Abstract
Dormant Bacillus subtilis spores can be induced to germinate by nutrients, as well as by nonmetabolizable chemicals, such as a 1:1 chelate of Ca2+ and dipicolinic acid (DPA). Nutrients bind receptors in the spore, and this binding triggers events in the spore core, including DPA excretion and rehydration, and also activates hydrolysis of the surrounding cortex through mechanisms that are largely unknown. As Ca2+-DPA does not require receptors to induce spore germination, we asked if this process utilizes other proteins, such as the putative cortex-lytic enzymes SleB and CwlJ, that are involved in nutrient-induced germination. We found that Ca2+-DPA triggers germination by first activating CwlJ-dependent cortex hydrolysis; this mechanism is different from nutrient-induced germination where cortex hydrolysis is not required for the early germination events in the spore core. Nevertheless, since nutrients can induce release of the spore's DPA before cortex hydrolysis, we examined if the DPA excreted from the core acts as a signal to activate CwlJ in the cortex. Indeed, endogenous DPA is required for nutrient-induced CwlJ activation and this requirement was partially remedied by exogenous Ca2+-DPA. Our findings thus define a mechanism for Ca2+-DPA-induced germination and also provide the first definitive evidence for a signaling pathway that activates cortex hydrolysis in response to nutrients.
Germination is the process by which dormant bacterial spores resume metabolism and growth, and it is generally triggered by the presence of nutrients, including amino acids, sugars, and nucleosides (8, 25). The germination process triggered by nutrients consists of a number of events whose precise temporal order has not been unequivocally determined. Some of those events occur in the spore core and include rehydration of the spore's somewhat dehydrated cytoplasm and excretion of its large (∼10% of the spore's dry weight) depot of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) and divalent cations, predominantly Ca2+, which are likely present as a 1:1 chelate (25). A third major event early in spore germination is the breakdown of the spore's cortex, which is a special peptidoglycan (PG) layer that surrounds the spore core and inner membrane and is responsible in some fashion for the core's relative dehydration and enzymatic dormancy (6, 25). It is currently thought that interaction of nutrients with their receptors in the spore's inner membrane (10, 24) induces some permeability change in that membrane leading to the release of DPA and cations from the spore core along with attendant water uptake (8, 29). It is also known that cortex hydrolysis is not needed for those events in the spore core but is absolutely necessary for subsequent steps in germination, including initiation of spore metabolism and growth of the germinated spore, that culminate in the formation of a viable cell (12, 26, 29). On that basis, nutrient-induced spore germination has been divided into stage I (29), which consists of events that occur in the absence of cortex hydrolysis, and stage II, which consists of all subsequent events, but how those various events are orchestrated by the nutrient receptors remains to be elucidated.
While spore germination is most commonly triggered by specific nutrients, it can also be induced by some chemicals that are not nutrients and by some physical treatments as well (8). The best-known example of a nonnutrient chemical germinant is a 1:1 chelate of Ca2+ and DPA which triggers the germination of spores in many species of endospore-forming bacteria. Although nutrient and nonnutrient germinant molecules are comparable in size, studies with Bacillus subtilis suggest that the two types of germinants act quite differently. In B. subtilis, a large body of work has shown that nutrient germinants bind to specific spore receptors that are encoded by the three expressed members of the gerA family of operons (17, 22); the germinant-receptor interaction then triggers the various germination events noted above by mechanisms that are largely unknown. The receptors are absolutely necessary for spore germination in response to nutrients because spores that lack all gerA operon homologs fail to germinate with nutrients (23). However, those mutant spores germinate readily with Ca2+-DPA, indicating that the receptors are dispensable for Ca2+-DPA-induced spore germination (23). Thus, the mechanisms by which nutrients and Ca2+-DPA induce spore germination appear to be different, at least with respect to the receptor-mediated step.
Another step in germination that has been studied in some detail is cortex hydrolysis. A number of different spore enzymes have been implicated in hydrolysis of the cortex during spore germination; these enzymes are called cortex-lytic enzymes (CLEs) and have been identified in various endospore-forming species through both biochemical and genomics methods (7, 12, 15, 19, 25). The CLEs specifically act on cortex PG because it contains muramic acid lactam (6), a component that is absent in growing-cell PG. In B. subtilis, two CLEs, named SleB and CwlJ, have been implicated in cortex hydrolysis during nutrient-induced spore germination (2, 12, 19). Whereas mutant spores lacking either sleB or cwlJ germinate relatively normally (12, 29), sleB cwlJ mutant spores are unable to degrade their cortex and consequently remain blocked in stage I of germination (12, 19, 29). The behavior of the sleB cwlJ spores suggests that SleB and CwlJ have redundant functions in cortex hydrolysis and also underscores the importance of cortex hydrolysis in germination (29). Consequently, it is expected that Ca2+-DPA also triggers cortex hydrolysis, but whether the same two CLEs are utilized during that process is not known.
In this study, we wanted to further compare the germination events triggered by the nutrient and nonnutrient germinants and thus investigated the role of SleB and CwlJ in Ca2+-DPA-induced spore germination by examining the response of spores lacking sleB and/or cwlJ to Ca2+-DPA. We also examined spores from a cotE mutant strain, which have a severe defect in the spore coat structure (5, 6), because previous work had shown that chemically decoated spores fail to germinate in response to Ca2+-DPA (23). Our studies showed that Ca2+-DPA triggers spore germination almost exclusively through activation of CwlJ-dependent cortex hydrolysis. We also found that endogenous spore DPA is critical for activation of CwlJ-dependent cortex hydrolysis during nutrient-induced germination. Putting those two findings together, we propose that endogenous spore DPA released in response to nutrients acts as a signal that triggers CwlJ-dependent cortex hydrolysis during nutrient-induced germination. Thus, in addition to defining a CwlJ axis for Ca2+-DPA-induced spore germination, our studies provide a putative mechanism by which germinant receptors in the inner membrane (10, 24) transduce the nutrient signal to CwlJ in the cortex during nutrient-induced germination.
MATERIALS AND METHODS
Strains and plasmids.
The B. subtilis strains used in this study are all derived from strain 168 and are listed in Table 1. Strains were constructed by transformation (1) with either plasmid or genomic DNA, and the genotype of each newly constructed strain was confirmed by Southern blot analysis (28). Where necessary, growth media were supplemented with (per liter) 100 mg of spectinomycin, 1 mg of erythromycin, 25 mg of lincomycin, 5 mg of chloramphenicol, 7 mg of kanamycin, or 20 mg of tetracycline (4). Standard molecular biology techniques were used in the construction of plasmids (28).
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| PS832 | Wild type | Laboratory stock |
| FB72 | ger-3 (ΔgerA::spc ΔgerB::cat ΔgerK::ermC) | 23 |
| FB106 | ΔspoVF::tet | pFE229→PS832; 21 |
| FB114 | FB72 ΔsleB::tet | pFE251→FB72 |
| FB115 | FB72 ΔcwlJ::tet | pFE243→FB72 |
| PS3329 | FB72 ΔcotE::tet | pPS3327→FB72 |
| FB110 | ΔsleB::tet | pFE251→PS832 |
| FB112 | ΔsleB::spc | pFE252→PS832 |
| FB111 | ΔcwlJ::tet | pFE243→PS832 |
| FB113 | ΔcwlJ::tet ΔsleB::spc | FB112→FB111 |
| PS3328 | ΔcotE::tet | pPS3327→PS832 |
| PS3330 | ΔcotE::tet ΔsleB::spc | FB112→PS3328 |
| FB122 | ΔspoVF::tet ΔsleB::spc | FB106→FB112 |
Plasmid pFE243, which was used to create the ΔcwlJ::tet mutant, was derived from plasmid pDG1515 (9). DNA upstream of the cwlJ gene (nucleotides [nt]−157 to +106 relative to the +1 cwlJ translation start site) was PCR amplified from wild-type genomic DNA by using appropriate primers (all of the primer sequences used in this work are available upon request). HindIII and EcoRI sites were introduced in the 5′ and 3′ primers, respectively, to facilitate subsequent cloning. The PCR product was cloned into plasmid pCR2.1 (Invitrogen) and sequenced, and one plasmid containing the correct insert was designated pFE240. DNA downstream of the cwlJ gene (nt +347 to +613) was similarly amplified and cloned into pCR2.1 to generate plasmid pFE241, except that BamHI and EagI sites were introduced into the 5′ and 3′ primers, respectively. The insert from plasmid pFE240 was excised by digestion with HindIII and EcoRI and cloned between the same sites in plasmid pDG1515 to create plasmid pFE242, after which the insert from plasmid pFE241 was excised as a BamHI-EagI fragment and cloned between the same sites in plasmid pFE242 to generate the ΔcwlJ::tet plasmid pFE243.
The ΔsleB::tet plasmid pFE251 was constructed by using a strategy similar to the one described above. The upstream (nt −139 to +100 relative to the +1 sleB translation start site) and downstream (nt +826 to +1013) regions flanking the sleB gene were PCR amplified from wild-type genomic DNA and cloned into plasmid pCR2.1 to generate plasmids pFE249 and pFE248, respectively. Specific restriction enzyme sites were introduced into the primers to facilitate subsequent cloning. The downstream fragment in plasmid pFE248 was excised by digestion with BamHI and EagI and cloned between the same sites in plasmid pDG1515 to generate plasmid pFE250. Following this, the upstream region in plasmid pFE249 was inserted as a HindIII-EcoRI fragment into plasmid pFE250 to create the ΔsleB::tet plasmid pFE251. The ΔsleB::spc plasmid pFE252 was derived from the ΔsleB::tet plasmid pFE251 by replacing the BamHI-EcoRI fragment, which contains the tet marker, with the BamHI-EcoRI fragment from plasmid pFE52 (22), which carries the spc marker.
The ΔcotE::tet plasmid was generated from plasmid pDG1515 (9). Upstream (from nt −261 to + 50 relative to the +1 cotE translation start site) and downstream (from nt +496 to +792) regions of the cotE gene were PCR amplified from wild-type genomic DNA. The upstream DNA fragment was cloned into the pCR2.1 vector, from where it was excised by digestion with BamHI and PstI (sites introduced in primers) and cloned between the same sites in plasmid pDG1515 to create plasmid pN′ cotE. The downstream DNA was also initially cloned into pCR2.1, from where it was excised by digestion with the EcoRI and HindIII (sites introduced in primers) enzymes and inserted between the same sites in plasmid pN′ cotE to produce the ΔcotE::tet plasmid pPS3327.
Growth and sporulation conditions.
B. subtilis strains were routinely grown at 37°C in rich (LB or 2×YT [28]) medium and sporulated at 37°C by nutrient exhaustion in 2×SG medium without antibiotics (20). Strains were routinely sporulated on 2×SG medium agar plates as previously described, and the spores were harvested by scraping them off the agar surface (21). Spores were cleaned by sonication and repeated washing with cold water and stored in distilled water at 4°C or, in the case of ΔspoVF spores, on ice (20, 21). All of the spores used in this work were free (>98%) of cells or germinated spores.
Chemical decoating and Ca2+-DPA treatment of spores.
Spores equivalent to an optical density at 600 nm (OD600) of 10 to 20 U were decoated by incubation for 30 min at 70°C in 0.1 M NaOH–0.1 M NaCl–1% sodium dodecyl sulfate–0.1 M dithiothreitol as previously described (30). The decoated spores were washed at least 10 times with distilled water to remove all traces of the decoating solution. This procedure removes large amounts of spore coat proteins and also largely, if not completely, removes the spore's outer membrane (3).
Ca2+-DPA treatment to induce spore germination consisted of incubating heat-activated (70°C for 30 min) spores at an OD600 of 1 in 60 mM Ca2+-DPA (pH 8.3) for 45 to 60 min at room temperature. Whereas the treated spores were used directly to determine titers, they were washed in water (10 × 1 ml) and incubated in water for 1.5 h at 37°C to allow the germination reactions to reach completion (23) before phase microscopy and the measurement of other spore germination parameters such as DPA content and buoyant density.
DPA assays, buoyant density gradients, and spore titers.
Spore DPA content was assayed as previously described after extraction of DPA from spores for 15 min in boiling water (20, 27). The buoyant density of spores was determined by equilibrium density centrifugation on a metrizoic acid gradient as described previously (14). As this assay was used only to distinguish dormant and germinated spores in this study, the spores were not decoated and consequently the spore core wet density could not be calculated. Nevertheless, the assay easily distinguished dormant spores from those that had proceeded at least through stage I of germination (26, 29).
To obtain spore titers, spore suspensions were heat activated at 70°C for 30 min and treated with Ca2+-DPA as described above, if necessary, and serial dilutions of the suspensions were spotted on Luria broth or 2×YT medium agar plates (23). Colony counts after 20 to 24 h of incubation at 30°C were used to calculate the titers as CFU per milliliter. Previous work (23) has shown that this assay gives good quantitation of spores that are capable of germinating fully within 4 to 8 h. However, spores that are blocked in stage I of germination or that germinate very slowly are not detected in this assay.
RESULTS
Effects of sleB and cwlJ mutations on Ca2+-DPA-induced spore germination.
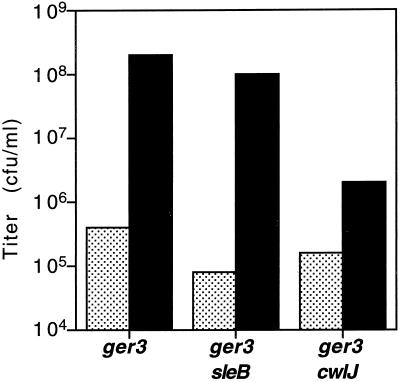
We had reported previously that Ca2+-DPA induction of spore germination does not require the gerA operon family of receptors that are necessary for nutrient induction of spore germination (23). To determine if Ca2+-DPA-induced spore germination required CwlJ and SleB, whose redundant cortex-hydrolytic function is essential for nutrient-induced spore germination (12, 19, 25), we examined colony formation by ger-3 cwlJ and ger-3 sleB spores after Ca2+-DPA treatment. Note that the designation ger-3 refers to strains lacking the gerA, -B, and -K operons, which encode the spore's three functional nutrient germinant receptors (23). Spores from ger-3, ger-3 cwlJ, and ger-3 sleB strains were incubated in water or 60 mM Ca2+-DPA and then spotted on 2×YT agar plates for determination of spore titers. The Ca2+-DPA-treated spore titers are a measure of spore germination in Ca2+-DPA because the ger-3 mutant spores do not respond to nutrients and thus give very low spore titers (≤0.1% of the total spores give rise to colonies in 24 h) when plated directly on rich medium (23). As expected from previous observations (23), the very low titer of untreated ger-3 spores was remedied after treatment with Ca2+-DPA (Fig. 1). The ger-3 sleB spores similarly responded to Ca2+-DPA (Fig. 1), showing that SleB was not essential for spore germination in response to Ca2+-DPA. In contrast, the titers of Ca2+-DPA-treated ger-3 cwlJ spores were only 1 to 3% of those of similarly treated ger-3 spores (Fig. 1), suggesting that deletion of cwlJ severely affected the ability of ger-3 spores to germinate in response to Ca2+-DPA. Note that mutations in cwlJ or sleB had very little effect on the low level of spontaneous spore germination events (titers obtained without Ca2+-DPA treatment) observed with ger-3 spores (Fig. 1) and that loss of either cwlJ or sleB has only a slight effect on otherwise wild-type spore titers (12, 19; see below).
FIG. 1.
Germination of spores in Ca2+-DPA. Spores from strains FB72 (ger-3), FB114 (ger-3 sleB), and FB115 (ger-3 cwlJ) were incubated in water (stippled) or 60 mM Ca2+-DPA (solid) at an OD600 of 1 for 45 min at room temperature, and serial dilutions were then spotted on 2×YT agar plates for titer determination. The values shown are averages of two experiments. The individual experimental values lay within 40% of the averages. The titer of ger-3 spores treated with Ca2+-DPA is essentially identical to that of wild-type spores plated directly.
As wild-type spores germinate and form colonies when plated on rich medium, the above-described method could not be used to determine if wild-type spores require CwlJ to germinate in response to Ca2+-DPA as described in Materials and Methods. Therefore, we used phase-contrast microscopy to distinguish between germinated spores, which appear phase dark, and dormant spores, which appear phase bright. Consistent with the above-described observations, phase-contrast microscopy showed that while most (>70%) of the Ca2+-DPA-treated ger-3 spores had become phase dark, the ger-3 cwlJ spores remained (>95%) phase bright after Ca2+-DPA treatment. Similar examination of wild-type and cwlJ single-mutant spores after Ca2+-DPA treatment showed that ≥70% of the wild-type spores had become phase dark, while <5% of the cwlJ spores were dark. Together, these observations show that CwlJ, but not SleB, allows spores to initiate germination in response to Ca2+-DPA.
Roles of CwlJ in nutrient- and Ca2+-DPA-induced spore germination.
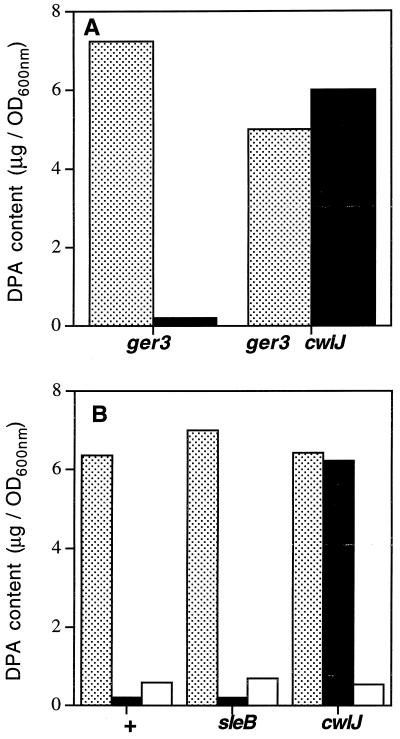
As CwlJ was necessary for Ca2+-DPA-induced spore germination and this protein was already implicated in nutrient germination, we wanted to compare the roles of CwlJ in the two germination pathways. CwlJ and its functionally redundant partner SleB are required for cortex hydrolysis during nutrient-induced spore germination. However, sleB cwlJ mutant spores release endogenous DPA and undergo significant core rehydration in response to nutrients, suggesting that CwlJ is not required for those processes during nutrient-induced spore germination (12, 26, 29). To determine if CwlJ plays a similar role during Ca2+-DPA-induced spore germination, we examined the effect of Ca2+-DPA treatment on the DPA contents of ger-3 and ger-3 cwlJ spores. Whereas ger-3 spores lost >90% of their DPA after treatment with exogenous Ca2+-DPA, there was no detectable difference between the DPA contents of water- and Ca2+-DPA-treated ger-3 cwlJ spores (Fig. 2A). A similar analysis of the DPA contents of spores from a wild-type strain and its sleB and cwlJ derivatives showed that wild-type and sleB spores lost >90% of their endogenous DPA after Ca2+-DPA treatment but, again, there was no detectable loss of DPA from Ca2+-DPA-treated cwlJ spores (Fig. 2B). This defect of cwlJ spores was specific to germination in Ca2+-DPA because those spores rapidly released >85% of their DPA in response to nutrients (2×YT medium) (Fig. 2B). Thus, in contrast to nutrient-induced spore germination, where CwlJ or SleB are required only for cortex hydrolysis, CwlJ is also required for the release of spore DPA during Ca2+-DPA-induced spore germination.
FIG. 2.
DPA contents of spores of several strains after various treatments. (A) Spores from strains FB72 (ger-3) and FB115 (ger-3 cwlJ) were incubated in water (stippled) or 60 mM Ca2+-DPA (solid), washed, incubated further in water, and assayed for DPA content as described in Materials and Methods. (B) Spores from strains PS832 (+), FB110 (sleB), and FB111 (cwlJ) were incubated in water (stippled), 60 mM Ca2+-DPA (solid), or 2×YT medium (open); washed; incubated further in water; and assayed for DPA content as described in Materials and Methods. All of the values shown are from a typical experiment. Repetitions of these experiments gave values within 20% of the values shown.
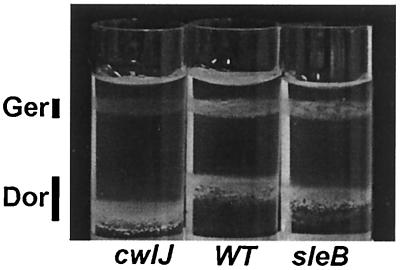
To further clarify the role of CwlJ in Ca2+-DPA-induced spore germination, we followed the change in spore buoyant density after Ca2+-DPA treatment because sleB cwlJ spores also initiate that event during nutrient-induced spore germination (13, 25, 26, 29). Aliquots of wild-type, cwlJ, and sleB spores were treated with water or 60 mM Ca2+-DPA; the two aliquots from each strain were then mixed, loaded on a metrizoic acid step gradient, and subjected to equilibrium density gradient centrifugation. The wild-type and sleB spore samples showed both lighter and denser bands of approximately equal intensities corresponding to the Ca2+-DPA-treated germinated spores and the water-treated dormant spores in the mixture (Fig. 3). In contrast, the spores in the cwlJ mixture were concentrated almost exclusively (>90%) in the denser band, showing that the Ca2+-DPA-treated cwlJ spores had not undergone the change in buoyant density which is characteristic of spores that have completed stage I of germination. Thus again, in contrast to what is seen during nutrient-induced spore germination, spores require CwlJ for the change in spore buoyant density, as well as for the release of endogenous DPA during Ca2+-DPA-induced spore germination.
FIG. 3.
Effect of Ca2+-DPA treatment on spore buoyant density. Spores from strains FB111 (cwlJ), PS832 (WT), and FB110 (sleB) were incubated in water or 60 mM Ca2+-DPA; the water- and Ca2+-DPA-treated spores from each strain were mixed, and the mixture was subjected to equilibrium density centrifugation on a metrizoic acid gradient. The expected positions of dormant (Dor) and germinated (Ger) spores are indicated by vertical bars. The drift in the position of the dormant spores is the result of minor variations in the gradient between experiments.
Effect of cotE deletion on spore response to Ca2+-DPA.
The effect of deletion of cwlJ on Ca2+-DPA-induced spore germination was similar to that reported previously for a decoating treatment that removes much of the spore coat (23). As CwlJ is synthesized in the mother cell at the same time as many of the spore coat proteins, it was plausible that the decoating treatment also removed or inactivated CwlJ. Before we investigated that possibility, we wanted to better define the role of the spore coat in the Ca2+-DPA-induced spore germination pathway and therefore examined germination in spores of a coat-defective cotE mutant strain. The CotE protein is essential for the assembly of the spore's outer coat, and its absence also results in defects in the inner coat structure (5, 6). Spores from ger-3 and ger-3 cotE strains were treated with water or Ca2+-DPA, and their titers were determined. As expected (23), Ca2+-DPA-treated ger-3 spores showed 100 to 150-fold higher titers than untreated ger-3 spores (Table 2). Ca2+-DPA treatment also enhanced the ger-3 cotE spore titers, but the effect (about 40-fold) was not as marked as that seen with ger-3 spores (Table 2, expt 1); nevertheless, the lower titer of ger-3 cotE spores with Ca2+-DPA treatment was seen in five independent experiments with two different spore preparations. Considering that cotE mutant spores retain much of the inner coat layer, albeit in a defective state, and that these spores have not lost as much coat protein as is removed by chemical decoating, the effect of the cotE mutation on ger-3 spore titers was consistent with the idea that an intact coat is necessary for Ca2+-DPA-induced germination. This conclusion was also supported by the observation that the effects of decoating and a cotE mutation were not additive, as measured by spore titers after Ca2+-DPA treatment in the ger-3 background (Table 2, expt 1).
TABLE 2.
Effects of mutations on Ca2+-DPA-induced spore germination
| Strain (genotype); treatment | Spore titer (CFU/ml)a
|
|
|---|---|---|
| Water | Ca2+-DPA | |
| Expt 1 | ||
| FB72 (ger-3); none | 1.0 × 105 | 3.6 × 107 |
| PS3329 (ger-3 cotE); none | 1.0 × 105 | 4.0 × 106 |
| FB72 (ger-3); decoated | 2.8 × 105 | 2.7 × 105 |
| PS3329 (ger-3 cotE); decoated | 3.0 × 105 | 4.0 × 105 |
| Expt 2 | ||
| FB122 (spoVF sleB) | 5.3 × 104 | 5.9 × 106 |
| FB122 (spoVF sleB); sporulated with DPAb | 5.2 × 107 | 1.3 × 108 |
Titers were determined by spotting serial dilutions of spores at an OD600 of 1 on 2×YT medium after incubation in water or 60 mM Ca2+-DPA as described in Materials and Methods. The values shown are averages of at least two experiments. The individual experimental values lay within 40% of the averages shown.
The sporulation medium was supplemented with DPA at 100 μg/ml.
The effect of the cotE mutation on Ca2+-DPA-induced spore germination was also tested in an otherwise wild-type background, where spore germination was followed by measuring the change in the spore buoyant density. Ca2+-DPA-treated wild-type spores (≥90%) migrated to the lower density characteristic of germinated spores, whereas a majority (∼75%) of the Ca2+-DPA-treated cotE spores migrated at the higher density typical of dormant spores (data not shown). Thus, like chemical decoating treatment (23), a cotE mutation interfered with Ca2+-DPA-induced spore germination, supporting the idea that an intact coat is required for germination in response to Ca2+-DPA.
Relationship between the role of the coat and CwlJ in spore germination.
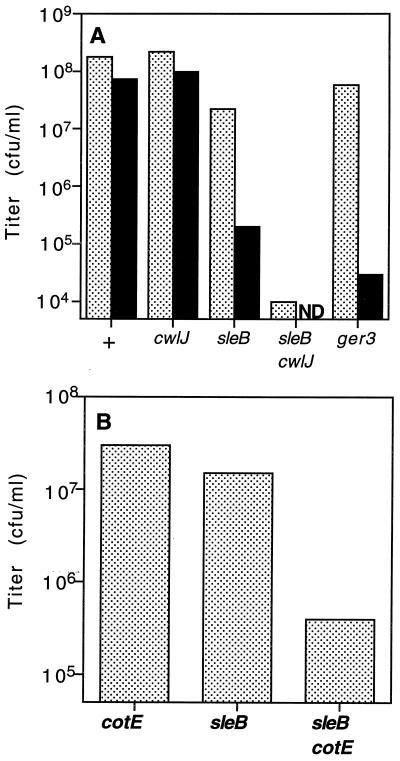
The results given above suggest either that CwlJ and the spore coat represent two independent requirements for Ca2+-DPA-induced spore germination or, alternatively, that CwlJ function is dependent upon an intact spore coat. If the latter scenario is correct, then treatments or mutations that disrupt the spore coat should also manifest the cwlJ-associated phenotypes that are not connected with Ca2+-DPA-induced spore germination. For example, decoated sleB spores should behave similarly to cwlJ sleB double mutant spores. To test this prediction, intact and decoated wild-type, cwlJ, sleB, and cwlJ sleB mutant spores were spotted on Luria broth agar plates for titer determination. As previously reported (12, 19, 23, 25), intact wild-type and cwlJ spores had similar titers, intact sleB spores had slightly reduced titers, while intact cwlJ sleB double-mutant spores had very low titers (Fig. 4A). Moreover, while decoating had no significant effect on the titers of wild-type and cwlJ spores, decoating of the sleB spores reduced their titers ∼500-fold, to a value only ∼20-fold higher than that of intact cwlJ sleB spores (Fig. 4A). A similar examination of cotE, sleB, and cotE sleB spores showed that the cotE mutation reduced nutrient-induced spore germination markedly in a sleB background but had a less severe effect in a wild-type background (5; Fig. 4B). Thus, disruption of the spore coat by either mutation or decoating produced cwlJ-associated spore germination phenotypes that are not connected with Ca2+-DPA germination.
FIG. 4.
Germination of sleB spores after decoating or in combination with cwlJ and cotE mutations. (A) Appropriate dilutions of intact (stippled) or decoated (solid) spores from strains PS832 (wild type) (+), FB111 (cwlJ), FB112 (sleB), FB113 (sleB cwlJ), and FB72 (ger-3) at an OD600 of 1 were spotted on 2×YT agar medium for titer determination. Note that decoated FB113 spores were not tested and the FB72 spores were spotted after Ca2+-DPA treatment. (B) Appropriate dilutions of spores from PS3328 (cotE), FB112 (sleB), and PS3330 (sleB cotE) strains at an OD600 of 1 were spotted on 2×YT medium for titer determination. Titers of wild-type spores at an OD600 of 1 were 1.3 × 108 CFU/ml. The values shown are averages of two experiments. The individual experimental values lay within 40% of the averages shown. ND, not determined.
To confirm that the decoating treatment mimicked a cwlJ mutation, we asked if decoated sleB spores were blocked in the same stage of nutrient-induced spore germination as were cwlJ sleB spores, which release DPA in response to nutrients but fail to complete cortex hydrolysis (12, 19, 26, 29). The release of core DPA in decoated wild-type, cwlJ, and sleB mutant spores was assayed before and after exposure to nutrients. After a 90-min incubation in rich medium, decoated spores from all three strains had lost >85% of the DPA present in spores treated with buffer alone (Fig. 5). Similarly, cotE and cotE sleB double-mutant spores also released ∼75% of their DPA after incubation in rich medium (Fig. 5). These observations show that disruption of the spore coat generates a phenocopy of a cwlJ mutation and therefore support the idea that the effects of a decoating treatment or a cotE mutation on Ca2+-DPA-induced spore germination are a consequence of their effects on CwlJ function.
FIG. 5.
DPA contents of spores of various strains with or without exposure to nutrients. Decoated spores from strains PS832 (wild type [WT]), FB110 (sleB), and FB111 (cwlJ) or intact spores from strains PS3328 (cotE) and PS3330 (sleB cotE) were incubated in water or 2×YT medium, washed, and assayed for DPA content. Since the DPA contents of the water-treated spores from all strains were similar, only the DPA contents of the 2×YT medium-treated spores are shown as percentages of the value of the water-treated spores. All of the values shown are from a typical experiment. Repetitions of these experiments gave values within 20% of the values shown.
Effect of endogenous spore DPA on CwlJ-dependent cortex hydrolysis.
Our studies suggested that Ca2+-DPA triggers germination by activating or modulating CwlJ function. In that case, one might expect the endogenous DPA that is released during stage I of nutrient-induced spore germination to activate subsequent CwlJ-dependent cortex hydrolysis. To study the effect of endogenous DPA on CwlJ function, we introduced a spoVF mutation that inactivates DPA synthase into a sleB strain, whose spores are solely dependent upon CwlJ for cortex hydrolysis during nutrient-induced germination. Previous work has shown that dormant spoVF spores cannot be isolated because they germinate spontaneously during sporulation (21); however, the presence of the ger-3 mutations partially suppresses this effect of a spoVF mutation and dormant ger-3 spoVF spores can be isolated (21). Consequently, it was noteworthy that dormant sleB spoVF spores were also stable. Indeed, since sleB spoVF spores do not germinate with nutrients (see below), they seem to be more stable than ger-3 spoVF spores, which do germinate with nutrients (21). As expected (21), sleB spoVF spores had <5% of the DPA content of either wild-type or sleB spores (data not shown). Spores from sleB single-mutant and sleB spoVF double-mutant strains were then tested for germination on nutrients by determination of their titer on a rich medium. While the sleB single-mutant spores showed slightly reduced titers of 3 × 107 CFU/ml (Fig. 4A) (19), the sleB spoVF double-mutant spores showed >1,000-fold lower titers (Table 2). Thus, sleB spoVF spores behaved similarly to sleB cwlJ spores (Table 2; expt 2; Fig. 4A), suggesting that CwlJ-dependent cortex hydrolysis was dependent upon endogenous DPA. Treatment of the sleB spoVF spores with 60 mM Ca2+-DPA increased their titer 100-fold (Table 2), showing that external Ca2+-DPA could partially alleviate the germination defect seen with these spores. Moreover, when the sleB spoVF strain was sporulated in the presence of DPA at 100 μg/ml, which restores the spore DPA content to ∼65% of wild-type levels (21; data not shown), the resultant spores behaved similarly to sleB single-mutant spores (Table 2; expt 2). Together, these findings suggest that the function of CwlJ in cortex hydrolysis is dependent upon endogenous spore DPA and that external Ca2+-DPA can at least partially make up for the absence of endogenous spore Ca2+-DPA.
DISCUSSION
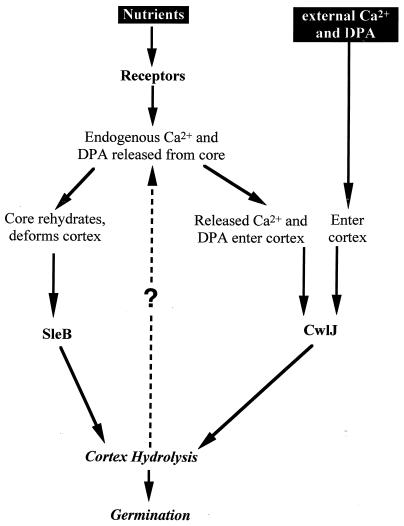
B. subtilis spores germinate in response to a variety of signals. Most commonly, spores can be induced to break dormancy by specific nutrients, but a 1:1 chelate of Ca2+ and DPA can also induce spore germination. In the studies presented here, we investigated the spore's response to Ca2+-DPA and found that this agent induces germination through a pathway which is distinct from that utilized by nutrient germinants but, significantly, shares some components with it. A model outlining these various pathways is shown in Fig. 6.
FIG. 6.
Model for nutrient- and Ca2+-DPA-induced spore germination. Nutrients activate receptors, and this results in the release of core Ca2+-DPA, which then triggers CwlJ action. SleB action may be triggered by both the receptor activation and changes in the properties of the spore core-cortex as a result of Ca2+-DPA release, but Ca2+-DPA does not activate SleB directly. SleB and CwlJ then catalyze cortex hydrolysis, which is sufficient for germination. External Ca2+-DPA might induce germination by triggering CwlJ-dependent cortex hydrolysis, which would be amplified by the consequent release of core Ca2+-DPA, as shown by the dashed arrow.
The germinant Ca2+-DPA induces spore germination through activation of the CwlJ protein. Previously, CwlJ, along with SleB, had been assigned a role in cortex hydrolysis during nutrient-induced spore germination. That idea was based on studies which showed that spores lacking both proteins initiated some germination reactions, such as release of DPA and the change in spore buoyant density, but failed to complete cortex hydrolysis and never formed colonies (12, 19, 26, 29). In contrast to this purely effector function during nutrient-induced spore germination, CwlJ seems to act as a part of the trigger mechanism during Ca2+-DPA-induced spore germination. Not only do cwlJ spores fail to form colonies in response to Ca2+-DPA, but they also show no detectable response to this chelate. The latter findings can be explained by the CwlJ protein having two distinct functions, one being cortex hydrolysis and the other being to trigger germination in response to Ca2+-DPA. Alternatively, as CwlJ is a small (∼16-kDa) protein, it is simpler to imagine that the cortex hydrolysis activity of CwlJ might be sufficient to account for its trigger-like function. Indeed, digestion of the spore cortex with exogenous enzymes such as lysozyme has been shown to trigger all of the events associated with spore germination, although exactly how that is accomplished is not known. Therefore, we propose that Ca2+-DPA, directly or indirectly, activates CwlJ-dependent cortex hydrolysis, which subsequently leads to spore germination. A critical aspect of this model that remains to be addressed is how Ca2+-DPA activates CwlJ. As Ca2+-DPA has been shown to allosterically activate a protease that acts during spore germination (11), it is not unreasonable that DPA alone or in a chelate with Ca2+ could directly activate CwlJ. Alternatively, Ca2+-DPA might activate some other protein(s) in the spore's outer layers that, in turn, activates CwlJ.
Regardless of how Ca2+-DPA activates CwlJ-dependent cortex hydrolysis, a decrease in that function best explains the effects of decoating and a cotE mutation on Ca2+-DPA-induced spore germination. Why is an intact coat necessary for CwlJ function? Although CwlJ has not yet been localized in the dormant spore, it is likely to be in the spore's outer layers, as it is synthesized exclusively in the mother cell compartment of the sporulating cell (12). Thus, it is possible that CwlJ would be removed or inactivated by decoating treatments that also remove the spore's outer membrane (3). It is, however, a bit more difficult to understand why a cotE mutation should affect CwlJ function. One possibility is that correct CwlJ localization depends upon CotE, and thus, CwlJ is largely mislocalized in cotE spores. Alternatively, as the cotE spore coat is permeable to large molecules, it is tempting to speculate that proteases in the sporulating culture might gain access to and degrade CwlJ in cotE spores or that the CwlJ molecules might themselves leach out through the defective spore coat.
Besides elucidating key steps in Ca2+-DPA-induced spore germination, our studies provide insight into a long-standing problem in nutrient-induced spore germination. The nutrient-receptor interaction likely occurs at the spore's inner membrane, as suggested by recent localization of the receptor proteins to that site (10, 24). In contrast, the SleB protein has been localized to the outer layers of the cortex (18), while the CwlJ protein is expected to be in the spore's outer layers, as noted above. So, how is a signal from the spore's inner membrane transmitted to the outer edge of the cortex? On the basis of our observations and the finding that nutrients trigger release of endogenous DPA in sleB cwlJ spores (12, 19, 29), we propose a mechanism for the activation of CwlJ during nutrient-induced spore germination: as endogenous Ca2+ and DPA released from the spore core in response to nutrients flow through the spore's external layers, they activate CwlJ located there (Fig. 6). The model is attractive both because its prediction that endogenous DPA is crucial for CwlJ function during nutrient-induced spore germination was experimentally confirmed and because it provides a paradigm for transduction of the nutrient signal to at least one CLE. One caveat of the experiment which tested the prediction is that the elimination of nutrient-induced spore germination in sleB spoVF spores, which lack DPA, might be due to an improper localization or level of CwlJ in these spores. That possibility is unlikely, given the ability of exogenous Ca2+-DPA to partially restore CwlJ function in sleB spoVF spores, but it still needs to be ruled out. Those studies would also clarify whether the partial effect of external Ca2+-DPA on DPA-less spores is due to a positive feedback loop (dashed line in Fig. 6) in which external Ca2+-DPA activates low levels of CwlJ-dependent cortex hydrolysis that, in turn, results in the release of endogenous DPA, which further activates CwlJ.
The above-described model only explains the activation of CwlJ and not that of SleB, which plays a redundant role, along with CwlJ, in nutrient-induced spore germination. While our study did not directly address the activation of SleB, our observation that the spontaneous germination of DPA-less (spoVF) spores was reduced when sleB was deleted suggests that SleB is active in the absence of spore DPA. Furthermore, deletion of sleB and that of all known gerA-like receptors had similar effects in retarding the spontaneous germination of DPA-less spores, suggesting that the core DPA inhibits or checks the nutrient receptor-dependent activation of SleB. One major effect of the absence of endogenous DPA is that DPA-less spore cores are more hydrated and consequently might be more turgid than wild-type spore cores. Intriguingly, as SleB activity is extremely sensitive to the precise physical state of its cortex substrate (7, 15), those ideas can be assimilated into a scenario in which the swollen DPA-less spore core deforms the cortex, makes it susceptible to SleB-dependent hydrolysis, and thus results in spontaneous germination. That idea can be further extended to nutrient-induced germination where core hydration, following release of core cations and DPA, activates SleB-dependent cortex hydrolysis through cortical deformation. The attractive aspect of this model is that a single event, namely, cation and DPA release from the core, needs to be triggered by the germinant-bound receptor to activate both CwlJ and SleB (Fig. 6). In this light, the homology (50%) between some of the Ger receptor proteins and SpoVAF, a protein implicated in DPA transport, and some amino acid transporters (16, 17, 31) raises the possibility that the germinant receptors are ligand (germinant)-activated cation or DPA transporters. Further studies are required to better explore and test these models of spore germination. Indeed, the overall picture of the signaling mechanisms operating during spore germination is not completely clear but there certainly are some breaks in the clouds.
ACKNOWLEDGMENTS
We thank members of our laboratory for their suggestions and criticisms regarding this work.
This work was supported by grant GM19698 from the National Institutes of Health.
REFERENCES
- 1.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:74–76. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boland F M, Atrih A, Chirakkal H, Foster S J, Moir A. Complete spore-cortex hydrolysis during germination of Bacillus subtilis 168 requires SleB and YpeB. Microbiology. 2000;146:57–64. doi: 10.1099/00221287-146-1-57. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan C E, Neyman S L. Correlation of penicillin-binding protein composition with different functions of two membranes in Bacillus subtilis forespores. J Bacteriol. 1986;165:498–503. doi: 10.1128/jb.165.2.498-503.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutting S M, Vander Horn P B. Genetic analysis. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley & Sons Ltd.; 1990. pp. 27–74. [Google Scholar]
- 5.Driks A. The Bacillus subtilis spore coat. Microbiol Mol Biol Rev. 1999;63:1–20. doi: 10.1128/mmbr.63.1.1-20.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driks A, Setlow P. Morphogenesis and properties of the bacterial spore. Washington, D.C.: American Society for Microbiology; 1999. [Google Scholar]
- 7.Foster S J, Johnstone K. Purification and properties of a germination-specific cortex-lytic enzyme from spores of Bacillus megaterium KM. Biochem J. 1987;242:573–579. doi: 10.1042/bj2420573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould G W. Germination. In: Gould G W, Hurst A, editors. The bacterial spore. New York, N.Y: Academic Press, Inc.; 1969. pp. 397–444. [Google Scholar]
- 9.Guerot-Fleury A-M, Shazand K, Frandsen N, Stragier P. Antibiotic-resistance cassettes for Bacillus subtilis. Gene. 1985;167:335–336. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 10.Hudson K D, Corfe B M, Kemp E H, Feavers I M, Coote P J, Moir A. Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore. J Bacteriol. 2001;183:4317–4322. doi: 10.1128/JB.183.14.4317-4322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Illades-Aguiar B, Setlow P. Autoprocessing of the protease that degrades small, acid-soluble proteins of spores of Bacillus species is triggered by low pH, dehydration, and dipicolinic acid. J Bacteriol. 1994;176:7032–7037. doi: 10.1128/jb.176.22.7032-7037.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa S, Yamane K, Sekiguchi J. Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J Bacteriol. 1998;180:1375–1380. doi: 10.1128/jb.180.6.1375-1380.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnstone K, Ellar D J. The role of cortex hydrolysis in the triggering of germination of Bacillus megaterium KM spores. Biochim Biophys Acta. 1982;714:185–191. [Google Scholar]
- 14.Lindsay J A, Beaman T C, Gerhardt P. Protoplast water content of bacterial spores determined by buoyant density sedimentation. J Bacteriol. 1985;163:735–737. doi: 10.1128/jb.163.2.735-737.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makino S, Ito N, Inoue T, Miyata S, Moriyama R. A spore-lytic enzyme released from Bacillus cereus spores during germination. Microbiology. 1994;140:1403–1410. doi: 10.1099/00221287-140-6-1403. [DOI] [PubMed] [Google Scholar]
- 16.Moir A, Kemp E H, Robinson C, Corfe B M. The genetic analysis of bacterial spore germination. J Appl Bacteriol Symp Suppl. 1994;76:9S–16S. [PubMed] [Google Scholar]
- 17.Moir A, Smith D A. The genetics of bacterial spore germination. Annu Rev Microbiol. 1990;44:531–553. doi: 10.1146/annurev.mi.44.100190.002531. [DOI] [PubMed] [Google Scholar]
- 18.Moriyama R, Fukuoka H, Miyata S, Kudoh S, Hattori A, Kozuka S, Yasuda Y, Tochikubo K, Makino S. Expression of a germination-specific amidase, SleB, of bacilli in the forespore compartment of sporulating cells and its localization on the exterior side of the cortex in dormant spores. J Bacteriol. 1999;181:2373–2378. doi: 10.1128/jb.181.8.2373-2378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moriyama R, Miyata S, Kudoh S, Hattori A, Makino S. A gene (sleB) encoding a spore cortex-lytic enzyme from Bacillus subtilis and response of the enzyme to l-alanine-mediated germination. J Bacteriol. 1996;178:6059–6063. doi: 10.1128/jb.178.20.6059-6063.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson W L, Setlow P. Sporulation, germination, and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley & Sons Ltd.; 1990. pp. 391–450. [Google Scholar]
- 21.Paidhungat M, Setlow B, Driks A, Setlow P. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol. 2000;182:5505. doi: 10.1128/jb.182.19.5505-5512.2000. 5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paidhungat M, Setlow P. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J Bacteriol. 1999;181:3341–3350. doi: 10.1128/jb.181.11.3341-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paidhungat M, Setlow P. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J Bacteriol. 2000;182:2513–2519. doi: 10.1128/jb.182.9.2513-2519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paidhungat M, Setlow P. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J Bacteriol. 2001;183:3982–3990. doi: 10.1128/JB.183.13.3982-3990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paidhungat, M., and P. Setlow. Spore germination and outgrowth. In J. A. Hoch, R. Losick, and A. L. Sonenshein (ed.), Bacillus subtilis and its relatives: from genes to cells, in press. American Society for Microbiology, Washington, D.C.
- 26.Popham D L, Helin J, Costello C E, Setlow P. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc Natl Acad Sci USA. 1996;93:15405–15410. doi: 10.1073/pnas.93.26.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotman Y, Fields M L. A modified reagent for dipicolinic acid analysis. Anal Biochem. 1967;22:168. doi: 10.1016/0003-2697(68)90272-8. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Setlow B, Melly E, Setlow P. Properties of spores of Bacillus subtilis blocked at an intermediate stage in spore germination. J Bacteriol. 2001;183:4894–4899. doi: 10.1128/JB.183.16.4894-4899.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vary J C. Germination of Bacillus megaterium spores after various extraction procedures. J Bacteriol. 1973;95:1327–1334. doi: 10.1128/jb.116.2.797-802.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuberi A R, Moir A, Feavers I M. The nucleotide sequence and gene organization of the gerA spore germination operon of Bacillus subtilis 168. Gene. 1987;51:1–11. doi: 10.1016/0378-1119(87)90468-9. [DOI] [PubMed] [Google Scholar]