To the Editor:
Coronavirus disease (COVID-19)-related acute respiratory distress syndrome (C-ARDS) is believed to be associated with prolonged mechanical ventilation (MV) (1), unlike non–severe acute respiratory syndrome coronavirus 2 (non–SARS-CoV-2) viral ARDS (NC-ARDS) (2), possibly because of weaning difficulty or unreadiness (too early to wean). To compare MV duration and its partitions (weaning unreadiness: from intubation to weaning start; weaning phase: from weaning start to successfully weaned) between C-ARDS and NC-ARDS, we conducted an observational study to assess probabilities of starting MV weaning and of being successfully weaned over time, using a multistate approach (3). Some data of the present cohort were reported in a previous study on ventilator-associated pneumonia (VAP) (4).
Methods
Setting and patients
All patients referred to the medical ICU of a French tertiary hospital between October 1, 2009, and April 29, 2020, for viral ARDS requiring MV for >48 hours were included. ARDS diagnosis satisfied the Berlin definition. Patients with C-ARDS were those with ARDS and a positive SARS-CoV-2 PCR test result. Patients with NC-ARDS had ARDS and a positive PCR test result for other ARDS-causing respiratory viruses (4). This study was approved by the institutional review board of the French Intensive Care Medicine Society (Comité d’éthique Société de Réanimation de Langue Française 20-45), and informed consent was waived.
MV and weaning
Patients with ARDS received MV following a standardized protective ventilation strategy, as well as rescue therapies following the national guidelines (5). The nurse-driven sedation and weaning protocols did not significantly change over the study period. Classical weaning readiness criteria were checked in our routine daily practice. Criteria of spontaneous breathing trial (SBT) failure were those recommended in international guidelines (6). Once they succeeded in the trial, patients were extubated if cough and alertness were deemed adequate (6). Weaning start and success were defined as per WIND (Weaning Outcome according to a New Definition) criteria (7). Start of weaning implies any kind of separation attempt from MV: for intubated patients, an SBT with or without extubation, or an extubation directly performed without identified SBT; for tracheotomized patients, one or several consecutive days with spontaneous nonmechanical ventilation through tracheostomy. Patients were followed up until Day 90 after ICU admission.
Statistical analysis
The sample size was not calculated a priori; we considered the number of patients treated during the study period instead. Results are reported as median and interquartile range (25th–75th percentiles) or numbers with percentages. Initial bivariate comparisons were conducted using χ2 or Fisher exact tests for categorical data and Mann-Whitney U test for continuous data. The primary endpoint analysis compared ventilation duration (until Day 90 after ICU admission) and its components (weaning unreadiness phase and weaning phase) between patients with C-ARDS and NC-ARDS using multistate models. As the risk of prolonged ventilation accrues over time of survival, death was deemed a competing risk for MV weaning. Univariate and multivariable multistate models were built for transition between weaning unreadiness, MV weaning, successfully weaned, and death, the competing risk. Cause-specific hazard regression models were then introduced to study the potential impact of factors on each transition hazard. Proportional hazards assumption for the covariate was evaluated, and a time-dependent correction coefficient was applied wherever appropriate (8). In the sensitivity analysis, weaning start was defined as the switch to pressure-support ventilation mode with FiO2 ⩽ 50% and positive end-expiratory pressure < 8 cm H2O.
Stacked prediction probabilities of transitions were plotted. Multistate analysis was performed using R 3.1.2 package mstate (9) (R Foundation for Statistical Computing). The other analyses were conducted on SPSS Base 21.0 statistics software package (SPSS Inc.). Two-sided P values < 0.05 were considered significant.
Results
Patients and outcomes
Ninety patients with C-ARDS and 82 patients with NC-ARDS (influenza [n = 48], respiratory syncytial virus [n = 15], influenza–respiratory syncytial virus coinfection [n = 2], endemic human coronavirus [n = 5], metapneumovirus [n = 5], parainfluenza [n = 5], and adenovirus [n = 2]) were included. At admission, bacterial coinfection was evidenced in 14 (16%) patients with C-ARDS and 39 (47%) patients with NC-ARDS (24 had influenza, and 15 had other viruses). Patients with C-ARDS, compared with NC-ARDS, showed longer intubation-to–weaning start intervals (16.0 [10.0–29.0] vs. 6.0 [4.0–10.0] d; P < 0.001) and intubation-to–successful weaning intervals (20.0 [13.0–43.0] vs. 9.0 [5.2–15.0] d; P < 0.001) and were often tracheostomized (14 [15.6%] vs. 4 [4.9%]; P = 0.02). Both groups had similar weaning start–to–successful weaning time (2.0 [1.0–9.0] vs. 2.0 [1.0–4.0] d; P = 0.5) (Figure 1). Patients with C-ARDS lived fewer days without MV than those with NC-ARDS, at Day 60 and Day 90: 7.0 [0–42.0] vs. 43.0 [0–52.0] d; P = 0.0001; and 37.0 [0–72.0] vs. 73.0 [0–82.0] d; P = 0.003, respectively. ICU length of stay was longer in C-ARDS survivors (30 [19–45] vs. 15 [10–20] d; P < 0.001), whereas Day 90 mortality was similar in both groups (36 [40%] in C-ARDS vs. 28 [34%)] in NC-ARDS; P = 0.4).
Figure 1.
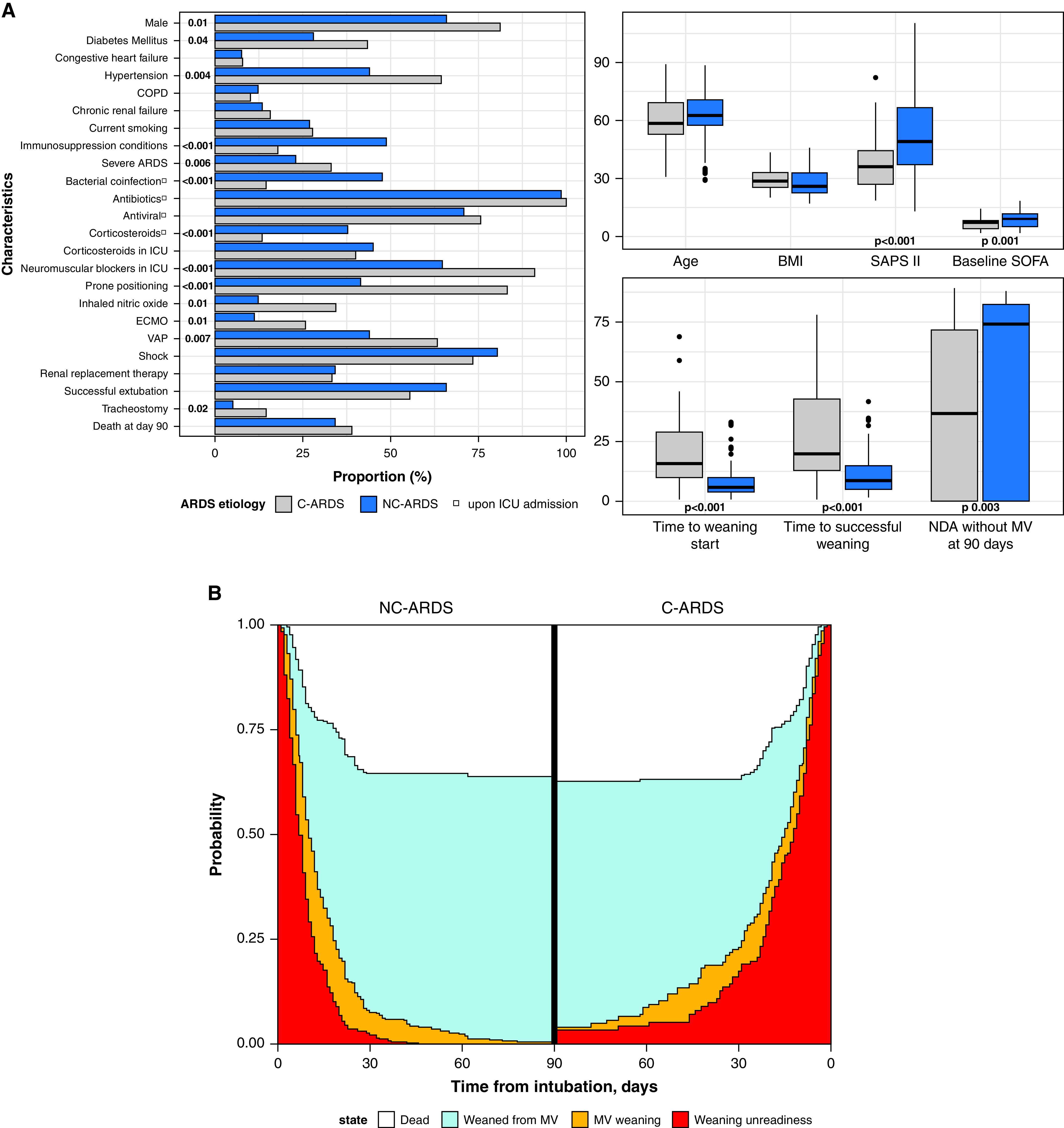
(A) Patient characteristics and (B) stacked predicted probabilities of state transition in patients with viral acute respiratory distress syndrome (ARDS), according to coronavirus disease (COVID-19) status. Values at the left of the stacked bars represent statistically significant P values. BMI = body mass index; C-ARDS = COVID-19–related ARDS; COPD = chronic obstructive pulmonary disease; ECMO = extracorporeal membrane oxygenation; MV = mechanical ventilation; NC-ARDS = non–COVID-19 related ARDS; NDA = number of days alive; SAPS II = Simplified Acute Physiology Score II; SOFA = Sequential Organ Failure Assessment; VAP = ventilator-associated pneumonia.
Multistate analysis
Stacked predicted probabilities of transitions in mechanically ventilated patients with viral ARDS are displayed in Figure 1. The probability of transition from weaning unreadiness to MV weaning was lower in patients with C-ARDS than in those with NC-ARDS, whereas the transition probability from MV weaning to successfully weaned was similar. In the multivariable analysis, C-ARDS was independently associated with a reduced likelihood of weaning unreadiness–to–MV weaning transition, hence the prolonged weaning unreadiness, together with higher VAP, use of proning, and extracorporeal membrane oxygenation (Table 1). Factors independently associated with a reduced likelihood of MV weaning–to–successfully weaned transition were VAP and corticosteroid use in the ICU (Table 1). In the sensitivity analysis, where switch to pressure support represented MV weaning start, C-ARDS was independently associated with a reduced likelihood of weaning unreadiness–to–MV weaning but not MV weaning–to–successfully weaned. Such associations persisted even upon forcing age and chronic obstructive pulmonary disease into the multivariable model or keeping oseltamivir/zanamivir as the only antiviral treatment or removing antiviral treatment from the model.
Table 1.
Statistically Significant Factors as Shown by Univariate and Multivariable Analysis of Transitions during Mechanical Ventilation in Patients with Viral Acute Respiratory Distress Syndrome
| Variable | Transition from Weaning Unreadiness to Weaning |
Transition from MV Weaning to Weaned |
||
|---|---|---|---|---|
| Univariate* | Multivariable | Univariate* | Multivariable | |
| C-ARDS† | 0.56 (0.45–0.69); P < 0.001 | 0.42 (0.27–0.67); P = 0.0002 | 0.71 (0.58–0.86); P < 0.001 | NS |
| Immunosuppression | 1.07 (1.02–1.13); P = 0.009 | NS | NS | — |
| SAPS II score | 1.01 (1.00–1.02); P = 0.02 | NS | NS | — |
| Bacterial coinfection† | NS | — | 1.07 (1.02–1.13); P = 0.006 | NS |
| Antiviral treatment‡ | 0.76 (0.58–0.99); P = 0.05 | NS | 0.64 (0.48–0.86); P = 0.003 | NS |
| Steroids at admission | 1.66 (1.08–2.55); P = 0.02 | NS | 0.45 (0.28–0.73); P = 0.001 | NS |
| Steroids in ICU | NS | — | 0.36 (0.24–0.55); P < 0.001 | 0.47 (0.27–0.79); P = 0.005 |
| VAP | 0.61 (0.49–0.77); P < 0.001 | 0.58 (0.38–0.89); P = 0.01 | 0.46 (0.35–0.61); P < 0.001 | 0.5 (0.37–0.68); P < 0.001 |
| Prone positioning† | 0.41 (0.31–0.54); P < 0.001 | 0.62 (0.44–0.87); P = 0.005 | 0.52 (0.4–0.68); P < 0.001 | NS |
| Neuromuscular blockers in ICU | 0.52 (0.36–0.75); P = 0.0004 | NS | 0.54 (0.36–0.81); P = 0.003 | NS |
| ECMO† | 0.79 (0.7–0.88); P < 0.001 | 0.82 (0.73–0.93); P = 0.002 | 0.91 (0.84–0.99); P = 0.03 | NS |
| RRT | 0.5 (0.33–0.75); P = 0.0008 | NS | 0.58 (0.37–0.92); P = 0.02 | NS |
| Shock† | 0.63 (0.47–0.85); P = 0.002 | NS | 0.54 (0.4–0.74); P = 0.0001 | NS |
Definition of abbreviations: C-ARDS = coronavirus disease–related acute respiratory distress syndrome; ECMO = extracorporeal membrane oxygenation; MV = mechanical ventilation; NS = not significant; RRT = renal replacement therapy; SAPS II = Simplified Acute Physiology Score II; VAP = ventilator-associated pneumonia.
Data are presented as hazard ratio (95% confidence interval); P value. — indicates not included in multivariable analysis (not significant in univariate analysis).
The following variables did not reach statistical significance in univariate analysis for the two transitions: male sex, age, chronic obstructive pulmonary disease, Charlson score (without age), McCabe score, diabetes, cardiac failure, atrial fibrillation, hypertension, chronic renal failure, chronic renal replacement therapy, stroke, cirrhosis (Child C), smoking, shock at ICU admission, antibiotics at ICU admission, nitric oxide, Sequential Organ Failure Assessment score at ICU admission, creatinine at ICU admission, and PaO2/FiO2 ratio at Day 1.
Correction for nonproportional hazard.
Including oseltamivir/zanamivir, remdesivir, lopinavir/ritonavir, or hydroxychloroquine.
Discussion
In this study, we have evidenced that patients with C-ARDS endured a longer MV duration and a lower likelihood of transition from weaning unreadiness to MV weaning than patients with NC-ARDS.
The prolonged MV we observed in C-ARDS is consistent with multicenter cohort results (1). During C-ARDS, the uncontrolled innate and impaired adaptive immune responses may impede recovery of lung injury (10) and trigger VAP (4), which jeopardizes weaning readiness. Other factors potentially involved in prolonging C-ARDS dependence on artificial ventilation include progressing fibrosis of lung injury and persistent abnormally high respiratory drive (11), which hinders switching to partial ventilatory mode. This prolongation could also in part be explained by the different managements of C-ARDS (e.g., higher doses of sedation or paralysis given independently of case severity).
Our study strength comes from the detailed characterization of weaning readiness and successfulness and the use of multivariable multistate models to properly partition MV duration. The study limitations include its monocentric design, limited sample size, and long duration (time between inclusion of patients with NC-ARDS and C-ARDS), with probable adaptations of ventilation care (e.g., less experienced bedside staff during COVID-19 pandemic). Moreover, missing values precluded the inclusion of neuromuscular blocking duration in the final model. Our seminal partition of MV into weaning unreadiness and weaning phases may require external validation, although the same effects of C-ARDS on transition from weaning unreadiness to MV weaning, and from MV weaning to successfully weaned, were observed upon considering earlier MV weaning start.
In conclusion, we have observed that the prolonged MV duration in patients with C-ARDS, compared with NC-ARDS, is related to weaning unreadiness rather than to weaning prolongation. Further research should be focused on factors hastening COVID-19 healing in patients on MV.
Acknowledgments
Acknowledgment
The authors thank Jean Hazebroucq, Clement Ourghanlian, Christian Brun-Buisson, and Francois Hemery for their invaluable help.
Footnotes
Author Contributions: S.G.: study design, data analysis, data interpretation, and script writing. B.B., S.T., and M.D.: data acquisition and data interpretation. G.C., N.d.P., and K.R.: data analysis and interpretation. A.M.D.: study design, data analysis, data interpretation, and script writing. All authors revised the drafted manuscript, and all read and approved its final version.
Originally Published in Press as DOI: 10.1164/rccm.202108-1963LE on April 8, 2022
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med . 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rouzé A, Martin-Loeches I, Povoa P, Makris D, Artigas A, Bouchereau M, et al. coVAPid Study Group Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med . 2021;47:188–198. doi: 10.1007/s00134-020-06323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bluhmki T, Allignol A, Ruckly S, Timsit J-F, Wolkewitz M, Beyersmann J. Estimation of adjusted expected excess length-of-stay associated with ventilation-acquired pneumonia in intensive care: a multistate approach accounting for time-dependent mechanical ventilation. Biom J . 2018;60:1135–1150. doi: 10.1002/bimj.201700242. [DOI] [PubMed] [Google Scholar]
- 4. Razazi K, Arrestier R, Haudebourg AF, Benelli B, Carteaux G, Decousser J-W, et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to Coronavirus 19 disease. Crit Care . 2020;24:699. doi: 10.1186/s13054-020-03417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Papazian L, Aubron C, Brochard L, Chiche J-D, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care . 2019;9:69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quintard H, l’Her E, Pottecher J, Adnet F, Constantin JM, De Jong A, et al. Experts’ guidelines of intubation and extubation of the ICU patient of French Society of Anaesthesia and Intensive Care Medicine (SFAR) and French-Speaking Intensive Care Society (SRLF): in collaboration with the pediatric Association of French-Speaking Anaesthetists and Intensivists (ADARPEF), French-Speaking Group of Intensive Care and Paediatric Emergencies (GFRUP) and Intensive Care Physiotherapy Society (SKR) Ann Intensive Care . 2019;9:13. doi: 10.1186/s13613-019-0483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Béduneau G, Pham T, Schortgen F, Piquilloud L, Zogheib E, Jonas M, et al. WIND (Weaning according to a New Definition) Study Group and the REVA (Réseau Européen de Recherche en Ventilation Artificielle) Network Epidemiology of weaning outcome according to a new definition: the WIND study. Am J Respir Crit Care Med . 2017;195:772–783. doi: 10.1164/rccm.201602-0320OC. [DOI] [PubMed] [Google Scholar]
- 8.Therneau T, Crowson C, Atkinson E. Using time dependent covariates and time dependent coefficients in the Cox model. 2022. Available from: https://cran.r-project.org/web/packages/survival/vignettes/timedep.pdf
- 9. de Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed . 2010;99:261–274. doi: 10.1016/j.cmpb.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 10. Hue S, Beldi-Ferchiou A, Bendib I, Surenaud M, Fourati S, Frapard T, et al. Uncontrolled innate and impaired adaptive immune responses in patients with COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med . 2020;202:1509–1519. doi: 10.1164/rccm.202005-1885OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med . 2020;46:606–618. doi: 10.1007/s00134-020-05942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


