Abstract
Novel molecular imaging opportunities to preoperatively diagnose renal cell carcinoma is under development and will add more value in limiting the postoperative renal function loss and morbidity. We aimed to comprehensively review the research on single photon emission computed tomography/computed tomography (SPECT/CT) and positron emission tomography computed tomography (PET-CT) molecular imaging and to enhance the urologists’ and radiologists’ knowledge of the current research pattern. We identified an increase in prospective and also retrospective studies that researched to distinguish between benign and malignant lesions and between different clear cell renal cell carcinoma subtypes, with small numbers of patients studied, nonetheless with excellent results on specificity, sensitivity and accuracy, especially for 99mTc-sestamibi SPECT/CT that delivers quick results compared to a long acquisition time for girentuximab PET-CT, which instead gives better image quality. Nuclear medicine has helped clinicians in evaluating primary and secondary lesions, and has lately returned with new and exciting insights with novel radiotracers to reinforce its diagnostic potential in renal carcinoma. To further limit the renal function loss and post-surgery morbidity, future research is mandatory to validate the results and to clinically implement the diagnostic techniques in the context of precision medicine.
Keywords: 99mTc-sestamibi SPECT, girentuximab PET-CT, molecular imaging, renal cancer
1. Introduction
In the United States, it is estimated that, in 2023, there will be approximately 81,800 new cases and 14,890 new cancer-related deaths related to kidney and renal pelvis cancers [1]. The recent widespread of imaging techniques designed to diagnose renal masses [2] lead to an incidental and small tumors identification with highly diverse imaging features with an increased variability of their histology [3,4]. The incidental small mass tumors identified among renal cancers are renal cell carcinoma (RCC) and are around 70–80% [5,6]. Subtypes of RCC have been identified to have different aggressiveness and metastatic potential [7,8]. Almost three quarters of the RCC encountered subtype is clear cell (cc) RCC (approx. 75%), then papillary (pap) RCC (approx. 15%), and the chromophobe (ch) RCC (approx. 5%) [6]. There are two subtypes that have potential for metastatic spread (ccRCC and type II papRCC). Type I papRCC and chRCC and hybrid oncocytic chromophobe tumors (HOCTs) are considered less aggressive [7]. Benign tumors (oncocytomas and angiomyolipomas (AMLs)) are also often incidentally identified in preoperative imaging (up to 20% of clinically localized renal masses) [9,10]. Lately, there has been a reduction in the postsurgical identification of benign lesions [11], and some of the predictors for benign histology have been identified as female gender, low body mass index and low volume tumor [12]. Renal biopsy can preoperatively distinguish between benign and cancerous lesions [13]. The presence of complications and some improved diagnostic rate for small tumors can still cause the urologist’s and patient’s decision to be affected by the poor negative-predictive value of renal biopsy [14], even if in some studies the complications, seeding rate [15] after biopsy and high diagnostic rate [16] are manageable by fine needle aspiration, computed tomography (CT) [17] or magnetic resonance imaging (MRI) [18] guidance and regardless of the hospital volume [19]. It is known that a percentage of renal masses surgically removed are related to benign tumors [10] or an indolent disease [20]. Therefore, a better characterization of aggressive diseases prior to surgery using novel imaging techniques, biomarkers and antibodies can reduce the number of surgeries limiting the burden of complications, morbidity and financial costs.
The aim of this review is to comprehensively assess the current evidence of the use of molecular imaging through novel devices such as SPECT and PET-CT and 99mTc-sestamibi and girentuximab, to discuss the results of the trials and to enhance urologists’ and radiologists’ knowledge of the current research pattern.
2. Materials and Methods
In order to have a clear picture of the current evidence on how radiopharmaceutical agents and imaging can diagnose renal cancer, can identify different subtypes of renal lesions, and can explore the near possibility of entering in clinical practice, we have developed a review focusing our research on the mentioned aspects. We have used PubMed database to identify original research on these topics from the last ten years. The keywords used were ”kidney neoplasm”, ”renal tumor”, ”renal mass”, ”molecular imaging”, ”positron emission tomography”, ”single photon emission tomography”, “99mTc-sestamibi SPECT” and “girentuximab PET-CT”. We have included articles up to November 2022 from the last ten years and the evidence exploring metastatic renal cancer or follow-up after treatment were excluded from the analysis. The evidence was screened by title and abstract by two independent reviewers (B.B. and F.C.) and the articles included were analyzed further following the approval of authors.
3. Results
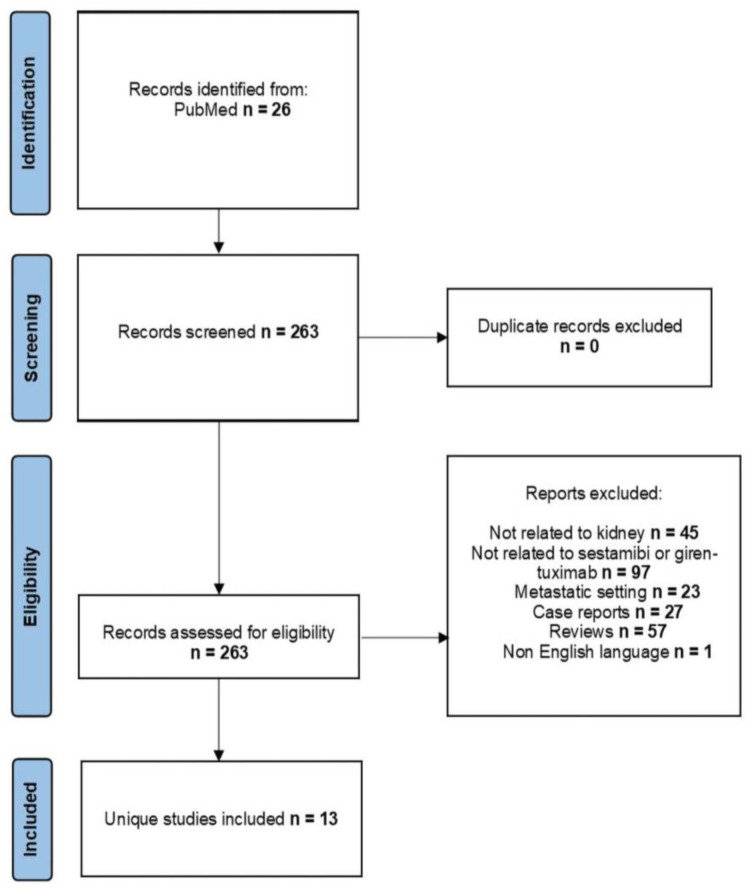
After searching the PubMed database, we identified 263 research articles, of which 13 were selected according to the set criteria and were published in the last ten years. Data from one ongoing clinical trial was also evaluated. In Figure 1, we have embedded the obtained results.
Figure 1.
Flowchart of identified studies for analysis.
3.1. Molecular Imaging
Molecular processes of the organism can be characterized by special molecules. These antibodies or peptides are used to carry radionuclides, making the targeted organ visible on imaging techniques such as positron emission tomography (PET) or single photon emission computed tomography/computed tomography (SPECT/CT). The combination of SPECT and PET with CT have raised the diagnostic accuracy of both PET and SPECT. There are differences among PET/CT and SPECT/CT that the clinicians will have to be aware of. PET/CT seems to bring more quantitative information than SPECT/CT and appears to have better results. PET/CT can provide better image resolution, less attenuation and disperse artifacts, higher sensitivity and better and multiple radiotracers. However, PET also comes with a higher cost than SPECT and the radionuclides used are cheaper, have a longer half-life, and this can lead to better targeting potential, resulting in a higher ability to describe the biological processes [21,22,23]. Through PET, the patient experiences radiation levels comparable to that of a CT scan [24]. The selection of the tracer is critical in terms of availability, physical and radiopharmaceuticals characteristics [24,25]. The stability (at certain pH, light, temperature) and the bio-distribution of the radionuclide are important factors that will influence its in vivo decomposition [26]. SPECT radionuclides are gamma-emitting radioisotopes. The sensitivity, resolution and fast acquisition of images due to the solid-state detector technology makes this technology have a great impact in nuclear diagnosis [27,28]. For comparison, SPECT is widely available and costs less. The research for new radionuclides is on the way to attenuate the big limitation of PET/CT (uptake localization) [21]. The advancements in radiometal-based radiopharmaceuticals for PET can lead to improvements in radiometal designing research for SPECT radiometals (e.g., technetium) [27]. An important aspect is dose optimization of radiation, and this will individualize the use of either PET or SPECT (uses less radiation doses) technology and will have to be evaluated considering the patients risks, in order to provide quality scan pictures for relevant clinical decisions [29,30].
The search for molecular imaging agents is a current area of great interest for the unique imaging and molecular characterization of renal tumors, beyond the well-known used and approved histology of renal lesions [3,31]. The antibodies carrying radionuclides are targeting surface tumor antigens. Other small molecules and peptides, target specific cellular processes and can provide data on the subsequent histology of tumors within the kidney [32]. This molecular characterization of tumors can overcome the limitations of conventional imaging in order to identify the histological heterogeneity of renal tumors. Recently, the radiolabeled molecular antibody girentuximab and a mitochondrial imaging agent (99mTc-MIBI) were heavily studied regarding the differentiation of renal lesions [33,34].
3.2. 99mTc-Sestamibi SPECT/CT
Prior to the introduction of PET in clinical use, sestamibi radiopharmaceuticals were used to detect other types of tumors, such as breast cancer [35,36,37], the evaluation of mediastinal involvement in lung cancer patients [38], in multiple myeloma [39], the follow-up of brain glioma after chemotherapy [40] and ongoing and present use to characterize parathyroid adenomas [41,42].
In 2015, an initial trial performed by Rowe et al. identified that all oncocytomas demonstrated radiotracer uptake, but not the RCCs tumors [43]. One of the first studies that prospectively assessed the accuracy for the differentiation of oncocytomas and hybrid oncocytic/chRCC from other kidney lesions identified an overall sensitivity of 87.5% (95% confidence interval [CI], 47.4–99.7%) and a specificity of 95.2% (95% CI, 83.8–99.4%) [44]. In a pilot study, Tzortzakakis et al. aimed to characterize solid kidney lesions and the differentiation of oncocytomas from RCCs, and found that 91.6% of oncocytomas displayed positive uptake of 99mTc-sestamibi [45]. Sheikhbahaei et al. assessed the addition of 99mTc-sestamibi SPECT in order to increase the degree of diagnostic confidence to distinguish between benign and cancerous tissue, obtaining an area under the curve (AUC) of 0.60 for conventional imaging alone and 0.85 after reviewing 99mTc-sestamibi (p for difference = 0.03) [46]. Due to the close uptake ratio cutoff for some of the tumors investigated so far, Jones et al. and Tzortzakakis et al. aimed to standardize uptake value both for the intra- and inter-observer agreement, to see if a quantitative SPECT/CT reconstruction method could be applied in the context of a clinical setting and if quantitative biomarkers could be validated for the evaluation of renal lesions. The improvement of separation between uptake ratios and a high intra-class correlation coefficient could discriminate further between different renal lesions [47,48]. Zhu et al. developed a study that aimed to assess if dual-phase 99mTc-sestamibi SPECT/CT could identify benign or cancerous renal masses in a cohort of 147 patients and showed that benign tissues demonstrated a significantly elevated early relative uptake value and delayed relative uptake value than cancerous lesions (p < 0.0001) with high degree of sensitivity, specificity and accuracy (100% sensitivity, 94.8% specificity 94.8%, accuracy 95.3% and sensitivity 100%, specificity 96.3%, accuracy of 96.6%, respectively). In the context of dual phasing, both can differentiate between the renal lesions and the delay phase points a higher diagnostic accuracy [33]. A cost effective study has been also performed for this imaging tool and identified a positive cost effectiveness when compared with existing strategies [49]. Lately, Warren et al. started a pilot diagnostic accuracy study to evaluate 99mTc-sestamibi SPECT/CT compared to a reference standard of histopathology and reported that the sensitivity and specificity to detect oncocytic/ch lesions from other RCCs was 100% (95% CI, 74–100%) and 100% (95% CI, 63–100%), respectively. Sensitivity and specificity to discriminate between benign versus malignant masses was 100% (95% CI, 54–100%) and 85.7% (95% CI, 57–98%), respectively [50]. Following the same rationale of detecting the accuracy of 99mTc-sestamibi SPECT/CT in the diagnosis of renal lesions, compared with contrast enhanced CT only, Parihar et al. showed a sensitivity and specificity of SPECT/CT for the diagnosis of tumors of low malignant potential or benign to be 66.7%, and 89.5%, respectively, compared to 10% and 75% for contrast-enhanced CT, respectively [51]. Sistani et al. demonstrated that the 99mTc-sestamibi SPECT/CT has the potential to characterize indeterminate renal lesions [52]. Viswambaram et al. determined the effectiveness of 99mTc-sestamibi SPECT/CT to differentiate between malignant and benign renal lesions having a sensitivity of 89% (95% CI, 77–95%) and a specificity of 73% (95% CI, 45–91%). On the other hand, the authors found that the diagnostic accuracy was not improved when compared to visual interpretation alone [53]. Taken altogether, non-concerning lesions defined as oncocytic or benign tumors have very high sensitivity and specificity of being diagnosed with 99mTc-sestamibi SPECT/CT when compared with only contrast-enhanced CT [51]. Asi et al. identified that all oncocytomas identified in their study had a high uptake of 99mTc-sestamibi. There were 64 (71.11%) RCC lesions that had no uptake of the labeled radiotracer, resulting in a positive predictive value (PPV) and negative predictive value (NPV) of 60% and 91.3%, respectively, for identifying benign lesions [54]. Warren et al. reported results from a pilot diagnostic accuracy trial in patients with renal lesions aiming to assess, with the help of expert radiologists, the quantitative uptake of the radiotracer in the lesions compared to the healthy renal parenchyma and between different lesions subtypes, with excellent results on sensitivity, specificity in identifying ch lesions from RCCs and to differentiate benign from cancerous lesions [50]. Sistani et al. found in a prospective comparative study on 29 patients that the benign or oncocytic tumors compared to RCCs had a higher mean and maximum relative lesion uptake (p = 0.016 and 0.012, respectively) [52].
Presently, the recommendations from the EAU guidelines are that CT and MRI can accurately make a diagnosis of RCC, but cannot reliably distinguish oncocytoma and fat-free AML from malignant renal neoplasms [55]. However, the new imaging technique 99mTc-sestamibi SPECT/CT show promising results to differentiate between benign and low grade RCC [56]. A summary of evidence is listed in Table 1.
Table 1.
Evaluation of studies assessing 99mTc-sestamibi SPECT/CT in the diagnosis of renal cancer.
| Authors | Study Type/No. Patients or Histological Diagnosis | Pathologic Diagnosis/Biopsy | Positive/Negative on 99mTc-Sestamibi SPECT/CT, n (%) | Results |
|---|---|---|---|---|
| Rowe et al. [43] | Evaluation prospective study/6 | Oncocytoma RCC |
Yes, 3 (100%) No, 3 (100%) |
Maximum radiotracer uptake similar/above adjacent renal parenchyma (average tumor uptake, 1.19; range, 0.85–1.78) Markedly photopenic relative to renal parenchyma average tumor uptake of 0.26 (range, 0.21–0.31) |
| Gorin et al. [44] | Prospective/50 | Oncocytoma HOCTs chRCC RCC |
Yes, 5 (83.3%) Yes, 2 (100%) Yes, falsely 2 (50%) No, 40 (100%) |
99mTc-sestamibi SPECT/CT correctly identified 5 of 6 (83.3%) oncocytomas and 2 of 2 (100%) HOCTs Good sensitivity of 87.5% (95% CI, 47.4–99.7%) and specificity of 95.2% (95% CI, 83.8–99.4%). |
| Tzortzakakis et al. [45] | Prospective/27 | Oncocytoma HOCTs AML ccRCC papRCC chRCC ch/papRCC |
Yes, 12 (91.6%) Yes, 3 (100%) Yes, 1 (100%) No, 0 No, 1 slightly positive (33.3%) No, 0 No, 0 |
91.6% of oncocytomas displayed positive uptake of 99mTc-sestamibi |
| Sheikhbahaei et al. [46] | Prospective/48 | Oncocytoma HOCTs AML RCC papRCC ccRCC Unclassified RCC chRCC |
Yes, 6; (12.5%) False-positive 1 Yes, 2 (4.2%) Yes 2, (4.2%) False-positive results 1 No, 25 (52%) No, 4 (8.3%) No, 2 (4.2%) No, 1 (2.1) No, 4; (8.3%) Yes 2 falsely high uptake |
AUC 0.60 to distinguish between benign and cancerous tissue for conventional imaging alone AUC 0.85 after reviewing 99mTc-sestamibi (p for difference = 0.03) |
| Zhu et al. [33] | Prospective/147 patients and 148 histopathology results | Oncocytoma AML Malignant tumors |
Yes, 4 (2.7%) Yes, 8 (5.4%) No, 124 (83.8%) Partial early uptake, 7 (5.1% from malignant tumors) Partial delayed uptake, 5 (3.7% from malignant tumors) |
Higher ERUV and DRUV than malignant renal tumors (n = 136; both p < 0.0001). ERUV cutoff value of 0.53 helped to differentiate benign from malignant renal tumors (DRUV of 0.50), with sensitivity of 100%, specificity of 94.8%, and accuracy of 95.3% (DRUV sensitivity 100%, specificity 96.3%, accuracy of 96.6%) for the diagnosis of benign renal tumors. |
| Parihar et al. [51] | Retrospective/36 lesions | non-concerning lesions (oncocytic or benign tumors) ccRCC |
Yes, 15 (41.7%) No, 13 (36.%) |
The sensitivity and specificity of SPECT/CT for discriminating oncocytic or benign tumors = 66.7%, and 89.5%, respectively, compared to 10%, and 75% for CECT, respectively. |
| Asi et al. [54] | Prospective/90 lesions | Oncocytoma AML Chronic sclerosis Fibroma Hydatic cyst chRCC ch/papRCC All RCC |
Yes, 10 (11.1%) No, 4 (4.5%) No, 2 (2.2%) No, 1 (1.1%) No, 1 (1.1%) Yes, 5 (5.6%) Yes, 3 (3.3%) No, 64 (71.1%) |
PPV and NPV of 60% and 91.3%, respectively for identifying benign lesions. |
| Warren et al. [50] | Prospective, pilot diagnostic accuracy/19 lesions | Oncocytoma Oncocytic RCC chRCC ccRCC papRCC histology unknown |
Yes, 6 (31.657%) Yes, 1 (5.3%) Yes, 1 (5.3%) No, 9 (47.4%) No, 2 (10.5%) No, 1 (5.2%) |
Qualitative assessment for sestamibi-renal images categorized as avid or photopenic has been identified as 100%. Sensitivity and specificity of sestamibi-renal to detect ch lesions from other RCCs was 100% (95% CI 74 100%) and 100% (95% CI 63–100%), respectively. Sensitivity and specificity for discriminating benign versus cancerous lesions was 100% (95% CI 54–100%) and 85.7% (95% CI 57–98%), respectively. |
| Sistani et al. [52] | Prospective, comparative study/29 patients | Oncocytoma HOCT chRCC ccRCC papRCC cc/papRCC chRCC |
Yes, 7 (24.1%) Yes, 1 (3.5%) Yes, 1 low uptake (3.5%) No, 15 (51.7%) No, 4 (13.8%) No, 2 (6.9%) No, 1 (3.4%) |
The benign or oncocytic tumors compared to RCCs had higher mean and maximum relative lesion uptake (p = 0.016 and 0.012, respectively). |
| Viswambaram et al. [53] | Prospective/74 patients | Accuracy to detect malignant or benign lesions | 49 (66.2%) 11 (14.8%) |
Sensitivity of 89% (95% CI 77–95%) and a specificity of 73% (95% CI 45–91%) for detecting malignant or benign tumors. |
AML = angiomyolipoma; AUC = area under the curve; ccRCC = clear cell renal cell carcinoma; CECT = contrast-enhanced computed tomography; chRCC = chromophobe renal cell carcinoma; CI = confidence interval; DRUV = delayed relative uptake value; ERUV = early relative uptake value; HOCT = renal hybrid oncocytic/chromophobe tumors; NPV = negative predictive value; pap = papillary; PPV = positive predictive value; PET-CT = positron emission tomography computed tomography; RCC = renal cell carcinoma; SPECT/CT = single photon emission computed tomography/computed.
The combination of presence or absence of sestamibi uptake and lesions’ density on SPECT/CT, the efforts to standardize the cut-off values, the very good results in terms of sensitivity, specificity and accuracy to distinguish between benign and malignant renal tissues could represent a novel imaging approach to stratify the risk of marginal/low risk [32] described kidney lesions on contrast-enhanced CT. The additional studies on health economics [49], additional validation [52,54] and the added value in renal lesions diagnosis (already approved for myocardial and parathyroid imaging [57]), 99mTc-sestamibi SPECT/CT can make a fast transition into clinical practice for evaluating kidney lesions, with minimal costs.
3.3. Girentuximab PET/CT
The carbonic anhydrase IX (CAIX) antibody, found to have the ability to link on an antigen expressed on the surface of ccRCC [58], is present in over 95% of the ccRCC tumors [34]. It is not expressed in normal kidney parenchyma, cysts, papRCC or chRCC subtypes [59]. Girentuximab, a chimeric IgG1 monoclonal antibody [60], developed to target the CAIX antigen was studied with the purpose of diagnostic imaging along with PET [32,34]. Several radionuclides were used to label girentuximab for diagnostic therapeutic purposes [61]. Divgi et al. aimed to preoperatively assess, in an open-label pilot study, the iodine-124-labelled antibody chimeric G250 (124I-cG250) PET/CT) to accurately detect ccRCC with a 94% sensitivity, a NPV of 90%, and specificity and positive predictive accuracy of 100% [62]. Due to these results, the author designed a clinical study to assess the characterization of renal masses provided by 124I-cG250) PET/CT and found very good sensitivity and specificity (86.2% and 85.9%, respectively) and very good inter-reader and intra-reader agreement [63]. A 2018 multi-center trial that is an ongoing study was developed using 89zirconium-girentuximab (89Zr-girentuximab) instead of I124-girentuximab, because it may have high sensitive ability to detect ccRCC (ZIRCON trial, registered on ClinicalTrials.gov: NCT03849118) [64,65,66].
4. Discussion
The need for more comprehensive, accurate preoperative diagnosis of renal lesions that have the potential to progress and metastasize, in the setting of almost one fifth localized lesions being identified as benign in nature [9,10], is of great importance to minimize morbidity and renal function loss after the surgery of benign tumors [67]. The current research holds a positive premise to the identification of radiotracers and antibodies linked to different antigens of cancer cells [68], which can better distinguish between benign lesions, cancerous lesions and also different histological subtypes of RCC [3]. Being a novel area of investigation, most of the works are presented as pilot studies, with a limited number of enrolled subjects. Although this limitation can be applied, the results of trials investigating 99mTc-sestamibi SPECT have very good accuracy in detection of benign tumors (83.3% up to 100% for oncocytoma) [33,44,54], showing that the technique has great diagnostic potential for these types of tumors. For AMLs, the accuracy was 100% [33,48], with one case of AML that had false-positive uptake [46] and 100% for HOCTs [44,47,48]. These AMLs and oncocytomas are rich in mitochondria [69], and therefore the uptake of the lipophilic 99mTc-sestamibi is high in oncocytomas and AMLs, compared to malignant tissues where the multidrug resistance pump (performs wash-out of 99mTc-sestamibi) is well expressed, making it good to discriminate oncocytomas from cancerous tissues [54,70].
For the chRCC subtype, we have identified a poorer performance in some studies (of just 50%) [44], but with excellent results in other research performed (100%) [50]. This high variance of results is probably due to the lack of standardization of protocols, time of exposure from injections that varies greatly between studies (from 1 to 7 days), and between molecules used (5 days for girentuximab or approximately 75 min for 99mTc-sestamibi) [43,63].
RCC and its subtypes do not uptake the radiotracer, with the exception of chRCC. Two studies reported 50% false uptake [44,46], and one study showed slightly positive uptake of the radiotracer [45]. What is more important is that the sensitivity and specificity is very high in detecting benign compared to malignant lesions (ranging from 87.5% to 100% for sensitivity and 73% to 94.8% for specificity) [33,44,53]. Almost all studies have shown the ability of 99mTc-sestamibi SPECT/CT to distinguish the benign lesions from malignant lesions, and this technique could be close to clinical implementation for diagnostic purposes in RCC, but there are some drawbacks to a generalized use. Girentuximab has a long stay in the main circulation of the body, therefore it is accumulated in the whole renal parenchyma [71], limiting the acquired quality of the images. The 99mTc-sestamibi SPECT/CT can be performed relatively quickly after injection [43], limiting the long period and logistics to scan the patients. PET/CT has a greater image quality and time for slice acquisition [72], and used with a new modified ligand (64Cu XYIMSR-06) can shorten acquisition time compared to SPECT/CT [73]. Taken altogether, the techniques of SPECT/CT and PET/CT combined with molecular antibodies and radiotracers give new insights for the possibility of preoperatively distinguishing between benign and malignant lesions, and in some proportion to differentiate between subtypes of RCC. 99mTc-sestamibi is approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) [74] to be used as a myocardial perfusion imaging molecule for use in SPECT for the identification of the risk of myocardial events [75], for assessment of breast, lung, thyroid, and head and neck benign or malignant tumors [76,77]. The girentuximab PET/CT holds great promise, but in this moment its use is limited to clinical trials [78]. A head-to-head comparison is not eligible due to the lack of data from clinical trials. Lately, there are new techniques evolving, such as the new long axial field-of-view PET/CT scanners, that are trying to overcome the disadvantage of the limited axial field of view of current scanners, and to provide an accurate dynamic scan of whole body tumors at the same time with short acquisition time [79,80,81].
There are no prospective, large, multi-center studies to validate the results obtained so far. There are no standardized protocols for image acquisition after the injection of radiotracers, no head-to-head comparison between SPECT/CT and PET/CT. These limit the application outside clinical trials. Validation and health economics studies will have to be performed before the worldwide clinical implementation of these techniques, but the future is here.
5. Conclusions
Nuclear medicine has helped clinicians in evaluating primary and secondary lesions and has lately returned with new and exciting insights with novel radiotracers to reinforce its diagnostic potential in renal carcinoma. The purpose is to limit the benign excised tumors and, subsequently, the morbidity and renal function loss in these patients. Further research is mandatory to validate the results and to clinically implement such diagnostic techniques in the context of precision medicine.
Author Contributions
Conceptualization, O.S.T. and M.F.; methodology, O.S.T., M.F., B.B. and F.C.; software, O.S.T. and M.F.; validation, O.S.T., M.F., M.M., F.C., B.B., G.L., F.D.G., A.V., G.I.R., A.L.G., G.M.B., S.L., M.L.P., G.M. and M.D.V.; formal analysis, O.S.T. and M.F.; investigation, O.S.T., M.F., B.B. and F.C.; resources, O.S.T.; data curation, O.S.T., M.F., M.M., F.C., B.B., G.L., F.D.G., A.V., G.I.R., A.L.G., G.M.B., S.L., M.L.P., G.M. and M.D.V.; writing—original draft preparation, O.S.T.; writing—review and editing, O.S.T., M.F., M.M., F.C., B.B., G.L., F.D.G., A.V., G.I.R., A.L.G., G.M.B., S.L., M.L.P., G.M. and M.D.V.; visualization, O.S.T.; supervision, O.S.T. and M.F.; project administration, O.S.T. and M.F.; funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J. Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Turner R.M., Morgan T.M., Jacobs B.L. Epidemiology of the Small Renal Mass and the Treatment Disconnect Phenomenon. Urol. Clin. N. Am. 2017;44:147–154. doi: 10.1016/j.ucl.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer A.R., Allaf M.E., Rowe S.P., Gorin M.A. The role of molecular imaging in the characterization of renal masses. Curr. Opin. Urol. 2018;28:159–165. doi: 10.1097/MOU.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 4.Williamson S.R. Clear cell papillary renal cell carcinoma: An update after 15 years. Pathology. 2021;53:109–119. doi: 10.1016/j.pathol.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Frank I., Blute M.L., Cheville J.C., Lohse C.M., Weaver A.L., Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: The SSIGN score. J. Urol. 2002;168:2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 6.Frank I., Blute M.L., Cheville J.C., Lohse C.M., Weaver A.L., Zincke H. Solid renal tumors: An analysis of pathological features related to tumor size. J. Urol. 2003;170:2217–2220. doi: 10.1097/01.ju.0000095475.12515.5e. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen D.P., Vertosick E.A., Corradi R.B., Vilaseca A., Benfante N.E., Touijer K.A., Sjoberg D.D., Russo P. Histological subtype of renal cell carcinoma significantly affects survival in the era of partial nephrectomy. Urol. Oncol. 2016;34:259.e1–259.e8. doi: 10.1016/j.urolonc.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren A.Y., Harrison D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: Standards and controversies. World J. Urol. 2018;36:1913–1926. doi: 10.1007/s00345-018-2447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutikov A., Fossett L.K., Ramchandani P., Tomaszewski J.E., Siegelman E.S., Banner M.P., Van Arsdalen K.N., Wein A.J., Malkowicz S.B. Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology. 2006;68:737–740. doi: 10.1016/j.urology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Johnson D.C., Vukina J., Smith A.B., Meyer A.-M., Wheeler S.B., Kuo T.-M., Tan H.-J., Woods M.E., Raynor M.C., Wallen E.M., et al. Preoperatively misclassified, surgically removed benign renal masses: A systematic review of surgical series and United States population level burden estimate. J. Urol. 2015;193:30–35. doi: 10.1016/j.juro.2014.07.102. [DOI] [PubMed] [Google Scholar]
- 11.Yoo S., You D., Song C., Hong B., Hong J.H., Kim C.-S., Ahn H., Jeong I.G. Declining incidence of benign lesions among small renal masses treated with surgery: Effect of diagnostic tests for characterization. Urol. Oncol. 2018;36:362.e9–362.e15. doi: 10.1016/j.urolonc.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Nandanan N., Veccia A., Antonelli A., Derweesh I., Mottrie A., Minervini A., Aron M., Simone G., Capitanio U., Simeone C., et al. Outcomes and predictors of benign histology in patients undergoing robotic partial or radical nephrectomy for renal masses: A multicenter study. Cent. Eur. J. Urol. 2020;73:33–38. doi: 10.5173/ceju.2020.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amaral B.S., Macek P., Arora A., Pazeto C.L., Zugail A.S., Mombet A., Fregeville A., Lefevre M., Sanchez-Salas R., Cathelineau X. Renal Tumor Biopsy: Rationale to Avoid Surgery in Small Renal Masses. Curr. Urol. Rep. 2021;22:46. doi: 10.1007/s11934-021-01064-8. [DOI] [PubMed] [Google Scholar]
- 14.Richard P.O., Jewett M.A.S., Tanguay S., Saarela O., Liu Z.A., Pouliot F., Kapoor A., Rendon R., Finelli A. Safety, reliability and accuracy of small renal tumour biopsies: Results from a multi-institution registry. BJU Int. 2017;119:543–549. doi: 10.1111/bju.13630. [DOI] [PubMed] [Google Scholar]
- 15.Renshaw A.A., Powell A., Caso J., Gould E.W. Needle track seeding in renal mass biopsies. Cancer Cytopathol. 2019;127:358–361. doi: 10.1002/cncy.22147. [DOI] [PubMed] [Google Scholar]
- 16.Garstka N., Shariat S.F., Remzi M. The evolving role of percutaneous biopsy in renal masses. Curr. Opin. Urol. 2018;28:364–368. doi: 10.1097/MOU.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 17.Velez-Torres J., Guido L.P., Jorda M. Adult Renal Neoplasms: Cytology, Immunohistochemistry, and Cytogenetic Characteristics. Surg. Pathol. Clin. 2018;11:611–631. doi: 10.1016/j.path.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Cazzato R.L., De Marini P., Auloge P., Leclerc L., Tricard T., Linder V., Jost M., Ramamurthy N., Lang H., Garnon J., et al. Diagnostic accuracy and safety of percutaneous MRI-guided biopsy of solid renal masses: Single-center results after 4.5 years. Eur. Radiol. 2021;31:580–590. doi: 10.1007/s00330-020-07160-6. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari M., Cartolari R., Barizzi J., Pereira Mestre R., D’Antonio E., Renard J. Percutaneous biopsy of small renal mass: Can diagnostic accuracy be affected by hospital volume? Cent. Eur. J. Urol. 2021;74:334–340. doi: 10.5173/ceju.2021.3.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klotz L. Overdiagnosis in urologic cancer: For World Journal of Urology Symposium on active surveillance in prostate and renal cancer. World J. Urol. 2022;40:1–8. doi: 10.1007/s00345-020-03523-2. [DOI] [PubMed] [Google Scholar]
- 21.Crișan G., Moldovean-Cioroianu N.S., Timaru D.-G., Andrieș G., Căinap C., Chiș V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022;23:5023. doi: 10.3390/ijms23095023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuocolo A., Petretta M. PET and SPECT Specialty Grand Challenge. When Knowledge Travels at the Speed of Light, Photons Take to the Field. Front. Nucl. Med. 2021;1:671914. doi: 10.3389/fnume.2021.671914. [DOI] [Google Scholar]
- 23.van der Meulen N.P., Strobel K., Lima T.V.M. New Radionuclides and Technological Advances in SPECT and PET Scanners. Cancers. 2021;13:6183. doi: 10.3390/cancers13246183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadsak W., Mitterhauser M. Basics and principles of radiopharmaceuticals for PET/CT. Eur. J. Radiol. 2010;73:461–469. doi: 10.1016/j.ejrad.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Lau J., Rousseau E., Kwon D., Lin K.-S., Bénard F., Chen X. Insight into the Development of PET Radiopharmaceuticals for Oncology. Cancers. 2020;12:1312. doi: 10.3390/cancers12051312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y.-S. Radiopharmaceuticals for molecular imaging. Open Nucl. Med. J. 2010;2:178–185. doi: 10.2174/1876388X01002010178. [DOI] [Google Scholar]
- 27.Duatti A. Review on 99mTc radiopharmaceuticals with emphasis on new advancements. Nucl. Med. Biol. 2021;92:202–216. doi: 10.1016/j.nucmedbio.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Israel O., Pellet O., Biassoni L., De Palma D., Estrada-Lobato E., Gnanasegaran G., Kuwert T., la Fougère C., Mariani G., Massalha S., et al. Two decades of SPECT/CT—The coming of age of a technology: An updated review of literature evidence. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:1990–2012. doi: 10.1007/s00259-019-04404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fahey F., Stabin M. Dose optimization in nuclear medicine. Semin. Nucl. Med. 2014;44:193–201. doi: 10.1053/j.semnuclmed.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Mettler F.A., Huda W., Yoshizumi T.T., Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: A catalog. Radiology. 2008;248:254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 31.Weissleder R., Mahmood U. Molecular imaging. Radiology. 2001;219:316–333. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 32.Roussel E., Capitanio U., Kutikov A., Oosterwijk E., Pedrosa I., Rowe S.P., Gorin M.A. Novel Imaging Methods for Renal Mass Characterization: A Collaborative Review. Eur. Urol. 2022;81:476–488. doi: 10.1016/j.eururo.2022.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu H., Yang B., Dong A., Ye H., Cheng C., Pan G., Zuo C. Dual-Phase 99mTc-MIBI SPECT/CT in the Characterization of Enhancing Solid Renal Tumors: A Single-Institution Study of 147 Cases. Clin. Nucl. Med. 2020;45:765–770. doi: 10.1097/RLU.0000000000003212. [DOI] [PubMed] [Google Scholar]
- 34.van Oostenbrugge T., Mulders P. Targeted PET/CT imaging for clear cell renal cell carcinoma with radiolabeled antibodies: Recent developments using girentuximab. Curr. Opin. Urol. 2021;31:249–254. doi: 10.1097/MOU.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 35.Schillaci O., Danieli R., Filippi L., Romano P., Cossu E., Manni C., Simonetti G. Scintimammography with a hybrid SPECT/CT imaging system. Anticancer Res. 2007;27:557–562. [PubMed] [Google Scholar]
- 36.Tiling R., Tatsch K., Sommer H., Meyer G., Pechmann M., Gebauer K., Münzing W., Linke R., Khalkhali I., Hahn K. Technetium-99m-sestamibi scintimammography for the detection of breast carcinoma: Comparison between planar and SPECT imaging. J. Nucl. Med. 1998;39:849–856. [PubMed] [Google Scholar]
- 37.Urbano N., Scimeca M., Bonanno E., Schillaci O. 99mTc sestamibi SPECT: A possible tool for early detection of breast cancer lesions with high bone metastatic potential. Future Oncol. 2019;15:455–457. doi: 10.2217/fon-2018-0735. [DOI] [PubMed] [Google Scholar]
- 38.Chiti A., Maffioli L.S., Infante M., Grasselli G., Incarbone M., Gasparini M.D., Savelli G., Bombardieri E. Assessment of mediastinal involvement in lung cancer with technetium-99m-sestamibi SPECT. J. Nucl. Med. 1996;37:938–942. [PubMed] [Google Scholar]
- 39.Mosci C., Pericole F.V., Oliveira G.B., Delamain M.T., Takahashi M.E.S., Carvalheira J.B.C., Etchebehere E.C.S.C., Santos A.O., Miranda E.C.M., Lima M.C.L., et al. 99mTc-sestamibi SPECT/CT and 18F-FDG-PET/CT have similar performance but different imaging patterns in newly diagnosed multiple myeloma. Nucl. Med. Commun. 2020;41:1081–1088. doi: 10.1097/MNM.0000000000001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prigent-Le Jeune F., Dubois F., Perez S., Blond S., Steinling M. Technetium-99m sestamibi brain SPECT in the follow-up of glioma for evaluation of response to chemotherapy: First results. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:714–719. doi: 10.1007/s00259-004-1463-7. [DOI] [PubMed] [Google Scholar]
- 41.Lavely W.C., Goetze S., Friedman K.P., Leal J.P., Zhang Z., Garret-Mayer E., Dackiw A.P., Tufano R.P., Zeiger M.A., Ziessman H.A. Comparison of SPECT/CT, SPECT, and planar imaging with single- and dual-phase (99m)Tc-sestamibi parathyroid scintigraphy. J. Nucl. Med. 2007;48:1084–1089. doi: 10.2967/jnumed.107.040428. [DOI] [PubMed] [Google Scholar]
- 42.Mallick R., Malik J., Yip L., Muthukrishnan A., Carty S.E., McCoy K.L. Novel Findings on SPECT-CT Tc-99 Sestamibi Imaging for Primary Hyperparathyroidism. J. Surg. Res. 2020;252:216–221. doi: 10.1016/j.jss.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Rowe S.P., Gorin M.A., Gordetsky J., Ball M.W., Pierorazio P.M., Higuchi T., Epstein J.I., Allaf M.E., Javadi M.S. Initial experience using 99mTc-MIBI SPECT/CT for the differentiation of oncocytoma from renal cell carcinoma. Clin. Nucl. Med. 2015;40:309–313. doi: 10.1097/RLU.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorin M.A., Rowe S.P., Baras A.S., Solnes L.B., Ball M.W., Pierorazio P.M., Pavlovich C.P., Epstein J.I., Javadi M.S., Allaf M.E. Prospective Evaluation of (99m)Tc-sestamibi SPECT/CT for the Diagnosis of Renal Oncocytomas and Hybrid Oncocytic/Chromophobe Tumors. Eur. Urol. 2016;69:413–416. doi: 10.1016/j.eururo.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 45.Tzortzakakis A., Gustafsson O., Karlsson M., Ekström-Ehn L., Ghaffarpour R., Axelsson R. Visual evaluation and differentiation of renal oncocytomas from renal cell carcinomas by means of 99mTc-sestamibi SPECT/CT. EJNMMI Res. 2017;7:29. doi: 10.1186/s13550-017-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheikhbahaei S., Jones C.S., Porter K.K., Rowe S.P., Gorin M.A., Baras A.S., Pierorazio P.M., Ball M.W., Higuchi T., Johnson P.T., et al. Defining the Added Value of 99mTc-MIBI SPECT/CT to Conventional Cross-Sectional Imaging in the Characterization of Enhancing Solid Renal Masses. Clin. Nucl. Med. 2017;42:e188–e193. doi: 10.1097/RLU.0000000000001534. [DOI] [PubMed] [Google Scholar]
- 47.Jones K.M., Solnes L.B., Rowe S.P., Gorin M.A., Sheikhbahaei S., Fung G., Frey E.C., Allaf M.E., Du Y., Javadi M.S. Use of quantitative SPECT/CT reconstruction in 99mTc-sestamibi imaging of patients with renal masses. Ann. Nucl. Med. 2018;32:87–93. doi: 10.1007/s12149-017-1222-z. [DOI] [PubMed] [Google Scholar]
- 48.Tzortzakakis A., Holstensson M., Hagel E., Karlsson M., Axelsson R. Intra- and Interobserver Agreement of SUV SPECT Quantitative SPECT/CT Processing Software, Applied in Clinical Settings for Patients with Solid Renal Tumors. J. Nucl. Med. Technol. 2019;47:258–262. doi: 10.2967/jnmt.118.223297. [DOI] [PubMed] [Google Scholar]
- 49.Su Z.T., Patel H.D., Huang M.M., Meyer A.R., Pavlovich C.P., Pierorazio P.M., Javadi M.S., Allaf M.E., Rowe S.P., Gorin M.A. Cost-effectiveness Analysis of 99mTc-sestamibi SPECT/CT to Guide Management of Small Renal Masses. Eur. Urol. Focus. 2021;7:827–834. doi: 10.1016/j.euf.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Warren H., Boydell A.-R., Reza A., Pencharz D., Holman B.F., El-Sheikh S., Wildgoose W.H., Barod R., Patki P., Mumtaz F., et al. Use of 99m Tc-sestamibi SPECT/CT for indeterminate renal tumours: A pilot diagnostic accuracy study. BJU Int. 2022;130:748–750. doi: 10.1111/bju.15879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parihar A.S., Mhlanga J., Ronstrom C., Schmidt L.R., Figenshau R.S., Dehdashti F., Wahl R.L. Diagnostic accuracy of 99mTc-Sestamibi SPECT/CT for characterization of solid renal masses. J. Nucl. Med. 2022;64:90–95. doi: 10.2967/jnumed.122.264329. [DOI] [PubMed] [Google Scholar]
- 52.Sistani G., Bjazevic J., Kassam Z., Romsa J., Pautler S. The value of 99mTc-sestamibi single-photon emission computed tomography-computed tomography in the evaluation and risk stratification of renal masses. Can. Urol. Assoc. J. 2021;15:197–201. doi: 10.5489/cuaj.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viswambaram P., Picardo A., Hohnen A., Pham K., Macdonald W., Hayne D., Hamid A. Technetium-99 m-sestamibi single-photon emission computerised tomography (CT)/CT in the prediction of malignant versus benign small renal masses. BJU Int. 2022;130:23–31. doi: 10.1111/bju.15737. [DOI] [PubMed] [Google Scholar]
- 54.Asi T., Tuncali M.Ç., Tuncel M., Alkanat N.E.İ., Hazir B., Kösemehmetoğlu K., Baydar D.E., Akdoğan B. Proceedings of the Urologic Oncology: Seminars and Original Investigations. Volume 38. Elsevier; Amsterdam, The Netherlands: 2020. The role of Tc-99m MIBI scintigraphy in clinical T1 renal mass assessment: Does it have a real benefit? pp. 937.e11–937.e17. [DOI] [PubMed] [Google Scholar]
- 55.Ljungberg B., Albiges L., Abu-Ghanem Y., Bedke J., Capitanio U., Dabestani S., Fernández-Pello S., Giles R.H., Hofmann F., Hora M., et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022;82:399–410. doi: 10.1016/j.eururo.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Wilson M.P., Katlariwala P., Murad M.H., Abele J., McInnes M.D.F., Low G. Diagnostic accuracy of 99mTc-sestamibi SPECT/CT for detecting renal oncocytomas and other benign renal lesions: A systematic review and meta-analysis. Abdom. Radiol. 2020;45:2532–2541. doi: 10.1007/s00261-020-02469-8. [DOI] [PubMed] [Google Scholar]
- 57.Rowe S.P., Gorin M.A., Solnes L.B., Ball M.W., Choudhary A., Pierorazio P.M., Epstein J.I., Javadi M.S., Allaf M.E., Baras A.S. Correlation of 99mTc-sestamibi uptake in renal masses with mitochondrial content and multi-drug resistance pump expression. EJNMMI Res. 2017;7:80. doi: 10.1186/s13550-017-0329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oosterwijk E., Ruiter D.J., Hoedemaeker P.J., Pauwels E.K., Jonas U., Zwartendijk J., Warnaar S.O. Monoclonal antibody G 250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int. J. Cancer. 1986;38:489–494. doi: 10.1002/ijc.2910380406. [DOI] [PubMed] [Google Scholar]
- 59.Stillebroer A.B., Mulders P.F.A., Boerman O.C., Oyen W.J.G., Oosterwijk E. Carbonic anhydrase IX in renal cell carcinoma: Implications for prognosis, diagnosis, and therapy. Eur. Urol. 2010;58:75–83. doi: 10.1016/j.eururo.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 60.Steffens M.G., Boerman O.C., Oosterwijk-Wakka J.C., Oosterhof G.O., Witjes J.A., Koenders E.B., Oyen W.J., Buijs W.C., Debruyne F.M., Corstens F.H., et al. Targeting of renal cell carcinoma with iodine-131-labeled chimeric monoclonal antibody G250. J. Clin. Oncol. 1997;15:1529–1537. doi: 10.1200/JCO.1997.15.4.1529. [DOI] [PubMed] [Google Scholar]
- 61.Lau J., Lin K.-S., Bénard F. Past, Present, and Future: Development of Theranostic Agents Targeting Carbonic Anhydrase IX. Theranostics. 2017;7:4322–4339. doi: 10.7150/thno.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Divgi C.R., Pandit-Taskar N., Jungbluth A.A., Reuter V.E., Gönen M., Ruan S., Pierre C., Nagel A., Pryma D.A., Humm J., et al. Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: A phase I trial. Lancet Oncol. 2007;8:304–310. doi: 10.1016/S1470-2045(07)70044-X. [DOI] [PubMed] [Google Scholar]
- 63.Divgi C.R., Uzzo R.G., Gatsonis C., Bartz R., Treutner S., Yu J.Q., Chen D., Carrasquillo J.A., Larson S., Bevan P., et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: Results from the REDECT trial. J. Clin. Oncol. 2013;31:187–194. doi: 10.1200/JCO.2011.41.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Telix International Pty Ltd A Confirmatory, Prospective, Open-label, Multi-centre Phase 3 Study to Evaluate Diagnostic Performance of Zirconium-labelled Girentuximab to Non-invasively Detect ccRCC by PET/CT Imaging in Patients with Indeterminate Renal Masses. [(accessed on 7 December 2022)]; Available online: clinicaltrials.gov.
- 65.Cheal S.M., Punzalan B., Doran M.G., Evans M.J., Osborne J.R., Lewis J.S., Zanzonico P., Larson S.M. Pairwise comparison of 89Zr- and 124I-labeled cG250 based on positron emission tomography imaging and nonlinear immunokinetic modeling: In vivo carbonic anhydrase IX receptor binding and internalization in mouse xenografts of clear-cell renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:985–994. doi: 10.1007/s00259-013-2679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merkx R.I.J., Lobeek D., Konijnenberg M., Jiménez-Franco L.D., Kluge A., Oosterwijk E., Mulders P.F.A., Rijpkema M. Phase I study to assess safety, biodistribution and radiation dosimetry for 89Zr-girentuximab in patients with renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:3277–3285. doi: 10.1007/s00259-021-05271-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bertolo R., Autorino R., Simone G., Derweesh I., Garisto J.D., Minervini A., Eun D., Perdona S., Porter J., Rha K.H., et al. Outcomes of Robot-assisted Partial Nephrectomy for Clinical T2 Renal Tumors: A Multicenter Analysis (ROSULA Collaborative Group) Eur. Urol. 2018;74:226–232. doi: 10.1016/j.eururo.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 68.de Campos N.S.P., Souza B.S., da Silva G.C.P., Porto V.A., Chalbatani G.M., Lagreca G., Janji B., Suarez E.R. Carbonic Anhydrase IX: A Renewed Target for Cancer Immunotherapy. Cancers. 2022;14:1392. doi: 10.3390/cancers14061392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crane P., Laliberté R., Heminway S., Thoolen M., Orlandi C. Effect of mitochondrial viability and metabolism on technetium-99m-sestamibi myocardial retention. Eur. J. Nucl. Med. 1993;20:20–25. doi: 10.1007/BF02261241. [DOI] [PubMed] [Google Scholar]
- 70.Tobe S.W., Noble-Topham S.E., Andrulis I.L., Hartwick R.W., Skorecki K.L., Warner E. Expression of the multiple drug resistance gene in human renal cell carcinoma depends on tumor histology, grade, and stage. Clin. Cancer Res. 1995;1:1611–1615. [PubMed] [Google Scholar]
- 71.Garousi J., Huizing F.J., Vorobyeva A., Mitran B., Andersson K.G., Leitao C.D., Frejd F.Y., Löfblom J., Bussink J., Orlova A., et al. Comparative evaluation of affibody- and antibody fragments-based CAIX imaging probes in mice bearing renal cell carcinoma xenografts. Sci. Rep. 2019;9:14907. doi: 10.1038/s41598-019-51445-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rahmim A., Zaidi H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008;29:193–207. doi: 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- 73.Minn I., Koo S.M., Lee H.S., Brummet M., Rowe S.P., Gorin M.A., Sysa-Shah P., Lewis W.D., Ahn H.-H., Wang Y., et al. [64Cu]XYIMSR-06: A dual-motif CAIX ligand for PET imaging of clear cell renal cell carcinoma. Oncotarget. 2016;7:56471–56479. doi: 10.18632/oncotarget.10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urbano N., Scimeca M., Di Russo C., Mauriello A., Bonanno E., Schillaci O. [99mTc]Sestamibi SPECT Can Predict Proliferation Index, Angiogenesis, and Vascular Invasion in Parathyroid Patients: A Retrospective Study. J. Clin. Med. 2020;9:2213. doi: 10.3390/jcm9072213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leung K. Molecular Imaging and Contrast Agent Database (MICAD) National Center for Biotechnology Information (US); Bethesda, MD, USA: 2004. 1-(9H-Carbazol-4-yloxy)-3-(2-(2-[11C]methoxyphenoxy)ethylamino)-propan-2-ol. [PubMed] [Google Scholar]
- 76.Leung K. Molecular Imaging and Contrast Agent Database (MICAD) National Center for Biotechnology Information (US); Bethesda, MD, USA: 2004. 6,7-Dimethoxy-2-{3-[4-[11C]methoxy-3,4-dihydro-2H-naphthalen-(1E)-ylidene]-propyl}-1,2,3,4-tetrahydro-isoquinoline. [PubMed] [Google Scholar]
- 77.Howard B.A. Molecular Imaging in the Head and Neck: Diagnosis and Therapy. Radiol. Clin. N. Am. 2020;58:1135–1146. doi: 10.1016/j.rcl.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 78.Smaldone M.C., Chen D.Y., Yu J.Q., Plimack E.R. Potential role of (124)I-girentuximab in the presurgical diagnosis of clear-cell renal cell cancer. Biologics. 2012;6:395–407. doi: 10.2147/BTT.S30413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brouwers A.H., van Sluis J., van Snick J.H., Schröder C.P., Baas I.O., Boellaard R., Glaudemans A.W.J.M., Borra R.J.H., Lammertsma A.A., Dierckx R.A.J.O., et al. First-time imaging of [89Zr]trastuzumab in breast cancer using a long axial field-of-view PET/CT scanner. Eur. J. Nucl. Med. Mol. Imaging. 2022;49:3593–3595. doi: 10.1007/s00259-022-05777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Filippi L., Dimitrakopoulou-Strauss A., Evangelista L., Schillaci O. Long axial field-of-view PET/CT devices: Are we ready for the technological revolution? Expert Rev. Med. Devices. 2022;19:739–743. doi: 10.1080/17434440.2022.2141111. [DOI] [PubMed] [Google Scholar]
- 81.Slart R.H.J.A., Tsoumpas C., Glaudemans A.W.J.M., Noordzij W., Willemsen A.T.M., Borra R.J.H., Dierckx R.A.J.O., Lammertsma A.A. Long axial field of view PET scanners: A road map to implementation and new possibilities. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:4236–4245. doi: 10.1007/s00259-021-05461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.