Abstract
Hyperactivation of the mechanistic target of rapamycin (mTOR) signaling pathway is linked to more than a dozen neurologic diseases, causing a range of pathologies, including excess neuronal growth, disrupted neuronal migration, cortical dysplasia, epilepsy and autism. The mTOR pathway also regulates angiogenesis. For the present study, therefore, we queried whether loss of Pten or Tsc2, both mTOR negative regulators, alters brain vasculature in three mouse models: one with Pten loss restricted to hippocampal dentate granule cells [DGC-Pten knock-outs (KOs)], a second with widespread Pten loss from excitatory forebrain neurons (FB-Pten KOs) and a third with focal loss of Tsc2 from cortical excitatory neurons (f-Tsc2 KOs). Total hippocampal vessel length and volume per dentate gyrus were dramatically increased in DGC-Pten knock-outs. DGC-Pten knock-outs had larger dentate gyri overall, however, and when normalized to these larger structures, vessel density was preserved. In addition, tests of blood-brain barrier integrity did not reveal increased permeability. FB-Pten KOs recapitulated the findings in the more restricted DGC-Pten KOs, with increased vessel area, but preserved vessel density. FB-Pten KOs did, however, exhibit elevated levels of the angiogenic factor VegfA. In contrast to findings with Pten, focal loss of Tsc2 from cortical excitatory neurons produced a localized increase in vessel density. Together, these studies demonstrate that hypervascularization is not a consistent feature of mTOR hyperactivation models and suggest that loss of different mTOR pathway regulatory genes exert distinct effects on angiogenesis.
Keywords: angiogenesis, focal cortical dysplasia, mTOR, mtoropathy, tuberous sclerosis, Vegf
Significance Statement
Here, we examined three mouse models to determine whether mechanistic target of rapamycin (mTOR) hyperactivation consistently drives brain hypervascularization. Both focal loss of Pten from dentate granule cells, and widespread loss from forebrain produced larger brain structures and corresponding increases in vascular growth, but normal vessel density. By contrast, focal cortical Tsc2 lesions exhibited significantly increased vessel density. Findings indicate that hypervascularization is not characteristic of all mTOR hyperactivation models and suggest vascular changes may be driven by gene-specific effects.
Introduction
Mutations in more than a dozen genes that regulate the mechanistic target of rapamycin (mTOR) pathway cause a variety of syndromes collectively known as mTORopathies (Crino, 2020). mTORopathies are characterized by brain structural defects, benign tumors, intellectual disability, autism and epilepsy (Mirzaa et al., 2016; Marsan and Baulac, 2018). Affected genes include mTOR itself, as well as negative regulators like tuberous sclerosis complex subunits 1 and 2 (TSC1/2) and phosphatase and tensin homolog (PTEN). Mutations in animal models directly increase mTOR signaling in affected neurons, leading to morphologic and physiological changes that include neuronal hypertrophy, dendritic and axonal sprouting and increased excitability (Zeng et al., 2009; Barrows et al., 2017; Switon et al., 2017; Skelton et al., 2020; Narvaiz et al., 2022).
Mutations in mTOR pathway genes can also lead to blood vessel abnormalities. In human cancers, where mTOR mutations are common, mutations contribute to tumor-induced angiogenesis (Wen et al., 2001; Tian et al., 2010; Karar and Maity, 2011). Increased vessel density has been described in focal cortical dysplasia (Wintermark et al., 2013), a condition commonly associated with mTOR mutations. Vascular abnormalities have also been observed in patients with tuberous sclerosis complex (Mühlebner et al., 2016; Chihi et al., 2019 Sun et al., 2021) and in animal models of the disease (Zhang et al., 2019; Kútna et al., 2020). Vascular abnormalities could be driven by several mechanisms. First, excess growth of neurons with mTOR pathway mutations can lead to enlarged brain structures, necessitating additional angiogenesis to support the larger structures. Second, mTOR pathway mutations commonly cause seizures that can disrupt the blood-brain barrier and induce vascular changes (van Vliet et al., 2012; Mendes et al., 2019). Finally, increased expression of genes involved in vascular remodeling has been observed in mTOR mutants and following pharmacological manipulation of mTOR signaling. Changes have been observed at the mRNA and protein levels in a variety of tissue types, including peripheral tumors and brain. Affected genes include vascular endothelial growth factor (Vegf), angiopoietin 1 and 2 (Ang-1, Ang-2), hypoxic inducible factor 1 (Hif-1alpha) and matrix metalloproteinases (Land and Tee, 2007; Parker et al., 2011; Feliciano et al., 2013; Xue et al., 2018; Broekaart et al., 2020).
Vascular abnormalities could be important disease-driving components for patients with mTOR mutations. Increased vascularity would provide better access to oxygen and nutrients for dysplastic neurons, facilitating aberrant growth akin to that observed in tumors. Vessel remodeling can also be associated with increased blood-brain barrier permeability. Increased permeability can initiate cascades of negative sequelae, including disrupted ion homeostasis, inflammatory effects of toxic serum proteins like albumin and seizures (Frigerio et al., 2012; Gorter et al., 2019).
Here, we queried whether loss of the mTOR pathway inhibitors Pten or Tsc2 would also produce vascular changes in the brain. Three genetic models were used: the 1st targeting hippocampal granule cell progenitors [DGC-Pten knock-out (KO)], the 2nd targeting excitatory forebrain neurons (FB-Pten KO) and the 3rd producing focal Tsc2 loss from cortical excitatory neurons. Neurons in both Pten models exhibit physiologic and morphologic abnormalities, while the animals exhibit spontaneous seizures (McMahon et al., 2012; Sperow et al., 2012; LaSarge et al., 2015, 2016, 2019; Santos et al., 2017; Arafa et al., 2019; Chen et al., 2019; White et al., 2020). These studies will help to establish whether vascular abnormalities are characteristic of mTORopathies.
Materials and Methods
Animals
All animal procedures were conducted in accordance with National Institutes of Health (NIH) and Institutional Animal Care and Use Committee (IACUC) guidelines. Gli1-CreERT2 (RRID:IMSR_JAX:007913), CamK2α-Cre (RRID:IMSR_JAX:005359), Ptenfl/fl (RRID:IMSR_JAX:006440), TdTomatofl/fl (TdTom; RRID:IMSR_JAX:007914), and Tsc2fl/fl (RRID:IMSR_JAX:027458) mice were obtained from the Jackson laboratory and maintained as colonies in our vivarium. All animals were maintained on a C57BL/6 background, including the Tsc2fl/fl mice that were obtained as B6129SF2/J and subsequently crossed with C57BL/6 for eight generations. Littermates were distributed across all time points and groups where possible. The ARRIVE guidelines were followed for study design and reporting (Kilkenny et al., 2010).
Labeling of vascular structure in DGC-Pten KOs
To examine the impact of deleting Pten from granule cells on hippocampal vascular structure, Gli1-CreERT2+/−, Ptenwt/wt, TdTomfl/wt (cre control) and Gli1-CreERT2+/−, Ptenfl/fl, TdTomfl/wt (DGC-Pten KO) mice were generated (Table 1, Experiment #1). DGC-Pten KO and control animals were given a subcutaneous injection of tamoxifen (Sigma-Aldrich, T5648; 250 mg/kg dissolved at 20 mg/ml in corn oil) on postnatal day 14 (P14). Blood vessel labeling and tissue collection was conducted when the animals were four, six and 10 weeks old. To label vessels, mice were briefly anesthetized with isoflurane and the left eye was treated with one drop of 0.5% proparacaine hydrochloride ophthalmic solution (AKORN). DyLight 649 labeled Lycopersicon esculentum lectin (Vector Laboratories #DL-1178; 0.1 ml of a 1 mg/ml solution in 10 mm HEPES, 0.1 mm Ca+2, 0.15 m NaCl, pH 7.5) was injected into the retro-orbital sinus of the anesthetized eye. Mice recovered for 10–15 min after lectin injections and were then given 0.1 ml of pentobarbital (65 mg/ml) intraperitoneally. When the animals were fully anesthetized, they were transcardially perfused for 1–2 min with 0.1 m PBS with 1 U/ml heparin, followed by a 10 min perfusion with 2.5% paraformaldehyde with 4% sucrose in 0.1 m PBS, pH 7.4. Brains were removed and incubated overnight in the same fixative at 4°C. Brains were cryoprotected in ascending 10%, 20% and 30% sucrose solutions in 0.1 m PBS before freezing. Sagittal cryosections were cut at 60 μm, mounted onto gelatin-coated slides and stored at −80°C.
Table 1.
Listing of animals used for the five main experiments in the study
| Experiment # | Readout | Model | Cellular target | Genotype/AAV | Group | Age (weeks) | Mouse # |
|---|---|---|---|---|---|---|---|
| 1 | Vascular structure |
DGC-Pten KO |
≈20% of dentate granule cells |
Gli1-CreERT2+/−, Ptenwt/wt, TdTomfl/wt |
Control | 4 | 6 (3 F, 3 M) |
| Gli1-CreERT2+/−, Ptenwt/wt, TdTomfl/wt |
Control | 6 | 7 (2 F, 5 M) | ||||
| Gli1-CreERT2+/−, Ptenwt/wt, TdTomfl/wt |
Control | 10 | 6 (3 F, 3 M) | ||||
| Gli1-CreERT2+/−, Ptenfl/fl, TdTomfl/wt |
KO | 4 | 7 (2 F, 5 M) | ||||
| Gli1-CreERT2+/−, Ptenfl/fl, TdTomfl/wt |
KO | 6 | 8 (4 F, 4 M) | ||||
| Gli1-CreERT2+/−, Ptenfl/fl, TdTomfl/wt |
KO | 10 | 11 (5 F,6 M) | ||||
| 2 | BBB Integrity | DGC-Pten KO |
≈20% of dentate granule cells |
Gli1-CreERT2−/−, Ptenfl/fl, tdTomwt/wt |
Control | 11 | 3 (3 F, 0 M) |
| Gli1-CreERT2−/−, Ptenfl/fl, tdTomfl/wt |
Control | 11 | 2 (1 F, 1 M) | ||||
| Gli1-CreERT2+/−, Ptenfl/fl, tdTomwt/wt |
KO | 11 | 4 (3 F, 1 M) | ||||
| 3 | Vascular structure |
FB-Pten KO |
Forebrain excitatory neurons |
CamK2α-Cre−/−, Ptenfl/fl (or Ptenfl/wt) |
Control | 6.5–7 | 4 (3 F, 1 M) |
| CamK2α-Cre+/−, Ptenwt/wt | Control | 6.5–7 | 3 (2 F, 1 M) | ||||
| CamK2α-Cre+/−, Ptenfl/fl | KO | 6.5–7 | 5 (3 F, 2 M) | ||||
| 4 | Western blotting |
FB-Pten KO |
Forebrain excitatory neurons |
CamK2α-Cre−/−, Ptenfl/fl | Control | 7–8 | 2 (1 F, 1 M) |
| CamK2α-Cre+/−, Ptenwt/wt | Control | 7–8 | 5 (3 F, 2 M) | ||||
| CamK2α-Cre+/−, Ptenfl/fl | KO | 7–8 | 7 (4 F, 3 M) | ||||
| 5 | Vascular structure |
f-Tsc2 KO |
Focal loss from cortical excitatory neurons |
Tsc2wt/wt + AAV9-CaMKII- mCherry-Cre |
Control | 7, 15, 17, 20 | 4 (2 F, 2 M) |
|
Tsc2fl/fl + AAV9-CaMKII- mCherry-Cre |
KO | 9, 14, 14,15,19 | 5 (3 F, 2 M) |
Experiment #1, DyLight 649 treatment of DGC-Pten KOs and controls to examine vascular structure. Experiment #2, AF488-BSA treatment of DGC-Pten KOs to assess blood-brain barrier permeability. Experiment #3, DyLight 649 treatment of FB-Pten KOs and controls to examine vascular structure. Experiment #4, Western blot analyses of FB-Pten KOs and controls. Experiment #5; CD31 immunostaining of focal Tsc2 KO mice to examine vascular structure.
Pten immunohistochemistry
Pten immunohistochemistry was performed on study animals to facilitate quantification of the percentage of hippocampal granule cells with Pten deletion. Two to four sections per mouse were immunostained with rabbit anti-Pten primary antibodies (1:250, Cell Signaling Technology catalog #9559 RRID:AB_390810), AF647 goat anti-rabbit secondary antibodies (1:750; ThermoFisher Scientific catalog #A32733 RRID: AB_2633282) followed by counterstaining with DAPI. Confocal optical sections of stained dentate gyri were scored to determine the percentage of Pten immunonegative granule cells in accord with established protocols (Arafa et al., 2019; LaSarge et al., 2019, 2021).
Confocal imaging of vascular structure in DGC-Pten KOs
Sagittal brain sections between medial-lateral coordinates 1.56 and 1.8 mm were selected for imaging. Slide-mounted brain sections were thawed, rehydrated, and coverslipped with Prolong Diamond with DAPI (#P36962, Thermo Fisher Scientific). Imaging was performed on a Nikon Ti-E inverted confocal microscope using a 20× objective (NA = 0.75). DyLight 649-lectin was excited with the 647-nm laser line. Sections were imaged through 24 μm of the z-depth of the tissue with a 1.0-μm step to create a confocal image stack. Image resolution was 0.63 μm/pixel in the xy plane. Images from one section/animal were tiled to capture the entire dentate gyrus.
Two-dimensional analysis of vascular structure in DGC-Pten KOs
To collect vessel area measurements from four-, six-, and 10-week-old control and DGC-Pten KO mice, confocal image stacks were converted into maximum projections using Nikon Elements software (AR 5.21.01). The hippocampal fissure and the hippocampal/thalamic border were used as dorsal and ventral boundaries of the dentate gyrus, respectively, while a straight line drawn between the tips of the upper and lower blades of the granule cell body layer set the anterior/posterior boundary. The boundaries of the dentate gyrus were manually encoded using Nikon software, while DyLight 649-lectin labeled vessels were detected automatically using Nikon software’s Object Count function. Identical threshold and parameter settings were used for all slides. Vessel density was calculated by normalizing vessel area to the volume of the dentate gyrus using the following equation: vessel area/(dentate area × 24-μm z-depth). Normalizing vessel area to dentate volume is not technically correct, but was felt to be more accurate than normalizing to dentate area, as vessel area measurements were collected from maximum projections of 24-μm-thick dentate image stacks, and thus encompass all vessels in the stack.
Three-dimensional analysis of vascular structure in DGC-Pten KOs
To better elucidate exactly how vessel structure changed in 10-week-old control and DGC-Pten KO mice, confocal image stacks through the 24-μm z-depth of tissue were imported into Neurolucida 360 software (Microbrightfield) for three-dimensional analysis. Volumetric reconstructions of lectin-stained vessels were used to quantify length, tortuosity, surface area, volume and diameter of hippocampal blood vessels. Quantitative measurements were extracted from the vessel reconstructions using Neurolucida Explorer.
Permeability assessment in DGC-Pten KOs
DGC-Pten KO and control mice were generated to determine whether loss of Pten alters blood-brain barrier permeability (Table 1, Experiment #2). All Pten KO mice and three of five flox control mice were treated with 250 mg/kg of tamoxifen at P14. Alexa Fluor 488 conjugated bovine serum albumin (AF488-BSA, 66,000 Da; Invitrogen #A13100) was given at 11 weeks of age to assess vascular permeability (Marcon et al., 2009; Di Pardo et al., 2017; Ahishali and Kaya, 2021). AF488-BSA was resuspended to 1 mg/ml in sterile normal saline and filtered to remove particulates. Mice were anesthetized with inhaled isoflurane, and 0.1 ml of AF488-BSA injected into the retro-orbital sinus of the left eye as described for tomato lectin. Mice recovered for 10–15 min before euthanizing, perfusing, postfixing and cryoprotecting as described for other animals. Coronal cryosections were cut at 40 μm, mounted onto gelatin-coated slides and stored at −80°C.
To generate positive control animals for blood-brain barrier assessment, pilocarpine status epilepticus was induced in two male 11-week-old Gli1-CreERT2−/−, Ptenfl/fl mice as described previously (Hester et al., 2016; Wulsin et al., 2021). Briefly, mice were injected subcutaneously with 1 mg/kg scopolamine followed 30 min later with 380 mg/kg pilocarpine. Three hours after the onset of status epilepticus, mice were treated with AF488-BSA and perfusion fixed as described for other animals.
Sections were rehydrated in 1× PBS and mounted using Prolong Glass Anti-fade with NucBlue (Thermofisher #P36981). Brain sections from dorsal (bregma = −1.58) and ventral (bregma = −3.1) hippocampi of AF488-BSA infused animals were imaged using a Nikon A1R inverted confocal microscope equipped with a 20× objective (NA = 0.75). Confocal optical sections of the dentate gyri in each section (four to seven dentate gyri/mouse) were collected 5 μm below the tissue surface. AF488-BSA was imaged using the 488 laser line to produce images with a resolution of 1.24 μm/pixel. Images were tiled to capture the entire dentate gyrus. All sections were imaged with identical confocal settings. Images were analyzed using Nikon Elements. A region of interest (ROI) was drawn around the entire dentate gyrus in each confocal optical section, as described for vessel area measurements. Average pixel intensity for regions of interest was calculated and averaged for each animal for statistical analysis.
Two-dimensional analysis of vascular structure in FB-Pten KO mice
To determine whether widespread loss of Pten from excitatory neurons in hippocampus and cortex would alter vascular structure, CamK2α-Cre+/−, Ptenfl/fl knock-out mice (FB-Pten KOs) were generated. CamK2α-Cre+/−, Ptenwt/wt and CamK2α-Cre−/−, Ptenfl/fl or fl/wt mice served as controls (Table 1, Experiment #3). Additionally, post hoc genotype confirmation identified a germline recombination event in two FB-Pten KO mice (one male, one female), such that these animals were germline Pten heterozygotes combined with CamK2α-Cre mediated recombination of the second Pten allele from forebrain excitatory neurons. As this unexpected recombination event might increase the severity of the Pten phenotype, potentially providing a more robust test of the role of Pten loss in driving vascular changes, these two animals were retained in the study for analysis.
FB-Pten KO mice were treated with DyLight 649 lectin and brains prepared for histologic studies when they were 6.5–7 weeks old. FB-Pten KO mice undergo significant mortality after 8–10 weeks, so only younger animals can be examined. Vascular labeling and tissue preparation were identical to the procedures described for the two-dimensional analysis of DGC-Pten KO mice except that quantification was conducted on the entire hippocampus in each brain slice, as Pten loss affects granule cells and pyramidal cells in these animals. The region of cortex immediately above the hippocampus was imaged to assess cortical vasculature. Confocal z-series images collected through 20 μm of tissue at a 1.0-μm step were used for this analysis.
SDS-PAGE and Western blot analysis in FB-Pten KOs
For Western blot studies, a second cohort of FB-Pten KO mice was generated (Table 1, Experiment #4). Mice were killed by CO2 inhalation between seven and eight weeks of age. Cortex was extracted and immediately frozen on dry ice. Samples were stored at −80°C before tissue lysis.
Tissue lysis was performed with lysis buffer [50 mm Tris (pH 7.4), 40 mm NaCl, 1 mm ethylenediaminetetraacetic acid (EDTA, pH 8), 0.5% Triton X-100, 50 mm NaF, 10 mm Na pyrophosphate, 10 mm Na β-glycerol phosphate, and 1× protease inhibitor; Sigma-Aldrich, catalog #11873580001]. Protein concentration was determined using Bio-Rad Protein Assay Dye (catalog #5000006). Samples were mixed with SDS sample buffer and equal amounts of proteins were run in duplicate on SDS-PAGE gels and transferred to PVDF Transfer Membranes (Millipore Sigma). Membranes were blocked using 5% milk for 1 h. The following antibodies were used for Western blotting: Pten (1:1000; catalog #9559, RRID: AB_390810), anti-β-Actin (1:40,000, Sigma #A1978, clone AC-15, RRID:AB_476692), and vascular endothelial growth factor A (VegfA; 1:1000; Abcam, catalog #ab46154, RRID: AB_2212642). Antibodies were diluted to the desired concentration in 1% Tween in 0.1M PBS and incubated at 4°C overnight. Membranes were then washed and incubated with secondary antibody; either Rabbit IgG HRP Linked Whole Antibody (1:2000; Millipore Sigma; catalog #GENA934) or Mouse IgG HRP Linked Whole Antibody (1:2000; Millipore Sigma; catalog #NXA931V). Signals were detected with enhanced chemiluminescence using Pierce ECL Blotting Substrate (Thermo Scientific, catalog #32106). If a second detection was needed, blots were stripped using Restore Western Blot Stripping buffer (Thermo Scientific, catalog #21059), blocked again in 5% milk, and incubated overnight with the desired antibody. Specific signals on Western blottings were quantified by densitometry using NIH ImageJ software. Signal intensities were normalized to β-Actin on the same blot. Duplicate samples were averaged for statistical analysis.
Two-dimensional analysis of vascular structure in cortical f-Tsc2 KO mice
To determine whether focal loss of Tsc2 from excitatory neurons in cortex would alter vascular structure, Tsc2fl/fl and Tsc2wt/wt mice were generated (Table 1, Experiment #5). All mice received two bilateral stereotaxic injections starting with the left hemisphere (four injections total at coordinates A/P, M/L, D/V: 0.5, ±0.5, −0.35 and −1.0, ±0.65, −0.35). Injections contained AAV9-CaMK2α_short>mCherry:T2A:Cre:WPRE vector with 0.05% Trypan Blue in sterile PBS (50 nl per site of 3.55x 109 gc/μl concentration; VectorBuilder, VB200130-1243kmp) and were conducted on P2 using hypothermia anesthesia (Kim et al., 2014). Pups were returned to the mother after the injection and weaned at P28. As part of another experiment, two Tsc2wt/wt mice and four Tsc2fl/fl underwent EEG implantation surgery between 12 and 13 weeks of age in accord with established protocols (LaSarge et al., 2021). All mice were perfused between seven and 20 weeks of age (control: 104 ± 18.9 d old; f-Tsc2 KOs: 100 ± 11.0 d; mean ± SEM; Table 1, Experiment #5). Tissue was postfixed, cryoprotected, frozen, cut, and stored as described for other animals.
Tsc2 immunohistochemistry
Tsc2 immunohistochemistry was performed on tissue from study animals to verify loss of Tsc2 in virally-infected (mCherry-expressing) cells. Sections were immunostained with rabbit anti-Tuberin/Tsc2 primary antibodies (1:750, Cell Signaling Technology catalog #4308 RRID:AB_10547134), AF647 goat anti-rabbit secondary antibodies (1:750; Thermo Fisher Scientific catalog #A32733 RRID: AB_2633282) and counterstained with NeuroTrace 500/525 (1:300; Invitrogen catalog #N21480). Slides were mounted with Prolong Glass Anti-fade with NucBlue (Thermofisher #P36981). Images were acquired using a Nikon AXR inverted confocal microscope equipped with a 10× (NA = 0.45) and 60× water immersion (NA = 1.20) objective.
Analysis of vascular structure in f-Tsc2 KO mice
To quantify blood vessel density, CD31 immunohistochemistry was conducted. Bregma-matched tissue sections from control and f-Tsc2 KO mice (A/P 1.4-2.4) were immunostained with rat-anti mouse CD31 primary antibody (1:500, BD Biosciences catalog #553370) and AF647 goat anti-rat secondary antibodies (1:750, Thermo Fisher Scientific catalog #A21247 RRID: AB_2633282). The slides were coverslipped with ProLong Glass Antifade Mountant with NucBlue (#P36981, Thermo Fisher Scientific). Slides were then screened to identify mCherry expressing regions in cortex. Injections in the left hemisphere consistently produced focal mCherry labeling in cortex, while mCherry labeling was rare in the right hemisphere, likely a consequence of the syringe clogging after the first one to two injections. Imaging was performed on a Nikon Ti-E inverted confocal microscope using a water immersion 20× objective (NA = 0.95). Serial channel imaging was used to capture mCherry-labeled cells (581 nm) and CD31 expression (647 nm) in a 635 × 635 (xy) × 12 (z-depth, 0.5-μm step) field centered on the region containing mCherry-labeled Tsc2 KO or control cells in the left hemisphere. An anatomically correspondent region of interest (ROI) in the right hemisphere lacking mCherry expression was also imaged using identical settings. Confocal image stacks were converted into maximum projections using Nikon Elements software (AR 5.21.01). Vessel density in the right hemisphere was determined using Nikon software’s Object Count function. For the left hemisphere, images were imported into Neurolucida 360 software to define a ROI surrounding mCherry-expressing Tsc2 KO cells, or mCherry-expressing wild-type cells in control mice. The user-guided tracing function was employed to trace the blood vessels within each ROI. Vessel area was normalized to ROI volume for statistical analyses, as described for the DGC-Pten KO model.
Statistical analysis
All data collection was conducted by investigators unaware of animal genotype or treatment group. Statistical significance was determined using Sigma Plot (version 14), R or GraphPad Prism (version 9.3.1) with α = 0.05. Sex effects were assessed by including sex as a factor in two-way or three-way ANOVAs. No effects of sex were found except as noted. Note as well that the study was not powered to detect sex differences. In the absence of differences, data from male and female animals was pooled. Data were tested for normality and equal variance, and parametric or nonparametric equivalents used as appropriate. Blood-brain barrier leakage was assessed using a linear mixed effect model with a random animal effect. Values presented are mean ± SD unless otherwise noted.
Results
To determine whether loss of the mTOR negative regulator Pten from a subset of dentate granule cells induces vascular changes in the dentate gyrus, a conditional, inducible mouse model approach was used to delete Pten from hippocampal dentate granule cell progenitors (DGC-Pten KOs; Table 1, Experiment #1). To induce Pten deletion, mice were treated with tamoxifen on P14, when many granule cell progenitors are still active. Tamoxifen-activated cre recombinase leads to the deletion of Pten and expression of tdTomato among hippocampal granule cell progenitors and all subsequent daughter cells (Pun et al., 2012; LaSarge et al., 2015, 2016). Among DGC-Pten KO animals, 22% (8 of 36) died before the experimental endpoint compared with 0 of 21 control animals. Mice were harvested at four, six, or 10 weeks of age (two, four, and eight weeks after tamoxifen treatment). On the day of harvest, mice were injected retro-orbitally with a fluorescent lectin (DyLight 649) to label the vasculature.
DGC-Pten KO cell load
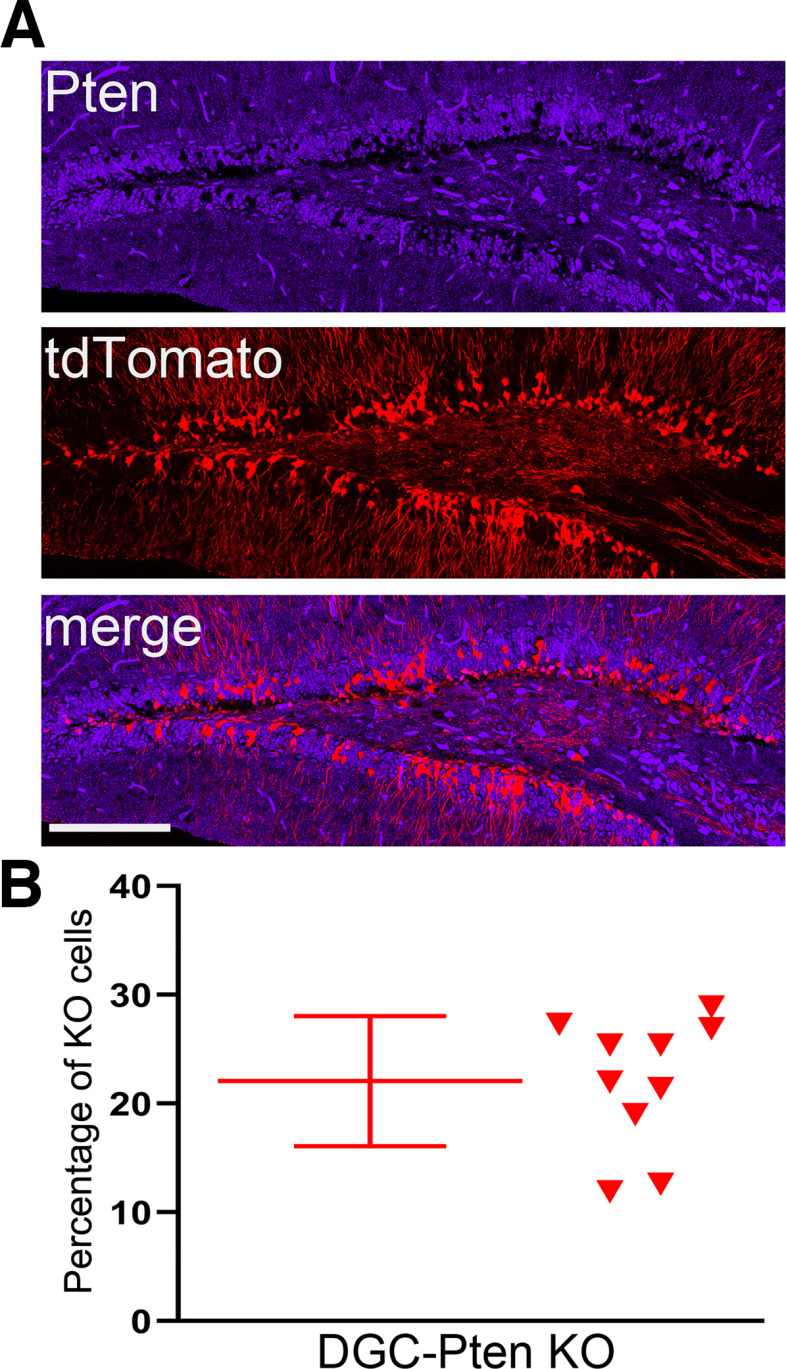
In prior studies, we have observed that animals with DGC-Pten KO cell loads >10% exhibit spontaneous cortical seizures as early as eight weeks (Pun et al., 2012). As seizures can drive changes in microvascular density (van Lanen et al., 2021), the current study was designed to produce animals with KO rates in excess of 10%. Quantification of DGC-Pten KO cell number in 10-week-old KO animals revealed that, on average, 22.1 ± 6.0% of granule cells lacked Pten protein (n = 10; range 11.9–28.9%; Fig. 1). Although seizures were not directly assessed here, animals in the current study were well above the threshold for spontaneous cortical seizures established in prior work (Pun et al., 2012; LaSarge et al., 2021).
Figure 1.
A, Pten immunostaining (blue) and tdTomato fluorescence (red) in a 10-week-old DGC-Pten knock-out (KO) mouse. Scale bar = 100 μm. B, Scatterplot showing the percentage of DGC-Pten KO cells for a subset of animals used in the present study.
DGC-Pten KO mice exhibit increased hippocampal vasculature but preserved vascular density
Hippocampi from four, six and 10-week-old control and DGC-Pten KO mice were examined to determine total vessel area in the dentate gyri of hippocampal sections (Fig. 2A,B). Among control animals, dentate vessel area was stable across all three time points (two-way ANOVA on log transformed data; 4 vs 6, p = 0.944; 4 vs 10, p = 0.999; 6 vs 10, p = 0.961; Fig. 2C). Among KOs, by contrast, vessel area was significantly greater at 10 weeks relative to four (p = 0.004) and six (p < 0.001) weeks. Vascular area in the 10-week KO group was 51.9% greater than the 10-week control group (p < 0.001). These data demonstrate that vascular area expands significantly in DGC-Pten KO animals between six and 10 weeks.
Figure 2.
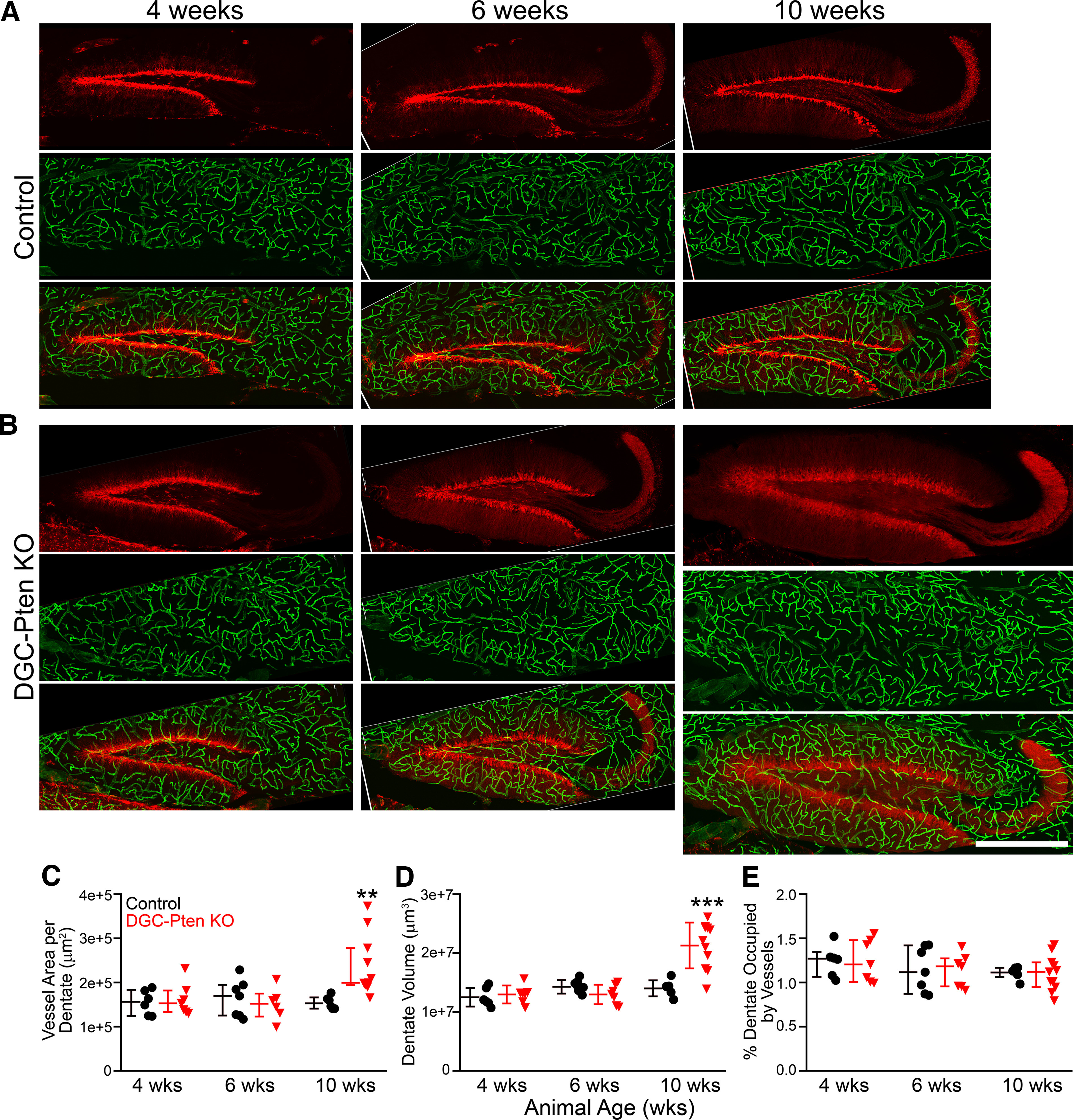
A, B, Confocal images of hippocampal dentate gyri showing granule cells expressing tdTomato (red) and blood vessels labeled with DyLight 649-lectin (green). Dentate gyri from four-, six-, and 10-week (wks) control (A) and DGC-Pten knock-out (KO) (B) animals are shown. All images are at the same scale, demonstrating the striking hypertrophy of the dentate in the 10-week KO. Scale bar = 500 μm. C, Scatterplots of individual animal scores and group medians ± interquartile range for vessel area in control (black) and DGC-Pten KO (red) animals. Area was calculated from maximum projections of images from 24-μm-thick hippocampal sections. D, Scatter plot and group means ± SD for the volume of the dentate gyrus in 24-μm-thick hippocampal sections. E, Vessel area (C) was normalized to dentate volume (D) to estimate the percentage of the dentate occupied by vessels in 24-μm-thick hippocampal slices. Values are group medians ± interquartile range. **p < 0.01, ***p < 0.001.
To determine whether the observed increase in vessel area also produced an increase in vessel density, we quantified dentate volume for each brain slice examined. Pten loss can increase brain size (Kwon et al., 2003) driven at least in part by somatic and dendritic hypertrophy of KO cells (LaSarge et al., 2015, 2019; Santos et al., 2017). Visual examination of dentate gyri from 10-week KO animals revealed that the dentate gyrus was indeed larger (Fig. 2A,B). Quantification of dentate volume in each brain slice revealed changes that paralleled vessel area. Dentate volume was stable across all three time points in control mice (two-way ANOVA on log transformed data; 4 vs 6, p = 0.198; 4 vs 10, p = 0.325; 6 vs 10, p = 0.966; Fig. 2D). Among KOs, by contrast, dentate volume was significantly greater at 10 weeks relative to four (p < 0.001) and six (p < 0.001) weeks. Dentate volume in the 10-week KO group was 51.8% greater than the 10-week control group (p < 0.001). To approximate vessel density, we normalized vessel area to dentate volume for each brain slice. Vessels occupied ∼1% of the dentate volume in this analysis. No differences in vessel density were evident among different ages (two-way ANOVA on log transformed data, p = 0.170; Fig. 2E) or genotypes (p = 0.941). Taken together, these findings indicate the DGC-Pten KO animals exhibit ∼50% increases in blood vessel area and dentate volume, but overall vessel density is preserved. Therefore, although DGC-Pten KO dentate gyri are larger in 10-week animals, the data suggest that the average distance between a neuron and the nearest vessel is unchanged.
Volumetric analyses of vascularity in DGC-Pten KO mice
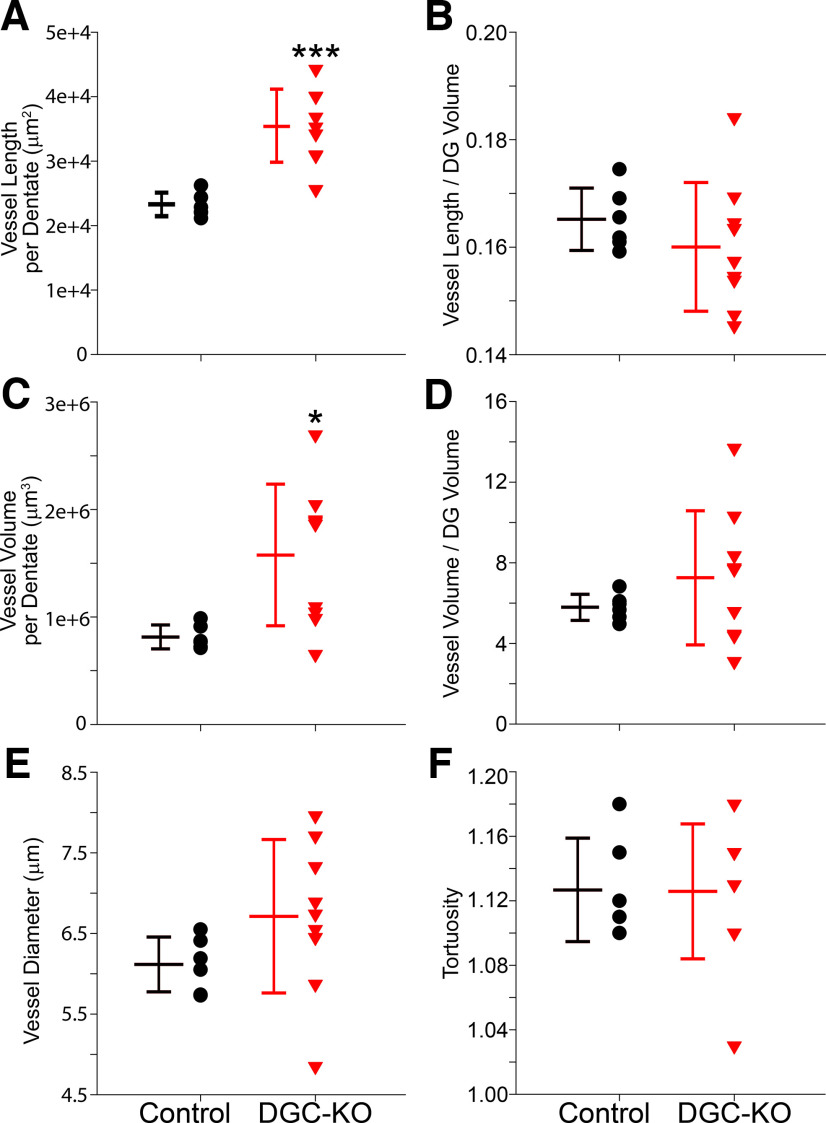
Given the robust changes in dentate structure in 10-week DGC-Pten KOs, we sought to analyze vessel structure in these animals in greater detail. Three dimensional reconstructions of vessels in each dentate were generated, encoding vessel length, volume, diameter, tortuosity and volume of the dentate slice sampled. This approach allows us to calculate a true vessel density relative to the area measurements and estimates shown in Figure 2.
Vessel length per dentate was significantly increased in 10-week DGC-Pten KOs relative to 10-week controls (control, n = 6; KO, n = 9; Welch’s t test, p < 0.0001; Fig. 3A). When normalized to dentate volume, however, no differences in overall vessel density were evident (t test, p < 0.346; Fig. 3B). Similar findings were evident when vessel volume was examined. Total vessel volume per dentate was significantly increased in KOs (Welch’s t test, p = 0.008; Fig. 3C), but vessel volume normalized to dentate volume was unchanged (Welch’s t test, p = 0.234; Fig. 3D). Vessel diameter was statistically similar between groups (t test, p = 0.170; Fig. 3E). Finally, we compared vessel tortuosity, a measure of how “twisted” a vessel’s path is. Tortuosity was not found to differ among groups (t test, p = 0.957; Fig. 3F). These findings confirm the increase in vessel extent per dentate observed in the prior analysis, and the preservation of overall vessel density. They further reveal that increasing vessel extent per dentate is driven by vessel lengthening, without overt changes in vessel diameter or path, at least at the time points examined.
Figure 3.
Graphs show scatterplots of individual animal scores and group means ± SD for 10-week control and DGC Pten-KO animals. All data were collected from a 24-μm-thick section of the hippocampal dentate gyrus (DG). A, Mean vessel length per dentate section. B, Mean vessel length per dentate brain section divided by the volume of the section X 100. C, Mean vessel volume per dentate section. D, Mean vessel volume per dentate brain section divided by the volume of the section X 100. E, Mean vessel diameter. F, Mean vessel tortuosity. *p < 0.05, ***p < 0.001.
Blood-brain barrier leakage
Seizures can induce blood-brain barrier leakage (Gorter et al., 2019). Angiogenesis can also be associated with impaired blood-brain barrier integrity (Rust, 2020). To determine whether the blood-brain barrier might be impaired in DGC-Pten KO mice, a new group of 11-week-old animals was treated with AF488-conjugated bovine serum albumin (BSA) before perfusion (Table 1, Experiment #2). The conjugated albumin is too large to pass through the wall of blood vessels, so its presence in the extra-vascular space is indicative of alterations in blood-brain barrier permeability. AF488-BSA is cleared from within vessels during perfusion, so only reagent that leaked out of the vessels is retained. Analyses focused on the dentate gyrus, where Pten KO cells are localized.
Gross examination of brain slices from DGC-Pten KO mice revealed no evidence of blood-brain barrier leakage relative to controls (Fig. 4A). In addition to gross analyses, mean AF488-BSA signal intensity was collected from four to seven hippocampi per DGC-Pten KO or control mouse. Although a trend toward slightly increased signal intensity was evident among DGC-Pten KO mice, the effect did not reach significance (Control, pixel intensity = 286 ± 93; Pten KO, 386 ± 95, Linear mixed effect model with a random animal effect, p = 0.1195; Fig. 4B).
Figure 4.
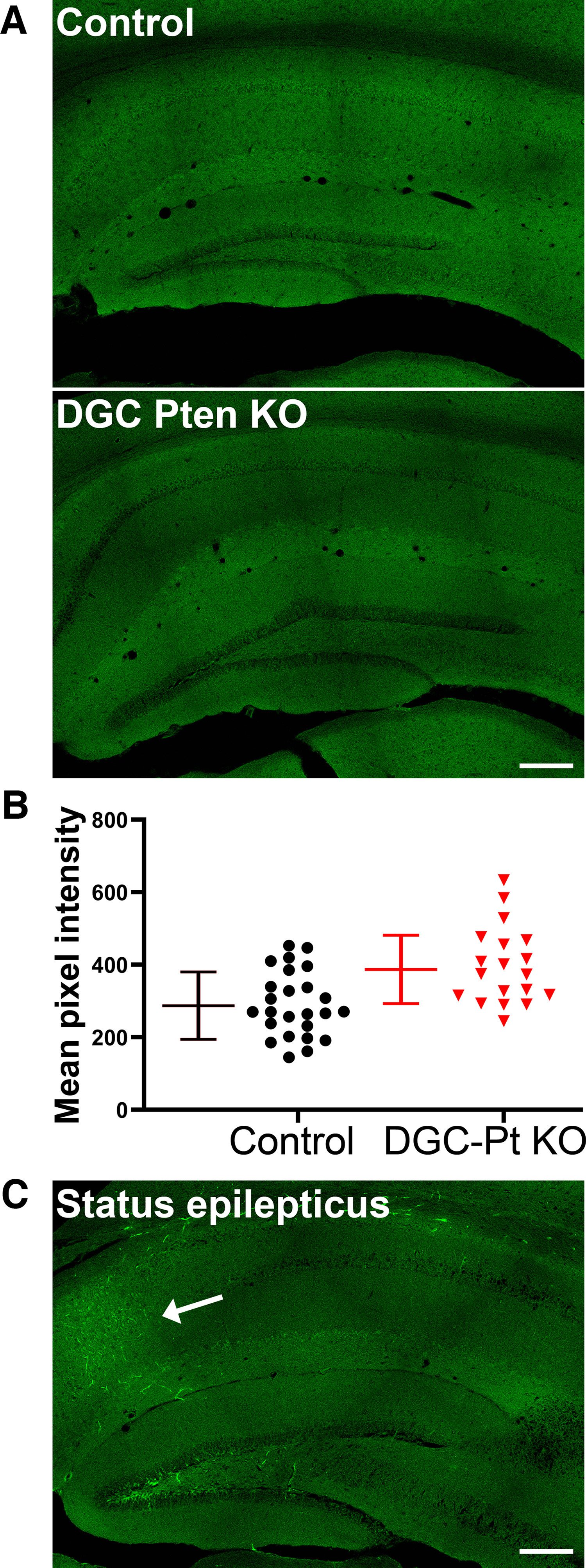
A, AF488-BSA fluorescence in the hippocampi of a control mouse and a DGC-Pten (Pt) KO mouse. No evidence of extravascular AF488-BSA was seen in either group. Scale bar = 200 μm. B, Symbols denote the mean pixel intensity of the AF488-BSA signal for each control and DGC-Pten KO dentate gyrus brain section. Bars show animal means and SD (N = 5 controls and 4 DGC-Pten KOs, 4–7 sections/mouse; p = 0.1195). C, AF488-BSA fluorescence in the hippocampus of a mouse that went through pilocarpine-induced status epilepticus. This positive control animal shows a clear increase in AF488-BSA labeling in the CA1 pyramidal cell layer (arrow). Scale bar = 200 μm.
To confirm the efficacy of AF488-conjugated BSA in detecting blood-brain barrier leakage, two DGC-Ptenfl/fl control mice were infused with the conjugate immediately after 3 h of pilocarpine-induced status epilepticus. Modest leakage was evident in the CA1 region and hilus of one of the mice (Fig. 4C), while the second exhibited patchy regions of intense AF488 BSA accumulation in cortex and thalamus. No leakage was evident in the dentate cell body layer in these animals, although it is noted that granule cells are relatively resistant to damage in this model. These findings demonstrate that AF488-conjugated BSA infusion can reveal blood-brain barrier leakage, and support the conclusion that increased vascular permeability is not a prominent or persistent feature of the DGC-Pten KO mouse model of epilepsy.
Vascular changes in forebrain-specific Pten KO mice
The absence of an effect on vascular density in DGC-Pten KO mice could reflect the relatively small population of affected neurons (22% of granule cells). We queried, therefore, whether Pten deletion from forebrain would impact vascular structure by examining 6.5- to 7-week-old CamK2α-Cre, Ptenfl/fl mice (FB-Pten KOs; Table 1, Experiment #3). CamK2α-Cre drives widespread Pten deletion from excitatory neurons in cortex and hippocampus, a much broader cellular distribution than the DGC-Pten KOs.
FB-Pten KO mice had enlarged overall brain weights relative to controls (control, 0.377 ± 0.030 g; FB-Pten KO, 0.447 ± 0.026 g; two-way ANOVA with genotype and sex as factors, p = 0.002). There was no significant effect of sex (p = 0.505) or interaction between factors (p = 0.094). When brain weight was expressed as a percentage of body weight, significant effects of genotype (p = 0.029) and sex (p = 0.016) were found (Control male, 1.7 ± 0.0%; FB-Pten KO male, 2.2 ± 0.1; Control female, 2.2 ± 0.2; FB-Pten KO female, 2.3 ± 0.2). There was no significant interaction between sex and genotype (p = 0.105). These findings demonstrate that Pten deletion from forebrain produces an overall gross disruption of brain size.
Quantification of vascular area was conducted for hippocampus and cortex, both regions with widespread Pten loss. Within the hippocampus, vessel area showed a significant 28.5% increase in FB-Pten KOs relative to controls (t test, p = 0.049; Fig. 5). Similarly, vessel area in cortex was increased by 33.1% in FB-Pten KOs relative to controls (t test, p = 0.045). As with DGC-Pten KOs, vessel area in FB-Pten KOs was normalized to the volume of tissue examined to determine whether there was a net increase in vessel density. Neither hippocampal (t test, p = 0.283; Fig. 5) nor cortical (Mann–Whitney rank-sum test, p = 0.106) volume was significantly increased, although results in cortex should be interpreted cautiously, as it was not possible to use the natural boundaries of the structure. When vessel area was normalized to the volume of each sample region (Fig. 5), no increase in vessel density was evident for either hippocampus (t test, p = 0.149) or cortex (t test, p = 0.176). In addition, values from two FB-Pten KOs with the unexpected germline loss of one Pten allele (Fig. 5, red squares) overlapped with values from animals with Pten loss restricted to forebrain (Fig. 5, red triangles), suggesting that global Pten heterozygosity does not amplify the phenotype. The findings indicate that even widespread loss of Pten from neurons is not sufficient to drive robust hypervascularization.
Figure 5.
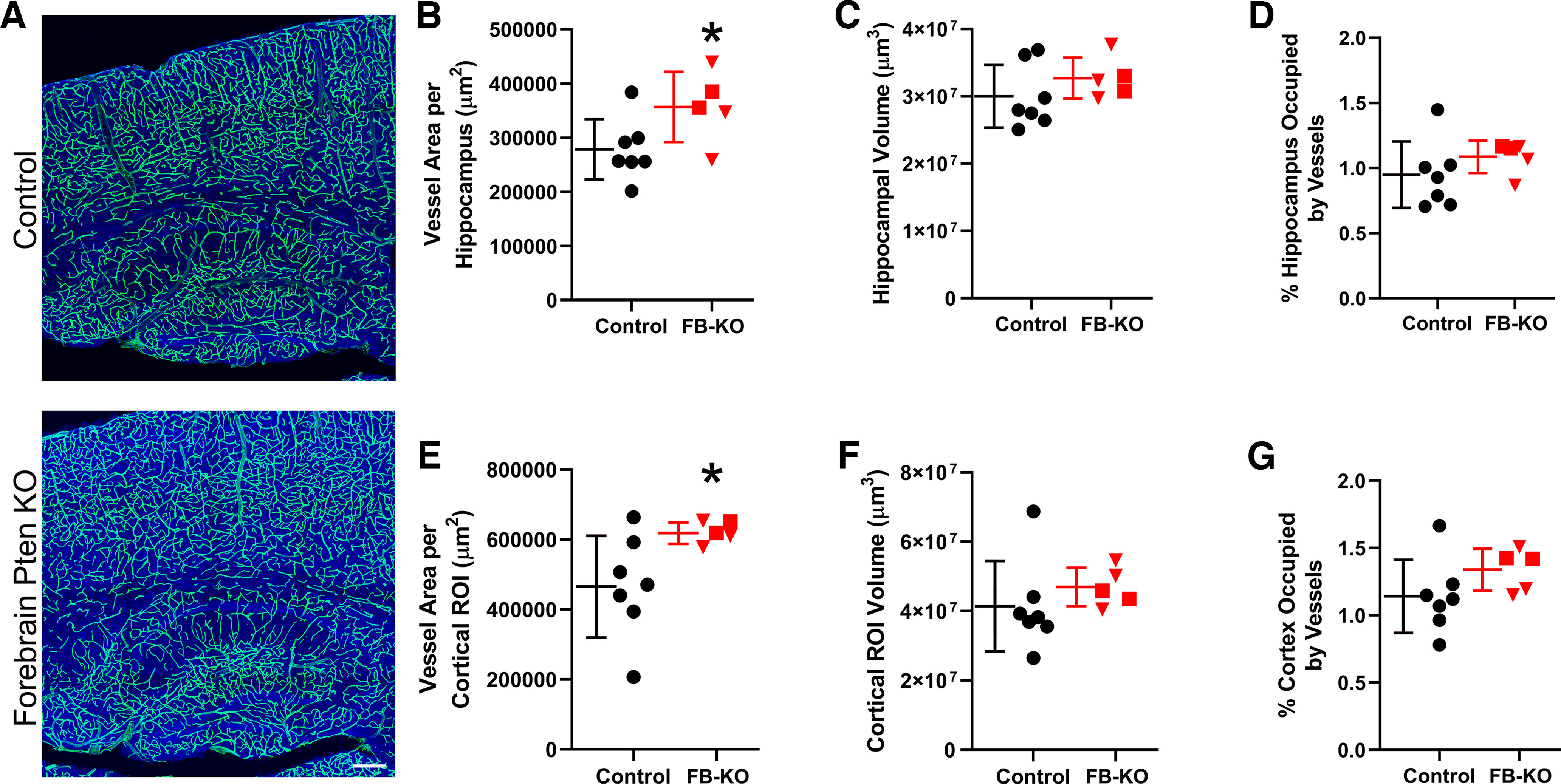
A, Confocal images of hippocampus and cortex showing blood vessels labeled with DyLight 649-lectin (green). The tissue is counterstained with nuclear blue. Scale bar = 200 μm. B, E, Graphs show scatterplots of individual animal scores and group means ± SD for vessel area in control (black) and Forebrain (FB)-Pten KO (red) animals for hippocampus (B) and cortex (E). Area was calculated from maximum projections of images from 20-μm-thick sections. C, F, Scatter plot and group means for the volume of the hippocampus (C) and cortex (F) in 20-μm-thick sections. D, G, Vessel area (B, E) was normalized to dentate volume (C, F) to estimate the percentage of the hippocampus (D) or cortex (G) occupied by vessels in 20-μm-thick slices. Red squares denote the two CamK2α-Cre+/−, Ptenfl/- mice with germline loss of one Pten allele. *p < 0.05.
Vascular endothelial growth factor (VegfA)
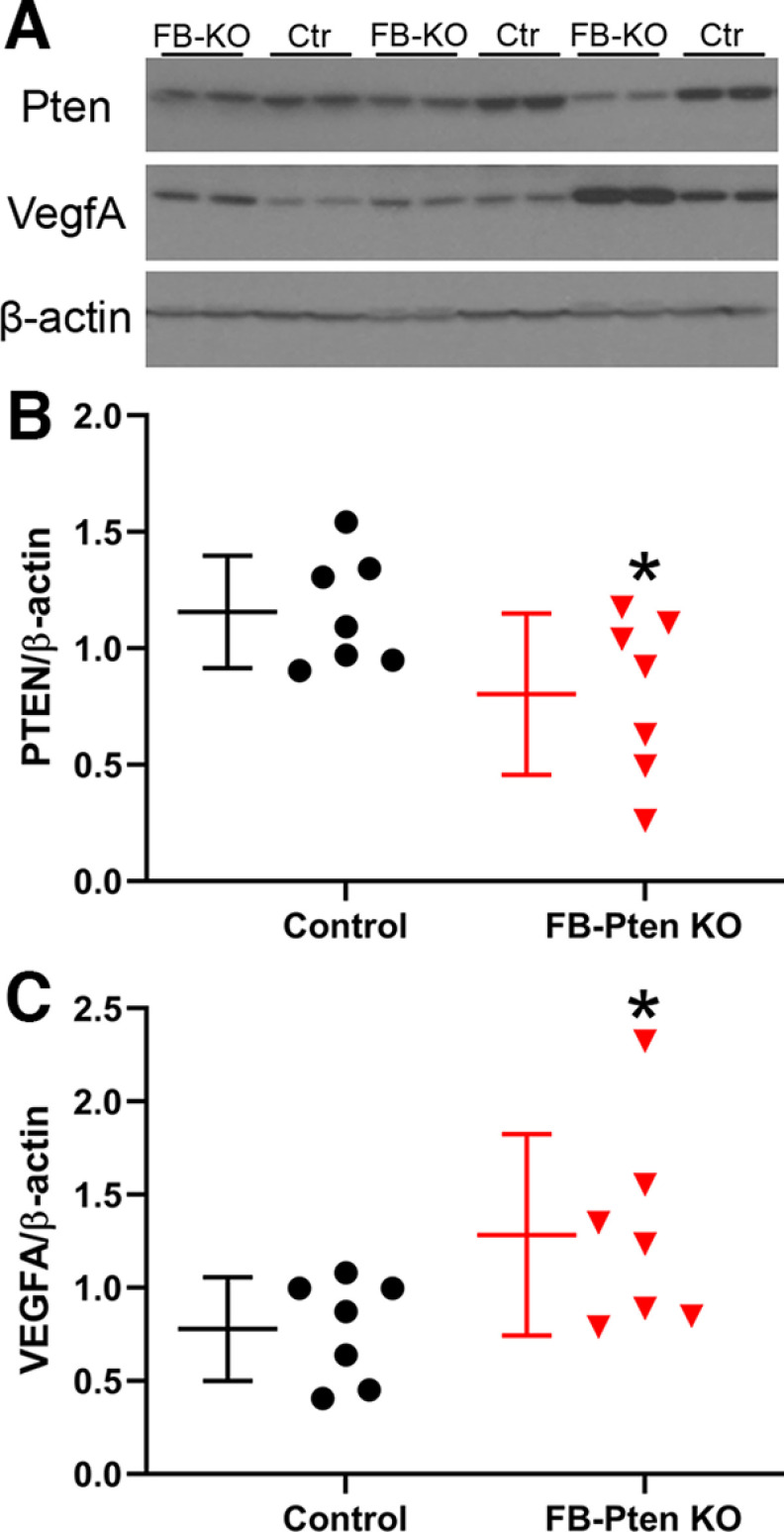
Vascular remodeling and angiogenesis can be driven by increased expression of angiogenic factors known to be downstream targets for mTOR, including VegfA. We queried, therefore, whether neuronal Pten loss drives increased VegfA expression in FB-Pten KO mice. Western blot analyses were conducted using a second cohort of control and FB-Pten KO mice (Table 1, Experiment #4). Western blot analyses confirmed a 31% decrease in Pten in the cortex of FB-Pten KO mice compared with littermate controls (t test, p = 0.047; Fig. 6). VegfA protein levels, on the other hand, were increased by 65% in FB-Pten KOs relative to controls (t test, p = 0.048; Fig. 6). Findings support the conclusion that neuronal Pten deletion can drive increased VegfA protein levels.
Figure 6.
VegfA is increased in mice with neuronal Pten loss from forebrain (FB-KO). A, Representative Western blottings of samples from FB-KO and littermate control (Ctr) cortex. B, Pten protein is reduced in the cortex of FB-KO mice. C, VegfA protein is increased in FB-KO mice. Each point represents averaged data from single mouse. *p < 0.05.
Focal Tsc2 loss from cortical excitatory neurons increases vascular density
To query whether focal loss of Tsc2 might produce a different effect than Pten loss, Tsc2fl/fl mice received bilateral cortical injections of AAV9-CaMKII-mCherry-T2A-Cre on postnatal day 2, leading to mCherry expression and cre-mediated focal loss of Tsc2 from cortical excitatory neurons in Tsc2fl/fl mice, and mCherry expression-only in Tsc2wt/wt control mice. Despite bilateral injections, mCherry expression was restricted to the left hemisphere in most animals, likely because of clogging of the syringe used to inject the AAV. In the left hemisphere, mCherry expression tended to be localized to upper layers of cortex, although variability among animals was noted (Fig. 7A,B). The volume occupied by mCherry-expressing neurons in the 12-μm-thick confocal image stacks examined averaged 1,594,609 μm3 in control animals, and 2,178,774 μm3 in f-Tsc2 KO animals (t test, p = 0.546, t test; Fig. 7C,D), confirming that viral injections were similar across groups. To determine whether Tsc2 KO lesions exhibited increased vascularity, within animal analyses comparing mCherry-expressing cortical regions in the left hemisphere to anatomically comparable mCherry-negative regions in the right hemisphere were conducted for both genotypes [f-Tsc2 KO and control (Tsc2wt/wt)], revealing a significant interaction between genotype and hemisphere [two-way repeated measures ANOVA controlling for animal, genotype and hemisphere (as the repeated measure); p = 0.008; Fig. 7E]. Post hoc tests showed significantly increased vascular density in the mCherry-expressing hemisphere relative to the mCherry-negative hemisphere within f-Tsc2 KOs (Bonferroni t test, p < 0.001) but not controls (p = 0.993). Comparisons across animals showed a similar pattern. Specifically, brain hemispheres lacking mCherry expression did not differ significantly between control and f-Tsc2 KO mice (p = 0.948), while a nonsignificant trend was evident when mCherry-expressing hemispheres from f-Tsc2 KOs were compared with mCherry-expressing hemispheres from control mice (p = 0.054).
Figure 7.
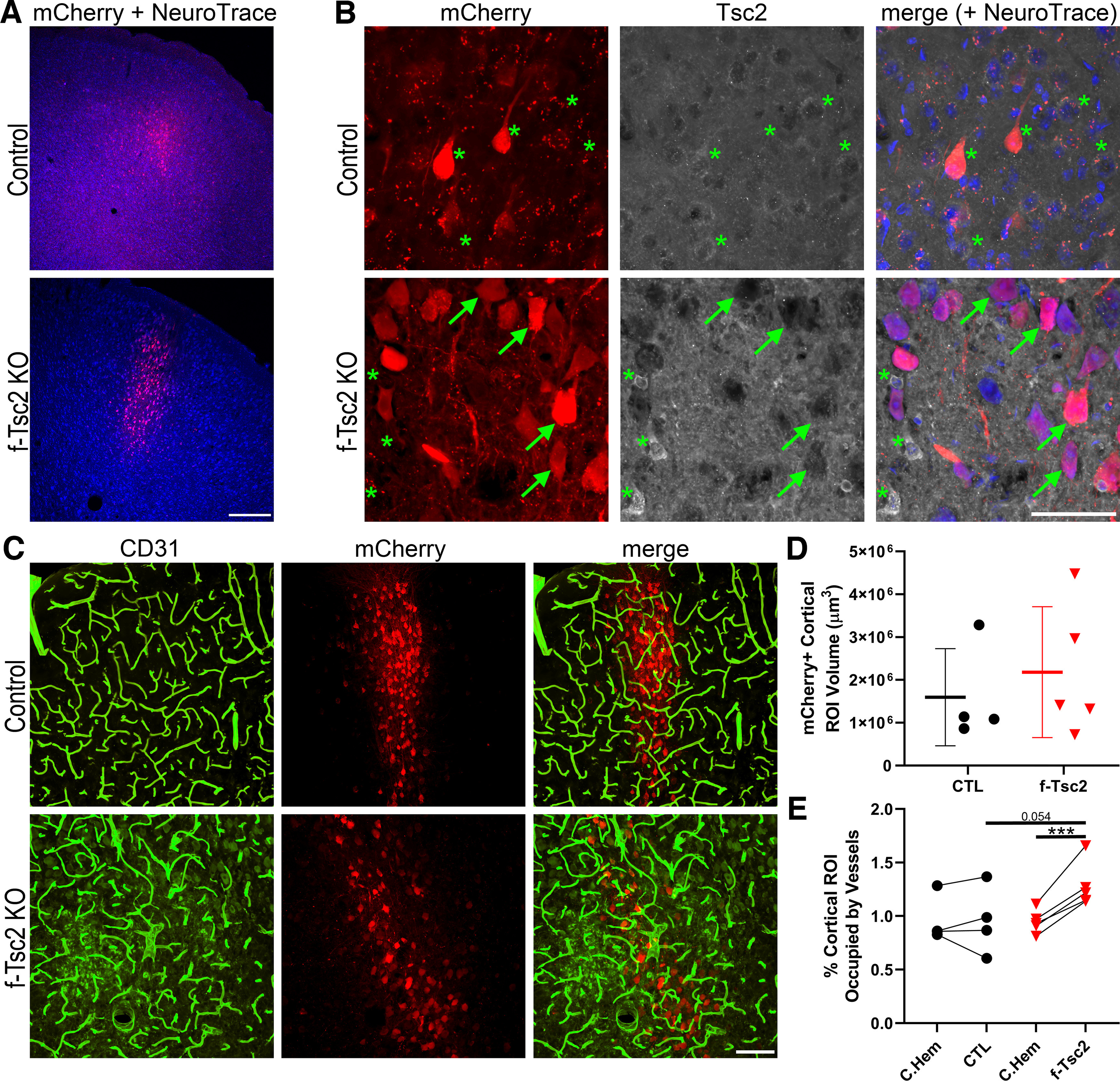
A, Confocal optical sections of cortex showing focal mCherry expression (red) with a NeuroTrace counterstain (blue) in control (Tsc2wt/wt) and f-Tsc2 KO (Tsc2fl/fl) mice injected with AAV9-Cre-mCherry. Scale bar = 300 μm. B, Confocal maximum projections (4-μm depth) of mCherry-expressing cells in control and f-Tsc2 KO cortical tissue immunostained for Tsc2. Cells in control animals, and mCherry negative cells in KOs (*) show cytoplasmic Tsc2 labeling, with exclusion of the protein from the nucleus. Staining is absent from mCherry-expressing cells in KOs (arrows). Scale bar = 50 μm. C, Blood vessels immunostained with CD31 (green) are shown around Tsc2 KO cells labeled with mCherry (red) in a f-Tsc2 KO mouse, while mCherry-labeled wild-type neurons are shown in the control mouse. Scale bar = 100 μm. D, Graph shows the area occupied by mCherry-expressing (Tsc2 KO, red) neurons in the cortex of f-Tsc2 KO mice relative to the area occupied by mCherry-expressing (wild-type, black) neurons in control (CTL) mice. E, Graph shows the percentage of cortical regions of interest (ROIs) occupied by blood vessels for control and f-Tsc2 KO mice [(vessel area/cortical ROI volume)*100]. Each paired set of points represents one animal, with the first point giving vessel density in the mCherry-negative right hemisphere (C. Hem), while the second point is the measure for the mCherry-expressing left hemisphere. ***p < 0.001.
Discussion
Mutations leading to excess mTOR pathway signaling have been associated with vascular abnormalities. Here, we examined two mouse models with either focal hippocampal or widespread forebrain loss of the mTOR negative regulator Pten, and a third model with focal cortical loss of the mTOR negative regulator Tsc2. In both Pten models, vessel area was increased. Pten deletion, however, also led to gross increases in brain volume, and when vessel areas were normalized to these larger volumes, overall vessel density was found to be preserved. In addition, although the angiogenic factor VegfA was increased in FB-Pten KOs, the absence of hypervascularization suggests this increase may simply reflect enhanced vessel growth to accommodate larger brain volumes. By contrast, focal loss of Tsc2 from excitatory cortical neurons significantly increased vessel density within the KO region. Together, these findings suggest that neuronal Tsc2 loss may exert a greater effect on tissue vascularity than Pten loss, however, further studies are needed to exclude potential model-specific differences. Regardless, studies demonstrate that hypervascularization is not a ubiquitous consequence of enhanced mTOR pathway signaling in brain.
Regulation of angiogenesis by different mTOR pathway genes
A plausible explanation for the different effects of Pten versus Tsc2 deletion is that distinct mTOR pathway genes differentially regulate angiogenesis. Tsc1, Tsc2 and Pten occupy different positions in the mTOR signaling cascade (Pten is upstream of Tsc1/2 and Rheb) and distinct proteins can have mTOR independent effects. Indeed, Tsc2 may be able to regulate VegfA through mTOR-independent mechanisms (Brugarolas et al., 2003). It would not be particularly surprising, therefore, for mutations in different mTOR pathway genes to produce distinct effects on angiogenesis. Pten, however, has been shown to directly regulate angiogenesis in glioma models (Wen et al., 2001) and patients with Pten mutations often exhibit vascular abnormalities (Dhamija and Hoxworth, 2020). To further query whether Pten deletion could drive angiogenesis, we examined VegfA protein levels in FB-Pten KO mice and found that Pten deletion did result in increased amounts of VegfA protein. The finding confirms that Pten deletion does increase this major angiogenic factor, but perhaps only to drive sufficient angiogenesis to accommodate the larger brain volumes. By contrast, the present findings with Tsc2 now add to an expanding body of research linking the Tsc1/Tsc2 complex to disrupted brain vascularity. Specifically, Tsc1 deletion from cortical excitatory neurons and astrocytes using Tsc1fl/fl, Emx1-Cre mice and germline loss of Tsc2 from Eker rats produces mTOR hyperactivation and vascular abnormalities (Zhang et al., 2019; Kútna et al., 2020). In utero electroporation of a constitutively active Rheb, the direct target of the Tsc1/Tsc2 complex, also causes hypervascularity (Zhang et al., 2019).
In addition to the genes themselves, the cell types targeted for gene deletion could also be a critical variable. Zhang and colleagues were able to target a variety of cortical neurons and some astrocytes with their approaches (Zhang et al., 2019), whereas the Gli1-CreERT2 driver used in DGC-Pten KO model targets hippocampal granule cells through the rostral caudal extent of both hippocampi, subventricular zone progenitors that populate olfactory bulb and small numbers of non-neuronal cells throughout the brain (Pun et al., 2012). FB-Pten KOs produce widespread Pten loss among excitatory neurons in cortex and hippocampus with minimal involvement of other cell types. Pten loss in astrocytes has been shown to drive angiogenesis (Xiao et al., 2005), so relative inclusion of this cell type could be a key variable. Notably, however, cortical excitatory neurons were also targeted in f-Tsc2 KOs, but with a more restricted distribution relative to the widespread targeting in the FB-Pten Kos, so at least in the f-Tsc2 KO model, mTOR hyperactivation in cortical neurons appears to be sufficient to drive hypervascularization.
The developmental timing and duration of gene deletion could also modulate angiogenic responses to mTOR hyperactivation. Pten deletion in DGC-Pten KOs occurs among neural progenitor cells following tamoxifen administration to two-week old mice, while Pten deletion in FB-Pten KOs is delayed until a few weeks after birth, when expression of the CamkII promoter is robust (Tsien et al., 1996). By contrast, Emx1-Cre-driven and in utero electroporation approaches with Tsc1 and Rheb both target immature cortical neurons, so gene deletion will occur earlier in development in these models (Zhang et al., 2019). The f-Tsc2 KO model, however, also uses a later-acting CamkII promoter to drive cre-recombination, denying an obvious developmental explanation for the difference with the FB-Pten Kos, although the many other difference between models could be important (surgery, viral exposure, etc.). Possible technical differences between our Pten and Tsc2 models are the use of DyLight 649-lectin to label the vessels in the Pten models and the within-animal analysis approach used in the Tsc2 model. The lectin provides superior signal-to-noise than CD31 immunostaining, but has to be given in vivo and could not be used on existing fixed tissue from f-Tsc2 KOs. On the other hand, the within-animal comparison approach used for the f-Tsc2 KOs cannot be conducted in the Pten KOs, where both hemispheres were affected. Within-animal approaches can be statistically more powerful by controlling for interanimal variability, and thus might detect more subtle changes. Finally, we suggest that the duration of mTOR hyperactivation would not appear to be a driving factor for differing results, as prior work with Tsc1 and Rheb found hypervascularization by P14 (Zhang et al., 2019), so animals in the present study harbored Pten KO cells for longer (albeit at later ages). Nonetheless, additional studies controlling for cell type, deletion extent and developmental timing with comparable techniques will be needed to establish whether the absence of hypervascularization in Pten KOs, and its presence in Tsc1/Tsc2 KOs, reflects distinct gene function effects or other factors.
Blood-brain barrier leakage
Failure of the blood-brain barrier is hypothesized to contribute to some forms of epilepsy (Dadas and Janigro, 2019). Introduction of serum proteins into the brain can directly evoke seizures and seizures can open the blood-brain barrier, creating the potential for an epileptogenic negative feedback loop (Gorter et al., 2019). Moreover, the mTOR antagonist rapamycin regulates vascular remodeling and blood-brain barrier leakage after status epilepticus (van Vliet et al., 2012, 2016a, b), indicating that a physiological function of the mTOR pathway (i.e., in the absence of any mutations) is to regulate the blood-brain barrier. This adds an additional level of complexity to understanding mTOR mutants, as mutations might drive initial pathology, such as seizures, and alter vascular remodeling responses to those seizures.
Systemic infusion with brain impermeant fluorescent tracers did not reveal significant evidence of leakage in DGC-Pten KO mice. Data with AF488-BSA is shown (Fig. 4), but pilot experiments with Evans Blue, various size dextran molecules and tomato lectin also showed no evidence of leakage (data not shown). By contrast, clear leakage was evident following pilocarpine-induced status epilepticus, an established cause of barrier failure (Ndode-Ekane et al., 2010; Bankstahl et al., 2018; Mendes et al., 2019). The findings suggest that ongoing and persistent blood-brain barrier failure is not a major contributor to seizure incidence in DGC-Pten KOs. We cannot exclude the possibility, however, that the leakage occurs only transiently. For example, immediately after seizure events or during specific developmental time windows. Notably, enhanced vessel growth, to accommodate the larger dentate gyri, was observed, and as new vessels have immature tight junctions and are thus more prone to leakage (Rust, 2020), it is possible that KO animals might exhibit leakage at earlier time points when vessel growth was maximal.
Implications for mTORopathies
The present findings suggest that hypervascularization is not a universal consequence of mTOR pathway mutations, even when associated with gross hypertrophy of brain structures. Additional studies of clinical samples are needed to determine whether, and under what conditions, hypervascularization is prominent. Hypervascularization is a feature of tuberous sclerosis complex lesions (Laviv et al., 2018), and vascular abnormalities are evident in a variety of other disorders caused by mTOR pathway mutations (Hashizume et al., 2004; Martinez-Lopez et al., 2019). In addition to the genetic diversity of mTOR activating mutations, human conditions also exhibit extensive cellular diversity. Human disease-causing mutations can be germline, mosaic or a combination of both in the case of two-hit lesions in tuberous sclerosis complex (Martin et al., 2017; Reyna-Fabián et al., 2020). Affected cell types, therefore, could be a key determinant in whether vascular abnormalities develop. As shown in the present study, neuronal Pten loss can regulate VegfA protein levels, which could induce vascular changes and sustain neuronal hypertrophy. Studies in the cancer literature, however, also show that Pten can act on endothelial cells and astrocytes to drive angiogenesis (Gomez-Manzano et al., 2003; Su et al., 2003; Bhattacharya et al., 2013). As vascular pathology could be a key component of neurologic deficits associated with mTORopathies, understanding the variables driving vascular pathogenesis remains an important area of research.
Acknowledgments
Acknowledgments: We thank Maria Ashton for editorial assistance and Dr. Lili Ding for help with statistical analyses. We also thank the Cincinnati Children’s Hospital Medical Center (CCHMC) Confocal Imaging Core for assistance with confocal imaging and analysis and CCHMCs Veterinary Services for assistance with animal colonies.
Synthesis
Reviewing Editor: William Stacey, University of Michigan
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below. The following reviewer(s) agreed to reveal their identity: Matthew Weston, Bryan Luikart.
We agreed that these experiments demonstrated that, in these models, although the vasculature grows to support the increased dentate size that is evident at 10 wks of age, density is preserved and there is no BBB leakage evidence. Earlier timepoints show no change. Corroborating results are observed in the CaMKIIa model, although only one time point is tested due to constraints of the model. The findings are somewhat unexpected given the prior work on TSC and RHEB models.
In general there were only minor concerns. However, one question is whether the impact of this paper could be improved with some additional data. We at eNeuro are averse to asking authors for additional experiments, so if the data are not readily available this is not required. However, the following comment explains one area in which we think this paper could be greatly improved, if perhaps this analysis could be run from the prior experiments:
1. The results leave several open questions, which are addressed by the authors in the discussion but not experimentally. These include, whether other mTOR gene deletions (e.g. Tsc1) have fundamentally different effects, or whether other cell types (e.g. glia, vascular endothelial cells), or time points would cause more dramatic vascular reorganization. Performing these experiments is probably too much to ask for this paper, as they would take several months to a year to complete. The paper does not contain a lot of data, however, so adding some experiments would improve the overall impact of the paper. Perhaps there are additional protein targets involved in vascular remodeling that could be assayed by either Western or immunostaining.
Minor points:
2. “512 μm/pixel” resolution in the section describing the AF488-BSA experiments is likely a typo/mistake.
3. It is good that you caught the germline recombination events in the CaMKIIa line, but what is the rationale for keeping these animals in the study, if that is what is meant by “retained in the study.”
4. It would be good to see the pixel values for AF488-BSA fluorescence after Status next to the other two groups in Figure 4.
5. Please add some references that use BSA to assess BBB permeability.
6. Figure legend, Figure 4. (hippocampi/mouse) should be (sections/mouse)
7. In the introduction “Finally, the mTOR pathway regulates genes ....” Can the authors state how these genes have been shown to be regulated. IE is it an effect on translation or transcription? In what cell type has it been shown?
8. In the results, “A correlation analysis of Pten and Vegf protein levels....” Our feeling is that you simply do not have the “n” to drive such a correlation analysis, so would suggest removing this so as not to draw incorrect interpretation of the negative data.
9. In the discussion “The present findings are consistent with the interpretation that different mTOR-pathway genes have distinct effects on angiogenesis” We don’t think that this is the only plausible interpretation. Differences in the cell types that are genetically manipulated via IUE vs the transgenic strategy in the present study is an equally plausible explanation. Later in the paper, cell type specificity and timing are discussed. I would suggest changing the above verbiage indicating something like “a plausible mechanism for differences between studies in that different mTOR-pathway genes...”
10. There is not a citation or methods on how the PTEN IHC was performed in figure 1.
11. In figure 4 can the data for the wild-type, Pten KO, and the pilocarpine-induced SE positive control all be quantitated and presented in 4B.
References
- Ahishali B, Kaya M (2021) Evaluation of blood-brain barrier integrity using vascular permeability markers: Evans blue, sodium fluorescein, albumin-Alexa Fluor conjugates, and horseradish peroxidase. Methods Mol Biol 2367:87–103. 10.1007/7651_2020_316 [DOI] [PubMed] [Google Scholar]
- Arafa SR, Lasarge CL, Pun RYK, Khademi S, Danzer SC (2019) Self-reinforcing effects of mTOR hyperactive neurons on dendritic growth. Exp Neurol 311:125–134. 10.1016/j.expneurol.2018.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankstahl M, Breuer H, Leiter I, Märkel M, Bascuñana P, Michalski D, Bengel FM, Löscher W, Meier M, Bankstahl JP, Härtig W (2018) Blood-brain barrier leakage during early epileptogenesis is associated with rapid remodeling of the neurovascular unit. eNeuro 5:ENEURO.0123-18.2018. 10.1523/ENEURO.0123-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrows CM, Mccabe MP, Chen H, Swann JW, Weston MC (2017) PTEN loss increases the connectivity of fast synaptic motifs and functional connectivity in a developing hippocampal network. J Neurosci 37:8595–8611. 10.1523/JNEUROSCI.0878-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Singh MK, Chaudhuri S, Acharya S, Basu AK, Chaudhuri S (2013) T11TS impedes glioma angiogenesis by inhibiting VEGF signaling and pro-survival PI3K/Akt/eNOS pathway with concomitant upregulation of PTEN in brain endothelial cells. J Neurooncol 113:13–25. 10.1007/s11060-013-1095-5 [DOI] [PubMed] [Google Scholar]
- Broekaart DWM, Van Scheppingen J, Anink JJ, Wierts L, Van Het Hof B, Jansen FE, Spliet WG, Van Rijen PC, Kamphuis WW, De Vries HE, Aronica E, van Vliet EA (2020) Increased matrix metalloproteinases expression in tuberous sclerosis complex: modulation by microRNA 146a and 147b in vitro. Neuropathol Appl Neurobiol 46:142–159. 10.1111/nan.12572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG Jr (2003) TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4:147–158. 10.1016/s1535-6108(03)00187-9 [DOI] [PubMed] [Google Scholar]
- Chen CJ, Sgritta M, Mays J, Zhou H, Lucero R, Park J, Wang IC, Park JH, Kaipparettu BA, Stoica L, Jafar-Nejad P, Rigo F, Chin J, Noebels JL, Costa-Mattioli M (2019) Therapeutic inhibition of mTORC2 rescues the behavioral and neurophysiological abnormalities associated with Pten-deficiency. Nat Med 25:1684–1690. 10.1038/s41591-019-0608-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihi M, Gembruch O, Darkwah Oppong M, Chen B, Dinger TF, Barthel L, Pierscianek D, Wrede KH, Özkan N, Sure U, Jabbarli R (2019) Intracranial aneurysms in patients with tuberous sclerosis complex: a systematic review. J Neurosurg Pediatr 24:174–183. 10.3171/2019.2.PEDS18661 [DOI] [PubMed] [Google Scholar]
- Crino PB (2020) mTORopathies: a road well-traveled. Epilepsy Curr 20:64s–66s. 10.1177/1535759720959320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadas A, Janigro D (2019) Breakdown of blood brain barrier as a mechanism of post-traumatic epilepsy. Neurobiol Dis 123:20–26. 10.1016/j.nbd.2018.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamija R, Hoxworth JM (2020) Imaging of PTEN-related abnormalities in the central nervous system. Clin Imaging 60:180–185. 10.1016/j.clinimag.2019.12.006 [DOI] [PubMed] [Google Scholar]
- Di Pardo A, Castaldo S, Capocci L, Amico E, Vittorio M (2017) Assessment of blood-brain barrier permeability by intravenous infusion of FITC-labeled albumin in a mouse model of neurodegenerative disease. J Vis Exp (129):56389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano DM, Zhang S, Quon JL, Bordey A (2013) Hypoxia-inducible factor 1a is a Tsc1-regulated survival factor in newborn neurons in tuberous sclerosis complex. Hum Mol Genet 22:1725–1734. 10.1093/hmg/ddt018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio F, Frasca A, Weissberg I, Parrella S, Friedman A, Vezzani A, Noé FM (2012) Long-lasting pro-ictogenic effects induced in vivo by rat brain exposure to serum albumin in the absence of concomitant pathology. Epilepsia 53:1887–1897. 10.1111/j.1528-1167.2012.03666.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Manzano C, Fueyo J, Jiang H, Glass TL, Lee HY, Hu M, Liu JL, Jasti SL, Liu TJ, Conrad CA, Yung WK (2003) Mechanisms underlying PTEN regulation of vascular endothelial growth factor and angiogenesis. Ann Neurol 53:109–117. 10.1002/ana.10396 [DOI] [PubMed] [Google Scholar]
- Gorter JA, Aronica E, van Vliet EA (2019) The roof is leaking and a storm is raging: repairing the blood-brain barrier in the fight against epilepsy. Epilepsy Curr 19:177–181. 10.1177/1535759719844750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume K, Tsuda H, Hodozuka A, Tanaka T (2004) Clinical and experimental studies of epilepsy associated with focal cortical dysplasia. Psychiatry Clin Neurosci 58:S26–S29. 10.1111/j.1440-1819.2004.01244_7.x [DOI] [PubMed] [Google Scholar]
- Hester MS, Hosford BE, Santos VR, Singh SP, Rolle IJ, Lasarge CL, Liska JP, Garcia-Cairasco N, Danzer SC (2016) Impact of rapamycin on status epilepticus induced hippocampal pathology and weight gain. Exp Neurol 280:1–12. 10.1016/j.expneurol.2016.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karar J, Maity A (2011) PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci 4:51. 10.3389/fnmol.2011.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne Wj, Cuthill Ic, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412. 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Grunke SD, Levites Y, Golde TE, Jankowsky JL (2014) Intracerebroventricular viral injection of the neonatal mouse brain for persistent and widespread neuronal transduction. J Vis Exp (91):51863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kútna V, Uttl L, Waltereit R, Krištofiková Z, Kaping D, Petrásek T, Hoschl C, Ovsepian SV (2020) Tuberous sclerosis (tsc2+/-) model Eker rats reveals extensive neuronal loss with microglial invasion and vascular remodeling related to brain neoplasia. Neurotherapeutics 17:329–339. 10.1007/s13311-019-00812-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Baker SJ (2003) mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci U S A 100:12923–12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land SC, Tee AR (2007) Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem 282:20534–20543. 10.1074/jbc.M611782200 [DOI] [PubMed] [Google Scholar]
- Lasarge CL, Santos VR, Danzer SC (2015) PTEN deletion from adult-generated dentate granule cells disrupts granule cell mossy fiber axon structure. Neurobiol Dis 75:142–150. 10.1016/j.nbd.2014.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasarge CL, Pun RY, Muntifering MB, Danzer SC (2016) Disrupted hippocampal network physiology following PTEN deletion from newborn dentate granule cells. Neurobiol Dis 96:105–114. 10.1016/j.nbd.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasarge CL, Pun RYK, Gu Z, Santos VR, Danzer SC (2019) Impact of mTOR hyperactive neurons on the morphology and physiology of adjacent neurons: do PTEN KO cells make bad neighbors? Exp Neurol 321:113029. 10.1016/j.expneurol.2019.113029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasarge CL, Pun RYK, Gu Z, Riccetti MR, Namboodiri DV, Tiwari D, Gross C, Danzer SC (2021) mTOR-driven neural circuit changes initiate an epileptogenic cascade. Prog Neurobiol 200:101974. 10.1016/j.pneurobio.2020.101974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviv Y, Kasper BS, Kasper EM (2018) Vascular hyperpermeability as a hallmark of phacomatoses: is the etiology angiogenesis comparable with mechanisms seen in inflammatory pathways? Part I: historical observations and clinical perspectives on the etiology of increased CSF protein levels, CSF clotting, and communicating hydrocephalus: a comprehensive review. Neurosurg Rev 41:957–968. 10.1007/s10143-017-0839-7 [DOI] [PubMed] [Google Scholar]
- Marcon J, Gagliardi B, Balosso S, Maroso M, Noé F, Morin M, Lerner-Natoli M, Vezzani A, Ravizza T (2009) Age-dependent vascular changes induced by status epilepticus in rat forebrain: implications for epileptogenesis. Neurobiol Dis 34:121–132. 10.1016/j.nbd.2008.12.018 [DOI] [PubMed] [Google Scholar]
- Marsan E, Baulac S (2018) Review: mechanistic target of rapamycin (mTOR) pathway, focal cortical dysplasia and epilepsy. Neuropathol Appl Neurobiol 44:6–17. 10.1111/nan.12463 [DOI] [PubMed] [Google Scholar]
- Martin KR, Zhou W, Bowman MJ, Shih J, Au KS, Dittenhafer-Reed KE, Sisson KA, Koeman J, Weisenberger DJ, Cottingham SL, Deroos ST, Devinsky O, Winn ME, Cherniack AD, Shen H, Northrup H, Krueger DA, Mackeigan JP (2017) The genomic landscape of tuberous sclerosis complex. Nat Commun 8:15816. 10.1038/ncomms15816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lopez A, Salvador-Rodriguez L, Montero-Vilchez T, Molina-Leyva A, Tercedor-Sanchez J, Arias-Santiago S (2019) Vascular malformations syndromes: an update. Curr Opin Pediatr 31:747–753. 10.1097/MOP.0000000000000812 [DOI] [PubMed] [Google Scholar]
- McMahon J, Huang X, Yang J, Komatsu M, Yue Z, Qian J, Zhu X, Huang Y (2012) Impaired autophagy in neurons after disinhibition of mammalian target of rapamycin and its contribution to epileptogenesis. J Neurosci 32:15704–15714. 10.1523/JNEUROSCI.2392-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes NF, Pansani AP, Carmanhães ERF, Tange P, Meireles JV, Ochikubo M, Chagas JR, Da Silva AV, Monteiro De Castro G, Le Sueur-Maluf L (2019) The blood-brain barrier breakdown during acute phase of the pilocarpine model of epilepsy is dynamic and time-dependent. Front Neurol 10:382. 10.3389/fneur.2019.00382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaa GM, et al. (2016) Association of MTOR mutations with developmental brain disorders, including megalencephaly, focal cortical dysplasia, and pigmentary mosaicism. JAMA Neurol 73:836–845. 10.1001/jamaneurol.2016.0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlebner A, et al. (2016) Novel histopathological patterns in cortical tubers of epilepsy surgery patients with tuberous sclerosis complex. PLoS One 11:e0157396. 10.1371/journal.pone.0157396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaiz DA, Sullens DG, Santana-Coelho D, Lugo JN (2022) Neuronal subset-specific phosphatase and tensin homolog knockout mice exhibit age and brain region-associated alterations in microglia/macrophage activation. Neuroreport 33:476–480. 10.1097/WNR.0000000000001808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndode-Ekane XE, Hayward N, Gröhn O, Pitkänen A (2010) Vascular changes in epilepsy: functional consequences and association with network plasticity in pilocarpine-induced experimental epilepsy. Neuroscience 166:312–332. 10.1016/j.neuroscience.2009.12.002 [DOI] [PubMed] [Google Scholar]
- Parker WE, Orlova KA, Heuer GG, Baybis M, Aronica E, Frost M, Wong M, Crino PB (2011) Enhanced epidermal growth factor, hepatocyte growth factor, and vascular endothelial growth factor expression in tuberous sclerosis complex. Am J Pathol 178:296–305. 10.1016/j.ajpath.2010.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun RY, Rolle IJ, Lasarge CL, Hosford BE, Rosen JM, Uhl JD, Schmeltzer SN, Faulkner C, Bronson SL, Murphy BL, Richards DA, Holland KD, Danzer SC (2012) Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron 75:1022–1034. 10.1016/j.neuron.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna-Fabián ME, Hernández-Martínez NL, Alcántara-Ortigoza MA, Ayala-Sumuano JT, Enríquez-Flores S, Velázquez-Aragón JA, Varela-Echavarría A, Todd-Quiñones CG, González-Del Angel A (2020) First comprehensive TSC1/TSC2 mutational analysis in Mexican patients with tuberous sclerosis complex reveals numerous novel pathogenic variants. Sci Rep 10:6589. 10.1038/s41598-020-62759-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust R (2020) Insights into the dual role of angiogenesis following stroke. J Cereb Blood Flow Metab 40:1167–1171. 10.1177/0271678X20906815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos VR, Pun RYK, Arafa SR, Lasarge CL, Rowley S, Khademi S, Bouley T, Holland KD, Garcia-Cairasco N, Danzer SC (2017) PTEN deletion increases hippocampal granule cell excitability in male and female mice. Neurobiol Dis 108:339–351. 10.1016/j.nbd.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton PD, Stan RV, Luikart BW (2020) The role of PTEN in neurodevelopment. Mol Neuropsychiatry 5:60–71. 10.1159/000504782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperow M, Berry RB, Bayazitov IT, Zhu G, Baker SJ, Zakharenko SS (2012) Phosphatase and tensin homologue (PTEN) regulates synaptic plasticity independently of its effect on neuronal morphology and migration. J Physiol 590:777–792. 10.1113/jphysiol.2011.220236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JD, Mayo LD, Donner DB, Durden DL (2003) PTEN and phosphatidylinositol 3'-kinase inhibitors up-regulate p53 and block tumor-induced angiogenesis: evidence for an effect on the tumor and endothelial compartment. Cancer Res 63:3585–3592. [PubMed] [Google Scholar]
- Sun K, Cui J, Xue R, Jiang T, Wang B, Zhang Z, Zhuo Y, Zhou Xj, Liang S, Yu X, Chen L (2021) New imaging features of tuberous sclerosis complex: a 7 T MRI study. NMR Biomed 34:e4565. [DOI] [PubMed] [Google Scholar]
- Switon K, Kotulska K, Janusz-Kaminska A, Zmorzynska J, Jaworski J (2017) Molecular neurobiology of mTOR. Neuroscience 341:112–153. 10.1016/j.neuroscience.2016.11.017 [DOI] [PubMed] [Google Scholar]
- Tian T, Nan KJ, Wang SH, Liang X, Lu CX, Guo H, Wang WJ, Ruan ZP (2010) PTEN regulates angiogenesis and VEGF expression through phosphatase-dependent and -independent mechanisms in HepG2 cells. Carcinogenesis 31:1211–1219. 10.1093/carcin/bgq085 [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S (1996) Subregion- and cell type-restricted gene knockout in mouse brain. Cell 87:1317–1326. 10.1016/s0092-8674(00)81826-7 [DOI] [PubMed] [Google Scholar]
- Van Lanen RH, Melchers S, Hoogland G, Schijns OE, Zandvoort MAV, Haeren RH, Rijkers K (2021) Microvascular changes associated with epilepsy: a narrative review. J Cereb Blood Flow Metab 41:2492–2509. 10.1177/0271678X211010388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet EA, Forte G, Holtman L, Den Burger JC, Sinjewel A, De Vries HE, Aronica E, Gorter JA (2012) Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood-brain barrier leakage but not microglia activation. Epilepsia 53:1254–1263. 10.1111/j.1528-1167.2012.03513.x [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Otte WM, Wadman WJ, Aronica E, Kooij G, De Vries HE, Dijkhuizen RM, Gorter JA (2016a) Blood-brain barrier leakage after status epilepticus in rapamycin-treated rats I: magnetic resonance imaging. Epilepsia 57:59–69. 10.1111/epi.13246 [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Otte WM, Wadman WJ, Aronica E, Kooij G, De Vries HE, Dijkhuizen RM, Gorter JA (2016b) Blood-brain barrier leakage after status epilepticus in rapamycin-treated rats II: potential mechanisms. Epilepsia 57:70–78. 10.1111/epi.13245 [DOI] [PubMed] [Google Scholar]
- Wen S, Stolarov J, Myers MP, Su JD, Wigler MH, Tonks NK, Durden DL (2001) PTEN controls tumor-induced angiogenesis. Proc Natl Acad Sci U S A 98:4622–4627. 10.1073/pnas.081063798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AR, Tiwari D, Macleod MC, Danzer SC, Gross C (2020) PI3K isoform-selective inhibition in neuron-specific PTEN-deficient mice rescues molecular defects and reduces epilepsy-associated phenotypes. Neurobiol Dis 144:105026. 10.1016/j.nbd.2020.105026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermark P, Lechpammer M, Warfield SK, Kosaras B, Takeoka M, Poduri A, Madsen JR, Bergin AM, Whalen S, Jensen FE (2013) Perfusion imaging of focal cortical dysplasia using arterial spin labeling: correlation with histopathological vascular density. J Child Neurol 28:1474–1482. 10.1177/0883073813488666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin AC, Kraus KL, Gaitonde KD, Suru V, Arafa SR, Packard BA, Herman JP, Danzer SC (2021) The glucocorticoid receptor specific modulator CORT108297 reduces brain pathology following status epilepticus. Exp Neurol 341:113703. 10.1016/j.expneurol.2021.113703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao A, Yin C, Yang C, Di Cristofano A, Pandolfi PP, Van Dyke T (2005) Somatic induction of Pten loss in a preclinical astrocytoma model reveals major roles in disease progression and avenues for target discovery and validation. Cancer Res 65:5172–5180. 10.1158/0008-5472.CAN-04-3902 [DOI] [PubMed] [Google Scholar]
- Xue L, Huang J, Zhang T, Wang X, Fu J, Geng Z, Zhao Y, Chen H (2018) PTEN inhibition enhances angiogenesis in an in vitro model of ischemic injury by promoting Akt phosphorylation and subsequent hypoxia inducible factor-1α upregulation. Metab Brain Dis 33:1679–1688. 10.1007/s11011-018-0276-5 [DOI] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M (2009) The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci 29:6964–6972. 10.1523/JNEUROSCI.0066-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Huang T, Teaw S, Bordey A (2019) Hypervascularization in mTOR-dependent focal and global cortical malformations displays differential rapamycin sensitivity. Epilepsia 60:1255–1265. 10.1111/epi.15969 [DOI] [PMC free article] [PubMed] [Google Scholar]