Abstract
Oxidative stress is a novel risk factor for chronic kidney disease (CKD). The oxidative balance score (OBS) was developed to represent the overall oxidative balance based on dietary and lifestyle pro-oxidant and antioxidant components. The aim of this study is to verify the relationship between the OBS and the incidence of CKD. Data from 5795 participants without CKD at the baseline survey of the Korean Genome and Epidemiology Study were analyzed. Participants were classified into sex-specific OBS tertiles. During the mean follow-up period of 13.6 years, 286 men and 382 women newly developed CKD. The Cox proportional hazard spline curve revealed an inverse dose–response association between the OBS and incident CKD in both men and women. Multiple Cox proportional hazard regression analysis revealed that the adjusted hazard ratios (95% confidence intervals) for sex-specific highest (T3) and middle (T2) OBS tertile groups were 0.80 (0.59–1.08) and 0.70 (0.51–0.95), respectively, in men and 0.76 (0.59–0.98) and 0.73 (0.55–0.96), respectively, in women, with the sex-specific lowest OBS tertile group (T1) as the reference. These results suggest that a healthy diet and lifestyle that increases the OBS may help prevent CKD in both men and women.
Keywords: oxidative balance score, chronic kidney disease, incidence, prospective cohort
1. Introduction
Chronic kidney disease (CKD) is a growing health issue worldwide that is associated with a high economic burden, including clinic visits, dialysis, and medical costs [1,2]. The global prevalence of CKD was estimated to be approximately 700 million in 2017, with a 29.3% increase since 1990 [3]. CKD is the 12th leading cause of death, with 1.2 million deaths globally [3]. Therefore, to reduce the health burden caused by CKD, it is important to develop effective screening tools to identify risk factors. Smoking, obesity, hypertension, and diabetes mellitus are well-known modifiable risk factors for CKD [4].
Recent studies have shown that oxidative stress, defined as an imbalance between the production of reactive oxygen species and antioxidant defense capacity, is a novel risk factor for CKD [5,6,7]. Oxidative stress causes damage to macromolecules and DNAs and abnormal cell signaling [8]. As the kidney is a mitochondria-rich organ with a high energy demand, it is more vulnerable to oxidative stress [9]. In addition, oxidative stress can progress to CKD and contribute to complications, including cardiovascular and neurological complications [10,11]. Several studies have demonstrated that pro-oxidant biomarker levels are elevated and antioxidant biomarker levels are reduced in patients with CKD [12,13,14,15]. However, pro- or antioxidant biomarkers have limitations in indicating the overall oxidative stress burden. Thus, the oxidative balance score (OBS) was developed to represent the overall oxidative balance based on dietary and lifestyle pro- and antioxidant components [16]. Since the OBS was first defined by Van Hoydonck et al. [17] in 2002, more than 20 modified versions of the OBS have been published by selecting different factors or applying different scoring systems to improve the quality of this particular scoring system [18]. In the OBS, antioxidant components contribute positively, whereas pro-oxidant components contribute negatively; thus, lower OBSs indicate a higher pro-oxidant burden.
To date, only one cohort study in the United States has shown an inverse association between the OBS and new-onset CKD [19]. However, there are racial/ethnic differences in the development and progression of CKD [20,21]. Therefore, the aim of this study was to evaluate the association between the OBS and the risk of new-onset CKD in East Asian middle-aged and older adults, using community-based, large-scale Korean cohort data.
2. Materials and Methods
2.1. Study Population
Data from the Korean Genome and Epidemiology Study (KoGES)_Ansan and Ansung cohorts were used. The KoGES_Ansan and Ansung cohorts were established to verify the genetic and epidemiologic risk factors for non-communicable diseases. The detailed study design and procedures have been described in a previous study [22]. A total of 10,030 community-dwelling adults aged 40–69 years participated in the baseline survey (2001–2002) and were followed up biennially to the eighth follow-up year (2017–2018).
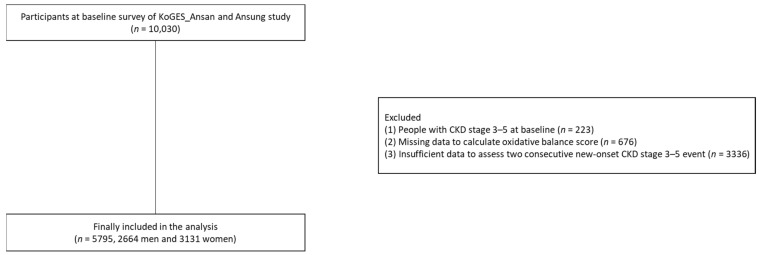
Among 10,030 participants at baseline, we excluded patients with (1) CKD stages 3–5 at baseline (n = 223), (2) insufficient data to calculate the OBS (n = 676), and (3) insufficient data to assess two consecutive events of new-onset CKD stages 3–5 during follow-up (n = 3336). Finally, 5795 participants were included in the analysis (Figure 1).
Figure 1.
Flowchart of the study population. Abbreviations: KoGES, Korean Genome and Epidemiology Study; CKD, chronic kidney disease.
Informed consent was obtained from all participants. In accordance with the 1964 Declaration of Helsinki and its later amendments, the study protocol was approved by the institutional review board (IRB) of Nowon Eulji Medical Center (IRB number: 2021-03-009).
2.2. Assessment of OBS
The OBS was totalized by calculating eight pro-oxidant and seven antioxidant factors selected based on previous studies [23,24,25,26]. Table 1 describes the OBS scheme [27]. Pro-oxidant factors included saturated fatty acid (SFA), omega-6 polyunsaturated fatty acid (PUFA), copper intake, total iron intake, cigarette smoking status, alcohol drinking status, obesity status, and abdominal obesity status. The sex-specific tertile values of SFA, omega-6 PUFA, total iron intake, and copper intake were scored corresponding to the lowest (score 2), middle (score 1), and highest (score 0) tertiles, respectively. Current smokers, former smokers, and never smokers were assigned scores of 0, 1, and 2 points, respectively. Regarding alcohol drinking status, heavy drinkers (≥30 g/day in men, ≥20 g/day in women), mild-to-moderate drinkers (1–29 g/day in men, 1–19 g/day in women), and non-drinkers were assigned 0, 1, and 2 points, respectively. As for obesity, overweight, and normal weight, the scores were 0, 1, and 2, respectively. For abdominal obesity, 0 points were assigned for abdominal obesity and 1 point for a normal abdomen. Antioxidant factors included the intake of omega-3 PUFA, vitamin C, vitamin E, selenium, beta-carotene, and zinc, as well as physical activity. The sex-specific tertile values of each variable, omega-3 PUFA, vitamin C, vitamin E, selenium, beta-carotene, and zinc intake were scored corresponding to the lowest (score 0), middle (score 1), and highest tertiles (score 2). Low-intensity physical activity received 0 points, moderate-intensity physical activity received 1 point, and high-intensity physical activity received 2 points. The sum of the OBS ranged from 0 to 29 points. Using the OBS, we divided the individuals into tertile groups in a sex-specific manner.
Table 1.
Oxidative balance score assignment scheme.
| OBS Components | Assignment Scheme * |
|---|---|
| 1. Saturated fatty acid [P] | 0 = high (3rd tertile), 1 = intermediate (2nd tertile), 2 = low (1st tertile) |
| 2. Omega-6 PUFA intake [P] | 0 = high (3rd tertile), 1 = intermediate (2nd tertile), 2 = low (1st tertile) |
| 3. Total iron intake [P] | 0 = high (3rd tertile), 1 = intermediate (2nd tertile), 2 = low (1st tertile) |
| 4. Copper intake [P] | 0 = high (3rd tertile), 1 = intermediate (2nd tertile), 2 = low (1st tertile) |
| 5. Smoking status [P] | 0 = current smoker, 1 = former smoker, 2 = never smoker |
| 6. Alcohol drinking status [P] | 0 = heavy drinker, 1 = mild-to-moderate drinker, 2 = non-drinker |
| 7. Overweight/obese [P] | 0 = obese, 1 = overweight, 2= normal |
| 8. Abdominal obesity [P] | 0 = abdominal obesity, 1 = normal |
| 9. Omega-3 PUFA intake [A] | 0 = low (1st tertile), 1 = intermediate (2nd tertile), 2 = high (3rd tertile) |
| 10. Vitamin C intake [A] | 0 = low (1st tertile), 1 = intermediate (2nd tertile), 2 = high (3rd tertile) |
| 11. Vitamin E intake [A] | 0 = low (1st tertile), 1 = intermediate (2nd tertile), 2 = high (3rd tertile) |
| 12. Selenium intake [A] | 0 = low (1st tertile), 1 = intermediate (2nd tertile), 2 = high (3rd tertile) |
| 13. Total beta-carotene intake [A] | 0 = low (1st tertile), 1 = intermediate (2nd tertile), 2 = high (3rd tertile) |
| 14. Zinc intake [A] | 0 = low (1st tertile), 1 = intermediate (2nd tertile), 2 = high (3rd tertile) |
| 15. Physical activity [A] | 0 = low intensity, 1 = moderate intensity, 2 = high intensity |
* Low, intermediate, and high categories correspond to sex-specific tertile values among participants in the KoGES at the baseline survey. Abbreviations: OBS, oxidative balance score; PUFA, polyunsaturated fatty acid. (Reprinted with permission of authors [27].)
2.3. Definition of CKD
The primary endpoint was new-onset CKD defined as two newly developed consecutive events of an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. The eGFR was calculated using the CKD Epidemiology Collaboration (CKD-EPI) equation [28].
2.4. Covariates
Variables from the baseline survey were used as covariates in the analysis. Each participant’s height (m) and body weight (kg) were measured to the nearest 0.001 m and 0.1 kg, respectively. A body mass index (BMI) ≥25 kg/m2 indicated obesity, and BMI ≥ 23 and <25 kg/m2 indicated overweight based on the definition of the 2018 Korean Society for the Study of Obesity [29]. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured, then we calculated mean blood pressure (MBP). Hypertension was defined as SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, or treatment with antihypertensive medications [30].
Blood samples were collected after ≥8 h of fasting and were analyzed with a chemical analyzer. Fasting plasma glucose, creatinine, total cholesterol, high-density lipoprotein cholesterol, triglyceride, and C-reactive protein levels were enzymatically analyzed (Hitachi 7600, Hitachi, Tokyo, Japan in August 2002 and ADVIA 1650, Siemens, Tarrytown, NY, USA from September 2002). Low-density lipoprotein cholesterol levels were calculated using the Friedewald equation for serum triglyceride levels <400 mg/dL [31].
A 103-item food frequency questionnaire (FFQ) was used to estimate the amount of macro-/micronutrient consumed by each participant. Well-trained dietitians conducted in-person interviews with participants for dietary surveillance. The total energy (kcal/day), protein (g/day), SFA (g/day), omega-6-PUFA (g/day), total iron (mg/day), copper (μg/day), omega-3 PUFA (g/day), vitamin C (mg/day), vitamin E (mg/day), selenium (μg/day), beta-carotene (μg/day), and zinc (mg/day) intake of each participant were calculated from the FFQ using a computer-aided nutritional analysis program (CAN-pro version 5.0, The Korean Nutrition Society, Seoul, Korea). The protein-to-total energy intake (%) was calculated as 100 × 4 (kcal/g) × protein intake (g/day)/total energy intake (kcal/day).
Participants also completed a self-report questionnaire regarding smoking, alcohol consumption, physical activity, level of education, and monthly household income. The status of smoker was classified as never smoker, former smoker, or current smoker. Those who had never smoked or had smoked less than 100 cigarettes in their lifetime were considered never smokers. Former smokers were defined as adults who had quit smoking at the time of the interview and had smoked at least 100 cigarettes in their lifetime. Current smokers were defined as adults who currently smoked and had smoked at least 100 cigarettes in their lifetime. Alcohol consumption per day (g/day) was calculated by multiplying the amount of alcohol consumption per time (glasses/time) by the alcohol consumption frequency (times/month) × 10 (g/per glass of drink)/30 (days/month). Physical activity levels were measured by asking people how many metabolic equivalent of tasks (MET) they perform on a daily basis (Met-h/day) using the International Physical Activity Questionnaire [32]. Low (<7.5 MET-h/day), moderate (7.5–30 MET-h/day), and high (>30 MET-h/day) were the categories categorized by physical activity [33]. The education level was classified as (1) elementary school or middle school, (2) high school, or (3) college or university. The monthly household income was divided into three groups: (1) <1 million Korean Won (KRW), (2) 1–2 million KRW, and (3) >2 million KRW.
2.5. Statistical Analysis
All data were analyzed separately according to sex. All data are presented as means ± standard deviations for continuous variables and numbers (percentages, %) for categorical variables. For continuous variables, analysis of variance was used to compare differences between sex-specific OBS tertile groups. For categorical variables, the chi-square test was used.
Cox proportional hazard spline curves were drawn to determine the dose–response relationship between the OBS and incident CKD in men and women. We used Kaplan–Meier curves with log-rank tests to determine the cumulative incidence rate of CKD according to the sex-specific OBS tertile groups. Multiple Cox proportional hazard regression analysis was performed to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for incident CKD in the sex-specific highest (T3) and middle (T2) OBS tertile groups compared with the sex-specific lowest (T1) OBS tertile group. The HR with 95% CI for incident CKD per increment of OBS was also verified. In the adjusted model, age, education level, monthly household income, ratio of protein intake to total energy intake, MBP, serum total cholesterol level, and serum C-reactive protein level were included as confounding variables.
We used SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC, USA) and R software (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria) to perform the statistical analyses. Statistical significance was set at a p value < 0.05.
3. Results
3.1. Characteristics of the Study Population
Table 2 presents the baseline characteristics of the study population across total and sex-specific OBS tertile groups. The T3 group showed the highest proportion of participants with the highest education level, household income in total population and percentage of women. The mean values of MBP, glucose, insulin, total cholesterol, and triglyceride levels in the total population and in both men and women were highest in the T1 group. The mean values of age were highest in the T1 group among total study population and women. The T3 group exhibited the highest mean values for energy intake and protein/total energy intake in total population and both sex. The values for the high-density lipoprotein cholesterol level and eGFR were highest in the T3 group across total group and women.
Table 2.
Baseline characteristics of the study population.
| Oxidative Balance Score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Men | Women | ||||||||||
| Variables | T1 (n = 1923) |
T2 (n = 1757) |
T3 (n = 2115) |
p * | T1 (n = 798) |
T2 (n = 779) |
T3 (n = 1087) |
p * | T1 (n = 1125) |
T2 (n = 978) |
T3 (n = 1028) |
p * |
| Men, n (%) | 798 (41.5%) | 779 (44.3%) | 1087 (51.4%) | <0.001 | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Age, years | 52.4 ± 8.4 | 51.8 ± 8.5 | 50.3 ± 8.2 | <0.001 | 50.5 ± 7.7 | 51.5 ± 8.4 | 50.8 ± 8.2 | 0.593 | 53.8 ± 8.6 | 52.1 ± 8.7 | 49.9 ± 8.1 | <0.001 |
| MBP, mmHg | 98.0 ± 13.2 | 96.7 ± 12.7 | 94.0 ± 12.6 | <0.001 | 98.4 ± 12.2 | 98.1 ± 11.9 | 96.2 ± 12.3 | <0.001 | 97.6 ± 13.8 | 95.6 ± 13.3 | 91.7 ± 12.6 | <0.001 |
| Glucose, mg/dL | 87.3 ± 18.8 | 86.6 ± 18.9 | 85.7 ± 20.6 | 0.008 | 90.1 ± 20.8 | 89.0 ± 18.6 | 88.4 ± 23.2 | 0.089 | 85.3 ± 17.0 | 84.6 ± 19.0 | 82.8 ± 16.8 | 0.001 |
| Insulin, µU/mL | 8.2 ± 5.5 | 7.7 ± 4.7 | 7.2 ± 4.0 | <0.001 | 7.4 ± 4.5 | 7.2 ± 4.0 | 6.8 ± 3.6 | <0.001 | 8.7 ± 6.1 | 8.1 ± 5.1 | 7.6 ± 4.3 | <0.001 |
| Total cholesterol, mg/dL | 194.7 ± 34.5 | 191.0 ± 35.7 | 187.8 ± 33.5 | <0.001 | 195.4 ± 34.9 | 193.4 ± 36.2 | 190.3 ± 34.3 | 0.002 | 194.1 ± 34.2 | 189.1 ± 35.2 | 185.0 ± 32.5 | <0.001 |
| Triglyceride, mg/dL | 176.6 ± 108.5 | 160.3 ± 102.7 | 147.8 ± 94.8 | <0.001 | 196.3 ± 121.9 | 180.2 ± 123.0 | 161.3 ± 101.9 | <0.001 | 162.6 ± 95.4 | 144.3 ± 79.6 | 133.5 ± 84.4 | <0.001 |
| HDL cholesterol, mg/dL | 43.3 ± 9.3 | 44.7 ± 10.2 | 45.1 ± 9.9 | <0.001 | 42.3 ± 9.2 | 43.3 ± 9.7 | 43.9 ± 10.0 | 0.001 | 44.0 ± 9.2 | 45.8 ± 10.4 | 46.4 ± 9.7 | <0.001 |
| CRP, mg/dL | 0.2 ± 0.4 | 0.3 ± 0.8 | 0.2 ± 0.4 | 0.016 | 0.2 ± 0.3 | 0.3 ± 0.8 | 0.2 ± 0.3 | 0.157 | 0.2 ± 0.5 | 0.2 ± 0.8 | 0.2 ± 0.4 | 0.041 |
| eGFR, mL/min/1.73 m2 | 91.8 ± 13.2 | 92.8 ± 13.2 | 94.2 ± 12.8 | <0.001 | 91.2 ± 13.4 | 91.5 ± 13.4 | 91.8 ± 13.0 | 0.321 | 92.2 ± 13.0 | 93.9 ± 12.9 | 96.6 ± 12.2 | <0.001 |
| Education level, n (%) | <0.001 | 0.928 | <0.001 | |||||||||
| Elementary/middle school | 1165 (60.9%) | 951 (54.5%) | 1018 (48.3%) | 312 (39.1%) | 303 (39.1%) | 437 (40.4%) | 853 (76.5%) | 648 (66.7%) | 581 (56.7%) | |||
| High school | 529 (27.7%) | 559 (32.0%) | 747 (35.5%) | 307 (38.5%) | 303 (39.1%) | 402 (37.1%) | 222 (19.9%) | 256 (26.4%) | 345 (33.7%) | |||
| College/university | 219 (11.4%) | 236 (13.5%) | 342 (16.2%) | 179 (22.4%) | 169 (21.8%) | 244 (22.5%) | 40 (3.6%) | 67 (6.9%) | 98 (9.6%) | |||
| Household income, n (%) | <0.001 | 0.159 | <0.001 | |||||||||
| <100 million Korean Won | 706 (37.2%) | 603 (34.7%) | 588 (28.1%) | 187 (23.6%) | 204 (26.3%) | 265 (24.5%) | 519 (46.9%) | 399 (41.5%) | 323 (31.9%) | |||
| 100–200 million Korean Won | 545 (28.7%) | 499 (28.7%) | 633 (30.2%) | 242 (30.6%) | 212 (27.4%) | 352 (32.5%) | 303 (27.4%) | 287 (29.8%) | 281 (27.8%) | |||
| >200 million Korean Won | 647 (34.1%) | 635 (36.6%) | 872 (41.7%) | 363 (45.8%) | 359 (46.3%) | 465 (43.0%) | 284 (25.7%) | 276 (28.7%) | 407 (40.3%) | |||
| Total energy intake, kcal/day | 1820.0 ± 528.8 | 2134.7 ± 755.2 | 2519.8 ± 868.2 | <0.001 | 1926.4 ± 507.4 | 2209.1 ± 762.0 | 2556.4 ± 769.6 | <0.001 | 1744.5 ± 531.0 | 2075.4 ± 744.8 | 2481.2 ± 960.4 | <0.001 |
| † Protein/total energy intake, % | 12.7 ± 2.0 | 13.3 ± 2.3 | 14.2 ± 2.4 | <0.001 | 13.0 ± 2.0 | 13.5 ± 2.3 | 14.1 ± 2.3 | <0.001 | 12.4 ± 2.0 | 13.2 ± 2.2 | 14.3 ± 2.5 | <0.001 |
* p value for the comparison of the baseline characteristics among sex-specific tertile groups of oxidative balance score at the baseline survey. † Protein/total energy intake was calculated as 100 × 4 (kcal/g) × protein intake (g/day)/total energy intake (kcal/day). Statistical significance was set at p < 0.05. Abbreviations: MBP, mean blood pressure; HDL, high-density lipoprotein; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate.
3.2. Individual Components of the OBS According to Sex-Specific OBS Tertile
Table 3 displays the clinical characteristics of the individual OBS components according to the OBS tertiles. The mean intake of SFA, omega-6 PUFA, total iron, total copper, omega-3 PUFA, vitamin C, vitamin E, beta-carotene, and zinc intake increased across the total, male and female tertiles of OBS. Participants with obesity, current smokers, heavy drinkers, and low-intensity physical activity were more prevalent among the T1 group for both sexes and total study population.
Table 3.
Individual components of the score according to oxidative balance score tertiles.
| Oxidative Balance Score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Men | Women | ||||||||||
| Variables | T1 (n = 1923) |
T2 (n = 1757) |
T3 (n = 2115) |
p * | T1 (n = 798) |
T2 (n = 779) |
T3 (n = 1087) |
p * | T1 (n = 1125) |
T2 (n = 978) |
T3 (n = 1028) |
p * |
| Saturated fatty acid, g/day | 8.3 ± 4.4 | 10.5 ± 6.0 | 13.3 ± 8.4 | <0.001 | 8.7 ± 4.0 | 10.6 ± 6.0 | 12.9 ± 7.4 | <0.001 | 7.9 ± 4.6 | 10.4 ± 6.0 | 13.8 ± 9.3 | <0.001 |
| omega-6 PUFA intake, g/day | 7.7 ± 4.2 | 8.8 ± 5.7 | 9.3 ± 5.4 | <0.001 | 7.6 ± 3.8 | 8.8 ± 5.6 | 8.9 ± 5.0 | <0.001 | 7.8 ± 4.4 | 8.8 ± 5.8 | 9.7 ± 5.9 | <0.001 |
| Total iron intake, mg/day | 15.3 ± 6.1 | 19.0 ± 8.6 | 23.8 ± 11.3 | <0.001 | 16.2 ± 6.2 | 19.2 ± 8.6 | 23.4 ± 10.0 | <0.001 | 14.7 ± 6.1 | 18.8 ± 8.5 | 24.3 ± 12.5 | <0.001 |
| Copper intake, μg/day | 1082.0 ± 821.5 | 1288.9 ± 892.7 | 1504.6 ± 916.4 | <0.001 | 1168.1 ± 856.4 | 1294.4 ± 926.5 | 1398.1 ± 840.4 | <0.001 | 1020.9 ± 790.5 | 1284.6 ± 865.3 | 1617.3 ± 978.3 | <0.001 |
| Smoking status, n (%) | <0.001 | <0.001 | <0.001 | |||||||||
| Current smoker | 520 (27.0%) | 390 (22.2%) | 366 (17.3%) | 466 (58.4%) | 374 (48.0%) | 363 (33.4%) | 54 (4.8%) | 16 (1.6%) | 3 (0.3%) | |||
| Former smoker | 259 (13.5%) | 257 (14.6%) | 353 (16.7%) | 242 (30.3%) | 250 (32.1%) | 350 (32.2%) | 17 (1.5%) | 7 (0.7%) | 3 (0.3%) | |||
| Never smoker | 1144 (59.5%) | 1110 (63.2%) | 1396 (66.0%) | 90 (11.3%) | 155 (19.9%) | 374 (34.4%) | 1054 (93.7%) | 955 (97.6%) | 1022 (99.4%) | |||
| Drinking status, n (%) | <0.001 | <0.001 | <0.001 | |||||||||
| Heavy drinker | 259 (13.5%) | 172 (9.8%) | 127 (6.0%) | 229 (28.7%) | 162 (20.8%) | 121 (11.1%) | 30 (2.7%) | 10 (1.0%) | 6 (0.6%) | |||
| Mild-to-moderate drinker | 790 (41.1%) | 654 (37.2%) | 738 (34.9%) | 448 (56.1%) | 417 (53.5%) | 547 (50.3%) | 342 (30.4%) | 237 (24.2%) | 191 (18.6%) | |||
| Non-drinker | 874 (45.4%) | 931 (53.0%) | 1250 (59.1%) | 121 (15.2%) | 200 (25.7%) | 419 (38.5%) | 753 (66.9%) | 731 (74.7%) | 831 (80.8%) | |||
| Obesity status, n (%) | <0.001 | <0.001 | <0.001 | |||||||||
| Obese | 1230 (64.0%) | 794 (45.2%) | 516 (24.4%) | 498 (62.4%) | 346 (44.4%) | 278 (25.6%) | 732 (65.1%) | 448 (45.8%) | 238 (23.2%) | |||
| Overweight | 452 (23.5%) | 443 (25.2%) | 632 (29.9%) | 176 (22.1%) | 198 (25.4%) | 319 (29.3%) | 276 (24.5%) | 245 (25.1%) | 313 (30.4%) | |||
| Normal weight | 241 (12.5%) | 520 (29.6%) | 967 (45.7%) | 124 (15.5%) | 235 (30.2%) | 490 (45.1%) | 117 (10.4%) | 285 (29.1%) | 477 (46.4%) | |||
| Abdominal obesity, n (%) | 909 (47.3%) | 533 (30.3%) | 292 (13.8%) | <0.001 | 274 (34.3%) | 190 (24.4%) | 113 (10.4%) | <0.001 | 635 (56.4%) | 343 (35.1%) | 179 (17.4%) | <0.001 |
| omega-3 PUFA intake, g/day | 1.1 ± 0.7 | 1.3 ± 0.9 | 1.5 ± 0.9 | <0.001 | 1.1 ± 0.6 | 1.3 ± 0.9 | 1.5 ± 0.9 | <0.001 | 1.1 ± 0.7 | 1.3 ± 0.9 | 1.6 ± 1.0 | <0.001 |
| Vitamin C intake, mg/day | 79.5 ± 59.3 | 123.7 ± 102.4 | 182.1 ± 133.8 | <0.001 | 74.2 ± 42.4 | 109.1 ± 83.8 | 158.4 ± 113.6 | <0.001 | 83.2 ± 68.6 | 135.3 ± 113.7 | 207.1 ± 148.2 | <0.001 |
| Vitamin E intake, mg/day | 9.7 ± 4.3 | 13.6 ± 6.8 | 18.3 ± 9.1 | <0.001 | 10.5 ± 4.2 | 13.7 ± 6.7 | 17.6 ± 7.7 | <0.001 | 9.1 ± 4.3 | 13.5 ± 7.0 | 19.0 ± 10.4 | <0.001 |
| Selenium intake, μg/day | 32.3 ± 19.4 | 47.2 ± 28.9 | 66.3 ± 41.6 | <0.001 | 37.1 ± 20.2 | 49.5 ± 30.8 | 65.7 ± 34.9 | <0.001 | 28.9 ± 18.0 | 45.3 ± 27.1 | 66.8 ± 47.8 | <0.001 |
| Beta-carotene intake, μg/day | 2074.7 ± 1488.3 | 3385.7 ± 2861.2 | 5062.3 ± 4270.2 | <0.001 | 2288.7 ± 1756.2 | 3508.6 ± 3073.5 | 4866.7 ± 3861.9 | <0.001 | 1922.9 ± 1242.9 | 3287.8 ± 2677.6 | 5269.2 ± 4656.2 | <0.001 |
| Zinc intake, mg/day | 10.5 ± 3.4 | 12.4 ± 4.7 | 14.8 ± 5.7 | <0.001 | 10.7 ± 3.1 | 12.5 ± 4.7 | 14.7 ± 5.1 | <0.001 | 10.3 ± 3.5 | 12.3 ± 4.7 | 15.0 ± 6.3 | <0.001 |
| Physical activity, n (%) | <0.001 | <0.001 | <0.001 | |||||||||
| Low (<7.5 METs-hr/wk) | 230 (12.0%) | 103 (5.9%) | 78 (3.7%) | 89 (11.2%) | 28 (3.6%) | 36 (3.3%) | 141 (12.5%) | 75 (7.7%) | 42 (4.1%) | |||
| Moderate (7.5–30 METs-hr/wk) | 1198 (62.3%) | 1098 (62.5%) | 1193 (56.4%) | 527 (66.0%) | 485 (62.3%) | 590 (54.3%) | 671 (59.6%) | 613 (62.7%) | 603 (58.7%) | |||
| High (>30 METs-hr/wk) | 495 (25.7%) | 556 (31.6%) | 844 (39.9%) | 182 (22.8%) | 266 (34.1%) | 461 (42.4%) | 313 (27.8%) | 290 (29.7%) | 383 (37.3%) | |||
* p value for the comparison of the baseline characteristics among sex-specific tertile groups of oxidative balance score at baseline survey. Statistical significance was set at p < 0.05. Abbreviations: PUFA, polyunsaturated fatty acid.
3.3. Relationship between Oxidative Balance Score and Incident Chronic Kidney Disease
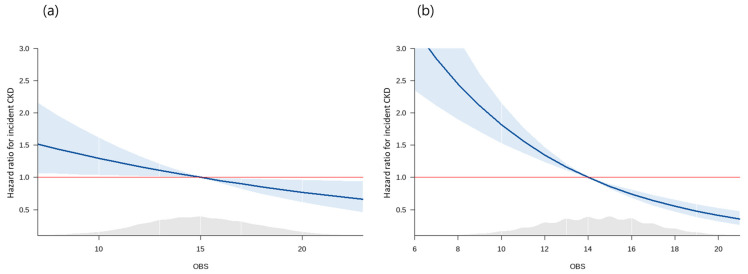
A total of 286 men and 382 women developed new-onset CKD during a mean follow-up period of 13.6 years. Cox proportional hazard spline curves showed that incident CKD was inversely associated with the OBS in a dose-dependent manner (Figure 2).
Figure 2.
Spline curves for the Cox proportional hazard model of incident CKD according to OBS in (a) men and (b) women. Abbreviations: CKD, chronic kidney disease; OBS, oxidative balance score.
Table 4 presents the independent association between new-onset CKD and the OBS using HRs and 95% CIs. Compared with T1, the HRs (95% CIs) for new-onset CKD in the T2 and T3 groups were 0.76 (0.63–0.91) and 0.57 (0.48–0.69) in the overall population, respectively, and 0.94 (0.71–1.25) and 0.74 (0.56–0.98), respectively, in men and 0.66 (0.53–0.84) and 0.48 (0.37–0.62), respectively, in women. After adjusting for age, education level, monthly household income, protein/total energy intake, MBP, serum total cholesterol level, and C-reactive protein level, the adjusted HRs (95% CIs) for T2 and T3 were 0.77 (0.63–0.93) and 0.71 (0.58–0.87), respectively, in overall population and 0.80 (0.59–1.08) and 0.70 (0.51–0.95), respectively, in men and 0.76 (0.59–0.98) and 0.73 (0.55–0.96), respectively, in women. The adjusted HRs and 95% CIs for incident CKD for each increment of 1 in the OBS were 0.94 (0.91–0.97) in overall population, 0.95 (0.90–0.99) in men and 0.93 (0.89–0.98) in women.
Table 4.
Cox proportional hazard regression analysis showing the relationship of the oxidative balance score with incident chronic kidney disease.
| Oxidative Balance Score Tertile | Numbers, n | New-Onset Cases, n | Follow-Up Period, Person–Year | Incidence Rate Per 1000 Person Years | Unadjusted | Adjusted |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||||
| Total | ||||||
| Continuous (per 1 increment) | 0.90 (0.87–0.93) | 0.94 (0.91–0.97) | ||||
| T1 | 1923 | 288 | 28,539.5 | 10.09 | 1 (reference) | 1 (reference) |
| T2 | 1757 | 203 | 26,507.1 | 7.66 | 0.76 (0.63–0.91) | 0.77 (0.63–0.93) |
| T3 | 2115 | 187 | 32,302.5 | 5.79 | 0.57 (0.48–0.69) | 0.71 (0.58–0.87) |
| p for trend | <0.001 | <0.001 | ||||
| Men | ||||||
| Continuous (per 1 increment) | 0.95 (0.91–0.99) | 0.95 (0.90–0.99) | ||||
| T1 | 798 | 97 | 12,012.2 | 8.08 | 1 (reference) | 1 (reference) |
| T2 | 779 | 90 | 11,802.2 | 7.63 | 0.94 (0.71–1.25) | 0.80 (0.59–1.08) |
| T3 | 1087 | 99 | 16,552.1 | 5.98 | 0.74 (0.56–0.98) | 0.70 (0.51–0.95) |
| p for trend | 0.031 | 0.022 | ||||
| Women | ||||||
| Continuous (per 1 increment) | 0.86 (0.83–0.90) | 0.93 (0.89–0.98) | ||||
| T1 | 1125 | 191 | 16,527.3 | 11.56 | 1 (reference) | 1 (reference) |
| T2 | 978 | 113 | 14,704.9 | 7.68 | 0.66 (0.53–0.84) | 0.76 (0.59–0.98) |
| T3 | 1028 | 88 | 15,750.4 | 5.59 | 0.48 (0.37–0.62) | 0.73 (0.55–0.96) |
| p for trend | <0.001 | 0.015 |
Adjusted for age, education level, monthly household income, protein/total energy intake (%), mean blood pressure, serum total cholesterol level, and serum C-reactive protein level. Abbreviations: HR, hazard ratio; CI, confidence interval.
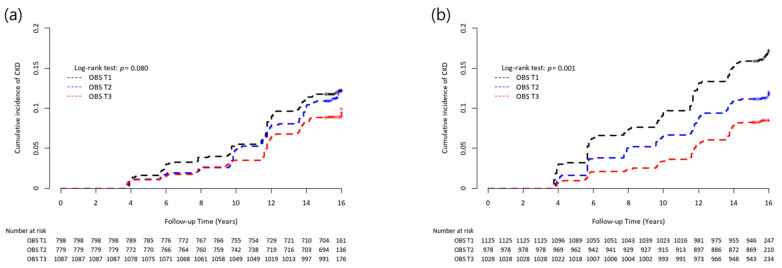
The Kaplan–Meier curve with the log-rank test showed the highest risk of cumulative CKD incidence in the T1 group, followed by the T2 and T3 groups in both men and women (Figure 3).
Figure 3.
Inverse Kaplan–Meier curves of incident CKD according to OBS tertile groups in (a) men and (b) women. Abbreviations: CKD, chronic kidney disease, OBS, oxidative balance score.
4. Discussion
This large-scale prospective study is one of the few to examine the association between the OBS and the risk of CKD. Incident CKD occurred in 10.7% of men and 12.2% of women during the follow-up period, showing a high prevalence in women. Although gender difference in CKD prevalence vary across the countries, the results of this study are similar to those in a meta-analysis of previous studies comparing CKD prevalence in Asian countries, where the prevalence was higher in women but the severity and mortality rates were higher in men [34]. Additionally, the results of this study suggested that a lower OBS at baseline was associated with an increased risk of CKD during the 16.8-year follow-up in both men and women. After adjustment, the OBS was still inversely related to the risk of new-onset CKD. The adjusted HRs and 95% Cis for incident CKD for each increment of 1 in the OBS were 0.95 (0.90–0.99) in men and 0.93 (0.89–0.98) in women, showing lower HRs in women. This discrepancy suggests that oxidative stress is physiologically different in men and women. There has been some evidence that females have a greater antioxidant capacity than males [35], possibly owing to sex differences in antioxidant enzyme expression and activity [36]. Particularly, estradiol decreases NADPH oxidase activity and expression, generating reactive oxygen species, whereas it enhances antioxidant enzymes such as superoxide dismutase and glutathione peroxidase [37]. Furthermore, estrogen scavenges free radicals via its phenolic hydroxyl group [38]. In this regard, the results of this study suggest that men without the antioxidant potential of estradiol need to follow more anti-oxidant lifestyles and diets to reduce the risk of CKD.
The mean values of SFA, omega-6, total iron, and copper intake showed opposite trends compared to other pro-oxidant components. This discrepancy may be due to the effect of total energy intake, which tends to increase with increasing OBS tertiles. Additionally, because OBS is the sum of its various components, each component may show an independent trend.
Our findings are in line with those of previous epidemiological studies that have shown a negative link between oxidative stress and CKD progression. The levels of various oxidative stress markers, such as plasma 8-isoprostane, malondialdehyde, and oxidized serum albumin, seem to increase as CKD progresses and are inversely associated with the glomerular filtration rate [5,39,40]. Another cross-sectional study conducted in 116 patients with CKD also showed similar results using plasma levels of 15-F2t-isoprostane, which is a highly accurate oxidative stress marker in vivo in humans [41]. In contrast, the levels of various antioxidant markers, such as glutathione peroxidase, plasma thiol, superoxide dismutase, and catalase, seemed to be reduced in CKD patients [5,42,43]. Because these biomarkers are not widely used in clinical practice and there is no single marker that reflects the overall oxidative stress burden, the OBS used in this study can be applied in clinical practice, as it provides a cost-effective method to evaluate the overall oxidative stress balance using only dietary and lifestyle questionnaires without blood or urine tests.
Ilori et al. [19] reported that higher OBS quartiles were significantly associated with a lower risk of CKD, whereas OBS quartiles were not significantly associated with albuminuria and incident ESRD. Although this study is the first to show a negative relationship between OBS and the risk of new-onset CKD, most of the study population included Caucasians. As dietary and lifestyle factors constituting OBS are highly influenced by race and culture [20,21], similar results from our study support previous evidence that a healthy diet and lifestyle, which lower oxidative stress, reduce the risk of incident CKD in Asian populations.
In a meta-analysis of 103 observational studies, modifiable lifestyle factors for preventing CKD included a high intake of fruits, vegetables, and potassium-rich food; higher physical activity levels; moderate alcohol consumption; low intake of sodium; and smoking cessation [44]. Most of these factors were included in the OBS used in this study. Although a meta-analysis described these factors as contributing to the prevention of CKD by reducing the risk of obesity and lowering blood pressure, reducing the oxidative stress burden is also thought to be relevant. In this respect, we believe that OBS can be a useful tool for educating the general population on healthy diet and lifestyle patterns to prevent CKD, in addition to its value as a screening tool to predict the risk of CKD.
Although the etiology of CKD is complex, the most likely explanation for the relationship between oxidative stress and incident CKD is endothelial dysfunction and chronic inflammation. High levels of oxidative stress and chronic inflammation decrease nitric oxide bioavailability, leading to endothelial dysfunction [45]. Endothelial dysfunction contributes to impaired renal function through anatomical alterations [46]. In addition, a recent study demonstrated that oxidative stress induces autophagy and apoptosis in podocytes, which maintain the glomerular filtration barrier [47].
This study has some limitations. First, because all the components used in the OBS were equally weighted, they may not have appropriately represented the actual biological contributions. However, studies indicated that there was no significant difference between weighted and unweighted OBS regarding the association with the risk of colorectal adenoma and prostate cancer [24,48]. Second, antioxidants have a threshold effect above which they can show toxic pro-oxidant activity at high doses, but OBS did not consider this and was defined under the assumption that the properties of all components linearly affect oxidative stress [49]. Third, we calculated each nutritional component based on the FFQ; therefore, recall and selection bias may exist. In addition, we could not analyze the changes in OBSs over time because we only had baseline FFQ data. Finally, the OBS used in this study does not include intake of medication, such as NSAIDs and aspirin that may affect oxidative stress levels due to lack of data. Despite these limitations, we believe that our finding of an inverse relationship between the OBS and incident CKD in a prospective cohort study is clinically valuable.
5. Conclusions
In this prospective cohort study, a higher OBS, which indicates a predominance of antioxidant exposure, was associated with a lower risk of incident CKD after adjustments for confounding factors. This study suggests that a healthy diet and lifestyle that increases OBS may help prevent CKD in both men and women.
Author Contributions
Conceptualization, D.-H.S. and J.-H.L.; methodology, D.-H.S. and J.-H.L.; software, D.-H.S., S.-Y.S. and J.-H.L.; validation, D.-H.S., H.S.L., Y.-J.L. and J.-H.L.; formal analysis, D.-H.S., S.-Y.S. and H.S.L.; investigation, J.-H.L.; resources, J.-H.L.; data curation, D.-H.S. and J.-H.L.; writing—original draft preparation, D.-H.S.; writing—review and editing, Y.-J.L. and J.-H.L.; visualization, D.-H.S. and Y.-J.L.; supervision, Y.-J.L. and J.-H.L.; project administration, D.-H.S. and J.-H.L.; funding acquisition, Y.-J.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Nowon Eulji Medical Center (IRB Number: 2021-03-009 and date of approval).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset used in this study can be provided after a review and evaluation of the research plan by the Korea Centers for Disease Control and Prevention (http://www.cdc.go.kr/CDC/eng/main.jsp (accessed on 2 September 2022)).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Research Foundation (NRF) grant funded by the Ministry of Science and ICT of Korea (No. NRF-2022R1A2C1013106).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Honeycutt A.A., Segel J.E., Zhuo X., Hoerger T.J., Imai K., Williams D. Medical costs of CKD in the Medicare population. J. Am. Soc. Nephrol. 2013;24:1478–1483. doi: 10.1681/ASN.2012040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manns B., Hemmelgarn B., Tonelli M., Au F., So H., Weaver R., Quinn A.E., Klarenbach S., Solutions C.S., Disease I.t.O.C.K. The cost of care for people with chronic kidney disease. Can. J. Kidney Health Dis. 2019;6:2054358119835521. doi: 10.1177/2054358119835521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikbov B., Purcell C.A., Levey A.S., Smith M., Abdoli A., Abebe M., Adebayo O.M., Afarideh M., Agarwal S.K., Agudelo-Botero M. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazancioğlu R. Risk factors for chronic kidney disease: An update. Kidney Int. Suppl. 2013;3:368–371. doi: 10.1038/kisup.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dounousi E., Papavasiliou E., Makedou A., Ioannou K., Katopodis K.P., Tselepis A., Siamopoulos K.C., Tsakiris D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. 2006;48:752–760. doi: 10.1053/j.ajkd.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Small D.M., Coombes J.S., Bennett N., Johnson D.W., Gobe G.C. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology. 2012;17:311–321. doi: 10.1111/j.1440-1797.2012.01572.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim B.H., Lee E.S., Choi R., Nawaboot J., Lee M.Y., Lee E.Y., Kim H.S., Chung C.H. Protective effects of curcumin on renal oxidative stress and lipid metabolism in a rat model of type 2 diabetic nephropathy. Yonsei Med. J. 2016;57:664–673. doi: 10.3349/ymj.2016.57.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kregel K.C., Zhang H.J. An integrated view of oxidative stress in aging: Basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007;292:R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 9.Che R., Yuan Y., Huang S., Zhang A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. -Ren. Physiol. 2014;306:F367–F378. doi: 10.1152/ajprenal.00571.2013. [DOI] [PubMed] [Google Scholar]
- 10.Chawla L.S., Eggers P.W., Star R.A., Kimmel P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumgaertel M.W., Kraemer M., Berlit P. Neurologic complications of acute and chronic renal disease. Handb. Clin. Neurol. 2014;119:383–393. doi: 10.1016/B978-0-7020-4086-3.00024-2. [DOI] [PubMed] [Google Scholar]
- 12.Liakopoulos V., Roumeliotis S., Gorny X., Dounousi E., Mertens P.R. Oxidative stress in hemodialysis patients: A review of the literature. Oxidative Med. Cell. Longev. 2017;2017:3081856. doi: 10.1155/2017/3081856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceballos-Picot I., Witko-Sarsat V., Merad-Boudia M., Nguyen A.T., Thévenin M., Jaudon M.C., Zingraff J., Verger C., Jingers P., Descamps-Latscha B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic. Biol. Med. 1996;21:845–853. doi: 10.1016/0891-5849(96)00233-X. [DOI] [PubMed] [Google Scholar]
- 14.Aveles P.R., Criminácio C.R., Gonçalves S., Bignelli A.T., Claro L.M., Siqueira S.S., Nakao L.S., Pecoits-Filho R. Association between biomarkers of carbonyl stress with increased systemic inflammatory response in different stages of chronic kidney disease and after renal transplantation. Nephron Clin. Pract. 2010;116:c294–c299. doi: 10.1159/000318792. [DOI] [PubMed] [Google Scholar]
- 15.Kuchta A., Pacanis A., Kortas-Stempak B., Çwiklińska A., Ziętkiewicz M., Renke M., Rutkowski B. Estimation of oxidative stress markers in chronic kidney disease. Kidney Blood Press. Res. 2011;34:12–19. doi: 10.1159/000321508. [DOI] [PubMed] [Google Scholar]
- 16.Goodman M., Bostick R.M., Dash C., Flanders W.D., Mandel J.S. Hypothesis: Oxidative stress score as a combined measure of pro-oxidant and antioxidant exposures. Ann. Epidemiol. 2007;17:394–399. doi: 10.1016/j.annepidem.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 17.Van Hoydonck P.G., Temme E.H., Schouten E.G. A dietary oxidative balance score of vitamin C, β-carotene and iron intakes and mortality risk in male smoking Belgians. J. Nutr. 2002;132:756–761. doi: 10.1093/jn/132.4.756. [DOI] [PubMed] [Google Scholar]
- 18.Hernández-Ruiz Á., García-Villanova B., Guerra-Hernández E.J., Carrión-García C.J., Amiano P., Sánchez M.-J., Molina-Montes E. Oxidative Balance Scores (OBSs) Integrating Nutrient, Food and Lifestyle Dimensions: Development of the NutrientL-OBS and FoodL-OBS. Antioxidants. 2022;11:300. doi: 10.3390/antiox11020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilori T.O., Ro Y.S., Kong S.Y., Gutierrez O.M., Ojo A.O., Judd S.E., Narayan K.V., Goodman M., Plantinga L., McClellan W. Oxidative balance score and chronic kidney disease. Am. J. Nephrol. 2015;42:320–327. doi: 10.1159/000441623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathur R., Dreyer G., Yaqoob M.M., Hull S.A. Ethnic differences in the progression of chronic kidney disease and risk of death in a UK diabetic population: An observational cohort study. BMJ Open. 2018;8:e020145. doi: 10.1136/bmjopen-2017-020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muntner P., Newsome B., Kramer H., Peralta C.A., Kim Y., Jacobs D.R., Kiefe C.I., Lewis C.E. Racial Differences in the Incidence of Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2012;7:101–107. doi: 10.2215/CJN.06450611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jo H., Kim J.-Y., Jung M.-Y., Ahn Y.-S., Chang S.-J., Koh S.-B. Leisure time physical activity to reduce metabolic syndrome risk: A 10-year community-based prospective study in Korea. Yonsei Med. J. 2020;61:218–228. doi: 10.3349/ymj.2020.61.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernández-Ruiz Á., García-Villanova B., Guerra-Hernández E., Amiano P., Ruiz-Canela M., Molina-Montes E. A review of a priori defined oxidative balance scores relative to their components and impact on health outcomes. Nutrients. 2019;11:774. doi: 10.3390/nu11040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakkur S., Goodman M., Bostick R.M., Citronberg J., McClellan W., Flanders W.D., Judd S., Stevens V.L. Oxidative balance score and risk for incident prostate cancer in a prospective US cohort study. Ann. Epidemiol. 2014;24:475–478.e474. doi: 10.1016/j.annepidem.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong S.Y., Goodman M., Judd S., Bostick R.M., Flanders W.D., McClellan W. Oxidative balance score as predictor of all-cause, cancer, and noncancer mortality in a biracial US cohort. Ann. Epidemiol. 2015;25:256–262.e251. doi: 10.1016/j.annepidem.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho A., Kwon Y.-J., Lim H.-J., Lee H.S., Kim S., Shim J.-Y., Lee H.-R., Lee Y.-J. Oxidative balance score and serum γ-glutamyltransferase level among Korean adults: A nationwide population-based study. Eur. J. Nutr. 2018;57:1237–1244. doi: 10.1007/s00394-017-1407-1. [DOI] [PubMed] [Google Scholar]
- 27.Lee J.H., Son D.H., Kwon Y.J. Association between oxidative balance score and new-onset hypertension in adults: A community-based prospective cohort study. Front. Nutr. 2022;9:1066159. doi: 10.3389/fnut.2022.1066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y., Castro III A.F., Feldman H.I., Kusek J.W., Eggers P., Van Lente F., Greene T. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo M.H., Lee W.-Y., Kim S.S., Kang J.-H., Kang J.-H., Kim K.K., Kim B.-Y., Kim Y.-H., Kim W.-J., Kim E.M. 2018 Korean Society for the Study of Obesity guideline for the management of obesity in Korea. J. Obes. Metab. Syndr. 2019;28:40. doi: 10.7570/jomes.2019.28.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., Clement D.L., Coca A., De Simone G., Dominiczak A. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) Eur. Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 31.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 32.Chun M.Y. Validity and reliability of Korean version of international physical activity questionnaire short form in the elderly. Korean J. Fam. Med. 2012;33:144. doi: 10.4082/kjfm.2012.33.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang M., Won C., Choi H., Kim S., Park W., Kim D., Jeong S., Kim B. Effects of physical activity on fractures in adults: A community-based Korean cohort study. Korean J. Sport. Med. 2017;35:97–102. doi: 10.5763/kjsm.2017.35.2.97. [DOI] [Google Scholar]
- 34.Hockham C., Bao L., Tiku A., Badve S.V., Bello A.K., Jardine M.J., Jha V., Toyama T., Woodward M., Jun M. Sex differences in chronic kidney disease prevalence in Asia: A systematic review and meta-analysis. Clin. Kidney J. 2022;15:1144–1151. doi: 10.1093/ckj/sfac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatia K., Elmarakby A.A., El-Remessey A., Sullivan J.C. Oxidative stress contributes to sex differences in angiotensin II-mediated hypertension in spontaneously hypertensive rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2012;302:R274–R282. doi: 10.1152/ajpregu.00546.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kander M.C., Cui Y., Liu Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017;21:1024–1032. doi: 10.1111/jcmm.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borrás C., Gambini J., Gómez-Cabrera M.C., Sastre J., Pallardó F.V., Mann G.E., Viña J. 17β-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2 [MAPK]/NFκB cascade. Aging Cell. 2005;4:113–118. doi: 10.1111/j.1474-9726.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 38.Prokai L., Prokai-Tatrai K., Perjési P., Simpkins J.W. Mechanistic insights into the direct antioxidant effects of estrogens. Drug Dev. Res. 2005;66:118–125. doi: 10.1002/ddr.20050. [DOI] [Google Scholar]
- 39.Witko-Sarsat V., Friedlander M., Nguyen Khoa T., Capeillère-Blandin C., Nguyen A.T., Canteloup S., Dayer J.M., Jungers P., Drüeke T., Descamps-Latscha B. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J. Immunol. 1998;161:2524–2532. doi: 10.4049/jimmunol.161.5.2524. [DOI] [PubMed] [Google Scholar]
- 40.Terawaki H., Yoshimura K., Hasegawa T., Matsuyama Y., Negawa T., Yamada K., Matsushima M., Nakayama M., Hosoya T., Era S. Oxidative stress is enhanced in correlation with renal dysfunction: Examination with the redox state of albumin. Kidney Int. 2004;66:1988–1993. doi: 10.1111/j.1523-1755.2004.00969.x. [DOI] [PubMed] [Google Scholar]
- 41.Karamouzis I., Sarafidis P.A., Karamouzis M., Iliadis S., Haidich A.B., Sioulis A., Triantos A., Vavatsi-Christaki N., Grekas D.M. Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am. J. Nephrol. 2008;28:397–404. doi: 10.1159/000112413. [DOI] [PubMed] [Google Scholar]
- 42.Dobashi K., Ghosh B., Orak J., Singh I., Singh A. Kidney ischemia-reperfusion: Modulation of antioxidant defenses. Mol. Cell. Biochem. 2000;205:1–11. doi: 10.1023/A:1007047505107. [DOI] [PubMed] [Google Scholar]
- 43.Lahera V., Goicoechea M., de Vinuesa S.G., Oubina P., Cachofeiro V., Gómez-Campderá F., Amann R., Luño J. Oxidative stress in uremia: The role of anemia correction. J. Am. Soc. Nephrol. 2006;17:S174–S177. doi: 10.1681/ASN.2006080911. [DOI] [PubMed] [Google Scholar]
- 44.Kelly J.T., Su G., Zhang L., Qin X., Marshall S., González-Ortiz A., Clase C.M., Campbell K.L., Xu H., Carrero J.-J. Modifiable lifestyle factors for primary prevention of CKD: A systematic review and meta-analysis. J. Am. Soc. Nephrol. 2021;32:239–253. doi: 10.1681/ASN.2020030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roumeliotis S., Mallamaci F., Zoccali C. Endothelial dysfunction in chronic kidney disease, from biology to clinical outcomes: A 2020 update. J. Clin. Med. 2020;9:2359. doi: 10.3390/jcm9082359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonetti P.O., Lerman L.O., Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003;23:168–175. doi: 10.1161/01.ATV.0000051384.43104.FC. [DOI] [PubMed] [Google Scholar]
- 47.Duni A., Liakopoulos V., Roumeliotis S., Peschos D., Dounousi E. Oxidative stress in the pathogenesis and evolution of chronic kidney disease: Untangling Ariadne’s thread. Int. J. Mol. Sci. 2019;20:3711. doi: 10.3390/ijms20153711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dash C., Goodman M., Flanders W.D., Mink P.J., McCullough M.L., Bostick R.M. Using pathway-specific comprehensive exposure scores in epidemiology: Application to oxidative balance in a pooled case-control study of incident, sporadic colorectal adenomas. Am. J. Epidemiol. 2013;178:610–624. doi: 10.1093/aje/kwt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young A.J., Lowe G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001;385:20–27. doi: 10.1006/abbi.2000.2149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used in this study can be provided after a review and evaluation of the research plan by the Korea Centers for Disease Control and Prevention (http://www.cdc.go.kr/CDC/eng/main.jsp (accessed on 2 September 2022)).