Abstract
We have tested the hypothesis that the autoamplification of two-component regulatory systems results in “learning” behavior, i.e., that bacteria respond faster or more extensively to a signal when a similar signal has been perceived in the past. Indeed, the induction of alkaline phosphatase activity upon phosphate limitation was faster if the cultures had been limited for phosphate previously, and this faster response correlated with the autoamplification of the cognate two-component system.
The adaptation of bacteria to fluctuating environmental conditions often proceeds via two-component regulatory systems, which usually consist of a sensor in the cytoplasmic membrane and a cytoplasmic response regulator (16, 24). Upon stimulation, the sensor autophosphorylates and the phosphoryl group is subsequently transferred to the cognate regulator, eventually resulting in a suitable response, e.g., the activation of the transcription of a specific set of genes. An example of a two-component regulatory system is found in the pho regulon of Escherichia coli (29). Growth of E. coli under inorganic phosphate (Pi) limitation results in the induction of the synthesis of many proteins, including the periplasmic enzyme alkaline phosphatase (28) and the high-affinity uptake systems for Pi (the Pst system) (26) and for sn-glycerol-3-phosphate and sn-glycerophosphoryl diesters (the Ugp system) (15). These proteins function to scavenge traces of Pi or phosphorylated compounds from the extracellular medium. The expression of the genes encoding these proteins is controlled by a two-component regulatory system consisting of the sensor PhoR and the transcriptional activator PhoB (11, 12). In addition to PhoR and PhoB, the Pst system plays a role in regulation, since disruption of any of the genes of the pstSCAB-phoU operon usually leads to the constitutive expression of the pho regulon (29).
The regulatory genes phoB and phoR form an operon, which is subject to autoamplification (6, 22), meaning that signal transfer through the PhoB-PhoR system stimulates its own expression. Such autoamplification of the regulatory genes has been reported for several two-component regulatory systems (for examples, see references 4 and 18). However, its physiological role has not been studied explicitly so far. Provided that the regulatory proteins PhoB and PhoR are stable, their amplification upon signal transduction could, among other possibilities, lead to some kind of “learning” behavior, i.e., it could allow the cells to respond faster or more extensively when the system is repeatedly triggered. In this study, we have evaluated this behavior of E. coli in its response to Pi limitation.
Isolation of a phoA(Ts) mutant.
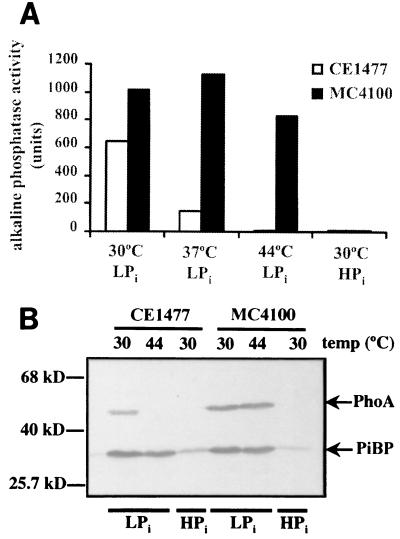
To determine whether autoamplification of signal transduction components leads to faster responses upon repeated stimulation, we wanted to measure the kinetics of the induction of the pho regulon. Expression of at least one component of the pho regulon, i.e., phoA encoding alkaline phosphatase, can be determined quantitatively (27). To ensure that the background enzyme activity is low even after repeated exposure to Pi limitation, we decided to isolate a temperature-sensitive phoA mutant. Strain MC4100 (3) was mutagenized with ethyl methanesulfonate (EMS) (14) and plated on HEPES-buffered synthetic medium (27) containing 0.5% glucose, supplemented with 40 μM K2HPO4 (low-Pi medium [LPi]) and with 40 μg of the alkaline phosphatase substrate 5-bromo-4-chloro-3-indolylphosphate (XP) (2) ml−1 and solidified with 2% agarose. After overnight incubation at 42°C, white colonies were picked, streaked on similar LPi plates, and incubated at either 30 or 44°C. One of the colonies that was blue after overnight incubation at 30°C but remained white after incubation at 44°C was designated CE1477 and studied in detail. Alkaline phosphatase was induced after growth of the strain in LPi medium at 30°C, induced only poorly at 37°C, and induced not at all at 44°C (Fig. 1A). Furthermore, the protein could be demonstrated on Western blots (1) after growth of the cells at 30°C but not at 44°C, whereas its detection was not influenced by the growth temperature in the case of the parental strain MC4100 (Fig. 1B). In contrast, induction of the synthesis of the Pi-binding protein (encoded by the pstS gene) (Fig. 1B) and of UgpC (results not shown) in strain CE1477 was not affected at the higher growth temperature, indicating that the temperature-sensitive mutation was located in the phoA gene rather than in a regulatory gene. This supposition was confirmed by PCR amplification of the phoA allele of strain CE1477 using primers phoA1 (5′-AAGCTTTGGAGATTATCGTC-3′) and phoA2 (5′-CCATGAGCGTATGCGCCC-3′) and its subsequent cloning in the HincII site of pUC18 (30). After introduction of the recombinant plasmid in phoA mutant strain DH5α (19) and plating on LPi medium containing XP, the colonies resulting after overnight growth at 30°C were blue, whereas they were white after incubation at 42°C. Sequencing of the phoA allele on two plasmids, obtained after independent PCRs, revealed a single mutation resulting in a Gly258Ser substitution in alkaline phosphatase in both cases. Thus, we have isolated a strain that will allow for the autoamplification of the signal transduction components under Pi limitation at 42°C without the concomitant increase of alkaline phosphatase activity.
FIG. 1.
Expression of alkaline phosphatase in strain MC4100 and its phoA(Ts) derivative, CE1477, at various temperatures. (A) Alkaline phosphatase activities. Cells were grown overnight at 30, 37, or 44°C in LPi medium or at 30°C in HPi medium before harvesting and determination of alkaline phosphatase activity. Two independent measurements were performed with essentially the same results, and the data from one of these experiment are shown. (B) Western blot analysis of total cell proteins. Expression of alkaline phosphatase (PhoA) and Pi- binding protein (PiBP) was detected using polyclonal antisera. Cells were grown at 30 or 44°C in LPi medium or at 30°C in HPi medium. Equal amounts of cells based on cell density were loaded on the gel. The positions of the molecular size marker proteins are shown on the left.
Stability of the signal transduction components.
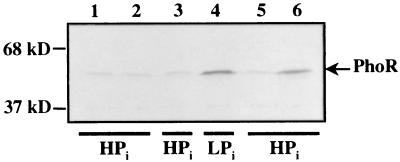
Regulatory proteins are often proteolytically unstable, and their susceptibility to proteolysis is presumed to play a key role in their regulatory function (13). However, learning effects can only be expected if the increased levels of the regulatory proteins PhoR and PhoB can still be detected at a considerable time after Pi limitation has been discontinued. To test the accumulation of PhoR and PhoB, exponentially growing cells (in HPi medium, which is LPi medium supplemented with 660 μM K2HPO4 to create Pi-replete conditions; the generation time in the logarithmic phase was approximately 56 min) of strain CE1477 were harvested and incubated for 45 min in LPi medium at 42°C. Western blot analysis confirmed induction of the synthesis of the PhoR protein (Fig. 2, lane 4) and the PhoB protein (results not shown) in these cells in comparison with control cells that were kept under Pi-replete conditions (Fig. 2, lane 3, and results not shown). When the cells were subsequently incubated in HPi medium at 30°C for 1 h, the increased amounts of PhoR protein (Fig. 2, lane 6) and of PhoB protein (results not shown) were still detectable. Apparently, PhoR and PhoB are rather stable proteins. Alternatively, the mRNA of the phoBR operon is very stable, but this explanation seems unlikely considering the short half-lives of mRNAs in E. coli (9).
FIG. 2.
Western blot analysis of total cell proteins of strain CE1477 using polyclonal antiserum directed against PhoR. The phoA(Ts) strain CE1477 was grown overnight in HPi medium at 37°C. This overnight culture was diluted 1:15 in HPi medium (optical density at 660 nm [OD660], ∼0.075) and grown at 37°C until the culture reached an OD660 of 0.4 (corresponding to 4 × 108 CFU ml−1) (lanes 1 and 2). Cells were harvested by centrifugation at 1,054 × g for 10 min at room temperature, resuspended in LPi medium, and subsequently incubated for 45 min at 42°C (lane 4). After addition of K2HPO4 (end concentration, 660 μM), the cells were grown for 1 h at 30°C (lane 6). A control culture was treated similarly, except that it was resuspended in HPi instead of LPi medium for the incubation at 42°C (lane 3) and no additional Pi was added thereafter during the 1 h of growth at 30°C (lane 5). Equal amounts of cells based on cell density were loaded on a sodium dodecyl sulfate-polyacrylamide gel (10). The positions of the molecular size marker proteins are shown on the left.
Kinetics of the induction of alkaline phosphatase synthesis.
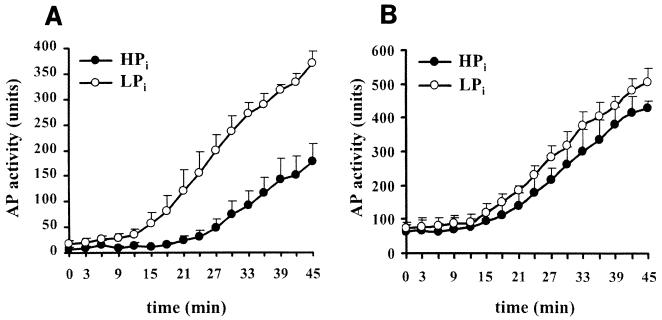
To test whether the accumulation of PhoB and PhoR during the period of Pi limitation leads to a faster or more extensive response when the cells encounter Pi limitation again, exponentially growing cells of the phoA(Ts) mutant strain CE1477 were first incubated for 45 min in LPi at 42°C and then for 1 h in HPi at 30°C as described above. Subsequently, the cells were transferred to LPi medium and incubated at 30°C, and the induction of alkaline phosphatase synthesis was measured as a function of time. The induction of alkaline phosphatase synthesis was clearly faster in the cells that had previously been limited for Pi than in the control cells that were constantly kept on high Pi during the preincubation procedure (Fig. 3A). The faster induction was still detectable when the high-Pi period between the two periods of Pi limitation was extended from 1 h to as much as 2 h (i.e., at early stationary phase), although the difference from the induction in the control culture had clearly diminished (results not shown). These results demonstrated that the bacteria respond faster after exposure to an initial stimulus in the recent past.
FIG. 3.
Induction kinetics of alkaline phosphatase synthesis. (A) Alkaline phosphatase synthesis in phoA(Ts) strain CE1477. Cells were grown as described in the legend to Fig. 2. Subsequently, cells from both cultures were harvested by centrifugation at 1,054 × g for 10 min at room temperature. Pellets were washed with 150 mM NaCl, resuspended in LPi medium, and incubated at 30°C, and alkaline phosphatase activities were measured over time. The alkaline phosphatase activities were determined using para-nitrophenyl phosphate as a substrate (27). The units are defined as nanomoles of para-nitrophenol released per minute per unit of optical density at 660 nm of cell culture. Data represent average values of six independent experiments, and standard deviations are shown. Open symbols represent the cultures that had been incubated for 45 min at 42°C in LPi medium during the preincubation; closed symbols represent the cultures that were not limited for Pi before. (B) Alkaline phosphatase synthesis in pstS phoA(Ts) strain CE1478. Cells were treated as described in the legend to Fig. 2 except that they were kept at 42°C during preincubation to prevent the synthesis of alkaline phosphatase. Symbols are as described for panel A. Data represent averages of three independent experiments with standard deviations.
To verify that the learning behavior described above correlated with the accumulation of the signal transduction components during the first period of Pi limitation, a pstS mutation was introduced by P1 transduction into the phoA(Ts) mutant strain CE1477, using strain CE1488 (8) as the donor strain. Mutations in pstS generally lead to the constitutive expression of the pho regulon. Hence, except for pstS and phoA, all genes of the pho regulon, including phoR and phoB, are expressed constitutively when this strain, designated CE1478, is grown at 42°C. When this strain was subjected to incubation procedures similar to those described above for strain CE1477, except that it was kept at 42°C for the entire preincubation, the kinetics of the appearance of alkaline phosphatase activity after the shift to 30°C in LPi were independent of the Pi history of the cells (Fig. 3B). Therefore, the different responses observed in the case of strain CE1477 (Fig. 3A) are not a consequence of unspecific physiological effects but appear to correlate with the autoamplification of the signal transduction components.
Previously, we have hypothesized that the various two-component systems that are present in a single bacterial cell may constitute a phospho-neural network (7). An important characteristic of a neural network is its ability to learn. Here we have demonstrated that autoamplification of regulatory components upon signal transduction indeed leads to learning behavior. The learning behavior is different from the growth advantage that results from the accumulation of the appropriate transporters and metabolic enzymes, which is observed when cells are preincubated on, for example, a particular carbon source like maltose or lactose (20). This latter effect is most appropriately called (physiological) adaptation. The learning behavior described in the present study is also, both mechanistically and effectively, different from the adaptation effects observed in chemotaxis, a process based on the methylation of methyl-accepting chemotaxis proteins (25). However, the response that we describe is strongly reminiscent of the boosting effects observed in the immune system, where the term “learning” is being used (5).
Besides the pho regulon of E. coli, many other two-component regulatory systems, including the BvgAS, PhoPQ, and CpxAR and NtrI-NtrII (or NtrB-NtrC) regulators of Bordetella, Salmonella, and E. coli, respectively (17, 18, 21, 23), have been reported to show autoregulation of the expression of the signal transduction components. Therefore, learning behavior might be a rather common feature in the response of bacteria to environmental signals. It should be noted, however, that additional physiological roles for the autoamplification of the regulatory proteins of the two-component systems cannot be excluded. For example, autoamplification may ensure that the target gene expression is activated only after a minimum threshold of the stimulus has been encountered (17), or it may establish a hierarchy of target operon expression. Future experiments will be directed to investigate such additional roles for autoamplification in the pho regulon.
REFERENCES
- 1.Agterberg M, Fransen R, Tommassen J. Expression of Escherichia coli PhoE protein in avirulent Salmonella typhimurium aroA and galE strains. FEMS Microbiol Lett. 1988;50:295–299. [Google Scholar]
- 2.Brickman E, Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and ϕ80 transducing phages. J Mol Biol. 1975;96:307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- 3.Casadaban M J. Transposition and fusion of the lac genes to select promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–544. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 4.Clarke H R G, Leigh J A, Douglas C J. Molecular signals in the interaction between plants and microbes. Cell. 1992;71:191–199. doi: 10.1016/0092-8674(92)90348-g. [DOI] [PubMed] [Google Scholar]
- 5.Eisen H N. Learning and memory in the immune response. Cancer Res. 1966;26:2005–2011. [PubMed] [Google Scholar]
- 6.Guan C-D, Wanner B L, Inouye H. Analysis of regulation of phoB expression using a phoB-cat fusion. J Bacteriol. 1983;156:710–717. doi: 10.1128/jb.156.2.710-717.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellingwerf K J, Postma P W, Tommassen J, Westerhoff H V. Signal transduction in bacteria: phospho-neural network(s) in Escherichia coli? FEMS Microbiol Rev. 1995;16:309–321. doi: 10.1111/j.1574-6976.1995.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoffer S M, Schoondermark P, van Veen H W, Tommassen J. Activation by gene amplification of pitB, encoding a third phosphate transporter of Escherichia coli K-12. J Bacteriol. 2001;183:4659–4663. doi: 10.1128/JB.183.15.4659-4663.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kushner S R. mRNA decay. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 849–860. [Google Scholar]
- 10.Lugtenberg B, Meijers J, Peters R, van der Hoek P, van Alphen L. Electrophoretic resolution of the “major outer membrane protein”of Escherichia coli into four bands. FEBS Lett. 1975;58:254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- 11.Makino K, Shinagawa H, Amemura M, Kimura S, Nakata A, Ishihama A. Regulation of the phosphate regulon of Escherichia coli. Activation of pstS transcription by PhoB protein in vitro. J Mol Biol. 1988;203:85–95. doi: 10.1016/0022-2836(88)90093-9. [DOI] [PubMed] [Google Scholar]
- 12.Makino K, Shinagawa H, Amemura M, Kawamoto T, Yamada M, Nakata A. Signal transduction in the phosphate regulon of Escherichia coli involves phosphotransfer between PhoR and PhoB proteins. J Mol Biol. 1989;210:551–559. doi: 10.1016/0022-2836(89)90131-9. [DOI] [PubMed] [Google Scholar]
- 13.Miller C G. Protein degradation and proteolytic modification. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 938–954. [Google Scholar]
- 14.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 15.Overduin P, Boos W, Tommassen J. Nucleotide sequence of the ugp genes of Escherichia coli K-12: homology to the maltose system. Mol Microbiol. 1988;2:767–775. doi: 10.1111/j.1365-2958.1988.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 16.Parkinson J S, Kofoid E C. Communication modules in bacterial signalling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 17.Raivio T L, Popkin D L, Silhavy T J. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J Bacteriol. 1999;181:5263–5272. doi: 10.1128/jb.181.17.5263-5272.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reitzer L J, Magasanik B. Isolation of the nitrogen assimilation regulator, NRI, the product of the glnG gene of Escherichia coli. Proc Natl Acad Sci USA. 1983;82:1979–1983. doi: 10.1073/pnas.80.18.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrígues-Quin̆ones F, Benedí V J. Escherichia coli strain DH5αTM is a suitable host for the study of phoA insertions. Focus. 1993;15:110–112. [Google Scholar]
- 20.Roseman S, Meadow N D. Signal transduction by the bacterial phosphotransferase system. J Biol Chem. 1990;265:2993–2996. [PubMed] [Google Scholar]
- 21.Roy C R, Miller J F, Falkow S. Autogenous regulation of the Bordetella pertussis bvgABC operon. Proc Natl Acad Sci USA. 1990;87:6753–6757. doi: 10.1073/pnas.87.10.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinagawa H, Makino M, Nakata A. Regulation of the pho regulon in Escherichia coli K-12. Genetic and physiological regulation of the positive regulatory gene phoB. J Mol Biol. 1983;168:477–488. doi: 10.1016/s0022-2836(83)80297-6. [DOI] [PubMed] [Google Scholar]
- 23.Soncini F C, Vescovi E G, Groismann E. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J Bacteriol. 1995;177:4364–4371. doi: 10.1128/jb.177.15.4364-4371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stock J B, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stock J B, Surette M G. Chemotaxis. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1103–1129. [Google Scholar]
- 26.Surin B P, Rosenberg H, Cox G B. Phosphate-specific transport system of Escherichia coli: nucleotide sequence and gene-polypeptide relationships. J Bacteriol. 1985;161:189–198. doi: 10.1128/jb.161.1.189-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tommassen J, Lugtenberg B. Outer membrane protein e of Escherichia coli is co-regulated with alkaline phosphatase. J Bacteriol. 1980;143:151–157. doi: 10.1128/jb.143.1.151-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torriani A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960;38:460–470. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- 29.Wanner B L. Phosphorus assimilation and control of the phosphate regulon. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1357–1381. [Google Scholar]
- 30.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]