Abstract
A family of 11 phosphatases can help to modulate the activity of response regulator proteins in Bacillus subtilis. Downstream of seven of the rap (phosphatase) genes are phr genes, encoding secreted peptides that function as phosphatase regulators. By using fusions to lacZ and primer extension analysis, we found that six of the seven phr genes are controlled by the alternate sigma factor sigma-H. These results expand the potential of sigma-H to contribute to the output of several response regulators by controlling expression of inhibitors of phosphatases.
A family of phosphatases and cognate regulators (Fig. 1) modulate the output of two-component signal transduction systems in Bacillus subtilis (19, 31, 34). Two-component systems generally consist of a histidine protein kinase that autophosphorylates on a histidine residue and a response regulator, often a transcription factor, whose activity is controlled by phosphorylation of an aspartate residue (10, 11). The response regulator obtains phosphate from its cognate kinase. Many cellular processes are controlled by two-component systems: the B. subtilis genome encodes 37 histidine kinases and 34 response regulators (5, 16).
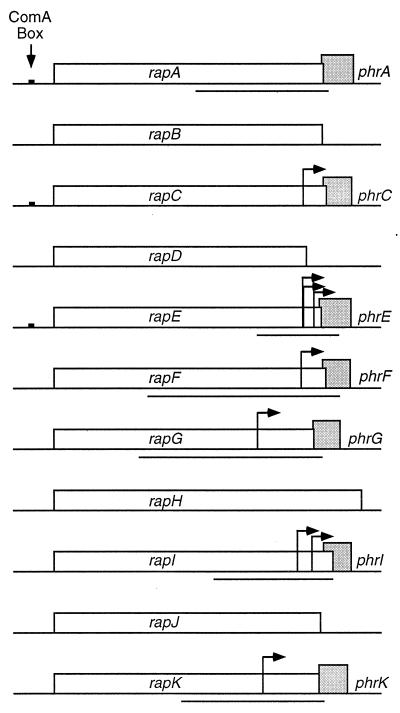
FIG. 1.
The family of rap phosphatases and phr peptide regulators. Putative transcriptional start sites, as determined by mapping 5′ ends by primer extension analysis, are indicated by arrows. To analyze expression, DNA fragments (denoted by solid lines underneath the genes) were cloned upstream of lacZ and integrated into the chromosome at amyE. The fragments were amplified by PCR with Vent polymerase and primers with restriction sites (EcoRI and BamHI) at the ends. These fragments were subcloned between the EcoRI and BamHI sites of pKS2 (23). The resulting plasmids were linearized and transformed into wild-type B. subtilis, selecting for Neor transformants. Transformants were screened for an amylase-deficient phenotype to confirm the plasmid had integrated into the chromosome at the amyE locus. For each phr gene, the fragment end points are indicated relative to the translation start site: phrA, 530 bp upstream, 30 bp downstream; phrE, 275 bp upstream, 73 bp downstream; phrF, 749 bp upstream, 62 bp downstream; phrG, 745 bp upstream, 30 bp downstream; phrI, 465 bp upstream, 37 bp downstream; phrK, 583 bp upstream, 22 bp downstream.
The phosphorylation state of many response regulators is negatively regulated by phosphatases. B. subtilis has a family of 11 genes encoding phosphatases (or putative phosphatases) that are homologous to each other (16, 29, 34). Several of these response regulator aspartyl phosphate phosphatases (Rap phosphatases) have been characterized. RapA and RapB (and to a lesser extent RapE) negatively regulate the initiation of sporulation (14, 25, 30, 31). They do so by dephosphorylating the response regulator protein Spo0F, which is part of the phosphorelay (1) that is required to activate the sporulation transcription factor Spo0A in response to multiple signals, including starvation (6, 12, 31).
The activities of RapA, RapB, and RapE are negatively regulated by specific pentapeptides. RapA and RapE are inhibited by the pentapeptides produced from precursors encoded by phrA and phrE, the genes downstream from rapA and rapE, respectively (14, 28, 33). RapB is inhibited by the pentapeptide that is produced from the precursor encoded by phrC, the gene downstream from rapC (28). These inhibitory peptides are exported, and at least some of them accumulate in culture supernatant. They are then imported by the oligopeptide permease (Opp [also known as Spo0K]) (20, 32, 38). In this way, the peptides can be indicators of population density (17, 18, 20, 33, 40), as well as the intracellular conditions necessary for their production (18, 28, 29).
The pentapeptide produced from the phrC gene product, called CSF (competence and sporulation-stimulating factor), was initially purified from culture supernatant as an activity that stimulates expression of genes activated by the transcription factor ComA (40, 41). comA encodes a response regulator required for the development of genetic competence (26, 45) and the general quorum response (17, 19). ComA is active in the phosphorylated form and obtains phosphate from the histidine kinase ComP (15, 36, 37, 46). RapC inhibits expression of genes activated by ComA∼P, most likely by dephosphorylating ComA∼P (40). Expression of genes activated by ComA∼P is increased by two different extracellular peptides, the ComX pheromone, which activates the kinase ComP (23, 36, 41), and CSF, which appears to inhibit the phosphatase RapC (41). In this way, two different extracellular signaling molecules contribute to activate a general quorum response controlled by ComA.
Transcription of phrC is controlled by two promoters: P1, which is upstream of rapC and directs transcription of rapC and phrC (18); and P2, which is internal to rapC and directs transcription of phrC (2, 18). Transcription from P2 is controlled by the alternate sigma factor sigma-H (the product of sigH [also known as spo0H]) and increases during entry into stationary phase (2, 18). phrE is also dependent on sigma-H for maximal expression (14).
A phr gene, encoding a putative or bona fide secreted peptide that functions as a phosphatase regulator, is found downstream from 7 of the 11 rap genes (Fig. 1). We have found that, with the exception of phrA, all of the phr genes have sigma-H promoters upstream of the gene and internal to the cognate rap gene. These findings indicate that the alternate sigma factor sigma-H controls production of a family of phosphatase regulators and that synthesis of these regulators is controlled by the nutritional conditions that modulate sigma-H.
Expression of phr-lacZ fusions.
A search of the B. subtilis genome found putative sigma-H promoters upstream of each phr gene, except phrA (19). To measure expression from the potential promoters upstream of the phr genes and to test the dependence on sigma-H, we constructed transcriptional fusions of the regions upstream of the phr genes to lacZ (Fig. 1 and Table 1). We amplified the putative promoter regions by PCR and cloned the fragments upstream from lacZ, and the resulting fusions were integrated into the chromosome at a heterologous site (amyE). We measured β-galactosidase specific activity for each fusion during growth and after entry into stationary phase in nutrient broth sporulation medium (Fig. 2) and defined minimal medium (data not shown) at 37°C. The patterns of expression were similar in both media. All of the fusions had an initially low level of expression that increased at or shortly before the transition to stationary phase (T0). The peak of β-galactosidase specific activity was between 1 and 3 h after the end of exponential growth. No expression was observed from a fusion made to the region upstream of phrA (data not shown), indicating that under the conditions tested, there is not a functional promoter in this region.
TABLE 1.
B. subtilis strains used in this study
| Strain | Relevant genotypea |
|---|---|
| JH642 | trpC2 pheA1 |
| IRN238 | amyE::(phrC-lacZΩ2 neo) |
| IRN243 | amyE::(phrC-lacZΩ2 neo) sigH::cat::spc |
| RSM106 | amyE::(phrE-lacZΩ2 neo) |
| RSM114 | amyE::(phrE-lacZΩ2 neo) sigH::cat::spc |
| RSM154 | amyE::(phrE-lacZΩ2 neo) spo0AD56N-cat::spc |
| RSM139 | amyE::(phrE-lacZΩ2 neo) abrB::Tn917 |
| RSM147 | amyE::(phrE-lacZΩ2 neo) spo0AD56N-cat::spc abrB::Tn917 |
| RSM128 | amyE::(phrF-lacZΩ7 neo) |
| RSM135 | amyE::(phrF-lacZΩ7 neo) sigH::cat::spc |
| RSM132 | amyE::(phrF-lacZΩ7 neo) spo0AD56N-cat::spc |
| RSM140 | amyE::(phrF-lacZΩ7 neo) abrB::Tn917 |
| RSM148 | amyE::(phrF-lacZΩ7 neo) spo0AD56N-cat::spc abrB::Tn917 |
| RSM129 | amyE::(phrG-lacZΩ8 neo) |
| RSM136 | amyE::(phrG-lacZΩ8 neo) sigH::cat::spc |
| RSM133 | amyE::(phrG-lacZΩ8 neo) spo0AD56N-cat::spc |
| RSM141 | amyE::(phrG-lacZΩ8 neo) abrB::Tn917 |
| RSM149 | amyE::(phrG-lacZΩ8 neo) spo0AD56N-cat::spc abrB::Tn917 |
| NC131 | amyE::(phrI-lacZΩ2 neo) |
| RSM165 | amyE::(phrI-lacZΩ2 neo) sigH::cat::spc |
| RSM166 | amyE::(phrI-lacZΩ2 neo) spo0AD56N-cat::spc |
| RSM167 | amyE::(phrI-lacZΩ2 neo) abrB::Tn917 |
| RSM168 | amyE::(phrI-lacZΩ2 neo) spo0AD56N-cat::spc abrB::Tn917 |
| RSM130 | amyE::(phrK-lacZΩ9 neo) |
| RSM137 | amyE::(phrK-lacZΩ9 neo) sigH::cat::spc |
| RSM134 | amyE::(phrK-lacZΩ9 neo) spo0AD56N-cat::spc |
| RSM142 | amyE::(phrK-lacZΩ9 neo) abrB::Tn917 |
| RSM150 | amyE::(phrK-lacZΩ9 neo) spo0AD56N-cat::spc abrB::Tn917 |
All strains are derived from JH642 and contain the trpC2 and pheA1 mutations.
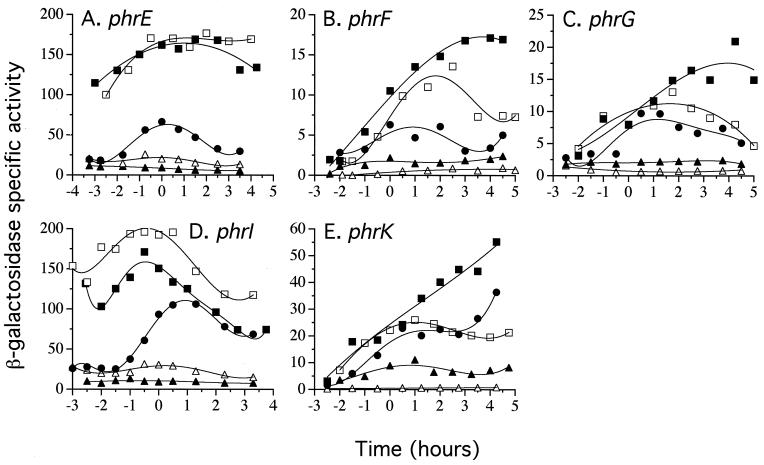
FIG. 2.
Expression of the phr-lacZ transcriptional fusions. β-Galactosidase specific activity was assayed as described previously (13, 24). The time indicated is relative to the transition to stationary phase (T0). (A) phrE-lacZ. (B) phrF-lacZ. (C) phrG-lacZ. (D) phrI-lacZ. (E) phrK-lacZ. Solid circles, wild type; open triangles, sigH; solid triangles, spo0A; solid squares, abrB; open squares, spo0A abrB. Strains are indicated in Table 1.
A sigH null mutation reduced the maximal level of expression to less than 1% of that of the wild type for all fusions, except phrE and phrI (Fig. 2). The phrE- and phrI-lacZ fusions retained a significant level of expression in a sigH null mutant (Fig. 2A and D), presumably due to transcription from the sigma-A promoters described below. The pattern of expression seen for the phrE-lacZ fusion is consistent with that described previously (14).
Spo0A and AbrB affect expression of many sigma-H-regulated promoters (6, 8, 35, 42, 47, 48). In a spo0A null mutant, transcription of phrCP2 is decreased ∼5-fold during stationary phase. This effect is relieved by a null mutation in abrB (2, 18). Similar effects were observed with the other phr-lacZ fusions (Fig. 2). In all cases, a spo0A null mutation caused a decrease in activity compared to that of the wild type, and this effect was relieved by a null mutation in abrB.
The expression of the phrE-lacZ and phrI-lacZ fusions was significantly higher in the abrB null mutant than in the wild type (Fig. 2A and D). There are sequences in these promoter regions that resemble known AbrB binding sites (42), and we suspect that AbrB acts directly to repress expression from phrEP1 and/or -P2 and phrIP1.
Primer extension mapping of phr promoters.
The lacZ fusions confirmed the existence of promoters upstream of the phr genes. To determine if the location of the promoters correlated with the sequences resembling sigma-H-dependent promoters, we used primer extension to map precisely the 5′ ends of transcripts of phrE, phrF, phrG, phrI, and phrK (Fig. 3). Cells were grown in sporulation medium (39) at 37°C, and RNA was prepared from cells 2 to 3 h after the end of exponential growth. Primer extension was performed essentially as described previously (43). The major primer extension products for all of the genes correlated to start sites downstream from the putative sigma-H promoters (Fig. 3). Furthermore, these products were not observed in a sigH null mutant (Fig. 3) (data not shown). phrE and phrI also had primer extension products that were not dependent on sigma-H (Fig. 3A and D), consistent with the pattern of expression of the phrE-lacZ and phrI-lacZ fusions (described above).
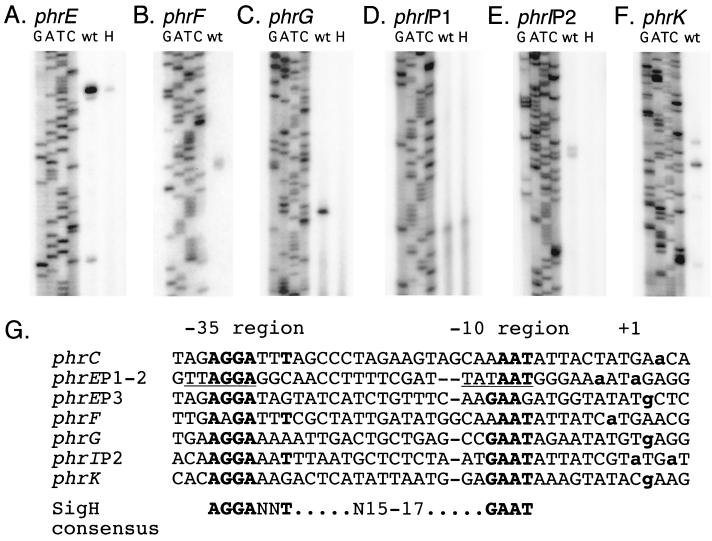
FIG. 3.
Primer extension mapping of the 5′ ends of phr mRNA and sequences of the promoter regions. (A to F) RNA for primer extension was prepared by the Qiagen RNeasy protocol. Approximately 50 μg of RNA was used per primer extension reaction. Each primer extension reaction is shown alongside a sequencing ladder (lanes labeled GATC) produced with the same end-labeled primer. RNA was prepared from wild-type (wt) cells (IRN238 or RSM128) and a sigH mutant (lane H [IRN243 or RSM135]). (A) phrE, primer phrE4 (5′-CCAATTAAAACGGCGGATAAACTGA-3′). (B) phrF, primer phrF5 (5′-GAGCCAGACAAGAGAGTAATAGTTTAGA-3′). (C) phrG, primer RM36 (5′-CTTACACTGTCATACTCTTTCTCGGAC-3′). (D) phrIP1, primer phrIPEU (5′-CGTCTTGCTTCAATACTCATACG-3′). (E) phrIP2, primer phrIPE (5′-CTATGCCCCTACCCGATCTGCAGC-3′). (F) phrK, primer RM38 (5′-GCTCGTTGCTTCTTCAAAAGCACC-3′). (G) Sigma-H promoters identified by primer extension analysis. Promoters were aligned by the −10 region with spaces added to align the −35 regions. Residues with identity to the sigma-H consensus sequence are indicated in boldface. The transcription start sites (+1) as determined by primer extension are indicated by boldface, lowercase letters. The sigma-A consensus site in phrEP1–2 is underlined.
phrE transcripts had three detectable 5′ ends (Fig. 3A). The level of each was reduced in a sigH null mutant, with the two downstream ends reduced below the limit of detection. The most upstream end (P1) corresponds to a potential sigma-A promoter, and the two downstream ends (P2 and P3) correspond to potential sigma-H promoters. We suspect that the effect of the sigH null mutation on P1 is due to the effects of sigH on expression of spo0A, which is required for full expression of phrE (Fig. 2A) (14).
phrI had two apparent 5′ ends corresponding to promoters of two different sigma factors (Fig. 3D to E). The upstream product was independent of sigma-H and corresponds to the start site for a potential sigma-A promoter (P1). The downstream product was not observed in a sigH null mutant and matches the start site of the sigma-H promoter (P2) predicted by sequence analysis.
Sigma-H and regulation of response regulators.
The characterized Phr peptides act as extracellular regulators of gene expression by inhibiting Rap phosphatases that act on response regulators (14, 20, 28, 40). The remaining phr genes are predicted to encode similar extracellular peptides that regulate the activity of other Rap phosphatases (34). The finding that all of the phr genes (except phrA) are transcribed by RNA polymerase holoenzyme containing sigma-H implies that many cellular processes and response regulators are regulated indirectly by sigma-H.
Sigma-H is regulated by many diverse signals, and this regulation occurs at the transcriptional and posttranslational levels. The activity of sigma-H is regulated by growth phase (9, 47), pH (3), members of the Clp protease family (21, 22, 27), and perhaps the stringent response (4). As cells experience conditions that increase activity of sigma-H, the amount of phr transcription relative to its upstream rap will increase. This will enhance the regulation (inhibition) of the Rap phosphatases by their respective Phr peptides.
The effects of sigma-H on control of expression of the phr genes most likely contribute to the effects of cell culture density on sporulation. Sporulation is more efficient at high than low cell densities (7, 12, 20, 44), due to the accumulation of signaling peptides in culture supernatant (7, 20). sigH mutants are defective in production of at least some of the signaling peptides (7, 20). We suspect that several other cellular responses are modulated by population density and that sigma-H contributes to these via its role in expression of the phr genes.
Acknowledgments
We thank members of the laboratory for useful discussions and especially R. A. Britton and E. Küster-Schöck for comments on the manuscript.
R.S.M. and N.C. were supported in part by National Institutes of Health (NIH) predoctoral training grants, and this work was supported in part by Public Health Service grant GM50895 from the NIH.
REFERENCES
- 1.Burbulys D, Trach K A, Hoch J A. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- 2.Carter H L, III, Tatti K M, Moran C P., Jr Cloning of a promoter used by sigma-H RNA polymerase in Bacillus subtilis. Gene. 1990;96:101–105. doi: 10.1016/0378-1119(90)90347-t. [DOI] [PubMed] [Google Scholar]
- 3.Cosby W M, Zuber P. Regulation of Bacillus subtilis ςH (Spo0H) and AbrB in response to changes in external pH. J Bacteriol. 1997;179:6778–6787. doi: 10.1128/jb.179.21.6778-6787.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eymann C, Mittenhuber G, Hecker M. The stringent response, ςH-dependent gene expression and sporulation in Bacillus subtilis. Mol Gen Genet. 2001;264:913–923. doi: 10.1007/s004380000381. [DOI] [PubMed] [Google Scholar]
- 5.Fabret C, Feher V A, Hoch J A. Two-component signal transduction in Bacillus subtilis: how one organism sees its world. J Bacteriol. 1999;181:1975–1983. doi: 10.1128/jb.181.7.1975-1983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grossman A D. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu Rev Genet. 1995;29:477–508. doi: 10.1146/annurev.ge.29.120195.002401. [DOI] [PubMed] [Google Scholar]
- 7.Grossman A D, Losick R. Extracellular control of spore formation in Bacillus subtilis. Proc Natl Acad Sci USA. 1988;85:4369–4373. doi: 10.1073/pnas.85.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn J, Roggiani M, Dubnau D. The major role of Spo0A in genetic competence is to downregulate abrB, an essential competence gene. J Bacteriol. 1995;177:3601–3605. doi: 10.1128/jb.177.12.3601-3605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Healy J, Weir J, Smith I, Losick R. Post-transcriptional control of a sporulation regulatory gene encoding transcription factor ςH in Bacillus subtilis. Mol Microbiol. 1991;5:477–487. doi: 10.1111/j.1365-2958.1991.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 10.Hoch J A. Two-component and phosphorelay signal transduction. Curr Opin Microbiol. 2000;3:165–170. doi: 10.1016/s1369-5274(00)00070-9. [DOI] [PubMed] [Google Scholar]
- 11.Hoch J A, Silhavy T J. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. [Google Scholar]
- 12.Ireton K, Rudner D Z, Siranosian K J, Grossman A D. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev. 1993;7:283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- 13.Jaacks K J, Healy J, Losick R, Grossman A D. Identification and characterization of genes controlled by the sporulation-regulatory gene spo0H in Bacillus subtilis. J Bacteriol. 1989;171:4121–4129. doi: 10.1128/jb.171.8.4121-4129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang M, Grau R, Perego M. Differential processing of propeptide inhibitors of Rap phosphatases in Bacillus subtilis. J Bacteriol. 2000;182:303–310. doi: 10.1128/jb.182.2.303-310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang M, Shao W, Perego M, Hoch J A. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol Microbiol. 2000;38:535–542. doi: 10.1046/j.1365-2958.2000.02148.x. [DOI] [PubMed] [Google Scholar]
- 16.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 17.Lazazzera B A, Grossman A D. The ins and outs of peptide signaling. Trends Microbiol. 1998;6:288–294. doi: 10.1016/s0966-842x(98)01313-4. [DOI] [PubMed] [Google Scholar]
- 18.Lazazzera B A, Kurtser I G, McQuade R S, Grossman A D. An autoregulatory circuit affecting peptide signaling in Bacillus subtilis. J Bacteriol. 1999;181:5193–5200. doi: 10.1128/jb.181.17.5193-5200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazazzera B A, Palmer T, Quisel J, Grossman A D. Cell density control of gene expression and development in Bacillus subtilis. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: ASM Press; 1999. pp. 27–46. [Google Scholar]
- 20.Lazazzera B A, Solomon J M, Grossman A D. An exported peptide functions intracellularly to contribute to cell density signaling in B. subtilis. Cell. 1997;89:917–925. doi: 10.1016/s0092-8674(00)80277-9. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Cosby W M, Zuber P. Role of Lon and ClpX in the post-translational regulation of a sigma subunit of RNA polymerase required for cellular differentiation in Bacillus subtilis. Mol Microbiol. 1999;33:415–428. doi: 10.1046/j.1365-2958.1999.01489.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Zuber P. The ClpX protein of Bacillus subtilis indirectly influences RNA polymerase holoenzyme composition and directly stimulates ςH-dependent transcription. Mol Microbiol. 2000;37:885–897. doi: 10.1046/j.1365-2958.2000.02053.x. [DOI] [PubMed] [Google Scholar]
- 23.Magnuson R, Solomon J, Grossman A D. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell. 1994;77:207–216. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 24.Miller J. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 25.Mueller J P, Sonenshein A L. Role of the Bacillus subtilis gsiA gene in regulation of early sporulation gene expression. J Bacteriol. 1992;174:4374–4383. doi: 10.1128/jb.174.13.4374-4383.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano M M, Zuber P. Cloning and characterization of srfB, a regulatory gene involved in surfactin production and competence in Bacillus subtilis. J Bacteriol. 1989;171:5347–5353. doi: 10.1128/jb.171.10.5347-5353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanamiya H, Ohashi Y, Asai K, Moriya S, Ogasawara N, Fujita M, Sadaie Y, Kawamura F. ClpC regulates the fate of a sporulation initiation sigma factor, ςH protein, in Bacillus subtilis at elevated temperatures. Mol Microbiol. 1998;29:505–513. doi: 10.1046/j.1365-2958.1998.00943.x. [DOI] [PubMed] [Google Scholar]
- 28.Perego M. A peptide export-import control circuit modulating bacterial development regulates protein phosphatases of the phosphorelay. Proc Natl Acad Sci USA. 1997;94:8612–8617. doi: 10.1073/pnas.94.16.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perego M. Self-signaling by Phr peptides modulates Bacillus subtilis development. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washingon, D.C.: ASM Press; 1999. pp. 243–258. [Google Scholar]
- 30.Perego M, Glaser P, Hoch J A. Aspartyl-phosphate phosphatases deactivate the response regulator components of the sporulation signal transduction system in Bacillus subtilis. Mol Microbiol. 1996;19:1151–1157. doi: 10.1111/j.1365-2958.1996.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 31.Perego M, Hanstein C, Welsh K M, Djavakhishvili T, Glaser P, Hoch J A. Multiple protein-aspartate phosphatases provide a mechanism for the integration of diverse signals in the control of development in B. subtilis. Cell. 1994;79:1047–1055. doi: 10.1016/0092-8674(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 32.Perego M, Higgins C F, Pearce S R, Gallagher M P, Hoch J A. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol Microbiol. 1991;5:173–185. doi: 10.1111/j.1365-2958.1991.tb01838.x. [DOI] [PubMed] [Google Scholar]
- 33.Perego M, Hoch J A. Cell-cell communication regulates the effects of protein aspartate phosphatases on the phosphorelay controlling development in Bacillus subtilis. Proc Natl Acad Sci USA. 1996;93:1549–1553. doi: 10.1073/pnas.93.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perego M, Hoch J A. Protein aspartate phosphatases control the output of two-component signal transduction systems. Trends Genet. 1996;12:97–101. doi: 10.1016/0168-9525(96)81420-x. [DOI] [PubMed] [Google Scholar]
- 35.Perego M, Spiegelman G B, Hoch J A. Structure of the gene for the transition state regulator, abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Mol Microbiol. 1988;2:689–699. doi: 10.1111/j.1365-2958.1988.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 36.Piazza F, Tortosa P, Dubnau D. Mutational analysis and membrane topology of ComP, a quorum-sensing histidine kinase of Bacillus subtilis controlling competence development. J Bacteriol. 1999;181:4540–4548. doi: 10.1128/jb.181.15.4540-4548.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roggiani M, Dubnau D. ComA, a phosphorylated response regulator protein of Bacillus subtilis, binds to the promoter region of srfA. J Bacteriol. 1993;175:3182–3187. doi: 10.1128/jb.175.10.3182-3187.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudner D Z, LeDeaux J R, Ireton K, Grossman A D. The spo0K locus of Bacillus subtilis is homologous to the oligopeptide permease locus and is required for sporulation and competence. J Bacteriol. 1991;173:1388–1398. doi: 10.1128/jb.173.4.1388-1398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaeffer P, Millet J, Aubert J P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomon J M, Lazazzera B A, Grossman A D. Purification and characterization of an extracellular peptide factor that affects two different developmental pathways in Bacillus subtilis. Genes Dev. 1996;10:2014–2024. doi: 10.1101/gad.10.16.2014. [DOI] [PubMed] [Google Scholar]
- 41.Solomon J M, Magnuson R, Srivastava A, Grossman A D. Convergent sensing pathways mediate response to two extracellular competence factors in Bacillus subtilis. Genes Dev. 1995;9:547–558. doi: 10.1101/gad.9.5.547. [DOI] [PubMed] [Google Scholar]
- 42.Strauch M A. Delineation of AbrB-binding sites on the Bacillus subtilis spo0H, kinB, ftsAZ, and pbpE promoters and use of a derived homology to identify a previously unsuspected binding site in the bsuB1 methylase promoter. J Bacteriol. 1995;177:6999–7002. doi: 10.1128/jb.177.23.6999-7002.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Triezenberg S J. Primer extension. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1992. pp. 4.8.1–4.8.5. [Google Scholar]
- 44.Vasantha N, Freese E. Enzyme changes during Bacillus subtilis sporulation caused by deprivation of guanine nucleotides. J Bacteriol. 1980;144:1119–1125. doi: 10.1128/jb.144.3.1119-1125.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinrauch Y, Guillen N, Dubnau D A. Sequence and transcription mapping of Bacillus subtilis competence genes comB and comA, one of which is related to a family of bacterial regulatory determinants. J Bacteriol. 1989;171:5362–5375. doi: 10.1128/jb.171.10.5362-5375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinrauch Y, Penchev R, Dubnau E, Smith I, Dubnau D. A Bacillus subtilis regulatory gene product for genetic competence and sporulation resembles sensor protein members of the bacterial two-component signal-transduction systems. Genes Dev. 1990;4:860–872. doi: 10.1101/gad.4.5.860. [DOI] [PubMed] [Google Scholar]
- 47.Weir J, Predich M, Dubnau E, Nair G, Smith I. Regulation of spo0H, a gene coding for the Bacillus subtilis ςH factor. J Bacteriol. 1991;173:521–529. doi: 10.1128/jb.173.2.521-529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuber P, Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987;169:2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]