Abstract
Structural diversity in heterocyclic chemistry is key to unlocking new properties and modes of action. In this regard, heterocycles embedding emerging fluorinated substituents hold great promise. Herein is described a strategy to access 2-SF5-(aza)indoles for the first time. The sequence relies on the radical addition of SF5Cl to the alkynyl π-system of 2-ethynyl anilines followed by a cyclization reaction. A telescoped sequence is proposed, making this strategy very appealing and reproducible on a gram scale. Downstream functionalizations are also demonstrated, allowing an easy diversification of N- and C3-positions. Ames test, pKa, log P, and differential scanning calorimetry measurements of several fluorinated 2-Rf-indoles are also disclosed. These studies highlight the strategic advantages that a C2-pentafluorosulfanylated motif impart to a privileged scaffold such as an indole.
Keywords: structural diversity, fluorinated indoles, heterocyclic chemistry, pentafluorosulfanyl
Introduction
The incorporation of a fluorinated motif in organic or inorganic molecules influences chemical and physical properties (metabolic stability, bioavailability, pKa, etc), and this strategy is nowadays widely used in medicinal chemistry.1 Among the so-called “emerging” fluorinated groups, the pentafluorosulfanyl group (SF5)2,3 is of growing interest in heterocyclic synthesis,4−7 materials science,8 and medicinal chemistry.9 The SF5 group has a volume of 55.4 Å3, between the t-Bu (76.9 Å3) and CF3 (34.6 Å3) groups, and its unique octahedral geometry allows a more selective interaction of SF5-containing molecules with biological receptors.10−12 The high lipophilicity of SF5, expressed by the Hansch parameter (π = 1.23),2,13 is greater than the ones of CF3 (0.88) or OCF3 (1.04) groups and may confer an enhanced cell membrane permeating ability. The high electronegativity of SF5 expressed by the Hammett constant (σp = 0.68, σm = 0.61)2,13 is also greater than that of CF3 (σp = 0.53, σm = 0.43)2,13 which, in turn, confers high metabolic stability. All of these properties make SF5 an interesting alternative to the CF3 group as a bioisostere, especially in drug development.9,14−16
However, synthetic routes to SF5-containing compounds and their structural diversity remain highly challenging. Two general methods are reported for accessing SF5-containing small molecules. The first method is an oxidative fluorination reaction of (hetero)aromatic disulfides, thiols, or, more recently, sulfenyl phthalimides which give access to ClF4S- and then SF5-(hetero)aromatic compounds after a final chloride–fluoride exchange step.17−23 The second method is a direct introduction of the SF5 group to an alkyne, an alkene, or an α-diazo carbonyl thanks to the use of SF5Cl gas24 under radical conditions to yield SF5-containing compounds.25−28 Although the use of commercially available gaseous SF5Cl is atom economical and quite straightforward from a practical point of view, recent efforts toward its preparation from sulfur powder, potassium fluoride, and trichloroisocyanuric acid have been disclosed.29,30 Recently, SF6 was used as an alternative source of SF5• in photoredox catalysis, but this method up to now is limited to reactions with styrene derivatives.31−33
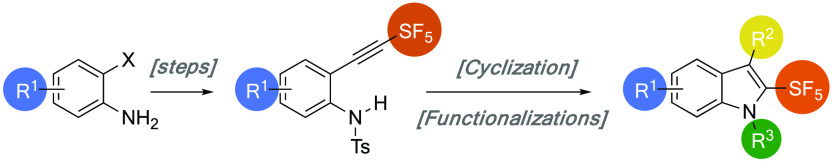
Indoles are privileged scaffolds in medicinal chemistry, and developing synthetic strategies to modulate their structures and physicochemical properties is of central importance.34−36 In this regard, combining indoles and original fluorinated moieties such as the pentafluorosulfanyl group is of interest as it would pave the way to structural and physicochemical studies that could have an impact in medicinal chemistry. Only a handful of 5-37−42 and 6-SF5-indoles43−45 are known and were obtained from commercially available SF5-anilines or nitrophenyls (Scheme 1B). However, introducing the SF5 group on other positions of the indole nucleus, and more precisely on the C2-position, which is as close as possible to the nitrogen atom, is highly challenging and still not reported. Among the different strategies to access C2-SF5-indoles, the intramolecular 5-endo-dig cyclization46−49 of an ortho-alkynylaniline appears to be the most promising (Scheme 1C). Indeed, SF5-substituted alkynes are easily prepared by the reaction between SF5Cl and a terminal alkyne under radical conditions followed by basic elimination, as demonstrated by Dolbier (Scheme 1A).25 In addition, Tsui reported that 2-CF3-indoles could be synthesized via a domino trifluoromethylation/5-endo-dig cyclization of ortho-alkynylanilines.49 Herein, we report that this strategic blueprint allows for a general synthesis of 2-SF5-indoles from readily available starting materials. Their thermal stabilities, pKa values, and lipophilicities were also studied and compared to more classical C2-fluorinated/fluoroalkylated indoles. Finally, evaluation of the mutagenic potential (Ames test) of a selection of 2-SF5-indoles was performed.
Scheme 1. State of the Art for the Introduction of the SF5 Group on Alkyne (A) and on the C5- or C6-Position of Indoles (B) and Proposed Synthetic Strategy for the Preparation of C2-SF5-Indole (C).
Results and Discussion
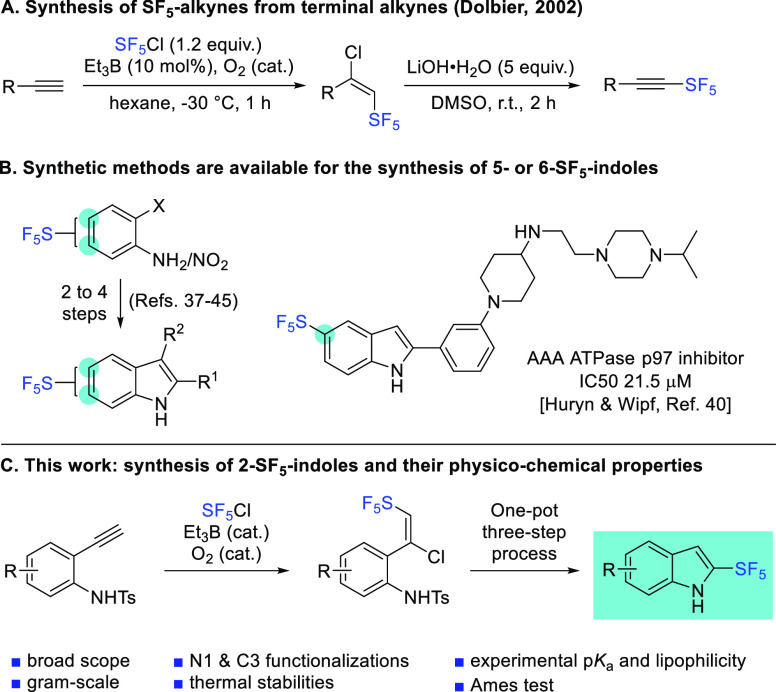
N-Tosyl-2-ethynylaniline 1a was selected as a model compound for the screening of chloropentafluorosulfanylation conditions (Scheme 2).50 Using catalytic amounts of triethylborane and oxygen,51,52 the reaction proceeded smoothly in ethyl acetate or dichloromethane (0.4 M) at −40 to −20 °C, delivering 2a in quantitative yield. Gratifyingly, a single regio- and stereoisomer was observed, with the structure of 2a being unambiguously confirmed by X-ray diffraction (CCDC 2073141).53 As reported by Paquin in 2019,54 several classical organic solvents are compatible with SF5Cl, and we found that ethyl acetate turned out to be the solvent of choice for the synthesis of 2a–p in terms of conversions and, more importantly, purity. Indeed, in most cases, no further purification of 2 is needed.55
Scheme 2. Scope and Limitations for the Addition of SF5Cl to 1.
Yields determined by 19F NMR and 1H NMR using trifluorotoluene as internal standard.
Reaction performed in CH2Cl2 instead of EtOAc.
Electron-donating (4-Me 2b, 4-OMe 2c, and 5-Me 2d) and electron-withdrawing (4-Cl 2e, 4-CO2Me 2f, 4-OCF32g, 4-CN 2h, 4-Br 2i, 5-Cl 2j, and 5-F 2k) substituents on the aromatic ring are well-tolerated and give high NMR yields (77–100%). Noteworthy, when the conversion is high, the crude product 2 is very clean and can be used for the next step without further purification. In a few cases, we noticed that with 6-F 2l,m and 6-Cl 2n aniline derivatives, very low conversion or no reaction was observed. Much to our delight, 2-aminopyridine derivatives are tolerated, and SF5 adducts 2o,p were obtained in 44 and 33% yields, respectively.
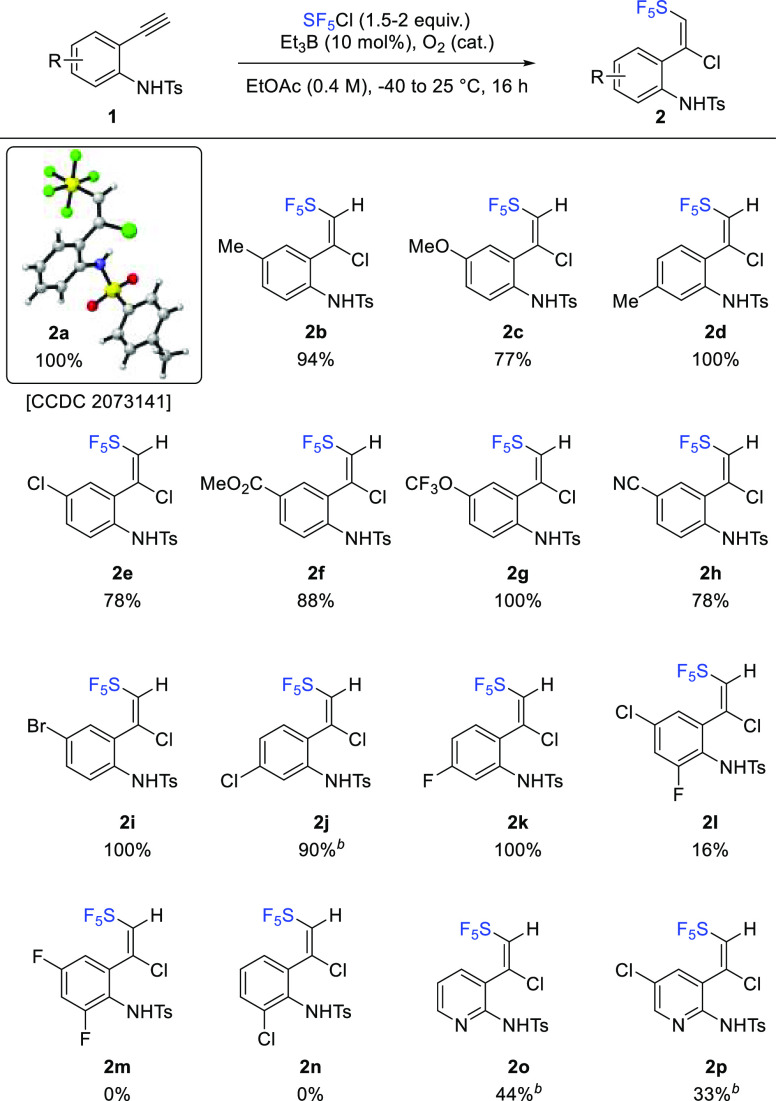
For the subsequent step, we first tested basic conditions described by Dolbier for the dehydrochlorination reaction (LiOH in DMSO).25 After 16 h at room temperature, we were pleased to observe full conversion of 2a to the expected SF5-alkyne 3a, along with N-Ts-2-SF5-indole 4a in a 50:50 ratio (as measured by 19F NMR, Scheme 3). Extended reaction times, up to 84 h, afforded a 47:53 mixture of N-Ts-2-SF5-indole 4a and 2-SF5-indole 5a (arising from the deprotection of 4a under basic conditions). Structures of 4a (CCDC 2073143) and 5a (CCDC 2073142) were unambiguously confirmed by X-ray diffraction.56 After careful optimization, it was found that full conversion of 2a into 5a was obtained after 40 h at 40 °C. This one-pot three-step sequence (dehydrochlorination, 5-endo-dig cyclization, and deprotection of the tosyl moiety) is general and proceeds smoothly with all substrates 2a–p independently of the substitution.
Scheme 3. Scope and Limitations for the Synthesis of 2-SF5-Indoles 5.
NMR yields determined by 19F NMR and 1H NMR using trifluorotoluene as internal standard. Isolated yields in brackets after purification on SiO2.
Good to excellent NMR yields ranging from 55% to quantitative are obtained. Noteworthy, functional groups such as ester 5f, nitrile 5h, halides 5e–5i,j, or even the more exotic OCF35g are well-tolerated. It should be noted that isolated yields of 2-SF5-indoles 5 are 34 ± 16% lower (after chromatography on silica gel) than NMR yields. Unfortunately, all of the purification media that were screened, such as deactivated silica gel, demetalated silica gel,57 C-18 reversed-phase silica, Florisil, or alumina did not improve yields further. However, 60–70% overall yields are still highly relevant considering that this is a formal three-step sequence. In addition, the reaction is easily scalable up to 1.8 g (4 mmol) in reproducible 66% isolated yield.
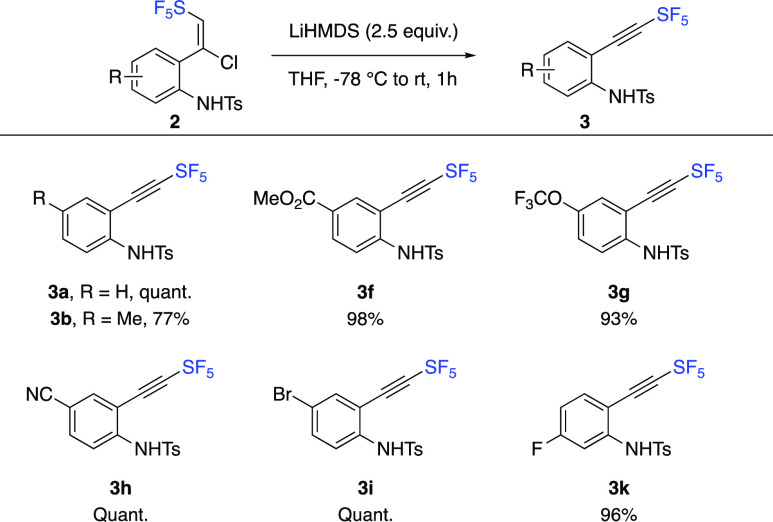
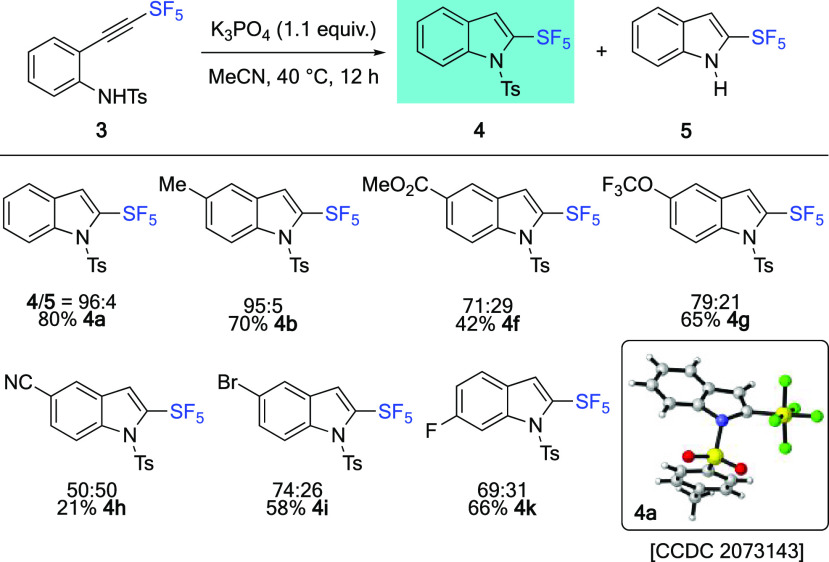
While the synthesis of N-unprotected 2-SF5-indoles 5 is of interest, keeping the N-tosyl protecting group would also be an asset. After an extensive screening of base, it was found that lithium hexamethyldisilazane (LiHMDS) led to a smooth dehydrochlorination reaction at −78 °C for 1 h (Scheme 4). SF5-Alkynes 3 were formed in 77–100% yield, with an excellent functional group tolerance. In addition, the reaction was very clean, and no purification was needed. Next, for the cyclization step, it was found that K3PO4 was able to convert SF5-alkynes 3 into the desired N-Ts-2-SF5-indoles 4 in acetonitrile at 40 °C for 12 h alongside the deprotected indole 5 (Scheme 5). As expected from an electronic point of view, electron-neutral or electron-donating substituents as in 3a,b gave high selectivity for the corresponding N-Ts-2-SF5-indoles 4a,b.
Scheme 4. Synthesis SF5-Alkynes 3.
Isolated yields after extraction; no purification needed.
Scheme 5. Synthesis of N-Ts-2-SF5-Indoles 4.
Isolated yields after purification on SiO2. Ratio of 4/5 determined by 19F NMR using trifluorotoluene as an internal standard.
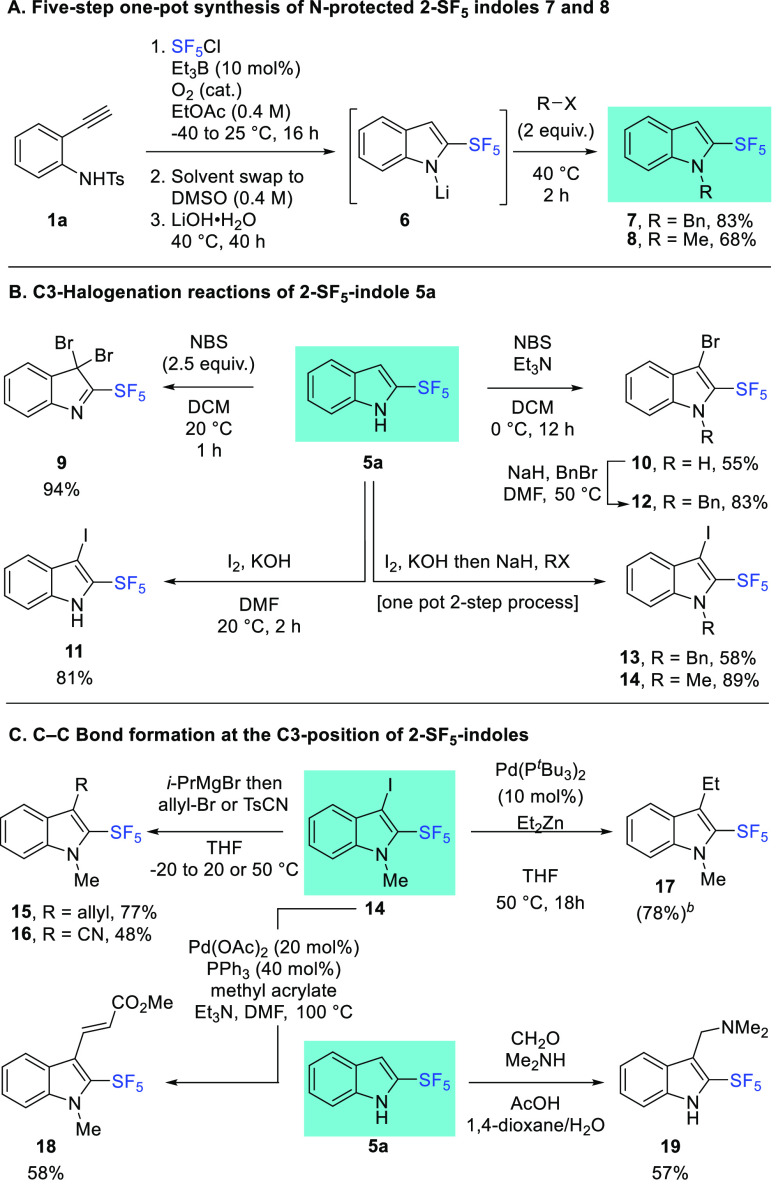
In contrast, with electron-withdrawing substituents, a non-negligible amount of 2-SF5-indoles 5f–i,k (R = Br, F, OCF3, CO2Me, CN) was observed ranging from 30% with R = CO2Me OCF3, Br, F) to 50% with R = CN. Quite interestingly, isolated yields of N-Ts-2-SF5-indoles 4 are much closer to NMR yields, which indicates that N-substituted 2-SF5-indoles possess improved stability toward purification. This was further confirmed by the independent preparation of N-Bn-2-SF5-indole 7 and N-Me-2-SF5-indole 8 via the interception of the intermediate 6 by the corresponding electrophiles (Scheme 6A). Clean reactions and excellent yields for five steps were obtained, in line with the NMR yields (92% for 7 and 73% for 8, a mean deviation of 7 ± 2% from the isolated yields).
Scheme 6. Downstream Functionalizations of 2-SF5-Indoles.
NMR yield determined by 19F NMR using trifluorotoluene as an internal standard.
Obtained as an inseparable mixture with indole 8.
C3-Functionalizations of 2-SF5-indoles 5 were next investigated (Scheme 6B,C). We first focused on the innate C3-nucleophilicity of 5a in halogenation reactions. Double bromination reaction58 with an excess of N-bromosuccinimide (NBS) is very efficient and yielded 9a in 94% yield. Monobromination is also possible using a slight excess (1.1 equiv) of NBS in the presence of triethylamine.59 The reaction proceeded quantitatively by NMR and 10 was isolated in 55% yield. Incorporation of a C3-iodine atom is also possible using molecular iodine in the presence of potassium hydroxide,60 delivering 11 in 81% yield. The iodination step can then be combined in a one-pot process with N-benzylation (13, 58%) or N-methylation (14, 89%). Finally, C–C bond formations were investigated (Scheme 6C). Iodine–magnesium exchange of 14 followed by trapping with an electrophile such as allyl bromide or tosyl cyanide delivered 15 (77%) and 16 (48%), respectively.61 Negishi cross-coupling with diethylzinc62 proved to be efficient with the formation of 17 in 78% NMR yield (along with the reduced indole 8 as an inseparable mixture). Heck cross-coupling63,64 with methyl acrylate is also productive, yielding 18 in 58% yield as a single E-stereoisomer. Finally, we evaluated the reactivity of the 2-SF5-indole 5a toward Eschenmoser salt for the synthesis of 19 (57%), the 2-SF5 analogue of the naturally occurring indole alkaloid gramine.65
Having designed a synthetic strategy toward 2-SF5-indoles and explored a selection of downstream functionalizations, we turned our attention to the investigation of their physicochemical properties and how they compare with differently C2-substituted indoles. Six indoles were selected: the 2-SF5-indoles 5a and 8 alongside four C2-substituted indoles, 2-H (20), 2-Me (21), 2-F (22), and 2-CF3 (23).
We started with differential scanning calorimetry (DSC)66−69 analysis to gain information about the thermal tolerance threshold of our process and the thermal stability of 2-SF5-indoles (Figure 1A).55 Both 2-SF5-indole 5a and 8 induce a strong release of energy (exothermic) when the threshold of thermal stability is reached, with enthalpies of −1180 kJ/kg with an onset above 165 °C for 5a and −1324 kJ/kg starting above 310 °C for 8.
Figure 1.
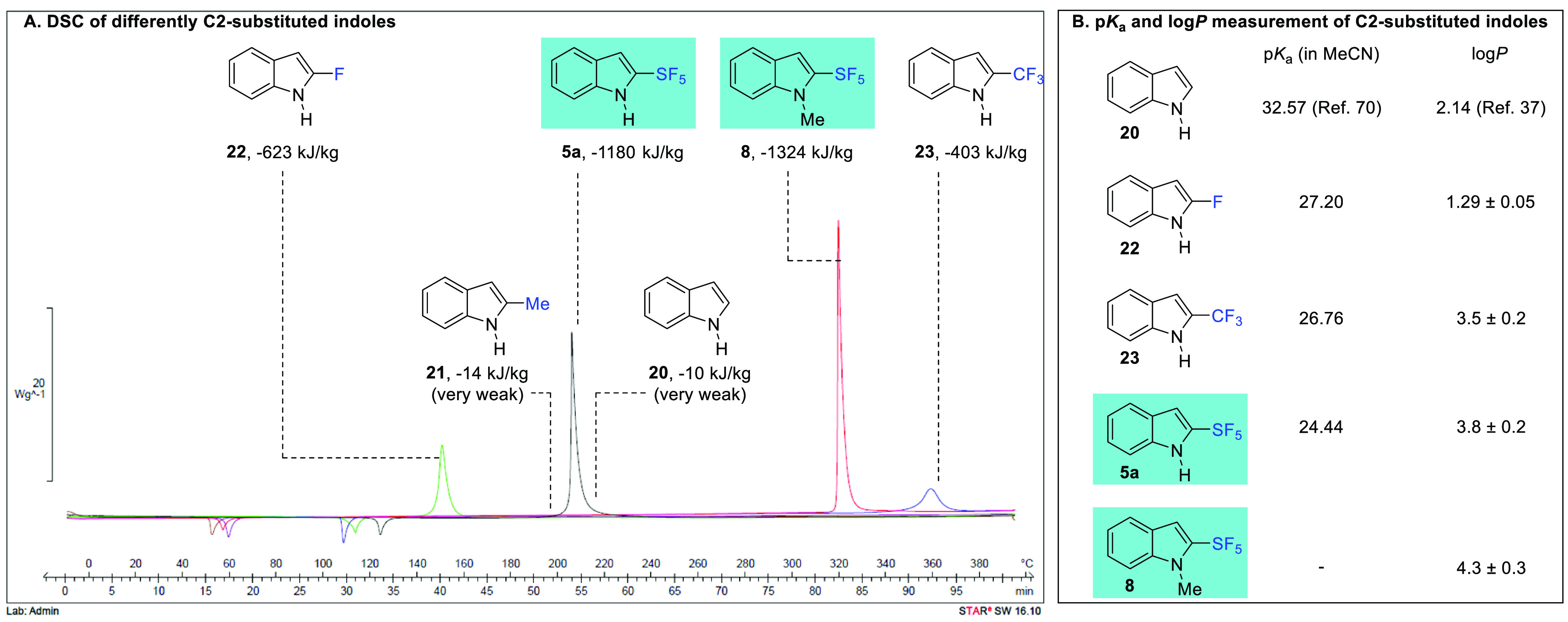
Differential scanning calorimetry (A) and pKa and log P (B) of C2-substituted indoles.
This highly exothermic event is characteristic of a violent decomposition. However, this threshold appears at relatively high temperatures (>165 °C for 5a, 310 °C for 8) and therefore much higher than the maximum temperatures used for the synthesis of 2-SF5-indoles (up to 40 °C) or their functionalization (up to 100 °C). This means that the synthetic methods devised in Schemes 2–6 are safe with a fairly large safety margin gap (>120 °C) between the reaction processes and the decomposition onsets. As expected, the protection of 2-SF5-indole 5a by a methyl group (8) significantly increases its thermal stability. Among the fluorinated indole analogues, 2-CF3 indole 23 is the most stable heterocycle with an exothermic degradation above 325 °C (−403 kJ/kg), whereas 2-F indole 22 degrades above 120 °C (−623 kJ/kg). It is important to note that, in comparison, the temperatures and degradation energies of 2-Me indole 21 (low exotherm above 165 °C, enthalpy of −14 kJ/kg) and indole 20 (low exotherm above 190 °C, enthalpy of −10 kJ/kg) are negligible.
Incorporation of a fluorine atom or a fluorinated group has a tremendous impact on physicochemical properties of molecules and nearby functional groups. In the specific case of the pentafluorosulfanyl moiety, physicochemical data are scarce2−6 and experimental measurements of the acidity (pKa)70 and lipophilicity (log P)71,72 imparted by the pentafluorosulfanyl moiety would be useful data for medicinal chemistry programs. We thus turned our attention to a subset of indoles substituted at C-2 by H (20), F (22), CF3 (23), or SF5 (5a/8), and the results are summarized in Figure 1B. Values of pKa were measured in acetonitrile by spectrophotometric titration.55 The pKa of indole 20 (32.57)70 decreases dramatically by 5.4 units upon fluorine and fluorine-containing substitution at C2, resulting in pKa 27.20 for 22. Swapping C2-F for a C2-CF3 substituent only slightly impacted the pKa by 0.44 units (pKa of 23: 26.76). On the other hand, a pronounced drop in pKa was measured for 5a possessing a C2-SF5 motif; with a pKa of 24.44, it stands 2.32 units lower than the pKa of 23 and is comparable to the pKa of 2-nitroindole (23.64).70
Fine modulation of lipophilicity is central to drug development,73−75 and fluorine-containing substituents play an important role in this regard, whether in the aromatic76 or aliphatic series.77−79 As a consequence, assessing the impact of the pentafluorosulfanyl motif at the C2-position of indoles on log P was of interest. The lipophilicities of the five indole derivatives were obtained by combining experimental and computational data.55 The average log P values are given in Figure 1B. Replacement of the C2-hydrogen atom of indole 20 by a fluorine atom (22) decreases the lipophilic character by 0.85 unit (from 2.14 to 1.29). Although lipophilicity classically increases upon H–F swap in the aromatic series, this drop in log P between 20 and 22 can be rationalized by the increased polarization of the N–H bond, leading to the increased hydrogen bond donating ability of 22 (favoring hydrophilicity) balanced by a small increase in hydrophobic surface area.76,80 On the other hand, the latter parameter dramatically increases in the case of 2-CF3-indole 23, overcompensating the increased hydrogen bonding ability. An increase of log P to 3.5 ± 0.2 was measured for 23. Replacing 2-CF3 with 2-SF5 substituent as in compound 5a further increases log P by roughly 0.3 units, to 3.8 ± 0.2. Finally, N-methylation of 5a logically led to an increased log P of 4.3 ± 0.3. Overall, these results allow the assessment of the impact of the pentafluorosulfanyl group compared to a fluorine atom or a trifluoromethyl group in the C2-position of indoles. A pronounced drop in pKa and a simultaneous increase in log P are unambiguously demonstrated, thereby modulating physicochemical properties of this relevant heterocycle in a unique fashion.
Finally, as indole is a privileged scaffold in drug discovery, we thought that the mutagenic character of the newly synthesized compounds 5a and 8 will be important to be determined and valuable information to be provided to the community. In silico assessment is typically done in the first place to estimate the mutagenic potential of a compound against databases. Indole is considered to be nonmutagenic, and therefore, there is no mutagenicity concern emerging from the indole moiety. However, uncovered fragment −SF5 was detected in the used systems (Derek, Sarah Nexus, and Case Ultra).55 Hence, due to incomplete coverage, it was recommended to perform further tests to evaluate potential mutagenic activity of the SF5 moiety. We thus performed the Ames test which is a classical biological test to determine the mutagenic potential of a chemical compound.55 Since cancers are often linked to damage to DNA, this rapid, reliable, and inexpensive test is used to estimate the carcinogenic and genetic activity at the nucleotide level, based on different histidine-requiring bacterial strains of Salmonella typhimurium carrying mutations in the genes in the absence and presence of a liver-metabolizing system.81,82 Over the years, a large database has been accumulated with this assay, confirming its ability to detect genetically active compounds of most chemical classes with around 80–90% sensitivity and specificity. The 2-SF5-indoles 5a and 8 have been tested, and no mutagenic evidence was observed over the different bacterial strains tested, which means that they can potentially be used for further development in drug discovery.55
Conclusion
In conclusion, we developed an efficient synthesis of 2-SF5-indoles and azaindoles from 2-ethynylaniline derivatives in a two-step telescoped procedure. This sequence consists of four formal synthetic steps: radical addition of SF5Cl followed by dehydrochlorination, 5-endo-dig cyclization and deprotection of the tosyl fragment in basic conditions. We then decomposed the full sequence into a stepwise synthesis allowing to keep the N-protecting group on the 2-SF5-indole. A selection of downstream functionalizations was demonstrated, including N-alkylation and benzylation, C3-halogenation, alkylation, allylation, cyanation, and alkenylation. Carcinogenic potential (Ames test) and relevant physicochemical properties (such as thermal stability (DSC), acidity (pKa), and lipophilicity (log P) were measured in order to highlight the strategic advantages that a C2-pentafluorosulfanylated motif could impart on the indole nucleus.
Acknowledgments
This work was supported by the French National Research Agency (Grant No. ANR-PRC-17-CE07-0008), Université de Haute-Alsace, Université de Strasbourg, CNRS, Région Grand-Est, and Foundation for Frontier Research in Chemistry (ICFRC). The authors thank Pascale Hoehn (safety lab, DSC) and Susanne Glowienke and Erika Udovic (preclinical safety, Ames test, in silico) from Novartis. Work at Tartu was supported by the Estonian Research Council grant (PRG690), by EU through the European Regional Development Fund under project TK141 “Advanced materials and high-technology devices for energy recuperation systems” (2014-2020.4.01.15-0011) and was carried out using the instrumentation at the Estonian Center of Analytical Chemistry (www.akki.ee). The authors thank Dr. F. Leroux, Dr. G. Hanquet, Dr. A. Panossian, Dr. C. Meyer, and Dr. S. Lakhdar for fruitful discussions. This article is dedicated to the memory of Francis Marty, Emeritus Professor at the University of Burgundy, France.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsorginorgau.1c00010.
Detailed procedures and characterization of all products; copies of 1H, 13C, and 19F NMR (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wang J.; Sánchez-Roselló M.; Aceña J. L.; del Pozo C.; Sorochinsky A. E.; Fustero S.; Soloshonok V. A.; Liu H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- Savoie P. R.; Welch J. T. Preparation and Utility of Organic Pentafluorosulfanyl-Containing Compounds. Chem. Rev. 2015, 115, 1130–1190. 10.1021/cr500336u. [DOI] [PubMed] [Google Scholar]
- von Hahmann C. N.; Savoie P. R.; Welch J. T. Reactions of Organic Pentafluorosulfanyl-containing Compounds. Curr. Org. Chem. 2015, 19, 1592–1618. 10.2174/1385272819666150601211131. [DOI] [Google Scholar]
- Kanishchev O. S.; Dolbier W. R.. Chapter One - SF5-Substituted Aromatic Heterocycles. In Advances in Heterocyclic Chemistry, Scriven E. F. V., Ramsden C. A., Eds.; Academic Press, 2016; Vol. 120, pp 1–42. [Google Scholar]
- Das P.; Tokunaga E.; Shibata N. Recent Advancements in the Synthesis of Pentafluorosulfanyl (SF5)-Containing Heteroaromatic Compounds. Tetrahedron Lett. 2017, 58, 4803–4815. 10.1016/j.tetlet.2017.11.015. [DOI] [Google Scholar]
- Cui B.; Shibata N. The Story of SF5-Substituted Pyridines. Phosphorus, Sulfur Silicon Relat. Elem. 2019, 194, 658–663. 10.1080/10426507.2019.1602624. [DOI] [Google Scholar]
- Beier P.Pentafluorosulfanylation of Aromatics and Heteroaromatics. In Emerging Fluorinated Motifs: Synthesis, Properties and Applications; Cahard D., Ma J.-A., Eds.: Wiley-VCH: Weinheim, Germany, 2020; Vol 2, pp 551–570. [Google Scholar]
- Chan J. M. W. Pentafluorosulfanyl Group: an Emerging Tool in Optoelectronic Materials. J. Mater. Chem. C 2019, 7, 12822–12834. 10.1039/C9TC01949A. [DOI] [Google Scholar]
- Sowaileh M. F.; Hazlitt R. A.; Colby D. A. Application of the Pentafluorosulfanyl Group as a Bioisosteric Replacement. ChemMedChem 2017, 12, 1481–1490. 10.1002/cmdc.201700356. [DOI] [PubMed] [Google Scholar]
- Altomonte S.; Zanda M. Synthetic Chemistry and Biological Activity of Pentafluorosulphanyl (SF5) Organic Molecules. J. Fluorine Chem. 2012, 143, 57–93. 10.1016/j.jfluchem.2012.06.030. [DOI] [Google Scholar]
- Meanwell N. A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. 10.1021/acs.jmedchem.7b01788. [DOI] [PubMed] [Google Scholar]
- Savoie P. R.; von Hahmann C. N.; Penger A.; Wei Z.; Welch J. T. The Control of Stereochemistry by the Pentafluorosulfanyl Group. Org. Biomol. Chem. 2018, 16, 3151–3159. 10.1039/C7OB03146G. [DOI] [PubMed] [Google Scholar]
- Sheppard W. A. The Electrical Effect of the Sulfur Pentafluoride Group. J. Am. Chem. Soc. 1962, 84, 3072–3076. 10.1021/ja00875a007. [DOI] [Google Scholar]
- Welch J. T.; Lim D. S. The Synthesis and Biological Activity of Pentafluorosulfanyl Analogs of Fluoxetine, Fenfluramine, and Norfenfluramine. Bioorg. Med. Chem. 2007, 15, 6659–6666. 10.1016/j.bmc.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Lim D. S.; Choi J. S.; Pak C. S.; Welch J. T. Synthesis and Herbicidal Activity of a Pentafluorosulfanyl Analog of Trifluralin. J. Pestic. Sci. 2007, 32, 255–259. 10.1584/jpestics.G06-50. [DOI] [Google Scholar]
- Gujjar R.; El Mazouni F.; White K. L.; White J.; Creason S.; Shackleford D. M.; Deng X.; Charman W. N.; Bathurst I.; Burrows J.; Floyd D. M.; Matthews D.; Buckner F. S.; Charman S. A.; Phillips M. A.; Rathod P. K. Lead Optimization of Aryl and Aralkyl Amine-Based Triazolopyrimidine Inhibitors of Plasmodium falciparum Dihydroorotate Dehydrogenase with Antimalarial Activity in Mice. J. Med. Chem. 2011, 54, 3935–3949. 10.1021/jm200265b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto T.; Garrick L. M.; Saito N. Discovery of Practical Production Processes for Arylsulfur Pentafluorides and Their Higher Homologues, Bis- and Tris(sulfur pentafluorides): Beginning of a New Era of “Super-Trifluoromethyl” Arene Chemistry and its Industry. Beilstein J. Org. Chem. 2012, 8, 461–471. 10.3762/bjoc.8.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobokov M.; Cui B.; Balia A.; Matsuzaki K.; Tokunaga E.; Saito N.; Shibata N. Importance of a Fluorine Substituent for the Preparation of meta- and para-Pentafluoro-λ6-sulfanyl-Substituted Pyridines. Angew. Chem., Int. Ed. 2016, 55, 10781–10785. 10.1002/anie.201605008. [DOI] [PubMed] [Google Scholar]
- Cui B.; Jia S.; Tokunaga E.; Saito N.; Shibata N. Silver-Induced Self-Immolative Cl-F Exchange Fluorination of Arylsulfur Chlorotetrafluorides: Synthesis of Arylsulfur Pentafluorides. Chem. Commun. 2017, 53, 12738–12741. 10.1039/C7CC07222H. [DOI] [PubMed] [Google Scholar]
- Cui B.; Kosobokov M.; Matsuzaki K.; Tokunaga E.; Shibata N. IF5 Affects the Final Stage of the Cl-F Exchange Fluorination in the Synthesis of Pentafluoro-λ6-Sulfanyl-Pyridines, Pyrimidines and Benzenes with Electron-Withdrawing Substituents. Chem. Commun. 2017, 53, 5997–6000. 10.1039/C7CC02802D. [DOI] [PubMed] [Google Scholar]
- Pitts C. R.; Bornemann D.; Liebing P.; Santschi N.; Togni A. Making the SF5 Group More Accessible: A Gas-Reagent-Free Approach to Aryl Tetrafluoro-λ6-sulfanyl Chlorides. Angew. Chem., Int. Ed. 2019, 58, 1950–1954. 10.1002/anie.201812356. [DOI] [PubMed] [Google Scholar]
- Wang L.; Cornella J. A Unified Strategy for Arylsulfur(VI) Fluorides from Aryl Halides: Access to Ar-SOF3 Compounds. Angew. Chem., Int. Ed. 2020, 59, 23510–23515. 10.1002/anie.202009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanagawa K.; Zhao Z.; Saito N.; Shibata N. AgBF4-mediated Chlorine-Fluorine Exchange Fluorination for the Synthesis of Pentafluorosulfanyl (Hetero)arenes. Bull. Chem. Soc. Jpn. 2021, 94, 1682. 10.1246/bcsj.20210109. [DOI] [Google Scholar]
- Burton D. J.; Wang Y.; Bizet V.; Cahard D.. Pentafluorosulfanyl Chloride. e-EROS Encyclopedia of Reagents for Organic Synthesis; Wiley, 2020. [Google Scholar]
- Aït-Mohand S.; Dolbier W. R. New and Convenient Method for Incorporation of Pentafluorosulfanyl (SF5) Substituents Into Aliphatic Organic Compounds. Org. Lett. 2002, 4, 3013–3015. 10.1021/ol026483o. [DOI] [PubMed] [Google Scholar]
- Dolbier W. R. Jr; Aït-Mohand S.; Schertz T. D.; Sergeeva T. A.; Cradlebaugh J. A.; Mitani A.; Gard G. L.; Winter R. W.; Thrasher J. S. A Convenient an Efficient Method for Incorporation of Pentafluorosulfanyl (SF5) Substituents Into Aliphatic Compounds. J. Fluorine Chem. 2006, 127, 1302–1310. 10.1016/j.jfluchem.2006.05.003. [DOI] [Google Scholar]
- Niina K.; Tanagawa K.; Sumii Y.; Saito N.; Shibata N. Pyridine Tetrafluoro-λ6-Sulfanyl Chlorides: Spontaneous Addition to Alkynes and Alkenes in the Presence or Absence of Photo-Irradiation. Org. Chem. Front. 2020, 7, 1276–1282. 10.1039/D0QO00339E. [DOI] [Google Scholar]
- Das P.; Takada M.; Tokunaga E.; Saito N.; Shibata N. Synthesis of Pyridine Trans-Tetrafluoro-λ6-Sulfane Derivatives via Radical Addition. Org. Chem. Front. 2018, 5, 719–724. 10.1039/C7QO00994A. [DOI] [Google Scholar]
- Pitts C. R.; Santschi N.; Togni A. WO Patent Appl. 2019229103, 2019.
- Shou J. Y.; Xu X. H.; Qing F. L. Chemoselective Hydro(Chloro)pentafluorosulfanylation of Diazo Compounds with Pentafluorosulfanyl Chloride. Angew. Chem., Int. Ed. 2021, 60, 15271–15275. 10.1002/anie.202103606. [DOI] [PubMed] [Google Scholar]
- Rombach D.; Wagenknecht H. A. Photoredox Catalytic Activation of Sulfur Hexafluoride for Pentafluorosulfanylation of α-Methyl- and α-Phenyl Styrene. ChemCatChem 2018, 10, 2955–2961. 10.1002/cctc.201800501. [DOI] [Google Scholar]
- Rombach D.; Wagenknecht H.-A. Photoredox Catalytic α-Alkoxypentafluorosulfanylation of α-Methyl- and α-Phenylstyrene Using SF6. Angew. Chem., Int. Ed. 2020, 59, 300–303. 10.1002/anie.201910830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombach D.; Birenheide B.; Wagenknecht H. A. Photoredox Catalytic Pentafluorosulfanylative Domino Cyclization of alpha-Substituted Alkenes to Oxaheterocycles by Using SF6. Chem. - Eur. J. 2021, 27, 8088–8093. 10.1002/chem.202100767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha N.; Silakari O. Indoles as Therapeutics of Interest in Medicinal Chemistry: Bird’s Eye View. Eur. J. Med. Chem. 2017, 134, 159–184. 10.1016/j.ejmech.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Usachev B. I. 1-/2-/3-Fluoroalkyl-Substituted Indoles, Promising Medicinally and Biologically Beneficial Compounds: Synthetic Routes, Significance and Potential Applications. J. Fluorine Chem. 2016, 185, 118–167. 10.1016/j.jfluchem.2016.02.006. [DOI] [Google Scholar]
- Sravanthi T. V.; Manju S. L. Indoles - A Promising Scaffold for Drug Development. Eur. J. Pharm. Sci. 2016, 91, 1–10. 10.1016/j.ejps.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Tatsuta K.; Morisawa Y. JP Patent Appl. 2004067524, 2004.
- Welch J. T.; Lim D.. Fluorinated Heterocycles; American Chemical Society, 2009; Vol. 1003, pp 165–181. [Google Scholar]
- Pettersson M. Y.; Johnson D. S.; Subramanyam C.; O’donnell C. J.; Am Ende C. W.; Green M. E.; Patel N. C.; Stiff C. M.; Tran T. P.; Kauffman G. W.; Stepan A. F.; Verhoest P. R. WO Patent Appl. 2015049616, 2015.
- Alverez C.; Arkin M. R.; Bulfer S. L.; Colombo R.; Kovaliov M.; LaPorte M. G.; Lim C.; Liang M.; Moore W. J.; Neitz R. J.; Yan Y.; Yue Z.; Huryn D. M.; Wipf P. Structure-Activity Study of Bioisosteric Trifluoromethyl and Pentafluorosulfanyl Indole Inhibitors of the AAA ATPase p97. ACS Med. Chem. Lett. 2015, 6, 1225–1230. 10.1021/acsmedchemlett.5b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huryn D. M.; Wipf P.; Laporte M. G.; Colombo R.; Kovaliov M.; Lim C.; Alverez C. N.; Yue Z.; Samahkumara L. P.; Chatterley A. J.; Yan Y.; Liang M.; Green N. J.; Moore W.; Baldwin E.; Arkin M. R.; Neitz J.; Bulfer S.; Ang K.-H.; Bryant C. WO Patent Appl. 2017070320, 2017.
- Bardiot D. A. M.-E.; Bonfanti J.-F.; Coesemans E.; Kesteleyn B. R. R.; Marchand A. D. M.; Raboisson P. J.-M. B. WO Patent Appl. 2017167952, 2017.
- Iakobson G.; Pošta M.; Beier P. Synthesis of Pentafluorosulfanyl-Containing Indoles and Oxindoles. Synlett 2013, 24, 855–859. 10.1055/s-0032-1318452. [DOI] [Google Scholar]
- Chen J.; Xu L.; Mi X. Palladium-Catalyzed Oxidative Synthesis of SF5-Indoles. Tetrahedron Lett. 2015, 56, 4204–4206. 10.1016/j.tetlet.2015.05.053. [DOI] [Google Scholar]
- Frischmuth A.; Unsinn A.; Groll K.; Stadtmüller H.; Knochel P. Preparations and Reactions of SF5-Substituted Aryl and Heteroaryl Derivatives via Mg and Zn Organometallics. Chem. - Eur. J. 2012, 18, 10234–10238. 10.1002/chem.201201485. [DOI] [PubMed] [Google Scholar]
- Alabugin I. V.; Gilmore K.; Manoharan M. Rules for Anionic and Radical Ring Closure of Alkynes. J. Am. Chem. Soc. 2011, 133, 12608–12623. 10.1021/ja203191f. [DOI] [PubMed] [Google Scholar]
- Gilmore K.; Alabugin I. V. Cyclizations of Alkynes: Revisiting Baldwin’s Rules for Ring Closure. Chem. Rev. 2011, 111, 6513–6556. 10.1021/cr200164y. [DOI] [PubMed] [Google Scholar]
- Gilmore K.; Mohamed R. K.; Alabugin I. V. The Baldwin Rules: Revised and Extended. WIREs Comput. Mol. Sci. 2016, 6, 487–514. 10.1002/wcms.1261. [DOI] [Google Scholar]
- Ye Y.; Cheung K. P. S.; He L.; Tsui G. C. Synthesis of 2-(Trifluoromethyl)indoles via Domino Trifluoromethylation/Cyclization of 2-Alkynylanilines. Org. Lett. 2018, 20, 1676–1679. 10.1021/acs.orglett.8b00509. [DOI] [PubMed] [Google Scholar]
- 2-Ethynylanilines protected with a tosyl group proved to be the best candidate for both SF5Cl addition and cyclization step; see Supporting Information for details.
- Miura K.; Ichinose Y.; Nozaki K.; Fugami K.; Oshima K.; Utimoto K. Triethylborane-Induced Hydrodehalogenation of Organic Halides by Tin Hydrides. Bull. Chem. Soc. Jpn. 1989, 62, 143–147. 10.1246/bcsj.62.143. [DOI] [Google Scholar]
- Ollivier C.; Renaud P. Organoboranes as a Source of Radicals. Chem. Rev. 2001, 101, 3415–3434. 10.1021/cr010001p. [DOI] [PubMed] [Google Scholar]
- All X-ray crystal structures are displayed using CYLview20; Legault C. Y.CYLview Visualization and Analysis Software for Computational Chemistry; www.cylview.org (accessed 2021-06-22).
- Gilbert A.; Paquin J.-F. Evaluation of the Compatibility of Pentafluorosulfanyl Chloride with Various solvents and Additives. J. Fluorine Chem. 2019, 221, 70–74. 10.1016/j.jfluchem.2019.04.003. [DOI] [Google Scholar]
- See Supporting Information for details.
- Control experiments were conducted to confirm that SF5-alkynes 3 and N-Ts-2-SF5-indoles 4 are synthetic intermediates in the reaction sequence described in Scheme 3: they were independently reacted with LiOH in DMSO. Complete conversion to a single product, 5, was obtained in each case.
- Hubbard J. S.; Harris T. M. Condensations at the 6 Position of the Methyl Ester and the Dimethylamide of 3,5-Dioxohexanoic Acid via 2,4,6-Trianions. J. Org. Chem. 1981, 46, 2566–2570. 10.1021/jo00325a026. [DOI] [Google Scholar]
- Parrick J.; Yahya A.; Jin Y. A Convenient Conversion of Indoles to 3,3-Dibromooxindoles and Then to Isatins. Tetrahedron Lett. 1984, 25, 3099–3100. 10.1016/0040-4039(84)80017-9. [DOI] [Google Scholar]
- Saikia I.; Borah A. J.; Phukan P. Use of Bromine and Bromo-Organic Compounds in Organic Synthesis. Chem. Rev. 2016, 116, 6837–7042. 10.1021/acs.chemrev.5b00400. [DOI] [PubMed] [Google Scholar]
- Bocchi V.; Palla G. High Yield Selective Bromination and Iodination of Indoles in N,N-Dimethylformamide. Synthesis 1982, 1982, 1096–1097. 10.1055/s-1982-30087. [DOI] [Google Scholar]
- Barl N. M.; Sansiaume-Dagousset E.; Karaghiosoff K.; Knochel P. Full Functionalization of the 7-Azaindole Scaffold by Selective Metalation and Sulfoxide/Magnesium Exchange. Angew. Chem., Int. Ed. 2013, 52, 10093–10096. 10.1002/anie.201303490. [DOI] [PubMed] [Google Scholar]
- Jones R. M.; Buzard D. J.; Kawasaki A. M.; Kim S. H.; Thoresen L.; Lehmann J.; Zhu X. US Patent Appl. 20110160243, 2011.
- Yue D.; Larock R. C. Synthesis of 3-Iodoindoles by Electrophilic Cyclization of N,N-Dialkyl-2-(1-alkynyl)anilines. Org. Lett. 2004, 6, 1037–1040. 10.1021/ol0498996. [DOI] [PubMed] [Google Scholar]
- Crisp G. T. Variations on a Theme—Recent Developments on the Mechanism of the Heck Reaction and Their Implications for Synthesis. Chem. Soc. Rev. 1998, 27, 427–436. 10.1039/a827427z. [DOI] [Google Scholar]
- Joule J. A.Science of Synthesis Knowledge Updates; Thieme Chemistry, 2010; Vol. 2. [Google Scholar]
- Frurip D. J.; Elwell T. Effective Use of Differential Scanning Calorimetry in Reactive Chemicals Hazard Evaluation. Process Saf. Prog. 2007, 26, 51–58. 10.1002/prs.10167. [DOI] [Google Scholar]
- Frurip D. J. Selection of the Proper Calorimetric Test Strategy in Reactive Chemicals Hazard Evaluation. Org. Process Res. Dev. 2008, 12, 1287–1292. 10.1021/op800121x. [DOI] [Google Scholar]
- Sheng M.; Valco D.; Tucker C.; Cayo E.; Lopez T. Practical Use of Differential Scanning Calorimetry for Thermal Stability Hazard Evaluation. Org. Process Res. Dev. 2019, 23, 2200–2209. 10.1021/acs.oprd.9b00266. [DOI] [Google Scholar]
- For a recent example from our group about DSC analysis, see:; Brach N.; Le Fouler V.; Bizet V.; Lanz M.; Gallou F.; Bailly C.; Hoehn P.; Parmentier M.; Blanchard N. Optimized Synthesis of 7-Azaindazole by a Diels-Alder Cascade and Associated Process Safety. Org. Process Res. Dev. 2020, 24, 776–786. 10.1021/acs.oprd.0c00184. [DOI] [Google Scholar]
- Kütt A.; Tshepelevitsh S.; Saame J.; Lõkov M.; Kaljurand I.; Selberg S.; Leito I. Strengths of Acids in Acetonitrile. Eur. J. Org. Chem. 2021, 2021, 1407–1419. 10.1002/ejoc.202001649. [DOI] [Google Scholar]
- Tshepelevitsh S.; Hernits K.; Jenčo J.; Hawkins J. M.; Muteki K.; Solich P.; Leito I. Systematic Optimization of Liquid-Liquid Extraction for Isolation of Unidentified Components. ACS Omega 2017, 2, 7772–7776. 10.1021/acsomega.7b01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tshepelevitsh S.; Kadam S. A.; Darnell A.; Bobacka J.; Rüütel A.; Haljasorg T.; Leito I. LogP Determination for Highly Lipophilic Hydrogen-Bonding Anion Receptor Molecules. Anal. Chim. Acta 2020, 1132, 123–133. 10.1016/j.aca.2020.07.024. [DOI] [PubMed] [Google Scholar]
- Young R. J.; Leeson P. D. Mapping the Efficiency and Physicochemical Trajectories of Successful Optimizations. J. Med. Chem. 2018, 61, 6421–6467. 10.1021/acs.jmedchem.8b00180. [DOI] [PubMed] [Google Scholar]
- Johnson T. W.; Gallego R. A.; Edwards M. P. Lipophilic Efficiency as an Important Metric in Drug Design. J. Med. Chem. 2018, 61, 6401–6420. 10.1021/acs.jmedchem.8b00077. [DOI] [PubMed] [Google Scholar]
- Shultz M. D. Two Decades under the Influence of the Rule of Five and the Changing Properties of Approved Oral Drugs. J. Med. Chem. 2019, 62, 1701–1714. 10.1021/acs.jmedchem.8b00686. [DOI] [PubMed] [Google Scholar]
- Böhm H.-J.; Banner D.; Bendels S.; Kansy M.; Kuhn B.; Müller K.; Obst-Sander U.; Stahl M. Fluorine in Medicinal Chemistry. ChemBioChem 2004, 5, 637–643. 10.1002/cbic.200301023. [DOI] [PubMed] [Google Scholar]
- Huchet Q. A.; Kuhn B.; Wagner B.; Fischer H.; Kansy M.; Zimmerli D.; Carreira E. M.; Müller K. On the Polarity of Partially Fluorinated Methyl Groups. J. Fluorine Chem. 2013, 152, 119–128. 10.1016/j.jfluchem.2013.02.023. [DOI] [Google Scholar]
- Jeffries B.; Wang Z.; Troup R. I.; Goupille A.; Le Questel J.-Y.; Fallan C.; Scott J. S.; Chiarparin E.; Graton J.; Linclau B. Lipophilicity Trends Upon Fluorination of Isopropyl, Cyclopropyl and 3-Oxetanyl groups. Beilstein J. Org. Chem. 2020, 16, 2141–2150. 10.3762/bjoc.16.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries B.; Wang Z.; Felstead H. R.; Le Questel J.-Y.; Scott J. S.; Chiarparin E.; Graton J.; Linclau B. Systematic Investigation of Lipophilicity Modulation by Aliphatic Fluorination Motifs. J. Med. Chem. 2020, 63, 1002–1031. 10.1021/acs.jmedchem.9b01172. [DOI] [PubMed] [Google Scholar]
- Liu K.; Kokubo H. Uncovering Abnormal Changes in logP After Fluorination Using Molecular Dynamics Simulations. J. Comput.-Aided Mol. Des. 2019, 33, 345–356. 10.1007/s10822-018-0183-1. [DOI] [PubMed] [Google Scholar]
- Curvall M.; Florin I.; Jansson T. Mutagenicity of Some Indoles and Related Compounds in the Ames Test. Toxicology 1982, 23, 1–10. 10.1016/0300-483X(82)90036-1. [DOI] [PubMed] [Google Scholar]
- Zeiger E. The Test That Changed the World: The Ames Test and the Regulation of Chemicals. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 2019, 841, 43–48. 10.1016/j.mrgentox.2019.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.