Abstract
We previously described a bacteriophage of the Lyme disease agent Borrelia burgdorferi designated φBB-1. This phage packages the host complement of the 32-kb circular plasmids (cp32s), a group of homologous molecules found throughout the genus Borrelia. To demonstrate the ability of φBB-1 to package and transduce DNA, a kanamycin resistance cassette was inserted into a cloned fragment of phage DNA, and the resulting construct was transformed into B. burgdorferi CA-11.2A cells. The kan cassette recombined into a resident cp32 and was stably maintained. The cp32 containing the kan cassette was packaged by φBB-1 released from this B. burgdorferi strain. φBB-1 has been used to transduce this antibiotic resistance marker into naive CA-11.2A cells, as well as two other strains of B. burgdorferi. This is the first direct evidence of a mechanism for lateral gene transfer in B. burgdorferi.
Borrelia burgdorferi, the causative agent of Lyme disease (5, 32), has a complex genome that contains both linear and circular DNA (7, 13). A growing number of tools have been developed for studying the molecular biology of this organism, although elaborating a convenient manipulatable genetic system will require further effort. Despite indirect evidence for lateral gene transfer in these bacteria (19, 33, 38), natural mechanisms for the transfer of genetic material (including conjugative plasmids and transducing bacteriophages) remain elusive for B. burgdorferi and related spirochetes.
In a number of other bacteria, bacteriophages are well characterized, naturally occurring mechanisms for lateral gene transfer (22). Bacteriophages have been observed in association with many spirochetes (reviewed in references 10 and 11), including Borrelia spp. (3, 12, 14, 23), Leptospira biflexa (29), and Brachyspira hyodysenteriae (15, 27). VSH-1, a generalized transducing phage of B. hyodysenteriae, has been used to demonstrate the transfer of antibiotic resistance markers between two different strains of this spirochete (16). Recently, a prophage from L. biflexa was developed into a shuttle vector (28). To our knowledge, no transduction or any other naturally occurring mechanism for the introduction of exogenous DNA has been demonstrated in B. burgdorferi or related species.
We previously reported the preliminary characterization of a temperate bacteriophage (Fig. 1) (10–12), designated φBB-1, which packages the 32-kb circular plasmids (cp32s) of the B. burgdorferi genome. The cp32s are a family of extrachromosomal elements, several of which can be maintained in a single cell (7, 8, 35). There are three regions of variability on these homologous plasmids: two that encode various lipoproteins, and one that may encompass a possible partitioning region (1, 6–8, 17, 24, 25, 33–36, 40, 42). Sequence analysis of these molecules suggests that they have evolutionarily undergone recombination, possibly from both endogenous and exogenous sources (6–8, 20, 33, 35, 38). Many features of the cp32 family are consistent with the hypothesis that these plasmids are temperate prophage genomes (7–11).
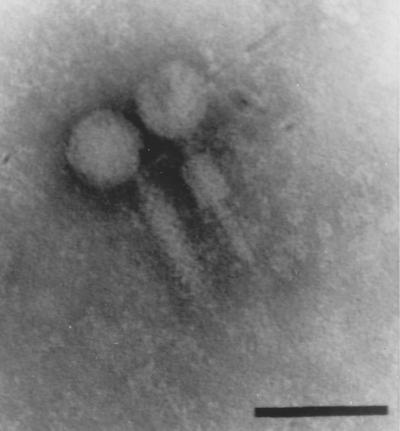
FIG. 1.
B. burgdorferi phage particles. Samples were collected from polyethylene glycol-precipitated cell supernatants of an MNNG-treated culture of B. burgdorferi CA-11.2A and viewed by transmission electron microscopy. Although previously reported as having a simple noncontractile tail (12), subsequent modifications to the purification and preparation protocol (10, 11) reveal that all phage particles observed have intact contractile sheaths, seen here either extended (left) or contracted (right). Phosphotungstic acid stain. Bar, 45 nm.
To elucidate a possible biological role for φBB-1 as well as evaluate its use as a potential molecular tool, we have extended our characterization of this phage. Using a DNA fragment from a partial library of φBB-1 DNA, we have constructed a recombinant cp32 (φBB-1 prophage) carrying a kanamycin resistance cassette. Subsequently, φBB-1 was used to transduce this recombinant cp32 to other strains of B. burgdorferi. The introduction of an antibiotic resistance marker into naive cells by φBB-1 is the first direct demonstration of lateral gene transfer in B. burgdorferi.
MATERIALS AND METHODS
Bacterial strains and bacteriophage recovery.
B. burgdorferi sensu stricto strains CA-11.2A (21) and high-passage B31-UM were part of our collection. High-passage B. burgdorferi sensu stricto strain 1A7, a clone of Sh2–82, was generously provided by Tom Schwan (Rocky Mountain Laboratories). Bacterial isolates were routinely cultivated in modified Barbour-Stoenner-Kelly (BSK-H) complete medium (Sigma, St. Louis, Mo.) at 34°C with a 5% CO2 atmosphere. Culture densities were determined, and φBB-1 particles were recovered from cultures induced with 1-methyl-3-nitro-1-nitrosoguanidine(MNNG) as described previously (12). Phage particles were concentrated, stained, and visualized by transmission electron microscopy using a protocol described elsewhere (10).
Agarose gel electrophoresis.
DNA for restriction digests was extracted as described previously (12) and digested with different restriction enzymes as instructed by the manufacturer (New England Biolabs, Beverly, Mass.). DNA samples were resolved in 0.8% agarose gels (SeaKem GTG; Bio*Whittaker Molecular Applications; Walkersville, Md.) in TBE (45 mM Tris-borate, 2 mM EDTA) at 80 V (4.3 V cm−1). Field-inversion gel electrophoresis (FIGE) was performed at room temperature using a PPI-200 programmable power inverter (MJ Research, Waltham, Mass.) with optimal programs and times for separating the desired range of fragments determined using the GelTimes software supplied by the manufacturer. The running buffer was supplemented with 100 mM glycine (as suggested by MJ Research), and the buffer was recirculated during electrophoresis. After electrophoresis, gels were stained with 0.5 μg of ethidium bromide (EtBr) ml−1 for 0.5 to 1 h and destained in water for 1 to 2 h. The DNA was visualized on a UV transilluminator, and images were captured on a Gel Doc 1000 system (Bio-Rad, Hercules, Calif.). Two-dimensional gel electrophoresis and genomic DNA extraction were performed essentially as described previously (12, 31).
Southern hybridization.
Agarose gels to be blotted were prepared and run as described above. After visualization, the gels were destained in water and then vacuum blotted to Immobilon-Ny+ (Millipore, Bedford, Mass.) as described elsewhere (18). Southern hybridization was performed as previously described (12). Approximate sizes of the major fragments were determined by comparison to markers of known sizes. Blots to be reprobed were stripped in increasingly stringent solutions as described elsewhere (2).
The conserved cp32-specific probes used for Southern hybridizations were probe 4 (8) and a probe that flanks an NdeI site (cp32SK12NdeI) in the paralogous family (PF) gene 50. Probes were generated from B. burgdorferi genomic DNA by PCR with Taq polymerase as described by the manufacturer (Sigma) using an annealing temperature of either 50°C (probe 4) or 44°C (probe cp32SK12NdeI). Additionally, the pOK12 plasmid was used as a probe for the kan cassette. All probes were labeled using the Prime-it II kit as instructed by the manufacturer (Stratagene, La Jolla, Calif.).
Variable region PCR.
A ClustalW alignment (MacVector 6.5.1; Oxford Molecular, Madison, Wis.) of the available B. burgdorferi B31 cp32 sequences from GenBank (7) was performed to identify possible diagnostic variable regions. Oligonucleotides to highly conserved sequences flanking one variable region, designated VR1, were designed using MacVector (Table 1). The VR1s were amplified from either cellular cp32s or phage DNA using Taq polymerase and the cp32-VR1 primers with 25 cycles of 92°C for 1 min, 50°C for 1 min, and 72°C for 3 min. The PCR products were resolved on 0.8% agarose gels subjected to FIGE, as described above. The PPI-200 power inverter was programmed with a maximum resolution in the range of 2 to 6 kb. The sizes of the amplified VR1s were determined using Multi-Analyst Software 1.0 (Bio-Rad).
TABLE 1.
Oligonucleotides generated for this study
| Oligonucleotide | Sequence (5′ to 3′) | Application |
|---|---|---|
| cp32-VR1F | AAATAAAACTTAGGAGTTGGTTTTGAA | Diagnostic PCR |
| cp32-VR1R | TAACTTTCCTAGCGTTAACTTCTGAT | Diagnostic PCR |
| cp32SK12NdeIF | ACTTTGTTGTAGTGATTATTTGTTC | Screening; probe |
| cp32SK12NdeIR | GGGGAAAGAATTGTTGAAG | Screening; probe |
| KanR1207F | ATTACGCTGACTTGACGGG | kan cassette screening |
Construction of pCE210.
HindIII-digested φBB-1 DNA was ligated into the HindIII site of pBluescript II SK+ (Stratagene, La Jolla, Calif.), generating a library that contained random phage DNA fragments of less than or equal to 4 kb. A plasmid with a 4-kb insert was selected and designated pCE100. The insert was found to contain one NdeI site approximately 1.1 kb from one end (Fig. 2A).
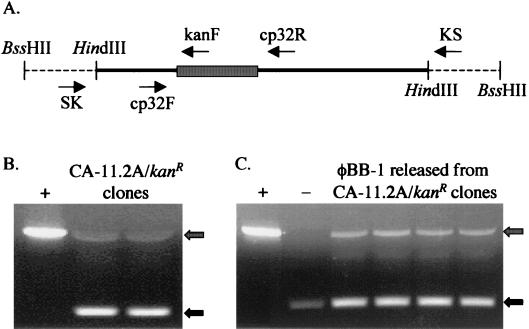
FIG. 2.
Kanamycin-resistant transformants of B. burgdorferi. (A) pCE210 construct. A 4-kb fragment of phage DNA (solid line) was cloned into the HindIII site of pBluescript SK+ (dashed line). The kan cassette (hatched box) was cloned into a unique NdeI site on the phage fragment 1.1 kb from the SK primer location. The locations of the SK, KS, cp32SK12NdeIF (cp32F), cp32SK12NdeIR (cp32R), and KanR1207F (kanF) oligonucleotides are shown (not drawn to scale). Arrows indicate oligonucleotide site and orientation. (B) Transformed CA-11.2A colonies were screened by PCR using the cp32SK12NdeI primers. PCR products were resolved on a 1% agarose gel and stained with EtBr. Both a small product (no kan cassette; black arrow) and a larger product (integrated kan cassette; hatched arrow) were amplified from the transformants. (C) Products consistent with a population of cp32s both containing the kan cassette (hatched arrow) and lacking the kan cassette (black arrow) were also amplified from phage DNA collected from CA-11.2A/kanR transformants. pCE210 DNA was amplified as a positive (+) control, and wild-type CA-11.2A phage DNA was used as a negative (−) control.
The 1.3-kb flg promoter-kan fusion conferring kanamycin resistance in B. burgdorferi was excised from pTAKanG (4) by EcoRI digestion. Both pCE100 linearized by digestion with NdeI and the purified kan cassette were treated with mung bean nuclease (New England Biolabs) to blunt the ends of the DNA as instructed by the manufacturer. The kan cassette was subsequently ligated into pCE100, resulting in pCE210 (Fig. 2A).
Transformation of B. burgdorferi CA-11.2A with the kan cassette.
pCE210 was digested with BssHII, and the insert containing the recombinant φBB-1 DNA-kan cassette was gel purified with a Qiaex gel extraction kit (Qiagen, Valencia, Calif.). Purified insert DNA (10 μl, ∼2 μg) was electroporated into competent B. burgdorferi CA-11.2A cells and plated in solid medium as described previously (30). Transformants were selected in solid BSK at 34°C in the presence of 500 μg of kanamycin per ml. Colonies were screened by PCR using the cp32SK12NdeI primers (Table 1 and Fig. 2) as described above. PCR products were resolved on 1% agarose gels and stained with GelStar nucleic acid stain (Bio*Whittaker Molecular Applications).
Transduction assays.
Phage particles were purified from the cell supernatants of the CA-11.2A/kanR transformant (12) and treated with an additional 10% chloroform (CHCl3) to eliminate possible cellular contamination, incubated at room temperature for 15 min, and then centrifuged at 14,000 × g, and the aqueous phase was recovered. Sterile 1 M MgCl2 was added to the sample to bring the final Mg2+ concentration to 16 mM, and 1 μl of RQ1 DNase (Promega, Madison, Wis.) was added per 250 μl of volume. After 0.5 h at 37°C, 100 μl of the prepared phage sample (∼35 ng of phage DNA; the equivalent of ∼109 phage) was mixed with 107 cells (100:1), and BSK-complete was added for a final volume of 1 ml.
After 16 h of incubation at 34°C, the phage-cell mixtures were plated, and potential transductants were selected on solid medium with 500 μg of kanamycin per ml. Colonies were picked and screened by PCR with the cp32SK12NdeIF and KanR1207F primers (Table 1 and Fig. 2A) using the same parameters as for the cp32SK12NdeI primer pair. Positive clones were transferred into 10 ml of BSK-complete with 500 μg of kanamycin per ml. The frequency of transduction was determined as the number of positive transductants divided by the total number of CFU.
When the putative transductants reached approximately 5 × 107 cells ml−1, DNA was extracted from the culture and the clones were screened again by PCR for the kan cassette using the above primers. Additionally, using the KanR1207F primer and the cp32-VR1R primer, a 7.5-kb region of DNA was amplified (25 cycles of 92°C for 30 s, 50°C for 30 s, and 72°C for 6 min) with TaqPlus long (Stratagene) from each transductant.
Analysis of recombinants.
Plasmid DNA was extracted from log-phase cultures of B. burgdorferi using Wizard Plus Midi preps (Promega). The concentration of DNA was determined by measuring the absorbance at 260 nm. A total of 500 ng of DNA was digested with either EcoRV or XbaI, and the DNA was resolved by FIGE. Following electrophoresis and staining, the gels were vacuum blotted, probed, stripped, and reprobed as described above.
RESULTS
Determining the minimum number of cp32s present in a population of cells or phage.
Specific probes to individual B. burgdorferi B31 cp32s (8) did not hybridize to B. burgdorferi CA-11.2A cp32s (data not shown). To determine an approximate number of cp32s in both B. burgdorferi cells and φBB-1 capsids, oligonucleotides that flank a diagnostic variable region (designated VR1) were designed (Table 1). VR1 encompasses a portion of each cp32 where the ospE/ospF/elp genes (erp loci) are located (1, 6, 7, 20, 25, 35). The cp32-VR1R primer (27 nucleotides) is conserved on all the B. burgdorferi B31 cp32s and the cp32-like molecule integrated into lp56 (7). The cp32-VR1F primer (26 nucleotides) has one mismatch on cp32–9 and two mismatches on the cp32-like molecule integrated into lp56, but is conserved on all the other B31 cp32s. PCR amplification of VR1 with these oligonucleotides generates products of different sizes for several of the B. burgdorferi B31 cp32s whose sequences are available (Table 2).
TABLE 2.
Predicted fragment sizesa of VR1 of the B31 cp32s
|
B. burgdorferi B31 plasmid |
Amplicon size (bp) |
|---|---|
| cp32-1 | 3429 
|
| cp32-3 | 2442 |
| cp32-4 | 3377 
|
| cp32-6 | 2507 |
| cp32-7 | 3575 |
| cp32-8 | 3429 
|
| cp32-9 | 3367 
|
| 1p56 | 2796 |
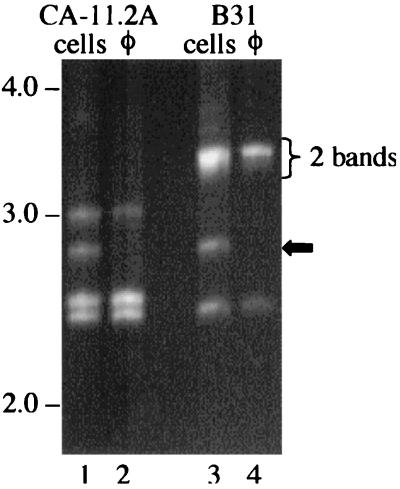
Using the cp32-VR1 primers and FIGE for maximum resolution, we identified a minimum of four different cp32s and homologous molecules (i.e., lp56) present in B. burgdorferi CA-11.2A (Table 3; Fig. 3, lane 1) and also a minimum of four different cp32s (or homologous molecules) present in B. burgdorferi strain B31-UM (Table 3; Fig. 3, lane 3). The φBB-1 phages released from CA-11.2A (Fig. 3, lane 2) and B31 cells (Fig. 3, lane 4) package cp32s that are represented by only three of the VR1s amplified from the respective hosts. The absence of the ∼2.8-kb band (Table 3) from both φBB-1 samples suggests that the phage does not package the cp32 that is integrated into lp56 (or any DNA that is packaged from the lp56 does not include the targeted region).
TABLE 3.
Amplification of the VR1s of CA-11.2A, B31, and φBB-1a
| Size (bp)
|
Predicted B31 plasmid | |||
|---|---|---|---|---|
| CA-11.2A
|
B31-UM
|
|||
| Total DNA | Phage DNA | Total DNA | Phage DNA | |
| 3,426 | 3,426 | cp32-1 or 8 | ||
| 3,343 | 3,343 | cp32-9 or 4 | ||
| 3,047 | 3,047 | |||
| 2,819 | 2,828 | 1p56 | ||
| 2,537 | 2,537 | |||
| 2,484b | 2,484 | cp32-3b | ||
| 2,455 | 2,455 | |||
Sizes determined by regression using Multi-Analyst software; predictions based on size estimates from ≥3 trials and applicable only to the B31 fragments.
Prediction confirmed by sequencing of VR1.
FIG. 3.
Determining the minimum number of cp32 molecules in B. burgdorferi cells and φBB-1 capsids by PCR amplification of VR1. Highly conserved primers that flank a variable region (VR1) of cp32 amplify four different size products from B. burgdorferi strains CA-11.2A and B31. The two VR1s from the cp32s of B31 at ∼3.3 kb can be resolved with extended electrophoresis times (see Fig. 7). PCR of φBB-1 released from both of these strains amplifies three of the VR1 products (lanes 2 and 4), but not the ∼2.8-kb amplicon (black arrow; see Table 2). Fragments were resolved on a 0.8% agarose gel by FIGE and stained with EtBr. Molecular sizes (in kilobase pairs) are indicated.
Inserting the kanamycin resistance cassette into a cp32.
A construct containing a 1.3-kb kan gene integrated into a fragment of phage DNA (see Fig. 2A) was transformed into B. burgdorferi CA-11.2A cells. Colonies selected in 500 μg of kanamycin per ml were screened by PCR using the cp32SK12NdeI primers (Table 1) that flank the site into which the kan cassette had been inserted (Fig. 2A and B). Partial sequence obtained from the fragment of phage DNA (data not shown) indicates that the kan gene was inserted into the PF50 paralog of cp32. The cp32SK12NdeI primers amplify a product from both the recombined cp32 (∼1.4 kb) and the other homologous cp32 loci in the cells that do not contain the insert (∼100 bp) (Fig. 2B). Both products are expected in a population of B. burgdorferi cells containing more than one cp32 if, as anticipated, the fragment bearing the kan cassette does not recombine with all of the cp32s. The φBB-1 capsids collected from the supernatants of these CA-11.2A transformants include genomes containing the kan gene as well as uninserted parental phage genomes (Fig. 2C). These data are consistent with the results from the variable-region analysis, indicating that more than one cp32 is packaged in a population of φBB-1 phage heads (Fig. 3, lanes 2 and 4).
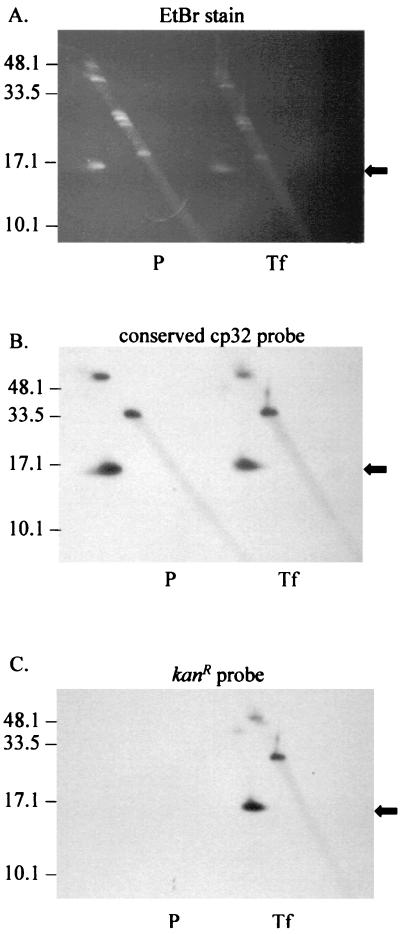
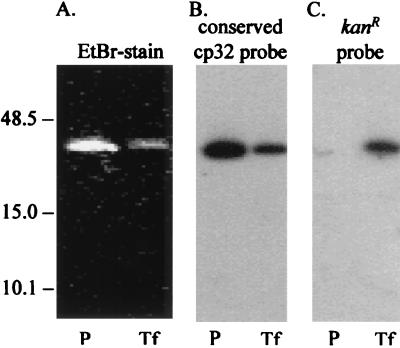
The genomic location of the integrated kan cassette was determined by Southern hybridization (Fig. 4B and C) of total DNA resolved by two-dimensional electrophoresis (Fig. 4A). The blot of the gel was probed with cp32-specific probe 4 to localize the circular 32-kb molecules (Fig. 4B). The membrane was also probed with pOK12, the original source of the kanamycin resistance gene (4, 39) (Fig. 4C). pOK12 (the kan probe) hybridizes only to the CA-11.2A/kanR transformant (Fig. 4C) and has the same hybridization pattern as the cp32-specific probe 4 (Fig. 4B), locating the integration site of the kan cassette to a cp32. Extracted phage DNA (Fig. 5A) was also analyzed. Probe 4 (Fig. 5B) hybridizes to phage DNA released from both the parental and the transformed CA-11.2A; however, the kan cassette probe hybridizes to only the φBB-1 DNA packaged and released by CA-11.2A/kanR (Fig. 5C).
FIG. 4.
Analysis of the genomic location of the kanamycin resistance cassette. Total DNA from both parental CA-11.2A (P) and a transformed clone of CA-11.2A/kanR (Tf) was extracted and resolved by two-dimensional gel electrophoresis. The gel was stained with EtBr (A), blotted to nylon, and probed with the cp32-specific probe 4 (B) or pOK12, the source of the kan gene (C). The kan probe has the same hybridization pattern as the cp32-specific probe. The supercoiled form of cp32 is indicated by the black arrow, while the other hybridization sites likely represent the nicked (or lp56) and linearized forms of cp32 generated during DNA isolation. Molecular sizes (in kilobase pairs) are indicated.
FIG. 5.
Analysis of kan cassette packaging by φBB-1. Phage particles were precipitated from both parental (P) and transformed (Tf) CA-11.2A cells. The DNA was extracted and resolved on a 0.5% agarose gel by conventional field electrophoresis. The gel was stained with EtBr (A), then blotted and probed with either probe 4 (cp32-specific; B) or pOK12 (kan; C). Molecular sizes (in kilobase pairs) are indicated.
Transduction.
To evaluate the ability of φBB-1 to transduce the kan gene into kanamycin-susceptible cells, bacteriophage from the CA-11.2A/kanR transformant were incubated with CA-11.2A cells at an approximately 100:1 multiplicity of infection. After an overnight incubation, the cells were plated in 500 μg of kanamycin per ml. We screened 10 of ∼100 colonies by PCR. All the colonies screened contained the kan gene integrated into a cp32 (Fig. 6A and data not shown).
FIG. 6.
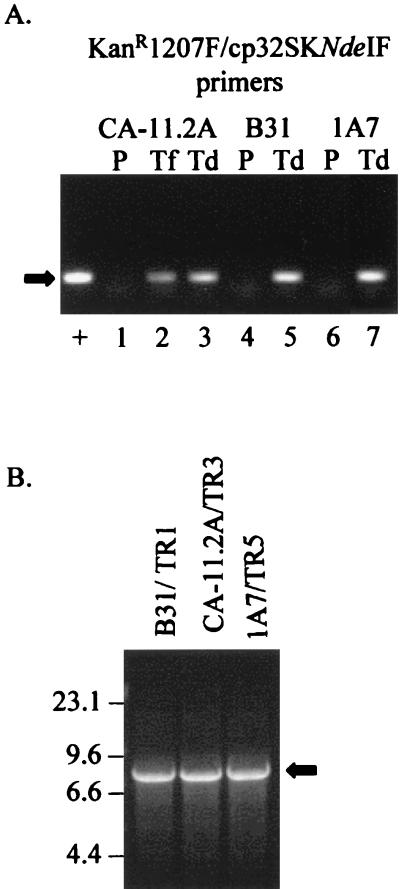
PCR analysis of kanamycin-resistant transductants of B. burgdorferi. (A) Putative transductants from B. burgdorferi strains CA-11.2A, B31, and 1A7 were screened by PCR using the KanR1207F and cp32SK12NdeIF primers. pCE210 was amplified as a positive control (+), while the parental cells (P) of each strain served as negative controls (lanes 1, 4, and 6). Lane 2 is the CA-11.2A/kanR transformant (Tf). PCR yields a ∼125-bp product from DNA containing the kan cassette (black arrow, lanes 2, 3, 5, and 7). The products were resolved on a 1% agarose gel and stained by EtBr. (B) The kan integration site in each transductant was verified by long-range PCR using the KanR1207F and cp32-VR1R primers.
The frequency of transduction of φBB-1 between CA-11.2A cells is ∼10−5 to 10−6 per cell. Alternatively, the frequency of transduction by φBB-1 is ∼10−7 per phage particle (∼100 transductants from ∼109 phage particles). No colonies grew when either CA-11.2A cells without φBB-1/kanR or φBB-1/kanR phage without cells were plated in kanamycin. The addition of proteinase K to φBB-1/kanR prior to incubation with cells abrogated the transduction of the antibiotic resistance marker. Incubating washed, CHCl3-killed CA-11.2A/kanR cells with live CA-11.2A cells resulted in 0 to 3 colonies. However, no colonies grew when proteinase K was added to the dead transformant cells prior to mixing them with susceptible cells, implying that even the small amount of transfer between dead CA-11.2A/kanR cells and live CA-11.2A cells requires protein. These data indicate that phage φBB-1 mediated the lateral gene transfer.
φBB-1/kanR isolated from the CA-11.2A transductant (CA-11.2A/TR3) was incubated with several other strains of B. burgdorferi. The kan cassette was transduced by φBB-1/kanR (CA-11.2A) into B. burgdorferi strains B31 and 1A7 (a high-passage clone of B. burgdorferi Sh2–82) (Fig. 6A). No demonstrable DNA transfer occurred when dead CA-11.2A/kanR cells were incubated with 1A7 and B31 cells. Long-range PCR using an internal kan primer (KanR1207F) and the cp32-VR1R primer, which lies outside the cloned fragment approximately 7.5 kb from the insertion site, was done to verify the integration of the kan cassette into the cp32 molecule (Fig. 6B). A partial sequence of the ospC gene from the transductant B31/TR1 confirmed that the strain (or at least the cp26) was derived from B31 (data not shown).
The frequency of transduction by φBB-1/kanR (CA-11.2A) into B31 is ∼10−6 to 10−7 and into 1A7 is ∼10−5 to 10−6 for individual clones of these strains. Neither the 1A7 transductant (1A7/TR5) nor the B31 transductant (B31/TR1) assumes the prolific CA-11.2A phage-producing phenotype, although both B31 and B31/TR1 spontaneously produce low levels of φBB-1. φBB-1 (B31) is also capable of packaging the kan cassette from B31/TR1 (data not shown). The kan marker can be efficiently transduced back into naive B31 using φBB-1/kanR (B31), but we have not yet been able to transduce the gene back into susceptible CA-11.2A cells using φBB-1/kanR (B31).
Variable-region analysis of the transduced cp32s.
We have demonstrated a mechanism for lateral gene transfer in B. burgdorferi via transduction by φBB-1. To analyze possible changes in the cp32 population of the transductants, the VR1s of the uncloned parent strains CA-11.2A, B31, and 1A7, as well as the transduced clones CA-11.2A/TR3, B31/TR1, and 1A7/TR5, were amplified and resolved by FIGE (Fig. 7A). The region of the cp32 that we have designated VR1 is located ∼5 kb from the site of the kan insertion (7).
FIG. 7.
Amplification of variable region VR1 from B. burgdorferi kan transductants. The cp32-VR1 primers were used to amplify the cp32 VR1s of parental CA-11.2A (lane 1), B31 (lane 3), and 1A7 (lane 5) as well as transductants CA-11.2A/TR3 (lane 2), B31/TR1 (lane 4), and 1A7/TR5 (lane 6). The 2,537-bp VR1 (black arrow) was found in all transductants. Amplification products were resolved on 0.8% agarose gels by FIGE and stained with EtBr. Molecular sizes are shown in kilobase pairs.
The fragments generated by PCR from the VR1 regions of the CA-11.2A transductant (TR3; Fig. 7A, lane 2) are identical to those of parental CA-11.2A (Fig. 7A, lane 1). The B31 transductant (TR1; Fig. 7A, lane 4) has lost the smallest VR1 (2,484 bp; corresponding to cp32–3) of the parental B31 (Fig. 7A, lane 3) and has gained a VR1 that is the same size as the second smallest CA-11.2A VR1 (2,537 bp; Fig. 7A, black arrow). The B31 transductant has also lost the VR1 corresponding to lp56 (2,828 bp). In addition, 1A7 has a small VR1 (2,373 bp; Fig. 7A, lane 5) that is missing from 1A7/TR5 (Fig. 7A, lane 6), and the transductant has gained a VR1 that is also the same size as the second smallest CA-11.2A VR1 (2,537 bp; Fig. 7A, black arrow). This analysis suggests that the VR1 from the introduced cp32, which contains the 2,537-bp VR1 (data not shown), has replaced the smallest VR1 fragment of both B31/TR1 and 1A7/TR5.
Whether the loss of the VR1 from the transductants is due to displacement of a resident plasmid, recombination by the kan gene into an extant plasmid, or the loss of a plasmid during cloning and subsequent replacement with the φBB-1/kanR (CA-11.2A) prophage is not known. We have confirmed that the 2,537-bp VR1 is linked to the kan cassette on the transduced φBB-1/kanR (CA-11.2A) genome by amplifying the VR1 region from the kanR/cp32-VR1R long-range PCR product (Fig. 6B and data not shown).
Restriction mapping of transductants.
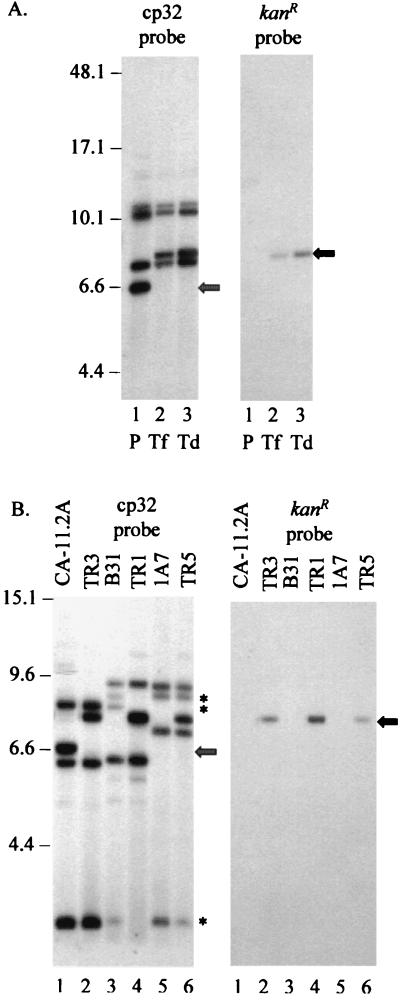
Plasmid DNA from CA-11.2A, CA-11.2A/kanR (transformant), and CA-11.2A/TR3 (transductant) was extracted and digested with EcoRV to analyze changes in the restriction pattern of the cp32 molecules. The location of the kan cassette on cp32 was mapped using Southern hybridization (Fig. 8A).
FIG. 8.
Restriction mapping of the cp32s of B. burgdorferi kan transductants. (A) Comparison of the restriction maps of parental, transformant, and transductant CA-11.2A DNA. Plasmid DNA was extracted from CA-11.2A (lane 1; P), the CA-11.2A/kanR transformant (lane 2; Tf), and CA-11.2A/TR3 (lane 3; Td), digested with EcoRV, and resolved by FIGE. A blot of the gel was probed either with the cp32SK12NdeI PCR product (left panel) or with kan (right panel). A unique ∼6.6-kb fragment of CA-11.2A parental DNA is indicated by the hatched arrow. A ∼8-kb band (black arrow) corresponding to the ∼6.6-kb band plus the 1.3-kb kan insert is present in both the transformant and the transductant. (B) Comparison of the restriction maps of the cp32s of B. burgdorferi kan transductants. Plasmid DNA from CA-11.2A (lane 1), CA-11.2A/TR3 (lane 2), B31 (lane 3), B31/TR1 (lane 4), 1A7 (lane 5), and 1A7/TR5 (lane 6) was digested with XbaI and resolved by FIGE. A blot of the gel was probed with either the cp32SK12NdeI probe (left panel) or the kan probe (right panel). A ∼8-kb band (black arrow) is found only in the transductants. The hatched arrow indicates the 6.6-kb fragment from the parental CA-11.2A strain (lacking the kan cassette). Three bands (∗) appear in parental B31 but not B31/TR1. Molecular sizes are shown in kilobase pairs.
There are four hybridization sites in the cellular cp32 population digested with EcoRV and probed with the cp32SK12NdeI probe (Fig. 8A, left panel). This includes a ∼6.6-kb band (hatched arrow) in the parental CA-11.2A (Fig. 8A, lane 1). This EcoRV fragment is not present in either the CA-11.2A/kanR transformant (Fig. 8A, lane 2) or the transductant, CA-11.2A/TR3 (Fig. 8A, lane 3). In the latter two strains, there is a ∼8-kb fragment (Fig. 8A, black arrow) that is not found in the parental strain. The size difference is the same size as the kan cassette (∼1.3 kb), suggesting that the kan cassette has been inserted into the 6.6-kb fragment. When the same blot is probed with the kan marker, the ∼8-kb fragments in the CA-11.2A/kanR transformant and in CA-11.2A/TR3 are the only hybridization sites (Fig. 8A, right panel, lanes 2 and 3, respectively). The ∼6.6-kb fragment also corresponds to the major band in an EcoRV digest of φBB-1 DNA probed with the cp32SK12NdeI probe (data not shown).
To compare the kan insertion site in the other transductants, plasmid DNA was extracted from parental CA-11.2A, B31, and 1A7 and the transductant CA-11.2A/TR3, B31/TR1, and 1A7/TR5 cells. The plasmids were digested with XbaI, and the DNA was resolved by FIGE. A blot of the gel was probed with both the cp32SK12NdeI PCR product and the kan cassette (Fig. 8B). When probed with the cp32SK12NdeI product (Fig. 8B, left panel), there is hybridization to a ∼6.6-kb XbaI fragment that is found exclusively in the parental CA-11.2A (Fig. 8B, lane 1; hatched arrow) and not in the parental B31 or 1A7. However, a ∼8-kb fragment is present in all the transductant lanes (Fig. 8B, lanes 2, 4, and 6, black arrow). Hybridization with kan (Fig. 8B, right panel) verified that the ∼8-kb fragment contained the kan cassette in all three transductants. This suggests that the kan cassette is on a cp32 in the transductants that is not a member of either of the parental B31 or 1A7 populations. The B31 parent has three bands that do not appear in B31/TR1 (Fig. 8B, left panel, lanes 3 and 4, asterisk), possibly due either to displacement of a cp32 or loss of plasmids (cp32 or lp56) during cloning. There are also three bands that are present in the B31 parent but absent from the transductant when plasmid DNA from these isolates is digested with EcoRV and probed with the cp32SK12NdeI probe (data not shown).
DISCUSSION
We recently identified φBB-1, a temperate bacteriophage of B. burgdorferi (Fig. 1), whose prophage is in the cp32 family of circular plasmids (10–12). In this study we demonstrated that φBB-1 can package all of the free cp32s within a population of cells (Fig. 3). To date, no known strain of B. burgdorferi carries fewer than three cp32s (7, 8, 26), and any or all of these cp32s may be prophages (associated with all of the necessary functions for lysogeny, progeny formation, and cellular lysis), or perhaps cryptic prophages, requiring other factors in trans for the successful propagation of φBB-1. The effect that the protein products of the various cp32s (such as repressors) may have on each other in trans has not been studied.
Prior to this work, no mechanism for lateral gene transfer had been demonstrated in Borrelia. Previous evidence of recombination in at least one locus (erp, containing the ospE, ospF, and elp homologs) on the cp32s (33, 38) suggested that lateral gene transfer has occurred among these plasmids. Gene transfer could be between different cp32s within the same cell, by phage transduction of alternate cp32s within a population of cells, or by some other, as yet undefined, mechanism. In this study, the kan cassette was inserted into a fragment of phage DNA and electroporated into CA-11.2A cells, generating a recombinant cp32. To our knowledge, this is the first such integration into a member of the cp32 plasmid family.
Mapping and sequence data (not shown) indicate that the site of the kan cassette insertion is within the cp32–3 PF50 paralog (BBS34) of a CA-11.2A cp32, located near the variable region that includes the putative paralogous partitioning genes of the cp32s (7, 8, 36, 41). The cp32 containing the kan gene is stable over 20 passages (∼150 generations) in the absence of selection, suggesting that the disrupted open reading frame (ORF) (BBS34 homolog) either has no critical role in the molecular metabolism of this plasmid or has a function that can be supplied in trans by another cellular cp32. The PF50 paralog (BBC02) of the 9-kb circular plasmid (cp9), a derivative of cp32, is critical for the replication of a cp9-based vector (37), suggesting that the replication requirements for this smaller plasmid are different than those of the cp32s.
We have demonstrated transduction of the kan cassette by φBB-1 into three different strains of B. burgdorferi (Fig. 6–8). This represents the first direct evidence of lateral gene transfer in B. burgdorferi. The ability of φBB-1 to transduce the antibiotic resistance marker into a strain is apparently unrelated to the ability of that strain to produce phage, since we have found that both low-passage B31 and high-passage 1A7 are transducible but not inducible (12; data not shown). Additionally, introducing the φBB-1/kanR (CA-11.2A) into these transducible strains does not confer the phage-producing phenotype of CA-11.2A on these strains.
The evidence suggests that the φBB-1/kanR genome is being introduced as a discrete plasmid into the cell during transduction. The host cell loses a VR1 marker concomitantly with gaining the φBB-1 (CA-11.2A) VR1, suggesting the displacement of a host plasmid (Fig. 7). However, there are no obvious candidates discernible from the restriction maps (Fig. 8B). The PF50 paralog into which the kan cassette has been inserted is most similar to the PF50 paralog on cp32–3 of B31, the plasmid that is apparently absent in the transductant as determined by VR1 analysis. We hypothesize that the transduced cp32/kanR from CA-11.2A phage displaced the resident cp32 homologs (carrying the smallest VR1s) in strains B31 and 1A7 (no cp32 data are currently available for the 1A7 parental strain Sh2–82). An alternate hypothesis is that prior loss of these cp32 homologs created populations of cells that were susceptible to transduction. B31 cp32–3 can be lost during cloning, and the loss of this plasmid has little or no effect on the infectivity of this isolate (26). Analysis of the plasmid profiles of several recent clones of CA-11.2A, B31, and 1A7 by PCR amplification of the VR1s suggests that the prior absence of the smallest VR1 is not required for transduction to occur, particularly in strain 1A7 (C. H. Eggers, B. J. Kimmel, and D. S. Samuels, unpublished data).
In this study, we have demonstrated a mechanism for lateral gene transfer that should be explored to establish its role within the infectious cycle or during the course of disease. Further analysis of φBB-1, the first bacteriophage of B. burgdorferi described at a molecular level, could play a critical role in the investigation of plasmid genetics, the development of a genetic system, and the analysis of metabolic processes in these bacteria as a whole.
ACKNOWLEDGMENTS
We thank Mike Minnick, Thad Stanton, Sherwood Casjens, and Sharyl Fyffe for thoughtful and critical reading of the manuscript; S. Casjens, Brian Stevenson, Kit Tilly, Fred Hayes, Claude Garon, Lori Lubke, Melissa Caimano, and Justin Radolf for useful discussions; B. Stevenson, Chris Damman, and Don Oliver for cp32-specific probes; Tom Schwan for bacterial strains; and Gary Hettrick for assistance with figure preparation.
This work was supported by grants from the National Institutes of Health (AI41559), Arthritis Foundation, National Science Foundation (MCB-9722408), and the University of Montana University Grant Program to D.S.S. C.H.E. is a recipient of a Predoctoral Honors Fellowship from the University of Montana.
REFERENCES
- 1.Akins D R, Caimano M J, Yang X, Cerna F, Norgard M V, Radolf J D. Molecular and evolutionary analysis of Borrelia burgdorferi 297 circular plasmid-encoded lipoproteins with OspE- and OspF-like leader peptides. Infect Immun. 1999;67:1526–1532. doi: 10.1128/iai.67.3.1526-1532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1999. [Google Scholar]
- 3.Barbour A G, Hayes S F. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bono J L, Elias A F, Kupko J J, Stevenson B, Tilly K, Rosa P. Efficient targeted mutagenesis in Borrelia burgdorferi. J Bacteriol. 2000;182:2445–2452. doi: 10.1128/jb.182.9.2445-2452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease–a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 6.Caimano M J, Yang X, Popova T G, Clawson M L, Akins D R, Norgard M V, Radolf J D. Molecular and evolutionary characterization of the cp32/18 family of supercoiled plasmids in Borrelia burgdorferi 297. Infect Immun. 2000;68:1574–1586. doi: 10.1128/iai.68.3.1574-1586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casjens S, Palmer N, van Vugt R, Huang W M, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson R J, Haft D, Hickey E, Gwinn M, White O, Fraser C M. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 8.Casjens S, van Vugt R, Tilly K, Rosa P A, Stevenson B. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J Bacteriol. 1997;179:217–227. doi: 10.1128/jb.179.1.217-227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damman C J, Eggers C H, Samuels D S, Oliver D B. Characterization of Borrelia burgdorferi BlyA and BlyB proteins: a prophage-encoded holin-like system. J Bacteriol. 2000;182:6791–6797. doi: 10.1128/jb.182.23.6791-6797.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eggers C H, Casjens S, Hayes S F, Garon C F, Damman C J, Oliver D B, Samuels D S. Bacteriophages of spirochetes. J Mol Microbiol Biotechnol. 2000;2:365–373. [PubMed] [Google Scholar]
- 11.Eggers C H, Casjens S, Samuels D S. Bacteriophages of Borrelia burgdorferi and other spirochetes. In: Saier M H Jr, García-Lara J, editors. The spirochetes: molecular and cellular biology. Wymondham, Norfolk, England: Horizon Press; 2001. [Google Scholar]
- 12.Eggers C H, Samuels D S. Molecular evidence for a new bacteriophage of Borrelia burgdorferi. J Bacteriol. 1999;181:7308–7313. doi: 10.1128/jb.181.23.7308-7313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete. Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 14.Hayes S F, Burgdorfer W, Barbour A G. Bacteriophage in the Ixodes dammini spirochete, etiological agent of Lyme disease. J Bacteriol. 1983;154:1436–1439. doi: 10.1128/jb.154.3.1436-1439.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphrey S B, Stanton T B, Jensen N S. Mitomycin C induction of bacteriophages from Serpulina hyodysenteriae and Serpulina innocens. FEMS Microbiol Lett. 1995;134:189–194. doi: 10.1111/j.1574-6968.1995.tb07921.x. [DOI] [PubMed] [Google Scholar]
- 16.Humphrey S B, Stanton T B, Jenson N S, Zuerner R L. Purification and characterization of VSH-1, a generalized transducing bacteriophage of Serpulina hyodysenteriae. J Bacteriol. 1997;179:323–329. doi: 10.1128/jb.179.2.323-329.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam T T, Nguyen T-P K, Montgomery R R, Kantor F S, Fikrig E, Flavell R A. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marconi R T, Samuels D S, Garon C F. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J Bacteriol. 1993;175:926–932. doi: 10.1128/jb.175.4.926-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marconi R T, Samuels D S, Landry R K, Garon C. Analysis of the distribution and molecular heterogeneity of the ospD gene among the Lyme disease spirochetes: evidence for lateral gene exchange. J Bacteriol. 1994;176:4572–4582. doi: 10.1128/jb.176.15.4572-4582.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marconi R T, Sung S Y, Norton-Hughes C A, Carlyon J A. Molecular and evolutionary analyses of a variable series of genes in Borrelia burgdorferi that are related to ospE and ospF, constitute a gene family, and share a common upstream homology box. J Bacteriol. 1996;178:5615–5626. doi: 10.1128/jb.178.19.5615-5626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margolis N, Rosa P. Regulation of expression of major outer surface proteins in Borrelia burgdorferi. Infect Immun. 1993;61:2207–2210. doi: 10.1128/iai.61.5.2207-2210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masters M. Generalized transduction. In: Neidhardt F, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 2421–2441. [Google Scholar]
- 23.Neubert U, Schaller M, Januschke E, Stolz W, Schmieger H. Bacteriophages induced by ciprofloxacin in a Borrelia burgdorferi skin isolate. Zentralbl Bakteriol. 1993;279:307–315. doi: 10.1016/s0934-8840(11)80363-4. [DOI] [PubMed] [Google Scholar]
- 24.Porcella S F, Fitzpatrick C A, Bono J L. Expression and immunological analysis of the plasmid-borne mlp genes of Borrelia burgdorferi strain B31. Infect Immun. 2000;68:4992–5001. doi: 10.1128/iai.68.9.4992-5001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porcella S F, Popova T G, Akins T G, Li M, Radolf J D, Norgard M V. Borrelia burgdorferi supercoiled plasmids encode multicopy tandem open reading frames and a lipoprotein gene family. J Bacteriol. 1996;178:3293–3307. doi: 10.1128/jb.178.11.3293-3307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purser J E, Norris S J. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci USA. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritchie A E, Robinson I M, Joens L A, Kinyon J M. A bacteriophage for Treponema hyodysenteriae. Vet Rec. 1978;103:34–35. doi: 10.1136/vr.103.2.34. [DOI] [PubMed] [Google Scholar]
- 28.Saint Girons I, Bourhy P, Ottone C, Picardeau M, Yelton D, Hendrix R W, Glaser P, Charon N. The LE1 bacteriophage replicates as a plasmid within Leptospira biflexa: construction of an L. biflexa-Escherichia coli shuttle vector. J Bacteriol. 2000;182:5700–5705. doi: 10.1128/jb.182.20.5700-5705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saint Girons I, Margarita D, Amouriaux P, Baranton G. First isolation of bacteriophages for a spirochete: potential genetic tools for Leptospira. Res Microbiol. 1990;143:615–621. doi: 10.1016/0923-2508(90)90086-6. [DOI] [PubMed] [Google Scholar]
- 30.Samuels D S. Electrotransformation of the spirochete Borrelia burgdorferi. In: Nickoloff J A, editor. Electroporation protocols for microorganisms. Vol. 47. Totowa, N.J: Humana Press; 1995. pp. 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samuels D S, Garon C F. Coumermycin A1 inhibits growth and induces relaxation of supercoiled plasmids in Borrelia burgdorferi, the Lyme disease agent. Antimicrob Agents Chemother. 1993;37:46–50. doi: 10.1128/aac.37.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steere A C, Grodzicki R L, Kornblatt A N, Craft J E, Barbour A G, Burgdorfer W, Schmid G P, Johnson E, Malawista S E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 33.Stevenson B, Casjens S, Rosa P. Evidence of past recombination events among the genes encoding the Erp antigens of Borrelia burgdorferi. Microbiology. 1998;144:1869–1879. doi: 10.1099/00221287-144-7-1869. [DOI] [PubMed] [Google Scholar]
- 34.Stevenson B, Casjens S, van Vugt R, Porcella S F, Tilly K, Bono J L, Rosa P. Characterization of cp18, a naturally truncated member of the cp32 family of Borrelia burgdorferi plasmids. J Bacteriol. 1997;179:4285–4291. doi: 10.1128/jb.179.13.4285-4291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevenson B, Tilly K, Rosa P A. A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J Bacteriol. 1996;178:3508–3516. doi: 10.1128/jb.178.12.3508-3516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson B, Zückert W, Akins D. Repetition, conservation, and variation: the multiple cp32 plasmids of Borrelia species. J Mol Microbiol Biotechnol. 2000;2:411–422. [PubMed] [Google Scholar]
- 37.Stewart P E, Thalken R, Bono J L, Rosa P. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol Microbiol. 2001;39:714–721. doi: 10.1046/j.1365-2958.2001.02256.x. [DOI] [PubMed] [Google Scholar]
- 38.Sung S Y, McDowell J V, Carlyon J A, Marconi R T. Mutation and recombination in the upstream homology box-flanked ospE-related genes of the Lyme disease spirochete result in the development of new antigenic variants during infection. Infect Immun. 2000;68:1319–1327. doi: 10.1128/iai.68.3.1319-1327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vieira J, Messing J. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene. 1991;100:189–194. doi: 10.1016/0378-1119(91)90365-i. [DOI] [PubMed] [Google Scholar]
- 40.Yang X, Popova T G, Hagman K E, Wikel S K, Shoeler G B, Caimano M J, Radolf J D, Norgard M V. Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect Immun. 1999;67:6008–6018. doi: 10.1128/iai.67.11.6008-6018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zückert W R, Filipuzzi-Jenny E, Meister-Turner J, Ståhlhammar-Carlemalm M, Meyer J. Repeated DNA sequences on circular and linear plasmids of Borrelia burgdorferi sensu lato. In: Axford J S, Rees D H E, editors. Lyme borreliosis. New York, N.Y: Plenum Press; 1994. pp. 253–260. [Google Scholar]
- 42.Zückert W R, Meyer J. Circular and linear plasmids of Lyme disease spirochetes have extensive homology: characterization of a repeated DNA element. J Bacteriol. 1996;178:2287–2298. doi: 10.1128/jb.178.8.2287-2298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]