Abstract
We have overexpressed and purified the Helicobacter pylori Fur protein and analyzed its interaction with the intergenic regions of divergent genes involved in iron uptake (frpB and ceuE) and oxygen radical detoxification (katA and tsaA). DNase I footprint analysis showed that Fur binds specifically to a high-affinity site overlapping the PfrpB promoter and to low-affinity sites located upstream from promoters within both the frpB-katA and ceuE-tsaA intergenic regions. Construction of an isogenic fur mutant indicated that Fur regulates transcription from the PfrpB promoter in response to iron. In contrast, no effect by either Fur or iron was observed for the other promoters.
In many bacteria iron-responsive gene expression is regulated by a transcriptional repressor called Fur (ferric uptake regulator) (9, 12). The DNA-binding activity of Fur is Fe dependent where Fe acts as a corepressor. Under iron-rich conditions Fur is complexed with Fe and binds target sequences called Fur boxes, located in the promoter regions of iron-regulated genes, thus preventing transcription. When iron is scarce, the Fur molecule loses the Fe corepressor and is released from DNA, allowing transcription to occur. Fur has been extensively characterized for Escherichia coli. Homologs of Fur have been identified in many gram-negative bacteria and more recently in gram-positive bacteria (7, 32).
The fur gene of Helicobacter pylori was first cloned by virtue of the Fur titration assay and was shown to be able to partially complement an E. coli Fur mutant in an iron-dependent way, indicating that Fe acts as a corepressor of the H. pylori Fur protein (4, 5). A modified Fur titration assay with an E. coli strain expressing H. pylori Fur only was used to identify Fur binding sites in the promoter regions of the fecA2 and ribBA genes of H. pylori (14). Furthermore, it has been shown that transcription of both of these genes is repressed by iron (14, 30). Recently, Fur has been shown to be necessary for the iron-dependent regulation of the pfr gene (6). The Fur protein has been implicated also in the regulation of genes involved in the detoxification of oxygen radicals (2, 10, 17, 18, 34).
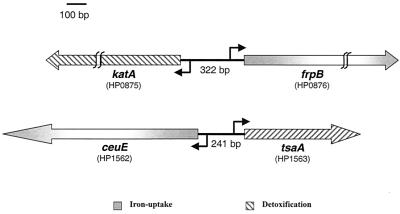
Analysis of the annotated genomes of H. pylori (1, 29) led us to the selection of two loci as candidate targets for Fur regulation. The structural organization of these loci is represented schematically in Fig. 1. Each locus is comprised of two genes that code for proteins expected to be involved in iron uptake (frpB and ceuE) and detoxification (katA and tsaA), that are oriented in different directions, and, therefore, that are expected to be transcribed from divergent promoters. The FrpB protein is a homolog of the Fe limitation-inducible outer membrane protein of Neisseria meningitidis (27.6% amino acid identity and 49.5% similarity) that belongs to the family of TonB-dependent receptors (25, 29). In Neisseria spp. FrpB, which is an iron-regulated, 76-kDa outer membrane protein, functions as an enterobactin receptor (8). The CeuE protein is a homolog of the iron(III) ABC transporter, periplasmic iron-binding protein (29). The other two genes, katA and tsaA, code for the catalase (24) and alkyl hydroperoxide reductase (AhpC) (21) proteins, respectively, which are involved in the detoxification of oxygen radicals in H. pylori.
FIG. 1.
Schematic representation of the two selected iron uptake and detoxification loci. Arrows indicate directions of transcription. Nomenclature is as described in the work of Tomb et al. (29).
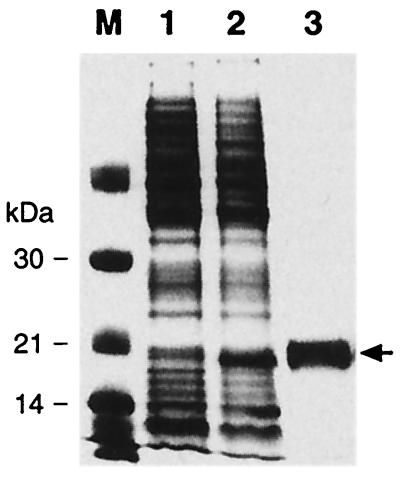
In order to investigate if Fur has a role in the regulation of these candidate loci, we purified a recombinant H. pylori Fur protein to use in DNase I footprinting experiments. The fur gene was amplified by PCR from H. pylori chromosomal DNA and cloned into the expression plasmid pET22b+, generating pETfur (Table 1), such that a tail encoding six histidines was added to the fur gene. E. coli BL21(DE3) was transformed with plasmid pETfur, and expression was induced by adding 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to exponentially growing cells. The recombinant protein was then purified under native conditions from the cell lysate by Ni-nitrilotriacetic acid affinity chromatography as described by the manufacturer (Qiagen). Figure 2 shows a sodium dodecyl sulfate (SDS)-polyacrylamide gel with cell lysates from the uninduced and induced E. coli expression culture and the purified Fur protein.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | supE44 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 16 |
| BL21 (DE3) | hsdS gal (λcIts857 ind-1 Sam7 nin-5 lacUV5-T7 gene 1) | 28 |
| H. pylori | ||
| G27 | Clinical isolate, wild type | 31 |
| G27fur::kan | G27 derivative in which 410 bp of the fur gene has been deleted and replaced by a kanamycin cassette, Kmr | This study |
| Plasmids | ||
| pGem3Z | Cloning vector, Ampr | Promega |
| pET22b+ | T7 promoter-based expression vector, Ampr | Novagen |
| pILL600 | Plasmid containing the kanamycin cassette from Campylobacter coli | 20 |
| pETfur | pET22b+ derivative containing a 452-bp NdeI-XhoI fragment obtained with oligonucleotides Fur-L1, gggaattccatATGAAAAGATTAGAAACTTTGGAA (NdeI) and Fur-R1, atccgctcgagACATTCACTCTCTTGGCATTC (BamHI), comprising the fur gene encoding the H. pylori Fur protein with a C-terminal 6-histidine tag; Ampr | This study |
| pGemfur::Km | Derivative of pGEM3z containing a 1,400-bp BamHI fragment with the kanamycin cassette from pILL600 flanked with a 735-bp EcoRI-BamHI fragment obtained with oligonucleotides HP1026L, aaaccggaatTCCTTACGGCCTCATAGCCTAAATG (EcoRI), and HP1026R, aaggccggatccTTCCAAAGTTTCTAATCTTTTCATGC (BamHI), comprising the 3′ end of the HP1026 gene, the HP1026 fur intergenic region, the first 24 bp of the fur gene, and a 524-bp BamHI-PstI fragment obtained with oligonucleotides HP1028L, aaacgcggatCCAAGAGAGTGAATGTTAAAAGATTTT (BamHI), and HP1028R, attggttctgcAGTCTTGTTATAGTTTTTTGTTGTT (PstI), comprising the last 15 bp of the fur gene and the 3′ end of the HP1028 gene; Ampr Kmr | This study |
| pGEMK-F | Derivative of pGEM3z containing a 447-bp EcoRI-PstI fragment obtained with primers 0875-L, cccgaattCCGTAATCACATTGTTGTCATCC (EcoRI), and 0875-R, gggttctgcAGTAGGGGGAAACATAAAGCG (PstI), comprising the intergenic region between katA and frpB and the 5′ end of each gene | This study |
| pGEMC-T | Derivative of pGEM3z containing a 449-bp EcoRI-PstI fragment obtained with primers Tsa-L, ggtgaaTTCTTGATTGGAAGCACTAGCCAC (EcoRI), and Tsa-R, ggaaactgCAGAAGAAAAGAATCGCACCGTTC (PstI), comprising the intergenic region between ceuE and tsaA and the 5′ end of each gene | This study |
In sequence, capital letters indicate H. pylori-derived sequences, lowercase letters indicate sequences added for cloning purposes, lowercase letters in bold indicate the site-directed mutations introduced by PCR, and underlined letters indicate restriction recognition sites. Restriction recognition sites appear in parentheses. Ampr, ampicillin resistance.
FIG. 2.
Expression and purification of a recombinant H. pylori Fur protein in E. coli and SDS-polyacrylamide gel electrophoresis analysis of protein samples. DNA manipulations were carried out routinely as described previously (26). Lanes 1 and 2 contain whole-cell extract of E. coli BL21(DE3) carrying the expression plasmid pETfur (Table 1) before and after 4 h of induction with 1 mM IPTG, respectively. Lane 3 contains the six-His-tagged Fur protein preparation after purification by affinity chromatography. Lane M contains protein size standards; molecular masses are indicated to the left. The arrow indicates the migration of the Fur protein. Fur was purified by Ni-nitrilotriacetic acid chromatography (Qiagen), dialyzed twice against 800 ml of buffer D (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1 mM MgCl2, 2 mM dithiothreitol) containing 10% glycerol, and dialyzed once against 200 ml of buffer D containing 50% glycerol. Protein concentration was determined by the Bradford method (Bio-Rad) as 1.8 mg/ml; the protein was aliquoted and stored at −80°C.
The H. pylori Fur protein binds to two loci involved in iron uptake and detoxification.
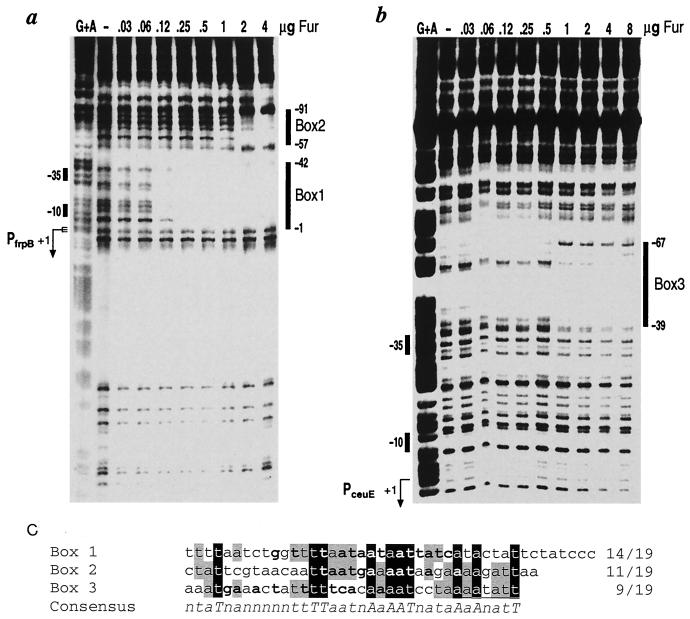
DNA fragments comprising the intergenic regions of the frpB-katA and ceuE-tsaA divergent gene loci were cloned (Table 1), 5′-end labeled at one extremity with [γ-32P]ATP (5,000 Ci/mmol; Amersham) and T4 polynucleotide kinase (New England Biolabs), and used as probes in DNase I footprinting experiments with the purified Fur protein. Protein-DNA complexes were formed in 50 μl of footprinting buffer (10 mM Tris-HCl [pH 8], 50 mM NaCl, 10 mM KCl, 1 mM dithiothreitol, 0.1% NP-40, 10% glycerol) containing approximately 10 ng (20,000 cpm) of the labeled probe and a 100-fold excess (1 μg) of sonicated salmon sperm DNA as the nonspecific competitor DNA for 15 min at room temperature. Following incubation for 1 min at room temperature with 0.01 U of DNase I and 5 mM CaCl2, the reaction was stopped by addition of 140 μl of stop buffer (192 mM Na-acetate, 32 mM Na2EDTA, 0.14% SDS, 64 μg of sonicated salmon sperm DNA per ml). Samples were phenol extracted, ethanol precipitated, resuspended in denaturing sample buffer, and fractionated on urea–6% acrylamide gels. Figure 3a and b show the results of DNase I footprinting with Fur on the frpB-katA and ceuE-tsaA probes, respectively. Addition of 0.12 μg of Fur protein resulted in one region of DNase I protection on the frpB-katA probe spanning positions −1 to −42 (box 1) with respect to the initiation of RNA transcription (see below) of the frpB gene (Fig. 3a). Another region with less affinity was evident only after addition of 1 μg of the Fur protein and spans positions −57 to −91 (box 2). On addition of 1 μg of Fur protein, the ceuE-tsaA probe exhibited one region of DNase I protection from positions −39 to −67 (box 3) upstream of the transcription initiation site of the ceuE gene (Fig. 3b). Alignment of the nucleotide sequences of the Fur-protected regions revealed 10 absolutely conserved nucleotides and 15 nucleotides conserved in two out of three of the sequences over a 35-nucleotide region (Fig. 3c). Analysis of the sequence of the box 1 binding site, which shows highest affinity for the protein, reveals the presence of overlapping sequences resembling that of the E. coli Fur box consensus (GATAATGATAATCATTATC) at 3-nucleotide intervals ranging from 14 matches to 12 matches out of 19 nucleotides. Figure 3c shows in bold the nucleotides matching the consensus of the first of the overlapping Fur boxes. Boxes 2 and 3 also contain sequences resembling the Fur box consensus, although to lesser extents, showing 11 and 9 out of 19 matches, respectively. Therefore, as reported for other bacteria (23, 32, 33), Fur from H. pylori binds to sequences sharing similarity to the E. coli Fur box.
FIG. 3.
DNase I footprints of Fur on the frpB-katA (a) and the ceuE-tsaA (b) probes. Probes derived from plasmids pGemK-F and pGemC-T (Table 1) were 5′-end labeled at their EcoRI sites and incubated with increasing microgram amounts of Fur as indicated above each lane. The vertical bars on the right of each panel indicate the areas of DNase I protection. Numbers indicate the distance from the initiation of transcription of the frpB gene (a) and the ceuE gene (b). The G+A lane is a G+A sequence reaction on the DNA probe used as a size marker (22). (c) Alignment of Fur binding sites. Absolutely conserved nucleotides have a black background, and nucleotides that are conserved in two out of three sequences have a gray background. Bold lettering indicates conservation with the consensus E. coli Fur box (GATAATGATAAGCATTATC), with the numbers to the left indicating numbers of conserved nucleotides over the total number of nucleotides. A consensus of the aligned sequences is shown below these sequences, with uppercase letters indicating absolutely conserved nucleotides and lowercase letters indicating nucleotides that are conserved in two out of three sequences. Quantification of the signals from extension products obtained was performed using a PhosphorImager and ImageQuant software (Molecular Dynamics).
These results showed that Fur is a DNA-binding protein that binds specifically to the two intergenic regions of the candidate loci. Fur binds, in vitro, to low-affinity binding sites in both intergenic regions and to a high-affinity site upstream of the frpB gene.
The divergent genes in the frpB-katA and ceuE-tsaA loci are transcribed by ς80-dependent promoters.
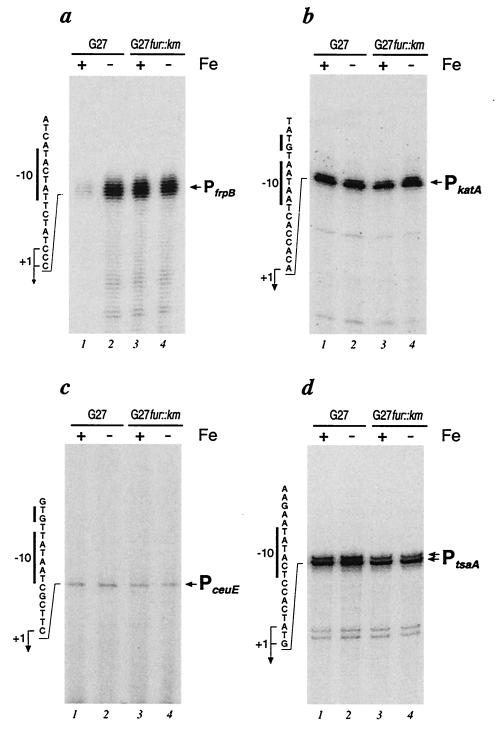
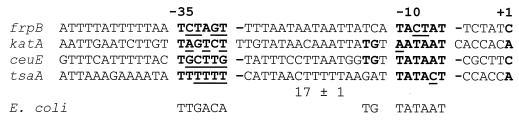
Total RNA was extracted from H. pylori strain G27, and the start point of RNA transcription was mapped by primer extension using specific primers (27). Autoradiographs of urea-acrylamide gels in Fig. 4 (lanes 1) show the results of the primer extension experiments. Extension of the RNA with katA-specific, ceuE-specific, and tsaA-specific primers gave rise to major bands placing the transcriptional start sites of the PkatA, PceuE, and PtsaA promoters at 55, 25, and 96 nucleotides upstream of the katA, ceuE, and tsaA translational start codons, respectively (Fig. 4b to d, lanes 1). Extension of RNA with an frpB-specific primer gave rise to a cluster of faint bands, which on longer exposure of the gel could be centered around position −78 with respect to the translational start codon of the frpB gene (Fig. 4a, lane 1). Nucleotide sequence analysis of the DNA regions upstream of the four transcriptional start sites revealed the presence of −10 hexamers with striking similarities (maximum of two mismatches) to the consensus sequence recognized by E(α2ββ′)ς70, the major RNA polymerase in E. coli (Fig. 5). By contrast, the −35 hexamers showed a high degree of sequence variability (minimum of three mismatches) compared to the E. coli consensus sequence and among themselves (Fig. 5). These results, taken together, suggest that the promoters transcribing the divergent genes in both loci are transcribed by RNA polymerase containing ς80, the H. pylori homolog of the vegetative sigma factor ς70 from E. coli. Previous work in our laboratory has shown that this sigma factor shares homology, promoter sequence recognition, and binding specificities with its E. coli counterpart (3).
FIG. 4.
Primer extension analysis of the promoters of the frpB-katA and ceuE-tsaA loci. Total RNAs isolated from H. pylori strains G27 (lanes 1 and 2) and G27fur::kan (lanes 3 and 4) were hybridized to the radiolabeled oligonucleotides Frp (CTCTTAAAAACATCCAAC) (a), Kat (CACATCTTTATTAACCAT) (b), Ceu (ACGATGAAACAAGAAGCG) (c), and Tsa (GGCAAGTTTTGTAACTAAC) (d) and elongated with reverse transcriptase (27). Elongated primers are indicated by arrows. To ensure correct mapping of the extension, we sequenced in parallel the respective cloned promoter region with the same primers used in the primer extension reactions (not shown). The nucleotide sequence of the sense strand upstream of the transcriptional initiations are shown to the left of the panels, with the −10 motifs indicated by a vertical bar and the nucleotides corresponding to +1 initiation sites indicated by bent arrows. Wild-type H. pylori G27 and the isogenic fur mutant G27fur:: kan were grown to logarithmic phase in liquid cultures and then harvested or further incubated for 15 min in the presence of 50 μM 2,2′-dipyridyl before being harvested (lanes 2 and 4, marked by −).
FIG. 5.
Nucleotide sequences of the promoters of the iron uptake and detoxification genes. An alignment of sequences with respect to their transcriptional start sites (+1) is shown. The −10 and −35 hexamers are indicated in bold. The consensus sequence for the promoter that recognized the E. coli ς70 sigma factor is shown below. Underlined nucleotides indicate substitutions from the consensus E. coli hexamers. It is worth noting that the PceuE and PkatA promoters show a TG motif at position −14 or −15 (also in bold) typical of so-called extended −10 promoters (19).
Mutation of the fur gene results in derepression of PfrpB.
To study the regulation of the PfrpB, PkatA, PceuE, and PtsaA promoters, we constructed an isogenic mutant strain with a mutated H. pylori fur gene. By homologous recombination with a suicide vector construct (pGemfur::km) (Table 1), most of the fur coding sequence of H. pylori strain G27 was replaced with a kanamycin resistance gene (27). Correct replacement of the wild-type sequence with the antibiotic resistance cassette was verified by means of PCR using oligonucleotides complementary to regions flanking the insertion site. Total RNA from the fur mutant strain (G27fur::kan) was isolated, and transcription from the promoters under study was investigated by primer extension assays (Fig. 4, lanes 3). Extension of the RNA at the iron uptake promoters gave different results. While the amount of RNA at the PfrpB promoter was increased over 10-fold in G27fur::kan compared to that in the wild-type strain (Fig. 4a, lane 3), suggesting that the PfrpB promoter is derepressed in the fur mutant, the amount of RNA at the PceuE promoter remained unchanged in the mutant strain (Fig. 4c, lane 3). Analysis at the detoxification promoters PkatA and PtsaA showed essentially the same amount of transcript resulting from both the wild-type and fur mutant RNA preparations (Fig. 4b and d, lanes 1 and 3). Therefore, inactivation of the fur gene causes derepression of the PfrpB promoter, indicating that the Fur protein of H. pylori negatively regulates PfrpB. Under the experimental conditions used, no effect of the mutation of fur was observed on transcription from the PceuE, PkatA, and PtsaA promoters, suggesting that these promoters are not Fur regulated.
Iron-dependent transcription of PfrpB is Fur mediated.
To study the transcriptional response of the Fur regulatory protein to various concentrations of iron, we isolated RNAs from liquid cultures of the wild-type and G27fur::kan strains that had been grown to logarithmic phase and subsequently incubated for a further 15 min in the presence or absence of 50 μM 2,2′-dipyridyl to deplete iron. Primer extension analysis of these RNAs showed that, in the wild-type strain, a 15-min treatment of cells with 2,2′-dipyridyl led to an increase (10-fold) in the amount of transcripts from the PfrpB promoter (Fig. 4a, compare lane 1 with lane 2); thus, depletion of iron from the growth medium resulted in induction of the promoter. The amount of transcript at the PfrpB promoter in the G27fur::kan mutant was unaffected by the depletion of iron with 2,2′-dipyridyl (Fig. 4a, lanes 3 and 4) and was comparable to the amount in the wild-type strain treated for 15 min with 2,2′-dipyridyl (lane 2). Primer extension analyses carried out on the PkatA, PceuE, and PtsaA promoters (Fig. 4b, c, and d, respectively) revealed no significant differences in the amounts of RNA in either the wild-type or the mutant strain under either of the growth conditions. Furthermore, a 15-min treatment with 100 μM Fe2SO4 to increase the iron concentration in wild-type or fur mutant cells had no effect on the amount of RNA transcribed from the PceuE, PkatA, and PtsaA promoters (data not shown).
These results showed that transcription from the PfrpB promoter is derepressed in the wild-type strain on depletion of iron from the growth medium, that treatment with 50 μM 2,2′-dipyridyl is sufficient to fully derepress the promoter to a level comparable to that in the fur mutant, and that this iron regulation is Fur mediated, as the response to iron is not present in the fur mutant. In addition, no iron regulation was observed for any of the PceuE, PkatA, and PtsaA promoters.
In conclusion, we have characterized the Fur protein of H. pylori to be a DNA-binding protein that binds to sequences resembling the E. coli consensus Fur box. We have characterized the promoter of an frpB gene predicted to be involved in iron uptake as iron regulated and demonstrated that Fur represses transcription from this promoter in an iron-dependent fashion by binding directly to the promoter sequences and, probably, denying access to RNA polymerase (11, 13). Furthermore, we found two low-affinity sites whose functions in vivo remain unclear. The biological function of these low-affinity binding sites may be in the regulation of genes in response to other environmental signals. Accordingly, Fur has been shown to respond to environmental signals other than iron concentration, such as in Salmonella enterica, where Fur was shown to mediate induction of acid tolerance genes in response to acid shock (15). In H. pylori, where regulators are few, it will be interesting to discover the full extent of the role of this protein.
Acknowledgments
We thank C. Mallia for editing the manuscript and G. Corsi for artwork.
This work has been supported partially by EU-TMR grant FMRX-CT980164, Chiron, and MURST. I.D. is the recipient of an EU-TMR fellowship (FMRX-CT980164), and A.B.F.P. was supported by a CAPES fellowship (Brazil).
REFERENCES
- 1.Alm R A, Ling L S, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jiang Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 2.Baillon M L, van Vliet A H, Ketley J M, Constantinidou C, Penn C W. An iron-regulated alkyl hydroperoxide reductase (AhpC) confers aerotolerance and oxidative stress resistance to the microaerophilic pathogen Campylobacter jejuni. J Bacteriol. 1999;181:4798–4804. doi: 10.1128/jb.181.16.4798-4804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beier D, Spohn G, Rappuoli R, Scarlato V. Functional analysis of the Helicobacter pylori principal sigma subunit of RNA polymerase reveals that the spacer region is important for efficient transcription. Mol Microbiol. 1998;30:121–134. doi: 10.1046/j.1365-2958.1998.01043.x. [DOI] [PubMed] [Google Scholar]
- 4.Bereswill S, Lichte F, Greiner S, Waider B, Fassbinder F, Kist M. The ferric uptake regulator (Fur) homologue of Helicobacter pylori: functional analysis of the coding gene and controlled production of the recombinant protein in Escherichia coli. Med Microbiol Immunol. 1999;188:3–40. doi: 10.1007/s004300050102. [DOI] [PubMed] [Google Scholar]
- 5.Bereswill S, Lichte F, Vey T, Fassbinder F, Kist M. Cloning and characterization of the fur gene from Helicobacter pylori. FEMS Microbiol Lett. 1998;159:193–200. doi: 10.1111/j.1574-6968.1998.tb12860.x. [DOI] [PubMed] [Google Scholar]
- 6.Bereswill S, Greiner S, van Vliet A H, Waidner B, Fassbinder F, Schiltz E, Kusters J G, Kist M. Regulation of ferritin-mediated cytoplasmic iron storage by the ferric uptake regulator homolog (Fur) of Helicobacter pylori. J Bacteriol. 2000;182:5948–5953. doi: 10.1128/jb.182.21.5948-5953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann J D. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol. 1998;29:189–198. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 8.Carson S D, Klebba P E, Newton S M, Sparling P F. Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J Bacteriol. 1999;181:2895–2901. doi: 10.1128/jb.181.9.2895-2901.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosa J H. Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol Mol Biol Rev. 1997;61:319–336. doi: 10.1128/mmbr.61.3.319-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubrac S, Touati D. Fur-positive regulation of iron superoxide dismutase in Escherichia coli: functional analysis of the sodB promoter. J Bacteriol. 2000;182:3802–3808. doi: 10.1128/jb.182.13.3802-3808.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escolar L, Perez-Martin J, de Lorenzo V. Coordinated repression in vitro of the divergent fepA-fes promoters of Escherichia coli by the iron uptake regulation (Fur) protein. J Bacteriol. 1998;180:2579–2582. doi: 10.1128/jb.180.9.2579-2582.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escolar L, Perez-Martin J, de Lorenzo V. Opening the iron box: transcriptional metalloregulation by the Fur protein. J Bacteriol. 1999;181:6223–6229. doi: 10.1128/jb.181.20.6223-6229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escolar L, de Lorenzo V, Perez-Martin J. Metalloregulation in vitro of the aerobactin promoter of Escherichia coli by the Fur (ferric uptake regulation) protein. Mol Microbiol. 1997;26:799–808. doi: 10.1046/j.1365-2958.1997.6211987.x. [DOI] [PubMed] [Google Scholar]
- 14.Fassbinder F, van Vliet A H, Gimmel V, Kusters J G, Kist M, Bereswill S. Identification of iron-regulated genes of Helicobacter pylori by a modified fur titration assay (FURTA-Hp) FEMS Microbiol Lett. 2000;184:225–229. doi: 10.1111/j.1574-6968.2000.tb09018.x. [DOI] [PubMed] [Google Scholar]
- 15.Hall H K, Foster J W. The role of fur in the acid tolerance response of Salmonella typhimurium is physiologically and genetically separable from its role in iron acquisition. J Bacteriol. 1996;178:5683–5691. doi: 10.1128/jb.178.19.5683-5691.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 17.Hassett D J, Howell M L, Ochsner U A, Vasil M L, Johnson Z, Dean G E. An operon containing fumC and sodA encoding fumarase C and manganese superoxide dismutase is controlled by the ferric uptake regulator in Pseudomonas aeruginosa: fur mutants produce elevated alginate levels. J Bacteriol. 1997;179:1452–1459. doi: 10.1128/jb.179.5.1452-1459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horsburgh M J, Ingham E, Foster S J. In Staphylococcus aureus, Fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J Bacteriol. 2001;183:468–475. doi: 10.1128/JB.183.2.468-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Malloch R A, Fujita N, Smillie D A, Ishihama A, Hayward R S. The minus 35-recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an “extended minus 10” promoter. J Mol Biol. 1993;232:406–418. doi: 10.1006/jmbi.1993.1400. [DOI] [PubMed] [Google Scholar]
- 20.Labigne-Roussel A, Courcoux P, Tompkins L. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J Bacteriol. 1988;170:1704–1708. doi: 10.1128/jb.170.4.1704-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundstrom A M, Bolin I. A 26 kDa protein of Helicobacter pylori shows alkyl hydroperoxide reductase (AhpC) activity and the mono-cistronic transcription of the gene is affected by pH. Microb Pathog. 2000;29:257–266. doi: 10.1006/mpat.2000.0388. [DOI] [PubMed] [Google Scholar]
- 22.Maxam A M, Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci USA. 1977;74:560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochsner U A, Vasil M L. Gene repression by the ferric uptake regulator in Pseudomonas aeruginosa: cycle selection of iron-regulated genes. Proc Natl Acad Sci USA. 1996;93:4409–4414. doi: 10.1073/pnas.93.9.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odenbreit S, Wieland B, Haas R. Cloning and genetic characterization of Helicobacter pylori catalase and construction of a catalase-deficient mutant strain. J Bacteriol. 1996;178:6960–6967. doi: 10.1128/jb.178.23.6960-6967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pettersson A, Maas A, van Wassenaar D, van der Ley P, Tommassen J. Molecular characterization of FrpB, the 70-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect Immun. 1995;63:4181–4184. doi: 10.1128/iai.63.10.4181-4184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Spohn G, Beier D, Rappuoli R, Scarlato V. Transcriptional analysis of the divergent cagAB genes encoded by the pathogenicity island of Helicobacter pylori. Mol Microbiol. 1997;26:361–372. doi: 10.1046/j.1365-2958.1997.5831949.x. [DOI] [PubMed] [Google Scholar]
- 28.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 29.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 30.Worst D J, Otto B R, de Graaff J. Iron-repressible outer membrane proteins of Helicobacter pylori involved in heme uptake. Infect Immun. 1995;63:4161–4165. doi: 10.1128/iai.63.10.4161-4165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang Z, Censini S, Bayeli P F, Telford J L, Figura N, Rappuoli R, Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995;63:94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong A, Singh V K, Cabrera G, Jayaswal R K. Molecular characterization of the ferric-uptake regulator, fur, from Staphylococcus aureus. Microbiology. 2000;146:659–668. doi: 10.1099/00221287-146-3-659. [DOI] [PubMed] [Google Scholar]
- 33.Zheleznova E E, Crosa J H, Brennan R G. Characterization of the DNA- and metal-binding properties of Vibrio anguillarum fur reveals conservation of a structural Zn2+ ion. J Bacteriol. 2000;182:6264–6267. doi: 10.1128/jb.182.21.6264-6267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou P, Borovok I, Ortiz de Orue Lucana D, Muller D, Schrempf H. The mycelium-associated Streptomyces reticuli catalase-peroxidase, its gene and regulation by FurS. Microbiology. 1999;145:549–559. doi: 10.1099/13500872-145-3-549. [DOI] [PubMed] [Google Scholar]