn2016520. [DOI] [PubMed] [Google Scholar]
- 4.Pileggi C., Mascaro V., Bianco A., Nobile C.G., Pavia M. Ventilator Bundle and Its Effects on Mortality Among ICU Patients: A Meta-Analysis. Crit. Care Med. 2018;46:1167–1174. doi: 10.1097/CCM.0000000000003136. [DOI] [PubMed] [Google Scholar]
- 5.Khan R., Al-Dorzi H.M., Al-Attas K., Ahmed F.W., Marini A.M., Mundekkadan S., Balkhy H.H., Tarnous J., Arabi Y.M. The impact of implementing multifaceted interventions on the prevention of ventilator-associated pneumonia. Am. J. Infect. Control. 2016;44:320–326. doi: 10.1016/j.ajic.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Lim K.P., Kuo S.W., Ko W.J., Sheng W.H., Chang Y.Y., Hong M.C., Sun C.C., Chen Y.C., Chang S.C. Efficacy of ventilator-associated pneumonia care bundle for prevention of ventilator-associated pneumonia in the surgical intensive care units of a medical center. J. Microbiol. Immunol. Infect. 2015;48:316–321. doi: 10.1016/j.jmii.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Moore K.R. Critical Care Nursing Interventions and Incidence of Ventilator Associated Pneumonia in the Trauma Population 2012, Nursing Theses and Capstone Projects.135. [(accessed on 1 September 2022)]. Available online: https://digitalcommons.gardner-webb.edu/cgi/viewcontent.cgi?article=1134&context=nursing_etd.
- 8.Resar R., Pronovost P., Haraden C., Simmond T., Rainey T., Nolan T. Using a Bundle Approach to Improve Ventilator Care Processes and Reduce Ventilator Associated Pneumonia. J. Qual. Patient Saf. 2005;31:243–248. doi: 10.1016/S1553-7250(05)31031-2. [DOI] [PubMed] [Google Scholar]
- 9.Munro N., Ruggiero M. Ventilator-Associated Pneumonia Bundle. Reconstruction for Best Care. AACN Adv. Crit. Care. 2014;25:163–175. doi: 10.4037/NCI.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 10.Kandeel N., Tantawy N. Current Nursing Practice for Prevention of Ventilator Associated Pneumonia in ICUs. Life Sci. J. 2012;9:966–975. [Google Scholar]
- 11.Chahoud J., Semaan A., Almoosa K. Ventilator-associated events prevention, learning lessons from the past: A systematic review. Heart Lung. 2015;44:251–259. doi: 10.1016/j.hrtlng.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Alecrim R.X., Taminato M., Belasco A., Longo M.C., Lusahara D.M., Fram D. Strategies for preventing ventilator-associated pneumonia: An integrative review. Rev. Bras. Enferm. 2019;72:521–530. doi: 10.1590/0034-7167-2018-0473. [DOI] [PubMed] [Google Scholar]
- 13.Kallet R.H. Ventilator Bundles in Transition: From Prevention of Ventilator Associated Pneumonia to Prevention of Ventilator-Associated Events. Respir. Care. 2019;8:994–1006. doi: 10.4187/respcare.06966. [DOI] [PubMed] [Google Scholar]
- 14.Thapa D., Liu T., Chair S.Y. Multifaceted interventions are likely to be more effective to increase adherence to the ventilator care bundle: A systematic review of strategies to improve care bundle compliance. Intensive Crit. Care Nurs. 2022;74:103310. doi: 10.1016/j.iccn.2022.103310. [DOI] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Hoboken, NJ, USA: 2019. [Google Scholar]
- 17.Berenholtz S.M., Pham J.C., Thompson D.A., Needham D.M., Lubomski L.H., Hyzy R.C., Welsh R., Cosgrove S.E., Sexton J.B., Colantuoni E., et al. Collaborative Cohort Study of an Intervention to Reduce Ventilator-Associated Pneumonia in the Intensive Care Unit. Infect. Control. Hosp. Epidemiol. 2011;32:305–314. doi: 10.1086/658938. [DOI] [PubMed] [Google Scholar]
- 18.Shaban A.M., El-Mokadem N.M., Abdallah S.E. Effectiveness of Implementing Ventilator Associated Pneumonia Prevention Bundle among Mechanically Ventilated Patients. Int. J. Nov. Res. Healthc. Nurs. 2021;8:329–342. [Google Scholar]
- 19.Marini A.L., Khan R., Mundekkadan S. Multifaceted bundle interventions shown effective in reducing VAP rates in our multidisciplinary ICUs. BMJ Qual. Improv. Rep. 2016;5:u205566-w2278. doi: 10.1136/bmjquality.u205566.w2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ismail R., Zahran E. The effect of nurses training on ventilator-associated pneumonia (VAP) prevention bundle on VAP incidence rate at a critical care unit. J. Nurs. Educ. Pract. 2015;5:42–48. doi: 10.5430/jnep.v5n12p42. [DOI] [Google Scholar]
- 21.AÁlvarez-Lerma F., Palomar-Martínez M., Sánchez-García M., Martínez-Alonso M., Álvarez-Rodríguez J., Lorente L., Arias-Rivera S., García R., Gordo F., Añón J.M., et al. Prevention of Ventilator-Associated Pneumonia: The Multimodal Approach of the Spanish ICU “Pneumonia Zero” Program. Crit. Care Med. 2018;46:181–188. doi: 10.1097/CCM.0000000000002736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cengiz H.O., Kanan N. The effectiveness of training given to nurses for reducing ventilator-associated pneumonia in intensive care patients. Dev. Health Sci. 2019;2:36–45. [Google Scholar]
- 23.Chen J.K., Chen T.H., Liu H.E., Kao C.C., Chen C.F., Ou T.Y., Tseng P.C., Kuo K.N., Lee W.S. Bundle Care for Preventing Ventilator-associated Pneumonia at a Medical Center: A Preliminary Report. J. Exp. Clin. Med. 2014;6:157–160. doi: 10.1016/j.jecm.2014.08.003. [DOI] [Google Scholar]
- 24.Eom J.S., Lee M.S., Chun H.K., Choi H.J., Jung S.Y., Kim Y.S., Yoon S.J., Kwak Y.G., Oh G.B., Jeon M.H., et al. The impact of a ventilator bundle on preventing ventilator-associated pneumonia: A multicenter study. Am. J. Infect. Control. 2014;42:34–37. doi: 10.1016/j.ajic.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Landelle C., NocquetBooyer V., Abbas M., Genevois E., Abidi N., Naimo S., Raulais R., Bouchoud L., Boroli F., Terrisse H., et al. Impact of a multifaceted prevention program on ventilator-associated pneumonia including selective oropharyngeal decontamination. Intensive Care Med. 2018;44:1777–1786. doi: 10.1007/s00134-018-5227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mogyorodi B., Dunai E., Gal J., Ivanyi Z. Ventilator—Associated pneumonia and the importance of education of ICU nurses on prevention—Preliminary results. Interv. Med. Appl. Sci. 2016;8:147–151. doi: 10.1556/1646.8.2016.4.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortaleza C.M.C.B., Filho S.P.F., Silva M.O., Queiroz S.M., Cavalcante R.S. Sustained reduction of healthcare-associated infections after the introduction of a bundle for prevention of ventilator-associated pneumonia in medical-surgical intensive care units. Braz. J. Infect. Dis. 2020;24:373–379. doi: 10.1016/j.bjid.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng W.P., Su H., Chen C.W., Cheng S.M., Chang L.F., Tzeng W.C., Tzeng B.H. Care Bundle for Ventilator-Associated Pneumonia in a Medical Intensive Care Unit in Northern Taiwan. J. Med. Sci. 2015;35:68–73. [Google Scholar]
- 29.Al-Tawfiq J.A., Abed M.S. Decreasing ventilator-associated pneumonia in adult intensive care units using the Institute for Healthcare Improvement bundle. Am. J. Infect. Control. 2010;38:552–556. doi: 10.1016/j.ajic.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Bird D., Zambuto A., O’Donnell C., Silva J., Korn C., Burke R., Burke P., Agarwal S. Adherence to Ventilator-Associated Pneumonia Bundle and Incidence of Ventilator-Associated Pneumonia in the Surgical Intensive Care Unit. Achieves Surg. 2010;145:465–470. doi: 10.1001/archsurg.2010.69. [DOI] [PubMed] [Google Scholar]
- 31.Branco A., Louvencone E.M., Monteiro A.B., Fonseca J.P., Blatt C.R., Caregnato R.C. Education to prevent ventilator-associated pneumonia in intensive care unit. Rev. Bras. De Enferm. 2020;73:e20190477. doi: 10.1590/0034-7167-2019-0477. [DOI] [PubMed] [Google Scholar]
- 32.Ban K.O. The effectiveness of an evidence-based nursing program to reduce ventilator-associated pneumonia in a Korean ICU. Intensive Crit. Care Nurs. 2011;27:226–232. doi: 10.1016/j.iccn.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Viana W.N., Bragazzi C., Couto de Castro J.E., Alves M.B., Rocco J.R. Ventilator-associated pneumonia prevention by education and two combined bedside strategies. Int. J. Qual. Health Care. 2013;25:308–313. doi: 10.1093/intqhc/mzt025. [DOI] [PubMed] [Google Scholar]
- 34.Alcan A.O., Korkmaz F.D., Uyar M. Prevention of ventilator—Associated pneumonia: Use of the care bundle approach. Am. J. Infect. Control. 2016;44:e173–e176. doi: 10.1016/j.ajic.2016.04.237. [DOI] [PubMed] [Google Scholar]
- 35.Dosher W.B., Loomis E.C., Richardson S.L., Crowell J.A., Waltman R.D., Miller L.D., Nazim M., Khasawneh F.A. The effect of a Nurse—Led Multidisciplinary Team on Ventilator—Associated Pneumonia Rates. Crit. Care Res. Pract. 2014;2014:682621. doi: 10.1155/2014/682621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallagher J.A. Implementation of VentilatorAssociated Pneumonia Clinical Guideline (Bundle) J. Nurse Pract. 2012;8:377–382. doi: 10.1016/j.nurpra.2012.02.017. [DOI] [Google Scholar]
- 37.Righi E., Aggazzotti G., Ferrari E., Giovanardi C., Busani S., Rinaldi L., Girardis M. Trends in ventilator-associated pneumonia: Impact of a ventilator care bundle in an Italian tertiary care hospital intensive care unit. Am. J. Infect. Control. 2014;42:1312–1316. doi: 10.1016/j.ajic.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Gatell M.R., Roig M.S., Vian O.H., Santin E.C., Duaso C.T., Moreno I.F., Daunis J.V. Assessment of a training programme for the prevention of ventilator-associated pneumonia. Br. Assoc. Crit. Care Nurses. 2012;17:285–292. doi: 10.1111/j.1478-5153.2012.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kao C.C., Chiang H.T., Chen C.Y., Hung C.T., Chen Y.C., Su L.H., Shi Z.Y., Liu J.W., Liu C.P., Chuang Y.C., et al. National bundle care program implementation to reduce ventilator-associated pneumonia in intensive care units in Taiwan. J. Microbiol. Immunol. Infect. 2019;52:592–597. doi: 10.1016/j.jmii.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Liu W., Yang Y., Zhang K., Hai Y., Li H., Xing H., Xu B., Bai H., Zhao H., Bao H., et al. Evaluation of the effects of applying the ventricular care bundle (VCB) method for reducing ventilator-associated pneumonia (VAP) in the intensive care unit of a general Chinese tertiary hospital. Ann. Palliat. Med. 2020;9:2853–2861. doi: 10.21037/apm-20-289. [DOI] [PubMed] [Google Scholar]
- 41.Guanche-Garcell H., Morales-Perez C., Rosenthal V.D. Effectiveness of a multidimensional approach for the prevention of ventilator-associated pneumonia in an adult intensive care unit in Cuba: Findings of the International Nosocomial Infection Control Consortium (INICC) J. Infect. Public Health. 2013;6:98–107. doi: 10.1016/j.jiph.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Micik S., Besic N., Johnson N., Han M., Harnlyn S., Ball H. Reducing risk for ventilator associated pneumonia through nursing sensitive interventions. Intensive Crit. Care Nurs. 2013;29:261–265. doi: 10.1016/j.iccn.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Burja S., Belec T., Bizjak N., Mori J., Markota A., Sinkovic A. Efficacy of a bundle approach in preventing the incidence of ventilator associated pneumonia (VAP) Bosn. J. Basic Med. Sci. 2018;18:105–109. doi: 10.17305/bjbms.2017.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouadma L., Mourvillier B., Deiler V., Le Corre B., Lolom I., Regnier B., Wolff M., Lucet J.C. A multifaceted program to prevent ventilator—Associated pneumonia: Impact on compliance with preventive measures. Crit. Care Med. 2010;38:789–796. doi: 10.1097/CCM.0b013e3181ce21af. [DOI] [PubMed] [Google Scholar]
- 45.Michelangelo H., Angriman F., Pizarro R., Bauque S., Kecskes C., Staneloni I., Garcia D., Espinola F., Mazer G., Ferrari C. Implementation of an experiential learning strategy to reduce the risk of ventilator-associated pneumonia in critically ill adult patients. J. Intensive Care Soc. 2020;21:320–326. doi: 10.1177/1751143719887285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris A.C., Hay A.W., Swann D.G., Everingham K., McCulloch C., McNulty J., Brooks O., Laurenson I.F., Cook B., Walsh T.S. Reducing ventilator-associated pneumonia in intensive care: Impact of implementing a care bundle. Crit. Care Med. 2011;39:2218–2224. doi: 10.1097/CCM.0b013e3182227d52. [DOI] [PubMed] [Google Scholar]
- 47.Ochoa-Hein E., Choi S.J., Gomez-Santillan J.A., Oyervides-Alvarado J.A., Galindo-Fraga A., Rivero-Sigarroa E., Hernandez-Gilsoul T., Dominguez-Cherit J.G. Near-zero ventilator-associated pneumonia rates after implementation of a multimodal preventive strategy in a Mexican hospital. Am. J. Infect. Control. 2020;48:446–447. doi: 10.1016/j.ajic.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Colombo S.M., Palomeque A.C., Bassi G. The zero-VAP sophistry and controversies surrounding prevention of ventilator-associated pneumonia. Intensive Care Med. 2020;46:368–371. doi: 10.1007/s00134-019-05882-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leblebicioglu H., Yalcin A.N., Rosenthal V.D., Koksal I., Sirmatel F., Unal S., Turgut H., Ozdemir D., Ersoz G., Uzun C. Effectiveness of a multidimensional approach for prevention of ventilator-associated pneumonia in 11 adult intensive care units from 10 cities of Turkey: Findings of the International Nosocomial Infection Control Consortium (INICC) Infection. 2013;41:447–456. doi: 10.1007/s15010-013-0407-1. [DOI] [PubMed] [Google Scholar]
- 50.Mehta Y., Jaggi N., Rosenthal V.D., Rodrigues C., Todi S.K., Saini N., Udwadia F.E., Karlekar A., Kothari V., Myatra S.N., et al. Effectiveness of a multidimensional approach for prevention of ventilator-associated pneumonia in 21 adult intensive-care units from 10 cities in India: Findings of the International Nosocomial Infection Control Consortium (INICC) Epidemiol. Infect. 2013;141:2483–2491. doi: 10.1017/S0950268813000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.