Abstract
Simple Summary
We herein reviewed the current evidence for the role of microRNAs (miRNAs) in the mechanism of chemoresistance in pancreatic cancer. Pancreatic cancer has an extremely poor prognosis due to its late discovery, aggressive nature, and chemoresistance. Recent accumulated reports proved that aberrant miRNAs could induce chemoresistance in pancreatic cancer. However, the exact underlying molecular mechanisms remain poorly understood. In this review, we discuss recently available and novel knowledge about overcoming chemoresistance in pancreatic cancer.
Abstract
Despite extensive research, pancreatic cancer remains a lethal disease with an extremely poor prognosis. The difficulty in early detection and chemoresistance to therapeutic agents are major clinical concerns. To improve prognosis, novel biomarkers, and therapeutic strategies for chemoresistance are urgently needed. microRNAs (miRNAs) play important roles in the development, progression, and metastasis of several cancers. During the last few decades, the association between pancreatic cancer and miRNAs has been extensively elucidated, with several miRNAs found to be correlated with patient prognosis. Moreover, recent evidence has revealed that miRNAs are intimately involved in gemcitabine sensitivity and resistance through epithelial-to-mesenchymal transition, the tumor microenvironment, and drug metabolism. Gemcitabine is the gold standard drug for pancreatic cancer treatment, but gemcitabine resistance develops easily after chemotherapy initiation. Therefore, in this review, we summarize the gemcitabine resistance mechanisms associated with aberrantly expressed miRNAs in pancreatic cancer, especially focusing on the mechanisms associated with epithelial-to-mesenchymal transition, the tumor microenvironment, and metabolism. This novel evidence of gemcitabine resistance will drive further research to elucidate the mechanisms of chemoresistance and improve patient outcomes.
Keywords: microRNA, gemcitabine, pancreatic cancer, biomarker, chemoresistance
1. Introduction
Pancreatic cancer remains a lethal disease and is the third leading cause of cancer-related deaths in the United States, with an incidence of 62,210 new cases, and an exceptionally high mortality rate of 49,830 deaths (80.1%) in 2022 [1]. Pancreatic cancer is expected to become the second leading cause of cancer-related deaths in the future [2]. Despite accumulated knowledge regarding pancreatic cancer etiology, patient prognosis has not significantly improved over the last decade [3]. The high mortality rate is rooted in the lack of specific symptoms, diagnostic tools, and effective chemotherapeutics, along with the increasing incidence of chemoresistance. Among these, chemoresistance is a well-known factor that contributes to a poor prognosis.
Compared with 5-fluorouracil, gemcitabine was found to be superior in relieving symptoms caused by progression in patients with pancreatic cancer [4]. This made gemcitabine the key drug for pancreatic cancer in combination with other chemotherapeutic agents such as nab-paclitaxel [5] or oxaliplatin [6]. However, the clinical benefits of these regimens are limited by chemoresistance. Thus, there is an urgent and crucial need to establish strategies for overcoming gemcitabine resistance to improve pancreatic cancer treatment. To date, the most clinically reliable marker for prognosis is CA19-9) (carbohydrate antigen 19-9 in pancreatic cancer [7]. Several other markers have been reported; however, they have not seen clinical use [8,9,10].
microRNAs (miRNAs) regulate gene expression by binding to the 3′-UTR (prime untranslated region) of their target mRNAs and inhibiting the production of those proteins. The association between miRNAs and chemoresistance has been reported in various types of cancer [11,12,13]. Thus, miRNAs are attractive molecules that retain the potential to overcome gemcitabine resistance. However, the scientific mechanism underlying this association remains unresolved despite extensive study. Recent evidence has shown that epithelial-to-mesenchymal transition (EMT) [14], tumor microenvironment (TME) [15,16], and drug metabolism [17] are major causes of the development of gemcitabine resistance via aberrantly expressed miRNAs. Therefore, it is important to identify innovative biomarkers and novel targets for intrinsic and acquired gemcitabine resistance in patients with pancreatic cancer, since CA19-9 is not a biomarker for gemcitabine resistance, though it is known to be a prognostic marker with high sensitivity and specificity (both 80%) [7]. EMT is a process that changes epithelial cells into mesenchymal-like cells, leading to the acquisition of cellular properties for invasiveness, proliferation, chemoresistance, and higher metastatic potential. Evidence has shown that expression of EMT-related genes such as those encoding vimentin, Snai1, and Zeb1 (zinc finger E-box binding homeobox 1) could further increase the risk of cancer cells acquiring a high-grade phenotype [18,19].
The TME is composed of interstitial tissues surrounding the pancreatic cancer cells and comprises immune cells, pancreatic stellate cells, stromal cells, blood vessels, fibroblasts, and the extracellular matrix (ECM). The TME can induce hypoxia, which confers several metabolic advantages for cancer cell protection and survival [20,21]. Recent review articles have shown that TME plays a pivotal role in the development of pancreatic cancer cells and gemcitabine resistance [22,23].
Finally, aberrant gemcitabine metabolism, including gemcitabine-related transporters and enzymes, is a cause of gemcitabine resistance [24,25,26,27]. Dysregulation of these transporters or enzymes results in gemcitabine resistance through complex, irregular factors and signaling pathways [28].
Based on these reports, the purpose of this review was to summarize the current evidence on the roles of miRNAs as potential biomarkers and therapeutic targets for gemcitabine resistance via EMT, TME, and drug metabolism in pancreatic cancer. Here, we systematically summarized recent published reports, including review articles, and focused on gemcitabine resistance in pancreatic cancer. A systematic search was performed using PubMed to identify original research studies mainly published in the last 20 years using the search terms pancreatic cancer and gemcitabine resistance. Finally, 1357 articles were chosen. Moreover, we targeted studies that satisfied the following criteria: (1) studied miRNA expression due to gemcitabine resistance in pancreatic cancer, (2) used human samples, and (3) were written in English. The full texts of the articles of interest were then evaluated. In this review, we focused on the mechanisms of gemcitabine resistance via EMT, TME, and gemcitabine metabolism.
2. Diverse Functions of miRNAs
miRNAs are short noncoding RNAs containing approximately 22 nucleotides, which can bind directly to specific targets within the 3′-untranslated regions of mRNAs [29,30]. In addition, miRNAs inhibit translation or enhance mRNA cleavage at the post-transcriptional level [31]. miRNAs are key regulators in the development, differentiation, and apoptosis of normal cells and may affect tumorigenesis and metastatic potential [32]. Remarkably, miRNAs exhibit tissue-specific and disease-specific expression, which suggests their potential as novel diagnostic and prognostic markers as well as therapeutic targets [33]. In addition, each miRNA has several different target genes, which results in the inhibition of the target genes for signaling pathways; therefore, miRNAs can function as both oncogenes and tumor suppressor genes [34]. Recent data have shown that frequently deregulated miRNAs are associated with detection, progression, and chemoresistance in pancreatic cancer [35,36]. Furthermore, several studies have documented that miRNAs influence gemcitabine resistance via EMT, TME, and drug metabolism [37,38,39].
3. Gemcitabine Resistance in Pancreatic Cancer
Gemcitabine remains the gold standard for the treatment of pancreatic cancer. Clinical studies, including combination treatment with gemcitabine, have been extensively performed; however, combination regimens were found to be insufficient compared with traditional regimens, such as gemcitabine plus nab-paclitaxel or erlotinib, or S-1 combination [40,41,42,43]. The major limitations in treatment effects include gemcitabine resistance and considerable adverse events [28]. An understanding of the mechanisms underlying gemcitabine resistance may lead to the discovery of promising targeted therapies. Numerous studies have reported the major molecular factors of gemcitabine resistance, such as: (1) cellular factors (cell signaling pathways, cancer stem cells, and EMT); (2) environmental factors (oxygen supply, stroma, ECM, and blood supply); and (3) drug metabolism (transporter, drug activation, and enzymes) in pancreatic cancer [22,44,45,46]. Moreover, these factors can result from molecular and cellular changes, including aberrant expression of mRNAs or miRNAs, and can interact in a complex manner [47]. It is therefore challenging to overcome gemcitabine resistance given the several different factors involved. Herein, we summarize the reported miRNA-mediated mechanisms that directly contribute to gemcitabine sensitivity and resistance in pancreatic cancer. Among them, we focus on EMT, TME, and metabolism-related mechanisms via miRNAs.
4. EMT-Mediated Mechanisms of Gemcitabine Resistance in Pancreatic Cancer
4.1. EMT and Gemcitabine Resistance
EMT is associated with gemcitabine resistance in pancreatic cancer [48,49]. It is characterized by the evolution of an epithelial phenotype into a mesenchymal phenotype, which can lead to cell proliferation, invasiveness, and metastasis [50,51]. This fundamental process is accompanied by morphological changes in the cancer cells. EMT is mediated by a variety of key genes and cellular signaling pathways; consequently, EMT results in higher proliferation, invasion, and chemoresistance. Molecular markers of EMT include increased expression of vimentin, Twist, Snail, Slug, and ZEB1 [52,53]. In contrast, mesenchymal-epithelial transition (MET) markers include Zo-1 and E-cadherin. Signaling pathways, such as the Notch and NFκB pathways, are critical for the induction of EMT [50]. Several studies have reported that gemcitabine-resistant cells show upregulated vimentin and downregulated E-cadherin expression associated with the activation of NFkB and c-MET tyrosine kinase, respectively [54,55,56]. Additionally, gemcitabine-resistant cells express higher SMAD2 or cancer stem cell (CSC) markers with EMT characteristics [57,58], because CSCs are evidently linked to the EMT process, which has been associated with gemcitabine resistance [59]. miR-34a recovers sensitivity to gemcitabine by inhibiting Notch 1, which is located upstream of the EMT pathways [60]. EMT can directly induce the CSC phenotype in pancreatic cancer. In contrast, gemcitabine induces EMT and CSC molecular marker expression [61]. Thus, EMT features overlap with molecular and morphological changes in CSC. In contrast, miR-17 is reduced in gemcitabine-resistant CSC by targeting the TGF-β1 signaling pathway and inhibiting the downstream targets p21, p57, and TBX3 [62]. Moreover, Ji et al. revealed that miR-34 may be involved in pancreatic cancer stem cell self-renewal, potentially via the direct modulation of downstream targets Bcl-2 and Notch [63]. In this review, we considered CSCs to be consistent with the EMT phenomenon. Conversely, inhibition of Notch signaling in gemcitabine-resistant cells could induce the MET phenotype from the EMT phenotype, indicating that gemcitabine resistance is reversible and associated with decreased EMT marker expression [58]. Hypoxia can also induce PLOD2-influenced gemcitabine resistance through EMT [64]. Moreover, ZEB1 can also mediate gemcitabine resistance and reduce E-cadherin expression. In contrast, reduced ZEB1 levels can restore gemcitabine sensitivity, indicating that ZEB1 is responsible for gemcitabine resistance [65]. Carrasco-Garcia et al. also showed that SOX9, which participates in the initiation of pancreatic cancer, correlated with EMT with high vimentin and low E-cadherin expression [66]. Recent evidence has shown that signaling pathways, including tumor necrosis factor (TNF) and hypoxia-inducible factor-1 (HIF-1), are associated with EMT in pancreatic cancer [66,67]. Moreover, Zhang et al. showed that DPEP1 induction enhanced gemcitabine sensitivity through the RAS-RAF-MEK-ERK and PI3K pathways [68]. EMT is also associated with tumor budding, which accounts for gemcitabine resistance; a study indicated that tumor budding with vimentin expression becomes a key process in pancreatic cancer and is responsible for progression and gemcitabine resistance [69]. Taken together, EMT induction is strongly correlated with the development of gemcitabine resistance in pancreatic cancer.
4.2. miRNAs Related to Gemcitabine and EMT
4.2.1. miRNAs Associated with Gemcitabine Sensitivity
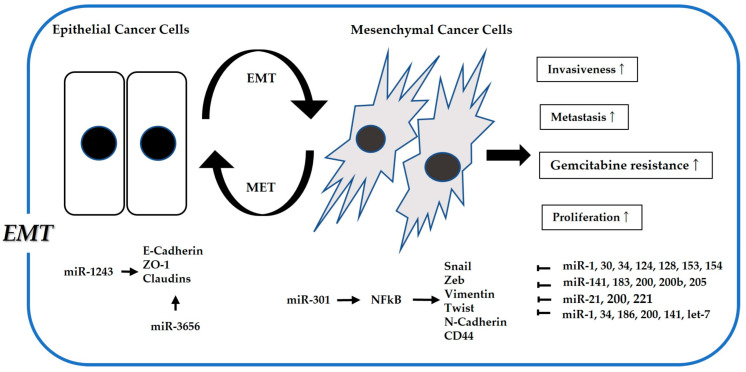
With regard to the association between gemcitabine sensitivity and miRNAs mediating EMT, several tumor suppressor miRNAs have been identified to regulate EMT-related genes and improve gemcitabine sensitivity in pancreatic cancer [70,71]. (See Figure 1 and Table 1). Wang et al. showed that miR-30a reverses gemcitabine resistance in pancreatic cancer by targeting the Snail-AKT signaling pathway [72]. Funamizu et al. revealed that miR-200b could restore gemcitabine sensitivity by inhibiting ZEB1, thereby upregulating E-cadherin [73]. In addition, Li et al. proved that naturally occurring agent-induced miR-200 and let-7 expression could reverse MET from the EMT phenotype in gemcitabine-resistant cells [74]. Liu et al. reported that miR-125a-3p can restore gemcitabine sensitivity and inhibit EMT through targeting Fyn [75]. Fu et al. also revealed that NEAT1, mediated by miR-506, could control ZEB2 expression [76]. Additionally, Li et al. showed that miR-506 suppressed sphingosine kinase 1, which was significantly associated with poor survival in a large cohort [77]. Hiramoto et al. demonstrated that miR-509 and miR-1243 improve gemcitabine sensitivity via E-cadherin expression [78]. Furthermore, Yang et al. reported that miR-3656 plays a significant role in gemcitabine sensitivity by inhibiting vimentin and Twist expression [79]. Two reports have shown that miR-153 can enhance gemcitabine sensitivity by inhibiting Snail [80,81]. Wang et al. revealed that upregulation of miR-183 and miR-200b improved gemcitabine sensitivity via ZEB1 inhibition caused by KLF4 [82]. Chaudhary et al. also reported that miR-205 impairs gemcitabine resistance by inhibiting the EMT phenotype [83]. More recently, Yang et al. revealed that exosomal miR-210 in CSCs mediates gemcitabine resistance by activating the PI3K/Akt/mTOR pathway [84].
Figure 1.
Association between microRNAs related to EMT (Epithelial–mesenchymal transition) and gemcitabine resistance. EMT is well known to increase drug resistance, cell proliferation, and invasiveness through acquiring properties like cancer stem cells.
4.2.2. miRNAs Associated with Gemcitabine Resistance
The reported miRNAs associated with gemcitabine resistance in pancreatic cancer have been summarized. Hasegawa et al. revealed that miR-1246 contributes to gemcitabine resistance and induces CSC-like properties through CCNG2 [85]. Xiong et al. demonstrated that miR-10a contributes to gemcitabine resistance by targeting TFAP2C, thereby resulting in increased Snail 1 expression and EMT induction [86]. Zhang et al. showed that miR-15b degrades SMURF2 and promotes TGF-β-mediated EMT in pancreatic cancer [87]. In addition, Yang and Funamizu et al. demonstrated that miR-301b induced EMT and enhanced gemcitabine resistance by reducing E-cadherin expression [54,88]. Zhang et al. also showed that activation of the miR-301/TP63 axis caused by hypoxia-induced EMT contributes to gemcitabine resistance [89]. Okazaki et al. suggested that miR-296-5p induces EMT and gemcitabine resistance [90]. Meanwhile, Ma et al. demonstrated that miR-223 enhances gemcitabine resistance by targeting FBXW7 through activating Notch signaling-mediated EMT [91]. Yu et al. revealed that miR-1206 enhances gemcitabine resistance through ESRP1 inhibition [92]. Furthermore, Yu et al. showed that miR-497 could inhibit gemcitabine resistance in CSCs by targeting NFκB [93]. Ma et al. indicated that miR-200-3p attenuates gemcitabine resistance in CSCs by modulating EMT and stemness [94].
Table 1.
miRNAs for EMT.
| Author | Ref. Number | miRNA | Target Gene |
|---|---|---|---|
| Sensitivity | |||
| Li Y | [74] | let-7 | NA |
| Cioffi M | [62] | miR-17 | NODAL |
| Wang T | [72] | miR-30a | SNAI1 |
| Ji Q | [63] | miR-34 | Bcl-2 |
| Liu G | [75] | miR-125a | Fyn |
| Liu F | [80] | miR-153 | Snail |
| Bai Z | [81] | miR-153 | Snail |
| Wang Z | [82] | miR-183 | ZEB1 |
| Li Y | [74] | miR-200 | NA |
| Funamizu N | [55] | miR-200b | ZEB1 |
| Wang Z | [82] | miR-200b | ZEB1 |
| Ma C | [94] | miR-200c | NA |
| Chaudhary AK | [83] | miR-205 | TUBB3 |
| Yu Q | [93] | miR-497 | NFKB1 |
| Fu X | [76] | miR-506 | NEAT1 |
| Hiramoto H | [78] | miR-509 | NA |
| Yu S | [92] | miR-1206 | ESRP1 |
| Hiramoto H | [78] | miR-1243 | NA |
| Yang RM | [79] | miR-3656 | EMT related genes |
| Resistance | |||
| Xiong G | [86] | miR-10a | TFAP2C |
| Zhang WL | [87] | miR-15b | SMURF2 |
| Yang Z | [84] | miR-210 | Rapamycin |
| Ma J | [91] | miR-223 | FBXW7 |
| Okazaki J | [90] | miR-296 | BOK |
| Zhang KD | [89] | miR-301 | TP63 |
| Funamizu N | [54] | miR-301b | TP63 |
| Yang S | [88] | miR-301b | NR3C2 |
| Hasegawa S | [85] | miR-1246 | CyclinG2 |
Ref: reference, NA: not applicable.
Despite this evidence, the role of miRNAs in gemcitabine sensitivity and resistance remains controversial because the complex and interacting networks underlying the phenomenon are challenging to elucidate. However, accumulating evidence implicates that aberrant expression of miRNAs modulates the responsiveness of gemcitabine sensitization.
5. TME-Mediated Mechanisms of Gemcitabine Resistance in Pancreatic Cancer
5.1. TME and Gemcitabine Resistance in Pancreatic Cancer
Pancreatic cancer has unique features to survive therapeutic strategies, characterized by the presence of an extensive desmoplastic stroma composed of ECM, cancer-associated fibroblasts (CAFs), pancreatic stellate cells (PSCs), inflammatory cells, immune cells (including tumor-associated macrophages [TAMs]), and other cell types (such as endothelial cells). The dense stroma facilitates the compression of blood vessels and leads to a hypoxic environment, which reduces the supply of chemotherapeutic agents and supports cancer progression [95]. Under these circumstances, the TME can induce immunosuppression to escape the immune system. The desmoplastic stroma and hypoxic environment have also been reported to promote EMT and cause gemcitabine resistance [96,97]. Accumulated evidence has revealed that desmoplastic stroma simply does not develop a physical barrier to gemcitabine; moreover, the respective components in the TME act to resist gemcitabine [98,99,100,101].
5.2. Role of ECM in Gemcitabine Resistance
ECM is defined as the physical support and material that fills the extracellular space and functions as a scaffold for cell adhesion, including fibronectin, collagen, and proteoglycan. ECM components, including collagen, fibronectin, and laminin, are secreted by PSCs and CAFs [95]. Recent evidence has shown that the ECM functions as a supporting tissue, regulating cancer cell proliferation and EMT [102]. Fibronectin is a major component of the ECM that Miyamoto et al. have demonstrated to be a contributor to gemcitabine resistance [103]. In addition, fibronectin plays a key role in gemcitabine resistance by activating the ERK1/2 pathway [104]. Topalovski et al. indicated that cooperation between TGF-β and fibronectin may establish coordinated EMT induction [105]. Furthermore, fibronectin can support cancer progression and reduce gemcitabine response [105]. In contrast, Dangi-Garimella et al. demonstrated that membrane type 1 matrix metalloproteinase (MMP) contributes to gemcitabine resistance and suggested that targeting MMP could be a novel approach to improving gemcitabine sensitivity [106]. Thus, ECM-targeted agents combined with gemcitabine chemotherapy are promising strategies for overcoming gemcitabine resistance. However, two clinical studies involving anti-ECM inhibitors did not show significant efficacy of these drugs [107,108].
5.3. Role of CAFs in Gemcitabine Resistance
CAFs constitute a major part of the tumor mass in pancreatic cancer. CAFs can enhance chemoresistance through ECM remodeling and immunological reprogramming [109]. Richards et al. recently reported that CAFs have the potential for gemcitabine resistance. They also showed that exosomes produced by CAFs promote EMT and gemcitabine resistance by inducing Snail [110]. In addition, Zhang et al. showed that CAFs activate NFκB and IL1 receptor-associated kinase 4, which results in enhanced tumor fibrosis, cell proliferation, and gemcitabine resistance [111]. Recent reports have revealed that CAFs promote gemcitabine resistance via the LIF/STAT3 or the TGF-β1/SMAD2/3 pathway [112,113], suggesting that targeting these pathways may be a novel strategy to reverse gemcitabine resistance. Thus, therapeutic agents controlling CAF function may play a vital role in sensitizing gemcitabine.
5.4. Role of PSCs in Gemcitabine Resistance
PSCs provide a solid foundation for the production of collagenous stroma for cancer development and survival [114,115]. Unfortunately, the role of PSCs in gemcitabine resistance has not yet been fully elucidated. However, recent evidence suggests that PSCs act as drivers of gemcitabine resistance and cancer progression. Cao et al. showed that the Notch pathway activated by PSCs promotes gemcitabine resistance and induces EMT [116]. Interestingly, PSCs have no tolerance to glucose adjustment; therefore, an increased number of PSCs in the pancreas allows the development of type 2 diabetes. Moreover, EMT-mediated higher glucose levels promote malignant potential [117]. It is well known that PSCs promote EMT in pancreatic cancer cells [118]. These reports suggest that targeting the Notch pathway may be an effective strategy for recovering gemcitabine tolerance.
5.5. Role of TAMs in Gemcitabine Resistance
TAMs are also abundantly present in the TME. TAMs innately play a role in the phagocytosis of apoptotic cells and affect cancer progression and gemcitabine resistance by secreting numerous factors, such as growth factors, proteolytic enzymes, and inflammatory cytokines [119,120]. Weiseman et al. revealed that TAMs contribute to gemcitabine resistance by reducing apoptosis and upregulating cytidine deaminase (CDA) expression. These effects augment the response to gemcitabine by activating caspase-3 [121]. Moreover, Guo et al. showed that miR-222, delivered by TAM, suppresses TSC1 and activates the PI3K/AKT/mTOR pathway, which results in the development of gemcitabine-resistant cancer cells [122]. Additionally, Nagathihalli et al. showed that a STAT3 pathway inhibitor combined with gemcitabine can enhance gemcitabine delivery and response by remodeling the tumor stroma [123]. In contrast, TAMs directly activate the STAT3 pathway to regulate CSCs [124]. Thus, STAT3-targeted therapy with gemcitabine may be a promising therapeutic strategy for pancreatic cancer.
5.6. miRNAs Involved in TME-Mediated Gemcitabine Resistance
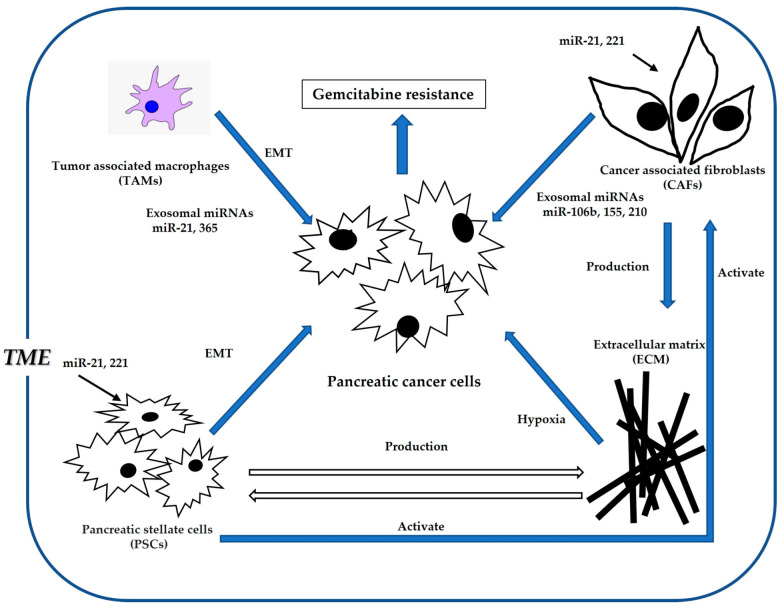
Hypoxia is an essential component of the TME for the survival of cancer cells in pancreatic cancer [125,126,127]. Extensive studies have revealed that miRNAs are strongly associated with TME function [128,129,130]. (Figure 2 and Table 2). Luo et al. revealed that miR-301a plays a critical role in gemcitabine resistance via the TME [131]. Liu et al. also showed that PVT1 and HIF-1 inhibition, mediated by miR-143, improves gemcitabine sensitivity [132]. Xin et al. proposed that nano-medically modified gemcitabine and miR-519c are effective therapeutic targets to treat desmoplasia and hypoxia-induced gemcitabine resistance in the TME [133]. In contrast, HIF-1 expression is a well-known inducible factor for gemcitabine resistance and EMT [134,135]. As such, HIF-1-targeted miRNA strategies have been described in the literature. Liu et al. showed that miR-3662 inhibits gemcitabine resistance by inhibiting HIF-1 in the TME [136]. In addition, Ni et al. showed that miR-210 controls HOXA9 expression and upregulates the HIF-1/NF-κB pathway, which promotes EMT and hypoxia [137].
Figure 2.
Association of microRNAs with TME (tumor microenvironment) and gemcitabine resistance. TME works to the advantage of cancer cell survival by, for example, inducing gemcitabine resistance and enhancing cell proliferation.
A recent report showed that miR-21 affects CAFs accumulation, and subsequently, CAFs release miR-21 that can induce gemcitabine resistance by downregulating PTEN [111,138,139]. Notably, hypoxia induces miR-21 expression [115]. Moreover, tumor associated fibrosis produces miR-21 that targets PTEN [140]. miR-21 is a powerful oncogenic gene that enhances gemcitabine resistance by targeting PTEN or FasL [141]. In contrast, Park et al. reported that miR-21 inhibition or miR-221 induction enhanced gemcitabine sensitivity in pancreatic cancer due to increased PTEN expression [142]. Fang et al. showed that CAF-derived exosomal miR-106b plays a significant role in gemcitabine resistance [143]. Thus, miR-21-mediated CAF-targeted therapy may be a promising strategy to overcome chemoresistance in TME.
Table 2.
miRNAs for TME.
| Author | Ref. Number | miRNA | Target Gene |
|---|---|---|---|
| Sensitivity | |||
| Liu YF | [132] | miR-143 | HIF-1 |
| Ni J | [137] | miR-210 | HOXA9 |
| Liu A | [136] | miR-3662 | HIF-1 |
| Resistance | |||
| Zhang L | [138] | miR-21 | PDCD4 |
| Kadera BE | [139] | miR-21 | NA |
| Fang Y | [143] | miR-106b | TP53INP1 |
| Masamune A | [144] | miR-221 | NA |
| Guo Y | [122] | miR-222 | TSC1 |
| Luo G | [131] | miR-301a | TP63 |
| Xin X | [133] | miR-519c | HIF-1 |
Ref: reference, NA: not applicable.
Finally, increased miR-221 expression in PSCs contributes to gemcitabine resistance by activating the MAPK signaling pathway [144]. These findings suggest the importance of miRNAs in the regulation of TME components and their potential role as novel targets for improving gemcitabine efficacy in pancreatic cancer.
6. Gemcitabine Metabolism-Mediated Mechanisms of Gemcitabine Resistance in Pancreatic Cancer
6.1. Gemcitabine Metabolism
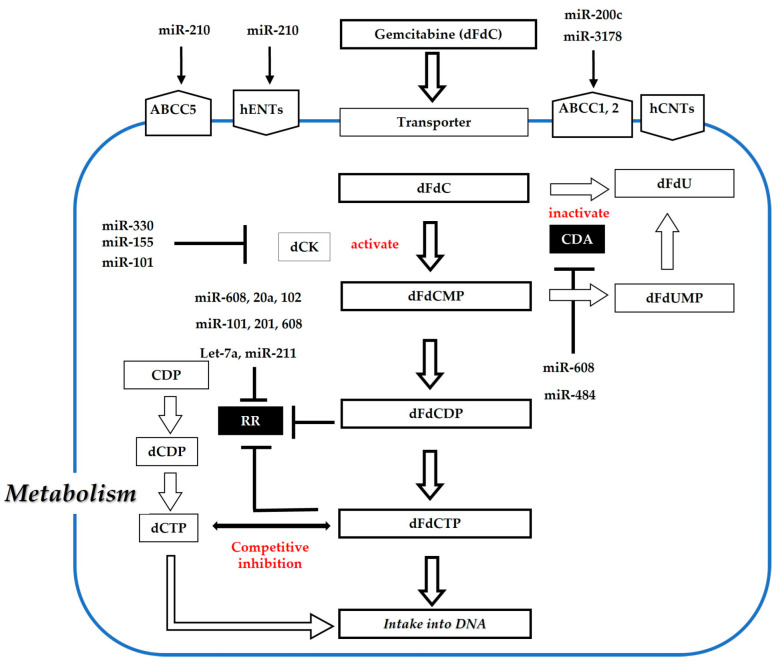
Previously, numerous investigations regarding the transport- and metabolism-related genes for gemcitabine were performed to elucidate the mechanism of gemcitabine resistance in pancreatic cancer cells [145,146,147]. Gemcitabine (dFdC) is a deoxycytidine nucleoside analog. Its metabolic pathways are shown in Figure 3. Gemcitabine is transported into the cells by nucleoside transporters, including concentrative nucleoside transporters (hCNTs) and equilibrative nucleoside transporters (hENT1 and hENT2). hENT1 is the main transporter for gemcitabine uptake in cancer cells. Gemcitabine is a prodrug that requires intracellular phosphorylation for its activation. dFdC is first phosphorylated to dFdCMP by deoxycytidine kinase (dCK), a key enzyme in the rate-limiting process. Subsequently, double phosphorylation results in the formation of the triphosphate form (dFdCTP). Finally, dFdCTP is incorporated into the DNA chain to produce a therapeutic effect. However, most dFdCs are inactivated by CDA. Additionally, dFdCDP and dFdCTP can inhibit ribonucleotide reductase (RR), which is responsible for converting ribonucleosides to deoxyribonucleoside triphosphates (dNTPs). Moreover, the dCTP produced by RR acts as a competitive inhibitor of dFdCTP. Therefore, overt changes in gemcitabine metabolism may generate gemcitabine resistance [148,149,150,151,152,153].
Figure 3.
Association of miRNAs with metabolism and gemcitabine resistance. Mainly, transporter and deoxycytidine kinase (dCK) are associated with the activation of gemcitabine. Conversely, cytidine deaminase (CDA) and ribonucleotide reductase (RR) are associated with the inactivation of gemcitabine. These transporters and enzymes are controlled by miRNAs, which results in gemcitabine resistance in pancreatic cancer.
6.2. Relation of Gemcitabine Metabolism to Gene Expression in Pancreatic Cancer
6.2.1. Transporters Associated with Gemcitabine Resistance
Alterations in hENT1, the major transporter for gemcitabine [153], contribute to the development of gemcitabine resistance [154]. Tsesmetzis et al. summarized that nucleoside analogs are principally transported by two membrane transporter families: hCNT1-3 and hENT1-4. Nucleoside analogs are transported by both hENTs and hCNTs, whereas nucleoside analogs are excreted by multidrug resistance proteins (MRP), also called ATP-binding cassette transporters (ABC transporters), which are classified into seven different subtypes (from ABCA- to ABCG) based on their gene structure [155]. Giovannetti et al. reported an association between hENT1 and gemcitabine sensitivity [156]. Mackey et al. also reported that hENT1 deficiency causes significant gemcitabine resistance [157]. In addition, recent data have shown that highly expressed hENT1 is a molecular and mechanistically relevant predictive marker for gemcitabine sensitivity [158,159]. In contrast, Hung et al. revealed that hCNT1 deficiency affects gemcitabine resistance [150]. Moreover, Skrypek et al. showed that MUC4-induced hcNT1 inhibition enhances gemcitabine resistance [160]. Qiu et al. described that inhibition of the PI3K/NFκB pathway could induce ABC transporter reduction by improving gemcitabine resistance [161]. Hagmann et al. also showed that ABC transporters are associated with gemcitabine resistance [149]. Because of the key roles played by hNT and ABC transporters in gemcitabine resistance, modulation of these transporters may lead to better efficacy of gemcitabine-based chemotherapeutic strategies.
6.2.2. CDA-Induced Gemcitabine Resistance
CDA is a catabolic enzyme that transforms gemcitabine into an inactivated metabolite [162]. Several studies have shown that the upregulation of CDA confers gemcitabine resistance and gemcitabine-induced toxicities [24,163,164,165,166]. Moreover, CDA expression levels can be used as a predictive marker for gemcitabine resistance [167]. Therefore, upregulated CDA expression may play an important role in gemcitabine resistance and patient prognosis in pancreatic cancer.
6.2.3. Relation of dCK and Gemcitabine Resistance
Gemcitabine is phosphorylated by dCK, resulting in its active product. dCK is the rate-limiting enzyme for nucleoside analogs [168,169]. Therefore, dCK inhibition is considered one of the causes of the development of gemcitabine resistance [25,170]. Ohmime et al. suggested that dCK expression is a prognostic factor in patients with pancreatic cancer [171]. Kamada et al. also reported that transduction of dCK could recover gemcitabine sensitivity in pancreatic cancer [172].
6.2.4. Relation of RR to Gemcitabine Resistance
Another potential factor in the mechanism for gemcitabine resistance is the overexpression of RR. RR is composed of the regulatory subunit M1 and the catalytic subunit M2, which are responsible for the conversion of ribonucleosides to deoxyribonucleoside triphosphates [173]. Duxbury et al. found that upregulated RRM2 closely participates in gemcitabine resistance by inhibiting the NF-κB signaling pathway [174]. Furthermore, previous studies have shown that increased RR is involved in the enhanced resistance to gemcitabine [24,175]. In contrast, Nakahira et al. revealed that RRM1 inhibition significantly reduces gemcitabine resistance [27]. Moreover, several studies have revealed that increased RR expression serves as a prognostic marker for patients with bile duct and pancreatic cancers [176,177].
6.3. Relation of Gemcitabine Metabolism to miRNAs
Amponsah et al. recently clarified the impact of miRNAs on gemcitabine metabolism, including transporters and gemcitabine-related enzymes. This study showed that miR-210 is responsible for gemcitabine sensitivity by targeting the ABCC5 gene [178]. Gu et al. indicated that miR-3178 promotes chemoresistance to gemcitabine by upregulating the ABC transporter-mediated RhoB/PI3K/Akt pathway [179]. Although Wang et al. did not mention gemcitabine resistance, they reported an association between miR-520h and ABCG2 [180]. Additionally, miR-93 enhances ABCB1 expression via targeting PTEN and increases Akt phosphorylation [181]. Furthermore, miR-331 induces ABCB1 expression by activating the Wnt/β-catenin pathway through ST7L in pancreatic cancer cells [182]. To the best of our knowledge, there are no reports regarding the association of hENT and hCNT with miR-mediated gemcitabine resistance. Recent data have shown that miR-101-3p and miR-211 enhance gemcitabine sensitivity by inhibiting RRM1 and RRM2, respectively [17,183]. Bhutia et al. revealed that let-7 negatively regulates RRM2 and sensitizes to gemcitabine [184]. Moreover, Rajabpour et al. showed that miR-608 leads to increased gemcitabine sensitivity, with decreased RRM1 and CDA expression [185]. Lu et al. suggested that miR-20a-5p upregulates gemcitabine sensitivity by targeting RRM2 [186]. Patel et al. also recently demonstrated that suppression of miR-155 induced dCK levels and restored gemcitabine sensitivity [187]. This demonstrates that miRNAs are connected with numerous targets involved in resistance to gemcitabine metabolism (Figure 3 and Table 3).
Table 3.
miRNAs for metabolism.
| Author | Ref. Number | miRNA | Target Gene |
|---|---|---|---|
| Sensitivity | |||
| Bhutia YD | [184] | let-7a | RRM2 |
| Lu H | [186] | miR-20a | RRM2 |
| Fan P | [183] | miR-101 | RRM1 |
| Amponsah PS | [178] | miR-210 | ABCC5 |
| Maftouh M | [17] | miR-211 | RRM2 |
| Rajabpour A | [185] | miR-608 | CDA, RRM1 |
| Gu J | [179] | miR-3178 | ABC transporter |
| Resistance | |||
| Wu Y | [181] | miR-93 | PTEN |
| Patel GK | [187] | miR-155 | dCK |
| Zhan T | [182] | miR-331 | ST7L |
| Xin X | [133] | miR-519c | ABCG2 |
Ref: reference.
7. Conclusions
We believe gemcitabine plus miRNA-based therapeutics can be expected to overcome the poor prognosis of pancreatic cancer, although further studies are needed to elucidate the molecular mechanism of chemoresistance caused by aberrantly expressed miRNAs. One of the major limitations of miRNA-based therapeutics is the lack of a delivery system capable of targeting tumors. Recent clinical trials of miR-34a liposomal-based therapy have been performed for advanced solid cancers [188]. Unfortunately, the trial was halted due to serious adverse events. However, miRNA-based strategies have the potential to deliver unprecedented value in pancreatic cancer treatment, as these may be able to control several target genes and signaling pathways. Moreover, recent studies showed that secreted microvesicles such as exosomes contain miRNAs, which play an important role in gemcitabine chemoresistance [110,189,190]. Comandatore et al. reviewed that exosomes, including oncogenic miRNAs, can affect gemcitabine resistance [191]. Thus, we suggest that miRNA-based strategies, involving regulation of miRNA expression, might contribute to overcoming gemcitabine resistance in the future. In contrast, exosomes, including specific miRNAs, must be a biomarker for gemcitabine resistance due to liquid biopsy.
Acknowledgments
The authors thank Noriko Funamizu (Department of Internal Medicine, Hirose Hospital, Ehime, Japan) for the invaluable advice and discussions regarding the manuscript.
Abbreviations
| Bcl-2 | B cell lymphoma-2 |
| ZEB1 | zinc finger E-box binding homeobox 1 |
| TUBB3 | tubulin-beta3 |
| NFKB1 | nuclear factor kappa B subunit 1 |
| NEAT1 | nuclear-enriched abundant transcript 1 |
| ESRP1 | epithelial splicing regulatory protein 1 |
| TFAP2C | transcription factor activating protein 2 gamma |
| SMURF2 | SMAD specific E3 ubiquitin protein ligase 2 |
| FBXW7 | F-Box and WD Repeat Domain Containing 7 |
| BOK | B-cell lymphoma 2 (BCL-2) ovarian killer |
| TP63 | tumor protein p63 |
| NR3C2 | nuclear receptor subfamily 3 group C member 2 |
| SHC1 | src homology domain-containing transforming protein 1 |
| Bax | bcl-2 associated X protein |
| RRM | ribonucleotide reductase regulatory subunit M |
| ABCC | ATP binding cassette C |
| CDA | cytidine deaminase |
| PTEN | phosphatase and tensin homolog |
| dCK | deoxycytidine kinase |
| ST7L | suppression of tumorigenicity 7-like |
| HIF-1 | hypoxia-inducible factor 1 |
| HOXA9 | homeobox A9 |
| PDCD4 | programmed cell death 4 |
| TP53INP1 | tumor protein 53-induced nuclear protein 1 |
| TSC1 | tuberous sclerosis complex 1 |
Author Contributions
Conceptualization, N.F. and Y.T.; methodology, N.F.; validation, M.H., K.T. and K.O.; investigation, N.F.; resources, M.H.; data curation, K.T.; writing—original draft preparation, N.F.; writing—review and editing, Y.T.; visualization, K.S.; supervision, K.O.; project administration, K.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest for this article.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Paulson A.S., Tran Cao H.S., Tempero M.A., Lowy A.M. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–1326. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 4.Burris H.A., Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., Cripps M.C., Portenoy R.K., Storniolo A.M., Tarassoff P., et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louvet C., Labianca R., Hammel P., Lledo G., Zampino M.G., André T., Zaniboni A., Ducreux M., Aitini E., Taïeb J., et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J. Clin. Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Barhli A., Cros J., Bartholin L., Neuzillet C. Prognostic stratification of resected pancreatic ductal adenocarcinoma: Past, present, and future. Dig. Liver Dis. 2018;50:979–990. doi: 10.1016/j.dld.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Ohuchida K., Mizumoto K., Kayashima T., Fujita H., Moriyama T., Ohtsuka T., Ueda J., Nagai E., Hashizume M., Tanaka M. MicroRNA expression as a predictive marker for gemcitabine response after surgical resection of pancreatic cancer. Ann. Surg. Oncol. 2011;18:2381–2387. doi: 10.1245/s10434-011-1602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhayat S., Mardin W.A., Mees S.T., Haier J. Epigenetic markers for chemosensitivity and chemoresistance in pancreatic cancer—A review. Int. J. Cancer. 2011;129:1031–1041. doi: 10.1002/ijc.26078. [DOI] [PubMed] [Google Scholar]
- 10.Chiou S.H., Dorsch M., Kusch E., Naranjo S., Kozak M.M., Koong A.C., Winslow M.M., Grüner B.M. Hmga2 is dispensable for pancreatic cancer development, metastasis, and therapy resistance. Sci. Rep. 2018;8:14008. doi: 10.1038/s41598-018-32159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mollah F., Varamini P. Overcoming therapy resistance and relapse in TNBC: Emerging technologies to target breast cancer-associated fibroblasts. Biomedicines. 2021;9:1921. doi: 10.3390/biomedicines9121921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos A., Sharma S., Obermair A., Salomon C. Extracellular vesicle-associated miRNAs and chemoresistance: A systematic review. Cancers. 2021;13:4608. doi: 10.3390/cancers13184608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos P., Almeida F. Role of exosomal miRNAs and the tumor microenvironment in drug resistance. Cells. 2020;9:1450. doi: 10.3390/cells9061450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wellner U., Schubert J., Burk U.C., Schmalhofer O., Zhu F., Sonntag A., Waldvogel B., Vannier C., Darling D., zur Hausen A., et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 15.Rice A.J., Cortes E., Lachowski D., Cheung B.C.H., Karim S.A., Morton J.P., Del Río Hernández A. Matrix stiffness induces epithelial-mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis. 2017;6:e352. doi: 10.1038/oncsis.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoepp M., Ströse A.J., Haier J. Dysregulation of miRNA expression in cancer associated fibroblasts (CAFs) and its consequences on the tumor microenvironment. Cancers. 2017;9:54. doi: 10.3390/cancers9060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maftouh M., Avan A., Funel N., Frampton A.E., Fiuji H., Pelliccioni S., Castellano L., Galla V., Peters G.J., Giovannetti E. miR-211 modulates gemcitabine activity through downregulation of ribonucleotide reductase and inhibits the invasive behavior of pancreatic cancer cells. Nucleosides Nucleotides Nucleic Acids. 2014;33:384–393. doi: 10.1080/15257770.2014.891741. [DOI] [PubMed] [Google Scholar]
- 18.Fazilaty H., Rago L., Kass Youssef K., Ocaña O.H., Garcia-Asencio F., Arcas A., Galceran J., Nieto M.A. A gene regulatory network to control EMT programs in development and disease. Nat. Commun. 2019;10:5115. doi: 10.1038/s41467-019-13091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregory P.A., Bert A.G., Paterson E.L., Barry S.C., Tsykin A., Farshid G., Vadas M.A., Khew-Goodall Y., Goodall G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 20.Lin H.J., Lin J. Seed-in-soil: Pancreatic cancer influenced by tumor microenvironment. Cancers. 2017;9:93. doi: 10.3390/cancers9070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunami Y., Böker V., Kleeff J. Targeting and reprograming cancer-associated fibroblasts and the tumor microenvironment in pancreatic cancer. Cancers. 2021;13:697. doi: 10.3390/cancers13040697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng S., Pöttler M., Lan B., Grützmann R., Pilarsky C., Yang H. Chemoresistance in pancreatic cancer. Int. J. Mol. Sci. 2019;20:4504. doi: 10.3390/ijms20184504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang J.H., Jiang Y., Pillarisetty V.G. Role of immune cells in pancreatic cancer from bench to clinical application: An updated review. Medicine. 2016;95:e5541. doi: 10.1097/MD.0000000000005541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funamizu N., Kamata Y., Misawa T., Uwagawa T., Lacy C.R., Yanaga K., Manome Y. Hydroxyurea decreases gemcitabine resistance in pancreatic carcinoma cells with highly expressed ribonucleotide reductase. Pancreas. 2012;41:107–113. doi: 10.1097/MPA.0b013e318224b5fb. [DOI] [PubMed] [Google Scholar]
- 25.Funamizu N., Okamoto A., Kamata Y., Misawa T., Uwagawa T., Gocho T., Yanaga K., Manome Y. Is the resistance of gemcitabine for pancreatic cancer settled only by overexpression of deoxycytidine kinase? Oncol. Rep. 2010;23:471–475. doi: 10.3892/or_00000657. [DOI] [PubMed] [Google Scholar]
- 26.Funamizu N., Lacy C.R., Fujita K., Furukawa K., Misawa T., Yanaga K., Manome Y. Tetrahydrouridine inhibits cell proliferation through cell cycle regulation regardless of cytidine deaminase expression levels. PLoS ONE. 2012;7:e37424. doi: 10.1371/journal.pone.0037424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakahira S., Nakamori S., Tsujie M., Takahashi Y., Okami J., Yoshioka S., Yamasaki M., Marubashi S., Takemasa I., Miyamoto A., et al. Involvement of ribonucleotide reductase M1 subunit overexpression in gemcitabine resistance of human pancreatic cancer. Int. J. Cancer. 2007;120:1355–1363. doi: 10.1002/ijc.22390. [DOI] [PubMed] [Google Scholar]
- 28.Amrutkar M., Gladhaug I.P. Pancreatic cancer chemoresistance to gemcitabine. Cancers. 2017;9:157. doi: 10.3390/cancers9110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 30.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 31.Bagga S., Bracht J., Hunter S., Massirer K., Holtz J., Eachus R., Pasquinelli A.E. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 32.Garofalo M., Romano G., Di Leva G., Nuovo G., Jeon Y.J., Ngankeu A., Sun J., Lovat F., Alder H., Condorelli G., et al. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat. Med. 2011;18:74–82. doi: 10.1038/nm.2577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Forterre A., Komuro H., Aminova S., Harada M. A comprehensive review of cancer microRNA therapeutic delivery strategies. Cancers. 2020;12:1852. doi: 10.3390/cancers12071852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan S., Ansarullah A., Kumar D., Jaggi M., Chauhan S.C. Targeting microRNAs in pancreatic cancer: Microplayers in the big game. Can. Res. 2013;73:6541–6547. doi: 10.1158/0008-5472.CAN-13-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uddin M.H., Al-Hallak M.N., Philip P.A., Mohammad R.M., Viola N., Wagner K.U., Azmi A.S. Exosomal microRNA in pancreatic cancer diagnosis, prognosis, and treatment: From bench to bedside. Cancers. 2021;13:2777. doi: 10.3390/cancers13112777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato S., Honda K. Use of biomarkers and imaging for early detection of pancreatic cancer. Cancers. 2020;12:1965. doi: 10.3390/cancers12071965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavlíková L., Šereš M., Breier A., Sulová Z. The roles of microRNAs in cancer multidrug resistance. Cancers. 2022;14:1090. doi: 10.3390/cancers14041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan G., Liu Y., Shang L., Zhou F., Yang S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun. 2021;41:199–217. doi: 10.1002/cac2.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwang G.R., Yuen J.G., Ju J. Roles of microRNAs in gastrointestinal cancer stem cell resistance and therapeutic development. Int. J. Mol. Sci. 2021;22:1624. doi: 10.3390/ijms22041624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pusceddu S., Ghidini M., Torchio M., Corti F., Tomasello G., Niger M., Prinzi N., Nichetti F., Coinu A., Di Bartolomeo M., et al. Comparative effectiveness of gemcitabine plus nab-paclitaxel and FOLFIRINOX in the first-line setting of metastatic pancreatic cancer: A systematic review and meta-analysis. Cancers. 2019;11:484. doi: 10.3390/cancers11040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y.S., Lee J.C., Kim J.H., Kim J., Hwang J.H. Pharmacoethnicity of FOLFIRINOX versus gemcitabine plus nab-paclitaxel in metastatic pancreatic cancer: A systematic review and meta-analysis. Sci. Rep. 2021;11:20152. doi: 10.1038/s41598-021-99647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Z.Y., Yuan J.Q., Di M.Y., Zheng D.Y., Chen J.Z., Ding H., Wu X.Y., Huang Y.F., Mao C., Tang J.L. Gemcitabine plus erlotinib for advanced pancreatic cancer: A systematic review with meta-analysis. PLoS ONE. 2013;8:e57528. doi: 10.1371/journal.pone.0057528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D., Chen C., Zhou Y., Chen R., Fan X., Bi Z., Li Z., Liu Y. Gemcitabine compared with gemcitabine and S-1 combination therapy in advanced pancreatic cancer: A systematic review and meta-analysis. Medicine. 2015;94:e1345. doi: 10.1097/MD.0000000000001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin C., Yang G., Yang J., Ren B., Wang H., Chen G., Zhao F., You L., Wang W., Zhao Y. Metabolism of pancreatic cancer: Paving the way to better anticancer strategies. Mol. Cancer. 2020;19:50. doi: 10.1186/s12943-020-01169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang C., Shi S., Meng Q., Liang D., Ji S., Zhang B., Qin Y., Xu J., Ni Q., Yu X. Complex roles of the stroma in the intrinsic resistance to gemcitabine in pancreatic cancer: Where we are and where we are going. Exp. Mol. Med. 2017;49:e406. doi: 10.1038/emm.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaianigo N., Melisi D., Carbone C. EMT and treatment resistance in pancreatic cancer. Cancers. 2017;9:122. doi: 10.3390/cancers9090122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Royam M.M., Ramesh R., Shanker R., Sabarimurugan S., Kumarasamy C., Gothandam K.M., Baxi S., Gupta A., Krishnan S., Jayaraj R. miRNA predictors of pancreatic cancer chemotherapeutic response: A systematic review and meta-analysis. Cancers. 2019;11:900. doi: 10.3390/cancers11070900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng X., Carstens J.L., Kim J., Scheible M., Kaye J., Sugimoto H., Wu C.C., LeBleu V.S., Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z., Li Y., Kong D., Banerjee S., Ahmad A., Azmi A.S., Ali S., Abbruzzese J.L., Gallick G.E., Sarkar F.H. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–2407. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhayat S.A., Traeger M.M., Rehkaemper J., Stroese A.J., Steinestel K., Wardelmann E., Kabar I., Senninger N. Clinical impact of epithelial-to-mesenchymal transition regulating microRNAs in pancreatic ductal adenocarcinoma. Cancers. 2018;10:328. doi: 10.3390/cancers10090328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wellner U., Brabletz T., Keck T. ZEB1 in Pancreatic Cancer. Cancers. 2010;2:1617–1628. doi: 10.3390/cancers2031617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polyak K., Weinberg R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 54.Funamizu N., Lacy C.R., Parpart S.T., Takai A., Hiyoshi Y., Yanaga K. MicroRNA-301b promotes cell invasiveness through targeting TP63 in pancreatic carcinoma cells. Int. J. Oncol. 2014;44:725–734. doi: 10.3892/ijo.2014.2243. [DOI] [PubMed] [Google Scholar]
- 55.Funamizu N., Hu C., Lacy C., Schetter A., Zhang G., He P., Gaedcke J., Ghadimi M.B., Ried T., Yfantis H.G., et al. Macrophage migration inhibitory factor induces epithelial to mesenchymal transition, enhances tumor aggressiveness and predicts clinical outcome in resected pancreatic ductal adenocarcinoma. Int. J. Cancer. 2013;132:785–794. doi: 10.1002/ijc.27736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah A.N., Summy J.M., Zhang J., Park S.I., Parikh N.U., Gallick G.E. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann. Surg. Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 57.Wu Q., Wang X., Liu J., Zheng J., Liu Y., Li Y., Su F., Ou W., Wang R. Nutlin-3 reverses the epithelial-mesenchymal transition in gemcitabine-resistant hepatocellular carcinoma cells. Oncol. Rep. 2016;36:1325–1332. doi: 10.3892/or.2016.4920. [DOI] [PubMed] [Google Scholar]
- 58.Quint K., Tonigold M., Di Fazio P., Montalbano R., Lingelbach S., Rückert F., Alinger B., Ocker M., Neureiter D. Pancreatic cancer cells surviving gemcitabine treatment express markers of stem cell differentiation and epithelial-mesenchymal transition. Int. J. Oncol. 2012;41:2093–2102. doi: 10.3892/ijo.2012.1648. [DOI] [PubMed] [Google Scholar]
- 59.Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y., Brooks M., Reinhard F., Zhang C.C., Shipitsin M., et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan Y., Li K., Tao X., Zhao Y., Chen Q., Li N., Liu J., Go V.L.W., Guo J., Gao G., et al. MicroRNA-34a alleviates gemcitabine resistance in pancreatic cancer by repression of cancer stem cell renewal. Pancreas. 2021;50:1260–1266. doi: 10.1097/MPA.0000000000001920. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y., Wei J., Wang H., Xue X., An Y., Tang D., Yuan Z., Wang F., Wu J., Zhang J., et al. Epithelial mesenchymal transition correlates with CD24+CD44+ and CD133+ cells in pancreatic cancer. Oncol. Rep. 2012;27:1599–1605. doi: 10.3892/or.2012.1681. [DOI] [PubMed] [Google Scholar]
- 62.Cioffi M., Trabulo S.M., Sanchez-Ripoll Y., Miranda-Lorenzo I., Lonardo E., Dorado J., Reis Vieira C., Ramirez J.C., Hidalgo M., Aicher A., et al. The miR-17-92 cluster counteracts quiescence and chemoresistance in a distinct subpopulation of pancreatic cancer stem cells. Gut. 2015;64:1936–1948. doi: 10.1136/gutjnl-2014-308470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ji Q., Hao X., Zhang M., Tang W., Yang M., Li L., Xiang D., Desano J.T., Bommer G.T., Fan D., et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS ONE. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okumura Y., Noda T., Eguchi H., Sakamoto T., Iwagami Y., Yamada D., Asaoka T., Wada H., Kawamoto K., Gotoh K., et al. Hypoxia-induced PLOD2 is a key regulator in epithelial-mesenchymal transition and chemoresistance in biliary tract cancer. Ann. Surg. Oncol. 2018;25:3728–3737. doi: 10.1245/s10434-018-6670-8. [DOI] [PubMed] [Google Scholar]
- 65.Arumugam T., Ramachandran V., Fournier K.F., Wang H., Marquis L., Abbruzzese J.L., Gallick G.E., Logsdon C.D., McConkey D.J., Choi W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carrasco-Garcia E., Lopez L., Moncho-Amor V., Carazo F., Aldaz P., Collado M., Bell D., Gaafar A., Karamitopoulou E., Tzankov A., et al. SOX9 triggers different epithelial to mesenchymal transition states to promote pancreatic cancer progression. Cancers. 2022;14:916. doi: 10.3390/cancers14040916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh M., Yelle N., Venugopal C., Singh S.K. EMT: Mechanisms and therapeutic implications. Pharmacol. Ther. 2018;182:80–94. doi: 10.1016/j.pharmthera.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 68.Zhang G., Schetter A., He P., Funamizu N., Gaedcke J., Ghadimi B.M., Ried T., Hassan R., Yfantis H.G., Lee D.H., et al. DPEP1 inhibits tumor cell invasiveness, enhances chemosensitivity and predicts clinical outcome in pancreatic ductal adenocarcinoma. PLoS ONE. 2012;7:e31507. doi: 10.1371/journal.pone.0031507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lawlor R.T., Veronese N., Nottegar A., Malleo G., Smith L., Demurtas J., Cheng L., Wood L.D., Silvestris N., Salvia R., et al. Prognostic role of high-grade tumor budding in pancreatic ductal adenocarcinoma: A systematic review and meta-analysis with a focus on epithelial to mesenchymal transition. Cancers. 2019;11:113. doi: 10.3390/cancers11010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krantz S.B., Shields M.A., Dangi-Garimella S., Bentrem D.J., Munshi H.G. Contribution of epithelial-mesenchymal transition to pancreatic cancer progression. Cancers. 2010;2:2084–2097. doi: 10.3390/cancers2042084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maier H.J., Wirth T., Beug H. Epithelial-mesenchymal transition in pancreatic carcinoma. Cancers. 2010;2:2058–2083. doi: 10.3390/cancers2042058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang T., Chen G., Ma X., Yang Y., Chen Y., Peng Y., Bai Z., Zhang Z., Pei H., Guo W. Mir-30a regulates cancer cell response to chemotherapy through snai1/irs1/akt pathway. Cell Death Dis. 2019;10:153. doi: 10.1038/s41419-019-1326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Funamizu N., Lacy C.R., Kamada M., Yanaga K., Manome Y. MicroRNA-200b and −301 are associated with gemcitabine response as biomarkers in pancreatic carcinoma cells. Int. J. Oncol. 2019;54:991–1000. doi: 10.3892/ijo.2019.4676. [DOI] [PubMed] [Google Scholar]
- 74.Li Y., Vandenboom T.G., Kong D., Wang Z., Ali S., Philip P.A., Sarkar F.H. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu G., Ji L., Ke M., Ou Z., Tang N., Li Y. miR-125a-3p is responsible for chemosensitivity in PDAC by inhibiting epithelial-mesenchymal transition via Fyn. Biomed. Pharmacother. 2018;106:523–531. doi: 10.1016/j.biopha.2018.06.114. [DOI] [PubMed] [Google Scholar]
- 76.Fu X., Deng X., Xiao W., Huang B., Yi X., Zou Y. Downregulation of NEAT1 sensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine through modulation of the miR-506-3p/ZEB2/EMT axis. Am. J. Cancer Res. 2021;11:3841–3856. [PMC free article] [PubMed] [Google Scholar]
- 77.Li J., Wu H., Li W., Yin L., Guo S., Xu X., Ouyang Y., Zhao Z., Liu S., Tian Y., et al. Downregulated miR-506 expression facilitates pancreatic cancer progression and chemoresistance via SPHK1/Akt/NF-κB signaling. Oncogene. 2016;35:5501–5514. doi: 10.1038/onc.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hiramoto H., Muramatsu T., Ichikawa D., Tanimoto K., Yasukawa S., Otsuji E., Inazawa J. miR-509-5p and miR-1243 increase the sensitivity to gemcitabine by inhibiting epithelial-mesenchymal transition in pancreatic cancer. Sci. Rep. 2017;7:4002. doi: 10.1038/s41598-017-04191-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang R.M., Zhan M., Xu S.W., Long M.M., Yang L.H., Chen W., Huang S., Liu Q., Zhou J., Zhu J., et al. miR-3656 expression enhances the chemosensitivity of pancreatic cancer to gemcitabine through modulation of the RHOF/EMT axis. Cell Death Dis. 2017;8:e3129. doi: 10.1038/cddis.2017.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu F., Liu B., Qian J., Wu G., Li J., Ma Z. miR-153 enhances the therapeutic effect of gemcitabine by targeting Snail in pancreatic cancer. Acta Biochim. Biophys. Sin. 2017;49:520–529. doi: 10.1093/abbs/gmx039. [DOI] [PubMed] [Google Scholar]
- 81.Bai Z., Sun J., Wang X., Wang H., Pei H., Zhang Z. MicroRNA-153 is a prognostic marker and inhibits cell migration and invasion by targeting SNAI1 in human pancreatic ductal adenocarcinoma. Oncol. Rep. 2015;34:595–602. doi: 10.3892/or.2015.4051. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Wang Z., Chen Y., Lin Y., Wang X., Cui X., Zhang Z., Xian G., Qin C. Novel crosstalk between KLF4 and ZEB1 regulates gemcitabine resistance in pancreatic ductal adenocarcinoma. Int. J. Oncol. 2017;51:1239–1248. doi: 10.3892/ijo.2017.4099. [DOI] [PubMed] [Google Scholar]
- 83.Chaudhary A.K., Mondal G., Kumar V., Kattel K., Mahato R.I. Chemosensitization and inhibition of pancreatic cancer stem cell proliferation by overexpression of microRNA-205. Cancer Lett. 2017;402:1–8. doi: 10.1016/j.canlet.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang Z., Zhao N., Cui J., Wu H., Xiong J., Peng T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cell. Oncol. 2020;43:123–136. doi: 10.1007/s13402-019-00476-6. [DOI] [PubMed] [Google Scholar]
- 85.Hasegawa S., Eguchi H., Nagano H., Konno M., Tomimaru Y., Wada H., Hama N., Kawamoto K., Kobayashi S., Nishida N., et al. MicroRNA-1246 expression associated with CCNG2-mediated chemoresistance and stemness in pancreatic cancer. Br. J. Cancer. 2014;111:1572–1580. doi: 10.1038/bjc.2014.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiong G., Huang H., Feng M., Yang G., Zheng S., You L., Zheng L., Hu Y., Zhang T., Zhao Y. Mir-10a-5p targets tfap2c to promote gemcitabine resistance in pancreatic ductal adenocarcinoma. J. Exp. Clin. Cancer Res. 2018;37:76. doi: 10.1186/s13046-018-0739-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang W.L., Zhang J.H., Wu X.Z., Yan T., Lv W. miR-15b promotes epithelial-mesenchymal transition by inhibiting SMURF2 in pancreatic cancer. Int. J. Oncol. 2015;47:1043–1053. doi: 10.3892/ijo.2015.3076. [DOI] [PubMed] [Google Scholar]
- 88.Yang S., He P., Wang J., Schetter A., Tang W., Funamizu N., Yanaga K., Uwagawa T., Satoskar A.R., Gaedcke J., et al. A novel MIF signaling pathway drives the malignant character of pancreatic cancer by targeting NR3C2. Cancer Res. 2016;76:3838–3850. doi: 10.1158/0008-5472.CAN-15-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang K.D., Hu B., Cen G., Yang Y.H., Chen W.W., Guo Z.Y., Wang X.F., Zhao Q., Qiu Z.J. Mir-301a transcriptionally activated by hif-2alpha promotes hypoxia-induced epithelial-mesenchymal transition by targeting tp63 in pancreatic cancer. World J. Gastroenterol. 2020;26:2349–2373. doi: 10.3748/wjg.v26.i19.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okazaki J., Tanahashi T., Sato Y., Miyoshi J., Nakagawa T., Kimura T., Miyamoto H., Fujino Y., Nakamura F., Takehara M., et al. MicroRNA-296-5p promotes cell invasion and drug resistance by targeting Bcl2-related ovarian killer, leading to a poor prognosis in pancreatic cancer. Digestion. 2020;101:794–806. doi: 10.1159/000503225. [DOI] [PubMed] [Google Scholar]
- 91.Ma J., Fang B., Zeng F., Ma C., Pang H., Cheng L., Shi Y., Wang H., Yin B., Xia J., et al. Down-regulation of mir-223 reverses epithelial-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Oncotarget. 2015;6:1740–1749. doi: 10.18632/oncotarget.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu S., Wang M., Zhang H., Guo X., Qin R. Circ_0092367 inhibits EMT and gemcitabine resistance in pancreatic cancer via regulating the miR-1206/ESRP1 axis. Genes. 2021;12:1701. doi: 10.3390/genes12111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu Q., Xiu Z., Jian Y., Zhou J., Chen X., Chen X., Chen C., Chen H., Yang S., Yin L., et al. microRNA-497 prevents pancreatic cancer stem cell gemcitabine resistance, migration, and invasion by directly targeting nuclear factor kappa B 1. Aging. 2022;14:5908–5924. doi: 10.18632/aging.204193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ma C., Huang T., Ding Y.C., Yu W., Wang Q., Meng B., Luo S.X. Microrna-200c overexpression inhibits chemoresistance, invasion and colony formation of human pancreatic cancer stem cells. Int. J. Clin. Exp. Pathol. 2015;8:6533–6539. [PMC free article] [PubMed] [Google Scholar]
- 95.Feig C., Gopinathan A., Neesse A., Chan D.S., Cook N., Tuveson D.A. The pancreas cancer microenvironment. Clin. Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deng D., Patel R., Chiang C.Y., Hou P. Role of the tumor microenvironment in regulating pancreatic cancer therapy resistance. Cells. 2022;11:2952. doi: 10.3390/cells11192952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Neesse A., Michl P., Frese K.K., Feig C., Cook N., Jacobetz M.A., Lolkema M.P., Buchholz M., Olive K.P., Gress T.M., et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 98.Deshmukh S.K., Tyagi N., Khan M.A., Srivastava S.K., Al-Ghadhban A., Dugger K., Carter J.E., Singh S., Singh A.P. Gemcitabine treatment promotes immunosuppressive microenvironment in pancreatic tumors by supporting the infiltration, growth, and polarization of macrophages. Sci. Rep. 2018;8:12000. doi: 10.1038/s41598-018-30437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahmad I.M., Dafferner A.J., O’Connell K.A., Mehla K., Britigan B.E., Hollingsworth M.A., Abdalla M.Y. Heme Oxygenase-1 inhibition potentiates the effects of nab-paclitaxel-gemcitabine and modulates the tumor microenvironment in pancreatic ductal adenocarcinoma. Cancers. 2021;13:2264. doi: 10.3390/cancers13092264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wei L., Ye H., Li G., Lu Y., Zhou Q., Zheng S., Lin Q., Liu Y., Li Z., Chen R. Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer. Cell Death Dis. 2018;9:1065. doi: 10.1038/s41419-018-1104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gu Z., Du Y., Zhao X., Wang C. Tumor microenvironment and metabolic remodeling in gemcitabine-based chemoresistance of pancreatic cancer. Cancer Lett. 2021;521:98–108. doi: 10.1016/j.canlet.2021.08.029. [DOI] [PubMed] [Google Scholar]
- 102.Park J., Schwarzbauer J.E. Mammary epithelial cell interactions with fibronectin stimulate epithelial-mesenchymal transition. Oncogene. 2014;33:1649–1657. doi: 10.1038/onc.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miyamoto H., Murakami T., Tsuchida K., Sugino H., Miyake H., Tashiro S. Tumor-stroma interaction of human pancreatic cancer: Acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28:38–44. doi: 10.1097/00006676-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 104.Amrutkar M., Aasrum M., Verbeke C.S., Gladhaug I.P. Secretion of fibronectin by human pancreatic stellate cells promotes chemoresistance to gemcitabine in pancreatic cancer cells. BMC Cancer. 2019;19:596. doi: 10.1186/s12885-019-5803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Topalovski M., Brekken R.A. Matrix control of pancreatic cancer: New insights into fibronectin signaling. Cancer Lett. 2016;381:252–258. doi: 10.1016/j.canlet.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dangi-Garimella S., Krantz S.B., Barron M.R., Shields M.A., Heiferman M.J., Grippo P.J., Bentrem D.J., Munshi H.G. Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA2. Cancer Res. 2011;71:1019–1028. doi: 10.1158/0008-5472.CAN-10-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Cutsem E., Tempero M.A., Sigal D., Oh D.Y., Fazio N., Macarulla T., Hitre E., Hammel P., Hendifar A.E., Bates S.E., et al. Randomized Phase III trial of Pegvorhyaluronidase Alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J. Clin. Oncol. 2020;38:3185–3194. doi: 10.1200/JCO.20.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ho W.J., Jaffee E.M., Zheng L. The tumour microenvironment in pancreatic cancer—Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020;17:527–540. doi: 10.1038/s41571-020-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 110.Richards K.E., Xiao W., Hill R., On Behalf Of The Usc Pancreas Research Team Cancer-associated fibroblasts confer gemcitabine resistance to pancreatic cancer cells through PTEN-targeting miRNAs in exosomes. Cancers. 2022;14:2812. doi: 10.3390/cancers14112812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang D., Li L., Jiang H., Li Q., Wang-Gillam A., Yu J., Head R., Liu J., Ruzinova M.B., Lim K.H. Tumor-stroma IL1beta-IRAK4 feedforward circuitry drives tumor fibrosis, chemoresistance, and poor prognosis in pancreatic cancer. Cancer Res. 2018;78:1700–1712. doi: 10.1158/0008-5472.CAN-17-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu C., Xia R., Zhang X., Li T., Ye Y., Li G., He R., Li Z., Lin Q., Zheng S., et al. circFARP1 enables cancer-associated fibroblasts to promote gemcitabine resistance in pancreatic cancer via the LIF/STAT3 axis. Mol. Cancer. 2022;21:24. doi: 10.1186/s12943-022-01501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wei L., Lin Q., Lu Y., Li G., Huang L., Fu Z., Chen R., Zhou Q. Cancer-associated fibroblasts-mediated ATF4 expression promotes malignancy and gemcitabine resistance in pancreatic cancer via the TGF-β1/SMAD2/3 pathway and ABCC1 transactivation. Cell Death Dis. 2021;12:334. doi: 10.1038/s41419-021-03574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ikenaga N., Ohuchida K., Mizumoto K., Akagawa S., Fujiwara K., Eguchi D., Kozono S., Ohtsuka T., Takahata S., Tanaka M. Pancreatic cancer cells enhance the ability of collagen internalization during epithelial-mesenchymal transition. PLoS ONE. 2012;7:e40434. doi: 10.1371/journal.pone.0040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng M., Li H., Sun L., Brigstock D.R., Gao R. Interleukin-6 participates in human pancreatic stellate cell activation and collagen I production via TGF-β1/Smad pathway. Cytokine. 2021;143:155536. doi: 10.1016/j.cyto.2021.155536. [DOI] [PubMed] [Google Scholar]
- 116.Cao F., Li J., Sun H., Liu S., Cui Y., Li F. HES 1 is essential for chemoresistance induced by stellate cells and is associated with poor prognosis in pancreatic cancer. Oncol. Rep. 2015;33:1883–1889. doi: 10.3892/or.2015.3789. [DOI] [PubMed] [Google Scholar]
- 117.Lee E., Ryu G.R., Ko S.H., Ahn Y.B., Song K.H. A role of pancreatic stellate cells in islet fibrosis and β-cell dysfunction in type 2 diabetes mellitus. Biochem. Biophys. Res. Commun. 2017;485:328–334. doi: 10.1016/j.bbrc.2017.02.082. [DOI] [PubMed] [Google Scholar]
- 118.Kikuta K., Masamune A., Watanabe T., Ariga H., Itoh H., Hamada S., Satoh K., Egawa S., Unno M., Shimosegawa T. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2010;403:380–384. doi: 10.1016/j.bbrc.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 119.LaRue M.M., Parker S., Puccini J., Cammer M., Kimmelman A.C., Bar-Sagi D. Metabolic reprogramming of tumor-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer. Proc. Natl. Acad. Sci. USA. 2022;119:e2119168119. doi: 10.1073/pnas.2119168119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Helm O., Held-Feindt J., Grage-Griebenow E., Reiling N., Ungefroren H., Vogel I., Krüger U., Becker T., Ebsen M., Röcken C., et al. Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int. J. Cancer. 2014;135:843–861. doi: 10.1002/ijc.28736. [DOI] [PubMed] [Google Scholar]
- 121.Weizman N., Krelin Y., Shabtay-Orbach A., Amit M., Binenbaum Y., Wong R.J., Gil Z. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene. 2014;33:3812–3819. doi: 10.1038/onc.2013.357. [DOI] [PubMed] [Google Scholar]
- 122.Guo Y., Wu H., Xiong J., Gou S., Cui J., Peng T. miR-222-3p-containing macrophage-derived extracellular vesicles confer gemcitabine resistance via TSC1-mediated mTOR/AKT/PI3K pathway in pancreatic cancer. Cell Biol. Toxicol. 2022 doi: 10.1007/s10565-022-09736-y. [DOI] [PubMed] [Google Scholar]
- 123.Nagathihalli N.S., Castellanos J.A., Shi C., Beesetty Y., Reyzer M.L., Caprioli R., Chen X., Walsh A.J., Skala M.C., Moses H.L., et al. Signal transducer and activator of transcription 3, mediated remodeling of the tumor microenvironment results in enhanced tumor drug delivery in a mouse model of pancreatic cancer. Gastroenterology. 2015;149:1932–1943.e9. doi: 10.1053/j.gastro.2015.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mitchem J.B., Brennan D.J., Knolhoff B.L., Belt B.A., Zhu Y., Sanford D.E., Belaygorod L., Carpenter D., Collins L., Piwnica-Worms D., et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oba T., Sato N., Adachi Y., Amaike T., Kudo Y., Koga A., Kohi S., Hirata K. Hypoxia increases KIAA1199/CEMIP expression and enhances cell migration in pancreatic cancer. Sci. Rep. 2021;11:18193. doi: 10.1038/s41598-021-97752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Keleg S., Kayed H., Jiang X., Penzel R., Giese T., Büchler M.W., Friess H., Kleeff J. Adrenomedullin is induced by hypoxia and enhances pancreatic cancer cell invasion. Int. J. Cancer. 2007;121:21–32. doi: 10.1002/ijc.22596. [DOI] [PubMed] [Google Scholar]
- 127.Chee N.T., Carriere C.H., Miller Z., Welford S., Brothers S.P. Activating transcription factor 4 regulates hypoxia inducible factor 1α in chronic hypoxia in pancreatic cancer cells. Oncol. Rep. 2023;49:14. doi: 10.3892/or.2022.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fan Y., Xu L.L., Shi C.Y., Wei W., Wang D.S., Cai D.F. MicroRNA-454 regulates stromal cell derived factor-1 in the control of the growth of pancreatic ductal adenocarcinoma. Sci. Rep. 2016;6:22793. doi: 10.1038/srep22793. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Bao B., Ali S., Ahmad A., Azmi A.S., Li Y., Banerjee S., Kong D., Sethi S., Aboukameel A., Padhye S.B., et al. Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS ONE. 2012;7:e50165. doi: 10.1371/journal.pone.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nong K., Zhang D., Chen C., Yang Y., Yang Y., Liu S., Cai H. MicroRNA-519 inhibits hypoxia-induced tumorigenesis of pancreatic cancer by regulating immune checkpoint PD-L1. Oncol. Lett. 2020;19:1427–1433. doi: 10.3892/ol.2019.11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luo G., Xia X., Wang X., Zhang K., Cao J., Jiang T., Zhao Q., Qiu Z. miR-301a plays a pivotal role in hypoxia-induced gemcitabine resistance in pancreatic cancer. Exp. Cell Res. 2018;369:120–128. doi: 10.1016/j.yexcr.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 132.Liu Y.F., Luo D., Li X., Li Z.Q., Yu X., Zhu H.W. PVT1 knockdown inhibits autophagy and improves gemcitabine sensitivity by regulating the MiR-143/HIF-1α/VMP1 axis in pancreatic cancer. Pancreas. 2021;50:227–234. doi: 10.1097/MPA.0000000000001747. [DOI] [PubMed] [Google Scholar]
- 133.Xin X., Kumar V., Lin F., Kumar V., Bhattarai R., Bhatt V.R., Tan C., Mahato R.I. Redox-responsive nanoplatform for codelivery of miR-519c and gemcitabine for pancreatic cancer therapy. Sci. Adv. 2020;6:eabd6764. doi: 10.1126/sciadv.abd6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kasuya K., Tsuchida A., Nagakawa Y., Suzuki M., Abe Y., Itoi T., Serizawa H., Nagao T., Shimazu M., Aoki T. Hypoxia-inducible factor-1α expression and gemcitabine chemotherapy for pancreatic cancer. Oncol. Rep. 2011;26:1399–1406. doi: 10.3892/or.2011.1457. [DOI] [PubMed] [Google Scholar]
- 135.Yang X., Yin H., Zhang Y., Li X., Tong H., Zeng Y., Wang Q., He W. Hypoxia-induced autophagy promotes gemcitabine resistance in human bladder cancer cells through hypoxia-inducible factor 1α activation. Int. J. Oncol. 2018;53:215–224. doi: 10.3892/ijo.2018.4376. [DOI] [PubMed] [Google Scholar]
- 136.Liu A., Zhou Y., Zhao T., Tang X., Zhou B., Xu J. MiRNA-3662 reverses the gemcitabine resistance in pancreatic cancer through regulating the tumor metabolism. Cancer Chemother. Pharmacol. 2021;88:343–357. doi: 10.1007/s00280-021-04289-z. [DOI] [PubMed] [Google Scholar]
- 137.Ni J., Zhou S., Yuan W., Cen F., Yan Q. Mechanism of miR-210 involved in epithelial-mesenchymal transition of pancreatic cancer cells under hypoxia. J. Recept. Signal Transduct. 2019;39:399–406. doi: 10.1080/10799893.2019.1683863. [DOI] [PubMed] [Google Scholar]
- 138.Zhang L., Yao J., Li W., Zhang C. Micro-RNA-21 regulates cancer-associated fibroblast-mediated drug resistance in pancreatic cancer. Oncol. Res. 2018;26:827–835. doi: 10.3727/096504017X14934840662335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kadera B.E., Li L., Toste P.A., Wu N., Adams C., Dawson D.W., Donahue T.R. MicroRNA-21 in pancreatic ductal adenocarcinoma tumor-associated fibroblasts promotes metastasis. PLoS ONE. 2013;8:e71978. doi: 10.1371/journal.pone.0071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mace T.A., Collins A.L., Wojcik S.E., Croce C.M., Lesinski G.B., Bloomston M. Hypoxia induces the overexpression of microRNA-21 in pancreatic cancer cells. J. Surg. Res. 2013;184:855–860. doi: 10.1016/j.jss.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang P., Zhuang L., Zhang J., Fan J., Luo J., Chen H., Wang K., Liu L., Chen Z., Meng Z. The serum miR-21 level serves as a predictor for the chemosensitivity of advanced pancreatic cancer, and miR-21 expression confers chemoresistance by targeting FASL. Mol. Oncol. 2013;7:334–345. doi: 10.1016/j.molonc.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Park J.K., Lee E.J., Esau C., Schmittgen T.D. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas. 2009;38:e190–e199. doi: 10.1097/MPA.0b013e3181ba82e1. [DOI] [PubMed] [Google Scholar]
- 143.Fang Y., Zhou W., Rong Y., Kuang T., Xu X., Wu W., Wang D., Lou W. Exosomal miRNA-106b from cancer-associated fibroblast promotes gemcitabine resistance in pancreatic cancer. Exp. Cell Res. 2019;383:111543. doi: 10.1016/j.yexcr.2019.111543. [DOI] [PubMed] [Google Scholar]
- 144.Masamune A., Nakano E., Hamada S., Takikawa T., Yoshida N., Shimosegawa T. Alteration of the microRNA expression profile during the activation of pancreatic stellate cells. Scand. J. Gastroenterol. 2014;49:323–331. doi: 10.3109/00365521.2013.876447. [DOI] [PubMed] [Google Scholar]
- 145.Liu M., Zhang Y., Yang J., Cui X., Zhou Z., Zhan H., Ding K., Tian X., Yang Z., Fung K.A., et al. ZIP4 Increases Expression of transcription factor ZEB1 to Promote integrin α3β1 Signaling and Inhibit Expression of the gemcitabine Transporter ENT1 in Pancreatic Cancer Cells. Gastroenterology. 2020;158:679–692.e1. doi: 10.1053/j.gastro.2019.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu Q., Qin Y., Zhang B., Liang C., Ji S., Shi S., Xu W., Xiang J., Liang D., Ni Q., et al. FBW7 increases the chemosensitivity of pancreatic cancer cells to gemcitabine through upregulation of ENT1. Oncol. Rep. 2017;38:2069–2077. doi: 10.3892/or.2017.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Michalski C.W., Erkan M., Sauliunaite D., Giese T., Stratmann R., Sartori C., Giese N.A., Friess H., Kleeff J. Ex vivo chemosensitivity testing and gene expression profiling predict response towards adjuvant gemcitabine treatment in pancreatic cancer. Br. J. Cancer. 2008;99:760–767. doi: 10.1038/sj.bjc.6604528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saiki Y., Hirota S., Horii A. Attempts to remodel the pathways of gemcitabine metabolism: Recent approaches to overcoming tumours with acquired chemoresistance. Cancer Drug Resist. 2020;3:819–831. doi: 10.20517/cdr.2020.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hagmann W., Faissner R., Schnölzer M., Löhr M., Jesnowski R. Membrane drug transporters and chemoresistance in human pancreatic carcinoma. Cancers. 2010;3:106–125. doi: 10.3390/cancers3010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hung S.W., Marrache S., Cummins S., Bhutia Y.D., Mody H., Hooks S.B., Dhar S., Govindarajan R. Defective hCNT1 transport contributes to gemcitabine chemoresistance in ovarian cancer subtypes: Overcoming transport defects using a nanoparticle approach. Cancer Lett. 2015;359:233–240. doi: 10.1016/j.canlet.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bhutia Y.D., Hung S.W., Patel B., Lovin D., Govindarajan R. CNT1 expression influences proliferation and chemosensitivity in drug-resistant pancreatic cancer cells. Cancer Res. 2011;71:1825–1835. doi: 10.1158/0008-5472.CAN-10-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maréchal R., Mackey J.R., Lai R., Demetter P., Peeters M., Polus M., Cass C.E., Young J., Salmon I., Devière J., et al. Human equilibrative nucleoside transporter 1 and human concentrative nucleoside transporter 3 predict survival after adjuvant gemcitabine therapy in resected pancreatic adenocarcinoma. Clin. Cancer Res. 2009;15:2913–2919. doi: 10.1158/1078-0432.CCR-08-2080. [DOI] [PubMed] [Google Scholar]
- 153.García-Manteiga J., Molina-Arcas M., Casado F.J., Mazo A., Pastor-Anglada M. Nucleoside transporter profiles in human pancreatic cancer cells: Role of hCNT1 in 2′,2′-difluorodeoxycytidine- induced cytotoxicity. Clin. Cancer Res. 2003;9:5000–5008. [PubMed] [Google Scholar]
- 154.Rauchwerger D.R., Firby P.S., Hedley D.W., Moore M.J. Equilibrative-sensitive nucleoside transporter and its role in gemcitabine sensitivity. Cancer Res. 2000;60:6075–6079. [PubMed] [Google Scholar]
- 155.Tsesmetzis N., Paulin C.B.J., Rudd S.G., Herold N. Nucleobase and nucleoside analogues: Resistance and re-sensitisation at the level of pharmacokinetics, pharmacodynamics and metabolism. Cancers. 2018;10:240. doi: 10.3390/cancers10070240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Giovannetti E., Del Tacca M., Mey V., Funel N., Nannizzi S., Ricci S., Orlandini C., Boggi U., Campani D., Del Chiaro M., et al. Transcription analysis of human equilibrative nucleoside transporter-1 predicts survival in pancreas cancer patients treated with gemcitabine. Cancer Res. 2006;66:3928–3935. doi: 10.1158/0008-5472.CAN-05-4203. [DOI] [PubMed] [Google Scholar]
- 157.Mackey J.R., Mani R.S., Selner M., Mowles D., Young J.D., Belt J.A., Crawford C.R., Cass C.E. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998;58:4349–4357. [PubMed] [Google Scholar]
- 158.Aughton K., Elander N.O., Evans A., Jackson R., Campbell F., Costello E., Halloran C.M., Mackey J.R., Scarfe A.G., Valle J.W., et al. hENT1 predicts benefit from gemcitabine in pancreatic cancer but only with low CDA mRNA. Cancers. 2021;13:5758. doi: 10.3390/cancers13225758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Farrell J.J., Elsaleh H., Garcia M., Lai R., Ammar A., Regine W.F., Abrams R., Benson A.B., Macdonald J., Cass C.E., et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187–195. doi: 10.1053/j.gastro.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 160.Skrypek N., Duchêne B., Hebbar M., Leteurtre E., van Seuningen I., Jonckheere N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative nucleoside Transporter family. Oncogene. 2013;32:1714–1723. doi: 10.1038/onc.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qiu F., Chen J., Cao J., Diao F., Huang P. Low-intensity low-frequency ultrasound enhances the chemosensitivity of gemcitabine-resistant ASPC-1 cells via PI3K/AKT/NF-κB pathway-mediated ABC transporters. Oncol. Rep. 2020;44:1158–1168. doi: 10.3892/or.2020.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mini E., Nobili S., Caciagli B., Landini I., Mazzei T. Cellular pharmacology of gemcitabine. Ann. Oncol. 2006;17((Suppl. S5)):v7–v12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 163.Neff T., Blau C.A. Forced expression of cytidine deaminase confers resistance to cytosine arabinoside and gemcitabine. Exp. Hematol. 1996;24:1340–1346. [PubMed] [Google Scholar]
- 164.Eliopoulos N., Cournoyer D., Momparler R.L. Drug resistance to 5-aza-2′-deoxycytidine, 2′,2′-difluorodeoxycytidine, and cytosine arabinoside conferred by retroviral-mediated transfer of human cytidine deaminase cDNA into murine cells. Cancer Chemother. Pharmacol. 1998;42:373–378. doi: 10.1007/s002800050832. [DOI] [PubMed] [Google Scholar]
- 165.Kanno S., Hiura T., Ohtake T., Koiwai K., Suzuki H., Ujibe M., Ishikawa M. Characterization of resistance to cytosine arabinoside (ara-C) in NALM-6 human B leukemia cells. Clin. Chim. Acta. 2007;377:144–149. doi: 10.1016/j.cca.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 166.Ohta T., Hori H., Ogawa M., Miyahara M., Kawasaki H., Taniguchi N., Komada Y. Impact of cytidine deaminase activity on intrinsic resistance to cytarabine in carcinoma cells. Oncol. Rep. 2004;12:1115–1120. doi: 10.3892/or.12.5.1115. [DOI] [PubMed] [Google Scholar]
- 167.Ludovini V., Floriani I., Pistola L., Minotti V., Meacci M., Chiari R., Garavaglia D., Tofanetti F.R., Flacco A., Siggillino A., et al. Association of cytidine deaminase and xeroderma pigmentosum group D polymorphisms with response, toxicity, and survival in cisplatin/gemcitabine-treated advanced non-small cell lung cancer patients. J. Thorac. Oncol. 2011;6:2018–2026. doi: 10.1097/JTO.0b013e3182307e1f. [DOI] [PubMed] [Google Scholar]
- 168.Blackstock A.W., Lightfoot H., Case L.D., Tepper J.E., Mukherji S.K., Mitchell B.S., Swarts S.G., Hess S.M. Tumor uptake and elimination of 2′, 2. Clin. Cancer Res. 2001;7:3263–3268. [PubMed] [Google Scholar]
- 169.Manome Y., Wen P.Y., Dong Y., Tanaka T., Mitchell B.S., Kufe D.W., Fine H.A. Viral vector transduction of the human deoxycytidine kinase cDNA sensitizes glioma cells to the cytotoxic effects of cytosine arabinoside in vitro and in vivo. Nat. Med. 1996;2:567–573. doi: 10.1038/nm0596-567. [DOI] [PubMed] [Google Scholar]
- 170.Ohhashi S., Ohuchida K., Mizumoto K., Fujita H., Egami T., Yu J., Toma H., Sadatomi S., Nagai E., Tanaka M. Down-regulation of deoxycytidine kinase enhances acquired resistance to gemcitabine in pancreatic cancer. Anticancer Res. 2008;28:2205–2212. [PubMed] [Google Scholar]
- 171.Ohmine K., Kawaguchi K., Ohtsuki S., Motoi F., Ohtsuka H., Kamiie J., Abe T., Unno M., Terasaki T. Quantitative targeted proteomics of pancreatic cancer: Deoxycytidine kinase protein level correlates to progression-free survival of patients receiving gemcitabine treatment. Mol. Pharm. 2015;12:3282–3291. doi: 10.1021/acs.molpharmaceut.5b00282. [DOI] [PubMed] [Google Scholar]
- 172.Kamada M., Akiyoshi K., Akiyama N., Funamizu N., Watanabe M., Fujioka K., Ikeda K., Manome Y. Cholangiocarcinoma cell line TK may be useful for the pharmacokinetic study of the chemotherapeutic agent gemcitabine. Oncol. Rep. 2014;32:829–834. doi: 10.3892/or.2014.3227. [DOI] [PubMed] [Google Scholar]
- 173.Elledge S.J., Zhou Z., Allen J.B. Ribonucleotide reductase: Regulation, regulation, regulation. Trends Biochem. Sci. 1992;17:119–123. doi: 10.1016/0968-0004(92)90249-9. [DOI] [PubMed] [Google Scholar]
- 174.Duxbury M.S., Ito H., Benoit E., Zinner M.J., Ashley S.W., Whang E.E. Retrovirally mediated RNA interference targeting the M2 subunit of ribonucleotide reductase: A novel therapeutic strategy in pancreatic cancer. Surgery. 2004;136:261–269. doi: 10.1016/j.surg.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 175.Vena F., Li Causi E., Rodriguez-Justo M., Goodstal S., Hagemann T., Hartley J.A., Hochhauser D. The MEK1/2 inhibitor Pimasertib enhances gemcitabine efficacy in pancreatic cancer models by altering ribonucleotide reductase subunit-1 (RRM1) Clin. Cancer Res. 2015;21:5563–5577. doi: 10.1158/1078-0432.CCR-15-0485. [DOI] [PubMed] [Google Scholar]
- 176.Nakamura J., Kohya N., Kai K., Ohtaka K., Hashiguchi K., Hiraki M., Kitajima Y., Tokunaga O., Noshiro H., Miyazaki K. Ribonucleotide reductase subunit M1 assessed by quantitative double-fluorescence immunohistochemistry predicts the efficacy of gemcitabine in biliary tract carcinoma. Int. J. Oncol. 2010;37:845–852. [PubMed] [Google Scholar]
- 177.Sierzega M., Pach R., Kulig P., Legutko J., Kulig J. Prognostic implications of expression profiling for gemcitabine-related genes (hENT1, dCK, RRM1, RRM2) in patients with resectable pancreatic adenocarcinoma receiving adjuvant chemotherapy. Pancreas. 2017;46:684–689. doi: 10.1097/MPA.0000000000000807. [DOI] [PubMed] [Google Scholar]
- 178.Amponsah P.S., Fan P., Bauer N., Zhao Z., Gladkich J., Fellenberg J., Herr I. MicroRNA, 210 overexpression inhibits tumor growth and potentially reverses gemcitabine resistance in pancreatic cancer. Cancer Lett. 2017;388:107–117. doi: 10.1016/j.canlet.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 179.Gu J., Huang W., Wang X., Zhang J., Tao T., Zheng Y., Liu S., Yang J., Chen Z.S., Cai C.Y., et al. Hsa-miR-3178/RhoB/PI3K/Akt, a novel signaling pathway regulates ABC transporters to reverse gemcitabine resistance in pancreatic cancer. Mol. Cancer. 2022;21:112. doi: 10.1186/s12943-022-01587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang F., Xue X., Wei J., An Y., Yao J., Cai H., Wu J., Dai C., Qian Z., Xu Z., et al. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br. J. Cancer. 2010;103:567–574. doi: 10.1038/sj.bjc.6605724. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 181.Wu Y., Xu W., Yang Y., Zhang Z. miRNA-93-5p promotes gemcitabine resistance in pancreatic cancer cells by targeting the PTEN-mediated PI3K/Akt signaling pathway. Ann. Clin. Lab. Sci. 2021;51:310–320. [PubMed] [Google Scholar]
- 182.Zhan T., Chen X., Tian X., Han Z., Liu M., Zou Y., Huang S., Chen A., Cheng X., Deng J., et al. MiR-331-3p links to drug resistance of pancreatic cancer cells by activating WNT/β-catenin signal via ST7L. Technol. Cancer Res. Treat. 2020;19:1533033820945801. doi: 10.1177/1533033820945801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fan P., Liu L., Yin Y., Zhao Z., Zhang Y., Amponsah P.S., Xiao X., Bauer N., Abukiwan A., Nwaeburu C.C., et al. MicroRNA-101-3p reverses gemcitabine resistance by inhibition of ribonucleotide reductase M1 in pancreatic cancer. Cancer Lett. 2016;373:130–137. doi: 10.1016/j.canlet.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 184.Bhutia Y.D., Hung S.W., Krentz M., Patel D., Lovin D., Manoharan R., Thomson J.M., Govindarajan R. Differential processing of let-7a precursors influences RRM2 expression and chemosensitivity in pancreatic cancer: Role of LIN-28 and SET oncoprotein. PLoS ONE. 2013;8:e53436. doi: 10.1371/journal.pone.0053436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rajabpour A., Afgar A., Mahmoodzadeh H., Radfar J.E., Rajaei F., Teimoori-Toolabi L. MiR-608 regulating the expression of ribonucleotide reductase M1 and cytidine deaminase is repressed through induced gemcitabine chemoresistance in pancreatic cancer cells. Cancer Chemother. Pharmacol. 2017;80:765–775. doi: 10.1007/s00280-017-3418-2. [DOI] [PubMed] [Google Scholar]
- 186.Lu H., Lu S., Yang D., Zhang L., Ye J., Li M., Hu W. MiR-20a-5p regulates gemcitabine chemosensitivity by targeting RRM2 in pancreatic cancer cells and serves as a predictor for gemcitabine-based chemotherapy. Biosci. Rep. 2019;39:BSR20181374. doi: 10.1042/BSR20181374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Patel G.K., Khan M.A., Bhardwaj A., Srivastava S.K., Zubair H., Patton M.C., Singh S., Khushman M., Singh A.P. Exosomes confer chemoresistance to pancreatic cancer cells by promoting ROS detoxification and miR-155-mediated suppression of key gemcitabine-metabolising enzyme, DCK. Br. J. Cancer. 2017;116:609–619. doi: 10.1038/bjc.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hong D.S., Kang Y.K., Borad M., Sachdev J., Ejadi S., Lim H.Y., Brenner A.J., Park K., Lee J.L., Kim T.Y., et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer. 2020;122:1630–1637. doi: 10.1038/s41416-020-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Richards K.E., Zeleniak K.E., Fishel M.L., Wu J., Littlepage L.E., Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;30:1770–1778. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mikamori M., Yamada D., Eguchi H., Hasegawa S., Kishimoto T., Tomimaru Y., Asaoka T., Noda T., Wada H., Kawamoto K., et al. MicroRNA-155 controls exosome synthess and promotes gemcitabine resistance in pancreatic ductal adenocarcinoma. Sci. Rep. 2017;7:42339. doi: 10.1038/srep42339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Comanedatore A., Immordino B., Balsano M., Capula M., Garajova I., Ciccolini J., Giovannetti E., Morelli L. Potenital role of exosomes in the chemoresistance to gemcitabine and nab-paclitaxel in pancreatic cancer. Diagnostics. 2022;12:286. doi: 10.3390/diagnostics12020286. [DOI] [PMC free article] [PubMed] [Google Scholar]