Abstract
Cardiovascular disease (CVD) is one of the most common comorbidities in breast cancer survivors. Recently, the target population and treatment period for aromatase inhibitor (AI) treatment in breast cancer patients has been expanding. However, information on adverse CVD events from the long-term use of AI is still lacking. The aim of this study was to investigate the CVD side effects of AI treatment and to evaluate the changes in lipid profile during AI treatment. A systematic search of PubMed (Medline), EMBASE, and Cochrane Library databases reporting on cardiovascular outcomes or lipid profiles change in adult female breast cancer patients (>19 years old) with AI was performed. The pooled analysis of 25 studies showed that the prevalence rate of any type of cardiovascular disease was 6.08 per 100 persons (95% CI 2.91–10.31). Angina was the most common type of heart-related cardiovascular event accounting for 3.85 per 100 persons, followed by any type of stroke (3.34) and venous thromboembolism (2.95). Ischemic stroke (OR 1.39, 95% CI 1.07–1.81) and myocardial infarction (OR 1.30, 95% CI 0.88–1.93) were more common in AI compared with tamoxifen, whereas the prevalence of venous thromboembolism (OR 0.61, 95% CI 0.37–1) was significantly lower in the AI group. In addition, treatment with AI for 6–12 months showed a decrease in HDL-cholesterol and an increase in LDL-cholesterol and total cholesterol. Various CVDs can occur when using AI, and in particular, the risk of MI and ischemic stroke increases in comparison with the adverse effect of tamoxifen. The occurrence of CVD might be related to the deterioration of the lipid profile after AI treatment. Therefore, a customized individualization strategy considering each patient’s CV risk factors is needed during AI treatment.
Keywords: aromatase inhibitor, cardiovascular risk, angina, stroke, thromboembolism
1. Introduction
Breast cancer is a disorder rapidly increasing in prevalence worldwide [1,2]. In the United States, breast cancer is one of the most common cancers in women after skin cancer. A woman’s lifetime chance of developing breast cancer is 12.9%, and the incidence of breast cancer is increasing at the rate of 0.5% per year [3]. Fortunately, due to the development of effective screening and various treatment methods, the mortality rate from breast cancer is decreasing every year, and the 5-year survival rate reaches more than 90% [4]. As of 2021, the number of breast cancer survivors in the United States amounted to 3.8 million. The management of these breast cancer survivors’ comorbidities is directly related to the long-term prognosis of breast cancer patients.
Cardiovascular disease (CVD) is one of the most common comorbidities in breast cancer survivors [5]. In fact, CVD and breast cancer share various risk factors, and breast cancer patients have a high prevalence of CVD compared with the general population. CVD and its risk factors should be treated well clinically, as poor management of these CVDs leads to CVD death in breast cancer survivors [6].
In 2022, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) reported that aromatase inhibitors (AI) such as anastrozole, exemestane, or letrozole had a lower recurrence rate than tamoxifen in patients with early premenopausal hormone receptor-positive breast cancer accompanied by ovarian suppression therapy [7]. As similar evidence has been accumulated, the AI treatment period has recently been lengthened, and the indications for AI treatment in breast cancer patients have been extended to include premenopausal. However, reports of adverse events from the long-term use of AI have also been increasingly notified. Several recent studies showed that the use of AI escalates the risk of cardiac-related events and strokes in breast cancer patients [8,9,10]. These side effects of AI can be clinically problematic, especially for those breast cancer patients with high life expectancy. In addition, several studies show that the use of AI worsens the lipid profile, which is presumed to affect the increase in cardiovascular disease risk.
Despite the recent trend recommending the long-term use of AI, studies on the side effects of AI are lacking. Therefore, this study aims to investigate the CVD side effects of AI treatment in breast cancer patients through a systematic review of the literature and meta-analysis. The prevalence of CVD side effects according to AI treatment, a risk analysis of CVD outcome compared with tamoxifen, and changes in the lipid profile after AI treatment was analyzed.
2. Materials and Methods
The protocol for this review was pre-registered with PROSPERO (International Prospective Register of Systematic Reviews), which is an international registry for systematic reviews, and was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist. The registration number is CRD42022357061.
2.1. Inclusion Criteria, Exclusion Criteria, and Study Outcomes
The studies included in this review were of different types, such as randomized controlled trials, cross-sectional studies, or cohort studies, which can be either prospective or retrospective in design and report on the cardiovascular outcomes of adult female breast cancer patients who were older than 19 years old and took AI as treatment. The exclusion criteria were as follows: (i) case reports, (ii) case series of fewer than five patients, and (iii) review articles. The primary outcome of the study was the prevalence of cardiovascular events after AI in patients with breast cancer. For cardiovascular outcome, the overall outcome, regardless of type, was investigated first and was then subdivided into heart, brain, or thromboembolism. The secondary outcome of the study was a change in the lipid profile after AI.
Classification by country was based on the country where the research was mainly conducted. In the case of multicenter studies, multicenter studies within one continent, such as those in Europe, were classified as European, and studies involving two or more continents were classified as ‘worldwide’.
2.2. Search Strategy
A search strategy was developed by searching for synonymous terms, and the keywords used in the patient/problem, intervention, comparison, and outcome (PI-CO) model can be found in the supplementary material and method section. The search was conducted on various databases such as PubMed (Medline), EMBASE, Cochrane Library, Web of Science, and KoreaMed, using medical subject headings (MeSH) and terms to identify studies that were published in English between 1 January 1990, and 31 March 2022. The search strategies and results for each database can be found in the supplementary material and method section. A professional librarian (EAJ) conducted all search processes. The search terms included index words related to breast cancer, index words related to aromatase inhibitors (AI), and index words related to cardiovascular outcomes. The lipid profile-related terms were not systematically searched as an outcome. Instead, additional information on lipid profiles was collected from articles that searched for cardiovascular outcomes.
2.3. Study Selection and Data Extraction
The screening process was conducted by two authors independently, who screened the titles and abstracts. Two reviewers (JJY and BYK) also independently evaluated the full-text articles for relevance. In the case of any discrepancy, it was resolved by ZSK after a discussion. Both researchers also independently assessed the risk of bias and extracted study data, including the characteristics and results, and recorded them in a standard form.
2.4. Methodological Quality and Risk of Bias Assessment
We used the Cochrane risk of bias tool for randomized trials [11] and the risk of bias assessment tool for non-randomized studies (RoBANS) [12] for cohort studies to assess the risk of bias; the overall results are shown in the Supplementary Material risk of bias section. Any discrepancy was resolved by two authors (JJY and BYK) after discussion. Publication bias was assessed using funnel plots (Supplementary materials). Publication bias was evaluated only when there were three or more integrated studies.
2.5. Statistical Analysis
We derived the pooled event rate using a random-effects model and the following method of calculation: (1) transform the event rate into a quantity (Freeman–Tukey variant of the arcsine square root transformed proportion), and (2) calculate the pooled event rate as the back-transformation of the weighted mean of the transformed event rate using the Mantel–Haenszel method and assuming the random-effect model. The comparison of event rates in the AI and tamoxifen group was calculated by a random-effects model as the mean difference for continuous variable and as a Freeman–Tukey variant of the arcsine square root transformed proportion for the binary variable. The comparative results were recorded as odds ratios with 95% confidence intervals. We analyzed the variation between studies using the I2 metric and the p-value from the Cochran Q test. The I2 metric, which is a ratio of the variance between studies to the total variance, can range from 0% to 100%. The statistical analysis was conducted using RevMan 5 (Cochrane Library) or the R programming language’s meta package, version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Characteristics of Included Studies
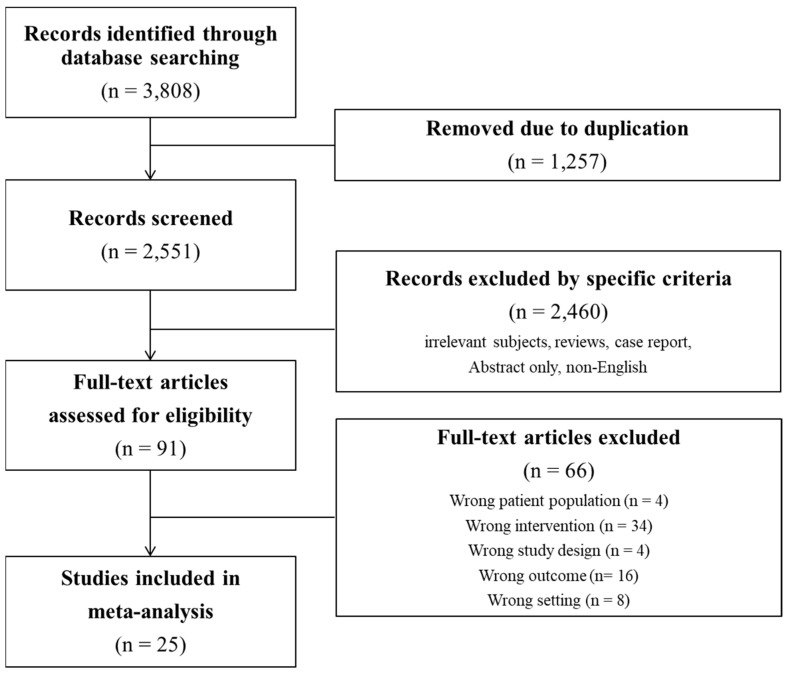
After reviewing the titles and abstracts, we found 91 studies that were potentially relevant. However, we eliminated 66 of these studies for specific reasons: wrong patient population (n = 4), wrong intervention (n = 34), wrong study design (n = 4), wrong outcome (n = 16), and wrong setting (n = 8). As a result, 25 studies were included in the meta-analysis (Figure 1). Information regarding the enrolled patients is presented in Table 1.
Figure 1.
Flow charts of the study.
Table 1.
Demographics and characteristics of studies included in the systematic review and meta-analysis.
| Study | Study Type | Country | Cancer Stage | Age (Median) |
Menopause | Treatment Duration | No. Group | Treatment | Control | No. Treatment | No. Control |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sawada 2005 [13] | Prospective RCT | Japan | early | 58.7 | postmenopausal | 12 wks | 2 | anastrozole | TAM | 22 | 22 |
| Lonning 2005 [14] | Prospective RCT | Norway | early | 60 | postmenopausal | 2 yrs | 2 | exemestane | placebo | 58 | 65 |
| Atalay 2004 [15] | Prospective RCT | Europe | non-metastatic | 64 | postmenopausal | 48 wks | 2 | exemestane | TAM | 36 | 36 |
| Khosrow-Khavar 2020 [8] | Retrospective cohort | UK | non-metastatic | 70.8 | postmenopausal | >1 year | 2 | AI | TAM | 8139 | 9783 |
| Wojtacki 2001 [16] | Retrospective cohort | Poland | non-metastatic | 61.6 | postmenopausal | 16.2 weeks | 1 | anastrozole | 44 | ||
| Markopoulos 2009 [17] | Prospective RCT | Greece | non-metastatic | 62.6 | postmenopausal | 5 years | 2 | exemestane | placebo | 211 | 200 |
| Tian 2018 [18] | Retrospective cohort | China | early breast cancer | 59.5 | postmenopausal | 2 years | 1 | letrozole | 38 | ||
| Santa-Maria 2016 [19] | Prospective cohort | USA | early breast cancer | 59 | postmenopausal | 3 months | 1 | AI | 422 | ||
| Abdel-Qadir 2016 [20] | Retrospective cohort | Canada | early breast cancer | 71 | postmenopausal | at least 1 year | 2 | AI | TAM | 7409 | 1941 |
| Pineda-Moncusi 2020 [21] | Retrospective cohort | UK, Spain | early breast cancer | 67 | postmenopausal | 29 months | 2 | AI | TAM | 18,455 | 3082 |
| Xu 2019 [22] | Prospective cohort | USA | non-metastatic | 65 | postmenopausal | 3.2 years | 2 | AI | TAM | 3837 | 4062 |
| Markopoulos 2005 [23] | Prospective RCT | Greece | early breast cancer | 65 | postmenopausal | 12 months | 2 | exemestane | TAM | 90 | 86 |
| Matthews 2021 [24] | Retrospective cohort | USA, UK | early breast cancer | 76 | postmenopausal | 2.2 years | 2 | AI | placebo | 15,074 | 4667 |
| Rabaglio 2021 [25] | Prospective RCT | Europe, USA | early breast cancer | NA | postmenopausal | 5 years | 2 | letrozole | TAM | 1535 | 1541 |
| Khosrow-Khavar 2020 [9] | Retrospective cohort | Canada | early breast cancer | 67.7 | postmenopausal | 5 years | 2 | AI | TAM | 1962 | 3874 |
| Seruga 2014 [26] | Retrospective cohort | Slovenia | early breast cancer | 69 | postmenopausal | NA | 2 | AI | TAM | 33 | 41 |
| Kamaraju 2019 [27] | Retrospective cohort | USA | early breast cancer | NA | postmenopausal | 12 months | 2 | AI | TAM | 4690 | 958 |
| Choi 2020 [28] | Retrospective cohort | Korea | all stage | 63.3 | postmenopausal | 3 years | 2 | AI | placebo | 19,584 | 18,807 |
| Ligibel 2012 [29] | Retrospective cohort | USA | all stage | 67 | NA | 30 months | 2 | AI | placebo | 9069 | 30,255 |
| Faiz 2021 [30] | Retrospective cohort | USA | all stage | 74.8 | postmenopausal | 2 years | 2 | AI | TAM | 64,384 | 22,042 |
| Franchi 2021 [31] | Retrospective cohort | Italy | early breast cancer | NA | postmenopausal | NA | 2 | AI | TAM | 7881 | 7881 |
| Chang 2022 [32] | Retrospective cohort | Taiwan | all stage | 62.53 | mixed | NA | 2 | AI | TAM | 11,728 | 16,730 |
| Thurlimann 2005 [33] | Prospective RCT | worldwide | early breast cancer | 61 | postmenopausal | 25.8 months | 2 | letrozole | TAM | 3975 | 3988 |
| Sund 2021 [10] | Retrospective cohort | Sweden | early breast cancer | 66 | postmenopausal | 46.8 months | 2 | AI | placebo | 1481 | 3668 |
| Haque 2016 [34] | Retrospective cohort | USA | all stage | 66.8 | postmenopausal | 2.3 years | 2 | AI | TAM | 3807 | 4207 |
Abbreviations: TAM, tamoxifen; AI, aromatase inhibitor; RCT, randomized controlled trial: NA, non-available; No., number.
As shown in Table 1, all 25 studies were conducted in various countries, mostly within Europe (n = 10), followed by America (n = 8), Asia (n = 4), and worldwide (n = 3). As for the study design, the most common type was a retrospective cohort study (16 studies), followed by seven prospective RCTs and two prospective cohort studies. Of the 25 studies, 20 studies were restricted to the analysis of non-metastatic stage breast cancer, and five studies included all stages of breast cancer. Twenty-three studies were conducted on postmenopausal women, and two studies did not record menopause status, but considering the average age (67 years, 62.5 years), they were more likely to be postmenopausal women. The duration of AI drug administration was at least 3 months. A total of 22 studies compared the results of AI with other treatments (tamoxifen or placebo), and three studies analyzed only the AI-only group without a comparator.
3.2. AI Use and the Prevalence of Various Cardiovascular Outcomes
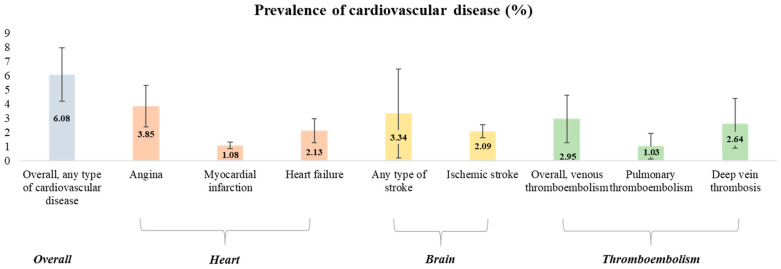
First, we calculated the prevalence of various cardiovascular events after AI in post-menopausal breast cancer patients (Table 2, Figure 2). The pooled prevalence rate of any type of cardiovascular disease from six studies was 6.08 per 100 persons (95% CI 2.91–10.31). Next, the cardiovascular event was sub-analyzed and divided into three categories: heart, brain, and thromboembolism. Angina was the most common type of heart-related cardiovascular event accounting for 3.85 per 100 persons (95% CI 1.48–7.18), followed by heart failure (pooled prevalence 2.13 per 100 persons, 95% CI 0.79–4.48), and myocardial infarction (pooled prevalence 1.08 per 100 persons, 95% CI 0.61–1.65). For brain-related cardiovascular events, any type of stroke and ischemic stroke were reported as 3.34 (95% CI 0–12.81) and 2.09 per 100 persons (95% CI 1.21–3.21), respectively. Finally, the overall prevalence rates of thromboembolism, pulmonary embolism, and deep vein thrombosis were 2.95 (95% CI 0.55–7.12), 1.03 (95% CI 0.01–3.61), and 2.64 per 100 persons (95% CI 0.3–7.19), respectively. The forest plots and funnel plots for each outcome are presented in Supplementary Figure S1.
Table 2.
Prevalence of various cardiovascular outcomes in patients with breast cancer with AI.
| Outcome | No. of Studies | No. of Patients Events/Total |
Pooled Event Rate (per 100 Person) |
95% CI | I 2 | p for Heterogeneity |
|---|---|---|---|---|---|---|
| Overall, any type of cardiovascular disease | 6 [21,25,31,32,33,34] |
2453/47,381 | 6.08 | 2.91 to 10.31 | 100% | <0.01 |
| Heart | ||||||
| Coronary artery disease including angina | 6 [21,24,25,26,33,34] |
1499/42,879 | 3.85 | 1.48 to 7.18 | 99% | <0.01 |
| Myocardial infarction | 9 [8,9,20,24,26,28,31,32,34] |
977/75,617 | 1.08 | 0.61 to 1.65 | 97% | <0.01 |
| Heart failure | 8 [8,9,24,25,31,32,33,34] |
2338/54,101 | 2.13 | 0.79 to 4.48 | 100% | 0 |
| Brain | ||||||
| Any type of stroke | 2 [24,28] |
1287/34,658 | 3.34 | 0 to 12.81 | 100% | <0.01 |
| Ischemic stroke | 5 [8,9,28,31,32] |
1293/49,294 | 2.09 | 1.21 to 3.21 | 98% | <0.01 |
| Thromboembolism | ||||||
| Overall, venous thromboembolism | 6 [21,22,24,25,30,33] |
7503/107,260 | 2.95 | 0.55 to 7.12 | 100% | 0 |
| Pulmonary thromboembolism | 4 [21,22,24,30] |
2701/101,750 | 1.03 | 0.01 to 3.61 | 100% | 0 |
| Deep vein thrombosis | 4 [21,22,24,30] |
5866/101,750 | 2.64 | 0.30 to 7.19 | 100% | 0 |
Figure 2.
Prevalence of overall cardiovascular outcomes in patients with AI.
3.3. Comparison of Various Cardiovascular Outcomes between AI and Tamoxifen
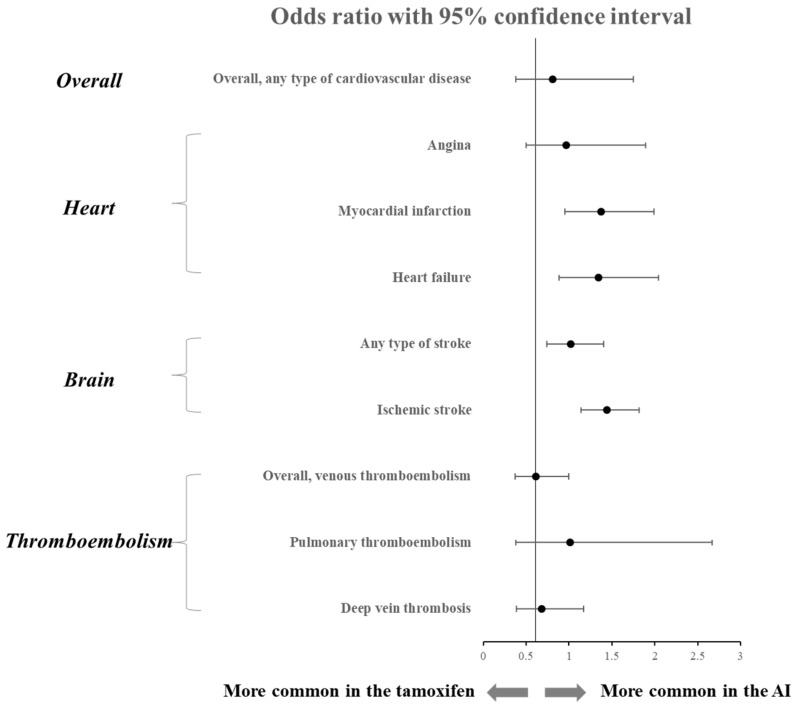
For studies comparing tamoxifen and AI, we analyzed how the two drugs differed in their influence on cardiovascular outcomes (Table 3). The overall cardiovascular outcome was slightly lower than that of tamoxifen in the AI group, with a pooled odds ratio (OR) of 0.81, but it was not statistically significant (95% CI 0.38–1.75). The prevalence of the ischemic stroke was OR 1.39, which was significantly higher in the AI group than tamoxifen (95% CI 1.07–1.81). In addition, the heart-related cardiovascular events, myocardial infarction (OR 1.30, 95% CI 0.88–1.93), and heart failure (OR 1.20, 95% CI 0.78–1.86) occurred relatively more frequently in the AI group but were not statistically significant. On the other hand, the prevalence of overall thromboembolism was significantly lower in the AI group than that of the tamoxifen group with an OR of 0.61 (95% CI 0.37–1.0), and the prevalence of DVT also showed a low prevalence tendency in the AI group (OR 0.68, 95% CI 0.39–1.17). Meanwhile, the occurrence of other cardiovascular events did not show a significant difference between the AI and the tamoxifen group. Pooled forest plots and forest plots of each outcome are presented in Figure 3 and Supplementary Figure S2, respectively.
Table 3.
Comparison of various cardiovascular outcomes between AI and tamoxifen.
| Outcome | No. of Studies | No. of Patients, Events/Total (AI) |
No. of Patients, Events/Total (Tamoxifen) |
OR | 95% CI | I 2 | p for Heterogeneity |
|---|---|---|---|---|---|---|---|
| Overall, any type of cardiovascular disease | 6 [21,25,31,32,33,34] |
2453/47,381 | 3361/37,429 | 0.81 | 0.38 to 1.75 | 99% | <0.01 |
| Heart | |||||||
| Coronary artery disease including angina | 6 [21,24,25,26,33,34] |
1499/42,879 | 523/15,145 | 0.97 | 0.50 to 1.89 | 95% | <0.01 |
| Myocardial infarction | 9 [8,9,20,24,26,28,31,32,34] |
977/75,617 | 412/53,824 | 1.30 | 0.88 to 1.93 | 88% | <0.01 |
| Heart failure | 8 [8,9,24,25,31,32,33,34] |
2338/54,101 | 1234/50,290 | 1.20 | 0.78 to 1.86 | 96% | <0.01 |
| Brain | |||||||
| Any type of stroke | 2 [24,28] |
1287/34,658 | 237/9367 | 1.02 | 0.74 to 1.40 | 69% | 0.07 |
| Ischemic stroke | 5 [8,9,28,31,32] |
1293/49,294 | 833/45,349 | 1.39 | 1.07 to 1.81 | 85% | <0.01 |
| Thromboembolism | |||||||
| Overall, venous thromboembolism | 6 [21,22,24,25,30,33] |
7503/107,260 | 2893/37,001 | 0.61 | 0.37 to 1.00 | 97% | <0.01 |
| Pulmonary thromboembolism | 4 [21,22,24,30] |
2701/101,750 | 991/31,472 | 1.01 | 0.38 to 2.67 | 94% | <0.01 |
| Deep vein thrombosis | 4 [21,22,24,30] |
5866/101,750 | 2060/31,472 | 0.68 | 0.39 to 1.17 | 96% | <0.01 |
Figure 3.
Comparison of cardiovascular outcomes between AI and tamoxifen.
3.4. Change in Lipid Profile during AI Treatment
Lastly, we investigated the change in the lipid profile after AI treatment (Table 4). After the use of AI, the decrease in HDL-cholesterol was significant (pooled mean difference −2.47 mg/dL, 95% CI −4.26–−0.69) at 6 months compared with the baseline value. In addition, a tendency to increase LDL-cholesterol and total cholesterol levels were observed after the use of AI, but it was not statistically significant. When AI and tamoxifen-treated patients were compared, a significant increase in LDL-cholesterol was observed at 6 months in the AI group (pooled mean difference +6.48 mg/dL, 95% CI 0.64–16.32). The forest plots and funnel plots of each indicator are presented in Supplementary Figure S3.
Table 4.
Change in lipid profile during AI treatment.
| Outcome | No. of Studies | Mean Difference | 95% CI | I 2 | p for Heterogeneity | |
|---|---|---|---|---|---|---|
| Comparison with baseline | ||||||
| HDL-cholesterol | 6-month | 5 [15,17,18,23] |
−2.47 | −4.26 to −0.69 | 81% | <0.01 |
| 12-month | 4 [17,18,23] |
1.16 | −3.18 to 5.49 | 94% | <0.01 | |
| LDL-cholesterol | 6-month | 5 [17,18,23] |
10.48 | −2.95 to 23.92 | 87% | <0.01 |
| 12-month | 5 [17,18,23] |
8.05 | −3.68 to 19.79 | 87% | <0.01 | |
| Total cholesterol | 6-month | 6 [15,17,18,23] |
5.16 | −2.07 to 12.40 | 83% | <0.01 |
| 12-month | 5 [17,18,23] |
4.62 | −4.00 to 13.24 | 99% | <0.01 | |
| Comparison with tamoxifen | ||||||
| HDL-cholesterol | 6-month | 2 [15,23] |
−3.67 | −12.31 to 4.97 | 99% | <0.01 |
| 12-month | 2 [17,23] |
−1.52 | −4.79 to 7.75 | 99% | <0.01 | |
| LDL-cholesterol | 6-month | 2 [17,23] |
6.48 | 0.64 to 16.32 | 99% | <0.01 |
| 12-month | 2 [17,23] |
11.87 | −15.77 to 39.50 | 100% | <0.01 | |
| Total cholesterol | 6-month | 3 [15,17,23] |
1.28 | −14.97 to 17.53 | 100% | <0.01 |
| 12-month | 2 [17,23] |
6.89 | −20.75 to 34.52 | 100% | <0.01 | |
4. Discussion
Through this meta-analysis, our study provided information about (i) the overall incidence of CVD outcomes after AI treatment, (ii) a comparison of the CVD outcome occurrence between the AI group and the tamoxifen group, and (iii) changes in the lipid profile after AI use. Because CVD is associated with the long-term mortality of patients, we assumed the importance of evaluating the CV risk even when patients had a clinical benefit to extending the duration of AI treatment in the perspective of cancer.
The first finding of our study is the incidence and types of CVD outcomes after AI treatment. Compared with the substantial meta-analysis results for the CVD outcome after tamoxifen, the research results for CVD outcome after AI are relatively insufficient [22,24,35,36]. Considering that the characteristics of the patient groups using AI or tamoxifen are different and that the two drugs are not often interchanged, the results of the AI alone group can provide a considerable amount of clinical information. To date, two meta-studies have been conducted on whether AI increases CVD in breast cancer patients [35,37]. However, the results of the two meta-analyses were inconsistent. In a meta-analysis published in 2017 [35], AI increased CVD by 19% compared to the control group, but in a meta-analysis published in 2019 [37], the increase in CVD in the AI group compared to the control group was not significant. On the other hand, in our study, certain types of CVDs, such as ischemic stroke or myocardial infarction, were analyzed to be increased in the AI group. This is probably because all meta-studies are different in terms of when the study was conducted (2017, 2019, 2022), how CVD was defined (including hypertension or dyslipidemia), and the type of study enrolled (randomized controlled trial only vs. cohort study included).
In our study, the most frequently reported CVD after AI treatment was angina, followed by any type of stroke and heart failure. However, the prevalence rates of angina, any type of stroke, and heart failure in the AI-using group were 3.85, 3.34, and 2.1 per 100 people, respectively, which was slightly lower than that of 6.2%, 20%, and 8.8% in the general women population [38,39,40]. We speculated that two factors may have contributed to the low prevalence of the AI group. First, the incidence rate might be underestimated due to the lack of interest of clinicians or the limitations of the retrospective study. Second, since the treatment period of most of the enrolled studies was less than 2 years, there is a possibility that sufficient data were not accumulated to evaluate the CVD outcome for the long-term use of AI. Nevertheless, conscientious attention from clinicians is required since it is consistently reported that AI treatment has a high CVD risk in well-designed cohort studies [8]. In particular, since the CVD outcome tends to increase with age, additional research on CVD risk in the long-term use of AI is still needed [41].
The second finding of our study was to compare the CVD prevalence between AI and tamoxifen. Although AI use had an increased risk of myocardial infarction and ischemic stroke compared with tamoxifen, there was no significant difference in heart failure or overall CVD events. Regarding the comparison of the CV risk of tamoxifen and AI, previous studies also showed inconsistent results. A population-based cohort study showed that AI slightly increased CV risk compared to tamoxifen, but it was not statistically significant as in our study [8]. On the other hand, other cohort studies reported AI to be associated more with increased risks of heart failure or cardiovascular mortality compared with tamoxifen [42]. The reasons for the inconsistency seem to be different in patient populations (ethnicity, cancer stage, prior comorbidity) or treatment durations for each study.
Meanwhile, venous thromboembolism had a higher risk in the tamoxifen-using group than in the AI-using group, which is very consistent with the previous results [43,44,45]. However, the prevalence of overall venous thromboembolism in the AI treatment group reached 2.95%, which is considerably high compared to 0.5% of the general population [46,47]. Therefore, the absolute risk of thromboembolism in the AI treatment group is never low, so this should not be overlooked in AI treatment. Heart failure was analyzed to be slightly (although not significant) higher in the AI group than tamoxifen, but it is difficult to clearly understand the mechanism by which AI treatment raises the risk of heart failure. So far, coronary artery disease is known to be the most common cause of heart failure [48]. Our results also showed a similar odds ratio between myocardial infarction and heart failure, which may provide a clue to the mechanism. Another hypothesis is that the deterioration of the lipid profile after AI treatment may exacerbate metabolic syndrome and increase the risk of heart failure as well. In fact, the risk of heart failure was 2.33 times higher in patients with metabolic syndrome consisting of high triglyceride and low HDL-cholesterol [49,50].
The third finding of our study is the change in the lipid profile after AI use. There are many related studies on how the lipid profile changes after AI use in breast cancer patients, but the results are different for each study. These differences are due to how long AI was used, in which the AI drug was used, breast cancer stage, or regularity of lipid profile data during follow-up. For example, regarding the duration of drug use, an increase in the total cholesterol and LDL-C was reported after 3 months of AI use, but there was no difference in the lipid level compared to the placebo after 36 months of use of AI [51]. In addition, among AI drugs, anastrozole and letrozole have reported lipid deterioration, but exemestane has a low rate of lipid deterioration [33,52,53]. Regarding the breast cancer stage, letrozole increased lipid levels in advanced breast cancer, but there was no significant change in the lipid before and after AI drug use in metastatic breast cancer [54,55]. Another confounding factor may be the method of collecting and recording lipid data. In the BIG 1-98 study, lipid data were collected regularly every 6 months, whereas, in the ATAC trial, data were collected irregularly on-demand [56,57].
In our study, the lipid profile after AI generally deteriorated compared to the baseline or the tamoxifen group. We thought that this change in the lipid profile might be related to the increase in CVD after AI treatment. The deterioration of the lipid profile can be explained by a phenomenon of the anti-estrogenic effect of AI. Whereas tamoxifen has an estrogenic agonistic effect on the lipid profile and CVD, AI has an anti-estrogenic effect on the lipid profile and CVD [58,59,60,61,62,63,64]. However, since there are also reports that the lipid profile deterioration of AI is comparable to tamoxifen and not significant compared to the control, well-designed follow-up studies are needed in the future. In our study, the lipid profile-related terms were not systematically searched as an outcome. Instead, additional information on the lipid profile was collected from articles that searched for cardiovascular outcomes. Therefore, the results of our meta-analysis alone are insufficient to draw accurate conclusions about the effect of AI on the lipid profile.
The strength of our study is that we analyzed well-designed studies reporting CV outcomes or lipid profile changes as primary endpoints. In particular, our meta-analysis first provided information on the lipid profile after AI. On the other hand, the limitations of our study are as follows. First, the AI treatment duration and breast cancer stage were different for each included study. Second, the majority of patients are postmenopausal women, and we cannot provide adequate information on the adverse effects of AI treatment in premenopausal women. Third, there is limited information on patients’ previous comorbidities or CV risk factors. Lastly, we could not perform in-depth investigations stratified by patients with and without lipid-lowering drugs. In these enrolled studies, detailed information on whether a statin was taken or not was disclosed to perform stratification, so integrated analysis could not be performed. This topic requires further research in the future.
5. Conclusions
In conclusion, various CVDs can occur when using AI, and in particular, the risk of MI and ischemic stroke increases compared with tamoxifen. On the other hand, the risk for venous thromboembolism is significantly lower in the AI group than in the tamoxifen group. The occurrence of CVD seems to be related to the deterioration of the lipid profile after AI. Therefore, customized individualization strategies considering each patient’s CV risk factors are needed when determining the duration of AI treatment. In addition, clinicians should pay attention to lipid management and educate patients on appropriate lifestyle modification when using AI.
Acknowledgments
We would like to thank Jae-Young Kim in Research Factory Inc. (www.rfactory.kr, accessed on 12 December 2022) for consulting the statistical analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol30020142/s1, Figure S1: Forest plots and funnel plots of Table 2; Figure S2: Forest plots and funnel plots of Table 3; Figure S3: Forest plots and funnel plots of Table 4.
Author Contributions
Conceptualization, Z.K. and B.-Y.K.; investigation, J.-J.Y. and E.-A.J.; data curation, J.-J.Y., Z.K. and B.-Y.K.; writing—original draft preparation, J.-J.Y.; writing—review and editing, J.-J.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was waived from the Soonchunhyang University Hospital due to the meta-analysis.
Informed Consent Statement
Informed consent was waived from the IRB due to the meta-analysis.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Soonchunhyang University Research Fund.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Huang J., Chan P.S., Lok V., Chen X., Ding H., Jin Y., Yuan J., Lao X.Q., Zheng Z.J., Wong M.C. Global incidence and mortality of breast cancer: A trend analysis. Aging. 2021;13:5748–5803. doi: 10.18632/aging.202502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lei S., Zheng R., Zhang S., Wang S., Chen R., Sun K., Zeng H., Zhou J., Wei W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021;41:1183–1194. doi: 10.1002/cac2.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 4.Hendrick R.E., Helvie M.A., Monticciolo D.L. Breast Cancer Mortality Rates Have Stopped Declining in U.S. Women Younger than 40 Years. Radiology. 2021;299:143–149. doi: 10.1148/radiol.2021203476. [DOI] [PubMed] [Google Scholar]
- 5.Mehta L.S., Watson K.E., Barac A., Beckie T.M., Bittner V., Cruz-Flores S., Dent S., Kondapalli L., Ky B., Okwuosa T., et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e30–e66. doi: 10.1161/CIR.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patnaik J.L., Byers T., DiGuiseppi C., Dabelea D., Denberg T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists’ Collaborative G. Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: A patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 2022;23:382–392. doi: 10.1016/S1470-2045(21)00758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khosrow-Khavar F., Filion K.B., Bouganim N., Suissa S., Azoulay L. Aromatase Inhibitors and the Risk of Cardiovascular Outcomes in Women With Breast Cancer: A Population-Based Cohort Study. Circulation. 2020;141:549–559. doi: 10.1161/CIRCULATIONAHA.119.044750. [DOI] [PubMed] [Google Scholar]
- 9.Khosrow-Khavar F., Bouganim N., Filion K.B., Suissa S., Azoulay L. Cardiotoxicity of Use of Sequential Aromatase Inhibitors in Women With Breast Cancer. Am. J. Epidemiol. 2020;189:1086–1095. doi: 10.1093/aje/kwaa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sund M., Garcia-Argibay M., Garmo H., Ahlgren J., Wennstig A.K., Fredriksson I., Lindman H., Valachis A. Aromatase inhibitors use and risk for cardiovascular disease in breast cancer patients: A population-based cohort study. Breast. 2021;59:157–164. doi: 10.1016/j.breast.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S.Y., Park J.E., Lee Y.J., Seo H.J., Sheen S.S., Hahn S., Jang B.H., Son H.J. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 2013;66:408–414. doi: 10.1016/j.jclinepi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Sawada S., Sato K., Kusuhara M., Ayaori M., Yonemura A., Tamaki K., Hiraide H., Mochizuki H., Ohsuzu F. Effect of anastrozole and tamoxifen on lipid metabolism in Japanese postmenopausal women with early breast cancer. Acta Oncol. 2005;44:134–141. doi: 10.1080/02841860510007585. [DOI] [PubMed] [Google Scholar]
- 14.Lonning P.E., Geisler J., Krag L.E., Erikstein B., Bremnes Y., Hagen A.I., Schlichting E., Lien E.A., Ofjord E.S., Paolini J., et al. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J. Clin. Oncol. 2005;23:5126–5137. doi: 10.1200/JCO.2005.07.097. [DOI] [PubMed] [Google Scholar]
- 15.Atalay G., Dirix L., Biganzoli L., Beex L., Nooij M., Cameron D., Lohrisch C., Cufer T., Lobelle J.P., Mattiaci M.R., et al. The effect of exemestane on serum lipid profile in postmenopausal women with metastatic breast cancer: A companion study to EORTC Trial 10951, ‘Randomized phase II study in first line hormonal treatment for metastatic breast cancer with exemestane or tamoxifen in postmenopausal patients’. Ann. Oncol. 2004;15:211–217. doi: 10.1093/annonc/mdh064. [DOI] [PubMed] [Google Scholar]
- 16.Wojtacki J., Kraszewski W., Leśniewski-Kmak K., Śliwińska M., Czyżewska K., Kruszewska E., Rachoń D. Short-term effects of anastrozole therapy on serum lipid profile in patients with breast cancer, previously treated with tamoxifen. Preliminary report. Nowotwory. J. Oncol. 2001;51:43. [Google Scholar]
- 17.Markopoulos C., Dafni U., Misitzis J., Zobolas V., Tzoracoleftherakis E., Koukouras D., Xepapadakis G., Papadiamantis J., Venizelos B., Antonopoulou Z., et al. Extended adjuvant hormonal therapy with exemestane has no detrimental effect on the lipid profile of postmenopausal breast cancer patients: Final results of the ATENA lipid substudy. Breast Cancer Res. 2009;11:R35. doi: 10.1186/bcr2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian W., Wu M., Deng Y. Comparison of Changes in the Lipid Profiles of Eastern Chinese Postmenopausal Women with Early-Stage Breast Cancer Treated with Different Aromatase Inhibitors: A Retrospective Study. Clin. Pharmacol. Drug Dev. 2018;7:837–843. doi: 10.1002/cpdd.420. [DOI] [PubMed] [Google Scholar]
- 19.Santa-Maria C.A., Blackford A., Nguyen A.T., Skaar T.C., Philips S., Oesterreich S., Rae J.M., Desta Z., Robarge J., Henry N.L., et al. Association of Variants in Candidate Genes with Lipid Profiles in Women with Early Breast Cancer on Adjuvant Aromatase Inhibitor Therapy. Clin. Cancer Res. 2016;22:1395–1402. doi: 10.1158/1078-0432.CCR-15-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdel-Qadir H., Amir E., Fischer H.D., Fu L., Austin P.C., Harvey P.J., Rochon P.A., Lee D.S., Anderson G.M. The risk of myocardial infarction with aromatase inhibitors relative to tamoxifen in post-menopausal women with early stage breast cancer. Eur. J. Cancer. 2016;68:11–21. doi: 10.1016/j.ejca.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Pineda-Moncusi M., Garcia-Giralt N., Diez-Perez A., Tusquets I., Servitja S., Albanell J., Prieto-Alhambra D., Nogues X. Thromboembolic, cardiovascular and overall mortality risks of aromatase inhibitors, compared with tamoxifen treatment: An outpatient-register-based retrospective cohort study. Ther. Adv. Med. Oncol. 2020;12:1758835920909660. doi: 10.1177/1758835920909660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X., Chlebowski R.T., Shi J., Barac A., Haque R. Aromatase inhibitor and tamoxifen use and the risk of venous thromboembolism in breast cancer survivors. Breast Cancer Res. Treat. 2019;174:785–794. doi: 10.1007/s10549-018-05086-8. [DOI] [PubMed] [Google Scholar]
- 23.Markopoulos C., Polychronis A., Zobolas V., Xepapadakis G., Papadiamantis J., Koukouras D., Lappas H., Gogas H. The effect of exemestane on the lipidemic profile of postmenopausal early breast cancer patients: Preliminary results of the TEAM Greek sub-study. Breast Cancer Res. Treat. 2005;93:61–66. doi: 10.1007/s10549-005-3783-0. [DOI] [PubMed] [Google Scholar]
- 24.Matthews A.A., Peacock Hinton S., Stanway S., Lyon A.R., Smeeth L., Lund J.L., Bhaskaran K. Endocrine therapy use and cardiovascular risk in postmenopausal breast cancer survivors. Heart. 2021;107:1327–1335. doi: 10.1136/heartjnl-2020-317510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabaglio M., Sun Z., Maibach R., Giobbie-Hurder A., Ejlertsen B., Harvey V.J., Neven P., Lang I., Bonnefoi H., Wardley A., et al. Cumulative incidence of cardiovascular events under tamoxifen and letrozole alone and in sequence: A report from the BIG 1-98 trial. Breast Cancer Res. Treat. 2021;185:697–707. doi: 10.1007/s10549-020-05981-z. [DOI] [PubMed] [Google Scholar]
- 26.Seruga B., Zadnik V., Kuhar C.G., Marinko T., Cufer T., Zakotnik B., Zorman D., Ocana A., Amir E. Association of aromatase inhibitors with coronary heart disease in women with early breast cancer. Cancer Investig. 2014;32:99–104. doi: 10.3109/07357907.2014.880452. [DOI] [PubMed] [Google Scholar]
- 27.Kamaraju S., Shi Y., Smith E., Nattinger A.B., Laud P., Neuner J. Are aromatase inhibitors associated with higher myocardial infarction risk in breast cancer patients? A Medicare population-based study. Clin. Cardiol. 2019;42:93–100. doi: 10.1002/clc.23114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi S.H., Kim K.E., Park Y., Ju Y.W., Jung J.G., Lee E.S., Lee H.B., Han W., Noh D.Y., Yoon H.J., et al. Effects of tamoxifen and aromatase inhibitors on the risk of acute coronary syndrome in elderly breast cancer patients: An analysis of nationwide data. Breast. 2020;54:25–30. doi: 10.1016/j.breast.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ligibel J.A., James O’Malley A., Fisher M., Daniel G.W., Winer E.P., Keating N.L. Risk of myocardial infarction, stroke, and fracture in a cohort of community-based breast cancer patients. Breast Cancer Res. Treat. 2012;131:589–597. doi: 10.1007/s10549-011-1754-1. [DOI] [PubMed] [Google Scholar]
- 30.Faiz A.S., Guo S., Kaveney A., Philipp C.S. Risk of venous thromboembolism and endocrine therapy in older women with breast cancer in the United States. Blood Coagul. Fibrinolysis. 2021;32:373–381. doi: 10.1097/MBC.0000000000001043. [DOI] [PubMed] [Google Scholar]
- 31.Franchi M., Tritto R., Tarantini L., Navazio A., Corrao G. Adjuvant Hormonotherapy and Cardiovascular Risk in Post-Menopausal Women with Breast Cancer: A Large Population-Based Cohort Study. Cancers. 2021;13:2254. doi: 10.3390/cancers13092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang W.T., Chen P.W., Lin H.W., Kuo Y.H., Lin S.H., Li Y.H. Risks of Aromatase Inhibitor-Related Cardiotoxicity in Patients with Breast Cancer in Asia. Cancers. 2022;14:508. doi: 10.3390/cancers14030508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breast International Group 1-98 Collaborative G., Thurlimann B., Keshaviah A., Coates A.S., Mouridsen H., Mauriac L., Forbes J.F., Paridaens R., Castiglione-Gertsch M., Gelber R.D., et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N. Engl. J. Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 34.Haque R., Shi J., Schottinger J.E., Chung J., Avila C., Amundsen B., Xu X., Barac A., Chlebowski R.T. Cardiovascular Disease after Aromatase Inhibitor Use. JAMA Oncol. 2016;2:1590–1597. doi: 10.1001/jamaoncol.2016.0429. [DOI] [PubMed] [Google Scholar]
- 35.Khosrow-Khavar F., Filion K.B., Al-Qurashi S., Torabi N., Bouganim N., Suissa S., Azoulay L. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: A systematic review and meta-analysis of randomized controlled trials. Ann. Oncol. 2017;28:487–496. doi: 10.1093/annonc/mdw673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Q., Xu Y., Yu E., Zheng Z. Risk of cardiovascular disease in breast cancer patients receiving aromatase inhibitors vs. tamoxifen: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2022;47:575–587. doi: 10.1111/jcpt.13598. [DOI] [PubMed] [Google Scholar]
- 37.He Y., Zhang J., Shen G., Liu L., Zhao Q., Lu X., Yang H., Hong D. Aromatase inhibitors and risk of cardiovascular events in breast cancer patients: A systematic review and meta-analysis. BMC Pharmacol. Toxicol. 2019;20:62. doi: 10.1186/s40360-019-0339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hemingway H., Langenberg C., Damant J., Frost C., Pyorala K., Barrett-Connor E. Prevalence of angina in women versus men: A systematic review and meta-analysis of international variations across 31 countries. Circulation. 2008;117:1526–1536. doi: 10.1161/CIRCULATIONAHA.107.720953. [DOI] [PubMed] [Google Scholar]
- 39.Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., Boehme A.K., Buxton A.E., Carson A.P., Commodore-Mensah Y., et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 40.Mehta P.A., Cowie M.R. Gender and heart failure: A population perspective. Heart. 2006;92((Suppl. 3)):iii14–iii18. doi: 10.1136/hrt.2005.070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.North B.J., Sinclair D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuppone F., Bria E., Verma S., Pritchard K.I., Gandhi S., Carlini P., Milella M., Nisticò C., Terzoli E., Cognetti F., et al. Do adjuvant aromatase inhibitors increase the cardiovascular risk in postmenopausal women with early breast cancer? Meta-analysis of randomized trials. Cancer. 2008;112:260–267. doi: 10.1002/cncr.23171. [DOI] [PubMed] [Google Scholar]
- 43.Meier C.R., Jick H. Tamoxifen and risk of idiopathic venous thromboembolism. Br. J. Clin. Pharmacol. 1998;45:608–612. doi: 10.1046/j.1365-2125.1998.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Decensi A., Maisonneuve P., Rotmensz N., Bettega D., Costa A., Sacchini V., Salvioni A., Travaglini R., Oliviero P., D’Aiuto G., et al. Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial. Circulation. 2005;111:650–656. doi: 10.1161/01.CIR.0000154545.84124.AC. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez R.K., Sorensen H.T., Pedersen L., Jacobsen J., Lash T.L. Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: A Danish population-based cohort study. Cancer. 2009;115:4442–4449. doi: 10.1002/cncr.24508. [DOI] [PubMed] [Google Scholar]
- 46.Heit J.A. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12:464–474. doi: 10.1038/nrcardio.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White R.H. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 48.Severino P., D’Amato A., Pucci M., Infusino F., Birtolo L.I., Mariani M.V., Lavalle C., Maestrini V., Mancone M., Fedele F. Ischemic Heart Disease and Heart Failure: Role of Coronary Ion Channels. Int. J. Mol. Sci. 2020;21:3167. doi: 10.3390/ijms21093167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mottillo S., Filion K.B., Genest J., Joseph L., Pilote L., Poirier P., Rinfret S., Schiffrin E.L., Eisenberg M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 50.Perrone-Filardi P., Paolillo S., Costanzo P., Savarese G., Trimarco B., Bonow R.O. The role of metabolic syndrome in heart failure. Eur. Heart J. 2015;36:2630–2634. doi: 10.1093/eurheartj/ehv350. [DOI] [PubMed] [Google Scholar]
- 51.Monnier A. Effects of adjuvant aromatase inhibitor therapy on lipid profiles. Expert Rev. Anticancer Ther. 2006;6:1653–1662. doi: 10.1586/14737140.6.11.1653. [DOI] [PubMed] [Google Scholar]
- 52.Boccardo F., Rubagotti A., Puntoni M., Guglielmini P., Amoroso D., Fini A., Paladini G., Mesiti M., Romeo D., Rinaldini M., et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: Preliminary results of the Italian Tamoxifen Anastrozole Trial. J. Clin. Oncol. 2005;23:5138–5147. doi: 10.1200/JCO.2005.04.120. [DOI] [PubMed] [Google Scholar]
- 53.Coombes R.C., Paridaens R., Jassem J., Van de Velde C.J., Delozier T., Jones S.E., Hall E., Kilburn L.S., Snowdon C.F., Bliss J.M. First mature analysis of the Intergroup Exemestane Study. J. Clin. Oncol. 2006;24:LBA527. doi: 10.1200/jco.2006.24.18_suppl.lba527. [DOI] [Google Scholar]
- 54.Elisaf M.S., Bairaktari E.T., Nicolaides C., Kakaidi B., Tzallas C.S., Katsaraki A., Pavlidis N.A. Effect of letrozole on the lipid profile in postmenopausal women with breast cancer. Eur. J. Cancer. 2001;37:1510–1513. doi: 10.1016/S0959-8049(01)00155-1. [DOI] [PubMed] [Google Scholar]
- 55.Zidan J., Chetver L., Hussein O., Zucker M. Effect of letrozole on plasma lipids, triglycerides, and estradiol in postmenopausal women with metastatic breast cancer. Oncologist. 2010;15:1159–1163. doi: 10.1634/theoncologist.2009-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruhstaller T., Giobbie-Hurder A., Colleoni M., Jensen M.B., Ejlertsen B., de Azambuja E., Neven P., Lang I., Jakobsen E.H., Gladieff L., et al. Adjuvant Letrozole and Tamoxifen Alone or Sequentially for Postmenopausal Women with Hormone Receptor-Positive Breast Cancer: Long-Term Follow-Up of the BIG 1-98 Trial. J. Clin. Oncol. 2019;37:105–114. doi: 10.1200/JCO.18.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuzick J., Sestak I., Baum M., Buzdar A., Howell A., Dowsett M., Forbes J.F., investigators A.L. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–1141. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 58.Dziewulska-Bokiniec A., Wojtacki J., Skokowski J., Kortas B. The effect of tamoxifen treatment on serum cholesterol fractions in breast cancer women. Neoplasma. 1994;41:13–16. [PubMed] [Google Scholar]
- 59.Thangaraju M., Kumar K., Gandhirajan R., Sachdanandam P. Effect of tamoxifen on plasma lipids and lipoproteins in postmenopausal women with breast cancer. Cancer. 1994;73:659–663. doi: 10.1002/1097-0142(19940201)73:3<659::AID-CNCR2820730325>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 60.Love R.R., Wiebe D.A., Feyzi J.M., Newcomb P.A., Chappell R.J. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women after 5 years of treatment. J. Natl. Cancer Inst. 1994;86:1534–1539. doi: 10.1093/jnci/86.20.1534. [DOI] [PubMed] [Google Scholar]
- 61.Grey A.B., Stapleton J.P., Evans M.C., Reid I.R. The effect of the anti-estrogen tamoxifen on cardiovascular risk factors in normal postmenopausal women. J. Clin. Endocrinol. Metab. 1995;80:3191–3195. doi: 10.1210/jcem.80.11.7593425. [DOI] [PubMed] [Google Scholar]
- 62.Decensi A., Bonanni B., Guerrieri-Gonzaga A., Gandini S., Robertson C., Johansson H., Travaglini R., Sandri M.T., Tessadrelli A., Farante G., et al. Biologic activity of tamoxifen at low doses in healthy women. J. Natl. Cancer Inst. 1998;90:1461–1467. doi: 10.1093/jnci/90.19.1461. [DOI] [PubMed] [Google Scholar]
- 63.Vrbanec D., Reiner Z., Belev B., Plestina S. Changes in serum lipid and lipoprotein levels in postmenopausal patients with node-positive breast cancer treated with tamoxifen. Tumori. 1998;84:687–690. doi: 10.1177/030089169808400615. [DOI] [PubMed] [Google Scholar]
- 64.Cushman M., Costantino J.P., Tracy R.P., Song K., Buckley L., Roberts J.D., Krag D.N. Tamoxifen and cardiac risk factors in healthy women: Suggestion of an anti-inflammatory effect. Arterioscler. Thromb. Vasc. Biol. 2001;21:255–261. doi: 10.1161/01.ATV.21.2.255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.