Abstract
Background
There is growing evidence of harm associated with trazodone and nonbenzodiazepine sedative hypnotics (e.g., zopiclone); however, their comparative risk of harm is unknown.
Methods
We conducted a retrospective cohort study with linked health administrative data, which enrolled older (≥66 years old) nursing home residents living in Alberta, Canada, between December 1, 2009, and December 31, 2018; the last follow-up date was June 30, 2019. We compared the rate of injurious falls and major osteoporotic fractures (primary outcome) and all-cause mortality (secondary outcome) within 180 days of first prescription of zopiclone or trazodone with cause-specific hazard models and inverse probability of treatment weights to control for confounding; primary analysis was intention-to-treat and secondary analysis was per-protocol (i.e., residents censored if dispensed the other exposure drug).
Results
Our cohort included 1,403 residents newly dispensed trazodone and 1,599 residents newly dispensed zopiclone. At cohort entry, the mean resident age was 85.7 (standard deviation [SD] 7.4), 61.6% were female, and 81.2% had dementia. New zopiclone use was associated with similar rates of injurious falls and major osteoporotic fractures (intention-to-treat-weighted hazard ratio 1.15, 95% confidence interval [CI] 0.90–1.48; per-protocol-weighted hazard ratio 0.85, 95% CI 0.60–1.21) and all-cause mortality (intention-to-treat-weighted hazard ratio 0.96, 95% CI 0.79–1.16; per-protocol-weighted hazard ratio 0.90, 95% CI 0.66–1.23) compared to trazodone.
Conclusions
Zopiclone was associated with a similar rate of injurious falls, major osteoporotic fractures, and all-cause mortality compared to trazodone—suggesting one medication should not be used in lieu of the other. Appropriate prescribing initiatives should also target zopiclone and trazodone.
Keywords: adverse drug event, trazodone, zopiclone, cohort studies
INTRODUCTION
Older adults living in nursing homes commonly experience neuropsychiatric symptoms such as sleep disturbances and agitation.(1) Pharmacologic interventions such as antipsychotics, antidepressants, benzodiazepines, and nonbenzodiazepines sedative hypnotics (i.e., z-drugs) are prescribed to treat these symptoms.(2) The pooled prevalence of chemical (e.g., antipsychotics, benzodiazepines) and physical (e.g., mittens, lap belt) restraint use in a systematic review of nursing home prevalence estimates was 32% and 33%, respectively.(3) Efficacious alternatives to chemical and physical restraints include massage therapy, music therapy, and multidisciplinary care plans; however, health-care provider knowledge of these interventions and resource limitations are perceived as barriers to their implementation.(4–6)
Time trend analyses conducted before the COVID-19 pandemic in Canada, the United States, and the United Kingdom suggested that antipsychotic and benzodiazepine use was stabilizing or decreasing, but use of alternative psychotropic medications such as antidepressants and anticonvulsants was increasing.(7–9) For example, 21.3% of older adults living in nursing homes in Ontario, Canada, were dispensed trazodone in 2013, compared to only 7.7% in 2002, which may have been in response to a growing body of literature describing harms associated with antipsychotic and benzodiazepine use, and initiatives targeting their appropriate prescribing.(10) From 2011 to 2014, 39.8% of older adults living in nursing homes in Oslo municipality, Norway, were prescribed nonbenzodiazepine sedative hypnotics (i.e., zopiclone or zolpidem); other studies have shown that nonbenzodiazepine sedative hypnotic use in people with dementia varies considerably by country.(2,11,12) Unlike benzodiazepines and antipsychotics, quality improvement work aimed at reducing potentially inappropriate prescribing of these alternative psychotropic medications has been limited.(13–15) This is important because there is growing evidence describing the risk of falls and fractures in older adults dispensed trazodone and nonbenzodiazepine sedative hypnotics (e.g., zopiclone).(16–18) Further, trazodone is associated with a similar risk of harm from injurious falls or fractures as benzodiazepines and atypical antipsychotics.(16,17)
Despite how commonly trazodone and zopiclone are prescribed in nursing homes, their comparative risk of harm in nursing home residents is unknown.(2,10) Our objective was to describe the risk of harm from injurious falls, fractures, and death associated with new use of zopiclone compared to new use of trazodone among older adults living in nursing homes in Alberta, Canada.
METHODS
This manuscript is reported in accordance with the STROBE (strengthening the reporting of observational studies in epidemiology) and RECORD-PE (reporting of studies conducted using observational routinely collected health data—pharmacoepidemiology) statements.(19, 20)
Setting and Data Sources
We created our cohort using linked health administrative databases in Alberta, Canada. Alberta has a largely publicly funded health-care system, in which individuals aged 65 years or older can access publicly funded nursing homes, when necessary, and receive universal coverage for physician services and most prescription medications. Between April 1, 2016, and March 31, 2017, there were 20,073 residents living in nursing homes in Alberta, and each resident received 10 different medications in the seven days before their Resident Assessment Instrument—Minimum Data Set, version 2.0 (RAI-MDS 2.0) assessment.(21) We linked patient-level data from these databases using each patient’s health insurance program number: National Ambulatory Care Reporting System, Provincial Registry, Discharge Abstract Database, Practitioner Claims Database, Pharmacy Information Network, and the Alberta Continuing Care Information System (see Appendix A for database details). These databases are accurate and reliable.(22–24)
Study Design
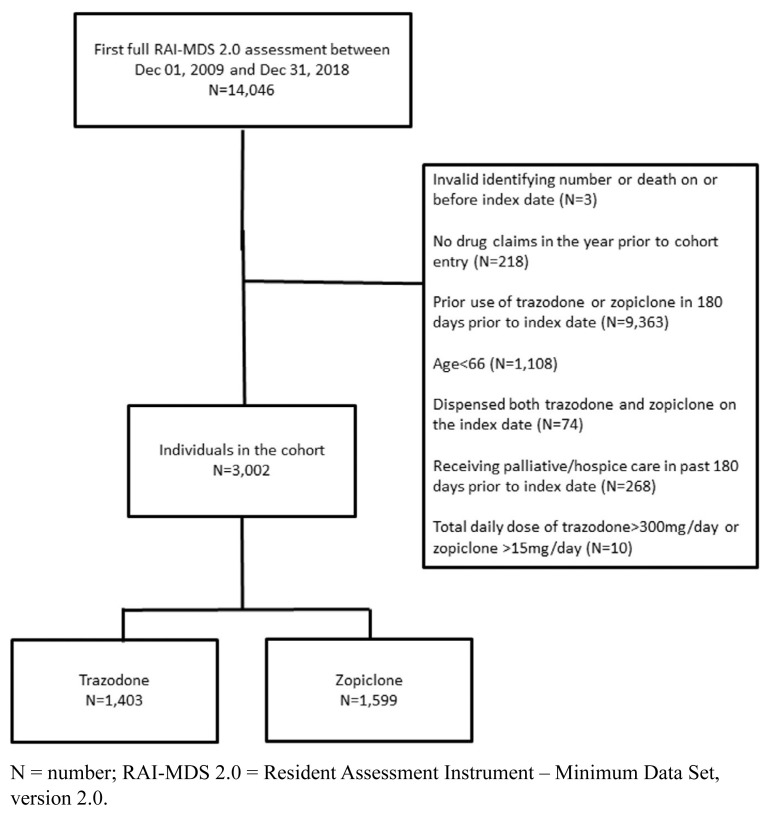
In this retrospective cohort study, the date of a new prescription for oral trazodone or zopiclone between December 1, 2009, and December 31, 2018, was our index date. We included nursing home residents, aged 66 years or older, who received a full RAI-MDS 2.0 assessment within 45 days before our index date. The RAI-MDS 2.0 is a validated assessment tool that records information about residents’ health status (e.g., independence in activities of daily living and cognitive impairment severity).(25) Choosing assessments within 45 days of the index date ensured a close temporal association between drug exposure and residents’ current health status. Our observation window was 180 days, which balanced the time required for event accrual against a need to minimize residual confounding over time. The maximum follow-up date was June 30, 2019. We excluded residents from our cohort if they: 1) did not have a complete Resident Assessment Instrument—Minimum Data Set, version 2.0 assessment within 45 days prior to cohort entry; 2) had an invalid identifying number or died on or before the index date; 3) had no drug claims in the year prior to cohort entry; 4) were dispensed trazodone or zopiclone in the 180 days prior to cohort entry; 5) were newly dispensed both trazodone and zopiclone on the index date; 6) received palliative care services in the 180 days prior to cohort entry; or 7) were dispensed trazodone at a dose greater than 300 mg/day or zopiclone at a dose greater than 15 mg/day (see Figure 1).
FIGURE 1.
Flow diagram of cohort creation
N = number; RAI-MDS 2.0 = Resident Assessment Instrument – Minimum Data Set, version 2.0.
We defined our primary outcome as a composite of fall-related emergency department visit (i.e., injurious fall) or major osteoporotic fracture, defined as a hip, pelvis, humerus, or forearm fracture (see Appendix B).(26) These outcomes are identified with a high positive predictive value in administrative databases and high level of agreement during medical chart re-abstraction.(22,26,27) Our secondary outcome was all-cause mortality. Where numbers permitted, we planned to report primary outcome components (i.e., fall, hip fracture, major osteoporotic fracture) as secondary outcomes. See Appendix A for all International Classification of Diseases, Tenth Revision (ICD-10) codes used to define residents’ baseline characteristics and study outcomes.(22,23,26)
Statistical Analysis
We summarized categorical baseline characteristics as frequencies (and percentages) and compared across exposure groups with chi-square tests. We summarized continuous baseline characteristics as means (and SDs) and compared across exposure groups with independent t-tests. We reported the proportion of residents missing data for individual baseline characteristics.
We derived inverse probability of treatment weights from an estimated propensity score, which we derived by regressing exposure status on baseline covariates (see Appendix B for covariates), including medications dispensed in the year before cohort entry. In our study, the propensity score was the probability that a resident would be dispensed trazodone or zopiclone, conditional on their baseline characteristics.(28) We included missing values for categorical variables as an additional category. There were no missing values for continuous variables. We modeled the average treatment effect because we could foresee any cohort member potentially receiving either exposure drug and we wished to understand the average effect of treatment in the entire cohort.(29) Treatment weights were inspected for outlying values (greater than 50).(30) Crude (i.e., unweighted) and weighted cause-specific hazard ratios comparing outcome rates associated with zopiclone or trazodone use were derived from cause-specific hazards models, because we wanted to understand the association between our exposure and outcome rate in residents who had not yet had an outcome and were, therefore, at risk of having an outcome.(31) Weighted cause-specific hazards models were adjusted for all baseline characteristics for which there were statistically significant differences (i.e., p<.05) between exposure groups. We verified that hazard ratios did not vary over time, and we used robust standard errors to account for within-subject homogeneity in outcomes induced by weighting.(32)
Our primary analyses were based on an intention-to-treat principle (i.e., residents who were newly dispensed either exposure drug remained in the cohort even if they were dispensed the other exposure drug during the follow-up period); residents were followed until the first of the outcome of interest, death, or 180 days after index date. In secondary analyses, we censored residents who were dispensed the other exposure drug during the 180-day follow-up period (i.e., per protocol). We reported weighted incidence rates as the number of events per 100 person-years. Where numbers permitted, we planned to conduct subgroup analyses based on residents’ age, sex, dementia severity, and concurrent antipsychotic prescription. Analyses were conducted with SAS (version 9.4; Cary, NC) and STATA SE (version 13.0; StataCorp LP, College Station, TX).
Ethics Approval
We obtained ethics approval for this study from the University of Alberta Research Ethics Board (Pro00091328).
RESULTS
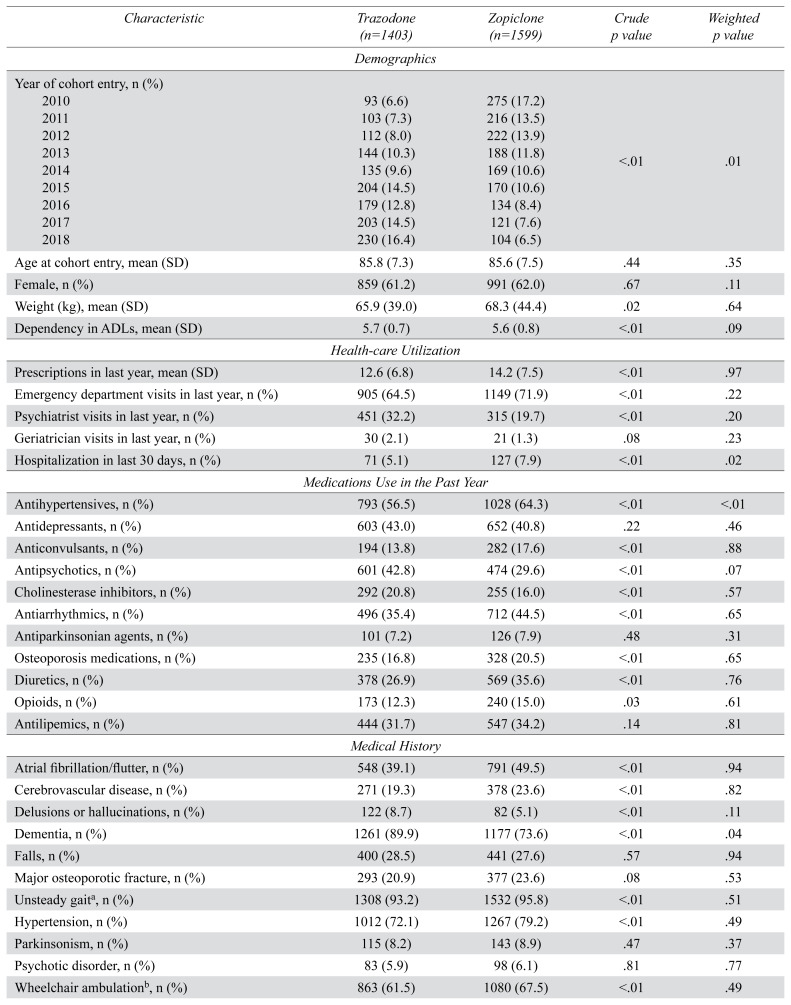
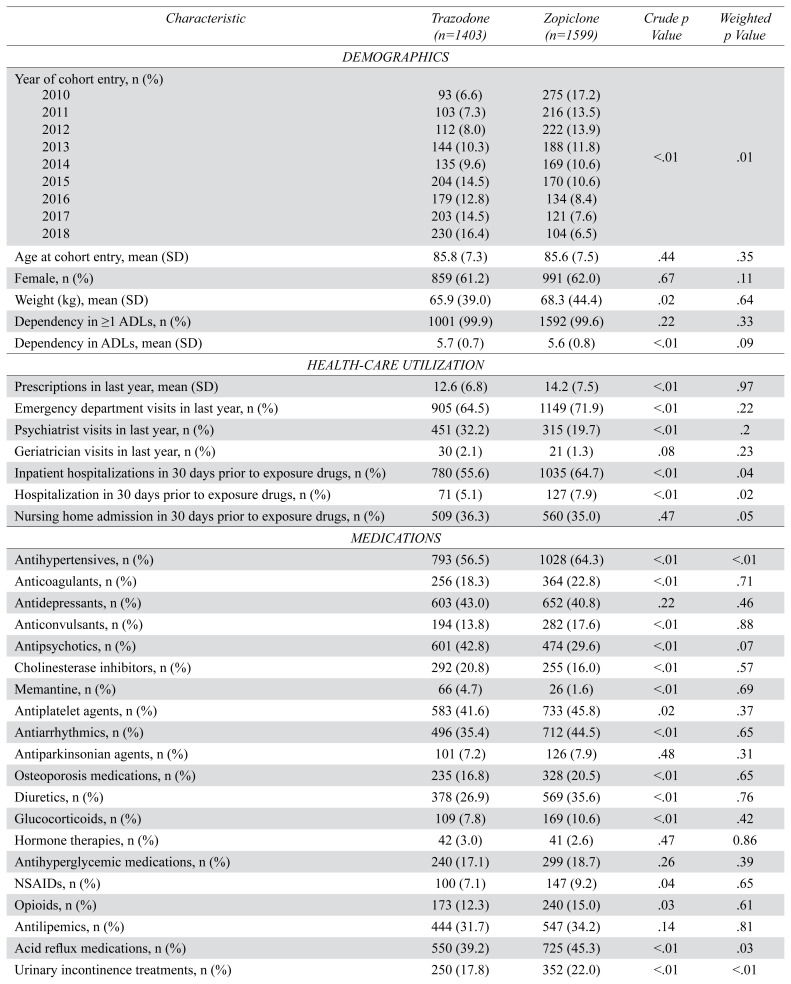
We included 3,002 residents in our study cohort: 1,403 residents were newly dispensed trazodone, and 1,599 residents were newly dispensed zopiclone (Figure 1). The median total daily dose of zopiclone was 7.5 mg (interquartile range 3.75 mg to 7.5 mg) and trazodone was 25 mg (interquartile range 25 mg to 50 mg). There were no outlying inverse probability of treatment weights. After applying inverse probability of treatment weights, exposure groups were similar at baseline (Table 1 and Table B1 in Appendix B). The mean age of residents on the date of cohort entry was 85.7 (SD 7.4), 61.6% were female, and 81.2% had a diagnosis of dementia.
TABLE 1.
Baseline characteristics of a cohort of older adults dispensed trazodone or zopiclone (number [n] =3,002)
| Characteristic | Trazodone (n=1403) | Zopiclone (n=1599) | Crude p value | Weighted p value |
|---|---|---|---|---|
| Demographics | ||||
|
| ||||
| Year of cohort entry, n (%) | ||||
| 2010 | 93 (6.6) | 275 (17.2) | <.01 | .01 |
| 2011 | 103 (7.3) | 216 (13.5) | ||
| 2012 | 112 (8.0) | 222 (13.9) | ||
| 2013 | 144 (10.3) | 188 (11.8) | ||
| 2014 | 135 (9.6) | 169 (10.6) | ||
| 2015 | 204 (14.5) | 170 (10.6) | ||
| 2016 | 179 (12.8) | 134 (8.4) | ||
| 2017 | 203 (14.5) | 121 (7.6) | ||
| 2018 | 230 (16.4) | 104 (6.5) | ||
|
| ||||
| Age at cohort entry, mean (SD) | 85.8 (7.3) | 85.6 (7.5) | .44 | .35 |
|
| ||||
| Female, n (%) | 859 (61.2) | 991 (62.0) | .67 | .11 |
|
| ||||
| Weight (kg), mean (SD) | 65.9 (39.0) | 68.3 (44.4) | .02 | .64 |
|
| ||||
| Dependency in ADLs, mean (SD) | 5.7 (0.7) | 5.6 (0.8) | <.01 | .09 |
|
| ||||
| Health-care Utilization | ||||
|
| ||||
| Prescriptions in last year, mean (SD) | 12.6 (6.8) | 14.2 (7.5) | <.01 | .97 |
|
| ||||
| Emergency department visits in last year, n (%) | 905 (64.5) | 1149 (71.9) | <.01 | .22 |
|
| ||||
| Psychiatrist visits in last year, n (%) | 451 (32.2) | 315 (19.7) | <.01 | .20 |
|
| ||||
| Geriatrician visits in last year, n (%) | 30 (2.1) | 21 (1.3) | .08 | .23 |
|
| ||||
| Hospitalization in last 30 days, n (%) | 71 (5.1) | 127 (7.9) | <.01 | .02 |
|
| ||||
| Medications Use in the Past Year | ||||
|
| ||||
| Antihypertensives, n (%) | 793 (56.5) | 1028 (64.3) | <.01 | <.01 |
|
| ||||
| Antidepressants, n (%) | 603 (43.0) | 652 (40.8) | .22 | .46 |
|
| ||||
| Anticonvulsants, n (%) | 194 (13.8) | 282 (17.6) | <.01 | .88 |
|
| ||||
| Antipsychotics, n (%) | 601 (42.8) | 474 (29.6) | <.01 | .07 |
|
| ||||
| Cholinesterase inhibitors, n (%) | 292 (20.8) | 255 (16.0) | <.01 | .57 |
|
| ||||
| Antiarrhythmics, n (%) | 496 (35.4) | 712 (44.5) | <.01 | .65 |
|
| ||||
| Antiparkinsonian agents, n (%) | 101 (7.2) | 126 (7.9) | .48 | .31 |
|
| ||||
| Osteoporosis medications, n (%) | 235 (16.8) | 328 (20.5) | <.01 | .65 |
|
| ||||
| Diuretics, n (%) | 378 (26.9) | 569 (35.6) | <.01 | .76 |
|
| ||||
| Opioids, n (%) | 173 (12.3) | 240 (15.0) | .03 | .61 |
|
| ||||
| Antilipemics, n (%) | 444 (31.7) | 547 (34.2) | .14 | .81 |
|
| ||||
| Medical History | ||||
|
| ||||
| Atrial fibrillation/flutter, n (%) | 548 (39.1) | 791 (49.5) | <.01 | .94 |
|
| ||||
| Cerebrovascular disease, n (%) | 271 (19.3) | 378 (23.6) | <.01 | .82 |
|
| ||||
| Delusions or hallucinations, n (%) | 122 (8.7) | 82 (5.1) | <.01 | .11 |
|
| ||||
| Dementia, n (%) | 1261 (89.9) | 1177 (73.6) | <.01 | .04 |
|
| ||||
| Falls, n (%) | 400 (28.5) | 441 (27.6) | .57 | .94 |
|
| ||||
| Major osteoporotic fracture, n (%) | 293 (20.9) | 377 (23.6) | .08 | .53 |
|
| ||||
| Unsteady gaita, n (%) | 1308 (93.2) | 1532 (95.8) | <.01 | .51 |
|
| ||||
| Hypertension, n (%) | 1012 (72.1) | 1267 (79.2) | <.01 | .49 |
|
| ||||
| Parkinsonism, n (%) | 115 (8.2) | 143 (8.9) | .47 | .37 |
|
| ||||
| Psychotic disorder, n (%) | 83 (5.9) | 98 (6.1) | .81 | .77 |
|
| ||||
| Wheelchair ambulationb, n (%) | 863 (61.5) | 1080 (67.5) | <.01 | .49 |
|
| ||||
| Performance Scales | ||||
|
| ||||
| Cognitive performance scale, n (%) | ||||
| mild | 284 (20.2) | 674 (42.2) | <.01 | .15 |
| moderate | 841 (59.9) | 721 (45.1) | ||
| severe | 278 (19.8) | 204 (12.8) | ||
|
| ||||
| Aggressive behavior scale, n (%) | ||||
| none | 473 (33.7) | 887 (55.5) | <.01 | .17 |
| mild-moderate | 591 (42.1) | 540 (33.8) | ||
| severe | 339 (24.2) | 172 (10.8) | ||
|
| ||||
| Depression rating scale, n (%) | ||||
| no depressive symptoms | 744 (53.0) | 1018 (63.7) | <.01 | .47 |
| depressive symptoms | 659 (47.0) | 581 (36.3) | ||
<1% missing data.
4% missing data.
ADL = activities of daily living; kg = kilogram; n = number; SD = standard deviation.
In both intention-to-treat and per-protocol analyses, residents newly dispensed zopiclone experienced a similar rate of falls and major osteoporotic fractures compared to residents newly dispensed trazodone (crude hazard ratio 0.89, 0.70 to 1.13; intention-to-treat-weighted hazard ratio 1.15, 95% CI 0.90 to 1.48; per-protocol-weighted hazard ratio 0.85, 95% CI 0.60 to 1.21) (Table 2). Similarly, residents newly dispensed zopiclone experienced a similar rate of all-cause mortality compared to residents newly dispensed trazodone (crude hazard ratio 0.96, 95% CI 0.82 to 1.13; intention-to-treat-weighted hazard ratio 0.96, 95% CI 0.79 to 1.16; per-protocol-weighted hazard ratio 0.90, 95% CI 0.66 to 1.23) (Table 2). We did not conduct subgroup analyses because there were too few outcomes in exposure groups.
TABLE 2.
Intention-to-treat and per-protocol analyses of the comparative risk of (i) fall or major osteoporotic fracture and (ii) all-cause mortality for new users of zopiclone vs. trazodone within 180 days
| Trazodone (n=1,403) | Zopiclone (n=1,599) | |
|---|---|---|
| Injurious fall or major osteoporotic fracture | ||
|
| ||
| Events per 100 person-yrs (95% CI) | ||
|
| ||
| Intention-to-treat | 17.5 (17.3,17.7) | 17.7 (17.5,17.8) |
|
| ||
| Per protocol | 9.83 (9.70,9.95) | 7.48 (7.37,7.59) |
| Hazard ratio (95% CI) | ||
| Crude | 1 (ref) | 0.89 (0.70, 1.13) |
| Intention-to-treat | 1 (ref) | 1.15 (0.90, 1.48) |
| Per protocol | 1 (ref) | 0.85 (0.60, 1.21) |
|
| ||
| All-cause Mortality | ||
|
| ||
| Events per 100 person-yrs (95% CI) | ||
|
| ||
| Intention-to-treat | 38.8 (38.6,39.1) | 38.4 (38.2,38.7) |
|
| ||
| Per protocol | 13.4 (13.3,13.6) | 15.5 (15.3,15.6) |
|
| ||
| Hazard ratio (95% CI) | ||
| Crude | 1 (ref) | 0.96 (0.82,1.13) |
| Intention-to-treat | 1 (ref) | 0.96 (0.79, 1.16) |
| Per protocol | 1 (ref) | 0.90 (0.66, 1.23) |
CI = confidence interval; HR = hazard ratio; n = number; ref = reference.
DISCUSSION
In our cohort of older nursing home residents, we found a similar rate of injurious falls, major osteoporotic fractures, and death in residents newly dispensed trazodone or zopiclone. Our findings are important because trazodone is associated with a similar risk of injurious falls and fractures compared to benzodiazepines and atypical antipsychotics, and these outcomes are common and important to residents and the health-care system: the mean incidence of falls in nursing homes is 1.5 falls per bed per year and 4% of falls result in a fracture.(16,17,33) Further, our findings provide direct estimates of the comparative risk of harm associated with trazodone or zopiclone use, which will inform quality improvement initiatives and highlight potential dangers associated with medication substitution as clinicians are discouraged from administering certain medications without having access to feasible and evidence-based alternatives.
There is mounting evidence of harm associated with trazodone and zopiclone use in older adults.(16–18) Trazodone is associated with a similar risk of harm from injurious falls or fractures as benzodiazepines or atypical antipsychotics.(16,17) Similarly, a systematic review and meta-analysis describing harms associated with nonbenzodiazepine sedative hypnotic use in older adults identified an increased risk of fractures and injuries, but it did not identify an increased risk of falls associated with their use.(18) However, a subsequent cohort study by Westerlind and colleagues identified an increased risk of falls associated with zopiclone or zolpidem use.(34) Our findings bridge the gap between these bodies of literature to show that trazodone and zopiclone are associated with a similar risk of harm in nursing home residents. Further, our findings, combined with the work of others, demonstrate that trazodone, zopiclone, atypical antipsychotics, and benzodiazepines are all associated with a similar risk of harm from injurious falls and fractures in this patient population.(16,17)
Unlike benzodiazepines and antipsychotics, there has been less research directed at reducing the potentially inappropriate prescribing of alternative psychotropic medications, such as trazodone and nonbenzodiazepines sedative hypnotics (i.e., z-drugs), despite growing evidence of harm associated these alternative psychotropic medications.(13–15,35) A multi-hospital study demonstrated that a sedative-hypnotic reduction quality improvement bundle, which consisted of order set changes, audit feedback, pharmacist-enabled medication reviews, sleep hygiene, daily sleep huddles, and education, decreased benzodiazepine and nonbenzodiazepine sedative hypnotic use.(35) Other potentially efficacious interventions that could be implemented instead of psychotropic medications include multidisciplinary care, massage and touch therapy, outdoor activities, and cognitive stimulation. However institution- and individual-level barriers, including perceived lack of effectiveness, lack of knowledge of the dangers associated with psychotropic medication use, inadequate staffing, and clinician attitudes towards neuropsychiatric symptom management, prevent their widespread use.(6,36–40) Institution-level (e.g., hospitals, non-profit organizations, nursing homes) policies that 1) establish an interprofessional team responsible for psychotropic medication stewardship; 2) agree on psychotropic medication appropriateness criteria, educate care staff; 3) inform and involve family and friend carers; 4) establish a regular medication review process; 5) discontinue potentially inappropriate medications; and 6) implement nonpharmacologic interventions are needed to ensure that inappropriate administration of psychotropic medications is minimized, and feasible nonpharmacologic alternative interventions are available.(41)
Our study has limitations. The nonrandomized study design means there are potential residual confounders that could influence our results; however, we 1) used comprehensive RAI-MDS 2.0 data containing information about resident health status, including functional and cognitive impairment; 2) restricted our cohort to a population of older nursing home residents (in Alberta, Canada, where residents are supported in completing basic and instrumental activities of daily living, which ensures they receive medications); and 3) utilized a propensity score to balance between-group differences. We also limited our definition of falls to those requiring transfer to hospital (i.e., injurious falls), which means our reported estimate of falls or major osteoporotic fractures per 100 person-years is likely an underestimate of true incidence.
We found that zopiclone use was associated with a similar rate of injurious falls, major osteoporotic fractures, and all-cause mortality compared to trazodone in older nursing home residents. Our findings suggest that quality improvement initiatives should target trazodone and nonbenzodiazepine sedative hypnotic use in older adults, in addition to benzodiazepine and antipsychotic use; trazodone and zopiclone, despite their potential for harm, are not currently targeted by many of these initiatives.(2,10,14,35,42) Our findings, combined with earlier research, suggest that 1) trazodone, zopiclone, benzodiazepines, and atypical antipsychotics are all similarly harmful in older adults with respect to a risk of falling or sustaining a fracture; 2) trazodone and zopiclone should also be targeted by quality improvement initiatives to decrease their inappropriate use; 3) further comparative safety research is needed on other psychotropic medications to inform decision-making for patients and caregivers that will help them understand the risks and benefits of choosing pharmacologic or nonpharmacologic interventions for reducing neuropsychiatric symptoms; and, 4) greater resources should be dedicated to implementing feasible nonpharmacologic interventions to antipsychotics and benzodiazepines in nursing homes so we can avoid inappropriate substitution of potentially harmful alternative medications.(16–18,34)
ACKNOWLEDGEMENTS
Not applicable.
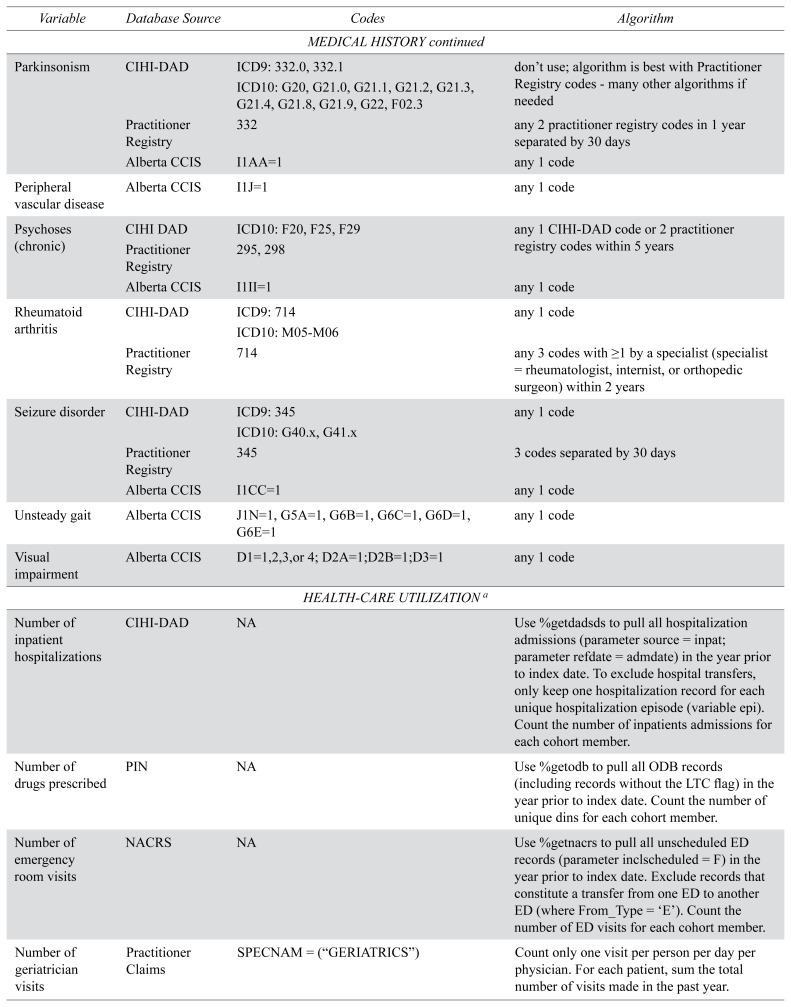
APPENDIX A. Variable definitions used for cohort creation
| Variable | Database Source | Codes | Algorithm |
|---|---|---|---|
| DEMOGRAPHICS | |||
|
| |||
| Age | Provincial Registry | NA | patient’s age at index date |
|
| |||
| Sex | Provincial Registry | NA | patient’s sex at index date |
|
| |||
| Year of cohort entry | PIN | NA | year of index date |
|
| |||
| Weight | Alberta CCIS | K2B | from most recent non-missing RAI assessment prior to index date |
|
| |||
| Receiving palliative care | CIHI-DAD | ICD10: Z515 or patserv=58 | any code in past 180 days prior to index date |
| Practitioner Registry | 03.05I, 03.05T, 03.05U | ||
| Alberta CCIS | P1AO=1 | ||
|
| |||
| Wheelchair ambulation | Alberta CCIS | G5B=1, G5C=1, G5D=1 | any 1 code from RAI assessment ≤45 days prior to index date |
|
| |||
| MEDICAL HISTORY | |||
|
| |||
| Alcohol use | CIHI-DAD | ICD9: 303,305 | any 1 code |
| ICD10: E244, E512, F10, G312, G621, G721, I426, K292, K70, K860, T510, X45, X65, Y15, Y573, Z502, Z714, Z721 | |||
| Practitioner Registry | 291, 303 | any 2 codes | |
|
| |||
| Atrial fibrillation/flutter | CIHI-DAD or NACRS | ICD9: 427.31, 427.32 | 1 CIHI-DAD/NACRS code OR 1 PIN claim for “Antiarrhythmics” (****correction: instead of 1 PIN claim) OR 1 practitioner registry claim for cardioversion [Z437] OR (PIN claim for anticoagulant + 1 practitioner registry code) |
| ICD10: I48 | |||
| Practitioner Registry | 427, 49.98X | ||
| PIN | see “Antiarrhythmics” and “Anticoagulants” | ||
|
| |||
| Cerebrovascular disease | CIHI-DAD | ICD10: 160.x (exclude 160.8), 161.x, 163.x (exclude 163.6), H34.1, 164.x, H34.0, G45.x (exclude G45.4) | any 1 code in DAD most responsible diagnosis position |
| Alberta CCIS | I1U | any 1 code | |
|
| |||
| Delusions/hallucinations | Alberta CCIS | J1E=1, J1I=1 | any 1 code (from most immediately antecedent RAI assessment prior to index date) |
|
| |||
| Dementia | CIHI-DAD | ICD9: 46.1, 290.0, 290.1, 290.2, 290.3, 290.4, 294.x, 331.0, 331.1, 331.5, 331.82 | 3 Practitioner registry codes at least 30 days apart in a 2 year period OR 1 PIN claim OR 1 CIHI-DAD code |
| ICD10: F00x, F01x, F02x, F03x, G30x | |||
| Practitioner Registry | 290, 331 | ||
| PIN | see “Cholinesterase Inhibitors” | ||
| Alberta CCIS | I1V=1, I1R=1, P1AN=1 | any 1 code | |
|
| |||
| Depression | CIHI-DAD | ICD10: F32.0, F32.1, F32.2, F32.3, F32.4, F32.5, F32.8, F32.9, F33.0, F33.1, F33.2, F33.3, F33.41, F33.42, F33.8, F33.9, F34.1, F34.8, F34.9, F38.0, F38.1, F38.8, F39, F41.2 | any 1 code |
| Practitioner Registry | 311 | ≥1 psychiatrist codes OR ≥2 family physician codes within 2 years for any ICD10 depression diagnoses in CIHI-DAD (see above) | |
| Alberta CCIS | I1GG=1 | any 1 code | |
|
| |||
| Diabetes mellitus | CIHI-DAD | ICD9: 250.x | any 1 code |
| Practitioner Registry | 250 | any 2 codes in a 2-year period | |
| Alberta CCIS | I1a=1 | any 1 code | |
|
| |||
| Injurious falls | NACRS or CIHI-DAD | ICD10: W00-W19 | Any 1 code in last year prior to index date |
|
| |||
| Hearing impairment | Alberta CCIS | C1=1,2,or3; C2A=1;C2B=1;C2C=1 | Any 1 code |
|
| |||
| Heart failure | CIHI-DAD | ICD9: 428 | Any 1 code |
| ICD10: I500, I501, I509 | Any 1 code | ||
| Practitioner Registry | 428 | Any 1 code + 2nd claim from CIHI-DAD or practitioner registry in 1 year | |
| Alberta CCIS | I1f=1 | Any 1 code | |
|
| |||
| Hypertension | Practitioner Registry | ICD9: 401x, 402x, 403x, 404x, 405x | Any 2 outpatient codes within 3 years |
| ICD10: I10x, I11x, I12x, I13x, I15x | |||
| CIHI-DAD | ICD9: 401x, 402x, 403x, 404x, 405x | ||
| ICD10: I10x, I11x, I12x, I13x, I15x | |||
| Alberta CCIS | I1i=1 | Any 1 code | |
|
| |||
| Ischemic heart disease | Practitioner Registry | 410, 412, 413, 48.12, 48.13, 48.14, 48.15, 51.61C, 51.59B, 51.59D, 51.59E, 51.59F | Any 2 codes (with 1 being in a hospital or emergency room setting) in a 1 year period |
| CIHI-DAD | CCI: 1IJ50, 1IJ57GQ, 1IJ76 | Any 1 code | |
|
| |||
| Kidney disease (chronic) | Alberta CCIS | P1AB=1 | any 2 codes |
|
| |||
| Liver disease (chronic) | Alberta CCIS | I1TT=1 | any 1 code |
|
| |||
| Major Osteoporotic Fracture | CIHI-DAD | ICD10: S321, S322, S323, S324, S325, S327, S328, S422, S432, S424, S720, S721; CCI 1VA53, 1VA73, 1VA74, 1VA80, 1VC73, 1VC74 | any 1 code |
| Practitioner Registry | 91.00A-E, 91.01, 91.04A-C, 91.08L, 91.08J, 91.10, 91.14, 91.30, 91.31, 91.34 | any 2 codes | |
| Alberta CCIS | J4C, I1M | any 1 code | |
|
| |||
| Malignancy | Alberta CCIS | I1RR=1, P1AA=1, P1AH=1 | any 1 code |
|
| |||
| Myocardial Infarction | CIHI-DAD or NACRS | ICD9: 410, 412 | any 3 practitioner registry codes in 1 year (with at least one being by a specialist [i.e., not a family doctor] OR within a hospital or emergency room) + any one CIHI-DAD code |
| ICD10: I21, I22, I25.2 | |||
| Practitioner Registry | ICD9: 410, 412 | ||
| ICD10: I21, I22, I25.2 | |||
|
| |||
| Osteoporosis | CIHI-DAD | ICD9: 733.01–733.09 | any 1 code in 5 years prior to index date |
| ICD10: M80, M81, M82 | |||
| PIN | See “Osteoporosis Medications” | any 1 code | |
| Practitioner Registry | 733 | any 1 code in 5 years prior to index date | |
| Alberta CCIS | I1O=1 | any 1 code | |
|
| |||
| Parkinsonism | CIHI-DAD | ICD9: 332.0, 332.1 | don’t use; algorithm is best with Practitioner Registry codes - many other algorithms if needed |
| ICD10: G20, G21.0, G21.1, G21.2, G21.3, G21.4, G21.8, G21.9, G22, F02.3 | |||
| Practitioner Registry | 332 | any 2 practitioner registry codes in 1 year separated by 30 days | |
| Alberta CCIS | I1AA=1 | any 1 code | |
|
| |||
| Peripheral vascular disease | Alberta CCIS | I1J=1 | any 1 code |
|
| |||
| Psychoses (chronic) | CIHI DAD | ICD10: F20, F25, F29 | any 1 CIHI-DAD code or 2 practitioner registry codes within 5 years |
| Practitioner Registry | 295, 298 | ||
| Alberta CCIS | I1II=1 | any 1 code | |
|
| |||
| Rheumatoid arthritis | CIHI-DAD | ICD9: 714 | any 1 code |
| ICD10: M05-M06 | |||
| Practitioner Registry | 714 | any 3 codes with ≥1 by a specialist (specialist = rheumatologist, internist, or orthopedic surgeon) within 2 years | |
|
| |||
| Seizure disorder | CIHI-DAD | ICD9: 345 | any 1 code |
| ICD10: G40.x, G41.x | |||
| Practitioner Registry | 345 | 3 codes separated by 30 days | |
| Alberta CCIS | I1CC=1 | any 1 code | |
|
| |||
| Unsteady gait | Alberta CCIS | J1N=1, G5A=1, G6B=1, G6C=1, G6D=1, G6E=1 | any 1 code |
|
| |||
| Visual impairment | Alberta CCIS | D1=1,2,3,or 4; D2A=1;D2B=1;D3=1 | any 1 code |
|
| |||
| HEALTH-CARE UTILIZATION a | |||
|
| |||
| Number of inpatient hospitalizations | CIHI-DAD | NA | Use %getdadsds to pull all hospitalization admissions (parameter source = inpat; parameter refdate = admdate) in the year prior to index date. To exclude hospital transfers, only keep one hospitalization record for each unique hospitalization episode (variable epi). Count the number of inpatients admissions for each cohort member. |
|
| |||
| Number of drugs prescribed | PIN | NA | Use %getodb to pull all ODB records (including records without the LTC flag) in the year prior to index date. Count the number of unique dins for each cohort member. |
|
| |||
| Number of emergency room visits | NACRS | NA | Use %getnacrs to pull all unscheduled ED records (parameter inclscheduled = F) in the year prior to index date. Exclude records that constitute a transfer from one ED to another ED (where From_Type = ‘E’). Count the number of ED visits for each cohort member. |
|
| |||
| Number of geriatrician visits | Practitioner Claims | SPECNAM = (“GERIATRICS”) | Count only one visit per person per day per physician. For each patient, sum the total number of visits made in the past year. |
|
| |||
| Number of psychiatrist visits | Practitioner Claims | SPECNAM = (“PSYCHIATRY”) | Count only one visit per person per day per physician. For each patient, sum the total number of visits made in the past year. |
|
| |||
| PERFORMANCE SCALES | |||
|
| |||
| Cognitive performance scale | Alberta CCIS | B1=0,1; B4=0,1,2,3; G1h=0,1,2; B2a=0,1; C4=0,1,2,3 | from RAI assessment ≤45 days prior to index date |
|
| |||
| Dependency in ≥1 activities of daily living (ADL) | Alberta CCIS | G1a=1,2,3, or 4; G1b=1,2,3, or 4; G1c=1,2,3, or 4; G1d=1,2,3, or 4; G1e=1,2,3, or 4; G1f=1,2,3, or 4; G1g=1,2,3, or 4; G1h=1,2,3, or 4; G1i=1,2,3, or 4; G1j=1,2,3, or 4; G2=1,2,3 or 4 | any 1 code equal to 1, 2, 3, or 4 from (one of G1a,G1b,G1c,G1d,G1e, G1f) OR G1g, G1h, G1i, G1j, G2 (from RAI assessment ≤30 days prior to index date) |
|
| |||
| Dependency in ADLs (mean, SD) | Alberta CCIS | G1a=1,2,3, or 4; G1b=1,2,3, or 4; G1c=1,2,3, or 4; G1d=1,2,3, or 4; G1e=1,2,3, or 4; G1f=1,2,3, or 4; G1g=1,2,3, or 4; G1h=1,2,3, or 4; G1i=1,2,3, or 4; G1j=1,2,3, or 4; G2=1,2,3 or 4 | if (G1a=1,2,3, or 4) OR (G1b=1,2,3, or 4) OR (G1c=1,2,3, or 4) OR (G1d=1,2,3, or 4) OR (G1e=1,2,3, or 4) OR (G1f=1,2,3, or 4) then patient is dependent for mobility (mobility=1); if G1g=1,2,3, or 4 then dressing=1; if G1h=1,2,3, or 4 then eating=1; if G1i=1,2,3, or 4 then toilet=1; if G1j=1,2,3, or 4 then hygiene=1; if G2=1,2,3 or 4 then bathing=1; sum of mobility + dressing + eating + toilet + hygiene + bathing = dependency in ADLs (from RAI assessment ≤30 days prior to index date) |
|
| |||
| Depression symptom scale | Alberta CCIS | E1a=0,1,2; E1d=0,1,2; E1f=0,1,2; E1h=0,1,2; E1i=0,1,2; E1l=0,1,2; E1m=0,1,2 | from RAI assessment ≤45 days prior to index date |
|
| |||
| Aggressive behaviour scale | Alberta CCIS | E4b=0,1,2,3; E4c=0,1,2,3; E4d=0,1,2,3; E4e=0,1,2,3 | from RAI assessment ≤45 days prior to index date |
|
| |||
| OUTCOMES | |||
|
| |||
| Major osteoporotic fracture | NACRS or CIHI-DAD | ICD 10: S321, S322, S323, S324, S325, S327, S328, S422, S432, S424, S720, S721; CCI 1VA53, 1VA73, 1VA74, 1VA80, 1VC73, 1VC74 | admission date (admdate) is within 180 days following index date; combine NACRS and DAD records and keep the record for the first (earliest) fracture within the 180 day follow-up period. |
| Practitioner Claims | 91.00A-E, 91.01, 91.04A-C, 91.08L, 91.08J, 91.10, 91.14, 91.30, 91.31, 91.34 | any 2 codes within the 180 day follow-up period | |
|
| |||
| Injurious fall | NACRS or CIHI-DAD | ICD 10: W00-W19 | admission date (admdate) is within 180 days following index date; combine NACRS and DAD records and keep the record for the first (earliest) fall within the 180 day follow-up period. |
For all hospitalizations using DAD defined above, use:
DAD source: inpatient; Diagnosis type: All diagnosis types except where specifically indicated otherwise;
Exclude suspected diagnoses.
Data Sources
The Alberta Continuing Care Information System (CCIS) contains comprehensive information about residents of nursing homes, including their functional dependence, severity of cognitive impairment, and symptoms relating to depression and pain. Dispensed prescription medications were identified in the Pharmacy Information Network (PIN). The Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD) was used to identify all inpatient hospitalizations. The National Ambulatory Care Reporting System (NACRS) database was used to identify all emergency department visits. All information relating to physician payment claims was obtained from the Practitioner Claims database. All patient demographic information (including date of death, if appropriate) was obtained from the Provincial Registry.
APPENDIX B.
TABLE B1.
Complete baseline characteristics of a cohort of older adults dispensed trazodone or zopiclone (number [n] =3,002)
| Characteristic | Trazodone (n=1403) | Zopiclone (n=1599) | Crude p Value | Weighted p Value |
|---|---|---|---|---|
| DEMOGRAPHICS | ||||
|
| ||||
| Year of cohort entry, n (%) | ||||
| 2010 | 93 (6.6) | 275 (17.2) | <.01 | .01 |
| 2011 | 103 (7.3) | 216 (13.5) | ||
| 2012 | 112 (8.0) | 222 (13.9) | ||
| 2013 | 144 (10.3) | 188 (11.8) | ||
| 2014 | 135 (9.6) | 169 (10.6) | ||
| 2015 | 204 (14.5) | 170 (10.6) | ||
| 2016 | 179 (12.8) | 134 (8.4) | ||
| 2017 | 203 (14.5) | 121 (7.6) | ||
| 2018 | 230 (16.4) | 104 (6.5) | ||
|
| ||||
| Age at cohort entry, mean (SD) | 85.8 (7.3) | 85.6 (7.5) | .44 | .35 |
|
| ||||
| Female, n (%) | 859 (61.2) | 991 (62.0) | .67 | .11 |
|
| ||||
| Weight (kg), mean (SD) | 65.9 (39.0) | 68.3 (44.4) | .02 | .64 |
|
| ||||
| Dependency in ≥1 ADLs, n (%) | 1001 (99.9) | 1592 (99.6) | .22 | .33 |
|
| ||||
| Dependency in ADLs, mean (SD) | 5.7 (0.7) | 5.6 (0.8) | <.01 | .09 |
|
| ||||
| HEALTH-CARE UTILIZATION | ||||
|
| ||||
| Prescriptions in last year, mean (SD) | 12.6 (6.8) | 14.2 (7.5) | <.01 | .97 |
|
| ||||
| Emergency department visits in last year, n (%) | 905 (64.5) | 1149 (71.9) | <.01 | .22 |
|
| ||||
| Psychiatrist visits in last year, n (%) | 451 (32.2) | 315 (19.7) | <.01 | .2 |
|
| ||||
| Geriatrician visits in last year, n (%) | 30 (2.1) | 21 (1.3) | .08 | .23 |
|
| ||||
| Inpatient hospitalizations in 30 days prior to exposure drugs, n (%) | 780 (55.6) | 1035 (64.7) | <.01 | .04 |
|
| ||||
| Hospitalization in 30 days prior to exposure drugs, n (%) | 71 (5.1) | 127 (7.9) | <.01 | .02 |
|
| ||||
| Nursing home admission in 30 days prior to exposure drugs, n (%) | 509 (36.3) | 560 (35.0) | .47 | .05 |
|
| ||||
| MEDICATIONS | ||||
|
| ||||
| Antihypertensives, n (%) | 793 (56.5) | 1028 (64.3) | <.01 | <.01 |
|
| ||||
| Anticoagulants, n (%) | 256 (18.3) | 364 (22.8) | <.01 | .71 |
|
| ||||
| Antidepressants, n (%) | 603 (43.0) | 652 (40.8) | .22 | .46 |
|
| ||||
| Anticonvulsants, n (%) | 194 (13.8) | 282 (17.6) | <.01 | .88 |
|
| ||||
| Antipsychotics, n (%) | 601 (42.8) | 474 (29.6) | <.01 | .07 |
|
| ||||
| Cholinesterase inhibitors, n (%) | 292 (20.8) | 255 (16.0) | <.01 | .57 |
|
| ||||
| Memantine, n (%) | 66 (4.7) | 26 (1.6) | <.01 | .69 |
|
| ||||
| Antiplatelet agents, n (%) | 583 (41.6) | 733 (45.8) | .02 | .37 |
|
| ||||
| Antiarrhythmics, n (%) | 496 (35.4) | 712 (44.5) | <.01 | .65 |
|
| ||||
| Antiparkinsonian agents, n (%) | 101 (7.2) | 126 (7.9) | .48 | .31 |
|
| ||||
| Osteoporosis medications, n (%) | 235 (16.8) | 328 (20.5) | <.01 | .65 |
|
| ||||
| Diuretics, n (%) | 378 (26.9) | 569 (35.6) | <.01 | .76 |
|
| ||||
| Glucocorticoids, n (%) | 109 (7.8) | 169 (10.6) | <.01 | .42 |
|
| ||||
| Hormone therapies, n (%) | 42 (3.0) | 41 (2.6) | .47 | 0.86 |
|
| ||||
| Antihyperglycemic medications, n (%) | 240 (17.1) | 299 (18.7) | .26 | .39 |
|
| ||||
| NSAIDs, n (%) | 100 (7.1) | 147 (9.2) | .04 | .65 |
|
| ||||
| Opioids, n (%) | 173 (12.3) | 240 (15.0) | .03 | .61 |
|
| ||||
| Antilipemics, n (%) | 444 (31.7) | 547 (34.2) | .14 | .81 |
|
| ||||
| Acid reflux medications, n (%) | 550 (39.2) | 725 (45.3) | <.01 | .03 |
|
| ||||
| Urinary incontinence treatments, n (%) | 250 (17.8) | 352 (22.0) | <.01 | <.01 |
|
| ||||
| MEDICAL HISTORY | ||||
|
| ||||
| Alcohol use disorder, n (%) | 90 (6.4) | 82 (5.1) | .13 | .06 |
|
| ||||
| Atrial fibrillation/flutter, n (%) | 548 (39.1) | 791 (49.5) | <.01 | .94 |
|
| ||||
| Cerebrovascular disease, n (%) | 271 (19.3) | 378 (23.6) | <.01 | .82 |
|
| ||||
| Delusions or hallucinations, n (%) | 122 (8.7) | 82 (5.1) | <.01 | .11 |
|
| ||||
| Depression, n (%) | 634 (45.2) | 723 (45.2) | .99 | .89 |
|
| ||||
| Dementia, n (%) | 1261 (89.9) | 1177 (73.6) | <.01 | .04 |
| Diabetes mellitus, n (%) | 384 (27.4) | 464 (29.0) | .32 | .45 |
| Injurious falls, n (%) | 400 (28.5) | 441 (27.6) | .57 | .94 |
|
| ||||
| Major osteoporotic fracture, n (%) | 293 (20.9) | 377 (23.6) | .08 | .53 |
|
| ||||
| Heart failure, n (%) | 251 (17.9) | 420 (26.3) | <.01 | .51 |
|
| ||||
| Unsteady gaita, n (%) | 1308 (93.2) | 1532 (95.8) | <.01 | .51 |
|
| ||||
| Hearing impairmentb, n (%) | 710 (50.6) | 792 (49.5) | .56 | .06 |
|
| ||||
| Myocardial Infarction, n (%) | 42 (3.0) | 102 (6.4) | <.01 | <.01 |
|
| ||||
| Hypertension, n (%) | 1012 (72.1) | 1267 (79.2) | <.01 | .49 |
|
| ||||
| Chronic liver disease, n (%) | 10 (0.7) | 12 (0.8) | .90 | .97 |
|
| ||||
| Malignancyb, n (%) | 108 (7.7) | 109 (6.8) | .35 | .52 |
|
| ||||
| Parkinsonism, n (%) | 115 (8.2) | 143 (8.9) | .47 | .37 |
|
| ||||
| Peripheral vascular diseaseb, n (%) | 45 (3.2) | 75 (4.7) | .04 | .83 |
|
| ||||
| Psychotic disorder, n (%) | 83 (5.9) | 98 (6.1) | .81 | .77 |
|
| ||||
| Rheumatoid arthritis, n (%) | 21 (1.5) | 32 (2.0) | .30 | .27 |
|
| ||||
| Seizure disorder, n (%) | 52 (3.7) | 65 (4.1) | .61 | .62 |
|
| ||||
| Visual impairmentb, n (%) | 1148 (81.8) | 1299 (81.2) | .68 | .59 |
|
| ||||
| Wheelchair ambulationb, n (%) | 863 (61.5) | 1080 (67.5) | <.01 | .49 |
|
| ||||
| Charlson comorbidity index, mean (SD) | 2.5 (2.1) | 3.1 (2.5) | <.01 | .1 |
|
| ||||
| PERFORMANCE SCALES | ||||
|
| ||||
| Cognitive performance scale, n (%) | ||||
| mild | 284 (20.2) | 674 (42.2) | <.01 | .15 |
| moderate | 841 (59.9) | 721 (45.1) | ||
| severe | 278 (19.8) | 204 (12.8) | ||
|
| ||||
| Aggressive behavior scale, n (%) | ||||
| none | 473 (33.7) | 887 (55.5) | <.01 | .17 |
| mild-moderate | 591 (42.1) | 540 (33.8) | ||
| severe | 339 (24.2) | 172 (10.8) | ||
|
| ||||
| Depression rating scale, n (%) | ||||
| no depressive symptoms | 744 (53.0) | 1018 (63.7) | <.01 | .47 |
| depressive symptoms | 659 (47.0) | 581 (36.3) | ||
<1% missing data.
4% missing data.
ADL = activities of daily living; kg = kilogram; NSAIDs = nonsteroidal anti-inflammatory drugs; n = number; SD = standard deviation.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood the Canadian Geriatrics Journal’s policy on conflicts of interest disclosure and declare there are no conflicts of interest.
FUNDING
This research did not receive any funding from agencies in the public, commercial, or not-for-profit sectors. In-kind support was provided by the Alberta SPOR Support Unit Data and Research Services team.
REFERENCES
- 1.Webster L, Costafreda Gonzalez S, Stringer A, Lineham A, Budgett J, Kyle S, et al. Measuring the prevalence of sleep disturbances in people with dementia living in care homes: a systematic review and meta-analysis. Sleep. 2020;43(4):zsz251. doi: 10.1093/sleep/zsz251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fog AF, Straand J, Engedal K, Blix HS. Drug use differs by care level. A cross-sectional comparison between older people living at home or in a nursing home in Oslo, Norway. BMC Geriatr. 2019;19(1):49. doi: 10.1186/s12877-019-1064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee DA, Robins LM, Bell JS, Srikanth V, Mohler R, Hill KD, et al. Prevalence and variability in use of physical and chemical restraints in residential aged care facilities: A systematic review and meta-analysis. Int J Nurs Stud. 2021;117:103856. doi: 10.1016/j.ijnurstu.2020.103856. [DOI] [PubMed] [Google Scholar]
- 4.Sivertsen B, Omvik S, Pallesen S, Bjorvatn B, Havik OE, Kvale G, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults. JAMA. 2006;295(24):2851–58. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 5.Brown CA, Wielandt P, Wilson D, Jones A, Crick K. Healthcare providers’ knowledge of disordered sleep, sleep assessment tools, and nonpharmacological sleep interventions for persons living with dementia: a national survey. Sleep Disord. 2014;2014:286274. doi: 10.1155/2014/286274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watt JA, Goodarzi Z, Veroniki AA, Nincic V, Khan PA, Ghassemi M, et al. Comparative efficacy of interventions for aggressive and agitated behaviors in dementia: a systematic review and network meta-analysis. Ann Intern Med. 2019;171(9):633–42. doi: 10.7326/M19-0993. [DOI] [PubMed] [Google Scholar]
- 7.Vasudev A, Shariff SZ, Liu K, Burhan AM, Herrmann N, Leonard S, et al. Trends in Psychotropic Dispensing Among Older Adults with Dementia Living in Long-Term Care Facilities: 2004–213. Am J Geriatr Psychiatry. 2015;23(12):1259–69. doi: 10.1016/j.jagp.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Gerlach LB, Kales HC, Kim HM, Bynum JPW, Chiang C, Strominger J, et al. Trends in antipsychotic and mood stabilizer prescribing in long-term care in the US: 2011–2014. J Am Med Dir Assoc. 2020;21(11):1629–35. doi: 10.1016/j.jamda.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donegan K, Black N, Livingston G, Banerjee S, Burns A. Trends in diagnosis and treatment for people with dementia in the UK from 2005 to 2015: a longitudinal retrospective cohort study. Lancet Public Health. 2017;2(3):e149–56. doi: 10.1016/S2468-2667(17)30031-2. [DOI] [PubMed] [Google Scholar]
- 10.Iaboni A, Bronskill SE, Reynolds KB, Wang X, Rochon PA, Herrmann N, et al. Changing pattern of sedative use in older adults: a population-based cohort study. Drugs Aging. 2016;33(7):523–33. doi: 10.1007/s40266-016-0380-3. [DOI] [PubMed] [Google Scholar]
- 11.Richardson K, Savva GM, Boyd PJ, Aldus C, Maidment I, Pakpahan E, et al. Non-benzodiazepine hypnotic use for sleep disturbance in people aged over 55 years living with dementia: a series of cohort studies. Health Technol Assess. 2021;25(1):1–202. doi: 10.3310/hta25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukacisinova A, Fialova D, Peel NM, Hubbard RE, Brkic J, Onder G, et al. The prevalence and prescribing patterns of benzodiazepines and Z-drugs in older nursing home residents in different European countries and Israel: retrospective results from the EU SHELTER study. BMC Geriatr. 2021;21(1):277. doi: 10.1186/s12877-021-02213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AMDA. Ten things clinicians and patients should question. Columbia, MD: AMDA Choosing Wisely; 2015. [Google Scholar]
- 14.Caswell L, Hoosen I, Vassilas CA, Haque S. Reducing hypnotic use on two older adult functional wards: an effective audit? Psychiatr Bull. 2006;30(3):95–97. doi: 10.1192/pb.30.3.95. [DOI] [Google Scholar]
- 15.Joglekar NN, Patel Y, Keller MS. Evaluation of clinical decision support to reduce sedative-hypnotic prescribing in older adults. Appl Clin Inform. 2021;12(3):436–44. doi: 10.1055/s-0041-1730030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bronskill SE, Campitelli MA, Iaboni A, Herrmann N, Guan J, Maclagan LC, et al. Low-dose trazodone, benzodiazepines, and fall-related injuries in nursing homes: a matched-cohort study. J Am Geriatr Soc. 2018;66(10):1963–71. doi: 10.1111/jgs.15519. [DOI] [PubMed] [Google Scholar]
- 17.Watt JA, Gomes T, Bronskill SE, Huang A, Austin PC, Ho JM, et al. Comparative risk of harm associated with trazodone or atypical antipsychotic use in older adults with dementia: a retrospective cohort study. CMAJ. 2018;190(47):E1376–83. doi: 10.1503/cmaj.180551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treves N, Perlman A, Kolenberg Geron L, Asaly A, Matok I. Z-drugs and risk for falls and fractures in older adults-a systematic review and meta-analysis. Age Ageing. 2018;47(2):201–08. doi: 10.1093/ageing/afx167. [DOI] [PubMed] [Google Scholar]
- 19.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–24. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Langan SM, Schmidt SA, Wing K, Ehrenstein V, Nicholls SG, Filion KB, et al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE) BMJ. 2018;363:k3532. doi: 10.1136/bmj.k3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Government of Alberta. Alberta Health Continuing Care. Alberta long-term care resident profile 2016/2017. 2018. Available from: https://open.alberta.ca/dataset/90c128a6-3a8e-4c6e-8591-58e88fe6b6f9/resource/894a3a9c-8999-4487-b7e5-2850b3bb1a2e/download/cc-ltc-resident-profile-2017.pdf.
- 22.Juurlink D, Preyra C, Croxford R, Chong A, Austin P, Tu J, et al. Canadian Institute for Health Information Discharge Abstract Database: a validation study. Toronto: Institute for Clinical Evaluative Sciences; 2006. [Google Scholar]
- 23.Wodchis WP, Naglie G, Teare GF. Validating diagnostic information on the minimum data set in ontario hospital-based long-term care. Med Care. 2008;46(8):882–87. doi: 10.1097/MLR.0b013e3181789471. [DOI] [PubMed] [Google Scholar]
- 24.Gibson D, Richards H, Chapman A. The national ambulatory care reporting system: factors that affect the quality of its emergency data. Int J Inform Qual. 2008;2(2):97–114. doi: 10.1504/IJIQ.2008.022958. [DOI] [Google Scholar]
- 25.Hirdes JP, Ljunggren G, Morris JN, Frijters DH, Finne Soveri H, Gray L, et al. Reliability of the interRAI suite of assessment instruments: a 12-country study of an integrated health information system. BMC Health Serv Res. 2008;8(1):277. doi: 10.1186/1472-6963-8-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welk B, McArthur E, Fraser LA, Hayward J, Dixon S, Hwang YJ, et al. The risk of fall and fracture with the initiation of a prostate-selective alpha antagonist: a population based cohort study. BMJ. 2015;351:h5398. doi: 10.1136/bmj.h5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamblyn R, Reid T, Mayo N, McLeod P, Churchill-Smith M. Using medical services claims to assess injuries in the elderly: sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol. 2000;53(2):183–94. doi: 10.1016/S0895-4356(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 28.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Austin PC, Stuart EA. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat Methods Med Res. 2017;26(4):1654–70. doi: 10.1177/0962280215584401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee BK, Lessler J, Stuart EA. Weight trimming and propensity score weighting. PLoS One. 2011;6(3):e18174. doi: 10.1371/journal.pone.0018174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–09. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642–55. doi: 10.1002/sim.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubenstein LZ, Josephson KR, Robbins AS. Falls in the nursing home. Ann Intern Med. 1994;121(6):442–51. doi: 10.7326/0003-4819-121-6-199409150-00009. [DOI] [PubMed] [Google Scholar]
- 34.Westerlind B, Ostgren CJ, Molstad S, Midlov P, Hagg S. Use of non-benzodiazepine hypnotics is associated with falls in nursing home residents: a longitudinal cohort study. Aging Clin Exp Res. 2019;31(8):1078–95. doi: 10.1007/s40520-018-1056-0. [DOI] [PubMed] [Google Scholar]
- 35.Soong C, Ethier C, Lee Y, Othman D, Burry L, Wu PE, et al. Reducing sedative-hypnotics among hospitalized patients: a multi-centered study. J Gen Intern Med. 2022;37:2345–50. doi: 10.1007/s11606-021-07292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmons SF, Bonnett KR, Hollingsworth E, Kim J, Powers J, Habermann R, et al. Reducing antipsychotic medication use in nursing homes: a qualitative study of nursing staff perceptions. Gerontologist. 2018;58(4):e239–e50. doi: 10.1093/geront/gnx083. [DOI] [PubMed] [Google Scholar]
- 37.Walsh KA, Sinnott C, Fleming A, Mc Sharry J, Byrne S, Browne J, et al. Exploring antipsychotic prescribing behaviors for nursing home residents with dementia: a qualitative study. J Am Med Dir Assoc. 2018;19(11):948–58. doi: 10.1016/j.jamda.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Kuntz J, Kouch L, Christian D, Peterson PL, Gruss I. Barriers and facilitators to the deprescribing of nonbenzodiazepine sedative medications among older adults. Perm J. 2018;22:17–157. doi: 10.7812/TPP/17-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watt JA, Goodarzi Z, Veroniki AA, Nincic V, Khan PA, Ghassemi M, et al. Comparative efficacy of interventions for reducing symptoms of depression in people with dementia: systematic review and network meta-analysis. BMJ. 2021;372:n532. doi: 10.1136/bmj.n532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halek M, Reuther S, Muller-Widmer R, Trutschel D, Holle D. Dealing with the behaviour of residents with dementia that challenges: a stepped-wedge cluster randomized trial of two types of dementia-specific case conferences in nursing homes (FallDem) Int J Nurs Stud. 2020;104:103435. doi: 10.1016/j.ijnurstu.2019.103435. [DOI] [PubMed] [Google Scholar]
- 41.BC Patient Safety & Quality Council. When psychosis isn’t the diagnosis: a toolkit for reducing inappropriate use of antipsychotics in long term care. 2019. Available from: https://bcpsqc.ca/resource/when-psychosis-isnt-the-diagnosis-a-toolkit-for-reducing-inappropriate-use-of-antipsychotics-in-long-term-care/
- 42.Farrell B, Tsang C, Raman-Wilms L, Irving H, Conklin J, Pottie K. What are priorities for deprescribing for elderly patients? Capturing the voice of practitioners: a modified delphi process. PLoS One. 2015;10(4):e0122246. doi: 10.1371/journal.pone.0122246. [DOI] [PMC free article] [PubMed] [Google Scholar]