Abstract
Simple Summary
Between 5% and 15% of colorectal cancers (CRC) show deficiencies in the mismatch repair machinery and high microsatellite instability (dMMR/MSI-H). dMMR/MSI-H CRC is characterized by a dysfunctional DNA repair system, which renders the tumor immune microenvironment more susceptible to immunotherapy. Currently, immunomodulating drugs are included in the therapeutic arsenal of dMMR/MSI-H CRC and have substantially improved cancer treatment. However, an important proportion of patients with dMMR/MSI-H CRC show primary or acquired resistance to immunotherapy due to molecular traits that are yet to be fully elucidated. Here, we review the current understanding of dMMR/MSI-H CRC molecular and clinical features and discuss their therapeutic implications for CRC patients.
Abstract
About 5 to 15% of all colorectal cancers harbor mismatch repair deficient/microsatellite instability–high status (dMMR/MSI-H) that associates with high tumor mutation burden and increased immunogenicity. As a result, and in contrast to other colorectal cancer phenotypes, a significant subset of dMMR/MSI-H cancer patients strongly benefit from immunotherapy. Yet, a large proportion of these tumors remain unresponsive to any immuno-modulating treatment. For this reason, current efforts are focused on the characterization of resistance mechanisms and the identification of predictive biomarkers to guide therapeutic decision-making. Here, we provide an overview on the new advances related to the diagnosis and definition of dMMR/MSI-H status and focus on the distinct clinical, functional, and molecular cues that associate with dMMR/MSI-H colorectal cancer. We review the development of novel predictive factors of response or resistance to immunotherapy and their potential application in the clinical setting. Finally, we discuss current and emerging strategies applied to the treatment of localized and metastatic dMMR/MSI-H colorectal tumors in the neoadjuvant and adjuvant setting.
Keywords: colorectal cancer, mismatch repair, microsatellite instability, immunotherapy
1. Introduction
Colorectal cancer (CRC) is one of the leading forms of malignant tumors worldwide with estimated rising incidence in the following decade [1]. Comprehensive analysis of CRC molecular biology has defined common features of patients with CRC that are used for clinical decision-making, such as RAS mutational status, BRAF V600E mutations, and hypermutant phenotypes, among others [2]. Of note, the hypermutant phenotype referred to as microsatellite instability high (MSI-H) status is associated with the inactivation of mismatch repair (MMR) genes resulting in MMR deficiency (dMMR). MMR genes MLH1, MSH2, MSH6, and PMS2 effectively control and correct errors introduced in short repeated sequences of 1–6 nucleotides and are widely distributed in the DNA, mostly near coding regions. These sequence motifs, also called microsatellites, are prone to polymerase slippage during replication, resulting in a higher rate of sequence alterations (i.e., loss or insertion of repeat units) [3]. Consequently, an impaired MMR machinery in dMMR/MSI-H tumors results in an increased mutational rate compared to MMR proficient/microsatellite stable (pMMR/MSS) CRC. Indeed, dMMR/MSI-H CRC commonly display 10 to 100-fold greater number of somatic mutations compared to pMMR/MSS tumors [4].
dMMR/MSI-H is a pan-tumor phenotype representing nearly 15% of all CRCs. It was firstly reported in inherited cancers associated with Lynch syndrome and was later described in sporadic CRC [5,6,7]. Yet, the dMMR/MSI-H phenotype occurs mostly in sporadic CRC through MLH1 promoter methylation [5,7,8]. dMMR/MSI-H CRCs have a distinct pathological profile including right-sided primary, mucinous, and poorly differentiated tumors as well as increased occurrence of BRAF mutations [9]. Approximately 20% of stage II, 12% of stage III, and 4% of stage IV CRC tumors are diagnosed as dMMR/MSI-H, thus suggesting an association of dMMR/MSI-H with earlier stages of CRC and with better prognosis [10,11]. This favorable outcome is likely related to the generation of large amounts of neoantigens associated with the mutational rate of dMMR/MSI-H tumors, which results into an immunogenic tumor microenvironment (TME) [12,13]. In contrast, retrospective series and pooled analyses pointed out the dMMR/MSI-H phenotype as a negative prognostic factor in the metastatic setting, potentially due to the coexistence of BRAF V600E mutation in around one third of cases [11,14]. However, these data were analyzed before the irruption of immunotherapy, which entailed profound changes in the natural evolution of advanced dMMR/MSI-H CRC and has thus become a new standard in this niche [15]. Even so, further research is needed to define the patterns of benefit or resistance to immunotherapy in dMMR/MSI-H CRC.
2. The Clinical Assessment of dMMR/MSI-H Phenotype
2.1. Microsatellite Instability Detection by Fluorescent Multiplex PCR
MSI testing is commonly performed by multiplex PCR using fluorescent primers and followed by capillary electrophoresis fragment analysis. The Bethesda revised microsatellites markers (commonly mononucleotide BAT-25, BAT-26, NR-21, NR-4 and NR-27/MONO-27) are the recommended PCR targets for the characterization of MSI/MSS status due to their stability between individuals [16,17]. Accordingly, a tumor is considered MSI-H when the size of two or more of these markers is altered, whereas only one or no alterations at all are characteristic for MSS status. Of note, new targets are continuously being evaluated. Among them, ACVR2A, BTBD7, DIDO1, MRE11, RYR3, SEC31A and SULF2 have been identified through genome sequencing of different MSI-H tumors [18]. These markers, selected for their applicability in different ethnicities and over cancers of distinct origins, are currently available for clinical testing [19,20].
2.2. Immunodetection of MMR Proteins
Immunohistochemical (IHC) detection of MMR proteins MLH1, PMS2, MSH2, and MSH6 is widely used in the clinical setting to assess the microsatellite status, mainly because of its robustness and the straightforward interpretation of results by well-trained pathologists [21]. Similar to MSH2 and MSH6, MLH1 and PMS2 form a heterodimer that recognizes and binds to mismatch base pairs. Therefore, the concurrent loss of both proteins is frequently detected in dMMR [4]. Furthermore, the detection of this panel of MMR-related biomarkers provides mechanistic insights on the dMMR/MSI-H phenotype [22]. The detection of all four MMR proteins expression defines proficient mismatch repair system (pMMR). In contrast, dMMR is characterized by the lack of expression of at least one of these markers [23].
2.3. Limitations of the Clinical Assessment of dMMR/MSI-H Status
Although dMMR and MSI-H status are mostly equivalent, some exceptions have been reported. For instance, dMMR caused by MSH6 germline mutation does not meet the criteria of MSI-H diagnosis [24]. Conversely, IHC does not segregate functional and nonfunctional proteins, thus limiting the diagnostic power of this approach. For instance, approximately 5% of the alterations observed in MMR genes are due to missense mutations (especially in MLH1) that lead to the expression of functionally impaired proteins. Therefore, the detection of MLH1, PMS2, MSH2, and MSH6 expression does not entirely exclude microsatellite instability. In this regard, it is worth noting that positive IHC staining, while being either homogeneous or heterogeneous, will be commonly considered as “intact” expression by clinical pathologists [25]. Staining positivity is usually contrasted with positive reaction in internal control cells (nuclei of stromal, inflammatory, or non-neoplastic epithelial cells). However, heterogeneous staining may challenge the clinical evaluation of MMR status since no clear guidelines are available to date [25,26,27]. In this line, misdiagnosis/failure to identify either MSI or dMMR is not rare. For instance, the assessment of MSI or dMMR status in patients with metastatic CRC associates with 5–10% false-positive cases [28]. Therefore, the sequential application of IHC followed by PCR is recommended to reduce incompatibility and minimize false negative results [20].
2.4. New Strategies for the Evaluation of dMMR/MSI-H Status
With the advent of next generation sequencing (NGS), new options are being developed to evaluate more accurately MSI status [18,29,30]. In this line, methods such as whole genome/exome sequencing or targeted gene sequencing allow the detection of alterations in thousands of microsatellite loci. MSI status assessment by NGS relies on computational algorithms able to eliminate the bias introduced by polymerase slippage [31]. In this line, MSIsensor was one of the first algorithms developed in paired tumor and matched normal samples [32]. In contrast, mSINGS is based on the comparison of tumor samples with normal population controls as baseline [30]. More recent and accurate algorithms include MOSAIC [29], MANTIS [33], the Cortes-Ciriano method [34], mSILICO [35], and MSINGB [36]. Alternatively, radiomics is emerging as a promising tool for the MSI diagnosis. Ensemble models based on PET/CT images have been constructed based on this approach, combining clinical risk factors and machine learning-based analysis models. Although still under development, this technique may provide a quantitative, efficient, and non-invasive method for the assessment of MSI status in the near future [37].
3. Molecular Features of dMMR/MSI-H CRC
3.1. Intrinsic Characteristics of dMMR/MSI-H Cancer Cells
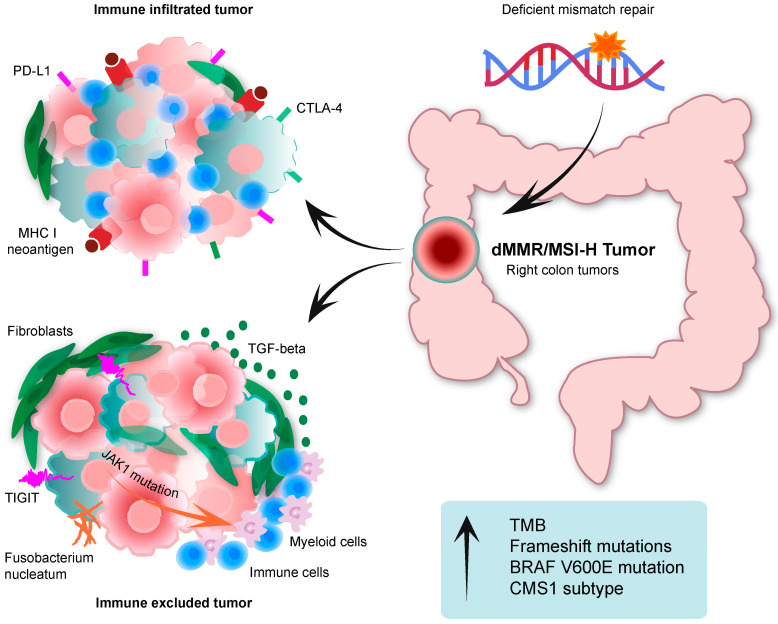
Seminal studies in CRC have pointed out the molecular idiosyncrasy of dMMR/MSI-H CRC (Figure 1). Exome sequencing analyses from The Cancer Genome Atlas (TCGA) project concluded that dMMR/MSI-H CRC, unlike pMMR/MSS tumors, is hypermutated, with a tumor mutational burden (TMB) above 12 mutations/Mb [2]. The TMB threshold was defined by the TCGA project itself, since there is no exact value that defines hypermutated tumors per se. In addition to the dMMR/MSI-H phenotype, mutations in POLE and POLD1 genes can also promote a hypermutated phenotype [2].
Figure 1.
Molecular features of immune infiltrated or immune excluded dMMR/MSI-H CRC. Immune infiltrated tumors are characterized by increased PD-L1 and CTLA-4 expression as well as elevated neoantigen presentation. Immune excluded tumors feature a TGF-beta enriched tumor microenvironment with abundant immunosuppressive myeloid cells and fibroblasts. Arrow in blue box means increased. dMMR: deficient mismatch repair. MSI-H: high microsatellite instability. PD-L1: programmed death ligand 1. CTLA-4: cytotoxic T-lymphocyte antigen. MHC I: major histocompatibility complex 1. TIGIT: T cell immunoglobulin and ITIM domain. TMB: tumor mutational burden. CMS1: consensus molecular subtype 1.
Frameshift mutations are one of the hallmarks of dMMR/MSI-H CRC. Of note, genes that include microsatellites in their coding areas are particularly vulnerable to frameshift mutations, which may appear at very early stages of tumor development in dMMR/MSI-H CRC. As a result, insertions or deletions of dinucleotides or trinucleotides in microsatellites are not repaired, which leads to the generation of highly immunogenic neoantigens [31]. Indeed, an in silico pan-tumor study using exome sequencing data from the TCGA showed that frameshift mutations generated almost three times more neoantigens that are recognized by T cells compared to any other non-synonymous mutations [38].
The study of the mutational profile in dMMR/MSI-H CRC is largely intricate due to the complexity of its hypermutability status [39]. Yet, massive genomic sequencing analyses have elucidated the most frequent molecular aberrations affecting the dMMR/MSI-H phenotype [39]. In particular, mutations in TP53 appear in 20% of dMMR/MSI-H CRC compared to a 60% of pMMR/MSS CRC, and APC in 51% compared to 81%, respectively [2]. Mutations in KRAS and BRAF V600E are among the most frequent mutations in advanced stages of dMMR/MSI-H CRC, and they are each found in approximately 30% of the cases compared to frequencies of around 40% and 8%, respectively, of pMMR/MSS CRC cases [2,40]. Importantly, BRAF V600E mutation is unique to sporadic dMMR/MSI-H CRC, and exclusive to the presence of Lynch Syndrome. Indeed, pre-clinical data suggest that BRAF V600E mutations promote dMMR/MSI-H phenotype through the activation of the MAPK pathway. In turn, MAPK signaling induces the expression of MAFG, a transcriptional repressor that binds to MLH1 promoter and recruits methyltransferases, resulting in promoter hypermethylation and inactivation [41]. ZBTB2 is another gene mutated in dMMR/MSI-H CRC. Mutations in ZBTB2 enhance the expression of MDM2, which regulates p53 degradation thus blocking its DNA repair effect [42,43]. Additional mutations are reported in the tumor suppressor RANBP2; in the mediator of p53 inhibition PSRC1; in MECOM, which encodes a transcriptional regulator of hematopoiesis, apoptosis, and proliferation; and in WNT pathway-related RNF43 [42,43]. Genes that associate with antigen presentation can also be mutated in dMMR/MSI-H CRC, although with variable frequency. These genes include the transcriptional regulators of MHC type I NLRC5 and RFX5; TAP1 and TAP2 involved in antigenic processing; as well as HLA-A, HLA-B, HLA-C, and B2M involved in antigen presentation [44]. In addition, genes encoding for subunits of epigenetic regulatory complexes, such as the SWI/SNF complex, also show a higher mutational incidence in dMMR/MSI-H tumors compared to pMMR/MSS [45].
3.2. Extrinsic Features of dMMR/MSI-H Tumors
In an effort to functionally stratify CRC tumors, Guinney and colleagues reconciled previous molecular classifications of CRC based on tumor transcriptomic profiles into four consensus molecular subtypes (CMS1 to 4). Remarkably, around 70% of dMMR/MSI-H CRCs are clustered in CMS1 [46], which is characterized by a strong influence of the TME. Consequently, this association indicates that dMMR/MSI-H CRC may be determined through their specific interactions with the TME in addition to the cancer cells intrinsic properties. Further transcriptomic analysis of the immune microenvironment revealed that CMS1 is also defined by genes associated with antigen presentation, interferon γ (IFNg) signaling, the T helper (Th) 1 phenotype, and chemoattractant cytokines such as CXCL9, CXCL10, and CXCL16 [47,48,49]. Accordingly, CMS1-dMMR/MSI-H CRCs exhibit higher abundance of immune cells in the tumor center and at the invasive margin, mainly consisting of cytotoxic T lymphocytes, natural killer (NK) cells, Th-1 and Th-2 lymphocytes, and M1-like macrophages [47,49,50]. Of note, the density of cytotoxic T-lymphocyte infiltration correlates with the number of frameshift mutations, thus indicating that lymphocytes may preferentially react to tumors with a higher frequency of neoantigen generation [51]. As a result, immunotherapies, particularly immune checkpoint inhibitors (ICI), have shown striking positive effects in dMMR/MSI-H mCRC [52,53].
Notwithstanding their favorable immune landscape, dMMR/MSI-H CRCs are also particularly prone to raise adaptive resistance to anti-cancer immunity (Figure 1). For instance, a dMMR/MSI-H status is also associated with increased expression of suppressive immune checkpoints such as indolamine 2,3-dioxygenae (IDO-1), cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed death receptor (PD-1) and its ligand (PD-L1), and lymphocyte activation gene 3 (LAG-3), among others [13,49,54]. In addition, recent studies have shown that a subset of dMMR/MSI-H is intrinsically characterized by low cytotoxic lymphocyte infiltration [55] or by increased abundance of immunosuppressive myeloid cells and fibroblasts [56] (Figure 1). These scenarios could be the result of similar activation of WNT or MAPK pathways to pMMR/MSS CRC, which lead to immune desertion [57].
3.3. Confounding Subsets of dMMR/MSI-H and pMMR/MSS CRC
In contrast to dMMR/MSI-H, pMMR/MSS CRCs are considered as immune excluded tumors. However, around 10% of pMMR/MSS tumors display an immunogenic profile similar to dMMR/MSI-H CRCs [58]. This was observed in the CCTG CO.26 trial, in which patients with refractory pMMR/MSS CRC were randomized to receive anti-PD-L1 (Durvalumab) and anti-CTLA-4 (Tremelimumab) monoclonal antibodies (mAbs) versus best supportive care. In this setting, patients with TMB above 28 mutations/Mb (21% of patients) experienced greater benefit from immunotherapy [59]. Remarkably, these high TMB levels were similar to the ones observed in dMMR/MSI-H tumors, thus suggesting that a subset of pMMR/MSS CRCs harbor hypermutant phenotype either per se or as a result of mutational footprints induced by chemotherapy [60]. Indeed, transcriptional analysis indicated that pMMR/MSS CRC responding to immunotherapy displayed a relative abundance of cytotoxic lymphocytes similar to dMMR/MSI-H CRC [61].
Conversely, a shift from pMMR/MSS to dMMR/MSI-H phenotype has been observed upon treatment with anti-EGFR mAbs and BRAF inhibitors in preclinical models of CRC [62]. This suggests that dMMR/MSI-H status is potentially acquired upon therapy as an adaptive mechanism of drug resistance. Along this line, alquilating agents such as Temozolamide are also able to induce hypermutational phenotype in pMMR/MSS CRC. Their combination with immune checkpoint inhibitors is currently evaluated in clinical trials as a promising strategy to sensitize unresponsive tumors to immunotherapy [63,64].
An increasing body of literature has reported a heterogeneous expression of MMR proteins within the same tumor, thus suggesting the existence of a MSS/MSI heterogeneous (MSS/MSI-het) subset of CRC [25,26,27]. These peculiar patterns are not artefactual since focal or zonal losses of MMR protein expression consistently correlate with MSI-H-associated molecular traits in the corresponding areas [26,27]. Even though this mixed MSS/MSI-het phenotype may be limited to a subset of CRCs, the coexistence of pMMR/MSS and dMMR/MSI-H malignant cells raises important questions that are not addressed by the current guidelines. For instance, the relative abundance of dMMR/MSI-H and pMMR/MSS cancer cells in a given tumor may both have an impact on diagnosis, treatment decision-making, and ultimately patient benefit from therapy. Yet, the penetrance of this MSS/MSI-het phenotype remains largely unknown and additional studies are still needed to elucidate its implication with CRC outcome.
4. Therapeutic Implication of dMMR/MSI-H Status
4.1. Initial Studies and Treatment in the Metastatic Setting
Early studies on the overall population of mCRC patients showed a very limited clinical benefit from ICIs. In a phase I trial that included 39 refractory solid tumors, only 1 patient presented a complete pathological response that lasted for more than 3 years. Interestingly, this mCRC patient displayed dMMR/MSI-H status [65]. In KEYNOTE-028 phase I trial, treatment with anti-PD-1 mAbs (Pembrolizumab) was evaluated in 23 solid tumors. One partial but long-lasting response was recorded. Here again, dMMR/MSI-H status was associated to this mCRC patient [66]. In line with these observations, the phase II KEYNOTE-016 trial evaluated the clinical efficacy of Pembrolizumab in patients with pMMR/MSS mCRC, dMMR/MSI-H mCRC, and dMMR/MSI-H non-colorectal cancer. A clear lack of response to therapy was observed in the cohort of 18 patients (0% ORR) displaying pMMR/MSS status. In contrast, the overall response rate (ORR) reached 40% in the 10 patients harboring dMMR/MSI-H mCRC. The updated results have shown a similar evolution, in which the disease control rate (DCR) and ORR were 89% and 50%, respectively, for dMMR/MSI-H mCRC (n = 28) versus a 16% and 0% for pMMR/MSS mCRC (n = 25). PFS and OS after 9 months median follow-up was not reached for dMMR/MSI-H mCRC compared to 2.4 and 6 months, respectively, for pMMR/MSS mCRC [67]. Pembrolizumab’s robust anti-tumor activity in mCRC was confirmed in the phase II KEYNOTE-164 in second or subsequent lines of treatment [68]. A similar positive effect was observed in the phase II Checkmate 142 trial, which evaluated the efficacy of another anti-PD-1 mAb (Nivolumab) in 74 chemorefractory patients with dMMR/MSI-H mCRC. Strikingly, PFS and OS at 12 months reached 50% and 73%, respectively [53]. Based on these promising results, US FDA approved Pembrolizumab and Nivolumab as the second-line treatment for dMMR/MSI-H mCRC in 2017. Since then, Pembrolizumab was evaluated in a phase III trial in dMMR/MSI-H mCRC as first line of treatment (KEYNOTE-177). Again, Pembrolizumab significantly improved both PFS and OS when compared to standard chemotherapy. In addition, the rates of Grade 3–5 treatment-related adverse events were 22% for Pembrolizumab (colitis, hepatitis) and 66% for chemotherapy (neutropenia, diarrhea, fatigue), thus indicating a reduced toxicity of the treatment [69].
It is worth mentioning that dual ICI regimen demonstrated even more benefit for dMMR/MSI-H CRC patients. For example, the phase II Checkmate 142 trial that included a cohort of 119 pretreated patients with dMMR/MSI-H mCRC showed an ORR of 55% and a 12-months OS of 83% upon anti-CTLA-4 (Ipilimumab) and Nivolumab dual therapy [70]. The same combination was administered as first line treatment to 45 patients with dMMR/MSI-H mCRC. In this setting, the ORR and OS at 12 months reached 60% and 83% respectively [71], thus suggesting that the dual inhibition of CTLA-4 and PD-1 synergistically promotes anti-tumor responses.
Currently, a great body of clinical research is exploring the potential benefit of combining ICIs with systemic and/or targeted therapies. In this line, the Checkmate 8HW (NCT04008030) phase III trial is currently evaluating the efficacy of Nivolumab monotherapy, Nivolumab plus Ipilimumab, or chemotherapy in patients with dMMR/MSI-H mCRC. On the other hand, preclinical data suggested that VEGF blockade induces an immune permissive TME improving the benefit from ICI [72,73,74]. On the basis of these results, the combination of ICI-anti-VEGF/VEGFR mAbs plus chemotherapy was evaluated in early clinical trial in dMMR/MSI-H mCRC. Treatment was well tolerated and associated with positive clinical activity [75]. Following on this, the COMMIT phase III trial (NCT02997228) is now evaluating the combination of anti-PD-L1 mAbs (Atezolizumab) with chemotherapy (FOLFOX) and anti-VEGF mAbs (Bevacizumab) in the first line treatment of dMMR/MSI-H mCRC [76]. Additionally, a single center phase II trial conducted in Chinese population is assessing chemotherapy (FOLFIRI)-Bevacizumab combination plus anti-PD-1 mAbs (Nivolumab or Pembrolizumab) as second-line therapy in dMMR/MSI-H mCRC (NCT05035381) [77]. Results from pivotal trials in patients with dMMR/MSI-H are summarized in Table 1.
Table 1.
Clinical outcome in pivotal trials involving dMMR/MSI-H mCRC patients.
| KEYNOTE-177 | Checkmate 142 | Keynote 164 | Keynote 016 | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment | 1st | 1st | 1st | >2nd | >2nd | >2nd | >3rd | >3rd |
| Phase | III | III | II | II | II | II | II | II |
| N patients | 153 | 154 | 45 | 74 | 119 | 63 | 61 | 10 |
| Schedule | Pembro | Chemo | Nivo + Ipi | Nivo | Nivo + Ipi | Pembro | Pembro | Pembro |
| ORR | 44% | 33% | 60% | 31% | 55% | 33% | 33% | 40% |
| DCR | 65% | 75% | 84% | 69% | 80% | 57% | 51% | 90% |
| mPFS | 16.5 m | 8.2 m | NR | 14.3 | NR | 4.1 m | 2.3 m | NR |
| 12 m PFS | 55% | 37% | 77% | 50 | 71% | 41% | 78% | |
| mOS | NR | NR | NR | NR | 31.4 m | NR | ||
| 12 m OS | 83% | 73 | 85% | 76% | 72% | NR | ||
dMMR/MSI-H, deficient mismatch repair, microsatellite instability-High; Pembro, Pembrolizumab; Nivo, Nivolumab; Ipi, Ipilimumab; Chemo, chemotherapy, ORR, overall response rate; DCR, disease control rate; PFS, progression-free survival; OS, overall survival; NR, not reached.
4.2. Adjuvant and Neoadjuvant Therapy in Localized CRC
While only ~5% of mCRC are dMMR/MSI-H, this subset represents about 10–12% of stage III CRC [78]. Consequently, the good results obtained from ICI-based therapies in dMMR/MSI-H mCRC have led to their evaluation in patients with non-metastatic disease. Two trials intended to test the potential efficacy of ICI as adjuvant treatment have been designed so far. On the one hand, ATOMIC phase III trial is evaluating the combination of mFOLFOX6 plus Atezolizumab compared to mFOLFOX alone (NCT02912559) [79]. On the other hand, POLEM trial aims to assess Avelumab (anti-PD-L1 mAb) treatment following adjuvant 5-FU based chemotherapy in dMMR/MSI-H and/or POLE exonuclease domain mutant CRC tumors [80]. However, while ATOMIC is currently recruiting patients, POLEM trial encountered significant technical challenges and was recently discontinued.
Additionally, the potential of ICI-based regimen for dMMR/MSI-H CRC is also being explored in the neoadjuvant setting. In this regard, the NICHE phase II trial assessed the efficacy of short course neoadjuvant treatment with Nivolumab and Ipilimumab. All 21 treated CRCs showed either major or complete pathological response. In contrast, only three major pathological responses were observed in the 15 pMMR/MSS CRCs [81]. In addition, a recent prospective phase II evaluated anti-PD-1 mAbs (Dostarlimab) as neoadjuvant treatment for dMMR/MSI-H stage II-III rectal adenocarcinoma. Strikingly, all 12 patients maintained clinical and radiological complete response during the 6-month follow-up [82]. Recently, the results of the NICHE-2 study were recently presented at the ESMO 2022 edition in Paris. In this study, neoadjuvant treatment with a cycle of Nivolumab plus Ipilimumab followed by another cycle with Nivolumab prior to surgery was assessed in 107 patients with locally advanced colon tumors (Stage II-III), with the objective of studying safety and response rate. With 13 months of median follow-up, a major pathological response rate (less than 10% residual viable tumor) of 95% was achieved, with a 67% of complete responses (0% residual viable tumor).
The use of immunotherapy as neoadjuvant treatment in CRC shows a great potential as a therapeutic strategy. However, there is still a need for more clinical studies to evaluate their benefits over current adjuvant strategies. Firstly, few clinical trials are evaluating the safety of immunotherapy and the possible surgical complications related to neoadjuvant treatment. Secondly, more efforts are needed to assess the effect of immunotherapy in the neoadjuvant setting. In this context, it remains unclear whether selected patients should follow an organ-preserving approach or a “watch and wait” strategy. Finally, the combinations of neoadjuvant immunotherapy with radiotherapy and/or chemotherapy, as well as the proper timing of incorporation (concomitant or sequential), should be investigated and optimized.
4.3. New Directions
Even though ICIs have demonstrated encouraging results, a substantial proportion of dMMR/MSI-H CRC patients still do not benefit from current treatment options. This calls for a more personalized therapy based on additional/original molecular markers. For example, while Pembrolizumab is indicated as first line therapy in unresectable dMMR/MSI-H mCRC, Encorafenib, a BRAF inhibitor used in combination with Cetuximab (anti-EGFR mAb) is only indicated in second line therapy for BRAF V600E-mutated mCRC patients. Therefore, and in the absence of established first line therapy, SEAMARK (NCT05217446) phase II trial was initiated in 2022 to evaluate the combination of Encorafenib, Cetuximab, and Pembrolizumab as first line treatment in in dMMR/MSI-H and BRAF V600E-mutated mCRC patients [83].
Alternatively, evidence indicates that dMMR/MSI-H tumors are enriched in NTRK fusions [84]. Indeed, Coco and colleagues identified up to 15% of dMMR/MSI-H mCRC harboring kinase fusions [85]. Remarkably, a recent study of dMMR/MSI-H mCRC patient with NTRK fusion treated with Larotrectinib, an inhibitor of tropomyosin kinase receptors TrkA, TrkB, and TrkC [86,87,88], reported a striking therapeutic response after ICI failure [89]. This suggests Larotrectinib as a potential therapeutic strategy in ICI-resistant dMMR/MSI-H tumors. Additional molecules inducing T cell activation or blocking T cell checkpoint inhibitors are currently evaluated in early clinical trials involving dMMR/MSI-H CRC patients [90]. In this line, studies have highlighted the expression of additional immune checkpoints markers such as LAG-3, T cell immunoglobulin mucin domain 3 (TIM-3), and IDO-1 in the MSI population, thus making it a rationale for the use of different ICIs [91]. Indeed, anti-TIM3 in combination with anti-PD-L1 mAbs showed promising clinical activity in dMMR/MSI-H tumors of distinct origins [92]. Therefore, the identification of new actionable targets may provide original treatment opportunities for dMMR/MSI-H CRC patients unresponsive to current ICI.
5. Predictive Factors of Therapeutic Outcome
5.1. Clinical Factors
As mentioned above, the important effect of ICIs in the clinical setting led to their approval as second-line treatment for dMMR/MSI-H mCRC. However, about 30–50% of dMMR/MSI-H mCRC patients still display intrinsic resistance to immunotherapy [53,69,93]. Hence, the identification of predictive biomarkers of response remains crucial to guide therapeutic decision-making [94]. In this sense, several studies have identified potential clinical predictors of response to ICIs. Among them, Pietrantonio and colleagues developed a nomogram that integrates five clinical variables to estimate the outcome of dMMR/MSI-H mCRC patients receiving ICIs [95]. The use of a multivariable model provides a dual scoring system for 12-month PFS and for time-independent event-free probability (EFP) [95]. However, a prospective validation of these methods is still required to establish their relevance in the clinical setting.
Clinical presentation has also been associated with ICIs treatment outcome. In this context, Fuca et al. retrospectively evaluated a cohort of patients with dMMR/MSI-H gastrointestinal cancers treated with anti-PD-1 ± anti-CTLA-4 agents to determine whether malignant ascites impacted treatment outcome [96]. Authors showed that peritoneal metastases manifested with ascites associate with unfavorable outcome upon anti-PD-1 mAbs treatment, possibly due to a link between malignant ascites with an immunosuppressive microenvironment [97].
5.2. Microbiome
Different studies have suggested that the administration of broad-spectrum antibiotics (ATBs) have a negative impact on ICI treatment outcome [98]. ATBs may induce intestinal dysbiosis, altering the normal gut microbiota with adverse consequences on the immune system [99], and it may take up to 3 months for the gut microbiota to recover [100]. In this context, the microbiome has emerged as a potential biomarker of immunotherapy effectiveness. For instance, increased abundance of Akkermansia muciniphila, Ruminoccoccus sp. and Faecalibacterium sp. have been observed in fecal samples from melanoma patients responding to anti-PD-1/PD-L1 or anti-CTLA-4 mAbs [101,102]. Fusobacterium nucleatum are other bacteria with potential immunomodulating properties (Figure 1). According to preclinical data in CRC cell lines, Fusobacterium nucleatum enhance oncogenic events such as WNT or MAPK pathway activation precluding immunogenic activation [103,104]. Alternatively, Fusobacterium nucleatum are also able to bind to negative regulatory immune checkpoints like TIGIT and CEACAM1 [105,106], thus leading to reduced CD4+/CD8+ T cells infiltration [107]. It is worth mentioning that a tumor-associated fecal bacterial profile has not been described for dMMR/MSI-H CRC in light of the benefit from immunotherapy. Yet, reports indicate that Fusobacterium nucleatum are particularly enriched in these tumors [108,109] and preliminary results point to improved response to immunotherapy in the presence of Fusobacterium nucleatum [110].
5.3. Molecular Factors
The overall improved responses of dMMR/MSI-H colorectal tumors to immunotherapy results largely from an increased immune infiltration compared to pMMR/MSS tumors [13,91]. As mentioned above, acquired mutations in the MMR machinery leads to increased TMB, resulting into a vigorous tumor immune microenvironment. Consequently, TMB is often used as a clinical biomarker of response to immune checkpoint inhibitors [111]. However, since a proportion of patients with a high mutational density are refractory to current immunotherapeutic strategies, TMB alone might not an accurate predictor of immunologic responses. Therefore, it is worth understanding additional molecular and cellular escape mechanisms to refine better biomarkers for clinical decision-making.
Mutations of key genes involved in immunological processes can be used as actionable biomarkers of therapeutic response in immuno-oncology, which could be relevant for dMMR/MSI-H tumors given their increased mutational rate. Of note, the elevated selective pressure posed by the immune surveillance often leads to damages in the MHC class I antigen presentation machinery [112,113,114]. Mutations of B2M, followed by the HLA-B heavy chain and HLA-C, are the most common alterations acquired by dMMR/MSI-H tumors preventing antigen presentation, and have been associated with negative responses to immunotherapy [112,114,115]. However, additional clinical studies have shown that mutations of B2M do not associate with resistance to immune checkpoint inhibition, as dMMR/MSI-H tumors with B2M alterations can respond to immunotherapy [116,117]. Therefore, the use of the MHC I antigen presentation machinery as a clinical biomarker remains controversial. Alternatively, resistant dMMR/MSI-H tumors may acquire additional alterations that ultimately govern immune cells infiltration and activity. For instance, mutations in JAK1, a kinase downstream the IFNg pathway, leads to a decreased anti-tumor immune activity. Consequently, these tumors could diminish the overall expression of PD-L1, which in turn renders them more resistant to anti-PD-L1 targeted therapies [118,119,120,121]. Conversely, BRAF mutated dMMR/MSI-H tumors seem to be associated with increased infiltration CD8 cytotoxic T cells infiltration and antigen-presenting cells such as dendritic cells and M1-like macrophages [122,123,124]. Nevertheless, neither BRAF V600E nor RAS mutations have been meaningfully associated with benefit to immunotherapy in the aforementioned clinical trials. [53]
Overall, tumors with a low presence of an immune tumor microenvironment, also named “cold tumors”, are correlated with poor responses to immunotherapy. In this regard, it is currently accepted that high levels of TGF-beta and stroma impair cytotoxic immunological responses in pMMR/MSS tumors [125,126,127,128]. However, a proportion of dMMR/MSI-H tumors may also contain a TGF-beta enriched tumor microenvironment, which could result into similar mechanisms of immune evasion [46,129] (Figure 1). On the other hand, recent advances in RNA sequencing techniques, particularly at the single cell level, and multiplex fluorescent labelling highlight the quality, rather than quantity, of immune infiltrates as a predictive biomarker for the clinical practice.
On the transcriptional level, Lal and colleagues defined in 2015 four categories of coordinate immune response clusters. In particular, cluster A, which is correlated with better responses to immunotherapy, is enriched in dMMR/MSI-H tumors [130]. In 2016, Becht and colleagues published the Microenvironment Cell Populations-counter (MCP-counter) method, which allows the quantification of stromal and immune cell populations using transcriptomic signatures [131]. A higher abundance of cytotoxic T cells as predicted by the MCP-counter is associated with improved prognosis in MSI CRC, and could be used to predict responses to ICI [132]. Recent research has pointed out several gene expression signatures associated with responses to ICIs, including CMS transcriptomes, IFNg signaling, and genes belonging to the cancer-immunity-cycle [57,133,134]. Alternatively, there is a yet nascent interest in the metabolomic biomarkers for CRC. Indeed, dysregulated metabolite markers including fatty acids, amino acids, and lysophosphatidylcholines have been associated with clinicopathological features of CRC, including therapeutic outcome [135,136,137]. Of note, and as recently discussed by Holbert and colleagues, the polyamine metabolism could potentially predict responses to ICIs [138].
On the histological level, seminal studies led by Jerome Galon have defined that the Immunoscore, a scoring system of immune cells with anti-tumor activity, robustly correlates with improved prognosis [139,140]. Interestingly, a study led by Mlecnik and colleagues shows that the Immunoscore outperforms the MSS/MSI status in predicting CRC outcome, and that a proportion of dMMR/MSI-H tumors show low Immunoscore values [47]. In this regard, a recent study by Pelka and colleagues suggested the existence of immunologic hubs that are shared between pMMR/MSS and dMMR/MSI-H colorectal tumors [56]. Supporting the evaluation of immune infiltrates and activity as prognostic predictors, Corti and colleagues proposed a Pan-Immune-Inflammation Value (PIV) assessment based on neutrophils, platelets, monocytes, and lymphocytes numeration in liquid biopsy. The authors from this study reported that the increase of PIV in MSI-H CRC patients observed 3–4 weeks after treatment with immunotherapy was an independent predictor of clinical benefit from ICI [141]. Overall, this body of research strengthens the fact that particular immune cell types may infiltrate CRC tumors regardless of the microsatellite status, and that the assessment of such cell types could be paramount for defining immunotherapeutic strategies for patients with dMMR/MSI-H tumors.
6. Conclusions and Future Perspectives
The emergence of immunotherapy has offered new treatment approaches for a subset of CRC patients with dMMR/MSI-H tumors. Compared to pMMR/MSS, dMMR/MSI-H CRC acquire a privileged immune landscape that can be targeted with state-of-the-art immunomodulators, showing outstanding response rates in patients with localized and metastatic disease. Despite the recent clinical advances, a proportion of patients within this subset are still refractory to the current immunotherapeutic strategies. Ongoing clinical trials are focusing on combinations of ICIs with different formats of chemotherapy and/or biological treatments to increase response rates in resistant dMMR/MSI-H CRC.
On the other hand, the realization that a subset of pMMR/MSS CRC patients may also benefit from immunotherapy as well as the existence of MSS/MSI-het CRCs call for the need of predictive markers beyond MSS/MSI status. For instance, the discovery and clinical use of original biomarkers could help the clinical decision-making by predicting benefit from immunotherapy not only in dMMR/MSI-H CRC patients but also in pMMR/MSS and MSS/MSI-het CRCs. In this line, factors such as the expression of immunomodulating proteins, the microbiome and the composition of immune infiltrates may shed light on resistance and response mechanisms, which could be leveraged to expand the population of responsive patients treated with immunotherapy and provide a rationale for the discovery of new druggable targets.
Author Contributions
N.M.-M., J.L., J.B.-R., M.J., C.S.M., J.L.M.M. and A.C. wrote the article. N.M.-M., J.L., J.B.-R. and A.C. reviewed and edited the article. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
A.C. is the recipient of a Miguel Servet research contract from Instituto de Salud Carlos III co-funded by the European Union (CPII21/00012). J.L. is the recipient of a Junior Clinician fellowship from the Scientific Foundation of the Spanish Association Against Cancer (FCAECC; CLJUN19004LINA).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Li J. Digestive Cancer Incidence and Mortality among Young Adults Worldwide in 2020: A Population-Based Study. World J. Gastrointest. Oncol. 2022;14:278–294. doi: 10.4251/wjgo.v14.i1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muzny D.M., Bainbridge M.N., Chang K., Dinh H.H., Drummond J.A., Fowler G., Kovar C.L., Lewis L.R., Morgan M.B., Newsham I.F., et al. Comprehensive Molecular Characterization of Human Colon and Rectal Cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishel R. Mismatch Repair. J. Biol. Chem. 2015;290:26395–26403. doi: 10.1074/jbc.R115.660142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vilar E., Gruber S.B. Microsatellite Instability in Colorectal Cancer-the Stable Evidence. Nat. Rev. Clin. Oncol. 2010;7:153–162. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boland C.R., Goel A. Microsatellite Instability in Colorectal Cancer. Gastroenterology. 2010;138:2073–2087.e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreira L., Muñoz J., Cuatrecasas M., Quintanilla I., Leoz M.L., Carballal S., Ocaña T., López-Cerón M., Pellise M., Castellví-Bel S., et al. Prevalence of Somatic Mutl Homolog 1 Promoter Hypermethylation in Lynch Syndrome Colorectal Cancer. Cancer. 2015;121:1395–1404. doi: 10.1002/cncr.29190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markowitz S.D., Bertagnolli M.M. Molecular Basis of Colorectal Cancer. N. Engl. J. Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalo V., Lozano J.J., Alonso-Espinaco V., Moreira L., Muñoz J., Pellisé M., Castellví-Bel S., Bessa X., Andreu M., Xicola R.M., et al. Multiple Sporadic Colorectal Cancers Display a Unique Methylation Phenotype. PLoS ONE. 2014;9:e91033. doi: 10.1371/journal.pone.0091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward R., Meagher A., Tomlinson I., O’Connor T., Norrie M., Wu R., Hawkins N. Microsatellite Instability and the Clinicopathological Features of Sporadic Colorectal Cancer. Gut. 2001;48:821. doi: 10.1136/gut.48.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinicrope F.A., Foster N.R., Thibodeau S.N., Marsoni S., Monges G., Labianca R., Yothers G., Allegra C., Moore M.J., Gallinger S., et al. DNA Mismatch Repair Status and Colon Cancer Recurrence and Survival in Clinical Trials of 5-Fluorouracil-Based Adjuvant Therapy. J. Natl. Cancer Inst. 2011;103:863–875. doi: 10.1093/jnci/djr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venderbosch S., Nagtegaal I.D., Maughan T.S., Smith C.G., Cheadle J.P., Fisher D., Kaplan R., Quirke P., Seymour M.T., Richman S.D., et al. Mismatch Repair Status and BRAF Mutation Status in Metastatic Colorectal Cancer Patients: A Pooled Analysis of the CAIRO, CAIRO2, COIN, and FOCUS Studies. Clin. Cancer Res. 2014;20:5322–5330. doi: 10.1158/1078-0432.CCR-14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannakis M., Mu X.J., Shukla S.A., Qian Z.R., Cohen O., Nishihara R., Bahl S., Cao Y., Amin-Mansour A., Yamauchi M., et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep. 2016;15:857–865. doi: 10.1016/j.celrep.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llosa N.J., Cruise M., Tam A., Wicks E.C., Hechenbleikner E.M., Taube J.M., Blosser R.L., Fan H., Wang H., Luber B.S., et al. The Vigorous Immune Microenvironment of Microsatellite Instable Colon Cancer Is Balanced by Multiple Counter-Inhibitory Checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tougeron D., Cohen R., Sueur B., Sefrioui D., Gentilhomme L., Lecomte T., Aparicio T., Des Guetz G., Artru P., de la Fouchardiere C., et al. A Large Retrospective Multicenter Study Evaluating Prognosis and Chemosensitivity of Metastatic Colorectal Cancer with Microsatellite Instability. Ann. Oncol. 2017;28:v180. doi: 10.1093/annonc/mdx393.059. [DOI] [Google Scholar]
- 15.Cervantes A., Adam R., Roselló S., Arnold D., Normanno N., Taïeb J., Seligmann J., De Baere T., Osterlund P., Yoshino T., et al. Metastatic Colorectal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022;34:10–32. doi: 10.1016/j.annonc.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Bacher J.W., Flanagan L.A., Smalley R.L., Nassif N.A., Burgart L.J., Halberg R.B., Megid W.M.A., Thibodeau S.N. Development of a Fluorescent Multiplex Assay for Detection of MSI-High Tumors. Dis. Markers. 2004;20:237–250. doi: 10.1155/2004/136734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buhard O., Suraweera N., Lectard A., Duval A., Hamelin R. Quasimonomorphic Mononucleotide Repeats for High-Level Microsatellite Instability Analysis. Dis. Markers. 2004;20:251–257. doi: 10.1155/2004/159347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H., Thienpont B., Yesilyurt B.T., Moisse M., Reumers J., Coenegrachts L., Sagaert X., Schrauwen S., Smeets D., Matthijs G., et al. Mismatch Repair Deficiency Endows Tumors with a Unique Mutation Signature and Sensitivity to DNA Double-Strand Breaks. eLife. 2014;3:e02725. doi: 10.7554/eLife.02725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Xu J., Li L., Mu X., Wang Y., Li X. Evaluation of a Fully Automated Idylla Test System for Microsatellite Instability in Colorectal Cancer. Clin. Colorectal Cancer. 2019;18:e316–e323. doi: 10.1016/j.clcc.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Velasco A., Tokat F., Bonde J., Trim N., Bauer E., Meeney A., de Leng W., Chong G., Dalstein V., Kis L.L., et al. Multi-Center Real-World Comparison of the Fully Automated IdyllaTM Microsatellite Instability Assay with Routine Molecular Methods and Immunohistochemistry on Formalin-Fixed Paraffin-Embedded Tissue of Colorectal Cancer. Virchows Arch. 2021;478:851–863. doi: 10.1007/s00428-020-02962-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li K., Luo H., Huang L., Luo H., Zhu X. Microsatellite Instability: A Review of What the Oncologist Should Know. Cancer Cell Int. 2020;20:16. doi: 10.1186/s12935-019-1091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umar A., Boland C.R., Terdiman J.P., Syngal S., de la Chapelle A., Rüschoff J., Fishel R., Lindor N.M., Burgart L.J., Hamelin R., et al. Revised Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. J. Natl. Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee C.T., Chow N.H., Chen Y.L., Ho C.L., Yeh Y.M., Lin S.C., Lin P.C., Lin B.W., Chu C.A., Tsai H.W., et al. Clinicopathological Features of Mismatch Repair Protein Expression Patterns in Colorectal Cancer. Pathol.-Res. Pract. 2021;217:153288. doi: 10.1016/j.prp.2020.153288. [DOI] [PubMed] [Google Scholar]
- 24.Radu O.M., Nikiforova M.N., Farkas L.M., Krasinskas A.M. Challenging Cases Encountered in Colorectal Cancer Screening for Lynch Syndrome Reveal Novel Findings: Nucleolar MSH6 Staining and Impact of Prior Chemoradiation Therapy. Hum. Pathol. 2011;42:1247–1258. doi: 10.1016/j.humpath.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Watson N., Grieu F., Morris M., Harvey J., Stewart C., Schofield L., Goldblatt J., Iacopetta B. Heterogeneous Staining for Mismatch Repair Proteins during Population-Based Prescreening for Hereditary Nonpolyposis Colorectal Cancer. J. Mol. Diagn. 2007;9:472. doi: 10.2353/jmoldx.2007.060162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarthy A.J., Capo-Chichi J.M., Spence T., Grenier S., Stockley T., Kamel-Reid S., Serra S., Sabatini P., Chetty R. Heterogenous Loss of Mismatch Repair (MMR) Protein Expression: A Challenge for Immunohistochemical Interpretation and Microsatellite Instability (MSI) Evaluation. J. Pathol. Clin. Res. 2019;5:115. doi: 10.1002/cjp2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joost P., Veurink N., Holck S., Klarskov L., Bojesen A., Harbo M., Baldetorp B., Rambech E., Nilbert M. Heterogenous Mismatch-Repair Status in Colorectal Cancer. Diagn. Pathol. 2014;9:126. doi: 10.1186/1746-1596-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratovomanana T., Cohen R., Svrcek M., Renaud F., Cervera P., Siret A., Letourneur Q., Buhard O., Bourgoin P., Guillerm E., et al. Performance of Next-Generation Sequencing for the Detection of Microsatellite Instability in Colorectal Cancer with Deficient DNA Mismatch Repair. Gastroenterology. 2021;161:814–826.e7. doi: 10.1053/j.gastro.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Hause R.J., Pritchard C.C., Shendure J., Salipante S.J. Classification and Characterization of Microsatellite Instability across 18 Cancer Types. Nat. Med. 2016;22:1342–1350. doi: 10.1038/nm.4191. [DOI] [PubMed] [Google Scholar]
- 30.Salipante S.J., Scroggins S.M., Hampel H.L., Turner E.H., Pritchard C.C. Microsatellite Instability Detection by next Generation Sequencing. Clin. Chem. 2014;60:1192–1199. doi: 10.1373/clinchem.2014.223677. [DOI] [PubMed] [Google Scholar]
- 31.Viguera E., Canceill D., Ehrlich S.D. Replication Slippage Involves DNA Polymerase Pausing and Dissociation. EMBO J. 2001;20:2587–2595. doi: 10.1093/emboj/20.10.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niu B., Ye K., Zhang Q., Lu C., Xie M., McLellan M.D., Wendl M.C., Ding L. MSIsensor: Microsatellite Instability Detection Using Paired Tumor-Normal Sequence Data. Bioinformatics. 2014;30:1015–1016. doi: 10.1093/bioinformatics/btt755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kautto E.A., Bonneville R., Miya J., Yu L., Krook M.A., Reeser J.W., Roychowdhury S. Performance Evaluation for Rapid Detection of Pan-Cancer Microsatellite Instability with MANTIS. Oncotarget. 2017;8:7452–7463. doi: 10.18632/oncotarget.13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortes-Ciriano I., Lee S., Park W.-Y., Kim T.-M., Park P.J. A Molecular Portrait of Microsatellite Instability across Multiple Cancers. Nat. Commun. 2017;8:15180. doi: 10.1038/ncomms15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y., Lee J.A., Park H.E., Han H., Kim Y., Bae J.M., Kim J.H., Cho N.Y., Kim H.P., Kim T.Y., et al. Targeted Next-Generation Sequencing-Based Detection of Microsatellite Instability in Colorectal Carcinomas. PLoS ONE. 2021;16:e0246356. doi: 10.1371/journal.pone.0246356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J., Wang M., Zhao D., Li F., Wu H., Liu Q., Li S. MSINGB: A Novel Computational Method Based on NGBoost for Identifying Microsatellite Instability Status from Tumor Mutation Annotation Data. Interdiscip. Sci. 2022:1–11. doi: 10.1007/s12539-022-00544-w. [DOI] [PubMed] [Google Scholar]
- 37.Ying M., Pan J., Lu G., Zhou S., Fu J., Wang Q., Wang L., Hu B., Wei Y., Shen J. Development and Validation of a Radiomics-Based Nomogram for the Preoperative Prediction of Microsatellite Instability in Colorectal Cancer. BMC Cancer. 2022;22:524. doi: 10.1186/s12885-022-09584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turajlic S., Litchfield K., Xu H., Rosenthal R., McGranahan N., Reading J.L., Wong Y.N.S., Rowan A., Kanu N., Al Bakir M., et al. Insertion-and-Deletion-Derived Tumour-Specific Neoantigens and the Immunogenic Phenotype: A Pan-Cancer Analysis. Lancet Oncol. 2017;18:1009–1021. doi: 10.1016/S1470-2045(17)30516-8. [DOI] [PubMed] [Google Scholar]
- 39.Campbell B.B., Light N., Fabrizio D., Zatzman M., Fuligni F., de Borja R., Davidson S., Edwards M., Elvin J.A., Hodel K.P., et al. Comprehensive Analysis of Hypermutation in Human Cancer. Cell. 2017;171:1042–1056.e10. doi: 10.1016/j.cell.2017.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farchoukh L., Kuan S.F., Dudley B., Brand R., Nikiforova M., Pai R.K. MLH1-Deficient Colorectal Carcinoma with Wild-Type BRAF and MLH1 Promoter Hypermethylation Harbor KRAS Mutations and Arise From Conventional Adenomas. Am. J. Surg. Pathol. 2016;40:1390–1399. doi: 10.1097/PAS.0000000000000695. [DOI] [PubMed] [Google Scholar]
- 41.Fang M., Ou J., Hutchinson L., Green M.R. The BRAF Oncoprotein Functions through the Transcriptional Repressor MAFG to Mediate the CpG Island Methylator Phenotype. Mol. Cell. 2014;55:904–915. doi: 10.1016/j.molcel.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gylfe A.E., Kondelin J., Turunen M., Ristolainen H., Katainen R., Pitkänen E., Kaasinen E., Rantanen V., Tanskanen T., Varjosalo M., et al. Identification of Candidate Oncogenes in Human Colorectal Cancers with Microsatellite Instability. Gastroenterology. 2013;145:540–543.e22. doi: 10.1053/j.gastro.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 43.Choi E.J., Kim M.S., Song S.Y., Yoo N.J., Lee S.H. Intratumoral Heterogeneity of Frameshift Mutations in MECOM Gene Is Frequent in Colorectal Cancers with High Microsatellite Instability. Pathol. Oncol. Res. 2017;23:145–149. doi: 10.1007/s12253-016-0112-3. [DOI] [PubMed] [Google Scholar]
- 44.Grasso C.S., Giannakis M., Wells D.K., Hamada T., Mu X.J., Quist M., Nowak J.A., Nishihara R., Qian Z.R., Inamura K., et al. Genetic Mechanisms of Immune Evasion in Colorectal Cancer. Cancer Discov. 2018;8:730–749. doi: 10.1158/2159-8290.CD-17-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tokunaga R., Xiu J., Goldberg R.M., Philip P.A., Seeber A., Battaglin F., Arai H., Lo J.H., Naseem M., Puccini A., et al. The Impact of ARID1A Mutation on Molecular Characteristics in Colorectal Cancer. Eur. J. Cancer. 2020;140:119–129. doi: 10.1016/j.ejca.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guinney J., Dienstmann R., Wang X., de Reyniès A., Schlicker A., Soneson C., Marisa L., Roepman P., Nyamundanda G., Angelino P., et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mlecnik B., Bindea G., Angell H.K., Maby P., Angelova M., Tougeron D., Church S.E., Lafontaine L., Fischer M., Fredriksen T., et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 48.Bindea G., Mlecnik B., Tosolini M., Kirilovsky A., Waldner M., Obenauf A.C., Angell H., Fredriksen T., Lafontaine L., Berger A., et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Becht E., de Reyniès A., Giraldo N.A., Pilati C., Buttard B., Lacroix L., Selves J., Sautès-Fridman C., Laurent-Puig P., Fridman W.H. Immune and Stromal Classification of Colorectal Cancer Is Associated with Molecular Subtypes and Relevant for Precision Immunotherapy. Clin. Cancer Res. 2016;22:4057–4066. doi: 10.1158/1078-0432.CCR-15-2879. [DOI] [PubMed] [Google Scholar]
- 50.Hamada T., Soong T.R., Masugi Y., Kosumi K., Nowak J.A., da Silva A., Mu X.J., Twombly T.S., Koh H., Yang J., et al. TIME (Tumor Immunity in the MicroEnvironment) Classification Based on Tumor CD274 (PD-L1) Expression Status and Tumor-Infiltrating Lymphocytes in Colorectal Carcinomas. Oncoimmunology. 2018;7:e1442999. doi: 10.1080/2162402X.2018.1442999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maby P., Tougeron D., Hamieh M., Mlecnik B., Kora H., Bindea G., Angell H.K., Fredriksen T., Elie N., Fauquembergue E., et al. Correlation between Density of CD8+ T-Cell Infiltrate in Microsatellite Unstable Colorectal Cancers and Frameshift Mutations: A Rationale for Personalized Immunotherapy. Cancer Res. 2015;75:3446–3455. doi: 10.1158/0008-5472.CAN-14-3051. [DOI] [PubMed] [Google Scholar]
- 52.Brahmer J.R., Tykodi S.S., Chow L.Q.M., Hwu W.-J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.J., Morse M.A., Desai J., Hill A., Axelson M., Moss R.A., et al. Nivolumab in Patients with Metastatic DNA Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer (CheckMate 142): An Open-Label, Multicentre, Phase 2 Study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Angelova M., Charoentong P., Hackl H., Fischer M.L., Snajder R., Krogsdam A.M., Waldner M.J., Bindea G., Mlecnik B., Galon J., et al. Characterization of the Immunophenotypes and Antigenomes of Colorectal Cancers Reveals Distinct Tumor Escape Mechanisms and Novel Targets for Immunotherapy. Genome Biol. 2015;16:64. doi: 10.1186/s13059-015-0620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung M., Lee J.A., Yoo S.Y., Bae J.M., Kang G.H., Kim J.H. Intratumoral Spatial Heterogeneity of Tumor-Infiltrating Lymphocytes Is a Significant Factor for Precisely Stratifying Prognostic Immune Subgroups of Microsatellite Instability-High Colorectal Carcinomas. Mod. Pathol. 2022;35:2011–2022. doi: 10.1038/s41379-022-01137-0. [DOI] [PubMed] [Google Scholar]
- 56.Pelka K., Hofree M., Chen J.H., Sarkizova S., Pirl J.D., Jorgji V., Bejnood A., Dionne D., Ge W.H., Xu K.H., et al. Spatially Organized Multicellular Immune Hubs in Human Colorectal Cancer. Cell. 2021;184:4734–4752.e20. doi: 10.1016/j.cell.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chida K., Kawazoe A., Suzuki T., Kawazu M., Ueno T., Takenouchi K., Nakamura Y., Kuboki Y., Kotani D., Kojima T., et al. Transcriptomic Profiling of MSI-H/DMMR Gastrointestinal Tumors to Identify Determinants of Responsiveness to Anti-PD-1 Therapy. Clin. Cancer Res. 2022;28:2110–2117. doi: 10.1158/1078-0432.CCR-22-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fabrizio D.A., George T.J., Dunne R.F., Frampton G., Sun J., Gowen K., Kennedy M., Greenbowe J., Schrock A.B., Hezel A.F., et al. Beyond Microsatellite Testing: Assessment of Tumor Mutational Burden Identifies Subsets of Colorectal Cancer Who May Respond to Immune Checkpoint Inhibition. J. Gastrointest. Oncol. 2018;9:610–617. doi: 10.21037/jgo.2018.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen E.X., Jonker D.J., Loree J.M., Kennecke H.F., Berry S.R., Couture F., Ahmad C.E., Goffin J.R., Kavan P., Harb M., et al. Effect of Combined Immune Checkpoint Inhibition vs. Best Supportive Care Alone in Patients with Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol. 2020;6:831–838. doi: 10.1001/jamaoncol.2020.0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pich O., Muiños F., Lolkema M.P., Steeghs N., Gonzalez-Perez A., Lopez-Bigas N. The Mutational Footprints of Cancer Therapies. Nat. Genet. 2019;51:1732–1740. doi: 10.1038/s41588-019-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Llosa N.J., Luber B., Tam A.J., Smith K.N., Siegel N., Awan A.H., Fan H., Oke T., Zhang J., Domingue J., et al. Intratumoral Adaptive Immunosuppression and Type 17 Immunity in Mismatch Repair Proficient Colorectal Tumors. Clin. Cancer Res. 2019;25:5250–5259. doi: 10.1158/1078-0432.CCR-19-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russo M., Crisafulli G., Sogari A., Reilly N.M., Arena S., Lamba S., Bartolini A., Amodio V., Magrì A., Novara L., et al. Adaptive Mutability of Colorectal Cancers in Response to Targeted Therapies. Science. 2019;366:1473–1480. doi: 10.1126/science.aav4474. [DOI] [PubMed] [Google Scholar]
- 63.Morano F., Raimondi A., Pagani F., Lonardi S., Salvatore L., Cremolini C., Murgioni S., Randon G., Palermo F., Antonuzzo L., et al. Temozolomide Followed by Combination with Low-Dose Ipilimumab and Nivolumab in Patients with Microsatellite-Stable, O6-Methylguanine-DNA Methyltransferase-Silenced Metastatic Colorectal Cancer: The MAYA Trial. J. Clin. Oncol. 2022;40:1562–1573. doi: 10.1200/JCO.21.02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crisafulli G., Sartore-Bianchi A., Lazzari L., Pietrantonio F., Amatu A., Macagno M., Barault L., Cassingena A., Bartolini A., Luraghi P., et al. Temozolomide Treatment Alters Mismatch Repair and Boosts Mutational Burden in Tumor and Blood of Colorectal Cancer Patients. Cancer Discov. 2022;12:1656–1675. doi: 10.1158/2159-8290.CD-21-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lipson E.J., Sharfman W.H., Drake C.G., Wollner I., Taube J.M., Anders R.A., Xu H., Yao S., Pons A., Chen L., et al. Durable Cancer Regression Off-Treatment and Effective Reinduction Therapy with an Anti-PD-1 Antibody. Clin. Cancer Res. 2013;19:462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Neil B.H., Wallmark J.M., Lorente D., Elez E., Raimbourg J., Gomez-Roca C., Ejadi S., Piha-Paul S.A., Stein M.N., Abdul Razak A.R., et al. Safety and Antitumor Activity of the Anti-PD-1 Antibody Pembrolizumab in Patients with Advanced Colorectal Carcinoma. PLoS ONE. 2017;12:e0189848. doi: 10.1371/journal.pone.0189848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S., et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diaz L.A., Marabelle A., Delord J.-P., Shapira-Frommer R., Geva R., Peled N., Kim T.W., Andre T., Van Cutsem E., Guimbaud R., et al. Pembrolizumab Therapy for Microsatellite Instability High (MSI-H) Colorectal Cancer (CRC) and Non-CRC. J. Clin. Oncol. 2017;35:3071. doi: 10.1200/JCO.2017.35.15_suppl.3071. [DOI] [Google Scholar]
- 69.André T., Shiu K.-K., Kim T.W., Jensen B.V., Jensen L.H., Punt C., Smith D., Garcia-Carbonero R., Benavides M., Gibbs P., et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 70.Overman M.J., Lonardi S., Wong K.Y.M., Lenz H.J., Gelsomino F., Aglietta M., Morse M.A., Van Cutsem E., McDermott R., Hill A., et al. Durable Clinical Benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 71.Lenz H.J., Van Cutsem E., Limon M.L., Wong K.Y.M., Hendlisz A., Aglietta M., García-Alfonso P., Neyns B., Luppi G., Cardin D.B., et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022;40:161–170. doi: 10.1200/JCO.21.01015. [DOI] [PubMed] [Google Scholar]
- 72.Kato Y., Tabata K., Kimura T., Yachie-kinoshita A., Ozawa Y., Yamada K., Ito J., Tachino S., Hori Y., Matsuki M., et al. Lenvatinib plus Anti-PD-1 Antibody Combination Treatment Activates CD8 + T Cells through Reduction of Tumor-Associated Macrophage and Activation of the Interferon Pathway. PLoS ONE. 2019;14:e0212513. doi: 10.1371/journal.pone.0212513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doleschel D., Hoff S., Koletnik S., Rix A., Zopf D., Kiessling F. Regorafenib Enhances Anti—PD1 Immunotherapy Efficacy in Murine Colorectal Cancers and Their Combination Prevents Tumor Regrowth. J. Exp. Clin. Cancer Res. 2021;40:288. doi: 10.1186/s13046-021-02043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saeed A., Park R., Sun W. The Integration of Immune Checkpoint Inhibitors with VEGF Targeted Agents in Advanced Gastric and Gastroesophageal Adenocarcinoma: A Review on the Rationale and Results of Early Phase Trials. J. Hematol. Oncol. 2021;14:13. doi: 10.1186/s13045-021-01034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bendell J.C., Powderly J.D., Lieu C.H., Eckhardt S.G., Hurwitz H., Hochster H.S., Murphy J.E., Funke R.P., Rossi C., Wallin J., et al. Safety and Efficacy of MPDL3280A (Anti-PDL1) in Combination with Bevacizumab (Bev) and/or FOLFOX in Patients (Pts) with Metastatic Colorectal Cancer (MCRC) J. Clin. Oncol. 2015;33:704. doi: 10.1200/jco.2015.33.3_suppl.704. [DOI] [Google Scholar]
- 76.Overman M.J., Yothers G., Jacobs S.A., Sanoff H.K., Cohen D.J., Guthrie K.A., Henry N.L., Ganz P.A., Kopetz S., Lucas P.C., et al. Colorectal Cancer Metastatic DMMR Immuno-Therapy (COMMIT) study: A randomized phase III study of Atezolizumab (Atezo) monotherapy versus MFOLFOX6/Bevacizumab/Atezo in the first-line treatment of patients (Pts) with deficient DNA mismatch repair (dMMR) or microsatellite instability high (MSI-H) metastatic colorectal cancer (mCRC)—NRG-GI004/SWOG-S1610. J. Clin. Oncol. 2021;39:TPS3618. doi: 10.1200/JCO.2021.39.15_suppl.TPS3618. [DOI] [Google Scholar]
- 77.Li H., Ning T., Zhang L., Ge S., Yang Y., Bai M., Wang X., Ji Z., Liu R., Deng T., et al. A Single Center Phase 2 Study of Anti-PD-1 Antibody plus Bevacizumab and FOLFIRI as Second-Line Treatment for Patients with MSI-H Metastatic Colorectal Cancer. J. Clin. Oncol. 2022;40:e15541. doi: 10.1200/JCO.2022.40.16_suppl.e15541. [DOI] [Google Scholar]
- 78.Koopman M., Mekenkamp L., Hoogerbrugge N. Deficient Mismatch Repair System in Patients with Sporadic Advanced Colorectal Cancer. Br. J. Cancer. 2009;100:266–273. doi: 10.1038/sj.bjc.6604867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sinicrope F.A., Ou F.-S., Zemla T., Nixon A.B., Mody K., Levasseur A., Dueck A.C., Dhanarajan A.R., Lieu C.H., Cohen D.J., et al. Randomized Trial of Standard Chemotherapy Alone or Combined with Atezolizumab as Adjuvant Therapy for Patients with Stage III Colon Cancer and Deficient Mismatch Repair (ATOMIC, Alliance A021502) J. Clin. Oncol. 2019;37:e15169. doi: 10.1200/JCO.2019.37.15_suppl.e15169. [DOI] [Google Scholar]
- 80.Lau D., Kalaitzaki E., Church D.N., Pandha H., Tomlinson I., Annels N., Gerlinger M., Sclafani F., Smith G., Begum R., et al. Rationale and Design of the POLEM Trial: Avelumab plus Fluoropyrimidine-Based Chemotherapy as Adjuvant Treatment for Stage III Mismatch Repair Deficient or POLE Exonuclease Domain Mutant Colon Cancer: A Phase III Randomised Study. ESMO Open. 2020;5:e000638. doi: 10.1136/esmoopen-2019-000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chalabi M., Fanchi L.F., Dijkstra K.K., Van den Berg J.G., Aalbers A.G., Sikorska K., Lopez-Yurda M., Grootscholten C., Beets G.L., Snaebjornsson P., et al. Neoadjuvant Immunotherapy Leads to Pathological Responses in MMR-Proficient and MMR-Deficient Early-Stage Colon Cancers. Nat. Med. 2020;26:566–576. doi: 10.1038/s41591-020-0805-8. [DOI] [PubMed] [Google Scholar]
- 82.Cercek A., Lumish M., Sinopoli J., Weiss J., Shia J., Lamendola-Essel M., El Dika I.H., Segal N., Shcherba M., Sugarman R., et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022;386:2363–2376. doi: 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kopetz S., Bekaii-Saab T.S., Yoshino T., Chung C.-H., Zhang X., Tabernero J. SEAMARK: Randomized Phase 2 Study of Pembrolizumab + Encorafenib + Cetuximab versus Pembrolizumab Alone for First-Line Treatment of BRAF V600E-Mutant and Microsatellite Instability-High (MSI-H)/Mismatch Repair Deficient (DMMR) Metastatic Colorectal Cancer. J. Clin. Oncol. 2022;40:TPS3634. doi: 10.1200/JCO.2022.40.16_suppl.TPS3634. [DOI] [Google Scholar]
- 84.Buchler T. Microsatellite Instability and Metastatic Colorectal Cancer—A Clinical Perspective. Front. Oncol. 2022;12:888181. doi: 10.3389/fonc.2022.888181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cocco E., Benhamida J., Middha S., Zehir A., Mullaney K., Shia J., Yaeger R., Zhang L., Wong D., Villafania L., et al. Colorectal Carcinomas Containing Hypermethylated MLH1 Promoter and Wild-Type BRAF / KRAS Are Enriched for Targetable Kinase Fusions. Cancer Res. 2019;79:1047–1053. doi: 10.1158/0008-5472.CAN-18-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berger S., Martens U.M., Bochum S. Larotrectinib (LOXO-101) Recent Results Cancer Res. 2018;211:141–151. doi: 10.1007/978-3-319-91442-8_10/COVER. [DOI] [PubMed] [Google Scholar]
- 87.Federman N., McDermott R. Larotrectinib, a Highly Selective Tropomyosin Receptor Kinase (TRK) Inhibitor for the Treatment of TRK Fusion Cancer. Expert Rev. Clin. Pharmacol. 2019;12:931–939. doi: 10.1080/17512433.2019.1661775. [DOI] [PubMed] [Google Scholar]
- 88.Scott L.J. Larotrectinib: First Global Approval. Drugs. 2019;79:201–206. doi: 10.1007/s40265-018-1044-x. [DOI] [PubMed] [Google Scholar]
- 89.Kasi P.M., Afghan M.K., Bellizzi A.M., Chan C.H.F. Larotrectinib in Mismatch-Repair-Deficient TRK Fusion-Positive Metastatic Colon Cancer After Progression on Immunotherapy. Cureus. 2022;14:6–10. doi: 10.7759/cureus.26648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ganesh K., Stadler Z.K., Cercek A., Mendelsohn R.B. Immunotherapy in Colorectal Cancer: Rationale, Challenges and Potential. Nat. Rev. Gastroenterol. Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hollebecque A., Chung H.C., Miguel M.J.D., Italiano A., MacHiels J.P., Lin C.C., Dhani N.C., Peeters M., Moreno V., Su W.C., et al. Safety and Antitumor Activity of α-PD-L1 Antibody as Monotherapy or in Combination with α-TIM-3 Antibody in Patients with Microsatellite Instability-High/Mismatch Repair-Deficient Tumors. Clin. Cancer Res. 2021;27:6393–6404. doi: 10.1158/1078-0432.CCR-21-0261. [DOI] [PubMed] [Google Scholar]
- 93.Le D.T., Kim T.W., Van Cutsem E., Geva R., Hara H., Burge M., Kavan P., Yoshino T., Guimbaud R., Taniguchi H., et al. Phase II Open-Label Study of Pembrolizumab in Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164 Abstract. J. Clin. Oncol. 2022;38:11–19. doi: 10.1200/JCO.19.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang D., Wu X., Sun Y. Therapeutic Targets and Biomarkers of Tumor Immunotherapy: Response versus Non-Response. Signal Transduct. Target. Ther. 2022;7:331. doi: 10.1038/s41392-022-01136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pietrantonio F., Lonardi S., Corti F., Infante G., Elez M.E., Fakih M., Jayachandran P., Shah A.T., Salati M., Fenocchio E., et al. Nomogram to Predict the Outcomes of Patients with Microsatellite Instability-High Metastatic Colorectal Cancer Receiving Immune Checkpoint Inhibitors. J. ImmunoTher. Cancer. 2021;9:e003370. doi: 10.1136/jitc-2021-003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fucà G., Cohen R., Lonardi S., Shitara K., Elez M.E., Fakih M., Chao J., Klempner S.J., Emmett M., Jayachandran P., et al. Ascites and Resistance to Immune Checkpoint Inhibition in DMMR/MSI-Metastatic Colorectal and Gastric Cancers. Cancer. 2022;10:e004001. doi: 10.1136/jitc-2021-004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chow A., Schad S., Green M.D., Wolchok J.D., Rudin C.M., Merghoub T., Chow A., Schad S., Green M.D., Hellmann M.D., et al. Article Tumor CD8 + T Cell Immunity Ll Ll Impair Anti-Tumor CD8 + T Cell Immunity. Cancer Cell. 2021;39:973–988.e9. doi: 10.1016/j.ccell.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Elkrief A., Derosa L., Kroemer G., Zitvogel L., Routy B. The Negative Impact of Antibiotics on Outcomes in Cancer Patients Treated with Immunotherapy: A New Independent Prognostic Factor? Incid. Mortal. Nasopharyng. Carcinoma. 2019;30:1572–1579. doi: 10.1093/annonc/mdz206. [DOI] [PubMed] [Google Scholar]
- 99.Routy B., Gopalakrishnan V., Daillère R., Zitvogel L., Wargo J.A., Kroemer G. The Gut Microbiota Influences Anticancer Immunosurveillance and General Health. Nat. Rev. Clin. Oncol. 2018;15:382–396. doi: 10.1038/s41571-018-0006-2. [DOI] [PubMed] [Google Scholar]
- 100.Raymond F., Déraspe M., Boissinot M., Bergeron M.G., Corbeil J., Raymond F., Déraspe M., Boissinot M., Bergeron M.G. Partial Recovery of Microbiomes after Antibiotic Treatment. Gut Microbes. 2016;7:428–434. doi: 10.1080/19490976.2016.1216747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy against Epithelial Tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 102.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C., et al. Gut Microbiome Modulates Response to Anti-PD-1 Immunotherapy in Melanoma Patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang Y., Weng W., Peng J., Hong L., Yang L., Toiyama Y., Gao R., Liu M., Yin M., Pan C., et al. Fusobacterium Nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-ΚB, and Up-Regulating Expression of MicroRNA-21. Gastroenterology. 2017;152:851–866.e24. doi: 10.1053/j.gastro.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rubinstein M.R., Baik J.E., Lagana S.M., Han R.P., Raab W.J., Sahoo D., Dalerba P., Wang T.C., Han Y.W. Fusobacterium Nucleatum Promotes Colorectal Cancer by Inducing Wnt/Β-catenin Modulator Annexin A1. EMBO Rep. 2019;20:e47638. doi: 10.15252/embr.201847638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gur C., Maalouf N., Shhadeh A., Berhani O., Singer B.B., Bachrach G., Mandelboim O. Fusobacterium Nucleatum Supresses Anti-Tumor Immunity by Activating CEACAM1. Oncoimmunology. 2019;8:e1581531. doi: 10.1080/2162402X.2019.1581531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gur C., Ibrahim Y., Isaacson B., Yamin R., Abed J., Gamliel M., Enk J., Bar-On Y., Stanietsky-Kaynan N., Coppenhagen-Glazer S., et al. Binding of the Fap2 Protein of Fusobacterium Nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity. 2015;42:344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen T., Li Q., Zhang X., Long R., Wu Y., Wu J., Fu X. TOX Expression Decreases with Progression of Colorectal Cancers and Is Associated with CD4 T-Cell Density and Fusobacterium Nucleatum Infection. Hum. Pathol. 2018;79:93–101. doi: 10.1016/j.humpath.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 108.Mima K., Nishihara R., Qian Z.R., Cao Y., Sukawa Y., Nowak J.A., Yang J., Dou R., Masugi Y., Song M., et al. Fusobacterium Nucleatum in Colorectal Carcinoma Tissue and Patient Prognosis. Gut. 2016;65:1973–1980. doi: 10.1136/gutjnl-2015-310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ito M., Kanno S., Nosho K., Sukawa Y., Mitsuhashi K., Kurihara H., Igarashi H., Takahashi T., Tachibana M., Takahashi H., et al. Association of Fusobacterium Nucleatum with Clinical and Molecular Features in Colorectal Serrated Pathway. Int. J. Cancer. 2015;137:1258–1268. doi: 10.1002/ijc.29488. [DOI] [PubMed] [Google Scholar]
- 110.Gao Y., Bi D., Xie R., Li M., Guo J., Liu H., Guo X., Fang J., Ding T., Zhu H., et al. Fusobacterium Nucleatum Enhances the Efficacy of PD-L1 Blockade in Colorectal Cancer. Signal Transduct. Target. Ther. 2021;6:398. doi: 10.1038/S41392-021-00795-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weng J., Li S., Zhu Z., Liu Q., Zhang R., Yang Y., Li X. Exploring Immunotherapy in Colorectal Cancer. J. Hematol. Oncol. 2022;15:95. doi: 10.1186/s13045-022-01294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cabrera C.M., Jiménez P., Cabrera T., Esparza C., Ruiz-Cabello F., Garrido F. Total Loss of MHC Class I in Colorectal Tumors Can Be Explained by Two Molecular Pathways: Β2-Microglobulin Inactivation in MSI-Positive Tumors and LMP7/TAP2 Downregulation in MSI-Negative Tumors. Tissue Antigens. 2003;61:211–219. doi: 10.1034/j.1399-0039.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 113.Janikovits J., Müller M., Krzykalla J., Körner S., Echterdiek F., Lahrmann B., Grabe N., Schneider M., Benner A., von Knebel Doeberitz M., et al. High Numbers of PDCD1 (PD-1)-Positive T Cells and B2M Mutations in Microsatellite-Unstable Colorectal Cancer. Oncoimmunology. 2017;7:e1390640. doi: 10.1080/2162402X.2017.1390640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ozcan M., Janikovits J., von Knebel Doeberitz M., Kloor M. Complex Pattern of Immune Evasion in MSI Colorectal Cancer. Oncoimmunology. 2018;7:e1445453. doi: 10.1080/2162402X.2018.1445453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Snahnicanova Z., Kasubova I., Kalman M., Grendar M., Mikolajcik P., Gabonova E., Laca L., Caprnda M., Rodrigo L., Ciccocioppo R., et al. Genetic and Epigenetic Analysis of the Beta-2-Microglobulin Gene in Microsatellite Instable Colorectal Cancer. Clin. Exp. Med. 2019;20:87–95. doi: 10.1007/s10238-019-00601-7. [DOI] [PubMed] [Google Scholar]
- 116.Middha S., Yaeger R., Shia J., Stadler Z.K., King S., Guercio S., Paroder V., Bates D.D.B., Rana S., Jr L.A.D., et al. Majority of B2M-Mutant and -Deficient Colorectal Carcinomas Achieve Clinical Benefit From Immune Checkpoint Inhibitor Therapy and Are Microsatellite Instability-High. JCO Precis. Oncol. 2019;3:1–14. doi: 10.1200/PO.18.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang B., Li X., Yin T., Qin D., Chen Y., Ma Q., Shu P., Wang Y. Neurotoxicity of Tumor Immunotherapy: The Emergence of Clinical Attention. J. Oncol. 2022;2022:4259205. doi: 10.1155/2022/4259205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sveen A., Johannessen B., Tengs T., Danielsen S.A., Eilertsen I.A., Lind G.E., Berg K.C.G., Leithe E., Meza-Zepeda L.A., Domingo E., et al. Multilevel Genomics of Colorectal Cancers with Microsatellite Instability-Clinical Impact of JAK1 Mutations and Consensus Molecular Subtype 1. Genome Med. 2017;9:46. doi: 10.1186/s13073-017-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garcia-Diaz A., Shin D.S., Moreno B.H., Saco J., Escuin-Ordinas H., Rodriguez G.A., Zaretsky J.M., Sun L., Hugo W., Wang X., et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017;19:1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stelloo E., Versluis M.A., Nijman H.W., de Bruyn M., Plat A., Osse E.M., van Dijk R.H., Nout R.A., Creutzberg C.L., de Bock G.H., et al. Microsatellite Instability Derived JAK1 Frameshift Mutations Are Associated with Tumor Immune Evasion in Endometrioid Endometrial Cancer. Oncotarget. 2016;7:39885–39893. doi: 10.18632/oncotarget.9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Picard E., Verschoor C.P., Ma G.W., Pawelec G. Relationships Between Immune Landscapes, Genetic Subtypes and Responses to Immunotherapy in Colorectal Cancer. Front. Immunol. 2020;11:369. doi: 10.3389/fimmu.2020.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim B.K., Cheon J., Kim H., Kang B., Ha Y., Kim D.Y., Hwang S.G., Chon Y.E., Chon H.J. Atezolizumab/Bevacizumab vs. Lenvatinib as First-Line Therapy for Unresectable Hepatocellular Carcinoma: A Real-World, Multi-Center Study. Cancers. 2022;14:1747. doi: 10.3390/cancers14071747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bolzacchini E., Libera L., Church S.E., Sahnane N., Bombelli R., Digiacomo N., Giordano M., Petracco G., Sessa F., Capella C., et al. Tumor Antigenicity and a Pre-Existing Adaptive Immune Response in Advanced BRAF Mutant Colorectal Cancers. Cancers. 2022;14:3951. doi: 10.3390/cancers14163951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cen S., Liu K., Zheng Y., Shan J., Jing C., Gao J., Pan H., Bai Z., Liu Z. BRAF Mutation as a Potential Therapeutic Target for Checkpoint Inhibitors: A Comprehensive Analysis of Immune Microenvironment in BRAF Mutated Colon Cancer. Front. Cell Dev. Biol. 2021;9:1988. doi: 10.3389/fcell.2021.705060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Batlle E., Massagué J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chakravarthy A., Khan L., Bensler N.P., Bose P., De Carvalho D.D. TGF-β-Associated Extracellular Matrix Genes Link Cancer-Associated Fibroblasts to Immune Evasion and Immunotherapy Failure. Nat. Commun. 2018;9:4692. doi: 10.1038/s41467-018-06654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tauriello D.V.F., Palomo-Ponce S., Stork D., Berenguer-Llergo A., Badia-Ramentol J., Iglesias M., Sevillano M., Ibiza S., Cañellas A., Hernando-Momblona X., et al. TGFβ Drives Immune Evasion in Genetically Reconstituted Colon Cancer Metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 128.Mariathasan S., Turley S.J., Nickles D., Castiglioni A., Yuen K., Wang Y., Kadel E.E., Koeppen H., Astarita J.L., Cubas R., et al. TGFβ Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee H.O., Hong Y., Etlioglu H.E., Cho Y.B., Pomella V., Van den Bosch B., Vanhecke J., Verbandt S., Hong H., Min J.W., et al. Lineage-Dependent Gene Expression Programs Influence the Immune Landscape of Colorectal Cancer. Nat. Genet. 2020;52:594–603. doi: 10.1038/s41588-020-0636-z. [DOI] [PubMed] [Google Scholar]
- 130.Lal N., Beggs A.D., Willcox B.E., Middleton G.W. An Immunogenomic Stratification of Colorectal Cancer: Implications for Development of Targeted Immunotherapy. Oncoimmunology. 2015;4:e976052. doi: 10.4161/2162402X.2014.976052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Becht E., Giraldo N.A., Lacroix L., Buttard B., Elarouci N., Petitprez F., Selves J., Laurent-Puig P., Sautès-Fridman C., Fridman W.H., et al. Estimating the Population Abundance of Tissue-Infiltrating Immune and Stromal Cell Populations Using Gene Expression. Genome Biol. 2016;17:218. doi: 10.1186/s13059-016-1070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dienstmann R., Villacampa G., Sveen A., Mason M.J., Niedzwiecki D., Nesbakken A., Moreno V., Warren R.S., Lothe R.A., Guinney J. Relative Contribution of Clinicopathological Variables, Genomic Markers, Transcriptomic Subtyping and Microenvironment Features for Outcome Prediction in Stage II/III Colorectal Cancer. Ann. Oncol. 2019;30:1622–1629. doi: 10.1093/annonc/mdz287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang H., Wang X., Xu L., Zhang J., Cao H. Analysis of the Transcriptomic Features of Microsatellite Instability Subtype Colon Cancer. BMC Cancer. 2019;19:605. doi: 10.1186/s12885-019-5802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hua H., He W., Chen N., He Y., Wu G., Ye F., Zhou X., Li Y., Ding Y., Zhong W., et al. Genomic and Transcriptomic Analysis of MSI-H Colorectal Cancer Patients with Targetable Alterations Identifies Clinical Implications for Immunotherapy. Front. Immunol. 2023;13:7038. doi: 10.3389/fimmu.2022.974793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Farshidfar F., Weljie A.M., Kopciuk K.A., Hilsden R., McGregor S.E., Buie W.D., MacLean A., Vogel H.J., Bathe O.F. A Validated Metabolomic Signature for Colorectal Cancer: Exploration of the Clinical Value of Metabolomics. Br. J. Cancer. 2016;115:848–857. doi: 10.1038/bjc.2016.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Long Z., Zhou J., Xie K., Wu Z., Yin H., Daria V., Tian J., Zhang N., Li L., Zhao Y., et al. Metabolomic Markers of Colorectal Tumor with Different Clinicopathological Features. Front. Oncol. 2020;10:981. doi: 10.3389/fonc.2020.00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gold A., Choueiry F., Jin N., Mo X., Zhu J. The Application of Metabolomics in Recent Colorectal Cancer Studies: A State-of-the-Art Review. Cancers. 2022;14:725. doi: 10.3390/cancers14030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Holbert C.E., Cullen M.T., Casero R.A., Stewart T.M. Polyamines in Cancer: Integrating Organismal Metabolism and Antitumour Immunity. Nat. Rev. Cancer. 2022;22:467–480. doi: 10.1038/s41568-022-00473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pagès F., Berger A., Camus M., Sanchez-Cabo F., Costes A., Molidor R., Mlecnik B., Kirilovsky A., Nilsson M., Damotte D., et al. Effector Memory T Cells, Early Metastasis, and Survival in Colorectal Cancer. N. Engl. J. Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 140.Galon J., Costes A., Sanchez-Cabo F., Kirilovsky A., Mlecnik B., Lagorce-Pages C., Tosolini M., Camus M., Berger A., Wind P., et al. Type, Density, and Location of Immune Cells within Human Colorectal Tumors Predict Clinical Outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 141.Corti F., Lonardi S., Intini R., Salati M., Fenocchio E., Belli C., Borelli B., Brambilla M., Prete A.A., Quarà V., et al. The Pan-Immune-Inflammation Value in Microsatellite Instability-High Metastatic Colorectal Cancer Patients Treated with Immune Checkpoint Inhibitors. Eur. J. Cancer. 2021;150:155–167. doi: 10.1016/j.ejca.2021.03.043. [DOI] [PubMed] [Google Scholar]