Abstract
Simple Summary
Through their regulatory effects on gene expression, histone acetyltransferases have been implicated in the normal physiological activities and genesis of cancer. Genetic aberrations of CREBBP/EP300 have been observed in various types of solid tumors and hematologic malignancies, making them serve as promising therapeutic targets. Here, this review discusses the critical role of CREBBP/EP300 in normal hematopoiesis and also provides a comprehensive overview of how they contribute to the genesis and progression of hematologic malignancies. The impact of different CREBBP/EP300 inhibitors and histone deacetylase inhibitors on targeting therapeutic potential, alleviating chemotherapy resistance, and enhancing immunotherapeutic potential has also been reviewed.
Abstract
Disordered histone acetylation has emerged as a key mechanism in promoting hematological malignancies. CREB-binding protein (CREBBP) and E1A-binding protein P300 (EP300) are two key acetyltransferases and transcriptional cofactors that regulate gene expression by regulating the acetylation levels of histone proteins and non-histone proteins. CREBBP/EP300 dysregulation and CREBBP/EP300-containing complexes are critical for the initiation, progression, and chemoresistance of hematological malignancies. CREBBP/EP300 also participate in tumor immune responses by regulating the differentiation and function of multiple immune cells. Currently, CREBBP/EP300 are attractive targets for drug development and are increasingly used as favorable tools in preclinical studies of hematological malignancies. In this review, we summarize the role of CREBBP/EP300 in normal hematopoiesis and highlight the pathogenic mechanisms of CREBBP/EP300 in hematological malignancies. Moreover, the research basis and potential future therapeutic implications of related inhibitors were also discussed from several aspects. This review represents an in-depth insight into the physiological and pathological significance of CREBBP/EP300 in hematology.
Keywords: CREBBP, EP300, acetylation, hematological malignancy, chemoresistance, immunotherapy
1. Introduction
Normal gene expression is indispensable for cells to perform hematopoiesis and other various physiological activities. Histone acetyltransferases (HATs) are a group of epigenetic modifying enzymes that play vital roles in the regulation of gene transcription. Among the HAT family, the acetyltransferase CREB-binding protein (CREBBP; also known as CBP or KAT2A) and E1A-binding protein P300 (EP300; also known as P300 or KAT2B) are two closely related regulators. Both are widely expressed within and outside the hematopoietic system and serve as tumor activators or suppressors depending on the situation [1,2]. CREBBP/EP300 regulate various key physiological functions, including cell apoptosis [3], proliferation and differentiation [4,5], DNA repair [6,7], and somatic cell reprogramming [8]. CREBBP/EP300 are essential for the maintenance of normal hematopoiesis. However, a growing number of studies have shown that aberrant expression of CREBBP/EP300 is associated with tumorigenesis and the progression of hematological malignancies. Recently, abundant CREBBP/EP300 inhibitors have been developed, representing emerging tools for clinical intervention in human diseases [9,10]. Here, after providing a brief overview of the molecular characteristics and functions of CREBBP/EP300, we summarize the current knowledge about the implications of CREBBP/EP300 in normal hematopoiesis and hematological malignancies. Finally, we discuss some prospective therapeutic strategies for CREBBP/EP300 inhibitors in hematological malignancies.
1.1. Molecular Domains and Characteristics of CREBBP/EP300
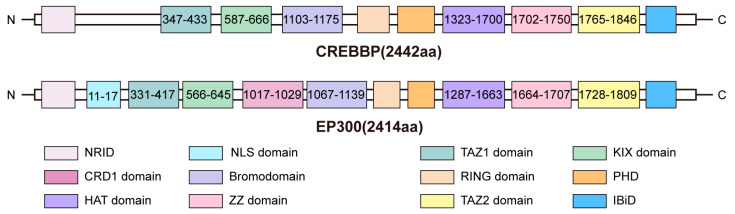
CREBBP and paralogous EP300 are two evolutionarily conserved and functionally related enzymes, and both share a similar domain architecture consisting of a diverse set of protein- and DNA-binding modules [10] (Figure 1). The histone acetyltransferase (HAT) domain of CREBBP/EP300 interacts with histone and is the site of acyl-CoA binding, which provides the basis for the acetylation state of multiple interacting proteins. A variety of short-chain acyl-CoA variants can serve as substrates for EP300, such as propionyl-CoA, crotonyl-CoA, and butyryl-CoA. The X-ray crystal structure of EP300 bound to these acyl-CoAs has been determined, suggesting that the active site of EP300 could accommodate longer acyl chains without major structural rearrangement [11]. Furthermore, CREBBP/EP300 and histone deacetylases (HDACs) antagonistically regulate mRNA or protein stability through acetylation or deacetylation. For example, CREBBP/EP300 acetylate the exoribonuclease CAF1, a catalytic subunit of the CCR4-CAF1-NOT deadenylase complex, thereby promoting deadenylation and degradation of poly(A) RNA, while HDAC-mediated deacetylation enhances mRNA stability [12].
Figure 1.
Molecular domains of the human CREBBP/EP300. CREBBP and EP300 have similar domain components and also share high sequence homology. Each domain is represented by a different color, and the number represents the location of the domain. NRID: nuclear receptor interaction domain; NLS: nuclear location signal; TAZ1: transcriptional-adaptor zinc-finger domain 1; KIX: kinase-inducible domain of CREB-interacting domain; CRD1: cell cycle regulatory domain 1; RING: really interesting new gene; PHD: plant homeodomain; HAT: histone acetyltransferase; ZZ: ZZ-type zinc finger; TAZ2: transcriptional-adaptor zinc-finger domain 2; IBiD: interferon-binding domain.
The bromodomain (BRD) of CREBBP/EP300 facilitates histone acetylation independent of chromatin localization. CREBBP/EP300 BRD inhibition leads to an enhancer-bias reduction in CDK9 at chromatin, indicating impaired positive transcription elongation factor (p-TEFb) complex recruitment and a concomitant reduction in RNA polymerase II at enhancers [13]. High-resolution X-ray crystal structures of the BRD-PHD tandem module of human CREBBP in complex with a lysine-acetylated histone H4 peptide have been solved at 1.9Å and 1.83Å resolution, respectively [14]. Furthermore, the autoacetylation of CREBBP is pivotal for the interaction of its BRD with H3K56ac on free histones with higher affinity than other monoacetylated binding partners. The interaction between CREBBP BRD and the histone chaperone anti-silencing function 1 (ASF1) is important for H3K56ac [15]. In diffuse large B-cell lymphoma (DLBCL), CREBBP/EP300 mutations have been found to primarily affect the HAT-lysine acetyltransferase 11 (KAT11) domain [16,17]. The cell cycle regulatory domain 1 (CRD1) domain of EP300 mediates transcriptional repression. The ZZ-type zinc finger (ZZ) domains of CREBBP and EP300 have different activities leading to different biological functions. The ZZ domain of EP300 has been identified as a novel member of the family of epigenetic readers, which selects the histone H3 tail to promote H3K27ac and H3K18ac [18]. Moreover, destruction of the CREBBP really interesting new gene (RING) domain is a recurrent pathogenic event caused by various types of somatic alterations in acute lymphocytic leukemia (ALL) [19]. It has been reported that the IRF3 and STAT1 dimerization enable trans-autoacetylation of EP300 in a highly conserved and intrinsically disordered autoinhibitory lysine-rich loop (AIL), further causing HAT activation. Ortega et al. also found that substrate access to the active site of HAT involved a rearrangement of an autoinhibitory RING domain [20]. The crystal structure of the ternary complex formed by the kinase-inducible domain of CREB-interacting (KIX) domain of CREBBP/EP300, the activation domain of human T-cell leukemia virus I basic leucine zipper protein (HTLV-1 HBZ), and the activation domain of c-Myb has been reported. This complex has been associated with hematopoietic differentiation and the development of adult T-cell leukemia [21].
1.2. Transcriptional Regulatory Activity of CREBBP/EP300
CREBBP/EP300 can act as transcriptional co-activators participating in the transcriptional regulation of multiple proteins [22,23,24]. Genes interacting with CREBBP/EP300 include the Krüppel-like factor family, the Gata family, and the Runx family, which have important functions in hematopoiesis, cell cycle regulation, and self-renewal of hematopoietic stem cells (HSCs) [24,25,26]. Ep300 deletion results in altered activity of specific enhancers in Tet2-deficient hematopoietic stem and progenitor cells (HSPCs) showing enrichment for key hematopoietic regulators, including Ets, Runx, Gata, Irf8, NF-κB, and Myb family members [27]. Inhibition of CREBBP/EP300 BRD reduces H3K27ac levels specifically at enhancers, and acute loss of H3K27ac from enhancers leads to suppression of nascent enhancer RNA (eRNA) production and significant downregulation of enhancer-driven oncogenes expressions, such as MYC, MYB, and IRF4, in several hematological cancer cells [13]. It leads to profound anti-proliferative effects in vitro and in vivo.
2. The Role of CREBBP/EP300 in Normal Hematopoiesis
CREBBP/EP300 act as tumor suppressors and maintain normal hematopoietic function. The CREBBP BRD mutation disrupts its interaction with ASF1 causing Rubinstein-Taybi syndrome, and patients are prone to develop cancer [15]. A full complement of functional Crebbp/Ep300 is required to maintain normal hematopoiesis, and mice with monoallelic inactivation of the Crebbp gene generate highly permeable multilineage defects in definitive hematopoietic differentiation and an increased incidence of hematological malignancies with age [28]. CREBBP/EP300 play different roles in the self-renewal of HSCs, with a full dose of CREBBP being essential for self-renewal and differentiation of HSCs, whereas EP300 is crucial for proper hematopoietic differentiation [25,29,30]. The interaction between CREBBP and c-Myb regulates HSCs and HSPCs function and is also critical for human acute myeloid leukemia (AML) [27,31,32]. The loss of Ep300EP300 upregulates Myb, and Myb depletion inhibits the proliferation of HSPCs and improves the survival of leukemia-bearing mice [27]. The activity of at least one acetyltransferase of CREBBP and EP300 is indispensable for B-cell development and survival [22]. CREBBP and EP300 regulate common as well as distinct transcriptional targets in sub-compartments of the germinal center (GC), a stage of the humoral immune response where B-cells undergo immunoglobulin affinity maturation. It has been reported that CREBBP loss occurs at different stages of lymphopoiesis resulting in different functions. Specifically, mice with the early loss of Crebbp in the HSPCs compartment are more likely to develop aggressive lymphomas. In contrast, loss of Crebbp in committed lymphoid cells inhibited tumor progression. Furthermore, Horton et al. identified CREBBP mutation in HSPCs of CREBBP-mutant lymphoma patients, which has profound implications for the underlying cellular origin and subsequent evolution of lymphoid malignancies [33].
CREBBP/EP300 are associated with HSC maintenance. EP300 and MED12 overlap in 80% of enhancers and >70% of super-enhancers, and both robustly interact on the chromatin of mouse isolated HSPCs and HPC-7 cells to maintain the active state of hematopoietic enhancers. Deletion of MED12 destabilizes EP300 binding at lineage-specific enhancers, resulting in H3K27ac depletion, enhancer inactivation, and consequent loss of HSCs stemness signatures, such as ERG, ETV6, RUNX1, and TCF7 [34]. CREBBP/EP300 are associated with hematopoietic microenvironment homeostasis. Myb/Ep300 deficiencies induce megakaryocytosis and thrombocytosis, which further leads to a decrease in circulating thrombopoietin concentrations and significant perturbations in the HSCs compartment. Crebbp and Brca1 functionally interact to maintain normal hematopoiesis. Crebbp haploinsufficiency in mice is deleterious for the bone marrow microenvironment, which results in myeloproliferation associated with an increase in splenic HSCs as well as a lethal systemic inflammatory disorder [35,36].
3. The Role of CREBBP/EP300 in Hematological Malignancies
3.1. Lymphoma
CREBBP/EP300 gene mutations frequently occur in hematological malignancies through a variety of different mechanisms, resulting in poor patient prognosis, including worse overall survival (OS), progression-free survival (PFS), and event-free survival (EFS) [37,38,39,40,41]. Genetic abnormalities of CREBBP/EP300 are frequent in hematological malignancies and include gene mutations, copy number variations, and structural variations (Table 1 and Table 2). For example, CREBBP/EP300 gene mutations are frequently detected in diverse lymphomas, such as follicular lymphoma (FL) [39,40], DLBCL [16,42,43], in situ follicular neoplasia [44], peripheral T-cell lymphoma (PTCL) [37,45], angioimmunoblastic T-cell lymphoma (AITL) [37], and plasmablastic lymphoma (PBL) [46]. The frequency and type of CREBBP/EP300 mutations vary significantly among lymphomas due to different geographic regions or subtypes. Firstly, EP300 mutations are the most common type of epigenetic mutation in North American Adult T-cell leukemia–lymphoma (ATLL) (20%), approximately three times more than in Japanese ATLL [41]. Secondly, EP300 mutations are more significant in AITL than in peripheral T-cell lymphoma–not otherwise specified (PTCL-NOS) (22.2% vs. 2.9%) [45]. Finally, Garcia-Ramirez et al. found a significant difference in CREBBP mutations between FL and DLBCL. CREBBP frameshift/nonsense mutations occurred more frequently in DLBCL compared to FL, but missense mutations were more frequently observed in FL. In addition, CREBBP mutations were associated with increased MYC expression in primary DLBCL tumors but not in FL [47]. Compared with EP300, mutational inactivation of CREBBP has additional cell-intrinsic engraftment and growth-promoting effects in orthotopic xenograft models of DLBCL [48]. Deletion of Ep300, but not Crebbp, impairs the fitness of GC B-cells in vivo. However, joint loss of Crebbp and Ep300 completely abolished GC formation, suggesting EP300-dependency in Crebbp-deficient lymphoma cells [49,50].
Table 1.
CREBBP/EP300 with high-frequency mutations in hematological malignancies.
| Tumor | Gene | Mutation Number | Case Number with Mutation | Percentage (Total Number) |
|---|---|---|---|---|
| Acute Lymphoblastic Leukemia | CREBBP | 2 | 1 | 1.1% (93) |
| Acute Myeloid Leukemia | CREBBP | 4 | 4 | 2.0% (200) |
| EP300 | 5 | 5 | 2.5% (200) | |
| Chronic Lymphocytic Leukemia | CREBBP | 2 | 2 | 1.3% (160) |
| EP300 | 1 | 1 | 1.0% (105) | |
| Cutaneous T-cell Lymphoma | CREBBP | 2 | 2 | 4.7% (43) |
| Diffuse Large B-cell Lymphoma | CREBBP | 35 | 28 | 20.7% (135) |
| EP300 | 4 | 4 | 7.5% (53) | |
| Hypodiploid Acute Lymphoid Leukemia | CREBBP | 7 | 7 | 15.9% (44) |
| Lymphoma Cell Lines | CREBBP | 10 | 9 | 26.5% (34) |
| EP300 | 10 | 6 | 17.6% (34) | |
| Lymphoid Neoplasm Diffuse Large B-cell Lymphoma | CREBBP | 6 | 6 | 12.5% (48) |
| EP300 | 3 | 3 | 3.6% (48) | |
| Mature B-cell malignancies | CREBBP | 229 | 180 | 23.8% (755) |
| EP300 | 57 | 56 | 7.4% (755) | |
| Multiple Myeloma | CREBBP | 1 | 1 | 0.5% (205) |
| EP300 | 1 | 1 | 0.5% (205) | |
| Non-Hodgkin Lymphoma | CREBBP | 3 | 2 | 14.3% (14) |
| EP300 | 1 | 1 | 7.1% (14) | |
| Pediatric Acute Lymphoid Leukemia | CREBBP | 6 | 6 | 4.0% (150) |
| EP300 | 2 | 2 | 1.3% (150) |
Notes: Data mainly come from the TCGA database.
Table 2.
CREBBP/EP300 with abnormal copy numbers and structural variants in hematological malignancies.
| Tumor | Gene | Type of CNA | Case Number with CNA | Percentage (Total Number) |
|---|---|---|---|---|
| Acute Myeloid Leukemia | CREBBP | AMP | 1 | 0.5% (191) |
| Diffuse Large B-Cell Lymphoma | CREBBP | AMP | 1 | 2.1% (48) |
| Lymphoid Neoplasm Diffuse Large B-cell Lymphoma | CREBBP | AMP | 2 | 4.2% (48) |
| HOMDEL | 1 | 2.1% (48) | ||
| Pediatric Acute Lymphoid Leukemia | CREBBP | AMP | 2 | 0.3% (764) |
| HOMDEL | 8 | 1.0% (764) | ||
| EP300 | AMP | 3 | 0.4% (764) | |
| HOMDEL | 3 | 0.4% (764) | ||
| Pediatric Acute Myeloid Leukemia | CREBBP | AMP | 1 | 0.4% (240) |
| EP300 | HOMDEL | 2 | 0.8% (240) | |
| Tumor | Gene | Structural variant number | Case number with mutation | Percentage (total number) |
| Acute Lymphoblastic Leukemia | CREBBP | 1 | 1 | 1.1% (93) |
| Acute Myeloid Leukemia | CREBBP | 1 | 1 | 0.5% (200) |
Notes: Cytoband: CREBBP (16p13.3); EP300 (22q13.2); CNA: copy number alteration; HOMDEL: homozygous deletion; AMP: amplification. The above data mainly come from the TCGA database.
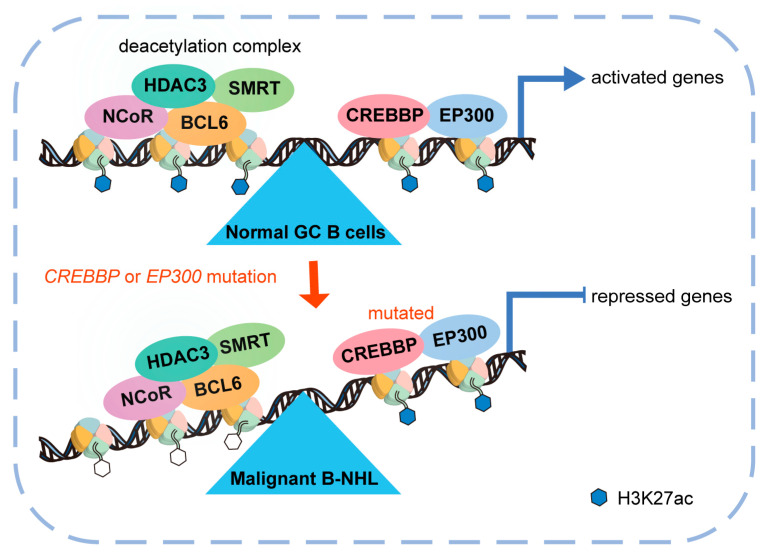
CREBBP/EP300 mutations as a major pathogenetic mechanism shared by common forms of B-cell non-Hodgkin lymphoma (B-NHL) have direct implications for the use of drugs targeting the acetylation/deacetylation mechanism. Pasqualucci et al. found that lymphomagenesis was related to inactivation or dose reduction of the HAT domain caused by the CREBBP/EP300 mutation [51,52]. CREBBP/EP300 and BCL6/SMRT/HDAC3 exert opposing functions by regulating enhancer/super-enhancer networks with key roles in the GC reaction, including signal transduction from the B-cell receptor and CD40 receptor, NF-κB and Galpha13 signaling, T-cell-mediated B-cell activation, plasma cell differentiation, and major histocompatibility complex class II (MHCII) antigen processing and presentation [53,54]. Genes disturbed by CREBBP mutation are direct targets of the BCL6/SMRT/HDAC3 tumor–repressor complex. CREBBP loss of function contributes to lymphomagenesis by promoting immune escape in vitro and in vivo [42,55,56] (Figure 2). Histone deacetylase inhibitors (HDACis) play positive roles in the treatment of patients with FL, especially those with nonfunctional CREBBP [57]. These findings suggest that HDACis are available for treating B-NHL patients, as they may contribute to re-establishing physiologic acetylation levels and subsequently contribute to the restoration of tumor immune surveillance. Certainly, efficacy and target specificity should be evaluated.
Figure 2.
The opposite function between CREBBP/EP300 and the BCL6/SMRT/HDAC3 tumor–repressor complex in the germinal center reaction. In normal germinal center B-cells, CREBBP/EP300-mediated H3K27ac and BCL6/SMRT/HDAC3 complex-mediated deacetylation keep in balance. However, CREBBP/EP300 mutation results in focal depletion of enhancer H3K27ac and aberrant transcriptional silencing of genes that regulate B-cell signaling and immune responses. It contributes to the development of HDAC3-dependent lymphomas by mediating immune escape. GC: germinal center; B-NHL: B-cell non-Hodgkin lymphoma.
3.2. Leukemia
Genetic abnormalities of CREBBP/EP300 are also common in leukemia. For example, CREBBP/EP300 mutations frequently occur in primary and relapsed pediatric ALL [58,59,60,61,62,63,64], aggressive NK-cell leukemia (ANKL) [65], and chronic-phase chronic myeloid leukemia (CML-CP) [66]. CREBBP mutation is associated with hyperdiploid karyotype and KRAS mutation in relapsed pediatric ALL. CREBBP knockdown enhances RAS/RAF/MEK/ERK signaling in ALL with Ras pathway mutation but remains sensitive to MEK inhibitors [67]. An analysis found that 18.3% of relapse ALL cases (n = 71) had sequence or deletion mutations of CREBBP, which resulted in truncated alleles or detrimental substitutions in conserved residues of the HAT domain [68]. In MLL1-rearranged acute leukemia, EZH2 binds directly to cMyc and EP300 via a hidden transactivation domain, thereby mediating gene activation and contributing to tumorigenesis [69]. CN470 has notable anti-tumor activity against MLL1-rearranged ALL by binding to the bromodomains (BRDs) of BRD4, CREBBP, and EP300 in vitro and in vivo [70].
CREBBP/EP300 play an important role in leukemogenesis by participating in the regulation of various fusion proteins and key proteins (Table 3). The gene expression profile of TCF3-ZNF384-positive ALL is related to CREBBP-ZNF384- and EP300-ZNF384-positive ALL, but not to other conventional genetic subtypes [71,72,73,74]. MOZ (MYST3)–CREBBP, t(8;16) (p11;p13), is a very rare abnormality in AML [75,76]. RUNX1 (AML1) is a common target of chromosomal translocations and has been suggested to be directly acetylated by EP300 at residue K43 in several types of AML and ALL [77,78]. MOZ regulates gene transcription by activating the RUNX1 transcription factor complex and is involved in the regulation of AML development and erythrophagocytosis [76,79,80].
Table 3.
CREBBP/EP300 and oncogenic protein complexes in leukemia and mechanisms.
| Complex Components | Tumors | Mechanisms | References |
|---|---|---|---|
| ZNF384-CREBBP t(12;16) (p13;p13) and ZNF384-EP300 t(12;22) | ALL | Upregulating JAK/STAT and cell adhesion pathways, downregulating cell cycle and DNA repair pathways, and enhancing oncogenic transformation | [81,82] |
| TCF3-HLF | ALL | Preferentially cooperating with ERG to recruit EP300 to activate the gene expression critical to ALL, which is also associated with chemoresistance | [83] |
| MAFB-ETS2 | T-ALL | Interacting with PCAF and EP300 to enhance NOTCH1 signaling, including MYC, NOTCH3, and HES1 | [84] |
| PML-RARα | APL | Recruiting abundant EP300 and HDAC1 to target genes, such as GFI1, exerts an activating effect by forming super-enhancers, while only sufficient HDAC1 is recruited to repressed target genes, such as CEBPE, which will exhibit repressive effects | [85] |
| MYB-C/EBPβ-EP300 | AML | GFI1, a target gene of MYB-C/EBPβ-EP300, is downregulated by C/EBPβ-inhibitory natural sesquiterpene lactones, further inhibiting cell proliferation | [86] |
| RUNX1-ETV6 | ALL | Inducing leukemogenesis through acetylation of RUNX1 by mTORC1 phosphorylated EP300 | [87] |
| RUNX1-ETO | AML | EP300 colocalizes in the regulatory regions of many RUNX1-ETO target genes and acetylates RUNX1-ETO to promote leukemogenesis | [88] |
| Recruiting EP300 to activate THAP10, which is a target and negatively regulated by microRNA-383 | [89] | ||
| E2A-PBX1 | ALL | Recruiting EP300, H3K27ac, and MED1 to E2A-PBX1-targeted RUNX1 sites | [90] |
| MOZ–CREBBP t(8;16)(p11;p13) | AML | Causing upregulation of HOXA family, PBX3, MEIS1, HNMT, etc., and inhibiting RUNX1-mediated differentiation of M1 myeloid cells into monocytes/macrophages | [79,80] |
| MOZ-TIF2 and Nup98-Hoxa9 | AML | Recruiting CREBBP/EP300 to induce leukemogenesis and serving as therapeutic targets for various human AML subtypes | [91] |
| MOZ-TIF2 and MLL-AFX | AML | Recruiting the AF4 family/ENL family/P-TEFb complex and activating CpG-rich promoters by CREBBP/EP300 | [92] |
Notes: ALL: acute lymphocytic leukemia; T-ALL: T-cell acute lymphocytic leukemia; APL: acute promyelocytic leukemia; AML: acute myeloid leukemia.
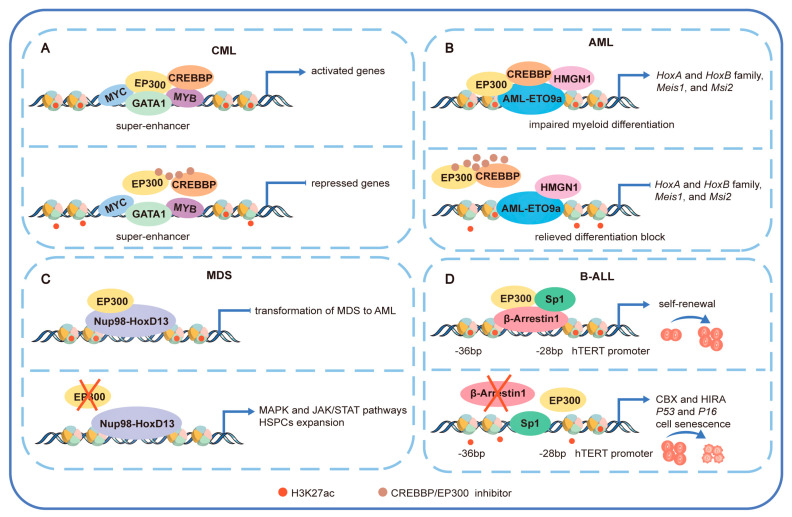
EP300 is abundant at super-enhancers and coincident with sites of GATA1 and MYC occupancy in chronic myeloid leukemia (CML) cell line K562. BRD inhibitors interfere with these oncogene-driven transcriptional programs, leading to cell cycle arrest in the G0/G1 phase [5] (Figure 3A). In AML, 21q22/HMGN1 amplification cooperates with the AML-ETO9a to impair myeloid differentiation and enhance leukemia stem cell activity. HMGN1 overexpression increases chromatin accessibility, expression, and H3K27ac at loci important for HSCs and leukemia, including many CREBBP/EP300 targets [93] (Figure 3B). Although the above data show the tumor-promoting role of CREBBP/EP300, they sometimes exert tumor-suppressive functions as well. For example, Ep300, but not Crebbp, plays a critical role in blocking the transformation of myelodysplastic syndrome (MDS) to AML and in controlling the balance between symmetric stem cell self-renewing divisions and stem cell depleting divisions in Nup98-HoxD13 transgenic mice, an animal model that phenotypically replicates human MDS [94] (Figure 3C). Promoting the senescence of malignant tumor cells is also a therapeutic strategy. Deletion of β-Arrestin1 promotes senescence of B-ALL initiating cells in vitro and in vivo by inhibiting the EP300/Sp1 interaction at −28 to −36 bp of the hTERT promoter [95,96] (Figure 3D).
Figure 3.
CREBBP/EP300 interact with multiple proteins to induce leukemogenesis. (A) In CML, CREBBP/EP300 are abundant in super-enhancer regions of oncogenes GATA1, MYC, and MYB. The CREBBP/EP300 BRD inhibitor reduces the levels of H3K27ac, inhibits the expression of GATA1, MYC, and their target genes, such as TET1, FOSL1, and CCND1, and causes cell cycle arrest. (B) HMGN1 overexpression cooperates with AML-ETO9a, which impairs myeloid differentiation and increases the expression of many CREBBP/EP300 targets, such as the HoxA and HoxB family, Meis1, and Msi2. CREBBP/EP300 inhibitors decrease H3K27ac in HMGN1-overexpression progenitors and relieve HMGN1-associated differentiation impairment. (C) EP300 plays a powerful role as a tumor suppressor in Nup98-HoxD13-driven progression of MDS to AML. Deletion of EP300 promotes activation of the MAPK and JAK/STAT pathways in the HSPC compartment. Loss of EP300 significantly triggers HSPCs’ expansion and accelerates MDS-associated leukemogenesis. (D) Depletion of β-Arrestin1 reduced the interaction of EP300 with Sp1 at the hTERT promoter, which enhanced the expression of proteins (CBX and HIRA) and genes (P53 and P16) associated with senescence, downregulated hTERT transcription, decreased telomerase activity, and shortened telomere length, thereby promoting senescence in leukemic cells. CML: chronic myeloid leukemia; AML: acute myeloid leukemia; MDS: myelodysplastic syndrome; B-ALL: B-cell acute lymphocytic leukemia.
3.3. Multiple Myeloma
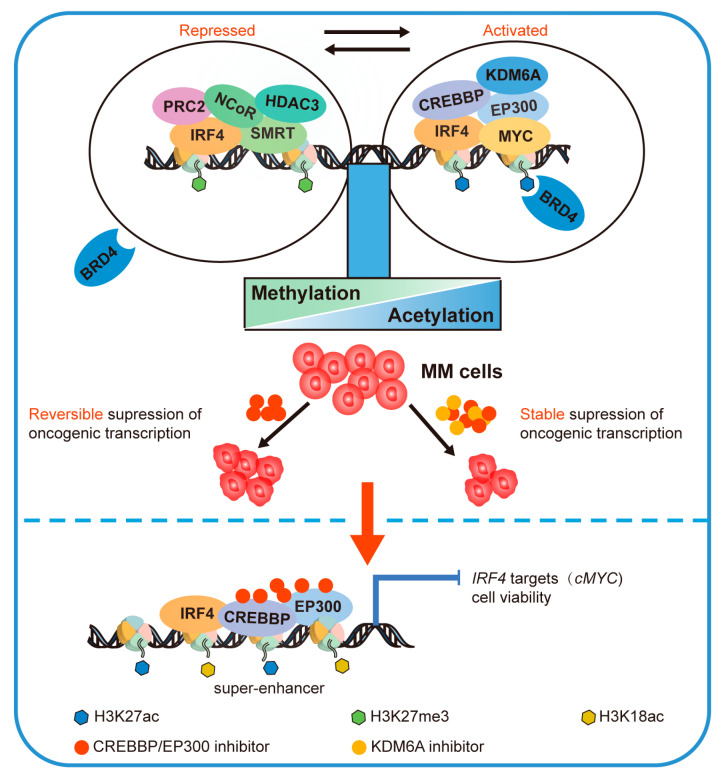
In a next-generation sequencing analysis of MM, EP300 (11.6%, n = 147) was one of the most commonly mutated genes in chromatin regulators [97]. CREBBP/EP300 exhibit evolutionary conservation in maintaining the core transcription program that is dynamically repressed following acute lysine acetyltransferase inhibition. Acute catalytic CREBBP/EP300 inhibition modulates transcription independently of DNA accessibility and selectively suppresses transcription of distinct oncogenic networks in different cancer types. Robust CREBBP/EP300 activity is required to maintain genome-wide histone acetylation and facilitates the recruitment of co-activators and RNA Pol II in the face of rapid deacetylation kinetics. HDAC3-dependent NCoR/SMRT co-repressor complexes and CREBBP/EP300 co-locate in active regions of chromatin, which are functionally antagonistic. Compensatory loss of NCoR/SMRT complexes perturbs genome-wide histone deacetylation rates and mitigates the transcriptional effects of CREBBP/EP300 inhibition. However, these effects remain highly reversible prior to compensatory histone methylation switching, and deacetylation of H3K27 provides nucleation sites for reciprocal methylation switching, a feature that can be treated by concomitant repression of KDM6A/UTX and CREBBP/EP300 in MM [54] (Figure 4). Shed syndecan-1 is a cell surface heparan sulfate proteoglycan that is translocated to the nucleus and binds to EP300 to inhibit histone acetylation in myeloma cells and bone-marrow-derived stromal cells, thereby facilitating communication within the tumor microenvironment [98].
Figure 4.
Oncogenic IRF4/MYC expression is regulated by histone acetylation and methylation switch. HDAC3-dependent NCoR/SMRT co-repressor complexes and PRC2-mediated methylation keep histone modifications in a hypoacetylated state and therefore oncogenes are repressed. However, CREBBP/EP300- and KDM6A-mediated demethylation activate oncogenes. The major effects of acute CREBBP/EP300 inhibition on acetylation and gene expression are highly reversible. The loss of H3K27ac and BRD4 binding represents transcriptional hypersensitivity to CREBBP/EP300 inhibition. CREBBP/EP300 inhibitors decrease H3K27ac and H3K18ac of the IRF4 super-enhancer, inhibiting IRF4 and its downstream target genes cMYC, further resulting in cell cycle arrest and apoptosis of MM cells. When combined with the CREBBP/EP300 inhibitor and the KDM6A inhibitor, the therapeutic effect was significantly improved. MM: multiple myeloma.
The IRF4/MYC axis is critical for MM progression. Inhibition of CREBBP/EP300 BRDs leads to a significant reduction in H3K18ac and H3K27ac at the IRF4 super-enhancer and transcription start site, which directly inhibits IRF4 and its downstream target genes such as cMYC, resulting in decreased viability of MM cell lines and causing cell cycle arrest and apoptosis (Figure 4). The ectopic expression of IRF4 and MYC antagonizes the phenotypic and transcriptional effects of CREBBP/EP300 BRD inhibition [99]. In MM, cereblon has been identified as a primary target of immunomodulatory drugs (IMiDs), such as lenalidomide [100]. Recently, IL6 upregulation and activation of STAT3 and its downstream genes (PIM2 and BIRC5) have been identified as a critical resistance mechanism to IMiDs. BRD inhibitors re-sensitize several IMiD-resistant human MM cell lines to lenalidomide by targeting the IRF4/MYC axis and also increase the sensitivity of IMiD-sensitive cell lines to lenalidomide. SGC-CBP30 and lenalidomide have a synergistic effect in reducing myeloma cell viability [101]. EP300/Sp1 interaction is also present in MM, and this complex promotes cell proliferation by regulating IQGAP1 transcription at the promoter [102].
3.4. Myelodysplastic Syndromes
MDS is a clonal bone marrow stem cell disorder and a third of patients develop AML. A large-scale genomics study found that the frequency of CREBBP/EP300 mutations in MDS patients is about 7% [103]. Several studies have shown that CREBBP is one of the genes affected by chromosomal translocations in patients with therapy-related MDS [24]. Previously, Kojima et al. reported a case of therapy-related MDS with chromosome t(10;16)(q22;p13), in which a MORF-CREBBP fusion variant was detected [104]. In addition, some MDS patients have t(11;16)(q23;p13) in which the MLL gene is fused to the CREBBP gene [105,106,107]. Crebbp+/− mice consistently develop MDS/myeloproliferative neoplasms (MPN) at 9–12 months of age and are hypersensitive to γ-radiation. Meanwhile, mice exhibit reduced numbers of HSCs and common myeloid progenitors and increased granulocyte/macrophage progenitors [108]. Interestingly, EP300 also has tumor suppressor effects in several different clinically relevant MDS models driven by mutations in epigenetic regulators TET2, ASXL1, and SRSF2, respectively, inhibiting the malignant transition of MDS to AML [27,94]. This suggests a potential therapeutic application of EP300 agonists in the treatment of MDS with the aforementioned inactivating mutations.
4. The Therapeutic Implications of CREBBP/EP300
4.1. Application of CREBBP/EP300 Small Molecule Compounds
4.1.1. CREBBP/EP300 Agonists
Aberrant expression of CREBBP/EP300 causes Rubinstein–Taybi syndrome and multiple hematologic malignancies by losing their activity [15,81,82]. CREBBP is a haploinsufficient tumor suppressor gene in GC B-cells. CREBBP deficiency promotes the development of B-cell lymphoma [42,47,48,53]. EP300 could suppress the transition of MDS to AML, suggesting the therapeutic potential of EP300 agonists in MDS patients with Tet2-inactivated mutations [27,94]. YF-2 is a highly selective CREBBP/EP300 HAT domain agonist, and it could improve the genome editing efficiency of cas9 nucleases [109]. CTB, an agonist of EP300, increases the expression of EP300 to induce the acetylation of P53 protein and subsequently leads to the death of breast cancer cells. Therefore, CTB can be used as an anticancer drug [110]. TTK21 is also a CREBBP/EP300 agonist that conjugates glucose-based carbon nanosphere (CSP) to cross the blood–brain barrier, which is beneficial for adult neurogenesis and long-term memory function [111]. Currently, the number of CREBBP/EP300 agonists is relatively rare compared to inhibitors, and more in-depth studies are required.
4.1.2. CREBBP/EP300 Inhibitors in Hematological Malignancies
Because of the BRD and acetyltransferase activity of CREBBP/EP300, HDACis, HAT domain inhibitors, and BRD inhibitors could inhibit their function. Researchers have summarized the development of CREBBP/EP300 inhibitors in recent years [9,10]. He et al. summarized the structures and molecular original names of 75 CREBBP/EP300 inhibitors from 2010 to 2021, including BRD inhibitors and HAT domain inhibitors, and they also discussed structure–activity relationship studies, bindings models, and biochemical data [10]. Xiong et al. discussed some candidates in clinical trials that could potentially inhibit CREBBP/EP300 function [9]. Chen et al. reviewed the structure and anticancer effects of small molecule compounds of CREBBP/EP300 [2]. In this review, we focus on summarizing several representative CREBBP/EP300 BRD inhibitors (CCS1477, CPI-637, and GNE272, etc.) and HAT inhibitors (A485, A-241, and C646, etc.) and their functions in hematological malignancies (Table 4).
Table 4.
Inhibitors of CREBBP/EP300 in hematological malignancies and their functions.
| Inhibitors | Targets | Tumor or Cells | Functions | Reference |
|---|---|---|---|---|
| CCS1477, CU329 | BRD/HAT | DLBCL cells | Reducing CREBBP/EP300 autoacetylation, H3K18ac, and H3K27ac, and significant negative enrichment in CREBBP- and EP300-regulated programs | [49] |
| CPI-637 | BRD | ALCL and HL | Inhibiting PD-L1 mediated tumor immune escape in vitro and in vivo | [112] |
| GNE272 | BRD | lymphoma and MM cells | Repressing expression of multiple oncogenes, including MYC, MYB, CCND1, BCL2, BCL-XL, MCL1, and BCL6, and exerting a significant antiproliferative effect on some tumor cells, such as Jurkat, Pfeiffer, KMS11, and U266B1 | [113] |
| GNE-781 | BRD | FL | Reducing differentiation of human CD4+ T-cells into Tregs, impairing proliferation of both naive activated T-cells and induced Tregs, and reducing cytokine secretion, such as prostacyclin, IL10, and IL2 | [114] |
| NEO2734, NEO1132 | BRD | MM | Effectively decreasing both cMyc and IRF4 protein expression and inducing G1 cell cycle arrest | [115] |
| SGC-CBP30 | BRD | AML | Reducing binding of BRD4 to the regulatory region of B7-H6 and decreasing expression of B7-H6 | [116] |
| SGC-CBP30 | BRD | MM | Inhibiting IL6 autocrine production and STAT3 activation, downregulating IRF4 (especially truncated IRF4) and MYC, and restoring immunomodulatory drugs sensitivity | [101] |
| SGC-CBP30, I-CBP112 | BRD | MM cells | Upregulating cell surface and mRNA expression of MICA and exhibiting an anti-myeloma effect by inhibiting the IRF4/MYC axis | [99,117,118] |
| SGC-CBP30, GNE-049 | BRD | AML cells | Blocking H3K27ac, eRNA production, and expression of enhancer-proximal genes | [13] |
| SGC-CBP30, GNE-272, CPI644 | BRD | CML cells | Inhibiting the expression of super-enhancer-associated genes such as MYC, GATA1, and MYB, as well as downregulating the expression of their target genes, such as TET1, FOSL1, and CCND1 | [5] |
| XDM-CBP | BRD | leukemia | Inhibiting cancer cells proliferation | [119] |
| A485 | HAT | ALL, MM, AML and B-NHL | Blocking TCF3-HLF-dependent gene expression, including an MYC-associated signature, and inhibiting tumor cell proliferation | [83,120] |
| A-485, A-241 | HAT | CLL and MM cells | Inducing potent apoptotic and cytostatic effects, suppressing IRF4-dependent MM signatures such as MYC, and PRDM1/BLIMP-1, and downregulating gene expression including those regulating B-cell activation and known oncogenic drivers in CLL, such as IRC3, ID3, and MYC | [54] |
| A485, C646 | HAT | AML | Relieving myeloid differentiation abnormalities associated with HMGN1 overexpression in hematopoietic progenitor cells and leukemia | [93] |
| B026 | HAT | Leukemia and lymphoma cells | Robustly decreasing MYC expression and growth of cell lines such as MV-4-11, Maver-1, K562, and Kasumi-1 | [121] |
| C646 | HAT | ALL | Attenuating GNAO1 expression upregulated by ETV6-RUNX1 | [87] |
| C646 | HAT | AML | Suppressing growth and colony formation in multiple AML cell lines and primary human AML samples | [91] |
| C646 | HAT | DLBCL cells | Inhibiting H3K9ac and H3K14ac and RUNX1-ETO while increasing THAP10 levels | [89] |
| C646 | HAT | MDS-derived AML cells | Increasing the sensitivity of AML cells to azacitidine | [122] |
| Salicylate, diflunisal | HAT | DLBCL | Inhibiting CREBBP/EP300 lysine acetyltransferase activity by direct competition with acyl-CoA at the catalytic site and suppressing the growth of EP300-dependent leukemia cell lines expressing RUNX1-ETO fusion protein in vitro and in vivo | [123] |
Notes: BRD: bromodomain; HAT: histone acetyltransferase; ALCL: anaplastic large cell lymphoma; HL: Hodgkin lymphoma; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; MM: multiple myeloma; CML: chronic myeloid leukemia; B-NHL: B-cell non-Hodgkin lymphoma; CLL: chronic lymphocytic leukemia; MDS: myelodysplastic syndrome.
To date, there is sufficient evidence that HDACis or other inhibitors of epigenetic modification molecules, alone or in combination, are effective against some hematologic malignancies with aberrant CREBBP/EP300 expression [124]. Frequently mutated EP300 in North American ATLL could be targeted by DNA methyltransferase inhibitor decitabine [41]. The phase II oral HDACi tucidinostat mitigates the negative prognostic impact of CREBBP/EP300 mutations on DLBCL (ClinicalTrials.gov: NCT02753647) [125,126]. CREBBP/EP300-mutated DLBCL cells are dependent on CARM1 arginine methylation activity. Inhibition of CARM1 leads to H3K27ac deletion in the CREBBP/EP300 chromatin binding region, downregulating CREBBP target genes and further inhibiting DLBCL cell growth. The combination of CARM1 inhibitors and CREBBP/EP300 inhibitors may have significant therapeutic potential for the treatment of DLBCL, MM, and AML [127,128,129].
In addition to direct inhibition of CREBBP/EP300, blocking the interaction of CREBBP/EP300 with other oncogenic molecules is also an important anti-cancer pathway. XX-650-23, a small molecule inhibitor of CREB, specifically disrupts the interaction of CREB to the KIX domain of CREBBP and inhibits CREB-driven gene expression in AML cells, further promoting apoptosis and cell cycle arrest and prolonging survival in mice injected with human AML cells [130]. ICG-001 and C-82/PRI-724 specifically inhibit the interaction between CREBBP/β-catenin, but not EP300/β-catenin, thereby rendering drug-resistant CML-initiating cells to be sensitive to BCR-ABL tyrosine kinase inhibitors [131,132,133]. PRI-724 is currently in phase I clinical trials for AML and phase II clinical trials for CML (ClinicalTrials.gov: NCT01606579) [134]. MYBMIM, a peptidomimetic inhibitor, possesses significant anti-leukemia efficacy in vivo and in vitro. It blocks the assembly of the MYB: CREBBP/EP300 complex, further promoting apoptosis and inhibiting the growth and survival of AML cells. In addition, it has been suggested that MYBMIM affects promoters and enhancers of several transcription factors, including ERG, PU.1, CEBPA, and RUNX1, and suppresses gene expression, including BCL2, MYC, GFI1, MTL5, and IKZF1 [135]. CRYBMIM, a second-generation version of MYBMIM, could specifically target the KIX domain of CREBBP/EP300 with a higher affinity for AML cells but less impact on normal hematopoietic progenitors [136]. Chetomin, a metabolite complex, could improve the poor prognosis of MM patients by targeting the HIF-1α/EP300 complex [137].
4.1.3. CREBBP/EP300 Inhibitors in Clinical Trials
In addition to the clinical non-CREBBP/EP300 inhibitors mentioned above (tucidinostat and PRI-724), two targeted inhibitors of CREBBP/EP300 are currently in clinical trials in cancer patients. CCS1477, a CREBBP/EP300 BRD inhibitor developed by CellCentric, is in phase I/IIa clinical trials (ClinicalTrials.gov: NCT03568656) for the treatment of metastatic castration-resistant prostate cancer, metastatic breast cancer, and non-small cell lung cancer [138]. CCS1477 is also being evaluated in phase I/IIa clinical trials (ClinicalTrials.gov: NCT04068597) for the treatment of B-NHL, AML, MM, and higher-risk MDS [139]. FT-7051, a CREBBP/EP300 BRD inhibitor, has been tested for its safety and tolerability in patients with metastatic castration-resistant prostate cancer (ClinicalTrials.gov: NCT04575766) [140]. In summary, inhibitors of CREBBP/EP300 are gradually increasing and they are becoming more selective, powerful, and efficient, such as UMB298 [141], dCBP-1 [142,143], and B026 [121]. The current inhibitors could be useful tools to study CREBBP/EP300-related diseases. However, there are still few clinically available inhibitors. These data are useful for the development of novel and more effective CREBBP/EP300 inhibitors and the treatment of patients with related tumors.
4.2. CREBBP/EP300 and Chemoresistance in Hematological Malignancies
Transcriptional or epigenetic dysregulation of CREBBP/EP300 in hematological malignancies may lead to chemoresistance. Zhou et al. found that CREBBP/EP300 inhibitors inhibited RTKs and the downstream activation of MAPK/ERK signaling, thereby overcoming mantle cell lymphoma (MCL) cells’ resistance to idelalisib in vitro and in vivo [144]. Downregulating CREBBP inhibits ALL cell proliferation and cell cycle progression and leads to daunorubicin resistance by interacting with E2F3a [145]. Furthermore, targeting CREBBP/EP300 restores the sensitivity of human MM cells to IMiDs [101].
Mutations in the histone modification gene may alter the function of proteins in the regulation of chromatin state, sensitizing tumor patients to HDACis, valproic acid, suberoylanilide hydroxamic acid, romidepsin, and chidamide. For example, PTCL patients bearing CREBBP/EP300 mutations may respond to chidamide, either alone or in combination with other chemotherapy such as decitabine [45]. Moreover, CREBBP inactivation could promote the sensitivity of drug-resistant DLBCL cells to chidamide by regulating the cell cycle. The combination of AURKA inhibitor and chidamide is a novel therapeutic strategy for the treatment of relapsed/refractory DLBCL [146]. DLBCL cell lines expressing high BCL6 levels or CREBBP/EP300 mutations are sensitive to GSK-J4, a histone demethylase KDM6B inhibitor [147]. A group of T-ALL cell lines with the mutated CREBBP allele were resistant to glucocorticoid dexamethasone treatment but sensitive to clinically useful concentrations of class I/II HDACi vorinostat [68]. However, Tamai et al. failed to find an association between the mutational status of the CREBBP gene and dexamethasone sensitivity in pre-B ALL. They suggested that most T-ALL cell lines were resistant to dexamethasone regardless of the mutational status of CREBBP [148]. Regardless of CREBBP mutation states and chromosomal aberrations, ICG-001 eradicated chemoresistance in vitro and significantly prolonged the survival of NOD/SCID mice engrafted with primary ALL [149]. Moreover, XX-650–23 and ICG-001 could synergize with dasatinib to enhance the sensitivity of pre-BCR+ ALL cells to dasatinib [150]. ICG-001 and HDACis have also been found to potentially relieve CML resistance to imatinib in vivo and in vitro [132,151]. In conclusion, patients with CREBBP/EP300 mutations are sensitive to HDACis, and HDACis may help alleviate chemoresistance to other drugs. More drug combination strategies still need to be explored, and the mechanism of CREBBP/EP300 inhibitors leading to chemotherapy resistance is also worth further research.
4.3. Implications of CREBBP/EP300 in Immunotherapy
CREBBP/EP300 participate in tumor immune regulation by modulating the function of immune cells through various pathways. T regulatory cells (Tregs) are a subset of CD4+ T-cells with significant immunosuppressive effects. CREBBP/EP300 regulate the differentiation of Tregs through transcriptional and non-transcriptional mechanisms [114]. Tregs are downregulated in FL patients carrying CREBBP/EP300 loss of function mutations. Inhibition of CREBBP/EP300 with GNE-781 impairs the differentiation of human CD4+ T-cells into Tregs, providing a therapeutic approach by promoting a pro-inflammatory tumor microenvironment [114]. In DLBCL, CREBBP/EP300 mutations promote tumor-associated macrophage M2 polarization, thereby facilitating tumor progression in vivo and in vitro [16]. Trim24, a CREBBP-associated E3 ligase, promotes the recruitment of CREBBP to STAT6 by catalyzing the ubiquitination of CREBBP at lysine 119. However, the loss of Trim24 promotes macrophage M2 polarization of mouse and human macrophages by inhibiting acetylation STAT6 at lysine 383, thus potentially suppressing anti-tumor immune responses [152]. The development of T helper 17 cells is regulated by the RORγt–SRC-3–EP300 axis, which is associated with T helper 17 cell-driven autoimmune diseases [153,154]. Tumor immune surveillance of NK cells is mediated by cytotoxicity receptor natural-killer group 2 member D (NKG2D), whose ligand NKG2D-ligand is not normally expressed in healthy cells. CREBBP/EP300 contribute to the upregulation of NKG2D ligands MICA/B and ULBP2 in humans and RAE-1 in mice. Inhibition of CREBBP/EP300 abrogates the sensitivity of stressed cells to NK-cell-mediated killing [22]. In MM, IRF4 is the transcription target of cMYC and the repressor of MICA promoter activity. CREBBP/EP300 bromodomain and extra-terminal domain inhibitors (BETi) enhance NK-cell-mediated cytotoxicity by downregulating IRF4 and cMyc expression [117,155]. Finally, CREBBP/EP300 also regulate myeloid-derived suppressor cell (MDSC)-associated genes and their function by modulating H3K27ac of STAT-associated genes. BRD of CREBBP/EP300 may be targeted to boost anti-tumor immune response [156].
CREBBP mutations have been identified as an early event in FL evolution that is enriched within lymphoma cell progenitors, which reduces the expression of MHC II on tumor B-cells, thereby promoting immune evasion by reducing antigen presentation [157]. Mice that lack Crebbp exhibit hyperproliferation of GC B-cells upon immunization, which tends to cause myc-driven lymphomagenesis [48]. HDAC3 inhibitors reverse the abnormal epigenetic program caused by CREBBP mutations and recover immune surveillance by inducing the BCL6-suppressed IFN pathway and antigen-presenting genes. The synergistic effect of HDAC3 inhibitors and PD-L1 blockade restores the ability of tumor-infiltrating lymphocytes to kill DLBCL cells in a major histocompatibility complex class I (MHCI) and MHCII-dependent manner [56]. In addition, Xian et al. found that CREBBP and CIITA co-deletions/mutations were related to immune evasion [17,158]. Taken together, these results suggest that CREBBP/EP300 are involved in tumor immune responses by regulating immune cell functions, including Tregs differentiation, macrophage M2 polarization, and immune surveillance and killing functions of NK cells. CREBBP/EP300 mutations lead to loss of MHCII expression in tumor cells and subsequently promote the development and progression of hematologic malignancies by facilitating immune escape. CERBBP/EP300 inhibitors and HDACis may help to restore and enhance the ability of immune cells to kill tumor cells.
5. Conclusions
As key acetyltransferase family members and transcriptional co-activators, CREBBP/EP300 regulate the acetylation levels of multiple substrates involved in the regulation of normal hematopoietic maintenance and the development of hematological malignancies. CREBBP/EP300 are involved in extensive hematopoietic regulatory networks at the transcriptome level [1]. The association between these two enzymes and other omics, such as metabolomics, still needs to be uncovered. Aberrant CREBBP/EP300 expression is common in hematological malignancies and is associated with chemoresistance. These abnormal cells are sensitive to targeted inhibitors of CREBBP/EP300 and other epigenetic inhibitors, such as HDACis, DNA methyltransferase inhibitors, and histone demethylase inhibitors. Proteolysis-targeting chimeras (PROTACs) are small molecule compounds to induce the degradation of targeted proteins via ubiquitin–proteasome system [159]. JQAD1 and dCBP-1 are chemical degraders of CREBBP/EP300, and both have higher activity compared with HAT domain and BRD inhibitors [143,160]. dCBP-1 hijacks E3 ubiquitin ligase CRBN to selectively target CREBBP/EP300 for degradation. Degradation of CREBBP/EP300 kills MM cells by suppressing the oncogenic enhancer activity that drives MYC expression [143]. The efficacy and mechanism of JQAD1 and dCBP-1 in hematologic malignancies need further exploration. Currently, although more targeted and potential CREBBP/EP300 inhibitors are gradually increasing, most of them are in the preclinical experimental stage and clinically available inhibitors are still lacking. CREBBP/EP300 also function as tumor suppressors, but the number of agonists and relevant preclinical studies is relatively rare, which requires further research. A more in-depth study of the crystal structure and domain function of CREBBP/EP300 may facilitate the development of new inhibitors and agonists.
Because of the high mutability of CREBBP/EP300, genome-scale CRISPR-Cas9-based synthetic lethality screens can be used to discover the genes that exert the synthetic lethal effect, which may enhance the therapeutic potential of CREBBP/EP300. EP300 recruits BRD4 to the BATF promoter region, and targeting the BRD4-EP300 signaling cascade supports the generation of superior antitumor T-cell engraftment for adoptive immunotherapy [161]. FL patients have been found to harbor competent CD8+ T-cells specific for recurrent mutations, such as CREBBPR1446C. Production of off-the-shelf mutation-specific TCR vectors for T-cell engineering may be practical to further mitigate the adverse effects of CD19-CAR T-cell therapy by targeting tumor-specific mutations rather than the pan-B-cell marker CD19 [162]. It is instructive to design personalized immunotherapy for hematological malignancies with CREBBP/EP300 driver mutations using next-generation sequencing technologies.
Abbreviations
| ALCL | anaplastic large cell lymphoma; |
| ALL | acute lymphocytic leukemia |
| AML | acute myeloid leukemia |
| AMP | amplification |
| APL | acute promyelocytic leukemia |
| ATLL | adult T-cell leukemia lymphoma |
| B-ALL | B-cell acute lymphocytic leukemia |
| B-NHL | B-cell non-Hodgkin lymphoma |
| BRD | bromodomain |
| CLL | chronic lymphocytic leukemia |
| CML | chronic myeloid leukemia |
| CNA | copy number alteration |
| CRD1 | cell cycle regulatory domain 1 |
| CREBBP | CREB-binding protein |
| DLBCL | diffuse large B-cell lymphoma |
| EP300 | E1A binding protein p300 |
| eRNA | enhancer RNA |
| ESC | embryonic stem cell |
| FL | follicular lymphoma |
| GC | germinal center |
| HAT | histone acetyltransferase |
| HATs | histone acetyltransferase |
| HDACs | histone deacetylases |
| HDACis | histone deacetylase inhibitors |
| HL | Hodgkin lymphoma |
| HOMDEL | homozygous deletion |
| HSCs | hematopoietic stem cells |
| HSPCs | hematopoietic stem and progenitor cells |
| IBiD | interferon-binding domain |
| IMiDs | immunomodulatory drugs |
| KIX | kinase-inducible domain of CREB interacting domain |
| LSTFL | limited-stage typical follicular lymphoma |
| MDS | myelodysplastic syndrome |
| MHC class II | major histocompatibility complex class II |
| MM | multiple myeloma |
| NLS | nuclear location signal |
| NKG2D | natural-killer group 2 member D |
| NRID | nuclear receptor interaction domain |
| PHD | plant homeodomain |
| PTCL | peripheral T-cell lymphoma |
| RING | really interesting new gene |
| T-ALL | T-cell acute lymphocytic leukemia |
| TAZ1 | transcriptional-adaptor zinc-finger domain 1 |
| TAZ2 | transcriptional-adaptor zinc-finger domain 2 |
| Tregs | T regulatory cells; ZZ: ZZ-type zinc finger. |
Author Contributions
Conceptualization, Y.Z., X.X. and J.Z.; methodology, Y.Z. and J.Z.; software, Y.Z. and Y.L.; validation, Z.W. and H.P.; formal analysis, X.X., J.L. and J.Z.; investigation, Y.Z., Z.W. and Y.L.; resources, H.P. and J.L.; data curation, Y.Z, H.P. and X.X.; writing—original draft preparation, Y.Z.; writing—review and editing, Y.Z., Z.W., Y.L., X.X., J.L. and J.Z.; visualization, Y.Z. and Y.L.; supervision, Z.W. and H.P.; project administration, X.X.; funding acquisition, X.X., J.L. and J.Z. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by grants from the National Natural Science Foundation of China (Nos.81970195, 82170138, 81920108004) and the National Key Research and Development Program of China (2018YFA0107800).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Blobel G.A. CREB-binding protein and p300: Molecular integrators of hematopoietic transcription. Blood. 2000;95:745–755. doi: 10.1182/blood.V95.3.745.003k05_745_755. [DOI] [PubMed] [Google Scholar]
- 2.Chen Q., Yang B., Liu X., Zhang X.D., Zhang L., Liu T. Histone acetyltransferases CBP/p300 in tumorigenesis and CBP/p300 inhibitors as promising novel anticancer agents. Theranostics. 2022;12:4935–4948. doi: 10.7150/thno.73223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogiwara H., Sasaki M., Mitachi T., Oike T., Higuchi S., Tominaga Y., Kohno T. Targeting p300 Addiction in CBP-Deficient Cancers Causes Synthetic Lethality by Apoptotic Cell Death due to Abrogation of MYC Expression. Cancer Discov. 2016;6:430–445. doi: 10.1158/2159-8290.CD-15-0754. [DOI] [PubMed] [Google Scholar]
- 4.Dutta R., Tiu B., Sakamoto K.M. CBP/p300 acetyltransferase activity in hematologic malignancies. Mol. Genet. Metab. 2016;119:37–43. doi: 10.1016/j.ymgme.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Carpizo V., Ruiz-Llorente S., Sarmentero J., Grana-Castro O., Pisano D.G., Barrero M.J. CREBBP/EP300 bromodomains are critical to sustain the GATA1/MYC regulatory axis in proliferation. Epigenet Chromatin. 2018;11:30. doi: 10.1186/s13072-018-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutto I., Scalera C., Prosperi E. CREBBP and p300 lysine acetyl transferases in the DNA damage response. Cell. Mol. Life Sci. 2018;75:1325–1338. doi: 10.1007/s00018-017-2717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding H., Zhao J., Zhang Y., Yu J., Liu M., Li X., Xu L., Lin M., Liu C., He Z., et al. Systematic Analysis of Drug Vulnerabilities Conferred by Tumor Suppressor Loss. Cell Rep. 2019;27:3331–3344.e3336. doi: 10.1016/j.celrep.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 8.Zorzan I., Pellegrini M., Arboit M., Incarnato D., Maldotti M., Forcato M., Tagliazucchi G.M., Carbognin E., Montagner M., Oliviero S., et al. The transcriptional regulator ZNF398 mediates pluripotency and epithelial character downstream of TGF-beta in human PSCs. Nat. Commun. 2020;11:2364. doi: 10.1038/s41467-020-16205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong Y., Zhang M., Li Y. Recent Advances in the Development of CBP/p300 Bromodomain Inhibitors. Curr. Med. Chem. 2020;27:5583–5598. doi: 10.2174/0929867326666190731141055. [DOI] [PubMed] [Google Scholar]
- 10.He Z.-X., Wei B.-F., Zhang X., Gong Y.-P., Ma L.-Y., Zhao W. Current development of CBP/p300 inhibitors in the last decade. Eur. J. Med. Chem. 2021;209:112861. doi: 10.1016/j.ejmech.2020.112861. [DOI] [PubMed] [Google Scholar]
- 11.Kaczmarska Z., Ortega E., Goudarzi A., Huang H., Kim S., Marquez J.A., Zhao Y., Khochbin S., Panne D. Structure of p300 in complex with acyl-CoA variants. Nat. Chem. Biol. 2017;13:21–29. doi: 10.1038/nchembio.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma S., Poetz F., Bruer M., Ly-Hartig T.B., Schott J., Seraphin B., Stoecklin G. Acetylation-Dependent Control of Global Poly(A) RNA Degradation by CBP/p300 and HDAC1/2. Mol. Cell. 2016;63:927–938. doi: 10.1016/j.molcel.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Raisner R., Kharbanda S., Jin L., Jeng E., Chan E., Merchant M., Haverty P.M., Bainer R., Cheung T., Arnott D., et al. Enhancer Activity Requires CBP/P300 Bromodomain-Dependent Histone H3K27 Acetylation. Cell Rep. 2018;24:1722–1729. doi: 10.1016/j.celrep.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 14.Plotnikov A.N., Yang S., Zhou T.J., Rusinova E., Frasca A., Zhou M.-M. Structural insights into acetylated-histone H4 recognition by the bromodomain-PHD finger module of human transcriptional coactivator CBP. Structure. 2014;22:353–360. doi: 10.1016/j.str.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das C., Roy S., Namjoshi S., Malarkey C.S., Jones D.N.M., Kutateladze T.G., Churchill M.E.A., Tyler J.K. Binding of the histone chaperone ASF1 to the CBP bromodomain promotes histone acetylation. Proc. Natl. Acad. Sci. USA. 2014;111:E1072–E1081. doi: 10.1073/pnas.1319122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y.H., Cai K., Xu P.P., Wang L., Huang C.X., Fang Y., Cheng S., Sun X.J., Liu F., Huang J.Y., et al. CREBBP/EP300 mutations promoted tumor progression in diffuse large B-cell lymphoma through altering tumor-associated macrophage polarization via FBXW7-NOTCH-CCL2/CSF1 axis. Signal Transduct. Target. Ther. 2021;6:10. doi: 10.1038/s41392-020-00437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamo A., Pischimarov J., Horn H., Ott G., Rosenwald A., Leich E. The exomic landscape of t(14;18)-negative diffuse follicular lymphoma with 1p36 deletion. Br. J. Haematol. 2018;180:391–394. doi: 10.1111/bjh.15041. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., Xue Y., Shi J., Ahn J., Mi W., Ali M., Wang X., Klein B.J., Wen H., Li W., et al. The ZZ domain of p300 mediates specificity of the adjacent HAT domain for histone H3. Nat. Struct. Mol. Biol. 2018;25:841–849. doi: 10.1038/s41594-018-0114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou X., Wang J., Patel J., Valentine M., Shao Y., Newman S., Sioson E., Tian L., Liu Y., Brady S.W., et al. Exploration of Coding and Non-coding Variants in Cancer Using GenomePaint. Cancer Cell. 2021;39:83–95.e84. doi: 10.1016/j.ccell.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortega E., Rengachari S., Ibrahim Z., Hoghoughi N., Gaucher J., Holehouse A.S., Khochbin S., Panne D. Transcription factor dimerization activates the p300 acetyltransferase. Nature. 2018;562:538–544. doi: 10.1038/s41586-018-0621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang K., Stanfield R.L., Martinez-Yamout M.A., Dyson H.J., Wilson I.A., Wright P.E. Structural basis for cooperative regulation of KIX-mediated transcription pathways by the HTLV-1 HBZ activation domain. Proc. Natl. Acad. Sci. USA. 2018;115:10040–10045. doi: 10.1073/pnas.1810397115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sauer M., Schuldner M., Hoffmann N., Cetintas A., Reiners K.S., Shatnyeva O., Hallek M., Hansen H.P., Gasser S., von Strandmann E.P. CBP/p300 acetyltransferases regulate the expression of NKG2D ligands on tumor cells. Oncogene. 2017;36:933–941. doi: 10.1038/onc.2016.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z., Wang P., Li Y., Peng H., Zhu Y., Mohandas N., Liu J. Interplay between cofactors and transcription factors in hematopoiesis and hematological malignancies. Signal Transduct. Target. Ther. 2021;6:24. doi: 10.1038/s41392-020-00422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedford D.C., Kasper L.H., Fukuyama T., Brindle P.K. Target gene context influences the transcriptional requirement for the KAT3 family of CBP and p300 histone acetyltransferases. Epigenetics. 2010;5:9–15. doi: 10.4161/epi.5.1.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan W.-I., Hannah R.L., Dawson M.A., Pridans C., Foster D., Joshi A., Göttgens B., Van Deursen J.M., Huntly B.J.P. The transcriptional coactivator Cbp regulates self-renewal and differentiation in adult hematopoietic stem cells. Mol. Cell. Biol. 2011;31:5046–5060. doi: 10.1128/MCB.05830-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan B., Yang J., Kim M.Y., Luo H., Cesari N., Yang T., Strouboulis J., Zhang J., Hardison R., Huang S., et al. HDAC1 is required for GATA-1 transcription activity, global chromatin occupancy and hematopoiesis. Nucleic Acids Res. 2021;49:9783–9798. doi: 10.1093/nar/gkab737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Man N., Mas G., Karl D.L., Sun J., Liu F., Yang Q., Torres-Martin M., Itonaga H., Martinez C., Chen S., et al. p300 suppresses the transition of myelodysplastic syndromes to acute myeloid leukemia. JCI Insight. 2021;6:e138478. doi: 10.1172/jci.insight.138478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kung A.L., Rebel V.I., Bronson R.T., Ch’ng L.E., Sieff C.A., Livingston D.M., Yao T.P. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 2000;14:272–277. doi: 10.1101/gad.14.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebel V.I., Kung A.L., Tanner E.A., Yang H., Bronson R.T., Livingston D.M. Distinct roles for CREB-binding protein and p300 in hematopoietic stem cell self-renewal. Proc. Natl. Acad. Sci. USA. 2002;99:14789–14794. doi: 10.1073/pnas.232568499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitsos C.M., Sankar U., Illario M., Colomer-Font J.M., Duncan A.W., Ribar T.J., Reya T., Means A.R. Calmodulin-dependent protein kinase IV regulates hematopoietic stem cell maintenance. J. Biol. Chem. 2005;280:33101–33108. doi: 10.1074/jbc.M505208200. [DOI] [PubMed] [Google Scholar]
- 31.Pattabiraman D.R., McGirr C., Shakhbazov K., Barbier V., Krishnan K., Mukhopadhyay P., Hawthorne P., Trezise A., Ding J., Grimmond S.M., et al. Interaction of c-Myb with p300 is required for the induction of acute myeloid leukemia (AML) by human AML oncogenes. Blood. 2014;123:2682–2690. doi: 10.1182/blood-2012-02-413187. [DOI] [PubMed] [Google Scholar]
- 32.Sandberg M.L., Sutton S.E., Pletcher M.T., Wiltshire T., Tarantino L.M., Hogenesch J.B., Cooke M.P. c-Myb and p300 regulate hematopoietic stem cell proliferation and differentiation. Dev. Cell. 2005;8:153–166. doi: 10.1016/j.devcel.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Horton S.J., Giotopoulos G., Yun H., Vohra S., Sheppard O., Bashford-Rogers R., Rashid M., Clipson A., Chan W.I., Sasca D., et al. Early loss of Crebbp confers malignant stem cell properties on lymphoid progenitors. Nat. Cell Biol. 2017;19:1093–1104. doi: 10.1038/ncb3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aranda-Orgilles B., Saldana-Meyer R., Wang E., Trompouki E., Fassl A., Lau S., Mullenders J., Rocha P.P., Raviram R., Guillamot M., et al. MED12 Regulates HSC-Specific Enhancers Independently of Mediator Kinase Activity to Control Hematopoiesis. Cell Stem Cell. 2016;19:784–799. doi: 10.1016/j.stem.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmstrom S.R., Wijayatunge R., McCrum K., Mgbemena V.E., Ross T.S. Functional Interaction of BRCA1 and CREBBP in Murine Hematopoiesis. iScience. 2019;19:809–820. doi: 10.1016/j.isci.2019.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmer S.N., Zhou Q., Zhou T., Cheng Z., Abboud-Werner S.L., Horn D., Lecocke M., White R., Krivtsov A.V., Armstrong S.A., et al. Crebbp haploinsufficiency in mice alters the bone marrow microenvironment, leading to loss of stem cells and excessive myelopoiesis. Blood. 2011;118:69–79. doi: 10.1182/blood-2010-09-307942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai M.C., Cheng S., Wang X., Hu J.D., Song Y.P., Huang Y.H., Yan Z.X., Jiang Y.J., Fang X.S., Zheng X.Y., et al. CEOP/IVE/GDP alternating regimen compared with CEOP as the first-line therapy for newly diagnosed patients with peripheral T cell lymphoma: Results from a phase 2, multicenter, randomized, controlled clinical trial. Genome Med. 2020;12:41. doi: 10.1186/s13073-020-00739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juskevicius D., Jucker D., Klingbiel D., Mamot C., Dirnhofer S., Tzankov A. Mutations of CREBBP and SOCS1 are independent prognostic factors in diffuse large B cell lymphoma: Mutational analysis of the SAKK 38/07 prospective clinical trial cohort. J. Hematol. Oncol. 2017;10:70. doi: 10.1186/s13045-017-0438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krysiak K., Gomez F., White B.S., Matlock M., Miller C.A., Trani L., Fronick C.C., Fulton R.S., Kreisel F., Cashen A.F., et al. Recurrent somatic mutations affecting B-cell receptor signaling pathway genes in follicular lymphoma. Blood. 2017;129:473–483. doi: 10.1182/blood-2016-07-729954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qu X., Li H., Braziel R.M., Passerini V., Rimsza L.M., Hsi E.D., Leonard J.P., Smith S.M., Kridel R., Press O., et al. Genomic alterations important for the prognosis in patients with follicular lymphoma treated in SWOG study S0016. Blood. 2019;133:81–93. doi: 10.1182/blood-2018-07-865428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah U.A., Chung E.Y., Giricz O., Pradhan K., Kataoka K., Gordon-Mitchell S., Bhagat T.D., Mai Y., Wei Y., Ishida E., et al. North American ATLL has a distinct mutational and transcriptional profile and responds to epigenetic therapies. Blood. 2018;132:1507–1518. doi: 10.1182/blood-2018-01-824607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Y., Ortega-Molina A., Geng H., Ying H.Y., Hatzi K., Parsa S., McNally D., Wang L., Doane A.S., Agirre X., et al. CREBBP Inactivation Promotes the Development of HDAC3-Dependent Lymphomas. Cancer Discov. 2017;7:38–53. doi: 10.1158/2159-8290.CD-16-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Intlekofer A.M., Joffe E., Batlevi C.L., Hilden P., He J., Seshan V.E., Zelenetz A.D., Palomba M.L., Moskowitz C.H., Portlock C., et al. Integrated DNA/RNA targeted genomic profiling of diffuse large B-cell lymphoma using a clinical assay. Blood Cancer J. 2018;8:60. doi: 10.1038/s41408-018-0089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt J., Ramis-Zaldivar J.E., Bonzheim I., Steinhilber J., Muller I., Haake A., Yu S.C., Raffeld M., Fend F., Salaverria I., et al. CREBBP gene mutations are frequently detected in in situ follicular neoplasia. Blood. 2018;132:2687–2690. doi: 10.1182/blood-2018-03-837039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji M.-M., Huang Y.-H., Huang J.-Y., Wang Z.-F., Fu D., Liu H., Liu F., Leboeuf C., Wang L., Ye J., et al. Histone modifier gene mutations in peripheral T-cell lymphoma not otherwise specified. Haematologica. 2018;103:679–687. doi: 10.3324/haematol.2017.182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramis-Zaldivar J.E., Gonzalez-Farre B., Nicolae A., Pack S., Clot G., Nadeu F., Mottok A., Horn H., Song J.Y., Fu K., et al. MAP-kinase and JAK-STAT pathways dysregulation in plasmablastic lymphoma. Haematologica. 2021;106:2682–2693. doi: 10.3324/haematol.2020.271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Ramirez I., Tadros S., Gonzalez-Herrero I., Martin-Lorenzo A., Rodriguez-Hernandez G., Moore D., Ruiz-Roca L., Blanco O., Alonso-Lopez D., Rivas J.L., et al. Crebbp loss cooperates with Bcl2 overexpression to promote lymphoma in mice. Blood. 2017;129:2645–2656. doi: 10.1182/blood-2016-08-733469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashwah H., Schmid C.A., Kasser S., Bertram K., Stelling A., Manz M.G., Muller A. Inactivation of CREBBP expands the germinal center B cell compartment, down-regulates MHCII expression and promotes DLBCL growth. Proc. Natl. Acad. Sci. USA. 2017;114:9701–9706. doi: 10.1073/pnas.1619555114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer S.N., Scuoppo C., Vlasevska S., Bal E., Holmes A.B., Holloman M., Garcia-Ibanez L., Nataraj S., Duval R., Vantrimpont T., et al. Unique and Shared Epigenetic Programs of the CREBBP and EP300 Acetyltransferases in Germinal Center B Cells Reveal Targetable Dependencies in Lymphoma. Immunity. 2019;51:535–547.e539. doi: 10.1016/j.immuni.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bannard O. Scoring a HAT-Trick against Lymphoma. Immunity. 2019;51:420–423. doi: 10.1016/j.immuni.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 51.Pasqualucci L., Dominguez-Sola D., Chiarenza A., Fabbri G., Grunn A., Trifonov V., Kasper L.H., Lerach S., Tang H., Ma J., et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471:189–195. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evrard S.M., Péricart S., Grand D., Amara N., Escudié F., Gilhodes J., Bories P., Traverse-Glehen A., Dubois R., Brousset P., et al. Targeted next generation sequencing reveals high mutation frequency of and in high-grade B-cell lymphoma with and and/or rearrangements. Haematologica. 2019;104:e154–e157. doi: 10.3324/haematol.2018.198572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J., Vlasevska S., Wells V.A., Nataraj S., Holmes A.B., Duval R., Meyer S.N., Mo T., Basso K., Brindle P.K., et al. The CREBBP Acetyltransferase Is a Haploinsufficient Tumor Suppressor in B-cell Lymphoma. Cancer Discov. 2017;7:322–337. doi: 10.1158/2159-8290.CD-16-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hogg S.J., Motorna O., Cluse L.A., Johanson T.M., Coughlan H.D., Raviram R., Myers R.M., Costacurta M., Todorovski I., Pijpers L., et al. Targeting histone acetylation dynamics and oncogenic transcription by catalytic P300/CBP inhibition. Mol. Cell. 2021;81:2183–2200.e2113. doi: 10.1016/j.molcel.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hopken U.E. Targeting HDAC3 in CREBBP-Mutant Lymphomas Counterstrikes Unopposed Enhancer Deacetylation of B-cell Signaling and Immune Response Genes. Cancer Discov. 2017;7:14–16. doi: 10.1158/2159-8290.CD-16-1285. [DOI] [PubMed] [Google Scholar]
- 56.Mondello P., Tadros S., Teater M., Fontan L., Chang A.Y., Jain N., Yang H., Singh S., Ying H.Y., Chu C.S., et al. Selective Inhibition of HDAC3 Targets Synthetic Vulnerabilities and Activates Immune Surveillance in Lymphoma. Cancer Discov. 2020;10:440–459. doi: 10.1158/2159-8290.CD-19-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desmots F., Roussel M., Pangault C., Llamas-Gutierrez F., Pastoret C., Guiheneuf E., Le Priol J., Camara-Clayette V., Caron G., Henry C., et al. Pan-HDAC Inhibitors Restore PRDM1 Response to IL21 in CREBBP-Mutated Follicular Lymphoma. Clin. Cancer Res. 2019;25:735–746. doi: 10.1158/1078-0432.CCR-18-1153. [DOI] [PubMed] [Google Scholar]
- 58.Ding L.W., Sun Q.Y., Tan K.T., Chien W., Mayakonda A., Yeoh A.E.J., Kawamata N., Nagata Y., Xiao J.F., Loh X.Y., et al. Mutational Landscape of Pediatric Acute Lymphoblastic Leukemia. Cancer Res. 2017;77:390–400. doi: 10.1158/0008-5472.CAN-16-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malinowska-Ozdowy K., Frech C., Schönegger A., Eckert C., Cazzaniga G., Stanulla M., zur Stadt U., Mecklenbräuker A., Schuster M., Kneidinger D., et al. KRAS and CREBBP mutations: A relapse-linked malicious liaison in childhood high hyperdiploid acute lymphoblastic leukemia. Leukemia. 2015;29:1656–1667. doi: 10.1038/leu.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mar B.G., Bullinger L.B., McLean K.M., Grauman P.V., Harris M.H., Stevenson K., Neuberg D.S., Sinha A.U., Sallan S.E., Silverman L.B., et al. Mutations in epigenetic regulators including SETD2 are gained during relapse in paediatric acute lymphoblastic leukaemia. Nat. Commun. 2014;5:3469. doi: 10.1038/ncomms4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma X., Edmonson M., Yergeau D., Muzny D.M., Hampton O.A., Rusch M., Song G., Easton J., Harvey R.C., Wheeler D.A., et al. Rise and fall of subclones from diagnosis to relapse in pediatric B-acute lymphoblastic leukaemia. Nat. Commun. 2015;6:6604. doi: 10.1038/ncomms7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oshima K., Khiabanian H., da Silva-Almeida A.C., Tzoneva G., Abate F., Ambesi-Impiombato A., Sanchez-Martin M., Carpenter Z., Penson A., Perez-Garcia A., et al. Mutational landscape, clonal evolution patterns, and role of RAS mutations in relapsed acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA. 2016;113:11306–11311. doi: 10.1073/pnas.1608420113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu S.X., Abdel-Wahab O. Genetic drivers of vulnerability and resistance in relapsed acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA. 2016;113:11071–11073. doi: 10.1073/pnas.1613836113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antic Z., Yu J., Van Reijmersdal S.V., Van Dijk A., Dekker L., Segerink W.H., Sonneveld E., Fiocco M., Pieters R., Hoogerbrugge P.M., et al. Multiclonal complexity of pediatric acute lymphoblastic leukemia and the prognostic relevance of subclonal mutations. Haematologica. 2021;106:3046–3055. doi: 10.3324/haematol.2020.259226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang L., Liu D., Wang N., Ling S., Tang Y., Wu J., Hao L., Luo H., Hu X., Sheng L., et al. Integrated genomic analysis identifies deregulated JAK/STAT-MYC-biosynthesis axis in aggressive NK-cell leukemia. Cell Res. 2018;28:172–186. doi: 10.1038/cr.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nteliopoulos G., Bazeos A., Claudiani S., Gerrard G., Curry E., Szydlo R., Alikian M., Foong H.E., Nikolakopoulou Z., Loaiza S., et al. Somatic variants in epigenetic modifiers can predict failure of response to imatinib but not to second-generation tyrosine kinase inhibitors. Haematologica. 2019;104:2400–2409. doi: 10.3324/haematol.2018.200220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dixon Z.A., Nicholson L., Zeppetzauer M., Matheson E., Sinclair P., Harrison C.J., Irving J.A. CREBBP knockdown enhances RAS/RAF/MEK/ERK signaling in Ras pathway mutated acute lymphoblastic leukemia but does not modulate chemotherapeutic response. Haematologica. 2017;102:736–745. doi: 10.3324/haematol.2016.145177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mullighan C.G., Zhang J., Kasper L.H., Lerach S., Payne-Turner D., Phillips L.A., Heatley S.L., Holmfeldt L., Collins-Underwood J.R., Ma J., et al. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature. 2011;471:235–239. doi: 10.1038/nature09727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J., Yu X., Gong W., Liu X., Park K.-S., Ma A., Tsai Y.-H., Shen Y., Onikubo T., Pi W.-C., et al. EZH2 noncanonically binds cMyc and p300 through a cryptic transactivation domain to mediate gene activation and promote oncogenesis. Nat. Cell Biol. 2022;24:384–399. doi: 10.1038/s41556-022-00850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imayoshi N., Yoshioka M., Tanaka K., Yang S.-M., Akahane K., Toda Y., Hosogi S., Inukai T., Okada S., Maloney D.J., et al. CN470 is a BET/CBP/p300 multi-bromodomain inhibitor and has an anti-tumor activity against MLL-rearranged acute lymphoblastic leukemia. Biochem. Biophys. Res. Commun. 2022;590:49–54. doi: 10.1016/j.bbrc.2021.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirabayashi S., Ohki K., Nakabayashi K., Ichikawa H., Momozawa Y., Okamura K., Yaguchi A., Terada K., Saito Y., Yoshimi A., et al. ZNF384-related fusion genes define a subgroup of childhood B-cell precursor acute lymphoblastic leukemia with a characteristic immunotype. Haematologica. 2017;102:118–129. doi: 10.3324/haematol.2016.151035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gocho Y., Kiyokawa N., Ichikawa H., Nakabayashi K., Osumi T., Ishibashi T., Ueno H., Terada K., Oboki K., Sakamoto H., et al. A novel recurrent EP300-ZNF384 gene fusion in B-cell precursor acute lymphoblastic leukemia. Leukemia. 2015;29:2445–2448. doi: 10.1038/leu.2015.111. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y.F., Wang B.Y., Zhang W.N., Huang J.Y., Li B.S., Zhang M., Jiang L., Li J.F., Wang M.J., Dai Y.J., et al. Genomic Profiling of Adult and Pediatric B-cell Acute Lymphoblastic Leukemia. EBioMedicine. 2016;8:173–183. doi: 10.1016/j.ebiom.2016.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jing Y., Li Y.-F., Wan H., Liu D.-H. Detection of EP300-ZNF384 fusion in patients with acute lymphoblastic leukemia using RNA fusion gene panel sequencing. Ann. Hematol. 2020;99:2611–2617. doi: 10.1007/s00277-020-04251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kayser S., Hills R.K., Langova R., Kramer M., Guijarro F., Sustkova Z., Estey E.H., Shaw C.M., Racil Z., Mayer J., et al. Characteristics and outcome of patients with acute myeloid leukaemia and t(8;16)(p11;p13): Results from an International Collaborative Study. Br. J. Haematol. 2021;192:832–842. doi: 10.1111/bjh.17336. [DOI] [PubMed] [Google Scholar]
- 76.Gervais C., Murati A., Helias C., Struski S., Eischen A., Lippert E., Tigaud I., Penther D., Bastard C., Mugneret F., et al. Acute myeloid leukaemia with 8p11 (MYST3) rearrangement: An integrated cytologic, cytogenetic and molecular study by the groupe francophone de cytogénétique hématologique. Leukemia. 2008;22:1567–1575. doi: 10.1038/leu.2008.128. [DOI] [PubMed] [Google Scholar]
- 77.Ito Y., Bae S.-C., Chuang L.S.H. The RUNX family: Developmental regulators in cancer. Nat. Rev. Cancer. 2015;15:81–95. doi: 10.1038/nrc3877. [DOI] [PubMed] [Google Scholar]
- 78.Yamaguchi Y., Kurokawa M., Imai Y., Izutsu K., Asai T., Ichikawa M., Yamamoto G., Nitta E., Yamagata T., Sasaki K., et al. AML1 is functionally regulated through p300-mediated acetylation on specific lysine residues. J. Biol. Chem. 2004;279:15630–15638. doi: 10.1074/jbc.M400355200. [DOI] [PubMed] [Google Scholar]
- 79.Kitabayashi I., Aikawa Y., Nguyen L.A., Yokoyama A., Ohki M. Activation of AML1-mediated transcription by MOZ and inhibition by the MOZ-CBP fusion protein. EMBO J. 2001;20:7184–7196. doi: 10.1093/emboj/20.24.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haferlach T., Kohlmann A., Klein H.U., Ruckert C., Dugas M., Williams P.M., Kern W., Schnittger S., Bacher U., Löffler H., et al. AML with translocation t(8;16)(p11;p13) demonstrates unique cytomorphological, cytogenetic, molecular and prognostic features. Leukemia. 2009;23:934–943. doi: 10.1038/leu.2008.388. [DOI] [PubMed] [Google Scholar]
- 81.Qian M., Zhang H., Kham S.K., Liu S., Jiang C., Zhao X., Lu Y., Goodings C., Lin T.N., Zhang R., et al. Whole-transcriptome sequencing identifies a distinct subtype of acute lymphoblastic leukemia with predominant genomic abnormalities of EP300 and CREBBP. Genome Res. 2017;27:185–195. doi: 10.1101/gr.209163.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McClure B.J., Heatley S.L., Kok C.H., Sadras T., An J., Hughes T.P., Lock R.B., Yeung D., Sutton R., White D.L. Pre-B acute lymphoblastic leukaemia recurrent fusion, EP300-ZNF384, is associated with a distinct gene expression. Br. J. Cancer. 2018;118:1000–1004. doi: 10.1038/s41416-018-0022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang Y., Mouttet B., Warnatz H.J., Risch T., Rietmann F., Frommelt F., Ngo Q.A., Dobay M.P., Marovca B., Jenni S., et al. The Leukemogenic TCF3-HLF Complex Rewires Enhancers Driving Cellular Identity and Self-Renewal Conferring EP300 Vulnerability. Cancer Cell. 2019;36:630–644.e639. doi: 10.1016/j.ccell.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 84.Pajcini K.V., Xu L., Shao L., Petrovic J., Palasiewicz K., Ohtani Y., Bailis W., Lee C., Wertheim G.B., Mani R., et al. MAFB enhances oncogenic Notch signaling in T cell acute lymphoblastic leukemia. Sci. Signal. 2017;10:eaam6846. doi: 10.1126/scisignal.aam6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan Y., Wang X., Song H., Zhang Y., Zhang R., Li S., Jin W., Chen S., Fang H., Chen Z., et al. A PML/RARα direct target atlas redefines transcriptional deregulation in acute promyelocytic leukemia. Blood. 2021;137:1503–1516. doi: 10.1182/blood.2020005698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yusenko M.V., Trentmann A., Casolari D.A., Abdel Ghani L., Lenz M., Horn M., Dorner W., Klempnauer S., Mootz H.D., Arteaga M.F., et al. C/EBPbeta is a MYB- and p300-cooperating pro-leukemogenic factor and promising drug target in acute myeloid leukemia. Oncogene. 2021;40:4746–4758. doi: 10.1038/s41388-021-01800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song L., Yu B., Yang Y., Liang J., Zhang Y., Ding L., Wang T., Wan X., Yang X., Tang J., et al. Identification of functional cooperative mutations of GNAO1 in human acute lymphoblastic leukemia. Blood. 2021;137:1181–1191. doi: 10.1182/blood.2020005622. [DOI] [PubMed] [Google Scholar]
- 88.Wang L., Gural A., Sun X.-J., Zhao X., Perna F., Huang G., Hatlen M.A., Vu L., Liu F., Xu H., et al. The leukemogenicity of AML1-ETO is dependent on site-specific lysine acetylation. Science. 2011;333:765–769. doi: 10.1126/science.1201662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y., Ning Q., Shi J., Chen Y., Jiang M., Gao L., Huang W., Jing Y., Huang S., Liu A., et al. A novel epigenetic AML1-ETO/THAP10/miR-383 mini-circuitry contributes to t(8;21) leukaemogenesis. EMBO Mol. Med. 2017;9:933–949. doi: 10.15252/emmm.201607180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pi W.-C., Wang J., Shimada M., Lin J.-W., Geng H., Lee Y.-L., Lu R., Li D., Wang G.G., Roeder R.G., et al. E2A-PBX1 functions as a coactivator for RUNX1 in acute lymphoblastic leukemia. Blood. 2020;136:11–23. doi: 10.1182/blood.2019003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giotopoulos G., Chan W.I., Horton S.J., Ruau D., Gallipoli P., Fowler A., Crawley C., Papaemmanuil E., Campbell P.J., Göttgens B., et al. The epigenetic regulators CBP and p300 facilitate leukemogenesis and represent therapeutic targets in acute myeloid leukemia. Oncogene. 2016;35:279–289. doi: 10.1038/onc.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miyamoto R., Okuda H., Kanai A., Takahashi S., Kawamura T., Matsui H., Kitamura T., Kitabayashi I., Inaba T., Yokoyama A. Activation of CpG-Rich Promoters Mediated by MLL Drives MOZ-Rearranged Leukemia. Cell Rep. 2020;32:108200. doi: 10.1016/j.celrep.2020.108200. [DOI] [PubMed] [Google Scholar]
- 93.Cabal-Hierro L., van Galen P., Prado M.A., Higby K.J., Togami K., Mowery C.T., Paulo J.A., Xie Y., Cejas P., Furusawa T., et al. Chromatin accessibility promotes hematopoietic and leukemia stem cell activity. Nat. Commun. 2020;11:1406. doi: 10.1038/s41467-020-15221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheng G., Liu F., Asai T., Lai F., Man N., Xu H., Chen S., Greenblatt S., Hamard P.J., Ando K., et al. Loss of p300 accelerates MDS-associated leukemogenesis. Leukemia. 2017;31:1382–1390. doi: 10.1038/leu.2016.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu S., Liu H., Qin R., Shu Y., Liu Z., Zhang P., Duan C., Hong D., Yu J., Zou L. The cellular senescence of leukemia-initiating cells from acute lymphoblastic leukemia is postponed by beta-Arrestin1 binding with P300-Sp1 to regulate hTERT transcription. Cell Death Dis. 2017;8:e2756. doi: 10.1038/cddis.2017.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shu Y., Zhou X., Qi X., Liu S., Li K., Tan J., Liu Z., Yu J., Zhang P., Zou L. β-Arrestin1 promotes the self-renewal of the leukemia-initiating cell-enriched subpopulation in B-lineage acute lymphoblastic leukemia related to DNMT1 activity. Cancer Lett. 2015;357:170–178. doi: 10.1016/j.canlet.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 97.Zhang L., Zhang R., Wang J., Chen Y., Qiao C., Shi Q., Jin Y., Shen X., Li J., Chen L. Identification of clinical implications and potential prognostic models of chromatin regulator mutations in multiple myeloma. Clin. Epigenetics. 2022;14:93. doi: 10.1186/s13148-022-01314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stewart M.D., Ramani V.C., Sanderson R.D. Shed syndecan-1 translocates to the nucleus of cells delivering growth factors and inhibiting histone acetylation: A novel mechanism of tumor-host cross-talk. J. Biol. Chem. 2015;290:941–949. doi: 10.1074/jbc.M114.608455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Conery A.R., Centore R.C., Neiss A., Keller P.J., Joshi S., Spillane K.L., Sandy P., Hatton C., Pardo E., Zawadzke L., et al. Bromodomain inhibition of the transcriptional coactivators CBP/EP300 as a therapeutic strategy to target the IRF4 network in multiple myeloma. eLife. 2016;5:e10483. doi: 10.7554/eLife.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ito T., Ando H., Suzuki T., Ogura T., Hotta K., Imamura Y., Yamaguchi Y., Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 101.Zhu Y.X., Shi C.X., Bruins L.A., Wang X., Riggs D.L., Porter B., Ahmann J.M., de Campos C.B., Braggio E., Bergsagel P.L., et al. Identification of lenalidomide resistance pathways in myeloma and targeted resensitization using cereblon replacement, inhibition of STAT3 or targeting of IRF4. Blood Cancer J. 2019;9:19. doi: 10.1038/s41408-019-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jin Z., Zhou S., Ye H., Jiang S., Yu K., Ma Y. The mechanism of SP1/p300 complex promotes proliferation of multiple myeloma cells through regulating IQGAP1 transcription. Biomed. Pharmacother. 2019;119:109434. doi: 10.1016/j.biopha.2019.109434. [DOI] [PubMed] [Google Scholar]
- 103.Papaemmanuil E., Gerstung M., Malcovati L., Tauro S., Gundem G., Van Loo P., Yoon C.J., Ellis P., Wedge D.C., Pellagatti A., et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616–3627. doi: 10.1182/blood-2013-08-518886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kojima K., Kaneda K., Yoshida C., Dansako H., Fujii N., Yano T., Shinagawa K., Yasukawa M., Fujita S., Tanimoto M. A novel fusion variant of the MORF and CBP genes detected in therapy-related myelodysplastic syndrome with t(10;16)(q22;p13) Br. J. Haematol. 2003;120:271–273. doi: 10.1046/j.1365-2141.2003.04059.x. [DOI] [PubMed] [Google Scholar]
- 105.Taki T., Sako M., Tsuchida M., Hayashi Y. The t(11;16)(q23;p13) translocation in myelodysplastic syndrome fuses the MLL gene to the CBP gene. Blood. 1997;89:3945–3950. doi: 10.1182/blood.V89.11.3945. [DOI] [PubMed] [Google Scholar]
- 106.Rowley J.D., Reshmi S., Sobulo O., Musvee T., Anastasi J., Raimondi S., Schneider N.R., Barredo J.C., Cantu E.S., Schlegelberger B., et al. All patients with the T(11;16)(q23;p13.3) that involves MLL and CBP have treatment-related hematologic disorders. Blood. 1997;90:535–541. doi: 10.1182/blood.V90.2.535. [DOI] [PubMed] [Google Scholar]
- 107.Lavau C., Du C., Thirman M., Zeleznik-Le N. Chromatin-related properties of CBP fused to MLL generate a myelodysplastic-like syndrome that evolves into myeloid leukemia. EMBO J. 2000;19:4655–4664. doi: 10.1093/emboj/19.17.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zimmer S.N., Lemieux M.E., Karia B.P., Day C., Zhou T., Zhou Q., Kung A.L., Suresh U., Chen Y., Kinney M.C., et al. Mice heterozygous for CREB binding protein are hypersensitive to γ-radiation and invariably develop myelodysplastic/myeloproliferative neoplasm. Exp. Hematol. 2012;40:295–306.e295. doi: 10.1016/j.exphem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu J., Li B., Yang L., Ren N., Xu M., Huang Q. Increasing Genome Editing Efficiency of Cas9 Nucleases by the Simultaneous Use of Transcriptional Activators and Histone Acetyltransferase Activator. CRISPR J. 2022;5:854–867. doi: 10.1089/crispr.2022.0001. [DOI] [PubMed] [Google Scholar]
- 110.Dastjerdi M.N., Salahshoor M.R., Mardani M., Hashemibeni B., Roshankhah S. The effect of CTB on P53 protein acetylation and consequence apoptosis on MCF-7 and MRC-5 cell lines. Adv. Biomed. Res. 2013;2:24. doi: 10.4103/2277-9175.108005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chatterjee S., Mizar P., Cassel R., Neidl R., Selvi B.R., Mohankrishna D.V., Vedamurthy B.M., Schneider A., Bousiges O., Mathis C., et al. A novel activator of CBP/p300 acetyltransferases promotes neurogenesis and extends memory duration in adult mice. J. Neurosci. 2013;33:10698–10712. doi: 10.1523/JNEUROSCI.5772-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wei W., Song Z., Chiba M., Wu W., Jeong S., Zhang J.-P., Kadin M.E., Nakagawa M., Yang Y. Analysis and therapeutic targeting of the EP300 and CREBBP acetyltransferases in anaplastic large cell lymphoma and Hodgkin lymphoma. Leukemia. 2022;37:396–407. doi: 10.1038/s41375-022-01774-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Crawford T.D., Romero F.A., Lai K.W., Tsui V., Taylor A.M., de Leon Boenig G., Noland C.L., Murray J., Ly J., Choo E.F., et al. Discovery of a Potent and Selective in Vivo Probe (GNE-272) for the Bromodomains of CBP/EP300. J. Med. Chem. 2016;59:10549–10563. doi: 10.1021/acs.jmedchem.6b01022. [DOI] [PubMed] [Google Scholar]
- 114.Castillo J., Wu E., Lowe C., Srinivasan S., McCord R., Wagle M.C., Jayakar S., Edick M.G., Eastham-Anderson J., Liu B., et al. CBP/p300 Drives the Differentiation of Regulatory T Cells through Transcriptional and Non-Transcriptional Mechanisms. Cancer Res. 2019;79:3916–3927. doi: 10.1158/0008-5472.CAN-18-3622. [DOI] [PubMed] [Google Scholar]
- 115.Ryan K.R., Giles F., Morgan G.J. Targeting both BET and CBP/EP300 proteins with the novel dual inhibitors NEO2734 and NEO1132 leads to anti-tumor activity in multiple myeloma. Eur. J. Haematol. 2021;106:90–99. doi: 10.1111/ejh.13525. [DOI] [PubMed] [Google Scholar]
- 116.Baragano Raneros A., Rodriguez R.M., Bernardo Florez A., Palomo P., Colado E., Minguela A., Suarez Alvarez B., Lopez-Larrea C. Bromodomain protein BRD4 is an epigenetic activator of B7-H6 expression in acute myeloid leukemia. Oncoimmunology. 2021;10:1897294. doi: 10.1080/2162402X.2021.1897294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abruzzese M.P., Bilotta M.T., Fionda C., Zingoni A., Soriani A., Vulpis E., Borrelli C., Zitti B., Petrucci M.T., Ricciardi M.R., et al. Inhibition of bromodomain and extra-terminal (BET) proteins increases NKG2D ligand MICA expression and sensitivity to NK cell-mediated cytotoxicity in multiple myeloma cells: Role of cMYC-IRF4-miR-125b interplay. J. Hematol. Oncol. 2016;9:134. doi: 10.1186/s13045-016-0362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Agnarelli A., Mitchell S., Caalim G., Wood C.D., Milton-Harris L., Chevassut T., West M.J., Mancini E.J. Dissecting the impact of bromodomain inhibitors on the Interferon Regulatory Factor 4-MYC oncogenic axis in multiple myeloma. Hematol. Oncol. 2022;40:417–429. doi: 10.1002/hon.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hugle M., Lucas X., Ostrovskyi D., Regenass P., Gerhardt S., Einsle O., Hau M., Jung M., Breit B., Gunther S., et al. Beyond the BET Family: Targeting CBP/p300 with 4-Acyl Pyrroles. Angew. Chem. Int. Ed. Engl. 2017;56:12476–12480. doi: 10.1002/anie.201705516. [DOI] [PubMed] [Google Scholar]
- 120.Lasko L.M., Jakob C.G., Edalji R.P., Qiu W., Montgomery D., Digiammarino E.L., Hansen T.M., Risi R.M., Frey R., Manaves V., et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature. 2017;550:128–132. doi: 10.1038/nature24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang Y., Zhang R., Li Z., Mei L., Wan S., Ding H., Chen Z., Xing J., Feng H., Han J., et al. Discovery of Highly Potent, Selective, and Orally Efficacious p300/CBP Histone Acetyltransferases Inhibitors. J. Med. Chem. 2020;63:1337–1360. doi: 10.1021/acs.jmedchem.9b01721. [DOI] [PubMed] [Google Scholar]
- 122.Diesch J., Le Pannérer M.-M., Winkler R., Casquero R., Muhar M., van der Garde M., Maher M., Herráez C.M., Bech-Serra J.J., Fellner M., et al. Inhibition of CBP synergizes with the RNA-dependent mechanisms of Azacitidine by limiting protein synthesis. Nat. Commun. 2021;12:6060. doi: 10.1038/s41467-021-26258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shirakawa K., Wang L., Man N., Maksimoska J., Sorum A.W., Lim H.W., Lee I.S., Shimazu T., Newman J.C., Schröder S., et al. Salicylate, diflunisal and their metabolites inhibit CBP/p300 and exhibit anticancer activity. eLife. 2016;5:e11156. doi: 10.7554/eLife.11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang P., Wang Z., Liu J. Role of HDACs in normal and malignant hematopoiesis. Mol. Cancer. 2020;19:5. doi: 10.1186/s12943-019-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang M.C., Fang Y., Wang L., Cheng S., Fu D., He Y., Zhao Y., Wang C.F., Jiang X.F., Song Q., et al. Clinical efficacy and molecular biomarkers in a phase II study of tucidinostat plus R-CHOP in elderly patients with newly diagnosed diffuse large B-cell lymphoma. Clin Epigenetics. 2020;12:160. doi: 10.1186/s13148-020-00948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.A Prospective, Single Arm, Open-Label, Phase II Study of Chidamide in Combination with R-CHOP in the Treatment of de Novo, Elderly, High-Risk Diffuse Large B-Cell Lymphoma. [(accessed on 1 February 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02753647.
- 127.Veazey K.J., Cheng D., Lin K., Villarreal O.D., Gao G., Perez-Oquendo M., Van H.T., Stratton S.A., Green M., Xu H., et al. CARM1 inhibition reduces histone acetyltransferase activity causing synthetic lethality in CREBBP/EP300-mutated lymphomas. Leukemia. 2020;34:3269–3285. doi: 10.1038/s41375-020-0908-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Drew A.E., Moradei O., Jacques S.L., Rioux N., Boriack-Sjodin A.P., Allain C., Scott M.P., Jin L., Raimondi A., Handler J.L., et al. Identification of a CARM1 Inhibitor with Potent In Vitro and In Vivo Activity in Preclinical Models of Multiple Myeloma. Sci. Rep. 2017;7:17993. doi: 10.1038/s41598-017-18446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Greenblatt S.M., Man N., Hamard P.-J., Asai T., Karl D., Martinez C., Bilbao D., Stathias V., Jermakowicz A.M., Duffort S., et al. CARM1 Is Essential for Myeloid Leukemogenesis but Dispensable for Normal Hematopoiesis. Cancer Cell. 2018;33:1111–1127.e1115. doi: 10.1016/j.ccell.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mitton B., Chae H.D., Hsu K., Dutta R., Aldana-Masangkay G., Ferrari R., Davis K., Tiu B.C., Kaul A., Lacayo N., et al. Small molecule inhibition of cAMP response element binding protein in human acute myeloid leukemia cells. Leukemia. 2016;30:2302–2311. doi: 10.1038/leu.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Emami K.H., Nguyen C., Ma H., Kim D.H., Jeong K.W., Eguchi M., Moon R.T., Teo J.-L., Oh S.W., Kim H.Y., et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proc. Natl. Acad. Sci. USA. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao Y., Masiello D., McMillian M., Nguyen C., Wu Y., Melendez E., Smbatyan G., Kida A., He Y., Teo J.L., et al. CBP/catenin antagonist safely eliminates drug-resistant leukemia-initiating cells. Oncogene. 2016;35:3705–3717. doi: 10.1038/onc.2015.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiang X., Mak P.Y., Mu H., Tao W., Mak D.H., Kornblau S., Zhang Q., Ruvolo P., Burks J.K., Zhang W., et al. Disruption of Wnt/beta-Catenin Exerts Antileukemia Activity and Synergizes with FLT3 Inhibition in FLT3-Mutant Acute Myeloid Leukemia. Clin. Cancer Res. 2018;24:2417–2429. doi: 10.1158/1078-0432.CCR-17-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.An Open-Label, Dose-Escalation Phase I/II Study of PRI-724 for Patients With Advanced Myeloid Malignancies. [(accessed on 1 December 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01606579.
- 135.Ramaswamy K., Forbes L., Minuesa G., Gindin T., Brown F., Kharas M.G., Krivtsov A.V., Armstrong S.A., Still E., de Stanchina E., et al. Peptidomimetic blockade of MYB in acute myeloid leukemia. Nat. Commun. 2018;9:110. doi: 10.1038/s41467-017-02618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takao S., Forbes L., Uni M., Cheng S., Pineda J.M.B., Tarumoto Y., Cifani P., Minuesa G., Chen C., Kharas M.G., et al. Convergent organization of aberrant MYB complex controls oncogenic gene expression in acute myeloid leukemia. eLife. 2021;10:e65905. doi: 10.7554/eLife.65905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Viziteu E., Grandmougin C., Goldschmidt H., Seckinger A., Hose D., Klein B., Moreaux J. Chetomin, targeting HIF-1α/p300 complex, exhibits antitumour activity in multiple myeloma. Br. J. Cancer. 2016;114:519–523. doi: 10.1038/bjc.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.An Open-Label Phase I/IIa Study to Evaluate the Safety and Efficacy of CCS1477 as Monotherapy and in Combination, in Patients With Advanced Solid/Metastatic Tumours. [(accessed on 1 December 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03568656.
- 139.An Open-Label Phase I/IIa Study to Evaluate the Safety and Efficacy of CCS1477 as Monotherapy and in Combination in Patients With Advanced Haematological Malignancies. [(accessed on 1 December 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04068597.
- 140.A Phase 1 Study of FT-7051 in Men With Metastatic Castration-Resistant Prostate Cancer. [(accessed on 2 February 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04575766.
- 141.Muthengi A., Wimalasena V.K., Yosief H.O., Bikowitz M.J., Sigua L.H., Wang T., Li D., Gaieb Z., Dhawan G., Liu S., et al. Development of Dimethylisoxazole-Attached Imidazo[1,2-a]pyridines as Potent and Selective CBP/P300 Inhibitors. J. Med. Chem. 2021;64:5787–5801. doi: 10.1021/acs.jmedchem.0c02232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lieberman W.K., Jing Y., Meier J.L. Chemical control of multidomain acetyltransferase activity. Cell Chem. Biol. 2021;28:433–435. doi: 10.1016/j.chembiol.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 143.Vannam R., Sayilgan J., Ojeda S., Karakyriakou B., Hu E., Kreuzer J., Morris R., Herrera Lopez X.I., Rai S., Haas W., et al. Targeted degradation of the enhancer lysine acetyltransferases CBP and p300. Cell Chem. Biol. 2021;28:503–514.e512. doi: 10.1016/j.chembiol.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 144.Zhou X.-R., Li X., Liao L.-P., Han J., Huang J., Li J.-C., Tao H.-R., Fan S.-J., Chen Z.-F., Li Q., et al. P300/CBP inhibition sensitizes mantle cell lymphoma to PI3Kδ inhibitor idelalisib. Acta Pharmacol. Sin. 2022;43:457–469. doi: 10.1038/s41401-021-00643-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gao C., Liu S.-G., Lu W.-T., Yue Z.-X., Zhao X.-X., Xing T.-Y., Chen Z.-P., Zheng H.-Y., Li Z.-G. Downregulating CREBBP inhibits proliferation and cell cycle progression and induces daunorubicin resistance in leukemia cells. Mol. Med. Rep. 2020;22:2905–2915. doi: 10.3892/mmr.2020.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sun Y., Gao Y., Chen J., Huang L., Deng P., Chen J., Chai K.X.Y., Hong J.H., Chan J.Y., He H., et al. CREBBP cooperates with the cell cycle machinery to attenuate chidamide sensitivity in relapsed/refractory diffuse large B-cell lymphoma. Cancer Lett. 2021;521:268–280. doi: 10.1016/j.canlet.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 147.Mathur R., Sehgal L., Havranek O., Kohrer S., Khashab T., Jain N., Burger J.A., Neelapu S.S., Davis R.E., Samaniego F. Inhibition of demethylase KDM6B sensitizes diffuse large B-cell lymphoma to chemotherapeutic drugs. Haematologica. 2017;102:373–380. doi: 10.3324/haematol.2016.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tamai M., Kasai S., Akahane K., Thu T.N., Kagami K., Komatsu C., Abe M., Watanabe A., Goi K., Miyake K., et al. Glucocorticoid receptor gene mutations confer glucocorticoid resistance in B-cell precursor acute lymphoblastic leukemia. J. Steroid Biochem. Mol. Biol. 2022;218:106068. doi: 10.1016/j.jsbmb.2022.106068. [DOI] [PubMed] [Google Scholar]
- 149.Gang E.J., Hsieh Y.T., Pham J., Zhao Y., Nguyen C., Huantes S., Park E., Naing K., Klemm L., Swaminathan S., et al. Small-molecule inhibition of CBP/catenin interactions eliminates drug-resistant clones in acute lymphoblastic leukemia. Oncogene. 2014;33:2169–2178. doi: 10.1038/onc.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Duque-Afonso J., Lin C.H., Han K., Morgens D.W., Jeng E.E., Weng Z., Jeong J., Wong S.H.K., Zhu L., Wei M.C., et al. CBP Modulates Sensitivity to Dasatinib in Pre-BCR(+) Acute Lymphoblastic Leukemia. Cancer Res. 2018;78:6497–6508. doi: 10.1158/0008-5472.CAN-18-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee S.M., Bae J.H., Kim M.J., Lee H.S., Lee M.K., Chung B.S., Kim D.W., Kang C.D., Kim S.H. Bcr-Abl-independent imatinib-resistant K562 cells show aberrant protein acetylation and increased sensitivity to histone deacetylase inhibitors. J. Pharmacol. Exp. Ther. 2007;322:1084–1092. doi: 10.1124/jpet.107.124461. [DOI] [PubMed] [Google Scholar]
- 152.Yu T., Gan S., Zhu Q., Dai D., Li N., Wang H., Chen X., Hou D., Wang Y., Pan Q., et al. Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat. Commun. 2019;10:4353. doi: 10.1038/s41467-019-12384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tanaka K., Martinez G.J., Yan X., Long W., Ichiyama K., Chi X., Kim B.-S., Reynolds J.M., Chung Y., Tanaka S., et al. Regulation of Pathogenic T Helper 17 Cell Differentiation by Steroid Receptor Coactivator-3. Cell Rep. 2018;23:2318–2329. doi: 10.1016/j.celrep.2018.04.088. [DOI] [PubMed] [Google Scholar]
- 154.Yahia-Cherbal H., Rybczynska M., Lovecchio D., Stephen T., Lescale C., Placek K., Larghero J., Rogge L., Bianchi E. NFAT primes the human RORC locus for RORgammat expression in CD4(+) T cells. Nat. Commun. 2019;10:4698. doi: 10.1038/s41467-019-12680-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Morelli E., Leone E., Cantafio M.E.G., Di Martino M.T., Amodio N., Biamonte L., Gullà A., Foresta U., Pitari M.R., Botta C., et al. Selective targeting of IRF4 by synthetic microRNA-125b-5p mimics induces anti-multiple myeloma activity in vitro and in vivo. Leukemia. 2015;29:2173–2183. doi: 10.1038/leu.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.de Almeida Nagata D.E., Chiang E.Y., Jhunjhunwala S., Caplazi P., Arumugam V., Modrusan Z., Chan E., Merchant M., Jin L., Arnott D., et al. Regulation of Tumor-Associated Myeloid Cell Activity by CBP/EP300 Bromodomain Modulation of H3K27 Acetylation. Cell Rep. 2019;27:269–281.e264. doi: 10.1016/j.celrep.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 157.Green M.R., Kihira S., Liu C.L., Nair R.V., Salari R., Gentles A.J., Irish J., Stehr H., Vicente-Dueñas C., Romero-Camarero I., et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc. Natl. Acad. Sci. USA. 2015;112:E1116–E1125. doi: 10.1073/pnas.1501199112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xian R.R., Xie Y., Haley L.M., Yonescu R., Pallavajjala A., Pittaluga S., Jaffe E.S., Duffield A.S., McCall C.M., Gheith S.M.F., et al. CREBBP and STAT6 co-mutation and 16p13 and 1p36 loss define the t(14;18)-negative diffuse variant of follicular lymphoma. Blood Cancer J. 2020;10:69. doi: 10.1038/s41408-020-0335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Békés M., Langley D.R., Crews C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022;21:181–200. doi: 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Durbin A.D., Wang T., Wimalasena V.K., Zimmerman M.W., Li D., Dharia N.V., Mariani L., Shendy N.A.M., Nance S., Patel A.G., et al. EP300 Selectively Controls the Enhancer Landscape of MYCN-Amplified Neuroblastoma. Cancer Discov. 2022;12:730–751. doi: 10.1158/2159-8290.CD-21-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kagoya Y., Nakatsugawa M., Yamashita Y., Ochi T., Guo T., Anczurowski M., Saso K., Butler M.O., Arrowsmith C.H., Hirano N. BET bromodomain inhibition enhances T cell persistence and function in adoptive immunotherapy models. J. Clin. Investig. 2016;126:3479–3494. doi: 10.1172/JCI86437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nielsen J.S., Sedgwick C.G., Shahid A., Zong Z., Brumme Z.L., Yu S., Liu L., Kroeger D.R., Treon S.P., Connors J.M., et al. Toward Personalized Lymphoma Immunotherapy: Identification of Common Driver Mutations Recognized by Patient CD8+ T Cells. Clin. Cancer Res. 2016;22:2226–2236. doi: 10.1158/1078-0432.CCR-15-2023. [DOI] [PubMed] [Google Scholar]