Abstract
Background and Methods: Severe COVID-19 is known to induce neurological damage (NeuroCOVID), mostly in aged individuals, by affecting brain-derived neurotrophic factor (BDNF), matrix metalloproteinases (MMP) 2 and 9 and the neurofilament light chain (NFL) pathways. Thus, the aim of this pilot study was to investigate BDNF, MMP-2, MMP-9, and NFL in the serum of aged men affected by COVID-19 at the beginning of the hospitalization period and characterized by different outcomes, i.e., attending a hospital ward or an intensive care unit (ICU) or with a fatal outcome. As a control group, we used a novelty of the study, unexposed age-matched men. We also correlated these findings with the routine blood parameters of the recruited individuals. Results: We found in COVID-19 individuals with severe or lethal outcomes disrupted serum BDNF, NFL, and MMP-2 presence and gross changes in ALT, GGT, LDH, IL-6, ferritin, and CRP. We also confirmed and extended previous data, using ROC analyses, showing that the ratio MMPs (2 and 9) versus BDNF and NFL might be a useful tool to predict a fatal COVID-19 outcome. Conclusions: Serum BDNF and NFL and/or their ratios with MMP-2 and MMP-9 could represent early predictors of NeuroCOVID in aged men.
Keywords: BDNF, NFL, NGF, MMP-2, MMP-9, NeuroCOVID, COVID-19, aging
1. Introduction
COVID-19 (coronavirus disease 19) is an acute respiratory syndrome induced by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) [1,2,3,4,5]. The COVID-19 infection has expanded all over the world since December 2019 and has rapidly become one of the greatest challenges of our century, responsible for enormous medical, social, and economic problems. Even though the number of cases and deaths is decreasing from the initial stage, COVID-19 is still a leading cause of death worldwide [6].
One thing we certainly understand about SARS-CoV-2 is that the virus is mutating constantly. Since the beginning of the pandemic, a number of noticeable variants, including Alpha, Beta, Delta, and Omicron have been discovered [7,8,9].
COVID-19 clinical characteristics differ from asymptomatic presence to severe respiratory distress syndrome. The main COVID-19 clinical outcomes are dyspnea, cough, and fever [10,11]. It is known that asymptomatic patients played a significantly crucial role in the transmission of COVID-19 [12]. The progression towards a bad ending is due to several risk factors: a hyper-inflammation state elicited by our immune system, the incidence of comorbidities, the advanced age of the affected individuals, and a higher risk of thrombosis [13,14,15,16].
During the paroxysm inflammation period, cytokine overproduction and the disrupted innate immune response may provoke parenchymal damage in vital organs and consequent failure in different tissues and organs [17]. COVID-19 is known to cause serious damage and greater mortality in aged individuals. However, COVID-19-associated casualties have also been observed in children, adolescents, and young adults [14,18,19]. Indeed, several pieces of evidence disclosed the occurrence of comorbidities, such as diabetes, cancer, and cardiovascular diseases that may influence the prognosis in older individuals with COVID-19 disease [15].
The potential target organs of COVID-19 also include the brain and, more generally, the central and peripheral nervous system (NeuroCOVID), which may be affected during and after the disease [7,8,9]. Neurotrophins are polypeptides recognized to control neuronal survival, growth, and morphology during development and in the adult brain [20,21,22]; they also regulate strategic nerve cell functions such as synaptogenesis, excitability, and brain aging [23,24]. The NGF (nerve growth factor), BDNF (brain-derived neurotrophic factor), neurotrophin-3 (NT-3), NT-4, NT-5, NT-6, and NT-7 are the important neurotrophins with neuroprotective and neurotrophic activities in several nerve cell populations, both in the peripheral and central nervous system. In physiological and/or pathological circumstances, NGF and BDNF, the most studied neurotrophins, influence not only neuronal cells but, likewise, play subtle functions in the endocrine, immune, and cardiometabolic systems to control biological homeostasis [25,26,27,28,29,30,31], crucially regulating inflammatory processes [32,33].
As for the relationship between NeuroCOVID and NGF/BDNF, to date, only a few papers discussed their potential role in this disease. Previous studies debated the role of NGF in pulmonary pathologies, alluding to the possibility of considering the NGF signaling as a potential diagnostic/therapeutic target in SARS-CoV-2-induced pulmonary complications [34], contributing to the antibody production in convalescent COVID-19 [35]. Other investigations proposed that serum BDNF content and the BDNF/adiponectin ratio may serve as predictors of a worsened prognosis in COVID-19, especially for adult male patients [36], with BDNF also playing a subtle role in the neurological and mental outcomes of COVID-19 [37,38].
Our previous data on children and adolescents [18] showing the long-lasting effects of the infection (long-COVID-19) revealed that NGF serum content was lower in post-infected-COVID-19 individuals when compared to healthy controls; interestingly, BDNF levels were found to be higher compared to healthy individuals only in post-infected-COVID-19 symptomatic and future long-COVID-19 girls, leaving the BDNF levels unchanged in asymptomatic individuals when compared to controls [18]. In a recent study, increased NGF and BDNF levels were quantified in saliva and serum during the acute phase of SARS-CoV-2 infection in hospitalized patients, but reduced levels were observed in the remission phase, never matching the baseline values [39]. Other data showed that the matrix metalloproteinase-9 (MMP-9)/BDNF ratio predicts more severe COVID-19 outcomes [40] as MMPs may contribute to the tissue damage induced by COVID-19 [41] as well as to the alteration of blood–brain barrier integrity [42]. NeuroCOVID is also characterized by neurodegeneration and elevations in the neurofilament light chain (NFL) in the blood, which may represent a useful tool to investigate the disease severity in COVID-19-affected individuals [43,44].
Thus, the aim of this pilot study was to investigate the NGF, BDNF, MMP-2, MMP-9, and NFL in the serum of aged men affected by COVID-19 at the beginning of the hospitalization period and characterized by different outcomes, i.e., a hospital ward group vs. an intensive care unit (ICU) group vs. a fatal outcome (deceased) group. As a control group we used, as the main novelty of the study, unexposed age-matched men. We predicted that BDNF and NFL presence in COVID-19 in aged individuals might be associated with severe morbidity and fatal outcomes. We also predicted that the ratios of BDNF and NFL with MMP-2 and MMP-9 could be considered early predictors of COVID-19 mortality. Furthermore, we correlated these findings with some routine laboratory analyses performed on COVID-19 patients such as amylase, lipase, aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transferase (GGT), lactate dehydrogenase (LDH), myoglobin (MGB), total creatine kinase (CK), isoenzyme MB of creatine kinase (CK-MB), high sensitivity troponin-T (TnT), interleukin-6 (IL-6), ferritin, C-reactive protein (CRP), plateletcrit (PCT), D-dimer, platelet count (PLT), prothrombin time (PT), the international normalized ratio (INR), the activated partial thromboplastin time (aPTT), fibrinogen (FBG), and white blood cell (WBC) number.
2. Materials and Methods
2.1. Participant Selection and Study Design
Blood samples were collected from 30 COVID-19-infected male aged patients (randomly chosen in a larger cohort group) at the beginning of their COVID-19 hospital ward permanence in the Sapienza University Hospital “Policlinico Umberto I” in Rome, Italy, between May 2020 and July 2020. At that time, vaccines were not yet available. Accordingly, the COVID-19 individuals were divided into three groups based on their outcomes. The first group included patients who entered the hospital wards and survived (hospital ward group, n = 10; age range in years 74–90). The second group included patients who after the COVID-19 hospital ward attended the COVID-19 intensive care unit and survived (ICU group, n = 10; age range in years 67–93). The third group included patients who after the COVID-19 hospital ward attended the COVID-19 intensive care unit but did not survive (deceased group, n = 10; age range in years 70–95). The real-time reverse-transcription polymerase chain reaction (RT-PCR) test on nasopharyngeal swab samples was used to identify SARS-CoV-2-infected individuals.
As controls, we used the blood samples of aged male healthy volunteers recruited at the Sapienza University blood donor group (control group, n = 10; age range in years 70–85). The main exclusion criteria to avoid any bias in the selection of controls belonging at the beginning of the recruitment to a much larger cohort included other ongoing pathologies and previous inflammatory, endocrine, and autoimmune disorders. We also excluded aged men with diagnosed cardiovascular pathologies that could have biased inflammatory analyses, and previous use of drugs or chemicals that can alter the serum levels of inflammation markers, such as antidepressants, anti-inflammatories, and immunosuppressants. The University Hospital ethical committee approved the study (Ref. 6536), and all study procedures followed the Helsinki Declaration of 1975, as revised in 1983, for human rights and experimentation.
2.2. Blood Withdrawal
According to methods previously described [45], peripheral blood samples of 5 mL were taken from each participant, collected in BD Vacutainer™ serum separation tubes, and centrifuged at 3000 rpm for 15 min to separate serum. Serum was then stored at −80 °C.
2.3. Data Collection
We extracted information on demographic characteristics, laboratory analytical results, and symptoms for each eligible patient. The results of the laboratory tests were collected during hospitalization at the Sapienza University Hospital “Policlinico Umberto I” in Rome. Laboratory analytical results included albumin, glycemia, pancreatic biomarkers (amylase and lipase), liver biomarkers (AST, ALT, and GGT), LDH, cardiac biomarkers (CK, MGB, and TnT), inflammatory biomarkers (IL-6, ferritin, CRP, and PCT), WBC, and coagulation profiles (PLT, PT, INR, PTT, FBG, and D-dimer). Data extracted from the patient medical records were related to cardiovascular diseases, heart failure, diabetes, chronic obstructive pulmonary disease, chronic hepatitis, chronic renal failure, solid neoplasm, leukemia, and possible transplants (Table 1). Recruited patients underwent the pharmacological treatments available at that time, such as antibiotics, anti-inflammatory and antipyretic drugs, and heparin.
Table 1.
Clinical characteristics of COVID-19 patients according to their medical records.
| Hospital Ward | ICU | Deceased | |
|---|---|---|---|
| Symptoms | n = 10 | n = 10 | n = 10 |
| Fever | 6 | 5 | 4 |
| Cough | 5 | 1 | 2 |
| Dyspnea | 2 | 8 | 7 |
| Nausea | 3 | 1 | 0 |
| Dysgeusia | 1 | 0 | 0 |
| Anosmia | 1 | 1 | 0 |
| Arthralgia | 0 | 0 | 0 |
| Diseases | |||
| Cardiovascular Diseases | 6 | 4 | 2 |
| Heart Failure | 2 | 2 | 2 |
| Diabetes | 2 | 3 | 4 |
| Chronic Renal Failure | 1 | 0 | 0 |
| COPD | 1 | 1 | 0 |
| Chronic Liver Diseases | 1 | 1 | 1 |
| Solid Neoplasm | 1 | 1 | 0 |
| Hematological Neoplasm | 1 | 0 | 0 |
| Transplant | 0 | 0 | 0 |
2.4. NGF and BDNF Serum Evaluation
NGF (Cat. No. DY256) and BDNF (Cat. No. DY248) were measured using a sandwich enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA), according to the protocols provided by the manufacturer [46]. Serum samples were diluted 2- and 100-fold with PBS for the detection of NGF and BDNF, respectively. The colorimetric reaction product was measured at 450 nm using a microplate reader (Dynatech MR 5000, PBI International, Washington, DC, USA). Data are represented as pg/mL, and all assays were performed in duplicate, which was averaged for statistical comparison [47].
2.5. NFL Serum Evaluation
As previously described [42,48], the evaluation of NFL levels in collected samples was assessed using the Simple PlexTM Ella assay (ProteinSimple, San Jose, CA, USA) on EllaTM microfluidic system (Bio-Techne, Minneapolis, MN, USA) according to the manufacturer’s instructions. EllaTM was calibrated using the in-cartridge factory standard curve. The limit of detection of NFL was 1.09 pg/mL, calculated by adding three standard deviations to the mean background signal determined from multiple runs.
2.6. MMP-2 and MMP-9 Serum Evaluation Using Zymography
As previously described [49], MMP-2 and MMP-9 serum activities were detected using zymography as white bands of digestion on the blue background of the gel and were identified by co-localization on the zymogram with human MMP-2 or MMP-9 standards (ALEXIS Biochemicals, San Diego, CA, USA). Quantitation of MMP-2 and MMP-9 serum activities were performed using computerized image analysis (Image Master 1D, Pharmacia Biotech, Buckinghamshire, UK) through one-dimensional scanning densitometry (Ultroscan XL, Pharmacia Biotech). MMP serum activities were expressed as optical density (OD) × mm2, representing the scanning area under the curves, which considers both brightness and width of the substrate lysis zone.
2.7. Laboratory Examination
The patient’s peripheral blood was collected in BD vacutainer® tubes for blood testing at the entrance of the hospital ward group and at the beginning of the intensive care period for both the ICU and deceased patient groups. The additives present in vacutainers were EDTA or sodium citrate as anticoagulants and separating gel from serum. Coagulation parameters were analyzed using a BCS XP System automatic hemostasis analyzer (Siemens Healthcare, Germany). Immunoturbidimetric assay and modified Claus method were used, respectively, for D-dimer (reference range: 50–420 μg/L) and fibrinogen (reference range: 1.5–4 g/L); the interassay coefficient of variation (CV), tested in normal and pathological control sera, was, respectively, between 2.2 and 4.3% and between 1.6 and 3.4%. PT (reference range: 11–16 s), INR (reference range: 0.8–1.2), and APTT (reference range: 25–35 s) were determined coagulometrically; the interassay coefficient of variation (CV), tested in normal and pathological samples, was between 1.5 and 2.2% for PT and between 0.3 and 2.8% for aPTT. The number of platelets (reference range: 150–450 cells/μL) and WBC (reference range: 4400–11,300 cells/L) was determined using ADVIA 2120i Hematology System (Siemens Healthcare, Germany). Tissue biomarkers included albumin (reference range: 35–55 g/L), glucose (reference range: 70.3–100.9 mg/dL), amylase (reference range: 28–100 U/L), lipase (reference range: 13–60 U/L), AST (reference range: 9–45 U/L), ALT (reference range: 10–40 U/L), GGT (reference range: 8–61 U/L), CK (reference range: 20–200 U/L), ferritin (reference range: male 30–400 μg/L; female 15–150 μg/L), CRP (reference range: 100–6000 μg/L), and LDH (reference range: 135–225 U/L) that were measured using standard colorimetric and enzymatic method on a Cobas C 501 analyzer with reagents supplied by Roche Diagnostics GmbH (Mannheim, Germany). CV was, respectively, 0.9% at serum albumin of 51.3 g/L, 1.1% at serum glucose of 95.1 mg/dL, 2.4% at a serum amylase of 35 U/L, 1.4% at a serum lipase of 48 U/L, 2.3% at a serum AST of 30 U/L, 2.6% at a serum ALT of 24 U/L, 3.2% at a serum GGT of 46.8 U/L, 3.2% at a serum CK of 18.7 U/L, 2.8% at serum ferritin of 26.1 μg/L, 1.3% at a serum CRP of 39.9 mg/L, and 2.7% at a serum LDH of 124 U/L. CK-MB (reference range: until 4.94 μg/L), MGB (reference range: 28–72 μg/L), TnT (reference range: until 0.014 μg/L), IL-6 (reference range: 1.5–7 pg/mL), and PCT (reference range: 0.02–0.064 ng/mL) were measured on a Cobas E 601 analyzer, using sandwich immunological methods with reagents supplied by Roche Diagnostics GmbH (Mannheim, Germany). The interassay CV was, respectively, 1.4% at a serum CK-MB of 5.34 ng/mL, 1.9% at a serum MGB of 60.5 ng/mL, 2.7% at a serum TnT of 0.017 μg/L, 3.1% at a serum IL-6 of 12.1 pg/mL, and 8.7% at a serum PCT of 0.08 ng/mL.
2.8. Statistical Analysis
According to methods previously described [50,51], data were analyzed to assess normality using Pearson’s chi-square test. The analysis of variance (ANOVA) was used to analyze the laboratory parameters (hospital ward vs. ICU vs. deceased vs. control groups). Post-hoc comparisons were carried out using Tukey’s HSD test [52]. The Spearman correlation test was used to investigate the correlation between the laboratory data and the age of the patients. A receiver operating characteristic (ROC) analysis was performed to measure the diagnostic/predictive accuracy of the ratios MMP-x/NGF-BDNF-NFL. Only area under the curve (AUC) values >9 were considered as predictive scores.
3. Results
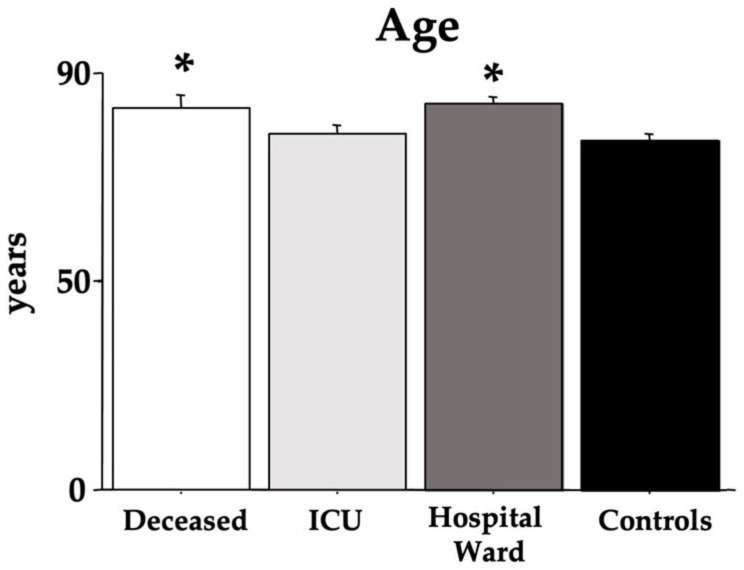
The age of the recruited COVID-19 individuals and controls is shown in Figure 1. ANOVA did evidence an effect of morbidity. Indeed, both the deceased and hospital ward groups had higher ages compared to the ICU and control groups [F(3,36) = 4.72, p < 0.01; see the post hoc differences in the picture].
Figure 1.
Age of the COVID-19 patients. The error bars indicate pooled standard error means (SEM) derived from the appropriate error mean square in the ANOVA. The asterisks (p < 0.05) indicate post hoc differences with controls.
The clinical characteristics of COVID-19 patients according to their medical records are shown in Table 1. As expected, in the deceased group, the presence of previous cardiovascular diseases and diabetes was observed. Vital signs (heart rate, temperature, blood pressure, respiratory rate, and oxygen saturation) were not considered and correlated with the blood parameters as these findings were not recorded for all patients at the moment of blood withdrawal.
Figure 2 shows the data on NGF and BDNF. ANOVA NGF data did not disclose differences between the groups [F(3,36) = 2.01, p = 0.12]. However, BDNF values were significantly lower in all COVID-19 groups (deceased, ICU, and hospital ward) compared to control individuals [F(3,36) = 11.34, p < 0.01; see the post hoc differences in the picture].
Figure 2.
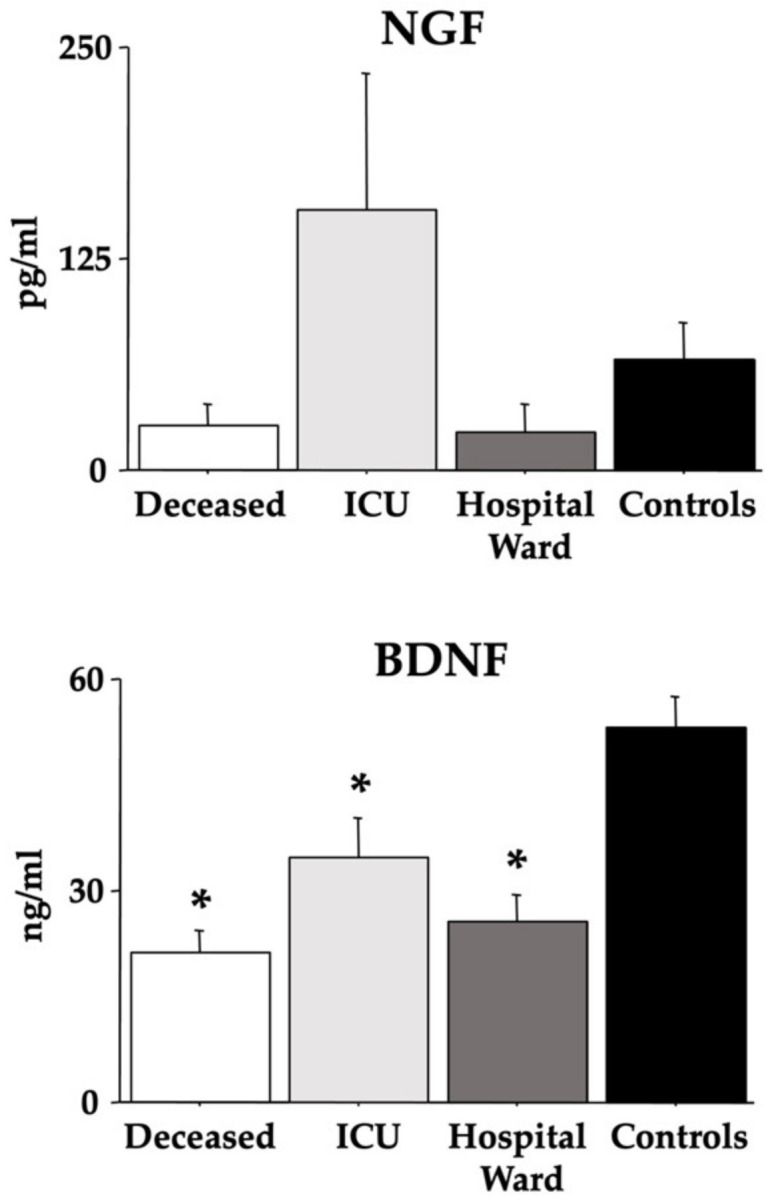
Serum NGF and BDNF in COVID-19 patients. The error bars indicate pooled standard error means (SEM) derived from the appropriate error mean square in the ANOVA. The asterisks (p < 0.05) indicate post hoc differences with controls.
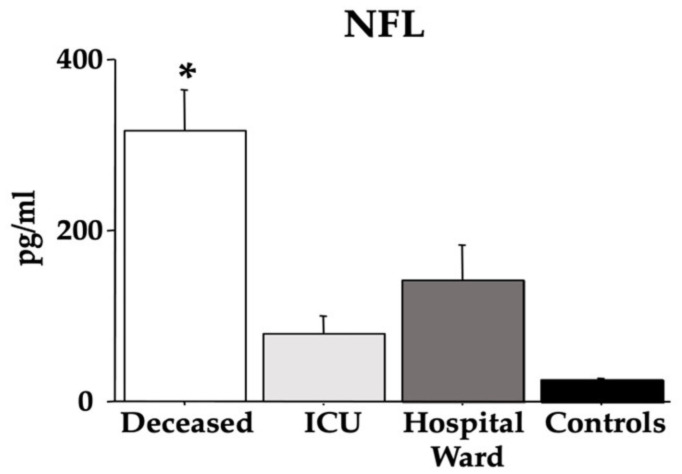
Figure 3 reports the results of the NFL. Quite interestingly, the deceased group displayed the highest values compared with the other groups of individuals (control, ICU, and hospital ward) [F(3,36) = 14.94, p < 0.01; see the post hoc differences in the picture].
Figure 3.
Serum NFL in COVID-19 patients. The error bars indicate pooled standard error means (SEM) derived from the appropriate error mean square in the ANOVA. The asterisk (p < 0.05) indicates post hoc differences between the deceased group and ICU, hospital ward, and control individuals.
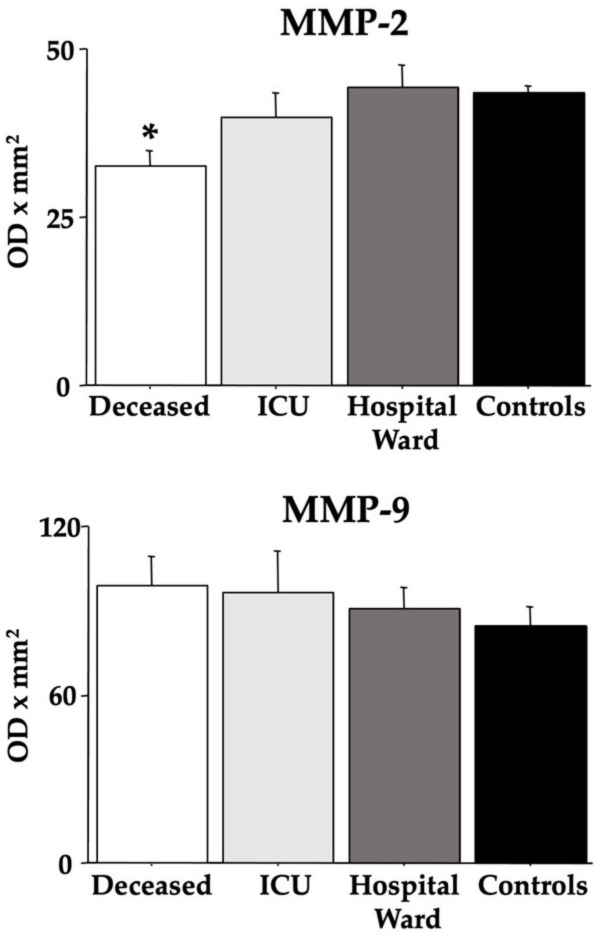
Figure 4 presents the MMP-2 and MMP-9 data. ANOVA revealed low MMP-2 values in the deceased group compared to both the control and hospital ward groups [F(3,36) = 3.74, p = 0.019; see the post hoc differences in the picture], but for the MMP-9 data, there were no differences between groups [F(3,36) = 0.38, p = 0.76].
Figure 4.
Serum MMP-2 and MMP-9 in COVID-19 patients. The error bars indicate pooled standard error means (SEM) derived from the appropriate error mean square in the ANOVA. The asterisk (p < 0.05) indicates post hoc differences with controls.
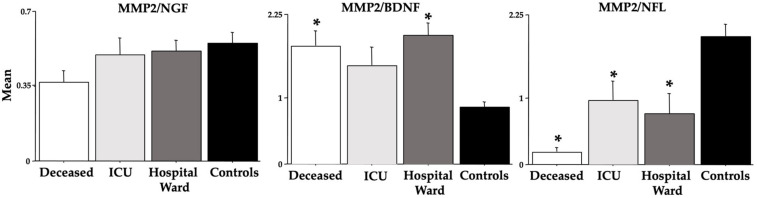
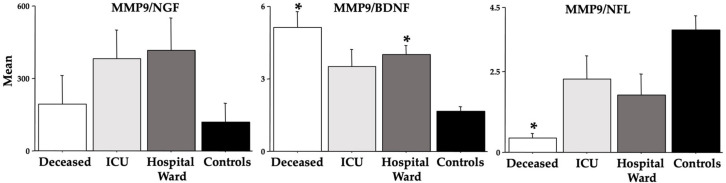
Figure 5 and Figure 6 show the ratios between the analyzed MMPs and NGF, BDNF, and NFL. Indeed, for the deceased group, the MMPs/BDNF ratios were higher [F(3,36) = 7.64 (MMP-9), 5.10 (MMP-2), ps < 0.01; see post hoc differences in the figure], but the MMPs/NFL ratios were lower [F(3,36) = 6.55 (MMP-9), 9.60 (MMP-2), ps < 0.01; see post hoc differences in the figure].
Figure 5.
MMP-2 ratios vs. NGF, BDNF, and NFL in COVID-19 patients. The error bars indicate pooled standard error means (SEM) derived from the appropriate error mean square in the ANOVA. The asterisk (p < 0.05) indicates post hoc differences with controls.
Figure 6.
MMP-9 ratios vs. NGF, BDNF, and NFL in COVID-19 patients. The error bars indicate pooled standard error means (SEM) derived from the appropriate error mean square in the ANOVA. The asterisk (p < 0.05) indicates post hoc differences with controls.
Table 2 shows the AUC scores of the ROC curves for the MMP-2/NGF, MMP-2/BDNF, MMP-2/NFL, MMP-9/NGF, MMP-9/BDNF, and MMP-9/NFL ratios. The highest scores (in bold) were disclosed in the deceased group, in particular for the MMP-2/BDNF, MMP-2/NFL, MMP-9/BDNF, and MMP-9/NFL ratios.
Table 2.
AUC scores for the MMP-2/NGF, MMP-2/BDNF, MMP-2/NFL, MMP-9/NGF, MMP-9/BDNF, and MMP-9/NFL ratios. The highest scores (in bold) were disclosed in the deceased group.
| Deceased vs. Controls | ICU vs. Controls | |||
|---|---|---|---|---|
| The Area under the Curve (AUC) |
95% CI for AUC | The Area under the Curve (AUC) |
95% CI for AUC | |
| MMP-2/NGF | 0.85 | 0.675–1 | 0.620 | 0.358–0.882 |
| MMP-2/BDNF | 0.940 | 0.848–1 | 0.755 | 0.499–1 |
| MMP-2/NFL | 1 | 1–1 | 0.835 | 0.620–1 |
| MMP-9/NGF | 0.710 | 0.467–0.953 | 0.620 | 0.333–0.907 |
| MMP-9/BDNF | 0.990 | 0.962–1 | 0.755 | 0.499–1 |
| MMP-9/NFL | 1 | 1–1 | 0.835 | 0.62–1 |
Table 3 shows the Spearman correlations between age and NGF, BDNF, NFL, MMP-9, and MMP-2). No correlations were disclosed for the COVID-19 individuals (all groups or in the deceased group), but a positive correlation with age was revealed for NFL in control individuals.
Table 3.
Spearman correlation values for the age parameters in all groups. Significant scores are shown in bold.
| Controls | All COVID-19 Groups | Deceased | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SSD | Rho | p-Value | SSD | Rho | p-Value | SSD | Rho | p-Value | |
| NGF | 267.000 | −0.618 | 0.063 | 4229.500 | 0.059 | 0.750 | 150.500 | 0.088 | 0.792 |
| BDNF | 234.500 | −0.421 | 0.206 | 6018.500 | −0.338 | 0.0680 | 148.500 | 0.100 | 0.7642 |
| NFL | 42.500 | 0.742 | 0.025 | 3067.000 | 0.318 | 0.0871 | 160.500 | 0.027 | 0.082 |
| MMP-2 | 96.500 | 0.415 | 0.213 | 4338.000 | 0.035 | 0.850 | 127.500 | 0.227 | 0.495 |
| MMP-9 | 165.500 | −0.003 | 0.992 | 4264.000 | 0.051 | 0.782 | 146.500 | 0.112 | 0.7366 |
Table 4 shows the ANOVA data on amylase, lipase, AST, ALT, GGT, LDH, MGB, CK, CK-MB, TnT, IL-6, ferritin, CRP, PCT, D-dimer, PLT, PT, INR, aPTT, FBG, and WBC. Data revealed the expected increasing effect of COVID-19 for ALT, GGT, LDH, ferritin, and CRP in the deceased group and for IL-6 in the ICU individuals. An elevation in ALT values was also observed in the hospital ward group compared to controls.
Table 4.
ANOVA data for some routine blood parameters in all groups. Significant scores are shown in bold. The asterisks indicate significant differences with controls.
| Deceased | ICU | Hospital Ward | Controls | p | F, dF | |
|---|---|---|---|---|---|---|
| Amylase in U/L | 99.6 ± 36.74 | 91.20 ± 9.77 | 51.10 ± 5.30 | 62.30 ± 6.61 | 0.25 | (3,36) = 1.40 |
| Lipase in U/L | 50.80 ± 9.00 | 42.50 ± 5.82 | 35.20 ± 5.52 | 34.60 ± 3.51 | 0.24 | (3,36) = 1.46 |
| AST in U/L | 39.80 ± 12.36 | 22.80 ± 3.38 | 30.60 ± 4.47 | 17.25 ± 1.25 | 0.16 | (3,36) = 1.80 |
| ALT in U/L | 36.70 ± 11.36 * | 29.5 ± 5.25 | 38.90 ± 8.11 * | 6.12 ± 0.51 | 0.033 | (3,36) = 3.32 |
| GGT in U/L | 73.60 ± 17.39 * | 46.90 ± 11.09 | 26.40 ± 5.34 | 17.20 ± 2.32 | 0.003 | (3,36) = 5.44 |
| LDH in U/L | 367.00 ± 80.37 * | 242.90 ± 26.18 | 245.70 ± 27.33 | 133.40 ± 6.01 | 0.008 | (3,36) = 4.56 |
| MGB in µg/L | 54.20 ± 7.66 | 77.59 ± 23.31 | 50.50 ± 8.69 | 57.36 ± 7.53 | 0.50 | (3,36) = 0.78 |
| CK in U/L | 103.20 ± 27.74 | 100.80 ± 28.44 | 102.80 ± 46.55 | 84.70 ± 8.58 | 0.96 | (3,36) = 0.08 |
| CK-MB | 3.39 ± 0.34 | 2.42 ± 0.20 | 3.12 ± 0.18 | 2.77 ± 0.25 | 0.062 | (3,36) = 2.66 |
| TnT in µg/L | 0.039 ± 0.018 | 0.042 ± 0.017 | 0.021 ± 0.013 | 0.013 ± 0.002 | 0.39 | (3,36) = 1.03 |
| IL-6 in pg/L | 65.54 ± 21.33 | 157.19 ± 70.13 * | 71.32 ± 20.09 | 3.71 ± 0.39 | 0.056 | (3,36) = 2.75 |
| Ferritin in µg/L | 1890.80 ± 733.13 * | 656.30 ± 149.31 | 1029.40 ± 337.89 | 130.00 ± 50.66 | 0.033 | (3,36) = 3.24 |
| CRP in µg/L | 53277 ± 14763 * | 38542 ± 14890 | 39440 ± 15520 | 1560 ± 361.23 | 0.049 | (3,36) = 2.88 |
| PCT in ng/L | 0.841 ± 0.423 | 2.120 ± 1.935 | 4.332 ± 2.559 | 0.046 ± 0.003 | 0.277 | (3,36) = 1.33 |
| D-dimer in µg/L | 2389 ± 513.44 | 2091 ± 406.04 | 2041 ± 476.70 | 1078 ± 258.6 | 0.166 | (3,36) = 1.79 |
| PLT in n/L | 202.90 ± 25.54 | 227.10 ± 26.28 | 199.30 ± 24.43 | 195.90 ± 12.45 | 0.766 | (3,36) = 0.38 |
| PT in sec | 12.41 ± 0.46 | 13.16 ± 1.47 | 11.60 ± 0.034 | 13.28 ± 1.89 | 0.756 | (3,36) = 0.39 |
| INR in (patient’s PT/control PT) | 1.06 ± 0.04 | 1.16 ± 0.14 | 1.00 ± 0.03 | 1.22 ± 0.19 | 0.620 | (3,36) = 0.59 |
| aPTT in sec | 33.13 ± 1.65 | 34.07 ± 6.67 | 34.79 ± 2.61 | 34.36 ± 3.43 | 0.992 | (3,36) = 0.03 |
| FBG in g/L | 3.85 ± 0.35 | 4.51 ± 0.43 | 4.03 ± 0.36 | 3.92 ± 0.46 | 0.667 | (3,36) = 0.52 |
| WBC | 9114 ± 2060 | 6389 ± 1073 | 7232 ± 1555 | 7791 ± 1145 | 0.636 | (3,36) = 0.57 |
Table 5 shows, for the individuals of the deceased group, the Spearman correlations between the blood parameters and NGF/BDNF/NFL/MMP-2/MMP-9, and the positive values are displayed in bold. Indeed, MGB correlates positively with BDNF, CK with NFL, and MMP-2 with different trends, and FBG correlates negatively with BDNF, MMP-2, and MMP-9. No correlations were disclosed for NGF.
Table 5.
Spearman correlations of the individuals of the deceased group between the blood parameters and NGF/BDNF/NFL/MMP-2/MMP-9. Significant scores are shown in bold.
| Spearman Correlation Data | Deceased Patients | |||||
|---|---|---|---|---|---|---|
| NGF | BDNF | NFL | MMP-2 | MMP-9 | ||
| Amylase | SSD Rho p-value |
156.00 | 88.00 | 194.00 | 125.50 | 118.00 |
| 0.055 | 0.467 | −0.176 | 0.239 | 0.285 | ||
| 0.870 | 0.161 | 0.598 | 0.472 | 0.392 | ||
| Lipase | SSD Rho p-value |
154.00 | 124.00 | 138.00 | 128.50 | 102.00 |
| 0.067 | 0.248 | 0.164 | 0.221 | 0.382 | ||
| 0.841 | 0.456 | 0.623 | 0.506 | 0.252 | ||
| AST | SSD Rho p-value |
247.50 | 102.50 | 181.50 | 201.50 | 118.50 |
| −0.500 | 0.379 | −0.100 | −0.221 | 0.282 | ||
| 0.133 | 0.255 | 0.764 | 0.506 | 0.397 | ||
| ALT | SSD Rho p-value |
255.00 | 202.00 | 110.00 | 262.50 | 205.00 |
| −0.545 | −0.224 | 0.333 | −0.591 | −0.242 | ||
| 0.101 | 0.501 | 0.317 | 0.076 | 0.467 | ||
| GGT | SSD Rho p-value |
250.00 | 162.00 | 152.00 | 226.50 | 216.00 |
| −0.515 | 0.018 | 0.079 | −0.373 | −0.309 | ||
| 0.122 | 0.956 | 0.813 | 0.263 | 0.353 | ||
| LDH | SSD Rho p-value |
226.00 | 216.00 | 150.00 | 202.50 | 180.00 |
| −0.370 | −0.309 | 0.091 | −0.227 | −0.091 | ||
| 0.267 | 0.353 | 0.785 | 0.495 | 0.785 | ||
| MGB | SSD Rho p-value |
180.00 | 32.00 | 254.00 | 58.50 | 70.00 |
| −0.091 | 0.806 | −0.539 | 0.645 | 0.576 | ||
| 0.785 | 0.156 | 0.105 | 0.052 | 0.084 | ||
| CK | SSD Rho p-value |
210.00 | 60.00 | 306.00 | 25.50 | 118.00 |
| −0.273 | 0.636 | −0.855 | 0.845 | 0.285 | ||
| 0.413 | 0.056 | 0.010 | 0.011 | 0.392 | ||
| CK-MB | SSD Rho p-value |
194.00 | 104.50 | 182.00 | 112.50 | 136.00 |
| −0.176 | 0.370 | −0.103 | 0.318 | 0.176 | ||
| 0.598 | 0.267 | 0.757 | 0.339 | 0.598 | ||
| TNT | SSD Rho p-value |
293 | 137.00 | 242.00 | 118.00 | 192.00 |
| −0.448 | 0.170 | −0.467 | 0.285 | −0.164 | ||
| 0.178 | 0.610 | 0.161 | 0.392 | 0.623 | ||
| IL-6 | SSD Rho p-value |
114.00 | 238.00 | 108.00 | 222.50 | 206.00 |
| 0.309 | −0.442 | 0.345 | −0.348 | −0.248 | ||
| 0.353 | 0.184 | 0.300 | 0.295 | 0.456 | ||
| Ferritin | SSD Rho p-value |
234.00 | 146.00 | 192.00 | 101.50 | 160.00 |
| −0.418 | 0.115 | −0.164 | 0.385 | 0.030 | ||
| 0.209 | 0.729 | 0.623 | 0.248 | 0.927 | ||
| CRP | SSD Rho p-value |
138.00 | 206.00 | 212.00 | 168.50 | 224.00 |
| 0.164 | −0.248 | −0.285 | −0.021 | −0.358 | ||
| 0.623 | 0.456 | 0.392 | 0.941 | 0.283 | ||
| PCT | SSD Rho p-value |
122.00 | 124.00 | 222.00 | 95.50 | 128.00 |
| 0.261 | 0.248 | −0.345 | 0.421 | 0.224 | ||
| 0.434 | 0.456 | 0.300 | 0.206 | 0.501 | ||
| PLT | SSD Rho p-value |
180.00 | 158.00 | 138.00 | 212.50 | 170.00 |
| −0.091 | 0.042 | 0.164 | −0.288 | −0.030 | ||
| 0.785 | 0.898 | 0.623 | 0.387 | 0.927 | ||
| PT | SSD Rho p-value |
107.50 | 161.50 | 118.50 | 189.00 | 111.50 |
| 0.348 | 0.021 | 0.282 | −0.145 | 0.324 | ||
| 0.295 | 0.949 | 0.397 | 0.662 | 0.330 | ||
| INR | SSD Rho p-value |
188.00 | 174.00 | 100.00 | 171.50 | 114.00 |
| −0.139 | −0.055 | 0.394 | −0.039 | 0.309 | ||
| 0.675 | 0.870 | 0.237 | 0.905 | 0.353 | ||
| aPTT | SSD Rho p-value |
150.00 | 156.00 | 176.00 | 141.50 | 146.00 |
| 0.091 | 0.055 | −0.067 | 0.142 | 0.115 | ||
| 0.785 | 0.870 | 0.841 | 0.669 | 0.729 | ||
| FBG | SSD Rho p-value |
158.00 | 282.00 | 90.00 | 238.50 | 276.00 |
| 0.042 | −0.709 | 0.455 | −0.718 | −0.673 | ||
| 0.898 | 0.033 | 0.172 | 0.031 | 0.043 | ||
| D-dimer | SSD Rho p-value |
200.50 | 231.50 | 154.50 | 171.00 | 215.50 |
| −0.215 | −0.430 | 0.064 | −0.036 | −0.306 | ||
| 0.518 | 0.226 | 0.848 | 0.913 | 0.358 | ||
| WBC | SSD Rho p-value |
122.00 | 196.00 | 114.00 | 225.50 | 206.00 |
| 0.261 | −0.188 | 0.309 | −0.367 | −0.248 | ||
| 0.434 | 0.573 | 0.353 | 0.271 | 0.456 | ||
4. Discussion
This is the first study to demonstrate that, by using healthy age-matched controls, severe or lethal COVID-19 in aged, hospitalized men heavily disrupted BDNF, NFL, and MMP-2 presence in the serum of infected individuals, whereas no differences between groups were found for NGF and MMP-9. We also confirmed and extended previous data [40] showing that the ratios of MMPs (2 and 9) versus BDNF and NFL might be a useful tool to predict a fatal COVID-19 outcome. As for the routine laboratory data, as previously shown in many studies, we found gross changes in ALT, GGT, LDH, IL-6, ferritin, and CRP are a consequence of severe COVID-19 in aged men.
Neurotrophins, such as NGF and BDNF, have multiple roles in different settings, including neuronal development and survival and function in both the central and peripheral nervous systems from early stages [53,54,55]. Alterations in NGF and/or BDNF have been associated with several pathologic manifestations, including behavioral aberrations, cognitive deficiencies, tumorigenesis, obesity, and epilepsy, as well as muscle-skeletal, inflammatory, and pain sensitivity diseases [56,57]. In previous studies, a correlation was found in COVID-19 patients between low BDNF presence and neurological or cognitive decline [58,59]. These findings are in line with the present study showing low BDNF in all COVID-19 individuals with no differences related to their outcome. However, another investigation revealed increased NGF and BDNF in saliva and serum during the acute COVID-19 phase, but reduced levels were observed 6 months after the acute phase [39].
Regarding the serum NFL, we found potentiated values only in individuals with a lethal outcome. Indeed, blood NFL has been proposed to act as an assessment of COVID-19 severity in hospitalized patients and a NeuroCOVID biomarker. It has been previously shown that NFL concentrations in the plasma of COVID-19 within 5 days of hospital admission were elevated [43]. This NFL elevation was associated with worse clinical outcomes, including the need for intensive care, prolonged hospitalization, mechanical ventilation, and higher functional disability at the discharge [43]. In a NeuroCOVID study, infected patients with severe neurological manifestations (encephalopathy, meningoencephalitis, disrupted movement disorder, or stroke) had increased NFL in the cerebrospinal fluid. Another investigation speculated that serum NFL could be associated with severe outcomes in geriatric patients [60].
As for the modifications we found in MMP-2 and the absence of alterations in MMP-9, it is well known that COVID-19 predominantly affected the respiratory tract leading to acute lung failure as the most severe outcome. It is also well established that pulmonary infection may be associated with hyper-inflammation and tissue damage. The MMPs may have a role in lung damage in many pathological situations leading also to the release of bioactive molecules with inflammatory action [61,62,63]. Previous COVID-19 findings showed elevated MMP-2 and MMP-9 levels in hospitalized patients [62,64]. Upregulated MMP-9 genes in COVID-19 patients, together with an increased level of MMP-9, was associated with a risk of respiratory failure [65]. Other researchers, however, found low levels of MMP-2 [66]. Changes in MMPs in severe COVID-19 patients may play subtle roles in disrupting the alveolar–capillary barrier, drawing inflammatory cells and injuring the lung parenchyma [67]. Indeed, the degradation in connexins, elastin, and extracellular collagen by MMPs might be potentiated by the stiffer collagen rapid replacement, which could induce fibrosis and other COVID-19 problems [67].
We also investigated the ratios MMP-2/-9 vs. NGF/BDNF/NFL because it was proposed as a novel method to predict COVID-19 morbidity and mortality [40]. We found significant differences due to COVID-19 disease severity for both BDNF and NFL but not for NGF in the ratios with MMP-2 or MMP-9 (see Figure 5 and Figure 6). In particular, high ratios which were severity-related were found between MMP-2-9 and BDNF, but low ratios which were severity-related were evidenced between MMP-2/-9 and NFL. In a study by Savic et al. [40], although no modifications in BDNF were disclosed, the MMP-9/BDNF ratio was significantly depleted in severely and critically COVID-19 patients, recommending this ratio as a predictor of COVID-19 severity. Based on the ratio data of the present research, we extend to MMP-2 and NFL as markers of COVID-19 morbidity and mortality as also shown by the AUC data of the ROC curves.
As for the laboratory data, we discovered in patients of the deceased group potentiations in ALT, GGT, LDH, ferritin, and CRP. In our previous investigation carried out always at the Sapienza University Hospital “Policlinico Umberto I” [1], we studied early blood routine parameters as crucial in predicting biomarkers of COVID-19 morbidity and mortality. We have demonstrated that already at admission in the emergency section, COVID-19 individuals who underwent a lethal outcome displayed higher concentrations of AST, ALT, MGB, LDH, ferritin, CRP, and D-dimer compared to patients who were discharged from the emergency room, confirming and extending the huge plethora of COVID-19 laboratory data available in the scientific literature [68,69,70]. Indeed, clinical laboratory professionals are continuously searching for reliable parameters associated with SARS-CoV(x) diseases for the proper clinical cure as blood biomarkers are commonly used to recognize, during the early stages of disease, patients at a higher risk of developing severe outcomes [1]. Spearman correlations scores (Table 5) underline the MGB and FBG ANOVA findings for the individuals of the deceased group.
5. Conclusions
The strength and novelty of this investigation were to study neuroinflammatory biomarkers in COVID-19-aged men according to their outcomes during the pandemic paroxysm wave and in absence of vaccines and compare these data with the findings obtained in healthy age-matched men. Of course, the absence of data on women and the relatively small number of the recruited individuals in this research could be putative limitations of the research. However, its strength depends on the choice of restricted recruitment guidelines.
Furthermore, with the purpose of showing other main potential COVID-19 biomarkers [1,50], this pilot study provides additional information aimed at disclosing further biomolecular events consequent to SARS-CoV-2 infection. Particularly, serum BDNF and NFL could represent a new tool as early predictors of NeuroCOVID effects in aged men associated with their ratios with MMP-2 and MMP-9. However, further investigations on the molecular and cellular link between neuroinflammation and neurodegeneration in COVID-19 are needed.
Acknowledgments
Authors thank IBBC-CNR and Sapienza University of Rome, Italy, for the financial and logistic support.
Author Contributions
M.F., M.A.Z., C.B., G.F., F.M.C., P.P., M.R.C., M.M., E.E., G.M.L., T.L., A.M. and C.P.: protocol/project development and data analysis; M.F., A.A., M.M., E.E., M.A.Z., C.B., G.F., F.M.C., P.P., M.R.C., G.M.L., T.L., A.M. and C.P: data collection or management; M.F.: subtle data analysis; C.B. and M.F.: manuscript writing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The University Hospital Policlinico Umberto I ethical committee approved the study (Prot. 6536), and all the study procedures followed the Helsinki Declaration of 1975, as revised in 1983, for human rights and experimentation.
Informed Consent Statement
As this is a retrospective study, no informed consent is needed for the COVID-19 patients with different outcomes. However, the healthy individuals, whose serum was used as control serum, signed an informed consent.
Data Availability Statement
Data are available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by grant Prot. N. 0000625 by BANCA D’ITALIA to A.M.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ceci F.M., Fiore M., Gavaruzzi F., Angeloni A., Lucarelli M., Scagnolari C., Bonci E., Gabanella F., Di Certo M.G., Barbato C., et al. Early Routine Biomarkers of SARS-CoV-2 Morbidity and Mortality: Outcomes from an Emergency Section. Diagnostics. 2022;12:176. doi: 10.3390/diagnostics12010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshimoto F.K. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2 or n-COV19), the Cause of COVID-19. Protein J. 2020;39:198–216. doi: 10.1007/s10930-020-09901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pillay T.S. Gene of the month: The 2019-nCoV/SARS-CoV-2 novel coronavirus spike protein. J. Clin. Pathol. 2020;73:366–369. doi: 10.1136/jclinpath-2020-206658. [DOI] [PubMed] [Google Scholar]
- 4.Sallard E., Halloy J., Casane D., Decroly E., van Helden J. Tracing the origins of SARS-CoV-2 in coronavirus phylogenies: A review. Environ. Chem. Lett. 2021;19:769–785. doi: 10.1007/s10311-020-01151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoccali F., Cambria F., Colizza A., Ralli M., Greco A., de Vincentiis M., Petrella C., Fiore M., Minni A., Barbato C. Sudden Sensorineural Hearing Loss after Third Dose Booster of COVID-19 Vaccine Administration. Diagnostics. 2022;12:2039. doi: 10.3390/diagnostics12092039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortaliza J., Amin K., Cox C. COVID-19 Leading Cause of Death Ranking—Peterson-KFF Health System Tracker n.d. [(accessed on 25 January 2023)]. Available online: https://www.healthsystemtracker.org/brief/covid-19-leading-cause-of-death-ranking/
- 7.Forchette L., Sebastian W., Liu T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr. Med. Sci. 2021;41:1037–1051. doi: 10.1007/s11596-021-2395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassine I.H. COVID-19 vaccines and variants of concern: A review. Rev. Med. Virol. 2022;32:e2313. doi: 10.1002/rmv.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araf Y., Akter F., Tang Y., Fatemi R., Parvez M.S.A., Zheng C., Hossain G. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2022;94:1825–1832. doi: 10.1002/jmv.27588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safari I., Elahi E. Evolution of the SARS-CoV-2 genome and emergence of variants of concern. Arch. Virol. 2022;167:293–305. doi: 10.1007/s00705-021-05295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He D., Zhao S., Lin Q., Zhuang Z., Cao P., Wang M.H., Yang L. The relative transmissibility of asymptomatic COVID-19 infections among close contacts. Int. J. Infect. Dis. 2020;94:145–147. doi: 10.1016/j.ijid.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbato C., Di Certo M.G., Gabanella F., Petrella C., Fiore M., Passananti C., Colizza A., Cavalcanti L., Ralli M., Greco A., et al. Staying tuned for post-COVID-19 syndrome: Looking for new research to sniff out. Eur. Rev. Med. Pharmacol. Sci. 2021;25:5318–5321. doi: 10.26355/eurrev_202108_26553. [DOI] [PubMed] [Google Scholar]
- 14.Niu S., Tian S., Lou J., Kang X., Zhang L., Lian H., Zhang J. Clinical characteristics of older patients infected with COVID-19: A descriptive study. Arch. Gerontol. Geriatr. 2020;89:104058. doi: 10.1016/j.archger.2020.104058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lian J., Jin X., Hao S., Cai H., Zhang S., Zheng L., Jia H., Hu J., Gao J., Zhang Y., et al. Analysis of epidemiological and clinical features in older patients with coronavirus disease 2019 (COVID-19) outside Wuhan. Clin. Infect. Dis. 2020;71:740–747. doi: 10.1093/cid/ciaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manson J.J., Crooks C., Naja M., Ledlie A., Goulden B., Liddle T., Khan E., Mehta P., Martin-Gutierrez L., Waddington K.E., et al. COVID-19-associated hyperinflammation and escalation of patient care: A retrospective longitudinal cohort study. Lancet Rheumatol. 2020;2:e594–e602. doi: 10.1016/S2665-9913(20)30275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustine J.N., Jones D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2021;191:4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrella C., Nenna R., Petrarca L., Tarani F., Paparella R., Mancino E., Di Mattia G., Conti M.G., Matera L., Bonci E., et al. Serum NGF and BDNF in Long-COVID-19 Adolescents: A Pilot Study. Diagnostics. 2022;12:1162. doi: 10.3390/diagnostics12051162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polat V., Bostancı G.İ. Sudden death due to acute pulmonary embolism in a young woman with COVID-19. J. Thromb. Thrombolysis. 2020;50:239–241. doi: 10.1007/s11239-020-02132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.West A.E., Pruunsild P., Timmusk T. Neurotrophins: Transcription and translation. Handb. Exp. Pharmacol. 2014;220:67–100. doi: 10.1007/978-3-642-45106-5_4. [DOI] [PubMed] [Google Scholar]
- 21.Dechant G., Neumann H. Neurotrophins. Adv. Exp. Med. Biol. 2002;513:303–334. doi: 10.5578/kvj.7239. [DOI] [PubMed] [Google Scholar]
- 22.Reichardt L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R Soc. B Biol. Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angelucci F., Piermaria J., Gelfo F., Shofany J., Tramontano M., Fiore M., Caltagirone C., Peppe A. The effects of motor rehabilitation training on clinical symptoms and serum BDNF levels in Parkinson’s disease subjects. Can. J. Physiol. Pharmacol. 2016;94:455–461. doi: 10.1139/cjpp-2015-0322. [DOI] [PubMed] [Google Scholar]
- 24.Tirassa P., Triaca V., Amendola T., Fiore M., Aloe L. EGF and NGF injected into the brain of old mice enhance BDNF and ChAT in proliferating subventricular zone. J. Neurosci. Res. 2003;72:557–564. doi: 10.1002/jnr.10614. [DOI] [PubMed] [Google Scholar]
- 25.Frohlich J., Chaldakov G.N., Vinciguerra M. Cardio- and Neurometabolic Adipobiology: Consequences and Implications for Therapy. Int. J. Mol. Sci. 2021;22:4137. doi: 10.3390/ijms22084137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carito V., Ceccanti M., Tarani L., Ferraguti G.N., Chaldakov G., Fiore M. Neurotrophins’ Modulation by Olive Polyphenols. Curr. Med. Chem. 2016;23:3189–3197. doi: 10.2174/0929867323666160627104022. [DOI] [PubMed] [Google Scholar]
- 27.Chaldakov G.N., Fiore M., Tonchev A.B., Aloe L. Neuroadipology: A novel component of neuroendocrinology. Cell Biol. Int. 2010;34:1051–1053. doi: 10.1042/CBI20100509. [DOI] [PubMed] [Google Scholar]
- 28.Lebrun B., Bariohay B., Moyse E., Jean A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: A minireview. Auton. Neurosci. 2006;126–127:30–38. doi: 10.1016/j.autneu.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Cohen-Cory S., Kidane A.H., Shirkey N.J., Marshak S. Brain-Derived Neurotrophic Factor and the Development of Structural Neuronal Connectivity. Dev. Neurobiol. 2011;70:271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulte-Herbruggen O., Braun A., Rochlitzer S., Jockers-Scherubl M.C., Hellweg R. Neurotrophic factors—A tool for therapeutic strategies in neurological, neuropsychiatric and neuroimmunological diseases? Curr. Med. Chem. 2007;14:2318–2329. doi: 10.2174/092986707781745578. [DOI] [PubMed] [Google Scholar]
- 31.Petrella C., Di Certo M.G., Gabanella F., Barbato C., Ceci F.M., Greco A., Ralli M., Polimeni A., Angeloni A., Severini C., et al. Mediterranean Diet, Brain and Muscle: Olive Polyphenols and Resveratrol Protection in Neurodegenerative and Neuromuscular Disorders. Curr. Med. Chem. 2021;28:7595–7613. doi: 10.2174/0929867328666210504113445. [DOI] [PubMed] [Google Scholar]
- 32.Minnone G., De Benedetti F., Bracci-Laudiero L. NGF and its receptors in the regulation of inflammatory response. Int. J. Mol. Sci. 2017;18:1028. doi: 10.3390/ijms18051028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carniel B.P., da Rocha N.S. Brain-derived neurotrophic factor (BDNF) and inflammatory markers: Perspectives for the management of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;108:110151. doi: 10.1016/j.pnpbp.2020.110151. [DOI] [PubMed] [Google Scholar]
- 34.Liu P., Li S., Tang L. Nerve growth factor: A potential therapeutic target for lung diseases. Int. J. Mol. Sci. 2021;22:9112. doi: 10.3390/ijms22179112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usai C., Gibbons J.M., Pade C., Li W., Jacobs S.R.M., McKnight Á., Kennedy P.T.F., Gill U.S. The β-NGF/TrkA Signalling Pathway Is Associated With the Production of Anti-Nucleoprotein IgG in Convalescent COVID-19. Front. Immunol. 2022;12:813300. doi: 10.3389/fimmu.2021.813300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minuzzi L.G., Seelaender M., Silva B.S.D.A., Cunha E.D.B.B., Deus M.D.C., Vasconcellos F.T.F., Marqueze L.F.B., Gadotti A.C., Baena C.P., Pereira T., et al. COVID-19 Outcome Relates With Circulating BDNF, According to Patient Adiposity and Age. Front. Nutr. 2021;8:784429. doi: 10.3389/fnut.2021.784429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehrabani M.M., Karvandi M.S., Maafi P., Doroudian M. Neurological complications associated with COVID-19; molecular mechanisms and therapeutic approaches. Rev. Med. Virol. 2022;32:e2334. doi: 10.1002/rmv.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motaghinejad M., Gholami M. Possible Neurological and Mental Outcomes of COVID-19 Infection: A Hypothetical Role of ACE-2\Mas\BDNF Signaling Pathway. Int. J. Prev. Med. 2020;11:84. doi: 10.4103/ijpvm.IJPVM_114_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biamonte F., Re A., Balzamino B.O., Ciasca G., Santucci D., Napodano C., Nocca G., Fiorita A., Marino M., Basile U., et al. Circulating and Salivary NGF and BDNF Levels in SARS-CoV-2 Infection: Potential Predictor Biomarkers of COVID-19 Disease—Preliminary Data. J. Pers. Med. 2022;12:1877. doi: 10.3390/jpm12111877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savic G., Stevanovic I., Mihajlovic D., Jurisevic M., Gajovic N., Jovanovic I., Ninkovic M. MMP-9/BDNF ratio predicts more severe COVID-19 outcomes. Int. J. Med. Sci. 2022;19:1903–1911. doi: 10.7150/ijms.75337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardy E., Fernandez-Patron C. Targeting mmp-regulation of inflammation to increase metabolic tolerance to COVID-19 pathologies: A hypothesis. Biomolecules. 2021;11:390. doi: 10.3390/biom11030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zingaropoli M.A., Iannetta M., Piermatteo L., Pasculli P., Latronico T., Mazzuti L., Campogiani L., Duca L., Ferraguti G., De Michele M., et al. Neuro-Axonal Damage and Alteration of Blood–Brain Barrier Integrity in COVID-19 Patients. Cells. 2022;11:2480. doi: 10.3390/cells11162480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erben Y., Prudencio M., Marquez C.P., Jansen-West K.R., Heckman M.G., White L.J., Dunmore J.A., Cook C.N., Lilley M.T., Qosja N., et al. Neurofilament light chain and vaccination status associate with clinical outcomes in severe COVID-19. IScience. 2022;25:105272. doi: 10.1016/j.isci.2022.105272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonetto V., Pasetto L., Lisi I., Carbonara M., Zangari R., Ferrari E., Punzi V., Luotti S., Bottino N., Biagianti B., et al. Markers of blood-brain barrier disruption increase early and persistently in COVID-19 patients with neurological manifestations. Front. Immunol. 2022;13:1070379. doi: 10.3389/fimmu.2022.1070379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarani L., Carito V., Ferraguti G., Petrella C., Greco A., Ralli M., Messina M.P., Rasio D., De Luca E., Putotto C., et al. Neuroinflammatory Markers in the Serum of Prepubertal Children with down Syndrome. J. Immunol. Res. 2020;2020:6937154. doi: 10.1155/2020/6937154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amendola T., Fiore M., Aloe L. Postnatal changes in nerve growth factor and brain derived neurotrophic factor levels in the retina, visual cortex, and geniculate nucleus in rats with retinitis pigmentosa. Neurosci. Lett. 2003;345:37–40. doi: 10.1016/S0304-3940(03)00491-9. [DOI] [PubMed] [Google Scholar]
- 47.Fiore M., Triaca V., Amendola T., Tirassa P., Aloe L. Brain NGF and EGF administration improves passive avoidance response and stimulates brain precursor cells in aged male mice. Physiol. Behav. 2002;77:437–443. doi: 10.1016/S0031-9384(02)00875-2. [DOI] [PubMed] [Google Scholar]
- 48.Ferrazzano G., Zingaropoli M.A., Costanzo M., Belvisi D., Dominelli F., Pasculli P., Ciardi M.R., Fabbrini G., Defazio G., Berardelli A., et al. Neurofilament assessment in patients with cervical dystonia. Park. Relat. Disord. 2022;98:70–71. doi: 10.1016/j.parkreldis.2022.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Iannetta M., Zingaropoli M.A., Latronico T., Pati I., Pontecorvo S., Prezioso C., Pietropaolo V., Cortese A., Frontoni M., D’Agostino C., et al. Dynamic changes of MMP-9 plasma levels correlate with JCV reactivation and immune activation in natalizumab-treated multiple sclerosis patients. Sci. Rep. 2019;9:311. doi: 10.1038/s41598-018-36535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Payán-Pernía S., Pérez L.G., Sevilla Á.F.R., Gil J.S., Canales S.N. Absolute Lymphocytes, Ferritin, C-Reactive Protein, and Lactate Dehydrogenase Predict Early Invasive Ventilation in Patients With COVID-19. Lab. Med. 2021;52:141–145. doi: 10.1093/labmed/lmaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aloe L., Fiore M. TNF-α expressed in the brain of transgenic mice lowers central tyroxine hydroxylase immunoreactivity and alters grooming behavior. Neurosci. Lett. 1997;238:65–68. doi: 10.1016/S0304-3940(97)00850-1. [DOI] [PubMed] [Google Scholar]
- 52.Aloe L., Fiore M., Probert L., Turrini P., Tirassa P. Overexpression of tumour necrosis factor alpha in the brain of transgenic mice differentially alters nerve growth factor levels and choline acetyltransferase activity. Cytokine. 1999;11:45–54. doi: 10.1006/cyto.1998.0397. [DOI] [PubMed] [Google Scholar]
- 53.Ceci F.M., Ferraguti G., Petrella C., Greco A., Ralli M., Iannitelli A., Carito V., Tirassa P., Chaldakov G.N., Messina M.P., et al. Nerve Growth Factor in Alcohol Use Disorders. Curr. Neuropharmacol. 2020;19:45–60. doi: 10.2174/1570159X18666200429003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durany N., Thome J. Neurotrophic factors and the pathophysiology of schizophrenic psychoses. Eur. Psychiatry. 2004;19:326–337. doi: 10.1016/j.eurpsy.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 55.Mondal A.C., Fatima M. Direct and indirect evidences of BDNF and NGF as key modulators in depression: Role of antidepressants treatment. Int. J. Neurosci. 2019;129:283–296. doi: 10.1080/00207454.2018.1527328. [DOI] [PubMed] [Google Scholar]
- 56.Ferraguti G., Terracina S., Micangeli G., Lucarelli M., Tarani L., Ceccanti M., Spaziani M., D’Orazi V., Petrella C., Fiore M. NGF and BDNF in pediatrics syndromes. Neurosci. Biobehav. Rev. 2023;145:105015. doi: 10.1016/j.neubiorev.2022.105015. [DOI] [PubMed] [Google Scholar]
- 57.Ceci F.M., Ferraguti G., Petrella C., Greco A., Tirassa P., Iannitelli A., Ralli M., Vitali M., Ceccanti M., Chaldakov G.N., et al. Nerve Growth Factor, Stress and Diseases. Curr. Med. Chem. 2020;28:2943–2959. doi: 10.2174/0929867327999200818111654. [DOI] [PubMed] [Google Scholar]
- 58.Asgarzadeh A., Fouladi N., Asghariazar V., Sarabi S.F., Khiavi H.A., Mahmoudi M., Safarzadeh E. Serum Brain-Derived Neurotrophic Factor (BDNF) in COVID-19 Patients and its Association with the COVID-19 Manifestations. J. Mol. Neurosci. 2022;72:1820–1830. doi: 10.1007/s12031-022-02039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Demir B., Beyazyüz E., Beyazyüz M., Çelikkol A., Albayrak Y. Long-lasting cognitive effects of COVID-19: Is there a role of BDNF? Eur. Arch. Psychiatry Clin. Neurosci. 2022:1–9. doi: 10.1007/s00406-022-01514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marchegiani F., Recchioni R., Marcheselli F., Di Rosa M., Sabbatinelli J., Matacchione G., Giuliani A., Ramini D., Stripoli P., Biscetti L., et al. Association of admission serum levels of neurofilament light chain and in-hospital mortality in geriatric patients with COVID-19. J. Neurol. 2022;270:37–43. doi: 10.1007/s00415-022-11373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.da Silva-Neto P.V., Do Valle V.B., Fuzo C.A., Fernandes T.M., Toro D.M., Fraga-Silva T.F.C., Basile P.A., de Carvalho J.C.S., Pimentel V.E., Pérez M.M., et al. Matrix Metalloproteinases on Severe COVID-19 Lung Disease Pathogenesis: Cooperative Actions of MMP-8/MMP-2 Axis on Immune Response through HLA-G Shedding and Oxidative Stress. Biomolecules. 2022;12:604. doi: 10.3390/biom12050604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duda I., Krzych Ł., Jędrzejowska-Szypułka H., Lewin-Kowalik J. Plasma matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 as prognostic biomarkers in critically ill patients. Open Med. 2020;15:50–56. doi: 10.1515/med-2020-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benlarbi M., Laroche G., Fink C., Fu K., Mulloy R.P., Phan A., Ariana A., Stewart C.M., Prévost J., Beaudoin-Bussières G., et al. Identification and differential usage of a host metalloproteinase entry pathway by SARS-CoV-2 Delta and Omicron. IScience. 2022;25:105316. doi: 10.1016/j.isci.2022.105316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Springall R., González-Flores J., García-Ávila C., Juárez-Vicuña Y., Hernández-Diazcouder A., Márquez-Velasco R., Cásares-Alvarado S., Sánchez-Muñoz F., Basilio-Gálvez E., Castillo-Salazar M., et al. Elevated Levels of Soluble CD147 are Associated with Hyperinflammation and Disease Severity in COVID-19: A Proof-of-Concept Clinical Study. Arch. Immunol. Ther. Exp. 2022;70:18. doi: 10.1007/s00005-022-00657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hazra S., Chaudhuri A.G., Tiwary B.K., Chakrabarti N. Matrix metallopeptidase 9 as a host protein target of chloroquine and melatonin for immunoregulation in COVID-19: A network-based meta-analysis. Life Sci. 2020;257:118096. doi: 10.1016/j.lfs.2020.118096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.D’Avila-Mesquita C., Couto A.E.S., Campos L.C.B., Vasconcelos T.F., Michelon-Barbosa J., Corsi C.A.C., Mestriner F., Petroski-Moraes B.C., Garbellini-Diab M.J., Couto D.M., et al. MMP-2 and MMP-9 levels in plasma are altered and associated with mortality in COVID-19 patients. Biomed. Pharmacother. 2021;142:112067. doi: 10.1016/j.biopha.2021.112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davey A., McAuley D.F., O’Kane C.M. Matrix metalloproteinases in acute lung injury: Mediators of injury and drivers of repair. Eur. Respir. J. 2011;38:959–970. doi: 10.1183/09031936.00032111. [DOI] [PubMed] [Google Scholar]
- 68.Carpenter C.R., Mudd P.A., West C.P., Wilber E., Wilber S.T. Diagnosing COVID-19 in the Emergency Department: A Scoping Review of Clinical Examinations, Laboratory Tests, Imaging Accuracy, and Biases. Acad. Emerg. Med. 2020;27:653–670. doi: 10.1111/acem.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vakili S., Savardashtaki A., Jamalnia S., Tabrizi R., Nematollahi M.H., Jafarinia M., Akbari H. Laboratory Findings of COVID-19 Infection are Conflicting in Different Age Groups and Pregnant Women: A Literature Review. Arch. Med. Res. 2020;51:603–607. doi: 10.1016/j.arcmed.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bivona G., Agnello L., Ciaccio M. Biomarkers for prognosis and treatment response in COVID-19 patients. Ann. Lab. Med. 2021;41:540–548. doi: 10.3343/alm.2021.41.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request.