Abstract

Polymer demonstrations have become increasingly important within science, technology, engineering, and math education curricula. These demonstrations not only afford the opportunity to introduce students to polymers but also provide an avenue to educate students about the impact polymers have on the planet. Despite the educational value and recreational enjoyment polymer demonstrations can provide, there are serious health and environmental hazards associated with some of the historically common polymer demonstrations that should be addressed. This Perspective describes the benefits and consequences of the historically common polymer “slime” and “silly putty” demonstrations and then details alternatives that can replace them. Finally, a Methods section of healthier and safer polymer demonstrations with detailed protocols is included.
Keywords: slime, polymers, borax, alginate, STEM education
Introduction
Since the introduction of the first synthetic polymer—Bakelite—in 1907, synthetic polymers and plastics have become commonplace in the daily lives of billions of people around the globe due to their wide-ranging properties and utility.1 From the pipes in massive buildings to corrective eyewear, polymers have become a necessary addition to the global landscape.2,3 In recent years, the downside and dangers of specific classes of polymers have been increasingly made known to both the scientific community and the general public.2,4 For example, Geyer et al. reported that, between the years 1950 and 2015, 6.3 Billion Metric Tons of plastic waste was produced, but only 9% of that waste has been recycled.4 Furthermore, 60% of all plastics ever produced are currently in landfills or can be found as waste elsewhere in the natural environment.4 This waste can do environmental damage,2 and it is also a threat to human health.5
As the world grapples with the problem of plastic waste, concerted efforts to utilize “green” methods in the making, processing, reuse, and/or disposal of polymer materials are being pursued. To this end, Paul Anastas and John Warner devised 12 Principles of Green Chemistry.6 Included in these principles are core ideals of preventing waste, carrying out less hazardous syntheses, and making use of renewable feedstocks. From a science, technology, engineering, and mathematics (STEM) education standpoint, in order to achieve such goals a rethinking of historically common polymer STEM education demonstrations is necessary.7,8 For this purpose, the Green Reagents and Sustainable Processes (GRASP) approach has been devised.9 GRASP is a method in which hazardous chemicals within common experiments are identified and replaced with less-hazardous chemicals, and the experimental procedures are updated to include those less-hazardous chemicals. In most instances this means repackaging old experiments utilizing different reagents, but in some instances it may mean a total discontinuation of a popular polymer demonstration. Included in the group of polymer demonstrations whose use warrants reconsideration is the making of slime. Although there has been a massive effort to improve recycling behaviors and to make polymers from less hazardous and more sustainable sources,10 the common slime demonstrations have remained a staple for those seeking to introduce polymers in educational settings despite their inherent dangers.
Herein, a Perspective is written to urge a movement by the STEM education community toward polymer demonstrations that still provide effective educational polymer demonstrations but also have a minimal negative impact on human health and the environment. This Perspective includes a Methods section that contains protocols for select polymer demonstrations that can be performed with grade-school students.
Discussion
The term “slime” is sometimes used interchangeably with the term “silly putty.” The trademarked toy Silly Putty belongs to Crayola LLC, and the product was originally developed as part of a program to find a cheaper substitute for rubber during World War II.11 Although the components used to make slime can also be used to make silly putty, silly putty is commonly understood to be thicker, less stretchy, and less sticky than slime. The playtime material known as slime was first introduced by the Mattel Company in the 1970s.12−14 It quickly became a popular toy, being found prominently placed on store shelves at major retailers as well as on several popular children’s television shows such as Nickelodeon’s “You Can’t Do That On Television” and “Double Dare.” With recipes similar to that of Mattel’s toy, making slime with and for children also became increasingly common within recreational and educational settings. Recently, on various social media platforms there has been a massive increase in videos and content that feature step-by-step instructions on how to make various types of homemade slime, with more than 1.2 million slime recipe videos featured on YouTube by 2017.15,16
The Borax Slime Demonstration: Benefits and Harms
Perhaps the most basic and common slime recipe is one that can be made from polyvinyl alcohol (PVA), sodium tetraborate, and water (Figure 1). This product is sometimes referred to as Flubber but is herein referred to as Borax Slime.12,17,18 The PVA is obtained via polyvinyl acetate from “Elmer’s Glue” or “school glue.” Sodium tetraborate, commonly called borax, is typically acquired from specific household cleaning agents. Although the ratios and reagent amounts used vary and are left up to the discretion of the person making the slime, making Borax Slime commonly requires adding borax to water in one container and separately adding school glue to water in another container. The borax solution and the glue solution are then combined and stirred until a uniform polymer material is formed (i.e., Borax Slime). Adjusting the ratios of the reagents gives slimes that vary in character from slippery and runny to stiff and taut. In addition to the borax, glue, and water, it is common to add water-based food coloring to dye the slime any desired color (Figure 1).
Figure 1.
(a) Reaction mechanism for Borax Slime (Adapted with permission from ref (18). Copyright 2015 John Wiley and Sons). (b) An image of Borax Slime dyed green.
Making this glue-based borax slime has become so popular that in 2017 Newell Brands, which owns Elmer’s glue, had to increase production of the glue due to consumer demand.15 As a result, it is common to find school glue for making slime being sold in dedicated sections of arts and crafts shops and toy sections at department stores as well as from online distributors. There are countless variations of the Borax Slime polymer synthesis, some of which include borax from sources other than household cleaners, such as contact lens solution. Additionally, homemade slime recipes may include shaving cream, shampoo, baking soda, dish detergent, toothpaste, glitter, iron shavings, glow-in-the-dark dyes, and shiny or glow-in-the-dark microbeads.
From an educational standpoint, there are numerous valuable concepts relevant to polymer science that can be demonstrated by making Borax Slime.17 The setting of the slime is indicative of a two-dimensional linear polymer being transformed into a three-dimensional network polymer upon addition of the borax. The chemical mechanism of borax cross-linking can lead to further exploration and a discussion of appropriate cross-linker-polymer combinations. Viscosity and other rheological properties can be studied through straightforward experiments in which students vary the amounts of borax and glue and evaluate the products.19 These types of hands-on learning examples are especially effective in teaching young students how to tailor reaction conditions to get the material property that they want: in this case, elastic or oozy. Hydrogen bonding plays a major role in the formation of Borax Slime, and this fact can be used as a means to discuss intermolecular forces and/or bond strengths. Finally, for more advanced students, the physics of fluid flow can be introduced by explaining that Borax Slime is a shear-thickening non-Newtonian fluid. Along with a discussion of what it means for a fluid to be non-Newtonian, the different categories of non-Newtonian fluids (e.g., shear-thickening, shear-thinning, thixotropic) can be described, and real-world examples of these types of fluids (e.g., corn starch in water, ketchup, yogurt) can be highlighted.
Unfortunately, for all the many wonderful educational aspects about making Borax Slime, there are significant consequential downsides to making it. There is a growing body of research that shows health consequences of making Borax Slime, including contact dermatitis and pediatric burns in children.20−28 For example, playing with slime was determined to be the cause of pruritic rash and skin discoloration of a healthy 4-year-old child.27 The symptoms lasted for several days after contact with the slime, and required a topical steroidal treatment to remedy.27 In another more severe instance, a 10-year-old child exhibited blisters on the child’s hand and fingers that grew progressively worse during the course of several days after making Borax Slime.28 The blisters resulted in limited motion in the child’s fingers. Ultimately an operation to apply cadaver skin was needed to repair the damage done to the child’s hand and fingers. Borax is often noted as the likely cause for these types of injuries due to its pH of 9 being great enough to cause skin burns.27,28 Although useful as a detergent, borax is not designed to be extensively handled by people and has itself been linked to allergic reactions in people outside of the context of making slime.27 Even more concerning, borax is listed as a category H360 health hazard in the Globally Harmonized System of Classification and Labeling of Chemicals (GHS) list. This classification means that borax is a threat to fertility and pregnancy according to the GHS.29 Thus, it has been recommended that gloves be worn when making and handling Borax Slime. Despite this recommendation, it is common for Borax Slime to be made with large groups of children without the use of gloves.
There have been modifications to the Borax Slime protocol that eliminate borax.30 However, not only is the borax within the slime a threat to cause physical harm, but also the glue itself is a threat to cause physical harm. This is because glue can contain Kathon, which is a combination of methylchloroisothiazolinone and methylisothiazolinone. These compounds are known contact allergens and irritants that can cause chemical burns.22,27,31 Therefore, the continued use of PVA in these types of borax-less slimes still results in polymers with a notable hazardous component. Although most occasions in which children make Borax Slime end without physical harm to the children, there have been enough reports of these adverse occurrences to warrant significant concern among parents, educators, and polymer scientists.32
Beyond the health concerns, there are substantial environmental concerns related to Borax Slime waste. Although the Borax Slime polymer network can be decomposed by adding hot water to it,19 the disposal of homemade slimes like Borax Slime typically consists of throwing the slime in the trash, meaning that the components go unrecycled. The amount of slime being made and later thrown away is not insignificant, as evidenced by posted YouTube videos featuring children as well as adult YouTube influencers throwing away gallons and gallons of slime by combining several pounds of slime that they made in the past that they no longer play with or have use for. These videos are sometimes accompanied by attempts to dissolve the slime with acidic solutions like vinegar and/or lemon juice in order to recover additives like beads, and so the slime can be “properly” disposed of. Despite these disposal efforts, almost all of the components still end up as waste in trash containers, and any acidic dissolving solutions also tend to become waste. The damage this slime waste produces is further complicated by the various additives (e.g., glitter and microbeads) that they release into the environment as they decompose in landfills. Both glitter and microbeads have been shown to be harmful to the environment and particularly to sea animals.33,34 It is also worth noting that, while making these slimes is popular with children, making slime is often unpopular with the adults that care for the children because slime can stain walls, damage furniture, get stuck in carpet, and get tangled in hair, and the slime fabrication processes waste valuable household items.
Safer Alternative to Borax Slime: Alginate Slime
With polymers being so prevalent, the opportunity to expand the library of common polymer demonstrations certainly exists. Not only can numerous other demonstrations be used, but all of them can be far less hazardous to the health of people and the environment. Beginning with Alginate Slime, the contents below highlight multiple viable alternatives to detrimental common polymer demonstrations (Table 1). Each alternative poses a minimal threat to human health or the environment, while yet presenting several key polymer-related educational opportunities.
Table 1. Primary Reagents for Educational Topics That Can Be Used in Safer Polymer STEM Demonstrations.
| primary reagents | educational topics | reference(s) |
|---|---|---|
| sodium alginate, calcium lactate | replacement reactions, tissue engineering | (35,36) |
| sodium alginate, chitosan | bioplastics, green chemistry | (37) |
| water, corn starch | non-Newtonian fluids, rheology | (43,44) |
| gummy candy, corn starch, powdered sugar, cooking oil | biopolymers, polymer fillers | (45−48) |
| oranges, lemon juice, white sugar, ethanol | waste valorization, rheology | (51) |
One alternative to Borax Slime uses sodium alginate cross-linked with calcium lactate (or calcium chloride) in water, and it is designated “Alginate Slime” herein (Figure 2).9,35,36 Sodium alginate is extracted from brown seaweed and has been used in numerous creative educational applications9,35−37 as well as a food additive more generally. In the laboratory, calcium lactate can be obtained by combining lactic acid with calcium complexes such as calcium carbonate. From a consumer perspective, calcium lactate is also a food additive and is sometimes used as a calcium supplement. Both sodium alginate and calcium lactate can be purchased in various grocery stores or online, where they are sometimes packaged together. The procedure to make Alginate Slime only requires dissolving sodium alginate in water and, in a separate container, dissolving calcium lactate in water. The gel (i.e., Alginate Slime) forms upon the addition of the sodium alginate solution to the calcium lactate solution. The gel formation occurs as a result of the interaction of the divalent calcium ions with guluronic acid residues from the sodium alginate.9,38,39 This interaction features calcium ions encapsulated by two guluronate helix chains in what is described as the “egg-box model.”40 The clear transparent Alginate Slime product can be altered by adding food coloring to the sodium alginate precursor solution. Depending on the method used to introduce the sodium alginate solution into the calcium lactate solution during the Alginate Slime synthesis, the resulting gel can take the form of a large bulk mass, spherical squishy balls, or stringy slime worms. Because the components are naturally occurring, damage to the environment is expected to be minimal, and the Alginate Slime can even be eaten. The Alginate Slime polymer network can also be decomposed upon addition of a trisodium citrate solution.
Figure 2.
(a) The basic scheme for Alginate Slime formation (Adapted with permission from ref (36). Copyright 2019 American Chemical Society). (b) Picture of Alginate Slime dyed pink.
From an educational standpoint many of the same concepts that have been historically taught alongside making Borax Slime can also be taught when making Alginate Slime. For example, similar to the transformation of poly(vinyl alcohol) from a linear two-dimensional polymer into a three-dimensional network polymer upon addition of the borax cross-linker, sodium alginate is a linear two-dimensional polymer that is converted into a three-dimensional network polymer once calcium lactate is introduced. Also, the cross-link density is affected by the amount of calcium lactate used, and this impacts the viscosity of the Alginate Slime. An additional teaching point that making Alginate Slime provides is the concept of replacement reactions, because the calcium ions are replacing the sodium ions during the Alginate Slime formation process. Furthermore, it is known that alginate gels are pH-responsive and that the interaction of alginate with calcium salts is pH -sensitive.41,42 This pH-responsive nature of alginate opens the door for experiments in which strong acidic liquids (e.g., lemon juice) can be introduced as one of the reagents, and students can attempt to make Alginate Slime. The presence of the strong acid limits calcium’s ability to cross-link the alginate, resulting in slimes that are softer than when the reaction solution has a more basic pH. This presents the opportunity to teach about cross-linker (i.e., calcium ion) inhibition due to the low pH protonation of the alginate by the acid. Finally, for advanced students, the differences between the dynamic-covalent Borax Slime and the supramolecular Alginate Slime networks can be explored in greater depth.
One notable difference between Borax Slime and Alginate Slime that can be used as a teaching tool is in their non-Newtonian character. Borax Slime is shear-thickening, which means that it hardens and behaves like a solid when a force is applied to it, while Alginate Slime is shear-thinning, which means it behaves more like a liquid when a force is applied to it. As these teaching points highlight, there is no loss in educational value in moving from making harmful Borax Slime to making safer Alginate Slime.
Safer Polymer Demonstrations: More Alternatives to Borax Slime
A well-known alternative to making Borax Slime is the addition of water to corn starch in certain proportions to make a slime that is sometimes referred to as “Oobleck” (Figure 3).43 Oobleck is a shear-thickening non-Newtonian fluid that creates an opportunity to teach about rheology and the physics of fluid flow as a broader concept.43,44 Oobleck is easy to make, and it can readily serve as either a teaching tool or a recreational activity. Of critical importance is the fact that the corn starch used can be recovered by drying out the Oobleck, making the corn starch available for reuse later, thus decreasing the waste from making this type of slime.
Figure 3.

Oobleck slime.
In addition to the aforementioned edible Alginate Slime, there are numerous other types of edible “slimes” that can be made from various confections including gummy candies, marshmallows, or peanut butter.45−48 The recipes to make these types of slime often require the use of vegetable oil, corn starch, and/or powdered sugar to alter the properties (e.g., viscosity) of the final product. The use of these types of slime may be more recreational than educational, but there are polymer education angles that can be discussed. For example, additives and fillers that alter polymer properties like tackiness can be discussed alongside industrial examples like additives and fillers that are used to make different types of rubber (a very common polymer). Lessons with undergraduate-level students could be focused on the gelatin used to make gummy candies. Gelatin is commonly derived from collagen, which is a polymer. This angle opens the door for discussions of polymers in biological systems and where biological polymers are used in the food industry. Although it is not advisible to consume large quantities of these edible confection-based slimes due to their sugar content, the waste level and negative environmental impact is mitigated by the fact that these slimes are edible and compostable.
Departing from the theme of making slime as a means to introduce polymers to students, there are other demonstrations that can be performed and important concepts that can be addressed therein. For example, making simple glues is a way in which students can learn about the importance of adhesives in their daily lives, while also learning about polymers (see Methods). As previously mentioned, gelatin is derived from the polymer collagen. Combining gelatin with sugar and water in certain proportions results in a sticky substance that can be used as an adhesive. Discussions about adhesive polymers affords the instructor the opportunity to discuss fluids that are polymers. Also, the time-dependent behavior of this and other glues can be discussed, as glues can harden on various time scales.
Polymers can be used to teach about osmosis and diffusion through a simple demonstration using gummy bear candies. In this demonstration, a gummy bear candy is placed in a liquid (e.g., water) and allowed to soak in the liquid for several hours. Once a certain amount of time has passed, the gummy bear will increase in size if it is permeable to the liquid it was submerged in. The polymer angle relies on the fact that gummy candies are largely made of gelatin, which is permeable to certain liquids. The gummy candies increase in size due to osmosis. Osmosis is a passive transport process by which a solvent can move from an area of high concentration into an area of low concentration by passing through a semipermeable membrane. Polymers that function as semipermeable membranes have tremendous importance in the real world, including in drug delivery and water purification applications.49,50 Submerging the gummy bears in different solvents and then carefully measuring and comparing the sizes highlights how effective semipermeable membranes can be at filtering various solvents. The educational discussions can be extended by defining diffusion and comparing diffusion with osmosis. Yet again the use of gelatin allows for the various discussions previously mentioned.
Waste valorization is the process of recycling waste materials and repackaging them into more useful materials that contribute back to society and/or the environment. A principle of waste valorization is that items historically seen as waste can be converted into items that have a greater value after their initial use than the amount of energy it took to produce them for their initial purpose. There are a number of accessible demonstrations that can be performed with students that demonstrate and teach about this important means for decreasing waste. This includes an undergraduate-level synthesis focused on making a polymer sol–gel from citrus fruit components and sugar.51 Oranges are common across a broad geographic portion of the planet, and processing them for human consumption (e.g., juice) results in the disposal of their peels as waste. In bulk, orange peel waste poses a threat to the environment and, in particular, to groundwater resources. This is in part due to the low pH that orange peel waste produces. Although orange peels are commonly disposed of as waste, they are composed of many valuable components (e.g., sugar, pectin, d-limonene), and valorizing this resource could decrease the groundwater contamination and greenhouse gas production where citrus waste is prevalent. In this experiment, students learn how to extract pectin from the orange peels and combine the pectin with a sugar solution to make marmalade. The polymer angle that can be explored with this process comes from the presence of pectin in the orange peels. Pectin is a polysaccharide that is used as a gelling component in the production of jelly. The process of making the marmalade includes opportunities to discuss the effect of acidic solutions on sugar extraction as well as rheology and non-Newtonian fluids as it pertains to gelation chemistry. Finally, a side benefit of performing this polymer demonstration is that the students can enjoy eating the oranges while collecting the orange peels. Although best suited for undergraduate-level students, the citrus sol–gel polymer synthesis represents a process that informs about natural and renewable resources, while demonstrating how these resources can be used to make useful materials. As more researchers give thoughtful consideration to creating polymer demonstrations that are not harmful to the body or the environment, a greater library of these types of “green” alternatives are likely to be reported and implemented at all levels of polymer education.
Methods
Included in this Methods section are select demonstrations/experiments that can be used to introduce various polymer-related concepts. Although informative at any age, the target age group for these examples is from 5 to 12 years old.
Alginate Slime
Safety
Neither eye protection nor gloves are required. Be careful not to get the electrical socket for the electric blender wet. The final product is edible and can be decomposed upon the addition of trisodium citrate solution.
Potential Discussion Topics
-
1.
Green polymers
-
2.
Single-replacement reactions
-
3.
Tissue engineering
-
4.
Non-Newtonian Fluids
-
5.
Rheology/Viscosity
Items Needed
1 cup of water in a small container
4 cups of cold water in a large container
Electric blender
1/4 teaspoon sodium alginate
1 teaspoon calcium lactate
Food coloring (optional)
Procedure
Add 1/4 teaspoon of sodium alginate to the small container with 1 cup of water in it. Stir this solution vigorously with the electric blender until the sodium alginate dissolves. Separately, add 1 teaspoon of calcium lactate to the large container that has 4 cups of cold water in it, and stir until the solution is clear. Dip a spoon/ladle into the sodium alginate solution until the spoon/ladle is nearly full with the sodium alginate solution. Slowly submerge the spoon/ladle that is holding the sodium alginate solution into the calcium lactate solution. Leave the spoon/ladle containing the sodium alginate solution submerged in the calcium lactate solution for at least 15 s. Finally, slowly empty the contents of the spoon/ladle into the calcium lactate solution. The Alginate Slime can now be retrieved from the large container with bare hands or kitchen utensils. For the optional use of food coloring, add a food coloring to the sodium alginate solution prior to submerging the sodium alginate into the calcium lactate. Note: Using an electric blender mitigates the difficulty of dissolving sodium alginate in water. This difficulty can also be mitigated by warming the water in the small container prior to adding the sodium alginate, making sure that the water does not boil. For large groups (>15 students) this demonstration may work best by breaking the group into smaller groups (e.g., three groups of five students) and then placing a bowl of sodium alginate and calcium lactate solutions at each table. This procedure can be completed in ∼10 min.
Oobleck
Safety
Neither eye protection nor gloves are required.
Potential Discussion Topics
-
1.
Non-Newtonian Fluids
-
2.
Rheology/Viscosity
Items Needed
1 cup corn starch
1/2 cup room-temperature water
Large bowl
Food coloring (optional)
Procedure
Add 1 cup of corn starch to a large bowl. Add 1/2 cup of room-temperature water to the corn starch in the bowl. Mix the water and corn starch together by hand or with a kitchen utensil (e.g., spoon). Food coloring can be added to the water to give the Oobleck any desired color. The corn starch can be recovered by evaporating the water from it. This procedure can be completed in ∼5 min.
Gummy Candy Slime
Safety
Burn hazard (items heated in the microwave will be very hot for several minutes). Safety glasses recommended.
Potential Discussion Topics
-
1.
Polymer materials in food science
-
2.
Biopolymers
-
3.
Polymer fillers
-
4.
Chemistry of sugars
Items Needed
1 cup of gummy worm/bear candies
Cooking oil
Powdered sugar
Corn starch
Large mixing bowl
Nonstick spatula
Procedure
Place 1 cup of gummy candy into a microwaveable mixing bowl (Figure 4). Place the bowl with the gummy candy in a microwave, and microwave for ∼30 s (or until gummy candy has softened significantly). Remove the bowl with the gummy candy from the microwave, and allow the gummy candy to cool for a few minutes. Using a nonstick spatula, blend in 2 tablespoons of corn starch. Also add 1 tablespoon of powdered sugar, and blend it in with the nonstick spatula. Add small amounts (1 teaspoon or less) of cooking oil to aid in the mixing. Once the gummy candy has cooled enough, knead it by hand to more thoroughly blend the components together. More cooking oil can be added to keep the gummy slime stretchy. The final product can be eaten, and leftover material can be composted. This procedure can be completed in ∼25 min.
Figure 4.
Making Gummy Candy Slime.
Marshmallow Slime
Safety
Burn hazard (items heated in the microwave will be very hot for several minutes). Safety glasses recommended.
Potential Discussion Topics
-
1.
Polymer materials in food
-
2.
Chemistry of sugars
Items Needed
1 and 1/2 tablespoons corn starch
1 and 1/2 tablespoons cooking oil
10 large marshmallows (or 1 cup mini-marshmallows)
Large mixing bowl
Nonstick spatula
Food coloring (optional)
Powdered/Confectioners’ Sugar (optional)
Procedure
Place 10 large marshmallows into a microwaveable mixing bowl (Figure 5). Add 1 and 1/2 tablespoons of cooking oil to the mixing bowl. Place the bowl with the marshmallows in a microwave, and microwave for ∼30 s (or until marshmallows have softened significantly). Remove the bowl with the marshmallows from the microwave, and allow the marshmallows to cool for ∼2 min. Using a nonstick spatula, blend in 1 and 1/2 tablespoons of corn starch. Food coloring (2–4 drops) can be added after the corn starch is added and while the marshmallows are still soft. After a few minutes, the marshmallows should be cool enough to be handled by hand, and the food coloring can be blended in more thoroughly. To alter the consistency of the marshmallows, a little corn starch can be added as the blending process continues. As an alternative (or in addition) to the corn starch, powdered sugar can be added to alter the consistency of the marshmallow slime. The final product can be eaten, and the leftover material can be composted. This procedure can be completed in ∼20 min.
Figure 5.
Making Marshmallow Slime.
Gummy Bear Osmosis
Safety
Gloves are not required. Safety glasses are recommended if fluids other than water (e.g., rubbing alcohol) are utilized.
Potential Discussion Topics
-
1.
Osmosis and diffusion
-
2.
Polymer membranes in real-world applications
Items Needed
Gummy bear candies
Room-temperature water
Clear transparent container(s)
Other fluids (e.g., vegetable oil, corn syrup, honey)
Procedure
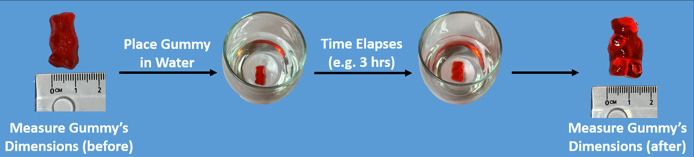
Using a ruler, measure the height, length, and width of a gummy bear candy and record these dimensions (Figure 6). Then place the gummy bear candy in a clear transparent container. Fill the container with room-temperature water and allow the container to sit at room temperature undisturbed for a specified amount of time (e.g., 3 h). Once the allotted time has passed, remove the gummy bear candy from the water and measure its dimensions. Compare the results with the initial measurements. A more in-depth variation involves placing gummy bear candies in differing fluids (e.g., water versus vegetable oil versus corn syrup) of the same temperature after measuring the dimensions of each gummy bear candy. In this variation, allow the gummy bear candies to stay in their respective fluids for the same amount of time, then measure their dimensions once the allotted time has passed. Compare the before and after dimensions for each individual gummy bear–fluid combination, then compare the results between gummy bear–fluid combinations. This experimental variation will give some indication as to which fluid the semipermeable gummy bear candy is most permeable to. The gummy bear candies can be composted. The time needed to complete this procedure will vary, based upon the time the gummy bear is submerged in fluid. Allotting at least 1 h to complete this procedure is recommended.
Figure 6.
Gummy Bear Osmosis demonstration.
Homemade Glue
Safety
Neither eye protection nor gloves are required. Although gloves are not required, they may be advisible due to the sticky nature of the material being made.
Potential Discussion Topics
-
1.
Adhesives/Glues (both naturally occurring and synthetic)
-
2.
Liquid polymers and viscosity time scales
Items Needed
2 teaspoons gelatin
1 teaspoon sugar
2 and 1/2 tablespoons lukewarm water
Small container
Stirring utensil
Measuring spoons
Paper/notecards/card stock
Procedure
Place 2 and 1/2 tablespoons of lukewarm water into a small container. Also add 1 teaspoon of sugar to the container. Next, stir in 2 teaspoons of gelatin using your stirring utensil. Stir vigorously until the mixture thickens and is uniform in texture and appearance (Figure 7). The mixture can then be removed from the container and spread onto a notecard using a utensil like a butter knife. Place another notecard on top of the mixture such that the mixture is sandwiched between two notecards. Firmly press the two notecards together for ∼10 s. An assessment of the quality of the adhesive mixture can now be made. The resulting adhesive mixture can be composted. This procedure can be completed in ∼10 min.
Figure 7.
Making Homemade Glue.
Outlook and Conclusions
Replacing common polymer demonstrations that result in harmful polymer materials (e.g., the making of Borax Slime) requires the attention and efforts of polymer scientists at all education levels and in all polymer science venues. This does not necessarily mean that harmful demonstrations need to be disposed of conceptually. Rather, they can sometimes be used as teaching vehicles to further promote healthier and more environmentally friendly polymer demonstrations. For example, an instructor can explain the polymeric transformation of Borax Slime (i.e., the conversion of a linear polymer into a three-dimensional polymer) while also explaining the reasons why making Borax Slime is harmful and why other slime demonstrations are now being utilized instead. The discussion could then be expanded to talk about safety concerns when handling certain synthetic materials like homemade slime, and a further discussion about the environmental hazards of glitter and microparticle additives can be had. And of course, comparisons between the chemistries used in the syntheses of Borax Slime and safer types of slime can be discussed and explained. Fortunately, as this Perspective details, polymer scientists are indeed devising healthier and more environmentally friendly means to insert polymer science into educational curricula and activities.
Note that this Perspective is not written to disparage anyone who has ever made harmful polymers like Borax Slime, whether in an educational setting or for fun. Neither is it meant to scuttle the slime-making fun of the thousands of youngsters who enjoy “slime time.” This Perspective is written to urge every individual and institution to move away from making physically harmful, nonreusable polymers that are environmentally damaging and to encourage a movement toward making polymer materials that yet provide educational opportunities while having a minimal negative impact on human health and the environment. This focus comes as part of a broader shift in STEM education in which the fun and engaging science demonstrations also present opportunities to educate about preserving and protecting the planet.52,53 Today we know that many types of homemade polymers (e.g., slimes) can be damaging to both the environment and to the physical well-being of people who make and handle them.54 Therefore, it is time to update the polymer education curriculum by using less harmful polymer recipes.
Biography
Dr. Darryl Boyd is the Co-Founder and Senior Scientist for Science Made Simple LLC, through which he offers his science knowledge to organizations throughout the United States via various forums as “Dr. Boyd The Chemist.” This includes his STEM-focused YouTube channel (https://youtube.com/c/drboydTheChemist). He is also a Research Chemist at the U.S. Naval Research Laboratory, where he develops novel infrared-transmitting optical polymers. His honors include being named to the Chemical & Engineering News “Talented 12” class of 2018.
The author declares no competing financial interest.
References
- Thompson R. C.; Swan S. H.; Moore C. J.; vom Saal F. S. Our plastic age. Philos. Trans. R. Soc., B 2009, 364 (1526), 1973–1976. 10.1098/rstb.2009.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millican J. M.; Agarwal S. Plastic Pollution: A Material Problem?. Macromolecules 2021, 54 (10), 4455–4469. 10.1021/acs.macromol.0c02814. [DOI] [Google Scholar]
- Andrady A. L.; Neal M. A. Applications and societal benefits of plastics. Philos. Trans. R. Soc., B 2009, 364 (1526), 1977–1984. 10.1098/rstb.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer R.; Jambeck J. R.; Law K. L. Production, use, and fate of all plastics ever made. Science Advances 2017, 3 (7), e1700782. 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. L.; Kelly F. J. Plastic and Human Health: A Micro Issue?. Environ. Sci. Technol. 2017, 51 (12), 6634–6647. 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- Anastas P. T.; Warner J. C., The Twelve Principles of Green Chemistry. Green Chemistry: Theory and Practice ;Oxford University Press: New York, 1998. [Google Scholar]
- Carraher C. E.; Berda E.; Blum F. D.; Droske J. P.; Ford W. T.; Howell B. A.; Long J. M.; Morgan S. E. History of Polymer Education in the United States through the Efforts of the Committee on Polymer Education and the Intersociety Polymer Education Council. J. Chem. Educ. 2017, 94 (11), 1607–1609. 10.1021/acs.jchemed.7b00614. [DOI] [Google Scholar]
- Ting J. M.; Ricarte R. G.; Schneiderman D. K.; Saba S. A.; Jiang Y.; Hillmyer M. A.; Bates F. S.; Reineke T. M.; Macosko C. W.; Lodge T. P. Polymer Day: Outreach Experiments for High School Students. J. Chem. Educ. 2017, 94 (11), 1629–1638. 10.1021/acs.jchemed.6b00767. [DOI] [Google Scholar]
- Garrett B.; Matharu A. S.; Hurst G. A. Using Greener Gels To Explore Rheology. J. Chem. Educ. 2017, 94 (4), 500–504. 10.1021/acs.jchemed.6b00584. [DOI] [Google Scholar]
- Billiet S.; Trenor S. R. 100th Anniversary of Macromolecular Science Viewpoint: Needs for Plastics Packaging Circularity. ACS Macro Lett. 2020, 9 (9), 1376–1390. 10.1021/acsmacrolett.0c00437. [DOI] [PubMed] [Google Scholar]
- Palucka T. Toying With a Ridiculous Material. MRS Bull. 2007, 32 (3), 283–283. 10.1557/mrs2007.31. [DOI] [Google Scholar]
- Casassa E. Z.; Sarquis A. M.; Van Dyke C. H. The gelation of polyvinyl alcohol with borax: A novel class participation experiment involving the preparation and properties of a ″slime″. J. Chem. Educ. 1986, 63 (1), 57. 10.1021/ed063p57. [DOI] [Google Scholar]
- Mattel, Slime
- Mak S. L.; Chiu W. H.; Tang W. F.; Li C. H. An Investigation and Optimization Study of Borax Content in the Slime Products. J. Test. Eval. 2021, 49 (3), 2175–2186. 10.1520/JTE20200212. [DOI] [Google Scholar]
- Scipioni J.Slime Craze forces Elmer’s Glue to Boost Production. Fox Business News 2017. [Google Scholar]
- Stretching out the slime fad. C&EN Global Enterprise 2018, 96 ( (25), ), 18–18. [Google Scholar]
- de Zea Bermudez V.; de Almeida P. P.; Seita J. F. How To Learn and Have Fun with Poly(Vinyl Alcohol) and White Glue. J. Chem. Educ. 1998, 75 (11), 1410. 10.1021/ed075p1410. [DOI] [Google Scholar]
- Alesanco Y.; Palenzuela J.; Viñuales A.; Cabañero G.; Grande H. J.; Odriozola I. Polyvinyl Alcohol–Borax Slime as Promising Polyelectrolyte for High-Performance, Easy-to-Make Electrochromic Devices. ChemElectroChem 2015, 2 (2), 218–223. 10.1002/celc.201402265. [DOI] [Google Scholar]
- Hurst G. A.; Bella M.; Salzmann C. G. The Rheological Properties of Poly(vinyl alcohol) Gels from Rotational Viscometry. J. Chem. Educ. 2015, 92 (5), 940–945. 10.1021/ed500415r. [DOI] [Google Scholar]
- Heller E.; Murthy A. S.; Jen M. V. A slime of the times: Two cases of acute irritant contact dermatitis from homemade slime. Pediatric dermatology 2019, 36 (1), 139–141. 10.1111/pde.13617. [DOI] [PubMed] [Google Scholar]
- Asher C.; Dalan R.; Aly M. I. ″Home-made slime″: A novel cause for paediatric burns’ referrals; do we need to raise awareness?. Burns 2018, 44 (6), 1613. 10.1016/j.burns.2018.04.020. [DOI] [PubMed] [Google Scholar]
- Salman A.; Demir G.; Apti O. Slime”: A trending cause of isothiazolinone contact allergy in children. Contact Dermatitis 2019, 80 (6), 409–411. 10.1111/cod.13237. [DOI] [PubMed] [Google Scholar]
- Aerts O.; De Fré C.; van Hoof T.; Ghys K.; Ortopelea R. A.; Lambert J. Slime”: A new fashion among children causing severe hand dermatitis. Contact Dermatitis 2018, 79 (6), 385–387. 10.1111/cod.13090. [DOI] [PubMed] [Google Scholar]
- Gittler J. K.; Garzon M. C.; Lauren C. T. ″Slime″ May Not be so Benign: A Cause of Hand Dermatitis. J. Pediatr. 2018, 200, 288. 10.1016/j.jpeds.2018.03.064. [DOI] [PubMed] [Google Scholar]
- Kondratuk K. E.; Norton S. A. "Slime” dermatitis, a fad-associated chronic hand dermatitis. Pediatric Dermatology 2019, 36 (1), e39–e40. 10.1111/pde.13729. [DOI] [PubMed] [Google Scholar]
- Zhang A. J.; Boyd A. H.; Asch S.; Warshaw E. M. Allergic contact dermatitis to slime: The epidemic of isothiazolinone allergy encompasses school glue. Pediatric dermatology 2019, 36 (1), e37–e38. 10.1111/pde.13681. [DOI] [PubMed] [Google Scholar]
- Brazen B. C.; Wehausen B.; Usmani A. A. Not All Fun and Games: A Case Report of Contact Dermatitis Related to Slime and Play-Doh. Cureus 2020, 12 (9), e10272–e10272. 10.7759/cureus.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman K.; Brea I. Do-it-yourself slime: A novel cause of pediatric burns. American journal of emergency medicine 2020, 38 (10), 2247. 10.1016/j.ajem.2020.05.067. [DOI] [PubMed] [Google Scholar]
- Winder C.; Azzi R.; Wagner D. The development of the globally harmonized system (GHS) of classification and labelling of hazardous chemicals. J. Hazard. Mater. 2005, 125 (1), 29–44. 10.1016/j.jhazmat.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Isokawa N.; Fueda K.; Miyagawa K.; Kanno K. Demonstration of the Coagulation and Diffusion of Homemade Slime Prepared Under Acidic Conditions without Borate. J. Chem. Educ. 2015, 92 (11), 1886–1888. 10.1021/acs.jchemed.5b00272. [DOI] [Google Scholar]
- Herman A.; Aerts O.; de Montjoye L.; Tromme I.; Goossens A.; Baeck M. Isothiazolinone derivatives and allergic contact dermatitis: a review and update. Journal of the European Academy of Dermatology and Venereology 2019, 33 (2), 267–276. 10.1111/jdv.15267. [DOI] [PubMed] [Google Scholar]
- Fenner J.; Hadi A.; Yeh L.; Silverberg N. Hidden risks in toys: A systematic review of pediatric toy contact dermatitis. Contact Dermatitis 2020, 82 (5), 265–271. 10.1111/cod.13500. [DOI] [PubMed] [Google Scholar]
- Green D. S.; Jefferson M.; Boots B.; Stone L. All that glitters is litter? Ecological impacts of conventional versus biodegradable glitter in a freshwater habitat. J. Hazard. Mater. 2021, 402, 124070. 10.1016/j.jhazmat.2020.124070. [DOI] [PubMed] [Google Scholar]
- Microplastic Beads Pollute Great Lakes. Chemical & Engineering News Archive 2013, 91 ( (37), ), 23–25. [Google Scholar]
- Bowles R. D.; Saroka J. M.; Archer S. D.; Bonassar L. J. Novel Model-Based Inquiry of Ionic Bonding in Alginate Hydrogels Used in Tissue Engineering for High School Students. J. Chem. Educ. 2012, 89 (10), 1308–1311. 10.1021/ed200651f. [DOI] [Google Scholar]
- Erdal N. B.; Hakkarainen M.; Blomqvist A. G. Polymers, Giant Molecules with Properties: An Entertaining Activity Introducing Polymers to Young Students. J. Chem. Educ. 2019, 96 (8), 1691–1695. 10.1021/acs.jchemed.8b00918. [DOI] [Google Scholar]
- Ward A. M.; Wyllie G. R. A. Bioplastics in the General Chemistry Laboratory: Building a Semester-Long Research Experience. J. Chem. Educ. 2019, 96 (4), 668–676. 10.1021/acs.jchemed.8b00666. [DOI] [Google Scholar]
- Gombotz W. R.; Wee S. Protein release from alginate matrices. Adv. Drug Delivery Rev. 1998, 31 (3), 267–285. 10.1016/S0169-409X(97)00124-5. [DOI] [PubMed] [Google Scholar]
- Morris E. R.; Rees D. A.; Thom D.; Boyd J. Chiroptical and stoichiometric evidence of a specific, primary dimerisation process in alginate gelation. Carbohydr. Res. 1978, 66 (1), 145–154. 10.1016/S0008-6215(00)83247-4. [DOI] [Google Scholar]
- Braccini I.; Pérez S. Molecular Basis of Ca2+-Induced Gelation in Alginates and Pectins: The Egg-Box Model Revisited. Biomacromolecules 2001, 2 (4), 1089–1096. 10.1021/bm010008g. [DOI] [PubMed] [Google Scholar]
- Zheng H.; Gao M.; Ren Y.; Lou R.; Xie H.; Yu W.; Liu X.; Ma X. An improved pH-responsive carrier based on EDTA-Ca-alginate for oral delivery of Lactobacillus rhamnosus ATCC 53103. Carbohydr. Polym. 2017, 155, 329–335. 10.1016/j.carbpol.2016.08.096. [DOI] [PubMed] [Google Scholar]
- Liang H.-F.; Hong M.-H.; Ho R.-M.; Chung C.-K.; Lin Y.-H.; Chen C.-H.; Sung H.-W. Novel Method Using a Temperature-Sensitive Polymer (Methylcellulose) to Thermally Gel Aqueous Alginate as a pH-Sensitive Hydrogel. Biomacromolecules 2004, 5 (5), 1917–1925. 10.1021/bm049813w. [DOI] [PubMed] [Google Scholar]
- Krishna V. S. R.; Hussain S.; Kiran C. H. R.; Kumar K. V. Experimental evaluation of impact energy on oobleck material (non-Newtonian fluid). Materials Today: Proceedings 2021, 45, 3609–3617. 10.1016/j.matpr.2020.12.1112. [DOI] [Google Scholar]
- Lin N. Y. C.; Guy B. M.; Hermes M.; Ness C.; Sun J.; Poon W. C. K.; Cohen I. Hydrodynamic and Contact Contributions to Continuous Shear Thickening in Colloidal Suspensions. Phys. Rev. Lett. 2015, 115 (22), 228304. 10.1103/PhysRevLett.115.228304. [DOI] [PubMed] [Google Scholar]
- Bayline J. L.; Tucci H. M.; Miller D. W.; Roderick K. D.; Brletic P. A. Chemistry of Candy: A Sweet Approach to Teaching Nonscience Majors. J. Chem. Educ. 2018, 95 (8), 1307–1315. 10.1021/acs.jchemed.7b00739. [DOI] [Google Scholar]
- Hani N. M.; Romli S. R.; Ahmad M. Influences of red pitaya fruit puree and gelling agents on the physico-mechanical properties and quality changes of gummy confections. Int. J. Food Sci. Technol. 2015, 50 (2), 331–339. 10.1111/ijfs.12638. [DOI] [Google Scholar]
- Tireki S.; Sumnu G.; Sahin S. Correlation between physical and sensorial properties of gummy confections with different formulations during storage. J. Food Sci. Technol. 2021, 58 (9), 3397–3408. 10.1007/s13197-020-04923-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartel R. W.; Hartel A. K. Candy Bites: The Science of Sweets 2014, 1 (XIV), 269. 10.1007/978-1-4614-9383-9. [DOI] [Google Scholar]
- Koide A.; Kishimura A.; Osada K.; Jang W.-D.; Yamasaki Y.; Kataoka K. Semipermeable Polymer Vesicle (PICsome) Self-Assembled in Aqueous Medium from a Pair of Oppositely Charged Block Copolymers: Physiologically Stable Micro-/Nanocontainers of Water-Soluble Macromolecules. J. Am. Chem. Soc. 2006, 128 (18), 5988–5989. 10.1021/ja057993r. [DOI] [PubMed] [Google Scholar]
- Pendergast M. M.; Hoek E. M. V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4 (6), 1946–1971. 10.1039/c0ee00541j. [DOI] [Google Scholar]
- Mackenzie L. S.; Tyrrell H.; Thomas R.; Matharu A. S.; Clark J. H.; Hurst G. A. Valorization of Waste Orange Peel to Produce Shear-Thinning Gels. J. Chem. Educ. 2019, 96 (12), 3025–3029. 10.1021/acs.jchemed.8b01009. [DOI] [Google Scholar]
- Fagnani D. E.; Hall A. O.; Zurcher D. M.; Sekoni K. N.; Barbu B. N.; McNeil A. J. Short Course on Sustainable Polymers for High School Students. J. Chem. Educ. 2020, 97 (8), 2160–2168. 10.1021/acs.jchemed.0c00507. [DOI] [Google Scholar]
- Cannon A. S.; Keirstead A. E.; Hudson R.; Levy I. J.; MacKellar J.; Enright M.; Anderson K. R.; Howson E. M. Safe and Sustainable Chemistry Activities: Fostering a Culture of Safety in K–12 and Community Outreach Programs. J. Chem. Educ. 2021, 98 (1), 71–77. 10.1021/acs.jchemed.0c00128. [DOI] [Google Scholar]
- Hurst G. A. Systems thinking approaches for international green chemistry education. Current Opinion in Green and Sustainable Chemistry 2020, 21, 93–97. 10.1016/j.cogsc.2020.02.004. [DOI] [Google Scholar]