Abstract
Antibodies raised against NdhH and NdhB detected these proteins in the thylakoid membrane of Synechocystis sp. strain PCC 6803, but not in a purified cytoplasmic membrane. We conclude that NAD(P)H dehydrogenase is largely, if not exclusively, confined to the thylakoid membrane.
Identification and localization of proteins are essential to elucidate their functions. Cyanobacteria possess two types of functionally distinct NAD(P)H dehydrogenases (NDH-1) (7). One type of NDH-1 plays a major role in photosystem 1 cyclic electron flow, and the other type is essential for active CO2 uptake. These NDH-1 complexes contain different types of NdhD and NdhF, but other subunits, including NdhB, NdhH, NdhJ, and NdhK, are present in both types of NDH-1 complexes. It has been considered that NDH-1 is present in the thylakoid membrane as well as in the cytoplasmic membrane, based on the result that the antibodies raised against NdhJ and NdhK detected these proteins in both types of membranes of Synechocystis sp. strain PCC 6803 (1, 9). However, there have been arguments about the purity of the membrane preparations used in these studies, and ambiguity about the location of NDH-1 remains (5, 6). Norling et al. have recently developed a new method of isolating highly purified cytoplasmic membrane and thylakoid membrane from Synechocystis sp. strain PCC 6803 by using aqueous polymer two-phase partitioning in combination with sucrose density gradient centrifugation (5). We attempted in this study to determine the location of NDH-1 by using this new method of isolating the two types of membranes.
Cells were grown at 30°C in BG11 medium (10) buffered with 20 mM N-Tris(hydroxy-methyl)methyl-2-aminoethane-sulfonic acid (TES)-KOH (pH 8.0) under air. The antibodies for NrtA (a subunit of an ABC-type nitrate transporter) and CP43 (a chlorophyll-binding protein) were kindly provided by T. Omata (Nagoya University) and M. Ikeuchi (Tokyo University), respectively. The antibody for NdhB was raised as described previously (8). A fragment of ndhH (651 bp, nucleotides 1510407 to 1511058 on Cyanobase; http://www.kazusa.or.jp/cyano/) was synthesized by PCR with primers containing NdeI and BamHI sites at their proximal ends, and the product was ligated to the expression vector (pET-a) after digestion with the two endonucleases. NdhH (partial, 217 amino acids) formed inclusion bodies in Escherichia coli, which were isolated, solubilized with 5% sodium dodecyl sulfate (SDS), and electrophoresed by SDS-polyacrylamide gel electrophoresis (PAGE). A prominent band at 23 kDa was cut out from the gels and was mashed with a pestle and mortar to be injected into rabbits. The antibody thus prepared specifically bound to NdhH and hardly cross-reacted with other proteins. Highly purified preparations of the cytoplasmic and thylakoid membranes were prepared from Synechocystis sp. strain PCC 6803 cells as described by Norling et al. (5). SDS-PAGE was performed with the system of Laemmli (4). Polypeptides were electrotransferred to nitrocellulose membrane and were detected with the antibodies. Goat anti-rabbit immunoglobulin G conjugated to peroxidase was used as the second antibody and was detected with an Amersham ECL enhanced chemiluminescence kit.
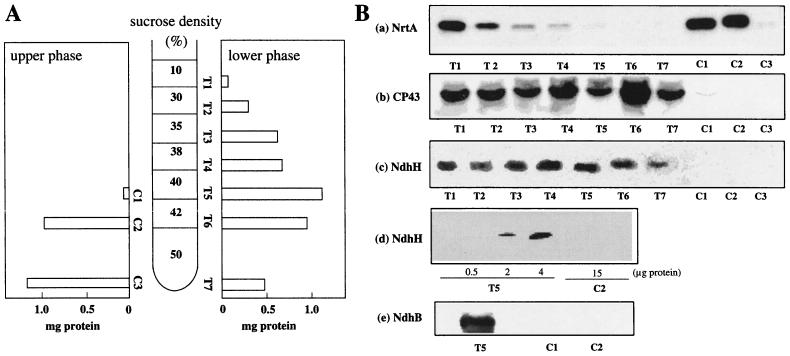
Figure 1A depicts the amounts of proteins in various fractions of thylakoid and cytoplasmic membranes after sucrose density gradient centrifugation of the upper and lower phases obtained by aqueous polymer two-phase partitioning. The separation of the thylakoid membrane (T1 to T7 in the right panel) was similar to that reported by Norling et al. (5). Sucrose density gradient centrifugation of the upper phase, however, gave only two cytoplasmic membrane bands (C1 and C2) with a precipitate (C3) (the left panel in Fig. 1A). We did not obtain light fractions, in contrast to their report (5). Perhaps the different growth conditions affect the density of the cytoplasmic membrane. There appear to be some differences between the two laboratories in the composition of growth medium and the way to supply CO2 to the cell suspension. We bubbled the cell suspension with air, whereas they shook the culture bottles.
FIG. 1.
(A) Schematic presentation of the distribution of membrane proteins after sucrose density gradient centrifugation of the lower- and upper-phase fractions obtained by aqueous polymer two-phase partitioning of Synechocystis sp. strain PCC 6803 membranes. The two-phase partitioning was carried out by the procedure reported by Norling et al. (5). (B) Immunodetection of NrtA (a), CP43 (b), NdhH (c and d), and NdhB (e) in various fractions obtained after sucrose gradient centrifugation. Each lane in panels a, b, c, and d was loaded with membranes containing 15 μg of protein. Various amounts of the thylakoid membrane (T5) and the cytoplasmic membrane (C2) were loaded in the lanes in panel e.
The cross-contamination of the two types of membranes in various fractions shown in Fig. 1A was tested by using the antibodies raised against NrtA and CP43, the marker proteins of the cytoplasmic membrane and thylakoid membrane, respectively (5). Panel a in Fig. 1B shows immunoblots of the membrane fractions with the antibody against NrtA. As expected, the antibody bound strongly to NrtA at 43 kDa in the cytoplasmic membrane fractions (C1 and C2) and produced a band decreasing in intensity from strong in fraction T1 to weak in fraction T5, whereas this band was not visible in fractions T6 and T7. Thus, the fractions T4 to T7 contain highly purified thylakoid membrane with little contamination of the cytoplasmic membrane. The C3 precipitate did not contain protein detected by the antibody and appears to be the cell wall. The antibody against CP43 bound to this protein in all of the thylakoid membrane fractions (T1 to T7 in panel b, Fig. 1B), but the band was not visible in the C2 fraction and was barely detectable in the C1 fraction. Thus, the C1 and C2 fractions contain highly purified cytoplasmic membrane. The antibodies raised against NdhH and NdhB recognized the proteins in the thylakoid membrane fractions (T1 to T7 for anti-NdhH and T5 for anti-NdhB), but not in the cytoplasmic membrane fractions (C1 and C2) (panels c and e in Fig. 1B). To test the detection limit of the antibody against NdhH, Western analysis was done with the gel loaded with various amounts of the C2 and T5 fractions. The immunoblot shown in panel d indicated that the antibody detected NdhH in the thylakoid membrane containing 2 μg of proteins, whereas no band was visible in the cytoplasmic membrane containing 15 μg of proteins. This result indicates that the amount of NdhH in fraction C2 was less than 10% of the amount in fraction T5, when normalized to the total amount of proteins.
The major fraction of the cytoplasmic membrane (C2) showed the same density as one of the major fractions of the thylakoid membrane (T6) (Fig. 1A). Thus, the two types of membranes of Synechocystis separated only by density gradient centrifugation are expected to have a significant cross-contamination, and the localization of proteins determined with such preparations would be meaningless. The present result obtained with highly purified membranes is consistent with the idea that NDH-1 is confined to the thylakoid membrane. The most important implication of this result is that we may be able to specify the site of a reaction or reactions involving NDH-1, such as electron transport, proton translocation, and active CO2 uptake. Schemes showing the role of NDH-1 in these reactions have been presented based on the assumption that the enzyme is present in both cytoplasmic and thylakoid membranes (2, 3). However, the present results suggested that the site of NDH-1-dependent reactions is the thylakoid membrane.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (B) (2)(12440228), a grant from the Research for the Future Program (JSPS-RFTF97R16001), and a grant from the Human Frontier Science Program (RG0051/1997 M).
REFERENCES
- 1.Berger S, Ellersiek U, Steinmüller K. Cyanobacteria contain a mitochondrial complex I-homologous NADH-dehydrogenase. FEBS Lett. 1991;286:129–132. doi: 10.1016/0014-5793(91)80957-5. [DOI] [PubMed] [Google Scholar]
- 2.Gant E. Supramolecular membrane organization. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer; 1994. pp. 119–138. [Google Scholar]
- 3.Kaplan A, Reinhold L. Annu. Rev Plant Physiol Plant Mol Biol. 1999;50:539–570. doi: 10.1146/annurev.arplant.50.1.539. [DOI] [PubMed] [Google Scholar]
- 4.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 5.Norling B, Zak E, Andersson B, Pakrasi H. 2D-isolation of pure plasma and thylakoid membranes from the cyanobacterium Synechocystis sp. PCC6803. FEBS Lett. 1998;436:189–192. doi: 10.1016/s0014-5793(98)01123-5. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa T. Identification and characterization of the ictA/ndhL gene product essential to inorganic carbon transport of Synechocystis PCC6803. Plant Physiol. 1992;99:1604–1608. doi: 10.1104/pp.99.4.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohkawa H, Pakrasi H B, Ogawa T. Two types of functionally distinct NAD(P)H dehydrogenases in Synechocystis sp. strain PCC 6803. J Biol Chem. 2000;182:31630–31634. doi: 10.1074/jbc.M003706200. [DOI] [PubMed] [Google Scholar]
- 8.Ohkawa H, Price G D, Badger M R, Ogawa T. Mutation of ndh genes leads to inhibition of CO2 uptake rather than HCO3− uptake in Synechocystis sp. strain PCC 6803. J Bacteriol. 2000;182:2591–2596. doi: 10.1128/jb.182.9.2591-2596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pieulle L, Guedeney G, Cassier-Chauvat C, Jeanjean R, Chauvat F, Peltier G. The gene encoding NdhH subunit of type 1 NAD(P)H dehydrogenase is essential to survival of Synechocystis PCC 6803. FEBS Lett. 2000;487:272–276. doi: 10.1016/s0014-5793(00)02369-3. [DOI] [PubMed] [Google Scholar]
- 10.Stanier R Y, Kunisawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]