Abstract
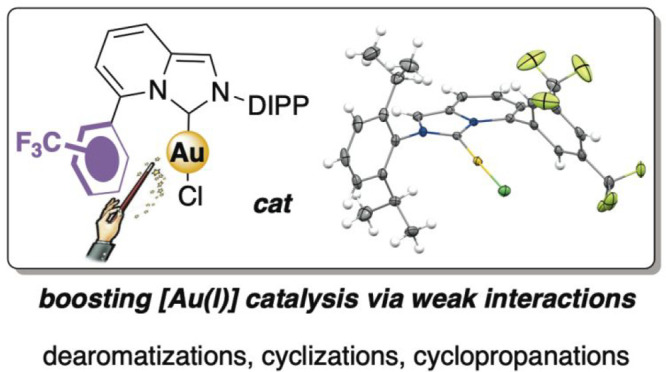
A series of modular ImPy–carbene–Au(I) complexes are synthesized and fully characterized both in the solid state and in solution. The presence of oligoaryl units (phenyl and thienyl rings) at the C5-position of the ImPy core (in close proximity to the gold center) imprints on the organometallic species fine-tunable and predictable catalytic properties. A marked accelerating effect was recorded in several [Au(I)]-catalyzed electrophilic activations of unsaturated hydrocarbons when a CF3-containing aromatic ring was accommodated at the ImPy core.
Keywords: carbene ligands, fine-tunability gold, homogeneous catalysis, secondary interactions
Homogenous gold catalysis has rapidly emerged as an established tool in synthetic organic methodologies.1−4 Despite its relatively young age (pioneering studies date back to early 2000), the use of gold(I) species in the electrophilic activation of unsaturated hydrocarbons and cross-coupling reactions has already begun to parallel the use of longer investigated and more consolidated noble metal catalysis. The robustness and adaptability of gold(I) catalysis in multifaceted applications is witnessed daily by its exploitation to enable synthetic methodologies such as photocatalysis, the total synthesis of structurally elaborated bioactive and photoactive molecular motifs, asymmetric catalysis, dual catalysis, electrosynthesis, and flow-chemistry.5−8 This scenario is the consequence of the ongoing request for dedicated gold complexes featuring a predesigned flexible and fine-tunable catalytic performance.9 Among others, the use of readily available N-heterocyclic carbenes (NHCs)10−13 as ligands in gold chemistry14−18 deserves a particular mention due to the strong σ-donating properties of the soft singlet carbon atom of NHCs, which results in Au complexes (soft metal) commonly featuring high stabilities and yet unique catalytic activities.19,20 Additionally, NHCs can rapidly access impressive organometallic diversity and complexity by incorporating a theoretically unlimited range of functionalities, a key requisite for the development of “on-demand” catalysts. In this wide and dynamic picture, the development of unsymmetrically substituted monodentate NHC ligands is receiving growing credit due to its prompt access to unlimited fine-tunable gold species and modular stereochemical environments for asymmetric transformations.21−23
The robust and readily accessible imidazo[1,5-a]pyridin-3-ylidene platform (ImPy) A (introduced almost simultaneously by Lassaletta24 and Glorius25) covers a prominent role in metal–carbene chemistry,26−33 allowing the installation of steric as well as electronic tools in close proximity to the metal center (i.e., C5-position of the ImPy core). In addition, ImPy shows a higher oxidation resistance and better synthetic accessibility with respect to P-based analogous ligands (Figure 1, top). As a consequence, over the past years fully characterized [Au(I)]- and [Au(III)]–ImPy complexes34−36 have been documented with ultimate applications in metal drug chemistry37 and catalysis.38−41 Modulating effects through steric constraints at the C5-position have been extensively investigated; however, the overall impact of electronic parameters have been far less systematically accounted so far and has been limited to the introduction of electron-rich aromatic pendants.42
Figure 1.
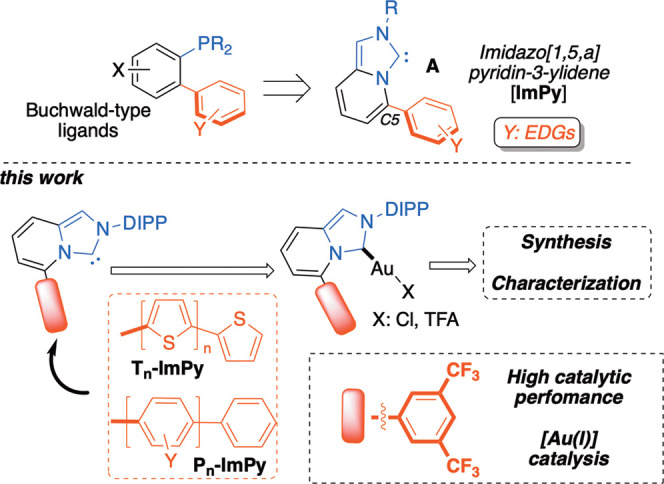
Chemical engineerization of fine-tunable C5-oligoaryl–ImPy gold complexes (DIPP, 2,5-diisopropyl-phenyl).
In the present work, a systematic investigation on a new family of C5-oligoaryl-ImPy gold complexes was carried out, disclosing an unprecedented role of the electron-deficient 3,5-(CF3)2-phenyl ring in modulating and enhancing the catalytic activity of cationic gold(I) complexes in several transformations involving carbocationic- or carbene-like organogold intermediates (Figure 1, bottom).19
In line with our ongoing interest in homogeneous gold catalysis43−50 and fine-tunable organometallic adducts via “secondary interactions”,51−56 we describe here two series of C5-oligoaryl imidazolium salts 6a–g, featuring phenyl- (Pn-ImPy) and 2-thienyl- (Tn-ImPy) based pendants (Scheme 1). The role of the electronic properties imprinted by the diversely functionalized π-clouds on the Pn-ImPy and Tn-ImPy carbene series was assessed in three different gold-catalyzed methodologies by a combined SC-XRD and DFT investigation of the corresponding [Au(I)] complexes.57,58
Scheme 1. Synthetic Sequence for the Preparation of Pn- and Tn-ImPy·HX 6a–g.
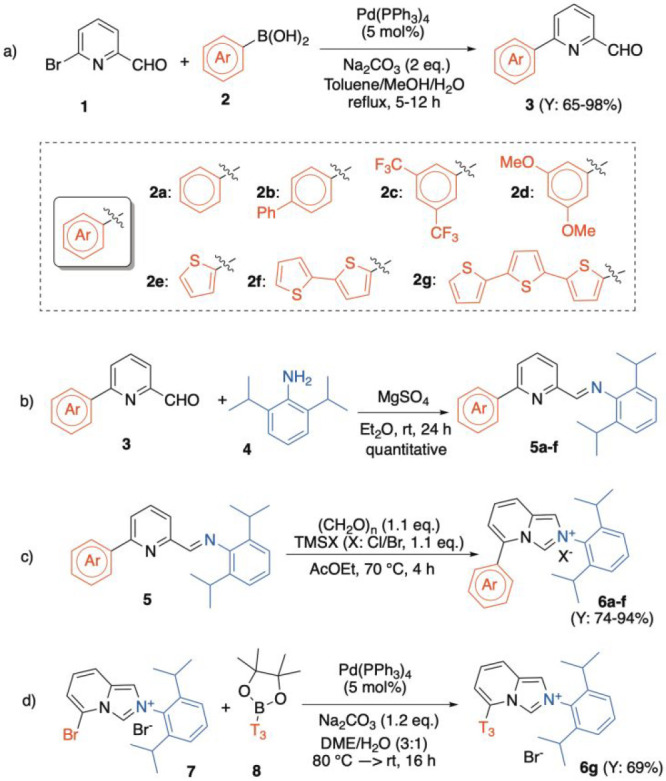
Imidazopyridinium salts were obtained via initial Suzuki–Miyaura cross-coupling reactions among the pyridyl aldehyde 1 and the corresponding aryl-boronic acids 2a–f. The 6-aryl pyridylcarbaldehydes 3 were isolated in excellent yields (65–98%) under optimal conditions (Scheme 1a). Then, the corresponding imines 5 were obtained quantitatively under dehydrative conditions (MgSO4, Et2O, rt) in the presence of 2,6-(iPr)2-aniline 4 (Scheme 1b). Finally, the desired imidazolium platforms 6a–f were achieved via a ring-closing strategy in the presence of (CH2O)n and TMSX (X = Br, Cl; 74–94%, Scheme 1c). Differently, for the synthesis of the T3-ImPy·HBr 6g, a Pd(0)-catalyzed Suzuki–Miyaura coupling between the Br derivative 7(40) and the commercially available terthienyl-boronate 8 (69%) was adopted.
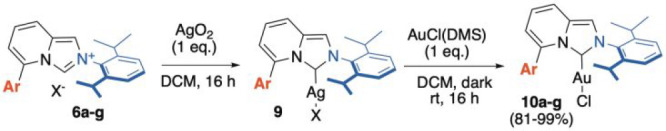
Subsequently, the C(5)-substituted imidazo[1,5-a]pyridinium halides 6a–g were subjected to the [Au(I)] complexation (10a–g) via metal metathesis through the corresponding Ag-complexes (9). The protocol (DCM, rt, dark, 16 h) proved highly competent in all cases, yielding the desired air-stable LAuCl complexes in excellent overall yields (81–99%, Scheme 2).
Scheme 2. Two-Step Procedure for the Synthesis of Tn- or PnImPy–AuCl complexes 10a–gvia a Metal Exchange Protocol of the Silver Analogues 9.
Some of us has recently documented the combined role of the cationic [Au(I)] center and basic anions59,60 in the chemo-, regio-, and stereoselective dearomatization of 2-naphthols61−67 with allenamides,68−70 discovering the synergistic role of multiple secondary interactions in controlling the mechanistic profile.71,72 In this line, we elected the dearomative process of dimethyl-naphthol 12 and allenamide 13 (Scheme 3) as a model control experiment for testing new [ImPyAuCl] complexes 10a–g.73 For these experiments, the corresponding trifluoroacetate complexes 11a–g were generated in situ via a chloride scavenging reaction of complexes 10 with AgTFA. The catalytic performances of the NHC–gold complexes are summarized in Scheme 3.
Scheme 3. Pn-ImPy–AuCl/AgTFA- and Tn-ImPy–AuCl/AgTFA-Catalyzed Dearomatization of Naphthol 12.
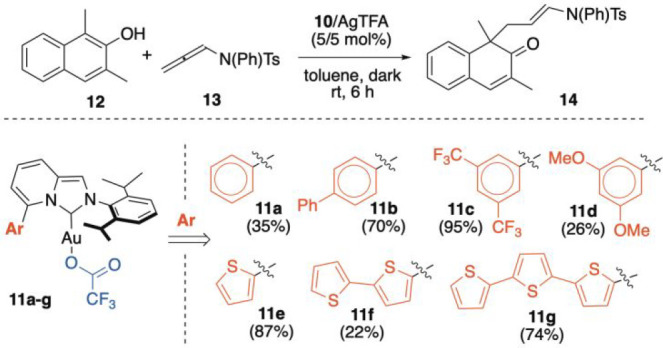
The yields are reported as an average of two runs.
From the data collected in the Scheme 3, several interesting conclusions can be drawn. The Pn-ImPyAuCl series provided product 14 from very low to almost quantitative yields (26–95%) with an unexpected correlation to the electronic properties of the pendant group at C5. As a matter of fact, while electron-rich pendants are generally accommodated in the organic ligand to stabilize cationic Au(I) intermediates via stacking interactions,74 in our case the incorporation of the 3,5-(CF3)2-C6H3 unit (11c) resulted as the best catalyst, providing 14 in a 95% yield.
Furthermore, complex 11c showed the highest initial reaction rate among the complexes tested (11a–11d); more precisely, the rate was ca. 2.8× higher than that of the 3,5-(OMe)2Ph-containing NHC–[Au(I)] complex 11d (see the SI for details).
To shed light on this intriguing and unexpected chemical outcome, a dedicated crystallographic investigation was carried out on both series of Pn- and Tn-ImPy–AuCl complexes. Suitable single crystals for the SC-XRD analysis of complexes 10a–g were obtained via vapor diffusion of n-hexane in DCM/toluene solutions of gold adducts. Some representative X-ray structures are depicted in Figure 2 (see the SI for further details).
Figure 2.
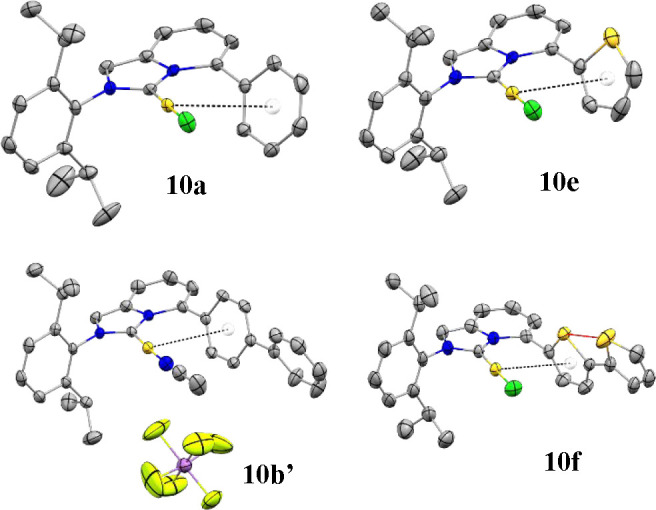
Representative molecular structures for complexes 10a, 10e, 10b′, and 10f. Hydrogen atoms were removed for clarity. The Arcentroid···Au interaction is shown as black dashed lines. The S1···S2 interaction (10f) is shown as a red line.
All synthesized [Au(I)] complexes showed a linear bicoordination geometry with the Cl atom trans to the NHC ligand and an almost perpendicular orientation of the bulky DIPP unit with respect to the imidazolyl ring (dihedral angles range from 83.84° to 87.79°, Table S11). This common arrangement is a consequence of the rotational restriction faced by the 2,6-diisopropyl substituents. Additionally, intramolecular Au···π contacts with the aryl pendants at the C5-position were observed.
In line also with recent findings by Toste and Sigman,75 who elegantly disclosed strong structure–catalytic activity correlations in regio-divergent [Au(I)]-catalyzed cycloaddition reactions, we targeted Au–Cl, Au–C1, and Au···Ar crystallographic distances as potential probe parameters to indirectly predict or rationalize the catalytic performances of the new ImPy–AuCl complexes. In this study, the cationic [10b–Au(CH3CN)](SbF6) complex 10b′ (Figure 2) was also taken into account, comparing the influence of the cationic metal center on the crystallographic data.
In Table 1, several structural parameters have been collected for a simplified comparative analysis.
Table 1. Structural Parameters Obtained by SC-XRD for ImPy–AuCl Complexes 10.
| 10 | Au–Cl (Å) | Au–C1 (Å) | Au···Ar (Å)a | Ar–ImPy (°)b |
|---|---|---|---|---|
| 10a | 2.304(3) | 1.983(7) | 3.511 | 56.95 |
| 10b | 2.2828(9) | 1.985(3) | 3.412 | 67.65 |
| 10b’ | 2.019(3)b | 1.974(3) | 3.384 | 61.62 |
| 10c | 2.287(1) | 1.982(5) | 3.352 | 64.63 |
| 10d | 2.2765(9) | 1.980(2) | 3.353 | 64.94 |
| 10e | 2.277(2) | 1.981(7) | 3.526 | 57.52 |
| 10f | 2.267(2) | 1.983(6) | 3.477 | 82.58 |
| 10g | 2.2932(6) | 1.970(3) | 3.456 | 73.20 |
The distance between [Au(I)] and the center of mass of the aromatic ring at C5, as calculated by Mercury.
The Au–N(ACN) distance.
Interestingly, the Au–C1 distances were scarcely affected by the electronic properties of the ligand (Au–C1 range of 1.970–1.983 Å). The truncated conjugation at C5 between the ImPy core and the aryl ring could be responsible for this trend. The large dihedral angles recorded in the solid state for the two aromatic systems (Ar and ImPy, 56.95–82.58°) are in good agreement with this hypothesis. Similarly, the Au–Cl distances (2.267–2.304 Å) showed only slight variations. In particular, while almost constant Au–Cl distances were observed in the Pn-ImPyAuCl series (10a–d), the Tn-ImPyAuCl family shows shorter distances for mono- and bithienyl substituents (10e and 10f) but a longer distance in the case of the tert-thienyl pendant (10g).
Therefore, Au···Ar contacts were examined across the two series Pn and Tn. First, by increasing the number of aryl units in both series, closer intramolecular contacts were identified. This trend nicely correlates with the electron-richness of oligoaryl cores that increases along with the number of repeating units. Second, a comparison between the 10b′ and 10b (entries 2 and 3, Table 1) revealed only a slight contraction in both the C1–Au bond and the Au···Ar contact for the cationic complex, supporting the suitability of the Au–Cl precursors 10a–g for the structure–activity relationship investigation. Interestingly, the introduction of arenes featuring opposite electronic properties such as 3,5-(OMe)2-Ph (10d) and 3,5-(CF3)2-Ph (10c) caused a shortening of the Au···Ar distances (3.353–3.352 Å) in both cases with respect to unsubstituted 10a (3.511 Å). However, a drastic change in the catalytic outcome was highlighted in the 10a–c series (95% yield with 10c and 26% yield with 10d).
Concerning the Tn-ImPyAuCl series, no direct S(thienyl)···Au contacts have emerged regardless the length of the oligothienyl.49,76 Surprisingly, 10f showed a quite uncommon cis-arrangement of the two thienyl sulfur atoms in the solid state (Figure 2, S1···S2 = 3.301 Å).77 This feature can be attributed to the fact that the S1···S2 forces play a stabilizing role in the packing efficiency.78 On the contrary, complex 10g bearing a terthienyl chain at C5 shows the conventional trans-trans-arrangement of the thienyl units (see the SI). The uncommon cis-arrangement observed for the T2 side arm could be tentatively targeted to rationalize the unsatisfactory catalytic performance of 10f–AgTFA (Y = 22%).79 Differently, T1 and T3 bearing gold complexes worked smoothly in the dearomative process, delivering 14 in 87% and 74% yields, respectively. The slight drop in the chemical outcome for 10g might be ascribed to the more electron-rich T3 and consequent “shielding” of the electrophilic properties of [Au(I)] center (Au···Ar = 3.536 and 3.456 for 10e and 10g, respectively). Additionally, the photophysical properties of the T3-ImPy series (6g and 10g) were investigated both in solution (DCM) and in solid state (see Figures S1 and S2 and Table S4). Here, a significant drop in the emission quantum yield of the T3 salt 6g (6.2%) was recorded when the metal center was introduced (0.2%, 10g). This phenomenon could be tentatively assigned to a higher efficiency of the nonradiative intersystem crossing deactivation of the fluorescent excited state promoted by the heavy atom effect.
To further analyze the electronic nature of such Au···Ar interactions and to provide a visual representation of them, we resorted to electronic structure calculations using density functional theory on complexes 10a, 10c, and 10d.80,81 Geometry optimizations and a topological analysis of the obtained electron density was performed using the atoms in molecules (AIM) and non-covalent interactions (NCIs) models.82,83 Calculations were performed at the B3LYP/6-31+G(d) level for main-group atoms, and the SDD basis set and electron core potential were used for the Au atom.84,85 We resorted to this compact basis set due to the large size of the complexes and the previous systematic benchmark showing that this particular combination of density functional and basis set, although compact, was able to reproduce gold chemistry with a comparable accuracy to triple-ζ and even larger basis sets.86,87 Actually, our calculations faithfully reproduced the experimentally recorded X-ray structures. Not only close steric contacts were observed from the NCIs analysis (Figure 3, right) but a full covalent bond-like topology was also detected under the AIM analysis. A bond critical point can be found between the gold atom and the aryl fragment, along with the expected ring critical point to fulfill the Poincaré–Hopf rule. Therefore, both analyses point toward intimate Au···aryl interactions in these complexes (Figure 3, left).
Figure 3.
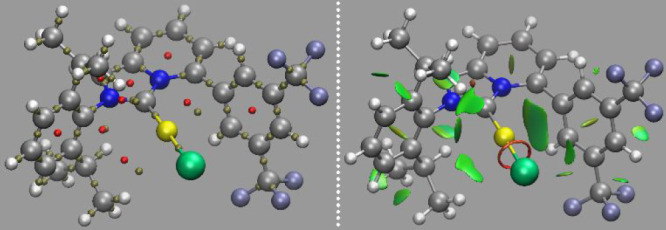
Ring and bond critical points of the electron density of 10c (left) and the close noncovalent Au···π-aryl steric contact (right).
Further support for this interpretation was gathered via a population analysis of the electron density calculated for intermediate A when the aryl C5-pendant was undecorated and also when it was meta-disubstituted with CF3 and OMe groups (Scheme 4). The partial charges found at the electrophilic end of the allenamide framework in these intermediates are fully compatible with the view of increased reactivity due to the electron-withdrawing effects of the C5-pendant when substituted with CF3. Gratifyingly, charge values at this reactive terminus (0.037, 0.035, and 0.041 au for the −OMe-substituted, unsubstituted, and −CF3-substituted systems, respectively) correlate well with the observed experimental yields.
Scheme 4. Proposed Reaction Intermediate A and Its Natural Bond Orbital (NBO) Partial Charge at the Electrophilic CH2 Terminus with Respect to the C5-Pendant Substitution.
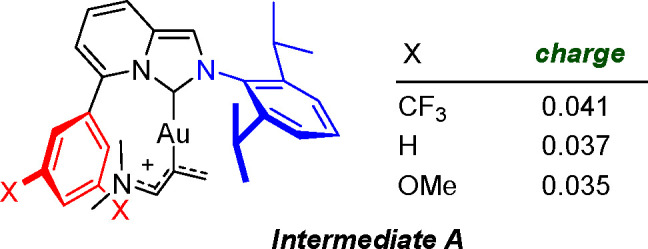
In addition, the marked deviation from planarity recorded for the NHC and aryl skeletal unit (see Table 1) precludes a major impact of the arene electronic features on the whole ImPy carbene fragment and elects the Ar···[Au] secondary contacts as responsible for the fine-tuning of the catalytic performance.
Finally, we decided to assess the extensibility of this unprecedent accelerating effect of the CF3-containg ring to other [Au(I)]-catalyzed manipulations of unsaturated hydrocarbons. In this regards, two chemical transformations based on carbocationic- or carbene-like organogold intermediates,19 namely olefin cyclopropanation88 and the cyclization of alkynylbenzaldehyde,89 were analyzed (Scheme 5).
Scheme 5. Olefin Cyclopropanations and Alkynylbenzaldehyde Cyclizations: Examples of the Accelerating Effect of CF3-Containing Carbene Ligands in [Au(I)] Catalysis.
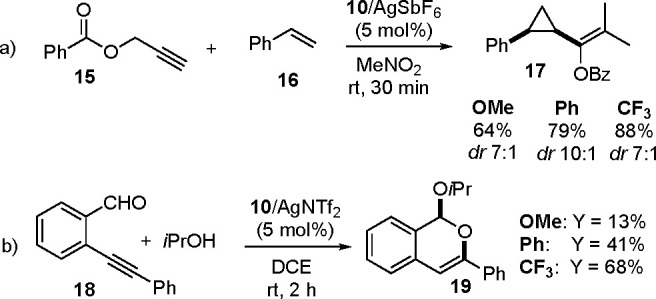
In this regard, the intermolecular olefin cyclopropanation introduced by Toste was first performed in the presence of complexes 10a,c–d/AgSbF6 (Scheme 5a). Interestingly, a marked increase of the chemical outcome toward the formation of the cyclopropane cis-17 (yield up to 88%) was observed when fluorinated complex 10c was adopted. The same trend was recorded also in the cyclization of the alkynylbenzaldehyde 18 in the presence of isopropanol. Here, 10c/AgNTf26 (5 mol %) provided the acetal 19 in a 68% yield, more than fivefold that obtained with the electron-rich complex 10d (Scheme 5b). Finally, it is worth mentioning that this reactivity trend was not affected by the loading of the gold complex. As a matter of fact, when catalysts 10 were employed in a 2.5 mol % loading, a similar chemical outcome relationship was recorded.90
In conclusion, we have documented the preparation, characterization (spectroscopic and crystallographic), and use in catalysis of a new class of ImPyAuX complexes featuring diversely substituted aromatic pendants at the C5-position. The impact of electronically different side-arms on the overall catalytic properties of the gold complexes (i.e., dearomatization of naphthols with allenamides) was examined through dedicated kinetic experiments, revealing an unusual accelerating action exerted by the 3,5-(CF3)2-Ph unit. Activating secondary interactions exerted by the ligand C5-pendant on the gold–alkylidene intermediate were accounted for the experimental outcome that resulted effective in several Au-mediated electrophilic activations of unsaturated hydrocarbons.
Acknowledgments
Acknowledgements are made to University of Bologna for financial support. M.B. is also grateful to the PRIN-2017 project 2017W8KNZW for financial support. C.S.L. is grateful to the Ministerio de Ciencia for financial support (PID2020-115789GB-C22) and CESGA for allocation of HPC time.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsorginorgau.1c00052.
General synthetic procedures; photophysical, crystallographic, and computational data; and NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Mato M.; Franchino A.; García-Morales C.; Echavarren A. M. Gold-Catalyzed Synthesis of Small Rings. Chem. Rev. 2021, 121, 8613–8684. 10.1021/acs.chemrev.0c00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.; Hashmi A. S. K. 1,2-Migrations onto Gold Carbene Centers. Chem. Rev. 2021, 121, 8948–8978. 10.1021/acs.chemrev.0c00811. [DOI] [PubMed] [Google Scholar]
- Campeau D.; Leon Rayo D. F.; Mansour A.; Muratov K.; Gagosz F. Gold-Catalyzed Reactions of Specially Activated Alkynes, Allenes, and Alkenes. Chem. Rev. 2021, 121, 8756–8867. 10.1021/acs.chemrev.0c00788. [DOI] [PubMed] [Google Scholar]
- Rocchigiani L.; Bochmann M. Recent Advances in Gold(III) Chemistry: Structure, Bonding, Reactivity, and Role in Homogeneous Catalysis. Chem. Rev. 2021, 121, 8364–8451. 10.1021/acs.chemrev.0c00552. [DOI] [PubMed] [Google Scholar]
- Hashmi A. S. K. Dual Gold Catalysis. Acc. Chem. Res. 2014, 47, 864–876. 10.1021/ar500015k. [DOI] [PubMed] [Google Scholar]
- Pflästerer D.; Hashmi A. S. K. Gold catalysis in total synthesis – recent achievements. Chem. Soc. Rev. 2016, 45, 1331–1367. 10.1039/C5CS00721F. [DOI] [PubMed] [Google Scholar]
- Zeitler K.; Neumann M. Synergistic visible light photoredox catalysis. Phys. Sci. Rev. 2019, 5, 20170173. 10.1515/psr-2017-0173. [DOI] [Google Scholar]
- Nijamudheen A.; Datta A. Gold-Catalyzed Cross-Coupling Reactions: An Overview of Design Strategies, Mechanistic Studies, and Applications. Chem. Eur. J. 2020, 26, 1442–1487. 10.1002/chem.201903377. [DOI] [PubMed] [Google Scholar]
- Zuccarello G.; Escofet I.; Caniparoli U.; Echavarren A. M. New-Generation Ligand Design for the Gold-Catalyzed Asymmetric Activation of Alkynes. ChemPlusChem. 2021, 86, 1283–1296. 10.1002/cplu.202100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez-González S.; Marion N.; Nolan S. P. N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. 10.1021/cr900074m. [DOI] [PubMed] [Google Scholar]
- Hopkinson M. N.; Richter C.; Schedler M.; Glorius F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. 10.1038/nature13384. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Zi G. N-heterocyclic carbene (NHC) complexes of group 4 transition metals. Chem. Soc. Rev. 2015, 44, 1898–1921. 10.1039/C4CS00441H. [DOI] [PubMed] [Google Scholar]
- Peris E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. 10.1021/acs.chemrev.6b00695. [DOI] [PubMed] [Google Scholar]
- Marion N.; Nolan S. P. N-Heterocyclic carbenes in gold catalysis. Chem. Soc. Rev. 2008, 37, 1776–1782. 10.1039/b711132k. [DOI] [PubMed] [Google Scholar]
- Nolan S. P. The Development and Catalytic Uses of N-Heterocyclic Carbene Gold Complexes. Acc. Chem. Res. 2011, 44, 91–100. 10.1021/ar1000764. [DOI] [PubMed] [Google Scholar]
- Gatineau D.; Goddard J.-P.; Mouriès-Mansuy V.; Fensterbank L. When NHC Ligands Make a Difference in Gold. Catalysis, Isr. J. Chem. 2013, 53, 892–900. 10.1002/ijch.201300059. [DOI] [Google Scholar]
- Tang X.-T.; Yang F.; Zhang T.-T.; Liu Y.-F.; Liu S.-Y.; Su T.-F.; Lv D.-C.; Shen W.-B. Recent Progress in N-Heterocyclic Carbene Gold-Catalyzed Reactions of Alkynes Involving Oxidation/Amination/Cycloaddition. Catalysts 2020, 10, 350. 10.3390/catal10030350. [DOI] [Google Scholar]
- Nahra F.; Tzouras N. V.; Collado A.; Nolan S. P. Synthesis of N-heterocyclic carbene gold(I) complexes. Nat. Prot. 2021, 16, 1476–1493. 10.1038/s41596-020-00461-6. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Muratore M. E.; Echavarren A. M. Gold Carbene or Carbenoid: Is There a Difference?. Chem. Eur. J. 2015, 21, 7332–7339. 10.1002/chem.201406318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Castro A.; Carey D. M.; Arratia-Perez R. Relativistic effects on dative carbon-coinage metal bond. Evaluation of NHC-MCl (M = Cu, Ag, Au) from relativistic DFT. Polyhedron 2021, 197, 115020. 10.1016/j.poly.2021.115020. [DOI] [Google Scholar]
- Zargaran P.; Wurm T.; Zahner D.; Schießl J.; Rudolph M.; Rominger F.; Hashmi A. S. K. A Structure-Based Activity Study of Highly Active Unsymmetrically Substituted NHC Gold(I) Catalysts. Adv.Synth.Catal. 2018, 360, 106–111. 10.1002/adsc.201701080. [DOI] [Google Scholar]
- Wurm T.; Hornung J.; O’Neill M.; Rudolph M.; Rominger F.; Hashmi A. S. K. Synthesis of Ester- and Phosphonate-Functionalized AuI–Imidazolylidene Chlorides through the Isonitrile Route. Chem. Eur. J. 2017, 23, 5143–5147. 10.1002/chem.201700214. [DOI] [PubMed] [Google Scholar]
- Kong L.; Morvan J.; Pichon D.; Jean M.; Albalat M.; Vives T.; Colombel-Rouen S.; Giorgi M.; Dorcet V.; Roisnel T.; Crévisy C.; Nuel D.; Nava P.; Humbel S.; Vanthuyne N.; Mauduit M.; Clavier H. From Prochiral N-Heterocyclic Carbenes to Optically Pure Metal Complexes: New Opportunities in Asymmetric Catalysis. J. Am. Chem. Soc. 2020, 142, 93–98. 10.1021/jacs.9b12698. [DOI] [PubMed] [Google Scholar]
- Alcarazo M.; Roseblade S. J.; Cowley A. R.; Fernández R.; Brown J. M.; Lassaletta J. M. A Versatile Architecture for Stable N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2005, 127, 3290–3291. 10.1021/ja0423769. [DOI] [PubMed] [Google Scholar]
- Burstein C.; Lehmann C. W.; Glorius F. Imidazo[1,5-a]pyridine-3-ylidenes—pyridine derived N-heterocyclic carbene ligands. Tetrahedron 2005, 61, 6207–6217. 10.1016/j.tet.2005.03.115. [DOI] [Google Scholar]
- Roseblade R. J.; Ros A.; Monge D.; Alcarazo M.; Álvarez E.; Lassaletta J. M.; Fernández R. Imidazo[1,5-a]pyridin-3-ylidene/Thioether Mixed C/S Ligands and Complexes Thereof. Organometallics 2007, 26, 2570–2578. 10.1021/om070063r. [DOI] [Google Scholar]
- Fürstner A.; Alcarazo M.; Krause H.; Lehmann C. W. Effective Modulation of the Donor Properties of N-Heterocyclic Carbene Ligands by “Through-Space” Communication within a Planar Chiral Scaffold. J. Am. Chem. Soc. 2007, 129, 12676–12677. 10.1021/ja076028t. [DOI] [PubMed] [Google Scholar]
- Nakano R.; Nozaki K. Copolymerization of Propylene and Polar Monomers Using Pd/IzQO Catalysts. J. Am. Chem. Soc. 2015, 137, 10934–10937. 10.1021/jacs.5b06948. [DOI] [PubMed] [Google Scholar]
- Iglesias-Sigüenza J.; Izquierdo C.; Diez E.; Fernández R.; Lassaletta J. M. Chirality and catalysis with aromatic N-fused heterobicyclic carbenes. Dalton Trans. 2016, 45, 10113–10117. 10.1039/C6DT01700B. [DOI] [PubMed] [Google Scholar]
- Park D.-A.; Ryu J. Y.; Lee J.; Hong S. Bifunctional N-heterocyclic carbene ligands for Cu-catalyzed direct C–H carboxylation with CO2. RSC Adv. 2017, 7, 52496–52502. 10.1039/C7RA11414A. [DOI] [Google Scholar]
- Tao W.; Akita S.; Nakano R.; Ito S.; Hoshimoto Y.; Ogoshi S.; Nozaki K. Copolymerisation of ethylene with polar monomers by using palladium catalysts bearing an N-heterocyclic carbene-phosphine oxide bidentate ligand. Chem. Commun. 2017, 53, 2630–2633. 10.1039/C7CC00002B. [DOI] [PubMed] [Google Scholar]
- Byun S.; Seo H.; Choi J.-H.; Ryu J. Y.; Lee J.; Chung W.-J.; Hong S. Fluoro-imidazopyridinylidene Ruthenium Catalysts for Cross Metathesis with Ethylene. Organometallics 2019, 38, 4121–4132. 10.1021/acs.organomet.9b00469. [DOI] [Google Scholar]
- Chen K.; Chen W.; Yi X.; Chen W.; Liu M.; Wu H. Sterically hindered N-heterocyclic carbene/palladium(ii) catalyzed Suzuki–Miyaura coupling of nitrobenzenes. Chem. Commun. 2019, 55, 9287–9290. 10.1039/C9CC04634H. [DOI] [PubMed] [Google Scholar]
- Kriechbaum M.; List M.; Berger R. J. F.; Patzschke M.; Monkowius U. Silver and Gold Complexes with a New 1,10-Phenanthroline Analogue N-Heterocyclic Carbene: A Combined Structural, Theoretical, and Photophysical Study. Chem. Eur. J. 2012, 18, 5506–5509. 10.1002/chem.201200465. [DOI] [PubMed] [Google Scholar]
- Espina M.; Rivilla I.; Conde A.; Díaz-Requejo M. M.; Pérez P. J.; Álvarez E.; Fernández R.; Lassaletta J. M. Chiral, Sterically Demanding N-Heterocyclic Carbenes Fused into a Heterobiaryl Skeleton: Design, Synthesis, and Structural Analysis. Organometallics 2015, 34, 1328–1338. 10.1021/acs.organomet.5b00046. [DOI] [Google Scholar]
- Grande-Carmona F.; Iglesias-Sigüenza J.; Álvarez E.; Díez E.; Fernández R.; Lassaletta J. M. Synthesis and Characterization of Axially Chiral Imidazoisoquinolin-2-ylidene Silver and Gold Complexes. Organometallics 2015, 34, 5073–5080. 10.1021/acs.organomet.5b00681. [DOI] [Google Scholar]
- Rana B. K.; Nandy A.; Bertolasi V.; Bielawski C. W.; Das Saha K.; Dinda J. Novel Gold(I)– and Gold(III)–N-Heterocyclic Carbene Complexes: Synthesis and Evaluation of Their Anticancer Properties. Organometallics 2014, 33, 2544–2548. 10.1021/om500118x. [DOI] [Google Scholar]
- Francos J.; Grande-Carmona F.; Faustino H.; Iglesias-Sigüenza J.; Díez E.; Alonso I.; Fernández R.; Lassaletta J. M.; López F.; Mascareñas J. L. Axially Chiral Triazoloisoquinolin-3-ylidene Ligands in Gold(I)-Catalyzed Asymmetric Intermolecular (4 + 2) Cycloadditions of Allenamides and Dienes. J. Am. Chem. Soc. 2012, 134, 14322–14325. 10.1021/ja3065446. [DOI] [PubMed] [Google Scholar]
- Varela I.; Faustino H.; Díez E.; Iglesias-Sigüenza J.; Grande-Carmona F.; Fernández R.; Lassaletta J. M.; Mascareñas J. L.; López F. Gold(I)-Catalyzed Enantioselective [2 + 2+2] Cycloadditions: An Expedient Entry to Enantioenriched Tetrahydropyran Scaffolds. ACS Catal. 2017, 7, 2397–2402. 10.1021/acscatal.6b03651. [DOI] [Google Scholar]
- Zhang J.-Q.; Liu Y.; Wang X.-W.; Zhang L. Synthesis of Chiral Bifunctional NHC Ligands and Survey of Their Utilities in Asymmetric Gold Catalysis. Organometallics 2019, 38, 3931–3938. 10.1021/acs.organomet.9b00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.; Benaissa I.; Huynh M.; Vendier L.; Lugan N.; Bastin S.; Belmont P.; César V.; Michelet V. An Original L-shape, Tunable N-Heterocyclic Carbene Platform for Efficient Gold(I) Catalysis. Angew. Chem., Int. Ed. 2019, 58, 7977–7981. 10.1002/anie.201901090. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Kim Y.; Hur M. Y.; Lee E. Efficient synthesis of bulky N-Heterocyclic carbene ligands for coinage metal complexes. J. Organomet. Chem. 2016, 820, 1–7. 10.1016/j.jorganchem.2016.07.023. [DOI] [Google Scholar]
- Cera G.; Chiarucci M.; Mazzanti A.; Mancinelli M.; Bandini M. Enantioselective Gold-Catalyzed Synthesis of Polycyclic Indolines. Org. Lett. 2012, 14, 1350–1353. 10.1021/ol300297t. [DOI] [PubMed] [Google Scholar]
- Chiarucci M.; di Lillo M.; Romaniello A.; Cozzi P. G.; Cera G.; Bandini M. Gold meets enamine catalysis in the enantioselective α-allylic alkylation of aldehydes with alcohols. Chem. Sci. 2012, 3, 2859–2863. 10.1039/c2sc20478a. [DOI] [Google Scholar]
- Cera G.; Piscitelli S.; Chiarucci M.; Fabrizi G.; Goggiamani A.; Ramón R. S.; Nolan S. P.; Bandini M. One-Pot Gold-Catalyzed Synthesis of Azepino[1,2-a]indoles. Angew. Chem., Int. Ed. 2012, 51, 9891–9895. 10.1002/anie.201205463. [DOI] [PubMed] [Google Scholar]
- Chiarucci M.; Mocci R.; Syntrivanis L.-D.; Cera G.; Mazzanti A.; Bandini M. Merging Synthesis and Enantioselective Functionalization of Indoles by a Gold-Catalyzed Asymmetric Cascade Reaction. Angew. Chem., Int. Ed. 2013, 52, 10850–10853. 10.1002/anie.201304619. [DOI] [PubMed] [Google Scholar]
- Jia M.; Monari M.; Yang Q.-Q.; Bandini M. Enantioselective gold catalyzed dearomative [2 + 2]-cycloaddition between indoles and allenamides. Chem. Commun. 2015, 51, 2320–2323. 10.1039/C4CC08736D. [DOI] [PubMed] [Google Scholar]
- Mastandrea M. M.; Mellonie N.; Giacinto P.; Collado A.; Nolan S. P.; Miscione G. P.; Bottoni A.; Bandini M. Gold(I)-Assisted α-Allylation of Enals and Enones with Alcohols. Angew. Chem. Int. Ed. 2015, 54, 14885–14889. 10.1002/anie.201507218. [DOI] [PubMed] [Google Scholar]
- An J.; Parodi A.; Monari M.; Reis M. C.; Lopez C. S.; Bandini M. Gold-Catalyzed Dearomatization of 2-Naphthols with Alkynes. Chem Eur. J. 2017, 23, 17473–17477. 10.1002/chem.201704942. [DOI] [PubMed] [Google Scholar]
- An J.; Pedrazzani R.; Monari M.; Marin-Luna M.; Lopez C. S.; Bandini M. Site-selective synthesis of 1,3-dioxin-3-ones via a gold(I) catalyzed cascade reaction. Chem. Commun. 2020, 56, 7734–7737. 10.1039/D0CC02703K. [DOI] [PubMed] [Google Scholar]
- Albano V. G.; Bandini M.; Melucci M.; Monari M.; Piccinelli F.; Tommasi S.; Umani-Ronchi A. Novel Chiral Diamino-Oligothiophenes as Valuable Ligands in Pd-Catalyzed Allylic Alkylations. On the “Primary” Role of “Secondary” Interactions in Asymmetric Catalysis. Adv. Synth. Catal. 2005, 347, 1507–1512. 10.1002/adsc.200505109. [DOI] [Google Scholar]
- Melucci M.; Gazzano M.; Barbarella G.; Cavallini M.; Biscarini F.; Piccinelli F.; Monari M.; Bandini M.; Bongini A.; Umani-Ronchi A.; Biscarini P. Synthesis, Multiphase Characterization, and Helicity Control in Chiral DACH-Linked Oligothiophenes. Chem. Eur. J. 2006, 12, 7304–7312. 10.1002/chem.200600312. [DOI] [PubMed] [Google Scholar]
- Albano V. G.; Bandini M.; Monari M.; Tommasi S.; Piccinelli F.; Umani-Ronchi A. Synthesis, structural characterization, and catalytic activity of chiral diamine and diimine Pd(II)-complexes. Inorg. Chim. Acta 2007, 360, 1000–1008. 10.1016/j.ica.2006.07.096. [DOI] [Google Scholar]
- Bandini M.; Eichholzer A.; Tommasi S.; Umani-Ronchi A. Practical Aspects in the Gram-Scale Synthesis of Chiral Diamino-Bithiophene ‘DAT2′ Ligand. Synthesis 2007, 2007, 1587–1588. 10.1055/s-2007-965953. [DOI] [Google Scholar]
- Albano V. G.; Bandini M.; Moorlag C.; Piccinelli F.; Pietrangelo A.; Tommasi S.; Umani-Ronchi A.; Wolf M. O. Electropolymerized Pd-Containing Thiophene Polymer: A Reusable Supported Catalyst for Cross-Coupling Reactions. Organometallics 2007, 26, 4373–4375. 10.1021/om070210l. [DOI] [Google Scholar]
- Bandini M.; Melucci M.; Piccinelli F.; Sinisi R.; Tommasi S.; Umani-Ronchi A. New chiral diamino-bis(tert-thiophene): an effective ligand for Pd- and Zn-catalyzed asymmetric transformations. Chem. Commun. 2007, 4519–4521. 10.1039/b711666g. [DOI] [PubMed] [Google Scholar]
- Schmidbaur H.; Schier A. Gold η2-Coordination to Unsaturated and Aromatic Hydrocarbons: The Key Step in Gold-Catalyzed Organic Transformations. Organometallics 2010, 29, 2–23. 10.1021/om900900u. [DOI] [Google Scholar]
- Ni Q.-L.; Jiang X.-F.; Huang T.-H.; Wang X.-J.; Gui L.-C.; Yang K.-G. Gold(I) Chloride Complexes of Polyphosphine Ligands with Electron-Rich Arene Spacer: Gold–Arene Interactions. Organometallics 2012, 31, 2343–2348. 10.1021/om201276w. [DOI] [Google Scholar]
- An J.; Lombardi L.; Grilli S.; Bandini M. PPh3AuTFA Catalyzed in the Dearomatization of 2-Naphthols with Allenamides. Org. Lett. 2018, 20, 7380–7383. 10.1021/acs.orglett.8b03018. [DOI] [PubMed] [Google Scholar]
- Pedrazzani R.; An J.; Monari M.; Bandini M. New Chiral BINOL-Based Phosphates for Enantioselective [Au(I)]-Catalyzed Dearomatization of β-Naphthols with Allenamides. Eur. J. Org. Chem. 2021, 2021, 1732–1736. 10.1002/ejoc.202100166. [DOI] [Google Scholar]
- Zhuo C.-X.; Zhang W.; You S.-L. Catalytic Asymmetric Dearomatization Reactions. Angew. Chem., Int. Ed. Engl. 2012, 51, 12662–12686. 10.1002/anie.201204822. [DOI] [PubMed] [Google Scholar]
- Zheng C.; You S.-L. Catalytic Asymmetric Dearomatization by Transition-Metal Catalysis: A Method for Transformations of Aromatic Compounds. Chem. 2016, 1, 830–857. 10.1016/j.chempr.2016.11.005. [DOI] [Google Scholar]
- You S.-L.Asymmetric Dearomatization Reactions; Wiley-VCH, 2016. [Google Scholar]; f Wu W.-T.; Zhang L.; You S.-L. Catalytic asymmetric dearomatization (CADA) reactions of phenol and aniline derivatives. Chem. Soc. Rev. 2016, 45, 1570–1580. 10.1039/C5CS00356C. [DOI] [PubMed] [Google Scholar]
- Wu W.-T.; Zhang L.; You S.-L. Recent Progress on Gold-catalyzed Dearomatization Reactions. Acta Chim. Sinica 2017, 75, 419–438. 10.6023/A17020049. [DOI] [Google Scholar]
- An J.; Bandini M. Gold-catalyzed Dearomatization Reactions. CHIMIA 2018, 72, 610–613. 10.2533/chimia.2018.610. [DOI] [PubMed] [Google Scholar]
- Xia Z.-L.; Xu-Xu Q.-F.; Zheng C.; You S.-L. Chiral phosphoric acid-catalyzed asymmetric dearomatization reactions. Chem. Soc. Rev. 2020, 49, 286–300. 10.1039/C8CS00436F. [DOI] [PubMed] [Google Scholar]
- An J.; Bandini M. Recent Advances in the Catalytic Dearomatization of Naphthols. Eur. J. Org. Chem. 2020, 2020, 4087–4097. 10.1002/ejoc.202000107. [DOI] [Google Scholar]
- Lu T.; Lu Z. J.; Ma Z. X.; Zhang Y.; Hsung R. P. Allenamides: A Powerful and Versatile Building Block in Organic Synthesis. Chem. Rev. 2013, 113, 4862–4904. 10.1021/cr400015d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoni E.; Bandini M. N-Allenyl Amides and O-Allenyl Ethers in Enantioselective Catalysis. Eur. J. Org. Chem. 2016, 2016, 3135–3142. 10.1002/ejoc.201600304. [DOI] [Google Scholar]
- Li X.; Liu Y.; Ding N.; Tan X.; Zhao Z. Recent progress in transition-metal-free functionalization of allenamides. RSC Adv. 2020, 10, 36818–36827. 10.1039/D0RA07119F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Gómez E.; Nieto-Oberhuber C.; López S.; Benet-Buchholz J.; Echavarren A. M. Cationic η1/η2-Gold(I) Complexes of Simple Arenes. Angew. Chem., Int. Ed. 2006, 45, 5455–5459. 10.1002/anie.200601688. [DOI] [PubMed] [Google Scholar]
- Touil M.; Bechem B.; Hashmi A. S. K.; Engels B.; Omary M. A.; Rabaâ H. Theoretical study of weak CC double bond coordination in a gold (I) catalyst precursor. J. Mol. Struct.: THEOCHEM 2010, 957, 21–25. 10.1016/j.theochem.2010.06.030. [DOI] [Google Scholar]
- The partial effectiveness of IPrAuCl/AgTFA (5 mol%) in promoting the dearomatization of 12 with 13 was already proven (55% yield, see ref (75)). On the contrary, dinuclear [{Au(IPr)}2(μ-OH)][BF4] (see ref (18) and references therein) proved inefficient in the present model dearomatization reaction
- Caracelli I.; Zukerman-Schpector J.; Tiekink E. R. T. Supra-molecular synthons based on gold···π(arene) interactions. Gold Bull. 2013, 46, 81–89. 10.1007/s13404-013-0088-7. [DOI] [Google Scholar]
- Christian A. H.; Niemeyer Z. L.; Sigman M. S.; Toste F. D. Uncovering Subtle Ligand Effects of Phosphines Using Gold(I) Catalysis. ACS Catal. 2017, 7, 3973–3978. 10.1021/acscatal.7b00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham J. H.; Cho J.; Nayab S.; Jeong J. H. Synthesis and structural characterisation of copper complexes containing methylthiophene and methylfuryl derivatives of (R,R)-1,2-diaminocyclohexane as precatalysts for polymerisation of rac-lactide. Inorg. Chim. Acta 2018, 480, 33–41. 10.1016/j.ica.2018.04.025. [DOI] [Google Scholar]
- Veracini A. A.; Macciantelli D.; Lunazzi L. Conformational analysis of bithienyl derivatives: a liquid crystal nuclear magnetic resonance approach. J. Chem. Soc., Perkin Trans. 1973, 2, 751–754. 10.1039/p29730000751. [DOI] [Google Scholar]
- Tsuzuki S.; Orita H.; Sato N. Intermolecular interactions of oligothienoacenes: Do S···S interactions positively contribute to crystal structures of sulfur-containing aromatic molecules?. J. Chem. Phys. 2016, 145, 174503. 10.1063/1.4966580. [DOI] [PubMed] [Google Scholar]
- We might speculate that the cis-thienyl conformations could also be present in the solution, with a consequent overall modification of both steric cage of the gold complex as well as electronic interactions of the thienyl sulfur atoms with the Au center during the reaction course.
- Hohenberg P.; Kohn W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. 10.1103/PhysRev.136.B864. [DOI] [Google Scholar]
- Kohn W.; Sham L. J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133. 10.1103/PhysRev.140.A1133. [DOI] [Google Scholar]
- Bader R. F. W.; Popelier P. L. A.; Keith T. A. Theoretical Definition of a Functional Group and the Molecular Orbital Paradigm. Angew. Chem., Int. Ed. 1994, 33, 620. 10.1002/anie.199406201. [DOI] [Google Scholar]
- Contreras-García J.; Johnson E. R.; Keinan S.; Chaudret R.; Piquemal J.-P.; Beratan D. N.; Yang W. A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. 10.1021/ct100641a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becke A. D. Density-functional exchange-energy approximation with correct asymptotic-behavior. Phys. Rev. A 1988, 38, 3098. 10.1103/PhysRevA.38.3098. [DOI] [PubMed] [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Faza O. N.; Rodríguez R. A.; López C. S. Performance of density functional theory on homogeneous gold catalysis. Theor. Chem. Acc. 2011, 128, 647–661. 10.1007/s00214-010-0843-2. [DOI] [Google Scholar]
- Faza O. N.; López C. S. Computational Approaches to Homogeneous Gold Catalysis. Top. Curr. Chem. 2014, 357, 213–284. 10.1007/128_2014_591. [DOI] [PubMed] [Google Scholar]
- Johansson M. J.; Gorin D. J.; Staben S. T.; Toste F. D. Gold(I)-Catalyzed Stereoselective Olefin Cyclopropanation. J. Am. Chem. Soc. 2005, 127, 18002–18003. 10.1021/ja0552500. [DOI] [PubMed] [Google Scholar]
- Handa S.; Slaughter L. M. Enantioselective Alkynylbenzaldehyde Cyclizations Catalyzed by Chiral Gold(I) Acyclic Diaminocarbene Complexes Containing Weak Au–Arene Interactions. Angew. Chem., Int. Ed. 2012, 51, 2912–2915. 10.1002/anie.201107789. [DOI] [PubMed] [Google Scholar]
- The cyclopropanation and the alkynylbenzaldehyde cyclization were also carried out in the presence of a 2.5 mol% catalyst loading. In these cases, a marked accelerating effect with 10c was also observed: cyclopropanation, 10a yield = 50%, 10c yield = 62%, 10d yield = 38%; alkynylbenzaldehyde, 10a yield = 32%, 10c yield = 45%, 10d yield = 17%.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


