Abstract
Salmonella requires genes on the Salmonella pathogenicity island 1 (SPI1) for the intestinal phase of infection in several models of pathogenesis. In Salmonella enterica serovar Typhimurium, most SPI1 genes are arranged in operons that are coordinately regulated by the SPI1-encoded protein HilA. In the past, it has been shown that HilA directly activates two promoters on SPI1, PinvF-1 and PprgH. PinvF-1 contains a HilA binding site, termed a HilA box, that is necessary and sufficient for activation by HilA. The HilA box is 17 nucleotides long and contains a direct repeat comprised of two hexamers separated by 5 nucleotides, centered at −45 relative to the start site of transcription. PprgH also contains a HilA box, and here we investigate its role at PprgH. We have found that the HilA box is necessary, but not sufficient, for HilA-dependent activation of PprgH. Instead, half-site-like hexamers outside the HilA box appear to be required for HilA-dependent activation of PprgH, even though HilA binds to the HilA box in the absence of these hexamers. Thus, although HilA-dependent activation of PinvF-1 and PprgH coordinates the expression of the structural genes for a type III secretion apparatus and the effectors secreted by that apparatus, it is also possible that mechanisms not apparent under in vitro inducing conditions could separate the expression of invFGEABC-spaMNOPQRS-sicA-sipBCDA-iacP-sicP-sptP and prgHIJK-orgABC.
The serovar Typhimurium of Salmonella enterica is a facultative intracellular parasite that can cause gastroenteritis and enteric fevers, depending on the particular host-parasite combination. One virulence determinant important for Salmonella's survival in (and exploitation of) the host intestine is the type III secretion system 1 (TTSS-1) (19, 31, 32, 34). Electron micrographs reveal that the type III secretion apparatus resembles the flagellar basal body (22). The appearance of the apparatus has also been likened to a syringe with a long, presumably hollow, needle passing from inside the basal structure and out into the supernatant. The effectors secreted by TTSS-1 contribute to a diverse array of in vitro phenotypes and are required for virulence in both the mouse model of typhoid fever and the bovine model of gastroenteritis (10, 42).
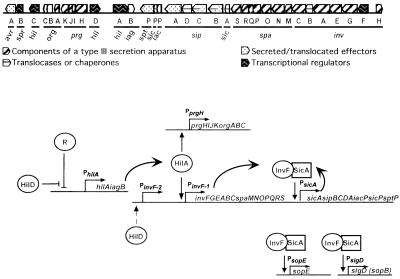
The more than 15 proteins that are thought to comprise the type III secretion apparatus of TTSS-1 are all encoded by genes of the Salmonella pathogenicity island 1 (SPI1) (Fig. 1A). The core of the complex, probably found embedded in the inner membrane and crossing the periplasmic space, is comprised of PrgH and PrgK, while the barrel of the syringe is predominantly PrgI, a protein that requires PrgJ for its stability (20). The genes encoding these proteins are in an operon (prgHIJK-orgABC) at one end of SPI1 (20–23) (Fig. 1A). All type III secretion systems include a secretin family outer membrane protein, possibly forming a channel through which the needle of the type III secretion apparatus passes. In the case of TTSS-1, the protein InvG probably serves this purpose (7, 8). InvG is also encoded by SPI1, but at the opposite end of the more than 40-kb pathogenicity island (Fig. 1A). InvG is encoded by an operon with 15 other genes: invFGEABC-spaMNOPQRS-sicA-sipBCDA-iacP-sicP-sptP (reviewed in reference 10) (C. A. Lee, unpublished data). With the exception of InvF and InvB, the other Inv and Spa proteins are probably directly involved in secretion (reviewed in reference 10).
FIG. 1.
HilA-dependent regulation of the TTSS-1. (A) A map of SPI1. Each gene is depicted, approximately to scale, as an arrow, with the direction of the arrow indicating the direction of transcription. The shading of each gene reflects the putative or demonstrated function(s) of its protein product. The functions of the proteins encoded by the unshaded genes are unknown. The part of the island containing sitABCD is not included in this depiction. The sit operon maps to the left of avrA. (B) HilA-dependent cascade of transcriptional activation. Under conditions that do not favor TTSS-1 gene expression, the hilA promoter is repressed by a putative regulatory protein (R). When environmental conditions become favorable for TTSS-1 gene expression, HilD derepresses the hilA promoter (14, 15, 37, 40, 41). HilA activates PinvF-1 (formerly PinvF) and PprgH and, thus, the expression of TTSS-1 structural genes (26 and this work). InvF, encoded by the first gene in the PinvF-1 transcript, interacts with SicA and activates the expression of effectors on and off SPI1 (9, 11, 12). Under inducing conditions, HilD also activates a second promoter far upstream of the invF translation start site, termed PinvF-2 (dotted arrow). PinvF-2 makes a minor contribution to invF production under inducing conditions in vitro (S. Akbar, unpublished data).
The production of TTSS-1 proteins is not constitutive in vitro and is probably regulated in vivo as well. In vitro inducing conditions that result in the optimal expression of TTSS-1 phenotypes include high osmolarity (10 g of NaCl/liter), low aeration, and slightly basic pH (2, 27). Transcriptional regulation of TTSS-1 genes is a primary mechanism for controlling the production of TTSS-1 factors in response to environmental and physiological cues (2, 10, 27, 28). The expression of SPI1 operons encoding components of the TTSS-1 apparatus and some of its effectors requires HilA, a transcription factor encoded by SPI1 (Fig. 1B) (1, 26).
HilA is an OmpR/ToxR family transcriptional regulator by virtue of the DNA binding and activation domain in its N terminus (1, 26). Consensus binding sites for OmpR/ToxR proteins have been difficult to agree upon, but the emerging trend is that they usually contain degenerate direct repeats (reviewed in reference 29). The OmpR DNA binding domain probably interacts with DNA as a dimer such that each monomer faces the same direction (18). Members of this family, however, can have binding sites that are more difficult to recognize as direct repeats, and they often make large footprints on DNA. Furthermore, in some cases they seem to require sites at different promoters that are completely unrelated in sequence (see, for example, references 25 and 35). Finally, many family members are posttranslationally modified or membrane associated, complicating biochemical analyses considerably (29).
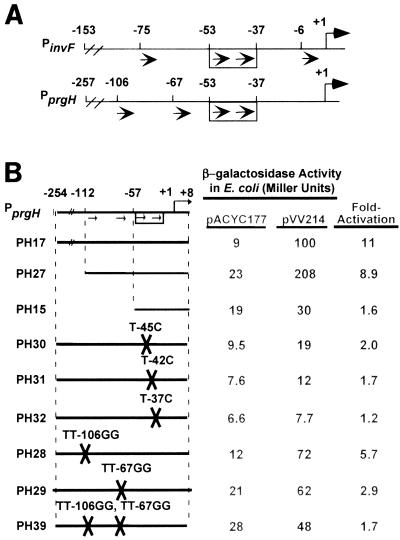
HilA activates transcription at PinvF-1 directly through binding to an element termed the HilA box (Fig. 1B) (26). The HilA box is a 17-nucleotide sequence comprised of two tandemly arranged hexamers (a direct repeat) separated by 5 nucleotides. PinvF-1 (−57 to +10), which contains only 3 nucleotides upstream of the HilA box, is sufficient for HilA-dependent activation at PinvF-1. Deletion of sequences upstream of −40 results in a complete loss of activation by HilA. Furthermore, two artificial promoters containing PinvF-1 (−57 to −30) sequences fused in frame to unrelated (−29 to +10) sequences are activated by HilA. Finally, double-stranded DNAs containing the HilA box bind to membrane-associated HilA in vitro. Thus, the HilA box in PinvF-1 is both necessary and sufficient for activation by HilA. Two additional hexamers similar in sequence to a half-site of the HilA box are found at −75 (TTTAAT) and −6 (TTACAT) in PinvF-1 (Fig. 2A). PinvF-1 (−78 to +10) is twofold more activated than a minimal PinvF-1 encoding only the HilA box through the +10, suggesting that the half-site-like hexamer at −75 may contribute to HilA-dependent transcription even though it is not required. In contrast, the half-site-like hexamer at −6 appears to be fortuitous and is not involved in HilA-dependent activation (26 and unpublished data).
FIG. 2.
The cis elements in PprgH required for activation by HilA. (A) PinvF-1 and PprgH each contain a HilA box at the same position relative to the +1 of transcription. The +1 accompanied by a bent arrow above the line indicates the transcription start site of each promoter. The arrows inside the rectangle between −53 and −37 show the HilA box, composed of a hexameric direct repeat (consensus TTTCAT) separated by 5 nucleotides. Two copies of a half-site-like hexamer are found in each promoter outside the HilA box, as indicated by the additional arrows below each line. (B) The HilA box is necessary, but not sufficient, for HilA-dependent activation of PprgH. PCR products or oligonucleotides carrying WT and mutant PprgH sequences were cloned into the pAH125oriT reporter system and then placed in the E. coli chromosome in single copy (see Materials and Methods). Strains were transformed with either pACYC177 or pVV214 (HilA+) and then grown to early log phase under oxygenating conditions on a culture wheel at 37°C. Values represent the average of at least two assays performed on three independent transformants of each reporter strain and were not necessarily performed on the same day. Fold activation was calculated as the ratio of units expressed in a pVV214-containing strain to that expressed in a pACYC177-containing strain and were calculated not from the average units reported in the Miller units columns but from raw data. All standard deviations are equal to or less than 15% of the reported values.
A second HilA-activated promoter, PprgH, also has a HilA box at the same position relative to the start (+1) of transcription. We have hypothesized that HilA activates PprgH through a direct interaction with its HilA box. Like PinvF-1, PprgH has two half-site-like hexamers outside the HilA box, but in this case they are located at −106 (TTTAAT) and −67 (TTTTAT) (Fig. 2A). In this paper, we report our investigations of the HilA box and the upstream half-site-like hexamers in PprgH. We have found that the HilA box is required for activation of PprgH. By using an in vivo binding assay, we found that the HilA box of PprgH is also likely to be the site of a direct interaction between HilA and PprgH DNA. Nevertheless, the HilA box is not sufficient for activation of PprgH. Curiously, our results indicate that sequences between the HilA box and the +1 of transcription affect the ability of the HilA box from PprgH to serve as an upstream activating site in the absence of distal PprgH sequences. In contrast, the HilA box from PinvF-1 is a strong upstream activating site in every promoter we have tested, irrespective of the sequences between the HilA box and +1. Although we cannot detect differential regulation of PinvF-1 and PprgH in vitro, we speculate that the two promoters could be regulated independently in vivo.
MATERIALS AND METHODS
Strains and growth of bacteria.
S. enterica serovar Typhimurium and Escherichia coli strains used in this study are listed in Table 1 (4, 5). Bacterial cultures were grown at 37°C in Luria-Bertani (LB) medium comprised of 0.5% Bacto-yeast extract, 1% Bacto-tryptone, and 1% NaCl. When appropriate, the medium was supplemented with antibiotics as follows: 100 to 200 μg of ampicillin/ml and 10 to 25 μg of kanamycin/ml. hilA-mychis expression was induced from pCH112 by adding 20% arabinose to a final concentration of 0.2% to early log cultures. β-Galactosidase assays were performed on Salmonella cultures grown under activating (limited oxygen, high salt) conditions as previously described (24) and on mid-log E. coli cultures grown with high aeration on a culture wheel; the β-galactosidase activities were then quantified by the Miller method (30).
TABLE 1.
S. enterica serovar Typhimurium and E. coli strains and plasmids used in this study
| Plasmid or strain | Description | Reference or source |
|---|---|---|
| Plasmids | ||
| pACYC177 | Low-copy cloning vector, Ampr Kanr | 5 |
| pVV214 | hilA cloned in pACYC177, Ampr | 1 |
| pAH125oriT | lacZ reporter vector, Kanr | 17 |
| pPH17 | PprgH −254 to +32 cloned in pAH125oriT | 26 |
| pPH27 | PprgH −112 to +8 cloned in pAH125oriT | This work |
| pPH15 | PprgH −57 to +8 cloned in pAH125oriT | This work |
| pPH30 | PprgH −254 to +32 cloned in pAH125oriT; T→ 45C mutation | This work |
| pPH31 | PprgH −254 to +32 cloned in pAH125oriT; T→ 42C mutation | This work |
| pPH32 | PprgH −254 to +32 cloned in pAH125oriT; T→ 37C mutation | This work |
| pPH28 | PprgH −254 to +32 cloned in pAH125oriT; TT→ 106GG mutation | This work |
| pPH29 | PprgH −254 to +32 cloned in pAH125oriT; TT→ 67GG mutation | This work |
| pPH39 | PprgH −254 to +32 cloned in pAH125oriT; TT→ 106GG, TT→ 67GG mutations | This work |
| pPH24 | PinvF (−57 to −30)/PprgH (−29 to +8) cloned in pAH125oriT | This work |
| pPH25 | PprgH (−57 to −30)/PinvF-1 (−29 to +8) cloned in pAH125oriT | This work |
| pPH26 | PprgH (−57 to +8) with five point mutations cloned in pAH125oriT | This work |
| pPH34 | DNA binding reporter; -35INVWT-10 cloned in pAH125oriT | This work |
| pPH35 | DNA binding reporter; -35INV(T→ 37C)-10 cloned in pAH125oriT | This work |
| pPH36 | DNA binding reporter; -35INV(T→ 42C)-10 cloned in pAH125oriT | This work |
| pPH37 | DNA binding reporter; -35INV(T→ 45C)-10 cloned in pAH125oriT | This work |
| pPH38 | DNA binding reporter; -35PRGWT-10 cloned in pAH125oriT | This work |
| pINT-ts | Temperature-sensitive Ampr plasmid that supplies integrase for pAH125oriT | 17 |
| pBAD-myc-his | Cloning vector to make C-terminal myc his-tagged proteins expressed | Invitrogen |
| under arabinose control, Ampr | ||
| pCH112 | hilA open reading frame cloned in pBAD-myc-his | 26 |
| Strains | ||
| S. enterica serovar Typhimurium | ||
| SL1344 | hisG Strr | |
| SL5283 | r− m+leu galE503 Strr | 4 |
| PL667 | EE637, relevant genotype SL1344 invF::lacZY pBAD-myc-his | 1 |
| PL673 | EE656, relevant genotype SL1344 prgH::lacZY pBAD-myc-his | 1 |
| E. coli K-12 | ||
| BW25142 | lacIqrrnBT14 ΔlacZWJ16 ΔphoBR580 hsdR514 ΔaraBADAH33 ΔrhaBADLD78galU95 endABT333uidA(ΔMluI)::pir-116 recA1 | 17 |
| BW21355 | F−ΔlacZ74 derivative of MG1655 | W. Jiang and B. Wanner, unpublished results |
| PL92 | BW21355 containing pINT-ts, Ampr | 26 |
| PL372 | pPH17 integrated at lambda att in BW21355 | This work |
| PL457 | pPH27 integrated at lambda att in BW21355 | This work |
| PL283 | pPH15 integrated at lambda att in BW21355 | This work |
| PL484 | pPH30 integrated at lambda att in BW21355 | This work |
| PL485 | pPH31 integrated at lambda att in BW21355 | This work |
| PL486 | pPH32 integrated at lambda att in BW21355 | This work |
| PL482 | pPH28 integrated at lambda att in BW21355 | This work |
| PL483 | pPH29 integrated at lambda att in BW21355 | This work |
| PL650 | pPH39 integrated at lambda att in BW21355 | This work |
| PL440 | pPH24 integrated at lambda att in BW21355 | This work |
| PL441 | pPH25 integrated at lambda att in BW21355 | This work |
| PL442 | pPH26 integrated at lambda att in BW21355 | This work |
| PL487 | pPH34 integrated at lambda att in BW21355 | This work |
| PL532 | pPH35 integrated at lambda att in BW21355 | This work |
| PL534 | pPH36 integrated at lambda att in BW21355 | This work |
| PL536 | pPH37 integrated at lambda att in BW21355 | This work |
| PL538 | pPH38 integrated at lambda att in BW21355 | This work |
Crossover PCR.
Crossover PCR was performed using a modified protocol from the thesis of Dereth Phillips from George Church's lab (D. Phillips and G. Church, personal communication; http://arep.med.harvard.edu/labgc/pko3_pcr.html). Two separate 50-μl reaction mixtures were used to amplify overlapping fragments of PprgH while at the same time introducing point mutations into the amplicon. The reaction mixtures contained 20 pmol of each primer (e.g., ebe89 and ebe91 or ebe90 and ebe96), 10 μl of 10× Extaq buffer, 4 μl of a deoxynucleoside triphosphate stock containing each nucleoside triphosphate at 2 mM (TaKaRa), 1 μl of genomic template DNA, exTaq polymerase (TaKaRa), and water. After these reactions, the flanks were crossed over by taking 1 μl from each of these PCRs and using them together as template in a second round of PCR, this time using ebe46 and ebe47 to add EcoRI and PstI sites, respectively, to clone the resulting product into pAH125oriT, as described previously (26).
Construction of strains.
Plasmid DNA was electroporated into either S. enterica serovar Typhimurium or E. coli by standard methods. Plasmids transferred from E. coli into S. enterica serovar Typhimurium were first passaged through the restriction-negative modification-positive strain listed in Table 1 (4).
pAH125oriT reporter construction.
To construct pAH125oriT reporter strains, oligonucleotides or PCR products (Table 2) were digested with EcoRI and PstI and ligated into pAH125oriT vector that had also been digested with EcoRI and PstI. Ligation mixes were transformed into E. coli BW25142, a strain with a pir-116 mutation that allows the plasmid to be maintained at high copy, and were plated on kanamycin (25 μg/ml) (17). Transformants containing proper inserts were identified by colony PCR using primers ebe20 and ebe22 (24), which flank the multiple cloning site in pAH125oriT. DNA was isolated from candidates using Qiagen miniprep columns or a Qiagen Biorobot, and then that DNA was sequenced by the Microbiology Core Facility to confirm the presence of correct inserts. Correct candidates were then electroporated into E. coli BW21355 (lac mutant) that already contained the plasmid pINT in order to construct chromosomal integrants and were plated on kanamycin (10 μg/ml) (17). Colonies were patched to identify clones that were ampicillin sensitive and so had lost pINT, and the presence of reporters in single copy was confirmed using primers P1, P2, P3, and P4 (17). Reporter strains with a single insertion were then transformed with either pACYC177, pVV214, pBAD vector, or pCH112 as indicated in Results.
TABLE 2.
Oligonucleotides used in this work
| Name | Sequence (5′ to 3′) | Purposea | Reference |
|---|---|---|---|
| ebe44 | GCACTTTTCATTCTATTTTCATCAGGAATCCCTGTGTCCTGTGCGGTAATCTGCTGCTATCGAGAAG | pPH15 top strand | This work |
| ebe45 | AATTCTTCTCGATAGCAGCAGATTACCGCACAGGACACAGGGATTCCTGATGAAAATAGAATGAAAAGTGCTGCA | pPH15 bottom strand | This work |
| ebe46 | GAACTGCAGTTCCTTACTGGTATCCTAC | PCR forward pPH17 | This work |
| ebe47 | CGGAATTCTTCTCGATAGCAGCAGATTACC | PCR reverse pPH17 | This work |
| ebe71 | GTCAGTTTCATAATGATTGCATCAGGAATCCCTGTGTCCTGTGCGGTAATCTGCTGCTATCGAGAAG | pPH24 top strand | This work |
| ebe72 | AATTCTTCTCGATAGCAGCAGATTACCGCACAGGACACAGGGATTCCTGATGCAATCATTATGAAACTGACTGCA | pPH25 bottom strand | This work |
| ebe73 | GCACTTTTCATTCTATTTTCATCAGGATTTTGCCACCCGCTCCCGGTATTGTTTACATATTAAAATGAG | pPH25 top strand | This work |
| ebe74 | AATTCTCATTTTAATATGTAAACAATACCGGGAGCGGGTGGCAAAATCCTGATGAAAATAGAATGAAAAGTGCTGCA | pPH25 bottom strand | This work |
| ebe75 | GCACTTTTCATTCTATTTTCATCAGGAATCCCTGTGTCCTGTGCGGTAATCTTTACATATCGAGAAG | pPH26 top strand | This work |
| ebe76 | AATTCTTCTCGATATGTAAAGATTACCGCACAGGACACAGGGATTCCTGATGAAAATAGAATGAAAAGTGCTGCA | pPH26 bottom strand | This work |
| ebe77 | GAACTGCAGGGTTCTTTTAATATGC | PCR forward pPH27 | This work |
| ebe83 | GTTGACATTTCATAATGATTGCATTATAATTTACATAG | pPH34 top strand | This work |
| ebe84 | AATTCTATGTAAATTATAATGCAATCATTATGAAATGTCAACTGCA | pPH34 bottom strand | This work |
| ebe85 | GGTTCTGGTAATATGTGTTG | PCR TT→ 106GG pPH28 | This work |
| ebe86 | CAACACATATTACCAGAACC | PCR TT→ 106GG pPH28 | This work |
| ebe87 | AAATTGAGGTTATTTCTCAC | PCR TT→ 67GG pPH29 | This work |
| ebe88 | GTGAGAAATAACCTCAATTT | PCR TT→ 67GG pPH29 | This work |
| ebe89 | ACTTTTAATTTGCTGCCGGG | PCR-387 prg for crossover | This work |
| ebe90 | TTTCATTCCATTTTCATCAGG | PCR TT→ 45C pPH30 | This work |
| ebe91 | CCTGATGAAAATGGAATGAAA | PCR TT→ 45C pPH30 | This work |
| ebe92 | CATTCTATCTTCATCAGGAATCC | PCR TT→ 42C pPH31 | This work |
| ebe93 | GGATTCCTGATGAAGATAGAATG | PCR TT→ 42C pPH31 | This work |
| ebe94 | TCTATTTTCACCAGGAATCCC | PCR TT→ 37C pPH32 | This work |
| ebe95 | GGGATTCCTGGTGAAAATAGA | PCR TT→ 37C pPH32 | This work |
| ebe96 | TTCACCGACCTGTATTGGCG | PCR +300 prg for crossover | This work |
| ebe102 | GTTGACATTTCATAATGATTGCACTATAATTTACATAG | pPH35 top strand | This work |
| dbe103 | AATTCTATGTAAATTATAGTGCAATCATTATGAAATGTCAACTGCA | pPH35 bottom strand | This work |
| ebe104 | GTTGACATTTCATAATGACTGCATTATAATTTACATAG | pPH36 top strand | This work |
| ebe105 | AATTCTATGTAAATTATAATGCAGTCATTATGAAATGTCAACTGCA | pPH36 bottom strand | This work |
| ebe106 | GTTGACATTTCATAACGATTGCATTATAATTTACATAG | pPH37 top strand | This work |
| ebe107 | AATTCTATGTAAATTATATAATGCAATCGTTATGAAATGTCAACTGCA | pPH37 bottom strand | This work |
| ebe108 | GTTGACATTTCATTCTATTTTCATTATAATTTACATAG | pPH38 top strand | This work |
| ebe109 | AATTCTATGTAAATTATAATGAAAATAGAATGAAATGTCAACTGCA | pPH38 bottom strand | This work |
| P1 | GGCATCACGGCAATATAC | 17 | |
| P2 | ACTTAACGGCTGACATGG | 17 | |
| P3 | ACGAGTATCGAGATGGCA | 17 | |
| P4 | TCTGGTCTGGTAGCAATG | 17 |
P1, P2, P3, and P4 are lambda att/pAH125oriT diagnostic primers.
Western blotting.
Polyclonal antiserum to HilA was raised by injecting rabbits with His-HilA purified from E. coli in the presence of sodium dodecyl sulfate (L. M. Schechter and C. A. Lee, unpublished data). The serum was diluted 1:500 and used as primary antibody to bind to HilA that had been transferred to polyvinylidene difluoride membranes following sodium dodecyl sulfate-polyacrylamide gel electrophoresis through a 10% gel, as described previously (26). Horseradish peroxidase-conjugated anti-rabbit antibody (Sigma) was used as the secondary antibody, and a chemiluminescent detection system was used to detect antibody-protein complexes using X-ray film in a standard protocol that was described elsewhere (38).
RESULTS
The HilA box is not sufficient for HilA-dependent activation of PprgH.
Based on the results with PinvF-1, we hypothesized that the HilA box at PprgH would be necessary and sufficient for activation by HilA. We first cloned three 5′ truncations of PprgH into a lacZ reporter system in E. coli (PH17, PH27, and PH15) (Fig. 2B). The shortest is in PH15 (PprgH, −57 to +8), which contains 3 bp upstream of the HilA box through 8 bp downstream of the start of transcription. The reporters were integrated in single copy in the E. coli chromosome. We then tested whether the reporters are responsive to HilA by transforming the reporter strains with pVV214 (hilA+) or pACYC177 (vector). We hypothesized that all three promoters would be activated by HilA, as is the case of an analogous series of PinvF-1 reporters (26). As predicted, full-length PprgH in PH17 is strongly activated by HilA (by about 11-fold) (Fig. 2B). The removal of sequences upstream of the hexamer at −106 has little effect on the fold activation by HilA (compare PH17 to PH27). In contrast, PH15 is barely activated by HilA (1.5-fold compared to 9- to 11-fold for the longer constructs). The simplest interpretation of these results is that the HilA box of PprgH is not sufficient to activate PprgH. This finding is dramatically different from our analysis of PinvF-1.
The HilA box is required for HilA-dependent activation of PprgH.
Because the HilA box was, unexpectedly, not sufficient for activation of PprgH, we tested whether the HilA box at PprgH is required for HilA-dependent activation. To do so we took advantage of knowledge gained from our previous work with PinvF-1. In full-length PinvF-1, mutations T→45C, T→42C, and T→37C in the HilA box result in promoters that are severely reduced in HilA-dependent activation (26). Thus, we constructed three full-length PprgH reporters with one of these three point mutations in the HilA box (Fig. 2B). At PprgH, these point mutations dramatically reduce activation by HilA, down from 11-fold to 2.0-, 1.7-, and 1.2-fold, respectively (see Fig. 2B, PH30, PH31, and PH32). From these results we conclude that the HilA box is required for activation of PprgH by HilA.
Mutations in the half-site-like hexamers upstream of the HilA box reduce HilA-dependent activation of PprgH.
To test whether the half-site-like hexamers at −106 and −67 are required for HilA-dependent activation, we tested three full-length PprgH promoters with mutations in one or both of these sequences (Fig. 2B). PH28, which has a TT-to-GG mutation in the first nucleotides of the hexamer at −106, loses half of its ability to respond to HilA, while PH29, which has a TT-to-GG mutation in the first nucleotides of the hexamer at −67, is even more severely compromised (Fig. 2B). PH39 has all of these mutations and is no longer activated by HilA. In fact, its phenotype is very similar to that of PH15 (PprgH, −57 to +8) This result strongly suggests that the phenotype of PH15 is owing to the absence of upstream sequences that include the initial T's in each of the half-site-like hexamers.
HilA can be converted to a repressor by interrupting the ς70 recognition hexamers with a HilA box.
The HilA box is necessary, but not sufficient, for activation of PprgH. This led us to hypothesize that HilA may bind less well to the HilA box in PprgH than it does to the HilA box in PinvF-1. We developed an in vivo binding assay to test this hypothesis. Genomic studies of E. coli K-12 have demonstrated that activator binding sites usually do not occur downstream of −30 (16). Moreover, forcing an activator to bind downstream of −30 can repress that promoter's activity (6, 13, 36, 43). In most cases, it appears that repression results from inhibiting the ability of RNA polymerase (RNAP) to bind the promoter by simple steric hindrance. To study HilA binding in vivo, we cloned the HilA box from PinvF-1 between consensus −35 and −10 hexamers that direct ς70-dependent transcription in E. coli. We cloned this construct into the same lacZ reporter system we used throughout this work, resulting in the construct designated -35INVWT-10 (Fig. 3A).
FIG. 3.
HilA behaves as a repressor when the HilA box from invF is cloned between the −35 and −10 hexamers of a ς70-dependent promoter (-35INVWT-10). (A) Diagram of -35INVWT-10. Using long oligonucleotides, the HilA box from PinvF-1 was artificially placed between consensus −35 and −10 hexamers that direct ς70-dependent transcription in E. coli, forming the promoter -35INVWT-10. This promoter was cloned into the pAH125oriT lacZ reporter system and then integrated in single copy in the E. coli chromosome. (B) HilA represses -35INVWT-10. -35INVWT-10 reporter strains containing either pBAD vector or pCH112 (HilA-MycHis+) were diluted 1:200 at time zero into LB containing 0.2% arabinose (see Materials and Methods). The Miller units produced by two independent transformants of each strain were determined for each time point, and a single representative experiment is shown. Strains grown in the absence of arabinose had approximately the same activity as the pBAD plus arabinose result shown (black boxes).
In vitro, HilA binds to the HilA box of PinvF-1, both at its native position and in a double-stranded oligonucleotide containing three consecutive copies of the HilA box separated by 10 nucleotides each (26). This being the case, HilA should bind to and repress -35INVWT-10 by preventing RNAP from binding to the −35 and −10 hexamers. If it does, fold repression is a measure of how well HilA occupies any site we place between the −35 and −10 hexamers. Thus, we made E. coli -35INVWT-10 reporter strains containing either pCH112 (pBAD-HilA- MycHis+) or pBAD vector. In the absence of arabinose, neither pBAD vector nor pCH112 (HilA-MycHis+) has any effect on the expression of -35INVWT-10 (data not shown). Reporter strains containing pBAD vector have a slight increase of activity after 4 h of induction. This increase occurs in -35INVWT-10 when HilA-MycHis+ is not expressed and may reflect the growth phase of the bacterium (data not shown). In contrast, in the presence of the inducer arabinose, pCH112 (HilA-MycHis+) causes a strong reduction of β-galactosidase activity (Fig. 3B).
We also found that repression of -35INVWT-10 by HilA could be titrated away by a high-copy plasmid (pBluescript) containing PinvF-1 promoter sequences. In the presence of a high-copy plasmid containing PinvF-1 (but not in the presence of a control vector), repression of -35INVWT-10 by HilA is significantly reduced (data not shown). Thus, it is the sequence in common between the titration plasmid and the -35INVWT-10 reporter construct, namely the HilA box itself, that is responsible for the HilA-dependent repression of -35INVWT-10.
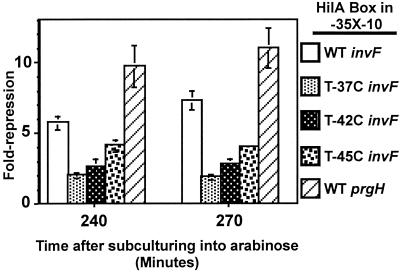
Mutations in the HilA box at positions −37, −42, and −45 cause reduced activation from both PinvF-1 and PprgH. The most severe reduction in activation at PinvF-1 and PprgH is caused by a T→37C change, followed by T→42C, and finally by T→45C (Fig. 2B) (26). To test if these mutations reduce activation by HilA by disrupting HilA binding, we assayed the ability of HilA to repress -35X-10 promoters, where X is a HilA box (derived from PinvF-1) containing a point mutation at one of these positions. To compare the activities of the different promoters, we calculated fold repression by dividing the units expressed by a strain containing pBAD vector by the units expressed by a strain containing pCH112 (HilA-MycHis+), both grown in the presence of arabinose. In all cases, fold repression peaked around 270 min following subculturing. As shown in Fig. 4, all of these mutations in the PinvF-1 HilA box led to a loss of repression by HilA and likely disrupt HilA binding.
FIG. 4.
The HilA box in PprgH is a functional HilA binding site. Strains were constructed and grown as described in the legend to Fig. 3. The nucleotides separating the −35 and −10 hexamers in the reporter encode a WT HilA box from PinvF-1, one of three point mutations in the HilA box from PinvF-1, or a WT copy of the HilA box from PprgH, as indicated in the legend. The mutations are numbered as they are when the HilA box is at its native position; e.g., T→37C is a mutation from T to C at the final T in the HilA box of PinvF-1 (see Fig. 5B for the sequence). Fold repression was calculated by growing the reporter strains, containing either pBAD vector or pCH112 (HilA-MycHis+) in the presence of arabinose, as for Fig. 3, and then dividing the units produced by a pBAD strain by those produced by a pCH112 strain. Values come from a single experiment on three independent colonies of each type. The standard deviations are indicated as error bars.
The prgH HilA box binds to HilA.
To test if HilA binds to the HilA box sequence in PprgH directly, we cloned the prgH HilA box into a repressor-reporter, -35PRGWT-10. As shown in Fig. 4, this reporter is strongly repressed by HilA. Using these results, we infer that the HilA box of PprgH is a functional HilA binding site. It is even possible that the HilA box at PprgH is a better HilA binding site than the HilA box at PinvF-1.
The HilA box from PprgH is a functional cis determinant for HilA-dependent activation.
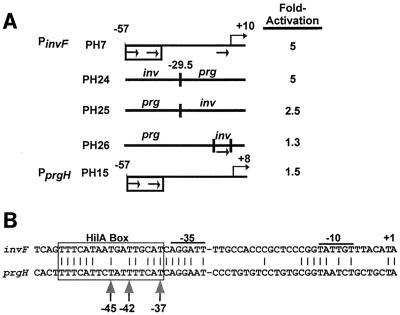
We tested whether the HilA box from PprgH can serve as an upstream activating site by creating heterologous hybrid promoters and testing their response to HilA (Fig. 5A). PH24 has the HilA box and the −35 region from PinvF-1 but has the −29 to +8 sequences from PprgH. It is activated to the same extent as PH7, which contains PinvF-1 (−57 to +10) (Fig. 3A, top line). This result indicates that the HilA box and the −35 region from PinvF-1 is sufficient to activate PprgH basal (−29 to +8) sequences (Fig. 5A). The reciprocal prg-inv hybrid in PH25 is also activated by HilA, demonstrating the HilA box and the −35 region from PprgH can also function as a HilA-dependent activating region (Fig. 5A).
FIG. 5.
The HilA box from PprgH is a functional cis element. (A) PprgH (−57 to −30) can confer HilA-dependent activation upon PinvF-1 (−29 to +10). Double-stranded oligonucleotides carrying the hybrid promoters were cloned into the pAH125oriT reporter system and then placed in the E. coli chromosome in single copy (see Materials and Methods). Strains were transformed with either pACYC177 or pVV214 (HilA+) and then grown to early log phase under oxygenating conditions on a culture wheel at 37°C. Fold activation is calculated as the ratio of units expressed in a pVV214-containing strain to that expressed in a pACYC177-containing strain. The value for PH7 is as reported in reference 26. All standard deviations are equal to or less than 15% of the reported values. (B) PprgH and PinvF-1 sequences are most dissimilar in the region between −29 and +1. The invF and prgH promoter sequences were aligned at their respective +1 start sites for transcription. Identical nucleotides are connected by a vertical line, and the −10 hexamers, −35 hexamers, and HilA boxes are indicated. T's at positions −37, −42, and −45 are indicated with vertical arrows.
We also tested if prg-inv is more strongly activated than native PprgH because of the half-site-like hexamer at −6 present in the inv portion of the hybrid promoter. We constructed PH26, containing PprgH (−57 to +8) with five point mutations, to convert the PprgH −6 region to the half-site-like hexamer present at that same position in PinvF-1 (Fig. 5A). It is no more activated than native PprgH (−57 to +8) (Fig. 5A, compare PH26 to PH15).
PprgH and PinvF-1 respond equally to HilA in E. coli and S. enterica serovar Typhimurium under in vitro culture conditions.
The cis requirements for HilA-dependent activation of PprgH and PinvF-1 are somewhat different, and hence the two promoters may respond differently to various amounts of HilA. We investigated this possibility in two ways. First, we examined the activity of PinvF-1 (−153 to +129) and PprgH (−254 to +8) in the presence of large or small amounts of HilA in E. coli. Small amounts of HilA expressed from uninduced pCH112 (HilA-MycHis+) activate full-length PinvF-1 2.9-fold and activate full-length PprgH 3.5-fold (Table 3). To test the promoters' responses to somewhat higher amounts of HilA, we expressed HilA from pVV214. Estimating from a Western blot, pVV214 produces two to three times more HilA than uninduced pCH112 (data not shown), and HilA expressed from pVV214 activates PinvF-1 7.5-fold and PprgH 11-fold (Table 3). So they respond about equally to both low and intermediate levels of HilA. To express very high levels of HilA, we induced pCH112 with 0.2% arabinose. After 2.5 h of induction, these populations of cells contain approximately 10 times more HilA than pVV214-expressing cells (data not shown), and PinvF-1 expression was activated 210-fold and PprgH was activated 237-fold. Thus, it appears that, at least in E. coli, PprgH and PinvF-1 have similar responses over a range of HilA levels.
TABLE 3.
PprgH and PinvF-1 respond identically to HilA in E. coli
| Promotera | Activity (in Miller units) with HilA expressed from the indicated plasmid and with or without arabinoseb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| No arabinose added
|
Arabinose added
|
|||||||
| pBAD | pCH112 | pACYC177 | pVV214 | pACYC177 | pVV214 | pBAD | pCH112 | |
| invF::lacZY | 37 (1.0) | 110 (2.9) | 38 (1.0) | 284 (7.5) | 38 (1.0) | 270 (7.3) | 32 (1.0) | 6,733 (210) |
| prgH::lacZY | 10 (1.0) | 35 (3.5) | 10 (1.0) | 112 (11) | 12 (1.0) | 122 (10) | 12 (1.0) | 2,843 (237) |
PprgH or PinvF-1 reporter strains containing the largest promoter pieces tested were transformed with either pACYC177, pVV214 (HilA+), pBAD vector, or pCH112 (HilA-MycHis+).
Cultures were subcultured into fresh LB and then grown to early log phase under oxygenating conditions on a culture wheel at 37°C in the absence or presence of 0.2% arabinose. Values represent the average of at least two assays performed on three independent transformants of each reporter strain. Fold-activation, reported in parentheses, was calculated as the ratio of units expressed in a HilA+ strain to that expressed in the appropriate vector-containing strain and were calculated from raw data. All standard deviations are equal to or less than 10% of the reported values. Levels of HilA were qualitatively assessed by Western blot (data not shown) and are indicated by − or + signs. For cultures grown without arabinose, the levels of HilA for each plasmid were as follows: pBAD, −; pCH112, −/+; pACYC177, −; pVV214, +. For cultures grown with arabinose, the levels of HilA were as follows: pACYC177, −; pVV214, +; pBAD, −; pCH112, +++ (see Results for details).
Secondly, we investigated the effects of large and small amounts of HilA in S. enterica serovar Typhimurium containing chromosomal invF::lacZY or prgH::lacZY reporter fusions in these two SPI1 genes at their native positions. In wild-type (WT) S. enterica serovar Typhimurium, when all other in vitro conditions are inducing (e.g., low aeration, near-neutral pH), levels of hilA expression can be modulated by varying the amount of NaCl in the LB broth (R. Lucas and C. A. Lee, unpublished data). As in E. coli, in S. enterica serovar Typhimurium prgH::lacZY and invF::lacZY appear to have identical responses to HilA over a wide range of concentrations. For example, at 2 g of NaCl /liter, prgH::lacZY expression is stimulated 2.6-fold and invF::lacZY expression is stimulated 3-fold. The responses, expressed as fold activation, were not different from one another at any of the NaCl concentrations we tested (Table 4). We performed Western blots using a polyclonal antiserum against HilA and found that levels of HilA increased as the concentration of NaCl increased. Furthermore, the invF and prgH reporter strains contained identical amounts of HilA when each strain was grown at the same concentration of NaCl.
TABLE 4.
PprgH and PinvF-1 respond identically to HilA in S. enterica serovar Typhimurium
| Promotera | Activity (in Miller units) in the presence of HilA and NaClb
|
|||
|---|---|---|---|---|
| HilA, −/+; NaCl, 0 | HilA, +; NaCl, 2 | HilA, ++; NaCl, 5 | HilA, +++; NaCl, 10 | |
| invF::lacZY | 177 (1.0) | 541 (3.0) | 1,508 (8.5) | 2,433 (14) |
| prgH::lacZY | 102 (1.0) | 270 (2.6) | 670 (6.6) | 1,084 (11) |
S. enterica serovar Typhimurium with lacZY transposon insertions in prgH or invF were grown in nearly full test tubes shaking upright at 150 rpm in LB containing the indicated amount of NaCl (in grams per liter).
Values represent the average of three assays performed on independent colonies of each reporter strain. Fold activation, reported in parentheses, was calculated as the ratio of units expressed in salt-containing broth to that expressed in the no-added-salt condition (column 1). All standard deviations are equal to or less than 10% of the reported values. Levels of HilA were qualitatively assessed by Western blot (data not shown) and are indicated by − and + signs.
DISCUSSION
We have observed that the HilA boxes in PinvF-1 and PprgH are required for activation at each promoter and that HilA binds to each of them (Fig. 2 through 4 as well as reference 26). The HilA boxes of PinvF-1 and PprgH are different from one another at only 5 of 17 positions and are in identical positions relative to the +1 of transcription at each promoter. The simplest model for activation by HilA is that the HilA box is a critical upstream activating sequence because it serves as the primary HilA binding site. Because positions −37, −42, and −45 are critical for binding and activation from both HilA boxes, we might assume that HilA makes the same base pair-specific contacts with the HilA box from PprgH and PinvF-1. By extension, we would simplistically hypothesize that the same protein-protein interaction(s) between HilA bound to the HilA box and RNAP serve(s) to activate transcription at PinvF-1 and PprgH.
But HilA-dependent activation may not be so straightforward. Our results demonstrate that the HilA box and −35 regions of PinvF-1 and PprgH have different capacities to serve as upstream activating sites in the context of other sequences both upstream and downstream of the HilA box. Strikingly, sequences downstream of the HilA box in PprgH appear to inhibit the prgH HilA box's activity in the absence of upstream sequences, including the half-site-like hexamers at −106 and −67 (Fig. 2). Changing the (−29 to +8) sequences in PprgH (−57 to +8) to PinvF-1 (−29 to +10) allows the prgH HilA box to serve as a HilA-dependent upstream activating site, even in the absence of the half-site-like hexamers (Fig. 5). In contrast, the PinvF-1 HilA box serves as a good upstream activating region when present in many (−57 to +8 [or +10]) promoters with different (−29 to +8 [or +10]) sequences (Fig. 5 and reference 26). In seeking to explain these results, we hypothesize that several molecular interactions influence the outcome of HilA bound to any given HilA box found between −53 and −37 at a promoter. We first discuss the role(s) the sequences upstream of the HilA box in PprgH might play in order to relieve poisoning by the (−29 to +8) sequences. Then we discuss how the sequences between −29 and +1 could affect transcriptional activation.
Sequences upstream of the HilA box in PprgH.
HilA activates PprgH very efficiently in the presence of upstream sequences that include the half-site-like hexamers at −106 and −67. The initiation of transcription is a complex process, so these sequences could be important for any of several reasons. What complicates the analysis of PprgH and PinvF-1 considerably is the fact that no matter what the molecular mechanism by which the half-site-like hexamers at −106 and −67 allow for activation of PprgH, they are required only in the presence of particular −29 to +1 sequences, so that they are dispensable at prg-inv but not at PprgH (−57 to +8). In contrast, PinvF-1 (−57 to +10), inv-phoE, inv-PRM, and inv-prg all have HilA box sequences from PinvF-1 and are activated strongly by HilA, regardless of the sequences between −29 and +1, and in the absence of any upstream half-site-like hexamers (Fig. 5 and reference 26).
The upstream sequences could affect the structure of the DNA and therefore the promoter's intrinsic kinetic properties. The half-site-like hexamers at −106 and −67 in PprgH are in the midst of a highly AT-rich region of DNA, and when they are present PprgH DNA is predicted to be sharply curved (bend.it; http://www2.icgeb.trieste.it/∼dna/bend_it.html) (33). In contrast, PinvF-1 DNA is not predicted to be as bent, regardless of the presence or absence of upstream sequences. Both the mild predicted bend in PinvF-1 and the severe predicted bend in PprgH center on the HilA box itself. If the upstream sequences in PprgH introduce a bend, it is conceivable that this bend distorts the DNA in a way that enhances the kinetics of RPinit formation at PprgH. HilA bound at the HilA box at PprgH may require the energetic enhancement caused by the bend to activate transcription from PprgH but not from PinvF-1.
If the predicted bend alone is sufficient to allow the HilA box to function as an upstream activating site in PprgH, a simple explanation for the effect of the two TT-to-GG mutations in the half-site-like hexamers at −106 and −67 is that they change the DNA bending. Similarly, the prg-inv hybrid, which contains the PprgH HilA box and is activated by HilA, should be bent more extensively than PprgH (−57 to +8) in PH15. According to bend.it, however, the promoters in PH17 (WT) and PH39 (double mutant) are predicted to have the same bend, and prg-inv is not predicted to be bent more than PprgH (−57 to +8). Still, the possibility that bent DNA might contribute to HilA-dependent activation at PprgH should be considered and investigated in the future.
The AT-rich sequences that include the half-site-like hexamers at −106 and −67 might also serve as binding sites for a second trans-acting factor important for activation of PprgH. This trans-acting factor could even be a subunit of RNAP. For example, up-elements, which are binding sites for the C-terminal domain of the α subunit of RNAP (α-CTD), are AT rich (39). The α-CTD has been found to protect sites upstream of other sites bound by activators centered around −41.5. So it is possible that HilA requires the α-CTD to contact DNA upstream of the HilA box at PprgH in order to activate transcription from those basal sequences. In this case, it could be that HilA contacts the α-CTD of RNAP to assist its binding to the AT-rich DNA. Further, two contacts between RNAP and HilA might be required to activate transcription of PprgH, one between the α-CTD and HilA and another between HilA and one of the subunits anchored to the −35 hexamer. Genetic studies using mutations and/or truncations in the α subunit of RNAP might be able to determine whether the α-CTD is required for activation of PprgH. If this model is correct, the α-CTD will be required for the activation of PprgH (−254 to +8) but not for the activation of PinvF-1.
It is also possible that HilA can bind to the −106 and/or −67 region of PprgH when those sequences are intact. However, the HilA box in PprgH is a good HilA binding site, and it can serve as an upstream activating region in the absence of the half-site-like hexamers (as in the prg-inv hybrid), so putative cooperative binding between HilA bound to distal half-site-like hexamers and HilA at the HilA box may have a more specialized role than simply increasing occupation of the HilA box. For example, the binding of HilA monomers to upstream half-site-like hexamers might position HilA bound at the HilA box in PprgH to allow it to make productive contact with RNAP. If this is true, this subtle positioning is required only for HilA to activate transcription in the presence of a particular combination of HilA box and basal sequences.
Another possibility is that a factor that is neither HilA nor a subunit of RNAP but that is present in both E. coli grown with high aeration and S. enterica serovar Typhimurium grown under inducing (low oxygen) conditions interacts with sequences upstream of the HilA box in PprgH. Candidates for such factors might be IHF, H-NS, HU, Fis, and/or other small nucleoid binding proteins likely to participate in the chromatin-like structure found at complex prokaryotic promoters (see, for example, reference 3). In this case, the particular architecture induced by the binding of such a factor would be required for HilA-dependent activation of PprgH but would be dispensable at PinvF-1.
The sequences downstream of the HilA box.
We determined that the differences in the activities of PinvF-1 (−57 to +10) and PprgH (−57 to +8) cannot be attributed to the half-site-like hexamer at −6 in PinvF-1. So some other sequences between (−29 and +8 [or +10]) must account for their different activities. The −10 hexamers play a large role in determining the intrinsic properties of ς70-dependent promoters, and the −10 hexamers of PinvF-1 and PprgH are different from one another at two of six positions (PinvF-1, TATTGT; PprgH, TAATCT; nonidentical positions are underlined). So we hypothesize that the −10 hexamers determine whether PprgH (−57 to −30) is able to serve as an upstream activating site in the absence of distal half-site-like hexamers. This hypothesis could be tested directly by systematically mutating every position of the −10 hexamers in PprgH and PinvF-1.
Traditionally, the sequence of the −10 hexamer is thought to govern kcat, the rate constant that governs the transition from RPc to RPo (reviewed in reference 3). At least two species of RPc and two species of RPo have been detected at different ς70-dependent test promoters (reviewed in reference 3). So it is possible that the particular sequence of the −10 hexamer may affect the stability of several complexes during the transition from RPc1 to RPo2. Given the differences between the −10 hexamers in PinvF-1 and PprgH, it is possible that the formation of a different RP complex is rate limiting in each case. If this is so, HilA bound to the HilA box of PinvF-1 might be able to stabilize more than one complex, while HilA bound to the HilA box of PprgH might be able to stabilize only one of them. So a simple model in which the same protein-protein contacts result in HilA-dependent activation at all HilA-dependent promoters may not account for all of our data.
Clearly, sequences both upstream and downstream of the HilA box influence the ability of HilA to activate a promoter. Much more experimentation will be needed to address the possible mechanisms by which these sequences determine the activity of HilA bound to the PprgH HilA box.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI33444.
We thank Lisa Schechter for purifying HilA under denaturing conditions and making polyclonal antiserum against HilA. Many thanks to Jane Lopilato for her help with the Western blottings. We thank the Microbiology Core Sequencing Facility at the Harvard Medical School for sequencing many of the constructs reported in this paper. We thank all members of the Lee laboratory, as well as Bob Kingston, Fred Winston, and Jon Beckwith for helpful discussion. We thank Sumita Jain for her critical review of the manuscript prior to submission. We thank Steve Busby for discussing the results section and Ann Hochschild for her critical review of the manuscript.
REFERENCES
- 1.Bajaj V, Hwang C, Lee C A. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj V, Lucas R L, Hwang C, Lee C A. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol. 1996;22:703–714. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- 3.Browning D F, Cole J A, Busby S J. Suppression of FNR-dependent transcription activation at the Escherichia coli nir promoter by Fis, IHF and H-NS: modulation of transcription initiation by a complex nucleo-protein assembly. Mol Microbiol. 2000;37:1258–1269. doi: 10.1046/j.1365-2958.2000.02087.x. [DOI] [PubMed] [Google Scholar]
- 4.Bullas L R, Ryu J I. Salmonella typhimurium LT2 strains which are r −m+ for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983;156:471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang A C, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collado-Vides J, Magasanik B, Gralla J D. Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev. 1991;55:371–394. doi: 10.1128/mr.55.3.371-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crago A M, Koronakis V. Salmonella InvG forms a ring-like multimer that requires the InvH lipoprotein for outer membrane localization. Mol Microbiol. 1998;30:47–56. doi: 10.1046/j.1365-2958.1998.01036.x. [DOI] [PubMed] [Google Scholar]
- 8.Daefler S, Russel M. The Salmonella typhimurium InvH protein is an outer membrane lipoprotein required for the proper localization of InvG. Mol Microbiol. 1998;28:1367–1380. doi: 10.1046/j.1365-2958.1998.00908.x. [DOI] [PubMed] [Google Scholar]
- 9.Darwin K H, Miller V L. InvF is required for expression of genes encoding proteins secreted by the SPI1 type III secretion apparatus in Salmonella typhimurium. J Bacteriol. 1999;181:4949–4954. doi: 10.1128/jb.181.16.4949-4954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darwin K H, Miller V L. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin Microbiol Rev. 1999;12:405–428. doi: 10.1128/cmr.12.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darwin K H, Miller V L. The putative invasion protein chaperone SicA acts together with InvF to activate the expression of Salmonella typhimurium virulence genes. Mol Microbiol. 2000;35:949–960. doi: 10.1046/j.1365-2958.2000.01772.x. [DOI] [PubMed] [Google Scholar]
- 12.Darwin K H, Miller V L. Type III secretion chaperone-dependent regulation: activation of virulence genes by SicA and InvF in Salmonella typhimurium. EMBO J. 2001;20:1850–1862. doi: 10.1093/emboj/20.8.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egland K A, Greenberg E P. Conversion of the Vibrio fischeri transcriptional activator, LuxR, to a repressor. J Bacteriol. 2000;182:805–811. doi: 10.1128/jb.182.3.805-811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichelberg K, Galan J E. Differential regulation of Salmonella typhimurium type III secreted proteins by pathogenicity island 1 (SPI-1)-encoded transcriptional activators InvF and HilA. Infect Immun. 1999;67:4099–4105. doi: 10.1128/iai.67.8.4099-4105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichelberg K, Hardt W D, Galán J E. Characterization of SprA, an AraC-like transcriptional regulator encoded within the Salmonella typhimurium pathogenicity island 1. Mol Microbiol. 1999;33:139–152. doi: 10.1046/j.1365-2958.1999.01458.x. [DOI] [PubMed] [Google Scholar]
- 16.Gralla J D, Collado-Vides J. Organization and function of transcription regulatory elements. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E E C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1232–1245. [Google Scholar]
- 17.Haldimann A, Daniels L L, Wanner B L. Use of new methods for construction of tightly regulated arabinose and rhamnose promoter fusions in studies of the Escherichia coli phosphate regulon. J Bacteriol. 1998;180:1277–1286. doi: 10.1128/jb.180.5.1277-1286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison-McMonagle P, Denissova N, Martinez-Hackert E, Ebright R H, Stock A M. Orientation of OmpR monomers within an OmpR:DNA complex determined by DNA affinity cleaving. J Mol Biol. 1999;285:555–566. doi: 10.1006/jmbi.1998.2375. [DOI] [PubMed] [Google Scholar]
- 19.Jones B, Pascopella L, Falkow S. Entry of microbes into the host: using M cells to break the mucosal barrier. Curr Opin Immunol. 1995;7:474–478. doi: 10.1016/0952-7915(95)80091-3. [DOI] [PubMed] [Google Scholar]
- 20.Kimbrough T G, Miller S I. Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc Natl Acad Sci USA. 2000;97:11008–11013. doi: 10.1073/pnas.200209497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein J R, Fahlen T F, Jones B D. Transcriptional organization and function of invasion genes within Salmonella enterica serovar Typhimurium pathogenicity island 1, including the prgH, prgI, prgJ, prgK, orgA, orgB, and orgC genes. Infect Immun. 2000;68:3368–3376. doi: 10.1128/iai.68.6.3368-3376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galán J E, Aizawa S I. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 23.Kubori T, Sukhan A, Aizawa S I, Galán J E. Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc Natl Acad Sci USA. 2000;97:10225–10230. doi: 10.1073/pnas.170128997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee C A, Jones B D, Falkow S. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci USA. 1992;89:1847–1851. doi: 10.1073/pnas.89.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C C, Crawford J A, DiRita V J, Kaper J B. Molecular cloning and transcriptional regulation of ompT, a ToxR-repressed gene in Vibrio cholerae. Mol Microbiol. 2000;35:189–203. doi: 10.1046/j.1365-2958.2000.01699.x. [DOI] [PubMed] [Google Scholar]
- 26.Lostroh C P, Bajaj V, Lee C A. The cis requirements for transcriptional activation by HilA, a virulence determinant encoded on SPI-1. Mol Microbiol. 2000;37:300–315. doi: 10.1046/j.1365-2958.2000.01991.x. [DOI] [PubMed] [Google Scholar]
- 27.Lucas R L, Lee C A. Unravelling the mysteries of virulence gene regulation in Salmonella typhimurium. Mol Microbiol. 2000;36:1024–1033. doi: 10.1046/j.1365-2958.2000.01961.x. [DOI] [PubMed] [Google Scholar]
- 28.Lucas R L, Lostroh C P, DiRusso C C, Spector M P, Wanner B L, Lee C A. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J Bacteriol. 2000;182:1872–1882. doi: 10.1128/jb.182.7.1872-1882.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Hackert E, Stock A M. Structural relationships in the OmpR family of winged-helix transcription factors. J Mol Biol. 1997;269:301–312. doi: 10.1006/jmbi.1997.1065. [DOI] [PubMed] [Google Scholar]
- 30.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- 31.Monack D M, Hersh D, Ghori N, Bouley D, Zychlinsky A, Falkow S. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J Exp Med. 2000;192:249–258. doi: 10.1084/jem.192.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munteanu M G, Vlahovicek K, Parthasarathy S, Simon I, Pongor S. Rod models of DNA: sequence-dependent anisotropic elastic modelling of local bending phenomena. Trends Biochem Sci. 1998;23:341–347. doi: 10.1016/s0968-0004(98)01265-1. [DOI] [PubMed] [Google Scholar]
- 34.Murray R A, Lee C A. Invasion genes are not required for Salmonella enterica serovar Typhimurium to breach the intestinal epithelium: evidence that Salmonella pathogenicity island 1 has alternative functions during infection. Infect Immun. 2000;68:5050–5055. doi: 10.1128/iai.68.9.5050-5055.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oropeza R, Sampieri C L, Puente J L, Calva E. Negative and positive regulation of the nonosmoregulated ompS1 porin gene in Salmonella typhi: a novel regulatory mechanism that involves OmpR. Mol Microbiol. 1999;32:243–252. doi: 10.1046/j.1365-2958.1999.01329.x. [DOI] [PubMed] [Google Scholar]
- 36.Raibaud O, Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- 37.Rakeman J L, Bonifield H R, Miller S I. A HilA-independent pathway to Salmonella typhimurium invasion gene transcription. J Bacteriol. 1999;181:3096–3104. doi: 10.1128/jb.181.10.3096-3104.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez C R, Cho E J, Keogh M C, Moore C L, Greenleaf A L, Buratowski S. Kin28, the TFIIH-associated carboxy-terminal domain kinase, facilitates the recruitment of mRNA processing machinery to RNA polymerase II. Mol Cell Biol. 2000;20:104–112. doi: 10.1128/mcb.20.1.104-112.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross W, Gosink K K, Salomon J, Igarashi K, Zou C, Ishihama A, Severinov K, Gourse R L. A third recognition element in bacterial promoters: DNA binding by the alpha subunit of RNA polymerase. Science. 1993;262:1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- 40.Schechter, L. M., S. M. Damrauer, and C. A. Lee. AraC/XylS family members, HilC and HilD, directly bind and derepress the S. typhimurium hilA promoter. Mol. Microbiol., in press. [DOI] [PubMed]
- 41.Schechter L M, Damrauer S M, Lee C A. Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol Microbiol. 1999;32:629–642. doi: 10.1046/j.1365-2958.1999.01381.x. [DOI] [PubMed] [Google Scholar]
- 42.Wallis T S, Galyov E E. Molecular basis of Salmonella-induced enteritis. Mol Microbiol. 2000;36:997–1005. doi: 10.1046/j.1365-2958.2000.01892.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Zhou Y, Ebright Y W, Ebright R H. Catabolite gene activator protein (CAP) is not an “acidic activating region” transcription activator protein. Negatively charged amino acids of CAP that are solvent-accessible in the CAP-DNA complex play no role in transcription activation at the lac promoter. J Biol Chem. 1992;267:8136–8139. [PubMed] [Google Scholar]