Abstract
Rationale
Pediatric-specific ventilator liberation guidelines are lacking despite the many studies exploring elements of extubation readiness testing. The lack of clinical practice guidelines has led to significant and unnecessary variation in methods used to assess pediatric patients’ readiness for extubation.
Methods
Twenty-six international experts comprised a multiprofessional panel to establish pediatrics-specific ventilator liberation clinical practice guidelines, focusing on acutely hospitalized children receiving invasive mechanical ventilation for more than 24 hours. Eleven key questions were identified and first prioritized using the Modified Convergence of Opinion on Recommendations and Evidence. A systematic review was conducted for questions that did not meet an a priori threshold of ⩾80% agreement, with Grading of Recommendations, Assessment, Development, and Evaluation methodologies applied to develop the guidelines. The panel evaluated the evidence and drafted and voted on the recommendations.
Measurements and Main Results
Three questions related to systematic screening using an extubation readiness testing bundle and a spontaneous breathing trial as part of the bundle met Modified Convergence of Opinion on Recommendations criteria of ⩾80% agreement. For the remaining eight questions, five systematic reviews yielded 12 recommendations related to the methods and duration of spontaneous breathing trials, measures of respiratory muscle strength, assessment of risk of postextubation upper airway obstruction and its prevention, use of postextubation noninvasive respiratory support, and sedation. Most recommendations were conditional and based on low to very low certainty of evidence.
Conclusions
This clinical practice guideline provides a conceptual framework with evidence-based recommendations for best practices related to pediatric ventilator liberation.
Keywords: airway extubation, clinical protocols, mechanical ventilators, pediatric intensive care units, ventilator weaning
Contents
Methods
-
Results
CORE Recommendations (Recommendations 1–3)
Systematic Review Recommendations (Recommendations 4–15)
Conclusions: Synthesizing These Recommendations into Clinical Practice
Pediatric critical care providers balance minimizing invasive mechanical ventilation (IMV) duration against the risk of extubation failure and its associated morbidities (1–3). Adult clinical practice guidelines for IMV liberation have been published (4). Although there have been several observational and interventional studies related to aspects of pediatric ventilator liberation, most of the pediatric literature is limited to narrative reviews and meta-analyses (5–9). There is also significant practice variation and limited adoption of ventilator liberation protocols in children (10). We sought to develop the first international pediatrics-specific ventilator liberation clinical practice guidelines focused on acutely hospitalized children receiving IMV for more than 24 hours.
Methods
Please refer to the online supplement for detailed methods and extensive justifications for all recommendations in this executive summary. The guidelines panel was a multiprofessional international group, including two cochairs (S.A.S. and R.G.K.), a lead methodologist (N.B.I.) and assistant methodologist (S.K.K.), and two medical librarians (E.C.W. and H.J.C.). The panel included 19 pediatric intensive care specialists, two respiratory therapists, four nurses, and one expert in human and translational physiology (14 from North America, three from South America, seven from Europe, and two from Asia). Panelists were chosen on the basis of their publications in the area of pediatric ventilator liberation in past 10 years. Panelists were divided into subgroups in charge of literature review, data extraction, and preparing draft recommendations and manuscripts for each clinical question. The committee identified clinical questions and outcomes of importance. As suggested by Grading of Recommendations Assessment, Development, and Evaluation (GRADE), only outcomes that were “critical” or “important” were used to formulate recommendations (11). Abbreviations and nomenclature are defined in detail in Table 1. As part of the modified Convergence of Opinion on Recommendations and Evidence (CORE) process, panelists were asked to select a recommendation for the intervention in each of the clinical questions: 1) in favor, 2) neither for nor against, or 3) against. Three questions had ⩾80% agreement on the direction of the recommendation, which were accepted as CORE recommendations without a formal systematic review (Figure 1) (12). For questions for which consensus was not reached, we used the GRADE approach (13, 14) to identify and summarize relevant evidence and to develop recommendations for clinical practice (Figure 1).
Table 1.
Nomenclature Used during the Guideline Development Process
| Term | Definition |
|---|---|
| Continuous positive airway pressure (CPAP) | Positive pressure with a single continuous distending pressure delivered through endotracheal tube, tracheostomy, or noninvasive interface (e.g., nasal mask, nasal pillows/prongs, full face mask or helmet) |
| Extubation failure | Need for reintubation typically within 72 h of extubation |
| Extubation readiness test (ERT) | A bundle of items that are used to assess the patient’s eligibility to be liberated from invasive mechanical ventilation |
| High-flow nasal cannula (HFNC) | Flow that is delivered through a heated humidified nasal cannula circuit and interface |
| Noninvasive ventilation (NIV) | Positive pressure with variable levels of pressure delivered without an artificial airway (e.g., nasal mask, nasal pillows/prongs, full face mask or helmet) |
| Noninvasive respiratory support (NRS) | HFNC, CPAP, or NIV |
| Spontaneous breathing trial (SBT) | A systematic method of reduction of ventilator support to assess patient’s ability to independently maintain gas exchange without excessive respiratory effort |
Figure 1.
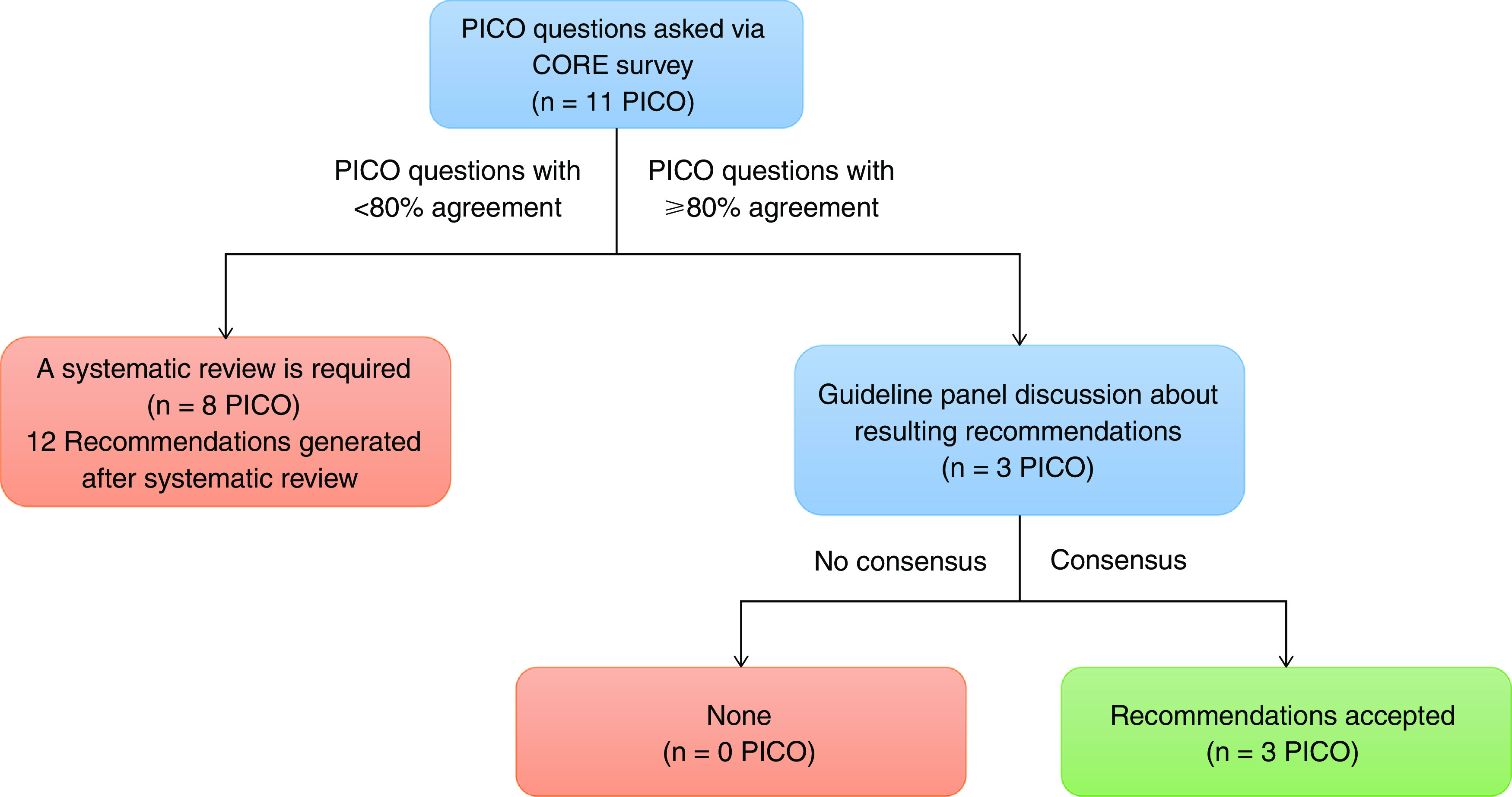
Guidelines development process. Adapted with permission from Reference 12. PICO = Population, Intervention, Comparator, Outcome.
Eight PICO (Population, Intervention, Comparator, Outcome) questions, encompassing five comprehensive literature searches, were run in MEDLINE (Ovid), Embase (Elsevier), and CINAHL Complete (EBSCOhost) in March 2021 and rerun in January 2022. Risk of bias was assessed using the Cochrane Risk of Bias-2 tool for randomized trials and the ROBINS-I tool for observational studies (15, 16). We used GRADEpro Guideline Development Tool online software to develop evidence profiles for each PICO question (13, 17, 18). To pool quantitative data, we performed meta-analyses using random effects models and Review Manager software (RevMan). For recommendations 9–12, we performed a random effects model network meta-analysis in a Bayesian framework (19).
When randomized controlled trials (RCTs) were available, only these were used to create the evidence profiles. Observational studies were used only when relevant outcome data were not available from RCTs (20). We used the GRADE framework to determine the certainty of evidence (21). For one question (recommendation 6), there was no direct or indirect evidence to inform the recommendation. To provide expert opinion using a systematic process, we used the RAND-UCLA Appropriateness Method to ascertain the panel’s judgment on different spontaneous breathing trial (SBT) durations for different extubation contexts (22). Recommendations were described as “strong” or “conditional” and the categorization was based on the GRADE’s Evidence to Decision framework (11). Recommendations developed using the CORE process were considered conditional because this method does not include the rating of the certainty of evidence. The guidelines PICO questions and summary of recommendations are provided in Table 2. The implication of the strength of recommendations for different stakeholders is provided in Table 3. We offered good practice statements in the absence of direct evidence, using guidelines provided by GRADE, when it was clear that implementing the recommendation will result in a large net positive effect (23). These guidelines apply to all children (aged 1 d–18 yr). Although many of these principles extend to preterm neonates and young adults, ventilator liberation in those populations is not specifically covered in these guidelines. This clinical practice guideline was endorsed by the Society of Critical Care Medicine on June 27, 2022, and by the American Thoracic Society on July 27, 2022.
Table 2.
Guidelines PICO Questions and Summary of Recommendations
| PICO Question | Recommendations | Strength of Recommendation | Certainty of Evidence |
|---|---|---|---|
| Should acutely hospitalized children receiving conventional mechanical ventilation for more than 24 h have protocolized screening to assess eligibility for ERT? | 1. We suggest the use of protocolized screening compared with no screening to assess eligibility for ERT. | CORE statement | N/A |
| Should acutely hospitalized children receiving conventional mechanical ventilation for more than 24 h have a protocolized extubation readiness bundle performed? | 2. We suggest using a protocolized ERT bundle compared with clinical assessment of extubation readiness. | CORE statement | N/A |
| In acutely hospitalized children receiving conventional mechanical ventilation for more than 24 h, should an SBT be included in determining extubation readiness? | 3. We suggest performing an SBT, as part of an ERT bundle to objectively assess the patient’s ability to independently maintain adequate minute ventilation and gas exchange without excessive respiratory effort if liberated from IMV. | CORE statement | N/A |
| In acutely hospitalized children receiving conventional mechanical ventilation for more than 24 h who are undergoing an SBT as part of extubation readiness assessments, should inspiratory pressure augmentation (i.e., PS or automatic tube compensation) be used? | 4. We suggest using either PS augmentation with CPAP or CPAP alone during SBTs in mechanically ventilated children at standard risk of extubation failure. | Conditional | Very low |
| 5. For children at higher risk of extubation failure, we suggest using CPAP without PS augmentation during SBTs for better assessment of extubation readiness. | Conditional | Very low | |
| In acutely hospitalized children receiving conventional mechanical ventilation for more than 24 h who are undergoing an SBT to assess for extubation readiness, should the SBT be conducted for 30 min or 60–120 min? | 6. We suggest the SBT be conducted for either 30 min or 60–120 min. | Conditional | Very low |
| In acutely hospitalized children receiving conventional mechanical ventilation for more than 24 h, should a measure of respiratory muscle strength during airway occlusion (i.e., NIF or PiMax) or function be included in determining extubation readiness? | 7. We suggest using PiMax as an element of the ERT bundle for critically ill children at risk for muscle weakness or at risk for extubation failure. | Conditional | Very low |
| In acutely hospitalized children receiving conventional mechanical ventilation for more than 24 h, should an endotracheal tube air leak test be measured before extubation to predict postextubation UAO? | 8. We suggest using the air leak test, in children with cuffed ETT, as part of the ERT bundle, to assess the risk for the development of postextubation UAO. | Conditional | Very low |
| In acutely hospitalized children receiving conventional mechanical ventilation for more than 24 h, should systemic corticosteroids be administered before extubation to prevent postextubation UAO? | 9. We suggest using dexamethasone at least 6 h before extubation in children at high risk of developing postextubation UAO. | Conditional | Very low |
| In acutely hospitalized children receiving conventional mechanical ventilation for more than 24 h, should planned noninvasive respiratory support (HFNC, CPAP, or NIV) be used after extubation? In acutely hospitalized children being extubated to planned noninvasive respiratory support (HFNC, CPAP, or NIV), would CPAP/NIV be superior to HFNC? |
10. For children at high risk for extubation failure, we suggest using NRS (which includes HFNC, CPAP, or NIV) over conventional oxygen therapy immediately after extubation. | Conditional | Very low |
| 11. For children developing respiratory distress while receiving conventional oxygen therapy after extubation, we suggest using NRS over continued use of conventional oxygen therapy. | Conditional | Very low | |
| 12. For children <1 yr of age who are being started on NRS (either planned or rescue), we suggest the use of CPAP over HFNC. | Conditional | Low | |
| In acutely hospitalized children receiving conventional mechanical ventilation for more than 24 h, should a goal-directed sedation protocol be used compared with nonprotocolized sedation management to guide sedation management during mechanical ventilation and endotracheal extubation? | 13. We recommend that the level of sedation, cough effectiveness, and capacity to manage oropharyngeal secretions be evaluated before extubation. | Good practice statement | N/A |
| 14. We recommend a targeted sedation management strategy using a validated, reliable tool to set sedation targets. | Good practice statement | N/A | |
| 15. We suggest either the use of a standardized sedation titration protocol or no standardized protocol to guide targeted sedation management during IMV and ERT. | Conditional | Moderate |
Definition of abbreviations: CPAP = continuous positive airway pressure; ERT = extubation readiness testing; ETT = endotracheal tube; HFNC = high-flow nasal cannula, IMV = invasive mechanical ventilation; NIF = negative inspiratory force; NIV = noninvasive ventilation; NRS = noninvasive respiratory support (HFNC, CPAP, or NIV); PiMax = maximal inspiratory pressure during airway occlusion; PS = pressure support; SBT = spontaneous breathing trial; UAO = upper airway obstruction.
Table 3.
Implications of Strength of Recommendations to Stakeholders
| Stakeholder | Strong Recommendation | Conditional Recommendation |
|---|---|---|
| Patients | Most individuals in this situation would want the recommended course of action, and only a small proportion would not. | The majority of individuals in this situation would want the suggested course of action, but many would not. |
| Clinicians | Most individuals should receive the recommended course of action. | Recognize that different choices will be appropriate for different patients and that you must help each patient arrive at a management decision consistent with her or his values and preferences. |
| Policy makers | The recommendation can be adapted as policy in most situations, including for use as performance indicators. | Policy making will require substantial debates and involvement of many stakeholders. Policies are also more likely to vary between regions. |
Results
CORE Recommendations (Recommendations 1–3)
Recommendation 1
We suggest the use of protocolized screening compared with no screening to assess eligibility for extubation readiness testing (ERT). (CORE statement, ungraded, 100% agreement)
Remarks
Protocolized screening for eligibility for ERT should be conducted at regular intervals to identify when a patient has met prespecified targets for physiologic parameters, ventilator settings, or pathology-specific milestones to safely conduct ERT.
Rationale
Panelists based this recommendation on data from five RCTs (24–28) and three quality improvement (QI) studies (29–31). Most studies identified a reduction in IMV duration or time of weaning for those undergoing systematic ERT screening, ranging from several hours to several days (24, 25, 28, 31). In addition, several studies identified lower rates of extubation failure (27, 29), although many studies do not specifically separate protocolized screening from other elements of the ERT bundle. There are likely no patient-related undesirable effects with judicious screening criteria. There are potential undesirable effects related to staff burden and screening fatigue that may contribute to low rates of compliance (30), although these effects can be minimized when screening is integrated into the clinical workflow (29, 31). Some studies have observed increased use of a postextubation high-flow nasal cannula (HFNC) (29–31) and noninvasive ventilation (NIV) (28, 30). Protocolized screening should include a series of physiologic parameters, ventilator targets, or pathology-specific milestones that are applied to all eligible patients at regular, periodic intervals to determine whether they have reached an appropriate point from which to proceed with ERT. Examples of ERT safety screening criteria are shown in Table E1 in the online supplement. Screening can be conducted by any qualified member of the care team.
Recommendation 2
We suggest using a protocolized ERT bundle compared with clinical assessment of extubation readiness. (CORE statement, ungraded, 88% agreement)
Remarks
This ERT bundle includes elements that are used to assess if the patient is ready to be liberated from IMV. In addition to an SBT, this may include factors such as assessment of sedation level, adequacy of neurologic control of the airway (i.e., cough and gag), likelihood of postextubation upper airway obstruction (UAO), assessment of respiratory muscle strength, magnitude of airway secretions, hemodynamic status, and a plan for postextubation respiratory support.
Rationale
Panelists based this recommendation on data from three QI studies (29–31). The implementation of a protocolized ERT bundle resulted in lower extubation failure rates (absolute risk reduction between 3.3% and 11.7%) (29, 31), with sensitivity and positive predictive value for extubation success with the use of an ERT bundle of 90% and 94%, respectively (31). No study demonstrated a significant difference with respect to IMV duration, but one study observed a significant reduction in pediatric ICU (PICU) length of stay (LOS) (31). Very few adverse effects were reported after the implementation of an ERT bundle (29), with similar rates of unplanned extubation between those subjects managed with and without extubation readiness protocols. There may be a risk of higher postextubation NIV use after ERT bundles are implemented (30). ERT bundles provide a systematic approach within the process of evaluating whether a pediatric patient is ready to be successfully liberated from IMV: a daily screening followed by an SBT and a series of pulmonary and nonpulmonary criteria to help with decision making.
Recommendation 3
We suggest performing an SBT as part of an ERT bundle to objectively assess the patient’s ability to independently maintain adequate minute ventilation and gas exchange without excessive respiratory effort if liberated from IMV. (CORE statement, ungraded, 96% agreement)
Rationale
Panelists based this recommendation on data from three RCTs (24, 28, 32), three QI studies (29–31), and two observational studies (27, 33). The use of SBTs was associated with lower extubation failure rates in several studies (28, 29, 32, 33), although others showed no difference in extubation failure rates (24, 30, 31). No studies showed higher extubation failure rates with the use of SBTs. The diagnostic accuracy of SBTs in predicting extubation success is high, with positive predictive value >90% (27, 33). Almost all studies have shown that IMV duration or length of the weaning phase is either shorter or no different in patients who receive an SBT compared with patients not subjected to an SBT. Reductions in IMV duration were as large as 30% (hazard ratio, 0.70; 95% confidence interval [CI], 0.53–0.9) (median, 1.2 d) (24) in some studies, although other studies report smaller differences (i.e., median of 6.1 h [28] or no difference [29, 31, 32]). No studies showed longer IMV duration with SBTs. There is no clear signal of increased harm with the use of SBTs identified in these studies. An additional risk relates to potential higher use of postextubation NIV or HFNC, although this finding is not consistent (24, 28, 29). Conduct of the SBT should include a procedure to reduce ventilator settings to prespecified values (see recommendations 4 and 5) with systematic evaluation by bedside providers of the patient’s ability to maintain adequate minute ventilation and gas exchange without excessive respiratory effort.
Systematic Review Recommendations (Recommendations 4–15)
Recommendations 4 and 5
-
•
We suggest using either pressure support (PS) augmentation with continuous positive airway pressure (CPAP) or CPAP alone during SBTs in mechanically ventilated children at standard risk for extubation failure (Table 4). (Conditional recommendation, very low certainty of evidence)
-
•
For children at higher risk of extubation failure (Table 4), we suggest using CPAP without PS augmentation during SBTs for better assessment of extubation readiness. (Conditional recommendation, very low certainty of evidence)
Table 4.
Populations to Consider as Potentially High Risk for Extubation Failure
| Younger age |
| Prolonged invasive mechanical ventilation (>14 d) |
| Chronic lung disease |
| Chronic critical illness |
| Preexisting CPAP/NIV use for any reason |
| Myocardial dysfunction |
| Neurologic impairment |
| Neuromuscular disease |
| Upper airway anomalies/surgical interventions |
| Trisomy 21 and other genetic syndromes |
| Previously failed extubation |
| Borderline passing SBT |
Definition of abbreviations: CPAP = continuous positive airway pressure; NIV = noninvasive ventilation; SBT = spontaneous breathing trial.
Rationale
One RCT evaluated critical outcomes related to extubation failure, mortality, or LOS (34) and showed no significant difference between PS-augmented and T-piece SBT. Three observational studies have shown that work/effort of breathing was significantly lower during PS-augmented SBTs versus CPAP alone and that PS augmentation significantly underestimates postextubation work/effort of breathing (35–37). Underestimation of effort of breathing may result in premature extubation and an increased extubation failure rate. Conversely, perceived high work of breathing on CPAP alone compared with PS with CPAP may result in delayed extubation for several patients who potentially could be extubated successfully, leading to longer IMV duration. This effect was not demonstrated in the only pediatric RCT. We considered avoidance of extubation failure and its associated sequelae as the most critical outcome for patients and therefore gave it the highest weight. On the basis of the available evidence, we are unable to state an overall benefit of one approach to SBTs over the other. In patients who may be at higher risk of extubation failure, the panel valued a higher degree of accuracy in predicting extubation failure (i.e., positive predictive value) and therefore recommended the use of CPAP only for SBTs in these subpopulations.
Recommendation 6
We suggest the SBT be conducted for either 30 minutes or 60–120 minutes, depending on the patient’s risk for extubation failure. (Conditional recommendation, very low certainty of evidence)
Remarks
For children at high risk of extubation failure (Table 4), the panel considered a longer SBT of 60–120 minutes as more appropriate.
Rationale
There were no studies directly comparing different SBT durations. Data from seven RCTs (24, 26, 28, 32, 34, 38, 39) and 11 observational cohort studies (29, 31, 33, 40–47) were used to provide indirect evidence about SBT duration. A shorter SBT (i.e., 30 min) is likely to result in more patients passing the SBT, potentially shortening the IMV duration. In contrast, a longer SBT (i.e., 60–120 min) is likely to result in a lower rate of extubation failure, although none of the studies were able to confirm these theoretical benefits. It is likely that a 60–120-minute SBT, when compared with a 30-minute SBT, can better approximate the effort of breathing after extubation, especially in patients at higher risk of extubation failure (e.g., cardiac disease, neuromuscular condition, prolonged IMV). We considered avoidance of extubation failure and its associated sequelae as the most critical outcome for patients and therefore weighted this outcome more importantly for patients at higher risk for extubation failure. Most panelists considered an SBT <30 minutes inappropriate for any mechanically ventilated child who has been ventilated for more than 24 hours. For standard-risk patients, SBT durations between 30 and 60 minutes were considered the most appropriate because lowering the already low risk of extubation failure does not clearly outweigh the benefit of a potentially more accurate SBT. For high-risk patients, SBT durations between 60 and 120 minutes were considered the most appropriate, given that preventing extubation failure is a higher priority, and a 60–120-minute SBT was considered to have higher diagnostic accuracy. Risk factors considered for high-risk patients are summarized in Table 4.
Recommendation 7
We suggest using measurement of maximal inspiratory pressure during airway occlusion (PiMax) as an element of the ERT bundle for critically ill children at risk for muscle weakness or at risk for extubation failure. (Conditional recommendation, very low certainty of evidence)
Remarks
Based on existing evidence, the optimal cutoff for PiMax cannot be recommended. A PiMax <20 cm H2O suggests increased risk of extubation failure due to inspiratory muscle weakness, whereas a PiMax >50 cm H2O suggests preserved inspiratory muscle strength and therefore reduced risk of extubation failure because of poor inspiratory muscle function.
Rationale
Nineteen studies assessing associations between respiratory muscle function before extubation and extubation outcomes were identified. Nine studies evaluated maximal inspiratory pressure (PiMax or an equivalent measure) (40, 48–55), seven studies evaluated diaphragmatic ultrasound (56–62), and three studies evaluated respiratory muscle electromyography (63–65). Compared with studies of PiMax, studies of diaphragmatic ultrasound and respiratory muscle electromyography recruited fewer participants, were more heterogeneous, and required technologies and expertise that are not readily available or easily implementable at most institutions. All but one of the included studies assessing PiMax showed an association between PiMax and extubation success. Studies report various PiMax thresholds (20–50 cm H2O) with wide ranges for sensitivity for extubation success (12.5–100%) and specificity (50–96%) (40, 48, 49, 51–55). In one study, a PiMax threshold of 20 cm H2O was associated with the lowest sensitivity but the highest specificity for extubation success (40), whereas other studies have shown that a PiMax of 50 cm H2O had higher sensitivities (50–100%) but variable specificities (50–94%) (51, 53, 55). Hence, PiMax measurement can be beneficial to improve the diagnostic accuracy of extubation failure risk and may be particularly important in children who have a higher baseline risk of extubation failure (Table E7). No studies reported any adverse events from PiMax measurement. Because the diagnostic accuracy of PiMax for predicting extubation success is variable, there is a potential that systematic measurement of respiratory muscle function may result in delayed extubation if PiMax is considered inadequate. Furthermore, we cannot recommend a specific PiMax threshold for discriminating children with respiratory muscle weakness. Although pediatric evidence is limited, risk factors of respiratory muscle weakness include prolonged IMV, neuromuscular disease, prolonged use of corticosteroids or neuromuscular blocking agents, sepsis, malnutrition, and chronic illnesses. Identification of respiratory muscle weakness was considered to be important for patients and clinicians because it could identify patients at higher risk of extubation failure and may prompt additional preventive or therapeutic strategies.
Recommendation 8
We suggest using the air leak test in children with a cuffed endotracheal tube (ETT) as part of the ERT bundle to assess the risk for the development of postextubation UAO. (Conditional recommendation, very low certainty evidence)
Remarks
For children with an uncuffed ETT, an air leak test is an unreliable method to assess the risk for the development of postextubation UAO.
Rationale
We identified eight observational studies (66–73) using air leak at the time of extubation. The diagnostic accuracy of air leak testing varies, depending on whether the ETT is cuffed or uncuffed. For children with cuffed ETTs, the presence of an air leak at the time of extubation (below 25–30 cm H2O) did not have a clear relationship with extubation failure (pooled sensitivity, 0.33 [95% CI, 0.13–0.60]; pooled specificity, 0.80 [95% CI, 0.54–0.93]). For the outcome of postextubation UAO, the presence of an air leak at the time of extubation had some diagnostic accuracy (pooled sensitivity, 0.57 [95% CI, 0.39–0.73]; pooled specificity, 0.91 [95% CI, 0.32–1.00]) (67, 70–72) (Table E11). For children with uncuffed ETTs, the presence of an air leak (below 25–30 cmH2O) at the time of extubation has no clear relationship with extubation failure (pooled sensitivity, 0.44 [95% CI, 0.27–0.62]; pooled specificity, 0.58 [95% CI, 0.32–0.80]) (69). The results were similar for the outcome of postextubation UAO (pooled sensitivity, 0.37 [95% CI, 0.23–0.54]; pooled specificity, 0.56 [95% CI, 0.40–0.71]) (66–68, 70, 73) (Table E11). The potential benefits of identifying patients at higher risk of postextubation UAO include administering dexamethasone (see recommendation 9) to prevent subglottic postextubation UAO. Although the risk of performing an air leak test itself at the time of extubation is negligible, the actions that may follow because of the air leak test could have unintended negative consequences. Given the low sensitivity, identifying patients who do not have an air leak could result in a delay in extubation to administer dexamethasone, which may prolong IMV duration.
Recommendation 9
We suggest using dexamethasone at least 6 hours before extubation in children at high risk of developing postextubation UAO. (Conditional recommendation, very low certainty of evidence)
Remarks
Although data from our network meta-analysis estimated a benefit with the use of dexamethasone to prevent UAO in all subgroups, there was unclear benefit in decreasing extubation failure caused by UAO. As such, the panel considered that extubation should not be delayed by administering a course of dexamethasone, particularly in standard-risk children.
Rationale
Data from eight RCTs (74–81) were used for pairwise and network meta-analyses (82). In the pairwise analysis, compared with placebo, prophylactic dexamethasone did not result in a statistically significant reduction in extubation failure rates (odds ratio [OR], 0.55 [95% CI, 0.21–1.46]; absolute risk reduction, 73 fewer per 1,000 patients [95% CI, 137 fewer reintubations to 63 more reintubations]) (Table E12). However, prophylactic dexamethasone did result in a decrease in the incidence of UAO (OR, 0.40 [95% CI, 0.21–0.73]; absolute risk reduction, 205 fewer per 1,000 patients [95% CI, 306 to 76 fewer]) (Table E12).
As part of the guidelines, Iyer and colleagues published a network meta-analysis which identified that early use of dexamethasone (⩾12 h before extubation) was likely the most important factor to consider, and, when started early, high- or low-dose regimens were associated with a similar likelihood of UAO prevention and were likely better than either high- or low-dose regimens started later (83). Similar results were seen when using >6 hours before extubation as the definition of early use, although the effect size was slightly smaller and credible intervals wider. When dexamethasone was administered within 6 hours of extubation, use of higher-dose dexamethasone (⩾0.5 mg/kg/dose) was likely to have some benefit for prevention of postextubation UAO, whereas lower-dose dexamethasone (<0.5 mg/kg/dose) within 6 hours of extubation appeared to have minimal impact on preventing extubation failure or postextubation UAO. Given the preference for early administration of dexamethasone, there is therefore a theoretical concern for delayed extubation when clinicians wait for dexamethasone administration before extubation.
For patients at high risk for postextubation UAO (Table 5), the benefits of prophylactic dexamethasone administered at least 6 hours before extubation for preventing extubation subglottic postextubation UAO and failure outweigh potential risks, including delaying extubation by up to 6 hours. However, the panel believed that in patients at standard risk for postextubation UAO, incremental benefits of dexamethasone are not outweighed by potential delays in extubation.
Table 5.
Populations to Consider as Potentially High Risk for Upper Airway Obstruction
| Multiple intubation attempts |
| Traumatic intubation |
| Use of large-for-age ETT |
| ETT air leak pressure >25 cm H2O for cuffed ETT |
| Anatomical anomaly of upper airways |
Definition of abbreviation: ETT = endotracheal tube.
Recommendations 10, 11, and 12
-
•
For children at high risk for extubation failure, we suggest using noninvasive respiratory support (NRS; including HFNC, CPAP, or NIV) over conventional oxygen therapy immediately after extubation (Table 4). (Conditional recommendation, very low certainty of evidence)
-
•
For children developing respiratory distress while receiving conventional oxygen therapy after extubation, we suggest using NRS over continued use of conventional oxygen therapy. (Conditional recommendation, very low certainty of evidence)
-
•
For children <1 year of age who are being started on NRS (either planned or rescue), we suggest the use of CPAP over HFNC. (Conditional recommendation, low certainty of evidence)
Remarks
-
•
For children >1 year of age who are started on NRS; CPAP, HFNC, and NIV are appropriate first-line therapies, and the choice will depend on the clinical setting and patient circumstances.
-
•
NIV can be considered if CPAP or HFNC does not relieve postextubation respiratory distress or for children who receive NIV for other chronic conditions.
Rationale
We identified two RCTs comparing the effectiveness of HFNC with that of CPAP after extubation as planned or rescue treatment (84, 85) and five RCTs comparing HFNC (86–88), CPAP (89), or NIV (90) against conventional oxygen therapy. Treatment with NRS versus conventional oxygen therapy had an OR for reducing extubation failure of 0.6 (95% CI, 0.31–1.14) (Figure E15). Treatment with NRS after extubation would result in 30 fewer extubation failures per 1,000 patients in a control population with an expected extubation failure rate of 8% and 83 fewer extubation failures in high-risk populations where the expected failure rate is 25%. To try to understand which NRS therapy was most effective (i.e., HFNC vs. CPAP/NIV), we conducted a network meta-analysis in which both HFNC (OR, 0.53; 95% credible interval, 0.23–1.2) and CPAP/NIV (OR, 0.49; 95% credible interval, 0.19–1.2) had better odds than conventional oxygen therapy of preventing extubation failure (Table E15). For preventing extubation failure, CPAP/NIV had the highest probability of being ranked the most effective therapy (60%), followed by HFNC (38%) (Table E15). For the combined outcome of treatment failure, CPAP/NIV also had the highest probability of being ranked the most effective therapy (69%), followed by HFNC (31%) (Table E15). In pairwise meta-analysis comparing HFNC with CPAP in mostly patients <1 year of age, CPAP had 5% less reintubations at any time after the first extubation (OR, 0.7; 95% CI, 0.47–1.04) and lower in-hospital mortality than HFNC (OR, 0.38; 95% CI, 0.15–0.97). In terms of risks, the use of NRS could result in a prolonged PICU and hospital LOS. In the few studies in which these outcomes were reported, conventional oxygen therapy was associated with a 0.74-day (95% CI, −0.72 to 2.19) reduction in PICU LOS and 9-day (95% CI, −0.97 to 18.9) reduction in hospital LOS, although there is significant imprecision in these estimates (88). Treatment with CPAP/NIV may be poorly tolerated in some children, but this outcome is rarely reported (85, 90).
Recommendations 13, 14, and 15
-
•
We recommend that the level of sedation, cough effectiveness, and capacity to manage oropharyngeal secretions be evaluated before extubation. (Ungraded, good practice statement)
-
•
We recommend a targeted sedation management strategy using a validated, reliable tool to set sedation targets. (Ungraded, good practice statement)
-
•
We suggest either the use of a standardized sedation titration protocol or no standardized protocol to guide targeted sedation management during IMV and ERT. (Conditional recommendation, moderate certainty of evidence)
Remarks
There were no studies specifically focused on sedation management in the periextubation period; the panel thus voted to examine the clinical impact of protocolized sedation over the entire course of IMV.
Rationale
We identified two RCTs (n = 11,292) (28, 91) that randomized by PICU. One study included mechanically ventilated children with acute respiratory failure with an expected length of IMV >24 hours (RESTORE [Randomized Evaluation of Sedation Titration for Respiratory Failure]) (91). The other RCT included all patients receiving IMV but reported a prespecified analysis of patients with expected duration of IMV >24 hours at the time of admission based on diagnosis (SANDWICH [Sedation AND Weaning In Children]) (28). Both RCTs compared usual PICU care with an intervention consisting of protocolized sedation assessment, targeted sedation goals, and ERT. Both studies used validated sedation tools to assess level of consciousness and the patient’s ability to comfortably accept ventilation, breathe spontaneously, and respond to stimulation and console. The SANDWICH trial demonstrated a statistically significant 0.25-day reduction in IMV duration (95% CI, −0.34 to −0.22 d) for patients receiving the intervention (Table E18) (28), although this difference did not meet the panel’s a priori threshold for clinical significance, which was 12 hours. The RESTORE trial demonstrated no difference in IMV duration (91). Absolute extubation failure rates were 0.5–0.6% lower in patients in the intervention groups in both RCTs, but neither was statistically different from the usual care group. The SANDWICH trial demonstrated a significantly shorter hospital LOS for the usual care group (median, 0.91 d shorter; interquartile range, 0.84–0.97) (28), increased use of NIV postextubation among intervention patients (adjusted relative risk, 1.22; 95% CI, 1.01–1.49), and a higher frequency of unplanned extubation (adjusted relative risk, 1.62; 95% CI, 1.05–2.51) (28). The RESTORE trial showed a higher rate of postextubation stridor among the intervention group (adjusted relative risk, 1.6; 95% CI, 1.15–2.22) (91). In addition to these potential harms, there is a potential burden on PICUs to incorporate protocolized sedation management, which may increase human costs and personnel. Although the benefits of a sedation titration protocol are not clear, critical care providers should work on strategies of incorporating the use of valid and reliable sedation assessment scales with a targeted goal in their daily workflow.
Conclusions: Synthesizing These Recommendations into Clinical Practice
As has been shown in several pediatric studies, extubation failure is often multifactorial. For this reason, extubation evaluation should consider multiple factors and requires clinical judgment. A systematic approach to evaluate parameters that characterize risk for extubation failure should be used and can be operationalized in an ERT bundle. We believe that the elements proposed as part of this guideline characterize the most important factors to consider before ventilator liberation in children. We synthesized these concepts in a flowchart (Figure 2) and provide more guidance on implementation considerations in the online supplement. Unfortunately, the certainty of evidence was low or very low for nearly all our recommendations, highlighting the need for high-quality research in each of these domains.
Figure 2.
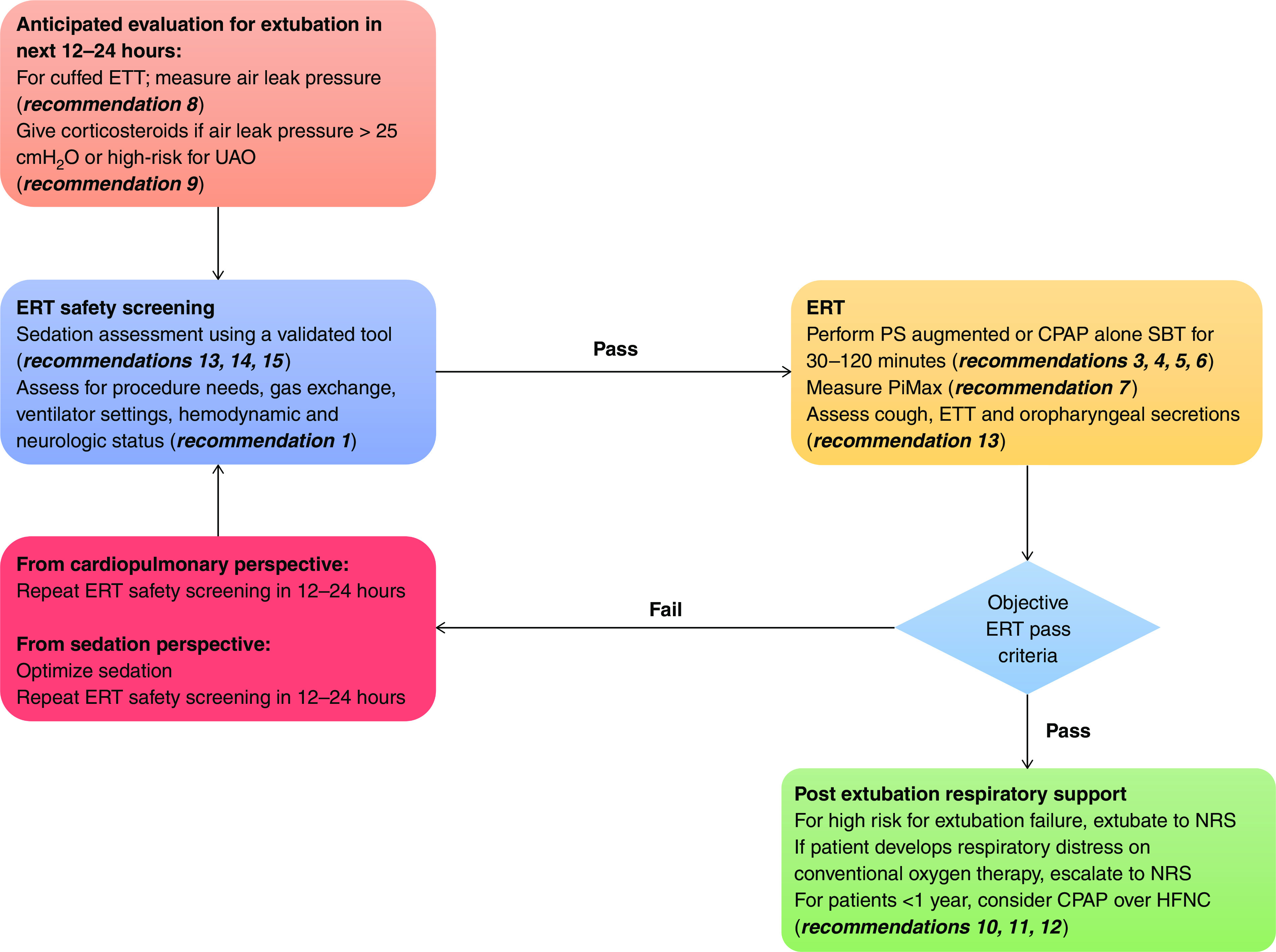
Extubation readiness testing conceptual framework and bundle elements. CPAP = continuous positive airway pressure; ERT = extubation readiness testing; ETT = endotracheal tube; HFNC = high-flow nasal cannula, NRS = noninvasive respiratory support (HFNC, CPAP, or NIV); PiMax = maximal inspiratory pressure during airway occlusion; PS = pressure support; SBT = spontaneous breathing trial; UAO = upper airway obstruction.
Footnotes
These guidelines were developed by the PALISI network and are not official guidelines from the American Thoracic Society. However, they were endorsed by the American Thoracic Society and the Society of Critical Care Medicine.
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute of the National Institutes of Health (R13HD102137), in addition to funds from the Department of Pediatrics at the Indiana University School of Medicine, Indianapolis, Indiana.
Author Contributions: All authors contributed to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work. All authors participated in drafting the work or revising it critically for important intellectual content and have approved and are responsible for the final version submitted for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202204-0795SO on August 15, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Kurachek SC, Newth CJ, Quasney MW, Rice T, Sachdeva RC, Patel NR, et al. Extubation failure in pediatric intensive care: a multiple-center study of risk factors and outcomes. Crit Care Med . 2003;31:2657–2664. doi: 10.1097/01.CCM.0000094228.90557.85. [DOI] [PubMed] [Google Scholar]
- 2. Kapnadak SG, Herndon SE, Burns SM, Shim YM, Enfield K, Brown C, et al. Clinical outcomes associated with high, intermediate, and low rates of failed extubation in an intensive care unit. J Crit Care . 2015;30:449–454. doi: 10.1016/j.jcrc.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 3. Gaies M, Tabbutt S, Schwartz SM, Bird GL, Alten JA, Shekerdemian LS, et al. Clinical epidemiology of extubation failure in the pediatric cardiac ICU: a report from the Pediatric Cardiac Critical Care Consortium. Pediatr Crit Care Med . 2015;16:837–845. doi: 10.1097/PCC.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fan E, Zakhary B, Amaral A, McCannon J, Girard TD, Morris PE, et al. Liberation from mechanical ventilation in critically ill adults. An official ATS/ACCP clinical practice guideline. Ann Am Thorac Soc . 2017;14:441–443. doi: 10.1513/AnnalsATS.201612-993CME. [DOI] [PubMed] [Google Scholar]
- 5. Newth CJ, Venkataraman S, Willson DF, Meert KL, Harrison R, Dean JM, et al. Eunice Shriver Kennedy National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med . 2009;10:1–11. doi: 10.1097/PCC.0b013e318193724d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khemani RG, Randolph A, Markovitz B. Corticosteroids for the prevention and treatment of post-extubation stridor in neonates, children and adults. Cochrane Database Syst Rev . 2009;(3):CD001000. doi: 10.1002/14651858.CD001000.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blackwood B, Murray M, Chisakuta A, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of invasive mechanical ventilation in critically ill paediatric patients. Cochrane Database Syst Rev . 2013:CD009082. doi: 10.1002/14651858.CD009082.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Sultaneh S, Mastropietro CW. In: Pediatric critical care: current controversies. Mastropietro CW, Valentine KM, editors. Cham, Switzerland: Springer; 2019. Weaning and extubation readiness assessment in pediatric patients; pp. 43–62. [Google Scholar]
- 9. Newth CJ, Hotz JC, Khemani RG. Ventilator liberation in the pediatric ICU. Respir Care . 2020;65:1601–1610. doi: 10.4187/respcare.07810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loberger JM, Campbell CM, Colleti J, Jr, Borasino S, Abu-Sultaneh S, Khemani RG. Ventilation liberation practices among 380 international PICUs. Crit Care Explor . 2022;4:e0710. doi: 10.1097/CCE.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Working Group GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ . 2016;353:i2016. doi: 10.1136/bmj.i2016. [DOI] [PubMed] [Google Scholar]
- 12. Wilson KC, Schoenberg NC, Raghu G, Idiopathic pulmonary fibrosis guideline recommendations. Need for adherence to Institute of Medicine methodology Ann Am Thorac Soc . 2019;16:681–686. doi: 10.1513/AnnalsATS.201812-871OC. [DOI] [PubMed] [Google Scholar]
- 13. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol . 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 14. Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. . 2013 [Google Scholar]
- 15. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ . 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ . 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 17. Schünemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, et al. ATS Documents Development and Implementation Committee An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med . 2006;174:605–614. doi: 10.1164/rccm.200602-197ST. [DOI] [PubMed] [Google Scholar]
- 18.Brożek J, Nowak A, Kunstman P, Schünemann HJ. GRADEpro guideline development tool. [Accessed 2021 Jun 1]. Available from: https://www.gradepro.org.
- 19. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods . 2012;3:285–299. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 20. Gershon AS, Lindenauer PK, Wilson KC, Rose L, Walkey AJ, Sadatsafavi M, et al. Informing healthcare decisions with observational research assessing causal effect. An official American Thoracic Society research statement. Am J Respir Crit Care Med . 2021;203:14–23. doi: 10.1164/rccm.202010-3943ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol . 2013;66:151–157. doi: 10.1016/j.jclinepi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lazaro P, et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: RAND Corporation; 2001. [Google Scholar]
- 23. Guyatt GH, Alonso-Coello P, Schünemann HJ, Djulbegovic B, Nothacker M, Lange S, et al. Guideline panels should seldom make good practice statements: guidance from the GRADE Working Group. J Clin Epidemiol . 2016;80:3–7. doi: 10.1016/j.jclinepi.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 24. Foronda FK, Troster EJ, Farias JA, Barbas CS, Ferraro AA, Faria LS, et al. The impact of daily evaluation and spontaneous breathing test on the duration of pediatric mechanical ventilation: a randomized controlled trial. Crit Care Med . 2011;39:2526–2533. doi: 10.1097/CCM.0b013e3182257520. [DOI] [PubMed] [Google Scholar]
- 25. Jouvet PA, Payen V, Gauvin F, Emeriaud G, Lacroix J. Weaning children from mechanical ventilation with a computer-driven protocol: a pilot trial. Intensive Care Med . 2013;39:919–925. doi: 10.1007/s00134-013-2837-8. [DOI] [PubMed] [Google Scholar]
- 26. Randolph AG, Wypij D, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, et al. Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA . 2002;288:2561–2568. doi: 10.1001/jama.288.20.2561. [DOI] [PubMed] [Google Scholar]
- 27. Faustino EV, Gedeit R, Schwarz AJ, Asaro LA, Wypij D, Curley MA, Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) Study Investigators Accuracy of an extubation readiness test in predicting successful extubation in children with acute respiratory failure from lower respiratory tract disease. Crit Care Med . 2017;45:94–102. doi: 10.1097/CCM.0000000000002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blackwood B, Tume LN, Morris KP, Clarke M, McDowell C, Hemming K, et al. SANDWICH Collaborators Effect of a sedation and ventilator liberation protocol vs usual care on duration of invasive mechanical ventilation in pediatric intensive care units: a randomized clinical trial. JAMA . 2021;326:401–410. doi: 10.1001/jama.2021.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abu-Sultaneh S, Hole AJ, Tori AJ, Benneyworth BD, Lutfi R, Mastropietro CW. An interprofessional quality improvement initiative to standardize pediatric extubation readiness assessment. Pediatr Crit Care Med . 2017;18:e463–e471. doi: 10.1097/PCC.0000000000001285. [DOI] [PubMed] [Google Scholar]
- 30. Krawiec C, Carl D, Stetter C, Kong L, Ceneviva GD, Thomas NJ. Challenges with implementation of a respiratory therapist-driven protocol of spontaneous breathing trials in the pediatric ICU. Respir Care . 2017;62:1233–1240. doi: 10.4187/respcare.05477. [DOI] [PubMed] [Google Scholar]
- 31. Loberger JM, Jones RM, Prabhakaran P. A respiratory therapist-driven pathway improves timeliness of extubation readiness assessment in a single PICU. Pediatr Crit Care Med . 2020;21:e513–e521. doi: 10.1097/PCC.0000000000002326. [DOI] [PubMed] [Google Scholar]
- 32. Ferreira FV, Sugo EK, Aragon DC, Carmona F, Carlotti APCP. Spontaneous breathing trial for prediction of extubation success in pediatric patients following congenital heart surgery: a randomized controlled trial. Pediatr Crit Care Med . 2019;20:940–946. doi: 10.1097/PCC.0000000000002006. [DOI] [PubMed] [Google Scholar]
- 33. Chavez A, dela Cruz R, Zaritsky A. Spontaneous breathing trial predicts successful extubation in infants and children. Pediatr Crit Care Med . 2006;7:324–328. doi: 10.1097/01.PCC.0000225001.92994.29. [DOI] [PubMed] [Google Scholar]
- 34. Farias JA, Retta A, Alía I, Olazarri F, Esteban A, Golubicki A, et al. A comparison of two methods to perform a breathing trial before extubation in pediatric intensive care patients. Intensive Care Med . 2001;27:1649–1654. doi: 10.1007/s001340101035. [DOI] [PubMed] [Google Scholar]
- 35. Willis BC, Graham AS, Yoon E, Wetzel RC, Newth CJ. Pressure-rate products and phase angles in children on minimal support ventilation and after extubation. Intensive Care Med . 2005;31:1700–1705. doi: 10.1007/s00134-005-2821-z. [DOI] [PubMed] [Google Scholar]
- 36. Khemani RG, Hotz J, Morzov R, Flink RC, Kamerkar A, LaFortune M, et al. Pediatric extubation readiness tests should not use pressure support. Intensive Care Med . 2016;42:1214–1222. doi: 10.1007/s00134-016-4387-3. [DOI] [PubMed] [Google Scholar]
- 37. van Dijk J, Blokpoel RGT, Koopman AA, Dijkstra S, Burgerhof JGM, Kneyber MCJ. The effect of pressure support on imposed work of breathing during paediatric extubation readiness testing. Ann Intensive Care . 2019;9:78. doi: 10.1186/s13613-019-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. El-Beleidy AS, Khattab AA, El-Sherbini SA, Al-Gebaly HF. Automatic tube compensation versus pressure support ventilation and extubation outcome in children: a randomized controlled study. ISRN Pediatr . 2013;2013:871376. doi: 10.1155/2013/871376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bairwa RC, Sagar H, Sapare AK, Aggarwal R. Comparison between continuous positive airway pressure and T piece as spontaneous breathing trial at a tertiary care pediatric intensive care unit: a pilot randomized control trial. J Pediatr Crit Care . 2021;8:123–127. [Google Scholar]
- 40. Farias JA, Alía I, Retta A, Olazarri F, Fernández A, Esteban A, et al. An evaluation of extubation failure predictors in mechanically ventilated infants and children. Intensive Care Med . 2002;28:752–757. doi: 10.1007/s00134-002-1306-6. [DOI] [PubMed] [Google Scholar]
- 41. Bilan N, Shva Sh, Ghaffari Sh. Survey of factors effective on outcome of weaning from mechanical ventilation. Pak J Biol Sci . 2009;12:83–86. doi: 10.3923/pjbs.2009.83.86. [DOI] [PubMed] [Google Scholar]
- 42. Riou Y, Chaari W, Leteurtre S, Leclerc F. Predictive value of the physiological deadspace/tidal volume ratio in the weaning process of mechanical ventilation in children. J Pediatr (Rio J) . 2012;88:217–221. doi: 10.2223/JPED.2190. [DOI] [PubMed] [Google Scholar]
- 43. Nascimento MS, Rebello CM, Vale LAPA, Santos É, Prado CD. Spontaneous breathing test in the prediction of extubation failure in the pediatric population. Einstein (Sao Paulo) . 2017;15:162–166. doi: 10.1590/S1679-45082017AO3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krasinkiewicz JM, Friedman ML, Slaven JE, Tori AJ, Lutfi R, Abu-Sultaneh S. Progression of respiratory support following pediatric extubation. Pediatr Crit Care Med . 2020;21:e1069–e1075. doi: 10.1097/PCC.0000000000002520. [DOI] [PubMed] [Google Scholar]
- 45. Hotz JC, Bornstein D, Kohler K, Smith E, Suresh A, Klein M, et al. Real-time effort driven ventilator management: a pilot study. Pediatr Crit Care Med . 2020;21:933–940. doi: 10.1097/PCC.0000000000002556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mahmoud NMS. Predicting successful extubation rate using modified spontaneous breathing trial in PICUs. J Compr Ped . 2021;12:e116602. [Google Scholar]
- 47. Krasinkiewicz JM, Friedman ML, Slaven JE, Lutfi R, Abu-Sultaneh S, Tori AJ. Extubation readiness practices and barriers to extubation in pediatric subjects. Respir Care . 2021;66:582–590. doi: 10.4187/respcare.08332. [DOI] [PubMed] [Google Scholar]
- 48. Shimada Y, Yoshiya I, Tanaka K, Yamazaki T, Kumon K. Crying vital capacity and maximal inspiratory pressure as clinical indicators of readiness for weaning of infants less than a year of age. Anesthesiology . 1979;51:456–459. doi: 10.1097/00000542-197911000-00017. [DOI] [PubMed] [Google Scholar]
- 49. Thiagarajan RR, Bratton SL, Martin LD, Brogan TV, Taylor D. Predictors of successful extubation in children. Am J Respir Crit Care Med . 1999;160:1562–1566. doi: 10.1164/ajrccm.160.5.9810036. [DOI] [PubMed] [Google Scholar]
- 50. Venkataraman ST, Khan N, Brown A. Validation of predictors of extubation success and failure in mechanically ventilated infants and children. Crit Care Med . 2000;28:2991–2996. doi: 10.1097/00003246-200008000-00051. [DOI] [PubMed] [Google Scholar]
- 51. Noizet O, Leclerc F, Sadik A, Grandbastien B, Riou Y, Dorkenoo A, et al. Does taking endurance into account improve the prediction of weaning outcome in mechanically ventilated children? Crit Care . 2005;9:R798–R807. doi: 10.1186/cc3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Harikumar G, Egberongbe Y, Nadel S, Wheatley E, Moxham J, Greenough A, et al. Tension-time index as a predictor of extubation outcome in ventilated children. Am J Respir Crit Care Med . 2009;180:982–988. doi: 10.1164/rccm.200811-1725OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johnston C, de Carvalho WB, Piva J, Garcia PC, Fonseca MC. Risk factors for extubation failure in infants with severe acute bronchiolitis. Respir Care . 2010;55:328–333. [PubMed] [Google Scholar]
- 54. Khemani RG, Sekayan T, Hotz J, Flink RC, Rafferty GF, Iyer N, et al. Risk factors for pediatric extubation failure: the importance of respiratory muscle strength. Crit Care Med . 2017;45:e798–e805. doi: 10.1097/CCM.0000000000002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Toida C, Muguruma T, Miyamoto M. Detection and validation of predictors of successful extubation in critically ill children. PLoS One . 2017;12:e0189787. doi: 10.1371/journal.pone.0189787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dionisio MT, Rebelo A, Pinto C, Carvalho L, Neves JF. Ultrasound assessment of ventilator-induced diaphragmatic dysfunction in paediatrics [in Portuguese] Acta Med Port . 2019;32:520–528. doi: 10.20344/amp.10830. [DOI] [PubMed] [Google Scholar]
- 57. Xue Y, Zhang Z, Sheng CQ, Li YM, Jia FY. The predictive value of diaphragm ultrasound for weaning outcomes in critically ill children. BMC Pulm Med . 2019;19:270. doi: 10.1186/s12890-019-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abdel Rahman DA, Saber S, El-Maghraby A. Diaphragm and lung ultrasound indices in prediction of outcome of weaning from mechanical ventilation in pediatric intensive care unit. Indian J Pediatr . 2020;87:413–420. doi: 10.1007/s12098-019-03177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. IJland MM, Lemson J, van der Hoeven JG, Heunks LMA. The impact of critical illness on the expiratory muscles and the diaphragm assessed by ultrasound in mechanical ventilated children. Ann Intensive Care . 2020;10:115. doi: 10.1186/s13613-020-00731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xue Y, Yang CF, Ao Y, Qi J, Jia FY. A prospective observational study on critically ill children with diaphragmatic dysfunction: clinical outcomes and risk factors. BMC Pediatr . 2020;20:422. doi: 10.1186/s12887-020-02310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Subhash S. Kumar V. Point-of-care ultrasound measurement of diaphragm thickening fraction as a predictor of successful extubation in critically ill children. J Pediatr Intensive Care . doi: 10.1055/s-0041-1730931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Valverde Montoro D, García Soler P, Hernández Yuste A, Camacho Alonso JM. Ultrasound assessment of ventilator-induced diaphragmatic dysfunction in mechanically ventilated pediatric patients. Paediatr Respir Rev . 2021;40:58–64. doi: 10.1016/j.prrv.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 63. Wolf GK, Walsh BK, Green ML, Arnold JH. Electrical activity of the diaphragm during extubation readiness testing in critically ill children. Pediatr Crit Care Med . 2011;12:e220–e224. doi: 10.1097/PCC.0b013e3181fe28fc. [DOI] [PubMed] [Google Scholar]
- 64. MacBean V, Jolley CJ, Sutton TG, Deep A, Greenough A, Moxham J, et al. Parasternal intercostal electromyography: a novel tool to assess respiratory load in children. Pediatr Res . 2016;80:407–414. doi: 10.1038/pr.2016.89. [DOI] [PubMed] [Google Scholar]
- 65. van Leuteren RW, de Waal CG, de Jongh FH, Bem RA, van Kaam AH, Hutten G. Diaphragm activity pre and post extubation in ventilated critically ill infants and children measured with transcutaneous electromyography. Pediatr Crit Care Med . 2021;22:950–959. doi: 10.1097/PCC.0000000000002828. [DOI] [PubMed] [Google Scholar]
- 66. Tamburro R, Bunitz M. Tracheal airleak as a predictor of post-extubation stridor in the paediatric intensive care unit. Clin Intensive Care . 1993;4:52–55. [Google Scholar]
- 67. Mhanna MJ, Zamel YB, Tichy CM, Super DM. The “air leak” test around the endotracheal tube, as a predictor of postextubation stridor, is age dependent in children. Crit Care Med . 2002;30:2639–2643. doi: 10.1097/00003246-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 68. Suominen PK, Tuominen NA, Salminen JT, Korpela RE, Klockars JG, Taivainen TR, et al. The air-leak test is not a good predictor of postextubation adverse events in children undergoing cardiac surgery. J Cardiothorac Vasc Anesth . 2007;21:197–202. doi: 10.1053/j.jvca.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 69. Wratney AT, Benjamin DK, Jr, Slonim AD, He J, Hamel DS, Cheifetz IM. The endotracheal tube air leak test does not predict extubation outcome in critically ill pediatric patients. Pediatr Crit Care Med . 2008;9:490–496. doi: 10.1097/PCC.0b013e3181849901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Khemani RG, Hotz J, Morzov R, Flink R, Kamerkar A, Ross PA, et al. Evaluating risk factors for pediatric post-extubation upper airway obstruction using a physiology-based tool. Am J Respir Crit Care Med . 2016;193:198–209. doi: 10.1164/rccm.201506-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schneider J, Mulale U, Yamout S, Pollard S, Silver P. Impact of monitoring endotracheal tube cuff leak pressure on postextubation stridor in children. J Crit Care . 2016;36:173–177. doi: 10.1016/j.jcrc.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 72. El Amrousy D, Elkashlan M, Elshmaa N, Ragab A. Ultrasound-guided laryngeal air column width difference as a new predictor for postextubation stridor in children. Crit Care Med . 2018;46:e496–e501. doi: 10.1097/CCM.0000000000003068. [DOI] [PubMed] [Google Scholar]
- 73. Veder LL, Joosten KFM, Schlink K, Timmerman MK, Hoeve LJ, van der Schroeff MP, et al. Post-extubation stridor after prolonged intubation in the pediatric intensive care unit (PICU): a prospective observational cohort study. Eur Arch Otorhinolaryngol . 2020;277:1725–1731. doi: 10.1007/s00405-020-05877-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tellez DW, Galvis AG, Storgion SA, Amer HN, Hoseyni M, Deakers TW. Dexamethasone in the prevention of postextubation stridor in children. J Pediatr . 1991;118:289–294. doi: 10.1016/s0022-3476(05)80505-0. [DOI] [PubMed] [Google Scholar]
- 75. Anene O, Meert KL, Uy H, Simpson P, Sarnaik AP. Dexamethasone for the prevention of postextubation airway obstruction: a prospective, randomized, double-blind, placebo-controlled trial. Crit Care Med . 1996;24:1666–1669. doi: 10.1097/00003246-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 76. Cesar RG, de Carvalho WB. L-epinephrine and dexamethasone in postextubation airway obstruction: a prospective, randomized, double-blind placebo-controlled study. Int J Pediatr Otorhinolaryngol . 2009;73:1639–1643. doi: 10.1016/j.ijporl.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 77. Malhotra D, Gurcoo S, Qazi S, Gupta S. Randomized comparative efficacy of dexamethasone to prevent postextubation upper airway complications in children and adults in ICU. Indian J Anaesth . 2009;53:442–449. [PMC free article] [PubMed] [Google Scholar]
- 78. Baranwal AK, Meena JP, Singhi SC, Muralidharan J. Dexamethasone pretreatment for 24 h versus 6 h for prevention of postextubation airway obstruction in children: a randomized double-blind trial. Intensive Care Med . 2014;40:1285–1294. doi: 10.1007/s00134-014-3358-9. [DOI] [PubMed] [Google Scholar]
- 79. Carvalho HT, Fioretto JR, Bonatto RC, Ribeiro CF, Martin JG, Carpi MF. Use of dexamethasone to prevent extubation failure in pediatric intensive care unit: a randomized controlled clinical trial. J Pediatr Intensive Care . 2020;11:41–47. doi: 10.1055/s-0040-1719044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ritu JU, Jhamb U. Dexamethasone in prevention of postextubation stridor in ventilated children: a randomized, double-blinded, placebo-controlled trial. Indian J Crit Care Med . 2020;24:1230–1235. doi: 10.5005/jp-journals-10071-23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Parajuli B, Baranwal AK, Kumar-M P, Jayashree M, Takia L. Twenty-four-hour pretreatment with low dose (0.25 mg/kg/dose) versus high dose (0.5 mg/kg/dose) dexamethasone in reducing the risk of postextubation airway obstruction in children: a randomized open-label noninferiority trial. Pediatr Pulmonol . 2021;56:2292–2301. doi: 10.1002/ppul.25388. [DOI] [PubMed] [Google Scholar]
- 82. Li T, Puhan MA, Vedula SS, Singh S, Dickersin K, Ad Hoc Network Meta-analysis Methods Meeting Working Group Network meta-analysis-highly attractive but more methodological research is needed. BMC Med . 2011;9:79. doi: 10.1186/1741-7015-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Iyer NP, Lopez-Fernandez YM, Gonzalez-Dambrauskas S, Baranwal AK, Hotz JC, Zhu M, et al. A network meta-analysis of dexamethasone for preventing post-extubation upper airway obstruction in children. Ann Am Thorac Soc . 2022 Aug;17 doi: 10.1513/AnnalsATS.202203-212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ramnarayan P, Lister P, Dominguez T, Habibi P, Edmonds N, Canter RR, et al. United Kingdom Paediatric Intensive Care Society Study Group (PICS-SG) FIRST-line support for Assistance in Breathing in Children (FIRST-ABC): a multicentre pilot randomised controlled trial of high-flow nasal cannula therapy versus continuous positive airway pressure in paediatric critical care. Crit Care . 2018;22:144. doi: 10.1186/s13054-018-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ramnarayan P, Richards-Belle A, Drikite L, Saull M, Orzechowska I, Darnell R, et al. FIRST-ABC Step-Down RCT Investigators and the Paediatric Critical Care Society Study Group Effect of high-flow nasal cannula therapy vs continuous positive airway pressure following extubation on liberation from respiratory support in critically ill children: a randomized clinical trial. JAMA . 2022;327:1555–1565. doi: 10.1001/jama.2022.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Testa G, Iodice F, Ricci Z, Vitale V, De Razza F, Haiberger R, et al. Comparative evaluation of high-flow nasal cannula and conventional oxygen therapy in paediatric cardiac surgical patients: a randomized controlled trial. Interact Cardiovasc Thorac Surg . 2014;19:456–461. doi: 10.1093/icvts/ivu171. [DOI] [PubMed] [Google Scholar]
- 87. Akyıldız B, Öztürk S, Ülgen-Tekerek N, Doğanay S, Görkem SB. Comparison between high-flow nasal oxygen cannula and conventional oxygen therapy after extubation in pediatric intensive care unit. Turk J Pediatr . 2018;60:126–133. doi: 10.24953/turkjped.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 88. Wijakprasert P, Chomchoey J. High-flow nasal cannula versus conventional oxygen therapy in post-extubation pediatric patients: a randomized controlled trial. J Med Assoc Thai . 2018;101:1331–1335. [Google Scholar]
- 89. Rodríguez JA, Von Dessauer B, Duffau G. Non-invasive continuous positive airways pressure for post-extubation laryngitis in pediatric patients [in Spanish] Arch Bronconeumol . 2002;38:463–467. doi: 10.1016/s0300-2896(02)75266-6. [DOI] [PubMed] [Google Scholar]
- 90. Fioretto JR, Ribeiro CF, Carpi MF, Bonatto RC, Moraes MA, Fioretto EB, et al. Comparison between noninvasive mechanical ventilation and standard oxygen therapy in children up to 3 years old with respiratory failure after extubation: a pilot prospective randomized clinical study. Pediatr Crit Care Med . 2015;16:124–130. doi: 10.1097/PCC.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 91. Curley MA, Wypij D, Watson RS, Grant MJ, Asaro LA, Cheifetz IM, et al. RESTORE Study Investigators and the Pediatric Acute Lung Injury and Sepsis Investigators Network Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA . 2015;313:379–389. doi: 10.1001/jama.2014.18399. [DOI] [PMC free article] [PubMed] [Google Scholar]


