Abstract
Simple Summary
In this study, we demonstrated that overall 51.2% of patients with hepatocellular carcinoma (HCC) had elevated alpha-fetoprotein (AFP) levels. The proportion of patients with elevated AFP levels was stationary in the period from 2011 to 2020. The proportion of patients with Barcelona Clinic Liver Cancer classification (BCLC) stages 0–A HCC decreased from 2011 to 2020, whereas the proportion of patients with non-HBV- and non-HCV (NBNC)-HCC increased in the same period. Furthermore, the proportion of patients with early-stage HCC (i.e., BCLC stages 0–A) was lower for NBNC-HCC than for HBV- or HCV-related HCC. Advanced tumor stage, severe underlying liver disease, viral etiology, and female gender are associated with elevated AFP levels in HCC patients.
Abstract
A recent study from the US showed a decreasing trend in the elevated serum alpha-fetoprotein (AFP) level (i.e., ≥20 ng/mL) in hepatocellular carcinoma (HCC) patients at the time of diagnosis. Furthermore, advanced tumor stage and severe underlying liver disease were associated with elevated AFP levels. We aimed to evaluate this issue in an area endemic for hepatitis B virus (HBV). Between 2011 and 2020, 4031 patients were newly diagnosed with HCC at our institution. After excluding 54 patients with unknown AFP data, the remaining 3977 patients were enrolled in this study. Elevated AFP level was defined as ≥20 ng/mL. Overall, 51.2% of HCC patients had elevated AFP levels; this proportion remained stationary between 2011 and 2020 (51.8% vs. 51.1%). Multivariate analysis showed that female gender (odds ratio (OR) = 1.462; p < 0.001), tumor size per 10 mm increase (OR = 1.155; p < 0.001), multiple tumors (OR = 1.406; p < 0.001), Barcelona Clinic Liver Cancer stages B–D (OR = 1.247; p = 0.019), cirrhosis (OR = 1.288; p = 0.02), total bilirubin > 1.4 mg/dL (OR = 1.218; p = 0.030), and HBV- or hepatitis C virus (HCV)-positive status (OR = 1.720; p < 0.001) were associated with elevated AFP levels. In conclusion, a stationary trend in elevated serum AFP level in HCC patients has been noted in the past 10 years. Advanced tumor stage, severe underlying liver disease, viral etiology, and female gender are associated with elevated AFP levels in HCC patients.
Keywords: alpha-fetoprotein, hepatocellular carcinoma, hepatitis B virus, hepatitis C virus
1. Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death worldwide [1]. A meta-analysis showed that HCC surveillance is associated with significant improvements in early-stage tumor detection, the receipt of curative therapy, and survival of cirrhotic patients [2]. The American Association for the Study of Liver Diseases (AASLD) guideline recommends HCC surveillance for high-risk populations. The modality recommended for surveillance is ultrasound with or without an alpha-fetoprotein (AFP) serum assay [3]. Ultrasound with an AFP serum assay is recommended for surveillance because a meta-analysis demonstrated that ultrasound alone had low sensitivity in detecting early-stage tumor in cirrhotic patients. The combination of AFP serum assay and ultrasound significantly increases the sensitivity of tumor detection [4]. Currently, the AASLD guideline recommends diagnostic multiphasic magnetic resonance imaging (MRI)/computed tomography (CT) for further evaluation when the AFP level is ≥20 ng/mL on surveillance [3].
Multiple factors, including advanced tumor stage and viral etiology of chronic liver disease, are associated with elevated AFP levels in HCC patients [5]. A recent study from the US found a downtrend in the percentage of HCC cases with elevated AFP levels at the time of diagnosis from 2010 to 2017 in a large cohort from the National Cancer Database. Elevated AFP was defined as ≥20 ng/mL. Furthermore, advanced tumor stage and severe underlying liver disease were associated with elevated AFP levels. The authors suggested that these changes in AFP values at HCC diagnosis were possibly related to the increasing trend in early-stage tumor detection and the shift from viral (i.e., hepatitis B virus (HBV) or hepatitis C virus (HCV)) to nonviral etiology. However, data on the etiology of liver disease were unavailable in the database analyzed in the study [6].
Approximately 90% of HCC cases are associated with a known underlying etiology [7]. In East Asia, the major risk factor is HBV, whereas, in the Western world, it is HCV [7]. The risk of HCC attributed to HCV infection has largely decreased owing to the eradication of the virus with direct-acting antiviral (DAA) agents [8]. Nonalcoholic fatty liver disease (NAFLD), which is usually associated with obesity, metabolic syndrome, or diabetes mellitus, is becoming the fastest growing etiology of HCC, not only in Western countries [9], but also in Asia [10].
Due to the different etiologies of HCC in the East and the West and viral etiology being associated with elevated AFP levels in HCC patients [5], we aimed to evaluate whether there is a downtrend in the percentages of HCC cases with elevated AFP levels at the time of diagnosis and the factors associated with elevated AFP levels in HCC patients in a country from East Asia, where the leading etiology of HCC is HBV.
2. Materials and Methods
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital (reference number: 202201189B0; date of approval: 8 August 2022).
The Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital waived the need for informed consent due to the retrospective and observational nature of the study design. Data were extracted from Kaohsiung Chang Gung Memorial Hospital’s HCC registry database, which holds prospectively collected and annually updated data.
From 2011 to 2020, 4031 patients were newly diagnosed with HCC at the institution. After excluding 54 patients with unknown AFP data, the remaining 3977 patients were enrolled in this study.
2.1. Variables of Interest
Patient demographics, tumor size and number, clinical tumor–node–metastasis (TNM) stage (seventh edition of the American Joint Committee on Cancer (AJCC)) [11], Barcelona Clinic Liver Cancer classification (BCLC) stage [12], AFP level, cirrhosis, Child–Pugh class [13], creatinine, bilirubin, international normalized ratio (INR), hepatitis B surface antigen (HBsAg), anti-HCV antibody, alcohol use disorder (AUD), and HCC diagnostic method (i.e., clinical vs. pathological diagnosis) were prospectively collected from the HCC registry data. Infection with HBV was defined as being HBsAg-positive. Infection with HCV was defined as being anti-HCV-antibody-positive, irrespective of viremia. An individual with AUD was defined as a habitual drinker. Demographic information included age, gender, height, and weight. Tumor size was determined according to the results of pathological examination of patients who underwent surgery, whereas it was determined according to the findings of imaging in patients who underwent nonsurgical treatments. Tumor number (solitary vs. multiple) was determined from the findings of imaging. The presence of cirrhosis was indicated by an Ishak score [14] of 5 or 6 in patients who underwent surgery, whereas it was determined according to the findings of imaging in patients who underwent nonsurgical treatments. Cirrhosis was indicated in imaging by small liver size, nodular liver surface, presence of regeneration nodules, left and right lobe liver volume redistribution, etc. [15]. The BCLC stages according to the original version and BCLC stage A were defined within Milan criteria [16].
The raw data for the cohort involved in this study are available via the following digital object identifier: https://www.dropbox.com/scl/fi/6hzj7a3hrqhp5yu4i17qu/afp-trend.xlsx?dl=0&rlkey=srqyaz7t1sqsli6heaoig4bk8 (accessed on 1 January 2023).
2.2. Statistical Analysis
Variables are presented as number and percentage or median and interquartile range. The Chi-square test was used to compare categorical variables. Mann–Whitney U test was used to compare continuous variables. Whether there was an increasing or decreasing trend of BCLC stages 0–A or non-HBV- and non-HCV (NBNC)-HCC according to the year of HCC diagnosis was examined for a linear trend using the Chi-square test. Univariate analyses were conducted to explore the association between elevated AFP levels and clinical variables. Variables with p-values ≤ 0.1 in univariate analyses were included in a multivariate logistic regression analysis. To avoid collinearity, we examined the correlation between two independent variables using Spearman’s correlation test. If two independent variables had a correlation coefficient above 0.5, then they were determined to be highly correlated with each other and thus, collinear. In this case, we only chose one of the variables for multivariate analysis. In this analysis, we used the following cutoff values for continuous variables: for bilirubin, the upper limit of the normal range (i.e., 1.4 mg/dL); for creatinine, the upper limit of the normal range (i.e., 1.2 mg/dL); and for INR, the upper limit of the normal range (i.e., 1.2). Relative risks are presented as odds ratio (OR) with a 95% confidence interval (CI). To compare with a recent US study [6], we used the same method adopted in that study [6] to interpret the temporal trend of elevated AFP levels. We estimated the percentage of elevated AFP using marginal effects (i.e., the average predicted probability) from a logistic regression model [17]. All statistical analyses were performed using SPSS version 22.0 and R statistical software version 4.0.5. Two-tailed significance values were applied, and the level of statistical significance was defined as p < 0.05.
3. Results
3.1. Trend in Elevated AFP Levels at the Time of HCC Diagnosis
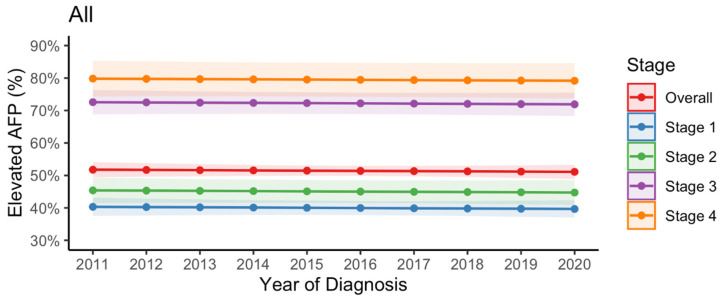
Overall, 2036 (51.2%) patients with HCC had elevated AFP levels. Between 2011 and 2020, the proportion of patients with elevated AFP levels was stationary in the case of all patients (51.8% [95% CI = 49.4–54.1%] in 2011 vs. 51.1% [95% CI = 48.9–53.3%] in 2020); patients in AJCC stage 1 (40.3% [95% CI = 37.5–43.1%] in 2011 vs. 39.7% [95% CI = 37.0–42.3%] in 2020); patients in AJCC stage 2 (45.4% [95% CI = 41.5–49.3%] in 2011 vs. 44.7% [95% CI = 40.9–48.6%] in 2020); patients in AJCC stage 3 (72.6% [95% CI = 68.8–76.3%] in 2011 vs. 71.9% [95% CI = 68.3–75.5%] in 2020); and patients in AJCC stage 4 (79.8% [95% CI = 74.3–85.3%] in 2011 vs. 79.2% [95% CI = 73.7–84.6%] in 2020) (Figure 1).
Figure 1.
Trend in elevated serum alpha-fetoprotein levels (i.e., ≥20 ng/mL) at the time of hepatocellular carcinoma diagnosis in all patients and at different stages (seventh edition American Joint Committee on Cancer).
3.2. Trends in Early-Stage Tumor Prevalence and Non-Viral Etiology at the Time of HCC Diagnosis
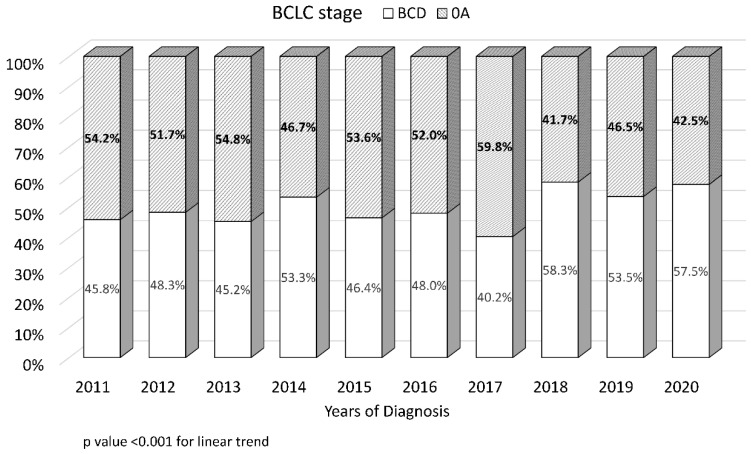
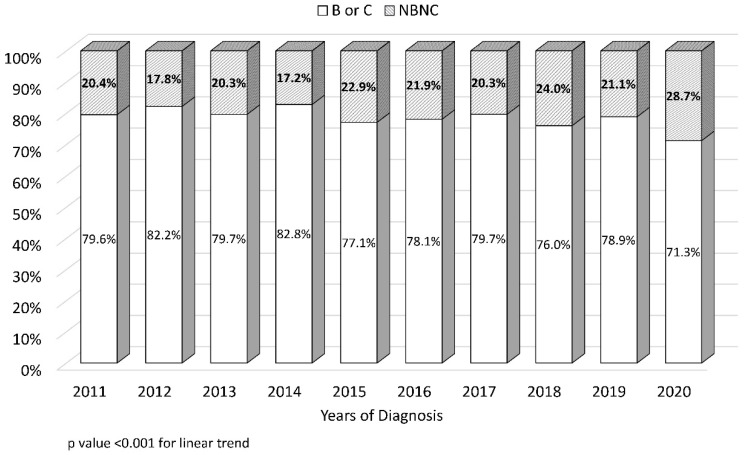
The proportion of patients with early-stage tumor (i.e., BCLC stages 0–A) decreased in the period from 2011 to 2020 (54.2% vs. 42.5%, p < 0.001) (Figure 2). The proportion of patients with non-HBV and non-HCV (NBNC)-HCC increased between the years 2011 and 2020 (from 20.4% to 28.7%, p < 0.001) (Figure 3).
Figure 2.
Trend in early-stage tumor at the time of hepatocellular carcinoma diagnosis. Early-stage tumor was defined as Barcelona Clinic Liver Cancer classification stages 0–A.
Figure 3.
Trend in nonviral etiology (i.e., patients negative for both hepatitis B and C viruses) at the time of hepatocellular carcinoma diagnosis.
3.3. Patients’ Characteristics Categorized by AFP Level
Compared to patients with normal AFP levels, a smaller proportion of patients with elevated AFP were male (p = 0.02), had a pathological diagnosis of HCC (p < 0.001), were in AJCC stage 1 or 2 HCC (p < 0.001), had a solitary tumor (p < 0.001), were in BCLC stage 0 or A HCC (p < 0.001), were in Child–Pugh class A (p < 0.001), and had a low body mass index (BMI) (p < 0.001). Furthermore, patients with elevated AFP levels had larger tumors (p < 0.001), a higher total bilirubin level (p < 0.001), and a higher INR (p < 0.001) and a higher proportion of them were cirrhotic (p = 0.001) and HBsAg-positive (p = 0.002). However, there were no significant differences in age, creatinine level, proportion with AUD, and proportion with anti-HCV-antibody-positive status between the two groups (Table 1).
Table 1.
Patients’ characteristics categorized by alpha-fetoprotein level.
| Characteristic | Elevated AFP (≥20 ng/mL), n = 2036 | Normal AFP (<20 ng/mL), n = 1941 | p |
|---|---|---|---|
| Age (years) | 63 (55–71) | 63 (56–71) | 0.286 |
| Male | 1437 (70.6%) | 1434 (73.9%) | 0.02 |
| BMI (kg/m2) | 24.2 (22.1–26.9) | 24.9 (22.5–27.8) | <0.001 |
| Diagnosis method | <0.001 | ||
| Clinical diagnosis | 896 (44.0%) | 593 (30.6%) | |
| Pathology diagnosis | 1140 (56.0%) | 1348 (69.4%) | |
| AUD | 0.460 | ||
| Yes | 260 (12.8%) | 225 (11.6%) | |
| No | 1762 (86.5%) | 1705 (87.8%) | |
| Not available | 11 (0.6%) | 14 (0.7%) | |
| HBsAg | 0.002 | ||
| Positive | 1006 (49.4%) | 866 (44.6%) | |
| Negative | 1030 (50.6%) | 1075 (55.4%) | |
| Anti-HCV | 0.327 | ||
| Positive | 741(36.4%) | 674 (34.7%) | |
| Negative | 1295 (63.6%) | 1266 (65.2%) | |
| Not available | 0 | 1 (0.1%) | |
| Cirrhosis | 0.001 | ||
| Yes | 1469 (72.2%) | 1294 (66.7%) | |
| No | 561 (27.6%) | 640 (33.0%) | |
| Not available | 7 (0.4%) | 6 (0.3%) | |
| Child–Pugh class | <0.001 | ||
| A | 1587 (77.9%) | 1627 (83.80%) | |
| B | 345 (16.9%) | 235 (12.1%) | |
| C | 70 (3.4%) | 51 (2.6%) | |
| Not available | 34 (1.7%) | 28 (1.4%) | |
| Creatinine (mg/dL) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 0.731 |
| Total bilirubin (mg/dL) | 1.1 (0.8–1.7) | 1.0 (0.7–1.4) | <0.001 |
| INR | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | <0.001 |
| Tumor size (mm) | 46 (26–98) | 28 (20–45) | <0.001 |
| Tumor number | <0.001 | ||
| Single | 1064 (52.3%) | 1360 (70.1%) | |
| Multiple | 972 (47.7%) | 581 (29.9%) | |
| 7th edition AJCC stage | <0.001 | ||
| 1 | 788 (38.7%) | 1168 (60.2%) | |
| 2 | 340 (16.7%) | 414 (21.3%) | |
| 3 | 617 (30.3%) | 233 (12.0%) | |
| 4 | 279 (13.7%) | 72 (3.7%) | |
| Not available | 12 (0.6%) | 54 (2.8%) | |
| BCLC stage | <0.001 | ||
| 0 | 206 (10.1%) | 293 (15.1%) | |
| A | 566 (27.3%) | 867 (44.7%) | |
| B | 446 (21.9%) | 362 (18.7%) | |
| C | 676 (33.2%) | 279 (14.4%) | |
| D | 124 (6.1%) | 81(4.2%) | |
| Not available | 28 (1.4%) | 59 (3.0%) |
AFP, alpha-fetoprotein; BMI, body mass index; AUD, alcohol use disorder; HBsAg, hepatitis B surface antigen; anti-HCV, anti-hepatitis C virus antibody; INR, international normalized ratio; AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer.
3.4. Variables Associated with Elevated AFP Level
Univariate analysis showed that the following variables were associated with elevated AFP levels: tumor size per 10 mm increase (OR = 1.167; 95% CI = 1.146–1.189; p < 0.001); using AJCC stage 1 as the reference, AJCC stage 2 (OR = 1.202; 95% CI = 1.013–1.426; p = 0.035), AJCC stage 3 (OR =3.909; 95% CI = 3.270–4.674; p < 0.001), and AJCC stage 4 (OR = 6.071; 95% CI = 4.553–8.096; p < 0.001); multiple tumors (OR = 2.076; 95% CI = 1.818–2.371; p < 0.001); using BCLC stages 0–A as the reference, BCLC stages B–D (OR = 2.640; 95% CI = 2.317–3.009; p < 0.001); cirrhosis (OR = 1.275; 95% CI = 1.110–1.465; p = 0.001); Child–Pugh class B or C (OR = 1.525; 95% CI = 1.287–1.806; p < 0.001); total bilirubin > 1.4 mg/dL (OR = 1.454; 95% CI = 1.262–1.676; p < 0.001); INR > 1.2 (OR = 1.238; 95% CI = 1.002–1.530; p = 0.048); and HBV- or HCV-positive status (OR = 1.355; 95% CI = 1.160–1.583; p < 0.001). Because of the strong correlation between AJCC and BCLC stages (correlation coefficient = 0.658, p < 0.001), we only selected the BCLC stage for multivariate analysis to avoid collinearity (Table 2).
Table 2.
Univariate and multivariate analyses of factors associated with elevated AFP levels.
| Variable | Univariate | p | Multivariate | p |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Age (per 10 years) | 0.955 (0.904–1.010) | 0.105 | ||
| Female vs. male | 1.20 (1.04–1.39) | 0.011 | 1.462 (1.256–1.701) | <0.001 |
| AUD | 1.077 (0.887–1.306) | 0.454 | ||
| HBsAg or anti-HCV-positive | 1.355 (1.160–1.583) | <0.001 | 1.720 (1.451–2.038) | <0.001 |
| Cirrhosis | 1.275 (1.110–1.465) | 0.001 | 1.288 (1.099–1.509) | 0.02 |
| Child–Pugh class B or C vs. A |
1.525(1.287–1.806) | <0.001 | 0.964 (0.770–1.205) | 0.745 |
| Creatinine >1.2 mg/dL |
0.983 (0.849–1.138) | 0.821 | ||
| Total bilirubin >1.4 mg/dL |
1.454 (1.262–1.676) | <0.001 | 1.218 (1.020–1.455) | 0.030 |
| INR >1.2 |
1.238 (1.002–1.530) | 0.048 | 0.897 (0.687–1.172) | 0.425 |
| Tumor size, per 10 mm increase | 1.167 (1.146–1.189) | <0.001 | 1.155 (1.127–1.183) | <0.001 |
| Multiple tumors | 2.076 (1.818–2.371) | <0.001 | 1.406 (1.205–1.641) | <0.001 |
| BCLC stage (O–A as reference) |
||||
| B–D | 2.640 (2.317–3.009) | <0.001 | 1.247 (1.037–1.500) | 0.019 |
| 7th edition AJCC Stage 1 as reference |
||||
| Stage 2 | 1.202 (1.013–1.426) | 0.035 | ||
| Stage 3 | 3.909 (3.270–4.674) | <0.001 | ||
| Stage 4 | 6.071 (4.553–8.096) | <0.001 |
AFP, alpha-fetoprotein; AUD, alcohol use disorder; HBsAg, hepatitis B surface antigen; anti-HCV, anti-hepatitis C virus antibody; INR, international normalized ratio; AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer.
Multivariate analysis showed that the following variables were associated with elevated AFP levels: female gender (OR = 1.462; 95% CI = 1.256–1.701; p < 0.001); tumor size per 10 mm increase (OR = 1.155; 95% CI = 1.127–1.183; p < 0.001); multiple tumors (OR = 1.406; 95% CI = 1.205–1.641; p < 0.001); BCLC stages B–D (OR = 1.247; 95% CI = 1.037–1.500; p = 0.019); cirrhosis (OR = 1.288; 95% CI = 1.099–1.509; p = 0.02); total bilirubin > 1.4 mg/dL (OR = 1.218; 95% CI = 1.020–1.455; p = 0.030); and HBV- or HCV-positive status (OR = 1.720; 95% CI = 1.451–2.038; p < 0.001) (Table 2).
3.5. Proportion of BCLC Stages 0–A Patients in NBNC-HCC vs. HBV- or HCV-Related HCC
Of the 870 patients with NBNC-HCC, 315 (36.2%) were in BCLC stages 0–A, 529 (60.8%) were in BCLC stages B–D, and 26 (0.3%) were of unknown BCLC stage. Of the 3107 HBV- or HCV-positive patients, 1607 (51.7%) were in BCLC stages 0–A, 1439 (46.3%) were in BCLC stages B–D, and 61 (0.2%) were of unknown BCLC stage. A significantly lower proportion of NBNC-HCC patients were in BCLC stages 0–A compared to HBV- or HCV-related HCC patients (p < 0.001).
4. Discussion
In this study, we demonstrated that overall 51.2% of patients with HCC had elevated AFP levels. The proportion of patients with an elevated AFP level was stationary in the period from 2011 to 2020. The proportion of patients with BCLC stages 0–A HCC decreased from 2011 to 2020, whereas the proportion of patients with NBNC-HCC increased in the same period. Furthermore, the proportion of patients with early-stage HCC (i.e., BCLC stages 0–A) was lower for NBNC-HCC than for HBV- or HCV-related HCC. Our previous study reported that the HCC cases in our institution accounted for 9.8% of the total cases at the national level [18].
Independent factors associated with elevated AFP levels included female gender, increased tumor size, multiple tumors, BCLC stages B–D, cirrhosis, total bilirubin > 1.4 mg/dL, and viral etiology. Advanced tumor stage and viral etiology were associated with elevated AFP levels. Between 2011 and 2020, the proportion of patients with NBNC-HCC increased (which would have led to a decreasing trend in AFP elevation), which was counterbalanced by the decreased proportion of patients with BCLC stages 0–A HCC (which would have led to an increasing trend in AFP elevation). Ultimately, the proportion of patients with elevated AFP levels was stationary between the years 2011 and 2020 in this study. Furthermore, the decreasing proportion of patients with BCLC stages 0–A HCC during this period may be due to the concurrent increase in the proportion of patients with NBNC-HCC, because the BCLC stages 0–A were less frequently found in NBNC-HCC patients compared to HBV- or HCV-related HCC patients, a result that agrees with the findings of a previous study [19]. In that study, patients with NBNC-HCC presented with larger tumors and at later stages of disease compared to patients with virus-related HCC [19]. This result may be due to the low rate of HCC surveillance in NBNC-HCC patients; furthermore, a significant proportion of NBNC-HCC patients could be NAFLD-related cases [20].
A recent study from the US reported an overall 62.6% of HCC patients with elevated AFP levels (i.e., ≥20 ng/mL) at the time of diagnosis. Between 2010 and 2017, there was a decline in the percentage of HCC patients with elevated AFP levels (68.2% vs. 57.5%). Furthermore, the decline was most evident among patients with early-stage tumors (i.e., seventh edition AJCC stage 1), from 55.7% in 2010 to 40.7% in 2017. However, the authors did not investigate the potential cause of these results. They assumed that these results were likely due to the increasing trend in early-stage tumor detection and the shift from viral to nonviral etiology [6]. In contrast, in this study, an overall 51.2% of patients with HCC had elevated AFP levels. The proportion of patients with elevated AFP levels was stationary in the period from 2011 to 2020 for all patients and across different AJCC stages in the present study. However, it is unclear why there is a discrepancy between this study and the US study [6].
Previous studies have shown that female gender, viral etiology, severe underlying liver disease, and advanced tumor stage are independently associated with elevated levels of AFP [5,6], findings that are compatible with those of the present study. Interestingly, female gender is associated with elevated AFP levels in the present study and previous large-scale studies [5,6]. However, the underlying mechanism is still unknown. It is well known that severe liver disease and advanced tumor stage are associated with elevated AFP levels [5,6,21]. The link between viral etiology and elevated AFP may be due to the former’s association with cirrhosis. A previous study demonstrated that NAFLD is the leading cause of non-cirrhotic HCC [22].
In Taiwan, HBV is the leading etiology of HCC [18]. In 1984, Taiwan established a universal HBV vaccination program for newborns [23,24]. HBV vaccination has reduced the incidence of HCC in children and adolescents [23,24]. Antiviral therapy for HBV and HCV has been widely used in Taiwan since 2003. Antiviral therapies reduce the incidence of HBV- and HCV-related HCC [25,26]. Consequently, the incidence of HBV- and HCV-related HCC was expected to decrease. Indeed, of the 3843 HCC patients from five medical centers in Taiwan enrolled by Chang et al. in their study during 2005–2011, only 10.7% had NBNC-HCC [27], in contrast to 20.4% in 2011 and the gradual rise to 28.7% in 2020 in the present study. Therefore, we infer that the universal HBV vaccination program for newborns and the widely employed antiviral therapy for HBV and HCV have resulted in the decreasing incidence of HBV- and HCV-related HCC in Taiwan.
The etiologies of NBNC-HCC may be AUD and NAFLD; other etiologies, such as autoimmune hepatitis, primary biliary cirrhosis, and primary sclerosing cholangitis, related to chronic liver disease are rare [22]. Patients with NBNC-HCC have an increased risk of metabolic comorbidities [28], which implies that a significant proportion of NBNC-HCC cases could be NAFLD-related.
The incidence of HCC attributed to a nonviral etiology (mainly NAFLD) is rising [29,30]; in addition, it is associated with a decline in the percentage of patients with elevated AFP levels. Other tumor markers more specific to NBNC-HCC are needed for HCC surveillance in this population. Another available tumor marker for HCC surveillance is des-γ-carboxyprothrombin (DCP), which is recommended in clinical practice guidelines by the Japan Society of Hepatology [31]. Previous studies have demonstrated that elevated DCP might be a diagnostic marker for NBNC-HCC [32,33].
Due to the obesity pandemic, the challenge is how to screen for HCC in patients with NAFLD. The American Gastroenterological Association (AGA) guidelines recommend that screening for HCC should be considered for cirrhotic patients due to NAFLD. When the quality of ultrasound is suboptimal for HCC screening (e.g., due to obesity), future surveillance should be performed using CT or MRI, with or without determining the AFP level, every 6 months [34]. However, the cost-effectiveness of HCC surveillance, if CT or MRI replaces ultrasound, remains unknown [34].
Modern molecular biology-based technologies (e.g., liquid biopsy) hold considerable promise for early diagnosis of HCC. To date, there are still no US Food and Drug Administration (FDA)-approved liquid biopsy assays for HCC, mainly due to the lack of survival benefit of such assays [35].
The strength of the present study is the use of a large cohort of patients with HCC with prospectively collected data and limited missing data. However, the study has several limitations. First, we did not use the Alcohol Use Disorders Inventory Test (AUDIT) [36] or AUDIT-C [37,38] (which is recommended by the European Association for the Study of the Liver (EASL) guidelines [39]) to screen HCC patients for AUD. In contrast, we reviewed medical records and used habitual drinking to define AUD, which may underestimate the prevalence of AUD in the present study. Second, the study lacked data on antiviral therapy for HBV and HCV, which can affect AFP levels. Previous studies have shown that the cutoff values of AFP for HCC surveillance are lower in HBV-related cirrhosis patients receiving nucleos(t)ide analogue therapy [40] and HCV-related cirrhosis patients treated with DAAs [41]. Third, the study lacked data on HCV ribonucleic acid (RNA). A proportion of patients with anti-HCV-antibody-positive status could have experienced a past episode of resolved HCV infection. Fourth, etiologies other than HBV, HCV, and AUD were unavailable. Furthermore, we also did not have data on hepatic steatosis and metabolic comorbidities [42]. Therefore, we could not define NAFLD in the present study. Finally, this is a retrospective study.
5. Conclusions
In the past 10 years, a stationary trend in elevated serum AFP levels in HCC patients was noted in this cohort from an HBV-endemic area. This result may be due to the proportion of patients with early-stage HCC decreasing and the proportion of patients with NBNC-HCC increasing during this period. Furthermore, advanced tumor stage, severe underlying liver disease, female gender, and viral etiology were associated with elevated AFP in HCC patients.
Acknowledgments
The authors thank the Cancer Center, Kaohsiung Chang Gung Memorial Hospital for the provision of HCC registry data. The authors thank Chih-Yun Lin and Nien-Tzu Hsu and the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistics work.
Author Contributions
Conceptualization, Y.-H.Y. and C.-C.W.; methodology, Y.-H.Y.; software, C.-Y.L.; validation, all authors; formal analysis, C.-Y.L.; investigation, Y.-H.Y.; resources, C.-C.W.; data curation, all authors; writing—original draft preparation, Y.-H.Y.; writing—review and editing, C.-C.W.; visualization, all authors; supervision, all authors; project administration, Y.-H.Y.; funding acquisition, Y.-H.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital approved this study (Reference number: 202201189B0) and waived the need for informed consent.
Informed Consent Statement
The Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital waived the need for informed consent due to the retrospective and observational nature of the study design.
Data Availability Statement
The data can be shared up on request.
Conflicts of Interest
The authors have no conflict of interest.
Funding Statement
This study was supported by Grant CMRPG8L0181 from the Kaohsiung Chang Gung Memorial Hospital, Taiwan.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Singal A.G., Pillai A., Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis. PLoS Med. 2014;11:e1001624. doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrero J.A., Kulik L.M., Sirlin C.B., Zhu A.X., Finn R.S., Abecassis M.M., Roberts L.R., Heimbach J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 4.Tzartzeva K., Obi J., Rich N.E., Parikh N.D., Marrero J.A., Yopp A., Waljee A.K., Singal A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706–1718.e1. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannini E.G., Sammito G., Farinati F., Ciccarese F., Pecorelli A., Rapaccini G.L., Di Marco M., Caturelli E., Zoli M., Borzio F., et al. Italian Liver Cancer (ITA.LI.CA) Group. Determinants of alpha-fetoprotein levels in patients with hepatocellular carcinoma: Implications for its clinical use. Cancer. 2014;120:2150–2157. doi: 10.1002/cncr.28706. [DOI] [PubMed] [Google Scholar]
- 6.Vipani A., Lauzon M., Luu M., Roberts L.R., Singal A.G., Yang J.D. Decreasing Trend of Serum α-Fetoprotein Level in Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2022;20:1177–1179.e4. doi: 10.1016/j.cgh.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global Burden of Disease Liver Cancer Collaboration. Akinyemiju T., Abera S., Ahmed M., Alam N., Alemayohu M.A., Allen C., Al-Raddadi R., Alvis-Guzman N., Amoako Y., et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanwal F., Kramer J., Asch S.M., Chayanupatkul M., Cao Y., El-Serag H.B. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996–1005.e1. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J., Zou B., Yeo Y.H., Feng Y., Xie X., Lee D.H., Fujii H., Wu Y., Kam L.Y., Ji F., et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019;4:389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 11.American Joint Committee on Cancer . In: American Joint Committee on Cancer Staging Manual. 7th ed. Edge S.B., Byrd D.R., Compton C.C., Fritz A.G., Greene F.L., Rotti A. III, editors. Springer; New York, NY, USA: 2010. p. 175. [Google Scholar]
- 12.Llovet J.M., Bru C., Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 13.Pugh R.N., Murray-Lyon I.M., Dawson J.L., Pietroni M.C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 14.Everhart J.E., Wright E.C., Goodman Z.D., Dienstag J.L., Hoefs J.C., Kleiner D.E., Ghany M.G., Mills A.S., Nash S.R., Govindarajan S., et al. Prognostic value of Ishak fibrosis stage: Findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology. 2010;51:585–594. doi: 10.1002/hep.23315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiha G., Ibrahim A., Helmy A., Sarin S.K., Omata M., Kumar A., Bernstien D., Maruyama H., Saraswat V., Chawla Y., et al. Asian-Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non-invasive assessment of hepatic fibrosis: A 2016 update. Hepatol. Int. 2017;11:1–30. doi: 10.1007/s12072-016-9760-3. [DOI] [PubMed] [Google Scholar]
- 16.Mazzaferro V., Regalia E., Doci R., Andreola S., Pulvirenti A., Bozzetti F., Montalto F., Ammatuna M., Morabito A., Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 17.Lüdecke D. sjPlot: Da ta Visualization for Statistics in Social Science. R Package Version 2.8.8. 2021. [(accessed on 1 January 2020)]. Available online: https://CRAN.R-project.org/package¼sjPlot.
- 18.Lin S.H., Lin C.Y., Hsu N.T., Yen Y.H., Kee K.M., Wang J.H., Hu T.H., Chen C.H., Hung C.H., Chen C.H., et al. Reappraisal of the roles of alpha-fetoprotein in hepatocellular carcinoma surveillance using large-scale nationwide database and hospital-based information. J. Formos. Med. Assoc. 2022;121:2085–2092. doi: 10.1016/j.jfma.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Jun T.W., Yeh M.L., Yang J.D., Chen V.L., Nguyen P., Giama N.H., Huang C.F., Hsing A.W., Dai C.Y., Huang J.F., et al. More advanced disease and worse survival in cryptogenic compared to viral hepatocellular carcinoma. Liver Int. 2018;38:895–902. doi: 10.1111/liv.13613. [DOI] [PubMed] [Google Scholar]
- 20.Chen V.L., Yeh M.L., Yang J.D., Leong J., Huang D.Q., Toyoda H., Chen Y.L., Guy J., Maeda M., Tsai P.C., et al. Effects of Cirrhosis and Diagnosis Scenario in Metabolic-Associated Fatty Liver Disease-Related Hepatocellular Carcinoma. Hepatol. Commun. 2020;5:122–132. doi: 10.1002/hep4.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson P., Duan Z., Kramer J., Davila J.A., Tyson G.L., El-Serag H.B. Determinants of serum alpha-fetoprotein levels in hepatitis C-infected patients. Clin. Gastroenterol. Hepatol. 2012;10:428–433. doi: 10.1016/j.cgh.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gawrieh S., Dakhoul L., Miller E., Scanga A., deLemos A., Kettler C., Burney H., Liu H., Abu-Sbeih H., Chalasani N., et al. Characteristics, aetiologies and trends of hepatocellular carcinoma in patients without cirrhosis: A United States multicentre study. Aliment. Pharmacol. Ther. 2019;50:809–821. doi: 10.1111/apt.15464. [DOI] [PubMed] [Google Scholar]
- 23.Chang M.H., Chen C.J., Lai M.S., Hsu H.M., Wu T.C., Kong M.S., Liang D.C., Shau W.Y., Chen D.S. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 24.Chang M.H., You S.L., Chen C.J., Liu C.J., Lee C.M., Lin S.M., Chu H.C., Wu T.C., Yang S.S., Kuo H.S., et al. Taiwan Hepatoma Study Group. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: A 20-year follow-up study. J. Natl. Cancer Inst. 2009;101:1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 25.Liaw Y.F., Sung J.J., Chow W.C., Farrell G., Lee C.Z., Yuen H., Tanwandee T., Tao Q.M., Shue K., Keene O.N., et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 26.Beste L.A., Green P., Berry K., Belperio P., Ioannou G.N. Hepatitis C-Related Hepatocellular Carcinoma Incidence in the Veterans Health Administration After Introduction of Direct-Acting Antivirals. JAMA. 2020;324:1003–1005. doi: 10.1001/jama.2020.10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang I.C., Huang S.F., Chen P.J., Chen C.L., Chen C.L., Wu C.C., Tsai C.C., Lee P.H., Chen M.F., Lee C.M., et al. The hepatitis viral status in patients with hepatocellular carcinoma: A study of 3843 patients from Taiwan Liver Cancer Network. Medicine. 2016;95:e3284. doi: 10.1097/MD.0000000000003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu P.Y., Hsu C.T., Yeh M.L., Huang C.F., Huang C.I., Liang P.C., Lin Y.H., Hsieh M.Y., Wei Y.J., Hsieh M.H., et al. Early Fibrosis but Late Tumor Stage and Worse Outcomes in Hepatocellular Carcinoma Patients without Hepatitis B or Hepatitis C. Dig. Dis. Sci. 2020;65:2120–2129. doi: 10.1007/s10620-019-05938-3. [DOI] [PubMed] [Google Scholar]
- 29.El-Serag H.B., Kanwal F., Feng Z., Marrero J.A., Khaderi S., Singal A.G. Risk factors for cirrhosis in contemporary hepatology practices-findings from the Texas hepatocellular carcinoma Consortium cohort. Gastroenterology. 2020;159:376–377. doi: 10.1053/j.gastro.2020.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang D.Q., El-Serag H.B., Loomba R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021;18:223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kokudo N., Takemura N., Hasegawa K., Takayama T., Kubo S., Shimada M., Nagano H., Hatano E., Izumi N., Kaneko S., et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 2019;49:1109–1113. doi: 10.1111/hepr.13411. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi M., Yamada S., Takano N., Okamura Y., Takami H., Inokawa Y., Sonohara F., Tanaka N., Shimizu D., Hattori N., et al. Different Characteristics of Serum Alfa Fetoprotein and Serum Des-gamma-carboxy Prothrombin in Resected Hepatocellular Carcinoma. In Vivo. 2021;35:1749–1760. doi: 10.21873/invivo.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taura N., Ichikawa T., Miyaaki H., Ozawa E., Tsutsumi T., Tsuruta S., Kato Y., Goto T., Kinoshita N., Fukushima M., et al. Frequency of elevated biomarkers in patients with cryptogenic hepatocellular carcinoma. Med. Sci. Monit. 2013;19:742–750. doi: 10.1016/S0168-8278(12)60771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loomba R., Lim J.K., Patton H., El-Serag H.B. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2020;158:1822–1830. doi: 10.1053/j.gastro.2019.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson P., Zhou Q., Dao D.Y., Lo Y.M.D. Circulating biomarkers in the diagnosis and management of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022;19:670–681. doi: 10.1038/s41575-022-00620-y. [DOI] [PubMed] [Google Scholar]
- 36.Saunders J.B., Aasland O.G., Babor T.F., de la Fuente J.R., Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 37.Bush K., Kivlahan D.R., McDonell M.B., Fihn S.D., Bradley K.A. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 38.Gual A., Segura L., Contel M., Heather N., Colom J. Audit-3 and audit-4: Effectiveness of two short forms of the alcohol use disorders identification test. Alcohol Alcohol. 2002;37:591–596. doi: 10.1093/alcalc/37.6.591. [DOI] [PubMed] [Google Scholar]
- 39.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J. Hepatol. 2018;69:154–181. doi: 10.1016/j.jhep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Su T.H., Peng C.Y., Chang S.H., Tseng T.C., Liu C.J., Chen C.L., Liu C.H., Yang H.C., Chen P.J., Kao J.H. Serum PIVKA-II and alpha-fetoprotein at virological remission predicts hepatocellular carcinoma in chronic hepatitis B related cirrhosis. J. Formos. Med. Assoc. 2022;121:703–711. doi: 10.1016/j.jfma.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Degasperi E., Perbellini R., D’Ambrosio R., Uceda Renteria S.C., Ceriotti F., Perego A., Orsini C., Borghi M., Iavarone M., Bruccoleri M., et al. Prothrombin induced by vitamin K absence or antagonist-II and alpha foetoprotein to predict development of hepatocellular carcinoma in Caucasian patients with hepatitis C-related cirrhosis treated with direct-acting antiviral agents. Aliment. Pharmacol. Ther. 2022;55:350–359. doi: 10.1111/apt.16685. [DOI] [PubMed] [Google Scholar]
- 42.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be shared up on request.