Abstract
As a major cause of morbidity and mortality globally, hypertension remains a serious threat to global public health. Despite the availability of many antihypertensive medications, several hypertensive individuals are resistant to standard treatments, and are unable to control their blood pressure. Regulation of the renin-angiotensin-aldosterone system (RAAS) controlling blood pressure, activation of the immune system triggering inflammation and production of reactive oxygen species, leading to oxidative stress and redox-sensitive signaling, have been implicated in the pathogenesis of hypertension. Thus, besides standard antihypertensive medications, which lower arterial pressure, antioxidant medications were tested to improve antihypertensive treatment. We review and discuss the role of oxidative stress in the pathophysiology of hypertension and the potential use of antioxidants in the management of hypertension and its associated organ damage.
Keywords: hypertension, cardiovascular diseases, oxidative stress, reactive oxygen species, antioxidants, antihypertensive therapy
1. Introduction
Hypertension is a chronic medical condition in which the blood pressure is elevated, which is a major cardiovascular risk factor. Cardiovascular diseases (CVDs) are associated with thirty-one percent of global mortality [1]. Known as a silent and an invisible killer, hypertension affects at least 1.4 billion people globally [1,2]. In 2015, 1 in 4 men and 1 in 5 women had hypertension. The majority of people with hypertension are unaware of it, and fewer than 1 in 5 hypertensive individuals have sufficient therapy to control their blood pressure [1]. Hypertension resulted in an estimated global death of 10.4 million people in 2017 [3]. It is a key risk factor for coronary heart disease, stroke and chronic kidney disease, resulting in premature mortality and morbidity [4]. Therefore, a global target of 25% relative reduction in the prevalence of hypertension by 2025 has been set by the W.H.O. [5].
The dysfunction of systems that modulate cardiac, vascular and/or renal physiology contribute to the development of hypertension [6]. Hypertension is associated with multiple organ damage, and causes impairment of cardiac, vascular, kidney and brain function. Thus, the assessment of hypertension-mediated organ damage (HMOD) in these organs is crucial for the risk of clinical complications in hypertensive patients [7]. Undetected in the early stages, the presence of HMOD is common, and several manifestations of HMOD can develop in a single hypertensive individual, further enhancing the risk of severe complications [7]. Although the central nervous system plays a fundamental role in the acute regulation of arterial blood pressure via sympathetic activation, regulation of vascular tone and peripheral resistance, the renin-angiotensin-aldosterone system (RAAS) is essential in the long-term regulation of arterial pressure. However, chronic activation of RAAS largely contributes to the development and progression of hypertension, due to the sustained production of angiotensin II. This also has important therapeutic implications. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are considered as gold standards in anti-hypertensive therapy worldwide [8,9]. Nevertheless, nearly 40% of hypertensive individuals are resistant to these standard therapies [6,10,11,12]. This indicates the critical role of other pathways such as inflammation and oxidative stress in the development and progression of hypertension, and underscores the innovative opportunity in the development of selective antioxidants as potential anti-hypertensive therapies.
In this review, we are focusing on the mechanisms of hypertension-mediated organ damage and the role of oxidative stress in the pathophysiology of hypertension. The therapeutic efficacy of antioxidants in the management of hypertension in animal and human studies is presented, and open questions are discussed.
2. Role of Oxidative Stress in Hypertension
Reactive oxygen species (ROS) play an important role in the regulation of vascular and cardiac function, and the development of cardiovascular diseases (Figure 1). Pathophysiologic processes in hypertension, leading to inflammation, fibrosis and end-organ damage, are linked to the risk factor oxidative stress [13,14]. Besides pathological conditions (such as hypertension), certain genetic variations in genes related to antioxidant function may increase the risk of ROS production and oxidative stress [15,16]. Additionally, ROS production can be influenced by multiple external factors such as certain medications (chemotherapy and non-steroidal anti-inflammatory drugs), nutrition (high-fat diet, black coffee), environmental stressors (pollution and UV radiation), harmful alcohol consumption and smoking [17,18]. Oxidative stress is characterized by an imbalance between oxidants and antioxidants (Figure 2), leading to impaired redox signaling and the oxidative modification of target molecules [19,20].
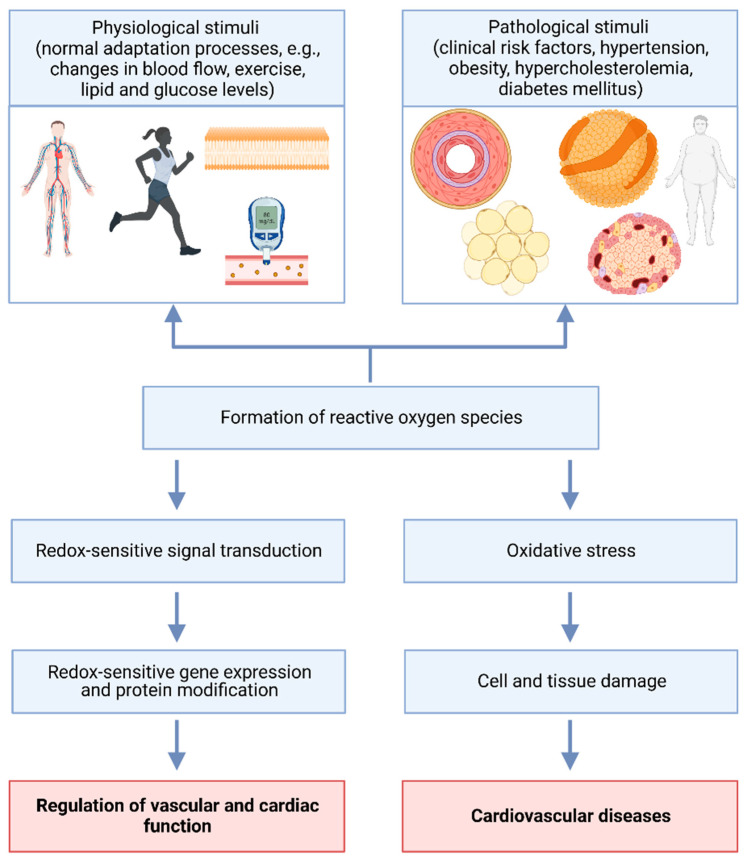
Figure 1.
Impact of physiological and pathophysiological stimuli on the regulation of vascular and cardiac function and cardiovascular diseases. Physiological stimuli like exercise can induce redox-sensitive signal transduction and the regulation of vascular and cardiac function. Redox-sensitive signal transduction, gene and protein expression and the regulation of vascular and cardiac function are also present in patients with cardiovascular diseases, despite being altered. Clinical risk factors and the metabolic syndrome can increase levels of vasoactive substances like angiotensin II, oxidative stress, cell and tissue damage, as well as in long-term cardiovascular diseases. Created with BioRender.com.
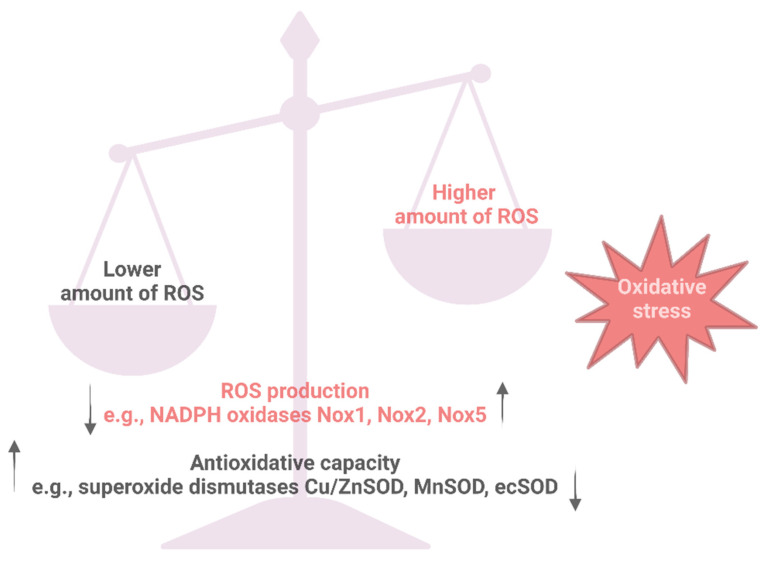
Figure 2.
Physiological redox balance and oxidative stress. Under physiological conditions, the production of reactive oxygen species (ROS) and the antioxidative capacity are in a balance. An increased production of ROS, e.g., by NADPH oxidase (NOX) isoforms 1, 2 and 5, which is not compensated by antioxidative defense mechanisms like the different superoxide dismutase (SOD) isoforms, can lead to oxidative stress. Created with BioRender.com.
The oxidative stress theory of disease is based on the idea that ROS involving (non)radicals reacts with cellular macromolecules such as DNA, RNA, proteins and lipids, causing cellular damage and cell death [21]. Interestingly, ROS have important physiologic functions. Low concentrations of ROS are important for redox regulation in maintaining endothelium integrity and vascular function [13,22,23]. The flowing blood can induce mechanical forces such as shear stress (e.g., laminar or oscillatory flow), acting on endothelial cells and affecting the formation and release of nitric oxide (NO) and ROS and the activation of signal transduction, as well as gene and protein expression that play important roles in vascular homeostasis [24,25]. Laminar flow increases endothelial nitric oxide synthase (eNOS) expression, activity and NO production, while during hypertension, oscillatory flow increases ROS formation and subsequent oxidative damage [26,27]. Exposure of endothelial cells to cigarette smoke extract can prevent the activation of the AKT/eNOS pathway, increased eNOS expression, phosphorylation and NO release in response to high laminar flow [28,29]. The type and degree of shear stress can differentially regulate the ROS- and NO-dependent signaling and remodeling of the vasculature [24,30].
Nitric oxide (NO) plays an important role in several physiological and pathophysiological processes. NO is produced by three major nitric oxide synthase (NOS) isoforms in the body. Neuronal NOS (nNOS, NOS1) is mainly found in neurons and the cardiovascular system, and is involved in the regulation of memory, learning and synaptic plasticity [16]. Inducible NOS (iNOS, NOS2) can be activated by various inflammatory stimuli and produce NO, which can mediate protective mechanisms against pathogens. Endothelial NOS (eNOS, NOS3) is mainly expressed in the cardiovascular system, and is responsible for the production of NO as the most important vasodilator [26,27]. NO can interact with superoxide anions, shifting the oxidant/antioxidant balance in favor of oxidants, increasing the formation of peroxynitrite and reducing NO bioavailability [31].
Oxidative stress has been implicated in the development of hypertension [32]. One initial hallmark is endothelial dysfunction with an impaired NO/ROS balance, supporting increased vasoconstriction, oxidation, inflammation, thrombosis and proliferation in the vessel wall [33]. The detailed molecular mechanisms of endothelial dysfunction during hypertension are still not fully resolved, even while our understanding of the role of endothelial cells in hypertension has been substantially improved in the last years [34,35]. One study found that antihypertensive therapy did not improve endothelial function in patients with essential hypertension [36], while in another study, treatment of hypertension with ACE inhibitors normalized blood pressure and restored vascular reactivity of patients [37]. In patients with essential hypertension, vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity [38]. This supports the hypothesis that nitric oxide inactivation by reactive oxygen species contributes to endothelial dysfunction in essential hypertension [39,40]. A role of ROS in the pathogenesis of hypertension is further supported by both clinical and experimental studies [33,41,42,43,44].
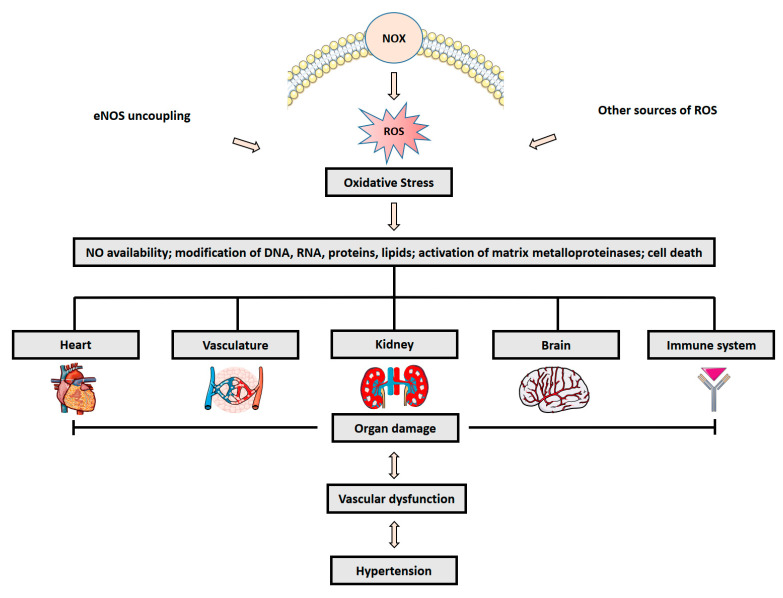
The ROS generation in the cardiovascular system is mainly mediated by nicotinamide adenine dinucleotide phosphate [NADPH] oxidases (NOX) [45,46] and other sources such as uncoupled eNOS [47], mitochondria [48] or xanthine oxidase [49] (Figure 3).
Figure 3.
ROS formation and hypertension. Activation of NADPH oxidases (NOX) and other sources of reactive oxygen species (ROS) promotes oxidative stress, and leads to organ damage, vascular dysfunction and hypertension. Parts of figure are adapted from SMART—Servier Medical Art, Servier: https://smart.servier.com. https://creativecommons.org/licenses/by/3.0/ (accessed on 22 September 2022).
Seven isoforms of the NOX family exist: NOX1, NOX2, NOX3, NOX4, NOX5, Duox1 and Doux2 [50]. Specific NOX isoforms have important functions in the cardiovascular system. They are expressed in vasculature, kidney, brain and heart [30]. Under pathophysiological conditions NOX1, 2 and 5 are upregulated and linked to oxidative stress in hypertension [50,51]. The NOX isoforms have 6 transmembrane α-helices, a heme region and a flavoprotein homology domain on the intracellular C terminal region that contains binding sites for flavin adenine dinucleotide (FAD) and NADPH [52]. Each NOX isoform has a catalytic core unit and a transmembrane domain. The specific NOX complexes can in addition contain the subunits p22phox, p47phox/NOXA1, p67phox/NOXO1 and p40phox. The cytosolic subunits p47phox/NOXA1, p67phox/NOXO1 can be phosphorylated and translocated to the cell membrane, forming an active NOX1 or NOX2 complex. NOX5 is independent of other NOX subunits, and can be activated by calcium binding [53,54]. The generation of superoxide anions, peroxynitrite, hydroxyl radicals, nitric oxide, hydrogen peroxide and hypochlorous acid can be regulated by the cell metabolism and scavenged by antioxidants. Increasing evidence also supports a role of NOX isoforms in the regulation of cardiac intermediary metabolism [55].
NOX4 has anti-atherosclerotic and vasoprotective properties in the endothelium and protects the vasculature against oxidative stress, angiotensin II-induced aortic inflammation, tunica media hypertrophy and endothelial dysfunction [56,57,58]. Interestingly, the vasoprotective properties of NOX4 are attributed to the NOX4-derived H2O2 that can cross the membrane and act as a signaling molecule or even endothelium-derived relaxation factor (EDRF), activating downstream effectors and inducing cardiac and vascular protection [56,58]. However, NOX isoforms have also been implicated in the development and progression of hypertension [42,59]. Mice deficient in NOX1 had preserved endothelium-dependent relaxation, blunted pressure response and reduced superoxide production. Consequently, overexpression of NOX1 led to increased oxidative stress, elevated blood pressure and hypertrophic response in Ang II-induced hypertension [60,61]. NOX2 in endothelial cells contributes to Ang II-induced endothelial dysfunction, vascular remodeling and hypertension by increasing ROS production and blood pressure in transgenic mice with endothelial-specific overexpression of NOX2 [62]. In human endothelial cells, NOX2 is induced by Ang II in a dose-dependent manner [63]. Furthermore, NOX2-stimulated the production of mitochondrial superoxide by activating reverse electron transfer in Ang II-induced hypertension [64]. Ang II induced mitochondrial dysfunction via a protein kinase C-dependent pathway by activating the endothelial cell NADPH oxidase and formation of peroxynitrite. Furthermore, mitochondrial dysfunction in response to Ang II modulates endothelial NO availability [65]. Mice with fibroblast-specific deficiency of NOX2 showed reduced vascular remodeling and hypertension in response to Ang II [66]. NOX3 expression and oxidative stress is increased in the brain of stroke-prone hypertensive rats [67]. Recently, a genome-wide associated study in Eastern Chinese Han population identified a variant of NOX3, rs6557421, to have a potential effect on individual susceptibility to pulmonary hypertension [68]. The NOX4-derived H2O2 can have dose-dependent effects [69]. NOX4 was shown to be involved in the development of hypertension in Dahl salt-sensitive (DSS) rats [70]. Salt-induced hypertension in DSS rats increased H2O2 release by NOX4, and contributed to renal injury by regulating the upstream target of mammalian target of rapamycin complex 1 (mTORC1), increasing immune cell infiltration and proliferation and subsequent renal oxidative stress [71]. In humans, NOX5 might also be involved in vascular redox signaling and remodeling during hypertension [72]. Recent studies further support a link between NOX5, oxidative stress, endothelial dysfunction and systolic hypertension. Mice expressing human Nox5 in endothelial cells developed-upon aging-severe systolic hypertension and impaired endothelium-dependent vasodilation due to uncoupled NO synthase [73].
Associated organ damage as a consequence of oxidative stress in hypertension have been investigated in the vasculature [64,74], kidney [75,76], brain [77,78] and immune system [79,80]. Thus, a balance between mediators of oxidative stress and antioxidants is essential to promote physiological organ function and enhance systemic defense mechanism.
3. Detection and Biomarkers of ROS
Accurate assessment and detection of ROS in biological samples is challenging, due to their high reactivity and instability. Oxidative modifications of a variety of probes allow the ROS detection in biological samples. Fluorescent protein-based probes can be used to monitor changes in the levels of cytoplasmic and mitochondrial H2O2. Experimental approaches might involve the transfection of cells with plasmids or adenoviruses, leading to the formation of chimeric proteins capable of detecting ROS [81,82]. Dihydroethidium (DHE) and mitochondrial-targeted probe mitoSOX has been used to efficiently detect cellular and mitochondrial superoxide. Addition of a triphenylphosphonium group facilitates the collection of O2− in the mitochondria, while the reaction of mitoSOX with O2− produces 2-hydroxy-mito-ethidium, which can be measured with high performance-liquid chromatography (HPLC) [82,83,84]. In addition, short-lived free radicals in living animals can be detected using X- and L-band electron spin resonance (ESR) spectroscopy. This allows the ex-vivo analysis of tissue or blood using ESR after the infusion of cyclic hydroxylamines or nitrone spin traps. Other direct methods for ROS detection include, but are not limited to, immunospin trapping, cyclic hydroxylamine spin and boronate-based fluorescent probes [82,85].
Besides the measurement of free-radical production, ROS-modified molecules have been identified as stable biomarkers which may precisely indicate the status of local or systemic oxidative stress. Oxidative modification of lipids, proteins, DNA and RNA have been used as important biomarkers for the assessment of the redox status of human samples [86,87]. Lipids are especially susceptible to ROS-induced oxidative damage, mainly due to the presence of unsaturated double bonds. The frequently studied end products of lipid peroxidation are 4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA) [88]. In spontaneously hypertensive rats, the severity of diastolic dysfunction was associated with elevated levels of 4-HNE, which can be measured with HPLC and immune-assays with specific anti-HNE antibodies [89]. Colorimetric or fluorimetric assays can be used to detect a pink adduct complex from the reaction of thiobarbituric acid (TBA) and thiobarbituric acid reactive substances (TBARS), which include MDA, alkadienals and alkenals [90]. The ROS-induced modification of proteins can lead to reversible or irreversible alteration of their biological function. Oxidative cleavage of protein backbones yielding in carbonylation is the most common irreversible oxidative modification of proteins [90]. Additionally, the relative stability and early formation of protein carbonyls has resulted in its frequent use as a biomarker of oxidative stress and protein damage in tissues. Measurement of carbonylated proteins with methods such as HPLC and ELISA has shown elevated levels in several cardiovascular diseases [90,91]. S-glutathionylation, S-sulfenylation and S-nitrosylation are reversible protein modifications which have been identified as key signaling pathways in cardiovascular health and diseases [92]. Current developments in mass spectrometry proteomics have allowed an accurate and specific identification and quantification of oxidized proteins in several tissues. Nucleotide oxidation, DNA strand breakage, loss of bases and adduct formation are ROS-induced DNA modification can lead to mutations and DNA damage [90].
In summary, the assessment of oxidative modification of molecules in biological samples can be a valuable tool in the clinical assessment of disease severity, and support the identification of new biomarkers and potential therapies of hypertension.
4. Mechanism of Antioxidants and Potential Therapeutic Strategy in Hypertension
The interaction of different ROS sources and its impact on redox signalling can significantly increase oxidative stress. In contrast, antioxidants play a key functional role in reversing the detrimental effect of ROS. This includes enzymatic and nonenzymatic antioxidants like different superoxide dismutases, catalase, glutathione peroxidase, α-lipoic acid, coenzyme Q10 and vitamins [32,44,93,94]. Deciphering the molecular complexities of antioxidant interactions will support our understanding of their role in oxidative stress-induced hypertension. In this section, we discuss selected antioxidants, their potential antihypertensive and antioxidative effects and their molecular interactions.
4.1. Vitamins
Vitamins can play a crucial role in the improvement of endothelial dysfunction. Vitamin C and E downregulates NADPH oxidase, a major source of ROS in the vascular wall, and upregulates eNOS, thus decreasing oxidative stress and lowing BP [95]. A study analysing the role of vitamin C and E in spontaneous hypertensive rats could show an effective modulation of vascular function through regulation of eNOS and NADPH oxidases [95]. Vitamin C acts directly as antioxidant in water-soluble environments. In lipids, the tocopheroxyl radical (formed when exogenous oxidants interact with alpha-tocopherol) can be reduced by vitamin C to generate the active form of vitamin E, alpha tocopherol, thus limiting lipid peroxidation in the cell membrane, mitochondria and endoplasmic reticulum, and preserving cell integrity [96].
4.2. Polyphenols
Polyphenols have hydrophobic and hydrophilic domains that enable them to interact with and diffuse through biological membranes. They bind to receptors, transcription factors and enzymes involved in intracellular signalling [97]. Such different effects of polyphenols enable them to exert biological activity via mechanisms that lead to free radical scavenging, mitochondrial protection, transcription factor regulation, membrane receptor modulation and inhibition of ROS and cellular proliferation [97,98]. In hypertensive patients, quercetin could reduce BP and improve endothelial function by inhibiting the activity of ACE and the ratio of circulating ET-1 to NO [99]. Quercetin reduced blood pressure by activating Na+-K+-2Cl− cotransporter 1 (NKCC1) in renal epithelial cells, elevating cytosolic Cl− concentration ([Cl−]c) and downregulating gene expression of epithelial Na+ channel (ENaC) [100,101]. Green tee supplementation could also regulate oxidative stress, inflammation, gene expression and serum levels of vasoactive substances and modulators of BP (as Ang II and aldosterone) [102]. Significant changes were evident in downregulating the mRNA expression of ACE and ET-1 and increasing mRNA expression of eNOS [102]. Since polyphenols become free radicals after ROS scavenging, they could lead to Kelch-like ech-associated protein-1 (keap-1) thiol group oxidation and promote translocation of Nuclear factor erythroid-2 related factor-2 (Nrf2) into the nucleus [103]. Nrf2 binding to antioxidant response elements leads to the transcription of genes that encode antioxidant proteins such as hemoxygenase-1 (HO-1), superoxide dismutase-2 and glutathione peroxidase [104]. Nrf2 activation could preserve endothelial function and prevent hypertension in Ang II-induced mice [105]. Thus, the Nrf2 pathway could represent a novel area of research to explore the pleiotropic and synergistic actions of different antioxidants in reducing oxidative damage while maintaining cardiovascular function.
4.3. α-Lipoic Acid
In signal transduction, α-lipoic acid induction of GSH (glutathione) via transcription factor Nrf2 leads to interaction with kinases and phosphatases [106,107]. α-lipoic acid acts as a metal chelator and free radical scavenger, reduces the oxidized forms of GSH and vitamin C and E and upregulates eNOS [106].
4.4. N-Acetylcysteine
N-acetylcysteine (NAC) as an antioxidant acts as a reductant of disulfide bonds, a scavenger of ROS and a precursor for glutathione biosynthesis [108]. As a precursor for glutathione, it exhibits its antioxidant action directly or indirectly by lowing oxidative stress, improving insulin resistance, altering glucose metabolism, improving NO bioavailability, modulating vasoactive molecules like Ang II and hydrogen sulfide and improving renal function [109,110]. In hypertension, maternal NAC therapy could protect offspring of spontaneously hypertensive rats through the regulation of the hydrogen sulphide-generating pathway [111,112]. NAC also improved NO-dependent, alpha-adrenergic and beta-adrenergic pathways in hypertensive rats [113,114]. In a recent review, Pedre et al. discussed a new mechanism of action involving the conversion of NAC into hydrogen sulfide and sulfane sulfur species. They argued that the steady but slow delivery of intracellular cysteine by NAC allows for a low level of hydrogen sulfide and sulfane sulfur production, leading to cytoprotection through stimulating mitochondrial bioenergetics, protecting cells against oxidative damage, modulating protein function and increasing scavenging capacity [108].
4.5. Coenzyme Q10
A component of the electron transport chain which accepts electrons from complexes I and II and the glyceraldehyde-3-phosphate shuttle is the Coenzyme Q10 (CoQ10) [115]. CoQ10 could reduce oxidative stress and the expression of the pro-inflammatory cytokine IL-1β, thereby increasing the scavenging activity of SOD and the anti-inflammatory cytokine IL-10 in salt-induced hypertensive rats [116]. A mitochondria-targeted CoQ10 formation given orally to hypertensive rats reduced blood pressure, increased NO bioavailability and reduced cardiac hypertrophy [117]. In older patients with hypertension, low levels of CoQ10 are prevalent [118], potentially due to the increase in ROS formation during the pathogenesis of hypertension. Interestingly, several human intervention studies with CoQ10 in hypertension have demonstrated a significant reduction in blood pressure [119,120,121].
4.6. Superoxide Dismutase
Superoxide dismutase acts as a defence against oxidative damage. Several SOD mimetics have been developed, and their therapeutic potential has been tested in renal and cardiovascular disease models [122,123]. SOD treatment with tempol mimetic could reduce blood pressure in experimental models of hypertension, partially via the vasodilating and antihypertensive effects of increased NO bioavailability [124,125]. Tempol could also reduce vascular remodelling and decrease superoxide anion formation in salt-loaded stroke-prone spontaneously hypertensive rats [126]. Savalia and colleagues have shown that administration of nanoformulated SOD, Poly-l-lysine (PLL50)-polyethylene glycerol (PEG) copper/zinc superoxide dismutase (CuZnSOD) could scavenge excessive superoxide anions and decrease blood pressure in a mouse model of Ang II-induced hypertension. In cultured cells, they showed that a non-reducible cross-linked CuZnSOD nanozyme could actively deliver CuZnSOD protein to neurons without significantly inducing toxicity [127]. These data suggest that tailored antioxidant therapy of specific targets could enhance their effectiveness. Blood pressure is markedly increased in Sirt3-knockout mice, even in response to low doses of Ang II, leading to increased oxidative stress and endothelial dysfunction [128]. SIRT3 is a key mitochondrial deacetylase, and activates cyclophilin D and the mitochondrial antioxidant SOD2 [128]. Ang II and inflammation can contribute to the decline in Sirt3 activity [129]. They can influence the antioxidant capacity of a SOD mimetic, since acetylation plays an important role in the post-translational regulation of SOD2 activity by inhibiting enzyme activity at the lysine residues (K68 and K122) of SOD2 [130].
4.7. Antioxidants and Hypertension
Antioxidants can reduce the formation of ROS. In many human and animal models of hypertension, antioxidant activity is markedly reduced [32,33,41,42,44,131]. Glutathione and thioredoxin are impaired in hypertension. Furthermore, mild increase of bilirubin concentration within physiological levels negatively correlates with the incidence of hypertension [41,132,133]. Although a higher intake of dietary carotenoid was associated in one study with a lower risk of hypertension [134], most clinical and experimental studies, except a few (see Table 1) did not show a clear benefit of antioxidant in hypertension, atherosclerosis and cardiovascular disease [135,136,137]. Many animal models of hypertension have shown promising effects of antioxidants; however, randomized clinical trials and population studies in hypertensive patients have shown disappointing outcomes [138,139,140,141,142]. Several factors may account for these differences. First, the trial design and type of antioxidants can affect the results. A long-term exposure to increased levels of pro-oxidant factors can cause structural defects at the mitochondrial DNA level, leading to the functional alteration of several enzymes, cellular structures and aberrations in gene expression [143]. Thus, patients with several additional cardiovascular risk factors may already have irreversible oxidative damage, and expecting to reverse this with antioxidants therapy within a few years during clinical studies may be unrealistic. In addition, a long-term unhealthy lifestyle may not be necessarily compensated by a rich antioxidant food administered. To achieve elevated steady state levels in biological membranes, supplementation with lipid-soluble antioxidants such as vitamin E may require several weeks [144]. Furthermore, the biological half-life of specific antioxidants can differ, which should also be considered in experimental studies. Natural antioxidants in food or synthetic antioxidants administered as supplements may significantly differ in their mode of uptake and action. For instance, some polyphenols tend to have very low concentration levels in the blood [145], yet they work very well in the body because they activate their own antioxidant mechanisms. Furthermore, the frequent use of antioxidants in the normal nutrition might affect the responses in control groups of clinical studies. Hence, due to the lack of evidence in antioxidant use, it is not recommended as a supplementation for hypertension treatment or prevention. Nevertheless, most dietary guidelines recommend the regular consumption of a diet with antioxidant-rich fruits and vegetables, whole grain, plant fibres, salmon, nuts and reduced salt intake as a low-sodium diet, which was shown to reduce oxidative stress and improve vascular function [146,147,148,149]. A high level of fruit consumption was associated with lower blood pressure and blood glucose levels, largely independent of these and other dietary and nondietary factors, with significant lower risks of cardiovascular diseases [150].
Table 1.
Studies analyzing the impact of antioxidants on hypertension in animal and human studies.
| Antioxidant | Model/Subject/Study Design | Outcome of Study |
|---|---|---|
| Vitamin C | Spontaneously hypertensive rats (SHR) [151,152]. | Blood pressure (BP) ⬇ [151,152]. |
| High salt-treated SHR [153]. | BP ⬇, endothelium-dependent relaxation ⬆ [153]. | |
| Hypertensive Wistar rats [154]. | BP ⬇ [154]. | |
| Stroke-prone SHR [155]. | BP ⬇ [155]. | |
| Humans, essential hypertension [38,156,157,158]. | Systolic blood pressure (SBP) ⬇, endothelial vasodilation ⬆, arterial stiffness ⬇ [38,156,157,158]. | |
| Humans, mild hypertension [159]. | SBP and diastolic BP (DBP) ⬇ [159]. | |
| Humans, elderly patients with hypertension [160]. | Small ⬇ in BP and antioxidant capacity ⬆ [160]. | |
| Humans, systemic review and meta-analysis [136]. | No consistent benefit for the prevention of CVD (hypertension) [136]. | |
| Humans, long-term risk of hypertension [138]. | No clear beneficial effect [138]. | |
| Humans, hypertension [161,162]. | SBP and mean BP ⬇, endothelial vasodilation ⬆ [161,162]. | |
| Humans, elderly patients, ambulatory BP [163]. | Modest effect on BP [163]. | |
| Humans, systemic review and meta-analysis [164]. | SBP ⬇ [164]. | |
| Vitamin E | SHR, Wistar-Kyoto (WKY) rats [165,166,167]. | BP ⬇ [165,166,167]. |
| High salt-treated Dahl salt-sensitive (DSS) rats [168]. | No effect on BP [168]. | |
| DSS rat [169]. | BP ⬇ [169]. | |
| Stroke-prone SHR [170]. | BP ⬇ [170]. | |
| Humans, hypertension and cerebral arteriosclerosis [171]. | SBP ⬇ [171]. | |
| Humans, mild essential hypertension [172]. | SBP, DBP and heart rate ⬇ [172]. | |
| Humans, sedentary elderly patients with mild systolic hypertension [173]. | SBP ⬇ [173]. | |
| Vitamin C and E | Stroke-prone SHR [155]. | BP ⬇ [155]. |
| Fructose-induced hypertensive WKY rats [174]. | BP ⬇ [174]. | |
| DOCA-salt-induced hypertensive rats [175]. | SBP ⬇ [175]. | |
| Humans, essential hypertension [157,158]. | SBP, DBP ⬇, endothelial vasodilation ⬆ and arterial stiffness ⬇ [157,158]. | |
| Humans, essential hypertension [176]. | SBP and DBP ⬇ [176]. | |
| Humans, essential hypertension [139]. | No effect on BP [139]. | |
| Polyphenols | ||
| Resveratrol | SHR, WKY rats [177,178]. | BP ⬇ [177,178]. |
| Rats with sucrose-induced hypertension [179]. | BP ⬇ [179]. | |
| Mice with Ang II-induced hypertension [180]. | BP ⬇ [180]. | |
| Fructose-induced hypertensive rats [181]. | BP ⬇ [181]. | |
| Hypertension induced in Wistar rats [182]. | SBP and DBP ⬇ [182]. | |
| Humans, essential hypertension [140]. | No significant change in BP [140]. | |
| Quercetin | Rats with sucrose-induced hypertension [179]. | BP ⬇ [179]. |
| DOCA-salt hypertensive rats [183]. | BP ⬇ [183]. | |
| L-NAME-induced hypertensive Wistar rats [184]. | BP ⬇ [184]. | |
| SHR [185]. | BP and heart rate ⬇ [185]. | |
| Humans, randomized controlled trial [186]. | DBP and mean arterial pressure ⬇ [186]. | |
| Humans, systemic review and meta-analysis [187]. | SBP and DBP ⬇ [187]. | |
| Humans, randomized controlled trial [188,189]. | SBP ⬇ [188,189]. | |
| Apocynin | SHR, WKY rats [190]. | BP ⬇ [190). |
| SHR [191]. | BP and heart rate ⬇ [191]. | |
| Fructose-treated hypertensive Sprague-Dawley rats [192]. | SBP ⬇ [192]. | |
| ANG II-induced hypertension in mice [193]. | SBP ⬇ [193]. | |
| DOCA-induced hypertensive rats [194]. | SBP ⬇ [194]. | |
| Dexamethasone-induced hypertension in SD rats [195]. | DBP ⬇ [195]. | |
| Green Tea | SHR [196]. | BP and heart rate ⬇ [196]. |
| Stroke-prone SHR [197]. | SBP and DBP ⬇ [197]. | |
| Salt-induced hypertensive Wistar rats [102]. | SBP and DBP ⬇ [102]. | |
| Humans, meta-analysis [198]. | SBP and DBP ⬇ [198]. | |
| Humans, systemic review and meta-analysis [199). | SBP and DBP ⬇ [199]. | |
| Humans, systemic review [200]. | SBP and DBP ⬇ [200]. | |
| Humans, systemic review and meta-analysis [201]. | SBP and DBP ⬇ [201]. | |
| (-)-Epicatechin | DOCA-salt hypertensive rats [202,203]. | SBP ⬇ [202,203]. |
| Borderline hypertensive rats [204]. | SBP ⬇ [204]. | |
| Fructose-induced hypertensive SD rats [205]. | SBP ⬇ [205]. | |
| L-NAME-induced hypertensive Wistar rats [206]. | No significant changes in SBP and heart rate [206]. | |
| SHR, WKY rats [207]. | SBP ⬇ [207]. | |
| Humans, meta-analysis [208,209]. | SBP and DBP ⬇ [208,209]. | |
| Humans, randomized controlled trial [210]. | SBP and DBP ⬇ [210]. | |
| Humans, randomized controlled trial [141]. | No significant changes in SBP [141]. | |
| N-acetyl cysteine | SHR [113,114,211,212,213]. | SBP, mean arterial pressure, heart rate ⬇, but not DBP [113,114,211,212,213]. |
| Fructose-treated hypertensive WKY rats [214]. | Attenuated increase in SBP [214]. | |
| Fructose-treated hypertensive SD rats [110]. | Prevented increases in SBP and DBP [110]. | |
| L-NAME-induced hypertensive SD rats [111]. | BP ⬇ [111]. | |
| Salt-induced hypertensive Wistar rats [215]. | No effect on BP [215]. | |
| Salt-induced hypertensive DSS rats [109]. | BP ⬇ [109]. | |
| Humans, essential hypertension [216]. | 24hr and daytime SBP and DBP ⬇ [216]. | |
| α-lipoic acid | Fructose-treated hypertensive WKY rats [217,218]. | Prevented increase in BP [217,218]. |
| SHR [219,220]. | BP ⬇ [219,220]. | |
| Salt-induced hypertensive WKY rats [221]. | Prevented increase in BP [221]. | |
| Salt-induced hypertensive Wistar rats [222]. | BP ⬇ [222]. | |
| High salt-induced hypertensive mice [223,224]. | BP ⬇ [223,224]. | |
| DSS rats [225]. | BP ⬇ [225]. | |
| Glucose-induced hypertensive SD rats [226,227]. | Prevented increase in BP [226,227]. | |
| Glucocorticoid-induced hypertension in SD rats [228]. | Partially ⬇ SBP [228]. | |
| Coenzyme Q10 | SHR [229]. | BP ⬇ in older animals [229]. |
| Stroke-prone SHR [230]. | SBP ⬇ [230]. | |
| Salt-induced hypertensive SD rats [116]. | BP ⬇ [116]. | |
| Humans, essential hypertension [119,120,231,232,233]. | SBP and DBP ⬇ [119,120,231,232,233]. | |
| Humans, hypertension with coronary artery disease [234]. | SBP and DBP ⬇ [234]. | |
| Humans, isolated systolic hypertension [121]. | SBP ⬇ [121]. | |
| Coenzyme Q10 therapy in humans, hypertensive with metabolic syndrome [142]. | No effect on SBP and DBP [142] | |
| Superoxide dismutases | Meta-analysis using SOD mimetic tempol in hypertensive animal models [235]. | BP ⬇ [235]. |
| EC-SOD in MCT-induced hypertensive rats [236]. | Improved right ventricular SBP [236]. | |
| Poly-l-lysine (PLL50)-polyethylene glycol (PEG) CuZn-SOD nanozyme in mice with Ang II-induced hypertension [127]. | BP ⬇ [127]. | |
| Melon SOD in SHR [237]. | BP ⬇ [237]. | |
| Tempol in hypertension of Wistar rats [124]. | SBP ⬇ [124]. | |
| Tempol in fructose-induced hypertensive SD rats [125]. | BP ⬇ [125]. | |
| Tempol in salt-loaded stroke-prone SHR [126]. | SBP ⬇ [126]. | |
| Tempol in advanced-stage stroke-prone SHR [238,239]. | No effect on SBP [238,239]. | |
| TAT-SOD in humans, essential hypertension [240]. | SBP and DBP ⬇ [240]. |
Abbreviations: ANG, angiotensin; BP, blood pressure; DBP, diastolic BP; DOCA, deoxycorticosterone acetate; DSS, Dahl salt-sensitive; EC-SOD, extracellular superoxide dismutase; L-NAME, L-NG-Nitro arginine methyl ester; MCT, monocrotaline; SBP, systolic BP; SD, Sprague-Dawley; SHR, spontaneous hypertensive rats; SOD, superoxide dismutase; WKY rats, Wistar-Kyoto rats. ⬇, decrease/d; ⬆, increase/d.
5. Open Questions
In many animal models, antioxidant treatments have proven efficacious in abrogating the development of hypertension, but human studies are mostly controversial and inconclusive, which could be attributed to several factors. These include multiple risk factors like aging, comorbidities and pharmacological treatments of patients, and the frequent use of antioxidative supplements in food preparation. Additionally, in most animal models, treatment with antioxidants starts at the onset of hypertension, which is contrary to testing the anti-hypertensive effects of these antioxidants in patients with pre-existing hypertension for several years [32,241]. Recently, the US Preventive Services Task Force concluded that the current evidence is insufficient to assess the balance of benefits, and harms of the use of single- or paired-nutrient supplements (other than beta carotene and vitamin E) for the prevention of cardiovascular disease or cancer [242]. Despite these challenges, dietary antioxidant intake and polyphenols could be beneficial to reduce and prevent hypertension [148]. Potential mechanisms might also involve redox sensitive signaling, epigenetic effects, reductive stress and more. Furthermore, oxidative stress and inflammation interacts in a vicious cycle, which exacerbates the progression of hypertension and targets organ damage. Hence, the development of more selective antioxidants will be a major challenge in the field, which would provide new tools to decrease specific ROS in the vessel wall.
6. Conclusions
In conclusion, many experimental and clinical studies support a causal role of ROS generation and oxidative stress in hypertension and its associated target organ damage. Physiological concentrations of ROS play an important role in the maintenance of endothelial integrity and vascular function, while elevated ROS formation leads to oxidative stress, the uncoupling of eNOS and reduced bioavailability of nitric oxide. This can cause endothelial dysfunction, which promotes the progression of hypertension. Antioxidants that are more selective could decrease specific ROS generation and subsequent inflammation, which might provide an attractive therapeutic strategy in the treatment of hypertension-associated organ damage.
Author Contributions
Conceptualization, M.A.-O., P.D.-N. and H.M.; design and composition, M.A.-O., P.D.-N. and H.M.; writing—original draft preparation, M.A.-O. and P.D.-N.; and writing—review and editing, M.A.-O., P.D.-N., S.S. and H.M. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors are supported by research grants of the German Academic Exchange Service (DAAD) and the Government of Ghana (to P.D.-N.), Deutsche Forschungsgemeinschaft (DFG) (Grants MO 1695/4-1 and 5-1; to H.M., IRTG 2251; to H.M.), German Centre for Cardiovascular Research (DZHK) (Grant 81X2800207 to H.M.) and by funding of the Excellence Initiative by the German Federal and State Governments (Institutional Strategy, measure ‘support the best’, 3-25 2, Grant F-03661-553-41B-1250000; to H.M.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mills K.T., Stefanescu A., He J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mowry F.E., Biancardi V.C. Neuroinflammation in hypertension: The renin-angiotensin system versus pro-resolution pathways. Pharmacol. Res. 2019;144:279–291. doi: 10.1016/j.phrs.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Stanaway J.D., Afshin A., Gakidou E., Lim S.S., Abate D., Abate K.H., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey R.M., Muntner P., Bosworth H.B., Whelton P.K. Prevention and Control of Hypertension: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018;72:1278–1293. doi: 10.1016/j.jacc.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dugani S., Gaziano T.A. 25 by 25: Achieving Global Reduction in Cardiovascular Mortality. Curr. Cardiol. Rep. 2016;18:10. doi: 10.1007/s11886-015-0679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drummond G.R., Vinh A., Guzik T.J., Sobey C.G. Immune mechanisms of hypertension. Nat. Rev. Immunol. 2019;19:517–532. doi: 10.1038/s41577-019-0160-5. [DOI] [PubMed] [Google Scholar]
- 7.Williams B., Mancia G., Spiering W., Rosei E.A., Azizi M., Burnier M., Clement D.L., Coca A., De Simone G., Dominiczak A., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) Eur. Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 8.Li E.C.K., Heran B.S., Wright J.M. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst. Rev. 2014;2014:CD009096. doi: 10.1002/14651858.CD009096.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oger E., Kerbrat S., Nowak E., Paillard F., Scarabin P., Happe A. Effectiveness of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on total and cardiovascular mortality and morbidity in primary prevention: A nationwide study based on French Health Insurance Data (SNDS) J. Clin. Hypertens. 2022;24:438–448. doi: 10.1111/jch.14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel S., Rauf A., Khan H., Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017;94:317–325. doi: 10.1016/j.biopha.2017.07.091. [DOI] [PubMed] [Google Scholar]
- 11.Mercier K., Smith H., Biederman J. Renin-angiotensin-aldosterone system inhibition: Overview of the therapeutic use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, mineralocorticoid receptor antagonists, and direct renin inhibitors. Prim. Care. 2014;41:765–778. doi: 10.1016/j.pop.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 12.de Mello W.C. Local Renin Angiotensin Aldosterone Systems and Cardiovascular Diseases. Med. Clin. N. Am. 2017;101:117–127. doi: 10.1016/j.mcna.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Rueckschloss U., Duerrschmidt N., Morawietz H. NADPH Oxidase in Endothelial Cells: Impact on Atherosclerosis. Antioxidants Redox Signal. 2003;5:171–180. doi: 10.1089/152308603764816532. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann A., Brunssen C., Morawietz H. Contribution of lectin-like oxidized low-density lipoprotein receptor-1 and LOX-1 modulating compounds to vascular diseases. Vasc. Pharmacol. 2018;107:1–11. doi: 10.1016/j.vph.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Egea G., Jiménez-Altayó F., Campuzano V. Reactive Oxygen Species and Oxidative Stress in the Pathogenesis and Progression of Genetic Diseases of the Connective Tissue. Antioxidants. 2020;9:1013. doi: 10.3390/antiox9101013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wigner P., Dziedzic A., Synowiec E., Miller E., Bijak M., Saluk-Bijak J. Variation of genes encoding nitric oxide synthases and antioxidant enzymes as potential risks of multiple sclerosis development: A preliminary study. Sci. Rep. 2022;12:10603. doi: 10.1038/s41598-022-14795-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krakowian D., Skiba D., Kudelski A., Pilawa B., Ramos P., Adamczyk J., Pawłowska-Góral K. Application of EPR spectroscopy to the examination of pro-oxidant activity of coffee. Food Chem. 2014;151:110–119. doi: 10.1016/j.foodchem.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 18.Juan C., de la Lastra J.P., Plou F., Pérez-Lebeña E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021;22:4642. doi: 10.3390/ijms22094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Touyz R.M., Rios F.J., Alves-Lopes R., Neves K.B., Camargo L.D.L., Montezano A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020;36:659–670. doi: 10.1016/j.cjca.2020.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghezzi P., Jaquet V., Marcucci F., Schmidt H.H. The oxidative stress theory of disease: Levels of evidence and epistemological aspects. Br. J. Pharmacol. 2017;174:1784–1796. doi: 10.1111/bph.13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paravicini T.M., Touyz R.M. Redox signaling in hypertension. Cardiovasc. Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Muller G., Morawietz H. NAD(P)H Oxidase and Endothelial Dysfunction. Horm. Metab. Res. 2009;41:152–158. doi: 10.1055/s-0028-1086023. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh H.-J., Liu C.-A., Huang B., Tseng A.H., Wang D.L. Shear-induced endothelial mechanotransduction: The interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci. 2014;21:3. doi: 10.1186/1423-0127-21-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan S. Molecular Mechanisms Responsible for the Atheroprotective Effects of Laminar Shear Stress. Antioxid. Redox Signal. 2009;11:1669–1682. doi: 10.1089/ars.2009.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison D.G., Widder J., Grumbach I., Chen W., Weber M., Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J. Intern. Med. 2006;259:351–363. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 27.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflug. Arch. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- 28.Giebe S., Cockcroft N., Hewitt K., Brux M., Hofmann A., Morawietz H., Brunssen C. Cigarette smoke extract counteracts atheroprotective effects of high laminar flow on endothelial function. Redox Biol. 2017;12:776–786. doi: 10.1016/j.redox.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giebe S., Hofmann A., Brux M., Lowe F., Breheny D., Morawietz H., Brunssen C. Comparative study of the effects of cigarette smoke versus next generation tobacco and nicotine product extracts on endothelial function. Redox Biol. 2021;47:102150. doi: 10.1016/j.redox.2021.102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller G., Morawietz H. Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid. Redox Signal. 2009;11:1711–1731. doi: 10.1089/ars.2008.2403. [DOI] [PubMed] [Google Scholar]
- 31.Karbach S., Wenzel P., Waisman A., Munzel T., Daiber A. eNOS Uncoupling in Cardiovascular Diseases—The Role of Oxidative Stress and Inflammation. Curr. Pharm. Des. 2014;20:3579–3594. doi: 10.2174/13816128113196660748. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigo R., González J., Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011;34:431–440. doi: 10.1038/hr.2010.264. [DOI] [PubMed] [Google Scholar]
- 33.Briones A.M., Touyz R.M. Oxidative Stress and Hypertension: Current Concepts. Curr. Hypertens. Rep. 2010;12:135–142. doi: 10.1007/s11906-010-0100-z. [DOI] [PubMed] [Google Scholar]
- 34.Ambrosino P., Bachetti T., D’Anna S.E., Galloway B., Bianco A., D’Agnano V., Papa A., Motta A., Perrotta F., Maniscalco M. Mechanisms and Clinical Implications of Endothelial Dysfunction in Arterial Hypertension. J. Cardiovasc. Dev. Dis. 2022;9:136. doi: 10.3390/jcdd9050136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu S., Ilyas I., Little P.J., Li H., Kamato D., Zheng X., Luo S., Li Z., Liu P., Han J., et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021;73:924–967. doi: 10.1124/pharmrev.120.000096. [DOI] [PubMed] [Google Scholar]
- 36.Panza J.A., Quyyumi A.A., Callahan T.S., Epstein S.E. Effect of antihypertensive treatment on endothelium-dependent vascular relaxation in patients with essential hypertension. J. Am. Coll. Cardiol. 1993;21:1145–1151. doi: 10.1016/0735-1097(93)90238-V. [DOI] [PubMed] [Google Scholar]
- 37.Souza-Barbosa L.A., Ferreira-Melo S.E., Ubaid-Girioli S., Nogueira E.A., Yugar-Toledo J.C., Moreno H., Jr. Endothelial vascular function in hypertensive patients after renin-angiotensin system blockade. J. Clin. Hypertens. 2006;8:803–811. doi: 10.1111/j.1524-6175.2006.05663.x. [DOI] [PubMed] [Google Scholar]
- 38.Taddei S., Virdis A., Ghiadoni L., Magagna A., Salvetti A. Vitamin C Improves Endothelium-Dependent Vasodilation by Restoring Nitric Oxide Activity in Essential Hypertension. Circulation. 1998;97:2222–2229. doi: 10.1161/01.CIR.97.22.2222. [DOI] [PubMed] [Google Scholar]
- 39.Dillon G.A., Greaney J.L., Shank S., Leuenberger U.A., Alexander L.M. AHA/ACC-defined stage 1 hypertensive adults do not display cutaneous microvascular endothelial dysfunction. Am. J. Physiol. Circ. Physiol. 2020;319:H539–H546. doi: 10.1152/ajpheart.00179.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kakabadze K., Megreladze I., Khvichia N., Mitagvaria N., Kipiani N., Dumbadze M., Sanikidze T. Some Aspects of Role of Nitric Oxide in the Mechanisms of Hypertension (Experimental Study) Cardiol. Res. 2021;12:16–24. doi: 10.14740/cr1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanito M., Nakamura H., Kwon Y.-W., Teratani A., Masutani H., Shioji K., Kishimoto C., Ohira A., Horie R., Yodoi J. Enhanced Oxidative Stress and Impaired Thioredoxin Expression in Spontaneously Hypertensive Rats. Antioxid. Redox Signal. 2004;6:89–97. doi: 10.1089/152308604771978381. [DOI] [PubMed] [Google Scholar]
- 42.Touyz R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigo R., Prat H., Passalacqua W., Araya J., Guichard C., Bächler J.P. Relationship between oxidative stress and essential hypertension. Hypertens. Res. 2007;30:1159–1167. doi: 10.1291/hypres.30.1159. [DOI] [PubMed] [Google Scholar]
- 44.Griendling K.K., Camargo L.L., Rios F.J., Alves-Lopes R., Montezano A.C., Touyz R.M. Oxidative Stress and Hypertension. Circ Res. 2021;128:993–1020. doi: 10.1161/CIRCRESAHA.121.318063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morawietz H. Endothelial NADPH oxidases: Friends or foes? Basic Res. Cardiol. 2011;106:521–525. doi: 10.1007/s00395-011-0188-6. [DOI] [PubMed] [Google Scholar]
- 46.Brandes R.P., Weissmann N., Schröder K. NADPH oxidases in cardiovascular disease. Free. Radic. Biol. Med. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 47.Kuzkaya N., Weissmann N., Harrison D.G., Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 48.Starkov A.A. The Role of Mitochondria in Reactive Oxygen Species Metabolism and Signaling. Ann. N. Y. Acad. Sci. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viel E.C., Benkirane K., Javeshghani D., Touyz R.M., Schiffrin E.L. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am. J. Physiol. Circ. Physiol. 2008;295:H281–H288. doi: 10.1152/ajpheart.00304.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brandes R.P., Weissmann N., Schröder K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free. Radic. Biol. Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 51.Schröder K. NADPH Oxidases in Redox Regulation of Cell Adhesion and Migration. Antioxid. Redox Signal. 2014;20:2043–2058. doi: 10.1089/ars.2013.5633. [DOI] [PubMed] [Google Scholar]
- 52.Takeya R., Ueno N., Kami K., Taura M., Kohjima M., Izaki T., Nunoi H., Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J. Biol. Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 53.Touyz R.M., Briones A.M., Sedeek M., Burger D., Montezano A.C. NOX Isoforms and Reactive Oxygen Species in Vascular Health. Mol. Interv. 2011;11:27–35. doi: 10.1124/mi.11.1.5. [DOI] [PubMed] [Google Scholar]
- 54.Rueckschloss U., Galle J., Holtz J., Zerkowski H.R., Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: Antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation. 2001;104:1767–1772. doi: 10.1161/hc4001.097056. [DOI] [PubMed] [Google Scholar]
- 55.Nabeebaccus A.A., Reumiller C.M., Shen J., Zoccarato A., Santos C.X., Shah A.M. The regulation of cardiac intermediary metabolism by NADPH oxidases. Cardiovasc. Res. 2023;118:3305–3319. doi: 10.1093/cvr/cvac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morawietz H. Cardiovascular protection by Nox4. Cardiovasc. Res. 2018;114:353–355. doi: 10.1093/cvr/cvx252. [DOI] [PubMed] [Google Scholar]
- 57.Langbein H., Brunssen C., Hofmann A., Cimalla P., Brux M., Bornstein S.R., Deussen A., Koch E., Morawietz H. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Eur. Heart J. 2016;37:1753–1761. doi: 10.1093/eurheartj/ehv564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schroeder K., Zhang M., Benkhoff S., Mieth A., Pliquett R., Kosowski J., Kruse C., Luedike P., Michaelis U.R., Weissmann N., et al. Nox4 Is a Protective Reactive Oxygen Species Generating Vascular NADPH Oxidase. Circ. Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 59.Paravicini T.M., Touyz R.M. NADPH oxidases, reactive oxygen species, and hypertension: Clinical implications and therapeutic possibilities. Diabetes Care. 2008;31:S170–S180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 60.Dikalova A., Clempus R., Lassègue B., Cheng G., McCoy J., Dikalov S., San Martin A., Lyle A., Weber D.S., Weiss D., et al. Nox1 Overexpression Potentiates Angiotensin II-Induced Hypertension and Vascular Smooth Muscle Hypertrophy in Transgenic Mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 61.Matsuno K., Yamada H., Iwata K., Jin D., Katsuyama M., Matsuki M., Takai S., Yamanishi K., Miyazaki M., Matsubara H., et al. Nox1 is involved in angiotensin II-mediated hypertension: A study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 62.Murdoch C.E., Alom-Ruiz S.P., Wang M., Zhang M., Walker S., Yu B., Brewer A., Shah A.M. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res. Cardiol. 2011;106:527–538. doi: 10.1007/s00395-011-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rueckschloss U., Quinn M.T., Holtz J., Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: Protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2002;22:1845–1851. doi: 10.1161/01.ATV.0000035392.38687.65. [DOI] [PubMed] [Google Scholar]
- 64.Dikalov S.I., Nazarewicz R.R., Bikineyeva A., Hilenski L., Lassègue B., Griendling K.K., Harrison D.G., Dikalova A.E. Nox2-Induced Production of Mitochondrial Superoxide in Angiotensin II-Mediated Endothelial Oxidative Stress and Hypertension. Antioxidants Redox Signal. 2014;20:281–294. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doughan A., Harrison D., Dikalov S. Molecular Mechanisms of Angiotensin II-Mediated Mitochondrial Dysfunction: Linking Mitochondrial Oxidative Damage and Vascular Endothelial Dysfunction. Circ. Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 66.Harrison C.B., Trevelin S.C., Richards D.A., Santos C.X., Sawyer G., Markovinovic A., Zhang X., Zhang M., Brewer A.C., Yin X., et al. Fibroblast Nox2 (NADPH Oxidase-2) Regulates ANG II (Angiotensin II)–Induced Vascular Remodeling and Hypertension via Paracrine Signaling to Vascular Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2021;41:698–710. doi: 10.1161/ATVBAHA.120.315322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michihara A., Oda A., Mido M. High Expression Levels of NADPH Oxidase 3 in the Cerebrum of Ten-Week-Old Stroke-Prone Spontaneously Hypertensive Rats. Biol. Pharm. Bull. 2016;39:252–258. doi: 10.1248/bpb.b15-00663. [DOI] [PubMed] [Google Scholar]
- 68.Yin C., Li K., Yu Y., Huang H., Yu Y., Wang Z., Yan J., Pu Y., Li Z., Li D., et al. Genome-wide association study identifies loci and candidate genes for non-idiopathic pulmonary hypertension in Eastern Chinese Han population. BMC Pulm. Med. 2018;18:158. doi: 10.1186/s12890-018-0719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Byon C.H., Heath J.M., Chen Y. Redox signaling in cardiovascular pathophysiology: A focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. 2016;9:244–253. doi: 10.1016/j.redox.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cowley A.W., Jr., Yang C., Zheleznova N.N., Staruschenko A., Kurth T., Rein L., Kumar V., Sadovnikov K., Dayton A., Hoffman M., et al. Evidence of the Importance of Nox4 in Production of Hypertension in Dahl Salt-Sensitive Rats. Hypertension. 2016;67:440–450. doi: 10.1161/HYPERTENSIONAHA.115.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumar V., Kurth T., Zheleznova N.N., Yang C., Cowley A.W., Jr. NOX4/H2O2/mTORC1 Pathway in Salt-Induced Hypertension and Kidney Injury. Hypertension. 2020;76:133–143. doi: 10.1161/HYPERTENSIONAHA.120.15058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montezano A.C., Tsiropoulou S., Dulak-Lis M., Harvey A., Camargo L.D.L., Touyz R.M. Redox signaling, Nox5 and vascular remodeling in hypertension. Curr. Opin. Nephrol. Hypertens. 2015;24:425–433. doi: 10.1097/MNH.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elbatreek M.H., Sadegh S., Anastasi E., Guney E., Nogales C., Kacprowski T., Hassan A.A., Teubner A., Huang P.-H., Hsu C.-Y., et al. NOX5-induced uncoupling of endothelial NO synthase is a causal mechanism and theragnostic target of an age-related hypertension endotype. PLoS Biol. 2020;18:e3000885. doi: 10.1371/journal.pbio.3000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martínez-Revelles S., García-Redondo A.B., Avendaño M.S., Varona S., Palao T., Orriols M., Roque F.R., Fortuño A., Touyz R.M., Martínez-González J., et al. Lysyl Oxidase Induces Vascular Oxidative Stress and Contributes to Arterial Stiffness and Abnormal Elastin Structure in Hypertension: Role of p38MAPK. Antioxid. Redox Signal. 2017;27:379–397. doi: 10.1089/ars.2016.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Araujo M., Wilcox C.S. Oxidative Stress in Hypertension: Role of the Kidney. Antioxid. Redox Signal. 2014;20:74–101. doi: 10.1089/ars.2013.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ratliff B.B., Abdulmahdi W., Pawar R., Wolin M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox Signal. 2016;25:119–146. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao L., Wang W., Li Y.-L., Schultz H.D., Liu D., Cornish K.G., Zucker I.H. Sympathoexcitation by central ANG II: Roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2271–H2279. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- 78.Chan S.H., Wu K.L., Chang A.Y., Tai M.-H., Chan J.Y. Oxidative Impairment of Mitochondrial Electron Transport Chain Complexes in Rostral Ventrolateral Medulla Contributes to Neurogenic Hypertension. Hypertension. 2009;53:217–227. doi: 10.1161/HYPERTENSIONAHA.108.116905. [DOI] [PubMed] [Google Scholar]
- 79.Youn J.-C., Yu H.T., Lim B.J., Koh M.J., Lee J., Chang D.-Y., Choi Y.S., Lee S.-H., Kang S.-M., Jang Y., et al. Immunosenescent CD8 + T Cells and C-X-C Chemokine Receptor Type 3 Chemokines Are Increased in Human Hypertension. Hypertension. 2013;62:126–133. doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 80.Abais-Battad J.M., Lund H., Dasinger J.H., Fehrenbach D.J., Cowley A.W., Jr., Mattson D.L. NOX2-derived reactive oxygen species in immune cells exacerbates salt-sensitive hypertension. Free. Radic. Biol. Med. 2020;146:333–339. doi: 10.1016/j.freeradbiomed.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malinouski M., Zhou Y., Belousov V.V., Hatfield D.L., Gladyshev V.N. Hydrogen peroxide probes directed to different cellular compartments. PLoS ONE. 2011;6:e14564. doi: 10.1371/journal.pone.0014564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panth N., Paudel K.R., Parajuli K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016;2016:9152732. doi: 10.1155/2016/9152732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dikalov S., Griendling K.K., Harrison D.G. Measurement of Reactive Oxygen Species in Cardiovascular Studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dikalova A.E., Bikineyeva A.T., Budzyn K., Nazarewicz R.R., McCann L., Lewis W., Harrison D.G., Dikalov S.I. Therapeutic Targeting of Mitochondrial Superoxide in Hypertension. Circ. Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang J., Liu K., Shi X., Swartz H. Detection of Short-Lived Free Radicals by Low-Frequency Electron Paramagnetic Resonance Spin Trapping in Whole Living Animals. Arch. Biochem. Biophys. 1995;319:570–573. doi: 10.1006/abbi.1995.1332. [DOI] [PubMed] [Google Scholar]
- 86.Arai H. Lipid Hydroperoxide-Derived Modification of Biomolecules. Vol. 77. Subcellular Biochemistry; Springer; Dordrecht, The Netherlands: 2014. Oxidative Modification of Lipoproteins; pp. 103–114. [DOI] [PubMed] [Google Scholar]
- 87.Lee R., Margaritis M., Channon K., Antoniades C. Evaluating Oxidative Stress in Human Cardiovascular Disease: Methodological Aspects and Considerations. Curr. Med. Chem. 2012;19:2504–2520. doi: 10.2174/092986712800493057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rodrigo R., Libuy M., Feliú F., Hasson D. Oxidative Stress-Related Biomarkers in Essential Hypertension and Ischemia-Reperfusion Myocardial Damage. Dis. Markers. 2013;35:773–790. doi: 10.1155/2013/974358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Asselin C., Shi Y., Clement R., Tardif J., Rosiers C.D. Higher circulating 4-hydroxynonenal–protein thioether adducts correlate with more severe diastolic dysfunction in spontaneously hypertensive rats. Redox Rep. 2007;12:68–72. doi: 10.1179/135100007X162202. [DOI] [PubMed] [Google Scholar]
- 90.Marrocco I., Altieri F., Peluso I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017;2017:6501046. doi: 10.1155/2017/6501046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dalle-Donne I., Giustarini D., Colombo R., Rossi R., Milzani A. Protein carbonylation in human diseases. Trends Mol. Med. 2003;9:169–176. doi: 10.1016/S1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 92.Pastore A., Piemonte F. Protein Glutathionylation in Cardiovascular Diseases. Int. J. Mol. Sci. 2013;14:20845–20876. doi: 10.3390/ijms141020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Packer L., Witt E.H., Tritschler H.J. Alpha-lipoic acid as a biological antioxidant. Free. Radic. Biol. Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-R. [DOI] [PubMed] [Google Scholar]
- 94.Frei B., Kim M.C., Ames B.N. Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc. Natl. Acad. Sci. USA. 1990;87:4879–4883. doi: 10.1073/pnas.87.12.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ulker S., McKeown P.P., Bayraktutan U. Vitamins Reverse Endothelial Dysfunction Through Regulation of eNOS and NAD(P)H Oxidase Activities. Hypertension. 2003;41:534–539. doi: 10.1161/01.HYP.0000057421.28533.37. [DOI] [PubMed] [Google Scholar]
- 96.Neuzil J., Thomas S.R., Stocker R. Requirement for, promotion, or inhibition by alpha-tocopherol of radical-induced initiation of plasma lipoprotein lipid peroxidation. Free Radic. Biol. Med. 1997;22:57–71. doi: 10.1016/S0891-5849(96)00224-9. [DOI] [PubMed] [Google Scholar]
- 97.Goszcz K., Duthie G.G., Stewart D., Leslie S.J., Megson I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017;174:1209–1225. doi: 10.1111/bph.13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Behl T., Bungau S., Kumar K., Zengin G., Khan F., Kumar A., Kaur R., Venkatachalam T., Tit D.M., Vesa C.M., et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020;130:110714. doi: 10.1016/j.biopha.2020.110714. [DOI] [PubMed] [Google Scholar]
- 99.Larson A., Witman M.A., Guo Y., Ives S., Richardson R.S., Bruno R.S., Jalili T., Symons J.D. Acute, quercetin-induced reductions in blood pressure in hypertensive individuals are not secondary to lower plasma angiotensin-converting enzyme activity or endothelin-1: Nitric oxide. Nutr. Res. 2012;32:557–564. doi: 10.1016/j.nutres.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 100.Marunaka Y., Marunaka R., Sun H., Yamamoto T., Kanamura N., Inui T., Taruno A. Actions of Quercetin, a Polyphenol, on Blood Pressure. Molecules. 2017;22:209. doi: 10.3390/molecules22020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakajima K., Niisato N., Marunaka Y. Quercetin stimulates NGF-induced neurite outgrowth in PC12 cells via activation of Na(+)/K(+)/2Cl(-) cotransporter. Cell. Physiol. Biochem. 2011;28:147–156. doi: 10.1159/000331723. [DOI] [PubMed] [Google Scholar]
- 102.Ye X., Tang X., Li F., Zhu J., Wu M., Wei X., Wang Y. Green and Oolong Tea Extracts With Different Phytochemical Compositions Prevent Hypertension and Modulate the Intestinal Flora in a High-Salt Diet Fed Wistar Rats. Front. Nutr. 2022;9:892801. doi: 10.3389/fnut.2022.892801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boots A.W., Kubben N., Haenen G., Bast A. Oxidized quercetin reacts with thiols rather than with ascorbate: Implication for quercetin supplementation. Biochem. Biophys. Res. Commun. 2003;308:560–565. doi: 10.1016/S0006-291X(03)01438-4. [DOI] [PubMed] [Google Scholar]
- 104.Chen B., Lu Y., Chen Y., Cheng J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015;225:R83–R99. doi: 10.1530/JOE-14-0662. [DOI] [PubMed] [Google Scholar]
- 105.Wang C., Luo Z., Carter G., Wellstein A., Jose P.A., Tomlinson J., Leiper J., Welch W.J., Wilcox C.S., Wang D. NRF2 prevents hypertension, increased ADMA, microvascular oxidative stress, and dysfunction in mice with two weeks of ANG II infusion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;314:R399–R406. doi: 10.1152/ajpregu.00122.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shay K.P., Moreau R.F., Smith E.J., Smith A.R., Hagen T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta. 2009;1790:1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tibullo D., Volti G.L., Giallongo C., Grasso S., Tomassoni D., Anfuso C.D., Lupo G., Amenta F., Avola R., Bramanti V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. 2017;66:947–959. doi: 10.1007/s00011-017-1079-6. [DOI] [PubMed] [Google Scholar]
- 108.Pedre B., Barayeu U., Ezeriņa D., Dick T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species. Pharmacol. Ther. 2021;228:107916. doi: 10.1016/j.pharmthera.2021.107916. [DOI] [PubMed] [Google Scholar]
- 109.Tian N., Rose R.A., Jordan S., Dwyer T.M., Hughson M.D., Manning R.D., Jr. N-Acetylcysteine improves renal dysfunction, ameliorates kidney damage and decreases blood pressure in salt-sensitive hypertension. J. Hypertens. 2006;24:2263–2270. doi: 10.1097/01.hjh.0000249705.42230.73. [DOI] [PubMed] [Google Scholar]
- 110.Song D., Hutchings S., Pang C.C. Chronic N-acetylcysteine prevents fructose-induced insulin resistance and hypertension in rats. Eur. J. Pharmacol. 2005;508:205–210. doi: 10.1016/j.ejphar.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 111.Tain Y.L., Lee C.T., Chan J.Y., Hsu C.N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal N(G)-Nitro-L-arginine-methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016;215:636.e1–636.e72. doi: 10.1016/j.ajog.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 112.Hsu C.-N., Hou C.-Y., Chang-Chien G.-P., Lin S., Tain Y.-L. Maternal N-Acetylcysteine Therapy Prevents Hypertension in Spontaneously Hypertensive Rat Offspring: Implications of Hydrogen Sulfide-Generating Pathway and Gut Microbiota. Antioxidants. 2020;9:856. doi: 10.3390/antiox9090856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Girouard H., Chulak C., LeJossec M., Lamontagne D., de Champlain J. Chronic antioxidant treatment improves sympathetic functions and beta-adrenergic pathway in the spontaneously hypertensive rats. J. Hypertens. 2003;21:179–188. doi: 10.1097/00004872-200301000-00028. [DOI] [PubMed] [Google Scholar]
- 114.Girouard H., Chulak C., Wu L., LeJossec M., De Champlain J. N-acetylcysteine improves nitric oxide and α-adrenergic pathways in mesenteric beds of spontaneously hypertensive rats. Am. J. Hypertens. 2003;16:577–584. doi: 10.1016/S0895-7061(03)00863-X. [DOI] [PubMed] [Google Scholar]
- 115.Kizhakekuttu T.J., Widlansky M.E. Natural Antioxidants and Hypertension: Promise and Challenges. Cardiovasc. Ther. 2010;28:e20–e32. doi: 10.1111/j.1755-5922.2010.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gao H.-L., Yu X.-J., Qi J., Yi Q.-Y., Jing W.-H., Sun W.-Y., Cui W., Mu J.-J., Yuan Z.-Y., Zhao X.-F., et al. Oral CoQ10 attenuates high salt-induced hypertension by restoring neurotransmitters and cytokines in the hypothalamic paraventricular nucleus. Sci. Rep. 2016;6:30301. doi: 10.1038/srep30301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Graham D., Huynh N.N., Hamilton C.A., Beattie E., Smith R.A., Cochemé H.M., Murphy M.P., Dominiczak A.F. Mitochondria-Targeted Antioxidant MitoQ10 Improves Endothelial Function and Attenuates Cardiac Hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 118.Overvad K., Diamant B., Holm L., Hølmer G., Mortensen S.A., Stender S. Coenzyme Q10 in health and disease. Eur. J. Clin. Nutr. 1999;53:764–770. doi: 10.1038/sj.ejcn.1600880. [DOI] [PubMed] [Google Scholar]
- 119.Langsjoen P., Willis R., Folkers K. Treatment of essential hypertension with Coenzyme Q10. Mol. Asp. Med. 1994;15((Suppl. 1)):s265–s272. doi: 10.1016/0098-2997(94)90037-X. [DOI] [PubMed] [Google Scholar]
- 120.Digiesi V., Cantini F., Oradei A., Bisi G., Guarino G., Brocchi A., Bellandi F., Mancini M., Littarru G. Coenzyme Q10 in essential hypertension. Mol. Asp. Med. 1994;15:s257–s263. doi: 10.1016/0098-2997(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 121.Burke B.E., Neuenschwander R., Olson R.D. Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in isolated systolic hypertension. South. Med. J. 2001;94:1112–1117. doi: 10.1097/00007611-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 122.Baker G.L.M., Corry R.J.M., Autor A.P. Oxygen Free Radical Induced Damage in Kidneys Subjected to Warm Ischemia and Reperfusion. Ann. Surg. 1985;202:628–641. doi: 10.1097/00000658-198511000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jolly S.R., Kane W.J., Bailie M.B., Abrams G.D., Lucchesi B.R. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ. Res. 1984;54:277–285. doi: 10.1161/01.RES.54.3.277. [DOI] [PubMed] [Google Scholar]
- 124.Nunes D.V., Costa C.A., De Bem G.F., Cordeiro V.S., Santos I.B., Carvalho L.C., Jordão A.K., Cunha A.C., Ferreira V.F., Moura R.S., et al. Tempol, a superoxide dismutase-mimetic drug, prevents chronic ischemic renal injury in two-kidney, one-clip hypertensive rats. Clin. Exp. Hypertens. 2018;40:721–729. doi: 10.1080/10641963.2018.1425423. [DOI] [PubMed] [Google Scholar]
- 125.Onuma S., Nakanishi K. Superoxide dismustase mimetic tempol decreases blood pressure by increasing renal medullary blood flow in hyperinsulinemic-hypertensive rats. Metabolism. 2004;53:1305–1308. doi: 10.1016/j.metabol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 126.Park J.B., Touyz R.M., Chen X., Schiffrin E.L. Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Pt 1Am. J. Hypertens. 2002;15:78–84. doi: 10.1016/S0895-7061(01)02233-6. [DOI] [PubMed] [Google Scholar]
- 127.Savalia K., Manickam D.S., Rosenbaugh E.G., Tian J., Ahmad I.M., Kabanov A.V., Zimmerman M.C. Neuronal uptake of nanoformulated superoxide dismutase and attenuation of angiotensin II-dependent hypertension after central administration. Free Radic. Biol. Med. 2014;73:299–307. doi: 10.1016/j.freeradbiomed.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dikalova A.E., Itani H.A., Nazarewicz R.R., McMaster W.G., Flynn C.R., Uzhachenko R., Fessel J.P., Gamboa J.L., Harrison D.G., Dikalov S.I., et al. Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ. Res. 2017;121:564–574. doi: 10.1161/CIRCRESAHA.117.310933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Capettini L.S., Montecucco F., Mach F., Stergiopulos N., Santos R.A., Da Silva R.F. Role of Renin-Angiotensin System in Inflammation, Immunity and Aging. Curr. Pharm. Des. 2012;18:963–970. doi: 10.2174/138161212799436593. [DOI] [PubMed] [Google Scholar]
- 130.Tao R., Vassilopoulos A., Parisiadou L., Yan Y., Gius D. Regulation of MnSOD Enzymatic Activity by Sirt3 Connects the Mitochondrial Acetylome Signaling Networks to Aging and Carcinogenesis. Antioxid. Redox Signal. 2014;20:1646–1654. doi: 10.1089/ars.2013.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Diaba-Nuhoho P., Cour M., Hadebe N., Marais D., Lecour S., Blackhurst D. Chronic and moderate consumption of reduced-alcohol wine confers cardiac benefits in a rat model of pulmonary arterial hypertension. BMC Res. Notes. 2021;14:324. doi: 10.1186/s13104-021-05738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chin H.J., Song Y.R., Kim H.S., Park M., Yoon H.J., Na K.Y., Kim Y., Chae D.-W., Kim S. The Bilirubin Level is Negatively Correlated with the Incidence of Hypertension in Normotensive Korean Population. J. Korean Med. Sci. 2009;24((Suppl. 1)):S50–S56. doi: 10.3346/jkms.2009.24.S1.S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Joles J.A., Wesseling S., Braam B. Renal glutathione S-transferase mu type 1 expression is already reduced in new-born spontaneously hypertensive rats. J. Hypertens. 2010;28:633–634. doi: 10.1097/HJH.0b013e328335fa99. [DOI] [PubMed] [Google Scholar]
- 134.Li Z., Chen J., Zhang D. Association between dietary carotenoid intakes and hypertension in adults: National Health and Nutrition Examination Survey 2007–2014. J. Hypertens. 2019;37:2371–2379. doi: 10.1097/HJH.0000000000002200. [DOI] [PubMed] [Google Scholar]
- 135.Schiffrin E.L. Antioxidants in Hypertension and Cardiovascular Disease. Mol. Interv. 2010;10:354–362. doi: 10.1124/mi.10.6.4. [DOI] [PubMed] [Google Scholar]
- 136.Jenkins D.J.A., Spence J.D., Giovannucci E.L., Kim Y.-I., Josse R., Vieth R., Blanco Mejia S., Viguiliouk E., Nishi S., Sahye-Pudaruth S., et al. Supplemental Vitamins and Minerals for CVD Prevention and Treatment. J. Am. Coll. Cardiol. 2018;71:2570–2584. doi: 10.1016/j.jacc.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 137.Jialal I., Devaraj S. Antioxidants and atherosclerosis: Don’t throw out the baby with the bath water. Circulation. 2003;107:926–928. doi: 10.1161/01.CIR.0000048966.26216.4C. [DOI] [PubMed] [Google Scholar]
- 138.Czernichow S., Bertrais S., Blacher J., Galan P., Briançon S., Favier A., Safar M., Hercberg S. Effect of supplementation with antioxidants upon long-term risk of hypertension in the SU.VI.MAX study: Association with plasma antioxidant levels. J. Hypertens. 2005;23:2013–2018. doi: 10.1097/01.hjh.0000187259.94448.8a. [DOI] [PubMed] [Google Scholar]
- 139.Kalpdev A., Saha S.C., Dhawan V. Vitamin C and E Supplementation Does Not Reduce the Risk of Superimposed PE in Pregnancy. Hypertens. Pregnancy. 2011;30:447–456. doi: 10.3109/10641955.2010.507840. [DOI] [PubMed] [Google Scholar]
- 140.Marques B., Trindade M., Aquino J.C.F., Cunha A.R., Gismondi R.O., Neves M.F., Oigman W. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin. Exp. Hypertens. 2018;40:218–223. doi: 10.1080/10641963.2017.1288741. [DOI] [PubMed] [Google Scholar]
- 141.Kirch N., Berk L., Liegl Y., Adelsbach M., Zimmermann B.F., Stehle P., Stoffel-Wagner B., Ludwig N., Schieber A., Helfrich H.-P., et al. A nutritive dose of pure (-)-epicatechin does not beneficially affect increased cardiometabolic risk factors in overweight-to-obese adults-a randomized, placebo-controlled, double-blind crossover study. Am. J. Clin. Nutr. 2018;107:948–956. doi: 10.1093/ajcn/nqy066. [DOI] [PubMed] [Google Scholar]
- 142.Young J.M., Florkowski C.M., Molyneux S., McEwan R.G., Frampton C.M., Nicholls M.G., Scott R.S., George P.M. A Randomized, Double-Blind, Placebo-Controlled Crossover Study of Coenzyme Q10 Therapy in Hypertensive Patients With the Metabolic Syndrome. Am. J. Hypertens. 2012;25:261–270. doi: 10.1038/ajh.2011.209. [DOI] [PubMed] [Google Scholar]
- 143.Sharifi-Rad M., Anil Kumar N.V., Zucca P., Varoni E.M., Dini L., Panzarini E., Rajkovic J., Tsouh Fokou P.V., Azzini E., Peluso I., et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roberts L.J., 2nd, Oates J.A., Linton M.F., Fazio S., Meador B.P., Gross M.D., Shyr Y., Morrow J.D. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic. Biol. Med. 2007;43:1388–1393. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fernández-García E., Carvajal-Lérida I., Jarén-Galán M., Garrido-Fernández J., Pérez-Gálvez A., Hornero-Méndez D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012;46:438–450. doi: 10.1016/j.foodres.2011.06.007. [DOI] [Google Scholar]
- 146.Fortuño A., Bidegain J., Robador P., Hermida J., López-Sagaseta J., Beloqui O., Díez J., Zalba G. Losartan metabolite EXP3179 blocks NADPH oxidase-mediated superoxide production by inhibiting protein kinase C: Potential clinical implications in hypertension. Hypertension. 2009;54:744–750. doi: 10.1161/HYPERTENSIONAHA.109.129353. [DOI] [PubMed] [Google Scholar]
- 147.Montezano A.C., Touyz R.M. Oxidative stress, Noxs, and hypertension: Experimental evidence and clinical controversies. Ann. Med. 2012;44((Suppl. 1)):S2–S16. doi: 10.3109/07853890.2011.653393. [DOI] [PubMed] [Google Scholar]
- 148.Appel L.J., Moore T.J., Obarzanek E., Vollmer W.M., Svetkey L.P., Sacks F.M., Bray G.A., Vogt T.M., Cutler J.A., Windhauser M.M., et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 149.Sacks F.M., Svetkey L.P., Vollmer W.M., Appel L.J., Bray G.A., Harsha D., Obarzanek E., Conlin P.R., Miller E.R., Simons-Morton D.G., et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N. Engl. J. Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 150.Du H., Li L., Bennett D., Guo Y., Key T.J., Bian Z., Sherliker P., Gao H., Chen Y., Yang L., et al. Fresh Fruit Consumption and Major Cardiovascular Disease in China. New Engl. J. Med. 2016;374:1332–1343. doi: 10.1056/NEJMoa1501451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yoshioka M., Aoyama K., Matsushita T. Effects of ascorbic acid on blood pressure and ascorbic acid metabolism in spontaneously hypertensive rats (SH rats) Int. J. Vitam. Nutr. Res. 1985;55:301–307. [PubMed] [Google Scholar]
- 152.Vasdev S., Ford C., Parai S., Longerich L., Gadag V. Dietary vitamin C supplementation lowers blood pressure in spontaneously hypertensive rats. Mol. Cell. Biochem. 2001;218:97–103. doi: 10.1023/A:1007234027421. [DOI] [PubMed] [Google Scholar]
- 153.Ettarh R.R., Odigie I.P., Adigun S.A. Vitamin C lowers blood pressure and alters vascular responsiveness in salt-induced hypertension. Can. J. Physiol. Pharmacol. 2002;80:1199–1202. doi: 10.1139/y02-147. [DOI] [PubMed] [Google Scholar]
- 154.Nishi E., Campos R.R., Bergamaschi C., De Almeida V.R., Ribeiro D.A. Vitamin C prevents DNA damage induced by renovascular hypertension in multiple organs of Wistar rats. Hum. Exp. Toxicol. 2010;29:593–599. doi: 10.1177/0960327109358267. [DOI] [PubMed] [Google Scholar]
- 155.Chen X., Touyz R.M., Park J.B., Schiffrin E.L. Antioxidant Effects of Vitamins C and E Are Associated With Altered Activation of Vascular NADPH Oxidase and Superoxide Dismutase in Stroke-Prone SHR. Hypertension. 2001;38:606–611. doi: 10.1161/hy09t1.094005. [DOI] [PubMed] [Google Scholar]
- 156.Duffy S., Gokce N., Holbrook M., Huang A., Frei B., Keaney J.F., Jr., Vita J.A. Treatment of hypertension with ascorbic acid. Lancet. 1999;354:2048–2049. doi: 10.1016/S0140-6736(99)04410-4. [DOI] [PubMed] [Google Scholar]
- 157.Plantinga Y., Ghiadoni L., Magagna A., Giannarelli C., Franzoni F., Taddei S., Salvetti A. Supplementation With Vitamins C and E Improves Arterial Stiffness and Endothelial Function in Essential Hypertensive Patients. Am. J. Hypertens. 2007;20:392–397. doi: 10.1016/j.amjhyper.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 158.Rodrigo R., Prat H., Passalacqua W., Araya J., Bächler J.P. Decrease in oxidative stress through supplementation of vitamins C and E is associated with a reduction in blood pressure in patients with essential hypertension. Clin. Sci. 2008;114:625–634. doi: 10.1042/CS20070343. [DOI] [PubMed] [Google Scholar]
- 159.Hajjar I.M., George V., Sasse E.A., Kochar M.S. A Randomized, Double-Blind, Controlled Trial of Vitamin C in the Management of Hypertension and Lipids. Am. J. Ther. 2002;9:289–293. doi: 10.1097/00045391-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 160.Ghosh S., Ekpo E., Shah I., Girling A., Jenkins C., Sinclair A. A Double-Blind, Placebo-Controlled Parallel Trial of Vitamin C Treatment in Elderly Patients with Hypertension. Gerontology. 1994;40:268–272. doi: 10.1159/000213595. [DOI] [PubMed] [Google Scholar]
- 161.Duffy S.J., Gokce N., Holbrook M., Hunter L.M., Biegelsen E.S., Huang A., Keaney J., Vita J. Effect of ascorbic acid treatment on conduit vessel endothelial dysfunction in patients with hypertension. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H528–H534. doi: 10.1152/ajpheart.2001.280.2.H528. [DOI] [PubMed] [Google Scholar]
- 162.Solzbach U., Hornig B., Jeserich M., Just H. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation. 1997;96:1513–1519. doi: 10.1161/01.CIR.96.5.1513. [DOI] [PubMed] [Google Scholar]
- 163.Fotherby M.D., Williams J.C., Forster L.A., Craner P., Ferns G.A. Effect of vitamin C on ambulatory blood pressure and plasma lipids in older persons. J. Hypertens. 2000;18:411–415. doi: 10.1097/00004872-200018040-00009. [DOI] [PubMed] [Google Scholar]
- 164.Guan Y., Dai P., Wang H. Effects of vitamin C supplementation on essential hypertension: A systematic review and meta-analysis. Medicine. 2020;99:e19274. doi: 10.1097/MD.0000000000019274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Newaz M.A., Nawal N., Rohaizan C., Muslim N., Gapor A. ?-tocopherol increased nitric oxide synthase activity in blood vessels of spontaneously hypertensive rats. Pt 1Am. J. Hypertens. 1999;12:839–844. doi: 10.1016/S0895-7061(99)00022-9. [DOI] [PubMed] [Google Scholar]
- 166.Pezeshk A., Dalhouse A.D. Vitamin E, membrane fluidity, and blood pressure in hypertensive and normotensive rats. Life Sci. 2000;67:1881–1889. doi: 10.1016/S0024-3205(00)00775-X. [DOI] [PubMed] [Google Scholar]
- 167.Vasdev S., Gill V., Parai S., Longerich L., Gadag V. Dietary vitamin E supplementation lowers blood pressure in spontaneously hypertensive rats. Mol. Cell. Biochem. 2002;238:111–117. doi: 10.1023/A:1019915306581. [DOI] [PubMed] [Google Scholar]
- 168.Atarashi K., Ishiyama A., Takagi M., Minami M., Kimura K., Goto A., Omata M. Vitamin E ameliorates the renal injury of Dahl salt-sensitive rats. Pt 2Am. J. Hypertens. 1997;10:116S–119S. doi: 10.1016/S0895-7061(97)00088-5. [DOI] [PubMed] [Google Scholar]
- 169.Vasdev S., Gill V., Parai S., Gadag V. Dietary Vitamin E Supplementation Attenuates Hypertension in Dahl Salt-Sensitive Rats. J. Cardiovasc. Pharmacol. Ther. 2005;10:103–111. doi: 10.1177/107424840501000204. [DOI] [PubMed] [Google Scholar]
- 170.Noguchi T., Ikeda K., Sasaki Y., Yamamoto J., Yamori Y. Effects of vitamin E and sesamin on hypertension and cerebral thrombogenesis in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2004;31((Suppl. 2)):S24–S26. doi: 10.1111/j.1440-1681.2004.04103.x. [DOI] [PubMed] [Google Scholar]
- 171.Iino K., Abe K., Kariya S., Kimura H., Kusaba T., Kusunoki R., Saku J., Soejima K., Nakakura S., Nakamura I., et al. A Controlled, Double-Blind Study of dl-Alpha-Tocopheryl Nicotinate (Juvela-Nicotinate®) for Treatment of Symptoms in Hypertension and Cerebral Arteriosclerosis. Jpn. Heart J. 1977;18:277–286. doi: 10.1536/ihj.18.277. [DOI] [PubMed] [Google Scholar]
- 172.Boshtam M., Rafiei M., Sadeghi K., Sarrafzadegan N. Vitamin E can Reduce Blood Pressure in Mild Hypertensives. Int. J. Vitam. Nutr. Res. 2002;72:309–314. doi: 10.1024/0300-9831.72.5.309. [DOI] [PubMed] [Google Scholar]
- 173.Jessup J.V., Horne C., Yarandi H., Quindry J. The effects of endurance exercise and vitamin E on oxidative stress in the elderly. Biol. Res. Nurs. 2003;5:47–55. doi: 10.1177/1099800403005001005. [DOI] [PubMed] [Google Scholar]
- 174.Vasdev S., Gill V., Parai S., Longerich L., Gadag V. Dietary vitamin E and C supplementation prevents fructose induced hypertension in rats. Mol. Cell. Biochem. 2002;241:107–114. doi: 10.1023/A:1020835229591. [DOI] [PubMed] [Google Scholar]
- 175.Seifi B., Kadkhodaee M., Karimian S.M., Zahmatkesh M., Shams S., Bakhshi E. Reduction of kidney damage by supplementation of vitamins C and E in rats with deoxycorticosterone-salt-induced hypertension. Iran. J. Kidney Dis. 2009;3:197–202. [PubMed] [Google Scholar]
- 176.Xu J., Su L., Chen L., Lin J. Protection from vascular endothelial dysfunction in acute glycemic load-induced primary hypertension by vitamin C and E. Genet. Mol. Res. 2014;13:7246–7255. doi: 10.4238/2014.September.5.9. [DOI] [PubMed] [Google Scholar]
- 177.Javkhedkar A.A., Quiroz Y., Rodriguez-Iturbe B., Vaziri N.D., Lokhandwala M.F., Banday A.A. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:R840–R846. doi: 10.1152/ajpregu.00308.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bhatt S.R., Lokhandwala M.F., Banday A.A. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur. J. Pharmacol. 2011;667:258–264. doi: 10.1016/j.ejphar.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 179.Castrejón-Téllez V., Villegas-Romero M., Rubio-Ruiz M.E., Pérez-Torres I., Carreón-Torres E., Díaz-Díaz E., Guarner-Lans V. Effect of a Resveratrol/Quercetin Mixture on the Reversion of Hypertension Induced by a Short-Term Exposure to High Sucrose Levels Near Weaning and a Long-Term Exposure That Leads to Metabolic Syndrome in Rats. Int. J. Mol. Sci. 2020;21:2231. doi: 10.3390/ijms21062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Prysyazhna O., Wolhuter K., Switzer C., Santos C., Yang X., Lynham S., Shah A., Eaton P., Burgoyne J. Blood Pressure-Lowering by the Antioxidant Resveratrol Is Counterintuitively Mediated by Oxidation of cGMP-Dependent Protein Kinase. Circulation. 2019;140:126–137. doi: 10.1161/CIRCULATIONAHA.118.037398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cheng P.-W., Lee H.-C., Lu P.-J., Chen H.-H., Lai C.-C., Sun G.-C., Yeh T.-C., Hsiao M., Lin Y.-T., Liu C.-P., et al. Resveratrol Inhibition of Rac1-Derived Reactive Oxygen Species by AMPK Decreases Blood Pressure in a Fructose-Induced Rat Model of Hypertension. Sci. Rep. 2016;6:25342. doi: 10.1038/srep25342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Franco J.G., Lisboa P.C., Lima N.S., Amaral T.A., Peixoto-Silva N., Resende A.C., Oliveira E., Passos M.C., Moura E.G. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. J. Nutr. Biochem. 2013;24:960–966. doi: 10.1016/j.jnutbio.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 183.Galisteo M., García-Saura M.F., Jiménez R., Villar I.C., Wangensteen R., Zarzuelo A., Vargas F., Duarte J. Effects of Quercetin Treatment on Vascular Function in Deoxycorticosterone Acetate-Salt Hypertensive Rats. Comparative Study with Verapamil. Planta Med. 2004;70:334–341. doi: 10.1055/s-2004-818945. [DOI] [PubMed] [Google Scholar]
- 184.Duarte J., Jimenez R., O’Valle F., Galisteo M., Pérez-Palencia R., Vargas F., Perez-Vizcaino F., Zarzuelo A., Tamargo J. Protective effects of the flavonoid quercetin in chronic nitric oxide deficient rats. J. Hypertens. 2002;20:1843–1854. doi: 10.1097/00004872-200209000-00031. [DOI] [PubMed] [Google Scholar]
- 185.Sánchez M., Galisteo M., Vera R., Villar I.C., Zarzuelo A., Tamargo J., Pérez-Vizcaíno F., Duarte J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 2006;24:75–84. doi: 10.1097/01.hjh.0000198029.22472.d9. [DOI] [PubMed] [Google Scholar]
- 186.Edwards R.L., Lyon T., Litwin S.E., Rabovsky A., Symons J.D., Jalili T. Quercetin Reduces Blood Pressure in Hypertensive Subjects. J. Nutr. 2007;137:2405–2411. doi: 10.1093/jn/137.11.2405. [DOI] [PubMed] [Google Scholar]
- 187.Serban M.C., Sahebkar A., Zanchetti A., Mikhailidis D.P., Howard G., Antal D., Andrica F., Ahmed A., Aronow W.S., Muntner P., et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016;5:e002713. doi: 10.1161/JAHA.115.002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Egert S., Bosy-Westphal A., Seiberl J., Kürbitz C., Settler U., Plachta-Danielzik S., Wagner A.E., Frank J., Schrezenmeir J., Rimbach G., et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009;102:1065–1074. doi: 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- 189.Zahedi M., Ghiasvand R., Feizi A., Asgari G., Darvish L. Does Quercetin Improve Cardiovascular Risk factors and Inflammatory Biomarkers in Women with Type 2 Diabetes: A Double-blind Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2013;4:777–785. [PMC free article] [PubMed] [Google Scholar]
- 190.Tain Y.-L., Hsu C.-N., Huang L.-T., Lau Y.-T. Apocynin attenuates oxidative stress and hypertension in young spontaneously hypertensive rats independent of ADMA/NO pathway. Free Radic. Res. 2012;46:68–76. doi: 10.3109/10715762.2011.639069. [DOI] [PubMed] [Google Scholar]
- 191.Perassa L.A., Graton M.E., Potje S.R., Troiano J.A., Lima M.S., Vale G.T., Pereira A.A.F., Nakamune A.C.M.S., Sumida D.H., Tirapelli C., et al. Apocynin reduces blood pressure and restores the proper function of vascular endothelium in SHR. Vasc. Pharmacol. 2016;87:38–48. doi: 10.1016/j.vph.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 192.Patil B.M., Unger B.S. Apocynin improves endothelial function and prevents the development of hypertension in fructose fed rat. Indian J. Pharmacol. 2009;41:208–212. doi: 10.4103/0253-7613.58508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Virdis A., Neves M.F., Amiri F., Touyz R.M., Schiffrin E.L. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J. Hypertens. 2004;22:535–542. doi: 10.1097/00004872-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 194.Beswick R.A., Dorrance A.M., Leite R., Webb R.C. NADH/NADPH Oxidase and Enhanced Superoxide Production in the Mineralocorticoid Hypertensive Rat. Hypertension. 2001;38:1107–1111. doi: 10.1161/hy1101.093423. [DOI] [PubMed] [Google Scholar]
- 195.Hu L., Zhang Y., Lim P.S., Miao Y., Tan C., McKenzie K.U., Schyvens C.G., Whitworth J.A. Apocynin but Not l-Arginine Prevents and Reverses Dexamethasone-Induced Hypertension in the Rat. Am. J. Hypertens. 2006;19:413–418. doi: 10.1016/j.amjhyper.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 196.Potenza M.A., Marasciulo F.L., Tarquinio M., Tiravanti E., Colantuono G., Federici A., Kim J.-A., Quon M.J., Montagnani M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1378–E1387. doi: 10.1152/ajpendo.00698.2006. [DOI] [PubMed] [Google Scholar]
- 197.Negishi H., Xu J.-W., Ikeda K., Njelekela M., Nara Y., Yamori Y. Black and Green Tea Polyphenols Attenuate Blood Pressure Increases in Stroke-Prone Spontaneously Hypertensive Rats. J. Nutr. 2004;134:38–42. doi: 10.1093/jn/134.1.38. [DOI] [PubMed] [Google Scholar]
- 198.Peng X., Zhou R., Wang B., Yu X., Yang X., Liu K., Mi M. Effect of green tea consumption on blood pressure: A meta-analysis of 13 randomized controlled trials. Sci. Rep. 2014;4:6251. doi: 10.1038/srep06251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xu R., Yang K., Ding J., Chen G. Effect of green tea supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Medicine. 2020;99:e19047. doi: 10.1097/MD.0000000000019047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li G., Zhang Y., Thabane L., Mbuagbaw L., Liu A., Levine M.A., Holbrook A. Effect of green tea supplementation on blood pressure among overweight and obese adults: A systematic review and meta-analysis. J. Hypertens. 2015;33:243–254. doi: 10.1097/HJH.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 201.Mahdavi-Roshan M., Salari A., Ghorbani Z., Ashouri A. The effects of regular consumption of green or black tea beverage on blood pressure in those with elevated blood pressure or hypertension: A systematic review and meta-analysis. Complement. Ther. Med. 2020;51:102430. doi: 10.1016/j.ctim.2020.102430. [DOI] [PubMed] [Google Scholar]
- 202.Jackson D., Connolly K., Batacan R., Ryan K., Vella R., Fenning A. (−)-Epicatechin Reduces Blood Pressure and Improves Left Ventricular Function and Compliance in Deoxycorticosterone Acetate-Salt Hypertensive Rats. Molecules. 2018;23:1511. doi: 10.3390/molecules23071511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gómez-Guzmán M., Jiménez R., Sánchez M., Zarzuelo M.J., Galindo P., Quintela A.M., López-Sepúlveda R., Romero M., Tamargo J., Vargas F., et al. Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension. Free. Radic. Biol. Med. 2012;52:70–79. doi: 10.1016/j.freeradbiomed.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 204.Kluknavsky M., Balis P., Skratek M., Manka J., Bernatova I. (–)-Epicatechin Reduces the Blood Pressure of Young Borderline Hypertensive Rats During the Post-Treatment Period. Antioxidants. 2020;9:96. doi: 10.3390/antiox9020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Litterio M.C., Prieto M.A.V., Adamo A.M., Elesgaray R., Oteiza P.I., Galleano M., Fraga C.G. (−)-Epicatechin reduces blood pressure increase in high-fructose-fed rats: Effects on the determinants of nitric oxide bioavailability. J. Nutr. Biochem. 2015;26:745–751. doi: 10.1016/j.jnutbio.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 206.Gómez-Guzmán M., Jiménez R., Sánchez M., Romero M., O’Valle F., Lopez-Sepulveda R., Quintela A.M., Galindo P., Zarzuelo M.J., Bailón E., et al. Chronic (−)-epicatechin improves vascular oxidative and inflammatory status but not hypertension in chronic nitric oxide-deficient rats. Br. J. Nutr. 2011;106:1337–1348. doi: 10.1017/S0007114511004314. [DOI] [PubMed] [Google Scholar]
- 207.Galleano M., Bernatova I., Puzserova A., Balis P., Sestakova N., Pechanova O., Fraga C.G. (-)-Epicatechin reduces blood pressure and improves vasorelaxation in spontaneously hypertensive rats by NO-mediated mechanism. IUBMB Life. 2013;65:710–715. doi: 10.1002/iub.1185. [DOI] [PubMed] [Google Scholar]
- 208.Ellinger S., Reusch A., Stehle P., Helfrich H.-P. Epicatechin ingested via cocoa products reduces blood pressure in humans: A nonlinear regression model with a Bayesian approach. Am. J. Clin. Nutr. 2012;95:1365–1377. doi: 10.3945/ajcn.111.029330. [DOI] [PubMed] [Google Scholar]
- 209.Hooper L., A Kroon P., Rimm E.B., Cohn J.S., Harvey I., Le Cornu K.A., Ryder J.J., Hall W.L., Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 210.Taubert D., Roesen R., Lehmann C., Jung N., Schomig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA. 2007;298:49–60. doi: 10.1001/jama.298.1.49. [DOI] [PubMed] [Google Scholar]
- 211.Vasdev S., Mian T., Ford C.A., Longerich L., Parai S. Role of endogenous aldehydes in spontaneously hypertensive and disulfiram-induced hypertensive rats. Nutr. Metab. Cardiovasc. Dis. 1996;6:130–140. [Google Scholar]
- 212.Cabassi A., Dumont E.C., Girouard H., Bouchard J.-F., Le Jossec M., Lamontagne D., Besner J.-G., de Champlain J. Effects of chronic N-acetylcysteine treatment on the actions of peroxynitrite on aortic vascular reactivity in hypertensive rats. J. Hypertens. 2001;19:1233–1244. doi: 10.1097/00004872-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 213.Girouard H., Denault C., Chulak C., de Champlain J. Treatment by N-acetylcysteine and melatonin increases cardiac baroreflex and improves antioxidant reserve. Am. J. Hypertens. 2004;17:947–954. doi: 10.1016/j.amjhyper.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 214.Vasdev S., Ford C.A., Longerich L., Gadag V., Wadhawan S. Role of aldehydes in fructose induced hypertension. Mol. Cell. Biochem. 1998;181:1–9. doi: 10.1023/A:1006844222963. [DOI] [PubMed] [Google Scholar]
- 215.Bourraindeloup M., Adamy C., Candiani G., Cailleret M., Bourin M.-C., Badoual T., Su J.B., Adubeiro S., Roudot-Thoraval F., Dubois-Rande J.-L., et al. N -Acetylcysteine Treatment Normalizes Serum Tumor Necrosis Factor-α Level and Hinders the Progression of Cardiac Injury in Hypertensive Rats. Circulation. 2004;110:2003–2009. doi: 10.1161/01.CIR.0000143630.14515.7C. [DOI] [PubMed] [Google Scholar]
- 216.Barrios V., Calderón A., Navarro-Cid J., Lahera V., Ruilope L.M. N -Acetylcysteine Potentiates the Antihypertensive Effect of ACE Inhibitors in Hypertensive Patients. Blood Press. 2002;11:235–239. doi: 10.1080/08037050213760. [DOI] [PubMed] [Google Scholar]
- 217.Vasdev S., Ford C.A., Parai S., Longerich L., Gadag V. Dietary lipoic acid supplementation prevents fructose-induced hypertension in rats. Nutr. Metab. Cardiovasc. Dis. 2000;10:339–346. [PubMed] [Google Scholar]
- 218.Thirunavukkarasu V., Nandhini A.T.A., Anuradha C.V. Lipoic acid attenuates hypertension and improves insulin sensitivity, kallikrein activity and nitrite levels in high fructose-fed rats. J. Comp. Physiol. B. 2004;174:587–592. doi: 10.1007/s00360-004-0447-z. [DOI] [PubMed] [Google Scholar]
- 219.Vasdev S., Ford C.A., Parai S., Longerich L., Gadag V. Dietary α-lipoic acid supplementation lowers blood pressure in spontaneously hypertensive rats. J. Hypertens. 2000;18:567–573. doi: 10.1097/00004872-200018050-00009. [DOI] [PubMed] [Google Scholar]
- 220.Louhelainen M., Merasto S., Finckenberg P., Lapatto R., Cheng Z.J., Mervaala E.M. Lipoic acid supplementation prevents cyclosporine-induced hypertension and nephrotoxicity in spontaneously hypertensive rats. J. Hypertens. 2006;24:947–956. doi: 10.1097/01.hjh.0000222766.37971.9f. [DOI] [PubMed] [Google Scholar]
- 221.Vasdev S., Gill V., Longerich L., Parai S., Gadag V. Salt-induced hypertension in WKY rats: Prevention by α-lipoic acid supplementation. Mol. Cell. Biochem. 2003;254:319–326. doi: 10.1023/A:1027354005498. [DOI] [PubMed] [Google Scholar]
- 222.Huang Y.-P., Jin H.-Y., Yu H.-P. Inhibitory effects of alpha-lipoic acid on oxidative stress in the rostral ventrolateral medulla in rats with salt-induced hypertension. Int. J. Mol. Med. 2017;39:430–436. doi: 10.3892/ijmm.2016.2846. [DOI] [PubMed] [Google Scholar]
- 223.de Queiroz T.M., Xia H., Filipeanu C.M., Braga V.A., Lazartigues E. alpha-Lipoic acid reduces neurogenic hypertension by blunting oxidative stress-mediated increase in ADAM17. Am. J. Physiol. Heart Circ. Physiol. 2015;241:H926–H934. doi: 10.1152/ajpheart.00259.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Su Q., Liu J.-J., Cui W., Shi X.-L., Guo J., Li H.-B., Huo C.-J., Miao Y.-W., Zhang M., Yang Q., et al. Alpha lipoic acid supplementation attenuates reactive oxygen species in hypothalamic paraventricular nucleus and sympathoexcitation in high salt-induced hypertension. Toxicol. Lett. 2015;241:152–158. doi: 10.1016/j.toxlet.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 225.Vasdev S., Gill V., Parai S., Gadag V. Dietary lipoic acid supplementation attenuates hypertension in Dahl salt sensitive rats. Mol. Cell. Biochem. 2005;275:135–141. doi: 10.1007/s11010-005-1095-7. [DOI] [PubMed] [Google Scholar]
- 226.Midaoui A.E., Elimadi A., Wu L., Haddad P.S., De Champlain J. Lipoic acid prevents hypertension, hyperglycemia, and the increase in heart mitochondrial superoxide production. Am. J. Hypertens. 2003;16:173–179. doi: 10.1016/S0895-7061(02)03253-3. [DOI] [PubMed] [Google Scholar]
- 227.El Midaoui A., de Champlain J. Prevention of hypertension, insulin resistance, and oxidative stress by alpha-lipoic acid. Hypertension. 2002;39:303–307. doi: 10.1161/hy0202.104345. [DOI] [PubMed] [Google Scholar]
- 228.Ong S.L.H., Vohra H., Zhang Y., Sutton M., Whitworth J.A. The Effect of Alpha-Lipoic Acid on Mitochondrial Superoxide and Glucocorticoid-Induced Hypertension. Oxid. Med. Cell. Longev. 2013;2013:517045. doi: 10.1155/2013/517045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Igarashi T., Nakajima Y., Tanaka M., Otake S. Effect of coenzyme Q10 on experimental hypertension in rats and dogs. J. Pharmacol. Exp. Ther. 1974;189:149–156. [PubMed] [Google Scholar]
- 230.Okamoto H., Kawaguchi H., Togashi H., Minami M., Saito H., Yasuda H. Effect of coenzyme Q10 on structural alterations in the renal membrane of stroke-prone spontaneously hypertensive rats. Biochem. Med. Metab. Biol. 1991;45:216–226. doi: 10.1016/0885-4505(91)90024-F. [DOI] [PubMed] [Google Scholar]
- 231.Yamagami T., Shibata N., Folkers K. Bioenergetics in clinical medicine. Studies on coenzyme Q10 and essential hypertension. Res. Commun. Chem. Pathol. Pharmacol. 1975;11:273–288. [PubMed] [Google Scholar]
- 232.Yamagami T., Shibata N., Folkers K. Bioenergetics in clinical medicine. VIII. Adminstration of coenzyme Q10 to patients with essential hypertension. Res. Commun. Chem. Pathol. Pharmacol. 1976;14:721–727. [PubMed] [Google Scholar]
- 233.Folkers K., Drzewoski J., Richardson P.C., Ellis J., Shizukuishi S., Baker L. Bioenergetics in clinical medicine. XVI. Reduction of hypertension in patients by therapy with coenzyme Q10. Res. Commun. Chem. Pathol. Pharmacol. 1981;31:129–140. [PubMed] [Google Scholar]
- 234.Singh R., Niaz M., Rastogi S., Shukla P., Thakur A. Effect of hydrosoluble coenzyme Q10 on blood pressures and insulin resistance in hypertensive patients with coronary artery disease. J. Hum. Hypertens. 1999;13:203–208. doi: 10.1038/sj.jhh.1000778. [DOI] [PubMed] [Google Scholar]
- 235.Dornas W.C., Silva M., Tavares R., de Lima W.G., dos Santos R.C., Pedrosa M.L., Silva M.E. Efficacy of the superoxide dismutase mimetic tempol in animal hypertension models: A meta-analysis. J. Hypertens. 2015;33:14–23. doi: 10.1097/HJH.0000000000000422. [DOI] [PubMed] [Google Scholar]
- 236.Kamezaki F., Tasaki H., Yamashita K., Tsutsui M., Koide S., Nakata S., Tanimoto A., Okazaki M., Sasaguri Y., Adachi T., et al. Gene Transfer of Extracellular Superoxide Dismutase Ameliorates Pulmonary Hypertension in Rats. Am. J. Respir. Crit. Care Med. 2008;177:219–226. doi: 10.1164/rccm.200702-264OC. [DOI] [PubMed] [Google Scholar]
- 237.Carillon J., Rugale C., Rouanet J.-M., Cristol J.-P., Lacan D., Jover B. Endogenous antioxidant defense induction by melon superoxide dismutase reduces cardiac hypertrophy in spontaneously hypertensive rats. Int. J. Food Sci. Nutr. 2014;65:602–609. doi: 10.3109/09637486.2014.893286. [DOI] [PubMed] [Google Scholar]
- 238.Sugama I., Kohagura K., Yamazato M., Nakamura T., Shinzato T., Ohya Y. Superoxide dismutase mimetic, tempol, aggravates renal injury in advanced-stage stroke-prone spontaneously hypertensive rats. J. Hypertens. 2014;32:534–541. doi: 10.1097/HJH.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 239.Pires P.W., Deutsch C., McClain J.L., Rogers C.T., Dorrance A.M. Tempol, a superoxide dismutase mimetic, prevents cerebral vessel remodeling in hypertensive rats. Microvasc. Res. 2010;80:445–452. doi: 10.1016/j.mvr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Guo J.-K., Xu J.-S., Chen T.-B., Xu M.-M., Liu S.-T., Zhang C.-X., Ke L.-J., Zhou J.-W., Wang Q., Rao P.-F. Effects of TAT-SOD at Acupoints on Essential Hypertension by Monitoring Meridians Electrical Potential. Chin. J. Integr. Med. 2020;26:694–700. doi: 10.1007/s11655-019-3173-9. [DOI] [PubMed] [Google Scholar]
- 241.Forman H.J., Zhang H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021;20:689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mangione C.M., Barry M.J., Nicholson W.K., Cabana M., Chelmow D., Coker T.R., Davis E.M., Donahue K.E., Doubeni C.A., Jaén C.R., et al. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327:2326–2333. doi: 10.1001/jama.2022.8970. [DOI] [PubMed] [Google Scholar]