Abstract
Context
Early prediction of hypothalamic-pituitary-adrenal (HPA) axis function following transsphenoidal surgery (TSS) can improve patient safety and reduce costs.
Objective
Systematic measurement of ACTH and cortisol at extubation following anesthesia to predict remission from Cushing's disease (CD) and HPA axis preservation following non-CD surgery.
Design
Retrospective analysis of clinical data between August 2015 and May 2022.
Setting
Referral center.
Patients
Consecutive patients (n = 129) undergoing TSS who had perioperative ACTH and cortisol measurements.
Interventions
ACTH and cortisol measurement at extubation. Further serial 6-hourly measurements in CD patients.
Main outcome measures
Prediction of future HPA axis status based on ACTH/cortisol at extubation.
Results
ACTH and cortisol increased sharply in all patients at extubation. CD patients (n = 101) had lower ACTH values than non-CD patients (110.1 vs 293.1 pg/mL; P < 0.01). In non-CD patients, lower plasma ACTH at extubation predicted the need for eventual corticosteroid replacement (105.8 vs 449.1 pg/mL, P < 0.01). In CD patients, the peak post-extubation cortisol at 6 hours was a robust predictor for nonremission (60.7 vs 219.2 µg/dL, P = 0.03). However, normalized early postoperative value (NEPV; the post-extubation values minus the peak preoperative CRH or desmopressin test values) of cortisol reliably distinguished nonremission earlier, at the time of extubation (−6.1 vs 5.9, P = 0.01), and later.
Conclusions
We found that at extubation following TSS, ACTH can predict the need for eventual steroid replacement in non-Cushing's patients. In patients with CD, we found a robust prediction of nonremission with NEPV cortisol at extubation and later.
Keywords: pituitary adenoma, hypercortisolism, Cushing's disease, transsphenoidal surgery, remission
The requirement for glucocorticoid replacement therapy after transsphenoidal surgery (TSS) depends on the preoperative diagnosis, intra-operative pituitary gland damage, and use of perioperative steroids [1–4]. Nearly all patients who achieve remission of Cushing's disease (CD) require substitution therapy until the hypothalamic-pituitary-adrenal (HPA) axis recovers from the suppression induced by previous hypercortisolism. By contrast, patients without Cushing's disease (non-CD) may require treatment if they received preoperative glucocorticoids or suffered significant pituitary damage.
We previously described normalized early postoperative values (NEPV) as a technique to predict postoperative remission after TSS for CD [5]. NEPV takes advantage of the observation that surgical stress mirrors the CRH or desmopressin (DDAVP)-induced increase in ACTH and cortisol. By subtracting postoperative cortisol and ACTH values from preoperative stimulated levels in the same patient, we demonstrated the ability to adjust for surgical stress response, minimize intrapatient variability, and unmask early postoperative cortisol and ACTH dynamics [5].
Whereas NEPV improved detection of remission status at a few hours postoperatively, we did not evaluate the exact timepoints. In this study, we sought to accurately map postsurgical stress hormone dynamics using measurements starting at the time of extubation following general anesthesia. We then applied an algorithmic approach to predict postoperative HPA axis function, including the presence of remission in CD and the need for postoperative steroid replacement in non-CD patients.
Materials and Methods
Patient Data
We performed a retrospective review of prospectively collected data on all consecutive patients who underwent sublabial endonasal TSS by the same surgeon (P.C.) between August 2016 and May 2022 at the National Institutes of Health (NIH) Clinical Center, which specializes in rare conditions including Cushing's disease. All patients were treated in a study approved by the Combined Neuroscience Institutional Review Board of the NIH, Bethesda (clinical trial identifier NCT00060541). Written informed consent was obtained from each patient for research study participation. Inclusion criteria included sellar lesions, TSS, and availability of perioperative stress hormone measurements.
Diagnosis of Cushing's Disease
Hypercortisolism was diagnosed as previously described [5] based on elevated late-night salivary cortisol [chemiluminescent enzyme immunoassay, Siemens Immulite 1000 analyzer, NIH Department of Laboratory Medicine (DLM), Bethesda, MD, USA; normal range <100 ng/dL], elevated 24-hour urine free cortisol (high performance liquid chromatography/tandem mass spectrometry, NIH DLM; normal range: 1.4-20 μg/24H for 3- to 8-year-olds, 2.6-37 for 9- to 12-year-olds, 4.0-56 μg/24H for 13- to 17-year-olds, and 3.5-45.0 μg/24H for ≥18-year-olds) or low-dose dexamethasone suppression testing (1 mg overnight or 2 mg over 48 hours) [6, 7]. Supplementary midnight plasma cortisol (normal range <7.5 mcg/dL) and 24-hour urinary 17- hydroxycorticosteroids excretion (Quest Diagnostics Nichols Institute, Chantilly VA; adult normal range 3-10 mg/24 hours for males and 2-6 mg/24 hours for females) [8] were used as needed. Pituitary ACTH production was verified using early morning ACTH levels (Nichols Advantage Immunochemiluminometric assay or chemiluminescence immunoassay, Siemens Immulite 2500 analyzer, NIH DLM, normal range 5-46 pg/mL) [5, 9], 8 mg overnight dexamethasone suppression testing and ovine CRH stimulation testing [10–12] (August 2016-June 2021), or DDAVP stimulation testing [13] (June 2021-May 2022) with >35% increase of the mean of 15- and 30-minute values above baseline for ACTH or >20% increase of the mean of 30- and 45-minute values above baseline for cortisol being consistent with a CD diagnosis. The average stimulated levels were defined as the mean of cortisol values at 30 and 45 minutes and the mean of the 15- and 30-minute ACTH values. Confirmatory inferior petrosal sinus sampling [14] was performed in all Cushing's disease patients, except where dexamethasone suppression testing and CRH or DDAVP testing were concordant and magnetic resonance imaging (MRI) showed unequivocal adenoma.
Diagnosis of non-Cushing's Disease Lesions
High resolution (1 to 1.5 mm slice thickness) MRI of the sella using spoiled gradient recalled acquisition sequences was performed preoperatively to identify sellar lesions. Patients with nonfunctioning pituitary adenomas and nonadenomatous lesions were evaluated with dedicated pituitary MRI imaging and a complete pituitary hormone panel (LH, FSH, alpha subunit, prolactin, TSH, GH, IGF1, ACTH) obtained at 8 Am in the morning [15, 16]. Patients with detectable pituitary adenomas on MRI and serum prolactin levels above 150 µg/L were diagnosed with likely prolactinomas [17]. To diagnose acromegaly, serum growth hormone levels and insulin like growth factor 1 levels were tested. Elevated IGF1 led to oral glucose tolerance test for GH at 2 hours (with documented hypoglycemia). GH levels of > 1 µg/L were diagnostic for acromegaly [18].
Postsurgical Outcomes
All patients had morning cortisol and ACTH measurements preoperatively and at extubation following surgery, with samples collected on ice. In CD patients, preoperative cortisol and ACTH levels were measured prior to CRH or DDAVP stimulation testing. Postoperatively, plasma cortisol and ACTH levels were obtained in CD patients at 6-hour intervals from postoperative day (POD) 0 through 3 (solid-phase competitive chemiluminescent enzyme immunoassay, Siemens Immulite 2500 analyzer, NIH DLM; normal cortisol range 5-25μg/dL; normal ACTH range 10-60 pg/mL). In CD patients who received exogenous corticosteroid replacement for adrenal insufficiency or in all CD patients after POD 3, daily morning cortisol levels were obtained until POD 10 or until patient discharge. Early remission from CD was defined as previously published [19] using a nadir plasma cortisol level of <5μg/dL within 10 days of surgery, prior to administration of exogenous glucocorticoids. NEPV for cortisol and ACTH were calculated for each postoperative value by subtracting the average post-CRH or post-DDAVP stimulation value [5]. In CD and non-CD patients, corticosteroid replacement was initiated if serial testing of single morning cortisol levels revealed cortisol suppression to levels <1 μg/dL or if the patients were symptomatic from hypocortisolism (tachycardia, dizziness, presyncope, hypotension, etc.). For non-CD patients, cosyntropin stimulation test was used to confirm the need for corticosteroid replacement [19] on POD 8 with a baseline of 7 μg/dL or an increase to 18 μg/dL 60 minutes after cosyntropin administration indicating need for replacement.
Statistical Analysis
Patient baseline clinical characteristics are presented as percentages or as means with SDs. Baseline characteristics were compared using 2-sample tests of proportions or 2-sample T-tests with unequal variance where appropriate, using Welch's approximation for degrees of freedom [20]. Normality for ACTH and cortisol at the time of extubation was verified using Shapiro-Wilks tests. Predictors of early nonremission (cortisol <5 ug/dL within 10 days of TSS) were identified by univariable and multivariable logistic regression with early nonremission as the dependent variable. Predictive ability was assessed using areas under the receiver operating characteristic (AUROC) curve and by Hosmer-Lemeshow χ2 statistics. Two-tailed P-values <0.05 were considered statistically significant. GraphPad Prism 6.0 software was used for figures (GraphPad Software, La Jolla, CA, USA). Statistical analyses were performed using STATA 14/IC software package (StataCorp LP, College Station, TX, USA).
Results
Patient Demographics
Of the 129 patients included in the study, 101 had CD. Among CD patients, early remission was confirmed in 90 (89%) patients. Five patients (5%) underwent reoperations within the same admission. Eleven CD patients (11%) presented with macroadenomas, while the rest had microadenomas. We found no major differences in demographics or endocrine presentation between patients in early remission vs nonremission (Table 1). The non-CD patients (n = 28) presented with sellar masses including nonfunctioning macroadenomas (n = 13), somatotropinomas (n = 8), prolactinomas (n = 5), a craniopharyngioma, and a meningioma (Table 1).
Table 1.
Patient demographics, endocrine characteristics, and result of immunohistochemical staining of resected tumors
| Diagnosis | CD | P-value | Non-CD | P-value* | |
|---|---|---|---|---|---|
| Endocrine remission | Remission | Nonremission | NA | NA | |
| Demographics | |||||
| Number of patients | 90 | 11 | NA | 28 | NA |
| Age in years | 22 (15) | 26 (21) | 0.43 | 44 (16) | <0.01 |
| BMI | 30 (8) | 33 (7) | 0.24 | 31 (6) | 0.88 |
| % Female | 53 | 69 | 0.31 | 46 | 0.4 |
| % White race | 76 | 54 | 0.12 | 48 | 0.02 |
| % re-operations | 10 | 46 | <0.01 | 7 | 0.39 |
| Endocrine characteristics | |||||
| Preoperative cortisol | 15.0 (6.1) | 14.0 (7.6) | 0.62 | 9.3 (4.4) | <0.01 |
| CRH-stimulated cortisol fold change | 0.8 (0.8) | 1.0 (0.4) | 0.19 | NA | NA |
| Preoperative ACTH | 54.1 (64.1) | 38.5 (19.9) | 0.09 | 29.6 (19.6) | <0.01 |
| CRH-stimulated ACTH fold change | 2.4 (3.5) | 2.3 (1.9) | 0.88 | NA | NA |
| Pathology | |||||
| ACTH stain | 90 | 11 | NA | 0 | NA |
| GH | 0 | 0 | NA | 8 | NA |
| PRL | 0 | 0 | NA | 5 | NA |
| Fsh/Lh | 0 | 0 | Na | 13 | Na |
Abbreviations: BMI, body mass index; CD, Cushing’s disease; PRL, prolactin.
HPA Axis at Extubation is Dependent on pre-operative Events
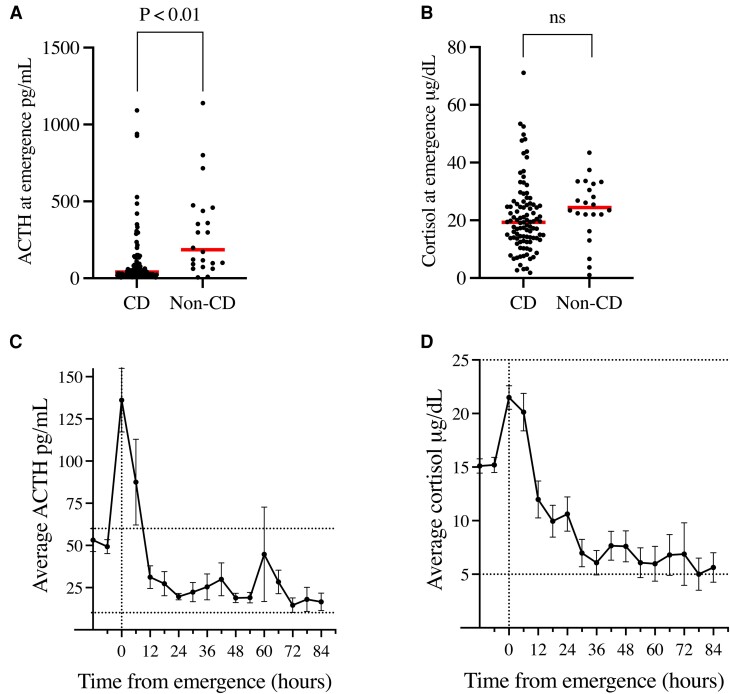
We measured plasma cortisol and ACTH at extubation after surgery. ACTH values were lower in CD patients compared to non-CD patients (110.3 ± 189.8 pg/mL vs 293.1 ± 289.4 pg/mL, P < 0.01; Fig. 1A). Despite this, there was no difference between the groups in cortisol values (CD: 20.9 ± 12.3 vs non-CD: 23.9 ± 10.7 μg/dL, P = 0.26; Fig. 1B). These data suggest that corticotrophs in CD patients are inhibited due to systemic hypercortisolism.
Figure 1.
Perioperative cortisol (ng/dL) and ACTH (pg/mL) levels in CD and non-CD patients. (A) ACTH levels at extubation were statistically lower in CD vs non-CD patients. (B) Cortisol at extubation was unchanged between CD and non-CD patients. (C) Average ACTH (normal range 10-60 pg/mL) in all patients (CD and non-CD) shows a postoperative peak at the time of extubation. Non-CD data was only available preoperatively and at extubation. (D) Average cortisol (normal range 5-25 μg/dL) in all patients (CD and non-CD) demonstrating a distinct peak between 0 and 6 hours from extubation from anesthesia. Non-CD data was only available preoperatively and at extubation.
Abbreviations: CD, Cushing's disease.
HPA Axis Elements Peak at Different Time-points Following Extubation
We found that serial (every 6 hours) monitoring of ACTH and cortisol in CD patients offered a powerful tool to analyze postoperative HPA axis dynamics. The marked increase in plasma ACTH levels at extubation was followed by a rapid fall over the next 12 hours (Fig. 1C). Plasma ACTH levels remained stable within the physiologic range beyond 18 hours. Plasma cortisol also peaked at extubation (21.5 ± 12.1 µg/dL) but showed a broader peak with levels remaining elevated at 12 hours (12.0 ± 17.0 µg/dL). Cortisol levels then fell slowly and stabilized only after 40 hours (Fig. 1D).
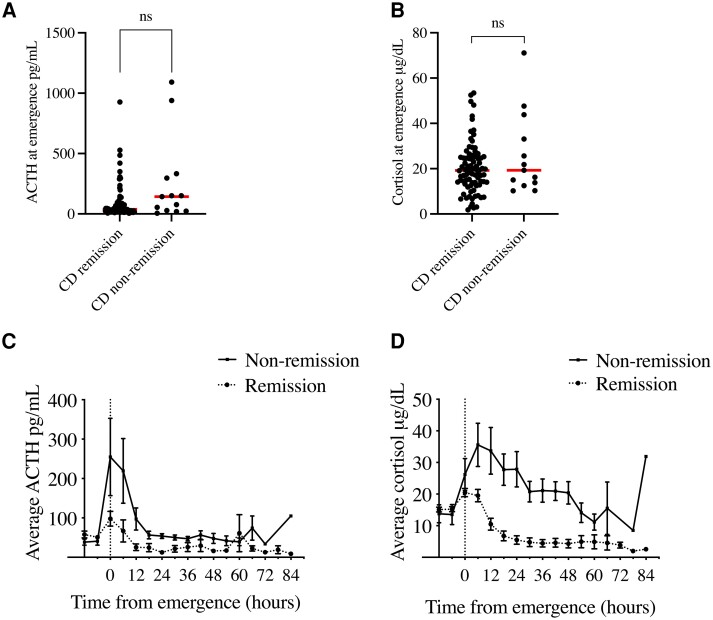
We suspected that nonremission due to corticotroph adenoma remnants in CD patients could significantly affect postoperative HPA ACTH and cortisol values. At extubation, there were no statistically significant differences between patients who later achieved early remission vs nonremission, either for ACTH (remission: 88.0 ± 140.6 vs 254.7 ± 354.3 pg/mL, P = 0.15; Fig. 2A) or cortisol (remission: 20.2 ± 11.1 vs 26.2 ± 18.2 μg/dL, P = 0.31; Fig. 2B). Plasma ACTH was highest at extubation for all CD patients but did not differ between the remission and nonremission patients till 6 hours post-extubation (60.7 ± 209.1 vs 219.2 ± 285.2 pg/mL, P = 0.03; Fig. 2C and Supplementary Table S1 [21]). Serum cortisol trends were also clearly different between CD patients in remission vs nonremission beginning at 6 hours after extubation (remission: 18.1 ± 15.7 vs 35.6 ± 23.7 µg/dL, P = 0.04; Fig. 2D) and remained different throughout the sampling period (Supplementary Table S2 [21]).
Figure 2.
Average perioperative cortisol and ACTH values in CD patients. (A)-(B). No differences in plasma ACTH (A) or cortisol (B) were identified CD patients in remission vs nonremission at the time of extubation. (C) In CD patients, the postoperative ACTH peak was blunted in patients achieving early remission. (D) CD patients achieving early remission also showed lower postoperative cortisol levels. Preoperative cortisol and ACTH levels (C and D) were measured just prior to CRH or DDAVP stimulation testing. Error bars = SEM. Abbreviations: CD, Cushing's disease; DDAVP, desmopressin.
NEPV for Cortisol Predicts non-remission at Extubation
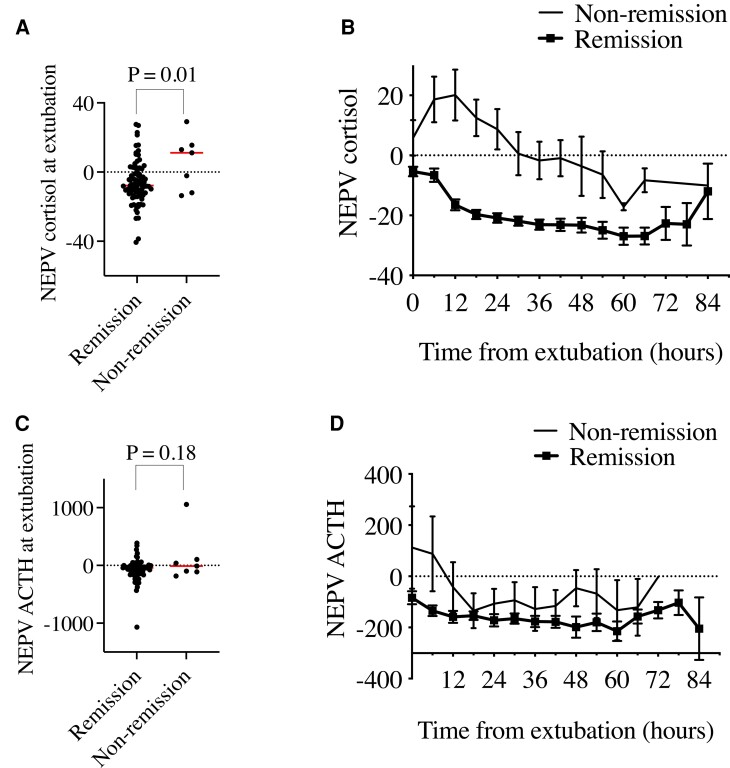
Previously we showed that in CD patients, postoperative ACTH/cortisol can be interpreted in the context of baseline CRH stimulation test results using NEPV [5]. NEPV for cortisol distinguished between patients in remission vs nonremission as early as at the time of extubation (−6.1 ± 12.9 vs 5.9 ± 15.7, P = 0.01; Fig. 3A) and later during hospitalization (Fig. 3B, Supplementary Table S2 [21]). In multivariable logistic regression adjusting for age, gender, and race, NEPV for cortisol at extubation remained a reliable predictor for nonremission [adjusted odds ratio (OR) 1.1, 95% CI, 1.0-1.2, P = 0.03; Table 2).
Figure 3.
Comparison of perioperative NEPV for cortisol and ACTH in CD patients in remission vs non-remission. (A) NEPV cortisol was significantly decreased CD patients in early remission vs nonremission. (B) NEPV cortisol improves early separation between CD patients in remission vs nonremission compared to plasma cortisol. (C) NEPV ACTH is not significantly different at extubation between CD patients in remission vs nonremission. (D) NEPV ACTH poorly separates CD patients in remission vs nonremission. Error bars = SEM. Abbreviations: CD, Cushing’s disease; NEPV, normalized early postoperative values.
Table 2.
Predictors of early nonremission following transsphenoidal surgery
| Variable | OR | 95% CI | P-value | Adj OR | Adj 95% CI | Adj P-value |
|---|---|---|---|---|---|---|
| Age in years | 1.0 | 1.0, 1.0 | 0.39 | 1.0 | 1.0, 1.0 | 0.55 |
| BMI | 1.0 | 1.0, 1.1 | 0.22 | 1.0 | 1.0, 1.1 | 0.41 |
| % Female | 2.0 | 0.6, 6.9 | 0.29 | 1.8 | 0.5, 6.6 | 0.35 |
| % White race | 0.4 | 0.1, 1.2 | 0.10 | 0.4 | 0.1, 1.5 | 0.17 |
| Preoperative cortisol | 1.0 | 0.8, 1.1 | 0.55 | 1.0 | 0.8, 1.1 | 0.63 |
| 0-hour cortisol | 1.0 | 1.0, 1.1 | 0.11 | 1.0 | 1.0, 1.1 | 0.19 |
| 6-hour cortisol | 1.0 | 1.0, 1.1 | 0.01 | 1.0 | 1.0, 1.1 | 0.02 |
| 12-hour cortisol | 1.1 | 1.0, 1.1 | 0.01 | 1.1 | 1.0, 1.1 | 0.02 |
| NEPV 0-hour cortisol | 1.1 | 1.0, 1.1 | 0.03 | 1.1 | 1.0, 1.2 | 0.03 |
| NEPV 6-hour cortisol | 1.1 | 1.0, 1.1 | 0.01 | 1.2 | 1.0, 1.3 | 0.02 |
| NEPV 12-hour cortisol | 1.1 | 1.0, 1.3 | 0.01 | 1.2 | 1.0, 1.3 | 0.01 |
| Preoperative ACTH | 1.0 | 1.0, 1.0 | 0.50 | 1.0 | 1.0, 1.0 | 0.58 |
| 0-hour ACTH | 1.0 | 1.0, 1.0 | 0.02 | 1.0 | 1.0, 1.0 | 0.07 |
| 6-hour ACTH | 1.0 | 1.0, 1.0 | 0.10 | 1.0 | 1.0, 1.0 | 0.28 |
| 12-hour ACTH | 1.0 | 1.0, 1.0 | 0.04 | 1.0 | 1.0, 1.0 | 0.13 |
| NEPV 0-hour ACTH | 1.0 | 1.0, 1.0 | 0.06 | 1.0 | 1.0, 1.0 | 0.04 |
| NEPV 6-hour ACTH | 1.0 | 1.0, 1.0 | 0.02 | 1.0 | 1.0, 1.0 | 0.02 |
| NEPV 12-hour ACTH | 1.0 | 1.0, 1.0 | 0.15 | 1.0 | 1.0, 1.0 | 0.16 |
Results of univariable logistic regression with nonremission as the dependent variable or multivariable logistic regression adjusting for age, female gender, and White race.
Abbreviations: Adj, adjusted; BMI, body mass index; NEPV, normalized early postoperative values; OR, odds ratio.
To further characterize NEPV for cortisol at extubation, we performed AUROC analysis. NEPV for cortisol predicted nonremission at extubation with AUROC of 0.72 (95% CI 0.48–0.95) indicating good model discrimination. We also tested model calibration using Hosmer-Lemeshow statistics, with P-values > 0.05 indicating good agreement. NEPV for cortisol predicted nonremission with Hosmer-Lemeshow χ2 11.73 using 10 groups, with P = 0.16, indicating good calibration. We ran sensitivity and specificity analysis and identified a cutoff of −2.1, above which NEPV for cortisol at extubation predicted nonremission with sensitivity of 71.4% and specificity of 68.8%. At 6 hours postoperatively, NEPV for cortisol predicted nonremission with AUROC of 0.85 (95% CI, 0.74-0.96), and the NEPV cortisol cutoff of −2.1 predicted nonremission with sensitivity of 100% and specificity of 66.3%. By 12 hours postoperatively, NEPV for cortisol predicted nonremission with AUROC of 0.95 (95% CI, 0.89-1.00), and, at a cutoff of −2.1, nonremission was predicted with sensitivity of 100% and specificity of 83.3%. This cutoff indicates that patients with postoperative serum cortisol levels near or above the preoperative CRH or DDAVP-stimulated levels are likely in nonremission.
NEPV for ACTH did not reliably distinguish between patients in early remission vs nonremission at extubation (−75.2 ± 185.2 vs 112.8 ± 426.8, P = 0.18; Fig. 3C) or later in the postoperative time-course (Fig. 3D, Supplementary Table S1 [21]). However, in multivariable logistic regression adjusting for age, gender, and race, NEPV for ACTH at extubation was a significant predictor of nonremission (adjusted OR 1.0, 95% CI, 1.0-1.0, P = 0.04; Table 2).
Secretagogue Choice Affects NEPV Analysis
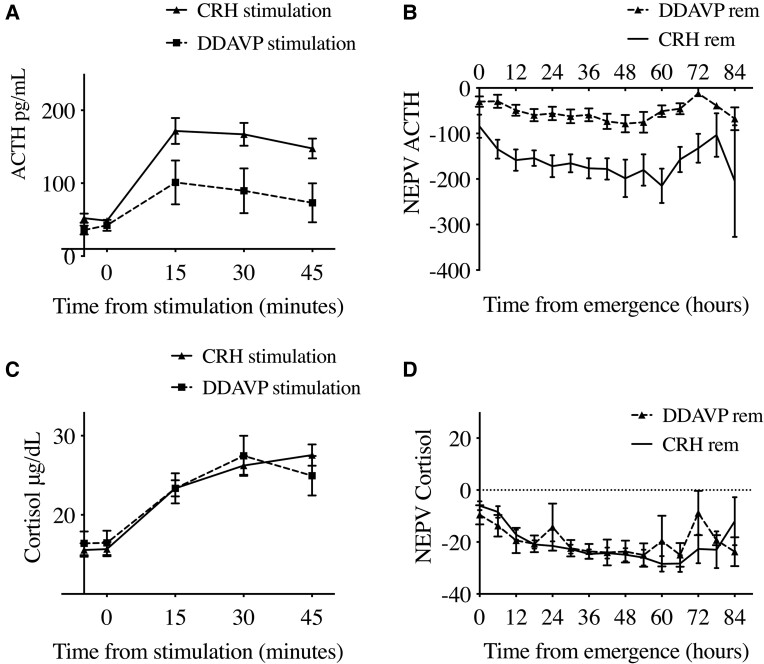
The basis of NEPV strategy is to compare HPA axis responses to an exogenous secretagogue (CRH or DDAVP) with an endogenous stressor (surgery). We evaluated whether the choice of exogenous secretagogue could affect the NEPV analysis. Of 89 patients with available preoperative stimulation testing, 72 patients underwent CRH stimulation while 15 patients underwent DDAVP stimulation testing. We found that CRH led to more robust ACTH stimulation compared to DDAVP (Fig. 4A, Table 3). This led to significantly lower CRH-calculated NEPV values compared to DDAVP-calculated NEPV values for ACTH at 6 hours (−125.5 ± 179.1 vs 14.3 ± 169.9, P < 0.01) and at 12 hours postoperatively (−155.1 ± 179.7 vs −26.2 ± 83.7, P < 0.01; Table 3) including patients in remission (Fig. 4B). These differences likely diminished the predictive ability of NEPV for ACTH at extubation and later. In contrast, we found no differences in the percent increase in cortisol or in NEPV cortisol calculated using CRH vs DDAVP stimulation at 0, 6, or 12 hours postoperatively (Table 3) particularly for patients in remission (Fig. 4C and 4D). These findings suggest that although the effect of secretagogue choice likely affected our ACTH results, there was no major effect on cortisol measurements in patients with CD.
Figure 4.
Comparison of perioperative cortisol and ACTH normalization using CRH stimulation vs DDAVP stimulation testing in CD patients in early remission. Values presented as means with SEM. No patients with DDAVP-based calculations were in early nonremission. (A) Higher ACTH levels after CRH vs DDAVP stimulation. (B) NEPV ACTH using CRH vs DDAVP-based calculations in patients in early remission. (C) Similar cortisol levels up to 30 minutes after CRH vs DDAVP stimulation. (D) Similar NEPV cortisol values between CRH and DDVAP-based calculations in patients in early remission. Error bars = SEM. Abbreviations: DDAVP, desmopressin; NEPV, normalized early postoperative values. hormone.
Table 3.
Comparison of preoperative cortisol and ACTH levels after CRH stimulation vs DDAVP stimulation
| CRH | DDAVP | P-value | |
|---|---|---|---|
| Number of patients | 72 | 15 | . |
| Percent increase in cortisol | 85% | 82% | 0.91 |
| 0 hour NEPV cortisol | −4.8 | −6.7 | 0.60 |
| 6 hour NEPV cortisol | −5.2 | −9.7 | 0.39 |
| 12 hour NEPV cortisol | −14.7 | −12.0 | 0.69 |
| Percent increase in ACTH | 250% | 200% | 0.56 |
| 0 hour NEPV ACTH | −80.5 | 53.5 | 0.12 |
| 6 hour NEPV ACTH | −125.5 | 14.3 | <0.01 |
| 12 hour NEPV ACTH | −155.1 | −26.2 | <0.01 |
Abbreviations: DDAVP, desmopressin; NEPV, normalized early postoperative values.
Plasma ACTH at Extubation Predicts Steroid Replacement in non-CD Patients
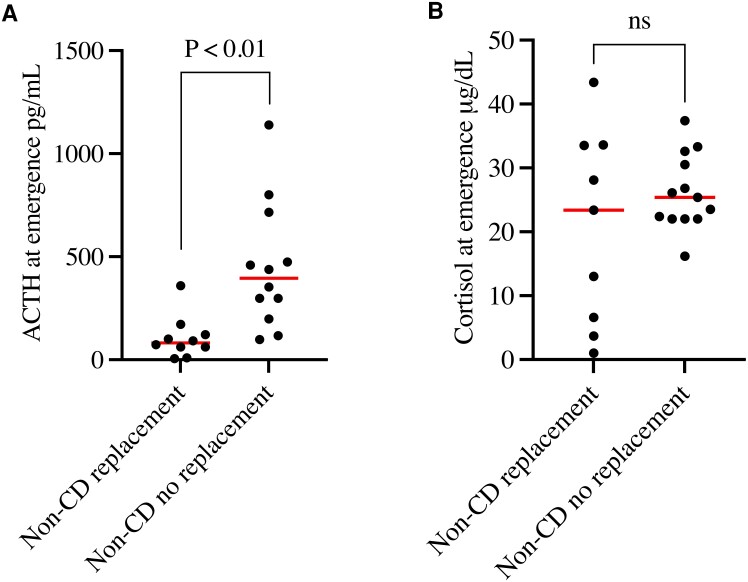
Previous studies have evaluated the utility of recovery room (within a few hours of extubation) cortisol in predicting the need for physiologic cortisol replacement [22]. Here, we asked if a single measure at extubation could provide similar information. Steroid replacement was required in 14 out of 28 non-CD patients (50%). ACTH at extubation was lower in non-CD patients requiring postoperative steroid replacement vs not (105.8 ± 101.8 vs 449.1 ± 304.9 pg/mL, P < 0.01; Fig. 5A). In contrast, cortisol at extubation was not significantly different in non-CD patients requiring steroid replacement vs not (glucocorticoid-requiring: 20.7 ± 15.2 vs 26.2 ± 5.9 μg/dL, P = 0.22; Fig. 5B). In univariable logistic regression, ACTH at extubation predicted the need for steroid replacement (OR 1.0, 95% CI, 1.0-1.0, P = 0.03; Table 4), whereas cortisol at extubation did not (OR 0.9, 95% CI, 0.9-1.0, P = 0.24; Table 4).
Figure 5.
Perioperative ACTH (a) and cortisol (b) in non-Cushing's disease patients requiring postoperative replacement steroids vs patients without replacement requirement. P < 0.05 2-tailed from 2-sample T-test with unequal variance and Welch's approximation for degrees of freedom. Abbreviations: ns, not significant.
Table 4.
Predictors of need for postoperative steroid hormone replacement in non-CD patients after TSS
| Variable | OR | 95% CI | P-value | Adj OR | Adj 95% CI | Adj P-value |
|---|---|---|---|---|---|---|
| Age | 1.0 | 1.0, 1.1 | 0.89 | 1.0 | 1.0, 1.1 | 0.76 |
| Female | 1.3 | 0.3, 5.9 | 0.71 | 0.9 | 0.2, 5.1 | 0.91 |
| White race | 0.4 | 0.1, 2.2 | 0.30 | 0.4 | 0.1, 2.2 | 0.29 |
| BMI | 1.2 | 1.0, 1.4 | 0.05 | 1.2 | 1.0, 1.6 | 0.07 |
| Cortisol at extubation | 0.9 | 0.9, 1.0 | 0.24 | 1.0 | 0.9, 1.1 | 0.44 |
| ACTH at extubation | 1.0 | 1.0, 1.0 | 0.03 |
Results of univariable or multivariable logistic regression with steroid replacement as the dependent variable. Multivariable analysis adjusted for age and gender.
Abbreviations: BMI, body mass index; non-CD, non-Cushing's disease; OR, odds ratio.
Finally, we quantified the ability of ACTH at extubation to predict hormone replacement in non-CD patients by calculating the area under the receiver operating characteristic (AUROC) curve. ACTH at extubation predicted need for hormone replacement with AUROC 0.91, 95% CI 0.79–1 indicating excellent model discrimination. ACTH at extubation also predicted hormone replacement with Hosmer-Lemeshow χ2 11.19, P = 0.19 indicating good model calibration. A criterion for plasma ACTH of 117 pg/mL below which need for steroid hormone replacement was predicted in non-CD patients had a sensitivity of 92% and specificity of 78%. These results suggest that pituitary gland function in non-CD patients can be reliably predicted by a single measure of ACTH at extubation from surgery.
Discussion
In this study, we measured cortisol and ACTH starting at the time of extubation from anesthesia for TSS to clarify the relationship between postoperative hormone dynamics and remission in CD or the need for postoperative glucocorticoid replacement in non-CD patients. Using normalized early postoperative values (NEPV = the post-extubation values minus the peak preoperative stimulation test values), we found that cortisol NEPVs greater than −2.1 at extubation predicted nonremission with sensitivity of 71.4% and specificity of 68.8%. Additionally, a plasma ACTH of less than 117 pg/mL predicted the need for glucocorticoid replacement in non-CD patients with a sensitivity of 92% and specificity of 78%.
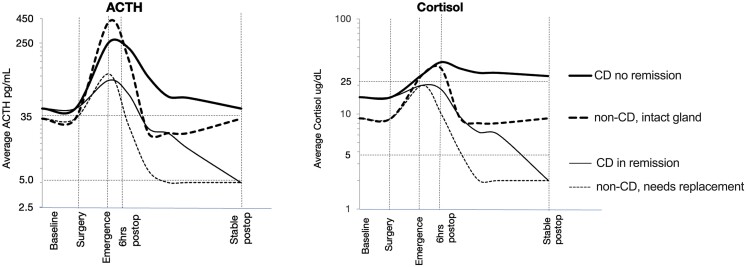
In mammals, stressors activate the HPA axis in part through CRH-mediated ACTH release from the anterior pituitary gland [23]. In CD patients, the source of ACTH is either the normal corticotrophs or the corticotroph adenoma. Complete surgical removal of the corticotroph adenoma unmasks the suppression of ACTH release from normal corticotrophs caused by previous hypercortisolism. Conversely, in non-CD patients, normal corticotrophs are not suppressed and the normal gland retains a full range of response to stress, resulting in a robust postoperative peak in ACTH [24, 25]. However, if the anterior pituitary gland in non-CD patients is damaged due to the tumor or surgery, the postoperative ACTH peak is reduced, similar to that of a CD patient in remission (Fig. 6). Therefore, careful interpretation of levels in the perioperative period can assist clinicians in predicting the long-term health of the HPA axis.
Figure 6.
Conceptual representation of perioperative ACTH and cortisol dynamics in CD (solid lines) and non-CD (dashed lines) patients plotted on a log10 scale. ACTH peak at extubation (approximately 400 pg/mL) distinguishes an intact pituitary gland (thick dashed line) from damaged gland (thin dashed line) and suppressed gland in CD (solid lines). A delayed cortisol peak similarly distinguishes CD patients in remission (thin solid line) from nonremission (thick solid line). Abbreviations: CD, Cushing's disease.
Although experts agree that TSS is the optimal treatment for CD [26], there is no consensus on how to quickly identify remission status after TSS to identify patients who may benefit from early reoperation [19, 27, 28]. Early reoperation is preferable due to lower risk of scarring and local tissue remodeling [27–29]. Several studies have evaluated postoperative cortisol and ACTH levels for prediction of postoperative remission [30, 31]. However, early perioperative hormone-based assessments have been limited by poor predictive accuracy [32, 33], due at least in part to intrapatient cortisol and ACTH variability.
In CD patients whose previous chronic hypercortisolism leads to suppression of CRH and ACTH [2, 32, 34], low cortisol and ACTH are generally reliable markers for postoperative remission [27, 29, 35–39]. However, patients who receive preoperative medical treatment to normalize cortisol levels, or those who have cyclic hypercortisolism, may not have suppressed levels. Postoperative peaks in cortisol and ACTH occur near the time of extubation from anesthesia [30, 34, 40] likely mediated by interleukin 1 [41–43] (Fig. 1C and 1D). The postoperative cortisol peak was present in CD patients who achieved early remission, suggesting that chronically suppressed normal corticotrophs in CD patients are able to mount a response to the stress of surgery and anesthesia. However, peak values were higher in patients without early remission, in whom a sizeable component of the postoperative peak is likely due to residual adenoma.
The intrapatient variability of postoperative cortisol and ACTH levels confound very early prediction of postoperative remission. We previously described a simple normalization strategy taking advantage of the observation that the stress of surgery is similar to preoperative CRH or DDAVP stimulation testing results [5]. Subtracting early postoperative values from preoperative CRH or DDAVP-stimulated values can uncover underlying postoperative cortisol and ACTH dynamics and reduce interpatient variability. Whereas recovery room cortisol has been successfully shown to predict remission status [44], we hypothesized that NEPV for cortisol would result in earlier prediction, possibly even at the time of extubation. We found a reliable separation between patients in remission vs nonremission using NEPV cortisol as early as the time of extubation, and this finding remained significant after multivariable analysis.
NEPV for ACTH poorly predicted early remission status in our study likely due to the differences we found in DDAVP-calculated vs CRH-calculated NEPV for ACTH. Preoperative DDAVP stimulates ACTH and cortisol release in most CD patients [13, 45, 46] via vasopressin receptor 2 signaling that has been reported in adenomatous but not in normal corticotrophs [47, 48]. Consequently, a diminished DDAVP response postoperatively has been used as a marker of early remission [49, 50]. Variable ACTH and cortisol responses to DDAVP stimulation have been reported [13, 45]; therefore a possible explanation is that the loss of V2 receptor signaling after successful adenectomy leads to a mismatch between the postoperative surgical stress response and preoperative DDAVP stimulation testing, although the reason why this effect is specific for ACTH remains obscure. DDAVP-calculated and CRH-calculated NEPV for cortisol were statistically indistinguishable in our study.
In non-CD patients undergoing TSS, plasma ACTH but not cortisol levels at extubation predicted need for postoperative hormone replacement. The half-life of ACTH is 22 minutes [51, 52]; therefore it is possible that acute anterior pituitary gland injury may not yet result in plasma cortisol changes at the time of extubation (Fig. 5). Furthermore, data indicated that higher ACTH values above 117 pg/mL at extubation predicted patients who would not require steroid hormone replacement postoperatively, likely because a normal postoperative ACTH peak indicates normal functioning corticotrophs in non-CD patients. This determination may promote earlier discharge for patients who are otherwise doing well postoperatively and also limit the need for serial lab draws in non-CD patients with gland injury or dysfunction who will ultimately require steroid hormone replacement postoperatively.
Our study has a number of limitations, including limited sample size, lack of long-term remission data, and patient accrual from a specialized clinical center that may not represent the US population at large. Our study benefits from uniform procedures performed by the same surgeon and rigorous endocrine assessment obtained at a single clinical center. Whereas NEPV for cortisol shows promise as a single predictor of remission status at the time of extubation, its sensitivity at the cutoff of −2.1 was 71.4% at extubation and 100% at 6 and 12 hours postoperatively. Larger studies may help further refine the NEPV cutoff and improve its clinical utility.
Summary and Conclusions
In this study, we measured cortisol and ACTH in CD and non-CD patients undergoing TSS, starting at the time of extubation from anesthesia, and clarified postoperative hormone dynamics in each group. NEPV cortisol predicted CD remission status as early as the time of extubation; however, its sensitivity at a single cutoff (−2.1) improved at 6 and 12 hours. By contrast, we found that plasma ACTH at extubation can predict the need for eventual steroid replacement in non-CD patients.
Contributor Information
David T Asuzu, Surgical Neurology Branch, National Institute of Neurological Diseases and Stroke, Bethesda, MD, USA; Neurosurgery Unit for Pituitary and Inheritable Diseases, National Institute of Neurological Diseases and Stroke, Bethesda, MD, USA; Department of Neurosurgery, University of Virginia, Charlottesville, VA, USA.
Shyama Bhatt, Neurosurgery Unit for Pituitary and Inheritable Diseases, National Institute of Neurological Diseases and Stroke, Bethesda, MD, USA.
Diana Nwokoye, Neurosurgery Unit for Pituitary and Inheritable Diseases, National Institute of Neurological Diseases and Stroke, Bethesda, MD, USA.
Christina Hayes, Surgical Neurology Branch, National Institute of Neurological Diseases and Stroke, Bethesda, MD, USA.
Michaela Cortes, Surgical Neurology Branch, National Institute of Neurological Diseases and Stroke, Bethesda, MD, USA.
Raven McGlotten, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA.
Meg Keil, Section on Endocrinology and Genetics, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Christina Tatsi, Section on Endocrinology and Genetics, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Lynnette Nieman, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA.
Prashant Chittiboina, Email: prashant.chittiboina@nih.gov, Surgical Neurology Branch, National Institute of Neurological Diseases and Stroke, Bethesda, MD, USA; Neurosurgery Unit for Pituitary and Inheritable Diseases, National Institute of Neurological Diseases and Stroke, Bethesda, MD, USA.
Disclosure
The authors have nothing to disclose.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Ciric I, Zhao J-C, Du H, et al. Transsphenoidal surgery for Cushing disease. Neurosurgery. 2012;70(1):70–81. doi: 10.1227/NEU.0b013e31822dda2c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hallén T, Olsson DS, Hammarstrand C, et al. Circulating brain injury biomarkers increase after endoscopic surgery for pituitary tumors. J Clin Neurosci. 2021;89:113–121. doi: 10.1016/j.jocn.2021.04.030 [DOI] [PubMed] [Google Scholar]
- 3. Marcet P, Paluzzi J, Morrison A, Vale FL. Endocrine outcomes of transsphenoidal surgery for pituitary apoplexy versus elective surgery for pituitary adenoma. Endocr Pract. 2019;25(4):353–360. doi: 10.4158/EP-2018-0488 [DOI] [PubMed] [Google Scholar]
- 4. Jahangiri A, Wagner JR, Han SW, et al. Improved versus worsened endocrine function after transsphenoidal surgery for nonfunctional pituitary adenomas: rate, time course, and radiological analysis. J Neurosurg. 2016;124(3):589–595. doi: 10.3171/2015.1.JNS141543 [DOI] [PubMed] [Google Scholar]
- 5. Asuzu D, Chatain GP, Hayes C, et al. Normalized early postoperative cortisol and ACTH values predict nonremission after surgery for Cushing disease. J Clin Endocrinol Metab. 2017;102(7):2179–2187. doi: 10.1210/jc.2016-3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing's syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. doi: 10.1210/jc.2008-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lonser RR, Nieman L, Oldfield EH. Cushing's disease: pathobiology, diagnosis, and management. J Neurosurg. 2017;126(2):404–417. doi: 10.3171/2016.1.JNS152119 [DOI] [PubMed] [Google Scholar]
- 8. Hsiao HP, Kirschner LS, Bourdeau I, et al. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J Clin Endocrinol Metab. 2009;94(8):2930–2937. doi: 10.1210/jc.2009-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papanicolaou DA, Yanovski JA, Cutler GB, Chrousos GP, Nieman LK. A single midnight serum cortisol measurement distinguishes Cushing's syndrome from pseudo-Cushing states. J Clin Endocrinol Metab. 2016;83(4):1163–1167. doi: 10.1210/jcem.83.4.4733 [DOI] [PubMed] [Google Scholar]
- 10. Günes M, Celik O, Kadioglu P. Reliability of the diagnostic tests for Cushing's syndrome performed in a tertiary referral center. Pituitary. 2013;16(2):139–145. doi: 10.1007/s11102-012-0387-7 [DOI] [PubMed] [Google Scholar]
- 11. Dichek HL, Nieman LK, Oldfield EH, Pass HI, Malley JD, Cutler GB. A comparison of the standard high dose dexamethasone suppression test and the overnight 8-mg dexamethasone suppression test for the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab. 1994;78(2):418–422. doi: 10.1210/jcem.78.2.8106630 [DOI] [PubMed] [Google Scholar]
- 12. Nieman LK, Oldfield EH, Wesley R, Chrousos GP, Loriaux DL, Cutler GB. A simplified morning ovine corticotropin-releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab. 1993;77(5):1308–1312. doi: 10.1210/jcem.77.5.8077325 [DOI] [PubMed] [Google Scholar]
- 13. Losa M, Mortini P, Dylgjeri S, et al. Desmopressin stimulation test before and after pituitary surgery in patients with Cushing's disease. Clin Endocrinol (Oxf). 2001;55(1):61–68. doi: 10.1046/j.1365-2265.2001.01324.x [DOI] [PubMed] [Google Scholar]
- 14. Oldfield EH, Doppman JL, Nieman LK, et al. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing's syndrome. N Engl J Med. 1991;325(13):897–905. doi: 10.1056/NEJM199109263251301 [DOI] [PubMed] [Google Scholar]
- 15. Galland F, Vantyghem M-C, Cazabat L, et al. Management of nonfunctioning pituitary incidentaloma. Ann Endocrinol (Paris). 2015;76(3):191–200. doi: 10.1016/j.ando.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 16. Chanson P, Raverot G, Castinetti F, Cortet-Rudelli C, Galland F, Salenave S. Management of clinically non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015;76(3):239–247. doi: 10.1016/j.ando.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 17. Casanueva FF, Molitch ME, Schlechte JA, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006;65(2):265–273. doi: 10.1111/j.1365-2265.2006.02562.x [DOI] [PubMed] [Google Scholar]
- 18. Katznelson L, Laws ER, Melmed S, et al. Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–3951. doi: 10.1210/jc.2014-2700 [DOI] [PubMed] [Google Scholar]
- 19. Ironside N, Chatain G, Asuzu D, et al. Earlier post-operative hypocortisolemia may predict durable remission from Cushing's disease. Eur J Endocrinol. 2018;178(3):255–263. doi: 10.1530/EJE-17-0873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Welch BL. The generalisation of student's problems when several different population variances are involved. Biometrika. 1947;34(1-2):28–35. doi: 10.1093/biomet/34.1-2.28 [DOI] [PubMed] [Google Scholar]
- 21. Asuzu DT, Bhatt S, Nwokoye D, et al. Supplementary Materials. Mendeley Data. Deposited January 21, 2023. doi: 10.17632/mrh46xn79n.1 [DOI]
- 22. Marko NF, Hamrahian AH, Weil RJ. Immediate postoperative cortisol levels accurately predict postoperative hypothalamic-pituitary-adrenal axis function after transsphenoidal surgery for pituitary tumors. Pituitary. 2010;13(3):249–255. doi: 10.1007/s11102-010-0227-6 [DOI] [PubMed] [Google Scholar]
- 23. Miller T, Gibbison B, Russell GM. Hypothalamic–pituitary–adrenal function during health, major surgery, and critical illness. BJA Educ. 2017;17(1):16–21. doi: 10.1093/bjaed/mkw042 [DOI] [Google Scholar]
- 24. Udelsman R, Holbrook NJ. Endocrine and molecular responses to surgical stress 6. CurrProblSurg. 1994;31:653–720. doi: 10.1016/0011-3840(94)90057-4 [DOI] [PubMed] [Google Scholar]
- 25. Udelsman R, Norton JA, Jelenich SE, et al. Responses of the hypothalamic-pituitary-adrenal and renin-angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. J Clin Endocrinol Metab. 1987;64(5):986–994. doi: 10.1210/jcem-64-5-986 [DOI] [PubMed] [Google Scholar]
- 26. Nieman LK, Biller BMK, Findling JW, et al. Treatment of Cushing's syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807–2831. doi: 10.1210/jc.2015-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ram Z, Nieman LK, Cutler GB, Chrousos GP, Doppman JL, Oldfield EH. Early repeat surgery for persistent Cushing's disease. J Neurosurg 1994;80(1):37–45. doi: 10.3171/jns.1994.80.1.0037 [DOI] [PubMed] [Google Scholar]
- 28. Locatelli M, Vance ML, Laws ER. Clinical review: the strategy of immediate reoperation for transsphenoidal surgery for Cushing's disease. J Clin Endocrinol Metab. 2005;90(9):5478–5482. doi: 10.1210/jc.2004-2436 [DOI] [PubMed] [Google Scholar]
- 29. Friedman RB, Oldfield EH, Nieman LK, et al. Repeat transsphenoidal surgery for Cushing's disease. J Neurosurg. 1989;71(4):520–527. doi: 10.3171/jns.1989.71.4.0520 [DOI] [PubMed] [Google Scholar]
- 30. Krikorian A, Abdelmannan D, Selman WR, Arafah BM. Cushing disease: use of perioperative serum cortisol measurements in early determination of success following pituitary surgery. Neurosurg Focus. 2007;23(3):E6. doi: 10.3171/foc.2007.23.3.8 [DOI] [PubMed] [Google Scholar]
- 31. Acebes JJ, Martino J, Masuet C, Montanya E, Soler J. Early post-operative ACTH and cortisol as predictors of remission in Cushing's disease. Acta Neurochir (Wien). 2007;149(5):471–477. doi: 10.1007/s00701-007-1133-1 [DOI] [PubMed] [Google Scholar]
- 32. Graham KE, Samuels MH, Raff H, Barnwell SL, Cook DM. Intraoperative adrenocorticotropin levels during transsphenoidal surgery for Cushing's disease do not predict cure. J Clin Endocrinol Metab. 1997;82(6):1776–1779. doi: 10.1210/jcem.82.6.4005 [DOI] [PubMed] [Google Scholar]
- 33. Nadezhdina E, Grigoriev A, Azizjan V, Ivashenko O, Rozhinskaya L, Ilyin A. The role of intraoperative hormone levels as predictors of Cushing's disease remission. Endocr Abstr. 2014.35:P730. doi: 10.1530/endoabs.35.P730 [DOI] [Google Scholar]
- 34. Monteith SJ, Starke RM, Jane JA, Oldfield EH. Use of the histological pseudocapsule in surgery for Cushing disease: rapid postoperative cortisol decline predicting complete tumor resection. J Neurosurg. 2012;116(4):721–727. doi: 10.3171/2011.12.JNS11886 [DOI] [PubMed] [Google Scholar]
- 35. Salassa RM, Laws ER, Carpenter PC, Northcutt RC. Transsphenoidal removal of pituitary microadenoma in Cushing's disease. Mayo Clin Proc. 1978;53(1):24–28. [PubMed] [Google Scholar]
- 36. Avgerinos PC, Chrousos GP, Nieman LK, Oldfield EH, Loriaux DL, Cutler GB. The corticotropin-releasing hormone test in the postoperative evaluation of patients with Cushing's syndrome. J Clin Endocrinol Metab. 1987;65(5):906–913. doi: 10.1210/jcem-65-5-906 [DOI] [PubMed] [Google Scholar]
- 37. Batista DL, Oldfield EH, Keil MF, Stratakis CA. Postoperative testing to predict recurrent Cushing disease in children. J Clin Endocrinol Metab. 2009;94(8):2757–2765. doi: 10.1210/jc.2009-0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Invitti C, Pecori Giraldi F, de Martin M, Cavagnini F. Diagnosis and management of Cushing's syndrome: results of an Italian multicentre study. Study Group of the Italian society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis. J Clin Endocrinol Metab. 1999;84(2):440–448. doi: 10.1210/jcem.84.2.5465 [DOI] [PubMed] [Google Scholar]
- 39. Lindsay JR, Oldfield EH, Stratakis CA, Nieman LK. The postoperative basal cortisol and CRH tests for prediction of long-term remission from Cushing's disease after transsphenoidal surgery. J Clin Endocrinol Metab. 2011;96(7):2057–2064. doi: 10.1210/jc.2011-0456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rollin GAFS, Ferreira NP, Junges M, Gross JL, Czepielewski MA. Dynamics of serum cortisol levels after transsphenoidal surgery in a cohort of patients with Cushing's disease. J Clin Endocrinol Metab. 2004;89(3):1131–1139. doi: 10.1210/jc.2003-031170 [DOI] [PubMed] [Google Scholar]
- 41. Woloski BM, Smith EM, Meyer WJ, Fuller GM, Blalock JE. Corticotropin-releasing activity of monokines. Science. 1985;230(4729):1035–1037. doi: 10.1126/science.2997929 [DOI] [PubMed] [Google Scholar]
- 42. Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79(1):1–71. doi: 10.1152/physrev.1999.79.1.1 [DOI] [PubMed] [Google Scholar]
- 43. Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ. Systemic cytokine response after major surgery. Br J Surg. 1992;79(8):757–760. doi: 10.1002/bjs.1800790813 [DOI] [PubMed] [Google Scholar]
- 44. Qaddoura A, Shalung TN, Meier MP, et al. Recovery room cortisol predicts long-term glucocorticoid need after transsphenoidal surgery for pituitary tumors. Clin Neurosurg. 2019;84(3):616–623. doi: 10.1093/neuros/nyy070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Terzolo M, Reimondo G, Alì A, et al. The limited value of the desmopressin test in the diagnostic approach to Cushing's syndrome. Clin Endocrinol (Oxf). 2001;54(5):609–616. doi: 10.1046/j.1365-2265.2001.01260.x [DOI] [PubMed] [Google Scholar]
- 46. Vassiliadi DA, Tsagarakis S. The role of the desmopressin test in the diagnosis and follow-up of Cushing's syndrome. Eur J Endocrinol. 2018;178(5):R201–R214. doi: 10.1530/EJE-18-0007 [DOI] [PubMed] [Google Scholar]
- 47. Wang F-F, Tang K-T, Yen Y-S, et al. Plasma corticotrophin response to desmopressin in patients with Cushing's disease correlates with the expression of vasopressin receptor 2, but not with that of vasopressin receptor 1 or 3, in their pituitary tumours. Clin Endocrinol (Oxf). 2012;76(2):253–263. doi: 10.1111/j.1365-2265.2011.04179.x [DOI] [PubMed] [Google Scholar]
- 48. Tsagarakis S, Tsigos C, Vasiliou V, et al. The desmopressin and combined CRH-desmopressin tests in the differential diagnosis of ACTH-dependent Cushing's syndrome: constraints imposed by the expression of V2 vasopressin receptors in tumors with ectopic ACTH secretion. J Clin Endocrinol Metab. 2002;87(4):1646–1653. doi: 10.1210/jcem.87.4.8358 [DOI] [PubMed] [Google Scholar]
- 49. Fleseriu M, Auchus R, Bancos I, et al. Consensus on diagnosis and management of Cushing's disease: a guideline update. Lancet Diabetes Endocrinol. 2021;9(12):847–875. doi: 10.1016/S2213-8587(21)00235-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cambos S, Mohammedi K, Castinetti F, et al. Persistent cortisol response to desmopressin predicts recurrence of Cushing's disease in patients with post-operative corticotropic insufficiency. Eur J Endocrinol. 2020;182(5):489–498. doi: 10.1530/EJE-19-0770 [DOI] [PubMed] [Google Scholar]
- 51. Veldhuis JD, Iranmanesh A, Naftolowitz D, Tatham N, Cassidy F, Carroll BJ. Corticotropin secretory dynamics in humans under low glucocorticoid feedback. J Clin Endocrinol Metab. 2001;86(11):5554–5563. doi: 10.1210/jcem.86.11.8046 [DOI] [PubMed] [Google Scholar]
- 52. Dorin RI, Qiao Z, Qualls CR, Urban FK. Estimation of maximal cortisol secretion rate in healthy humans. J Clin Endocrinol Metab. 2012;97(4):1285–1293. doi: 10.1210/jc.2011-2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.