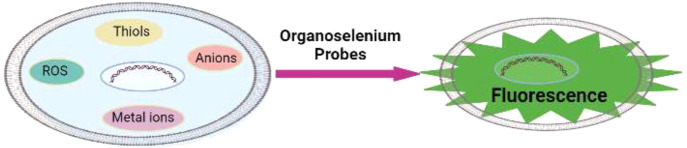
Abstract
Chemistry of organoselenium reagents have now become an important tool of synthetic organic and medicinal chemistry. These reagents activate the olefinic double bonds and used to archive the number of organic transformations under mild reaction conditions. A number of organoselenium compounds have been identified as potent oxidants. Recently, various organoselenium species have been employed as chemical sensors for detecting toxic metals. Moreover, a number of selenium-based fluorescent probes have been developed for detecting harmful peroxides and ROS. In this review article, the synthesis of selenium-based fluorescent probes will be covered including their application in the detection of toxic metals and harmful peroxides including ROS.
Keywords: Organoselenium compounds, Fluorescence probes, ROS, Metal ions, Thiols
1. Introduction
Since the discovery of the first organoselenium compound in 1836,1 various organoselenium reagents have been developed as important motifs for the synthesis of numerous biologically important synthetic and naturally occurring scaffolds.2−8 The scope of the biochemistry of selenium has been expanded with the discovery of selenocysteine in a number of mammalian enzymes. At the present date, 25 selenoproteins such as glutathione peroxidaes,9 iodothyronine deiodinase,10 and thioredoxin reductase11 have been reported. Selenium is an essential dietary element, and various organoselenium reagents are known for GPx and deiodinase activity.12−15 Insufficient selenium in the diet inactivates selenoproteins, which results in oxidative stress and further to cognitive decline that contributes to the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease. Selenium, when incorporated into the catalytic site of glutathione peroxidase, inhibits lipid peroxidation caused by ferroptosis. The organoselenium compounds have also been known to protect neurons in ischemic stroke and other brain illnesses where ferroptosis is associated.16,17
Selenium is usually found in the divalent state with two covalent bonds along with two lone pairs of valence electrons. In the chalcogen series, selenium exhibits a soft/hard donor property, due to different oxidation states and the polalizability (which depends on its chemical environment). The hard/soft nature of the selenium helps it to recognize specific analytes, and thus organoselenium compounds have been explored as fluorophores. Several bioactive molecules such as thiols, reactive oxygen species (ROS), reactive nitrogen species (RNS), etc., play an important role in the biological system.18−20 These analytes have been detected using various methods, but the most simple, inexpensive, rapid, and sensitive method of detection is fluorimetry. Recently, a number of selenium-containing fluorescent probes have been developed as potential sensors for the selective detection of bioactive molecules (metal ions, carboxylate anions, ROS, RNS, thiols).21−34 These fluorescent probes include various fluorophores including rhodamine, extended cyanine groups, coumarin, napthalimide, benzimidazole, boron-dipyrromethene (BODIPY) systems, and dipyrazolopyridine. The probes work through various photomechanisms including photo-induced electron transfer (PET) and internal charge transfer (ICT). This review article highlights the recent developments of selenium-cored fluorescence probes and their applications as a sensor for small molecules in the living cells and tissues.
2. Detection of Hg2+ Ions
Mercury metal ions have been recognized as highly toxic candidates even at very low concentrations and are responsible for some serious biological disorders in human body.35−39 These metal ions can easily pass through cell membranes via epidermal, respiratory, and gastrointestinal tissues. The long-term exposure to mercury metal ion causes irreversible damage to the central neurological and endocrine systems.40 Recently, Parkin and co-workers have demonstrated that the selenophilicity of mercury plays an important role to detect Hg2+ ions in biological samples.41 Although there are few analytical methods such as plasma spectroscopy, atomic absorption, atomic emission, and electrochemical methods are available to detect Hg2+ ions, but the fluorescence method showed obvious advantages in biological systems in term of high sensitivity and specificity.42−44
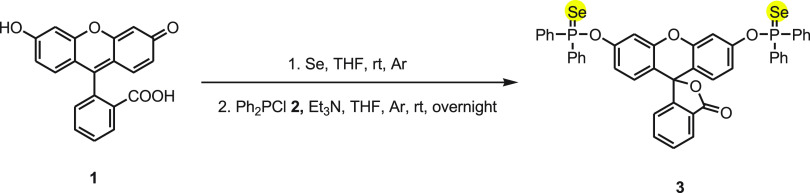
In 2009, Tang and co-workers investigated an organoselenium fluorescent probe 3 based on a deselenation reaction in aqueous solution to detect mercury ion.45 The probe 3 mainly consists of two distinguishable chemical units, fluorescein as a fluorophore and p-phenyphosphinoselenoic group as a reactive target for Hg2+ ions. The synthesis of fluorescent probe 3 was achieved by the reaction of fluorescein 1 with chlorodiphenylphosphine 2 and selenium powder in the presence of triethylamine (Scheme 1).
Scheme 1. Synthesis of Fluorescent Probe 3 from Fluorescein 1.
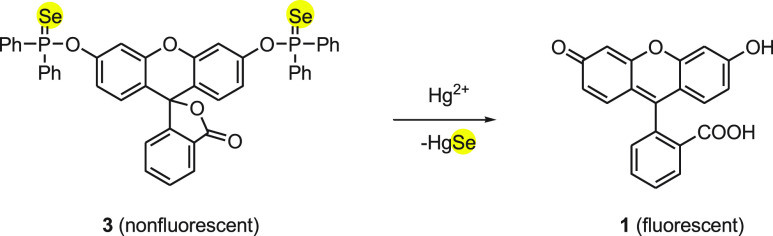
The probe 3 exhibited high selectivity for Hg2+ ions over many other relevant metal ions as well as high sensitivity with a very low detection limit of 1.0 nm. The mechanism for the detection of Hg2+ by probe 3 can be explained on the basis of the deselenylation reaction (Scheme 2). According to the mechanism, a nonfluorescent probe 3 reacts with Hg2+ ions to form fluorescent fluorescein 1 and HgSe. The formation of HgSe was confirmed by X-ray analysis. After the stoichiometric response of selenoprobe 3 to mercury ions, the intensity of fluorescence at λem was found to be directly proportional to the amount of mercury ion, up to 2 equiv. Furthermore, the probe was found to be highly effective, sensitive, and selective in mercury ions imaging in RAW 264.7 cells. This would be helpful in studying the toxicity of Hg2+ ions along with its bioactivity in the living organisms.
Scheme 2. Deselenation of Fluorescent Probe 3 with Mercury Ions.
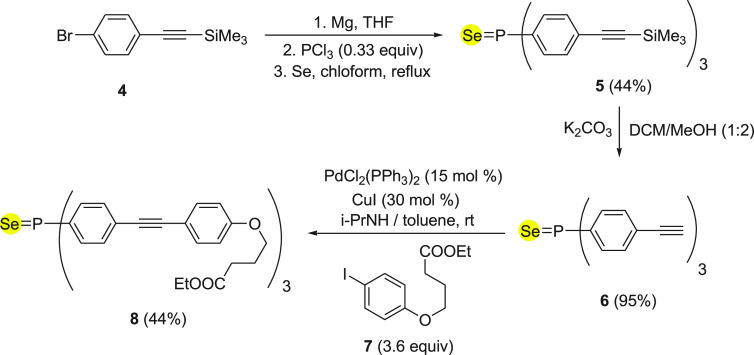
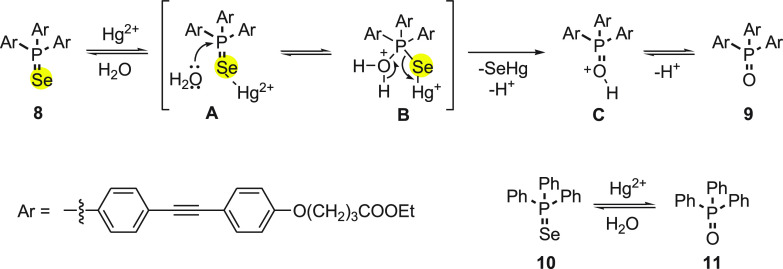
In 2011, Leray and co-workers46 reported a fluorescent probe 8 to detect Hg2+ ions, based on a phosphorus selenium linkage. The synthesis of probe 8 was achieved by an easily accessible precursor (4-bromophenyl)ethynyl)trimethylsilane 4 in three steps as described in Scheme 3. Initially, the starting material 4 was converted into selenium-containing compound 5, which was then transferred to corresponding terminal alkyne 6 with the removal of trimethylsilyl functionality under basic conditions. Finally, terminal alkyne 6 was treated with iodobenzene derivative 7 under coupling reaction conditions to form probe 8 in 44% yields (Scheme 3).
Scheme 3. Synthesis of Fluorescent Probe 8 Starting from Functionalized Alkyne 4.
The fluorescence was increased with the increase in the concentration of Hg2+ ions. The effect of change in fluorescence of probe 8 with the addition of Hg2+ ions in aqueous solution was because of mercury-induced irreversible deselenization reaction (Scheme 4). The fluorescent phosphane oxide 9 is formed as a result of this reaction.47
Scheme 4. Mechanism for the Deselenation of Compound 8 with Mercury Ions.
Furthermore, to prove the conversion of phosphane selenide to oxide, a known triphenylphosphane selenide 9 was prepared and treated with mercury salt (Scheme 4). The formation of phosphane oxide 11 was confirmed by 31P NMR spectroscopy. It was clear from mechanistic studies that the turn on of the fluorescence was due to the formation of phosphane oxide from phosphane selenide. The selectivity and sensibility toward mercury were estimated to be 0.18 ppb, which is comparable to the targeted level of the World Health Organization.
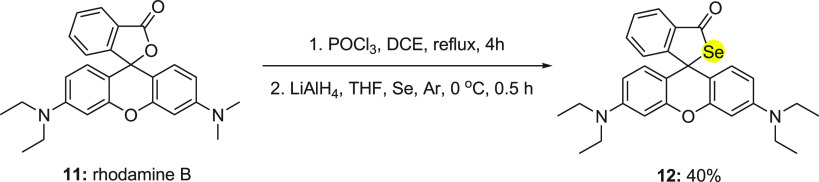
In 2010, Ma and co-workers48 investigated a fluorescent probe 12 with a unique selenospirocyclic structure, which can produce fluorescence with the reaction of both Hg2+ and Ag+ ions through deselenation. The synthesis of fluorescence probe 12 was achieved in single step by the reaction of rhodamine B 11 with phosphorus oxychloride followed by the addition of selenium powder in the presence of LiAlH4 (Scheme 5).
Scheme 5. Synthesis of Fluorescent Probe (RBse) 12 from Rhodamine 11.
The possible reaction mechanism for “‘OFF-ON’” fluorescence of 12 with both Hg2+ and Ag+ ions can be explained on basis of irreversible deselenation (Scheme 6). According to the mechanism, the selenium atom of 12 binds with Hg2+ and Ag+ ions to form an intermediate A. Furthermore, the intermediate A could lead to hydrolytic cleavage of the selenolactone bond to form 13 with emission of fluorescence (Scheme 6). The mechanism was supported by electrospray ionization mass spectral analysis of reaction products.
Scheme 6. Deselenation of RBSe 12 with Mercury or Silver Ions.
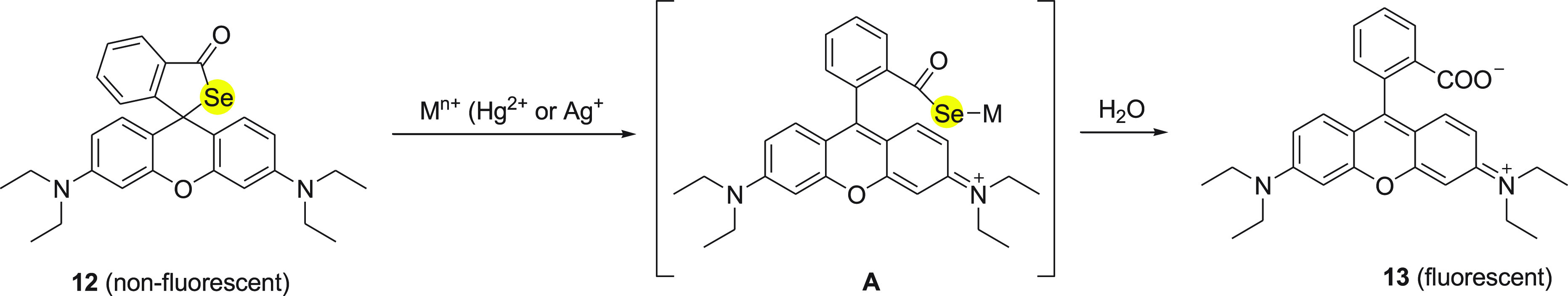
The probe 13 is advantageous over the other fluorescent probes because of (a) its membrane-permeable capability and (b) its reaction to both Ag+ and Hg2+ ions in the presence of Cl–. These properties make it potentially useful for imaging not only Hg2+ ions but also Ag+ ions in live cells. The behavior of probe 13 with Ag+ ions could be explained on the basis of higher affinity of Se than Cl– for Ag+, because the solubility product of Ag2Se (Ksp = 1.6 × 10–56) is much lower than that of AgCl (Ksp = 1.56 × 10–10).49−51 Furthermore, Hg2+ and Ag+ ion imaging in HeLa cells demonstrated its applicability. The detection limits were found to be 23 nM for Hg2+ and 52 nM for Ag+ ions. The reaction of Ag+ ions with the probe inside the cells is much slower than that of Hg2+ ions, which can be attributed to the high concentration of chloride ions present in the cell inhibiting the formation of sufficient free Ag+ ions.
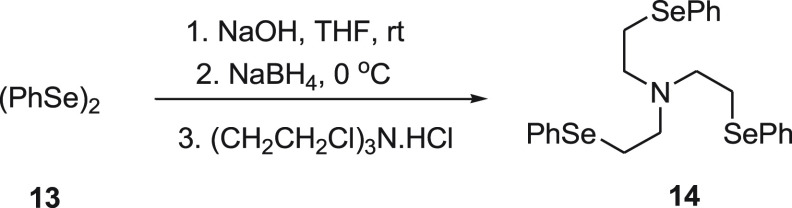
Singh and his research group52 investigated an organoselenium-based NSe3 type of tripodal system 14 as a Hg2+ ion selective fluorescence “turn-on” probe. The “turn-on” fluorescence behavior of selenotripod 14 is important because it is dependent on Hg–Se bonds and serves as reporting unit for this system (Scheme 7). Even in the presence of several other chalcophilic metal ions, the selenotripod 14 was found to be highly sensitive and selective toward Hg2+ ions. The system responds immediately and has shown a subnanomolar detection limit (0.1 nM) for Hg2+ ions. The same probe was sensing efficiently both aqueous and nonaqueous Hg2+ ions at 2 nM concentration.
Scheme 7. Synthesis of NSe3-type Tripodal System 14 from Diphenyl Diselenide 13.
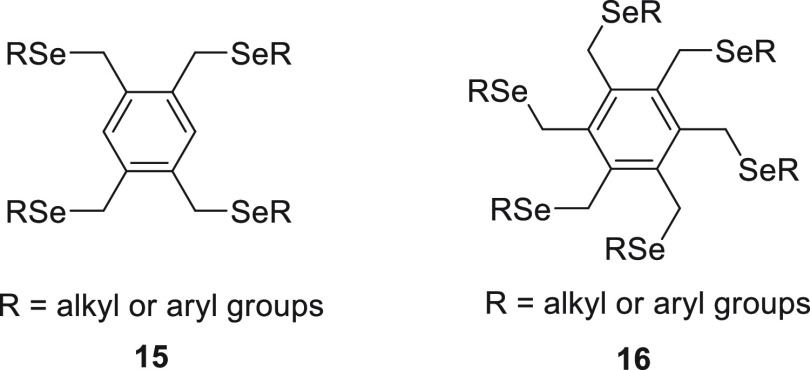
Furthermore, the same research group reported the synthesis of sterically encumbered tetra-substituted organoselenium species 15(53) and hexakis(alkylseleno)benzenes 16,54 respectively, and their application in sensing Hg2+ ions specifically (Figure 1).
Figure 1.
Showing tetra-substituted organoselenium species 15 and hexakis(alkylseleno)benzenes 16.
3. Detection of Reactive Oxygen Species
During aerobic respiration and cellular metabolism, oxygen is used to produce energy in the form of adenosine triphosphate (ATP) in cells. During the oxidative metabolism, oxygen is partially reduced to reactive oxygen species (ROS). ROS include peroxynitrite (ONOO–), superoxide (O2·–), hypochlorite/hypochlorous acid/ (−OCl/HOCl), hypobromite/hypobromous acid (−OBr/HOBr), hydrogen peroxide (H2O2), hydroxyl radical (OH–), and peroxyl radical (ROO·). The endogenously produced ROS are quite essential for biological functions like signal transduction, neurotransmission, and blood pressure modulation. However, an enhanced level of ROS or low level of antioxidants induces oxidative stress resulting in damage of biomolecules such as DNA, proteins, and lipids of cellular membranes. In long-term damage, redox perturbation leads to inflammatory diseases, cardiovascular diseases, cancer, neurodegenerative disorders, HIV activation, and aging. In short, the intracellular redox balance is very crucial for biological processes. Selenium is an important element in the human body. Under physiological conditions, a rapid and reversible redox transformation can easily happen between selenide and its selenoxide derivative. Many selenide-based fluorescent probes were synthesized to check intracellular redox status due to its sensitivity toward biological redox systems.27,55,56
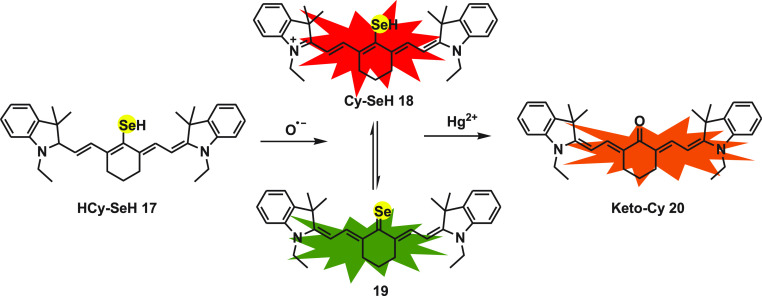
Lingxin Chen et al.57 designed a near-infrared three-channel fluorescent probe HCy-SeH 17 for the associated detection of mercury (Hg2+) and super oxide (O2•–). As the cytotoxicity of heavy metal ion Hg2+ is discussed in an earlier section, it is well-known that it also induces severe oxidative stress. While mercury poisoning can be treated with many different drugs such as sodium selenite, it still continuously damages human health. The anticipated reason behind this is intracellular oxidative stress caused by Hg2+ ion. To synthesize the probe HCy-SeH, heptamethine cyanine is used as fluorophore and a selenol group as a response unit. Heptamethine cyanine dye Cy.7.Cl on reacting with disodium selenide gives an intermediate Cy.SeH 18, which is further reduced by NaBH4 to obtain HCy-SeH as the final product. The response toward the probe in biological samples is generated because of two chemical reactions, hydrogen abstraction reaction in the presence of O2•– and another selenium antagonism reaction.
The π-electron system of polymethine in cyanine is distributed during the formation of hydrocyanine, which results in no fluorescence emission from HCy-SeH 17. After the oxidation of probe by O2•– Cy-SeH 18 recovers its conjugated system and emits fluorescence. Cy-SeH shows red emission with an absorption peak centered at 755 nm and emission peak centered at 800 nm. Cy-SeH coexists with its Lewis conjugated base Cy=Se 19, which exhibits green emission with an absorption peak and emission peak centered at 405 and 567 nm, respectively. Finally, Hg2+ on reacting with the intermediates Cy-SeH 18 and Cy=Se 19 gives Keto-Cy 20 with a max absorption at 510 nm and an emission peak at 607 nm. The probe displayed high sensitivity and selectivity for the detection of super oxides and mercury ions (Scheme 8).
Scheme 8. Mechanism for the Detection of Mercury Ions and Super Oxide Species by HCy-SeH 17.
The flow cytometry analysis and fluorescence imaging together established that the probe can be useful to detect in situ O2•– and Hg2+ in HEK 293 cells. The results established that Hg2+ ion accumulation disturbs the cellular antioxidant system and encourages overproduction of O2•–. The probe upon application to mice models for chronic mercuration confirmed the kidney damage leading to renal fibrosis due to mercury poisoning. On using sodium selenite as Hg2+ ion antagonist, the chronic mercuration was observed to reduce, but the damage due to increased level of O2•– were not diminished. The probe is a powerful tool for associated in vitro and in vivo detection of O2•– and Hg2+ ions. It can further be used for clinical surgery pre-evaluation.
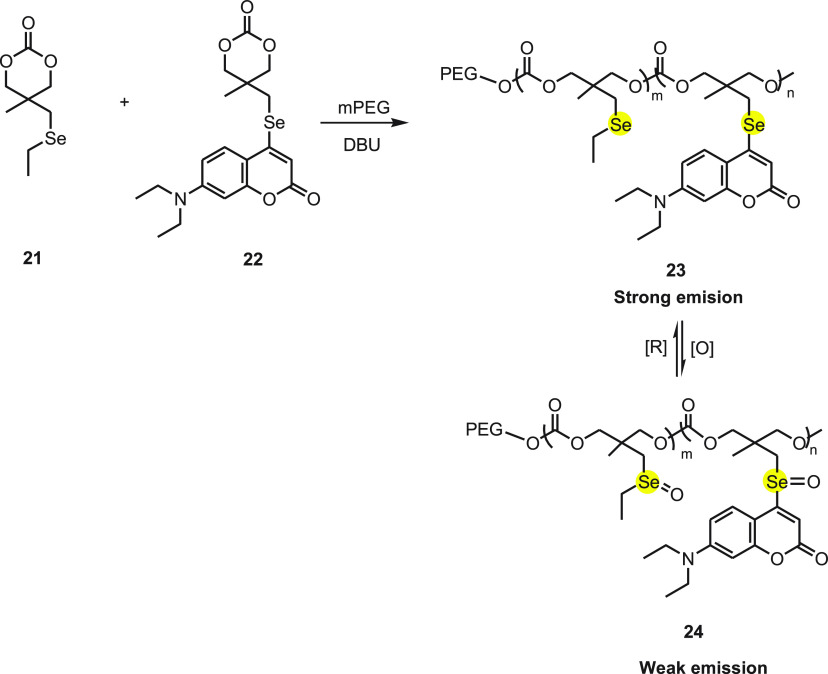
In 2019, Li et al.58 reported an amphiphilic copolymer nanoparticle 23 as a redox-responsive vehicle together for the chemotherapy and photodynamic therapy. The amphiphilic block copolymers were synthesized by the anionic ring-opening copolymerization of the cyclic carbonate attached to an ethylseleno group monomer M1 21 with a small fraction of the coumarin-based chromophore monomer F1 22, using PEG as the macroinitiator (Scheme 9). The copolymer forms micellar nanoparticles with the diameter of 40–110 nm and were found to be sensitive toward H2O2 present in cells under physiological condition with no cytotoxicity against human breast epithelial HBL-100 cells. The ROS and the GSH in the cell trigger a reversible transformation between the selenide and selenoxide inducing fluorescence. The cells treated with block copolymer (PF) nanoparticles exhibits qualitative correlation between the level of ROS in the cells and intensity of the fluorescence.
Scheme 9. Synthesis of Copolymer 23 by the Reaction of Selenium-Based Compounds 21 and 22 in the Presence of a Base.
Besides, PTX and cisplatin were loaded in the copolymer nanoparticles concurrently, and by adding cross-links through Se–Pt coordination the nanoparticles loaded with both drugs were stabilized. On triggering the oxidation/reduction reaction, the selenide and its selenoxide derivative 24 were easily transformed to each other, leading to the reversible changes in morphology of the PF nanoparticles. Under diverse redox stimulation, the dual drug-loaded PF nanoparticles exhibit a pulsatile drug release profile. However, at relatively high ROS levels, the continuous drug release was detected. The reversible redox response aspect makes dual drug-loaded PF nanoparticles appropriate for killing malignant cells without damaging the healthy cells. Thus, a redox-sensitive fluorescent, amphiphilic copolymer is considered to be a good theranostic nanoplatform which can release drugs in the targeted cells with high level of ROS and thus simultaneously can be used for monitoring the redox status of the cell.
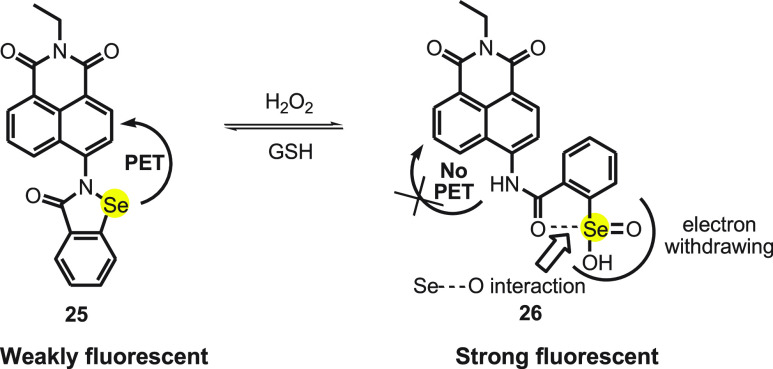
Mugesh and co-workers59 designed naphthalamide-based ebselen fluorescent probe 25. The probe NapEp 25 was synthesized by reacting 4-bromo-N-ethyl-1,8-naphthalimide to benzo[d][1,2]-selenazol-3(2H)-one. The probe is nonfluorescent in aqueous solution, but on adding H2O2 of various concentrations (50–500 nM), a strong concentration-dependent fluorescence is observed at 465 nm (Scheme 10). It is well reported that the selenium moiety in ebselen can be oxidized with ROS such as H2O2, super oxide (O2•–), and peroxynitrite (ONOO–). Although the probe showed fluorescence in the presence of O2•– and ONOO–, the increase was much lower than that observed for H2O2 within the given time frame.
Scheme 10. Change in Florescence of Probe NapEp 25 with H2O2 and GSH.
Various spectroscopic techniques confirmed that in the presence of H2O2 the selenium unit oxidizes to seleninic acid 26. On adding GSH, the regeneration of probe NapEp 25 was observed by HPLC. The NapEp 25 probe shows weak fluorescence due to the PET process, and on reacting with H2O2, the seleninic acid is introduced in the probe. The interaction of carbonyl oxygen of amide with selenium and the electron withdrawing property of seleninic acid prevents the PET process (Scheme 10). The probe was also found to be sensitive toward the change in intracellular H2O2 concentration which was increased in the HepG2 cells by using 3-amino-1,2,4-triazole, a catalase enzyme inhibitor. A significant increase in fluorescence was also observed when the concentration of super oxide increased by inhibiting superoxide dismutase (SOD), using diethyldithiocarbamate (DDC). Thus, the probe can be useful in detecting severe oxidative stress in the cells.
Recently, in 2020 Laursen et al.60 reported a novel reversible, redox-sensitive fluorescent probe, ebselen-azadioxatriangulenium (Ebselen-ADOTA). The probe is made up of two entities a photostable triangulenium-based fluorophore and a redox active ebselen motif as a modulator. The emission maxima of the probe were observed at 564 nm and its intensity depends on the oxidation and reduction. On oxidation, the intensity increases 10 times. The reaction mechanism confirms that oxidation of the selenium is followed by decrease in PET efficacy and can be observed as an increase of fluorescence. Although no clear response was detected for the increased level of ROS within mammalian cells, but by exposing anaerobe bacteria to O2, an increase in fluorescence was observed as an indication of oxidative stress.
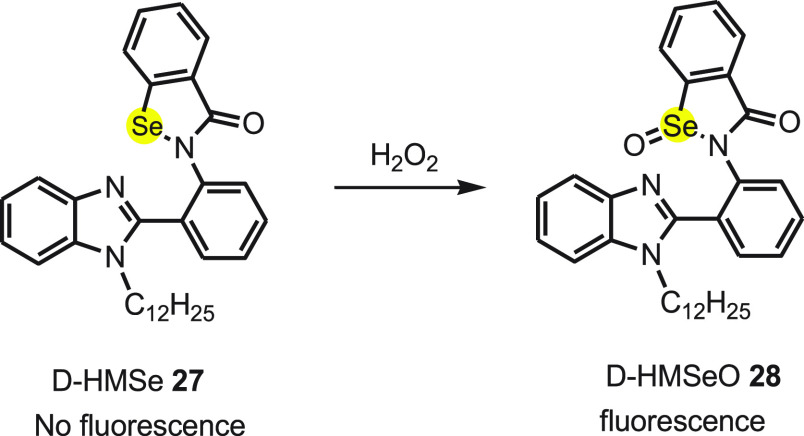
Yu et al.61 designed a novel selenium-containing aggregation-induced “turn-on” fluorescent probe to detect H2O2. The D-HMSe 27 probe consists of 2-phenyl-1H-benzimidazole as a fluorophore and selenium unit as modulator. To make the probe hydrophobic and to block the interaction between adjacent probe molecules in aggregated form, the hydrogen atom of the secondary amine of benzimidazole was substituted with bulky dodecyl chain. The probe D-HMSe 27 exhibits very weak fluorescence due to the PET process, and after treating with H2O2 a strong fluorescence enhancement was detected because of oxidation of selenium to D-HMeSeO 28 at ambient temperature (Scheme 11). The high selectivity of D-HMeSeO for H2O2 was concluded after screening the response toward different ROS under physiological condition. It was observed that the fluorescence enhanced 110-fold on treating the probe with 200 μM H2O2. The strong fluorescence became stronger on increasing the water fraction to 99% in the water–DMSO volume ratio. The phenomenon is credited to aggregation-induced emission (AIE) of D-HMSeO 28 (Scheme 11).
Scheme 11. Oxidation of Nonfluorescent Probe D-HMSe 27 to Fluorescent Compound D-HMSeO 28 by Hydrogen Peroxide.
A long dodecyl chain present on the benzimidazole makes D-HMSeO strongly hydrophobic, and as a result it aggregates in water. This restricts intramolecular rotation resulting in the aggregation-caused quenching and notable fluorescence enhancement together with blocking the PET process. No fluorescence is exhibited in other solvents because the dissolution and dispersion of the probe molecule allows free intramolecular rotation as a nonradiative relaxation pathway. The aggregation trend of D-HMSE after the oxidation by H2O2 was proven by the results obtained from dynamic light scattering and SEM.
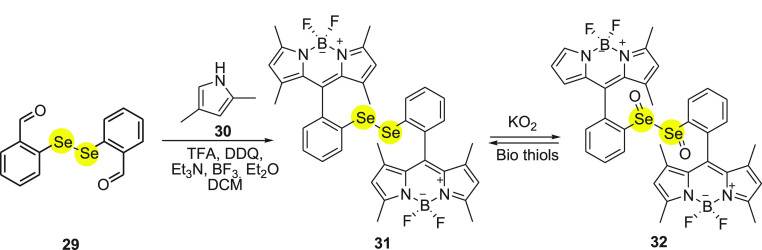
In 2014, Manjare and group62 synthesized first-time diselenide-based BODIPY probe 31 for the detection of ROS. The probe was obtained by reacting bis(O-formyl-phenyl)diselenide 29 dissolved in DCM with 2,4-dimethylpyrrole 30 in the presence of catalytic amounts of trifluoroacetic acid, DDQ, triethyl amine and BF3·Et2O under inert atmosphere (Scheme 12). On testing the probe with different ROS, it was observed that the probe showed green fluorescence in the presence of superoxide (O2•–) due to the oxidation of selenium 32. The λmax values of absorption and emission for probe 31 were observed at 504 and 514 nm, respectively. On testing the probe in the presence of KO2, it was observed that the intensity of fluorescence increases with the increase in the concentration of KO2, and the limit of detection was found to be 12.9 μM. Furthermore, in the presence of biothiols selenium oxide 32 reverts back to its original reduced state 31. From the above study, it was concluded that the probe can be used to monitor the dynamic variation of superoxide in living systems. Treating the MCF-7/ADR cells with 1 μg/mL of phorbol 12-myristate 13-acetate PMA (lipid that promote intracellular superoxide production) and 10 μM of probe 31 resulted in good fluorescence imaging. The finding indicates that probe 31 has cell permeability and is selective for the intracellular superoxide detection.
Scheme 12. Synthesis of Diselenide-Based BODIPY Probe 31 from Bis(O-formyl-phenyl)diselenide 29.
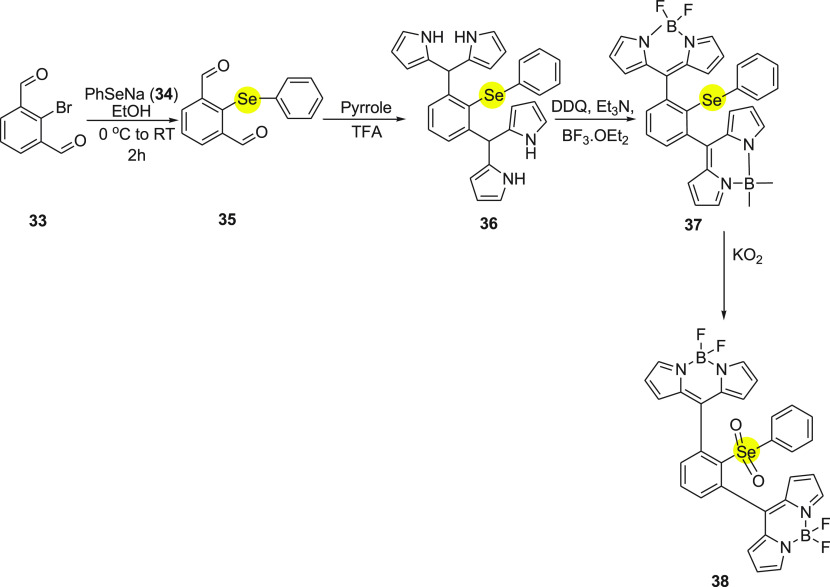
Further, in 2018 Manjare and co-workers63 synthesized monoselenium containing diBODIPY-based probe as an ROS detector. In probe 37, the phenyl selenide group was chosen as a commutator. The syntheses of probe 37 include addition of 2-bromoisophthalaldehyde 33 to the in situ generated PhSeNa 34 by the reaction of diphenyl diselenide and sodium borohydride to obtain 2-(phenylselenyl)isophthalaldehyde 35. Further, the intermediate compound 35 reacts with pyrrole 30 in the presence of catalytic amounts of trifluoroacetic acid to give the corresponding dipyrromethene 36. On treating the dipyrromethene derivative 36 with 2,3-dichloro-5,6-dicynobenzoquinone (DDQ) followed by triethylamine and borontrifluoride ethyletharate (BF3·Et2O) in DCM yields BODIPY probe 37 (Scheme 13). The study shows probe highly selective and sensitive toward superoxide in comparison to other reactive oxygen species. In the presence of superoxide, the probe turns on and produces an 11-fold increase in green fluorescence intensity at 526 nm. Probe 37 was found to have no cytotoxicity along with a detection limit of 4.42 μM. The MCF cells when treated with probe 4 on further addition of Phorbol-12-myristate 13-acetate (PMA) exhibit intense green fluorescence. This shows good permeability of probe 37 which can be used to detect superoxide in living cells.
Scheme 13. Synthesis of Monoselenium diBODIPY Probe 37 Starting with 2-Bromoisophthalaldehyde 33.
Peroxynitrite (ONOO–) is a powerful oxidizing agent which plays an important role in biological processes, especially physiological and pathological.64−67 The change in concentration of ONOO– can lead to useful as well as adverse effects in the cell. A balanced level of ONOO– regulates some important cellular functions such as organ homeostasis and maintains cellular integrity, while an increased level of ONOO– leads to DNA damage and induces lipid peroxidation in cell membranes.68 The fluorescent probes have some advantages over the other reported methods to detect the ONOO–; for example, few fluorescent probes can be used for selective detection of ONOO–. Additionally, these fluorescent probes can respond reversibly to the change in ONOO– concentration which would help determine the generation and metabolism of ONOO– as well as its dynamic damaging effect on living cells.
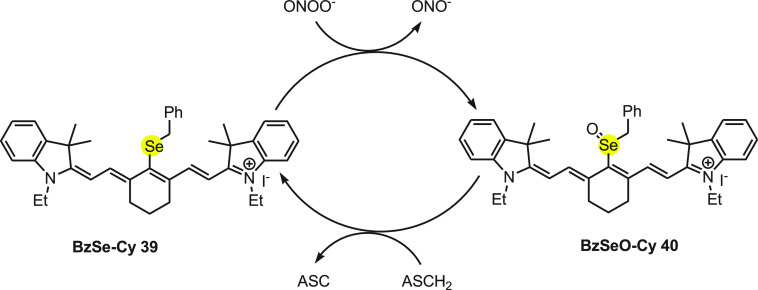
Tang and co-workers69 have developed a near-infrared reversible organoselenium fluorescent probe 39 (BzSe-Cy) for sensing ONOO– and imaging of redox cycles in living cells. BzSe-Cy 39 is a selenium containing molecule that responds reversibly to the change in concentration of ONOO– or ASCH2. On reacting with ONOO–, BzSe-Cy 39 shows substantial decrease in the fluorescence intensity, while in the presence of ASCH2 the fluorescence intensity increases. The same fluorescence probe was also employed for other reactive oxygen and nitrogen species but exhibited only high sensitivity and selectivity with ONOO–. The mechanism for the change in the fluorescence of probe with ONOO– and ASCH2 can be explained on the basis of redox cycle as depicted in Scheme 14. Initially, BzSe-Cy 39 reacts with ONOO– and oxidized to BzSeO-Cy 40, which is weakly fluorescent upon 770 nm excitation due to a photoinduced electron transfer process. Further, BzSeO-Cy 40 was reconverted to BzSe-Cy 39 with the reaction of reduced ascorbate to continue the redox cycle (Scheme 14).
Scheme 14. Redox Cycle for the Change in the Fluorescence of Probe BzSe-Cy 39 with ONOO– and ASCH2.
Furthermore, the probe was effective in imaging several cycles of oxidative stress and reductive repair in RAW 264.7 cells. This fluorescent probe could be used as important tool to study the generation, metabolism, and the dynamic damaging procedure of ONOO– in living cells.
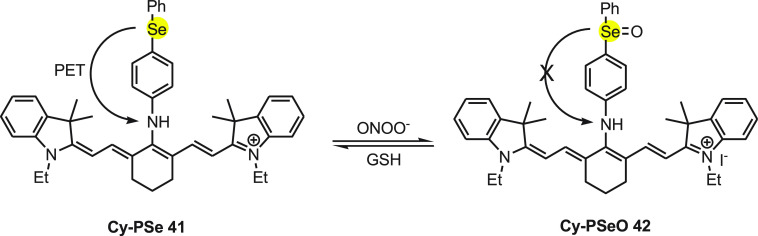
Furthermore, Han and co-workers70 have developed an NIR reversible fluorescent probe 41 containing cyanine (Cy), an NIR fluorescent dye, as signal transducer and 4-(phenylselenyl)aniline as modulator. The NIR reversible fluorescent probe 41 is highly sensitive and selective in monitoring peroxynitrite redox events. Under stimulated physiological conditions, probe 41 exhibited absorption and emission λmax at 758 and 800 nm, respectively. On adding 1 equiv of ONOO– to the buffer solution with Cy-PSe, the fluorescence intensity increases by 23.3-fold and the quantum yield increased from 0.05 to 0.12. The mechanism for the mode of action of fluorescent probe 41 can be explained on the basis of the ping-pong mechanism.71,72 The probe 41 (Cy-PSe) detects ONOO– in living cells through a fast PET process.
The PET process between the modular and the transducer quenches the fluorescence of Cy-PSe, but the oxidation of Se prevents the PET, which “switched on” the fluorescence emission (Scheme 15). Furthermore, selenoxide Cy-PSeO 42 can be reduced to selenide by GSH effectively and rapidly; at this point, the probe begins to function. The probe does not show the influence of autofluorescence in biological systems and has no cytotoxicity toward cells. The study shows that it can be useful in real-time imaging of living cells.
Scheme 15. Change in Florescence of Probe Cy-PSe 41 with Peroxy Nitrite Species and GSH.
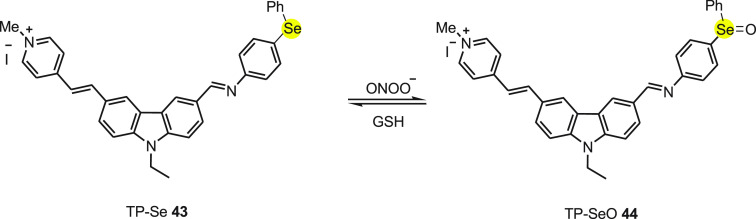
Wenjun Xie et al.73 synthesized a single fluorescent Two Photon-Selenium probe (TP-Se) 43 for reversible detection of ONOO– in mitochondria. An electron donating carbazole and an electron withdrawing cationic pyridinium moiety were chosen as the TP fluorophore due to the excellent intermolecular charge transfer. The methylpyridinium moiety was used as mitochondrial targeted functional group to find the location of mitochondria as well as to increase the solubility of probe.
On reacting with peroxynitrite, the probe switch on the fluorescence emission due to the formation of selenoxide, TP-SeO 44 (Scheme 16). The Se oxidation prevents the PET between the modulator and the transducer. Before reacting with ONOO–, the fluorescence of TP-Se probe was quenched as a consequence of PET. TP-Se 43 loaded RAW 264.7 cells in the presence of 3-morpholinosydnonimine, a peroxynitrite donor resulted in a considerable increase in intracellular fluorescence within 10 min with the detection limit as low as 31 nM. When cells were treated with GSH S-transferace as the ROS scavenger, the intracellular fluorescence decreased as the TP-Se sense the redox reaction. The subcellular localization of TP-Se, HeLa cells, and a MitoTracker Red FM mitochondrial dye upon colocalization study exhibited mitochondrial ONOO– under TP excitation in living cells. As a result, the TP-Se probe can be used to visualize mitochondrial peroxynitrite levels with minimal background fluorescence and cytoxicity.
Scheme 16. Change in florescence of Probe TP-Se 43 with Peroxy Nitrite and GSH.
Hypochlorous acid (HOCl), a kind of ROS, is a powerful antimicrobial agent and works as an important architect in the immune system.74 Hypochlorous acid is mainly produced in various leukocytes by the myeloperoxidase catalyzed reaction of a chloride ion with hydrogen peroxide.75 Generally, HOCl is produced during the defense mechanism in the immune system, but its excess amount leads various diseases including arthritis,76 cancer,77 and neurodegeneration.78
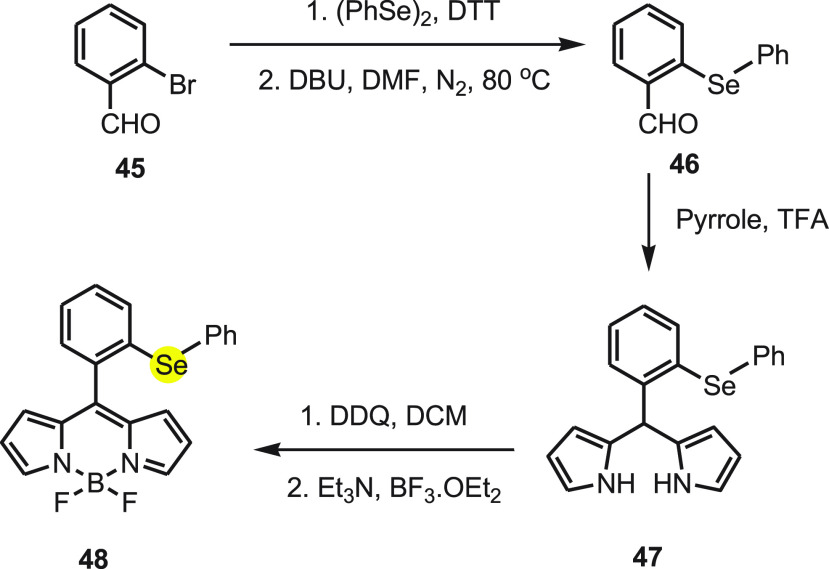
Wu and co-workers79 have reported a highly sensitive and selective fluorescent probe HCSe to detect HOCl based on specific HOCl-mediated oxidation of diphenyl selenide functionality. The probe can be synthesized in three steps, starting with easily available 2-bromobenzaldehyde 45 (Scheme 17). In the first step, 2-(phenylselenyl) benzaldehyde 46 was synthesized by the reaction of 2-bromobenzaldehyde 45 with diphenyl diselenide in the presence of dithiothreitol (DTT). Further, 2-(phenylselenyl) benzaldehyde 46 reacts with pyrrole in the presence of trifluoroacetic acid (TFA) to form corresponding dipyrromethane. Finally, probe 48 was obtained by the oxidation of 2-(phenylselenyl)phenyldipyrromethane 47 using DDQ followed by the reaction of BF3·OEt2 in the presence of triethylamine.
Scheme 17. Synthesis of Probe 48 Starting from 2-Bromobenzaldehyde 45 in Two Chemical Steps.
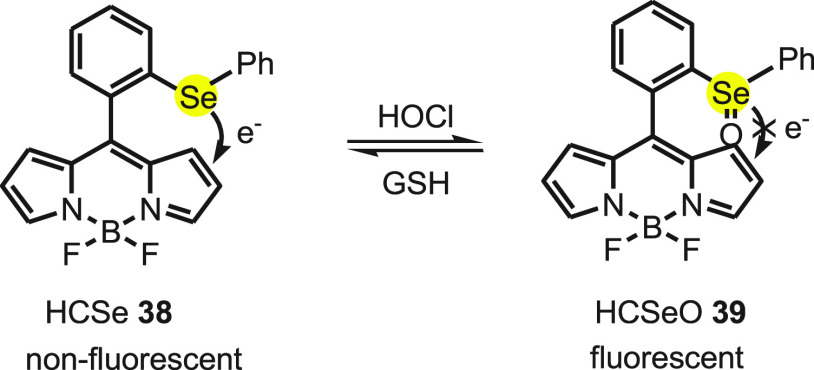
In probe 48, BODIPY (borondipyrromethane, a fluorescent dye) was used as signal transduction unit and diphenyl selenide as modulator to respond the amount of HOCl. The “ON-OFF” fluorescence of probe 48 can be explained by the redox cycle of selenium-based probe with HOCl and GSH. The probe 48 exhibits a strong green fluorescence immediately with addition of HOCl because it oxidizes the selenide functionality of 48 to the selenoxide functionality 49. Furthermore, the fluorescence of 49 was quenched with the addition of GSH (glutathione) because 49 is reduced to nonfluorescent 48 (Scheme 18).
Scheme 18. Redox Cycle of Selenium-Based Fluorescent Probe 48 with HOCl and GSH.
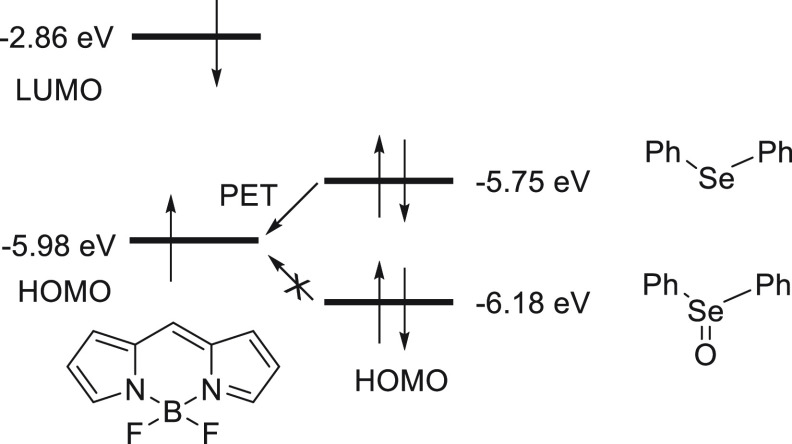
The mechanism for the detection of hypochlorous acid by fluorescence probe 48 can be explained on the density functional theory (DFT) calculations. As shown in Scheme 19, the HOMO energy level (−5.75 eV) of the diphenyl selenide moiety is higher than that of the fluorophore BODIPY (−5.98 eV), meaning the intramolecular electron transfer from the diphenyl selenide moiety to the BODIPY moiety is energetically favorable. As a result, the fluorescence of the BODIPY moiety is quenched via a PET process.
Scheme 19. Showing the Energy Diagram for the Reaction of Selenium-Based Probe 4\8 with HOCl.
Next, the oxidation of selenide moiety (HCSe) to selenoxide moiety (HCSeO) occurs in the presence of HOCl. Since the diphenyl selenoxide moiety shows a HOMO energy level (−6.18 eV) lower in comparison to the BODIPY unit, the PET process is restricted and BODIPY restored the fluorescence.
Furthermore, the probe was successfully applied for imaging hypochlorous acid in RAW264.7 cells by confocal fluorescence microscope. The detection limit of HCSe was found to be 7.98 nM, which makes it sufficiently sensitive to be used to evaluate the important roles of HOCl in biological systems.
Xiaojun Peng et al.80 developed a selective near-infrared (NIR) selenium containing fluorescent probe Se-Cy7 50 on the aggregation behavior of a heptamethine cyanine dye for selective and rapid response for HClO. In aqueous solution, the probe exhibits weak fluorescence, but on addition of NaClO, the emission spectra are obtained at 786 nm. On addition of 2.0 equiv of NaClO, 9.4-fold fluorescence enhancement was observed and the process finished within few seconds. At the same time, the detection limit of 0.31 mM for HClO (3s/k) makes the probe suitable for quantitative detection of HClO.
It was found that in aqueous solution SeCy 50 showed weak fluorescence due to the stern aggregation, but on reacting with HClO it was converted to SeOCy 51. With the formation of SeOCy 51, the aggregated probe is gradually released and enhance the fluorescence intensity (Scheme 20). The probe was found to be effective in visualizing HClO in living animals.
Scheme 20. Reaction of Fluorescent Probe Se-Cy 50 with NaClO to Form Highly Fluorescent Selenoxide 51.
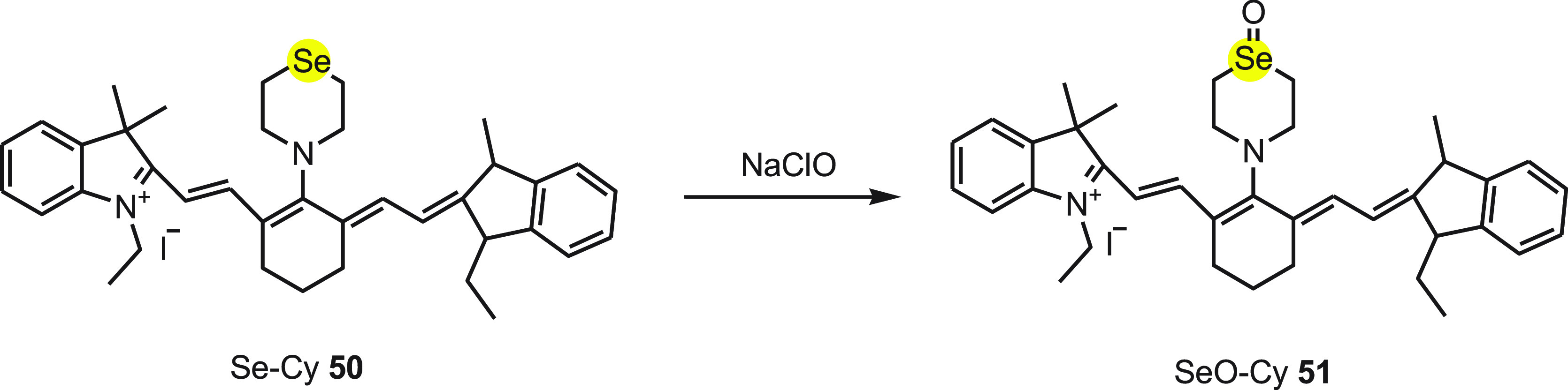
The mouse injected with SeCy 50, when injected with 2 equiv of NaClO in the same region, showed enhancement in fluorescence intensity gradually within 40 min. Thus, it was established that SeCy 50 can detect NaClO in vivo without interference of background signals.
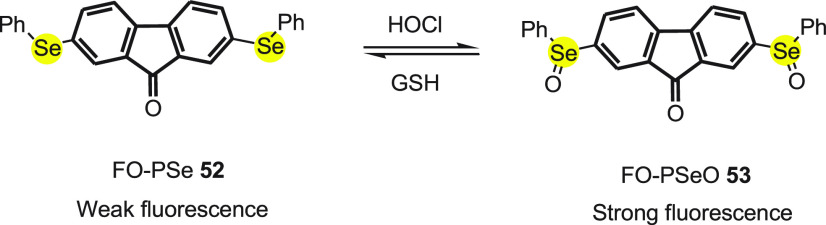
Tang et al.81 designed (FO-PSe) 52 a novel turn-on probe for the detection of HOCl. A two-photon (TP) fluorophore, 9-fluorenone was covalently conjugated with two selenium-containing moieties. The two-photon (TP) imaging procedure is considered better in comparison to their one-photon (OP) counterparts, as it can penetrate deep without photodamaging the sample by the use of two lower energy photons for excitation. The probe 52 exhibits weak fluorescence due to photoinduced electron transfer (PET) from the diphenyl selenide group to 9-fluorenone, but when HOCl is added, the selenium of the probe is oxidized to FO-PSeO 53 preventing the PET and thus turn-on fluorescence (Scheme 21).
Scheme 21. Change in Fluorescence of Probe FO-PSe 52 with HOCl and GSH.
The FO-PSe 52 shows an absorption band centered at 415 nm, while the emission maximum corresponding to it appeared at near 520 nm; the excitation maximum was found to be 415 and 800 nm for OP and TP, respectively. The probe was reported to be highly selective to HOCl, as other ROS and RNS hardly showed any significant fluorescence. The detection limit of HOCl was estimated to be 0.35 μM, which indicates that the probe can be used to determine the quantitative level of HOCl. The reversibility of the probe was established when cells on pretreating with glutathione (GSH) show weaker fluorescence compared to the cells stimulated with HOCl. The probe was also found to monitor HOCl in living cells under physiological conditions. The mouse microphage cell line RAW 264.7 shows almost no fluorescence in the absence of stimulant, whereas strong fluorescence was detected after treatment with phorbol 12-myristate 13-acetate (PMA). This indicates that probe 52 can be used to selectively visualize HOCl in cells. The application of FO-PSe was exhibited in intravital imaging in zebrafish. A strong response was observed when HOCl was added to the probe preloaded zebrafish, and after further treatment with vitamin C, fluorescence of the zebrafish decreased sharply. The probe FO-PSe also tracked the changes in HOCl levels deep inside mice and zebrafish. The probe is an ideal tool for investigating HOCl levels in live cells as well as in vivo, by using TP deep tissue imaging.
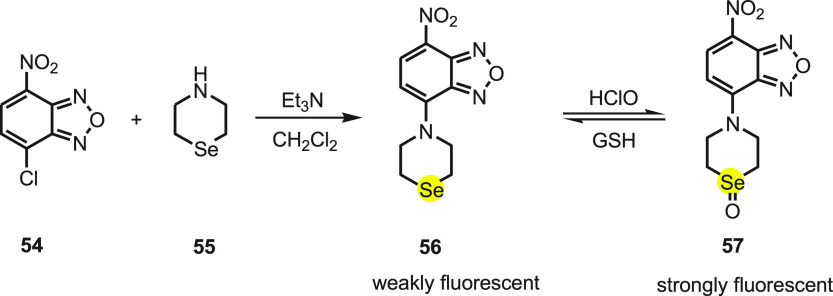
Shouzhuo Yao82 et al. synthesized a simple and reversible fluorescent probe for ClO– by reacting 7-nitrobenz-2-oxa-1,3-diazole (NBD) 54, a fluorophore, and selenomorpholine 55 as the recognition unit. The NBD-selenide probe 56 shows a weak fluorescence due to the PET process from the selenide to NBD in the excited state. In the presence of hypochlorite, the selenide oxidizes to selenoxide 57, which hampers the PET process and thus boosts the fluorescence (Scheme 22). The probe when tested against other ROS was found to be a highly selective, sensitive, and fast (<10 s) visual-based fluorescent probe for the detection of hypochlorite even at the concentration of 3.3 nM.
Scheme 22. Synthesis of Fluorescent Probe 56 and Its Reaction with HClO and GSH.
The sensor’s biological application is the real-time imaging of cellular redox cycles in HeLa cells. On incubating the HeLa cells with probe (10 μM) at 37 °C for 30 min, a dark fluorescence was recorded. On adding 30 μM NaClO into the medium and on incubating for an extra 10 min a strong fluorescence appeared. Further, the cells on loading with 100 μM GSH for 10 min exhibit no fluorescence. These findings indicate that the probe is a chemical sensor with good permeability and good reversibility that could monitor real-time redox status in cells.
Peng Li et al.83 designed Lyso-NI-Se, a lysosome-targetable fluorescent probe for imaging HOCl in living cells. The probe is synthesized using napthalamide as fluorophore, the selenide group as fluorescent modular, and the (aminoethyl)morpholine moiety as a function that led the probe to lysosomes. In phosphate buffer, Lyso-NI-Se exhibits a broad absorption band centered at 452 nm. Upon addition of NaClO, a fluorescence band rises significantly with maximum emission wavelength of 540 nm in a few seconds. The detection limit for HOCl was calculated to be 1.85 × 10–8 M. The probe turns on due to the restricted PET from the selenide pendant to the naphthalimide fluorophore. The intensity of the fluorescence decreases sharply on addition of GSH. The probe does not show any enhancement in the presence of other biologically relevant ROS or RNS. The Lyso-NI-Se highlighted a fast, selective, and sensitive fluorescence response to HOCl and was successfully used to monitor exogenous HClO in lysosomes of living cells.
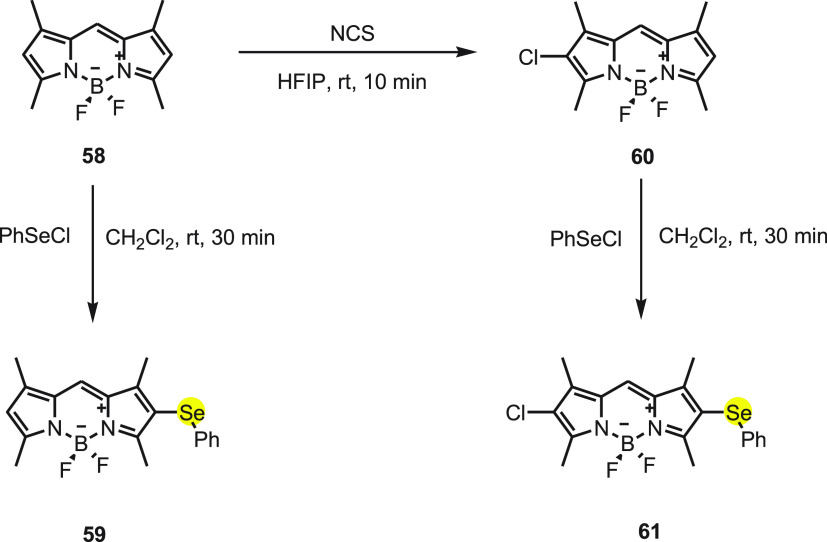
Churchil and co-workers84,85 synthesized two correlated “turn-on” fluorescent probes selectively for HOCl by incorporating phenyl selenide moiety to meso-unsubstituted BODIPY. On substituting BODIPY at the 2 and 6 positions with heavy metal, the fluorescence quantum yield decreases. Thus, in probe 59 the 2 position of BODIPY was substituted with phenyl selenide, and in probe 61, along with phenyl selenide at 2 position, Cl at the 6 position was introduced. In another probe Mes-BOD-SePh, the metsitylene at the 9 position of BODIPY along with a chloro and phenyl selenide at the 2 and 6 positions were also synthesized and studied via live cell imaging.
Probe 59 was synthesized by reacting BODIPY compound 58 with 1.0 equiv of phenyl selenyl chloride. The monochlorination of BODIPY with N-chlorosuccinimide (NCS) occurs in the presence of hexafluoro-2 propanol (HFIP) to provide 60, which on further reaction with phenylselenyl chloride gives probe 61 (Scheme 23). Both probes show absorbance maxima at 512 and 526 nm, respectively, and after adding 5.0 equiv of NaOCl, the absorption peak shifts toward longer wavelengths, i.e., 492 and 511 nm, respectively. The detection limits reported were 30.9 nm for 59 and 4.5 nm for 60.
Scheme 23. Synthesis of Fluorescent Probes 59 and 61 from Compound 58.
Density function theory (DFT) and time dependent DFT (TD-DFT) calculation explained the photomechanism of the probes. The electrons transfer from selenium to BODIPY core is efficient due to the direct attachment of both. The largest intense transition of probe 61 from HOMO–1 to LUMO (configuration interaction CI = 61.5%), together with oscillator strength (f) of 0.4984, is demarcated as the dominant transition. In the case of the oxidized state of probe 61, the largest intensity transition from HOMO to LUMO (CI = 51.9) had an oscillator strength (f) of 0.4086. The electronic distribution of HOMO and HOMO–1 of 61 is displayed on the phenylselenium moiety that delivers a PET to the BODIPY core. On the other hand, for the oxidized form of 61, the HOMO and LUMO level showed overlapped electronic distribution on the BODIPY core resulting in fluorescence enhancement. Thus, it was concluded that fluorescence enhancement is due to the oxidation of selenium to selenoxide, which blocks the PET process. The probe can be applied as a “turn-on” response selectively, as real-time imaging for hypochlorous acid at very low concentration in RAW264.7 and MCF-7 confirmed by fluorescence and confocal microscopy imaging.
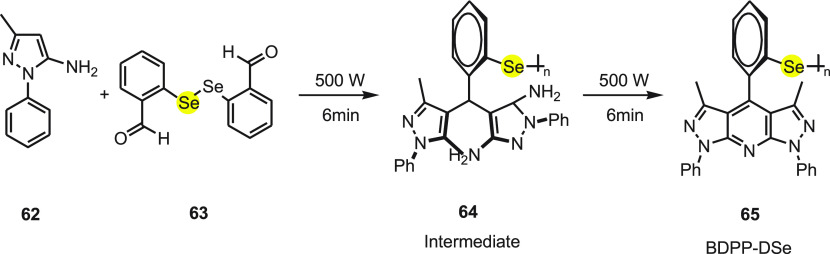
Along with aforementioned probes, Churchill et al.86 reported for the first time a dipyrazolopyridine (DPP) based probe bearing a diselenide group for chemosensing. Under solvent-free condition 5-amino-3 methyl-1-phenylpyrazole 62 reacts with bis(ortho-formylphenyl)diselenide 63 to give compound 64 as an intermediate, and on further irradiating the intermediate compound 64 at 500 W for 15 min, the final BDPP-DSe 65 was obtained (Scheme 24).
Scheme 24. Synthesis of Fluorescent Probe BDPP-DSe 65 in Two Chemical Steps.
The absorption and emission spectra of BDPP-DSe probe 65 were 331 and 436 nm, respectively. The probe was found to be sensitive toward the HOCl in comparison to other ROS, and the detection limit was calculated to be 3.6 × 10–7 M. Probe 65 shows a turn-on response in the presence of NaOCl along with a 180-fold increase in fluorescence intensity. The turn-on response is attributed to the oxidation of selenium to form BDPP-DSeO. This chemical change halts the PET mechanism between phenyl selenide group and BDPP, thus resulting in the fluorescence. Upon testing with other competitive ROS, the probe does not show any optical change and backs the selectivity for the NaOCl. A confocal microscopy imaging study using BDPP-DSe 65 was carried out in living breast cancer cells that demonstrated detection of HClO in living cells; thus the study concludes that the probe is cell membrane permeable. The probe can be potentially applied in various fields such as neurosciences, health sciences, as well as cancer and brain related diseases.
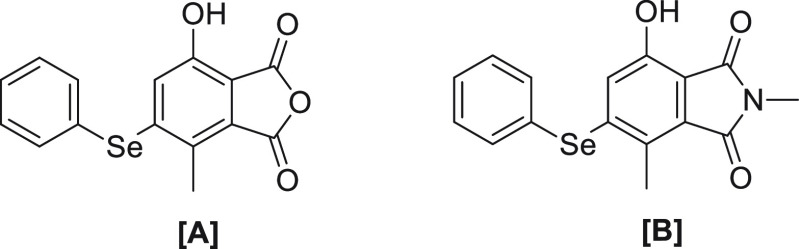
In another work, Churchill et al. developed two new probes, Probe [A] and Probe [B] (Figure 2) using anhydride and amide phenyl selenide conjugates.87 Both probes show no fluorescence due to the presence of the phenyl selenium group at the 2 position, as it is involved in the PET process, but as soon as OCl– is added, selenoxide is formed which blocks electron transfer from the phenyl selenium donor that results in blocking the PET process and turns on fluorescence in less than 1 s.
Figure 2.
Structures of probes [A] and [B].
The HRMS data backed the formation of selenoxide after addition of OCl– to the Probe [B]. Upon addition of l-cysteine into oxidized probe [B], turn-off fluorescence within 5 min is observed. Density functional theory (DFT) geometry optimization calculations confirmed the photo mechanism. The oxidized probe [A] was found to degrade in cell assays, whereas probe [B] shows high stability at physiological pH. The UV–Vis absorption spectrum for [B] shows absorbance maxima at 396 nm, and upon addition of 1 equiv of OCl– the absorption peak undergoes a red shift to 416 nm. No major change in absorption maxima is observed upon addition of other ROS/RNS. The probe was confirmed to be nontoxic after studying U-2 OS cells and HeLa cells. It was found that probe [B] undergoes aggregation induced emission (AIE) in lipid droplets (LDs); thus it can be used as a probe for LDs.
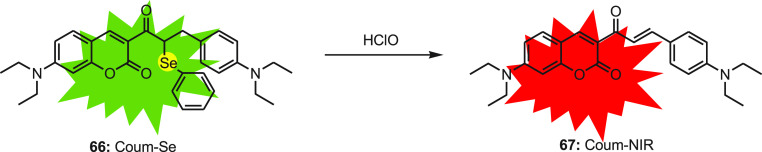
Tang et al.88 designed an innovative fluorescent probe Coum-Se 66 composed of an α-phenylseleno carbonyl moiety and coumarin as the fluorophore. The Coum-Se probe displays a single emission band at around 495 nm. In the presence of HClO, the initial band gradually disappears, and concurrently a new peak appears at 618 nm. The fluorescence ratio (I618/I495) depends on the dose and increases 241-fold in the presence of 5 equiv of HClO. The Coum-Se 66 probe emits green fluorescence, while in the presence of HClO, selenium oxidizes to selenoxide and further undergoes a spontaneous syn elimination to generate Coum-NIR 67, with an extended conjugation system which releases striking red fluorescence (Scheme 25). Upon adding HClO, both the intensities rapidly change and reach a plateau in 5 s as a response time. The probe is highly sensitive toward HOCl, as it shows no fluorescence ratio variation in the presence of bio-related ROS and RNS other than HClO. The calculated detection limit of the probe was found to be 4.6 nM.
Scheme 25. Elimination of Selenide Moiety from Probe 66 as Selenoxide Elimination with HClO.
The two-photon excitation Coum-Se 66 probe can be used for bioanalytical application in live cells, as it has shown minimal cytotoxicity against RAW264.7. It visualizes HClO fluctuation in RAW 264.7 macrophages upon stimulation with lipopolysaccharide (LPS) and phorbol myristate. The ratiometric response was further analyzed by ratiometric visualization of biogenic HClO in live animals. During LPS-induced acute hepatitis, the Coum-Se 66 was employed to monitor the real-time HClO bust. The result showed remarkable red fluorescence and a drop in green fluorescence with increasing dose of LPS in the mouse liver. The Coum-Se 66 can be used to map the ratiometric levels of HClO in live animals during any biomedical process.
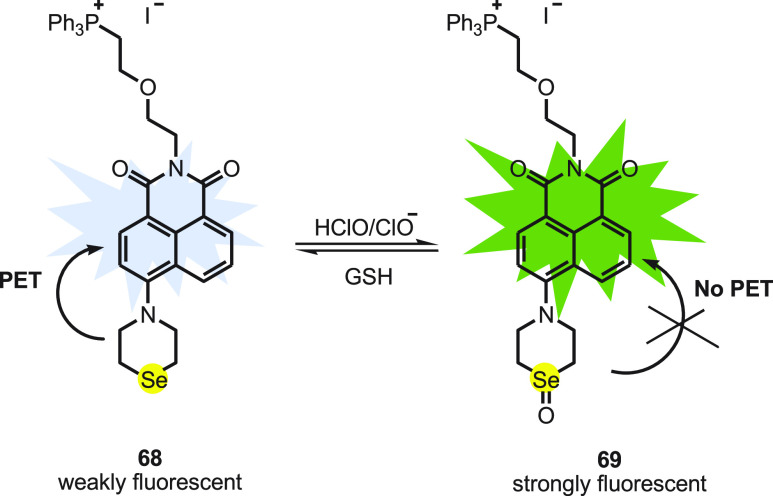
Wei Guo and co-workers89 reported a napthalimide-based reversible fluorescent probe Nap-Se 68 by integrating napthalimide with a fluorophore and selenomorpholine. The triphenylphosphonium group attached to the napthalimide unit improves the water solubility and guides the probe to mitochondria. The probe works on the principle of PET; thus Nap-Se 68 itself displays weak fluorescence. Upon addition of 1.0 equiv of ClO–, the PET process is hindered due to the formation of selenoxide 69 and is observed as a surge in fluorescence intensity (Scheme 26). Within 2 s a plateau was reached suggesting a fast response of the probe toward ClO–. Upon addition of 1 equiv of GSH, the intensity of the probe decreases gradually to its original state in 30 min showing reversibility of the probe. The estimated detection limit of the probe toward ClO– was reported to be 4.8 nM.
Scheme 26. Redox Cycle of Selenium-Based Fluorescent Probe 68 with HClO with GSH.
Raw 264.7 macrophages loaded with Nap-Se 68 display weak green emission. The cells stimulated with LPS and PMA followed by addition of Nap-Se 68 display enhanced emission in the green channel. Further, imaging the costained HeLa cells with Nap-Se and Mito Tracker Red FM using confocal laser scanning microscopy shows that green fluorescence from Nap-Se 68 was well overlapped with the red fluorescence signal from Mito Tracker. The study indicates that the Nap-Se was found to be primarily located in mitochondria. The probe can be explored for further study of redox biology.
4. Detection of Carboxylate Anions and Carboxylic Acids
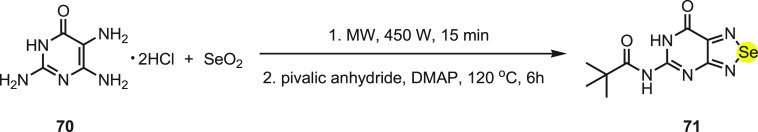
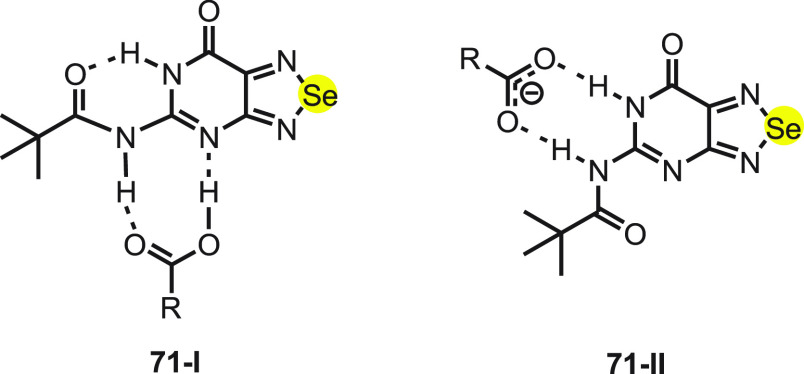
In past two decades, the recognition of carboxylic acids and carboxylate anions by synthetic receptors has been receiving particular attention due to their applications in biological pharmaceutical science.90−92 Recently, various sensors have been developed for recognizing a specific anion over a wide range of anions and other guests.93−96 Goswami and his co-workers97 have reported the synthesis of selenium-cored chromogenic fluorescent sensor 71 to distinguish carboxylate anions from carboxylic acids and other anions. The synthesis of sensor 71 was achieved in the solid phase by irradiating 2,5,6-triamino-3H-pyrimidin-4-one dihydrochloride 70 and the selenium dioxide mixture in a microwave oven followed by pivaloylation using pivalic anhydride (Scheme 27).
Scheme 27. Synthesis of Sensor 71 by the Reaction of Pyridine 70 with Selenium Dioxide.
The receptor 71 mostly exists in the 71-I state in which the lactam NH proton of the pyrimidine moiety forms a strong intramolecular hydrogen bonding with the carbonyl moiety of the amide. Furthermore, in the presence of carboxylate anions the intramolecular hydrogen bonds break, and the flexible amide proton present in the same direction as lactam NH recognizes carboxylate (71-II) (Scheme 28).
Scheme 28. Mode of Binding of Sensor 71 to (I) a Carboxylic Acid Moiety and (II) the Carboxylate Moiety.
The fluorescence behavior of 71 was recorded with the addition of carboxylic acid and carboxylate ions. There was no change in the fluorescence intensity with the addition of monocarboxylic acid, while a significant increase in the fluorescence was observed with the addition of carboxylate anions. These studies clearly indicate that 71 can be used as a fluorescence probe for detection of carboxylate anions over the other interfering anions and carboxylic acids. Furthermore, these results were supported by the NMR studies.
5. Detection of Thiols
Thiols are important constituents of cellular proteins and other simple molecules such as glutathione (GSH), cysteine (Cys), and homocysteine (Hcy), which are responsible for several biological events.98,99 The abnormal behavior of cellular thiols leads to a number of diseases including cancer, AIDS, and Alzheimer’s and cardiovascular diseases. In addition, cysteine (Cys) is a nonessential amino acid, and its deficiency is responsible for some syndromes such as slow growth in children and liver damage.21,100−102 The elevated level of homocysteine (HCy) in plasma leads to Alzheimer’s disease and cobalamin (vitamin B12) deficiency.103,104 More importantly, glutathione (GSH) is the most abundant intracellular nonprotein thiol105 which exist in reduced (GSH) as well as oxidized (GSSG) states and plays a vital role in maintaining intracellular redox balance.106
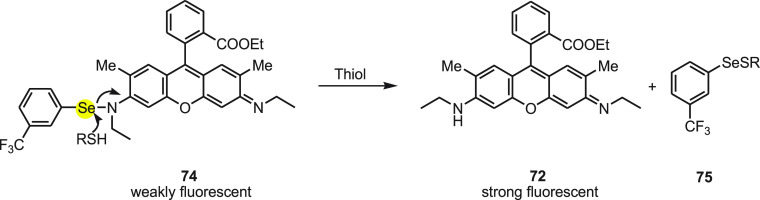
In 2007, Tang and co-workers107 developed a rhodamine-based fluorescent probe 74 containing a Se–N bond for the detection of thiols. The synthesis of the rhodamine-based fluorescent probe 74 was achieved by the reaction of rhodamine 6G 72, 3-bromobenzotrifluoride 73, and KSeCN in the presence of CuI using triethylamine as the base under inert atmosphere (Scheme 29).
Scheme 29. Synthesis of Fluorescent Probe 74 by the Reaction of Rhodamine 72 and Arylbromide 73 with KSeCN Followed by CuI/Et3N.
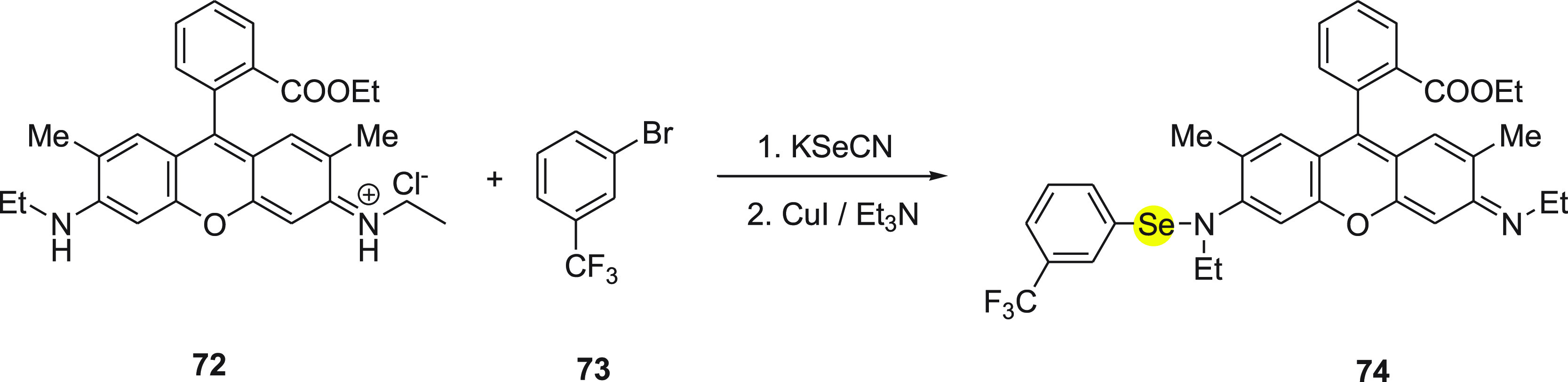
The mechanism to detect thiols by rhodamine-cored probe 74 depends on the strong nucleophilicity of the sulfhydryl to cleave the Se–N bond of probe 74. The probe in aqueous solution is weakly fluorescent, while after the Se–N bond breaks in the presence of sulfhydryl it gives the corresponding selenenyl sulfide 75 and the strongly fluorescent dye Rhodamine 6G 72 (Scheme 30). Probe 74 exhibited high selectivity toward GSH in the presence of other analytes. In addition, the probe was reacted with both nonprotein and protein thiols and has shown 2–3 times higher sensitivity for protein thiols than nonprotein thiols. The detection limit was calculated to be 1.4 nM, which indicated that the probe could detect GSH qualitatively and quantitatively. Furthermore, the probe was effective for thiols imaging in both HL-7702 cells and HepG2 cells with high selectivity and sensitivity.
Scheme 30. Reaction of Fluorescent Probe 74 with Thiol.
In 2012, Wang and co-workers108 developed two highly sensitive and selective near-infrared (NIR) fluorescent probes 80 (Cy-NiSe) and 81 (Cy-TfSe) for the detection of thiols on the basis of Se–N bond cleavage in cells and tissues. Initially, intermediate 77 (Cy7-MeAm) was synthesized by the reaction of 76 (Cy7-Cl) with methanamine hydrochloride in 87% yield. The other intermediate o-nitrophenyl selenochloride 76 was obtained by diselenide 78 using thionyl chloride.
In the next step, selenochloride 79 reacts with 77 (Cy7-MeAm) to form probe 80 (Cy-NiSe) in the presence of triethylamine. Finally, intermediate 77 (Cy7-MeAm) reacts with 3-bromobenzotrifluoride 73 and KSeCN to form the other probe 81 (Cy-TfSe) in the presence of triethylamine and CuI (Scheme 31).
Scheme 31. Synthesis of Fluorescent Probes 77, 80, and 81.
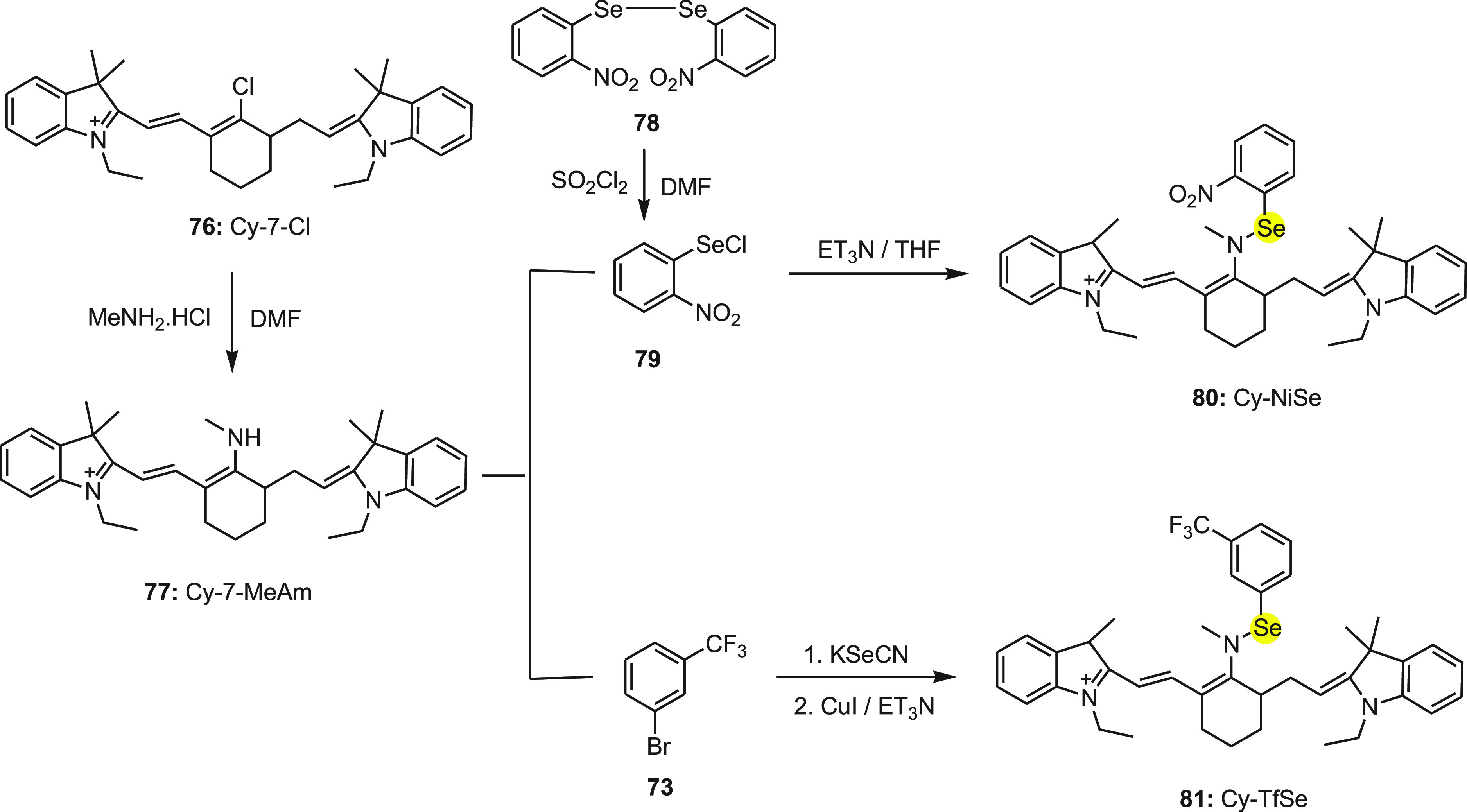
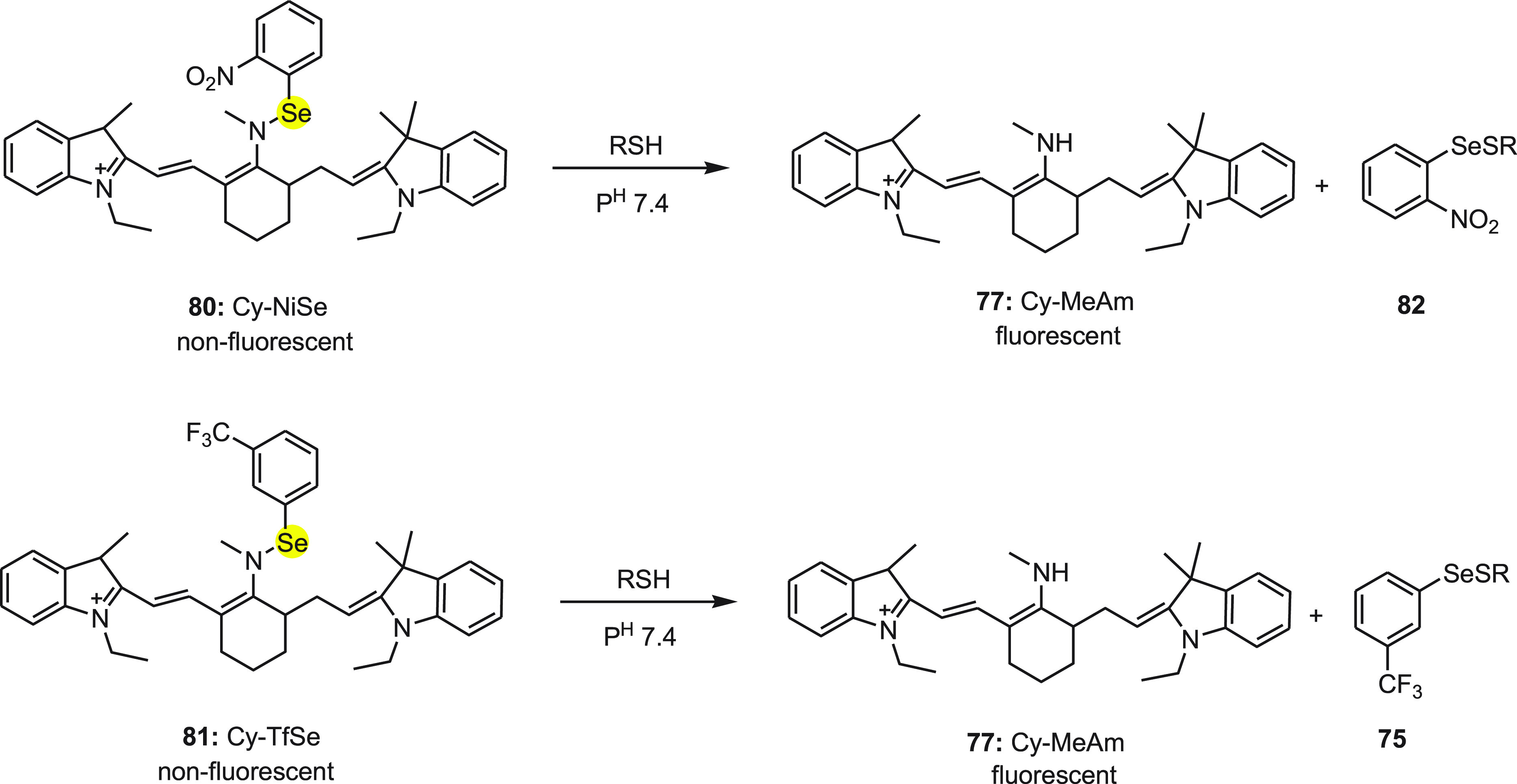
The mechanism for change in the fluorescence of probes 80 and 81 with the addition of thiols is depicted in Scheme 32. In the probes, cyanine dye was used as the signal transducer while strongly electron-withdrawing 2-nitrophenylselane and 3-(trifluoro-methyl)phenylselane groups were used as modulators. Initially, probes did not show any fluorescence because of the donor-excited photoinduced electron transfer (d-PET) between fluorophore and modulators. Further, both probes 80 and 81 exhibited strong fluorescence with the addition of thiol because of the restriction of the d-PET process between the fluorophore and modulators due the cleavage of the Se–N bond to form cyanine dye 77. Furthermore, both probes 80 and 81 have been applied successfully for the detection of thiols in living RAW 264.7 cells and fresh rat liver tissue. These findings will be of great benefit for biomedical researchers investigating the effects of thiols in biological systems.
Scheme 32. Conversion of Nonfluorescent Probes Cy-NiSe 80 and Cy-TfSe 81 to Fluorescent Probe Cy-MeAm upon Treatment with Thiol.
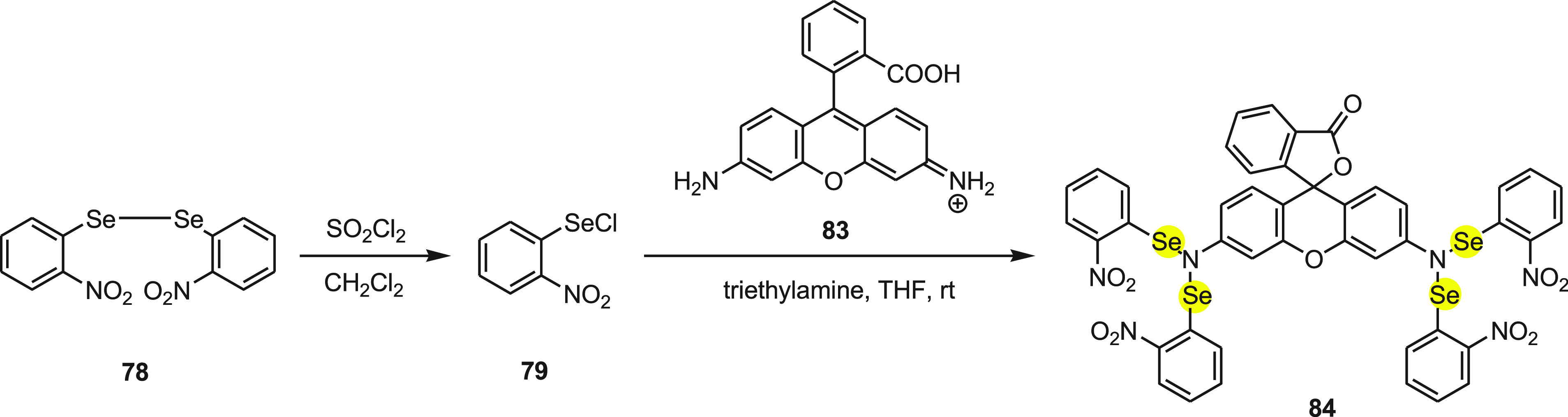
Tang and co-workers109 have reported a highly sensitive and specific organoselenium fluorescent probe 84 for the detection of thiols. The probe 84 (Rh-Se-2) was synthesized by the reaction of diselenide 78 and thionyl chloride followed by the reaction of 83 (rhodanine) in the presence of triethylamine (Scheme 33).
Scheme 33. Synthesis of Fluorescent Probe 84 Starting from Diselenide 78 in Two Chemical Steps.
The mechanism to detect thiols by rhodamine-cored probe 84 was quite similar to Rhodamine 6G-cored probe 74. Probe 84 did not show fluorescence originally in the aqueous solution but exhibited strong fluorescence with the addition of thiols due to the formation of 83 (Rhodamine, fluorescent dye) by the nucleophilic attack of thiol sulfur to cleave the Se–N bond (Scheme 34). Since glutathione is abundantly present in cells, it was selected as the representative thiol for the spectral experiments.
Scheme 34. Conversion of Weak Fluorescent Probe 84 to Strong Fluorescent Rhodamine 83 on the Treatment with Thiol.
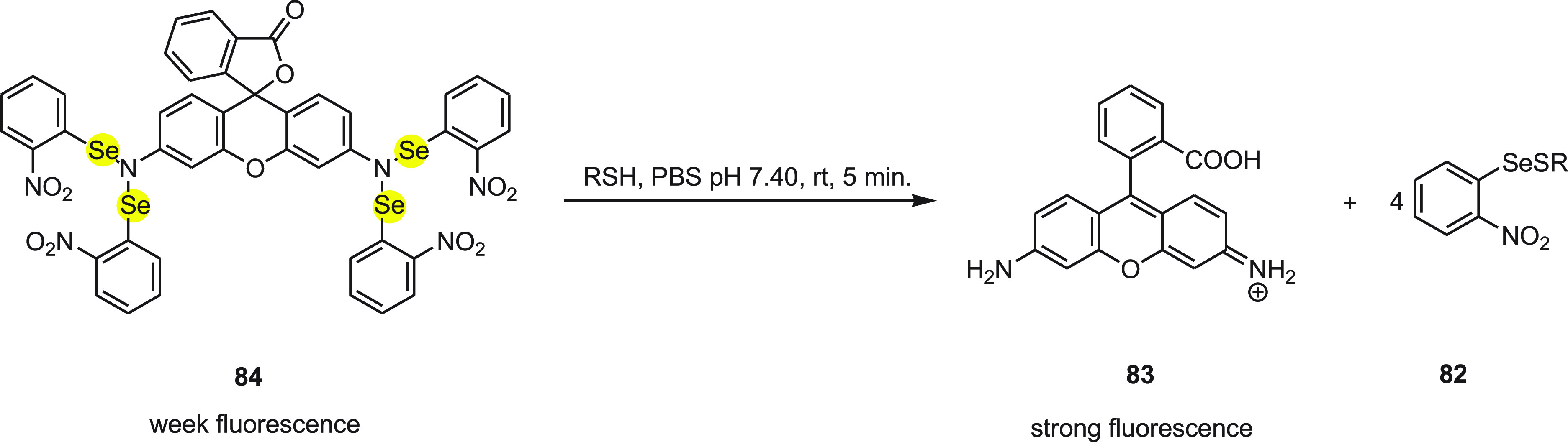
Interestingly, probe 84 can even differentiate between thiols and nonthiols with a clear change in color, easily observed by the naked eye. Furthermore, the confocal microscopy experiments using HL-7702 cells and HepG2 cells confirm that the probe 84 can visualize the variation in concentration of thiol in both normal and aberrant cell types. Thus, this can be considered a simple and dependable fluorometric system for the analysis of intracellular thiol.
Han and co-workers110 reported a fluorescent probe 85 (FSeSeF) based on diselenide for the fast detection of thiols and redox changes occurring due to the presence of thiols and ROS in living cells. This probe works according to the ping–pong mechanism of the GPx activity of diselenide compounds (Scheme 35). Probe 85 with a diselenide bond is a weak fluorescent molecule. This diselenide bond is considered a rapid, reversible recognition center for thiols and two fluoresceins as signal reporters.
Scheme 35. Change in Fluorescence of Diselenide-Based Probe FSeSeF 85 with GSH and H2O2.
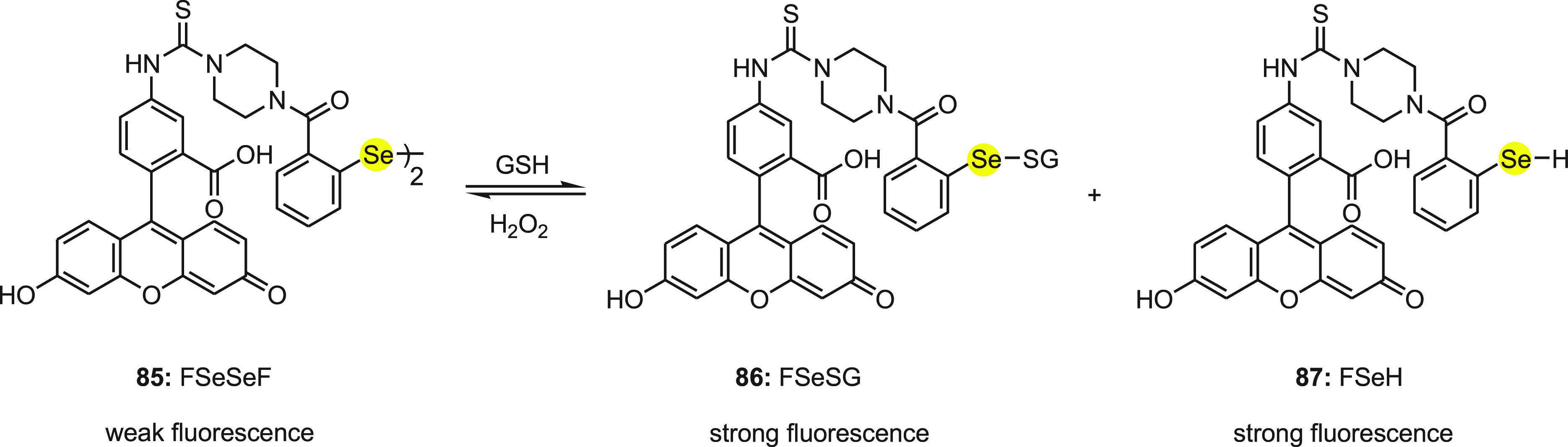
Interestingly, 85 exhibited strong fluorescence with the addition of GSH due to the cleavage of diselenide bond of 85 to form both selenenyl sulfide 86 (FSeSG) and selenol 87 (FSeH) containing one fluorescein unit. According to the ping–pong mechanism of GPx activity of the diselenide compounds, in the presence of H2O2 molecules 86 (FSeSG) and 87 (FSeH) can be restored back to 85 (FSeSeF); thus the redox changes in the cells due to the presence of thiols and ROS can be easily detected. These mechanistic studies were supported by API-ES mass spectroscopy and 77Se NMR. The detection limit for H2O2 was found to be 2.25 × 10–7 M. Furthermore, probe 85 was successfully applied for the detection of thiols in living cells (HeLa cells).
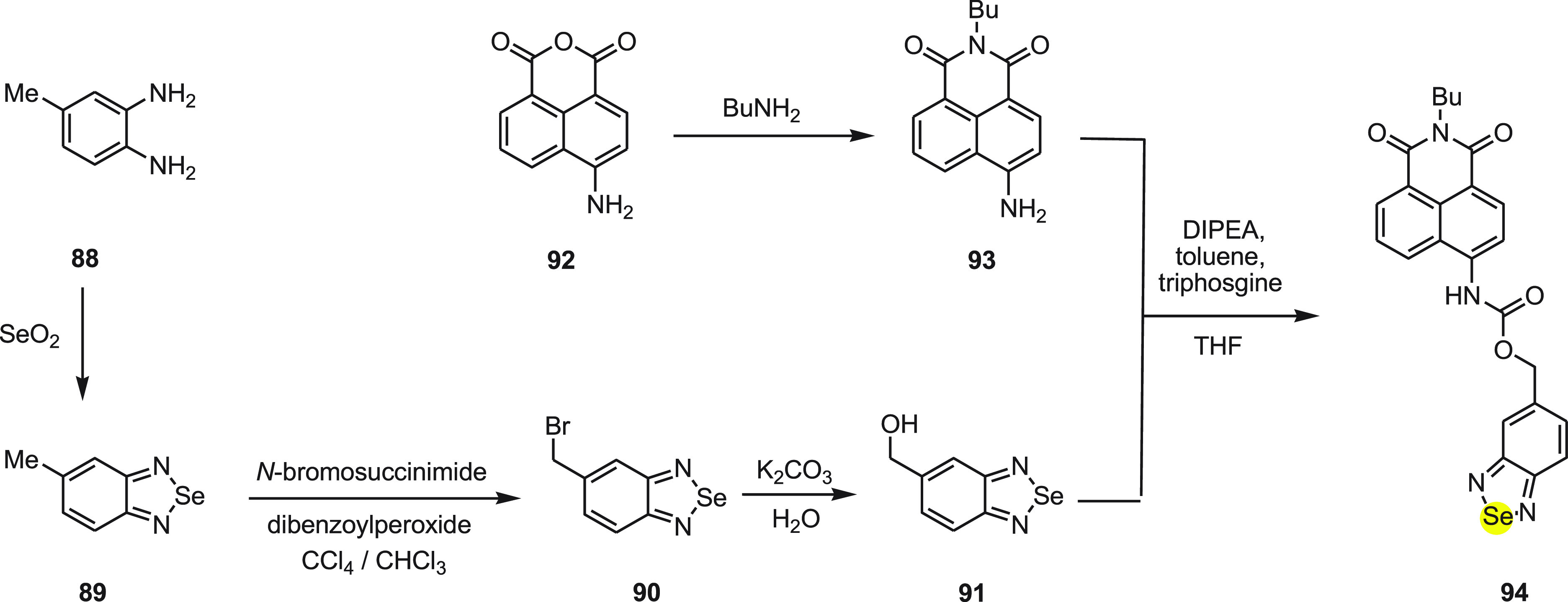
1,4-Dithiothreitol (DTT) is a synthetic thiol111,112 and is commonly used as a powerful reducing agent.113,114 DTT is a highly toxic substance which in the presence of transition metals lead to oxidative damage of biomolecules.115,116 It also increases the toxicity of some arsenic and mercury containing compounds.117,118 In 2010, a highly selective fluorescent probe 94 was developed for the detection of DTT using the internal charge transfer (ICT) mechanism.119
Probe 94 can be divided into three distinct parts—(a) a fluorophore, 4-aminonaphthalimide, (b) an organoselenium moiety as DTT receptor, and (c) the 3,4-diaminophenyl methanol, a self-immolative spacer selected for connecting the fluorophore with the receptor. In order to synthesize probe 94, two different intermediates 90 and 91 were synthesized and then reacted in the presence of DIPEA and triphosgine in toluene to form probe 94 (Scheme 36).
Scheme 36. Synthesis of Fluorescent Probe 94 Starting from ortho-Diamine 88.
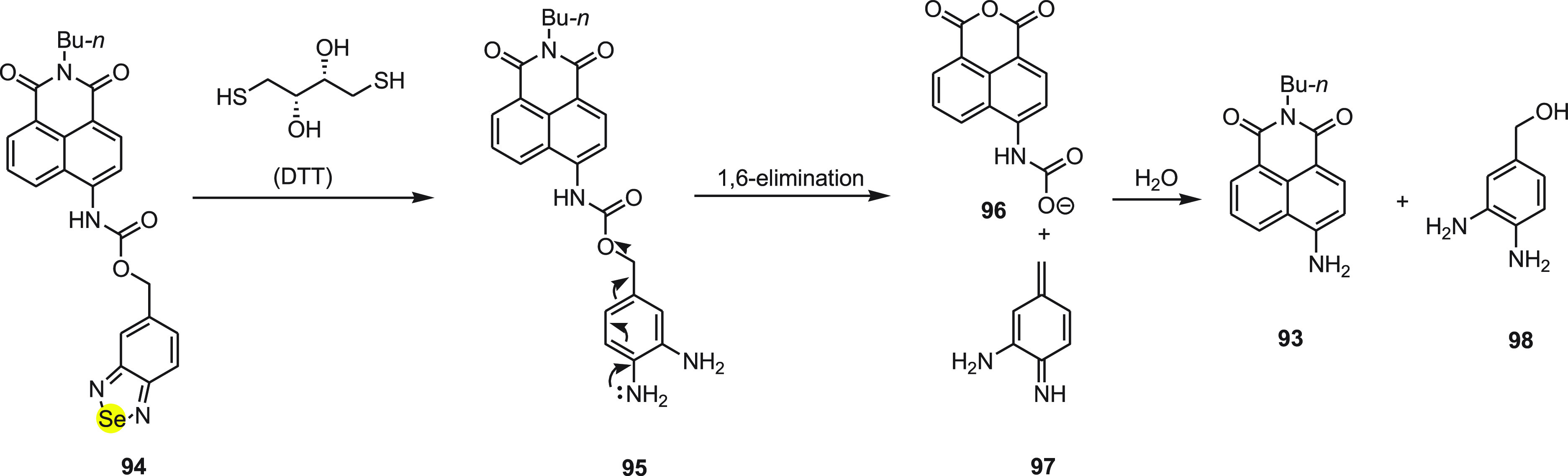
The mechanism for the change in fluorescence of probe 94 to detect DTT has been depicted in Scheme 37. According to the mechanism, nonfluorescent probe 94 reacts with DTT and cleaves the piazselenole-based carbamate protecting group via an internal charge transfer process and regenerates 4-aminonaphthalimide 93 with green fluorescence. Interestingly, the probe has a high selectivity for DTT when compared to other biothiols such as cysteine and glutathione, which was attributed to DTT’s strong reducing ability. The probe displays a 66 nm red-shift of fluorescence emission, and upon adding DTT, the color changes from colorless to jade-green and thus acts as a “naked-eye” probe for DTT. The live cell image experiments further demonstrate its importance in sensing DTT as well as changing the redox environment in living cells.
Scheme 37. Mechanism for the Change in Fluorescence of the Probe 94 to Detect DTT.
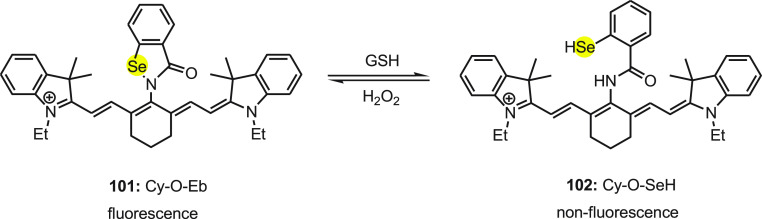
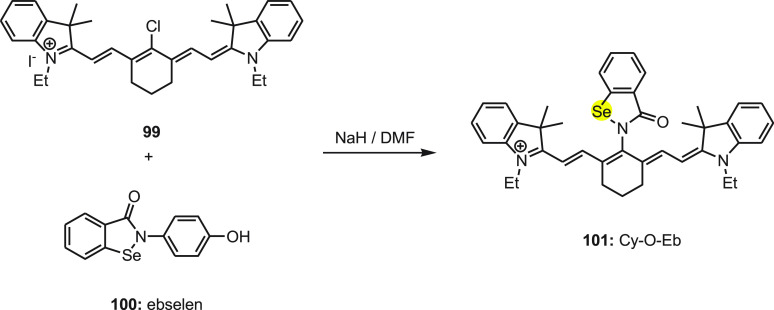
Tang and co-workers120 developed a fluorescent probe 101 for the detection of intracellular GSH/H2O2, illustrated in Scheme 39. In 101 the heptamethine cyanine platform (Cy) was used as a fluorophore since it has a high extinction coefficient, low cytotoxicity, and good compatibility with cells. The ebselen (Eb) moiety was integrated as a fluorescent modulator in the probe due to its unique response toward GSH/H2O2. The probe was synthesized by the reaction of 99 with ebselen 100 in the presence of sodium hydride in DMF (Scheme 38).
Scheme 39. Mechanism of Change in Fluorescence of Probe 101 with GSH/H2O2.
Scheme 38. Synthesis of Fluorescent Probe Cy-O-Eb 101 by the Reaction of Ebselen 100 with Compound 99 in the Presence of a Base.
The mechanism of change in fluorescence of probe 101 with GSH/H2O2 can be explained by redox cycle (Scheme 39). The strong fluorescent probe 101 can be reduced with the treatment of GSH by cleavage of Se–N which exhibits weak fluorescence. Furthermore, the reduced product 102 is quite unstable and reacts with H2O2 to oxidize to more fluorescent 101. In addition, the mechanism was also supported by mass spectroscopic analysis.
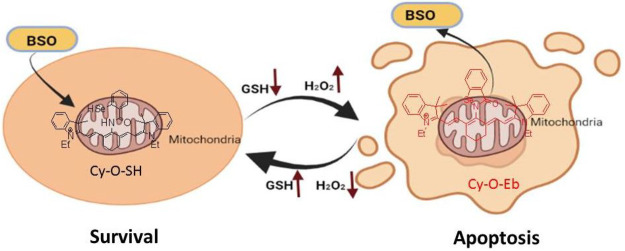
Finally, the probe was successfully implemented for real-time imaging of intracellular redox state changes during the apoptosis process carried out in mitochondria. The results showed that HepG2 and HL7702 cells exhibited better endurance toward H2O2 in comparison to normal cells when H2O2 was gradually increased by BSO stimuli. BSO-induced tumor apoptosis and apoptosis reversal in HepG2 cells are schematized in Figure 3. Furthermore, upon applying the probe on the wound margin in zebrafish the H2O2 changes in the affected area were successfully monitored.
Figure 3.
Illustration of the BSO-induced tumor apoptosis and the reversal of apoptosis in HepG2 cells.
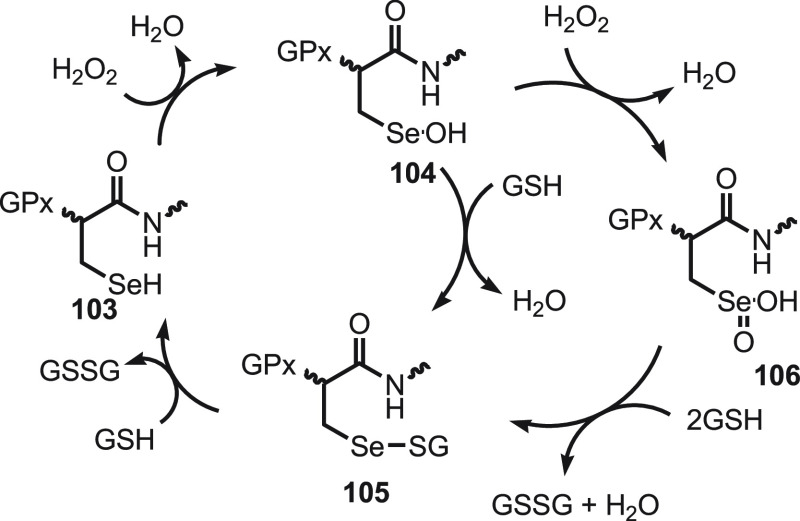
Glutathione peroxidizes enzyme (GPx) in the presence of glutathione (GSH) and catalyzes the reduction of harmful peroxides.121−124 Mugesh and his research group125−133 have developed various selenium-cored probes to regulate the reversible redox cycle with peroxides and thiols. The catalytic cycle initiates with the oxidation of active selenol 103 by peroxides resulting in corresponding selenenic acid 104, which reacts with a thiol to generate an important intermediate selenenyl sulfide 105. The Se–S bond in the selenenyl sulfide 105 is further attacked by a second GSH to give active selenol 103 along with the thiol cofactor (GSSG) in its oxidized form (Scheme 40). With the decrease in thiol level, the selenenic acid oxidizes to seleninic acid 106 which interferes with the main catalytic pathway. In this cycle the fast reaction of the selenenic acid and GSH results in selenenyl sulfide 105 which further reacts with another GSH to give selenol. These reactions ensure that the selenium moiety in the enzyme is not irreversibly inactivated.
Scheme 40. Mechanism for the Reduction of Harmful Peroxides in the Presence of Glutathione (GSH).
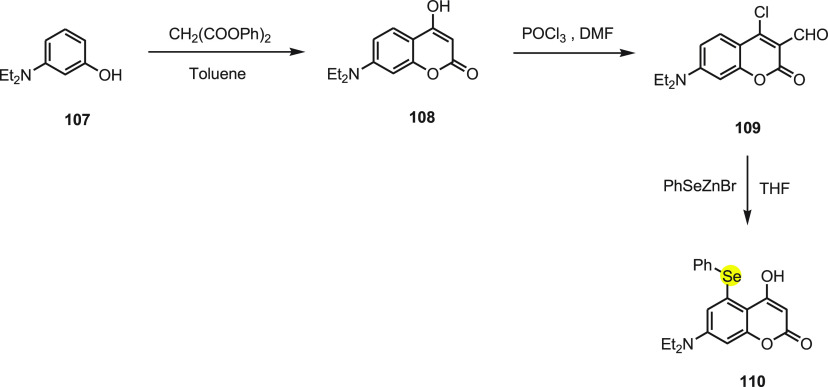
Churchill et al.134 developed a phenyl-selenium substituted coumarin probe 110 for rapid and highly selective detection of glutathione (GSH) compared to cysteine (Cys)/homocysteine (Hcy) without background fluorescence. Probe 110 was synthesized from 3-(diethylamino)phenol 107 by treating with diphenyl malonate to get 7-(diethylamino)-4-hydroxy-2H-chromen-2-one 108, which upon further reaction with dimethylformamide and phosphorus oxychloride gives 4-chloro-7-(diethylamino)-2-oxo-2H-chromene-3-carbaldehyde 109. Upon reacting intermediate 109 with phenylselenyl zinc bromide, probe 110 is obtained (Scheme 41).
Scheme 41. Synthesis of Fluorescent Probe 110 Starting from 3-Hydroxyaniline 107 in Three Chemical Steps.
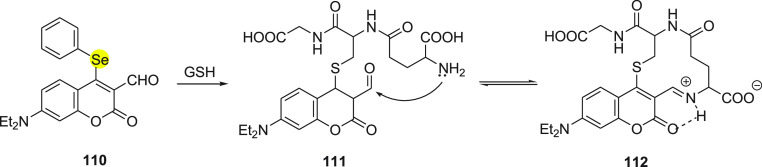
The phenylselenide group at the fourth position of coumarin has the capability to quench the fluorescence via the PET process as well as also being a good leaving group. The adjacent aldehyde group increases the electrophilicity at the fourth position as a Michael acceptor and helps to cyclize in the presence of sulfhydryl and primary amine groups of biothiols resulting in fluorescence (Scheme 42). The substitution reaction at the fourth position of coumarin with sulfhydryl of GSH occurs so fast that maximum fluorescence intensity is attained in a subsecond time frame and exhibits a 100-fold fluorescent increase in comparison to the fluorescence generated from other amino acids, including Cys and Hcy.
Scheme 42. Reaction of Fluorescent Probe 110 with GSH.
To envision the optimized structures and to calculate molecular orbital energy levels, density functional theory (DFT) calculations were performed for 110, 111, and 112. The results showed that 112 forms a stable hydrogen bond between the adjacent carbonyl group and iminium, resulting in a red-shift. The calculated frontier orbital energy difference for 112 was found to be smaller in comparison to that for 110 and 111. Confocal microscopy imaging of living cell systems indicates that the probe identifies GSH in Hep3B cells quickly and explicitly, and the limit of detection was determined to be 2.7 × 10–7 M. Furthermore, cell viability testing showed low cytotoxicity toward the probe; thus the probe has the potential for biological uses.
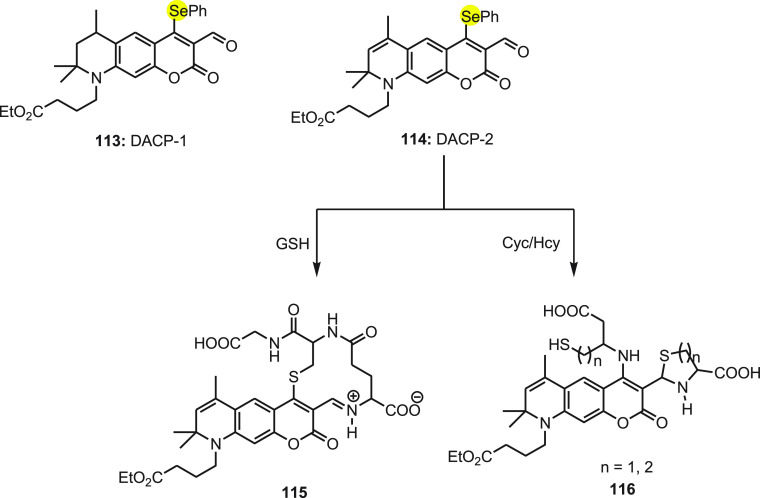
In 2018, Churchill and co-workers135 in continuation of the above work developed an improved version of coumarin-based fluorescent probes to detect biothiols. To block the rotational freedom, the alkylamino group of the above probe was replaced with N-heterocyclic derivative (DACP-1) 113. This improved the fluorescence emission intensity; however the emission wavelength remains the same. To get a more bathochromic shift in absorption and emission wavelength, an additional π-conjugation was introduced in the newly attached N-heterocyclic ring (DACP-2) 114. The new double bond gives DACP-2 114 better optical attributes for chemosensing in comparison to DACP-1 113. An ethyl butanoate group at the amino position improves water solubility of both probes. A phenylselenium group at the 4-position of both probes completely quenches the fluorescence via PET to give a nonfluorescent species. The probe exhibits selectively in the red region for GSH and in the green region for Cys/Hcy (Scheme 43). In comparison to closely related species such as amino acids and Cys/Hcy, DSCP-1 113 and DACP-2 114 showed a strong improvement in fluorescence when treated with GSH. The fluorescence quantum yields increase for the red channel (GSH) [<0.001 to 0.52 (DACP-1) and 0.48 (DACP-2)] and the green channel (Cys) [<0.001 to 0.030 (DACP-1) and 0.026 (DACP-2)]. Selective detection was further confirmed by confocal microscopy imaging experiment on live human lung cancer cells and fibroblasts as well as mice tumor model imaging. The detection limits of DACP-1 and DACP-2 for Cys were found to be 0.31 and 1.27 μM, respectively.
Scheme 43. Reaction of Fluorescent Probe 114 with GSH and Cys/Hcy.
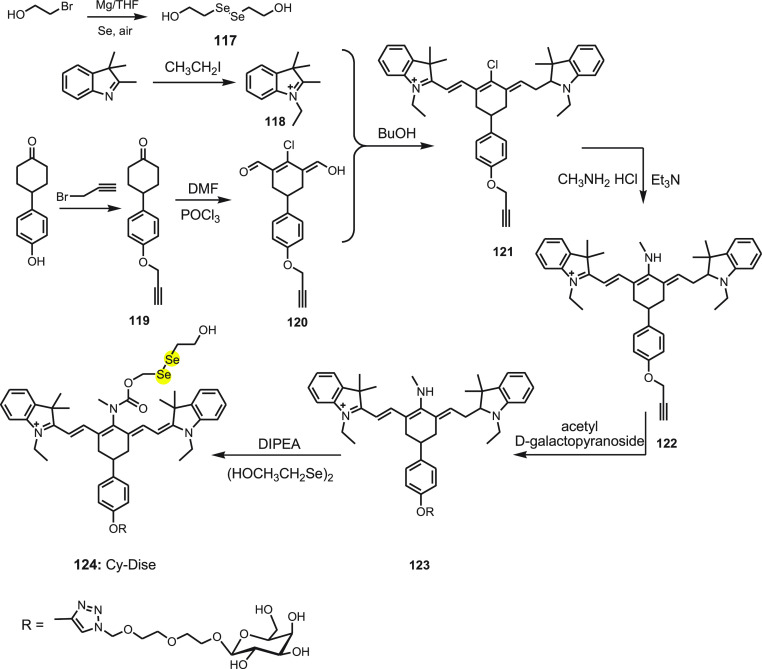
For the first time, Chen and co-workers136 synthesized a novel near-infrared fluorescent probe Cy-Dise 124 for selectively detecting cysteine hydropersulfide (Cys-SSH) in living cells and in vivo. Cy-Dise 124 involves three moieties: bis(2-hydroxyethyl) diselenide, a response module; heptamethine cyanine, a signal transducer; and d-galactose, a targeting unit. The response module bis(2-hydroxyethyl) diselenide 117 was synthesized by reacting selenium and 2-bromoethanol. The heptamethine cyanine dye 121 was synthesized using propargyl bromide and 4-(4-hydroxyphenyl) cyclohexanone 119. Further, reacting compound 119 with DMF and POCl3 yields 120. An intermediate compound, 120, and 1-ethyl-2,3,3 trimethyl-3H-indolenium iodide salt 118 afford compound 121. A nucleophilic substitution reaction by methylamine hydrochloride at the meso position of compound 121 results in compound 122. Acetyl-d-galactopyranoside is attached to compound 122 via click chemistry yielding compound 123. In the last step, compound 123 is treated with triphosgene subsequently with the addition of 117 to obtain Cy-Dise 124 (Scheme 44).
Scheme 44. Synthesis of Fluorescent Probe Cy-Dise 124.
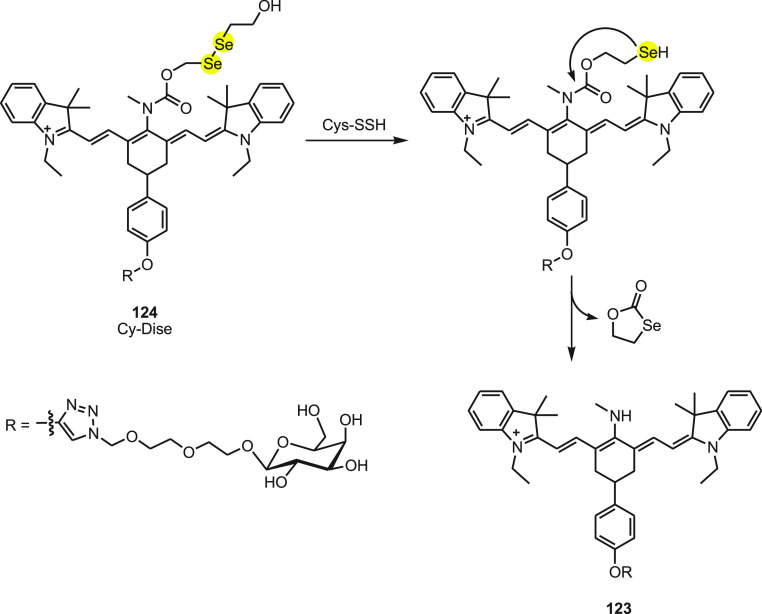
The integration of bis(2-hydroxyethyl) diselenide with a carbony group in the central nitrogen atom results in a red shift in the emission spectrum. Exchanging the response modulator by Cys-SSH leads to a blue shift in the emission spectrum. The ICT-based red–blue shift in the emission spectrum helps in detection of Cys-SSH in living cells and in vivo. The release of fluorophore 123 occurs due to the intermediate formed immediately upon reduction of diselenide and fast intramolecular cyclization along with the cleavage of the neighboring carbamate bond (Scheme 45). In the presence of Cys-SSH, the maximum absorbance changes from 790 to 614 nm accompanied by a color change from green to blue. The emission max wavelength also shifts from 797 to 749 nm. The intensity of fluorescence is directly related to the Cys-SSH concentration and thus has outstanding sensitivity and selectivity. The quantitative detection capability of Cy-Dise for Cys-SSH has been established by a ratio imaging analysis of HL-7702 cells, HepG2 cells, and primary hepatocytes. The experimental detection limit was determined to be 0.12 μM. The investigations in Sprague-Dawley (SD) rats further exhibited the potential use of the probe for recognizing Cys-SSH in the liver. The galactose part helps the probe to aim at the liver. The role of Cys-SSH in biological functions can be studied by using Cy-Dise 124.
Scheme 45. Reaction of Fluorescent Probe Cy-Dise 124 with Cys-SSH.
Following the previous work on Cy-Dise, Chen et al.137 developed another NIR fluorescent probe, CyO-Dise for qualitative and quantitative detection of GSH, during hyperthermia and hypothermia processes in vitro and in vivo. The CyO-Dise probe consist of bis(2-hydroxyethyl) diselenide, heptamethine cyanine, and d-galactose, and instead of an amide junction as in Cy-Dise, a more reactive ester bridge was used to improve the reaction kinetics and thermodynamics of diselenide toward GSH. In the presence of GSH, the selenium–sulfur reaction starts and the nucleophilic addition reactivation of the −SeH group is accelerated leading to the release of fluorophore. Based on this, the probe CyO-Dise detected GSH within 35 s. With the help of CyO-Dise, it was observed that short-term temperature stress increases cellular GSH concentration while long-term temperature stress results in an irregular decrease in cellular GSH. Thus, temperature stress can be useful to overcome the drug resistance of the HepG2/DDP cells by dipping the drug efflux and triggering mitochondrial apoptosis pathway. The probe was found useful for imaging the GSH levels of HepG2 and HepG2/DDP xenografts in vivo. The adjuvant therapy of a chemotherapy drug and temperature stress has been found to be effective for the inhibition of cancer cells. The strategy may be applied for the development of new chemical tools for accurate cancer diagnosis and evaluation of the efficacy of treatment.
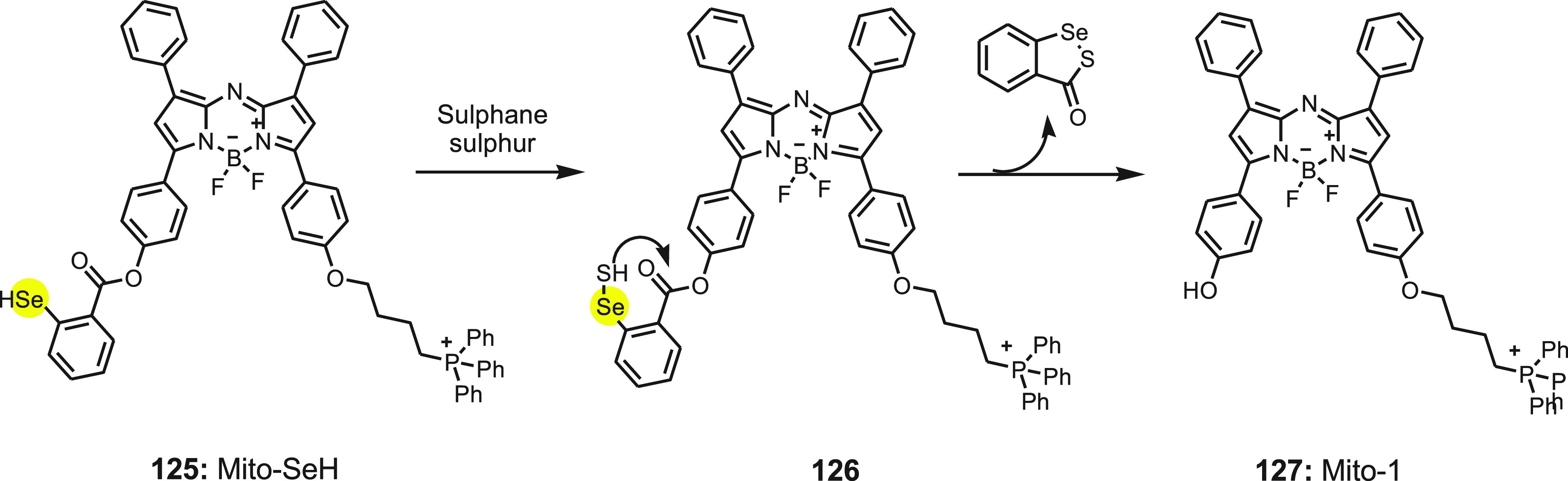
Recently, Chen et al.138 reported an NIR fluorescent probe Mito-SeH 125 for in vivo and in vitro selective imaging of sulfane sulfur in mitochondira under hypoxia stress. Probe 125 comprises of three moieties: azo-BODIPY fluorophore as module, selenol group 2-hydroselenobenzoate as a strong sulfur-acceptor, and a lipophilic alkyltriphenyl phosphonium cation as the mitochondrial targeting unit. The probe was synthesized by reacting Mito-1 with 2,2′-diselanediyldibenzoic acid in the presence of 1-(3-(dimethylamino)propyl)-3-ethylcarbodiimide hydrochloride (EDC) and 4-dimethylaminopyridine (DMAP). After stirring for 12 h, the mixture was neutralized with dilute HBr and extracted with DCM. After evaporating the organic solvent, the residue was dissolved in ethanol, and dithiothreitol was added and stirred for 5 h. The product was obtained after evaporating the solution.
Sulfane sulfur generally tautomerizes to the thiosulfoxide in biological systems and is known to be used in extraordinary regulatory functions. The reactive sulfur atom in thiosulfoxide, in the presence of probe 125, form a Se–SH adduct 126, and undergoes a fast intramolecular cyclization to liberate the fluorophore Mito-1 127 (Scheme 46). Mito-SeH 125 was found to be selective and highly sensitive with the detection limit of 3.1 nM toward sulfane sulfur. The probe shows low cytotoxicity, and the flow cytometry study confirmed the potential of the probe to detect endogenous sulfane sulfur in cells. The probe also has the capability of specifically locating and responding to the mitochondrial sulfane sulfur. The correlation between degrees of hypoxia and fluorescent intensities of Mito-SeH reveals that the sulfane sulfur stress response is highly dependent on oxygen levels. The probe was used to detect real-time hypoxia-induced sulfane sulfur changes in vivo and in vitro. The Mito-SeH can be used to further investigate the biological roles of sulfane sulfur in cells and in vivo.
Scheme 46. Reaction of NIR Fluorescent Probe Mito-SeH 125 with Sulfane Sulfur.
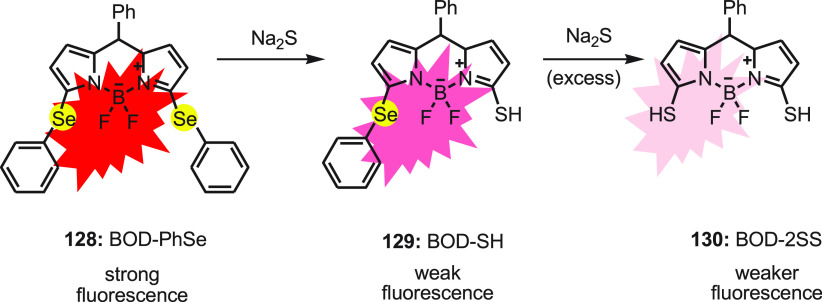
Hydrogen sulfide (H2S) has emerged as an endogenous gaseous transmitter of signaling molecules that regulate various functions like cardiovascular, neuronal, and immune system in human body. The concentration of sulfide in biological systems ranges from 10 to 100 μM in blood plasma, and a small change in the concentration leads to diseases such as Down’s syndrome, diabetes, arterial and pulmonary hypertension, and Alzheimer’s disease. Guo et al. developed a phenylselenium-substituted BODIPY fluorescent turn-off sensor for the detection of H2S.139 The probe BOD-PhSe 128 was synthesized by reacting 2,5-dichloro BODIPY to benzeneselenol in the presence of base. The reaction completed in 10 h, and the product was reported in good yield.
The detection of H2S is based on the substitution reaction of the phenylselenide group of the probe at the 3-position with H2S to form BOD-SH 129. In excess H2S, further substitution of the phenylselenide group at the 5-position of the probe occurs, forming BOD-2SH 130 with the decrease in fluorescence emission intensity (Scheme 47). The probe displayed an excellent selectivity and sensitivity with a 0.0025 μM detection limit toward H2S. The probe with good membrane permeability as well as no cytoxicity demonstrates the intracellular H2S monitoring and imaging via fluorescence microscopy in living cells.
Scheme 47. Change in Fluorescence of Probe BOD-PhSe 128 with Na2S.
In 2020, Yu and co-workers140 reported two red-emitting turn-on fluorescence probes SNARF-SSPy 131 and SNARF-SeSPy 133. Both probes contain semi-naphthorhodafluor (SNARF) attached to pyridyl disulfide (eSSPy) and pyridyl selenenyl sulfide (eSeSPy) by an ester linker. Both probes could undergo nucleophilic reaction to liberate the SNARF 132 fluorophore. However, in the presence of high concentrations of thiols, SNARF-SSPy 131 with a disulfide (S–S−) linkage undergoes the first nucleophilic substitution to form intermediate 134. SNARF-SeSPy 133 which contains a selenenyl sulfide (−Se–S−) linkage reacts with thiols to produce intermediate 135 and then further reacts with H2S to produce −Se–SH intermediate 136, and finally the intramolecular cyclization takes place to release the SNARF 132 fluorophore (Scheme 48). The major reaction products of SNARF-SeSPy 133 with H2S were found to be fluorophore SNARF 132 and cyclic acyl selenylsulfides.
Scheme 48. Sensing Mechanism of Probes SNARF-SSPy 131 and SNARF-SeSPy 133 toward H2S.
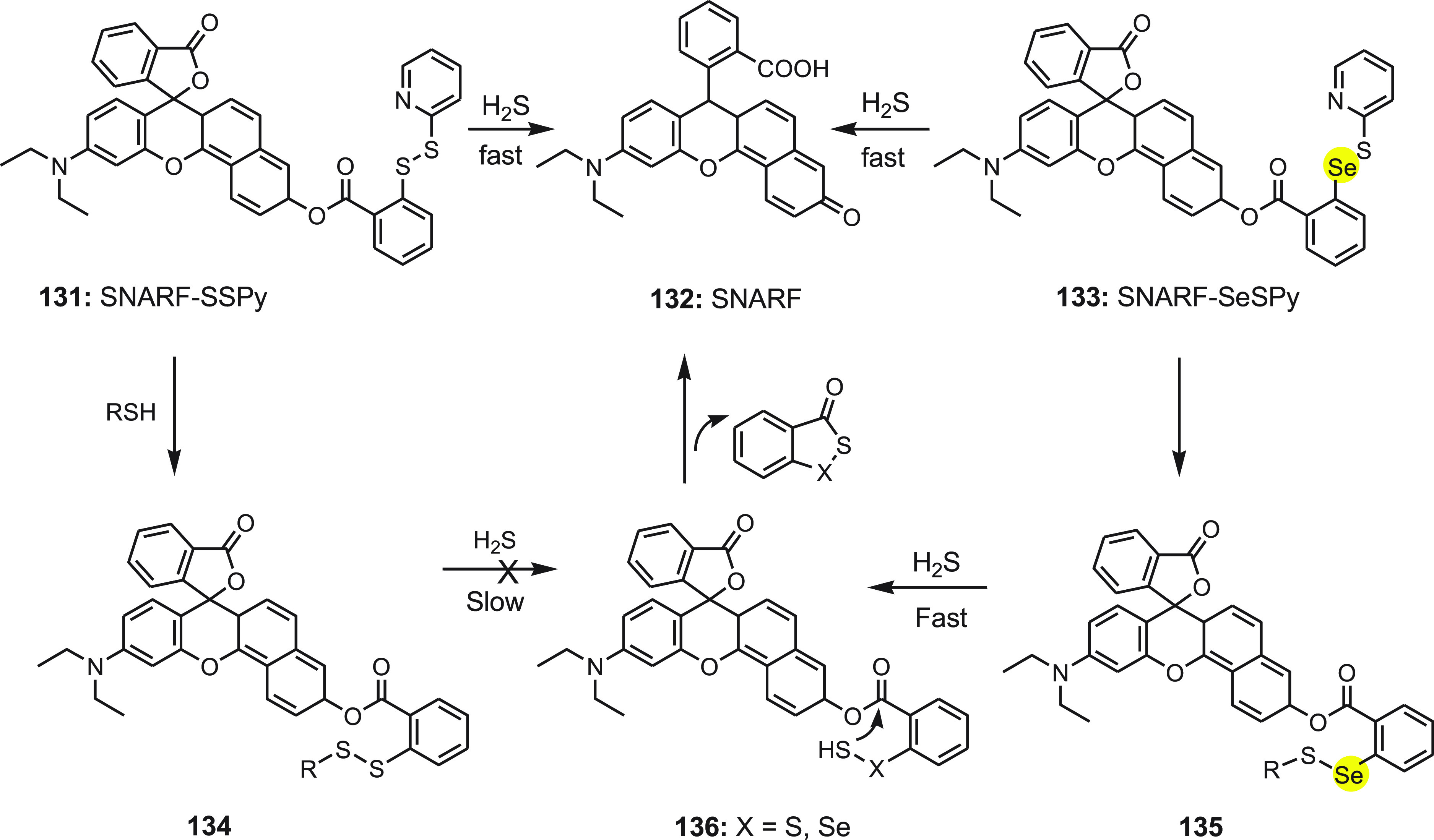
When both probes were treated with high concentrations of thiols (Cys, Hcy, GSH) and H2S, SNARF-SSPy exhibited an insignificant fluorescence change while SNARF-SeSPy showed a significant increase in fluorescence. This observation shows that SNARF-SeSPy could detect H2S in the pool of biological thiols and exhibit good anti-interference. It was found to respond H2S with high selectivity and sensitivity with the detection limit of 34 nM along with low cytotoxicity. H2S imaging in living cells and zebrafish confirmed that SNARF-SeSPy can track exogenous and endogenous H2S in vitro and in vivo; thus it could be applied for monitoring the same.
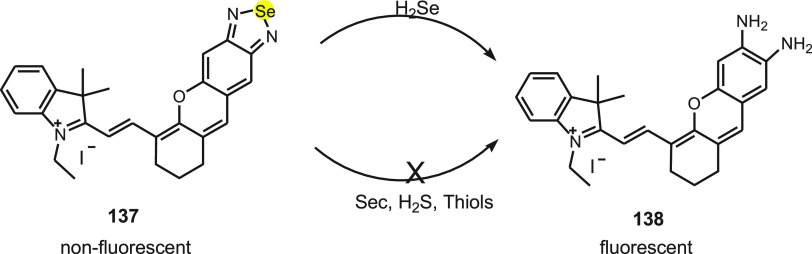
H2Se is a vital metabolite found in the reduced form of selenium and obtained from dietary Se compounds. Endogenous H2Se is involved in several biological and pathological processes. It is produced by reducing selenite with the help of GSH and other reduction systems. Tang et al.141 developed NIR-H2Se 137, a novel fluorescent probe for detecting H2Se. The probe consists of a 2,1,3-benzoselenadiazole (BS) moiety and near-infrared (NIR) merocyanine dye 138. The NIR-H2Se exhibits emission maximum at 735 nm with feeble fluorescence due to the quenching of BS owing to the heavy atom effect of Se (Scheme 49).
Scheme 49. Conversion of Nonfluorescent Probe 137 to Fluorescent Probe 138 by the Reaction with H2Se.
Under physiological conditions, NIR-H2Se selectively recognizes H2Se over selenocysteine (sec), H2S, Cys, Hcy, GSH, ROS, and metal ions. The probe also exhibits a rapid response and sensitivity toward H2Se with the calculated detection of the limit to be 7.0 nM. The study shows that under hypoxic condition the H2Se content increases and ROS level decreases, whereas in normoxic conditions, the H2O2 content increases and H2Se level rises slightly during HepG2 cell apoptosis induced by Na2SeO2. This suggests that non-oxidative stress is the pathway by which Se acts as an antitumor agent in hypoxic solid tumors. The probe can be applied to study the further role of H2Se and Se in the anticancer mechanism.
7. Conclusion and Future Vision
The organoselenium probes are gaining the attention of researchers because of their selectivity and sensitivity to recognize different analytes such as metal ions, biologically important anions, ROS, and thiols. In this review article, the synthesis and application of various selenium-based fluorescent probes have been summarized. The review article includes the detection of toxic metals such as mercury and silver ions by selenium-cored fluorescent probes. The scope of the selenium-based probes is not limited to the detection of toxic metals but successfully employed for the detection of various reactive oxygen species ROS and thiols.
The different selenium fluorophores are synthesized either by changing the fluorophore or by altering the Se-containing active reaction site. These changes improve the specificity of recognition and the “on–off” property of the probes. The different recognation modes disscused in this review include deselenation of fluorophore in the presence of analytes and the reaction of fluorophore with analytes. Organoselenium compounds exhibit high reactivity specifically toward Se–N, Se–O, and Se–P bonds during oxidation and elimination reactions and on reaction with nucleophiles and electrophiles, which make the probes a desirable sensor for neutral molecules (thiols) and ions (metal ions and anions). The selenium in organoselenium compounds is of low oxidation potential, which makes it sensitive toward various ROS. Superoxides, hydrogen peroxides, hypochlorous acid, and so forth which come under ROS exhibit the same chemical properties; however their reactivity depends on the electronic structure and chemical nature. Thus, by changing the steric and electronic environment around Se, selectivity and sensitivity of selenoprobes for ROS can be explored. Along with selectivity and sensitivity, water solubility and cell permeability are also important in the application of the probe in biological systems. This review article would provide insight about the synthesis of different molecular probes for the recognation of various analytes. The review would be quite helpful for researchers who are working in different areas of material and medicinal chemistry.
Acknowledgments
R.M. acknowledges the support from the Department of Science and Technology, New Delhi, India, for WOS-A project SR/WOS-A/CS-107/2018. F.V.S. is thankful to VIT Chennai for providing infrastructure for preparing the review article.
The authors declare no competing financial interest.
References
- Löwig C. J. Constitution of the organic compounds and comparison with the inorganics. Poggendorff’s Ann. Phys. 1836, 37, 552–561. [Google Scholar]
- Singh F. V.; Wirth T.. Selenium compounds as ligands and catalysts. In Organoselenium Chemistry: Synthesis and Reactions, Wirth T., Ed.; Wiley-VCH, 2011; pp 321–360. [Google Scholar]
- Singh F. V.; Wirth T.. Stereoselective reactions of organoselenium reagents including catalysis. In In Organic Selenium and Tellurium Compounds, Rappoport Z., Ed.; Wiley: Chichester, 2012; Vol.3, pp 303–355. [Google Scholar]
- Singh F. V.; Wirth T. Selenium-Catalyzed Regioselective Cyclization of Unsaturated Carboxylic Acids Using Hypervalent Iodine Oxidants. Org. Lett. 2011, 13, 6504–6507. 10.1021/ol202800k. [DOI] [PubMed] [Google Scholar]
- Gabriele E.; Singh F. V.; Freudendahl D. M.; Wirth T. Selenenylations of Alkenes with Styrene Nucleophiles. Tetrahedron 2012, 68, 10573–10576. 10.1016/j.tet.2012.08.034. [DOI] [Google Scholar]
- Singh F. V.; Wirth T.. Synthesis of organoselenium compounds with potential biological activities. In Organoselenium Compounds in Biology and Medicine, Jain V. K., Ed.; RSC, 2018; p 77–119. 10.1039/9781788011907-00077. [DOI] [Google Scholar]
- Singh F. V.; Wirth T.. Organoselenium chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd: Chichester, 2018; p 1–32. 10.1002/9781119951438.eibc2727. [DOI] [Google Scholar]
- Singh F. V.; Wirth T. Selenium Reagents as Catalysts. Catal. Sci. Technol. 2019, 9, 1073–1091. 10.1039/C8CY02274G. [DOI] [Google Scholar]
- Rotruck J. T.; Pope A. L.; Ganther H. E.; Swanson A. B.; Hafeman D. G.; Hoekstra W. G. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–90. 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Behne D.; Kyriakopoulos A.; Meinhold H.; Kohrle J. Identification of type I iodothyronine 5′-deiodinase as a selenoenzyme. Biochem. Biophys. Res. Commun. 1990, 173, 1143–49. 10.1016/S0006-291X(05)80905-2. [DOI] [PubMed] [Google Scholar]
- Tamura T.; Stadtman T. C. A new selenoprotein from human lung adenocarcinoma cells: purification, properties, and thioredoxin reductase activity. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 1006–1011. 10.1073/pnas.93.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhabak K. P.; Mugesh G. Functional Mimics of Glutathione Peroxidase: Bioinspired Synthetic Antioxidants. Acc. Chem. Res. 2010, 43, 1408–1419. 10.1021/ar100059g. [DOI] [PubMed] [Google Scholar]
- Bhowmick D.; Srivastava S.; D’Silva P.; Mugesh G. Highly Efficient Glutathione Peroxidase and Peroxiredoxin Mimetics Protect Mammalian Cells against Oxidative Damage. Angew. Chem., Int. Ed. 2015, 54, 8449–8453. 10.1002/anie.201502430. [DOI] [PubMed] [Google Scholar]
- Mondal S.; Mugesh G. Dehalogenation of halogenated nucleobases and nucleosides by organoselenium compounds. Chem.—Eur. J. 2019, 25, 1773–1780. 10.1002/chem.201805112. [DOI] [PubMed] [Google Scholar]
- Arai K.; Matsunaga T.; Ueno H.; Akahoshi N.; Sato Y.; Chakrabarty G.; Mugesh G.; Iwaoka M. Modeling Thioredoxin Reductase-like Activity Using Cyclic Selenenyl Sulfides: Participation of an NH···Se Hydrogen Bond through Stabilization of the Mixed Se–S Intermediate. Chem.—Eur. J. 2019, 25, 12751–12760. 10.1002/chem.201902230. [DOI] [PubMed] [Google Scholar]
- Cardoso B. R.; Roberts B. R.; Malpas C. B.; Vivash L.; Genc S.; Saling M. M.; Desmond P.; Steward C.; Hicks R. J.; Callahan J.; Brodtmann A.; Collins S.; Macfarlane S.; Corcoran N. M.; Hovens C. M.; Velakoulis D.; O’Brien T. J.; Hare D. J.; Bush A. I. Supranutritional Sodium Selenate Supplementation Delivers Selenium to the Central Nervous System: Results from a Randomized Controlled Pilot Trial in Alzheimer’s Disease. Neurotherapeutics 2019, 16, 192. 10.1007/s13311-018-0662-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo Q. Z.; Masaldan S.; Southon A.; Mawal C.; Ayton S.; Bush A. I.; Lei P.; Belaidi A. A. Characterization of Selenium Compounds for Anti-ferroptotic Activity in Neuronal Cells and After Cerebral Ischemia-Reperfusion Injury. Neurotherapeutics 2021, 18, 2682. 10.1007/s13311-021-01111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley C. T.; Dore T. M.; Hanson G. T.; Jackson W. C.; Remington S. J.; Tsien R. Y. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators:. J. Biol. Chem. 2004, 279, 22284–22293. 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- D W. Jr.; Ronai Z.; Tew K. D. Cellular thiols and reactive oxygen species in drug-induced apoptosis. J. Pharmacol. Exp. Ther. 2001, 296, 1–6. [PubMed] [Google Scholar]
- Lu T.; Pan Y.; Kao S. Y.; Li C.; Kohane I.; Chan J.; Yankner B. A. Gene regulation and DNA damage in the ageing human brain. Nature 2004, 429, 883–91. 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Chen X.; Zhou Y.; Peng X.; Yoon J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–35. 10.1039/b925092a. [DOI] [PubMed] [Google Scholar]
- Chen X.; Tian X.; Shin I.; Yoon J. Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2011, 40, 4783–4804. 10.1039/c1cs15037e. [DOI] [PubMed] [Google Scholar]
- Peng H.; Chen W.; Cheng Y.; Hakuna L.; Strongin R.; Wang B. Thiol Reactive Probes and Chemosensors. Sensor 2012, 12, 15907–15946. 10.3390/s121115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L.; Lin W.; Zheng K.; He L.; Huang W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2013, 42, 622–661. 10.1039/C2CS35313J. [DOI] [PubMed] [Google Scholar]
- Zheng H.; Zhan X.-Q.; Bian Q.-N.; Zhang X.-J. Advances in modifying fluorescein and rhodamine fluorophores as fluorescent chemosensor. Chem. Commun. 2013, 49, 429–447. 10.1039/C2CC35997A. [DOI] [PubMed] [Google Scholar]
- Manjare S. T.; Kim Y.; Churchill D. G. Selenium- and tellurium-containing fluorescent molecular probes for the detection of biologically important analytes. Acc. Chem. Res. 2014, 47, 2985–2998. 10.1021/ar500187v. [DOI] [PubMed] [Google Scholar]
- Lou Z.; Li P.; Han K. Redox-responsive fluorescent probes with different design strategies. Acc. Chem. Res. 2015, 48, 1358–1368. 10.1021/acs.accounts.5b00009. [DOI] [PubMed] [Google Scholar]
- Panda S.; Panda A.; Zade S. S. Organoselenium compounds as fluorescent probes. Coord. Chem. Rev. 2015, 300, 86–100. 10.1016/j.ccr.2015.04.006. [DOI] [Google Scholar]
- Wu D.; Chen L.; Kwon N.; Yoon J. Fluorescent probes containing selenium as a guest or host. Chem. 2016, 1, 674–698. 10.1016/j.chempr.2016.10.005. [DOI] [Google Scholar]
- Hoover G. C.; Seferos D. S. Photoactivity and optical applications of organic materials containing selenium and tellurium. Chem. Sci. 2019, 10, 9182–9188. 10.1039/C9SC04279B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Yang L.; Wang Y.; Cao T.; Sun Z.; Xu J.; Liu Y.; Chen G. Ebselen-agents for sensing, imaging and labeling: facile and full-featured application in biochemical analysis. ACS Appl. Bio Mater. 2021, 4, 2217–2230. 10.1021/acsabm.0c01561. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Yu Y.; Zhao Q.; Tang C.; Zhang H.; Qin Y.; Feng X.; Zhang J. Fluorescent probes based on nucleophilic aromatic substitution reactions for reactive sulfur and selenium species: Recent progress, applications, and design strategies. Coord. Chem. Rev. 2021, 427, 213601. 10.1016/j.ccr.2020.213601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdillah A.; Sonawane P. M.; Kim D.; Mametov D.; Shimodaira S.; Park Y.; Churchill D. G. Discussions of fluorescence in selenium Chemistry: Recently reported probes, particles, and a clearer Biological Knowledge. Molecules. 2021, 26, 692. 10.3390/molecules26030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirillo J.; De Simone B. C.; Russo N. Photophysical properties prediction of selenium- and tellurium-substituted thymidine as potential UVA chemotherapeutic agent. Theor. Chem. Acc. 2016, 135 (1), 1–5. 10.1007/s00214-015-1744-1. [DOI] [Google Scholar]
- Wolfe M. F.; Schwarzbach S.; Sulaiman R. A. Effects of mercury on wildlife: A comprehensive review. Environ. Toxicol. Chem. 1998, 17, 146–160. 10.1002/etc.5620170203. [DOI] [Google Scholar]
- Boening D. W. Ecological effects, transport, and fate of mercury: a general review. Chemosphere 2000, 40, 1335–1351. 10.1016/S0045-6535(99)00283-0. [DOI] [PubMed] [Google Scholar]
- Clarkson T. W.; Magos L.; Myers G. J. The Toxicology of Mercury–Current exposures and clinical manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- Carvalho C. M. L.; Chew E.-H.; Hashemy S. S.; Lu J.; Holmgren A. Inhibition of the Human Thioredoxin System. J. Biol. Chem. 2008, 283, 11913–11923. 10.1074/jbc.M710133200. [DOI] [PubMed] [Google Scholar]
- Nolan E. M.; Lippard S. J. Tools and Tactics for the optical detection of Mercuric Ion. Chem. Rev. 2008, 108, 3443–3480. 10.1021/cr068000q. [DOI] [PubMed] [Google Scholar]
- Gutknecht J. Inorganic mercury (Hg2+) transport through lipid bilayer membranes. J. Membr. Biol. 1981, 61, 61–66. 10.1007/BF01870753. [DOI] [Google Scholar]
- Melnick J. G.; Yurkerwich K.; Parkin G. On the chalcogenophilicity of Mercury: Evidence for a strong Hg-Se bond in [TmBut]HgSePh and its relevance to the toxicity of Mercury. J. Am. Chem. Soc. 2010, 132, 647–655. 10.1021/ja907523x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.-S.; Hong J.; Ogunseitan O. A.; Olson B. H. Interaction of mercuric ions with the bacterial growth medium and its effects on enzymic reduction of mercury. Biotechnol. Prog. 1993, 9, 526–32. 10.1021/bp00023a012. [DOI] [Google Scholar]
- Sekowski J. W.; Malkas L. H.; Wei Y.; Hickey R. J. Mercuric ion inhibits the activity and fidelity of the human cell DNA synthesom. Toxicol. Appl. Pharmacol. 1997, 145, 268–76. 10.1006/taap.1997.8185. [DOI] [PubMed] [Google Scholar]
- Baum C. R. Treatment of mercury intoxication. Curr. Opin. Pediatr 1999, 11, 265–268. 10.1097/00008480-199906000-00018. [DOI] [PubMed] [Google Scholar]
- Tang B.; Ding B.; Xu K.; Tong L. Use of Selenium to detect mercury in water and cells: An enhancement of the sensitivity and specificity of a seleno fluorescent probe. Chem.—Eur. J. 2009, 15, 3147–3151. 10.1002/chem.200802165. [DOI] [PubMed] [Google Scholar]
- Samb I.; Bell J.; Toullec P. Y.; Michelet V.; Leray I. Fluorescent Phosphane Selenide as Efficient Mercury Chemodosimeter. Org. Lett. 2011, 13, 1182–85. 10.1021/ol200066p. [DOI] [PubMed] [Google Scholar]
- Gulyas H.; Bacsik Z.; Szollosy A.; Bakos J. Facile synthesis of a monosulfonated triphenylphosphane (TPPMS) derived ligand having strong π-acceptor character. Adv. Synth. Catal 2006, 348, 1306–1310. 10.1002/adsc.200606003. [DOI] [Google Scholar]
- Shi W.; Sun S.; Li X.; Ma H. Imaging Different Interactions of Mercury and Silver with Live Cells by a Designed Fluorescence Probe Rhodamine B Selenolactone. Inorg. Chem. 2010, 49, 1206–1210. 10.1021/ic902192a. [DOI] [PubMed] [Google Scholar]
- Mehra M. C.; Gubeli A. O. The complexing characteristics of insoluble selenides. 1. Silver selenide. Can. J. Chem. 1970, 48, 3491–3497. 10.1139/v70-584. [DOI] [Google Scholar]
- Cypionka H. Characterization of sulfate transport in. Desulfovibrio desulfuricans. Arch. Microbiol 1989, 152, 237–243. 10.1007/BF00409657. [DOI] [PubMed] [Google Scholar]
- Tsipouras N.; Rix C. J.; Brady P. H. Solubility of silver sulfadiazine in physiological media and relevance to treatment of thermal burns with silver sulfadiazine cream. Clin. Chem. 1995, 41, 87–91. 10.1093/clinchem/41.1.87. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Singh J. D. An Organoselenium-based highly sensitive and selective fluorescent “Turn-On” probe for the Hg2+ Ion. Inorg. Chem. 2012, 51, 772–774. 10.1021/ic2023902. [DOI] [PubMed] [Google Scholar]
- Maheshwari M.; Khan S.; Singh J. D. Synthesis of sterically encumbered organoselenium species and their selectivity towards Hg(II) ions. Tetrahedron Lett. 2007, 48, 4737–4741. 10.1016/j.tetlet.2007.05.020. [DOI] [Google Scholar]
- Singh J. D.; Maheshwari M.; Khan S.; Butcher R. J. Sterically encumbered hexakis(alkylseleno)benzenes: conformational behavior of hexakis(iso-propylselenomethyl)benzene toward Hg2+ ions on selective recognition. Tetrahedron Lett. 2008, 49, 117–121. 10.1016/j.tetlet.2007.11.007. [DOI] [Google Scholar]
- Dharmaraja A. T. Role of reactive oxygen species (ROS) in therapeutics and drug resistance in cancer and bacteria. J. Med. Chem. 2017, 60, 3221–3240. 10.1021/acs.jmedchem.6b01243. [DOI] [PubMed] [Google Scholar]
- Khodade V. S.; Chandra S. M.; Banerjee A.; Lahiri S.; Pulipeta M.; Rangarajan R.; Chakrapani H. Bioreductively activated reactive oxygen species (ROS) generators as MRSA inhibitors. ACS Med. Chem. Lett. 2014, 5, 777–781. 10.1021/ml5001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Gao M.; Chen Q.; Yu F.; Jiang G.; Chen L. Associated detection of superoxide anion and Mercury(II) under chronic Mercury exposure in cells and mice models via a three-channel fluorescent probe. Anal. Chem. 2018, 90, 9769–9778. 10.1021/acs.analchem.8b01442. [DOI] [PubMed] [Google Scholar]
- Yu L.; Ke H. L.; Du F. S.; Li Z. C. Redox-responsive fluorescent polycarbonates based on selenide for chemotherapy of triple-negative breast cancer. Biomacromolecules. 2019, 20, 2809–2820. 10.1021/acs.biomac.9b00583. [DOI] [PubMed] [Google Scholar]
- Ungati H.; Govindaraj V.; Narayanan M.; Mugesh G. Probing the formation of a Seleninic Acid in Living Cells by a Fluorescence Switching of a Glutathione Peroxidase Mimetic. Angew. Chem. Int. Ed 2019, 58, 8156–8160. 10.1002/anie.201903958. [DOI] [PubMed] [Google Scholar]
- Koren K.; Salinas N. K. G.; Santella M.; Moshammere M.; Muller M. C.; Dmitriev R. I.; Borisov S. M.; Kuhl M.; Laursen B. W. Evaluation of Ebselen-azadioxatriangulenium as redox-sensitive fluorescent intracellular probe and as indicator within a planar redox optode. Dyes Pigm. 2020, 173, 107866. 10.1016/j.dyepig.2019.107866. [DOI] [Google Scholar]
- Liao Y. X.; Li K.; Wu M. Y.; Wu T.; Yu X. Q. A selenium-contained aggregation-induced “turnon” fluorescent probe for hydrogen peroxide. Org. Biomol. Chem. 2014, 12, 3004–3008. 10.1039/c4ob00206g. [DOI] [PubMed] [Google Scholar]
- Manjare S. T.; Kim S.; Heo W. D.; Churchill D. G. Selective and Sensitive Superoxide Detection with a New Diselenide-Based Molecular Probe in Living Breast Cancer Cells. Org. Lett. 2014, 16, 410–412. 10.1021/ol4033013. [DOI] [PubMed] [Google Scholar]
- Deshmukh P. P.; Navalkar A.; Maji S. K.; Manjare S. T. Phenylselenyl containing turn-on dibodipy probe for selective detection of superoxide in mammalian breast cancer cell line. Sens. Actuators, B 2019, 281, 8–13. 10.1016/j.snb.2018.10.072. [DOI] [Google Scholar]
- Radi R.; Peluffo G.; Alvarez M. N.; Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radical Biol. Med. 2001, 30, 463–488. 10.1016/S0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- Ferrer-Sueta G.; Radi R. Chemical biology of peroxynitrite: Kinetics, diffusion, and radicals. ACS Chem. Biol. 2009, 4, 161–177. 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- Pacher P.; Beckman J. S.; Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–428. 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C.; Ischiropoulos H.; Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug. Discovery 2007, 6, 662–680. 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- Beckman J. S.; Beckman T. W.; Chen J.; Marshall P. A.; Freeman B. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. A. Proc. Natl. Acad. Sci. U. S. A. 1990, 87, 1620–1624. 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K.; Chen H.; Tian J.; Ding B.; Xie Y.; Qiang M.; Tang B. A near-infrared reversible fluorescent probe for peroxynitrite and imaging of redox cycles in living cells. Chem. Commun. 2011, 47, 9468–9470. 10.1039/c1cc12994e. [DOI] [PubMed] [Google Scholar]
- Yu F.; Li P.; Li G.; Zhao G.; Chu T.; Han K. A Near-IR reversible fluorescent probe modulated by selenium for monitoring peroxynitrite and imaging in living cells. J. Am. Chem. Soc. 2011, 133, 11030–11033. 10.1021/ja202582x. [DOI] [PubMed] [Google Scholar]
- Wendel A.; Pilz W.; Ladenstein R.; Sawatzki G.; Weser U. Substrate-induced redox change of selenium in glutathione peroxidase studied by X-ray photoelectron spectroscopy. Biochim. Biophys. Acta 1975, 377, 211–115. 10.1016/0005-2744(75)90303-4. [DOI] [PubMed] [Google Scholar]
- Masumoto H.; Kissner R.; Koppenol W. H.; Sies H. Kinetic study of the reaction of ebselen with peroxynitrite. FEBS Lett. 1996, 398, 179–82. 10.1016/S0014-5793(96)01237-9. [DOI] [PubMed] [Google Scholar]
- Sun C.; Du W.; Wang P.; Wu Y.; Wang B.; Wang J.; Xie W. A novel mitochondria-targeted two-photon fluorescent probe for dynamic and reversible detection of the redox cycles between peroxynitrite and glutathione. Biochem. Biophys. Res. Commun. 2017, 494, 518–525. 10.1016/j.bbrc.2017.10.123. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C.; Hampton M. B.; Livesey J. H.; Kettle A. J. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J. Biol. Chem. 2006, 281, 39860–39869. 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- Yap Y. W.; Whiteman M.; Cheung N. S. Chlorinative stress: an under appreciated mediator of neurodegeneration?. Cell Signal. 2007, 19, 219–228. 10.1016/j.cellsig.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Steinbeck M. J.; Nesti L. J.; Sharkey P. F.; Parvizi J. Myeloperoxidase and chlorinated peptides in osteoarthritis: potential biomarkers of the disease. J. Orthop. Res. 2007, 25, 1128–1135. 10.1002/jor.20400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison D. I.; Davies M. J. Evidence for Rapid Inter- and Intramolecular Chlorine Transfer Reactions of Histamine and Carnosine Chloramines: Implications for the Prevention of Hypochlorous-Acid-Mediated Damage. Biochemistry 2006, 45, 8152–8162. 10.1021/bi060348s. [DOI] [PubMed] [Google Scholar]
- Pattison I.; Davies M. J. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem. Res. Toxicol. 2001, 14, 1453–1464. 10.1021/tx0155451. [DOI] [PubMed] [Google Scholar]
- Liu S.-R.; Wu S. P. Hypochlorous acid turn-on fluorescent probe based on oxidation of diphenyl selenide. Org. Lett. 2013, 15, 878–881. 10.1021/ol400011u. [DOI] [PubMed] [Google Scholar]
- Cheng G.; Fan J.; Sun W.; Cao J.; Hu C.; Peng X. A Near-infrared fluorescent probe for selective detection of HClO based on Se-sensitized aggregation of heptamethine cyanine dye. Chem. Commun. 2014, 50, 1018–1020. 10.1039/C3CC47864E. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Liu W.; Li P.; Kang J.; Wang J.; Wang H.; Tang B. Reversible two-photon fluorescent probe for imaging of hypochlorous acid in live cells and. In vivo. Chem. Commun. 2015, 51, 10150–10153. 10.1039/C5CC02537K. [DOI] [PubMed] [Google Scholar]
- Shen Y.; Zhang X.; Huang X.; Wen S.; Liu M.; Deng Y.; Zhang Y.; Zhang C.; Jin J.; Li H.; Yao S. A simple and reversible fluorescent probe based on NBD for rapid detection of hypochlorite and its application for bioimaging. RSC Adv. 2015, 5, 79519–79524. 10.1039/C5RA15373E. [DOI] [Google Scholar]
- Qu Z.; Ding J.; Zhao M.; Li P. Development of a selenide-based fluorescent probe for imaging hypochlorous acid in lysosomes. J. Photochem. Photobiol. A: Chem. 2015, 299, 1–8. 10.1016/j.jphotochem.2014.10.015. [DOI] [Google Scholar]
- Mulay S. V.; Choi M.; Jang Y. J.; Kim Y.; Jon S.; Churchill D. G. Enhanced fluorescence turn-on imaging of hypochlorous acid in living immune and cancer cells. Chem.—Eur. J. 2016, 22, 9642–9648. 10.1002/chem.201601270. [DOI] [PubMed] [Google Scholar]
- Mulay S. V.; Yudhistira T.; Choi M.; Kim Y.; Kim J.; Jang Y. J.; Jon S.; Churchill D. G. Substituent effects in BODIPY in live cell imaging. Chem. Asian J. 2016, 11, 3598–3605. 10.1002/asia.201601400. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Choi M.; Manjare S. T.; Jon S.; Churchill D. G. Diselenide-based probe for the selective imaging of hypochlorite in living cancer cells. RSC Adv. 2016, 6, 32013–32017. 10.1039/C6RA04257K. [DOI] [Google Scholar]
- Halle M. B.; Yudhistira T.; Lee K. J.; Choi J. H.; Kim Y.; Park H.-S.; Churchill D. G. Overriding phthalate decomposition when exploring mycophenolic acid intermediates as selenium-based ROS biological probes. ACS Omega 2018, 3, 13474–13483. 10.1021/acsomega.8b01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X.; Wu T.; Wang X.; Li Y.; Wang K.; Zhao Z.; Jiao X.; Tang B. A two-photon fluorescent probe for ratiometric visualization of hypochlorous acid in live cells and animals based on a selenide oxidation/elimination tandem reaction. Chem. Commun. 2018, 54, 11965–11968. 10.1039/C8CC07312K. [DOI] [PubMed] [Google Scholar]
- Lv X.; Yuan X.; Wang Y.; Guo W. A naphthalimide based fast and selective fluorescent probe for hypochlorous acid/hypochlorite and its application for bioimaging. New J. Chem. 2018, 42, 15105–10. 10.1039/C8NJ03208D. [DOI] [Google Scholar]
- Gale P. A. Anion and ion-pair receptor chemistry: highlights from 2000 and 2001. Coord. Chem. Rev. 2003, 240, 191–221. 10.1016/S0010-8545(02)00258-8. [DOI] [Google Scholar]
- Fitzmaurice R. J.; Kyne G. M.; Douheret D.; Kilburn J. D. Synthetic receptors for carboxylic acids and carboxylates. J. Chem. Soc., Perkin Trans. I 2002, 841–861. 10.1039/b009041g. [DOI] [Google Scholar]
- Martinez-Manez R.; Sancenon F. Fluorogenic and chromogenic chemosensors and reagents for anions. Chem. Rev. 2003, 103, 4419–4476. 10.1021/cr010421e. [DOI] [PubMed] [Google Scholar]
- Gale P. A.; Garcia-Garrido S. E.; Garric J. Anion receptors based on organic frameworks: highlights from 2005 and 2006. Chem. Soc. Rev. 2008, 37, 151–190. 10.1039/B715825D. [DOI] [PubMed] [Google Scholar]
- Sessler J. L.; Seidel D. Synthetic expanded porphyrin chemistry. Angew. Chem., Int. Ed. 2003, 42, 5134–5175. 10.1002/anie.200200561. [DOI] [PubMed] [Google Scholar]
- Gunnlaugsson T.; Davis A. P.; O’Brien J. E.; Glynn M. Synthesis and photophysical evaluation of charge neutral thiourea or urea based fluorescent PET sensors for bis-carboxylates and pyrophosphate. Org. Biomol. Chem. 2005, 3, 48–56. 10.1039/b409018g. [DOI] [PubMed] [Google Scholar]
- Lin Y.-S.; Tu G.-M.; Lin C.-Y.; Chang Y.-T.; Yen Y.-P. Colorimetric anion chemosensors based on anthraquinone: Naked-eye detection of isomeric dicarboxylate and tricarboxylate anions. New J. Chem. 2009, 33, 860–867. 10.1039/b811172c. [DOI] [Google Scholar]
- Goswami S.; Hazra A.; Chakrabarty R.; Fun H.-K. Recognition of carboxylate anions and carboxylic acids by selenium-based new chromogenic fluorescent sensor: A remarkable fluorescence enhancement of hindered carboxylates. Org. Lett. 2009, 11, 4350–4353. 10.1021/ol901737s. [DOI] [PubMed] [Google Scholar]
- Shimada K.; Mitamura K. J. Derivatization of thiol-containing compounds. Chromatogr., B: Biomed. Sci. Appl. 1994, 659, 227–241. 10.1016/0378-4347(93)E0444-U. [DOI] [PubMed] [Google Scholar]
- Rahman I.; MacNee W. Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches. Free Radical Biol. Med. 2000, 28, 1405–1420. 10.1016/S0891-5849(00)00215-X. [DOI] [PubMed] [Google Scholar]
- Hong R.; Han G.; Fernández J. M.; Kim B. J.; Forbes N. S.; Rotello V. M. J. Am. Chem. Soc. 2006, 128, 1078–1079. 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- Kemp M.; Go Y. M.; Jones D. P. Nonequilibrium thermodynamics of thiol/disulfide redox systems: A perspective on redox systems biology. Free Radical Biol. Med. 2008, 44, 921–937. 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T.; Holbrook N. J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Seshadri S.; Beiser A.; Selhub J.; Jacques P. F.; Rosenberg I. H.; D’Agostino R. B.; Wilson P. W. F.; Wolf P. A. Plasma Homocysteine as a Risk Factor for Dementia and Alzheimer’s Disease. N. Engl. J. Med. 2002, 346, 476–83. 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- Savage D. G.; Lindenbaum J.; Stabler S. P.; Allen R. H. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am. J. Med. 1994, 96, 239–246. 10.1016/0002-9343(94)90149-X. [DOI] [PubMed] [Google Scholar]
- Hwang C.; Sinskey A. J.; Lodish H. F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 1992, 257, 1496–1502. 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I.; Rossi R.; Colombo G.; Giustarini D.; Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem. Sci. 2009, 34, 85–96. 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Tang B.; Xing Y.; Li P.; Zhang N.; Yu F.; Yang G. A Rhodamine-based fluorescent probe containing a Se-N bond for detecting thiols and its application in living cells. J. Am. Chem. Soc. 2007, 129, 11666–11667. 10.1021/ja072572q. [DOI] [PubMed] [Google Scholar]
- Wang R.; Chen L.; Liu P.; Zhang Q.; Wang Y. Sensitive near-infrared fluorescent probes for thiols based on Se-N bond cleavage: imaging in living cells and tissues. Chem.—Eur. J. 2012, 18, 11343–11349. 10.1002/chem.201200671. [DOI] [PubMed] [Google Scholar]
- Tang B.; Yin L.; Wang X.; Chen Z.; Tong L.; Xu K. A fast-response, highly sensitive and specific organoselenium fluorescent probe for thiols and its application in bioimaging. Chem. Commun. 2009, 45, 5293–5295. 10.1039/b909542j. [DOI] [PubMed] [Google Scholar]
- Lou Z.; Li P.; Sun X.; Yang S.; Wang B.; Han K. A fluorescent probe for rapid detection of thiols and imaging of thiols reducing repair and H2O2 oxidative stress cycles in living cells. Chem. Commun. 2013, 49, 391–393. 10.1039/C2CC36839K. [DOI] [PubMed] [Google Scholar]
- Evans R. M.; Fraser J. B.; Owen L. N. 61. Dithiols. Part III. Derivatives of polyhydric alcohols. J. Chem. Soc. 1949, 248. 10.1039/jr9490000248. [DOI] [Google Scholar]
- Cleland W. W. Dithiothreitol, a New Protective Reagent for SH Group. Biochemistry 1964, 3, 480–482. 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Alliegro M. C. Effects of dithiothreitol on protein activity unrelated to thiol–disulfide exchange: for consideration in the analysis of protein function with cleland’s reagent. Anal. Biochem. 2000, 282, 102–106. 10.1006/abio.2000.4557. [DOI] [PubMed] [Google Scholar]
- Ates B.; Ercal B. C.; Manda K.; Abraham L.; Ercal N. Determination of glutathione disulfide levels in biological samples using thiol–disulfide exchanging agent, dithiothreitol. Biomed. Chromatogr. 2009, 23, 119–123. 10.1002/bmc.1083. [DOI] [PubMed] [Google Scholar]
- Spear N.; Aust S. D. The Effects of Different Buffers on the Oxidation of DNA by Thiols and Ferric Iron. J. Biochem. Mol. Toxicol. 1998, 12, 125–132. . [DOI] [PubMed] [Google Scholar]
- Taatjes D. J.; Gaudiano G.; Koch T. H. Production of formaldehyde and dna-adriamycin or dna-daunomycin adducts, initiated through redox chemistry of dithiothreitol/iron, xanthine oxidase/nadh/iron, or glutathione/iron. Chem. Res. Toxicol. 1997, 10, 953–961. 10.1021/tx970064w. [DOI] [PubMed] [Google Scholar]
- Gurr J. R.; Bau D. T.; Liu F.; Lynn S.; Jan K. Y. Dithiothreitol enhances arsenic trioxide-induced apoptosis in NB4 cells. Mol. Pharmacol. 1999, 56, 102–109. 10.1124/mol.56.1.102. [DOI] [PubMed] [Google Scholar]
- Hultberg B.; Andersson A.; Isaksson A. Interaction of metals and thiols in cell damage and glutathione distribution: potentiation of mercury toxicity by dithiothreitol. Toxicology 2001, 156, 93–100. 10.1016/S0300-483X(00)00331-0. [DOI] [PubMed] [Google Scholar]
- Zhu B.; Zhang X.; Jia H.; Li Y.; Liu H.; Tan W. A highly selective ratiometric fluorescent probe for 1,4-dithiothreitol (DTT) detection. Org. Biomol. Chem. 2010, 8, 1650–1654. 10.1039/b923754b. [DOI] [PubMed] [Google Scholar]
- Xu K.; Qiang M.; Gao W.; Su R.; Li N.; Gao Y.; Xie Y.; Kong F.; Tang B. A near-infrared reversible fluorescent probe for real-time imaging of redox status changes. In vivo. Chem. Sci. 2013, 4, 1079–1086. 10.1039/c2sc22076h. [DOI] [Google Scholar]
- Rotruck J. T.; Pope A. L.; Ganther H. E.; Swanson A. B.; Hafeman D. G.; Hoekstra W. G. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 1973, 179, 588–590. 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Epp O.; Ladenstein R.; Wendel A. The refined structure of the selenoenzyme glutathione peroxidase at 0.2-nm resolution. Eur. J. Biochem. 1983, 133, 51–69. 10.1111/j.1432-1033.1983.tb07429.x. [DOI] [PubMed] [Google Scholar]
- Mugesh G.; du Mont W.-W. Structure-activity correlation between natural glutathione peroxidase (GPx) and mimics: A biomimetic concept for the design and synthesis of more efficient GPx mimics. Chem.—Eur. J. 2001, 7, 1365–1370. . [DOI] [PubMed] [Google Scholar]
- Jacob C.; Giles G. I.; Giles N. M.; Sies H. Sulfur and Selenium: The Role of Oxidation State in Protein Structure and Function. Angew. Chem., Int. Ed. 2003, 42, 4742–4748. 10.1002/anie.200300573. [DOI] [PubMed] [Google Scholar]
- Sarma B. K.; Mugesh G. Glutathione peroxidase (GPx)-like antioxidant activity of the organoselenium drug ebselen: Unexpected complications with thiol exchange reactions. J. Am. Chem. Soc. 2005, 127, 11477–11485. 10.1021/ja052794t. [DOI] [PubMed] [Google Scholar]
- Bhabak K. P.; Mugesh G. Synthesis, characterization, and antioxidant activity of some ebselen analogues. Chem.—Eur. J. 2007, 13, 4594–4601. 10.1002/chem.200601584. [DOI] [PubMed] [Google Scholar]
- Bhabak K. P.; Mugesh G. A Simple and efficient strategy to enhance the antioxidant activities of amino-substituted glutathione peroxidase mimics. Chem.—Eur. J. 2008, 14, 8640–8651. 10.1002/chem.200800963. [DOI] [PubMed] [Google Scholar]
- Sarma B. K.; Mugesh G. Antioxidant activity of the anti-inflammatory compound ebselen: a reversible cyclization pathway via selenenic and seleninic acid intermediates. Chem.—Eur. J. 2008, 14, 10603–10614. 10.1002/chem.200801258. [DOI] [PubMed] [Google Scholar]
- Bhabak K. P.; Mugesh G. Amide-based glutathione peroxidase mimics: effect of secondary and tertiary amide substituents on antioxidant activity. Chem. Asian. J. 2009, 4, 974–983. 10.1002/asia.200800483. [DOI] [PubMed] [Google Scholar]
- Bhabak K. P.; Mugesh G. Synthesis and structure-activity correlation studies of secondary- and tertiary-amine-based glutathione peroxidase mimics. Chem.—Eur. J. 2009, 15, 9846–9854. 10.1002/chem.200900818. [DOI] [PubMed] [Google Scholar]
- Sarma B. K.; Manna D.; Minoura M.; Mugesh G. Synthesis, structure, spirocyclization mechanism, and glutathione peroxidase-like antioxidant activity of stable spirodiazaselenurane and spirodiazatellurane. J. Am. Chem. Soc. 2010, 132, 5364–5374. 10.1021/ja908080u. [DOI] [PubMed] [Google Scholar]
- Bhowmick D.; Mugesh G. Tertiary amine-based glutathione peroxidase mimics: some insights into the role of steric and electronic effects on antioxidant activity. Tetrahedron 2012, 68, 10550–10560. 10.1016/j.tet.2012.09.020. [DOI] [Google Scholar]
- Lamani D. S.; Bhowmick D.; Mugesh G. Spirodiazaselenuranes: synthesis, structure and antioxidant activity. Org. Biomol. Chem. 2012, 10, 7933–7943. 10.1039/c2ob26156a. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Mulay S. V.; Choi M.; Yu S. B.; Jon S.; Churchill D. G. Exceptional time response, stability and selectivity in doubly-activated phenyl selenium-based glutathione-selective platform. Chem. Sci. 2015, 6, 5435–5439. 10.1039/C5SC02090E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulay S. V.; Kim Y.; Choi M.; Lee D. Y.; Choi J.; Lee Y.; Jon S.; Churchill D. G. Enhanced Doubly Activated Dual Emission Fluorescent Probes for Selective Imaging of Glutathione or Cysteine in Living Systems. Anal. Chem. 2018, 90, 2648–2654. 10.1021/acs.analchem.7b04375. [DOI] [PubMed] [Google Scholar]
- Han X.; Yu F.; Song X.; Chen L. Quantification of cysteine hydropersulfide with a ratiometric near-infrared fluorescent probe based on selenium–sulfur exchange reaction. Chem. Sci. 2016, 7, 5098–5107. 10.1039/C6SC00838K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X.; Song X.; Yu F.; Chen L. A ratiometric fluorescent probe for imaging and quantifying anti-apoptotic effects of GSH under temperature stress. Chem. Sci. 2017, 8, 6991–7002. 10.1039/C7SC02888A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M.; Wang R.; Yu F.; Chen L. Evaluation of sulfane sulfur bioeffects via a mitochondria-targeting selenium-containing near-infrared fluorescent probe. Biomaterial 2018, 160, 1–14. 10.1016/j.biomaterials.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Gong D.; Zhu X.; Tian Y.; Han S.-C.; Deng M.; Iqbal A.; Liu W.; Qin W.; Guo H. A phenylselenium-substituted BODIPY fluorescent turn-off probe for fluorescence imaging of hydrogen sulfide in living cells. Anal. Chem. 2017, 89, 1801–1807. 10.1021/acs.analchem.6b04114. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Qu W.; Liu H.; Ma Y.; Wang L.; Sun Q.; Yu F. Visualizing hydrogen sulfide in living cells and zebrafish using a redemitting fluorescent probe via selenium-sulfur exchange reaction. Anal. Chim. Acta 2020, 1109, 37–43. 10.1016/j.aca.2020.02.061. [DOI] [PubMed] [Google Scholar]
- Kong F.; Ge L.; Pan X.; Xu K.; Liu X.; Tang B. A highly selective near-infrared fluorescent probe for imaging H2Se in living cells and in vivo. Chem. Sci. 2016, 7, 1051–1056. 10.1039/C5SC03471J. [DOI] [PMC free article] [PubMed] [Google Scholar]