Abstract
Germination of mutant spores of Bacillus subtilis unable to degrade their cortex is accompanied by excretion of dipicolinic acid and uptake of some core water. However, compared to wild-type germinated spores in which the cortex has been degraded, the germinated mutant spores accumulated less core water, exhibited greatly reduced enzyme activity in the spore core, synthesized neither ATP nor reduced pyridine or flavin nucleotides, and had significantly higher resistance to heat and UV irradiation. We propose that the germinated spores in which the cortex has not been degraded represent an intermediate stage in spore germination, which we term stage I.
Dormant spores of Bacillus species initiate germination in response to a variety of nutrients, with the precise nature of the nutrient dependent on the species and strain (14, 21). These nutrients, termed germinants, bind to one or more receptors in the spore, and this binding somehow triggers both permeability changes in the spore's inner membrane and activation of enzymes that initiate hydrolysis of the spore's peptidoglycan cortex (14, 21). The permeability changes in the spore's inner membrane allow excretion of the spore core's large depots of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) and divalent cations and uptake of a significant amount of water into the spore core (14, 21). More core water is then taken up as the spore core expands once the restraining cortex has been acted upon by cortex-lytic enzymes (CLEs) (14, 21). However, spore cortex hydrolysis does not appear to be essential for at least the initial permeability changes in response to interaction of germinants with their receptor (10, 22, 27).
It appears likely that spores in which germination has been initiated but cortex degradation has not taken place represent an intermediate stage in spore germination, since cortex hydrolysis is normally significantly slower than DPA excretion and initial water uptake (21). However, this intermediate stage is impossible to study during wild-type spore germination because of the rapidity of cortex hydrolysis and the asynchrony of germination in spore populations. Therefore, we have analyzed the properties of mutant spores in which germination has been initiated but cortex hydrolysis cannot take place in order to characterize this intermediate stage in spore germination that we propose to call stage I.
Bacillus subtilis was used for this work, and all strains were isogenic, except as noted otherwise, and were PS832 (wild type), PS2307 (cwlD and also carrying a chloramphenicol resistance marker) (22), and FB113 (cwlJ sleB and also carrying spectinomycin and tetracycline resistance markers) (19); all three strains are derivatives of strain 168. In strain PS2307, the cwlD mutation blocks the formation of muramic acid lactam in the spore cortex (22). Since muramic acid lactam is necessary for the action of CLEs on the spore cortex, there is no cortex degradation during the germination of spores of strain PS2307, although DPA is released, albeit slightly more slowly than during the germination of wild-type spores (22, 27). The cwlJ and sleB genes encode the two CLEs that play overlapping roles in hydrolysis of the cortex during B. subtilis spore germination; DPA is released during the germination of cwlJ sleB spores, even though there is no detectable cortex hydrolysis (2, 10, 15; see below). As would be predicted, neither cwlD nor cwlJ sleB spores give many colonies when plated directly on nutrient medium (≤0.003 to 0.05% of the total spores) (10, 22, 27); however, viable organisms can be recovered (30 to 80% of the total spores) if spores are first decoated and then the cortex is degraded with lysozyme in hypertonic medium (22; data not shown). Derivatives of strains PS832, PS2307, and FB113 which also carry the luxAB genes from Vibrio harveyi under the control of the strong forespore-specific sspB promoter were prepared by transforming these strains to erythromycin resistance with plasmid pSB357 (9), giving strains PS3379 (luxAB), PS3380 (cwlD luxAB), and PS3383 (cwlJ luxAB sleB). Spores of the strains without luxAB fusions were prepared on 2×SG medium agar plates (18, 20) without antibiotics by incubation for ∼48 h at 37°C, and spores with luxAB fusions were prepared in liquid 2×SG medium; in all cases, the spores were purified by extensive washing with water as previously described and stored in water at 5°C (18). All of the spore preparations used in this work were free (>97%) of sporulating cells, germinated spores, or cell debris, as seen in the phase-contrast microscope.
Spores were routinely germinated after a heat shock (30 min; 70°C) of spores in water, or in 50 mM KPO4 (pH 7.4) for experiments measuring light production (4). In some experiments, dormant spores were decoated prior to the heat shock by incubation at an optical density at 600 nm of 20 for 30 min at 65°C in 0.1 M NaOH–0.1 M NaCl–0.1 M dithiothreitol–0.5% sodium dodecyl sulfate, and the treated spores were washed as previously described (36). This decoating allowed determination of spore core wet densities and assessment of either spore resistance by using viability assays after digestion of the spore cortex by lysozyme in hypertonic medium or spore metabolism again after cortex digestion with lysozyme (13, 18, 22, 35). Spore germination was at 37°C and, unless otherwise noted, at an optical density at 600 nm of 1 in either (i) 10 mM Tris-HCL (pH 8.3) plus 8 mM l-alanine, (ii) 2×YT medium (6 g of tryptone per liter, 10 g of yeast extract per liter, 5 g of NaCl per liter) plus 4 mM l-alanine, (iii) 2×SG medium (18) without CaNO3 and glucose but with 10 mM l-alanine, or (iv) Spizizen's minimal medium (33) with 0.1% Casamino Acids and 4 mM l-alanine. Determination of spore DPA release, the core wet density of dormant and germinated spores, levels of 3-phosphoglyceric acid (3PGA), and ATP and light production from spores with luxAB genes were done as previously described (4, 9, 13, 25, 32, 35). The various small, acid-soluble spore proteins (SASP), which make up much of the protein in the spore core (21, 31), were extracted, aliquots were subjected to polyacrylamide gel electrophoresis at low pH, and the gel was stained with Coomassie blue as previously described (18).
As found previously (10, 22, 27), spores of cwlD and cwlJ sleB strains released ≥95% of their DPA by 45 min after initiation of spore germination (Table 1). The core wet densities of germinated cwlD and cwlJ sleB spores were similar but significantly lower than that of their dormant spore counterparts, as shown previously for cwlD spores (Table 1) (22); this finding is consistent with the loss of DPA from the core of these germinated spores. Since the core wet density of germinated cwlD and cwlJ sleB spores was also lower than that of dormant spores which lack DPA (1.297 g/ml) (20), this further indicates that the core of germinated cwlD and cwlJ sleB spores has taken up significant water, perhaps replacing the DPA excreted. However, the germinated cwlD and cwlJ sleB spores had a significantly higher core wet density than did germinated wild-type spores, again as noted previously for cwlD spores (22) (Table 1). This indicates that the core water content of germinated spores whose cortex has not been degraded is significantly less than that of germinated spores in which cortex degradation has taken place and the spore core has expanded, and it can be calculated (22) that the core or protoplast of wild-type germinated spores contains ≥15% more of its wet weight as water than do the cores of germinated cwlD or cwlJ sleB spores.
TABLE 1.
Properties of dormant and germinated spores of several B. subtilis strainsa
| Strain | % DPA remaining | Core wet density (g/ml) | 3PGA concn (nmol/mg of dry dormant spores) | ATP concn (pmol/mg of dry dormant spores) |
|---|---|---|---|---|
| PS832 (wild type) | ||||
| Dormant | 100b | 1.353 | 4 | <2 |
| Germinated | <3c | 1.194c | <0.15d | 3,225g |
| PS3207 (cwlD) | ||||
| Dormant | 100b | 1.348 | 4 | <2 |
| Germinated | <5c | 1.246c | 3.7e | <2g |
| FB113 (cwlJ sleB) | ||||
| Dormant | 100b | 1.351 | NDf | <2 |
| Germinated | 5c | 1.250c | ND | <2g |
Spores were germinated as described in the text at 37°C in either 2×YT medium plus 4 mM l-alanine (DPA and core wet density analyses) or Spizizen's minimal medium (33) plus 0.1% Casamino Acids and 4 mM l-alanine (3PGA and ATP analyses).
Value set at 100%, but wild-type, cwlD and cwlJ sleB dormant spores had essentially (within 7%) identical DPA levels.
Spores analyzed after 45 min of germination.
Spores analyzed after 30 min of germination.
Spores analyzed after 90 min of germination.
ND, not determined.
Spores analyzed after 35 min of germination; no ATP (<2 pmol/mg of original dry dormant spores) was found in germinated cwlD and cwlJ sleB spores at 6, 10, 17, and 60 min of germination as well.
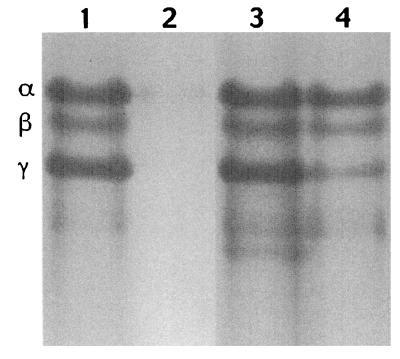
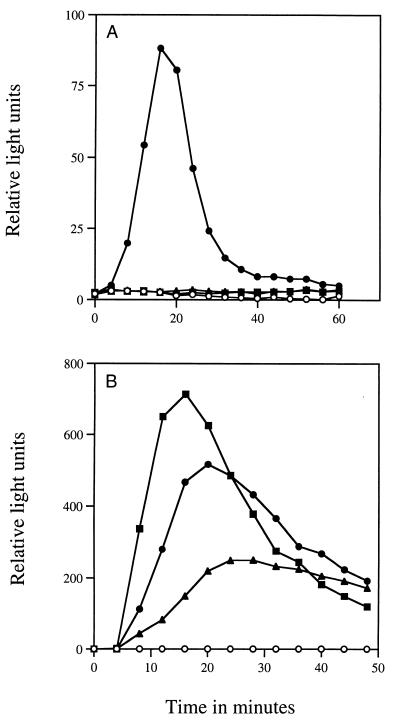
Further analysis of the germinated cwlD or cwlJ sleB spores showed that the various SASP that make up a large percentage of the protein in the dormant spore core and are normally rapidly degraded in spore germination (21, 30, 31) are degraded only very slowly during the germination of cwlD and cwlJ sleB spores (Fig. 1; data not shown). Indeed, there was only a very slight loss of DNA binding SASP-α and -β by 120 min after initiation of germination of cwlD or cwlJ sleB spores, although there was some loss of SASP-γ (Fig. 1, lanes 3 and 4); the latter protein does not bind to DNA and is thus not protected against proteolysis by the SASP-specific protease GPR in spores (21, 30, 31). In contrast, both types of SASP were almost completely degraded after 60 min of germination of wild-type spores (Fig. 1, lanes 1 and 2). Dormant spores also contain a large depot of 3PGA that is rapidly utilized in the first minute of spore germination to generate ATP (Table 1) (17, 21, 30). While >90% of the 3PGA depot was lost by 30 min after initiation of germination of wild-type spores, <10% of the 3PGA depot had been utilized by 90 min after initiation of cwlD spore germination (Table 1). Possibly most striking of all was the absence of detectable ATP in germinated cwlD and cwlJ sleB spores, while germinated wild-type spores accumulated up to 3 orders of magnitude more ATP in the same period (Table 1). In addition, in the assay which measures the reduction of a colorless tetrazolium dye to a red formazan product as a test for germination of spores in colonies on agar plates (14, 26), wild-type sporulated colonies turned red, as expected, while sporulated cwlD and cwlJ sleB colonies did not (data not shown). The latter result indicates that, in contrast to wild-type spores, neither cwlD nor cwlJ sleB spores were able to generate readily accessible reducing eqivalents (e.g., reduced pyridine nucleotides or flavin nucleotides) after initiation of germination. This was shown directly by analysis of light production during germination of spores of strains carrying the V. harveyi luxAB genes under the control of the forespore-specific sspB promoter, which results in significant levels of LuxA and -B in dormant spores (9). Previous work has shown that dormant wild-type spores carrying the luxAB genes under the control of the sspB promoter give no light production, presumably due to the lack of reduced flavin mononucleotide in dormant spores (9, 30), but that light production begins early in spore germination when metabolism begins, although light output then decreases (4, 9). We obtained similar results with spores of the wild-type strain carrying the luxAB genes under control of the sspB promoter, but cwlD and cwlJ sleB spores carrying these luxAB genes gave no detectable light production upon initiation of spore germination (Fig. 2A). One explanation for the lack of light production with the spores of the cwlD and cwlJ sleB derivatives is that neither the exogenous dodecanal nor the O2 needed for light production by LuxA and -B penetrated the inner membrane of these germinated spores; however, these compounds should penetrate the inner membrane of even dormant spores (6). That the absence of light production during germination of spores of cwlD and cwlJ sleB derivatives was due to the continued presence of the cortex in these germinated spores was shown by treatment of decoated dormant spores with lysozyme in a hypertonic medium (22) in which spores remain viable after cortex digestion as noted above; decoated spores of the cwlD luxAB and cwlJ luxAB sleB derivatives, as well as their luxAB counterpart, all exhibited rapid light production after lysozyme addition, indicating that spore cortex removal results in rapid initiation of spore metabolism (Fig. 2B). These observations, as well as the lack of ATP in germinated cwlD and cwlJ sleB spores, indicate that the latter spores do not resume metabolism after initiation of germination, and this is consistent with work that has shown that upon initiation of spore germination, metabolism begins only after at least some cortex hydrolysis (11).
FIG. 1.
SASP levels in dormant and germinated spores of various strains. Spores were germinated for 60 (wild type) or 120 (cwlD mutant) min in 2×YT medium plus 4 mM l-alanine, SASP were extracted from dormant and germinated spores, aliquots equivalent to ∼1.3 mg of original dormant spores were subjected to polyacrylamide gel electrophoresis at low pH, and the gel was stained as described in the text. The top of the gel is shown at the top, and the symbols on the left denote the positions of SASP-α, -β, and -γ. Lanes: 1, dormant wild-type spores; 2, germinated wild-type spores; 3, dormant cwlD spores; 4, germinated cwlJ spores. Results essentially identical to those obtained with cwlD spores were also obtained with cwlJ sleB spores (data not shown).
FIG. 2.
Light production from spores of various strains carrying luxAB under the control of the sspB promoter. (A) Spores were germinated at an optical density at 600 nm of 1.67 in 3 ml of 2×SG medium plus 0.01% dodecanal (Sigma) as described in the text. (B) Spores were decoated and incubated at 37°C and an optical density at 600 nm of 2.25 in 2 ml of hypertonic medium (22) plus 10 mM l-alanine and 0.01% dodecanal, and at time zero, lysozyme was added to 12.5 μg/ml. In both experiments, light production was measured in a Turner TD-20/20 Luminometer; note that the luminometer sensitivity settings were different in panels A and B, which caused the apparently higher values of relative light units in panel B. Symbols: ○, wild-type spores without the luxAB genes (PS832); ●, luxAB spores (PS3379); ▴, cwlD luxAB spores (PS3380); ■, cwlJ luxAB sleB spores (PS3383).
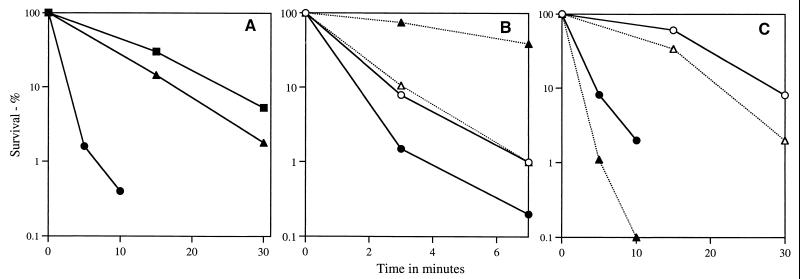
Since the germinated cwlD and cwlJ sleB spores had a lower core water content than germinated wild-type spores, it was expected that the germinated mutant spores would be more resistant to heat than germinated wild-type spores; this was the case when heat resistance was determined as previously described (20) (Fig. 3A). Similarly, the continued presence of α/β-type SASP in the germinated cwlD and cwlJ sleB spores led to the prediction that germinated cwlD and cwlJ sleB spores should retain the UV resistance of dormant spores (31). Indeed, since the germinated cwlD and cwlJ sleB spores had not only retained their UV-protective α/β-type SASP but had also lost their DPA, which sensitizes spores to killing by 254-nm UV radiation (28, 29), it seemed likely that these germinated spores would be significantly more UV radiation resistant than dormant spores. This was indeed the case when spore resistance to 254-nm UV radiation was determined as previously described (20), while germinated wild-type spores were less UV radiation resistant than dormant spores, as expected (Fig. 3B) (31). Analysis of spore hydrogen peroxide resistance as previously described (20) showed that spores of the wild-type, cwlD, and cwlJ sleB strains all lost significant hydrogen peroxide resistance upon germination (Fig. 3C; data not shown). This was not unexpected, since the germinated spores have a higher core water content than dormant spores and core water content is inversely related to spore hydrogen peroxide resistance (23). However, it was surprising that germinated cwlD and cwlJ sleB spores were less hydrogen peroxide resistant than germinated wild-type spores (Fig. 3C; data not shown), as the continued presence of α/β-type SASP in germinated cwlD and cwlJ sleB spores, as well as their lower core water content than that of germinated wild-type spores would have been expected to provide significant hydrogen peroxide resistance (23, 30, 31). One reason for the latter anomaly was revealed when the decomposition of hydrogen peroxide by germinated spores was measured. Wild-type spores germinated in Tris-HCl plus l-alanine for 30 min and washed and suspended at an optical density at 600 nm of 1 in 0.1 M NaCl–25 mM KPO4 (pH 7.5) decomposed >90% of the 0.75% hydrogen peroxide present in 30 min at 24°C (data not shown). This is presumably due to the action of catalases A and X, which are present in germinated spores, with catalase X largely responsible for germinated spore resistance to hydrogen peroxide (1, 3). In contrast, germinated cwlD spores (cwlJ sleB spores were not tested) incubated similarly decomposed <5% of the 0.75% hydrogen peroxide present in 60 min at 24°C (data not shown). Thus, the relative inactivity of catalases in germinated spores which have not undergone cortex degradation may have eliminated a major component of germinated spore hydrogen peroxide resistance and the presence of α/β-type SASP on germinated cwlD spore DNA and the slightly lower core water content of these spores were presumably not sufficient to compensate for the lack of catalase activity.
FIG. 3.
Resistance of dormant and germinated spores of various strains to heat (A) UV radiation (B), and hydrogen peroxide (C). Dormant or germinated spores (30 min in 2×YT medium plus 4 mM l-alanine) were suspended in 10 mM NaPO4–2 mM KPO4 (pH 7.4)–140 mM NaCl–3 mM KCl and either incubated at 60°C (A), irradiated with a short-wave length UV lamp (B) or incubated in 5% hydrogen peroxide (C). Survivors were determined after various incubation times as described in the text, and all values are ±25%. Symbols; ○ and ●, wild-type spores; ▵ and ▴, cwlD spores; ■, cwlJ sleB spores; ○ and ▵, dormant spores; ● and ▴, ■, germinated spores.
The results obtained in this work indicate that spores which have initiated germination but have not degraded their cortex are in an intermediate stage of spore germination, which we propose to call stage I. These spores have released their DPA and presumably much of their divalent cations, and these components appear to have been replaced with some increased core water content, although this is not as high as in germinated wild-type spores, presumably because of the restriction on core expansion due to the continued presence of the cortex. In some other respects, however, these spores held in stage I of germination are more like dormant spores. Thus SASP, in particular α/β-type SASP, are degraded extremely slowly, as is also the case for 3PGA, and the stage I germinated spores have neither ATP nor readily accessible reducing equivalents, as is also the case in dormant spores (30, 32).
The latter observations indicate that there is extremely low activity of a number of enzymes within the core of stage I germinated spores, and this is also suggested by the lack of reduction of a tetrazolium dye and the lack of decomposition of hydrogen peroxide by these spores. Calculations of the relative rates of enzymes within germinated wild-type and cwlD spores from data in Table 1 and Fig. 1 indicate that in wild-type germinated spores, 3PGA utilization is ≥30-fold faster, catalase is ≥40-fold more active, and GPR is ≥4-fold faster on SASP-γ and ≥10-fold faster on SASP-α and -β. The simplest explanation for the low activity of these enzymes or pathways (all of which are in the spore core) in stage I germinated spores is that this is due to the reduced level of core water in these germinated spores, relative to the level of core water in germinated spores which have progressed beyond stage I. Previous work has shown that enzymes in the core of dormant spores are not active, and this appears to be due, in large part, to the low water content of the domant spore (30). Even in dormant spores in which the core water content has been elevated significantly due to loss of DPA, enzyme activity in the dormant spore remains low or absent (20). Since the core water content of spores blocked in stage I of germination is only slightly higher than that in DPA-less dormant spores, as noted above, it seems most likely that the low water content in the core of these germinated spores is what greatly slows or blocks enzyme action. This lower core water content in stage I germinated spores also explains the higher heat resistance of these germinated spores compared to that of germinated wild-type spores, as there is an inverse correlation between core water content and spore heat resistance (5).
During sporulation, cortex synthesis around the developing forespore precedes DPA accumulation, as do loss of forespore ATP and accumulation of 3PGA and SASP, with enzyme action on the latter two compounds being very low in forespores even prior to DPA accumulation (20, 30, 32). Forespores at this stage of development are also more UV radiation resistant than are dormant spores and have acquired some heat resistance, although they are by no means as heat resistant as dormant spores (7, 16, 29). While there are no direct measurements of the core water content of these forespores, several analyses indicate that their core water content is lower than in the forespore compartment when the latter is first established (16). Thus, even though forespores which have acquired a cortex have not yet acquired their mature spore coat, the general properties of forespores at this intermediate stage of sporulation are very similar to those of spores in stage I of germination. If stage I germinated spores are, indeed, functionally equivalent to forespores in an intermediate stage of development, it is tempting to speculate that the latter stage was once the endpoint of the sporulation process, and it was only through further evolution that full spore dormancy and resistance were acquired, with these being attained only upon evolution of the capacity for mother cell synthesis and forespore acquisition of DPA and the attendant further reduction in spore water content. Many bacteria form quiescent, somewhat resistant forms in response to starvation (12), but it is the acquisition of DPA and the extreme degree of core (protoplast) dehydration that sets spores of Bacillus species and those of related genera apart from the quiescent forms of other bacteria.
The ability to isolate large quantities of stage I germinated spores may also provide an opportunity to analyze the state of the inner membrane in these spores. It has been suggested that the spore's inner membrane is in a nearly crystalline state due to its compression in dormant spores prior to the significant (approximately twofold) core volume expansion without any new membrane synthesis that accompanies cortex hydrolysis during spore germination (21, 34). Since this volume increase does not take place in germinated cwlD or cwlJ sleB spores (22; data not shown), the state of the membrane in germinated spores of these strains may be similar or even identical to that in dormant spores. Indeed, this could be one factor lowering catalase activity in germinated cwlD spores, as the state of the stage I germinated spore membrane might slow hydrogen peroxide uptake into the spore core, although this small molecule can, and indeed is expected to, penetrate the dormant spore core (6, 30). However, a number of larger molecules that readily enter the core region of wild-type germinated spores do not enter the core region of spores blocked in stage I of germination, since acridine orange, carbolfuchsin, and 4′,6′-diamino-2-phenylindole, which readily stain the core of wild-type germinated spores, do not stain the core regions of germinated cwlD or cwlJ sleB spores (8, 24; data not shown). While the core of stage I germinated spores is not accessible to stains, it is possible that these spores' inner membrane is accessible to appropriate probes, allowing direct analysis of the state of this membrane; this analysis will be facilitated, since large amounts of stage I germinated spores can be isolated and are stable (>75% survival after 24 h in 0.1 M NaCl–25 mM KPO4 [pH 7.5] at 24°C [data not shown]). Clearly, further analysis of the membrane structure and internal environment of stage I germinated spores will likely prove extremely rewarding.
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM19698) and the Army Research Office.
We are grateful to J. A. Grasso for the use of the luminometer and to P. J. Hill for the gift of plasmid pSB357.
REFERENCES
- 1.Bagyan I, Casillas-Martinez L, Setlow P. The katX gene, which codes for the catalase in spores of Bacillus subtilis, is a forespore-specific gene controlled by ςF, and KatX is essential for hydrogen peroxide resistance of the germinating spore. J Bacteriol. 1998;180:2057–2062. doi: 10.1128/jb.180.8.2057-2062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boland F M, Atrih A, Chirakkal H, Foster S J, Moir A. Complete spore-cortex hydrolysis during germination of Bacillus subtilis 168 requires SleB and YpeB. Microbiology. 2000;146:57–64. doi: 10.1099/00221287-146-1-57. [DOI] [PubMed] [Google Scholar]
- 3.Casillas-Martinez L, Driks A, Setlow P. Lack of a significant role for the PerR regulator in Bacillus subtilis spore resistance. FEMS Microbiol Lett. 2000;188:203–208. doi: 10.1111/j.1574-6968.2000.tb09194.x. [DOI] [PubMed] [Google Scholar]
- 4.Ciarciaglini G, Hill P J, Davies K, McClure P J, Kilsby D, Brown M H, Coote P J. Germination-induced bioluminescence, a route to determine the inhibitory effect of a combination preservation treatment on bacterial spores. Appl Environ Microbiol. 2000;66:3735–3742. doi: 10.1128/aem.66.9.3735-3742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerhardt P, Marquis R E. Spore thermoresistance mechanisms. In: Smith I, Slepecky R A, Setlow P, editors. Regulation of prokaryotic development. Washington, D.C.: American Society for Microbiology; 1989. pp. 43–63. [Google Scholar]
- 6.Gerhardt P, Scherrer R, Black S H. Molecular sieving by dormant spore structures. In: Halvorson H O, Hanson R, Campbell L L, editors. Spores V. Washington, D.C.: American Society for Microbiology; 1972. pp. 68–74. [Google Scholar]
- 7.Germaine G R, Cogglia E, Murrell W G. Development of ultraviolet resistance in sporulating Bacillus cereus T. J Bacteriol. 1973;116:823–831. doi: 10.1128/jb.116.2.823-831.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould G W. Germination. In: Gould G W, Hurst A, editors. The bacterial spore. New York, N.Y: Academic Press, Inc.; 1969. pp. 397–444. [Google Scholar]
- 9.Hill P J, Hall L, Vinicombe D A, Soper C J, Setlow P, Waites W M, Denyer S, Stewart G S A B. Bioluminescence and spores as biological indicators of inimical processes. J Appl Bacteriol. 1994;76:129S–134S. doi: 10.1111/j.1365-2672.1994.tb04364.x. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa S, Yamane K, Sekiguchi J. Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J Bacteriol. 1998;180:1375–1380. doi: 10.1128/jb.180.6.1375-1380.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnstone K, Ellar D J. The role of cortex hydrolysis in the triggering of germination of Bacillus megaterium KM endospores. Biochim Biophys Acta. 1982;714:185–191. [Google Scholar]
- 12.Kolter R, Siegele D A, Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 13.Lindsay J A, Beaman T C, Gerhardt P. Protoplast water content of bacterial spores determined by buoyant density gradient sedimentation. J Bacteriol. 1985;163:735–737. doi: 10.1128/jb.163.2.735-737.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moir A, Smith D A. The genetics of bacterial spore germination. Annu Rev Microbiol. 1990;44:531–553. doi: 10.1146/annurev.mi.44.100190.002531. [DOI] [PubMed] [Google Scholar]
- 15.Moriyama R, Hattori A, Miyata S, Kudoh S, Makino S. A gene (sleB) encoding a spore cortex-lytic enzyme from Bacillus subtilis and response of the enzyme to l-alanine-mediated germination. J Bacteriol. 1996;178:6059–6063. doi: 10.1128/jb.178.20.6059-6063.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murrell W G. Biophysical studies on the molecular mechanisms of spore heat resistance and dormancy. In: Levinson H S, Sonenshein A L, Tipper D J, editors. Sporulation and germination. Washington, D.C.: American Society for Microbiology; 1981. pp. 64–77. [Google Scholar]
- 17.Nelson D L, Spudich J A, Bonsen P P M, Bertsch L L, Kornberg A. Biochemical studies of bacterial sporulation and germination. XVI. Small molecules in spores. In: Campbell L L, editor. Spores IV. Washington, D.C.: American Society for Microbiology; 1969. pp. 59–71. [Google Scholar]
- 18.Nicholson W L, Setlow P. Sporulation, germination, and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley & Sons; 1990. pp. 391–450. [Google Scholar]
- 19.Paidhungat M, Ragkousi K, Setlow P. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J Bacteriol. 2001;183:4886–4893. doi: 10.1128/JB.183.16.4886-4893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paidhungat M, Setlow B, Driks A, Setlow P. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol. 2000;182:5505–5512. doi: 10.1128/jb.182.19.5505-5512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paidhungat, M., and P. Setlow. Spore germination and outgrowth. In J. A. Hoch, R. Losick, and A. L. Sonenshein (ed.), Bacillus subtilis and its relatives: from genes to cells, in press. American Society for Microbiology, Washington, D.C.
- 22.Popham D L, Helin J, Costello C E, Setlow P. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc Natl Acad Sci USA. 1996;93:15405–15410. doi: 10.1073/pnas.93.26.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popham D L, Sengupta S, Setlow P. Heat, hydrogen peroxide, and UV resistance of Bacillus subtilis spores with increased core water content and with or without major DNA binding proteins. Appl Environ Microbiol. 1995;61:3633–3638. doi: 10.1128/aem.61.10.3633-3638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ragkousi K, Cowan A E, Ross M A, Setlow P. Analysis of nucleoid morphology during germination and outgrowth of spores of Bacillus species. J Bacteriol. 2000;182:5556–5562. doi: 10.1128/jb.182.19.5556-5562.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotman Y, Fields M L. A modified reagent for dipicolinic acid analysis. Anal Biochem. 1967;22:168. doi: 10.1016/0003-2697(68)90272-8. [DOI] [PubMed] [Google Scholar]
- 26.Sammons R. Isolation and characterization of germination mutants. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley and Sons; 1990. pp. 418–424. [Google Scholar]
- 27.Sekiguchi J, Akeo K, Yamamoto H, Khasanov F K, Alonso J C, Kuroda A. Nucleotide sequence and regulation of a new putative cell wall hydrolase gene, cwlD, which affects germination in Bacillus subtilis. J Bacteriol. 1995;177:5582–5589. doi: 10.1128/jb.177.19.5582-5589.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setlow B, Setlow P. Absence of transient elevated UV resistance during germination of Bacillus subtilis spores lacking small, acid-soluble spore proteins α and β. J Bacteriol. 1988;170:2858–2859. doi: 10.1128/jb.170.6.2858-2859.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setlow B, Setlow P. Dipicolinic acid greatly enhances the production of spore photoproduct in bacterial spores upon ultraviolet irradiation. Appl Environ Microbiol. 1993;59:640–643. doi: 10.1128/aem.59.2.640-643.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setlow P. Mechanisms which contribute to the long-term survival of spores of Bacillus species. J Appl Bacteriol. 1994;76:49S–60S. doi: 10.1111/j.1365-2672.1994.tb04357.x. [DOI] [PubMed] [Google Scholar]
- 31.Setlow P. Resistance of bacterial spores. In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, D.C.: American Society for Microbiology; 2000. pp. 217–230. [Google Scholar]
- 32.Singh R P, Setlow B, Setlow P. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol. 1977;130:1130–1138. doi: 10.1128/jb.130.3.1130-1138.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spizizen J. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci USA. 1958;44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart G S A B, Eaton M W, Johnstone K, Barratt M D, Ellar D J. An investigation of membrane fluidity changes during sporulation and germination of Bacillus megaterium KM measured by electron spin and nuclear magnetic resonance spectroscopy. Biochim Biophys Acta. 1980;600:270–290. doi: 10.1016/0005-2736(80)90432-0. [DOI] [PubMed] [Google Scholar]
- 35.Tennen R, Setlow B, Davis K L, Loshon C A, Setlow P. Mechanisms of killing of spores of Bacillus subtilis by iodine, glutaraldehyde and nitrous acid. J Appl Microbiol. 2000;89:330–338. doi: 10.1046/j.1365-2672.2000.01114.x. [DOI] [PubMed] [Google Scholar]
- 36.Vary J C. Germination of Bacillus megaterium spores after various extraction procedures. J Bacteriol. 1973;95:1327–1334. doi: 10.1128/jb.116.2.797-802.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]