Abstract
Obesity is a worldwide epidemic that is on the rise, with approximately 30% of the world population classified as either overweight or obese. The United States has some of the highest rates of obesity, and in most countries in the world, obesity now poses more of a serious health concern than malnutrition. Obesity is a chronic, relapsing disorder that is both preventable and treatable; however, traditional interventions that target eating less and exercising more have low success rates, especially in the long term. Therefore, identifying the neurobehaviors that predict obesity is important to help identify targets to decrease BMI and improve obesity outcomes. Using the Competing Neurobehavioral Decisions System (CNDS) Theory, we hypothesized that individuals with obesity compared to individuals without obesity would display neurobehaviors marked by a hyperactive impulsive system and a hypoactive executive system. To test this hypothesis, we collected data from a battery of self-reported measures and neurocognitive assessments through Amazon Mechanical Turk from n = 178 obese (BMI ≥ 30) and n = 198 non-obese controls who were weight stable for the past 3 months. We found that compared to the non-obese control group, individuals with obesity showed heightened delay discounting (a marker of CNDS imbalance), impaired motivation, poor self-image, decreased affective state, and impaired executive function. Using a Bayesian network approach, we established a neurobehavioral model that predicts obesity with 64.4% accuracy and indicates an imbalance between impulsive and executive neural systems. Results from our study suggest that interventions targeting neurobehaviors may be integral to help improve obesity outcomes.
Keywords: obesity, Competing Neurobehavioral Decisions Systems Theory, delay discounting, impulsivity, executive function
Obesity is defined as an abnormal or excessive fat accumulation, with the World Health Organization classifying individuals with obesity as those with a body mass index (BMI) of 30 or above. Obesity is a worldwide epidemic with rates increasing 300% since 1975. Currently, an estimated 39% of adults aged 18 years and over are classified as overweight (BMI 25–30), and 13% are classified as obese. In the United States, a staggering 42% of adults are obese. In most countries in the world, obesity now poses more of a serious health concern than malnutrition (Murray et al., 2012). Additionally, obesity is placing individuals at greater risk for the development of multiple fatal serious health conditions including hypertension, heart disease, stroke, Type 2 diabetes, cancer, and premature death, a phenomenon known as multimorbidity (Ezzati, 2017). Because of this, obesity has profound implications for the healthcare system, with this epidemic leading to an estimated annual medical cost of $147 billion (Carlson et al., 2015).
Obesity is a preventable disease that results from engagement in maladaptive health behaviors, namely overeating and lack of physical activity. That is, the outcome of obesity is driven by a series of maladaptive decision-making choices. Contrarily, achieving a healthy weight requires balanced decision-making abilities that are geared toward healthy eating and engagement in physical activity. Because we live in an environment that affords us the ability to easily obtain high caloric foods without being physically active, we need to establish tremendous self-control when making decisions about our eating and exercise behaviors. First, when presented with ample food choices, we need to inhibit the behavior of choosing high calorically dense foods. Second, when presented with the opportunity to remain sedentary, we need to make an effort to become physically active.
From the perspective of the field of health neuroscience, the brain “affects and is affected by” these health-related behaviors; that is, the body and brain are interdependent (Erickson et al., 2014). Stemming from this idea, previous studies have examined obesity from a cognitive neuroscience perspective using technologies such as functional magnetic resonance imaging, electroencephalography, and transcranial magnetic stimulation (Batterink et al., 2010; Camus et al., 2009; De Ridder et al., 2016; Ha et al., 2020; Imperatori et al., 2015; Kim et al., 2018; Makaronidis & Batterham, 2018; Martin et al., 2010; Nijs et al., 2010; Park et al., 2017; Schmidt et al., 2018; Stice et al., 2008, 2011; Uher et al., 2005; Wang et al., 2001). The expensive and lengthy procedures of these technical experiments prohibit the inclusion of large sample sizes that are needed for population-based studies. Therefore, others have taken the approach of neurobehavioral assessments to examine the multivariate associates and predictors of obesity. Here, the term neurobehavior refers to an expansive range of behaviors, including both state and trait personality measures as well as cognitive abilities, that are emergent from the brain. Systematic reviews of these neurobehavioral studies have identified several areas of cognition that have been examined in relation to obesity including executive function, time judgment, motor behaviors, attention, visuospatial abilities, language, memory, and food motivation (Emery & Levine, 2017; Vainik et al., 2013; Yang et al., 2018). Of those, executive function and food motivation displayed the most robust relationship to BMI and eating behaviors. Additionally, systematic reviews have identified several areas of personality that have been examined in relation to obesity including neuroticism, extraversion, openness/intellect, agreeableness, and conscientiousness (i.e., the Five-Factor Model of Personality; Vainik et al., 2013). Of those, the most significant predictor of obesity was impulsiveness, a subdomain of neuroticism.
Several brain regions and circuits emerge as being critically involved in obesity. First, cortical structures such as the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex regulate executive functions such as future planning and self-control, which are needed to drive healthy eating behaviors (Gluck et al., 2017; Lowe et al., 2019). Second, subcortical structures such as the nucleus accumbens and amygdala regulate our motivation, reward, and emotional reactivity, which drive us to act on our immediate needs or desires (e.g., eating dessert in the absence of hunger; Berthoud et al., 2017; Mietus-Snyder & Lustig, 2008). These higher and lower brain centers have reciprocal connections with one another such that altered top–down and bottom–up processing may be a likely neurobiological mechanism of obesity (Kaisari et al., 2019; Naets et al., 2018; Scarpina et al., 2016). These higher and lower circuits are then regulated by hormones and neuromodulators, such as ghrelin and leptin, that are released from hypothalamic centers and mediate signals of hunger and satiety (Timper & Brüning, 2017).
In this context, we examined obesity from the perspective of the Competing Neurobehavioral Decisions System (CNDS) Theory, which is inspired by the field of Neuroeconomics and posits that decisions are governed by the higher and lower systems described above (Bickel et al., 2018). The higher executive system is mediated by prefrontal and temporal brain regions (e.g., DLPFC and hippocampus) and governs long-term, future-oriented choices; whereas the lower impulsive system is mediated by limbic (e.g., midbrain, amygdala, habenular commissure, and dorsal striatum) and paralimbic (e.g., insula and ventral striatum) brain regions and governs reward-based choices. When the two systems are in homeostatic balance, individuals exhibit flexible decision-making processes that result in healthy choices. However, when the impulsive system is hyperactive and the executive system is hypoactive, a dysregulation in behavior occurs, resulting in maladaptive behaviors and pathological states such as addiction. Recent research has shown that obesity, which some conceptualize as a food addiction (though controversy exists with this theory and new data sheds doubt on this comparison) (Avena et al., 2012; Blumenthal & Gold, 2010; Fletcher & Kenny, 2018; Vainik et al., 2020; Volkow et al., 2017; Wieland, 2019), may be governed by a similar imbalance between the two systems (Bickel et al., 2014; Foxall, 2016; Volkow et al., 2017). Specifically, individuals with obesity show hyperactivity in the nucleus accumbens and hypoactivity in the prefrontal cortex (Lowe et al., 2019; Martin & Davidson, 2014; Rapuano et al., 2017).
Here, we used both neurocognitive assessments as well as self-reported questionnaires in a large, population study to assess the most significant predictive measures of obesity. Though systematic reviews have assessed this area of inquiry previously, the novelty and value of the present study are that all neurobehavioral measures were assessed in the same sample. Alongside these neurobehavioral measures, we simultaneously collected measures of eating and exercise behaviors, motivations, and attitudes, which is yet another novelty of the current data set as many previous studies have not taken this approach. To assess the CNDS balance, we primarily used the neuroeconomic task of delay discounting (DD), which measures the rate of decline in the present value of a monetary reward based on its delay to receipt (Bickel et al., 2018). Other measures of executive tone include executive function, affective state, and self-evaluation, all of which have been mapped to the executive system (e.g., prefrontal cortex; Beer et al., 2010; Funahashi & Andreau, 2013; Gray et al., 2002; Hare & Duman, 2020; Liu et al., 2017; Perlstein et al., 2002; Yang et al., 2016). Additional measures of impulsive tone include the motivations for eating and exercise behaviors, which have been mapped to the impulsive system (e.g., nucleus accumbens; Basso & Morrell, 2015; Castro et al., 2015; Clithero et al., 2011; Herrera et al., 2016; Ikemoto & Panksepp, 1999; Reynolds & Berridge, 2002; Salamone et al., 2016). As these neurobehaviors are multifaceted, we acknowledge that interactions between these brain structures as well as other brain structures are also involved in their regulation, and we discuss our findings in this context (Genon et al., 2018; Ito et al., 2020; Salzman & Fusi, 2010). We explored the hypothesis that compared to non-obese controls, individuals with obesity demonstrate neurobehaviors that are weighted toward impulsive over executive decisions. We then used Bayesian network modeling to determine the most prominent neurobehavioral factors that drive obesity. Our results indicate that neurobehaviors related to CNDS functioning (e.g., DD) may be important factors affecting obesity outcomes and possible targets for future weight-loss programs.
Method
Recruitment, Participants, and Data Collection
Data were collected through Amazon Mechanical Turk (mTurk). The study was available to mTurk workers in the United States with a 90% or greater Human Intelligence Task (HIT) approval rate, indicating they provided good quality data in at least 90% of previously completed HITs. Participants were screened to ensure they met the inclusion criteria. In order to complete the questionnaire, participants needed to be between the ages of 18 and 45, have English as their primary language, be weight-stable for at east 3 months, not be pregnant or experiencing menopause, and not be colorblind. After screening, a total of 727 records were collected. Participants were compensated for the completion of the questionnaire and received bonus compensation if the data passed quality checks. All methods were approved by the Virginia Tech Committee on Activities Involving Human Subjects and were performed in accordance with the relevant guidelines and regulations.
Using mTurk, self-report questionnaires and neurocognitive assessments were administered in a randomized order to assess health behaviors, DD, executive functioning, affective state, eating motivations and attitudes, exercise motivations and attitudes, and self-evaluation (i.e., body image). All self-reported questionnaires were adapted to be administered in an online format and presented through Qualtrics, whereas the neurocognitive assessment was administered through Inquisit Web.
Records that were incomplete (i.e., progress <100%) or provided an invalid BMI were excluded from the analysis. A BMI of less than 15 or greater than 57 was considered invalid. In the case of multiple submissions, one submission from each mTurk Worker ID was included in the analysis. Additionally, we used a food purchasing task to screen for nonsystematic data (Epstein et al., 2010; Stein et al., 2015; Sze et al., 2017). Data were deemed systematic if they met the criteria of (a) trend, meaning purchasing decreased as the price increased, (b) bounce, meaning few local increases in purchasing occurred with price increases, and (c) reversal from zero using an algorithm developed by Stein et al. (2015). Data that were deemed unsystematic were excluded from the analysis. After data cleaning, 376 records were included in the analysis. The sample for analysis consisted of 198 participants without obesity (BMI < 30) and 178 participants with obesity (BMI ≥ 30). Though BMI is a continuous variable, the classification of obesity via BMI is an important clinical construct (Gutin, 2018; Samuel et al., 2004); as such, we utilized this clinical categorization to investigate neurobehavioral differences between body weight groups.
Health Behaviors
Health behaviors included eating and drinking behaviors as well as engagement in physical activities. Eating behaviors were assessed using the Fat Intake Screener, and Fruit and Vegetable Intake Screener (Block et al., 2000). These food screeners are brief questionnaires asking about the frequency of intake of fatty foods, and fruits and vegetables that were validated against a longer 100-item food frequency questionnaire. The Fat Intake Screener consists of 17 items scored on a 5-point Likert scale and summed for a total score. The Fruit and Vegetable Intake Screener consists of seven items scored on a 6-point Likert scale and summed for a total score. Drinking behaviors were assessed using the Beverage Intake Questionnaire (BEVQ-15) to estimate habitual intake of beverages, which was validated through comparison with three 24-hr dietary recalls (Hedrick et al., 2012). The BEVQ-15 is a 15-item quantitative questionnaire providing an estimate of habitual beverage intake (grams and kcals).
Exercise behavior was assessed using the Global Physical Activity Questionnaire (GPAQ), a 16-item interview measure that assesses physical activity during work, travel, and recreation as well as sedentary time (World Health Organization [WHO], n.d.). Time spent in moderate and vigorous physical activities are assigned four and eight Metabolic Equivalents (METs), respectively, to estimate weekly energy expenditure (MET minutes). Data cleaning was done per the World Health Organization GPAQ analysis guidelines (WHO, n.d.); due to this additional data cleaning, the analysis of GPAQ had a reduced sample size (N = 373).
Behavioral Economics Measures to Assess Balance Between the Impulsive and Executive Systems
The five-trial adjusting-DD task, which measures both impulsivity and future valuation, was used as our primary measure of the balance between the impulsive and executive systems (Koffarnus & Bickel, 2014). For this task, participants were asked if they would prefer an immediate $500 reward or a $1,000 reward at different time delays, increasing or decreasing the time delay based on the previous response. The natural log-transformed rate of monetary discounting (lnk) is reported.
Probability discounting was used as a control measure for our DD task and therefore we hypothesized that we would not see a significant difference between body weight groups (Bickel et al., 2014). For this task, participants were asked to choose between a smaller reward or a larger but less probable reward.
Additional Measures to Assess Impulsive Tone
Eating Motivations and Attitudes
Eating motivations and attitudes were evaluated using three different scales; namely, the Power of Food Scale (PFS); the Three-Factor Eating Questionnaire (TFEQ-R18); and the Eating Attitudes Test (EAT). Hedonic hunger or appetite for palatable foods was assessed using the PFS, a questionnaire that assesses the psychological impact of living in a food-rich environment (Lowe et al., 2009). The PFS has been validated in a general population as well as in a population with obesity, and has been found to be reliable (Cronbach’s α = 0.81–0.91) (Cappelleri et al., 2009; Lowe et al., 2009). The PFS consists of 15 items scored on a 5-point Likert scale and summed for a total score as well as three subscale scores including food available, food present, and food tasted. Eating behaviors were assessed using the TFEQ-R18, which is a validated and reliable questionnaire to assess one’s cognitive and emotional relationship to eating (de Lauzon et al., 2004; Karlsson et al., 2000). The TFEQ-R18 was originally developed for use in a population with obesity and has been validated against a food frequency questionnaire in a general population (de Lauzon et al., 2004). The TFEQ-R18 consists of 18 items that are scored on a 4-point Likert scale and summed for three different factor scores including cognitive restraint, emotional eating, and uncontrolled eating. Disordered eating behaviors were assessed using the EAT-26, which is a valid and reliable screening questionnaire originally developed as a screening tool for eating disorders (Garner et al., 1982). The EAT has been previously used in a variety of populations including adults, adolescents, and different cultural populations (Garfinkel & Newman, 2001). The EAT consists of 26 items that are scored on a 6-point Likert scale and summed for a total score (EAT score), and three subscales including dieting, oral control, and bulimia food preoccupation.
Exercise Motivations and Attitudes
Exercise motivations and attitudes were evaluated using two scales, the Behavioral Regulation of Exercise Questionnaire (BREQ-3) and the Subjective Exercise Experiences Scale (SEES). Motivation to engage in the exercise was assessed using the BREQ-3, which is a valid and reliable questionnaire (Cid et al., 2018). The BREQ-3 consists of 24 items that are scored on a 5-point Likert scale and averaged for 6 subscale scores including amotivation, external regulation, introjected regulation, identified regulation, integrated regulation, and intrinsic regulation. The Relative Autonomy Index (RAI) is an index indicating the degree to which individuals are self-determined to exercise, and is calculated as a sum of weighted subscale scores (Ryan & Connell, 1989):
| (1) |
Psychological responses to exercise were assessed using the SEES, which is a valid and reliable (Cronbach’s α = 0.84–0.92) questionnaire (McAuley & Courneya, 1994). The SEES consists of 12 items that are scored on a 7-point Likert scale and summed for 3 different factor scores including positive well-being, psychological distress, and fatigue.
Additional Measures to Assess Executive Tone
Executive Function
Response inhibition and attention were assessed using the Stroop Color-Word task (Stroop, 1935). Participants were presented with stimuli that were color words or rectangles written in colored font (e.g., the word “red” written in green font), and instructed to indicate the color of the word rather than its meaning using a keyboard press. Stimuli appeared on the screen in a randomized order and were displayed until the participant responded or for 400 ms with no response, which was followed by a 200-ms interval before the next stimulus was displayed. The task included stimuli of four different colors, and congruent (color and meaning the same), incongruent (color and meaning different), and control (colored rectangles) trials. Each color-congruency combination was repeated 7 times for a total of 84 trials presented in one test block. Percent correct and reaction time are reported. Stimuli were presented and responses were recorded using the Inquisit software (https://www.millisecond.com/). Participants were required to install the Inquisit software on their device to complete this task.
Affective State
Affective state measures were included to assess depression, anxiety, and positive and negative affect. Depression was assessed using the Beck Depression Inventory (BDI), which is a valid and reliable (Cronbach’s α = 0.81–0.86) measure to assess symptoms of depression (Beck et al., 1988). The BDI consists of 21 items that are scored on a 4-point Likert scale and summed for a total score. Anxiety was assessed using the Beck Anxiety Inventory (BAI), which is a valid and reliable (Cronbach’s α = 0.94) questionnaire assessing symptoms of anxiety (Beck et al., 1988; Fydrich et al., 1992). The BAI consists of 21 items scored on a 4-point Likert scale and summed for a total score. Positive and negative affect were assessed using the Positive and Negative Affect Schedule (PANAS), which is a valid and reliable (Cronbach’s α = 0.85–0.89 in a nonclinical sample) questionnaire that assesses mood (Crawford & Henry, 2004; Watson et al., 1988). The PANAS includes 20 items that are scored on a 5-point Likert scale and summed for a positive affect score and a negative affect score.
Self-Evaluation
Self-evaluation or perceived body image was assessed using the Body Attitudes Test (BAT), which is a valid and reliable (Cronbach’s α = 0.93) questionnaire that assesses body experience (Probst et al., 1995). The BAT includes 20 items that are scored on a 6-point Likert scale and summed for a total score (BAT score) as well as four subscales including negative appreciation, lack of familiarity, body dissatisfaction, and a rest factor.
Power Analysis and Statistics
To determine the sample size to sufficiently power our study, an a priori power analysis was conducted using G * Power 3.1 (Faul et al., 2009). The power analysis was based on a t-test difference between two independent means with a medium effect size (d = 0.5). In order to account for multiple testing as a result of several neurobehavioral measures, we used an alpha of 1 E-4 and 80% power, with results indicating a sample size of n = 183 per group. We compared demographic measures between groups using a χ2 test of independence (household income, education, employment status, sex, race, and ethnicity) or an independent samples t-test (age). For χ2 tests, all expected cell frequencies were greater than five. If reported personal income was greater than household income, the value for household income was replaced with the value for personal income. Household income was then stratified into low- (<$40,000 per year), middle- ($40,000–$125,000 per year), and high- (>$125,000 per year) income (Semega et al., 2017).
Between-group as well as continuous analyses were performed on all variables of interest. All self-report and cognitive task scores were compared between body weight groups using independent samples t-tests. For variables with unequal variances between groups, as determined by Levene’s test for homogeneity of variance, Welch’s t-test was used. Additionally, the relationships among all self-report and cognitive task scores and BMI were examined using Pearson correlations. A supplementary correlation analysis was conducted in a subset of the data, excluding participants with a BMI in the underweight (BMI < 18.5) range. For comparisons that were considered part of a grouping (e.g., health behaviors; affective state), Bonferroni correction was applied and significance was determined based on the resulting alpha value. The Bonferroni-corrected alpha value is reported for each grouping in the results.
Finally, an exploratory analysis was performed using a Bayesian network approach to create a model that describes the relationship between BMI and five neurobehaviors (i.e., DD, affective state, eating attitudes, exercise attitudes, and self-evaluation). Bayesian network modeling was selected because this approach seeks to identify a directed acyclic graph that infers the conditional dependencies among the features. This directed acyclic graph consists of nodes representing variables of interest and paths between nodes representing probabilistic dependencies between them (Friedman et al., 1997; Pearl & Russell, 2003). Conditional probabilities represent the strength of the relationships between each cluster of nodes. Bayesian networks may be particularly useful in examining neurocognitive functions (Bielza & Larrãnaga, 2014). Here, we used Bayesian networks to examine the role of neurobehaviors in predicting obesity. Specifically, composite scores of five neurobehavioral measures were derived as follows:
affective state composite: mean of z-normalized BAI and z-normalized BDI;
eating attitudes and motivations composite: mean of z-normalized TFEQ Cognitive Restraint (reverse scored), z-normalized TFEQ Uncontrolled Eating, z-normalized TFEQ Emotional Eating, z-normalized PFS, and z-normalized EAT;
exercise attitudes and motivation: mean of z-normalized BREQ RAI (reverse scored), z-normalized SEES Positive Wellbeing (reverse scored), z-normalized SEES Fatigue, and z-normalized SEES Psychological Distress;
self-evaluation: BAT; and
DD: monetary discount rate (lnk).
Appropriate variables were reverse-scored, as indicated above, to ensure higher scores reflected more negative outcomes. For analysis, these five composite measures were binned into ordinal categories of equal width and used as precipitants for predicting obesity. We estimated the Bayesian network using the tree augmented naïve approach using 10-fold cross-validation (note that the Markov blanket nd Markov blanket with feature selection methods did not achieve as high of a correct classification rate). The optimal network in each iteration was determined by minimizing the posterior classification error, that is, the number of incorrectly classified cases. Edge strengths in the Bayesian network are determined as the change in Bayesian Information Criterion (BIC) after removal of edge from the network. Analyses were completed using SPSS Statistics 26.0, GraphPad Prism, and RVersion 4.0.2 (June 22, 2020) using the bnlearn package (Scutari, 2010).
Results
Participants completed the study in an average of 56.4 min (±1.6 min SEM); however, this time includes other self-reported measures not included in this study.
Participant Demographics
No significant differences were observed between body weight groups in age, t(374) = −1.443, p = .150, sex, χ2(1) = 0.100, p = .752, household income, χ2(2) = .582, p = .747, education, χ2(3) = 4.477, p = .214, employment status, χ2(2) = 0.671, p = .715, race, χ2(3) = 5.405, p = .144, or ethnicity, χ2(1) = 0.113, p = .737. Table 1 summarizes participant demographic information.
Table 1.
Demographics Comparison Between Participants Without Obesity (n = 178) and Participants With Obesity (n = 198)
| (A) Frequency Table |
(B) Contingency Table |
|||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Non-obese | Obese | X2 / t | P | Measure | Non-obese | Obese | Total n |
| Mean age | 30.89 (0.45) | 31.84 (0.48) | −1.443 | 0.150 | n | 198 | 178 | 376 |
| Household income | 0.582 | 0.747 | Household income | |||||
| % Low income | 25.8 | 27.5 | Low income | 51 (52.66) [0.05] | 49 (47.34) [0.06] | 100 | ||
| % Middle income | 66.2 | 66.3 | Middle income | 131 (131.12) [0.00] | 118 (117.88) [0.00] | 249 | ||
| % High income | 8.1 | 6.2 | High income | 16 (14.22) [0.22] | 11 (12.78) [0.25] | 27 | ||
| Education | 4.477 | 0.214 | Education | |||||
| % High school/GED or lower | 8.1 | 8.4 | High school/GED or lower | 16 (16.32) [0.01] | 15 (14.68) [0.01] | 31 | ||
| % Some college | 27.8 | 21.9 | Some college | 55 (49.50) [0.61] | 39 (44.50) [0.68] | 94 | ||
| % College degree | 53.5 | 52.2 | College degree | 106 (104.79) [0.01] | 93 (94.21) [0.02] | 199 | ||
| % Advanced degree | 10.6 | 17.4 | Advanced degree | 21 (27.38) [1.49] | 31 (24.62) [1.66] | 52 | ||
| Employment status | 0.671 | 0.715 | Employment status | |||||
| % Working full-time | 75.3 | 78.1 | Working full-time | 149 (151.66) [0.05] | 139 (136.34) [0.05] | 288 | ||
| % Working part-time | 14.6 | 11.8 | Working part-time | 29 (26.33) [0.27] | 21 (23.67) [0.30] | 50 | ||
| % Not working | 10.1 | 10.1 | Not working | 20 (20.01) [0.00] | 18 (17.99) [0.00] | 38 | ||
| Sex | 0.100 | 0.752 | Sex | |||||
| % Female | 44.4 | 46.1 | Female | 88 (89.52) [0.03] | 82 (80.48) [0.03] | 170 | ||
| % Male | 55.6 | 53.9 | Male | 110 (108.48) [0.02] | 96 (97.52) [0.02] | 206 | ||
| Race | 5.405 | 0.144 | Race | |||||
| % White/Caucasian | 73.7 | 69.1 | White/Caucasian | 146 (141.65) [0.13] | 123 (127.35) [0.15] | 169 | ||
| % Black/African American | 17.7 | 15.7 | Black/African American | 35 (33.18) [0.10] | 28 (29.82) [0.11] | 63 | ||
| % Asian | 6.1 | 7.9 | Asian | 12 (13.69) [0.21] | 14 (12.31) [0.23] | 26 | ||
| % Other | 2.5 | 7.3 | Other | 5 (9.48) [2.12] | 13 (8.52) [2.35] | 18 | ||
| Ethnicity | 0.113 | 0.737 | Ethnicity | |||||
| % Hispanic | 9.1 | 10.1 | Hispanic | 18 (18.96) [0.05] | 18 (17.04) [0.05] | 36 | ||
| % Non-Hispanic | 90.9 | 89.9 | Non-Hispanic | 180 (179.04) [0.01] | 160 (160.96) [0.01] | 340 | ||
Note. (A) Demographic information is reported as the percentage of individuals belonging to each group. (B) Demographic information reported as the observed cell totals, (the expected cell totals), and [the χ2 statistic for each cell] “other” race includes those who answered American Indian/Alaska Native, Pacific Islander, or other. “Not working” includes those who answered not working, laid off, or homemaker.
Health Behaviors
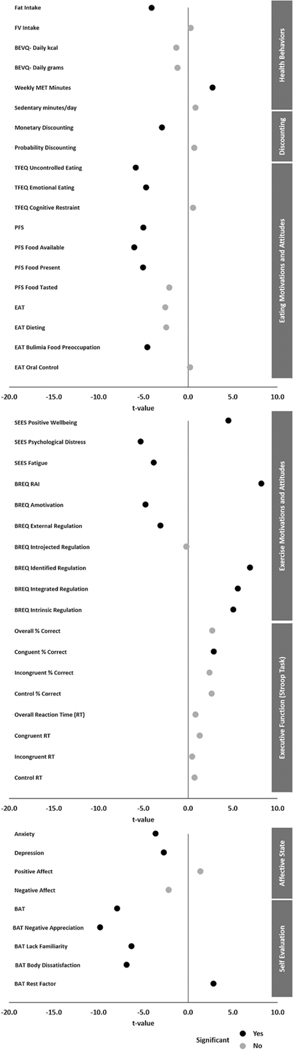
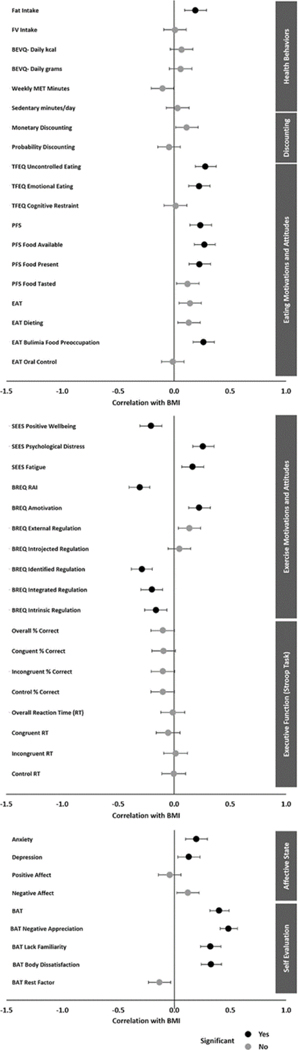
The Bonferroni-corrected critical alpha value for health behaviors measures is 0.0083. Individuals with obesity had a significantly higher fat intake, t(374) = −4.051, p < .001, N = 376, than individuals without obesity. No significant differences were found between groups for fruit and vegetable intake, t(374) = 0.290, p = .772, N = 376, or beverage intake in kcal, t(374) = −1.314, p = .190, N = 376. Individuals with obesity reported significantly lower levels of weekly physical activity, t(343.843) = 2.749, p = .006, N = 373, than individuals without obesity, while no significant difference was found between groups for daily sedentary time, t(371) = 0.820, p = .413, N = 373; Figure 1, Supplemental Table 1. Additionally, continuous BMI was found to be positively correlated with fat intake (r = .189, p < .001, N = 376). No significant correlations were found between BMI and fruit and vegetable intake, weekly physical activity, or daily sedentary time (Figure 2, Supplemental Table 8).
Figure 1.
Plot of t-Statistic for Differences Between Body Weight Groups on All Measures
Note. Bonferroni-corrected significance values: p < .0083 (health behaviors); p < .025 (discounting); p < .0045 (eating motivations and attitudes); p < .005 (exercise motivations and attitudes); p < .00625 (executive function); p < .0125 (affective state); p < .01 (self-evaluation).
Figure 2.
Plot of Correlations Between BMI and All Measures. Error Bars Represent 95% Confidence Intervals
Note. Bonferroni-corrected significance values: p < .0083 (health behaviors); p < .025 (discounting); p < .0045 (eating motivations and attitudes); p < .005 (exercise motivations and attitudes); p < .00625 (executive function); p < .0125 (affective state); p < .01 (self-evaluation).
Behavioral Economics Measures to Assess Balance Between the Impulsive and Executive Systems
Discounting Measures
The Bonferroni-corrected critical alpha value for discounting measures is 0.025. Individuals with obesity had a significantly higher rate of monetary discounting, t(374) = −2.909, p = .004, N = 376, than individuals without obesity on the five-trial adjusting DD task (Figure 1, Supplemental Table 2). No significant difference was found between groups for probability discounting, t(374) = 0.678, p = .498, N = 376; Figure 1, Supplemental Table 2. No significant correlation was found between continuous BMI and monetary discounting (r = .112, p = .031, N = 376) nor probability discounting (r = −.045, p = .379, N = 376) (Figure 2, Supplemental Table 8).
Additional Measures to Assess Impulsive Tone
Eating Motivations and Attitudes
The Bonferroni-corrected critical alpha value for eating motivation and attitude measures is 0.0045. Individuals with obesity reported significantly greater uncontrolled eating, t(374) = −5.831, p < .001, N = 376, and emotional eating, t(374) = −4.684, p < .001, N = 376, than individuals without obesity (Figure 1; Supplemental Table 3). No significant difference was found between groups for cognitive restraint (t(374) = 0.541, p = .589, N = 376). Individuals with obesity reported significantly higher appetites for palatable foods, t(346.924) = −4.974, p < .001, N = 376, including when food is available, t(343.362) = −5.989, p < .001, N = 376, and food is present, t(374) = −5.004, p < .001, N = 376, than individuals without obesity. No significant difference was found between groups when food is tasted, t(374) = −2.096, p = .037, N = 376. Individuals with obesity reported significantly greater bulimia food preoccupation, t(348.149) = −4.528, p < .001, N = 376, than individuals without obesity. No significant difference was found between groups for disordered eating (EAT total t(364.451) = −2.534, p = .012, N = 376), including dieting, t(374) = −2.431, p = .016, N = 376, and oral control, t(374) = 0.218, p = .828, N = 376. Neither group scored above the screening threshold for eating disorder evaluation. BMI was found to be positively correlated with uncontrolled eating (r = .280, p < .001, N = 376), emotional eating (r = .223, p < .001, N = 376), bulimia food preoccupation (r = .262, p < .001, N = 376), and appetite for palatable foods (r = .235, p < .001, N = 376), including when food is available (r = .270, p < .001, N = 376) and when food is present (r = .227, p < .001, N = 376) (Figure 2, Supplemental Table 8).
Exercise Motivations and Attitudes
The Bonferroni-corrected critical alpha value for exercise motivation and attitude measures is 0.005. Individuals with obesity reported significantly lower positive well-being, t(374) = 4.513, p < .001, N = 376, and significantly greater psychological distress (t(346.275) = −5.282, p < .001, N = 376) and fatigue, t(374) = −3.830, p < .001, N = 376, in response to exercise compared to individuals without obesity (Figure 1; Supplemental Table 4). Additionally, individuals with obesity reported significantly lower self-determination for exercise, t(370.578) = 8.198, p < .001, N = 376, than individuals without obesity. Individuals with obesity reported higher amotivation, t(361.150) = −4.735, p < .001, N = 376, and external regulation, t(374) = −3.085, p = .002, N = 376, and lower identified regulation, t(374) = 6.931, p < .001, N = 376, integrated regulation, t(374) = 5.572, p < .001, N = 376, and intrinsic regulation, t(374) = 5.074, p < .001, N = 376, compared to individuals without obesity. No significant differences were found between groups for introjected regulation (Figure 1, Supplemental Table 4). BMI was found to be positively correlated with psychological distress (r = .258, p < .001, N = 376) and fatigue (r = .165, p = .001, N = 376) in response to exercise, and amotivation for exercise (r = .223, p < .001, N = 376). BMI was found to be negatively correlated with positive well-being in response to exercise (r = −.206, p < .001, N = 376), self-determination for exercise (r = −0.307, p < .001, N = 376), identified regulation (r = −.287, p < .001, N = 376), integrated regulation (r = −.199, p < .001, N = 376), and intrinsic regulation (r = −.162, p = .002, N = 376) (Figure 2, Supplemental Table 8).
Additional Measures to Assess Executive Tone
Executive Function
The Bonferroni-corrected critical alpha value for executive function measures is 0.00625. On the Stroop task, individuals with obesity had a significantly lower percent correct on congruent trials, t(241.930) = 2.888, p = .004, N = 340, compared to individuals without obesity (Figure 1, Supplemental Table 5). No significant differences were found between groups in overall percent correct, t(250.335) = 2.726, p = .007, N = 340, percent correct on incongruent trials, t(269.392) = 2.402, p = .017, N = 340, and control trials, t(254.241) = 2.651, p = .009, N = 340, or in overall, t(338) = 0.827, p = .409, N = 340, congruent, t(338) = 1.312, p = .190, N = 340, incongruent, t(338) = 0.438, p = .661, N = 340, or control, t(338) = 0.739, p = .460, N = 340, reaction times (Figure 1, Supplemental Table 5). No significant correlations were found between continuous BMI and executive function measures (Figure 2, Supplemental Table 8).
Affective State
The Bonferroni-corrected critical alpha value for affective state measures is 0.0125. Individuals with obesity reported significantly higher levels of anxiety, t(356.127) = −3.623, p < .001, N = 376, and depression, t(374) = −2.693, p = .007, N = 376, than individuals without obesity (Figure 1, Supplemental Table 5). The group without obesity reported mild anxiety while the group with obesity reported moderate anxiety. Additionally, the group without obesity reported minimal depression while those with obesity reported mild depression. No significant differences were found between groups for positive, t(374) = 1.354, p = .177, N = 376, or negative affect, t(374) = −2.186, p = .029, N = 376. BMI was found to be positively correlated with anxiety (r = .197, p < .001, N = 376) and depression (r = .129, p = .012, N = 376) (Figure 2, Supplemental Table 8).
Self-Evaluation
The Bonferroni-corrected critical alpha value for self-evaluation measures is 0.01. Individuals with obesity reported significantly more negative overall self-image (BAT total t(341.750) = −7.899, p < .001, N = 376) including greater negative appreciation (t(342.615) = −9.809, p < .001, N = 376), higher lack of familiarity, t(341.436) = −6.315, p < .001, N = 376, and greater body dissatisfaction, t(374) = −6.859, p < .001, N = 376, than individuals without obesity (Figure 1, Supplemental Table 7). BMI was found to be positively correlated with more negative self-image (r = .401, p < .001, N = 376), including negative appreciation (r = .484, p < .001, N = 376), lack of familiarity (r = .323, p < .001, N = 376), and body dissatisfaction (r = .328, p < .001, N = 376) (Figure 2, Supplemental Table 8).
Bayesian Network Modeling of the Relationship Between BMI and Neurobehaviors
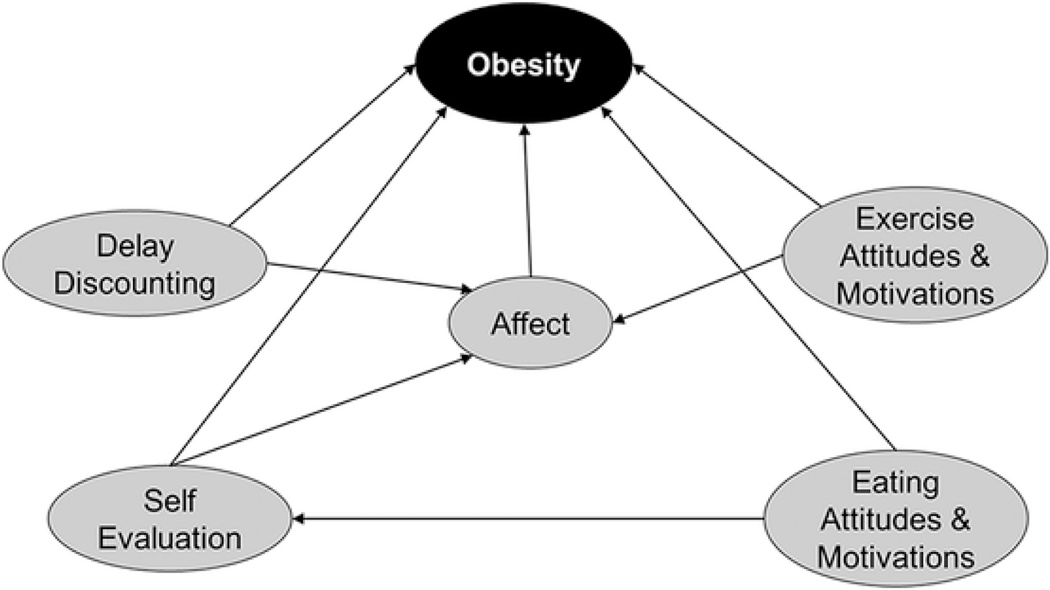
An exploratory analysis using a tree augmented naive Bayesian network with 10-fold cross-validation resulted in a model that accurately predicted obesity outcomes in 64.4% of cases (Figure 3). Testing of the model, with obesity defined as the “positive” condition, resulted in a sensitivity of 57.3%, and a specificity of 70.7%. The network revealed that all five neurobehaviors (DD, exercise attitudes, eating attitudes, affective state, and self-evaluation) were direct predictors of the obesity outcome. The strongest direct relationship to obesity was from DD (arc strength = 46.36), followed by eating attitudes and motivations (arc strength = 41.44), exercise attitudes and motivations (arc strength = 31.53), self-evaluation (arc strength = 31.40), and affective state (arc strength = 4.62) (Table 2). Additionally, DD, exercise attitudes and motivations, and self-evaluation were predictors of affective state; and eating attitudes and motivations was a predictor of self-evaluation (Figure 3).
Figure 3.
Tree Augmented Naïve Bayesian Network Model of Neurobehaviors Accurately Predicting 64.4% Cases of Obesity
Table 2.
Bayesian Network Model Arc Strength Arc Strength Represents the Change in Bayesian Information Criterion (BIC) After Removal of the Arc in the Bayesian Network
| Arc |
||
|---|---|---|
| From (predictor) | To (outcome) | Strength |
| Delay discounting | Affect | 58.616361 |
| Delay discounting | Obesity | 46.361231 |
| Eating attitudes and motivations | Obesity | 41.437493 |
| Exercise attitudes and motivations | Affect | 31.668234 |
| Exercise attitudes and motivations | Obesity | 31.527408 |
| Self-evaluation | Obesity | 31.403465 |
| Affect | Obesity | 4.616077 |
Discussion
Obesity is a chronic, relapsing, and progressive disease that is both preventable and treatable (Bray et al., 2017; De Lorenzo et al., 2020). Treatments for this disease include lifestyle change interventions, weight-loss medications and devices, and in extreme cases, bariatric surgery. However, treatment success rates, especially for programs implementing behavioral change, are low. This lack of success may be because obesity is not viewed from a holistic lens. New research indicates that obesity may be a more complex disorder stemming not from just eating too much or exercising too little, but actually a result of an imbalance in key brain circuits, namely the impulsive and executive systems. Using the framework of the CNDS theory and the statistical method of Bayesian network modeling, we have shown that obesity is predicted by a profile of neurobehaviors including enhanced impulsivity, impaired affective state, lower levels of motivation, and poor self-image. Several previous correlational and meta-analytic studies have established that overweight/obesity are related to impaired neurobehaviors including cognitive flexibility, short-term memory, fluid intelligence, impulsivity, and social functioning (Emery & Levine, 2017; Gray et al., 2020; Hovens et al., 2019; Mazza et al., 2020; Olivo et al., 2019; Vainik et al., 2013, 2018; Wood et al., 2019; Yang et al., 2018). The novelty of our data set is that we have provided a side-by-side comparison of a variety of neurobehaviors along with eating and exercise outcomes, establishing a model that predicts obesity with an accuracy of 64.4%. Our findings support the link between the body (e.g., BMI) and mind (e.g., neurobehavioral state) and suggest that neurobehaviors may be a target to enhance obesity treatment outcomes.
Impaired Neurobehavioral Profile in Obesity
Delay Discounting: A Task to Identify CNDS Balance
We have demonstrated that individuals with obesity have a significantly higher discount rate than individuals without obesity. Previous research has demonstrated mixed findings in this area; however, the most robust methodological study designs demonstrate a positive and significant association between DD and obesity (Tang et al., 2019), with BMI showing a significant positive correlation to discounting rate (Epstein et al., 2014). One recent study using data from the Human Connectome Project investigated cognitive dysfunction in obesity using a battery of 20 neurocognitive assessments and identified DD as the strongest predictor of obesity (Hovens et al., 2019), which our findings corroborate. DD is the behavioral tendency to undervalue rewards when the receipt of the reward is postponed in time. This behavior may have proven evolutionarily advantageous as the probability of actually receiving a reward often decreases as a temporal delay is introduced (Kagel et al., 1986). However, excessive discounting has been linked to impaired health behaviors and clinical issues such as addiction, and now, obesity (Bickel et al., 2019). We have previously proposed that DD is a candidate behavioral marker to establish the functioning of and balance between the executive and impulsive systems (Bickel et al., 2012, 2019). In this light, the present results demonstrate that individuals with obesity show a hyperactive impulsive system and a hypoactive executive system, indicating that DD may be a biomarker for obesity (Califf, 2018; Strimbu & Tavel, 2010).
Additional Findings That Indicate an Overactive Impulsive System in Obesity
Eating Motivations and Attitudes
We have shown that individuals with obesity report altered eating motivations compared to individuals without obesity. Namely, individuals with obesity report higher levels of uncontrolled and emotional eating, heightened appetite for palatable foods, and greater disordered eating including bulimia food preoccupation. Previous research using principal component analysis or meta-analytic techniques have indicated that various eating-related traits assessed through scales such as the PFS, TFEQ-R18, and EAT measure a similar construct, namely uncontrolled eating, and can be considered interchangeably (Price et al., 2015; Vainik et al., 2015, 2019). We, therefore, interpret our results as indicating that obesity is associated with uncontrolled eating, which is in line with previous findings showing that heightened motivation for consuming highly palatable foods is a hallmark of obesity (Campana et al., 2019; Ferrario, 2017; Lerma-Cabrera et al., 2016). Specifically, individuals with obesity compared to those without obesity display higher levels of hedonic hunger, or the motivational drive to eat in the absence of hunger cues (Lowe & Butryn, 2007). Additionally, weight loss driven by either behavioral or surgical (i.e., gastric bypass surgery) interventions in individuals with obesity has been shown to decrease levels of hedonic hunger (Schulte et al., 2020; Schultes et al., 2010). Disordered eating, especially as it relates to undercontrolled eating such as binge eating disorder (BED), has also emerged as an issue in obesity (de Zwaan, 2001; McCuen-Wurst et al., 2018). The prevalence of BED in the adult U.S. population is approximately 5% but rises to 50% in adults with obesity seeking weight-loss treatment (Hudson et al., 2007). In line with our theoretical framework, these food-addiction-like behaviors in populations with obesity have been linked to pathological heightened activity of the impulsive system, namely the nucleus accumbens (Castro et al., 2015; Coccurello & Maccarrone, 2018; Yang et al., 2020).
Exercise Motivations and Attitudes
Surprisingly few studies have examined the motivation for physical activity in populations with obesity in a cross-sectional format such as this one. Using the framework of self-determination theory (Deci & Ryan, 2012), we have newly shown that individuals with obesity report lower levels of motivation for physical activity than individuals without obesity, specifically reporting higher levels of amotivation and lower levels of intrinsic regulation. Our results indicate that individuals with obesity rely on external pressure to exercise (external regulation) and find that exercise is less important (identified regulation) and contributes less to their sense of self (integrated regulation) than individuals without obesity. That is, on the spectrum of self-determination, individuals with obesity are non-self-determined to exercise compared to individuals without obesity. Additionally, we have newly shown that individuals with obesity report lower levels of positive well-being and greater levels of psychological distress and fatigue in response to exercise. This affective response to exercise may contribute to the lack of motivation to engage in exercise, especially considering that the level of exercise motivation positively correlates to the level of positive well-being and negatively correlates to the level of psychological distress and fatigue experienced with exercise (Supplemental Table 9; Supplemental Figure 1). Similarly, in individuals with obesity, acute exercise has little to no impact on improving mood states (Unick et al., 2012, 2015), and sedentary activity is more reinforcing than physical activity (Carr & Epstein, 2020; Epstein et al., 1991). Additionally, previous studies have shown that weight-loss interventions in individuals who are overweight and obese can increase the motivation to engage in physical activity (Silva et al., 2008, 2010; Verloigne et al., 2011). We have previously demonstrated that regions integral to both the executive (i.e., medial prefrontal cortex) and impulsive (i.e., nucleus accumbens) systems are necessary for motivation to engage in physical activity (Basso & Morrell, 2015), suggesting that these regions may be involved in the amotivation to exercise in individuals with obesity.
Additional Findings That Indicate an Underactive Executive System in Obesity
Cognitive Functioning
We have shown that individuals with obesity, compared to individuals without obesity, demonstrate impaired executive function as measured by accuracy on the congruent trials of the Stroop Task. That is, individuals with obesity show a deficit in the ability to identify the color when the color and the meaning of the word match, a process referred to as the Stroop facilitation effect. Facilitation is a measure of attention and is a cognitive-behavioral process that relies on the anterior cingulate cortex, a region of the executive system (Carter et al., 1995; Lindsay & Jacoby, 1994). A meta-analysis that examined inhibitory control in obesity using several neurocognitive measures including the Stroop task, the stop-signal task, and the go/no-go task, found that individuals with obesity showed impairment in these prefrontal cortex-dependent behaviors compared to healthy weight controls (Lavagnino et al., 2016). Additionally, studies have shown that worsened executive functioning (i.e., working memory) is predictive of lower levels of weight loss in behavioral treatment programs for individuals with obesity (Dassen et al., 2018). Further, neuroimaging studies have identified that inhibitory control-related activity of the prefrontal cortex is negatively associated with both BMI and subsequent weight gain (Batterink et al., 2010; Hendrick et al., 2012; Kishinevsky et al., 2012; Weygandt et al., 2013). Our findings are in line with this body of work showing that impairment of the executive system is predictive of high BMI, continued weight gain, and obesity outcomes.
Affective State
Similar to what others have shown (Avila et al., 2015), we have demonstrated that individuals with obesity compared to individuals without obesity report higher levels of depression and anxiety. Research has found that obesity is a risk factor for serious mental health conditions including depression and anxiety (Avila et al., 2015). In fact, obesity is associated with an approximately 25% increase in odds of mood and anxiety disorders, with the strongest association between obesity and depression (Rajan & Menon, 2017; Simon et al., 2006). These mental health issues only exacerbate the impaired quality of life and levels of disability, morbidity, and mortality that accompany obesity (Avila et al., 2015). Collectively, this research indicates that a bidirectional link exists between obesity and mental health disorders, and as such, the treatment of one can improve the course of the other (Amiri & Behnezhad, 2019; Jantaratnotai et al., 2017; Luppino et al., 2010; McElroy et al., 2004; Rao et al., 2020). Dysfunction in regions of the executive system, such as the ventromedial prefrontal cortex, has been linked to mental health issues such as depression and anxiety (Hare & Duman, 2020; Hiser & Koenigs, 2018), suggesting another area of the executive system that may be targeted in obesity.
Self-Evaluation
We have shown that individuals with obesity report a more negative body image than individuals without obesity, including greater levels of negative appreciation, lack of familiarity, and body dissatisfaction. Previous research has shown that individuals with obesity report greater levels of impaired body image than individuals without obesity. This negative self-evaluation is an integral part of Body Dysmorphic Disorder (BDD), which is a clinical disorder characterized by excessive thoughts and repetitive behaviors regarding a preoccupation with physical appearance and is common in individuals with obesity (Sarwer et al., 2005, 1998). Body image disturbance is often a driving force for weight loss, and individuals who are overweight and obese are more motivated to lose weight to improve their physical appearance than to improve their health (Delgado et al., 2002; Latner & Wilson, 2011; Sarwer et al., 2005). Research shows that negative self-evaluation may persist even with weight loss because the individuals express dissatisfaction over some of the body shape changes that occur with weight loss (e.g., skin folds; Sarwer et al., 2005). Recent functional neuroimaging studies have implicated the executive system (medial PFC) in self-image, including self-referential and self-evaluative thought or the processing of information about the self (D’Argembeau et al., 2007; Mitchell et al., 2005; Owens et al., 2010).
Our Bayesian Network Model in Relation to the Competing Neurobehavioral Decisions System Theory
The CNDS Theory posits that our decisions are governed by two brain systems, namely the impulsive system and the executive system. As an example, in the case of eating, the impulsive system governs our approach to and consumption of palatable foods; whereas the executive system helps limit the overconsumption of said palatable foods. When the two systems are in balance, we can engage in a variety of behavioral choices that result in healthy weight status (i.e., normal BMI). However, when the two systems are out of balance, our behavioral choices are more limited (e.g., eating only high-density caloric foods and/or limited participation in physical activity) and lead to overweight or obese outcomes. Specifically, the results of our model suggest that obesity manifests as a result of an imbalance between the impulsive and executive systems and namely, a hyperactive impulsive system and a hypoactive executive system.
Limitations and Future Directions
The main limitation of this study exists in the fact that we have collected data from a sample of individuals on Amazon mTurk. Therefore, our participants could be distinctly different from that of the general population and so the results might not directly translate. Additionally, participant inattention may affect the quality of data collected through mTurk (Goodman et al., 2013). However, we used several techniques including the inclusion of mTurk workers with a 90% or greater HIT approval rate, open answered questions, and both automatic and visual inspection of our data for systematic and reasonable data entry. Despite these limitations, data collections through mTurk afforded us the ability to collect a large enough sample size to sufficiently power our study for analysis of the differences of 46 variables between populations with and without obesity. Another limitation of this study is the cross-sectional study design, which does not allow for causal determination. However, this type of study is needed to first identify the neurobehavioral factors that predict obesity. Previous findings from longitudinal studies suggest that the relationship between body weight and neurobehaviors is bidirectional. That is, certain neurobehaviors (e.g., impaired executive function) can predict future body weight outcomes (Guxens et al., 2009; Hartanto et al., 2019; Hofmann et al., 2014), and likewise changes in body weight can lead to changes in neurobehavioral outcomes (Alosco, Galioto, et al., 2014; Alosco, Spitznagel, et al., 2014; Hartanto et al., 2019). Additionally, while the current study investigates a comprehensive range of neurobehaviors related to obesity, it does not account for additional factors, such as genetic susceptibility (Loos, 2012; Loos & Janssens, 2017) or environmental influences (Garfinkel-Castro et al., 2017; Popkin, 2006), that contribute to the development of obesity. The lack of these additional measures may limit the predictive power of the current model, which has a 64.4% accuracy. Given this level of accuracy, this model is limited in its usefulness for individual prediction or diagnosis. Nonetheless, the results of this investigation provide evidence for the direct and indirect relationships of neurobehaviors to the development of obesity and suggest that improving neurobehaviors may aid in the prevention and/or treatment of obesity. Future studies should now focus on interventional strategies to support weight loss by targeting some of the factors identified in the present study.
At the individual level, combating obesity means significant weight loss brought about by decreasing food consumption and/or increasing physical activity. Altering and accurately tracking these health behaviors (i.e., eating and exercise) has proven challenging in the past, especially for individuals with clinical issues such as those with obesity, diabetes, or heart disease (Vanstone et al., 2013). A benefit of measures such as the ones utilized in the present study is that they can be quickly and easily collected in an office setting, much unlike the tracking of eating and exercise behaviors (Grandjean, 2012; Shim et al., 2014), and repeatedly measured over the course of time. For example, the self-reported measures assessed in the model would take approximately 30 min to complete and could be easily collected via phone or iPad in the waiting room. Our findings regarding the interconnected nature of neurobehaviors and obesity suggest that a strategy that directly targets the neurobehaviors behind overeating and lack of exercises such as cognition, mood, motivation, or impulsivity, might be an effective strategy for combating obesity. Previous literature on interventional strategies supports this idea. One recent meta-analysis and systematic review examined 66 studies that utilized cognitive raining to affect eating behaviors and weight loss, finding that inhibition training, attention bias modification training, and episodic future thinking training were the most effective interventions (Yang et al., 2019). Our lab has successfully utilized episodic future thinking, which directly targets DD through the vivid imagining of positive future events, to decrease the demand for food in a hypothetical food purchasing task (Sze et al., 2017), and we are currently using this strategy to improve health behaviors in individuals with type 2 diabetes. Additionally, cognitive-behavioral therapy, which targets mood regulation and self-evaluation, has been recommended as a treatment for obesity and has been shown effective for improving eating behaviors, sustained weight loss, and psychological improvement in individuals with obesity (Braet et al., 2004; Cheroutre et al., 2020; Cooper et al., 2003; Kang & Kwack, 2020; Pekkarinen et al., 1996). We hypothesize that an interventional approach combining episodic future thinking with cognitive-behavioral therapy or other strategies known to improve mood and self-image may be excellent strategies for tackling obesity.
At a societal level, we exist in an environment where engaging in overconsumption of highly palatable, calorically dense foods and remaining sedentary is the easy choice. Contrarily, engaging in healthy eating and exercise behaviors requires both energetic and financial resources. The choice to make the healthy decision in this food-rich environment may be even more challenging for individuals with obesity compared to healthy weight controls. Our Bayesian Network Model suggests that individuals with obesity have an imbalance of key neural circuitry; namely, a hyperactive impulsive decision system and a hypoactive executive decision system. That is, the brains of individuals with obesity respond to the obesogenic environment in a fundamentally different way marked by a heightened response to the available highly palatable foods and a decreased ability to inhibit the response to consume the food. Our data additionally suggest that targeting neurobehavioral factors such as DD, mood, or cognition may help individuals with obesity to make healthy food and exercise choices and decrease their BMI.
Conclusions
Using the framework of the CNDS theory, we hypothesized that individuals with obesity compared to non-obese controls would display neurobehaviors marked by a hyperactive impulsive system and a hypoactive executive system. Results from our Bayesian Network Model supported this hypothesis, showing that obesity is predicted by a distinct neurobehavioral profile that includes heightened DD, impaired motivation, decreased affective state, and poor self-image. Considering that treatment success rates for obesity are low, understanding the neurobehavioral profile that predicts obesity is important to help identify targets to decrease BMI and improve outcomes for this chronic, relapsing, progressive disease. Our results suggest that targeting neurobehaviors such as mood, motivation, impulsivity, and executive function may help to improve obesity outcomes. In order to tackle obesity, which affects approximately 30% of the world population, we must look at this disease from a holistic perspective. Treatments must not simply be about eating less and exercising more. For example, future studies targeting neuromodulation of the prefrontal cortex might be helpful to initiate and sustain weight loss in populations with obesity. In fact, studies have shown promise with techniques such as repetitive transcranial magnetic stimulation (rTMS) or repetitive direct current stimulation (rDCS) of the DLPFC causing a decrease in food craving and calorie consumption and an increase in weight loss, especially long-term protocols (e.g., eight treatments over a course of 4 weeks; Higuera-Hernández et al., 2018; Jáuregui-Lobera & Martínez-Quiñones, 2018; Kim, Chung, et al., 2019; Kim et al., 2018; Kim, Park, et al., 2019; Lee et al., 2018; Song et al., 2019). Experimental studies using single sessions of neuromodulation on executive control networks have even proven effective at decreasing food craving and consumption in individuals with eating disorders (e.g., BED, overweight, and obesity), suggesting that these techniques could serve as targeted treatments, and complementary treatments to traditional psychotropic medications, to enhance executive functioning in service of optimizing healthy behavioral choices (Fregni et al., 2008; Kekic et al., 2014, 2017; Lowe et al., 2017). Additionally, behavioral treatment interventions that include cognitive-behavioral therapy, episodic future thinking, meditation, cognitive training, or other alternative therapies that have been shown to enhance the functioning of the executive network may be the way of the future for tackling obesity.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH) Science of Behavior Change Common Fund Program through an award administered by the National Institute of Diabetes and Digestive and Kidney Diseases (UH2/UH3DK109543) and the Fralin Biomedical Research Institute at Virginia Tech Carilion.
Footnotes
We have no conflict of interests to report.
References
- Alosco ML, Galioto R, Spitznagel MB, Strain G, Devlin M, Cohen R, Crosby RD, Mitchell JE,&Gunstad J. (2014). Cognitive function after bariatric surgery: Evidence for improvement 3 years after surgery. American Journal of Surgery, 207(6), 870–876. 10.1016/j.amjsurg.2013.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Spitznagel MB, Strain G, Devlin M, Cohen R, Paul R, Crosby RD, Mitchell JE, & Gunstad J. (2014). Improved memory function two years after bariatric surgery. Obesity, 22(1), 32–38. 10.1002/oby.20494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri S, & Behnezhad S. (2019). Obesity and anxiety symptoms: A systematic review and meta-analysis. Neuropsychiatrie: Klinik, Diagnostik. Therapie Und Rehabilitation: Organ Der Gesellschaft Osterreichischer Nervenarzte Und Psychiater, 33(2), 72–89. 10.1007/s40211-019-0302-9 [DOI] [PubMed] [Google Scholar]
- Avena NM, Gearhardt AN, Gold MS, Wang G-J, & Potenza MN (2012). Tossing the baby out with the bathwater after a brief rinse? The potential downside of dismissing food addiction based on limited data [Review of Tossing the baby out with the bathwater after a brief rinse? The potential downside of dismissing food addiction based on limited data]. Nature Reviews. Neuroscience, 13(7), 514. 10.1038/nrn3212-c1 [DOI] [PubMed] [Google Scholar]
- Avila C, Holloway AC, Hahn MK, Morrison KM, Restivo M, Anglin R, & Taylor VH (2015). An overview of links between obesity and mental health. Current Obesity Reports, 4(3), 303–310. 10.1007/s13679-015-0164-9 [DOI] [PubMed] [Google Scholar]
- Basso JC,& Morrell JI (2015). The medial prefrontal cortex and nucleus accumbens mediate the motivation for voluntary wheel running in the rat. Behavioral Neuroscience, 129(4), 457–472. 10.1037/bne0000070 [DOI] [PubMed] [Google Scholar]
- Batterink L, Yokum S, & Stice E. (2010). Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. NeuroImage, 52(4), 1696–1703. 10.1016/j.neuroimage.2010.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, & Steer RA (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. 10.1037//0022-006X.56.6.893 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA,&Carbin MG (1988). Psychometric properties of the beck depression inventory: Twenty-five years of evaluation. Clinical Psychology Review, 8(1), 77–100. 10.1016/0272-7358(88)90050-5 [DOI] [Google Scholar]
- Beer JS, Lombardo MV, & Bhanji JP (2010). Roles of medial prefrontal cortex and orbitofrontal cortex in self-evaluation. Journal of Cognitive Neuroscience, 22(9), 2108–2119. 10.1162/jocn.2009.21359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H-R, Münzberg H,&Morrison CD (2017). Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology, 152(7), 1728–1738. 10.1053/j.gastro.2016.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Athamneh LN, Basso JC, Mellis AM, DeHart WB, Craft WH, & Pope D. (2019). Excessive discounting of delayed reinforcers as a trans-disease process: Update on the state of the science. Current Opinion in Psychology, 30, 59–64. 10.1016/j.copsyc.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, George Wilson A, Franck CT, Terry Mueller E, Jarmolowicz DP, Koffarnus MN, & Fede SJ (2014). Using crowdsourcing to compare temporal, social temporal, and probability discounting among obese and non-obese individuals. Appetite, 75, 82–89. 10.1016/j.appet.2013.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, & Gatchalian KM (2012). Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: Emerging evidence. Pharmacology & Therapeutics, 134(3), 287–297. 10.1016/j.pharmthera.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Mellis AM, Snider SE, Athamneh LN, Stein JS, & Pope DA (2018). 21st century neurobehavioral theories of decision making in addiction: Review and evaluation. Pharmacology, Biochemistry and Behavior, 164, 4–21. 10.1016/j.pbb.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Moody L, Quisenberry AJ, Ramey CT, & Sheffer CE (2014). A competing Neurobehavioral decision systems model of SES related health and behavioral disparities. Preventive Medicine, 68, 37–43. 10.1016/j.ypmed.2014.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielza C, & Larranaga P. (2014). Bayesian networks in neuroscience: A survey. Frontiers in Computational Neuroscience, 8, 131. 10.3389/fncom.2014.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block G, Gillespie C, Rosenbaum EH, & Jenson C. (2000). A rapid food screener to assess fat and fruit and vegetable intake. American Journal of Preventive Medicine, 18(4), 284–288. 10.1016/s0749-3797(00)00119-7 [DOI] [PubMed] [Google Scholar]
- Blumenthal DM, & Gold MS (2010). Neurobiology of food addiction. Current Opinion in Clinical Nutrition and Metabolic Care, 13(4), 359–365. 10.1097/MCO.0b013e32833ad4d4 [DOI] [PubMed] [Google Scholar]
- Braet C, Tanghe A, Decaluwé V, Moens E, & Rosseel Y. (2004). Inpatient treatment for children with obesity: Weight loss, psychological well-being, and eating behavior. Journal of Pediatric Psychology, 29(7), 519–529. 10.1093/jpepsy/jsh054 [DOI] [PubMed] [Google Scholar]
- Bray GA, Kim KK, Wilding JPH, & World Obesity Federation. (2017). Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obesity Reviews, 18(7), 715–723. 10.1111/obr.12551 [DOI] [PubMed] [Google Scholar]
- Califf RM (2018). Biomarker definitions and their applications. Experimental Biology and Medicine, 243(3), 213–221. 10.1177/1535370217750088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana B, Brasiel PG, de Aguiar AS, & Dutra SCPL (2019). Obesity and food addiction: Similarities to drug addiction. Obesity Medicine, 16, Article 100136. 10.1016/j.obmed.2019.100136 [DOI] [Google Scholar]
- Camus M, Halelamien N, Plassmann H, Shimojo S, O’Doherty J, Camerer C, & Rangel A. (2009). Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex decreases valuations during food choices. European Journal of Neuroscience, 30(10), 1980–1988. 10.1111/j.1460-9568.2009.06991.x [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Gerber RA, Leidy NK, Sexton CC, Karlsson J, & Lowe MR (2009). Evaluating the power of food scale in obese subjects and a general sample of individuals: Development and measurement properties. International Journal of Obesity, 33(8), 913–922. 10.1038/ijo.2009.107 [DOI] [PubMed] [Google Scholar]
- Carlson SA, Fulton JE, Pratt M, Yang Z, & Adams EK (2015). Inadequate physical activity and health care expenditures in the United States. Progress in Cardiovascular Diseases, 57(4), 315–323. 10.1016/j.pcad.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KA, & Epstein LH (2020). Choice is relative: Reinforcing value of food and activity in obesity treatment. American Psychologist, 75(2), 139–151. 10.1037/amp0000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Mintun M,&Cohen JD (1995). Interference and facilitation effects during selective attention: An H215O PET study of Stroop task performance. NeuroImage, 2(4), 264–272. 10.1006/nimg.1995.1034 [DOI] [PubMed] [Google Scholar]
- Castro DC, Cole SL, & Berridge KC (2015). Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: Interactions between homeostatic and reward circuitry. Frontiers in Systems Neuroscience, 9, 90. 10.3389/fnsys.2015.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheroutre C, Guerrien A, & Rousseau A. (2020). Contributing of cognitive-behavioral therapy in the context of bariatric surgery: A review of the literature. Obesity Surgery, 30(8), 3154–3166. 10.1007/s11695-020-04627-9 [DOI] [PubMed] [Google Scholar]
- Cid L, Monteiro D, Teixeira D, Teques P, Alves S, Moutão J, Silva M, & Palmeira A. (2018). The behavioral regulation in exercise questionnaire (BREQ-3) portuguese-version: Evidence of reliability, validity and invariance across gender. Frontiers in Psychology, 9, 1940. 10.3389/fpsyg.2018.01940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Reeck C, Carter RM, Smith DV, & Huettel SA (2011). Nucleus accumbens mediates relative motivation for rewards in the absence of choice. Frontiers in Human Neuroscience, 5, 87. 10.3389/fnhum.2011.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccurello R, & Maccarrone M. (2018). Hedonic eating and the “delicious circle”: From lipid-derived mediators to brain dopamine and back. Frontiers in Neuroscience, 12, 271. 10.3389/fnins.2018.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper Z, Fairburn CG, & Hawker DM (2003). Cognitive-behavioral treatment of obesity: A clinician’s guide (pp. 232). The Guilford Press. https://psycnet.apa.org/fulltext/2003-07132-000.pdf [Google Scholar]
- Crawford JR, & Henry JD (2004). The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. The British Journal of Clinical Psychology/the British Psychological Society, 43(Pt 3), 245–265. 10.1348/0144665031752934 [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, & Salmon E. (2007). Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience, 19(6), 935–944. 10.1162/jocn.2007.19.6.935 [DOI] [PubMed] [Google Scholar]
- Dassen FCM, Houben K, Allom V, & Jansen A. (2018). Self-regulation and obesity: The role of executive function and delay discounting in the prediction of weight loss. Journal of Behavioral Medicine, 41(6), 806–818. 10.1007/s10865-018-9940-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lauzon B, Romon M, Deschamps V, Lafay L, Borys J-M, Karlsson J, Ducimetière P, Charles MA, & The Fleurbaix Laventie Ville Sante Study Group. (2004). The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. Journal of Nutrition, 134(9), 2372–2380. 10.1093/jn/134.9.2372 [DOI] [PubMed] [Google Scholar]
- De Lorenzo A, Romano L, Di Renzo L, Di Lorenzo N, Cenname G, & Gualtieri P. (2020). Obesity: A preventable, treatable, but relapsing disease. Nutrition (Burbank, Los Angeles County, Calif.), 71, Article 110615. 10.1016/j.nut.2019.110615 [DOI] [PubMed] [Google Scholar]
- De Ridder D, Manning P, Leong SL, Ross S, Sutherland W, Horwath C, & Vanneste S. (2016). The brain, obesity and addiction: An EEG neuroimaging study. Scientific Reports, 6, Article 34122. 10.1038/srep34122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zwaan M. (2001). Binge eating disorder and obesity. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity, 25(Suppl. 1), S51–S55. 10.1038/sj.ijo.0801699 [DOI] [PubMed] [Google Scholar]
- Deci EL, & Ryan RM (2012). Self-determination theory. In Higgins E. (Ed.), Handbook of theories of social psychology (Vol. 1, pp. 416–436). SAGE Publications LTD. 10.4135/9781446249215.n21 [DOI] [Google Scholar]
- Delgado CC, Morales MJG, & Maruri IC (2002). Eating behavior, body attitudes and psychopathology in morbid obesity. Actas Espanolas de Psiquiatria. https://europepmc.org/abstract/med/12487948 [PubMed]
- Emery RL, & Levine MD (2017). Questionnaire and behavioral task measures of impulsivity are differentially associated with body mass index: A comprehensive meta-analysis. Psychological Bulletin, 143(8), 868–902. 10.1037/bul0000105 [DOI] [PubMed] [Google Scholar]
- Epstein LH, Dearing KK, & Roba LG (2010). A questionnaire approach to measuring the relative reinforcing efficacy of snack foods. Eating Behaviors, 11(2), 67–73. 10.1016/j.eatbeh.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Jankowiak N, Fletcher KD, Carr KA, Nederkoorn C, Raynor HA, & Finkelstein E. (2014). Women who are motivated to eat and discount the future are more obese. Obesity, 22(6), 1394–1399. 10.1002/oby.20661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Smith JA, Vara LS, & Rodefer JS (1991). Behavioral economic analysis of activity choice in obese children. Health Psychology:Official Journal of the Division of Health Psychology, American Psychological Association, 10(5), 311–316. 10.1037/0278-6133.10.5.311 [DOI] [PubMed] [Google Scholar]
- Erickson KI, Creswell JD, Verstynen TD, & Gianaros PJ (2014). Health neuroscience: Defining a new field. Current Directions in Psychological Science, 23(6), 446–453. 10.1177/0963721414549350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M. (2017). Excess weight and multimorbidity: Putting people’s health experience in risk factor epidemiology [Review of Excess weight and multimorbidity: putting people’s health experience in risk factor epidemiology]. The Lancet Public Health, 2(6), e252–e253. 10.1016/S2468-2667(17)30093-2 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, & Lang A-G (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- Ferrario CR (2017). Food addiction and obesity. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 42(1), 361. 10.1038/npp.2016.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, & Kenny PJ (2018). Food addiction: A valid concept?. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 43(13), 2506–2513. 10.1038/s41386-018-0203-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxall GR (2016). Metacognitive control of categorical neurobehavioral decision systems. Frontiers in Psychology, 7, 170. 10.3389/fpsyg.2016.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FAM, Nitsche MA, Mecca T, Macedo EC, Pascual-Leone A, & Boggio PS (2008). Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite, 51(1), 34–41. 10.1016/j.appet.2007.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman N, Geiger D, & Goldszmidt M. (1997). Bayesian network classifiers. Machine Learning, 29(2), 131–163. 10.1023/A:1007465528199 [DOI] [Google Scholar]
- Funahashi S, & Andreau JM (2013). Prefrontal cortex and neural mechanisms of executive function. Journal of Physiology, Paris, 107(6), 471–482. 10.1016/j.jphysparis.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Fydrich T, Dowdall D,&Chambless DL (1992). Reliability and validity of the beck anxiety inventory. Journal of Anxiety Disorders, 6(1), 55–61. 10.1016/0887-6185(92)90026-4 [DOI] [Google Scholar]
- Garfinkel PE, & Newman A. (2001). The eating attitudes test: Twenty-five years later. Eating and Weight Disorders, 6(1), 1–24. 10.1007/BF03339747 [DOI] [PubMed] [Google Scholar]
- Garfinkel-Castro A, Kim K, Hamidi S, & Ewing R. (2017). Obesity and the built environment at different urban scales: Examining the literature. Nutrition Reviews, 75(suppl 1), 51–61. 10.1093/nutrit/nuw037 [DOI] [PubMed] [Google Scholar]
- Garner DM, Olmsted MP, Bohr Y,& Garfinkel PE (1982). The eating attitudes test: Psychometric features and clinical correlates. Psychological Medicine, 12(4), 871–878. 10.1017/S0033291700049163 [DOI] [PubMed] [Google Scholar]
- Genon S, Reid A, Langner R, Amunts K,&Eickhoff SB (2018). How to characterize the function of a brain region. Trends in Cognitive Sciences, 22(4), 350–364. 10.1016/j.tics.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck ME, Viswanath P, & Stinson EJ (2017). Obesity, appetite, and the prefrontal cortex. Current Obesity Reports, 6(4), 380–388. 10.1007/s13679-017-0289-0 [DOI] [PubMed] [Google Scholar]
- Goodman JK, Cryder CE,&Cheema A. (2013). Data collection in a flat world: The strengths and weaknesses of mechanical Turk samples: Data collection in a flat world. Journal of Behavioral Decision Making, 26(3), 213–224. 10.1002/bdm.1753 [DOI] [Google Scholar]
- Grandjean AC (2012). Dietary intake data collection: Challenges and limitations. Nutrition Reviews, 70(Suppl 2), S101–S104. 10.1111/j.1753-4887.2012.00545.x [DOI] [PubMed] [Google Scholar]
- Gray JC, Schvey NA, & Tanofsky-Kraff M. (2020). Demographic, psychological, behavioral, and cognitive correlates of BMI in youth: Findings from the Adolescent Brain Cognitive Development (ABCD) study. Psychological Medicine, 50(9), 1539–1547. 10.1017/S0033291719001545 [DOI] [PubMed] [Google Scholar]
- Gray JR, Braver TS, & Raichle ME (2002). Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 99(6), 4115–4120. 10.1073/pnas.062381899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutin I. (2018). In BMI we trust: Reframing the body mass index as a measure of health. Social Theory & Health, 16(3), 256–271. 10.1057/s41285-017-0055-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, Mendez MA, Julvez J, Plana E, Forns J, Basagãna X, Torrent M, & Sunyer J. (2009). Cognitive function and overweight in preschool children. American Journal of Epidemiology, 170(4), 438–446. 10.1093/aje/kwp140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha O-R, Lim S-L, & Bruce AS (2020). Neural mechanisms of food decision-making in children. Current Nutrition Reports, 9(3), 236–250. 10.1007/s13668-020-00321-5 [DOI] [PubMed] [Google Scholar]
- Hare BD, & Duman RS (2020). Prefrontal cortex circuits in depression and anxiety: Contribution of discrete neuronal populations and target regions. Molecular Psychiatry, 25, 2742–2758. 10.1038/s41380-020-0685-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartanto A, Yong JC, & Toh WX (2019). Bidirectional associations between obesity and cognitive function in midlife adults: A longitudinal study. Nutrients, 11(10), 2343. 10.3390/nu11102343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick VE, Savla J, Comber DL, Flack KD, Estabrooks PA, Nsiah-Kumi PA, Ortmeier S, & Davy BM (2012). Development of a brief questionnaire to assess habitual beverage intake (BEVQ-15): Sugar sweetened beverages and total beverage energy intake. Journal of the Academy of Nutrition and Dietetics, 112(6), 840–849. 10.1016/j.jand.2012.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick OM, Luo X, Zhang S, & Li C-SR (2012). Saliency processing and obesity: A preliminary imaging study of the stop signal task. Obesity, 20(9), 1796–1802. 10.1038/oby.2011.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera JJ, Fedynska S, Ghasem PR, Wieman T, Clark PJ, Gray N, Loetz E, Campeau S, Monika Fleshner M, & Greenwood BN (2016). Neurochemical and behavioural indices of exercise reward are independent of exercise controllability. European Journal of Neuroscience, 43(9), 1190–1202. 10.1111/ejn.13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuera-Hernández MF, Reyes-Cuapio E, Gutiérrez-Mendoza M, Rocha NB, Veras AB, Budde H, Jesse J, Zaldívar-Rae J, Blanco-Centurión C, Machado S, & Murillo-Rodríguez E. (2018). Fighting obesity: Non-pharmacological interventions. Clinical Nutrition ESPEN, 25, 50–55. 10.1016/j.clnesp.2018.04.005 [DOI] [PubMed] [Google Scholar]
- Hiser J, & Koenigs M. (2018). The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biological Psychiatry, 83(8), 638–647. 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, Adriaanse M, Vohs KD, & Baumeister RF (2014). Dieting and the self-control of eating in everyday environments: An experience sampling study. British Journal of Health Psychology, 19(3), 523–539. 10.1111/bjhp.12053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovens IB, Dalenberg JR, & Small DM (2019). A brief neuropsychological battery for measuring cognitive functions associated with obesity. Obesity, 27(12), 1988–1996. 10.1002/oby.22644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG Jr., & Kessler RC (2007). The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry, 61(3), 348–358. 10.1016/j.biopsych.2006.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, & Panksepp J. (1999). The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Research Reviews, 31(1), 6–41. 10.1016/S0165-0173(99)00023-5 [DOI] [PubMed] [Google Scholar]
- Imperatori C, Fabbricatore M, Innamorati M, Farina B, Quintiliani MI, Lamis DA, Mazzucchi E, Contardi A, Vollono C, & Della Marca G. (2015). Modification of EEG functional connectivity and EEG power spectra in overweight and obese patients with food addiction: An eLORETA study. Brain Imaging and Behavior, 9(4), 703–716. 10.1007/s11682-014-9324-x [DOI] [PubMed] [Google Scholar]
- Ito T, Hearne LJ, & Cole MW (2020). A cortical hierarchy of localized and distributed processes revealed via dissociation of task activations, connectivity changes, and intrinsic timescales. NeuroImage, 221, Article 117141. 10.1016/j.neuroimage.2020.117141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantaratnotai N, Mosikanon K, Lee Y, & McIntyre RS (2017). The interface of depression and obesity. Obesity Research & Clinical Practice, 11(1), 1–10. 10.1016/j.orcp.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Jáuregui-Lobera I,& Martínez-Quiñones JV (2018). Neuromodulation in eating disorders and obesity: A promising way of treatment?. Neuropsychiatric Disease and Treatment, 14, 2817–2835. 10.2147/NDT.S180231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagel JH, Green L, & Caraco T. (1986). When foragers discount the future: Constraint or adaptation?. Animal Behaviour, 34, 271–283. 10.1016/0003-3472(86)90032-1 [DOI] [Google Scholar]
- Kaisari P, Kumar S, Hattersley J, Dourish CT, Rotshtein P, & Higgs S. (2019). Top-down guidance of attention to food cues is enhanced in individuals with overweight/obesity and predicts change in weight at one year follow up. International Journal of Obesity, 43(9), 1849–1858. 10.1038/s41366-018-0246-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang NR, & Kwack YS (2020). An update on mental health problems and cognitive behavioral therapy in pediatric obesity. Pediatric Gastroenterology, Hepatology & Nutrition, 23(1), 15–25. 10.5223/pghn.2020.23.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J, Persson L-O, Sjöström L, & Sullivan M. (2000). Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. International Journal of Obesity, 24(12), 1715–1725. 10.1038/sj.ijo.08014427 [DOI] [PubMed] [Google Scholar]
- Kekic M, McClelland J, Bartholdy S, Boysen E, Musiat P, Dalton B, Tiza M, David AS, Campbell IC, & Schmidt U. (2017). Single session transcranial direct current stimulation temporarily improves symptoms, mood, and self-regulatory control in bulimia nervosa: A randomized controlled trial. PLOS ONE, 12(1), Article e0167606. 10.1371/journal.pone.0167606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekic M, McClelland J, Campbell I, Nestler S, Rubia K, David AS,& Schmidt U. (2014). The effects of prefrontal cortex transcranial direct current stimulation (tDCS) on food craving and temporal discounting in women with frequent food cravings. Appetite, 78, 55–62. 10.1016/j.appet.2014.03.010 [DOI] [PubMed] [Google Scholar]
- Kim S-H, Chung J-H, Kim T-H, Lim SH, Kim Y, Eun Y-M, & Lee Y-A (2019). The effects of repetitive transcranial magnetic stimulation on body weight and food consumption in obese adults: A randomized controlled study. Brain Stimulation, 12(6), 1556–1564. 10.1016/j.brs.2019.07.020 [DOI] [PubMed] [Google Scholar]
- Kim S-H, Chung J-H, Kim T-H, Lim SH, Kim Y, Lee Y-A, & Song S-W (2018). The effects of repetitive transcranial magnetic stimulation on eating behaviors and body weight in obesity: A randomized controlled study. Brain Stimulation, 11(3), 528–535. 10.1016/j.brs.2017.11.020 [DOI] [PubMed] [Google Scholar]
- Kim S-H, Park B-Y, Byeon K, Park H, Kim Y, Eun Y-M,& Chung J-H (2019). The effects of high-frequency repetitive transcranial magnetic stimulation on resting-state functional connectivity in obese adults. Diabetes, Obesity & Metabolism, 21(8), 1956–1966. 10.1111/dom.13763 [DOI] [PubMed] [Google Scholar]
- Kishinevsky FI, Cox JE, Murdaugh DL, Stoeckel LE, Cook EW III, & Weller RE (2012). fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite, 58(2), 582–592. 10.1016/j.appet.2011.11.029 [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, & Bickel WK (2014). A 5-trial adjusting delay discounting task: Accurate discount rates in less than one minute. Experimental and Clinical Psychopharmacology, 22(3), 222–228. 10.1037/a0035973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latner JD, & Wilson RE (2011). Obesity and body image in adulthood. In Cash TF & Smolak L. (Eds.), Body image: A handbook of science, practice, and prevention (Vol. 2, 2nd ed., pp. 189–197), The Guilford Press. https://psycnet.apa.org/fulltext/2011-20792-021.pdf [Google Scholar]
- Lavagnino L, Arnone D, Cao B, Soares JC, & Selvaraj S. (2016). Inhibitory control in obesity and binge eating disorder: A systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neuroscience and Biobehavioral Reviews, 68, 714–726. 10.1016/j.neubiorev.2016.06.041 [DOI] [PubMed] [Google Scholar]
- Lee DJ, Elias GJB, & Lozano AM (2018). Neuromodulation for the treatment of eating disorders and obesity. Therapeutic Advances in Psychopharmacology, 8(2), 73–92. 10.1177/2045125317743435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma-Cabrera JM, Carvajal F, & Lopez-Legarrea P. (2016). Food addiction as a new piece of the obesity framework. Nutrition Journal, 15, 5. 10.1186/s12937-016-0124-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay DS, & Jacoby LL (1994). Stroop process dissociations: The relationship between facilitation and interference. Journal of Experimental Psychology: Human Perception and Performance, 20(2), 219–234. 10.1037/0096-1523.20.2.219 [DOI] [PubMed] [Google Scholar]
- Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, & Cui R. (2017). The role of neural plasticity in depression: From hippocampus to prefrontal cortex. Neural Plasticity, 2017, Article 6871089. 10.1155/2017/6871089 [DOI] [PMC free article] [PubMed]
- Loos RJF (2012). Genetic determinants of common obesity and their value in prediction. Best Practice & Research. Clinical Endocrinology & Metabolism, 26(2), 211–226. 10.1016/j.beem.2011.11.003 [DOI] [PubMed] [Google Scholar]
- Loos RJF, & Janssens ACJW (2017). Predicting polygenic obesity using genetic information. Cell Metabolism, 25(3), 535–543. 10.1016/j.cmet.2017.02.013 [DOI] [PubMed] [Google Scholar]
- Lowe CJ, Reichelt AC, & Hall PA (2019). The prefrontal cortex and obesity: A health neuroscience perspective. Trends in Cognitive Sciences, 23(4), 349–361. 10.1016/j.tics.2019.01.005 [DOI] [PubMed] [Google Scholar]
- Lowe CJ, Vincent C, & Hall PA (2017). Effects of noninvasive brain stimulation on food cravings and consumption: A meta-analytic review. Psychosomatic Medicine, 79(1), 2–13. 10.1097/PSY.0000000000000368 [DOI] [PubMed] [Google Scholar]
- Lowe MR, & Butryn ML (2007). Hedonic hunger: A new dimension of appetite?. Physiology & Behavior, 91(4), 432–439. 10.1016/j.physbeh.2007.04.006 [DOI] [PubMed] [Google Scholar]
- Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, Ochner CN, Coletta MC, Bellace D, Wallaert M, & Halford J. (2009). The power of food scale. A new measure of the psychological influence of the food environment. Appetite, 53(1), 114–118. 10.1016/j.appet.2009.05.016 [DOI] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, & Zitman FG (2010). Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Archives of General Psychiatry, 67(3), 220–229. 10.1001/archgenpsychiatry.2010.2 [DOI] [PubMed] [Google Scholar]
- Makaronidis JM, & Batterham RL (2018). Obesity, body weight regulation and the brain: Insights from fMRI. The British Journal of Radiology, 91(1089), Article 20170910. 10.1259/bjr.20170910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AA, & Davidson TL (2014). Human cognitive function and the obesogenic environment. Physiology & Behavior, 136, 185–193. 10.1016/j.physbeh.2014.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, Butler MG, & Savage CR (2010). Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity, 18(2), 254–260. 10.1038/oby.2009.220 [DOI] [PubMed] [Google Scholar]
- Mazza GL, Smyth HL, Bissett PG, Canning JR, Eisenberg IW, Enkavi AZ, Gonzalez O, Kim SJ, Metcalf SA, Muniz F, Pelham WE III, Scherer EA, Valente MJ, Xie H, Poldrack RA, Marsch LA, & MacKinnon DP (2020). Correlation database of 60 cross-disciplinary surveys and cognitive tasks assessing self-regulation. Journal of Personality Assessment, 103(2), 1–8. 10.1080/00223891.2020.1732994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley E, & Courneya KS (1994). The Subjective Exercise Experiences Scale (SEES): Development and preliminary validation. Journal of Sport & Exercise Psychology, 16, 163–177. http://epl.illinois.edu/sites/epl.illinois.edu/themes/adl/pdf/SEES_article.pdf [Google Scholar]
- McCuen-Wurst C, Ruggieri M,&Allison KC (2018). Disordered eating and obesity: Associations between binge-eating disorder, night-eating syndrome, and weight-related comorbidities. Annals of the New York Academy of Sciences, 1411(1), 96–105. 10.1111/nyas.13467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, & Nemeroff CB (2004). Are mood disorders and obesity related? A review for the mental health professional. The Journal of Clinical Psychiatry, 65(5), 634–651. 10.4088/JCP.v65n0507 [DOI] [PubMed] [Google Scholar]
- Mietus-Snyder ML, & Lustig RH (2008). Childhood obesity: Adrift in the “limbic triangle. Annual Review of Medicine, 59, 147–162. 10.1146/annurev.med.59.103106.105628 [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, & Macrae CN (2005). The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience, 17(8), 1306–1315. 10.1162/0898929055002418 [DOI] [PubMed] [Google Scholar]
- Murray CJL, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, Naghavi M, Salomon JA, Shibuya K, Vos T, Wikler D, & Lopez AD (2012). GBD 2010: Design, definitions, and metrics. Lancet, 380(9859), 2063–2066. 10.1016/S0140-6736(12)61899-6 [DOI] [PubMed] [Google Scholar]
- Naets T, Vervoort L, Verbeken S, & Braet C. (2018). Enhancing childhood multidisciplinary obesity treatments: The power of self-control abilities as intervention facilitator. Frontiers in Psychology, 9, 1956. 10.3389/fpsyg.2018.01956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs IMT, Muris P, Euser AS, & Franken IHA (2010). Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite, 54(2), 243–254. 10.1016/j.appet.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Olivo G, Gour S, & Schiöth HB (2019). Low neuroticism and cognitive performance are differently associated to overweight and obesity: A cross-sectional and longitudinal UK Biobank study. Psychoneuroendocrinology, 101, 167–174. 10.1016/j.psyneuen.2018.11.014 [DOI] [PubMed] [Google Scholar]
- Owens TE, Allen MD,& Spangler DL (2010). An fMRI study of self-reflection about body image: Sex differences. Personality and Individual Differences, 48(7), 849–854. 10.1016/j.paid.2010.02.012 [DOI] [Google Scholar]
- Park B-Y, Hong J, & Park H. (2017). Neuroimaging biomarkers to associate obesity and negative emotions. Scientific Reports, 7(1), 7664. 10.1038/s41598-017-08272-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl J, & Russell S. (2003). Bayesian networks. In Arbib MA (Ed.), The Handbook of brain theory and neural networks (Vol. 2, pp. 157–160). The MIT Press. https://ftp.cs.ucla.edu/pub/stat_ser/r216-reprint.pdf [Google Scholar]
- Pekkarinen T, Takala I, & Mustajoki P. (1996). Two year maintenance of weight loss after a VLCD and behavioural therapy for obesity: Correlation to the scores of questionnaires measuring eating behaviour. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity, 20(4), 332–337. https://www.ncbi.nlm.nih.gov/pubmed/8680460 [PubMed] [Google Scholar]
- Perlstein WM, Elbert T, & Stenger VA (2002). Dissociation in human prefrontal cortex of affective influences on working memory-related activity. Proceedings of the National Academy of Sciences of the United States of America, 99(3), 1736–1741. 10.1073/pnas.241650598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin BM (2006). Global nutrition dynamics: The world is shifting rapidly toward a diet linked with noncommunicable diseases. The American Journal of Clinical Nutrition, 84(2), 289–298. 10.1093/ajcn/84.2.289 [DOI] [PubMed] [Google Scholar]
- Price M, Higgs S, & Lee M. (2015). Self-reported eating traits: Underlying components of food responsivity and dietary restriction are positively related to BMI. Appetite, 95, 203–210. 10.1016/j.appet.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Probst M, Vandereycken W, Van Coppenolle H, & Vanderlinden J. (1995). The Body Attitude Test for patients with an eating disorder: Psychometric characteristics of a new questionnaire. Eating Disorders: The Journal of Treatment & Prevention, 3(2), 133–144. 10.1080/10640269508249156 [DOI] [Google Scholar]
- Rajan TM, & Menon V. (2017). Psychiatric disorders and obesity: A review of association studies. Journal of Postgraduate Medicine, 63(3), 182–190. 10.4103/jpgm.JPGM_712_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao W-W, Zong Q-Q, Zhang J-W, An F-R, Jackson T, Ungvari GS, Xiang Y, Su Y-Y, D’Arcy C, & Xiang Y-T (2020). Obesity increases the risk of depression in children and adolescents: Results from a systematic review and meta-analysis. Journal of Affective Disorders, 267, 78–85. 10.1016/j.jad.2020.01.154 [DOI] [PubMed] [Google Scholar]
- Rapuano KM, Zieselman AL, Kelley WM, Sargent JD, Heatherton TF, & Gilbert-Diamond D. (2017). Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proceedings of the National Academy of Sciences of the United States of America, 114(1), 160–165. 10.1073/pnas.1605548113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, & Berridge KC (2002). Positive and negative motivation in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABAelicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22(16), 7308–7320. 10.1523/JNEUROSCI.22-16-07308.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RM, & Connell JP (1989). Perceived locus of causality and internalization: Examining reasons for acting in two domains. Journal of Personality and Social Psychology, 57(5), 749–761. 10.1037/0022-3514.57.5.749 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Yohn SE, López-Cruz L, San Miguel N, & Correa M. (2016). Activational and effort-related aspects of motivation: Neural mechanisms and implications for psychopathology. Brain: A Journal of Neurology, 139(Pt 5), 1325–1347. 10.1093/brain/aww050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman CD, & Fusi S. (2010). Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annual Review of Neuroscience, 33, 173–202. 10.1146/annurev.neuro.051508.135256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel K, Burke LE, Bray GA, Steven B, Allison DB, Xavier P-S, & Eckel RH (2004). Clinical implications of obesity with specific focus on cardiovascular disease. Circulation, 110(18), 2952–2967. 10.1161/01.CIR.0000145546.97738.1E [DOI] [PubMed] [Google Scholar]
- Sarwer DB, Thompson JK, & Cash TF (2005). Body image and obesity in adulthood. The Psychiatric Clinics of North America, 28(1), 69–87, viii. 10.1016/j.psc.2004.09.002 [DOI] [PubMed] [Google Scholar]
- Sarwer DB, Wadden TA, & Foster GD (1998). Assessment of body image dissatisfaction in obese women: Specificity, severity, and clinical significance. Journal of Consulting and Clinical Psychology, 66(4), 651–654. 10.1037/0022-006X.66.4.651 [DOI] [PubMed] [Google Scholar]
- Scarpina F, Migliorati D, Marzullo P, Mauro A, Scacchi M, & Costantini M. (2016). Altered multisensory temporal integration in obesity. Scientific Reports, 6, Article 28382. 10.1038/srep28382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Sebert C, Kösling C, Grunwald M, Hilbert A, Hübner C, & Schäfer L. (2018). Neuropsychological and neurophysiological indicators of general and food-specific impulsivity in children with overweight and obesity: A pilot study. Nutrients, 10(12), Article 1983. 10.3390/nu10121983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte EM, Tuerk PW, Wadden TA, Garvey WT, Weiss D, Hermayer KL, Aronne LJ, Becker LE, Fujioka K, Miller-Kovach K, Kushner RF, Malcolm RJ, Raum WJ, Rost SL, Rubino DM, Sora ND, Veliko JL, & O’Neil PM (2020). Changes in weight control behaviors and hedonic hunger in a commercial weight management program adapted for individuals with Type 2 diabetes. International Journal of Obesity, 44, 990–998. 10.1038/s41366-020-0530-x [DOI] [PubMed] [Google Scholar]
- Schultes B, Ernst B, Wilms B, Thurnheer M, & Hallschmid M. (2010). Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. The American Journal of Clinical Nutrition, 92(2), 277–283. 10.3945/ajcn.2009.29007 [DOI] [PubMed] [Google Scholar]
- Scutari M. (2010). Learning bayesian networks with the bnlearn R package. Journal of Statistical Software, 35(3), 1–22. 10.18637/jss.v035.i0321603108 [DOI] [Google Scholar]
- Semega JL, Fontenot KR, & Kollar MA (2017). Income and poverty in the United States: 2016. Current population reports. Series P-28, Special Censuses (pp. 60–259). US Government Printing Office. https://www.census.gov/content/dam/Census/Library/publications/2019/demo/p60-266.pdf [Google Scholar]
- Shim J-S, Oh K, & Kim HC (2014). Dietary assessment methods in epidemiologic studies. Epidemiology and Health, 36, Article e2014009. 10.4178/epih/e2014009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MN, Markland D, Minderico CS, Vieira PN, Castro MM, Coutinho SR, Santos TC, Matos MG, Sardinha LB, & Teixeira PJ (2008). A randomized controlled trial to evaluate self-determination theory for exercise adherence and weight control: Rationale and intervention description. BMC Public Health, 8, Article 234. 10.1186/1471-2458-8-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MN, Vieira PN, Coutinho SR, Minderico CS, Matos MG, Sardinha LB, & Teixeira PJ (2010). Using self-determination theory to promote physical activity and weight control: A randomized controlled trial in women. Journal of Behavioral Medicine, 33(2), 110–122. 10.1007/s10865-009-9239-y [DOI] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, & Kessler RC (2006). Association between obesity and psychiatric disorders in the US adult population. Archives of General Psychiatry, 63(7), 824–830. 10.1001/archpsyc.63.7.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Zilverstand A, Gui W, Li H-J, & Zhou X. (2019). Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: A meta-analysis. Brain Stimulation, 12(3), 606–618. 10.1016/j.brs.2018.12.975 [DOI] [PubMed] [Google Scholar]
- Stein JS, Koffarnus MN, Snider SE, Quisenberry AJ, & Bickel WK (2015). Identification and management of nonsystematic purchase task data: Toward best practice. Experimental and Clinical Psychopharmacology, 23(5), 377–386. 10.1037/pha0000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C,&Small DM (2008). Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science, 322(5900), 449–452. 10.1126/science.1161550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, & Small DM (2011). Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(12), 4360–4366. 10.1523/JNEUROSCI.6604-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strimbu K, & Tavel JA (2010). What are biomarkers?. Current Opinion in HIV and AIDS, 5(6), 463–466. 10.1097/COH.0b013e32833ed177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18(6), 643–662. 10.1037/h0054651 [DOI] [Google Scholar]
- Sze YY, Stein JS, Bickel WK, Paluch RA,& Epstein LH (2017). Bleak present, bright future: Online episodic future thinking, scarcity, delay discounting, and food demand. Clinical Psychological Science, 5(4), 683–697. 10.1177/2167702617696511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Chrzanowski-Smith OJ, Hutchinson G, Kee F, & Hunter RF (2019). Relationship between monetary delay discounting and obesity: A systematic review and meta-regression. International Journal of Obesity, 43(6), 1135–1146. 10.1038/s41366-018-0265-0 [DOI] [PubMed] [Google Scholar]
- Timper K, & Brüning JC (2017). Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Disease Models & Mechanisms, 10(6), 679–689. 10.1242/dmm.026609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Yoganathan D, Mogg A, Eranti SV, Treasure J, Campbell IC, McLoughlin DM, & Schmidt U. (2005). Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biological Psychiatry, 58(10), 840–842. 10.1016/j.biopsych.2005.05.043 [DOI] [PubMed] [Google Scholar]
- Unick JL, Michael JC, & Jakicic JM (2012). Affective responses to exercise in overweight women: Initial insight and possible influence on energy intake. Psychology of Sport and Exercise, 13(5), 528–532. 10.1016/j.psychsport.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unick JL, Strohacker K, Papandonatos GD, Williams D, O’Leary KC, Dorfman L, Becofsky K, & Wing RR (2015). Examination of the consistency in affective response to acute exercise in overweight and obese women. Journal of Sport & Exercise Psychology, 37(5), 534–546. 10.1123/jsep.2015-0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainik U, Baker TE, Dadar M, Zeighami Y, Michaud A, Zhang Y, Alanis JCG, Misic B, Collins DL, & Dagher A. (2018). Neurobehavioral correlates of obesity are largely heritable. PNAS: Proceedings of the National Academy of Sciences of the United States of America, 115(37), 9312–9317. 10.1073/pnas.1718206115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainik U, Dagher A, Dubé L,&L. K (2013). Neurobehavioural correlates of body mass index and eating behaviours in adults: A systematic review. Neuroscience and Biobehavioral Reviews, 37(3), 279–299. 10.1016/j.neubiorev.2012.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainik U, García-García I, & Dagher A. (2019). Uncontrolled eating: A unifying heritable trait linked with obesity, overeating, personality and the brain. European Journal of Neuroscience, 50(3), 2430–2445. 10.1111/ejn.14352 [DOI] [PubMed] [Google Scholar]
- Vainik U, Misic B, Zeighami Y, Michaud A, Mõttus R, & Dagher A. (2020). Obesity has limited behavioural overlap with addiction and psychiatric phenotypes. Nature Human Behaviour, 4(1), 27–35. 10.1038/s41562-019-0752-x [DOI] [PubMed] [Google Scholar]
- Vainik U, Neseliler S, Konstabel K, Fellows LK,&Dagher, A. (2015). Eating traits questionnaires as a continuum of a single concept. Uncontrolled eating. Appetite, 90, 229–239. 10.1016/j.appet.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Vanstone M, Giacomini M, Smith A, Brundisini F, DeJean D, & Winsor S. (2013). How diet modification challenges are magnified in vulnerable or marginalized people with diabetes and heart disease: A systematic review and qualitative meta-synthesis. Ontario Health Technology Assessment Series, 13(14), 1–40. https://www.ncbi.nlm.nih.gov/pubmed/24228077 [PMC free article] [PubMed] [Google Scholar]
- Verloigne M, De Bourdeaudhuij I, Tanghe A, D’Hondt E, Theuwis L, Vansteenkiste M, & Deforche B. (2011). Self-determined motivation towards physical activity in adolescents treated for obesity: An observational study. The International Journal of Behavioral Nutrition and Physical Activity, 8, Article 97. 10.1186/1479-5868-8-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA, & Baler R. (2017). The dopamine motive system: Implications for drug and food addiction. Nature Reviews Neuroscience, 18(12), 741–752. 10.1038/nrn.2017.130 [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusll N, & Fowler JS (2001). Brain dopamine and obesity. Lancet, 357(9253), 354–357. 10.1016/s0140-6736(00)03643-6 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA,&Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Weygandt M, Mai K, Dommes E, Leupelt V, Hackmack K, Kahnt T, Rothemund Y, Spranger J, & Haynes J-D (2013). The role of neural impulse control mechanisms for dietary success in obesity. NeuroImage, 83, 669–678. 10.1016/j.neuroimage.2013.07.028 [DOI] [PubMed] [Google Scholar]
- Wieland DM (2019). Food addiction: A new mental health disorder?. Journal of Psychosocial Nursing and Mental Health Services, 57(12), 3–5. 10.3928/02793695-20191112-01 [DOI] [PubMed] [Google Scholar]
- Wood AC, Vainik U, Engelhardt LE, Briley DA, Grotzinger AD, Church JA, Paige Harden K, & Tucker-Drob EM (2019). Genetic overlap between executive functions and BMI in childhood. The American Journal of Clinical Nutrition, 110(4), 814–822. 10.1093/ajcn/nqz109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (n.d.). Global Physical Activity Questionnaire (GPAQ) Analysis Guide. https://www.who.int/ncds/surveillance/steps/resources/GPAQ_Analysis_Guide.pdf
- Yang AK, Mendoza JA, Lafferty CK, Lacroix F, & Britt JP (2020). Hippocampal input to the nucleus accumbens shell enhances food palatability. Biological Psychiatry, 87(7), 597–608. 10.1016/j.biopsych.2019.09.007 [DOI] [PubMed] [Google Scholar]
- Yang J, Xu X, Chen Y, Shi Z, & Han S. (2016). Trait self-esteem and neural activities related to self-evaluation and social feedback. Scientific Reports, 6, Article 20274. 10.1038/srep20274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shields GS, Guo C, & Liu Y. (2018). Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neuroscience and Biobehavioral Reviews, 84, 225–244. 10.1016/j.neubiorev.2017.11.020 [DOI] [PubMed] [Google Scholar]
- Yang Y, Shields GS, Wu Q, Liu Y, Chen H, & Guo C. (2019). Cognitive training on eating behaviour and weight loss: A meta-analysis and systematic review. Obesity Reviews, 20(11), 1628–1641. 10.1111/obr.12916 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.