To the Editor:
Pirfenidone and nintedanib, antifibrotic medications approved for the treatment of patients with mild to moderate idiopathic pulmonary fibrosis (IPF), have been shown to slow the decline in lung function and are recommended by international treatment guidelines (1).
Meta-analyses of randomized trials of this treatment have investigated their effects on reducing mortality in patients with IPF, with rather divergent conclusions (2–6). Indeed, whereas a meta-analysis concluded that neither pirfenidone nor nintedanib is associated with lower mortality (2), others found reduced mortality only with nintedanib but not pirfenidone (3), or vice-versa (6). Meta-analyses conducted specifically among trials for only one of the antifibrotic drugs concluded a mortality benefit (4, 5).
On the other hand, observational studies have consistently reported remarkable reductions in mortality with antifibrotic medications (7). Such remarkable effects from observational studies are often the result of time-related biases, such as immortal time bias that tends to considerably exaggerate the benefit of drugs, including those used to treat respiratory diseases (8).
Given these inconsistencies, we reviewed the observational studies examining the effect of antifibrotics on mortality in IPF, focusing on time-related biases that could explain these discrepancies.
The Observational Studies
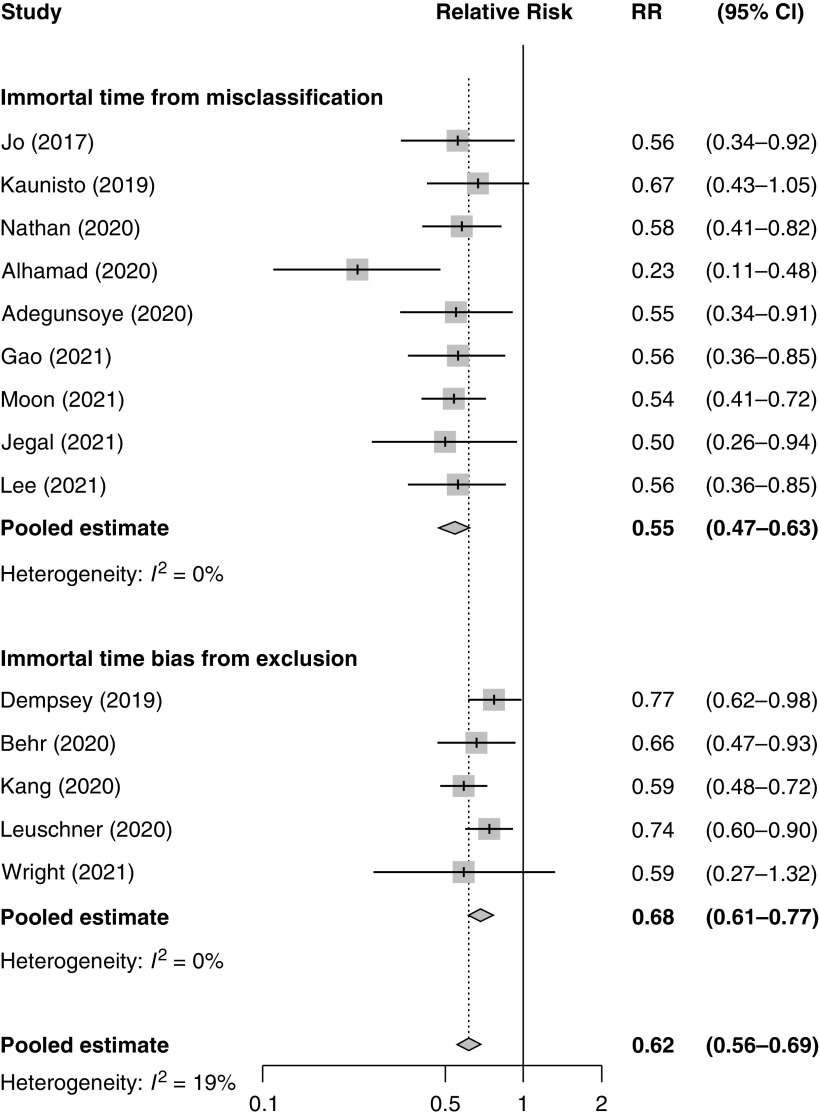
We searched the literature using MEDLINE and Embase for all observational studies of any antifibrotic reporting on mortality in patients with IPF (until January 24, 2022) and identified 14 studies reporting relative risks of death associated with antifibrotic use (9–22). The pooled relative risk of all-cause mortality with antifibrotic use was 0.62 (95% confidence interval [CI], 0.56–0.69) compared with nonuse (Figure 1). We found that all 14 studies used definitions of exposure and follow-up that lead to immortal time bias.
Figure 1.
Forest plot of relative risks of mortality associated with antifibrotic use from the 14 observational studies, with pooled estimates by a random effects model, according to studies affected by immortal time bias from misclassification and from exclusion. CI = confidence interval; RR = relative risk.
Immortal time refers to a period of cohort follow-up during which the outcome under study cannot occur, usually because it involves the time from cohort entry to the start of the treatment under study (23). In essence, the patient necessarily must be alive at the time they initiate treatment and thus “immortal” during this period. Misclassifying or excluding this immortal time period in the design or analysis of an observational study when defining exposure will introduce immortal time bias (24).
For immortal time bias from misclassification, the immortal period is misclassified as exposed rather than unexposed (24). This bias is introduced in a cohort study by classifying patients as exposed to the antifibrotic treatment from the day of cohort entry, even if they only started their treatment later during follow-up. This bias is intensified if the definition of exposure also includes a minimum duration of antifibrotic use, which will increase the length of the immortal time, thus also augmenting the magnitude of the bias.
For immortal time bias by exclusion, the immortal period is differentially excluded from the treated and untreated groups (24). This occurs when the start of follow-up is defined as the initiation of treatment for the exposed group and the date of diagnosis or entry into the registry for the nonusers. Consequently, the immortal period from the date of diagnosis (or of registry entry) to the initiation of treatment is differentially excluded from the analysis in one group, which leads to a spurious protective effect.
Studies with Immortal Time Bias from Misclassification
Immortal time bias resulting from misclassification of the follow-up period from cohort entry to the initiation of antifibrotic treatment as “exposed” was noted in nine studies (9, 11, 13, 15, 16, 18, 20–22). The nine studies affected by this form of immortal time bias had a pooled relative risk of all-cause mortality with antifibrotic use of 0.55 (95% CI, 0.47–0.63) (Figure 1).
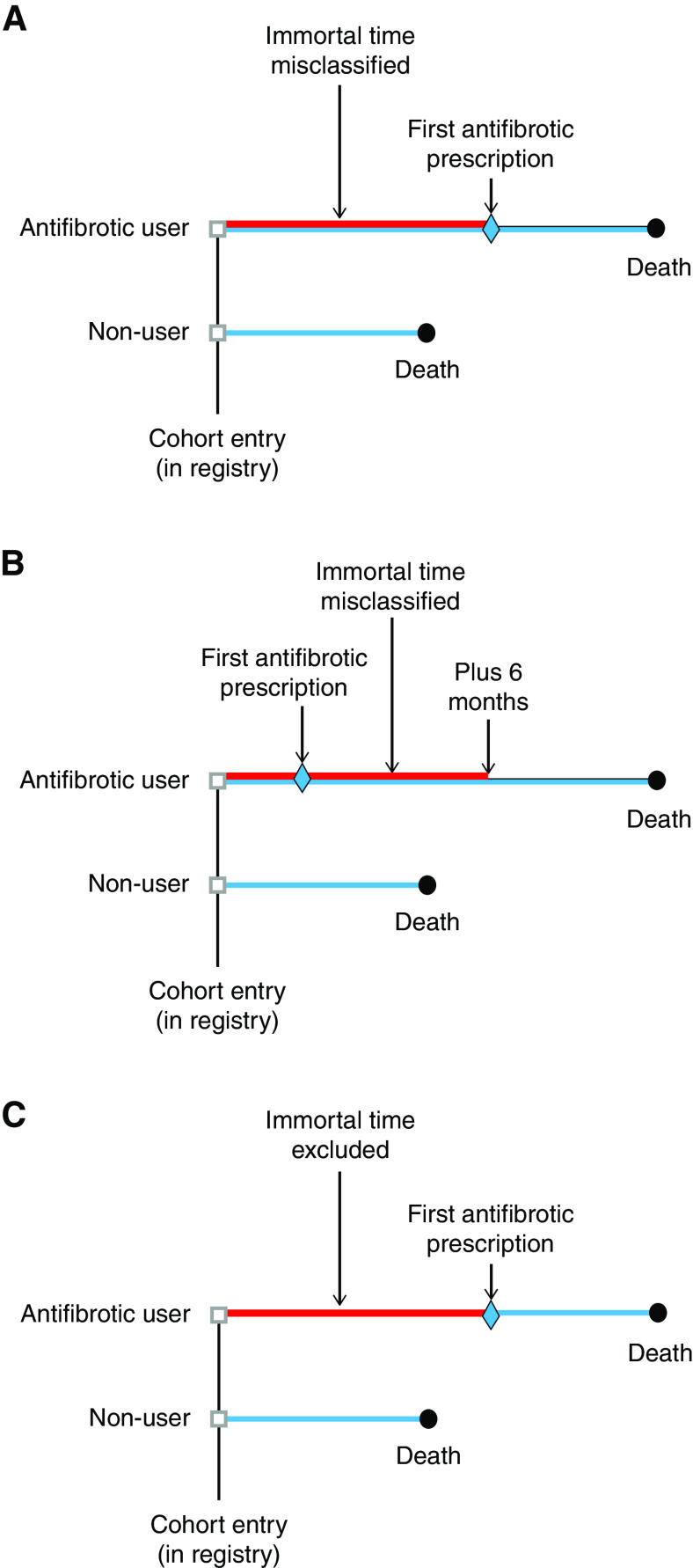
An example of immortal time misclassification is the study using the AIPFR (Australian IPF Registry), a national registry of 647 patients with IPF launched in 2012 (9). For the statistical analysis of mortality, a time to event approach was used, defined as the “time from AIPFR baseline until either death or censoring at last date the patient was known to be alive”. Immortal time bias was introduced in this study by considering the patients as exposed to antifibrotics from the day of AIPFR enrollment, even if they only filled their first prescription afterward, so that the time between cohort entry and the first antifibrotic treatment during follow-up is immortal, as the patient must survive to receive this treatment. Figure 2A depicts this bias by comparing the survival times between two typical cohort patients in which the antifibrotics “users” will necessarily have longer survival, artificially created by this added immortal time. This bias is intensified if exposure is also defined by a minimum duration of antifibrotic use (Figure 2B), which will increase the length of the immortal time. Such immortal time bias from exposure misclassification, with or without an imposed minimum duration of treatment, will result in a biased and exaggerated “protective” effect of antifibrotics exposure.
Figure 2.
Illustration of immortal time bias from cohort studies, with the red line representing misclassified or excluded immortal time and the blue line indicating follow-up period for the outcome. (A) In the “users”, the immortal time period between cohort entry and the first antifibrotic prescription is misclassified as “exposed to antifibrotic” when in fact, the patient is unexposed (9). (B) The immortal time period also includes the additional 6 months of required use to define exposure (16). (C) The immortal time bias from exclusion in cohort studies, which should not be excluded but included as unexposed, with the blue line starting at the first antifibrotic prescription.
Studies with Immortal Time Bias from Exclusion
Immortal time bias resulting from the exclusion of the time period from cohort entry to the initiation of antifibrotic treatment was noted in five studies (10, 12, 14, 17, 19). The five studies affected by this form of immortal time bias had a pooled relative risk of all-cause mortality with antifibrotic use of 0.68 (95% CI, 0.61–0.77) (Figure 1).
This form of immortal time bias, depicted in Figure 2C, was previously noted in the analysis of the Investigating Significant Health Trends in Idiopathic Pulmonary Fibrosis (INSIGHTS-IPF) registry of patients with IPF (12, 25). It reported a significantly lower risk of death (hazard ratio, 0.63; 95% CI, 0.45–0.87) in antifibrotic users compared with nonusers. The INSIGHTS-IPF cohort included 588 patients with IPF, of which 298 received an antifibrotic treatment at some time. Follow-up for mortality started at the treatment initiation for the antifibrotic users and at the registry enrollment date for the nonusers, so the time span between their registry enrollment and treatment initiation dates for the antifibrotic users was excluded from the analysis. This time span is immortal and should be included in the nonuser group up until the time “users” start their antifibrotic treatment; else, its omission introduces immortal time bias (24).
Conclusions
Observational studies are now widely used to evaluate the real-world effectiveness of drugs, especially to study major outcomes, such as mortality, which are rarely available in randomized controlled trials (26). We examined methodological aspects of 14 observational studies reporting a remarkable pooled 40% reduction in all-cause mortality with antifibrotics in the treatment of IPF. We showed that all were affected by immortal time bias, which systematically exaggerates the benefits of drugs.
Immortal time bias has been previously shown to affect many observational studies in the context of respiratory diseases (8), including those suggesting an important benefit of proton pump inhibitors on mortality in treating IPF (27). Observational studies that properly avoided this bias found no or little effect of these drugs on the major outcomes studied (28).
Although observational studies are important to assess the real-world effects of medications on major outcomes, proper design and analysis are essential to minimize bias. The observational studies reporting significantly decreased mortality with antifibrotic use cannot at this time be used as reliable evidence because all were affected by immortal time bias. Indeed, studies with such “critical risk of bias” are too problematic to provide useful evidence and should be excluded from any synthesis (29).
However, immortal time bias, which tends to greatly exaggerate the benefit of drugs, is correctable with proper study design or data analysis. For example, immortal time bias can be avoided by using a time-dependent definition of exposure, such as with the Cox proportional hazards model with time-dependent exposure that allows a patient to move from a period of nonexposure to a period of exposure during the follow-up period (24). One could also use study design approaches such as the prevalent new-user design, which would match antifibrotic initiators with nonusers at the same time point in the disease course, thus avoiding immortal time bias (28, 30). The authors of these 14 publications are urged to repeat the analysis of their studies using such revised approaches that avoid immortal time bias.
Footnotes
Author Contributions: S.S. and K.S. contributed to the conception, analysis, and writing of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202207-1301LE on August 11, 2022
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Raghu G, Selman M. Nintedanib and pirfenidone. New antifibrotic treatments indicated for idiopathic pulmonary fibrosis offer hopes and raises questions. Am J Respir Crit Care Med. 2015;191:252–254. doi: 10.1164/rccm.201411-2044ED. [DOI] [PubMed] [Google Scholar]
- 2. Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE. Drug treatment of idiopathic pulmonary fibrosis: systematic review and network meta-analysis. Chest . 2016;149:756–766. doi: 10.1016/j.chest.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 3. Rogliani P, Calzetta L, Cavalli F, Matera MG, Cazzola M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Pulm Pharmacol Ther . 2016;40:95–103. doi: 10.1016/j.pupt.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 4. Nathan SD, Albera C, Bradford WZ, Costabel U, Glaspole I, Glassberg MK, et al. Effect of pirfenidone on mortality: pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir Med . 2017;5:33–41. doi: 10.1016/S2213-2600(16)30326-5. [DOI] [PubMed] [Google Scholar]
- 5. Lancaster L, Crestani B, Hernandez P, Inoue Y, Wachtlin D, Loaiza L, et al. Safety and survival data in patients with idiopathic pulmonary fibrosis treated with nintedanib: pooled data from six clinical trials. BMJ Open Respir Res . 2019;6:e000397. doi: 10.1136/bmjresp-2018-000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di Martino E, Provenzani A, Vitulo P, Polidori P. Systematic review and meta-analysis of pirfenidone, nintedanib, and pamrevlumab for the treatment of idiopathic pulmonary fibrosis. Ann Pharmacother . 2021;55:723–731. doi: 10.1177/1060028020964451. [DOI] [PubMed] [Google Scholar]
- 7. Petnak T, Lertjitbanjong P, Thongprayoon C, Moua T. Impact of antifibrotic therapy on mortality and acute exacerbation in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Chest . 2021;160:1751–1763. doi: 10.1016/j.chest.2021.06.049. [DOI] [PubMed] [Google Scholar]
- 8. Suissa S, Dell’Aniello S. Time-related biases in pharmacoepidemiology. Pharmacoepidemiol Drug Saf . 2020;29:1101–1110. doi: 10.1002/pds.5083. [DOI] [PubMed] [Google Scholar]
- 9. Jo HE, Glaspole I, Grainge C, Goh N, Hopkins PM, Moodley Y, et al. Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian Idiopathic Pulmonary Fibrosis Registry. Eur Respir J . 2017;49:1601592. doi: 10.1183/13993003.01592-2016. [DOI] [PubMed] [Google Scholar]
- 10. Dempsey TM, Sangaralingham LR, Yao X, Sanghavi D, Shah ND, Limper AH. Clinical effectiveness of antifibrotic medications for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2019;200:168–174. doi: 10.1164/rccm.201902-0456OC. [DOI] [PubMed] [Google Scholar]
- 11. Kaunisto J, Salomaa ER, Hodgson U, Kaarteenaho R, Kankaanranta H, Koli K, et al. Demographics and survival of patients with idiopathic pulmonary fibrosis in the FinnishIPF registry. ERJ Open Res . 2019;5:00170-2018. doi: 10.1183/23120541.00170-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Behr J, Prasse A, Wirtz H, Koschel D, Pittrow D, Held M, et al. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: long-term results of the INSIGHTS-IPF registry. Eur Respir J . 2020;56:1902279. doi: 10.1183/13993003.02279-2019. [DOI] [PubMed] [Google Scholar]
- 13. Nathan SD, Brown AW, Mogulkoc N, Soares F, Collins AC, Cheng J, et al. The association between white blood cell count and outcomes in patients with idiopathic pulmonary fibrosis. Respir Med . 2020;170:106068. doi: 10.1016/j.rmed.2020.106068. [DOI] [PubMed] [Google Scholar]
- 14. Kang J, Han M, Song JW. Antifibrotic treatment improves clinical outcomes in patients with idiopathic pulmonary fibrosis: a propensity score matching analysis. Sci Rep . 2020;10:15620. doi: 10.1038/s41598-020-72607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alhamad EH, Cal JG, Alrajhi NN, Aharbi WM, AlRikabi AC, AlBoukai AA. Clinical characteristics, comorbidities, and outcomes in patients with idiopathic pulmonary fibrosis. Ann Thorac Med . 2020;15:208–214. doi: 10.4103/atm.ATM_230_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adegunsoye A, Alqalyoobi S, Linderholm A, Bowman WS, Lee CT, Pugashetti JV, et al. Circulating plasma biomarkers of survival in antifibrotic-treated patients with idiopathic pulmonary fibrosis. Chest . 2020;158:1526–1534. doi: 10.1016/j.chest.2020.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leuschner G, Klotsche J, Kreuter M, Prasse A, Wirtz H, Pittrow D, et al. INSIGHTS-IPF Registry Group Idiopathic pulmonary fibrosis in elderly patients: analysis of the INSIGHTS-IPF observational study. Front Med (Lausanne) . 2020;7:601279. doi: 10.3389/fmed.2020.601279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao J, Kalafatis D, Carlson L, Pesonen IHA, Li CX, Wheelock Å, et al. Baseline characteristics and survival of patients of idiopathic pulmonary fibrosis: a longitudinal analysis of the Swedish IPF Registry. Respir Res . 2021;22:40. doi: 10.1186/s12931-021-01634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wright WA, Crowley LE, Parekh D, Crawshaw A, Dosanjh DP, Nightingale P, et al. Real-world retrospective observational study exploring the effectiveness and safety of antifibrotics in idiopathic pulmonary fibrosis. BMJ Open Respir Res . 2021;8:e000782. doi: 10.1136/bmjresp-2020-000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moon SW, Kim SY, Chung MP, Yoo H, Jeong SH, Kim DS, et al. Longitudinal changes in clinical features, management, and outcomes of idiopathic pulmonary fibrosis. A nationwide cohort study. Ann Am Thorac Soc . 2021;18:780–787. doi: 10.1513/AnnalsATS.202005-451OC. [DOI] [PubMed] [Google Scholar]
- 21. Jegal Y, Park JS, Kim SY, Yoo H, Jeong SH, Song JW, et al. Clinical features, diagnosis, management, and outcomes of idiopathic pulmonary fibrosis in Korea: analysis of the Korea IPF cohort (KICO) Tuberc Respir Dis (Seoul) . 2022;85:185–194. doi: 10.4046/trd.2021.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee EG, Lee TH, Hong Y, Ryoo J, Heo JW, Gil BM, et al. Effects of low-dose pirfenidone on survival and lung function decline in patients with idiopathic pulmonary fibrosis (IPF): results from a real-world study. PLoS One . 2021;16:e0261684. doi: 10.1371/journal.pone.0261684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf . 2007;16:241–249. doi: 10.1002/pds.1357. [DOI] [PubMed] [Google Scholar]
- 24. Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol . 2008;167:492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 25. Suissa S, Assayag D. Mortality benefit with antifibrotics in idiopathic pulmonary fibrosis: real world evidence or bias? Eur Respir J . 2021;57:2004562. doi: 10.1183/13993003.04562-2020. [DOI] [PubMed] [Google Scholar]
- 26. Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence - what is it and what can it tell us? N Engl J Med . 2016;375:2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 27. Tran T, Suissa S. The effect of anti-acid therapy on survival in idiopathic pulmonary fibrosis: a methodological review of observational studies. Eur Respir J . 2018;51:1800376. doi: 10.1183/13993003.00376-2018. [DOI] [PubMed] [Google Scholar]
- 28. Tran T, Assayag D, Ernst P, Suissa S. Effectiveness of proton pump inhibitors in idiopathic pulmonary fibrosis: a population-based cohort study. Chest . 2021;159:673–682. doi: 10.1016/j.chest.2020.08.2080. [DOI] [PubMed] [Google Scholar]
- 29. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ . 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suissa S, Moodie EE, Dell’Aniello S. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf . 2017;26:459–468. doi: 10.1002/pds.4107. [DOI] [PubMed] [Google Scholar]