Abstract
Simple Summary
At Istituto Oncologico Veneto we are providing a geriatric assessment to all patients aged 70 years and older since 2003. Soft tissue sarcoma are really rare neoplasm and we, as a referral centre, evaluate a high volume of patients, so we decided to conduct this study to describe the geriatric multidisciplinary management and also the role the geriatric tools in the decision making and in assessing the prognosis.
Abstract
Background: Incidences of soft tissue sarcomas (STS) steadily increase with age. Yet, despite the high prevalence in advanced age, older patients (pts) are underrepresented in sarcoma clinical trials and evidence-based guidelines for chemotherapy are lacking. International oncological societies suggest using geriatric tools to evaluate older patients with cancer to optimise treatment indication. Comprehensive geriatric assessment (CGA) is a multidimensional assessment of older subjects, based on which pts can be classified as fit, vulnerable or frail. Onco-MPI (multidimensional prognostic index) is a CGA-based score which also considers tumour characteristics, classifying pts into three risk groups of death at one year: high-risk, intermediate-risk and low-risk. Methods: This is a single-centre retrospective study which aims at describing real-word management and outcomes of older pts with advanced stage STS and at assessing the ability of CGA and onco-MPI to predict survival in these pts. Consecutive pts with advanced stage STS aged 70 years or older and treated at the Istituto Oncologico Veneto from January 2009 to June 2020 were retrieved from a prospectively maintained database. Pts’ demographics, CGA assessments and tumour characteristics were analysed. Statistical analysis was performed with R version 3.4.3 Results: Out of 101 pts, with a median age of 77 years, 76 received chemotherapy (75.3%), which was anthracycline-based for 46 pts (60.5%). Anthracyclines were used in a higher proportion in fit pts (58.9% fit vs. 45.1% vulnerable vs. 12.5% frail pts). Frail pts and pts in the onco-MPI high-risk group experienced a higher rate of chemotherapy-related toxicities. Median OS was 13.8 months (95% CI 11.3–17.7 months). According to CGA, the median OS was 19.53 months (95% CI 15.23–36.8) for fit pts, 12.83 months (95% CI 9.7–17.5) for vulnerable and 7.75 months (95% CI 2.73–30) for frail pts (p = 0.005). Onco-MPI confirmed a predictive value for 1-year survival with intermediate risk pts not reaching a median OS at 1 year, and high-risk pts having a median one-year OS of 11.5 months (95%CI 9.7–NA), p = 0.02. In multivariate analysis, onco-MPI and CGA were associated with survival (high risk onco-MPI: HR 5.5, 95%CI 1.25–24.7 p = 0.02; fitness at CGA HR 0.552 95% 0.314–0.973; p = 0.040) as well as chemotherapy use (HR 0.24, 95% CI 0.11–0.51, p < 0.005). Conclusions: Both CGA and onco-MPI retain prognostic value for survival in pts with metastatic STS. Pts frail/vulnerable at CGA and pts within the onco-MPI high risk category should be offered an oncogeriatric management approach in order to optimise treatment-related survival and reduce toxicity.
Keywords: onco-MPI, CGA, geriatric assessment, elderly, sarcoma, chemotherapy
1. Introduction
Soft tissue sarcomas (STS) are rare neoplasms accounting for approximately 1% of cancer diagnoses in the adult population [1]. The incidence steadily increases with age, showing a first peak in the adolescent and young adult population and a second peak above 75 years of age [2].
Currently, more than 60% of all cancer diagnoses and 70% of cancer-related deaths occur in elders [3]. With regard to soft tissue and bone sarcomas, older subjects commonly present with a higher prevalence of biologically aggressive subtypes, with higher grade and more advanced disease at diagnosis, and one-year mortality due to sarcoma increases with age [4,5].
Despite the high prevalence in advanced age, older patients are underrepresented in sarcoma clinical trials and evidence-based guidelines are lacking [6].
Older patients with advanced STS, even if highly selected to enter clinical trials, have worse outcomes compared to younger patients; indeed, there is evidence that in routine clinical practice, chemotherapy is denied to a high proportion of patients older than 75 years with advanced STS. Older age (80 y), performance status ≥2 and a high Charlson comorbidity index (≥10) are characteristics associated with the choice of best supportive care [6,7].
Both European and American oncological society guidelines suggest using geriatric tools to evaluate older patients undergoing chemotherapy, and the International Society of Geriatric Oncology (SIOG) has been recommending some form of geriatric assessment since 2005 [8,9,10].
A comprehensive geriatric assessment (CGA) is a multidimensional assessment of an older person which considers health and wellbeing and formulates a plan to address issues which are of concern to the older subject, as well as to their family and caregivers when relevant, and arranges interventions according to the plan. Though there is no standard CGA, most of the used approaches evaluate patients’ abilities in the daily life and instrumental daily life activities, cognitive status, the presence of mood disorders, nutritional status, concomitant medications and comorbidities, the presence and the role of the caregiver and classify patients in either fit or unfit and who might be vulnerable or frail, according to performance in such domains (Figure 1) [11]. CGA adds crucial information on functional assessment, emotional and social aspects of older patients which may compromise quality of life and cancer treatments, and it has long known to be more performant than classical Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) [12,13].
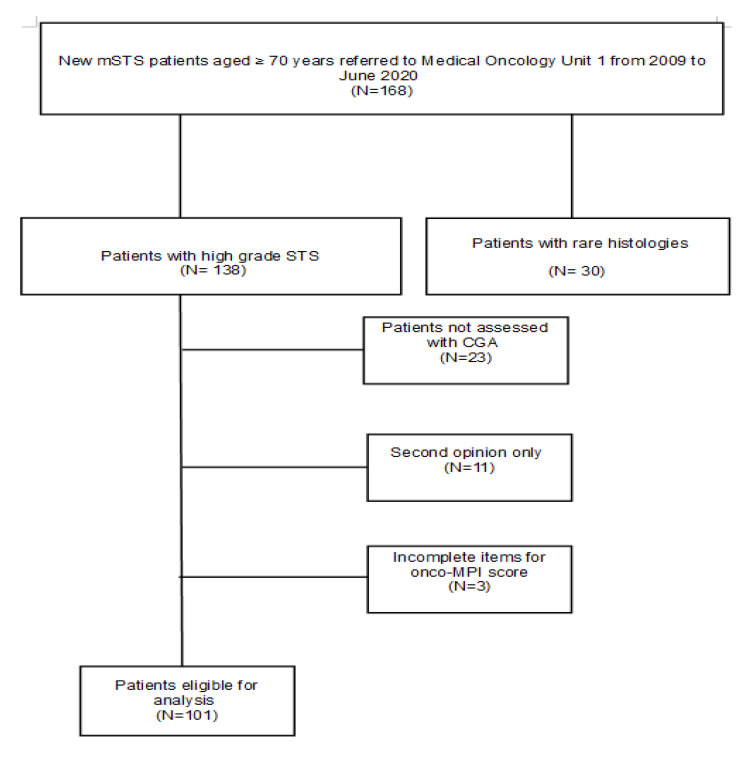
Figure 1.
CONSORT flow diagram.
Single items of CGA, such as functional impairment, malnutrition, depressive symptoms, comorbidities, showed to be independently associated to toxicity from chemotherapy and overall survival [14].
Indeed, assessing life expectancy is crucial to help clinicians make fully informed clinical decisions, especially in older patients for whom chronic conditions are competing risk factors for mortality [15].
More recently, an oncological multidimensional prognostic index (onco-MPI) has been developed and validated on the basis of a CGA, which takes into account age, sex, body mass index (BMI), functional impairment, comorbidities, cancer stage, ECOG PS, social status and tumour site. Onco-MPI classifies older patients into three prognostic categories: low, medium and high risk (Figure 2). Onco-MPI has been demonstrated to predict survival probability at one year with a very good discriminatory power and calibration [16].
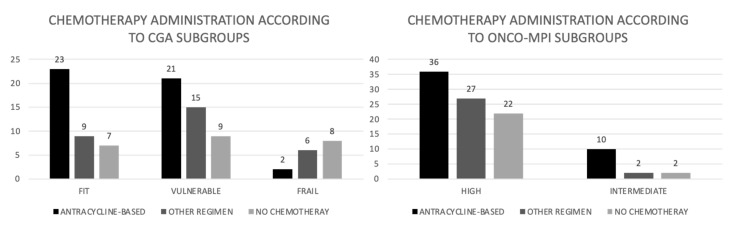
Figure 2.
Chemotherapy administration according to CGA and onco-MPI subgroups (absolute numbers).
The predictive and prognostic role of CGA-derived scores has been assessed in several specific cancer types but, as of today, no data on the role of geriatric assessment in decision making for older patients with soft tissue sarcomas are available [17,18,19,20,21].
Our institution has been providing a geriatric assessment to all patients aged 70 years and older, seen as new patients, since 2003. For patients deemed vulnerable or frail at CGA, as well as for fit patients with specific needs, patients are referred to the geriatric service in order to provide geriatric intervention, mainly focused on managing specific impairments, such as polipharmacology, comorbidities and involvement of caregivers for cognitive impairment, as well as for general supportive care.
In light of these considerations, we investigated real-word management and outcomes of older patients with advanced stage soft tissue sarcoma treated at our institution and the prognostic role of CGA and onco-MPI in these patients.
2. Patients and Methods
This is a single-centre retrospective study whose aims are describing real-word management and outcomes of older patients with advanced stage soft tissue sarcoma and at assessing the ability of CGA and onco-MPI to predict overall survival and one-year survival in these patients. Consecutive patients with advanced stage STS aged 70 years or older, assessed by means of CGA as per institutional practice and treated at the Istituto Oncologico Veneto from January 2009 to June 2020, were eligible.
The exclusion criteria were: STS pathological subtypes which are not routinely treated with chemotherapy, missing CGA data, single consultation and pts lost to follow-up. Date of diagnosis, age at diagnosis, histological data, PS, site of primary tumour and metastases, first treatment approach, response to treatments, treatment-related toxicities and social, nutritional, psychological and functional aspects were collected from electronic health records and prospectively maintained.
The following CGA domains were considered along with tests used to assess the domain: functional status, through activity of daily living (ADL) and instrumental activity of daily living (IADL), number of comorbid conditions and their severity, through the cumulative illness rating scale (CIRS), living conditions and presence of caregiver, cognitive status through the mini-mental state examination (MMSE) questionnaire, emotional status through the geriatric depression scale (GDS), polipharmacy, nutritional status through the BMI and mini-nutritional assessment (MNA) and the presence of geriatric syndromes [22,23,24,25,26,27,28]. Patients were classified into risk categories according to both Balducci’s criteria and onco-MPI, as previously published (Table 1), with the three categories of fit, vulnerable and frail for Balducci’s criteria and the three categories of low risk (score 0.0–0.46), intermediate risk (score 0.47–0.63) and high risk (score 0.64–1.0) for the onco-MPI (Table 2) [11,16].
Table 1.
Classification of patients according to Balducci’s criteria.
| Fit | Vulnerable | Frail |
|---|---|---|
|
|
|
Legend: ADL = Activities of Daily Living; IADL = Instrumental Activities of Daily Living.
Table 2.
Onco-MPI algorithm [16].
| Domains Onco-MPI | Category | Coefficient |
|---|---|---|
| Age (≥70 years) | continuous variable | 0.0473 |
| Sex | 0 F | 0 |
| 1 M | 0.01706 | |
| BMI (Kg/m2) | continuous variable | −0.09782 |
| ADL | continuous variable | −0.07717 |
| IADL | continuous variable | 0.04983 |
| Performance status | continuous variable | 0.70607 |
| N° of severe comorbidities (CIRS) | continuous variable | −0.1296 |
| Cancer stage | 1 | 0 |
| 2 | 1.11712 | |
| 3 | 0.74957 | |
| 4 | 1.80828 | |
| Tumour site | other | 0 |
| Breast | −1.93081 | |
| Colorectal | −1.03025 | |
| Lung | 0.36265 | |
| Prostate | −1.57998 | |
| other GU | 0.19956 | |
| MMSE | <24 | 0 |
| ≥24 | 0.0627 | |
| N° of drugs | continuous variable | −0.01218 |
| Caregiver | No | 0 |
| Yes | 0.21035 |
Legend: BMI = Body Mass Index; ADL = Activities of daily living; IADL = Instrumental activities of daily living; CIRS = Cumulative illness rating scale; GU = genitourinary; MMSE = Mini-mental state examination.
Overall survival (OS) was estimated with the Kaplan–Meier method and compared with the log-rank test and Cox proportional hazards method for multivariate analysis. Cox’s proportional hazard assumptions have been graphically verified and are respected for the variables considered in the model. OS was calculated from diagnosis of metastatic disease to death for any cause. The survival status of patients lost to follow-up was obtained through demographic registries.
The study was approved by the Ethics Committee of the Istituto Oncologico Veneto. Statistical analysis was performed with R software version 3.4.3.
3. Results
A total of 168 patients with a diagnosis of advanced/metastatic or locally advanced STS, aged 70 years or older, were identified, of whom 30 were excluded due to disease characteristics. Other patients (N = 37) were excluded because of missing data for CGA and/or onco-MPI, or because of a single access to the institution for consultation. In total, one hundred and one patients were eligible (Figure 1).
Patients’ characteristics are shown in Table 3.
Table 3.
Patient’s characteristics (N = 101).
| Characteristics | Categories | N (%) |
|---|---|---|
| Age | 77 (±4.3) | |
| Sex | Male/female | 51/42 |
| Histology | Liposarcoma | 26 (27.9%) |
| Leiomyosarcoma | 24 (25.8%) | |
| UPS | 14 (15.0%) | |
| Other | 29 (33.1%) | |
| Primary site | Extremities/trunk | 38 (40.8%) |
| Abdomen | 29 (31.1%) | |
| Chest | 6 (0.6%) | |
| Other | 20 (21.5%) | |
| Metastatic sites | Lung | 24 (25.8%) |
| Other | 35 (37.6%) | |
| Lung and other | 34 (36.6%) | |
| PS ECOG | 0–1 | 76 (75.2%) |
| ≥2 | 25 (24.8%) | |
| CIRS | 0–2 | |
| >2 | ||
| N. of medications | ≤3 | 52 (51.4%) |
| >3 | 49 (48.6%) | |
| 1st line chemotherapy | Anthracycline-based | 44 (47.3%) |
| CM | 11 (11.8%) | |
| Other | 15 (16.1%) | |
| CGA | Fit | 39 (38.6%) |
| Vulnerable | 46 (45.6%) | |
| Frail | 16 (15.8%) | |
| Onco-MPI | Low | 0 (0%) |
| Intermediate | 14 (13.9%) | |
| High | 87 (86.1%) |
Legend: ADL = Activities of daily living; CGA = Comprehensive geriatric assessment; CIRS = Cumulative illness rating scale; ECOG = Eastern cooperative oncology group; PS = Performance status.
The median age was 77 years (range 70–91 years), with 66 patients (65.3%) being aged 75 years and older. Primary tumour sites were the extremities or trunk for 44 patients (43.5%); the most frequent histological subtypes were liposarcoma (27 patients) and leiomyosarcoma (26 patients) and the most frequent metastatic site was the lungs (64 patients, 63.3%).
Out of 101 patients, 76 received chemotherapy (75.3%), which was anthracycline-based for 46 patients.
According to the CGA categories, 39 patients were fit (38.6%), 46 were vulnerable (45.6%), 16 were frail (15.8%); according to onco-MPI, 87 patients (86.1%) were in the high-risk category, 14 (13.9%) were in the intermediate risk and no patients were in the low-risk category.
Chemotherapy was administered to 82% of the patients in the fit group, in 80% of patients in the vulnerable group and 43% of frail patients (p = 0.016). Anthracyclines were used in 23 (58.9%), 21 (45.1%) and 2 (12.5%) patients of the fit, vulnerable and frail group, respectively (p = 0.07) (Figure 2). Toxicities and upfront dose reductions due to toxicities are reported in Table 4.
Table 4.
Rates of severe toxicities, upfront dose reduction and access to second line treatment according to CGA category and onco-MPI risk groups (% of pts).
| N. Patients Receiving First Line CT | FIT (32 pts) | Vulnerable (36 pts) | Frail (8 pts) | Intermediate Risk (12 pts) | High Risk (63 pts) |
|---|---|---|---|---|---|
| G3-G4 Toxicity | 40.6% | 33.3% | 51.1% | 58.3% | 36.5% |
| Upfront Dose Reduction | 34.4% | 44.4% | 42.8% | 66% | 39.4% |
| Dose Reduction due to Toxicity | 12.5% | 8.3% | 12.5% | 33.3% | 15.8% |
| Second Line CT | 38.4% | 34.7% | 12.5% | 66% | 58.7% |
Legend: CGA = Comprehensive geriatric assessment; CT = Chemotherapy; G3–G4 = Grade 3 or 4 according to Common Terminology Criteria for Adverse Events.
First line chemotherapy was administered to 85.7% of patients in the intermediate risk group, according to onco-MPI, and in 74.1% of high-risk group; an anthracycline- based chemotherapy was administered in 71.4% of patients in the intermediate risk group and in 42.3% in the high-risk group (p = 0.046) (Figure 2).
Toxicities and dose reduction are also reported in Table 4.
The median follow-up was 32.4 months (95% CI 0–202); median OS was 13.8 months (95% CI 11.3–17.7).
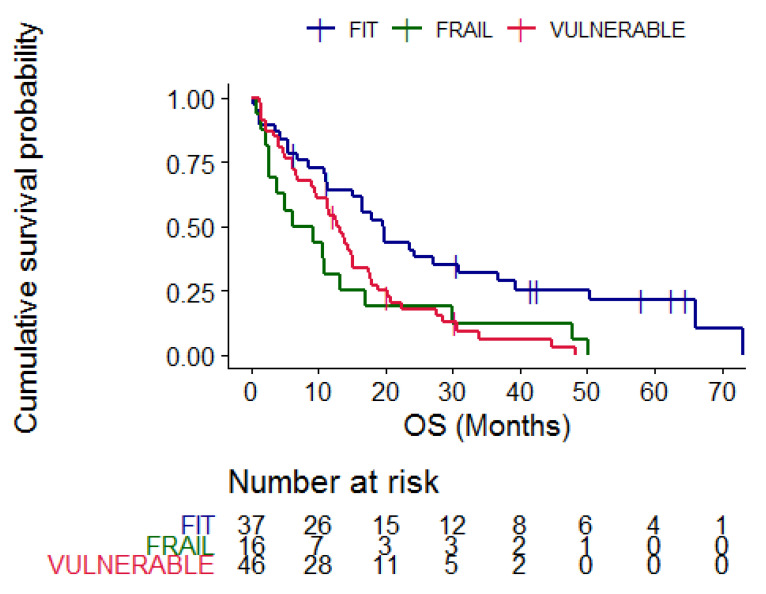
According to CGA categories, the median OS of fit patients was 19.53 months (95% CI 15.23–36.8), compared to 12.83 months (95% CI 9.7–17.5) in vulnerable and 7.75 months (95% CI 2.73–30) in frail patients (p = 0.005) (Figure 3).
Figure 3.
Kaplan–Meier curves representing overall survival according to CGA categories, blue = fit patients, red = vulnerable patients and green = frail patients. p = 0.005.
In univariate analysis, patients with ECOG PS ≥ 2 showed the worst survival, HR 2.34 p < 0.001; fit patients (HR 0.47, 95% CI 0.29–0.75) and patients who received first line chemotherapy (HR 0.25, 95% CI 0.15–0.4) had better survival (p < 0.005) (Table 5). Receiving anthracycline-based chemotherapy was not associated with an advantage in survival (HR = 0.84, CI 0.5459–1.294, p = 0.429).
Table 5.
Univariate and multivariate analysis for overall survival.
| Univariate Analysis for Overall Survival | |||||
| Variable | HR | Confidence Interval | p Value | ||
| Sex | M | 0.378 | 0.948 | 12.250 | 0.0858 |
| Histology | Leiomyosarcoma | 0.681 | 0.418 | 1.111 | 0.124 |
| Age | 75–80 | 1.163 | 0.702 | 1.927 | 0.557 |
| Age | ≥80 | 1.025 | 0.605 | 1.736 | 0.928 |
| Location metastasis | Lung | 0.822 | 0.502 | 1.347 | 0.437 |
| Location of primary tumour | Extremities/trunk | 0.940 | 0.581 | 1.521 | 0.800 |
| Location of primary tumour | Other | 0.327 | 0.549 | 1.761 | 0.955 |
| PS at metastatic disease | ≥2 | 2.345 | 1.449 | 3.794 | <0.001 |
| Comorbidity grade according to CIRS | 3–4 | 1.511 | 0.898 | 2.543 | 0.120 |
| CGA | Fit | 0.471 | 0.293 | 0.755 | 0.001 |
| Onco-MPI | High-risk | 1.300 | 0.670 | 2.526 | 0.438 |
| First line chemotherapy | Yes | 0.251 | 0.153 | 0.414 | <0.001 |
| Multivariate Analysis for Overall Survival | |||||
| Variable | HR | Confidence Interval | p-Value | ||
| Sex | Male | 1.4047 | 0.826 | 2.388 | 0.2096 |
| Histology | Leiomyosarcoma | 0.7135 | 0.396 | 1.285 | 0.2604 |
| Age | ≥80 | 0.718 | 0.396 | 1.301 | 0.2746 |
| Age | 75–80 | 0.7542 | 0.406 | 1.399 | 0.3707 |
| Location of metastasis | Lung | 0.774 | 0.441 | 1.357 | 0.3713 |
| Location of primary tumour | Retroperitoneum | 1.0266 | 0.565 | 1.866 | 0.9313 |
| Location of primary tumour | other | 0.9905 | 0.516 | 1.900 | 0.9771 |
| PS at metastatic disease | ≥2 | 1.5004 | 0.760 | 2.962 | 0.2424 |
| Comorbidity grade according to CIRS | 3–4 | 1.2159 | 0.664 | 2.226 | 0.5263 |
| First line chemotherapy | Yes | 0.3634 | 0.195 | 0.676 | 0.0014 |
| CGA | Fit | 0.5527 | 0.314 | 0.973 | 0.040 |
Legend: CGA = Comprehensive geriatric assessment; CIRS = Cumulative illness rating scale; ECOG = Eastern cooperative oncology group; PS = Performance status.
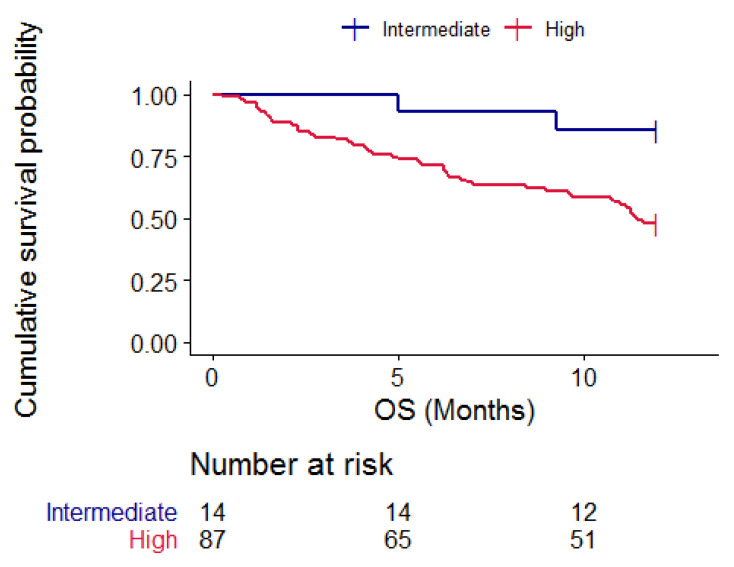
The onco-MPI score was a predictor of one-year survival; in fact, patients with intermediate risk onco-MPI did not reach a median one-year OS, while patients in the high-risk group had a median OS of 11.5 months (95% CI 9.7–NA); log rank test 5.7, p = 0.02. Figure 4.
Figure 4.
Kaplan–Meier curves representing one-year OS according to onco-MPI categories, blue = intermediate risk patients and red = high-risk patients. p = 0.02.
In the multivariate analysis, fitness at CGA and chemotherapy receipt were associated with better survival (HR 0.55, 95% CI 0.3–0.97, p = 0.04; and HR 0.36, 95% CI 0.19–0.67, p = 0.001, respectively), as shown in Table 6.
Table 6.
Univariate analysis and multivariate analysis for one-year survival.
| Univariate Analysis | |||||
| Variable | HR | Confidence Interval | p Value | ||
| Sex | M | 1.481 | 0.822 | 2.667 | 0.191 |
| Histology | Leiomyosarcoma | 0.633 | 0.306 | 1.309 | 0.218 |
| Age | 75–80 | 0.641 | 0.322 | 1.280 | 0.208 |
| Age | ≥80 | 0.710 | 0.356 | 1.416 | 0.331 |
| Location metastasis | Lung | 0.761 | 0.387 | 1.496 | 0.428 |
| Location of primary tumour | Retroperitoneum | 1.687 | 0.852 | 3.341 | 0.134 |
| Location of primary tumour | Other | 2.086 | 1.017 | 4.275 | 0.045 |
| PS at metastatic disease | ≥2 | 2.754 | 1.526 | 4.971 | <0.001 |
| Comorbidity grade according to CIRS | 3–4 | 1.043 | 0.502 | 2.171 | 0.91 |
| Onco-MPI | High-risk | 4.743 | 1.150 | 19.56 | 0.03 |
| First line chemotherapy | Yes | 0.193 | 0.106 | 0.354 | <0.001 |
| Multivariate Analysis | |||||
| Variable | HR | Confidence Interval | p Value | ||
| Sex | Male | 1.4579 | 0.666 | 3.188 | 0.3450 |
| Histology | Leiomyosarcoma | 0.5210 | 0.211 | 1.287 | 0.1574 |
| Age | ≥80 | 0.3968 | 0.174 | 0.906 | 0.0283 |
| Age | 75–80 | 0.5406 | 0.249 | 1.175 | 0.1203 |
| Location of metastasis | Lung | 0.5860 | 0.268 | 1.280 | 0.1802 |
| Location of primary tumour | Extremities/trunk | 2.0642 | 0.952 | 4.478 | 0.0666 |
| Location of primary tumour | Other | 2.2186 | 0.959 | 5.129 | 0.0624 |
| PS at metastatic disease | ≥2 | 2.0196 | 0.889 | 4.586 | 0.093 |
| Comorbidity grade according to CIRS | 3–4 | 0.8535 | 0.366 | 1.989 | 0.7136 |
| First line chemotherapy | YES | 0.2405 | 0.113 | 0.512 | 0.0002 |
| Onco-MPI | High-risk | 5.5682 | 1.251 | 24.793 | 0.0242 |
Legend: CGA = Comprehensive geriatric assessment; CIRS = Cumulative illness rating scale ECOG = Eastern cooperative oncology group; PS = Performance status.
Despite its good predictiveness of one-year survival, onco-MPI was not associated with global OS in the multivariate analysis.
When analysing the predictivity of one-year survival, onco-MPI and chemotherapy receipt were correlated with survival in the univariate analysis. The multivariate analysis confirmed the correlation between the onco-MPI high-risk group and worse survival (HR 5.5, 95%CI 1.25–24.7 p = 0.02) as well as the correlation of chemotherapy receipt and better survival (HR 0.24, 95% CI 0.11–0.51, p < 0.005), as shown in Table 6.
4. Discussion
This study provides data on the treatment and outcomes in an unselected real-world population of older patients with advanced/metastatic STS. To the best of our knowledge, the present study is the first to investigate the role of CGA and of the CGA-derived onco-MPI as prognosticators in older patients with metastatic STS. In the setting of advanced stage disease, chemotherapy has a palliative intent. In fact, chemotherapy has been shown to improve survival in patients with STS, though older patients experience a higher rate of toxicity [29,30] and benefits need to be thoroughly weighed against the risks in the frame of competing risks for mortality. Indeed, life expectancy estimation in older patients is crucial and tools to improve prognosis assessment in older patients with cancer are of utmost importance.
Age has been shown to be among the predictors of toxicity from doxorubicin in a retrospective analysis of the European Organization for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group database [31].
Moreover, for older patients, quality of life might be more worthwhile than the prolongation of life. Indeed, a recent Dutch study showed that patients with advanced stage sarcoma, aged less than 40 years, prioritised length of life, whereas two-thirds of patients aged ≥65 years felt that quality of life was equally or more important than length of life [32].
In our cohort of unselected patients with metastatic STS in the real-world practice, with median age being 77 years, global median OS was 13.8 months.
Our study showed CGA to be predictive of overall survival, and confirmed Onco-MPI to predict one-year survival.
Palliative chemotherapy may have a role for some older patients, as an American and French study also showed, with the mOS of patients managed with systemic therapy being 10.9 months versus 5.3 months for patients managed with best supportive care [7]. Our data show that fit patients may reach their median OS as high as 19.5 months. These figures are comparable to those derived from randomised trials of chemotherapy in patients with sarcoma, in which the median age is lower, such as in the Announce trial, in which the median age was 56.9 years [33]. Even in randomised clinical trials, specifically designed for older patients with sarcoma, with the age cut-off set nonetheless at 65 years, mOS ranged from 16.7 months (evofosfamide vs. doxorubicin trial) to 12.3 months (trofosfamide vs. doxorubicin trial) to 14.3 months (pazopanib vs. doxorubicin trial) [34,35,36].
Frail patients had a significantly shorter mOS of 7.75 months, as well as vulnerable patients, whose survival was 12.83 months. These data in frail older patients compare favourably with the literature data from retrospective studies, such as the English experience, in which the mOS is 6.5 months [37].
Our results therefore confirm the strong prognostic value of CGA for mOS in older patients with advanced STS, as already demonstrated for patients treated with chemotherapy for many other solid tumour types.
Unlike results from a previous study, in which age and PS were independently associated with survival, in our cohort of patients, these two variables, not other tumour single characteristics, were independent prognostic factors, confirming that a full assessment is more predictive than single variables, in line with studies in other cancer types [7,21,38,39,40]
Better survival rates observed in fit patients may have multiple explanations: fit patients are expected to be in better general conditions with less comorbidities, and this more likely influences a clinician’s decision to propose first line chemotherapy. The receipt of chemotherapy is the only variable that retains significance in the multivariate analysis in our study. Fit patients were treated with chemotherapy in a significantly higher proportion compared to frail patients (82% vs. 43%), and more often the regimen included anthracyclines (58.9% vs. 12.5%). The proportion of patients receiving chemotherapy is not significantly different when comparing fit and vulnerable patients (82% vs. 78.2%), yet the use of anthracycline-based regimens is significantly higher in fit patients (58.9% vs. 45.6%). Interestingly, our data showed that anthracycline-based chemotherapy did not provide a clear-cut benefit in older patients, and this is the rationale for ongoing randomised trials assessing first line use of doxorubicin vs. metronomic cyclophosphamide in older patients with metastatic STS (METROPHOLYS trial NCT04656262, TOLERANCE trial NCT04780464).
Our data also showed that vulnerable patients did not experience a higher rate of toxicity and dose reduction compared to fit patients, despite the evidence from some trials in which chemotherapy toxicity rates increased with geriatric impairments [38,41]. Such findings might be due to a higher proportion of patients in the fit group receiving anthracyclines in relation to comorbidity. Moreover, geriatric interventions offered to patients with impaired items at CGA might have had an impact in reducing the toxicity among vulnerable and frail patients.
As stated, vulnerable or frail patients at CGA were more often offered geriatric intervention to manage specific impairments, such as polipharmacology, comorbidities and cognitive impairment, as well as more general supportive care.
Indeed, geriatric interventions have been shown to reduce chemo-related toxicities in two recently published trials, randomising older patients to either receive chemotherapy as standard practice or to receive chemotherapy and CGA-driven geriatric intervention (GAIN study; GAP70+ study) [42,43] and to improve quality of life (INTEGERATE study) [44].
Onco-MPI intermediate and high-risk categories are the most represented in our study cohort, and this is likely due to metastatic stage conferring a higher risk in the stage onco-MPI domain. Moreover, patients with sarcoma were a small number in the development cohort of the onco-MPI, thus included under the “other” category. Nonetheless, onco-MPI in this study confirmed its role as a one-year survival predictor in patients with advanced stage STS, with patients in the intermediate risk category who did not reach the median survival and those in the high-risk category having a median survival of 11.5 months (p = 0.02).
The onco-MPI score in this population allowed a better prediction of mortality at one year, while the CGA impact is higher in distinguishing the unfit group of patients as vulnerable and frail. Therefore, the use of both CGA and onco-MPI could better stratify older patients as candidates for first line chemotherapy.
This study has some limitations residing mainly in its retrospective design, long accrual period, low number of patients due to the rarity of disease and the single centre experience. The low number of patients and the large number of variables could also have caused a moderate overfitting of the model, despite the number of events being consistent. Large multicentric and prospective trials, either randomised or observational, might therefore provide more solid and robust data.
As a matter of fact, decision making for vulnerable and frail patients remains an unmet need. Further prospective studies within this target population are needed, evaluating the role of geriatric intervention on chemo-related toxicities, quality of life and survival, though setting up and conducting such trials is challenging due to the rarity of the disease and the not-so-widespread geriatrisation of medical oncology units.
5. Conclusions
Benefit/risk balance in the approach to older patients with advanced or metastatic STS must be accurately considered. In our study, geriatric assessment has been confirmed to be prognostic and predictive also in rare diseases, such as sarcoma, and can better inform clinicians compared to simply considering chronological age or PS. Prospective studies incorporating geriatric parameters in the decision making for older STS are ongoing.
Author Contributions
Conceptualisation, B.C., A.B. (Antonella Brunello) and V.Z.; methodology, B.C., A.B. (Antonella Brunello), V.Z. and M.G.; software, M.G.; formal analysis, M.G.; investigation, B.C., I.T., A.G., F.M., S.A.-D., G.T., E.B., A.B. (Alberto Banzato), A.D.M., G.S., M.R., M.S., V.Z. and A.B. (Antonella Brunello); data curation, B.C., I.T., A.G., F.M., S.A.-D., G.T., E.B., A.B. (Alberto Banzato), A.D.M., G.S., M.R, M.S., V.Z. and A.B. (Antonella Brunello); writing—original draft preparation, B.C.; writing—review and editing, V.Z. and A.B. (Antonella Brunello); supervision, V.Z. and A.B. (Antonella Brunello); funding acquisition, A.B. (Antonella Brunello) and V.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Istituto Oncologico Veneto on 12 October 2015 with code 17/2015.
Informed Consent Statement
The requirement for informed consent was waived in view of the retrospective nature of the research and anonymity of the data.
Data Availability Statement
Data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Italian Ministero della Salute Grant GR-2016-02364834 and Ricerca Corrente funding from the Italian Ministry of Health.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Ressing M., Wardelmann E., Hohenberger Jakob J., Kasper B., Emrich K., Eberle A., Blettner M., Zeissig S.R. Strengthening health data on a rare and heterogeneous disease: Sarcoma incidence and histological subtypes in Germany. BMC Public Health. 2018;18:235. doi: 10.1186/s12889-018-5131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terret C., Zulian G.B., Naiem A., Albrand G. Multidisciplinary approach to the geriatric oncology patient. J. Clin. Oncol. 2007;25:1876–1881. doi: 10.1200/JCO.2006.10.3291. [DOI] [PubMed] [Google Scholar]
- 4.Kasper B., Hohenberger P. The challenge of treating elderly patients with advanced bone and soft tissue sarcomas. Crit. Rev. Oncol. Hematol. 2020;155:103108. doi: 10.1016/j.critrevonc.2020.103108. [DOI] [PubMed] [Google Scholar]
- 5.Nandra R., Hwang N., Matharu G.S., Reddy K., Grimer R. One-year mortality in patients with bone and soft tissue sarcomas as an indicator of delay in presentation. Ann. R. Coll. Surg. Engl. 2015;97:425–433. doi: 10.1308/003588415X14181254790284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younger E., Litière S., Le Cesne A., Mir O., Gelderblom H., Italiano A., Marreaud S., Jones R.L., Gronchi A., van der Graaf W.T.A. Outcomes of Elderly Patients with Advanced Soft Tissue Sarcoma Treated with First-Line Chemotherapy: A Pooled Analysis of 12 EORTC Soft Tissue and Bone Sarcoma Group Trials. Oncologist. 2018;23:1250–1259. doi: 10.1634/theoncologist.2017-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garbay D., Maki R.G., Blay J.Y., Isambert N., Piperno Neumann S., Blay C., Zanardi E., Boudou-Rouquette P., Bozec L., Duffaud F., et al. Advanced soft-tissue sarcoma in elderly patients: Patterns of care and survival. Ann. Oncol. 2013;24:1924–1930. doi: 10.1093/annonc/mdt059. [DOI] [PubMed] [Google Scholar]
- 8.Mohile S.G., Dale W., Somerfield M.R., Schonberg M.A., Boyd C.M., Burhenn P.S., Canin B., Cohen H.J., Holmes H.M., Hopkins J.O., et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J. Clin. Oncol. 2018;36:2326–2347. doi: 10.1200/JCO.2018.78.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decoster L., Van Puyvelde K., Mohile S., Wedding U., Basso U., Colloca G., Rostoft S., Overcash J., Wildiers H., Steer C., et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: An update on SIOG recommendations. Ann. Oncol. 2015;26:288–300. doi: 10.1093/annonc/mdu210. [DOI] [PubMed] [Google Scholar]
- 10.Marosi C., Köller M. Challenge of cancer in the elderly. ESMO Open. 2016;1:e000020. doi: 10.1136/esmoopen-2015-000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balducci L., Extermann M. Management of Cancer in the Older Person: A Practical Approach. Oncol. 2000;5:224–237. doi: 10.1634/theoncologist.5-3-224. [DOI] [PubMed] [Google Scholar]
- 12.Repetto L., Fratino L., Audisio R.A., Venturino A., Gianni W., Vercelli M., Parodi S., Dal Lago D., Gioia F., Monfardini S., et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J. Clin. Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 13.Basso U., Monfardini S. Multidimensional geriatric evaluation in elderly cancer patients: A practical approach. Eur. J. Cancer Care. 2004;13:424–433. doi: 10.1111/j.1365-2354.2004.00551.x. [DOI] [PubMed] [Google Scholar]
- 14.Caillet P., Laurent M., Bastuji-Garin S., Liuu E., Culine S., Lagrange J., Canoui-Poitrine F., Paillaud E. Optimal management of elderly cancer patients: Usefulness of the comprehensive geriatric assessment. Clin. Interv. Aging. 2014;9:1645–1660. doi: 10.2147/CIA.S57849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill T.M., Allore H.G., Gahbauer E.A., Murphy T.E. Change in Disability After Hospitalization or Restricted Activity in Older Persons. JAMA. 2010;304:1919–1928. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunello A., Fontana A., Zafferri V., Panza F., Fiduccia P., Basso U., Copetti M., Lonardi S., Roma A., Falci C., et al. Development of an oncological-multidimensional prognostic index (Onco-MPI) for mortality prediction in older cancer patients. J. Cancer Res. Clin. Oncol. 2016;142:1069–1077. doi: 10.1007/s00432-015-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierantoni F., Basso U., Maruzzo M., Lamberti E., Bimbatti D., Tierno G., Bergo E., Brunello A., Zagonel V. Comprehensive geriatric assessment is an independent prognostic factor in older patients with metastatic renal cell cancer treated with first-line Sunitinib or Pazopanib: A single center experience. J. Geriatr. Oncol. 2021;12:290–297. doi: 10.1016/j.jgo.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Okonji D.O., Sinha R., Phillips I., Fatz D., Ring A. Comprehensive geriatric assessment in 326 older women with early breast cancer. Br. J. Cancer. 2017;117:925–931. doi: 10.1038/bjc.2017.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barthélémy P., Heitz D., Mathelin C., Polesi H., Asmane I., Litique V., Rob L., Bergerat J.-P., Kurtz J.-E. Adjuvant chemotherapy in elderly patients with early breast cancer. Impact of age and comprehensive geriatric assessment on tumor board proposals. Crit. Rev. Oncol. Hematol. 2011;79:196–204. doi: 10.1016/j.critrevonc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Corre R., Greillier L., Audigier-Valette C., Baize N., Bérard H., Falchero L., Monnet I., Dansin E., Vergnenègre A., Marcq M., et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients with Advanced Non-Small-Cell Lung Cancer: The Phase III Randomized ESOGIA- GFPC-GECP 08-02 Study. J. Clin. Oncol. 2016;34:1476–1483. doi: 10.1200/JCO.2015.63.5839. [DOI] [PubMed] [Google Scholar]
- 21.Lombardi G., Bergo E., Caccese M., Padovan M., Bellu L., Brunello A., Zagonel V. Validation of the Comprehensive Geriatric Assessment as a predictor of mortality in elderly Glioblastoma patients. Cancers. 2019;11:1509. doi: 10.3390/cancers11101509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz S., Ford A.B., Moskowitz R.W., Jackson B.A., Jaffe M.W. Studies of Illness In The Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 23.Lawton M.P., Brody E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- 24.Conwell Y., Forbes N.T., Cox C., Caine E.D. Validation of a measure of physical illness burden at autopsy: The Cumulative Illness Rating Scale. J. Am. Geriatr. Soc. 1993;41:38–41. doi: 10.1111/j.1532-5415.1993.tb05945.x. [DOI] [PubMed] [Google Scholar]
- 25.Weitzner M.A., Haley W.E., Chen H. The family caregiver of the older cancer patient. Hematol. Oncol. Clin. N. Am. 2000;14:269–281. doi: 10.1016/S0889-8588(05)70288-4. [DOI] [PubMed] [Google Scholar]
- 26.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Sheikh J.L., Yesavage J.A. Geriatric depression scale (GDS): Recent evidence and development of a shorter version. In: Brink T.L., editor. Clinical Gerontology: A Guide to Assessment and Intervention. Hawthorne Press; New York, NY, USA: 1986. [Google Scholar]
- 28.Guigoz Y. The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J. Nutr. Health Aging. 2006;10 [PubMed] [Google Scholar]
- 29.Judson I., Verweij J., Gelderblom H., Hartmann J.T., Schöffski P., Blay J.Y., Kerst J.M., Sufliarsky J., Whelan J., Hohenberger P., et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 30.Hurria A., Togawa K., Mohile S.G., Owusu C., Klepin H.D., Gross C.P., Lichtman S.M., Gajra A., Bhatia S., Katheria V., et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J. Clin. Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sleijfer S., Rizzo E., Litière S., Mathijssen R.H.J., Judson I.R., Gelderblom H., Van Der Graaf W.T.A., Gronchi A. Predictors for doxorubicin-induced hematological toxicity and its association with outcome in advanced soft tissue sarcoma patients; A retrospective analysis of the EORTC-soft tissue and bone sarcoma group database. Acta Oncol. 2018;57:1117–1126. doi: 10.1080/0284186X.2018.1449248. [DOI] [PubMed] [Google Scholar]
- 32.Younger E., Jones R.L., den Hollander D., Soomers V.L.M.N., Desar I.M.E., Benson C., Young R.J., Oosten A.W., de Haan J.J., Miah A., et al. Priorities and preferences of advanced soft tissue sarcoma patients starting palliative chemotherapy: Baseline results from the HOLISTIC study. ESMO Open. 2021;6:100258. doi: 10.1016/j.esmoop.2021.100258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tap W.D., Jones R.L., Van Tine B.A., Chmielowski B., Elias A.D., Adkins D., Agulnik M., Cooney M.M., Livingston M.B., Pennock G., et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: An open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388:488–497. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Younger E., Ballman K., Lu Y., Pápai Z., Van Tine B.A., Attia S., Schöffski P., Reinke D., Tap W.D., Jones R.L. Subgroup analysis of older patients treated within the randomized phase 3 doxorubicin versus doxorubicin plus evofosfamide (SARC021) trial. J. Geriatr. Oncol. 2020;11:463–469. doi: 10.1016/j.jgo.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Hartmann J.T., Kopp H.G., Gruenwald V., Piperno-Neumann S., Kunitz A., Hofheinz R., Mueller L., Geissler M., Horger M., Fix P., et al. Randomised phase II trial of trofosfamide vs. doxorubicin in elderly patients with untreated metastatic soft-tissue sarcoma. Eur. J. Cancer. 2020;124:152–160. doi: 10.1016/j.ejca.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Grünwald V., Karch A., Schuler M., Schöffski P., Kopp H.G., Bauer S., Kasper B., Lindner L.H., Chemnitz J.M., Crysandt M., et al. Randomized Comparison of Pazopanib and Doxorubicin as First-Line Treatment in Patients with Metastatic Soft Tissue Sarcoma Age 60 Years or Older: Results of a German Intergroup Study. J. Clin. Oncol. 2020;38:3555–3564. doi: 10.1200/JCO.20.00714. [DOI] [PubMed] [Google Scholar]
- 37.Yousaf N., Harris S., Martin-Liberal J., Stanway S., Linch M., Ifijen M., Al Muderis O., Khabra K., Fisher C., Noujaim J., et al. First line palliative chemotherapy in elderly patients with advanced soft tissue sarcoma. Clin. Sarcoma Res. 2015;5:10. doi: 10.1186/s13569-015-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamaker M.E., Seynaeve C., Wymenga A.N.M., van Tinteren H., Nortier J.W.R., Maartense E., de Graaf H., de Jongh F.E., Braun J.J., Los M., et al. Baseline comprehensive geriatric assessment is associated with toxicity and survival in elderly metastatic breast cancer patients receiving single-agent chemotherapy: Results from the OMEGA study of the Dutch breast cancer trialists’ group. Breast. 2014;23:81–87. doi: 10.1016/j.breast.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Brugel L., Laurent M., Caillet P., Radenne A., Durand-Zaleski I., Martin M., Baron M., de Kermadec H., Bastuji-Garin S., Canouï-Poitrine F., et al. Impact of comprehensive geriatric assessment on survival, function, and nutritional status in elderly patients with head and neck cancer: Protocol for a multicentre randomised controlled trial (EGeSOR) BMC Cancer. 2014;14:427. doi: 10.1186/1471-2407-14-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stotter A., Reed M.W., Gray L.J., Moore N., Robinson T.G. Comprehensive Geriatric Assessment and predicted 3-year survival in treatment planning for frail patients with early breast cancer. Br. J. Surg. 2015;102 doi: 10.1002/bjs.9755. [DOI] [PubMed] [Google Scholar]
- 41.Aparicio T., Jouve J.L., Teillet L., Gargot D., Subtil F., Le Brun-Ly V., Cretin J., Locher C., Bouché O., Breysacher G., et al. Geriatric factors predict chemotherapy feasibility: Ancillary results of FFCD 2001-02 phase III study in first-line chemotherapy for metastatic colorectal cancer in elderly patients. J. Clin. Oncol. 2013;31:1464–1470. doi: 10.1200/JCO.2012.42.9894. [DOI] [PubMed] [Google Scholar]
- 42.Li D., Sun C.L., Kim H., Soto-Perez-de-Celis E., Chung V., Koczywas M., Fakih M., Chao J., Cabrera Chien L., Charles K., et al. Geriatric Assessment-Driven Intervention (GAIN) on Chemotherapy-Related Toxic Effects in Older Adults With Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021;7:e214158. doi: 10.1001/jamaoncol.2021.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohile S.G., Mohamed M.R., Xu H., Culakova E., Loh K.P., Magnuson A., Flannery M.A., Obrecht S., Gilmore N., Ramsdale E., et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): A cluster-randomised study. Lancet. 2021;398:1894–1904. doi: 10.1016/S0140-6736(21)01789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soo W.K., King M.T., Pope A., Parente P., Dārziņš P., Davis I.D. Integrated Geriatric Assessment and Treatment Effectiveness (INTEGERATE) in older people with cancer starting systemic anticancer treatment in Australia: A multicentre, open-label, randomized controlled trial. Lancet Healthy Longev. 2022;3:e617–e627. doi: 10.1016/S2666-7568(22)00169-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data presented in this study are available on request from the corresponding author.