Abstract
Rationale
Outdoor air pollution is a potential risk factor for lower lung function and chronic obstructive pulmonary disease (COPD). Little is known about how airway abnormalities and lung growth might modify this relationship.
Objectives
To evaluate the associations of ambient air pollution exposure with lung function and COPD and examine possible interactions with dysanapsis.
Methods
We made use of cross-sectional postbronchodilator spirometry data from 1,452 individuals enrolled in the CanCOLD (Canadian Cohort Obstructive Lung Disease) study with linked ambient fine particulate matter (PM2.5) and nitrogen dioxide (NO2) air pollution estimates. Dysanapsis, or the ratio of the airway-to-lung volume calculated from thoracic computed tomography images, was used to examine possible interactions.
Measurements and Main Results
In adjusted models, 101.7 ml (95% confidence interval [CI], −166.2 to −37.2) and 115.0 ml (95% CI, −196.5 to −33.4) lower FEV1 were demonstrated per increase of 2.4 ug/m3 PM2.5 and 9.2 ppb NO2, respectively. Interaction between air pollution and dysanapsis was not statistically significant when modeling the airway-to-lung ratio as a continuous variable. However, a 109.8 ml (95% CI, −209.0 to −10.5] lower FEV1 and an 87% (95% CI, 12% to 213%) higher odds of COPD were observed among individuals in the lowest, relative to highest, airway-to-lung ratio, per 2.4 μg/m3 increment of PM2.5.
Conclusions
Ambient air pollution exposure was associated with lower lung function, even at relatively low concentrations. Individuals with dysanaptic lung growth might be particularly susceptible to inhaled ambient air pollutants, especially those at the extremes of dysanapsis.
Keywords: chronic airflow obstruction, chronic obstructive pulmonary disease, air quality, pulmonary function test, computed tomography
At a Glance Commentary
Scientific Knowledge on the Subject
Air pollution is now recognized by the World Health Organization as the single biggest environmental health risk. Long-term air pollution exposure has been linked to reduced lung growth in children, as well as lung function decline and increased chronic obstructive pulmonary disease (COPD) prevalence and incidence in adults. Recent studies show that a mismatch between airway tree caliber and lung size (i.e., dysanaptic lungs) is associated with a higher risk of COPD. Few studies on COPD have been conducted in locations with relatively low pollution concentrations, and little is known about how air pollution exposure interacts with host factors such as abnormalities of airway and lung growth.
What This Study Adds to the Field
This is the first study in Canada to examine associations of long-term ambient air pollution exposure with lung function and spirometrically confirmed COPD. This study shows statistically and clinically significant effects of ambient air pollution exposure on lung function, even at low concentrations compared to many countries around the world. For the first time, this study also shows that individuals with dysanaptic lungs may be more susceptible to the long-term effects of air pollution exposure on lung function and COPD.
Chronic obstructive pulmonary disease (COPD) is the most common cause of hospital admissions among chronic illnesses in Canada (1). In 2015, 3.2 million people lost their lives to COPD globally, making it one of the world’s leading causes of death (2). Although tobacco smoke is recognized as the single most important risk factor for the development and progression of COPD, 25–45% of individuals with COPD have never smoked (3). Considering the global burden of COPD, identifying risk factors beyond tobacco smoking and determining interactions among them are of utmost importance to inform preventive strategies, clinical diagnosis, and management.
COPD is a complex heterogeneous disease resulting from interactions between environment, lifestyle, and genotype (4). Numerous nontobacco COPD risk factors have been proposed (5), and it has been demonstrated that heterogeneity in COPD prevalence is partly explained by variations in associated risk factors (6). Ambient air pollution has been identified as a potential risk factor for COPD (7). Outdoor air pollution is estimated to have caused 4.2 million deaths in 2015, more than 800,000 of which were attributable to COPD (8). The biological plausibility of air pollution as a risk factor for COPD has been demonstrated (9), and exposure to ambient air pollution such as fine particulate matter (PM2.5) and nitrogen dioxide (NO2) has been linked to slower lung function growth in children and adolescents (10, 11) and to lower lung function as well as higher chronic bronchitis, emphysema, and COPD prevalence and incidence in adults (7, 12–16). Although the effects of air pollution on lung health appear modest relative to tobacco use, its ubiquitous nature leads to a substantial population burden (8).
A better understanding of how air pollution exposure interacts with other individual-level characteristics can shape strategies to mitigate its impact. A recent observational study involving 6,529 Canadian and U.S. adults showed that dysanapsis, or a mismatch of airway tree caliber to lung size, appears to be a significant risk factor for COPD (17). Small airway size has also been shown to increase the susceptibility of respiratory symptoms amongst teenagers without asthma after exposure to wildfire smoke (18). Particle deposition modeling suggests that airway tree anatomy plays a vital role in deposition patterns in the lung (19) and could therefore influence health risks associated with inhaled particle pollution.
The primary objective of the current study was to evaluate the relationship of PM2.5 and NO2 ambient air pollution exposure with lung function and COPD in the Canadian population, making use of data from the CanCOLD (Canadian Cohort Obstructive Lung Disease) study. The secondary objective of the study was to assess if individuals characterized by smaller airways relative to lung size (i.e., dysanaptic lungs) are more susceptible to the impacts of ambient air pollution on lung function and COPD. We hypothesized that exposure to PM2.5 and NO2 air pollution is associated with lower lung function and higher prevalence of COPD, even at relatively low concentrations typically seen in Canada, and that abnormalities in airway and lung growth increase disease susceptibility.
Methods
Study Population
We used spirometry, chest computed tomography scan, and covariates from the first (i.e., baseline) assessment of the CanCOLD study (20), a population-based cohort of noninstitutionalized adults aged 40 years and older. CanCOLD participants were recruited from the COLD (Canadian Obstructive Lung Disease) prevalence study in two groups: 1) individuals with airflow obstruction on the basis of the Global Initiative for Chronic Obstructive Lung Disease criteria (4) (i.e., postbronchodilator [post-BD] FEV1/FVC ratio of <0.70; and 2) sex- and age-matched (±2 years) peers without COPD (i.e., post-BD FEV1/FVC ⩾ 0.70) split between ever- and never-smokers. Between 2009 and 2015, 1,561 individuals completed their baseline assessment in data collection sites in nine Canadian cities: Vancouver (n = 435), Montreal (n = 355), Kingston (n = 136), Halifax (n = 127), Calgary (n = 129), Ottawa (n = 116), Saskatoon (n = 104), Québec City (n = 85), and Toronto (n = 74). Most CanCOLD participants lived within the boundaries of each city rather than in the urban periphery. Details on the study sampling methodology can be found elsewhere (20). Informed consent was obtained from all participants, and the CanCOLD study protocol was approved by the institutional review boards of each site. CanCOLD is registered at ClinicalTrials.gov under ID: NCT00920348.
Outcome Assessment
Baseline lung function was assessed for each participant using spirometry (before and 15 min after inhalation of 200 μg salbutamol) by trained technicians according to American Thoracic Society and European Respiratory Society guidelines (21). The main outcomes for our study were post-BD FEV1, FVC, FEV1/FVC ratio, and COPD prevalence (defined as a post-BD FEV1/FVC ratio of <0.70) at baseline assessment. In separate analyses, COPD prevalence was defined using NHANES III (National Health and Nutrition Examination Survey) reference values for the lower limit of normal (LLN) (22). Individuals with an FEV1/FVC ratio below the LLN were classified as having COPD.
Ambient Air Pollution Exposure
PM2.5 and NO2 data distributed by CANUE (Canadian Urban Environmental Health Research Consortium) (23) were linked to CanCOLD participants’ six-digit residential postal codes at baseline assessment as indicators of long-term, chronic exposure. PM2.5 is inhalable particles with a diameter of 2.5 micrometers or less. Key emission sources for PM2.5 are industry, wildfire smoke, residential wood heating, cooking, agriculture, and vehicle traffic. On average, about half of PM2.5 is a result of atmospheric chemistry forming from gaseous emissions of sulfur dioxide, nitrogen oxides, ammonia, and volatile organic compounds. Three-year running averages of PM2.5 concentrations before baseline assessment were estimated across a 1 × 1-kilometer grid covering North America using NASA Moderate resolution Imaging Spectroradiometer, Multi-angle Imaging SpectroRadiometer, and Sea-viewing Wide Field-of-view Sensor satellite instruments, with aerosol vertical profiles and scattering properties simulated by the Goddard Earth Observing System chemical transport model (GEOS-Chem) (24). To adjust for any residual bias in the satellite-derived PM2.5 estimates, a geographically weighted regression incorporating ground-based observations was then applied (24). Good agreement was found with cross-validated surface observations across North America (R2 = 0.70). Annual average NO2 concentrations in parts per billion were estimated for each postal code location using a national land-use regression model for the year 2006 (25) and adjusted for the year of baseline assessment up to 2012 using air quality monitoring station data. NO2 is considered an indicator of traffic-related air pollution, which is a complex mixture of gases and particles, including ultrafine particles (diameter of 0.1 µm or less). The land-use regression NO2 model included road length, 2005–2011 satellite NO2 estimates, area of industrial land use within 2 km, and summer rainfall as predictors of regional NO2 variation (25). Deterministic gradients were used to model local scale variation related to roads (i.e., traffic) (25). The final NO2 model showed good performance, explaining 73% of the variation in measurements from national air pollution surveillance monitoring data with a root mean square error of 2.9 ppb. For both pollutants, we used two variables in our main analyses: 1) interquartile range (IQR) increases; and 2) categorical air pollution concentrations (low, medium, and high) on the basis of the range of exposure in the population.
Computed Tomography Scan for Dysanapsis Measurement
CanCOLD participants underwent inspiratory chest computed tomography scans at baseline assessment. Dysanapsis was determined by measuring the airway-to-lung ratio from thoracic computed tomography images or the geometric mean of airway lumen diameters (cm) measured at 19 standard anatomic locations divided by the cube-root of total lung volume in cubic centimeters (17). A lower airway-to-lung ratio indicates smaller airways relative to lung size, and higher values indicate larger airways relative to lung size. We used a continuous airway-to-lung ratio variable as well as a quartiles-based variable for analyses.
Covariates, Potential Confounders, and Subgroups
Predefined covariates included age (yr), sex, body mass index (BMI), educational attainment (high school or lower vs. postsecondary education), tobacco status (never, former, current smokers), pack-years smoking, environmental tobacco smoke at home, biomass fuel exposure, and respiratory medication intake in the past year. Detailed definitions of these variables are found in the online supplement. In sensitivity analyses, we also adjusted for neighborhood-level socioeconomic status by including a material deprivation index (26) linked to residential postal codes of participants and modeled as quintiles on the basis of its distribution across Canada. Finally, to identify population subgroups that might be susceptible to the long-term effects of air pollution exposure on lung function and COPD, sex, smoking status, self-reported physician diagnosis of asthma, and history of childhood hospitalization for a breathing problem before the age of 10 years were used.
Statistical Analyses
We generated summary statistics for outcomes, covariates, confounders, and subgroup variables, as well as for ambient air pollution concentrations. Cross-sectional linear regression models were used to assess the associations between ambient PM2.5 and NO2 air pollution exposure and post-BD FEV1, FVC, and FEV1/FVC ratio after adjusting for age, sex, BMI, educational attainment, tobacco status, pack-years of smoking, environmental tobacco smoke, biomass exposure, and current respiratory medication intake. To control for the potential effects of unmeasured confounders which vary by assessment site and to assess the impact of intraurban (as opposed to interurban) air pollution exposure, the CanCOLD data collection site was used as an additional adjustment factor. In sensitivity analyses, associations of air pollution concentrations with pre-BD lung function were also assessed. Adjusted logistic regression models were used to assess the relationship between air pollution exposure and odds of COPD prevalence, defined using the fixed ratio (FEV1/FVC < 0.7; main analyses) and LLN (sensitivity analyses) thresholds. In sensitivity analyses, we also adjusted for neighborhood material deprivation to adjust for other community-level confounders. Subgroup analyses and tests for interactions were conducted by stratifying analyses by 1) sex (men vs. women); 2) smoking status (current vs. former or never); 3) physician diagnosis of asthma; and 4) hospitalization as a child for a breathing problem. We assessed interactions between air pollution and dysanapsis on FEV1 and COPD by modeling the airway-to-lung ratio as a continuous variable as well as quartiles. Mean baseline difference in FEV1 and the relative difference in odds of COPD per IQR increase in air pollutant concentrations were estimated, adjusting for the same covariates as listed above. Interactions were assessed using product terms between air pollutant concentrations and airway-to-lung ratio. The explanatory power of the interactions between each air pollutant and dysanapsis (as quartiles) on FEV1 was also assessed using F tests. Finally, all analyses were conducted without CanCOLD data collection site adjustment to assess its impact on results. The R statistical software version 3.6.0 (27) was used throughout.
Results
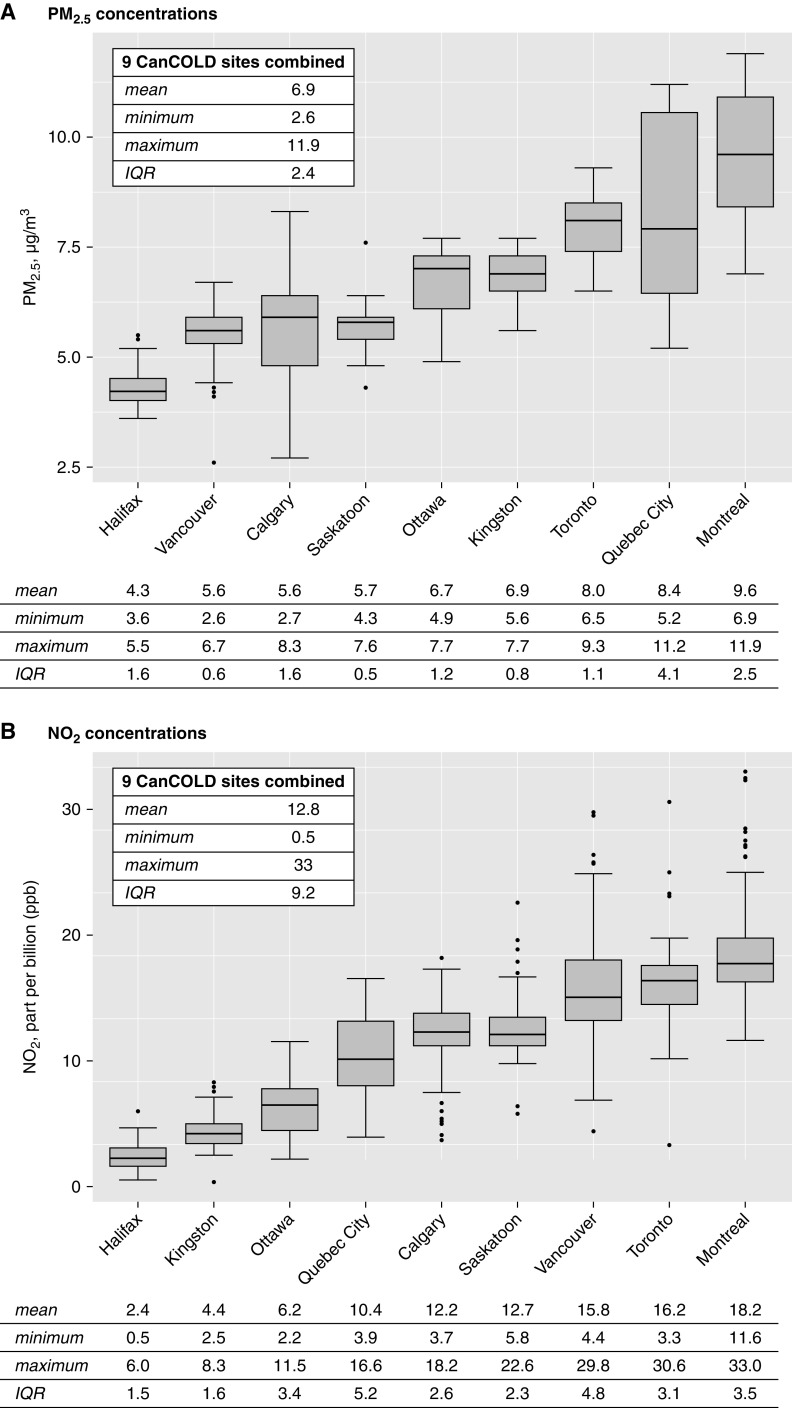
Figure 1 shows the study population flow diagram and final analytical samples. Study participants had a mean age of 66.6 years and BMI of 27.7 kg/m2, 56% were men, and fewer than one in four participants did not have postsecondary education (Table 1). Approximately one-third of participants had never smoked, two out of five participants reported environmental tobacco smoke exposure, and 14% reported biomass fuel exposure at home. Baseline post-BD FEV1, FVC, and FEV1/FVC ratio were 2.55 L (SD = 0.81), 3.68 L (SD = 1.07), and 0.69 (SD = 0.10), respectively. Just fewer than half of the individuals had COPD, the majority of whom were classified as having mild COPD (GOLD1). Approximately 1 in 4 participants reported respiratory medication intake in the past 12 months or a physician diagnosis of asthma, and fewer than 1 in 10 had been hospitalized before the age of 10 years for a breathing problem. In all CanCOLD cities combined, mean ambient PM2.5 and NO2 concentrations were 6.9 μg/m3 (IQR, 2.4 μg/m3) and 12.8 ppb (IQR, 9.2 ppb), respectively (Figure 2). Within-city pollutant concentration IQRs ranged from 0.5 to 4.1 PM2.5 μg/m3 and from 1.5 to 5.2 NO2 ppb (Figure 2).
Figure 1.

Study population, missing data, and exclusions flow diagram. CanCOLD = Canadian Cohort Obstructive Lung Disease; CT = computed tomography.
Table 1.
Baseline Characteristics of Canadian Cohort Obstructive Lung Disease Study Participants*
| Characteristic | Total (N = 1452) | COPD (n = 691; 47.6%) | Non-COPD (n = 761; 52.4%) |
|---|---|---|---|
| Sociodemographic factor | |||
| Sex, n (%) | |||
| Male | 815 (56.1) | 412 (59.6) | 403 (53.0) |
| Female | 637 (43.9) | 279 (40.4) | 358 (47.0) |
| Age, mean (SD), yr | 66.6 (9.8) | 67.3 (10.1) | 66.0 (9.5) |
| BMI, mean (SD), kg/m2 | 27.7 (5.3) | 27.5 (5.31) | 27.8 (5.24) |
| Education, n (%) | |||
| No postsecondary | 328 (22.6) | 179 (25.9) | 149 (19.6) |
| Some postsecondary | 1124 (77.4) | 512 (74.1) | 612 (80.4) |
| Tobacco and biomass smoke exposure | |||
| Tobacco status, n (%) | |||
| Never smoker | 514 (35.4) | 192 (27.8) | 322 (42.3) |
| Ex-smoker | 718 (49.4) | 370 (53.5) | 348 (45.7) |
| Current smoker | 220 (15.2) | 129 (18.7) | 91 (12.0) |
| Pack-years smoking, mean (SD) | 26.1 (23.8) | 31.6 (24.3) | 19.8 (21.6) |
| Environmental tobacco smoke, n (%) | |||
| Never | 836 (57.6) | 370 (53.5) | 466 (61.2) |
| Ever | 616 (42.4) | 321 (46.5) | 295 (38.8) |
| Lifetime biomass exposure, n (%), yr | |||
| <10 | 1249 (86.0) | 596 (86.3) | 653 (85.8) |
| ⩾10 | 203 (14.0) | 95 (13.7) | 108 (14.2) |
| Respiratory health | |||
| FEV1, L | 2.55 (0.81) | 2.33 (0.78) | 2.76 (0.77) |
| FEV1 (% predicted) | 91.7 (20.2) | 82.3 (19.3) | 100 (17.1) |
| FVC, L | 3.68 (1.07) | 3.78 (1.11) | 3.59 (1.02) |
| FVC (% predicted) | 99.0 (17.2) | 100 (17.9) | 97.8 (16.4) |
| FEV1/FVC (%) | 69.5 (10.3) | 61.1 (8.14) | 77.1 (4.63) |
| Airflow limitation severity, n (%) | |||
| Mild (GOLD 1) | — | 388 (56.2) | N/A |
| Moderate (GOLD 2) | — | 264 (38.2) | N/A |
| Severe (GOLD 3) | — | 38 (5.5) | N/A |
| Very severe (GOLD 4) | — | 1 (0.1) | N/A |
| Respiratory medication, n (%) | |||
| None | 1124 (77.4) | 446 (64.5) | 678 (89.1) |
| ⩾1 | 328 (22.6) | 245 (35.5) | 83 (10.9) |
| Asthma, n (%) | |||
| Nonasthmatic | 1107 (76.2) | 470 (68.0) | 637 (83.7) |
| Asthmatic | 345 (23.8) | 221 (32.0) | 124 (16.3) |
| Childhood hospitalization for respiratory illness, n (%) | |||
| No | 1352 (93.2) | 630 (91.3) | 722 (94.9) |
| Yes | 99 (6.8) | 60 (8.7) | 39 (5.1) |
| Missing | 1 | 1 | 0 |
| Dysanapsis | |||
| Airway-to-lung ratio | |||
| Fourth quartile (highest) | 296 (24.3) | 87 (14.6) | 209 (33.5) |
| Third quartile | 305 (25.0) | 117 (19.6) | 188 (30.2) |
| Second quartile | 305 (25.0) | 165 (27.7) | 140 (22.5) |
| First quartile (lowest) | 313 (25.7) | 227 (38.1) | 86 (13.8) |
| Missing | 233 | 95 | 138 |
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; N/A = not applicable.
For 1,452 participants with complete data for baseline age, BMI, education, tobacco status, pack-years smoking, environmental tobacco smoke at home, biomass fuel exposure, respiratory medication intake, spirometry, and ambient nitrogen dioxide exposure.
Figure 2.
Fine particulate matter (PM2.5) and nitrogen dioxide (NO2) ambient air pollution concentrations at the residences of the CanCOLD study participants. CanCOLD = Canadian Cohort Obstructive Lung Disease; IQR = interquartile range.
Air Pollution Relationship with Lung Function and COPD
Associations of air pollution with lung function and COPD are presented in Table 2. Higher concentrations of both PM2.5 and NO2 air pollution exposure were associated with statistically significant lower FEV1. Positive though not statistically significant associations were found between exposure to pollutants and COPD prevalence. Additionally, adjusting models for neighborhood material deprivation attenuated PM2.5 associations with FEV1, but all other associations remained similar (see Table E1 in the online supplement). When defining COPD using the LLN threshold, statistically significant positive associations were found for COPD prevalence and ambient NO2 exposure (Table E2). We also found statistically significant lower FVC amongst individuals living in areas with high compared to low PM2.5 and NO2 (Table E3). Associations of ambient air pollutant concentrations with pre-BD and post-BD lung function were very similar (Table E4).
Table 2.
Relationship of Air Pollution Exposure with Lung Function and Chronic Obstructive Pulmonary Disease
| Lung Function | COPD | ||||
|---|---|---|---|---|---|
| Exposure | n | FEV1 (ml) β (95% CI) |
FEV1/FVC (%) β (95% CI) |
Cases/Noncases | OR (95% CI) |
| PM2.5 (continuous) (per IQR increase = 2.4 μg/m3) |
1,451 | −101.7 (−166.2 to −37.2) | −0.6 (−1.7 to 0.5) | 691/760 | 1.17 (0.89 to 1.54) |
| PM2.5 (categorical), μg/m3 | |||||
| Low (2.6 to 5.6) | 404 | Reference | Reference | 199/205 | Reference |
| Medium (5.7 to 8.7) | 768 | −108.0 (−188.8 to −27.3) | −1.1 (−2.4 to 0.3) | 361/407 | 1.13 (0.80 to 1.59) |
| High (8.8 to 11.9) | 279 | −261.9 (−391.7 to −132.1) | −2.3 (−4.5 to −0.1) | 131/148 | 1.57 (0.90 to 2.73) |
| NO2 (per IQR increase = 9.2 ppb) | 1,452 | −115.0 (−196.5 to −33.4) | −1.1 (−2.5 to 0.3) | 691/761 | 1.19 (0.84 to 1.68) |
| NO2 (categorical), ppb | |||||
| Low (0.5 to 11.3) | 507 | Reference | Reference | 227/280 | Reference |
| Medium (11.4 to 22.1) | 881 | −87.1 (−190.9 to 16.8) | −0.8 (−2.5 to 1.0) | 431/450 | 1.30 (0.84 to 2.03) |
| High (22.2 to 33) | 64 | −268.84 (−434.21 to −103.47) | −2.6 (−5.4 to 0.2) | 33/31 | 1.58 (0.78 to 3.18) |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; IQR = interquartile range; NO2 = nitrogen dioxide; OR = odds ratio; PM2.5 = fine particulate matter.
All models are adjusted for age, sex, body mass index, educational level (high school or lower vs. postsecondary education), tobacco status (never smoker, former smoker, or current smoker), pack-years smoking, environmental tobacco smoke at home, biomass fuel exposure, respiratory medication intake, and Canadian Cohort Obstructive Lung Disease study site. Statistically significant results are shown in bold.
Analyses by sex, smoking status, asthmatic status, and childhood hospitalization for a breathing problem did not reveal any notable differences across subgroups or statistically significant interactions (Tables E5–E8). In models without CanCOLD site adjustment, associations between air pollution exposure and lung function were attenuated (see Tables E9 and E10).
Interaction of Air Pollution Exposure and Dysanapsis on Lung Function and COPD
Tables 3 and 4 show interactions between air pollution exposure and dysanapsis on FEV1 and COPD prevalence. No statistically significant interactions were found when modeling the airway-to-lung ratio as a continuous linear term (Table 3). However, there was evidence of interaction between PM2.5 air pollution exposure and dysanapsis for both FEV1 (P-value interaction = 0.030) and COPD (P-value interaction = 0.015) when comparing lowest to highest airway-to-lung ratio quartiles (Table 4). After accounting for confounding factors, FEV1 was 109.8 ml (95% confidence interval [CI], −209.0 to −10.5 ml) lower amongst individuals in the lowest compared to highest airway-to-lung ratio quartile, per IQR increase in PM2.5. An IQR increment in PM2.5 concentrations was also associated with 87% (95% CI, 12% to 213%) higher odds of COPD amongst individuals in the lowest relative to highest airway-to-lung ratio quartile. Interactions between PM2.5 concentrations and the second and third quartiles of airway-to-lung ratio (relative to the highest quartile) were nonsignificant (Table E11). A significant interaction between PM2.5 and dysanapsis on FEV1 was found when using the F test (P-value = 0.042) (Table E12). Finally, no significant interactions were found between NO2 exposure and dysanapsis.
Table 3.
Interactions between Air Pollution and Airway-to-Lung Ratio on FEV1 and Chronic Obstructive Pulmonary Disease Prevalence
| Difference in FEV1, β (95% CI), ml |
||
|---|---|---|
| Airway-to-Lung Ratio (per IQR Decrease) | P Value Interaction | |
| PM2.5 (per IQR increase = 2.4 μg/m3) | −38.3 (−82.2 to 5.6) | 0.087 |
| NO2 (per IQR increase = 9.2 ppb) | 19.4 (−37.7 to 76.4) | 0.505 |
| Difference in COPD OR (95% CI) |
||
|---|---|---|
| Airway-to-Lung Ratio (per IQR Decrease) | P Value Interaction* | |
| PM2.5 (per IQR increase = 2.4 μg/m3) | 1.26 (0.98 to 1.62) | 0.065 |
| NO2 (per IQR increase = 9.2 ppb) | 1.03 (0.77 to 1.40) | 0.826 |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; IQR = interquartile range; NO2 = nitrogen dioxide; OR = odds ratio; PM2.5 = fine particulate matter.
Airway-to-lung ratio modeled using continuous linear terms. All models are adjusted for age, sex, body mass index, educational level (high school or lower vs. postsecondary education), tobacco status (never smoker, former smoker, or current smoker), pack-years smoking, environmental tobacco smoke at home, biomass fuel exposure, respiratory medication intake, dysanapsis (continuous), and Canadian Cohort Obstructive Lung Disease study site. Statistically significant results are shown in bold.
To test for interactions, a product term was included between air pollution and continuous airway-to-lung ratio.
Table 4.
Interactions between Air Pollution and Airway-to-Lung Ratio on FEV1 and Chronic Obstructive Pulmonary Disease Prevalence
| Difference in FEV1 (ml), β (95% CI) |
|||||
|---|---|---|---|---|---|
| All Participants | Highest Quartile of Airway-to-Lung Ratio | Lowest Quartile of Airway-to-Lung Ratio | Difference between Highest and Lowest Quartile | P Value Interaction* | |
| PM2.5 (per IQR increase = 2.4 μg/m3) |
−107.0 (−175.9 to −38.0) | −70.8 (−160.5 to 18.9) | −180.5 (−273.9 to −87.2) | −109.8 (−209.0 to −10.5) | 0.030 |
| NO2 (per IQR increase = 9.2 ppb) |
−149.5 (−234.7 to −64.2) | −185.0 (−306.5 to −63.4) | −145.3 (−254.8 to −35.7) | 39.7 (−89.9 to 169.4) | 0.548 |
| Difference in COPD OR (95% CI) |
|||||
|---|---|---|---|---|---|
| All Participants | Highest Quartile of Airway-to-Lung Ratio | Lowest Quartile of Airway-To-Lung Ratio | Relative Difference between Highest and Lowest Quartile | P Value Interaction* | |
| PM2.5 (per IQR increase = 2.4 μg/m3) |
1.22 (0.89 to 1.69) | 0.96 (0.62 to 1.48) | 1.79 (1.11 to 2.88) | 1.87 (1.12 to 3.13) | 0.015 |
| NO2 (per IQR increase = 9.2 ppb) |
1.18 (0.8 to 1.75) | 1.04 (0.58 to 1.89) | 1.55 (0.90 to 2.65) | 1.48 (0.78 to 2.81) | 0.220 |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; IQR = interquartile range; NO2 = nitrogen dioxide; OR = odds ratio; PM2.5 = fine particulate matter.
Highest versus lowest airway-to-lung ratio quartiles. All models are adjusted for age, sex, body mass index, educational level (high school or lower vs. postsecondary education), tobacco status (never smoker, former smoker, or current smoker), pack-years smoking, environmental tobacco smoke at home, biomass fuel exposure, respiratory medication intake, dysanapsis (quartiles), and Canadian Cohort Obstructive Lung Disease study site. Statistically significant results are shown in bold.
To test for interactions, a product term was included between air pollution and airway-to-lung ratio quartiles.
Discussion
In this cross-sectional analysis of the CanCOLD study, a pan-Canadian population-based cohort, we demonstrated associations between ambient air pollution exposure and lower lung function. Notably, ambient PM2.5 and NO2 air pollution exposure were associated with lower FEV1, even at low concentrations relative to many countries across the world. Positive associations were also seen between outdoor air pollution exposure and COPD prevalence, though results did not reach statistical significance. No statistically significant interactions between air pollution and dysanapsis were found when modeling the airway-to-lung ratio as a continuous variable. However, individuals in the lowest quartile of the airway-to-lung ratio were more susceptible to the impacts of PM2.5 exposure on lung function and COPD than those in the highest airway-to-lung ratio quartile.
To our knowledge, this is the first study in Canada to examine associations of long-term ambient air pollution exposure with lung function and spirometrically confirmed COPD. Our findings are supported by other Canadian studies that used health administrative data to indirectly ascertain COPD. A recent study by Shin and colleagues following 5.1 million adults in the province of Ontario for a period of 15 years found positive associations between PM2.5 and NO2 and incident COPD identified from health administrative data (14). Positive associations between long-term ambient air pollution exposure and incident COPD derived from health administrative database records have also been reported amongst individuals living in Toronto (28) and Vancouver (29).
The current study showed a 101.7 ml (95% CI, −166.2 to −37.2) lower FEV1 per 2.4 μg/m3 increase in ambient PM2.5 concentrations and positive though nonsignificant associations with COPD prevalence. Associations between PM2.5 and FEV1 are consistent, though generally larger when compared to results from previous studies. A recent cross-sectional study of United Kingdom Biobank participants found an 83.13 ml lower FEV1 per 5 μg/m3 increase in PM2.5 concentrations (13). This study also showed higher odds of COPD with increases in PM2.5 (13). A meta-analysis of five European cohorts by Adam and colleagues found a 13.98 ml lower FEV1 per 10 μg/m3 increment in NO2 but no significant associations with PM2.5 (30). Using four of these same cohorts, a meta-analysis by Schikowski and colleagues did not find any statistically significant associations between either NO2 or PM2.5 air pollution exposure and the prevalence or incidence of COPD (31). Finally, using data from the U.S. Framingham Heart Study, Rice and colleagues found a 13.5 ml lower FEV1 per 2 μg/m3 increase in PM2.5 but no associations between PM2.5 concentrations and COPD (32). The higher associations found in our study might partially be explained by the older population in the CanCOLD cohort relative to previous studies. Research has shown that older individuals are more susceptible to the long-term effects of air pollution exposure on lung function (33), respiratory symptoms (34), and respiratory mortality (35, 36). Adjustment for the CanCOLD site might also have contributed to stronger associations; when the CanCOLD site variable was removed from our models, results were attenuated and overlapped with CIs of previous studies. Weaker associations when leaving out site adjustment might indicate that within-city contrasts in air pollution are responsible for most of the impact of air pollution on lung health. This, in turn, highlights the need for more research on local traffic-related air pollution exposure, which is the dominant contributor to intraurban variations in pollutant concentrations in these cities.
To minimize the health impacts of air pollution, the World Health Organization (WHO) established a threshold of 10 µg/m3 for annual mean concentrations of PM2.5 in 2005. Mean annual PM2.5 concentrations in the CanCOLD population were below 2005 WHO air quality guidelines and well below the mean annual concentrations seen in many international cities (Figure 3). This indicates that even at relatively low concentrations, ambient air pollution exposure impacts lung health. Consistent with our results, a recent pooled analysis of COPD hospital discharge data from three cohorts in Denmark and Sweden found statistically significant positive associations between long-term exposure to PM2.5 and NO2 and COPD development, even at concentrations below the current European Union, United States, and WHO guidelines (37). Similar conclusions of adverse effects of low-concentration PM2.5 air pollution on COPD risk have been reported in the Canadian study by Shin and colleagues (14). Such findings have informed revisions to WHO air quality guidelines. In September 2021, the WHO released new air quality guidelines, recommending annual average thresholds of 5 µg/m3 for PM2.5 (down from 10 µg/m3) and 10 µg/m3 for NO2 (down from 40 µg/m3). This study supports the decision to strengthen WHO guidelines as our results suggest efforts to adhere to these stricter air quality guidelines will help reduce the impacts of ambient air pollution on accelerated lung function decline and COPD burden.
Figure 3.
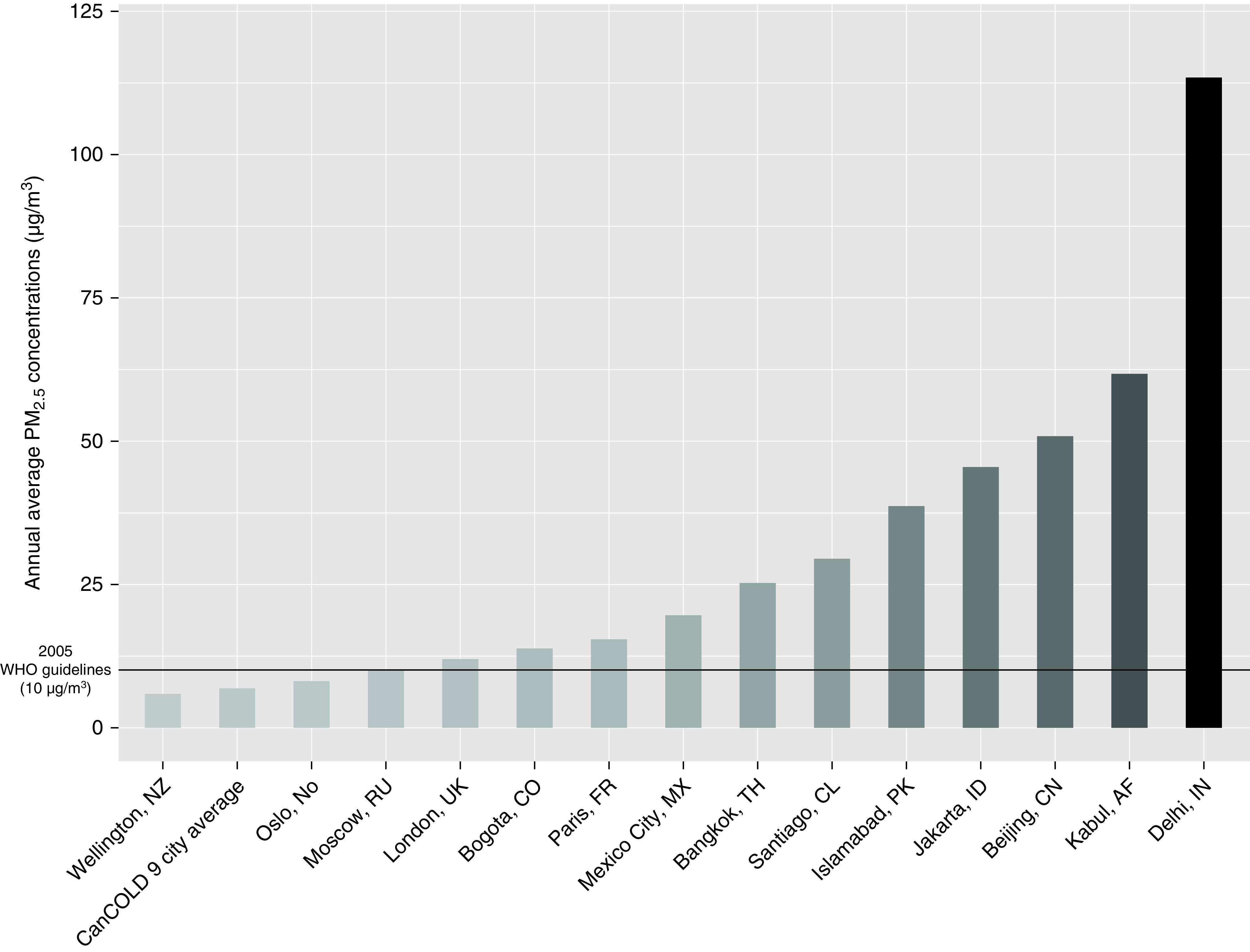
Annual average PM2.5 concentrations, CanCOLD study nine-city average and international capital cities. Adapted from IQAir (42). CanCOLD = Canadian Cohort Obstructive Lung Disease; PM2.5 = fine particulate matter; WHO = World Health Organization.
A recent analysis of the CanCOLD data with two other cohorts from the United States showed that a lower airway-to-lung ratio was associated with a significantly higher risk of COPD, even among never-smokers (17). For the first time, our results show that in addition to being a COPD risk factor, dysanapsis might also modify the impact of inhaled outdoor air pollutants on lung function and COPD. Lung morphology and fluid dynamics are major factors affecting particle deposition in the airways (19). Increased particulate velocities and greater airway impaction in individuals with smaller and narrower airways in proportion to lung volume might help explain the increased susceptibility to air pollution exposure amongst individuals with dysanaptic lungs seen in our study. Although air pollution exposure in the perinatal period and during childhood and adolescence has been shown to adversely affect lung function and lung development (10, 38), our study suggests that early-life lung development could also play a role in protecting from or increasing susceptibility to air pollution–induced lower lung function and COPD in adulthood.
The current study has many strengths, starting with the fact that it is the first to use a population-based cohort sample to investigate the relationship of ambient air pollution exposure with lung function and spirometrically defined COPD in Canada. Past Canadian studies exploring associations between air pollution exposure and COPD have generally made use of administrative health data, which have the advantage of very large sample sizes but can lead to over or underdiagnosis of COPD. Furthermore, such studies do not allow controlling for important individual-level confounders such as tobacco use. In addition, although most international studies with spirometry data have used prebronchodilator lung function, postbronchodilator spirometry testing in our study allowed us to better discriminate participants with reversible obstruction from those with limited airway reversibility, which mirrors the population of individuals most likely to have COPD. This is also the first study to date to examine interactions between air pollution and dysanapsis on lung function and COPD prevalence.
Among the limitations of this study, the cross-sectional analyses conducted herein do not allow the drawing of causal inferences concerning associations. Although CanCOLD has conducted two follow-up visits since the baseline assessment used in our study, the median follow-up time remains relatively short, at approximately 3 years. Longitudinal analyses will be conducted once sufficient follow-up time is accrued via a fourth insite assessment. Second, statistically significant interactions were observed only for the highest versus lowest quartiles of airway-to-lung ratio but not for intermediate quartiles or when modeling interactions as continuous linear terms. This may be because of a nonlinear or threshold of dysanapsis modifying the PM2.5 association with lung function or a chance observation, and therefore requires validation in an independent cohort. Also, our study only ascertained air pollution at the time of recruitment and residential location. No data on residential mobility in the years before enrollment were available. However, we do not believe this is a major limitation in the current study of older Canadians. According to Canadian census data, individuals aged 15 to 40 years old constitute the majority of internal migration in Canada, whereas those aged 40 years or more show relatively low residential mobility rates (39). Nonetheless, although we assumed exposure at the baseline residential address of CanCOLD participants is representative of recent past exposures, this is not necessarily the case, as some participants might have moved shortly before study enrollment.
Cumulative air pollution exposures over many years and assessment of time-activity patterns of study participants would help reduce the potential for exposure misclassification. Further, the 1 × 1-kilometer grids used to estimate PM2.5 concentrations are likely the coarsest resolution useful in characterizing within-city variations in ambient PM2.5 concentrations in larger Canadian cities. Although modeling PM2.5 at a finer spatial scale could sharpen the assessment of within-city contrasts, at this scale, it would be less clear what aspects of the urban air pollution mixture such an empirical model is actually representing (i.e., some specific component of PM2.5 and/or some covarying gaseous pollutants). Finally, we cannot rule out the possibility that the association between the presence of COPD and our index of dysanapsis was, in part, artifactual with our indices caused by the anatomic impact of COPD. We think this is unlikely, however, as the index of dysanapsis used in the study has 1) been shown to be associated with COPD independent of tobacco smoking and other standard COPD risk factors (17); and 2) extends to the peripheral airway tree (40) (the primary site of noxious particulate-associated COPD pathobiology) (41). Although COPD is associated with larger lung volumes, most individuals with COPD in our study had mild disease (56%) (Table 1) and a low likelihood of hyperinflation.
Conclusions
Chronic exposure to ambient air pollution is associated with clinically relevant lower lung function, even at relatively low concentrations. We also observed stronger relationships between ambient PM2.5 exposure and both FEV1 and COPD among those in the lowest than highest airways relative to lung size quartiles, indicating that there is a positive interaction between dysanapsis and air pollution. Our results, therefore, suggest that individuals with dysanaptic lung growth might be particularly susceptible to inhaled air pollutants. These new findings highlight the need to further reduce ambient air pollution concentrations and can help inform clinical management by identifying individuals with increased susceptibility to air pollutants. External validation of our results using other cohorts with computed tomography scans and spirometry data should be pursued. Longitudinal analyses are also needed to further examine these relationships.
Acknowledgments
Acknowledgment
The authors thank CanCOLD (Canadian Cohort Obstructive Lung Disease) study participants and individuals in the CanCOLD Collaborative Research Group. Fine particulate matter, nitrogen dioxide, and neighborhood material deprivation data indexed to DMTI Spatial Inc. postal codes were provided by the CANUE (Canadian Urban Environmental Health Research) Consortium.
CanCOLD Collaborative Research Group: Executive Committee: Jean Bourbeau, McGill University, Montreal, Québec, Canada; Wan C. Tan, the University of British Columbia (UBC), Vancouver, British Columbia, Canada; J. Mark FitzGerald, UBC, Vancouver, British Columbia, Canada; Don D. Sin, UBC, Vancouver, British Columbia, Canada; Darcy D. Marciniuk, University of Saskatoon, Saskatoon, Saskatchewan, Canada; Denis E. O’Donnell, Queen's University, Kingston, Ontario, Canada; Paul Hernandez, Dalhousie University, Halifax, Nova Scotia, Canada; Kenneth R. Chapman, University of Toronto, Toronto, Ontario, Canada; Brandie Walker, University of Calgary, Calgary, Alberta, Canada; Shawn Aaron, University of Ottawa, Ottawa, Ontario, Canada; and François Maltais, University of Laval, Québec City, Québec, Canada. International Advisory Board: Jonathon Samet, Keck School of Medicine of the University of Southern California, Los Angeles, California; Milo Puhan, Johns Hopkins School of Public Health, Baltimore, Maryland; Qutayba Hamid, McGill University, Montreal, Québec, Canada; and James C. Hogg, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada. Operations Center: Jean Bourbeau, University of McGill, Montreal, Québec, Canada; Dany Doiron, University of McGill, Montreal, Québec, Canada; Palmina Mancino, University of McGill, Montreal, Québec, Canada; Pei Zhi Li, University of McGill, Montreal, Québec, Canada; Dennis Jensen, University of McGill, Montreal, Québec, Canada; Carolyn Baglole, University of McGill, Montreal, Québec, Canada; Yvan Fortier, Laboratoire Telematique Respiratory Health Network, FRQS, Montreal, Québec, Canada; Wan C. Tan (co-PI), James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Don Sin, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Julia Yang, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Jeremy Road, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Joe Comeau, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Adrian Png, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Kyle Johnson, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Harvey Coxson, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Miranda Kirby, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Jonathon Leipsic, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; and Cameron Hague, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada. Economic Core: Mohsen Sadatsafavi, UBC, Vancouver, British Columbia, Canada. Public Health Core: Teresa To, University of Toronto, Toronto, Ontario, Canada; and Andrea Gershon, University of Toronto, Toronto, Ontario, Canada. Data Management and Quality Control: Wan C. Tan, UBC, Vancouver, British Columbia, Canada; Harvey Coxson, UBC, Vancouver, British Columbia, Canada; Jean Bourbeau, McGill University, Montreal, Québec, Canada; Pei Zhi Li, McGill University, Montreal, Québec, Canada; Zhi Song, McGill University, Montreal, Québec, Canada; Andrea Benedetti, McGill University, Montreal, Québec, Canada; Dennis Jensen, McGill University, Montreal, Québec, Canada; and Yvan Fortier, Laboratoire Telematique Respiratory Health Network, FRQS, Montreal, Québec, Canada. Field Centers: Wan C. Tan (PI), James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Christine Lo, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Sarah Cheng, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Elena Un, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Cynthia Fung, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Wen Tiang Wang, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Liyun Zheng, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Faize Faroon, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Olga Radivojevic, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Sally Chung, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Carl Zou, James Hogg Research Centre, UBC, Vancouver, British Columbia, Canada; Jean Bourbeau (PI), University of Toronto, Toronto, Ontario, Canada; Palmina Mancino, University of Toronto, Toronto, Ontario, Canada; Jacinthe Baril, University of Toronto, Toronto, Ontario, Canada; Laura Labonté, McGill University, Montreal, Québec, Canada; Kenneth Chapman (PI), University of Toronto, Toronto, Ontario, Canada; Patricia McClean, University of Toronto, Toronto, Ontario, Canada; Nadeen Audisho, University of Toronto, Toronto, Ontario, Canada; Brandie Walker (PI), University of Calgary, Calgary, Alberta, Canada; Curtis Dumonceaux, University of Calgary, Calgary, Alberta, Canada; Lisette Machado, University of Calgary, Calgary, Alberta, Canada; Paul Hernandez (PI), Dalhousie University and Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia, Canada; Scott Fulton, Dalhousie University and Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia, Canada; Kristen Osterling, Dalhousie University and Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia, Canada; Denise Wigerius, Dalhousie University and Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia, Canada; Shawn Aaron (PI), University of Ottawa, Ottawa, Ontario, Canada; Kathy Vandemheen, University of Ottawa, Ottawa, Ontario, Canada; Gay Pratt, University of Ottawa, Ottawa, Ontario, Canada; Amanda Bergeron, University of Ottawa, Ottawa, Ontario, Canada; Denis O'Donnell (PI), Queen's University, Kingston, Ontario, Canada; Sandra Vincent, Queen's University, Kingston, Ontario, Canada; Matthew McNeil, Queen's University, Kingston, Ontario, Canada; Kate Whelan, Queen's University, Kingston, Ontario, Canada; François Maltais (PI), University of Laval, Québec City, Québec, Canada; Cynthia Brouillard, University of Laval, Québec City, Québec, Canada; Darcy Marciniuk (PI), University of Saskatchewan, Saskatoon, Saskatchewan, Canada; Ron Clemens, University of Saskatchewan, Saskatoon, Saskatchewan, Canada; Janet Baran, University of Saskatchewan, Saskatoon, Saskatchewan, Canada; and Candice Leuschen, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
Footnotes
A complete list of CanCOLD Collaborative Research Group members may be found before the beginning of the References.
Supported by the Canadian Institutes of Health Research (453225) and GlaxoSmithKline (213021).
Author Contributions: J.B. and D.D. developed the protocol, analyzed the data, and drafted the original version of the manuscript. J.B., D.D., S.B., B.M.S., A.B., J.R.B., S.D.A., K.R.C., P.H., F.M., D.D.M., D.O’D., D.D.S., B.W., L.D., G.N., V.C., C.C., B.E.M., and W.C.T. made substantial contributions to the design of the study, interpretation of the data, and revision of the manuscript for important intellectual content and approved the final version submitted for publication. All authors have given agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the study are appropriately investigated and resolved.
Data Sharing: The CanCOLD (Canadian Cohort Obstructive Lung Disease) study makes deidentified data available for research on respiratory health. Information on how to submit a data access application can be found on the CanCOLD website at www.cancold.ca.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202106-1439OC on April 5, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
for the CanCOLD Collaborative Research Group and the Canadian Respiratory Research Network:
Jean Bourbeau, Wan C. Tan, J. Mark FitzGerald, Don D. Sin, Darcy D. Marciniuk, Denis E. O’Donnell, Paul Hernandez, Kenneth R. Chapman, Brandie Walker, Shawn Aaron, François Maltais, Jonathon Samet, Milo Puhan, Qutayba Hamid, James C. Hogg, Dany Doiron, Palmina Mancino, Pei Zhi Li, Dennis Jensen, Carolyn Baglole, Yvan Fortier, Julia Yang, Jeremy Road, Joe Comeau, Adrian Png, Kyle Johnson, Harvey Coxson, Miranda Kirby, Jonathon Leipsic, Cameron Hague, Mohsen Sadatsafavi, Teresa To, Andrea Gershon, Zhi Song, Andrea Benedetti, Christine Lo, Sarah Cheng, Elena Un, Cynthia Fung, Wen Tiang Wang, Liyun Zheng, Faize Faroon, Olga Radivojevic, Sally Chung, Carl Zou, Jacinthe Baril, Laura Labonté, Patricia McClean, Nadeen Audisho, Brandie Walker, Curtis Dumonceaux, Lisette Machado, Paul Hernandez, Scott Fulton, Kristen Osterling, Denise Wigerius, Shawn Aaron, Kathy Vandemheen, Gay Pratt, Amanda Bergeron, Sandra Vincent, Matthew McNeil, Kate Whelan, François Maltais, Cynthia Brouillard, Ron Clemens, Janet Baran, and Candice Leuschen
References
- 1.Canadian Institutes for Health Information. Inpatient hospitalization, surgery, newborn, alternate level of care and childbirth statistics, 2017–2018. Ottawa, Ontario, Canada: Canadian Institutes for Health Information; 2019. [Google Scholar]
- 2. Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al. GBD 2015 Chronic Respiratory Disease Collaborators Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med . 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet . 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 4.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020
- 5. Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, et al. Committee on Nonsmoking COPD, Environmental and Occupational Health Assembly An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 6. Leung C, Bourbeau J, Sin DD, Aaron SD, FitzGerald JM, Maltais F, et al. CanCOLD Collaborative Research Group The prevalence of chronic obstructive pulmonary disease (COPD) and the heterogeneity of risk factors in the Canadian population: results from the Canadian Obstructive Lung Disease (COLD) study. Int J Chron Obstruct Pulmon Dis . 2021;16:305–320. doi: 10.2147/COPD.S285338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schikowski T, Mills IC, Anderson HR, Cohen A, Hansell A, Kauffmann F, et al. Ambient air pollution: a cause of COPD? Eur Respir J . 2014;43:250–263. doi: 10.1183/09031936.00100112. [DOI] [PubMed] [Google Scholar]
- 8. Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet . 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kulkarni N, Pierse N, Rushton L, Grigg J. Carbon in airway macrophages and lung function in children. N Engl J Med . 2006;355:21–30. doi: 10.1056/NEJMoa052972. [DOI] [PubMed] [Google Scholar]
- 10. Gauderman WJ, McConnell R, Gilliland F, London S, Thomas D, Avol E, et al. Association between air pollution and lung function growth in southern California children. Am J Respir Crit Care Med . 2000;162:1383–1390. doi: 10.1164/ajrccm.162.4.9909096. [DOI] [PubMed] [Google Scholar]
- 11. Milanzi EB, Koppelman GH, Smit HA, Wijga AH, Oldenwening M, Vonk JM, et al. Air pollution exposure and lung function until age 16 years: the PIAMA birth cohort study. Eur Respir J . 2018;52:1800218. doi: 10.1183/13993003.00218-2018. [DOI] [PubMed] [Google Scholar]
- 12. Götschi T, Heinrich J, Sunyer J, Künzli N. Long-term effects of ambient air pollution on lung function: a review. Epidemiology . 2008;19:690–701. doi: 10.1097/EDE.0b013e318181650f. [DOI] [PubMed] [Google Scholar]
- 13. Doiron D, de Hoogh K, Probst-Hensch N, Fortier I, Cai Y, De Matteis S, et al. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J . 2019;54:1802140. doi: 10.1183/13993003.02140-2018. [DOI] [PubMed] [Google Scholar]
- 14. Shin S, Bai L, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, et al. Air pollution as a risk factor for incident chronic obstructive pulmonary disease and asthma. A 15-year population-based cohort study. Am J Respir Crit Care Med . 2021;203:1138–1148. doi: 10.1164/rccm.201909-1744OC. [DOI] [PubMed] [Google Scholar]
- 15. Wang M, Aaron CP, Madrigano J, Hoffman EA, Angelini E, Yang J, et al. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function. JAMA . 2019;322:546–556. doi: 10.1001/jama.2019.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doiron D, Bourbeau J, de Hoogh K, Hansell AL. Ambient air pollution exposure and chronic bronchitis in the Lifelines cohort. Thorax . 2021;76:772–779. doi: 10.1136/thoraxjnl-2020-216142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith BM, Kirby M, Hoffman EA, Kronmal RA, Aaron SD, Allen NB, et al. Mesa Lung, CanCOLD, and SPIROMICS Investigators Association of dysanapsis with chronic obstructive pulmonary disease among older adults. JAMA . 2020;323:2268–2280. doi: 10.1001/jama.2020.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mirabelli MC, Künzli N, Avol E, Gilliland FD, Gauderman WJ, McConnell R, et al. Respiratory symptoms following wildfire smoke exposure: airway size as a susceptibility factor. Epidemiology . 2009;20:451–459. doi: 10.1097/EDE.0b013e31819d128d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hofmann W. Modelling inhaled particle deposition in the human lung—a review. J Aerosol Sci . 2011;42:693–724. [Google Scholar]
- 20. Bourbeau J, Tan WC, Benedetti A, Aaron SD, Chapman KR, Coxson HO, et al. Cancold Study Group Canadian Cohort Obstructive Lung Disease (CanCOLD): fulfilling the need for longitudinal observational studies in COPD. COPD . 2014;11:125–132. doi: 10.3109/15412555.2012.665520. [DOI] [PubMed] [Google Scholar]
- 21. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J . 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 22. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med . 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 23. Brook JR, Setton EM, Seed E, Shooshtari M, Doiron D, et al. CANUE – The Canadian Urban Environmental Health Research Consortium The Canadian Urban Environmental Health Research Consortium - a protocol for building a national environmental exposure data platform for integrated analyses of urban form and health. BMC Public Health . 2018;18:114. doi: 10.1186/s12889-017-5001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Donkelaar A, Martin RV, Li C, Burnett RT. Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ Sci Technol . 2019;53:2595–2611. doi: 10.1021/acs.est.8b06392. [DOI] [PubMed] [Google Scholar]
- 25. Hystad P, Setton E, Cervantes A, Poplawski K, Deschenes S, Brauer M, et al. Creating national air pollution models for population exposure assessment in Canada. Environ Health Perspect . 2011;119:1123–1129. doi: 10.1289/ehp.1002976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pampalon R, Hamel D, Gamache P, Philibert MD, Raymond G, Simpson A. An area-based material and social deprivation index for public health in Québec and Canada. Can J Public Health . 2012;103:S17–S22. doi: 10.1007/BF03403824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team. R: a language and environment for statistical computing. 3.6.0 ed. Vienna, Austria: 2019. [Google Scholar]
- 28. Weichenthal S, Bai L, Hatzopoulou M, Van Ryswyk K, Kwong JC, Jerrett M, et al. Long-term exposure to ambient ultrafine particles and respiratory disease incidence in Toronto, Canada: a cohort study. Environ Health . 2017;16:64. doi: 10.1186/s12940-017-0276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gan WQ, FitzGerald JM, Carlsten C, Sadatsafavi M, Brauer M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med . 2013;187:721–727. doi: 10.1164/rccm.201211-2004OC. [DOI] [PubMed] [Google Scholar]
- 30. Adam M, Schikowski T, Carsin AE, Cai Y, Jacquemin B, Sanchez M, et al. Adult lung function and long-term air pollution exposure. ESCAPE: a multicentre cohort study and meta-analysis. Eur Respir J . 2015;45:38–50. doi: 10.1183/09031936.00130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schikowski T, Adam M, Marcon A, Cai Y, Vierkötter A, Carsin AE, et al. Association of ambient air pollution with the prevalence and incidence of COPD. Eur Respir J . 2014;44:614–626. doi: 10.1183/09031936.00132213. [DOI] [PubMed] [Google Scholar]
- 32. Rice MB, Ljungman PL, Wilker EH, Dorans KS, Gold DR, Schwartz J, et al. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am J Respir Crit Care Med . 2015;191:656–664. doi: 10.1164/rccm.201410-1875OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Forbes LJL, Kapetanakis V, Rudnicka AR, Cook DG, Bush T, Stedman JR, et al. Chronic exposure to outdoor air pollution and lung function in adults. Thorax . 2009;64:657–663. doi: 10.1136/thx.2008.109389. [DOI] [PubMed] [Google Scholar]
- 34. Doiron D, de Hoogh K, Probst-Hensch N, Mbatchou S, Eeftens M, Cai Y, et al. Residential air pollution and associations with wheeze and shortness of breath in adults: a combined analysis of cross-sectional data from two large European cohorts. Environ Health Perspect . 2017;125:097025. doi: 10.1289/EHP1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Naess Ø, Nafstad P, Aamodt G, Claussen B, Rosland P. Relation between concentration of air pollution and cause-specific mortality: four-year exposures to nitrogen dioxide and particulate matter pollutants in 470 neighborhoods in Oslo, Norway. Am J Epidemiol . 2007;165:435–443. doi: 10.1093/aje/kwk016. [DOI] [PubMed] [Google Scholar]
- 36. Fischer P, Hoek G, Brunekreef B, Verhoeff A, van Wijnen J. Air pollution and mortality in the Netherlands: are the elderly more at risk? Eur Respir J Suppl . 2003;40:34s–38s. doi: 10.1183/09031936.03.00402503. [DOI] [PubMed] [Google Scholar]
- 37. Liu S, Jørgensen JT, Ljungman P, Pershagen G, Bellander T, Leander K, et al. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: the ELAPSE project. Environ Int . 2021;146:106267. doi: 10.1016/j.envint.2020.106267. [DOI] [PubMed] [Google Scholar]
- 38. Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med . 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 39.Statistics Canada. Internal migration: overview, 2015/2016. Ottawa, Ontario, Canada: Statistics Canada; 2018. [Google Scholar]
- 40. Vameghestahbanati M, Kirby M, Tanabe N, Vasilescu DM, Janssens W, Everaerts S, et al. Central airway tree dysanapsis extends to the peripheral airways. Am J Respir Crit Care Med . 2021;203:378–381. doi: 10.1164/rccm.202007-3025LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med . 1968;278:1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 42.IQAir. 2018 World air quality report: region & city PM2.5 ranking; 2018 [accessed 2021 Mar]. https://www.iqair.com/world-most-polluted-cities/world-air-quality-report-2018-en.pdf [Google Scholar]