Abstract
Rationale
Although interstitial lung abnormalities (ILA), specific patterns of incidentally-detected abnormal density on computed tomography, have been associated with abnormal lung function and increased mortality, it is unclear if a subset with incidental interstitial lung disease (ILD) accounts for these adverse consequences.
Objectives
To define the prevalence and risk factors of suspected ILD and assess outcomes.
Methods
Suspected ILD was evaluated in the COPDGene (Chronic Obstructive Pulmonary Disease Genetic Epidemiology) study, defined as ILA and at least one additional criterion: definite fibrosis on computed tomography, FVC less than 80% predicted, or DLCO less than 70% predicted. Multivariable linear, longitudinal, and Cox proportional hazards regression models were used to assess associations with St. George's Respiratory Questionnaire, 6-minute-walk test, supplemental oxygen use, respiratory exacerbations, and mortality.
Measurements and Main Results
Of 4,361 participants with available data, 239 (5%) had evidence for suspected ILD, whereas 204 (5%) had ILA without suspected ILD. In multivariable analyses, suspected ILD was associated with increased St. George's Respiratory Questionnaire score (mean difference [MD], 3.9 points; 95% confidence interval [CI], 0.6–7.1; P = 0.02), reduced 6-minute-walk test (MD, −35 m; 95% CI, −56 m to −13 m; P = 0.002), greater supplemental oxygen use (odds ratio [OR], 2.3; 95% CI, 1.1–5.1; P = 0.03) and severe respiratory exacerbations (OR, 2.9; 95% CI, 1.1–7.5; P = 0.03), and higher mortality (hazard ratio, 2.4; 95% CI, 1.2–4.6; P = 0.01) compared with ILA without suspected ILD. Risk factors associated with suspected ILD included self-identified Black race (OR, 2.0; 95% CI, 1.1–3.3; P = 0.01) and pack-years smoking history (OR, 1.2; 95% CI, 1.1–1.3; P = 0.0005).
Conclusions
Suspected ILD is present in half of those with ILA in COPDGene and is associated with exercise decrements and increased symptoms, supplemental oxygen use, severe respiratory exacerbations, and mortality.
Keywords: interstitial lung disease, interstitial lung abnormalities, pulmonary fibrosis
At a Glance Commentary
Scientific Knowledge on the Subject
Interstitial lung abnormalities (ILA) are incidental computed tomography findings suggestive of interstitial lung disease (ILD) and have been found to be associated with reduced lung function, functional capacity, and survival. It is not currently known if there is a subset of ILA with suspected ILD that accounts for the increase in adverse outcomes.
What This Study Adds to the Field
This study demonstrates that approximately half of those with ILA have evidence for a suspected ILD defined by the presence of advanced radiologic findings, reduced pulmonary function, or both, and this group has reduced exercise capacity and increased symptoms, need for supplemental oxygen, respiratory exacerbations, and mortality. These findings have important implications for smokers in general and the evaluation and monitoring of those with ILA. Future studies are needed to follow this group longitudinally and to assess the effectiveness of further monitoring and potentially targeted interventions.
Interstitial lung abnormalities (ILA) are defined as incidental chest computed tomography (CT) imaging abnormalities suggestive of underlying interstitial lung disease (ILD) or early stage of pulmonary fibrosis in those without a clinical diagnosis (1–3). Evidence indicating that some people with ILA may have an incidental but clinically significant ILD includes work that has demonstrated associations between ILA and adverse clinical outcomes such as significantly reduced lung function (4), exercise capacity (5), and decreased survival (6). Although these studies support the idea that ILA, beyond simply representing an imaging abnormality, may represent an undiagnosed form of ILD in some people (7), few studies have risk-stratified ILA using CT characteristics and measures of pulmonary function to define a subset with suspected ILD (8).
We hypothesized that measures of CT imaging and pulmonary function could be combined to identify a group of ILA with suspected ILD among people previously unsuspected of having ILD and that many adverse outcomes attributable to those with ILA would be concentrated within this group. To test this hypothesis, we evaluated demographic, clinical, pulmonary function, and radiologic data from participants in the COPDGene (Genetic Epidemiology of Chronic Obstructive Pulmonary Disease) study. Some of the results of these studies have been previously reported in the form of an abstract (9).
Methods
Study Population
Participants enrolled in phase 2 (5-year follow-up) of COPDGene were included. Protocols for participant enrollment have been described previously (10). In brief, COPDGene is a multicenter, longitudinal cohort study that was designed to identify the epidemiologic and genetic risk factors of chronic obstructive pulmonary disease (COPD). Non-Hispanic White and African American participants aged 45–80 years with at least a 10–pack-year smoking history were enrolled between January 2008 and June 2011 at 21 clinical centers. Those with a known history of lung diseases other than asthma (including ILD) were excluded. Participants completed a protocol that included an assessment of medical history, respiratory questionnaires, pulmonary function tests, and chest CT scan; participants have since been followed with repeat assessments at 5-year increments. The COPDGene study was approved by the institutional review boards of all participating centers. Five-year follow-up data for these participants (phase 2) was analyzed to include assessments of DLCO, which were not measured at enrollment (phase 1). Participants without complete data were excluded from this analysis unless otherwise specified. Mortality was ascertained as of August 2020.
Chest CT Acquisition and Quantitative Analysis
Participants underwent volumetric CT scans of the chest at full inspiration (total lung capacity) and end-tidal expiration (functional residual capacity) as part of the COPDGene study according to previously published protocols (10). Computerized image analyses were performed with Thirona quantitative image analysis software. These analyses included assessments of the percentage of emphysema (quantified as the percentage of lung parenchyma ⩽−950 Hounsfield Units) and total lung capacity.
Visual Chest CT Analysis to Define ILA and Fibrosis
Chest CT scans were assessed for ILA using a sequential method by up to three readers blinded to clinical information as previously described (4). ILA were defined per Fleischner Society recommendations as nondependent changes affecting more than 5% of any lobar region, including ground–glass or reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, or traction bronchiectasis (but not those with centrilobular nodularity alone) (1). CT scans with focal or unilateral ground–glass attenuation or reticulation and patchy ground–glass abnormalities were considered indeterminate. Chest CT scans demonstrating ILA were also categorized as having definite fibrosis, defined by the presence of pulmonary parenchymal architectural distortion (e.g., traction bronchiectasis and honeycombing) as previously reported (11).
Spirometry and Lung Function Measurements
Pre- and postbronchodilator spirometry (FEV1 and FVC) in COPDGene phase 1 and phase 2 were performed with the EasyOne system in accordance with American Thoracic Society/European Respiratory Society recommendations (12). DLCO in phase 2 was measured using the EasyOne Pro (ndd Medizintechnik AG). The percentage of predicted values were calculated using Third National Health and Nutrition Examination Survey reference values for FEV1 and FVC (13, 14) and Global Lung Initiative reference equations for DLCO (15). Although recent data demonstrate that increases in the extent of quantitative measures of emphysema have a minimal effect on FVC among those with ILA, the effect on DLCO remains (16). Therefore, in an attempt to remove the effect of emphysema on reduced DLCO when defining suspected ILD, DLCO for each COPDGene participant was adjusted using calculated coefficients for the effect of percent emphysema obtained from multiple linear regression models (see supplemental methods in the online supplement). These adjusted values were then used for the categorization of suspected ILD.
Six-Minute-Walk Test (6MWT)
The 6MWT was performed in accordance with American Thoracic Society guidelines (17) without practice, indoors, on a flat course supervised by trained study staff. Subjects were instructed and encouraged with standardized phrases to push themselves to achieve maximal distance.
Clinical Data and Definitions
Clinical data were collected through standardized questionnaires (available at www.copdgene.org) to assess demographic information, smoking history, medical history, exacerbation history, supplemental oxygen use, and the Saint George’s Respiratory Questionnaire (SGRQ). Respiratory exacerbation frequency was quantified on the basis of the number of times in the last year the participant had a worsening of their respiratory status. A severe exacerbation was defined as a report of visiting an emergency room or hospitalization for an acute worsening respiratory status in the last year. Because comprehensive clinical evaluations and multidisciplinary discussions were not possible, suspected ILD was defined on the basis of previous work (8) as ILA and at least one of the following at phase 2: definite fibrosis on CT, postbronchodilator FVC less than 80% predicted, or DLCO less than 70% predicted after adjustment for emphysema.
Statistical Analysis
Multivariable linear and logistic regression models were used to determine the associations between suspected ILD and selected outcomes. Multivariable Cox proportional hazards models were used to evaluate mortality. Adjusted models included a priori covariates of age, sex, race, body mass index, pack-years of smoking, current smoking status, and GOLD (Global Initiative for Obstructive Lung Disease) stage. Cox proportional hazard models also included adjustment for clinical center given variable follow-up. All P values are two-sided, and values less than 0.05 were considered statistically significant. All analyses were performed using Statistical Analysis Software version 9.4 (SAS Institute) and R (Version 4.0.3).
Results
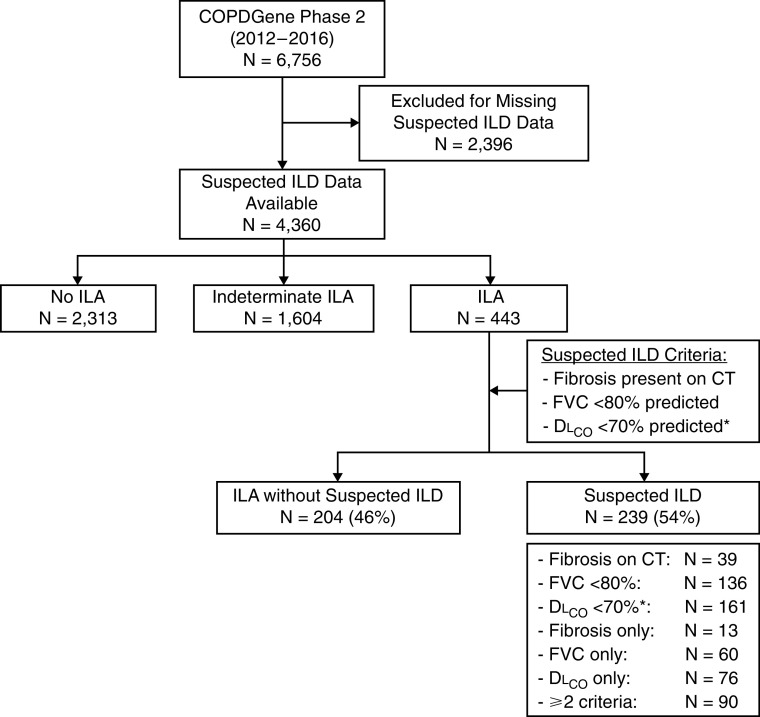
Of the 6,756 COPDGene phase 2 participants, 4,360 (65%) had complete data available to make an assessment of suspected ILD. Differences between those included and excluded from our analyses are included in Table E1 in the online supplement. Of those included, 443 (10%) participants had ILA, 2,313 (53%) did not have ILA, and 1,604 (37%) were indeterminate, similar to previously published results (16). Applying the three criteria to the 443 participants with ILA, 239 (54%) were found to have suspected ILD (5% of the phase 2 cohort), and 204 (46%) of those with ILA did not meet the criteria for suspected ILD. Of those with suspected ILD, 39 (16%) had evidence of definite fibrosis on CT, 136 (57%) had an FVC less than 80% predicted, and 161 (67%) had a DLCO less than 70% predicted after adjustment for emphysema. Of those with suspected ILD, 149 (62%) met only one of the three criteria for suspected ILD, including 13 (5%) with definite fibrosis, 60 (25%) with a reduced FVC, and 76 (32%) with a reduced DLCO; 83 (35%) met two criteria; and 7 (3%) met all three criteria (Figures 1 and E1).
Figure 1.
Flowchart depicting the cohort assembly. *DLCO percent predicted was adjusted for the percent emphysema on computed tomography. COPDGene = Chronic Obstructive Pulmonary Disease Genetic Epidemiology study; ILA = interstitial lung abnormality; ILD = interstitial lung disease.
Characteristics and Risk Factors
Characteristics of study participants stratified by ILA status, and further stratified by the presence or absence of suspected ILD, are presented in Table 1 (ILA status, including indeterminate category, is presented in Table E2). As expected, numerous differences were noted between participants with and without ILA. There were also several differences between participants with ILA with and without suspected ILD (Table 1). Compared with participants with ILA without suspected ILD, participants with suspected ILD were more likely to be of self-identified Black or African American race, have a higher pack-year smoking history, different GOLD stage, lower FEV1/FVC ratio and FEV1, and lower FVC and DLCO (as expected on the basis of criteria used to define suspected ILD). In multivariable analyses, when compared with those with ILA without suspected ILD, suspected ILD remained associated with race, pack-year smoking history, and GOLD stage, although not with a diagnosis of COPD (Table 2). For instance, self-identified Black or African American race had two times the odds of suspected ILD compared with non-Hispanic White race (odds ratio [OR], 2.0; 95% confidence interval [CI], 1.1–3.3; P = 0.01) and an additional 10 pack-years of smoking history increased the odds of suspected ILD by 18% (OR, 1.2; 95% CI, 1.1–1.3; P = 0.0005). After adjusting for covariates, there was no association between suspected ILD and COPD diagnosis, emphysema, and FEV1/FVC ratio compared with ILA without suspected ILD. Those with suspected ILD at phase 2, compared with those with ILA without suspected ILD, were more likely to have abnormalities present in phase 1, including ILA (34% vs. 23%; P = 0.006), FVC less than 80% predicted (45% vs. 7%; P = <0.0001), and definite fibrosis on imaging (24% vs. 7%; P = 0.02) (Table E3). Figure 2 shows an example of participants with ILA with and without suspected ILD at both phase 1 and phase 2.
Table 1.
Characteristics for No Interstitial Lung Abnormalities, Interstitial Lung Abnormalities without Suspected Interstitial Lung Disease, and Suspected Interstitial Lung Disease
| Variable | No ILA (n = 2313) | ILA without Suspected ILD (n = 204) | Suspected ILD (n = 239) |
|---|---|---|---|
| Age (yr), mean (SD) | 63.8 (8.2) | 68.4 (8.7) | 68.5 (8.3) |
| Sex, n (%) | |||
| Male | 1163 (50) | 108 (53) | 118 (49) |
| Female | 1150 (50) | 96 (47) | 121 (51) |
| Race, n (%) | |||
| White | 1671 (72) | 167 (82) | 176 (74) |
| Black or African American | 642 (28) | 37 (18) | 63 (26) |
| BMI (kg/m2), mean (SD) | 29.1 (6.4) | 29.9 (6.2) | 30.2 (6.7) |
| Pack-years smoking, median (IQR)* | 37.6 (25.0–50.1) | 40.0 (25.5–52.2) | 47.5 (32.8–64.5) |
| Smoking status, n (%) | |||
| Never | 35 (1.5) | 4 (2.0) | 2 (0.8) |
| Former | 1425 (62) | 127 (62) | 135 (56) |
| Current | 853 (37) | 73 (36) | 102 (43) |
| COPD, n (%)†‡ | 798 (35) | 62 (30) | 93 (39) |
| GOLD stage, n (%)ठ| |||
| PRISm | 246 (11) | 9 (4.4) | 56 (24) |
| Stage 0 | 1259 (55) | 133 (65) | 89 (37) |
| Stage 1 | 197 (8.6) | 26 (13) | 21 (8.8) |
| Stage 2 | 358 (16) | 31 (15) | 51 (21) |
| Stage 3 | 208 (9.0) | 5 (2.5) | 21 (8.8) |
| Stage 4 | 35 (1.5) | 0 (0) | 0 (0) |
| Emphysema (%), mean (SD) | 5.1 (8.6) | 3.7 (5.9) | 3.7 (5.8) |
| FEV1/FVC ratio (%), mean (SD) | 69.2 (14.2) | 71.4 (11.4) | 68.9 (11.8) |
| FEV1 % predicted, mean (SD) | 82.0 (24.3) | 91.2 (17.2) | 74.1 (18.8) |
| FVC % predicted, mean (SD) | 89.3 (17.4) | 96.1 (11.1) | 81.0 (16.9) |
| DLCO % predicted, mean (SD) | 82.9 (22.7) | 83.4 (16.2) | 63.0 (18.7) |
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Obstructive Lung Disease; ILA = interstitial lung abnormality; ILD = interstitial lung disease; IQR = interquartile range; PRISm = preserved ratio, impaired spirometry; SD = standard deviation.
Comparison of categorical variables was made using Fisher exact tests, continuous variables with two-tailed Student’s t test.
Missing pack-years smoking history for two participants.
COPD category includes participants with GOLD stage 1 or greater.
Missing COPD and GOLD stage data for 11 participants.
For the purposes of assessing differences in GOLD stage among groups, PRISm was included in GOLD stage 0.
Table 2.
Risk Factors Associated with Suspected Interstitial Lung Disease Compared with Interstitial Lung Abnormalities without Suspected Interstitial Lung Disease
| Risk Factor | Unadjusted Analysis |
Adjusted Analysis |
||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Age* | 1.0 (0.8–1.3) | 0.9 | 1.2 (0.9–1.6) | 0.2 |
| Male sex | 0.87 (0.6–1.3) | 0.5 | 0.77 (0.5–1.1) | 0.2 |
| Black or African American race | 1.6 (1.0–2.6) | 0.04 | 2.0 (1.1–3.3) | 0.01 |
| BMI* | 1.1 (0.8–1.4) | 0.7 | 1.1 (0.8–1.5) | 0.6 |
| Pack-years smoking* | 1.2 (1.1–1.3) | <0.0001 | 1.2 (1.1–1.3) | 0.0005 |
| Current smoking status† | 1.3 (0.89–1.9) | 0.2 | 1.4 (0.8–2.2) | 0.2 |
| COPD diagnosis‡ | 1.5 (0.99–2.2) | 0.06 | 1.3 (0.8–1.9) | 0.3 |
| GOLD stage§ | 1.4 (1.1–1.6) | 0.003 | 1.3 (1.0–1.6) | 0.04 |
| Percent emphysema on CT*‡ | 1.0 (0.7–1.4) | 1.0 | 0.8 (0.6–1.2) | 0.4 |
| FEV1/FVC ratio*‡ | 0.8 (0.7–0.98) | 0.03 | 0.9 (0.7–1.0) | 0.1 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Obstructive Lung Disease.
For unadjusted analyses, logistic regression models were used with the single variable of interest. For adjusted analyses, logistic regression models were used with the covariates of age, race, sex, BMI, pack-years smoking, current smoking status, and GOLD stage except where otherwise indicated.
Odds ratio per unit of 10.
Odds ratio and P value for current smokers compared with former smokers.
Adjusted analysis did not include the GOLD stage given colinearity.
Odds ratio per GOLD stage above 0.
Figure 2.
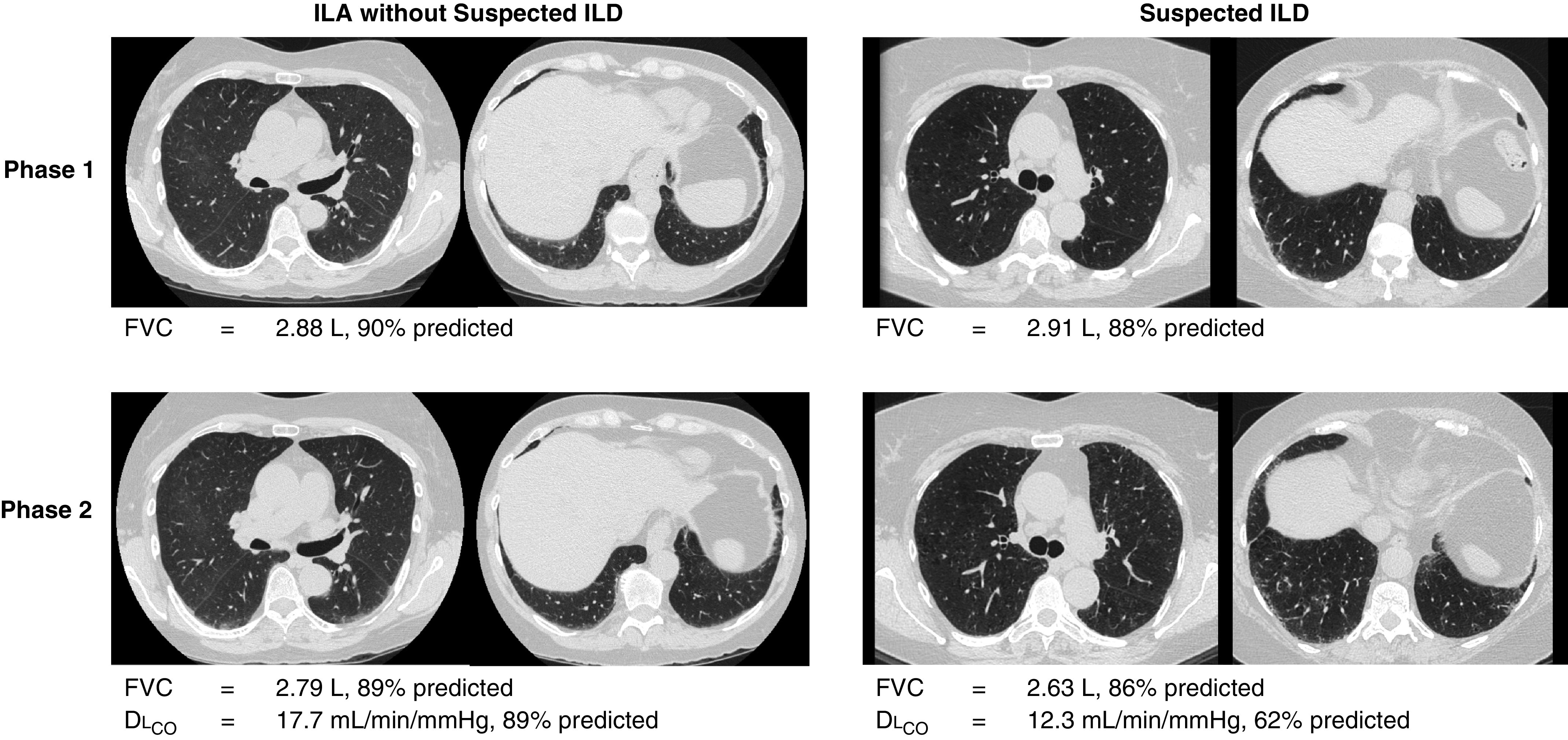
Serial chest computed tomography scans from two participants with interstitial lung abnormalities (ILA). The participant with ILA without suspected interstitial lung disease (ILD) had ILA in both phase 1 and phase 2 but did not have fibrosis or reduced pulmonary function, whereas the participant with suspected ILD had ILA in phase 1 and phase 2 with definite fibrosis and DLCO below 70% predicted in phase 2 as well as a relative decrease in FVC of over 5% in phase 2 compared with phase 1.
Clinical Endpoints: Symptoms, Functional Status, Oxygen Use, and Respiratory Exacerbations
Compared with participants with ILA without suspected ILD in adjusted analyses, participants with suspected ILD were more likely to have worse clinical endpoints, including an increased SGRQ score (mean difference [MD], 3.9 points; 95% CI, 0.6–7.1; P = 0.02), reduced 6MWT (MD, −35 m; 95% CI, −56 m to −13 m; P = 0.002), a greater percentage of supplemental oxygen use (OR, 2.3; 95% CI, 1.1–5.1; P = 0.03), and an increased percentage of participants that experienced a severe respiratory exacerbation (OR, 2.9; 95% CI, 1.1–7.5; P = 0.03) (Table 3). Results were similar in sensitivity analyses when using quantitative measures of total lung capacity to define suspected ILD in place of FVC (Table E4), without adjustment of DLCO for the percentage of emphysema (Table E5), without definite fibrosis as a criterion for suspected ILD (Table E6), and without DLCO as a criterion (Table E7). When participants with COPD were excluded, suspected ILD remained associated with decreased functional capacity as measured by 6MWT after adjustment for covariates (Table E8). Those with suspected ILD that met two or more criteria had significant differences in endpoints compared with those that only met one criterion (Table E9). Although the addition of each individual criterion to ILA demonstrated significant associations with some endpoints, ILA plus a reduced DLCO of less than 70% of predicted was the most consistent across a range of outcomes (Table E10). There were no significant differences between the group with ILA without suspected ILD and the group with no ILA (Table E11).
Table 3.
Outcomes for Suspected Interstitial Lung Disease Compared with Interstitial Lung Abnormalities without Suspected Interstitial Lung Disease
| Clinical Endpoint | ILA without Suspected ILD (n = 204) | Suspected ILD (n = 239) | Unadjusted Analysis |
Adjusted Analysis |
||
|---|---|---|---|---|---|---|
| Effect Estimate | P Value | Effect Estimate | P Value | |||
| Mean Difference (95% CI) | Mean Difference (95% CI) | |||||
| SGRQ (points), mean (SD) | 18.5 (16.3) | 25.9 (20.5) | 7.4 (4.0 to 10.9) | <0.0001 | 3.9 (0.6 to 7.1) | 0.02 |
| 6MWT (m), mean (SD)* | 415 (121) | 358 (119) | −57 (−80 to −34) | <0.0001 | −35 (−56 to −13) | 0.002 |
| Exacerbation frequency (n per yr), mean (SD) | 0.16 (0.46) | 0.30 (0.80) | 0.14 (0.02 to 0.26) | 0.02 | 0.10 (−0.03 to 0.22) | 0.1 |
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | |||||
| Supplemental oxygen, n (%) | 11 (5.4) | 36 (15) | 3.1 (1.5 to 6.3) | 0.001 | 2.3 (1.1 to 5.1) | 0.03 |
| Severe exacerbation, n (%)† | 6 (2.9) | 23 (9.7) | 3.5 (1.4 to 8.8) | 0.006 | 2.9 (1.1 to 7.5) | 0.03 |
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | |||||
| Mortality, n (%) | 13 (6.4) | 36 (15) | 2.7 (1.4 to 5.1) | 0.002 | 2.4 (1.2 to 4.6) | 0.01 |
Definition of abbreviations: 6MWT = 6-minute-walk test; CI = confidence interval; ILA = interstitial lung abnormality; ILD = interstitial lung disease; SD = standard deviation; SGRQ = St. George’s Respiratory Questionnaire.
For unadjusted analyses, a comparison of categorical variables was made using Fisher exact tests unless otherwise specified, continuous variables with two-tailed Student’s t test, and mortality with univariable Cox proportional hazards model. For adjusted analyses, regression models were used with age, race, sex, BMI, pack-years smoking, current smoking status, and GOLD stage as covariates. For mortality, the Cox proportional hazards model also included a clinical center.
Missing 6MWT data for eight participants.
Missing severe respiratory exacerbation data for one participant.
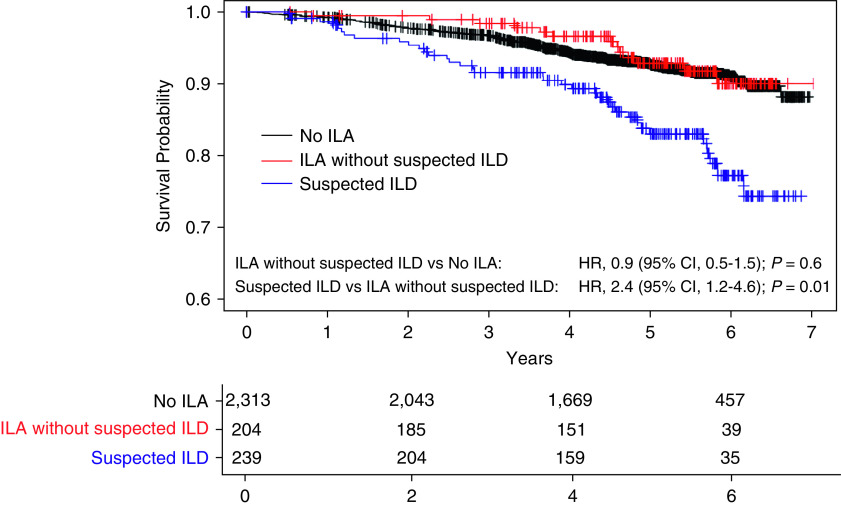
Mortality
After the phase 2 visit, participants were followed for a median of 4.9 years (interquartile range, 3.7– 5.8) for vital status. The unadjusted mortality rates were significantly higher among participants with suspected ILD when compared with those with ILA without suspected ILD. A total of 15% (36) of participants with suspected ILD died compared with 6% (13) of participants with ILA without suspected ILD (Table 2 and Figure 3). After adjustment for covariates, when compared with participants who had ILA without suspected ILD, suspected ILD was associated with a higher risk of death (hazard ratio, 2.4; 95% CI, 1.2–4.6; P = 0.01). Results were similar when done without definite fibrosis as a criterion for suspected ILD (Table E6), without DLCO as a criterion (Table E7), and when participants with COPD were excluded (Table E8). Similar to the other outcomes above, there were no significant differences between the group with ILA without suspected ILD and the group with no ILA (Table E11 and Figure 3).
Figure 3.
Kaplan-Meier survival curves showing percent survival for participants with suspected interstitial lung disease (ILD) (blue), interstitial lung abnormalities (ILA) without suspected ILD (red), and no ILA (black). After adjustment for covariates using Cox proportional hazards models, participants with suspected ILD had a higher risk of death compared with those with ILA without suspected ILD (hazard ratio [HR], 2.4; 95% CI, 1.2–4.6; P = 0.01), whereas there was no statistically significant difference between those with ILA without suspected ILD and those with no ILA (HR, 0.9; 95% CI, 0.5–1.5; P = 0.6).
Discussion
In this study, we have attempted to define and assess the prevalence and outcomes of suspected ILD among a large cohort of smokers in which known ILD was excluded at the time of enrollment. These findings demonstrate that approximately half of COPDGene participants with ILA have further evidence for suspected ILD, and adverse outcomes such as reduced functional status, increased symptoms, supplemental oxygen use, severe respiratory exacerbations, and mortality are more strongly associated with the group of those with ILA who have suspected ILD. These findings also suggest that suspected ILD was more common among those with self-identified Black or African American race within the context of COPDGene, and may have broader implications.
This study demonstrates an effective method for risk-stratifying patients with ILA. ILA are present in 2–7% of older adults in the general population and 4–9% of smokers (1), and efforts to detect progressive pulmonary fibrosis at an earlier stage have begun to focus more on the groups with ILA most likely to experience adverse outcomes (18). For example, a prior study demonstrated that ILA imaging patterns are more consistent with a usual interstitial pneumonia pattern, or those with definite fibrosis experience increased rates of radiologic progression and mortality (11). Our study provides some of the most comprehensive assessments to date, showing that those with imaging abnormalities can be further risk stratified using radiologic features and pulmonary function to account for a population at the highest risk for poor outcomes. Although a number of studies have demonstrated associations between those with ILA and adverse outcomes when compared with those without ILA (4–6, 19), this study shows that these poor outcomes are driven by the subset with fibrosis and/or lung function abnormalities and that in this cohort of smokers, those with ILA that do not have fibrosis or reduced lung function have outcomes similar to those without ILA (Table E11).
The findings presented in our study add to previous reports and strengthen recommendations made by the Fleischner Society Position Paper (1), as well as those made by an ILD expert consensus panel (20), that pulmonary function testing (including measures of DLCO) should be obtained in patients initially identified with ILA. Furthermore, this study demonstrates that radiologic characterization alone is not sufficient to account for the differences in outcomes but that the combination of radiologic and physiologic abnormalities allows for a more informative delineation of high-risk subsets. Further proof of this statement includes the fact that 84% of suspected ILD was identified by reduced lung function alone (without further evidence for definite fibrosis) and this subset was associated with adverse outcomes even when imaging evidence of definite fibrosis was removed as a criterion (Table E6).
This study adds weight to the idea that an undetected form of ILD may be more common than current prevalence estimates suggest (21, 22). The subset of ILA with suspected ILD identified in this cohort was 54%, which is similar to the estimate from a recently published cohort of first-degree relatives of patients with IPF in which 58% of those with ILA had ILD defined similarly (8). Future studies characterizing suspected ILD in diverse populations will be needed to determine additional estimates of population-specific prevalence. Although it is not known if targeted screening or interventions in specific populations will definitively improve clinical outcomes, given the numerous adverse outcomes experienced by those with suspected ILD in our study, the case to do so is becoming much stronger.
Although these results provide further evidence that screening in high-risk populations (e.g., smokers) may be helpful in identifying those with ILA who are at risk of experiencing adverse outcomes, our data also demonstrate that a single screening evaluation is likely not sufficient. In support of the latter statement, only 33% of those with suspected ILD in COPDGene at phase 2 had ILA on their chest CTs at phase 1, approximately 5 years earlier. This suggests that some form of repeated measurement (e.g., pulmonary function testing) will likely be necessary to adequately capture those who may be at risk, comparable to longitudinal screening evaluations for cancer and other age-related diseases (23–26).
This study also highlights groups in which likely clinically significant ILD may be unrecognized or misdiagnosed. It is perhaps not surprising that among smokers with COPD, clinicians may not consider the possibility that some physiologic limitations may be attributable to an additional or alternate lung disease. Future studies should consider providing more guidance on when screening for ILD among patients with COPD is most appropriate. In addition, these findings suggest that in this subset of those with ILA in COPDGene, participants who self-identified as Black or African American were more likely to have suspected ILD. Future work is needed to further define this group, identify any unique risk factors for the development of ILD, and importantly, confirm this finding. Prior studies have suggested that IPF may be less likely to be a cause of death among African Americans (27), but it is less clear whether this finding is the result of a reduced population-based prevalence, misdiagnosis, or both (28). It is also important to note that racial disparities are known to contribute to adverse outcomes in other respiratory conditions (29–32) and that underdiagnosis and/or misdiagnosis are common for many diseases in Black and African American populations (33, 34), highlighting the need for additional studies in ILD focusing on Black and African American populations.
Although we present findings among a large cohort of smokers, many of whom have COPD, there are a number of reasons why the presence of COPD or the amount of emphysema are not likely to explain our findings. First, multivariable analyses were all adjusted for COPD diagnosis and GOLD stage. Second, thresholds using DLCO to define suspected ILD were reweighted to account for the effect of emphysema. Finally, the associations with adverse outcomes we present are similar to sensitivity analyses that excluded patients with COPD (although smaller sample sizes may have limited the power to detect some differences) (Table E10) and removed DLCO as a criterion for suspected ILD (Table E7).
Our study has several potential limitations. First, as there is no current standardized approach to define ILD when it is unsuspected in a research cohort (in which comprehensive clinical evaluations and multidisciplinary diagnostic conferences are not available), we urge caution in interpreting the prevalence of suspected ILD in this cohort. It is also important to note that suspected ILD is not a diagnosis and likely represents a collection of heterogeneous disorders. Given the association with poor clinical outcomes, those who are found to have suspected ILD should undergo a comprehensive clinical evaluation to help determine the appropriate underlying diagnosis. Because our study suggests that the prevalence of suspected ILD may not be low, future work to develop consensus on the definitions and nomenclature would be helpful to refine and add confidence to what we termed as “suspected ILD”. Second, we cannot rule out the possibility that some of these findings are limited to smokers with and without COPD. However, it is worth noting that the findings of this cohort may be particularly relevant to those with ILA identified through lung cancer screening CTs because of their significant smoking history (35–37). Third, because DLCO was not available from the phase 1 data in COPDGene, the time for longitudinal follow-up was limited. Future studies will be needed to determine how often those with ILA but without suspected ILD progress to suspected ILD and over what time frame. Fourth, it remains unclear the extent to which measures of diffusion capacity are required to make accurate assessments of adverse outcomes. It is also possible that additional measures of pulmonary function, genetic risk factors, or other biomarkers could help improve risk stratification. We cannot rule out that the number of participants excluded from this analysis because of missing data could have created bias that influenced these results. Lastly, although we attempted to control for potential confounders that could affect measures of pulmonary function (e.g., body mass index and emphysema), we cannot rule out the possibility that other clinical conditions could have contributed, such as chest wall restriction for FVC and incidental pulmonary hypertension for DLCO in some participants.
Conclusions
Our study demonstrates that approximately half of those with ILA in COPDGene have suspected ILD, and most adverse outcomes attributable to those with ILA appear to be limited to this group. Although future longitudinal assessments of those with ILA will be required, our work demonstrates that those with suspected ILD have adverse clinical consequences, including reduced functional status and increased respiratory symptoms, supplemental oxygen use, severe respiratory exacerbations, and mortality. These findings demonstrate the important need to consider ILD among our patients with COPD and those with a significant smoking history and suggest that further monitoring and studies assessing the effectiveness of interventions might be warranted.
Footnotes
Supported by the Foundation for the National Institutes of Health (NIH) grants R01 HL153248, R01 HL135142, R01 HL149861, R01 HL137927, and R01 HL137148 (M.H.C.); NIH grant T32 HL007633 (J.A.R.); NIH grant R01 CA203636 (M.N.); NIH grant U01 HL133232 (I.O.R.); NIH grants R01 HL130275 and U01 HL131022 (S.E.-C.); NIH grants R01 HL130974, R01 HL118455, P01 HL132825, and U01 TR001810 (B.A.R.); NIH grant K08 HL145118 (S.Y.A.); NIH grant T32 HL007633 (B.C.); NIH grants R01 HL116473 and U01 HL146408 (G.R.W.); NIH grants R01 HL152728, U01 HL089856, R01 HL147148, R01 HL137927, and R01 HL133135 (E.K.S.); NIH grants R01 CA203636, U01CA209414, R01 HL111024, R01 HL135142, and R01 HL130974 (H.H.); NIH grants U01 HL089897 and U01 HL089856 (D.A.L.); NIH grants K08 HL140087 (R.K.P.); and NIH grants R01 HL111024, R01 HL130974, and R01 HL135142 (G.M.H.). COPDGene (Chronic Obstructive Pulmonary Disease Genetic Epidemiology study) is supported by NIH grant numbers U01 HL089897 and U01 HL089856. COPDGene is also supported by the COPD Foundation through contributions made to an Industry Advisory Board that has included AstraZeneca, Bayer Pharmaceuticals, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, and Sunovion.
Author Contributions: Study design: J.A.R., A.A.M., R.K.P., and G.M.H. Acquisition, analysis, or interpretation of the data: J.A.R., A.A.M., T.H., A.H., M.N., D.A.L., I.O.R., S.E.-C., B.A.R., S.Y.A., B.C., G.R.W., E.K.S., M.H.C., H.H., R.K.P., and G.M.H. Critical revision of the manuscript for important intellectual content: J.A.R., A.A.M., T.H., A.H., M.N., D.A.L., I.O.R., S.E.-C., B.A.R., S.Y.A., B.C., G.R.W., E.K.S., M.H.C., H.H., R.K.P., and G.M.H. Statistical analysis: J.A.R., A.A.M., R.K.P., and G.M.H. Obtained funding: G.R.W., E.K.S., M.H.C., and G.M.H.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202203-0550OC on August 5, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Hatabu H, Hunninghake GM, Richeldi L, Brown KK, Wells AU, Remy-Jardin M, et al. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner Society. Lancet Respir Med . 2020;8:726–737. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hatabu H, Hunninghake GM, Lynch DA. Interstitial lung abnormality: recognition and perspectives. Radiology . 2019;291:1–3. doi: 10.1148/radiol.2018181684. [DOI] [PubMed] [Google Scholar]
- 3. Hata A, Schiebler ML, Lynch DA, Hatabu H. Interstitial lung abnormalities: state of the art. Radiology . 2021;301:19–34. doi: 10.1148/radiol.2021204367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. COPDGene Investigators Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med . 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doyle TJ, Washko GR, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. COPDGene Investigators Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med . 2012;185:756–762. doi: 10.1164/rccm.201109-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators; COPDGene Investigators Association between interstitial lung abnormalities and all-cause mortality. JAMA . 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doyle TJ, Hunninghake GM, Rosas IO. Subclinical interstitial lung disease: why you should care. Am J Respir Crit Care Med . 2012;185:1147–1153. doi: 10.1164/rccm.201108-1420PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hunninghake GM, Quesada-Arias LD, Carmichael NE, Martinez Manzano JM, Poli De Frías S, Baumgartner MA, et al. Interstitial lung disease in relatives of patients with pulmonary fibrosis. Am J Respir Crit Care Med . 2020;201:1240–1248. doi: 10.1164/rccm.201908-1571OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose JA, Mennon AA, Hino T, Hata A, Nishino M, Lynch DA, et al. 2022.
- 10. Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD . 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Putman RK, Gudmundsson G, Axelsson GT, Hida T, Honda O, Araki T, et al. Imaging patterns are associated with interstitial lung abnormality progression and mortality. Am J Respir Crit Care Med . 2019;200:175–183. doi: 10.1164/rccm.201809-1652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Thoracic Society. Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med . 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 13. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med . 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 14. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stanojevic S, Graham BL, Cooper BG, Thompson BR, Carter KW, Francis RW, et al. Global Lung Function Initiative TLCO working group; Global Lung Function Initiative (GLI) TLCO Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J . 2017;50:1700010. doi: 10.1183/13993003.00010-2017. [DOI] [PubMed] [Google Scholar]
- 16. Menon AA, Putman RK, Sanders JL, Hino T, Hata A, Nishino M, et al. Interstitial lung abnormalities, emphysema and spirometry in smokers. Chest . 2021;161:999–1010. doi: 10.1016/j.chest.2021.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med . 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 18. Hunninghake GM. Interstitial lung abnormalities: erecting fences in the path towards advanced pulmonary fibrosis. Thorax . 2019;74:506–511. doi: 10.1136/thoraxjnl-2018-212446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, et al. Development and progression of interstitial lung abnormalities in the Framingham heart study. Am J Respir Crit Care Med . 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunninghake GM, Goldin JG, Kadoch MA, Kropski JA, Rosas IO, Wells AU, et al. Detection and early referral of patients with interstitial lung abnormalities: an expert survey initiative. Chest . 2021;161:470–482. doi: 10.1016/j.chest.2021.06.035. [DOI] [PubMed] [Google Scholar]
- 21. Kwon BS, Choe J, Chae EJ, Hwang HS, Kim YG, Song JW. Progressive fibrosing interstitial lung disease: prevalence and clinical outcome. Respir Res . 2021;22:282. doi: 10.1186/s12931-021-01879-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaul B, Cottin V, Collard HR, Valenzuela C. Variability in global prevalence of interstitial lung disease. Front Med (Lausanne) . 2021;8:751181. doi: 10.3389/fmed.2021.751181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siu AL, U.S. Preventive Services Task Force Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med . 2016;164:279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 24. Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. US Preventive Services Task Force Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA . 2021;325:1965–1977. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 25. Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. US Preventive Services Task Force Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA . 2021;325:962–970. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 26. Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, et al. US Preventive Services Task Force Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA . 2018;319:2521–2531. doi: 10.1001/jama.2018.7498. [DOI] [PubMed] [Google Scholar]
- 27. Swigris JJ, Olson AL, Huie TJ, Fernandez-Perez ER, Solomon J, Sprunger D, et al. Ethnic and racial differences in the presence of idiopathic pulmonary fibrosis at death. Respir Med . 2012;106:588–593. doi: 10.1016/j.rmed.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adegunsoye A, Oldham JM, Bellam SK, Chung JH, Chung PA, Biblowitz KM, et al. African-American race and mortality in interstitial lung disease: a multicentre propensity-matched analysis. Eur Respir J . 2018;51:1800255. doi: 10.1183/13993003.00255-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schraufnagel DE, Blasi F, Kraft M, Gaga M, Finn PW, Rabe KF, ATS/ERS Committee on Disparities in Respiratory Health An official American Thoracic Society/European Respiratory Society policy statement: disparities in respiratory health. Am J Respir Crit Care Med . 2013;188:865–871. doi: 10.1164/rccm.201308-1509ST. [DOI] [PubMed] [Google Scholar]
- 30. Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med . 2006;354:333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 31. Forno E, Celedón JC. Health disparities in asthma. Am J Respir Crit Care Med . 2012;185:1033–1035. doi: 10.1164/rccm.201202-0350ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thakur N, Lovinsky-Desir S, Bime C, Wisnivesky JP, Celedón JC. The structural and social determinants of the racial/ethnic disparities in the U.S. COVID-19 pandemic. What’s our role? Am J Respir Crit Care Med . 2020;202:943–949. doi: 10.1164/rccm.202005-1523PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim EJ, Kim T, Conigliaro J, Liebschutz JM, Paasche-Orlow MK, Hanchate AD. Racial and ethnic disparities in diagnosis of chronic medical conditions in the USA. J Gen Intern Med . 2018;33:1116–1123. doi: 10.1007/s11606-018-4471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin PJ, Daly AT, Olchanski N, Cohen JT, Neumann PJ, Faul JD, et al. Dementia diagnosis disparities by race and ethnicity. Med Care . 2021;59:679–686. doi: 10.1097/MLR.0000000000001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin GY, Lynch D, Chawla A, Garg K, Tammemagi MC, Sahin H, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology . 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pompe E, de Jong PA, Lynch DA, Lessmann N, Išgum I, van Ginneken B, et al. Computed tomographic findings in subjects who died from respiratory disease in the National Lung Screening Trial. Eur Respir J . 2017;49:1601814. doi: 10.1183/13993003.01814-2016. [DOI] [PubMed] [Google Scholar]
- 37. Hoyer N, Wille MMW, Thomsen LH, Wilcke T, Dirksen A, Pedersen JH, et al. Interstitial lung abnormalities are associated with increased mortality in smokers. Respir Med . 2018;136:77–82. doi: 10.1016/j.rmed.2018.02.001. [DOI] [PubMed] [Google Scholar]