Abstract
Background
Dilated cardiomyopathy (DCM) is a leading cause of heart transplantation (HTx) in children. Surgical pulmonary artery banding (PAB) is used worldwide to achieve functional heart regeneration and remodelling.
Case summary
We report for the first-time successful bilateral transcatheter implantation of bilateral pulmonary artery flow restrictors in a case series of three infants with severe DCM based on left-ventricular non-compaction morphology associated with Barth syndrome in one and a non-classified syndrome in another. Functional cardiac regeneration was observed in two patients after almost 6 months of endoluminal banding, and in the neonate with Barth syndrome already after 6 weeks. Accompanied by an improvement in functional class (Class IV to Class I), the left ventricular end-diastolic dimensions z-score normalized, as did the elevated serum brain natriuretic peptide levels. A listing for HTx could be avoided.
Discussion
Percutaneous bilateral endoluminal PAB is a novel minimally invasive approach that enables functional cardiac regeneration in infants with severe DCM and preserved right ventricular function. Interruption of the ventriculo-ventricular interaction, the key mechanism for recovery, is avoided. Intensive care for these critically ill patients is reduced to a minimum. However, investing in ‘heart regeneration to avoid transplantation’ remains a challenge.
Keywords: Paediatric dilated cardiomyopathy, Endovascular pulmonary artery banding, Pulmonary flow restrictors, Functional cardiac regeneration, Case series
Learning points.
Paediatric dilated cardiomyopathy has tremendous regenerative potential even at its advanced stages, particularly in infants, yet it will result in transplantation in facilities where cardiac transplantation is available.
Surgical pulmonary artery banding together with medication that promotes regeneration offers a realistic chance of functional regeneration and the avoidance of transplantation, especially in infants.
Transcatheter-assisted endovascular banding by placing pulmonary flow restrictors (PFRs) in the left and right pulmonary branch arteries is a minimally invasive procedure with less risk and less intensive care compared with an open-chest approach.
Investing in bespoke equipment such as PFRs sized for all ages is long overdue. However, investing in heart regeneration seems less lucrative than investing in a heart replacement, which of course is also necessary.
Introduction
Paediatric dilated cardiomyopathy (DCM) is the most common cause of heart failure and an indication for heart transplantation (HTx). The incidence is reported at 0.58–0.73 per 100 000 and the risk of death or transplantation is ∼26% within the first year after diagnosis. The highest annual incidence is observed in the first year of life.1
Pulmonary artery banding (PAB) has been proposed to induce functional regeneration (FR) in young patients with extensive left ventricular (LV)-DCM and preserved right ventricular (RV) function.2,3 The effectiveness of PAB is reciprocal to the patient’s age. As a surgical measure, PAB is now used worldwide as an alternative or as a bridge to HTx. Short- and long-term results are encouraging.2,4,5 The surgical approach involves a central PAB that requires an open chest and pericardium, challenging the ventriculo-ventricular interaction (VVI) and postoperative intensive care of these critically ill patients.
Here we report a novel transcatheter procedure to replace the surgical approach and ease the chance for FR. The technique of placing bilateral pulmonary arterial blood flow restrictors has recently been described in newborns with hypoplastic left heart syndrome and HLHS complex to avoid neonatal Norwood or Hybrid surgery.6
Timeline
| Endovascular bilateral pulmonary banding (ePAB) | ||||
|---|---|---|---|---|
| Pt. no | DCM | Procedure | Functional regeneration | Functional Ross I–IV change (last follow-up) with ePAB + BLS |
| Age at diagnosis months | Age at PAB months | Age (months) after endoPAB | ||
| 1 | 3 | 4.5 | 6 | IV to I (4 years) |
| 2 | 1.5 | 3 | 3–6 | IV to I (14 months) |
| 3 | 0.5 | 1.5 | 2 | IV to I (8 months) |
B, bisoprolol; DCM, dilated cardiomyopathy; ePAB, endoluminal pulmonary arterial banding; L, lisinopril; S, spironolactone.
Patient 1
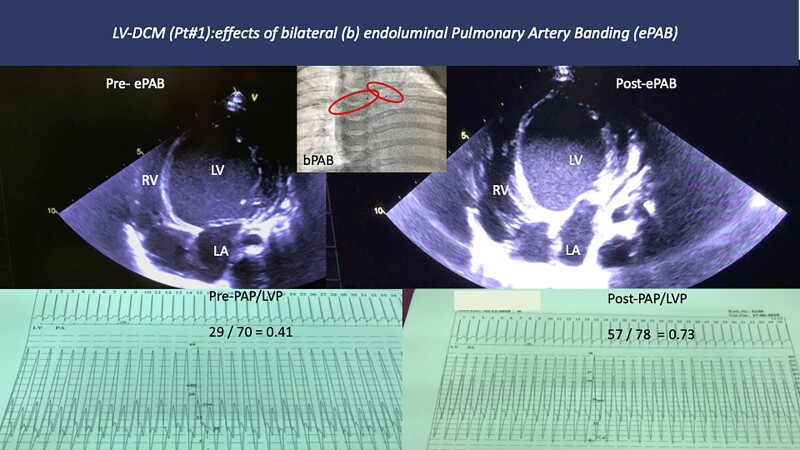
A 6-month-old female infant (body weight 6.1 kg, body length 65 cm) was referred to the paediatric intensive care unit of our Center for Tertiary Pediatric Cardiology due to acute symptoms of heart failure. Because of recurrent lung infections, persistent malnutrition, and tachypnoea, a chest x-ray was already taken at the referring hospital, which showed an enlarged heart. Echocardiography revealed a dilated LV with a reduced ejection fraction (EF). At admission, Ross-functional class correlated with NT-proBNP values of 19 920 pg/mL (age-related norm, <125 pg/mL). Because of the decompensated LV-DCM, nasal continuous positive airway pressure (CPAP) support with oxygen supplementation was administered with co-medication of analgosedatives as necessary. After placement of an IV-line, continuous intravenous infusions of epinephrine (0.05 µg/kg/min), norepinephrine (0.1 µg/kg/min) and milrinone (0.075 µg/kg/min) were introduced. Furosemide was administered in repeated single dosages of 5 mg, if necessary. The girl of consanguineous parents with negative genetic analyses classified as having an unknown syndrome showed echocardiographic LV-DCM with a non-compaction morphology of the (LV-NC). The LV end-diastolic diameter (LVEDD) was 43 mm, corresponding to a z-score of +6.7. The calculated LVEF was 18% (Table 1). The left atrium and pulmonary veins were dilated. Due to the persistent congestion symptoms, a first cardiac catheterization was performed 3 days after admission to establish restrictive atrial communication, as previously described in detail.7 Coronary anomalies were additionally ruled out by an overview aortogram. After this transcatheter procedure, the treatment of acute heart failure could be de-escalated. Continuous positive airway pressure was removed, catecholamines were weaned, but milrinone infusion was continued at a dosage of 1 µg/kg/min. In addition, levosimendan was administered at a dose of 0.1 µg/kg/min without a loading dose over 24 h as a continuous infusion every 5–7 days. Oral tube feeding also enabled the establishment of our standard oral treatment protocol for chronic heart failure, consisting of the beta1-specific blocker bisoprolol (B) in a single daily dose of (0.1–0.2 mg/kg), followed and combined by the even long-acting ACE-inhibitor lisinopril (L) in the same single dosage, starting with 0.6125 mg/day. Spironolactone (S) was even administered only once per day at a dosage of 1 × 6.25 mg/day (1 mg/kg). Withdrawal of catecholamines and treatment with bisoprolol reduced resting heart rate from above 165/min to <110/min (Table 1). Oral heart failure therapy, including adjuvant medication, has been established as previously described.8 While the clinical condition stabilized, but the echocardiogram of a severe LV-DCM did not change despite the treatment described, PAB was considered based on our entry criteria.3 Approval for HTx has already been ruled out for a number of reasons. Some of these reasons also support semi-invasive endo-luminal PAB branches rather than a central PAB as a surgical approach in the open-chest and pericardium. Percutaneous placement of the endoluminal PAB was approved as compassionate treatment according to local institutional ethics committee rules, and full written parental consent was obtained through a native translator. Twelve hours before the planned cardiac catheterization, levosimendan (0.1 µg/kg/min) was again administered in addition to milrinone that was still running. Both drugs even run through the catheter procedure. The orally administered drugs were discontinued 6 h beforehand. The child remained to breath spontaneously. Sedation consisted of small intravenous dosages of diazepam, ketamine, and propofol as needed. Femoral vascular access was used in aseptic conditions after local anaesthesia. A 6 Fr Terumo® sheath was placed in the femoral vein and a 4 Fr sheath in the femoral artery. Heparin was administered intravenously at an initial dose of 100 IU/kg, followed by 50 IU/kg after placement of the endo-pulmonary flow restrictors (PFRs). After a brief haemodynamic assessment (Figure 1), angiocardiography was performed through a 4 Fr pigtail-catheter placed in the pulmonary trunk to visualize the pulmonary branch arteries. Based on a right pulmonary artery (RPA) branch diameter of 8 mm and a left pulmonary artery (LPA) branch diameter of 7–8 mm, only the currently largest MVP-9Q devices (Medtronic Micro Vascular Plug™) were suitable for the manually fabricated endovascular PFRs. Considering that we had just begun the transcatheter technique of bilateral pulmonary artery branch banding, as described later,6 we removed the thin PTFE (polytetrafluoroethylene) membrane from 2 of the 10 coated end-cells of the micro vascular plug (MVP) to create the PFR for the RPA, and only 1 of the 10 end-cells to create a PFR for the LPA. The created fenestrations with different diameters of ∼4.5–5 mm and 4 × 4.5 mm was made on the basis of the still limited experience to minimize the risk of a thrombus formation. Selective RPA angiography at 20° cranial and 20–30° right anterior oblique (RAO) angulation and LPA angiography at 20° cranial and 20–30° left anterior oblique (LAO) angulation were performed at the site of the PFRs to be implanted. The manually modified MVPs were loaded into a 5 Fr Terumo® Cobra-shaped catheter pre-existing at the LPA and RPA, respectively. After each implantation of the PFR, selective angiography by hand-injection of contrast medium through the Cobra catheter visualized the correct position of the device and confirmed the fully perfused pulmonary artery branch. Finally, the pulmonary artery pressure was measured relative to the LV-pressure obtained through the remaining 4 Fr pigtail catheter. After the placement of the PFR’s, the RV/LV pressure ratio increased from 0.41 to 0.73. The echocardiographic assessment of the VVI before and after the endoPAB procedure, which was still carried out in the cardiac catheter laboratory, is also shown in Figure 1 (see Supplementary material online, Videos S1A and B). The intervention was without complications. The procedure time was 108 min, the total fluoroscopy time was 11.5 min, and the total area dose 410 cGy × cm2. However, the patient had to remain in the hospital for a further 2 months until both the cardiovascular system was sufficiently stabilized and the parents were finally instructed to care for their baby, including the administration of oral medication. In the regular follow-up, another catheter intervention was necessary when the echocardiographic assessment showed questionable effectiveness, especially of the right-sided PFR. The PFR within the RPA, originally created by removing 2 of 10 PTFE-coated end-cells, was removed uneventfully by sling technique, followed by replacement of MVP-9Q-based PFR, removing only one end-cell covering. The now 4-year-old girl has developed remarkably well, both physically and neurologically. The endo-PABs have remained unchanged since the last catheter intervention, as has the long-term oral therapy with doses adapted to the increasing body weight.
Table 1.
Patient data at admission (see also text)
| LV-DCM pts number (sex) | Further diagnoses | BW (kg) | Age (mo.) at ePAB | HR initial/min | TAPSE (mm) | LV-EDD (mm) (z-score) | LV-EF (%) initial | Ross-functional class (I–IV) | Initial Th. E, NE, M, Levo CPAP/CV tc rASD |
|---|---|---|---|---|---|---|---|---|---|
| 1. (f) |
LV-NC,
MR I-II consanguine |
6.1 | 6 | 165 | 15 |
43
(+6.7) |
18 | IV | E, NE, M, Levo CPAP tc rASD |
| 2. (f) |
LV-NC, MRI CRP rASD |
4.9 | 3 | 170 | 13 |
40
(+6,5) |
19 | IV | E, NE, M, Levo CV |
|
3.
(m) |
LV-NC,-Barth synd.
PFO |
3.5 | 1.5 | 158 | 11 |
32
(+5.1) |
15 | IV | E, M, L |
Abbreviations: ASA, acetyl-salicylic-acid; B, bisoprolol; Barth-Syndrome; BW, body weight; CV, controlled ventilation; CRP, cardiopulmonary resuscitation; DCM, dilated cardiomyopathy; E, epinephrine; EDD, enddiastolic diameter; EF, ejection fraction; ePAB, endo-pulmonary artery banding; f, female, HR, heart rate; L, lisinopril; Levo, levosimendan; LV, left ventricle; LV-NC, left ventricular non-compaction; m, male; M, milrinone; mo., months; MR, mitral regurgitation; NC, non-compaction; NE, norepinephrine; pts, patients; PFO, patent foramen ovale; rASD, restrictive atrial septum defect; S, spironolactone; TAPSE, tricuspid annular plane systolic excursion; tc, transcatheter.
Figure 1.
Pre- an and immediate post-interventional results after bilateral (b) endoluminal (e) pulmonary artery banding of Patient #1. Transcatheter placed bilateral pulmonary flow restrictors resulted in an immediate increase of the pulmonary artery pressure, while the ratio of the systolic pulmonary artery pressure to the left ventricular systolic pressure increased from 0.41 to 0.73. Note, that the ventricular septum shifted from right to left with functional improvement of the left ventricular function.
Patient 2
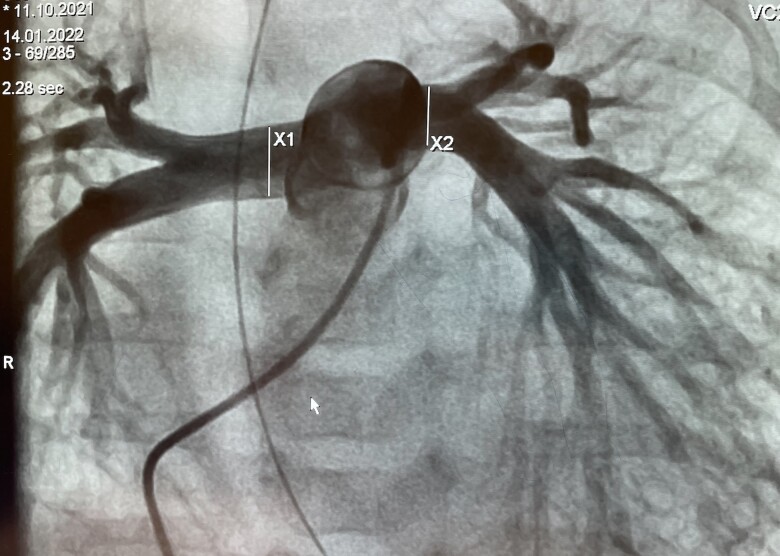
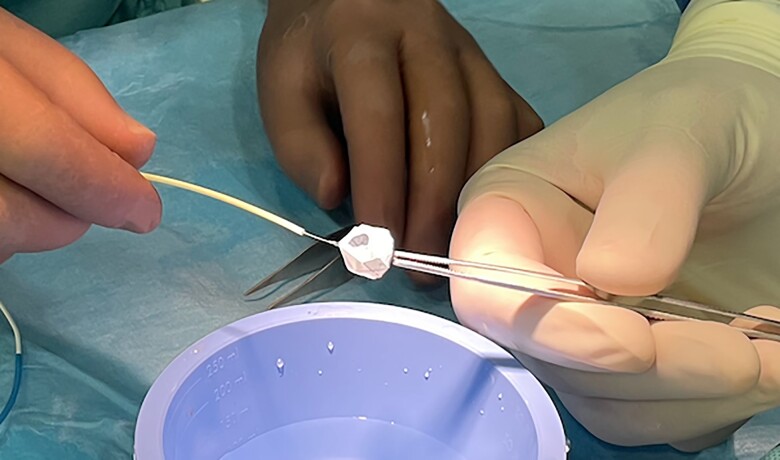
A 3-month-old female infant with previously diagnosed DCM was referred for assessment of whether she would be a candidate for PAB rather than HTx listing. The girl was born at term and the first 6 weeks of her life were uneventful. She was admitted to the referring hospital with symptoms of acute heart failure, namely poor feeding, pallor, and respiratory distress, present for at least 2 days. Bodyweight on admission was 4.8 kg and body length 59 cm. Ross clinical functional status of IV corresponded to NT-proBNP values of 65 350 pg/mL (age-related norm, <125 pg/mL). Upon admission, she presented with tachy-dyspnoea, prolonged capillary refill time, and tachycardia. After admission to the paediatric intensive care unit, she was immediately intubated, ventilated, and briefly resuscitated. A central line catheter was placed and echocardiography performed at the same time revealed the diagnosis of severe DCM. Epinephrine at a dosage of 0.1 µg/kg/min was infused intravenously together with sodium nitroprusside (15 µg/kg/min) and milrinone at 0.5 µg/kg/min. Levosimendan was administered intermittently at a dose of 0.1 µg/kg/min without a loading dose. Diuretic treatment consisted of intravenous furosemide in repeated single dosages of 1 mg/kg, six times daily. Spironolactone and hydrochlorothiazide were each administered at a dose of 5 mg twice daily. The heart rate at rest ranged between 160 and 170/min. The patient remained slightly stable. Detailed echocardiography showed a LV-DCM with LV-NC morphology. The measured LVEDD was 40 mm (z-score +6.5), the LV-EF was initially 19%. The atrial septum showed a small restrictive communication with a mean pressure gradient of 9–11 mmHg. Angiography ruled out a coronary anomaly and confirmed the LV-NC morphology of the low-contracting LV. An additional MRT was not performed. Medical de-escalating was initiated in view of the ‘Banana’-shaped RV with preserved function with an age-related normal TAPSE of 10–13 mm and a nearly collapsed inferior caval vein despite a LV that continued to be low contracting. An intravascular volume deficit was definitively ruled out by an additional intravenous volume challenge of 50 mL (10 mL/kg) of physiological Ringer® solution and the discontinuation of furosemide treatment the day before. Bisoprolol (B) was administered orally in a single 0.6125 mg dose, followed by lisinopril (L) in the same single oral dose. Spironolactone (S) was administered only once daily at a dose of 6.25 mg. She was extubated, the epinephrine infusion was stopped, but milrinone was continued at an intravenous dosage of 1 µg/kg/min. In addition, the intermittent 24 h infusion of levosimendan was also continued at an interval of 5–7 days. After transfer for PAB assessment, cardiac therapy was continued. Bisoprolol together with clonidine 3–6×/day (1–2 µg/kg/per dosage) lowered the heart rate to <120/min at rest and improved baby’s discomfort and partial oral feeding became possible instead. Based on our met entry criteria for PAB in LV DCM with preserved RV function,3 patient size, and echocardiographically measured right and LPA branches with diameters <8 mm, we also offered the parents the transcatheter endoluminal PAB approach as well as an alternative to surgical PAB. After detailed explanations, including the previously described transcatheter techniques,6 bilateral endoluminal PAB was decided as compassionate treatment according to the rules of the local institutional ethics committees. Full written parental consent was also obtained after information about alternative treatment strategies including HTx and abstention. According of our protocol,8 12 h before the planned cardiac catheterization, levosimendan (0.1 µg/kg/min) was again intravenously infused, combined with the still-infused milrinone. The orally applicated drugs were stopped 6 h before the intervention. The child remained spontaneously breathing and was sedated as needed with repeated small single intravenous doses of diazepam, ketamine, and propofol. The femoral vascular access was used. After local anaesthesia, a 5 Fr Terumo® sheath was placed in the femoral vein and a 4 Fr sheath for the femoral artery. Heparin was administered intravenously at an initial dose of 100 IU/kg, followed by 50 IU/kg after placement of the endo-PFRs. Following a brief haemodynamic assessment, an angiocardiography was performed via a 4 Fr pigtail catheter in the pulmonary trunk to visualize the pulmonary arteries and by the passage of contrast medium to show the morphology, dimensions, and function of the left atrium and LV (see Supplementary material online, Video S2A). After the final decision on the placement of endovascular PFRs, contrast medium injections were performed manually through a haemostat valve attached to the 4 Fr Terumo Cobra catheter when positioned at the entrance to the RPA or LPA and passed with a 0.014 inch coronary wire through whose distal tip has been stabilized deep in the periphery of the pulmonary vessels. Selective RPA angiography was performed at 20° cranial and 20–30° RAO angulation, and LPA angiography at 20° cranial and 20–30° LAO angulation. Considering the individual diameters of the pulmonary artery branches (Figure 2) and the distance from the entrance of the main pulmonary artery branch to the peripheral bifurcation of the right or LPA, an MVP-7Q for the LPA and an MVP-9Q for the RPA were manually prepared as PFRs. Again, the thin PTFE membrane was carefully removed with a scalpel from 1 of the 10 coated end-cells creating a fenestration of nearly 3.5 × 4 mm in the MVP-7Q and ∼4 × 4.5 mm of the MVP-9Q (Figure 3). The modified MVPs were first drawn underwater into the attached loading catheter, then loaded through the haemostat valve into the Cobra catheter, which was securely positioned distally in a pulmonary artery branch. The distal marker of the MVP-based PFR was advanced to the tip of the catheter under fluoroscopy, and then the catheter with the loaded PFR in ensemble was gently withdrawn to the peripheral bifurcation. The PFR marked by two markers was held in place and the Terumo®-catheter was slowly withdrawn until the nitinol-based self-expanding device was fully deployed. After briefly warming the nitinol frame to central body temperature, contrast medium was again injected through the catheter while the device was still screwed on. After confirming the correct position and demonstrating sufficient blood flow through the PFR, the device was unscrewed.
Figure 2.
Pulmonary artery angiography of Patient #2 with measurements of right and left pulmonary artery branch diameters.
Figure 3.
Medtronic vascular plug (MVP-7Q) manually prepared as a pulmonary flow restrictor by removal of one polytetrafluoroethylene covered end cell (Patient #2).
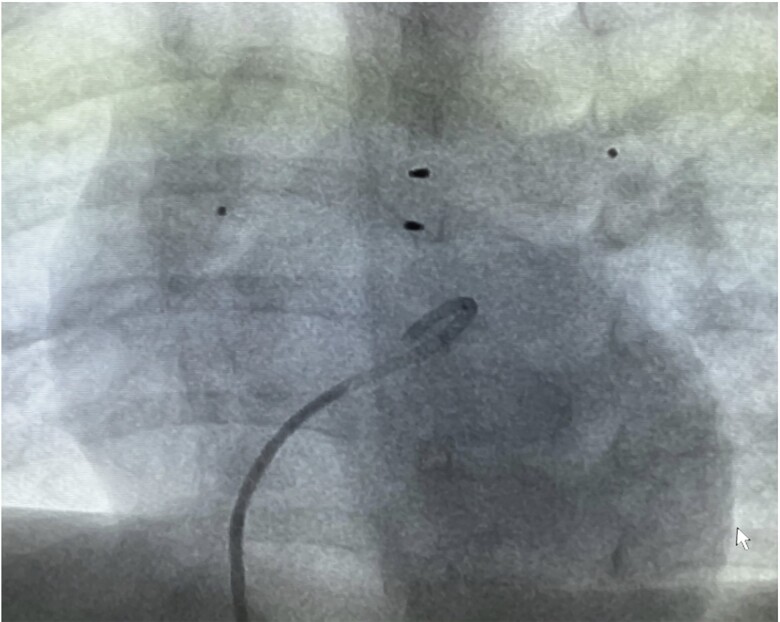
After implantation of the bilateral PFRs (Figure 4), pulmonary artery angiography was repeated to demonstrate well-perfused pulmonary artery branches (see Supplementary material online, Video S2B). Finally, an echocardiographic assessment of LV function and pulmonary flow characteristics, including pressure gradients across the PFR’s was analysed before the vascular sheaths were removed. The procedure time was 86 min, the total fluoroscopy time was 6.05 min, the total area dose 310 μGym2. A continuous infusion of heparin (300 E/kg/day) was given for another 48 h, followed by oral anti-aggregation with clopidogrel (0.2, later 0.5 mg/kg) combined with acetylsalicylic acid (ASA, 1–2 mg/kg). Milrinone was tapered daily in increments of 0.1 µg/kg/min and stopped at a dose of 0.3 µg/kg/min. Oral heart failure therapy was continued as previously described,8 supplemented by clopidogrel and ASA. Two weeks after placement of endoluminal PAB procedure, she was discharged home with intensive parental education on how to monitor their baby at home, specifically to observe her respiratory rate at rest. The outpatient follow-up checks were initially every 8–14 days, later at intervals of 6 weeks, which included a clinical examination, echocardiography, and an electrocardiogram. Functional regeneration was documented with a normalized z-score of LVEDD along with marked improvement of LV-EF after 3–6 months (see Table 2). Ross-functional Class I correlated with age-dependent normal serum NT-proBNP values. At 7 months of age, the girl developed an infection with elevated C-reactive protein (CRP) and leukocyte count, along with elevated fibrinogen and D-dimers. COVID-19 has been ruled out. However, the flow pattern along the right PFR was significantly reduced in suspected thrombus formation, while cardiac function was echocardiographically preserved. The re-indicated percutaneous catheterization was also performed under analgosedation. Angiographically, a reduced flow through the MVP-9Q based PFR placed in the RPA could be documented. Gradual balloon dilation was then performed, beginning with a 3.5 × 15 mm coronary balloon followed by a 5 × 20 mm Tyshak-Mini® balloon. Blood flow through the device improved immediately. Finally, LV-angiography confirmed the well-contracting LV with a normalized dimension (see Supplementary material online, Video S2C). Intravenous antibiotics in combination with low molecular weight heparin were continued for 10 days. Due to the controlled infection with normalized d-dimer levels, removal of the device could be avoided. Pressure gradients across the PFR’s remained in the 30–35 mmHg range. The patient, currently over 1 year old, is developing normally, with residual functional cardiac regeneration and normalized echocardiographic parameters. Oral treatment consists of B-L-S, Clopidogrel and ASA, and supplements as previously described.8
Figure 4.
Depicted are the pulmonary flow restrictors positioned within the right and left pulmonary branch arteries of Patient #2.
Table 2.
Patient data during follow-up (see also text)
| LV-DCM pts number (sex) | Further diagnoses ROSS/NYHA FC (I–IV) | BW (kg) (percentile) | Age (mo) | HR (/min) at rest | LV-EDD (mm) z-score | LV-EF (%) | RPA/LPA MVP-7 or -9 dP (mmHG) | Anti-cong. Anti. aggreg. therapy B-L-S + ASA, C | Remarks in term of FU |
|---|---|---|---|---|---|---|---|---|---|
| 1. (f) |
Improved, FR,
FC-I |
20
(98) |
42 | 87 |
40
(+1.4) |
48 | MVP-9/-9 30/45 |
B-L-S + ASA + C ++ |
RPA-PFR exchange 4 mo. after ePAB |
| 2. (f) |
Improved
FR FC-I |
6.5
(55) |
7 | 112 |
27
(+1.5) |
57 | MVP-9/-7 30/32 |
B-L-S + ASA + C | 6 mo FU Infect/RPA thrombus? RPA-PFR partial-de-banding |
| 3. (m) |
Improved
FR FC-I |
4.5
(30) |
4 | 118 |
24
(+1.5) |
55 | MVP-7/-7 32/36 |
B-L-S + ASA + C | Barth-S. related intermittent diarrhea |
Abbreviations: Anti-cong., congestive; Anti-aggreg., aggregative; ASA, acetyl-salicylic-acid; B, bisoprolol; BW, body weight; C, clopidogrel; CV, controlled ventilation; DCM, dilated cardiomyopathy; EDD, enddiastolic diameter; EF, ejection fraction; ePAB, endo-pulmonary artery banding; FC, functional class; FC-I, functional class I; FR, functional Ross-class; FU, follow-up; HR, heart rate; L, lisinopril; Le, levosimendan; LPA, left pulmonary artery; LV, left ventricle; M, milrinone; mo., months; MVP, Medtronic® Vascular Plug; pts, patients; PFR, pulmonary flow restrictor; RPA, right pulmonary artery; S, spironolactone; -S., syndrome.
Patient 3
A male newborn with a body weight of 3.3 kg and a body length of 53 cm was admitted to a neonatal intensive care unit on his first day of life because of a suspected neonatal infection. Persistent poor feeding together with tachypnoea despite antibiotic treatment led to a chest x-ray showing an enlarged heart. Echocardiography showed a dilated LV with reduced function. Because neonatal myocarditis was suspected, the newborn was treated with intravenous immunoglobulin and heart failure drugs consisting of epinephrine, milrinone, hydrochlorothiazide, and spironolactone. Since LV function did not improve with treatment, the newborn was referred to our tertiary paediatric cardiology centre at the age of 3 weeks. In our paediatric cardiology intensive care unit, the patient received additionally a levosimendan infusion due to persistently impaired LV function with a LV-EF of 24%. A coronary artery anomaly was excluded by cardiac catheterization. Cardiac magnetic resonance imaging (MRI) confirmed LV-NC with severely compromised LV and preserved RV function.
Despite heart failure medications consisting of epinephrine, milrinone, spironolactone and lisinopril, the boy continued to show clinical signs of heart failure (malnutrition, failure to thrive, tachypnoea) and LV function deteriorated with an EF now 15% (Table 1). The boy was treated for tachypnoea with intermittent high-flow respiratory support via a nasal cannula. Since conservative treatment did not result in sufficient improvement in LV function to allow the boy’s transfer to the normal ward and eventual discharge from the hospital, alternative options such as assist device therapy for bridging to HTx and finally the concept of PAB with the aim of heart regeneration were discussed in detail with the parents. Given the shortage of donor organs in Germany and the possible complications of therapy with assist devices, the parents consented to catheter-based implantation of pulmonary flow-restrictors, resulting in bilateral endo-PAB.
The procedure was performed under conscious sedation with midazolam and ketamine in a spontaneously breathing patient. After the application of local anaesthesia to the right groin, a 5 Fr Terumo Sheath was inserted into the right femoral vein and a 3 Fr catheter (Microseld) was placed in the right femoral artery for continuous blood pressure monitoring. Heparin was applied as a bolus of 100 IU/kg of body weight. Next, a 4 Fr guiding catheter (Judkins right, Cordis) was advanced into the RPA via the femoral vein using a 0.014 inch guidewire (balance middleweight, Abbott) and the guidewire tip firm was placed in the distalRPA. The 4 Fr Judkins right catheter was exchanged for a 5 Fr Terumo Cobra catheter and an angiography of the RPA was obtained, showing a diameter of the RPA branch of 4.4 mm. After a careful review of the angiography, the MVP 7Q device was selected for a self-made PFR as described in detail (see Patient 2). Finally, the modified MVP 7Q device was implanted via the 5 Fr Cobra catheter into the RPA. Control-angiography demonstrated the optimal position of the device and sufficient perfusion of the distal RPA branches. Likewise, a modified MVP 7Q device was implanted into the LPA. The optimal device position within the LPA was also angiographically confirmed. The systolic pressure of the main pulmonary artery increased from 17 before to 48 mmHg after implantation of both PFRs while the systolic femoral artery pressure was 54 mmHg. Transthoracic echocardiographic assessment of LV function and pulmonary flow characteristics, including pressure gradients across the PFR’s was performed before the vascular sheaths were removed (see Supplementary material online, Video S3). Left ventricular ejection fraction was 45% on unchanged treatment of continuous infusion of inodilators. The total procedure time was 79 min, the total fluoroscopy time was 5.09 min, and the total area dose was 116 μGym2. Inotropic support was weaned over the following 10 days. Anticoagulation was achieved with heparin (200 IU/kg/day) over 48 h following the procedure and was then changed to oral ASA (2–3 mg/kg/day) and clopidogrel (0.2 mg/kg/day). Additionally, the boy was put on bisoprolol, lisinopril, and spironolactone. Left ventricular function improved further and 19 days after transcatheter intervention, the boy was discharged home. He was followed weekly in our outpatient clinic. Already 3 months after PFR implantation, LV-EF further improved to low-normal levels of 57%, while RV function was always unaffected (Table 2). The boy thrived properly and initially elevated natriuretic peptides of 13 968 pg/mL went back to normal values during follow-up. Cardiac MRI 5 months later showed normal biventricular function and dimensions (right ventricular ejection fraction [RV-EF] 75%, left ventricular ejection fraction [LV-EF] 59%, right ventricular ediastolic volume index [RVEDVI] 31 mL/m2, and left ventricular enddiastolic volume index [LVEDVI] 49 mL/m2). Meanwhile, 8 months after the implantation of bilateral PFRs, the boy is doing well and thriving appropriately on a normal diet without calory supplementation. The last LVEF was 62% and LVEDD was 24 mm (z-score +0.5). Genetic testing, meanwhile, revealed a pathogenic mutation within the TAFAZZIN-Gen, resulting in the phenotype of Barth syndrome as the underlying disease for LV NC cardiomyopathy in this boy.
Discussion
Surgical PAB is now becoming a more integrated part of the therapeutic arsenal for the treatment of paediatric DCM, whether for cardiac recovery and FR or bridging to transplantation.2–4,9–12 Based on current clinical3,4,13 and bench-to-bedside experiences,14,15 it can be hypothesized that 70–80% of infants with LV-DCM first still have preserved RV function, and thus secondly have the entry criteria for a PAB. Currently, many of these infants are still receiving an assist device and are listed for HTx.16,17 They just have a chance of functional cardiac regeneration through the use of a PAB. Here we have shown for the first time that functional heart regeneration can also be achieved with a minimally invasive transcatheter procedure, provided that effective bilateral pulmonary arterial endo-banding can be established along with medication that promotes cardiac regeneration. However, due to the current limitations in the size of the MVPs, this transcatheter method is currently only applicable to selected very young infants with still small diameters of the pulmonary branch arteries. Due to this limitation, only three patients could be treated with the transcatheter technique so far. Therefore, our brief feasibility report on transcatheter endo-PAB in three infants should also be considered as the next step forward to improve the outcome of infants with LV-DCM by using a less invasive approach.
The first patient to be treated with surgical PAB at 2 months of age2 is now a 16-year-old boy with Ross functional Class I. This patient was free of NC morphology, and transcatheter-based PA de-banding was successfully performed at the age of 6 years. From the originally reported group of 12 patients,3 only one child of the ‘PAB-responders’ suffered a recurrence of relevant impairment of LV function due to DCM 10 years after PAB and subsequent interventional de-banding.
Although experience is limited, the endoluminal PAB techniques presented here are of significant importance: (i) The transcatheter technique simplifies the therapeutic approach of PAB in DCM. It significantly reduces the perioperative risk associated with anaesthesia, open-chest surgery, and postoperative intensive care while performed on spontaneously breathing patients. (ii) The key mechanism for inducing FR of the diseased LV, the VVI is instantaneously preserved upon transcatheter placement of the bilateral endo-PFR’s. Impairment of RV function by increasing RV afterload by PAB is less pronounced after endoluminal treatment compared with open-chest surgery. Periinterventional treatment, including the use of inodilators, seems more like a prophylactic tool than a therapeutic necessity. Of course, technical and long-term questions remain when using bilateral intraluminal self-made flow restrictors instead of a balloon-dilatable surgical PAB. Based on our experience with the off-label use of MVP for a variety of indications, the thin PTFE surface covering of the devices can also be easily punctured and expanded by balloon dilation, whether the device is still fully intact or not and already prepared as a PFR. As a proof of concept, one of our patients required gradual balloon dilation of the device, which could be performed without any problems. The devices can be surgically removed, but also—as has already been done in one of the patients, 4 months after implantation—by the catheter snare technique. Finally, obstructed devices are amenable to stent expansion, as demonstrated in in-vitro studies. Additional questions remain to be answered: (i) the medium- and long-term risk of PFRs placed into the pulmonary branches, (ii) the risk of thrombus-formation, (iii) the risk of infection as seen in one of our patients, and (iv) the impact of flow restriction on lung development. However, at present, infants with severe DCM carry a very high morbidity and mortality risk when the route of assist devices and HTx is followed. Along this route, HTx is the only option for medium-term survival, including the risks of rejection, immunosuppression, and opportunistic infections.17 In our view, the chance for heart regeneration and preserving the patient’s own heart is the preferable option. The demonstrated less invasive approach combined with the development of new devices could pave the way for improved future treatments of babies with severe DCM.
Lead author biography
 Dietmar Schranz, based on training as a pediatrician, neonatologist, paediatric intensivist and cardiologist focused on clinical research in heart failure, PAH, trans-catheter Interventions. I had the honour to lead the Department of the Pediatric Cardiology at the University Clinic Giessen for over 20 years. Currently, I am a senior consultant on the Department of Pediatric Cardiology, at the University Clinic Frankfurt.
Dietmar Schranz, based on training as a pediatrician, neonatologist, paediatric intensivist and cardiologist focused on clinical research in heart failure, PAH, trans-catheter Interventions. I had the honour to lead the Department of the Pediatric Cardiology at the University Clinic Giessen for over 20 years. Currently, I am a senior consultant on the Department of Pediatric Cardiology, at the University Clinic Frankfurt.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for the submission and publication of this case report, including images and associated text, has been obtained from the patients’ parents in line with COPE guidance.
Statement of ethics: Surgical pulmonary banding (PAB) is an ethically proofed procedure for treating end-stage dilated cardiomyopathy in young children, particularly infants. Parents signed the declaration of consent for the transcatheter procedure of bilateral endovascular PAB and publishing this case report. Ethical approval for publication was not additionally obtained, but publishing medical novelties belongs to the institutional rules.
Funding: None declared.
Supplementary Material
Contributor Information
Dietmar Schranz, Pediatric Cardiology, Children’s Hospital, Johann Wolfgang Goethe University, Theodor-Stern-Kai 7, Frankfurt D-60596, Germany.
Ulrich Krause, Department of Pediatric Cardiology, Georg-August-University Medical Center, Göttingen, Germany.
Gunter Kerst, Department of Pediatric Cardiology, University Hospital RWTH Aachen, Aachen, Germany.
Anoosh Esmaeili, Pediatric Cardiology, Children’s Hospital, Johann Wolfgang Goethe University, Theodor-Stern-Kai 7, Frankfurt D-60596, Germany.
Thomas Paul, Department of Pediatric Cardiology, Georg-August-University Medical Center, Göttingen, Germany.
References
- 1. Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, et al. Incidence, causes, and outcome of dilated cardiomyopathy in children. JAMA Cardiol 2006;296:1867–1876. [DOI] [PubMed] [Google Scholar]
- 2. Schranz D, Veldman A, Bartram U, Michel-Behnke I, Bauer J, Akintürk H. Pulmonary artery banding for idiopathic dilative cardiomyopathy: a novel therapeutic strategy using an old surgical procedure. J Thorac Cardiovasc Surg 2007;134:796–797. [DOI] [PubMed] [Google Scholar]
- 3. Schranz D, Rupp S, Muller M, Schmidt D, Bauer A, Valeske K, et al. Pulmonary artery banding in infants and young children with left ventricular dilated cardiomyopathy: a novel therapeutic strategy before heart transplantation. J Heart Lung Transplant 2013;32:475–481. [DOI] [PubMed] [Google Scholar]
- 4. Schranz D, Akintuerk H, Bailey L. Pulmonary artery banding for functional regeneration of end-stage dilated cardiomyopathy in young children: world network report. Circulation 2018;137:1410–1412. [DOI] [PubMed] [Google Scholar]
- 5. Di Candia A, Castaldi B, Bordin G, Cerutti A, Reffo E, Biffanti R, et al. Pulmonary artery banding for ventricular rehabilitation in infants with dilated cardiomyopathy: early results in a single-center experience. Front Pediatr 2020;168:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schranz D, Esmaeili A, Schrewe R, Kerst G, Akintuerk H. Hypoplastic left heart stage-I: no norwood, no hybrid. Circulation 2020;142:1402–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bauer A, Khalil M, Schmidt D, Recla S, Bauer J, Esmaeili A, et al. Transcatheter left atrial decompression in patients with dilated cardiomyopathy: bridging to cardiac transplantation or recovery. Cardiol Young 2019;29:355–362. [DOI] [PubMed] [Google Scholar]
- 8. Schranz D, Recla S, Malcic I, Kerst G, Mini N, Akintuerk H. Pulmonary artery banding in dilative cardiomyopathy of young children: review and protocol based on the current knowledge. Transl Pediatr 2019;8:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mets G, Panzer J, De Wolf D, Bové T. An alternative strategy for bridge-to-transplant/recovery in small children with dilated cardiomyopathy. Pediatr Cardiol 2017;38:902–908. [DOI] [PubMed] [Google Scholar]
- 10. Liu YH, Chen YS, Lin MT, Chen CA. Improved left ventricular strain and dys-synchrony after pulmonary artery banding in an infant with end-stage dilated cardiomyopathy: insights from three-dimensional speckle tracking. Pediatr Cardiol 2019;40:1317–1319. [DOI] [PubMed] [Google Scholar]
- 11. Spigel ZA, Razzouk A, Nigro JJ, Karamlou TB, Kavarana MN, Roeser ME, et al. Pulmonary artery banding for children with dilated cardiomyopathy: US experience. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2020;23:69–76. [DOI] [PubMed] [Google Scholar]
- 12. Ponzoni M, Frigo AC, Castaldi B, Cerutti A, Di Salvo G, Vida VL, et al. Surgical strategies for the management of end-stage heart failure in infants and children: a 15-year experience with a patient-tailored approach. Artif Organs 2021;45:1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ponzoni M, Castaldi B, Padalino MA. Pulmonary artery banding for dilated cardiomyopathy in children: returning to the bench from bedside. Children (Basel) 2022;9:1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Traister A, Patel R, Huang A, Patel S, Plakhotnik J, Lee JE, et al. Cardiac regenerative capacity is age- and disease-dependent in childhood heart disease. PLoS One 2018;13:e0200342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicin L, Abplanalp WT, Schänzer A, Sprengel A, John D, Mellentin H, et al. Single nuclei sequencing reveals novel insights into the regulation of cellular signatures in children with dilated cardiomyopathy. Circulation 2021;143:1704–1719. [DOI] [PubMed] [Google Scholar]
- 16. Godown J, Smith AH, Thurm C, Hall M, Dodd DA, Soslow JH, et al. Mechanical circulatory support costs in children bridged to heart transplantation—analysis of a linked database. Am Heart J 2018;201:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conway J, Cantor R, Koehl D, Spicer R, Gupta D, McCulloch M, et al. Survival after heart transplant listing for infants on mechanical circulatory support. J Am Heart Assoc 2020;9:e011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.