Abstract
Telomerase is a ribonucleoprotein reverse transcriptase that extends the ends of chromosomes. The two telomerase subunits essential for catalysis in vitro are the telomerase reverse transcriptase (TERT) and the telomerase RNA. Using truncations and site-specific mutations, we identified sequence elements of TERT and telomerase RNA required for catalytic activity and protein-RNA interaction for Tetrahymena thermophila telomerase. We found that the TERT amino and carboxyl termini, although evolutionarily poorly conserved, are nonetheless important for catalytic activity. In contrast, high-affinity telomerase RNA binding requires only a small region in the amino terminus of TERT. Surprisingly, the TERT region necessary and sufficient for telomerase RNA binding is completely separable from the reverse transcriptase motifs. The minimal Tetrahymena TERT RNA binding domain contains two sequence motifs with ciliate-specific conservation and one TERT motif with conservation across all species. With human TERT, we demonstrate that a similar region within the TERT amino terminus is essential for human telomerase RNA binding as well. Finally, we defined the Tetrahymena telomerase RNA sequences that are essential for TERT interaction. We found that a four-nucleotide region 5′ of the template is critical for TERT binding and that the 5′ end of telomerase RNA is sufficient for TERT binding. Our results reveal at least one evolutionarily conserved molecular mechanism by which the telomerase reverse transcriptase is functionally specialized for obligate use of an internal RNA template.
Telomeres are the structures at chromosome ends that distinguish chromosome termini from double-stranded DNA breaks, protecting natural chromosome ends against fusion, recombination, and degradation (reviewed in reference 35). In most eukaryotes, telomeric DNA is composed of a tandem array of simple sequence repeats. Some of these repeats are lost with each round of chromosome duplication, requiring a separate DNA synthesis mechanism to compensate for this loss. Most commonly, this task is accomplished by the enzyme telomerase. Telomerase is a reverse transcriptase (RT) that can add telomeric repeats to chromosome ends de novo by copying a template region within its integral RNA (reviewed in reference 13). Telomerase activity is required for the sustained growth of most single-celled eukaryotes and immortal cell lines, including human tumor cells (15, 16, 37). Insufficient telomerase activity induces the replicative senescence of primary human cells in culture (3) and may contribute to age-dependent decreases in the renewal capacity of certain human tissues (27).
One of the unique features of telomerase as an RT is that the assembly of a stable ribonucleoprotein (RNP) complex is required for polymerase activity. Thus, unlike viral RTs, telomerase recognizes a template containing RNA with sequence specificity. The roles of the telomerase RNA component in enzyme function have been most thoroughly studied in ciliate telomerases (reviewed in reference 8). Template changes alter the sequence of the synthesized telomeric repeat, as expected. In addition, sequence substitutions both inside and outside the template affect RNP assembly, overall activity level, and specific features of activity such as nucleotide and repeat addition processivities. These results suggest that the RNA plays roles in addition to simply providing the template sequence. Ciliate telomerase RNAs have limited primary sequence homology but do share elements of evolutionarily conserved secondary structure (20, 22, 23, 29).
Only one protein, the telomerase RT (TERT), is known to be common to telomerase RNPs of all species (reviewed in reference 4). The central region of the TERT polypeptide contains RT active site motifs which are essential for activity in vivo and in vitro (21, 33). Aside from these RT motifs, only limited patches of TERT-specific sequence similarity have been identified (6, 24, 28, 34). It is possible to assemble an active recombinant telomerase RNP containing only TERT and telomerase RNA, bypassing the cellular requirement for other telomerase proteins and a specific telomerase RNP assembly pathway (reviewed in reference 9). Expression of TERT and telomerase RNA from human, mouse, or the ciliate Tetrahymena thermophila in rabbit reticulocyte lysate is sufficient for telomerase activity (10, 12, 33). This catalytic core of telomerase faithfully positions the template to direct accurate telomeric repeat synthesis. Notably, the assembly of recombinant human or Tetrahymena telomerase RNPs involves the participation of other activities in reticulocyte lysate (17, 19). Recombinant Tetrahymena telomerase RNP assembly occurs with high specificity and is robust enough to allow detection of telomerase RNA coimmunoprecipitated with TERT (19).
Here, we have used recombinant Tetrahymena telomerase reconstituted in rabbit reticulocyte lysate to investigate the functional significance of regions within TERT and the telomerase RNA. We find that telomerase RNP activity in vitro requires even the TERT extreme N and C termini, which lack any substantial evolutionary sequence conservation. In contrast, the stable interaction of TERT and telomerase RNA requires a surprisingly small region of the TERT N terminus, completely separable from the RT motifs. The Tetrahymena TERT RNA binding domain contains one motif present in all TERTs (T motif) and two motifs that are ciliate specific (CP and CP2 motifs). We find that a similar region of human TERT (hTERT), preceding the RT motifs and containing the T motif, also mediates human telomerase RNA (hTR) binding. For the Tetrahymena telomerase RNA, TERT interaction requires only residues at the 5′ end of the RNA, including four nucleotides that are predicted to be unpaired. These results have implications for the evolution of telomerase functional specialization and for the novel mechanism of template definition within the telomerase RNP.
MATERIALS AND METHODS
Expression constructs.
N-terminal TERT truncation expression constructs were created by PCR of the desired TERT coding region followed by cloning into pCITE4a (Novagen). C-terminal truncation expression constructs were created by internal deletions of the N-terminally His-hemagglutinin (HA) double epitope-tagged TERT expression construct (19), using restriction enzymes that recognized a unique site in the plasmid polylinker and a unique internal site in the synthetic TERT gene. Combined N- and C-terminal truncation constructs were created by PCR of the desired coding region, followed by replacement cloning into the His-HA double epitope-tagged TERT expression construct digested with NdeI and BamHI.
All RNA expression constructs were designed for transcription by T7 RNA polymerase. Expression constructs for telomerase RNAs containing nucleotide substitutions and deletions were created from pT7159 (1) by site-specific mutagenesis of double-stranded DNA, using linear amplification with Pfu DNA polymerase followed by parental plasmid removal with DpnI. Wild-type telomerase RNA and most telomerase RNA variants were linearized with FokI before transcription to produce a wild-type (WT) 3′ end. For telomerase RNA deletion construct 1–107, an EcoRI site was created at nucleotides (nt) 107 to 112; for deletion construct 1–74, an EcoRI site was created at nt 70 to 75; and for deletion construct 1–59, an XbaI site was created at nt 55 to 60. The expression construct for the 3′ tagged telomerase RNA (pKW1) was created by insertion of annealed oligonucleotides at the BamHI site of pT7159 to produce a 31-nt 3′ tag by transcription after digestion with HindIII. Telomerase RNAs expressed from pT7159 derivatives possess a 5′ leader of three guanosines.
Recombinant telomerase production.
Rabbit reticulocyte lysate expression of TERT polypeptides was performed in the presence of [35S]methionine following the manufacturer's instructions (Promega TNT). For production of RNA added back to TERT expression lysates, transcription by purified T7 RNA polymerase was performed in vitro using standard protocols (Stratagene). RNAs were purified by DNase I treatment, organic extraction, and precipitation. All RNAs were examined by gel electrophoresis to confirm length and purity, and RNA concentration was determined by fluorometry and comparative dot blot hybridization. To reconstitute a telomerase RNP by purified telomerase RNA addition, TERT expression lysate was combined with telomerase RNA, with 10 μg of bovine serum albumin (BSA) and 10 μg of tRNA or total yeast RNA, and incubated at 30°C for 30 min.
Telomerase activity assay.
For each activity assay, up to 3 μl of rabbit reticulocyte lysate reaction mixture containing TERT and telomerase RNA, brought to 10 μl in T2MG buffer (20 mM Tris-HCl [pH 8.0], 1 mM MgCl2, 10% glycerol), was used. Each 20-μl activity assay sample also contained final concentrations of 50 mM Tris-acetate (pH 8.0), 10 mM spermidine, 5 mM β-mercaptoethanol, and 2 mM MgCl2 as buffer, with 200 μM dTTP, 4 μM unlabeled dGTP, 1 μM [32P]dGTP (800 Ci/mmol), and 1 μM primer (TG)8TTG. Reaction mixtures were incubated at 30°C for 1 h followed by phenol-chloroform extraction and ethanol precipitation, and then product DNA was resolved in a 9% denaturing gel (19:1 acrylamide:bis, 7 M urea, 0.6× Tris-borate-EDTA).
Immunoprecipitation of Tetrahymena TERT-telomerase RNA complexes.
For HA antibody immunoprecipitation, GammaBind Protein G-Sepharose resin (Pharmacia) was bound to HA antibody and preblocked with 20 μg of tRNA or total yeast RNA per ml and 20 μg of BSA per ml in binding buffer (20 mM Tris-HCl [pH 8.0], 1 mM MgCl2, 10% glycerol, 0.1 M NaCl). For immunoprecipitation with the TERT C-terminal antibody, the same procedure was used but with substituted Protein A-Sepharose resin (Pharmacia) and antibody. TERT reticulocyte lysate samples were combined with telomerase RNA, 10 μg of BSA, 10 μg of total yeast RNA, and 15 μl of a bead slurry in a 350-μl final volume and mixed for 1 h at room temperature or overnight at 4°C. After resin washing in binding buffer, one-third of the sample was mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, boiled, and analyzed by electrophoresis on SDS–15% PAGE gels. The remaining two-thirds of the sample was extracted with phenol-chloroform and precipitated with ethanol. Purified nucleic acid was resolved by denaturing gel electrophoresis in a 6 or 9% denaturing gel (19:1 acrylamide:bis, 7 M urea, 0.6× Tris-borate-EDTA) and transferred to Hybond N+ (Amersham) for hybridization analysis. The RNA blots shown in Fig. 3 and 4B and C were probed with oligonucleotide 8: GAAGGTTATATCAGCACTAGATTT. The RNA blots shown in Fig. 6 and 7 were probed with oligonucleotide 11: TGACAGTTCTATTACAGATCTG. The RNA blot shown in Fig. 4D was probed for the 3′ tag of the telomerase RNA with the oligonucleotide CTACGCCCTTCTCAGAATTCAATA. The quantification of 35S protein on SDS-PAGE gels and the quantification of RNA on RNA blots was performed by phosphorimager (Fuji) analysis.
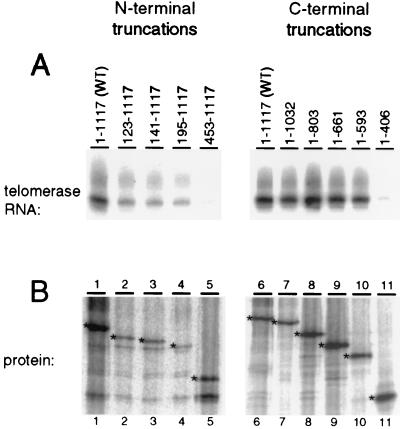
FIG. 3.
A region within the N terminus of TERT is required for telomerase RNA binding. Each TERT truncation was expressed in a 15-μl rabbit reticulocyte lysate reaction mixture and then mixed with 20 ng of telomerase RNA. The N-terminal TERT truncations were immunoprecipitated with TERT C-terminal antibody resin, and the C-terminal TERT truncations (with an N-terminal HA epitope tag) were immunoprecipitated with HA antibody resin. (A) Two-thirds of the immunoprecipitate was analyzed for telomerase RNA. The RNA coimmunoprecipitated with each TERT was purified and analyzed by RNA blot hybridization. Lanes 1 and 6, full-length TERT; lanes 2 to 5, N-terminal TERT truncations; lanes 7 to 11, C-terminal TERT truncations. (B) The remaining one-third of the immunoprecipitate was analyzed by electrophoresis on an SDS–15% PAGE gel and subsequent autoradiography. Intended protein products are indicated by asterisks.
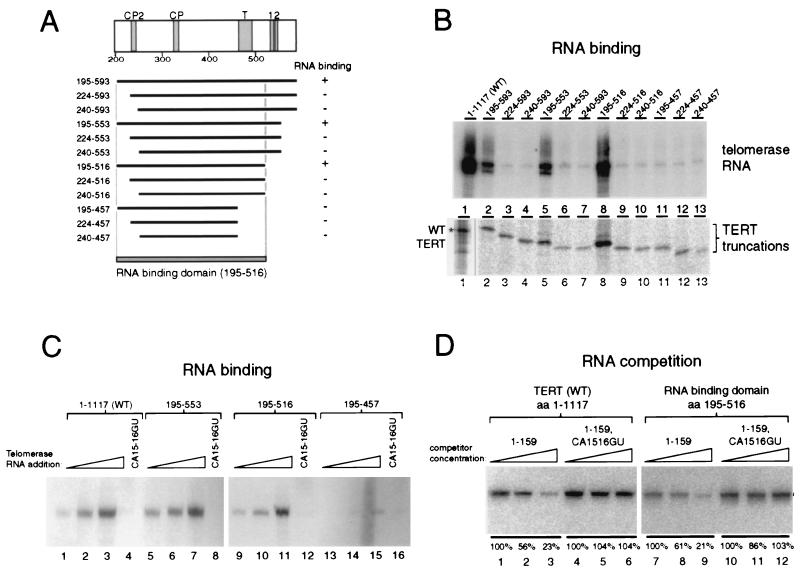
FIG. 4.
Mapping of the telomerase RNA binding domain in TERT. (A) TERT structure from amino acids 200 to 600 is illustrated with the conserved motifs in this region shaded. A summary of the 12 TERT double truncations is shown. The RNA binding domain of TERT maps to amino acids 195 to 516. (B) The ability of full-length TERT and TERT fragments to bind to 20 ng of telomerase RNA was assayed by coimmunoprecipitation. Two-thirds of each immunoprecipitate was extracted for telomerase RNA analysis by RNA blot hybridization (top), and one-third of each reaction mixture was analyzed for protein by SDS-PAGE and autoradiography (bottom). Different regions of the same SDS-PAGE gel are shown for full-length TERT (marked with an asterisk) and the TERT truncations. (C) Full-length TERT (lanes 1 to 4, 1–1117) and three TERT double truncations (lanes 5 to 8, 195–553; lanes 9 to 12, 195–516; lanes 13 to 16, 195–457) were synthesized in rabbit reticulocyte lysate. The expression levels of all TERT constructs were approximately equal (data not shown). To each protein synthesis reaction was added 2 ng (lanes 1, 5, 9, and 13), 10 ng (lanes 2, 6, 10, and 14), or 50 ng (lanes 3, 7, 11, and 15) of WT telomerase RNA or 50 ng of a CA15-16GU telomerase RNA variant (lanes 4, 8, 12, and 16). The RNA coimmunoprecipitated with each TERT was purified and analyzed by RNA blot hybridization. (D) Twenty nanograms of tagged telomerase RNA and various competitors were added to N-terminally HA epitope-tagged full-length TERT (lanes 1 to 6) or the N-terminally HA epitope-tagged RNA binding domain of TERT (lanes 7 to 12). Competitors, added as a titration of 1-, 5-, and 25-fold the molar amount of tagged RNA, were untagged full-length telomerase RNA (lanes 1 to 3 and 7 to 9) or untagged full-length telomerase RNA with a CA15-16GU substitution (lanes 4 to 6 and 10 to 12). The RNA coimmunoprecipitated with each TERT on HA antibody resin was purified and analyzed by RNA blot hybridization with an oligonucleotide recognizing the 3′ tag of the tagged telomerase RNA. Quantitation of competition is shown within each set relative to the lowest concentration of competitor.
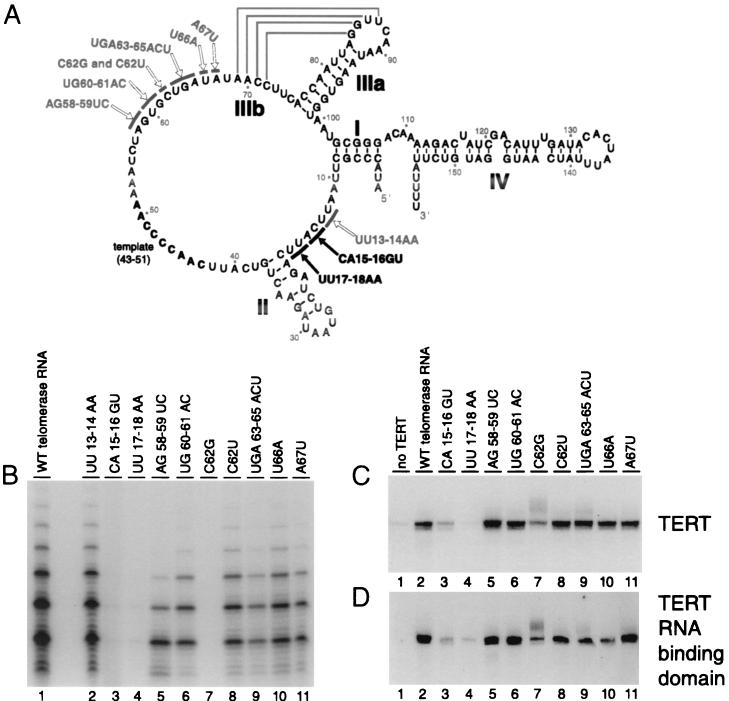
FIG. 6.
Mutational analysis of telomerase RNA. (A) The secondary structure of T. thermophila telomerase RNA is shown based on previous studies (29, 30). Stems I, II, IIIa, IIIb, and IV are indicated, and the template region is shown in bold. Arrows point to nucleotides which were mutated in this study. Black arrows indicate nucleotides of telomerase RNA which when substituted induced a strong inhibition of TERT association. (B) Full-length TERT was expressed in rabbit reticulocyte lysate, mixed with 50 ng of the indicated telomerase RNA, and then assayed for telomerase activity. (C and D) Thirty nanograms of WT telomerase RNA (lanes 1 and 2) or the telomerase RNA variant indicated (lanes 3 to 11) was coimmunoprecipitated without TERT (lanes 1) or with N-terminally HA-tagged full-length TERT (C) or TERT 195–516 (D). Coimmunoprecipitated RNA was purified and analyzed by RNA blot hybridization.
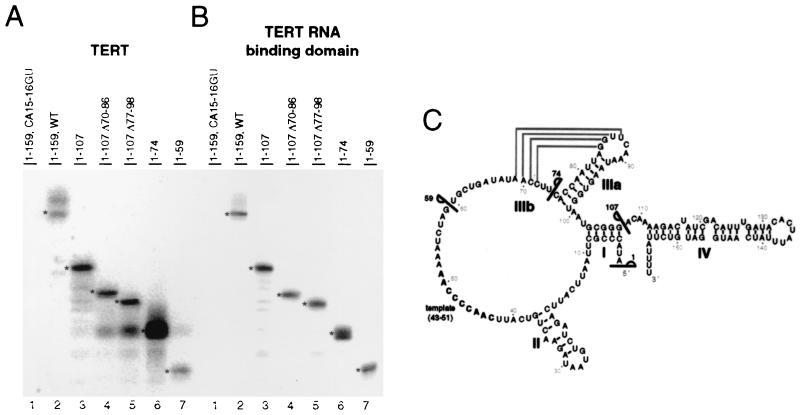
FIG. 7.
Truncation analysis of telomerase RNA. Full-length TERT (A) or the RNA binding domain of TERT (B) was expressed and mixed with 20 ng of a full-length telomerase RNA variant compromised for TERT binding (lanes 1), WT telomerase RNA (lanes 2), or telomerase RNA deletions (lanes 3 to 7). Coimmunoprecipitated RNA was purified and analyzed by RNA blot hybridization. RNAs of predicted sizes are indicated by an asterisk. (C) The secondary structure of telomerase RNA. Lines indicate the boundaries of telomerase RNA deletions.
Immunoprecipitation of hTERT-hTR complexes.
Calcium phosphate-mediated transient transfection of adenovirus-transformed human embryonic kidney (293) cells, freeze-thaw lysis and extract preparation, total RNA preparation by acid guanidine thiocyanate-phenol-chloroform extraction, RNA blot hybridization, immunoprecipitation on Flag antibody resin (Sigma), and hTERT immunoblots were performed as described previously (25, 27). Constructs for full-length hTERT expression were created by subcloning a KpnI-SalI restriction fragment containing an N-terminal Flag epitope tag into the HindIII site of pRc/CMV (Invitrogen). The 1–656, 1–617, and 1–540 hTERT expression constructs were created by subcloning restriction fragments digested with KpnI and XhoI, AatII, or BstYI, respectively, into the same pRc/CMV vector. The 326–631 construct was generated by PCR amplification with primers containing a 5′ terminal EcoRI restriction site and two 3′ terminal stop codons; this fragment was subcloned in frame behind an N-terminal Flag epitope tag in the pCR3 vector (Invitrogen).
RESULTS
N-terminally and C-terminally truncated TERTs are catalytically inactive.
TERT contains seven active site motifs (1, 2, A, B′, C, D, and E) (Fig. 1) that are conserved among RTs (21, 28). Based upon the presence of these conserved motifs, the central region of the TERT polypeptide is thought to adopt a structure similar to those of other polymerases. By analogy, the nucleotide addition active site of TERT could be viewed as a right hand, with finger, palm, and thumb regions surrounding the primer-template hybrid and positioning the primer 3′ end for nucleotidyl transfer. Due to the specialized features of telomerase, TERT should also contain regions that are responsible for stable association with the telomerase RNA and for template-independent association with primer DNA. Moreover, TERT is likely to harbor additional sites of interaction with proteins that direct telomerase assembly, localization, and regulation in vivo. Comparison of amino acids in TERT and a more typical RT, human immunodeficiency virus type 1 (HIV-1) RT, illustrates that TERT has long N-terminal and C-terminal extensions flanking the conserved RT motifs and also a larger number of amino acids separating some motifs (Fig. 1). Previous work (6, 24, 28, 34) has identified TERT-specific motifs in the N-terminal region that are conserved in all TERTs (T, T2) or specifically among ciliate TERTs (CP, CP2). These features, as well as the sequence differences in the RT motifs, are candidates for establishing telomerase-specific functions.
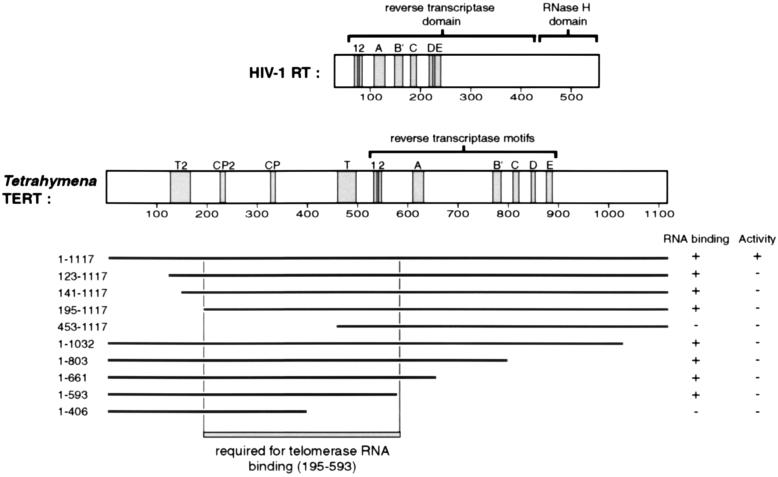
FIG. 1.
TERT truncation mapping. TERT and HIV-1 RT proteins are indicated as rectangles with the length scaled to the number of amino acids (amino acid numbering is shown). Evolutionarily conserved sequence motifs are shaded. Motifs 1, 2, and A to E are conserved among RTs. In the N-terminal TERT extension, all TERTs have motifs T and T2, whereas only ciliate TERTs have motifs CP and CP2. Below the Tetrahymena TERT structure is a summary of the N- and C-terminal truncations of TERT. Full-length TERT is 1,117 amino acids, and each TERT truncation is designated by the numbers of its first amino acid and its last amino acid. The results of telomerase RNA binding and telomerase activity assays performed on the N-terminal and C-terminal truncations are represented as follows. +, >25% of the full-length TERT level; −, <5% of the full-length TERT level. The region of TERT which is required for telomerase RNA binding as defined by this truncation analysis is boxed (amino acids 195 to 593).
To investigate the potential functions of different regions of TERT, we created a series of expression constructs that progressively truncated TERT N-terminal or C-terminal sequences. As shown in Fig. 1, N-terminal truncations were made up to motif T (to amino acid 453) and C-terminal truncations were made through the RT motif region of the protein (to amino acid 406). The N-terminal truncations were not epitope tagged but could be immunoprecipitated using antibody raised against a peptide of the C terminus of TERT, which has previously been shown not to interfere with telomerase activity (10). Each C-terminal truncation was constructed with an N-terminal HA epitope tag, which has been shown to be effective for immunoprecipitation of active recombinant TERT in vitro. Addition of this HA epitope tag to TERT has no apparent effect on telomerase activity or telomerase RNA binding (19).
The N-terminal and C-terminal TERT truncations were first tested for the ability to direct telomerase nucleotide addition activity. Following coexpression of each N-terminally or C-terminally truncated protein and telomerase RNA in rabbit reticulocyte lysate in the presence of [35S]methionine, a portion of each protein expression reaction mixture was analyzed by SDS-PAGE and autoradiography to quantitate the amount of protein synthesized. Each expression reaction produced a polypeptide of the expected molecular mass, and each protein was expressed at a comparable level (Fig. 2B). Various products smaller than the expected size were produced in each reaction. These small products were able to be immunoprecipitated using antibody against the TERT C terminus but could not be immunoprecipitated by antibody against the N-terminal epitope tag, suggesting that they are produced by translation initiation at internal ATG codons (see below). The remainder of the lysate-expressed TERT and telomerase RNA sample was assayed for its ability to elongate the single-stranded DNA primer (TG)8TTG in the presence of dTTP and radiolabeled dGTP (Fig. 2A). In a single round of template copying, telomerase will add up to six nucleotides to this primer, synthesizing a G3T2G repeat. Additional repeats of six nucleotides can also be added without product dissociation if a product 3′ end extended to the end of the template repositions at the template start.
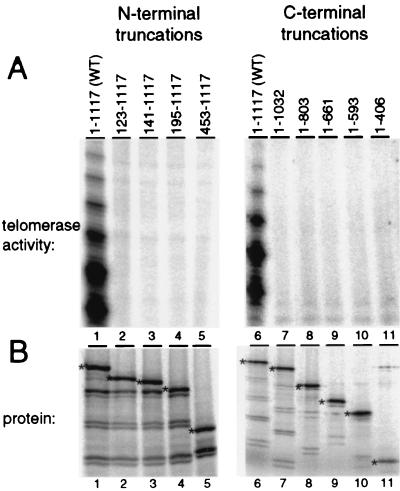
FIG. 2.
The N and C termini of TERT are necessary for telomerase activity. (A) Telomerase activity assays were performed with equal amounts (3 μl) of rabbit reticulocyte lysate reaction mixtures coexpressing TERT or the TERT truncation indicated and WT telomerase RNA. Activity was monitored by the incorporation of radiolabeled dGTP and unlabeled dTTP to elongate the primer (TG)8TTG. (B) Equal amounts (0.5 μl) of each [35S]methionine-labeled TERT and telomerase RNA coexpression reaction were analyzed by electrophoresis on an SDS–15% PAGE gel and subsequent autoradiography. Protein products of intended sizes are indicated by an asterisk.
While full-length TERT (amino acids 1 to 1117, WT) displays strong telomerase activity (Fig. 2A, lanes 1 and 6), no activity was detected using any of the N-terminal or C-terminal truncations (Fig. 2A, lanes 2 to 5 and 7 to 11). The weakly radiolabeled bands observed in the N- and C-terminal truncation lanes were observed even with the C-terminal truncation that removed the predicted polymerase domain of TERT (Fig. 2A, lane 11) and were also observed when the telomerase activity assay was performed on rabbit reticulocyte lysate not expressing TERT (data not shown). An additional, less extensive N-terminal truncation which removed only the first 53 amino acids of TERT also eliminated any detectable telomerase activity (data not shown). The least extensive TERT N- and C-terminal truncations were tested in activity assays with other primers, including an entirely telomeric sequence DNA primer (G4T2)3 and a nontelomeric sequence primer T18 that can anneal to the telomerase RNA 3′ of the template. No catalytic activity was detected with the truncated TERT proteins under any reaction condition tested, despite sensitivity to less than 1% of the WT activity level (data not shown). We assume that many of the truncated TERT proteins are likely to fold correctly when expressed in rabbit reticulocyte lysate based upon their ability to bind to telomerase RNA (see below). Thus, we conclude that the extreme N and C termini of TERT are necessary for recombinant Tetrahymena telomerase activity despite the lack of recognizable evolutionary sequence conservation within these regions of TERT.
The TERT N-terminal extension mediates stable association with telomerase RNA.
To identify which regions of TERT are required for interaction with telomerase RNA, we tested the TERT truncations using a telomerase RNA coimmunoprecipitation assay. Each TERT truncation was expressed in rabbit reticulocyte lysate in the presence of [35S]methionine and then mixed with telomerase RNA. The N-terminal truncations were immunoprecipitated using an antibody raised against a TERT C-terminal peptide (10). The HA epitope-tagged C-terminal truncations were immunoprecipitated using HA antibody. A portion of the immunoprecipitated material was analyzed by SDS-PAGE and autoradiography to visualize 35S-radiolabeled protein (Fig. 3B). Full-length TERT (Fig. 3B, lanes 1 and 6) and the TERT truncations (Fig. 3B, lanes 2 to 5 and 7 to 11) were synthesized in rabbit reticulocyte lysate and immunoprecipitated at comparable levels. Each TERT truncation protein was equally capable of being immunoprecipitated, demonstrating that all of the TERT truncations expressed were similarly soluble.
To determine the amount of RNA associated with each TERT truncation, nucleic acid in the remainder of the immunoprecipitated material was purified, resolved by denaturing gel electrophoresis, and then analyzed by RNA blot hybridization (Fig. 3A). An approximately 1,000-fold excess of nonspecific competitor RNA was added to each coimmunoprecipitation reaction to inhibit nonspecific association of telomerase RNA with the antibody resin. Alterations in the amount or type of competitor RNA added to the binding reaction did not strongly affect the amount of telomerase RNA coimmunoprecipitated, although a high concentration of total yeast RNA could slightly diminish telomerase RNA binding (data not shown).
Many of the TERT truncations, although incapable of generating a telomerase enzyme active for nucleotide addition, retained the ability to interact with telomerase RNA (Fig. 3A). The shortest C-terminally truncated TERT capable of binding telomerase ends at amino acid 593 (Fig. 3A, lane 10). The shortest N-terminal truncation capable of binding to telomerase RNA begins at amino acid 195 (Fig. 3A, lane 4). The decreased level of RNA precipitated for TERT truncations 123–1117, 141–1117, and 195–1117 (Fig. 3A, lanes 2 to 4) compared to that for wild-type TERT (Fig. 3A, lane 1) is probably due to the reduced expression levels of these truncations (Fig. 3B, lanes 2 to 4). Telomerase RNA was not immunoprecipitated with the most extensive TERT truncations (Fig. 3A, lanes 5 and 11) or in samples lacking TERT (data not shown). These results delineate a region of TERT between amino acids 195 and 593 that is necessary for telomerase RNA binding (Fig. 1). Surprisingly, this eliminates most of the central TERT RT motif region and therefore most of the evolutionarily conserved TERT residues.
The TERT RNA binding domain is entirely separable from the RT motifs.
To finely map the region of TERT which is necessary for stable telomerase RNA binding, and to determine the minimum sufficient region, we created a new set of TERT expression constructs which lacked both the TERT N and C termini. Twelve new TERT truncations were designed to include all combinations of proteins that begin at amino acids 195, 224, or 240 and end at amino acids 457, 516, 553, or 593 (Fig. 4A). The locations of the start and stop sites for these double truncations were chosen to accomplish a fairly regular increment of mass loss and to avoid disrupting regions of strong sequence conservation. Each new TERT truncation, designed to include an HA epitope tag at its N terminus, was expressed by in vitro translation and then mixed with telomerase RNA. The interaction of TERT truncations with telomerase RNA was assayed using the same coimmunoprecipitation protocol used for the C-terminal truncations. After purification on HA antibody resin, bound protein was analyzed by SDS-PAGE and autoradiography (Fig. 4B). Each protein was immunopurified with similar efficiency and migrated at approximately the expected molecular mass. Full-length TERT (amino acids 1 to 1117, WT) and the TERT truncations containing amino acids 195 to 593, 195 to 553, and 195 to 516 were able to immunoprecipitate telomerase RNA (Fig. 4B, lanes 2, 5, and 8). Notably, both N-terminal truncation to amino acid 224, up to the border of motif CP2, and truncation to amino acid 240, which completely removes motif CP2, dramatically inhibited the RNA binding activity of TERT. Motif CP2 has been implicated in the accurate definition of the template 5′ end (24). The removal of amino acids 516 to 458, a region that contains the TERT conserved motif T, was also inhibitory for RNA binding.
To confirm that the minimal TERT binding domain does bind to telomerase RNA with a similar efficiency as full-length TERT, we titrated telomerase RNA in our binding assay. After lysate expression of TERT protein, 2, 10, or 50 ng of purified telomerase RNA and an excess of nonspecific total yeast RNA were added to equal volumes of the TERT expression lysates before immunoprecipitation. We found, as before, that full-length TERT (Fig. 4C, lanes 1 to 3) and TERT fragments including amino acids 195 to 553 (Fig. 4C, lanes 5 to 7) or 195 to 516 (Fig. 4C lanes 9 to 11) coimmunoprecipitated telomerase RNA effectively. Removing amino acids 457 to 516 diminished RNA binding activity substantially (Fig. 4C, lanes 13 to 15).
A previous study demonstrated that a substitution of two nucleotides, CA15-16GU, 5′ of the T. thermophila RNA template inhibited catalytic activity and TERT interaction (19). We used this telomerase RNA variant to test whether the TERT N-terminal fragments retained a binding specificity parallel to that of full-length TERT. Indeed, we found that the CA15-16GU telomerase RNA substitution inhibited the interaction of telomerase RNA with the TERT fragments as it did with full-length TERT (Fig. 4C, lanes 4, 8, 12, and 16). Therefore, RNA association with the minimized RNA binding domain of TERT shows the same requirement for CA15-16 as the full-length protein.
We also demonstrated this specificity using a competition assay rather than a direct binding assay (Fig. 4D). With full-length TERT or the RNA binding domain of TERT expressed in lysate, we assayed for coimmunoprecipitation of a full-length telomerase RNA bearing a 3′ sequence tag. Extension of the telomerase RNA 3′ end does not inhibit activity (data not shown) or TERT interaction (see below). TERT protein was mixed with 20 ng of tagged RNA plus a 1-, 5-, or 25-fold molar excess of competitor RNA relative to the tagged RNA, which itself was in molar excess of the protein. As a positive control, untagged full-length telomerase RNA competed the coimmunoprecipitation of the tagged RNA by TERT (Fig. 4D, lanes 1 to 3) or the RNA binding domain of TERT (Fig. 4D, lanes 7 to 9). Telomerase RNA with the CA15-16GU substitution did not compete the immunoprecipitation of tagged telomerase RNA by TERT (Fig. 4D, lanes 4 to 6) or the RNA binding domain of TERT (Fig. 4D, lanes 10 to 12). We conclude that the interaction of the minimized TERT RNA binding domain (amino acids 195 to 516) with the telomerase RNA is specific and reflects interactions required for stable protein-RNA association in the WT RNP.
The N terminus of hTERT also mediates telomerase RNA association.
We next asked if separation of an independently functional telomerase RNA binding domain from the RT active site motifs is an evolutionarily conserved feature of TERTs. To do this, we analyzed the telomerase RNA binding properties of TERT from human cells. We created a set of constructs for hTERT expression in vivo (Fig. 5A). Because in vitro reconstitution of hTERT and hTR in rabbit reticulocyte lysate is strikingly inefficient compared to reconstitution of Tetrahymena TERT and telomerase RNA, in vivo reconstitution of the human RNP was used to provide a more robust assay. Each hTERT expression construct was engineered to include a Flag epitope tag at the hTERT N terminus. We tested three progressive C-terminal truncations which removed RT motifs A to E (amino acids 1 to 656), RT motifs 1 and 2 (amino acids 1 to 617), and motif T (amino acids 1 to 540). One additional construct was tested which removed the first 325 amino acids as well as all of the RT motifs, while leaving motif T intact (amino acids 326 to 613). Flag-tagged full-length hTERT (amino acids 1 to 1132) and hTERT truncations were expressed by transient transfection of immortal human 293 cells, which contain abundant hTR and telomerase activity. The interaction of the epitope-tagged hTERT proteins with endogenous hTR was assayed by Flag antibody immunoprecipitation followed by RNA blot hybridization. As with Tetrahymena TERT, we found that all of the conserved RT motifs are dispensable for hTERT-hTR interaction (Fig. 5B, hTR 1–451, lanes 2, 4, and 6). Removal of motif T, however, abrogated detectable hTERT-hTR association (Fig. 5B, lane 8). In contrast, removal of the N-terminal 325 amino acids of hTERT, including the conserved motif T2, did not similarly inhibit hTERT-hTR association (Fig. 5B, lane 10). The amount of hTR immunoprecipitated by hTERT 326–613 (Fig. 5B, lane 10) is reduced relative to the other hTERT fragments (Fig. 5B, lanes 2, 4, and 6). This may be due to the reduced expression and recovery of this hTERT fragment by immunoprecipitation (Fig. 5C, lane 5). The expression and immunoprecipitation efficiencies of the other hTERT fragments were comparable (Fig. 5C, lanes 1 to 4, and data not shown).
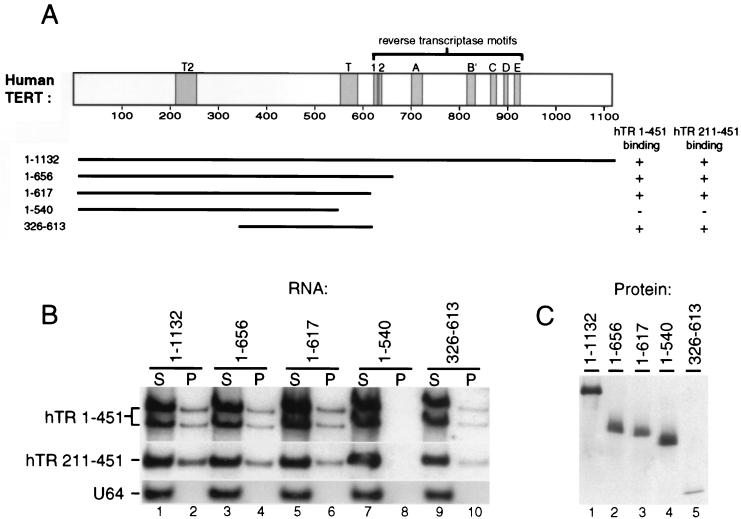
FIG. 5.
Evolutionary conservation of TERT-telomerase RNA interaction. (A) hTERT sequence is indicated as a rectangle scaled to amino acid length with conserved RT and TERT motifs shaded. C- and N-terminal truncations are indicated below; 1–1132 is the full-length protein. The results of in vivo association assays between hTR and hTERT truncations are indicated by a + or − indicating detectable or undetectable association, respectively. (B and C) Extracts of human 293 cells expressing Flag epitope-tagged full-length hTERT or hTERT truncations were immunoprecipitated with Flag antibody resin. (B) Total RNA remaining in the immunoprecipitation supernatant (S) and coimmunoprecipitated with each hTERT (P) was analyzed by RNA blot hybridization for full-length endogenous hTR (hTR 1–451), a coexpressed domain of recombinant hTR (hTR 211–451), and the endogenous H/ACA snRNA U64. Immunoprecipitates were loaded at a 2× concentration relative to supernatants. (C) Ten percent of each immunoprecipitate was analyzed by immunoblotting with a polyclonal antibody against an hTERT peptide (27); all truncations contained this hTERT peptide sequence.
We have previously demonstrated that two separable regions of hTR each interact independently with hTERT in vivo and in vitro (26). One of these regions is contained within the H/ACA domain at the 3′ end of hTR (nt 211 to 451), which can accumulate in vivo at a level comparable to the full-length molecule (nt 1 to 451). We therefore could test whether the hTR H/ACA domain alone interacts equally well with full-length hTERT and each of the hTERT truncations using coexpression by transient transfection (Fig. 5B, hTR 211–451). We found that the specificity of association of the recombinant hTR H/ACA domain alone with the hTERT fragments closely parallels that of full-length endogenous hTR. Thus, the T motif, but not amino acids 1 to 325, appears to be required for association of the hTR H/ACA domain with hTERT. As a control for the specificity of RNAs immunoprecipitated with the truncated hTERTs, we assayed for the immunoprecipitation of the box H/ACA small nucleolar RNA, U64. No detectable U64 was precipitated in any of the reactions (Fig. 5B, lanes 2, 4, 6, 8, and 10).
Telomerase RNA substitutions define the TERT binding site.
Finally, we sought to map the region of Tetrahymena telomerase RNA that interacts with the TERT RNA binding domain. The secondary structure of the 159-nt T. thermophila telomerase RNA is depicted in Fig. 6A, derived from phylogenetic sequence comparison studies (29, 30) and consistent with in vitro and in vivo chemical modification studies (2, 36). In addition to nucleotides CA15-16, previous work has revealed a requirement for the C19-G37 base pair at the end of stem II as important for TERT binding (19). In contrast with this sequence 5′ of the template, stem loops IIIa, IIIb, and IV, the template sequence (nt 43 to 51), and the adjacent nucleotides UCA38-40, UU41-42, UCU55-57, and C62, can all be substituted or deleted without substantial impact on stable TERT interaction (19 and data not shown). To determine if any other telomerase RNA residues are necessary for TERT binding, we created telomerase RNA variants with substitutions within the remaining residues between stem loops I and IIIb.
Depending on the extent of phylogenetic sequence conservation and susceptibility to chemical or enzymatic modification, we substituted one, two, or three positions at a time to the complementary base(s). Each of these telomerase RNA variants was transcribed in vitro, purified, and then added to full-length TERT synthesized in reticulocyte lysate. The reconstituted samples were assayed for telomerase activity. The previously described CA15-16GU substitution and the adjacent UU17-18AA substitution both inhibited telomerase activity (Fig. 6B, lanes 3 and 4). Although the C62G substitution (Fig. 6B, lane 7) was inhibitory as previously described (19), the more conservative C62U telomerase RNA had activity close to that of WT (Fig. 6B, lane 8). Repeat addition processivity appeared reduced with the substitution AG58-59UC (Fig. 6B, lane 5), the same phenotype previously observed with the adjacent substitution UCU55-57AGA (19).
We next tested whether these telomerase RNA variants retained the ability to interact with TERT and the RNA binding domain of TERT. N-terminally HA epitope-tagged full-length TERT expressed in reticulocyte lysate was combined with 30 ng of each purified telomerase RNA and then immunopurified using HA antibody resin. A low background level of telomerase RNA was immunoprecipitated by antibody resin alone (Fig. 6C and D, lanes 1). The CA15-16GU and UU17-18AA telomerase RNAs were reduced in interaction with TERT or the TERT RNA binding domain compared with the WT RNA (Fig. 6C and D, compare lanes 3 and 4 to lane 2), approaching the background level of RNA associated with antibody resin in the absence of TERT. The C62G telomerase RNA was also slightly compromised in TERT interaction, although not as substantially as CA15-16GU or UU17-18AA RNAs, and none of the other substitutions had any detectable effect (Fig. 6C and D, lanes 5 to 11). This is consistent with previous results (19). These mutational analyses suggest that the CAUU15-18 sequence in particular is important for high-affinity TERT binding.
The 5′ end of telomerase RNA is sufficient for stable TERT interaction.
Truncation analysis of telomerase RNA has demonstrated that removal of stem loop IV (nt 108 to 159), stem loop IIIa (nt 77 to 98), or stem loop IIIb (nt 70 to 86) or substitution of distal stem loop II (nt 22 to 34) with a tetraloop each individually did not affect binding of telomerase RNA to TERT (19). Based upon this previous study and the implication of nt 15 to 18 as critical for TERT binding, it seemed that the 3′ end of the telomerase RNA might be dispensable for TERT binding.
To test this directly, we created expression constructs for a series of 3′ truncations of telomerase RNA. Each telomerase RNA truncation was transcribed in vitro, gel purified, and then tested for TERT binding by coimmunoprecipitation. As positive and negative controls for this assay, we found that full-length telomerase RNA bound to both TERT and the RNA binding domain of TERT (Fig. 7A and B, lanes 2), whereas binding of the CA15-16GU variant was strongly inhibited (Fig. 7A and B, lanes 1). Telomerase RNA with deletion of stem loop IV (Fig. 7A and B, lanes 3) or simultaneous deletion of stem loops IV and alternate stem loop IIIa and IIIb (Fig. 7A and B, lanes 4 and 5) all bound TERT at levels comparable to full-length telomerase RNA. Next, two even more substantial telomerase RNA truncations were tested which included only nt 1 to 74 and 1 to 59 of the 159-nt full-length sequence. Both these shorter RNAs retained high-affinity TERT binding, although there was a possible decrease in the amount of the shortest 1–59 truncation bound to at least the full-length TERT protein (Fig. 7A and B, lanes 6 and 7). As expected, all of the telomerase RNA deletions bound much more poorly to a TERT fragment lacking the T motif (amino acids 195 to 457) (data not shown) than to full-length TERT or the RNA binding domain of TERT. This truncation analysis demonstrates that the 5′ end of Tetrahymena telomerase RNA, up to nt 59, is sufficient for high-affinity RNA binding to full-length TERT or the isolated TERT RNA binding domain.
DISCUSSION
Catalytically, telomerase is an RT: it copies an RNA template to synthesize one strand of DNA (14). Structurally as well, the TERT subunit of the telomerase RNP has primary sequence homology with RTs (21). These similarities aside, telomerase is a highly atypical polymerase. Telomerase has a unique substrate specificity for chromosome ends, and unlike all other RTs, it utilizes only a precisely defined internal RNA template. Both the template function and the as yet incompletely defined nontemplate functions of the telomerase RNA make the telomerase enzyme of particular interest as a model system for understanding the roles of RNA and protein in a catalytically codependent RNP complex.
We found that even the extreme TERT N and C termini are required for the catalytic activity of recombinant Tetrahymena telomerase. The shortest N-terminal and C-terminal truncations tested here had 53 and 85 amino acids of TERT removed, respectively. This result is somewhat surprising given the minimal evolutionary sequence conservation between even ciliate TERTs in these regions. Also, truncation of the entire C terminus up to RT motif E in the Saccharomyces cerevisiae TERT Est2p does not prevent telomere maintenance in vivo (11). One possible explanation for this difference in the impact of C-terminal truncation could be that other components of the yeast telomerase RNP rescue a catalytic deficiency imposed by C-terminal TERT truncation in vivo. It is also possible that our C-terminal truncations impacted some aspect of global TERT protein folding, compromising telomerase RNA binding. However, it seems most likely that the function of the TERT C-terminal region varies among TERTs of different species. A unigenic evolution approach (11) and a directed mutagenesis approach (34) investigating the Est2p sequences required for function in vivo revealed large regions of the Est2p N terminus that were less tolerant of sequence substitution or deletion. The correspondence of these regions of Est2p with specific amino acids in the Tetrahymena TERT N terminus is difficult to define, given the relative uncertainty of global sequence alignments in this region.
The main focus of the study described here was to elucidate how the catalytic telomerase protein and RNA subunits interact. Within the Tetrahymena telomerase RNA, only the RNA 5′ end including nt 1 to 59 is necessary for TERT interaction. Thus, the high-affinity TERT binding site appears to require a surprisingly small part of the full-length sequence. Our results, combined with those of a previous study (19), suggest that the CAUU15-18 sequence of telomerase RNA is the primary site for TERT binding, with a direct or indirect requirement for at least the C19-G37 base pair of adjacent stem II. Both full-length TERT and the TERT RNA binding domain require the CAUU15-18 sequence in telomerase RNA for high-affinity interaction. The CA15-16 dinucleotide is preferentially protected from chemical modification in the endogenous RNP relative to purified or deproteinized telomerase RNA alone (36). Our results indicate that this protection is likely to be the direct consequence of protein association. Notably, a CA(U/C)U sequence is conserved among telomerase RNAs from Tetrahymena and Paramecium species (22, 23, 29).
Utilizing a truncational analysis, we identified a 322-amino-acid region in the amino terminus of Tetrahymena TERT that is necessary and sufficient for telomerase RNA binding in vitro. Although there are potential limitations to our mutagenesis approach, including the potential for false-negative results due to misfolding of truncated recombinant proteins, our findings overall are highly consistent. Full-length TERT and the 322-amino-acid TERT RNA binding domain both bind telomerase RNA with high apparent affinity and similar specificity. We also identified a 288-amino-acid region of hTERT that is necessary and sufficient for association with hTR in vivo. Our mapping of Tetrahymena and hTERT telomerase RNA binding domains represents the first identification of independently functional subregions of TERTs from any species. Notably, both N-terminal TERT RNA binding domains lack any overlap with the central region of TERT containing the RT active site motifs. The reiterative use of a fixed internal template thus appears likely to be programmed from elsewhere than within the boundaries of a typical polymerase active site.
For Tetrahymena TERT, the telomerase RNA binding domain encompasses two sequence motifs that are conserved among ciliate TERTs (motifs CP and CP2) and one motif which is present in all TERTs (motif T). Truncation of either motif CP2 or motif T inhibited telomerase RNA association. In a contemporary study, alanine substitution of amino acids in Tetrahymena TERT motifs CP and T was also found to decrease telomerase RNA binding (5). It is particularly interesting to us that the RNA binding domain of TERT includes both of the ciliate-specific N-terminal motifs, CP and CP2. First, because the secondary structures of ciliate telomerase RNAs are similar, ciliate TERTs are likely to interact with their respective telomerase RNAs in a related fashion. Second, results from a site-specific mutagenesis study of Tetrahymena TERT indicate that motif CP2 plays a pivotal role in defining boundaries of the internal RNA template (24).
For hTERT, we defined a telomerase RNA binding domain of similar size to the Tetrahymena TERT RNA binding domain, which also precedes the RT active site motifs and contains the conserved motif T. Interestingly, our results predict that a catalytically inactive hTERT isoform, derived from mRNA alternative splicing, should contain a completely functional RNA binding domain (18, 32). Thus, the hTERT RNA binding domain alone may function as a physiologically expressed dominant-negative inhibitor, capable of sequestering hTR from interaction will full-length hTERT. The similarity between the Tetrahymena and human telomerase RNA binding domains is surprising considering the substantial differences between ciliate and human telomerase RNPs (26). Most obviously, ciliate and vertebrate telomerase RNAs share no conservation of primary sequence and only limited conservation of secondary structure (7). For the Tetrahymena telomerase RNA, there appears to be a single TERT binding site that is 5′ of the template. In contrast, the human telomerase RNA contains two separable regions which interact independently with hTERT (26) and has no sequence 5′ of the template required for hTERT-hTR interaction (31 and our unpublished observations). It is therefore particularly striking that removal of motif T in both Tetrahymena and human TERTs has a similar impact on telomerase RNA binding. It is possible that the conserved residues of motif T direct the stable folding of a novel RNA binding motif and that evolutionarily variable residues outside of this core are responsible for the sequence specificity of RNA binding.
ACKNOWLEDGMENTS
We thank Jill Licht and Keren Witkin for telomerase RNA expression constructs and Carla Schultz for the epitope-tagged TERT expression construct. We also thank Donald Rio, James Berger, and members of the Collins lab for discussion on the manuscript.
This work was funded by a grant from the National Institutes of Health (GM54198) and a Burroughs Wellcome Fund New Investigator Award to K.C.
REFERENCES
- 1.Autexier C, Greider C W. Functional reconstitution of wild-type and mutant Tetrahymena telomerase. Genes Dev. 1994;8:563–575. doi: 10.1101/gad.8.5.563. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya A, Blackburn E H. Architecture of telomerase RNA. EMBO J. 1994;13:5721–5731. doi: 10.1002/j.1460-2075.1994.tb06910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C-P, Morin G, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 4.Bryan T M, Cech T R. Telomerase and the maintenance of chromosome ends. Curr Opin Cell Biol. 1999;11:318–324. doi: 10.1016/S0955-0674(99)80043-X. [DOI] [PubMed] [Google Scholar]
- 5.Bryan T M, Goodrich K J, Cech T R. Telomerase RNA bound by protein motifs specific to telomerase reverse transcriptase. Mol Cell. 2000;6:493–499. doi: 10.1016/s1097-2765(00)00048-4. [DOI] [PubMed] [Google Scholar]
- 6.Bryan T M, Sperger J M, Chapman K B, Cech T R. Telomerase reverse transcriptase genes identified in Tetrahymena thermophila and Oxytricha trifallax. Proc Natl Acad Sci USA. 1998;95:8479–8484. doi: 10.1073/pnas.95.15.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J-L, Blasco M A, Greider C W. Secondary structure of vertebrate telomerase RNA. Cell. 2000;100:503–514. doi: 10.1016/s0092-8674(00)80687-x. [DOI] [PubMed] [Google Scholar]
- 8.Collins K. Ciliate telomerase biochemistry. Annu Rev Biochem. 1999;68:187–218. doi: 10.1146/annurev.biochem.68.1.187. [DOI] [PubMed] [Google Scholar]
- 9.Collins K. Mammalian telomeres and telomerase. Curr Opin Cell Biol. 2000;12:378–383. doi: 10.1016/s0955-0674(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 10.Collins K, Gandhi L. The reverse transcriptase component of the Tetrahymena telomerase ribonucleoprotein complex. Proc Natl Acad Sci USA. 1998;95:8485–8490. doi: 10.1073/pnas.95.15.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman K L, Cech T R. Essential functions of amino-terminal domains in the yeast telomerase catalytic subunit revealed by selection for viable mutants. Genes Dev. 1999;13:2863–2874. doi: 10.1101/gad.13.21.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg R A, Allsopp R C, Chin L, Morin G B, DePinho R A. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene. 1998;16:1723–1730. doi: 10.1038/sj.onc.1201933. [DOI] [PubMed] [Google Scholar]
- 13.Greider C W. Telomerase biochemistry and regulation. In: Blackburn E H, Greider C W, editors. Telomeres. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 35–68. [Google Scholar]
- 14.Greider C W, Blackburn E H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 15.Hahn W C, Stewart S A, Brooks M W, York S G, Eaton E, Kurachi A, Beijersbergen R L, Knoll J H M, Meyerson M, Weinberg R A. Inhibition of telomerase limits the growth of human cancer cells. Nat Med. 1999;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 16.Herbert B-S, Pitts A E, Baker S I, Hamilton S E, Wright W E, Shay J W, Corey D R. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt S E, Aisner D L, Baur J, Tesmer V M, Dy M, Ouellette M, Trager J B, Morin G B, Toft D O, Shay J W, Wright W E, White M A. Functional requirement of p23 and Hsp90 in telomerase complexes. Genes Dev. 1999;13:817–826. doi: 10.1101/gad.13.7.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilian A, Bowtell D D L, Abud H E, Hime G R, Venter D J, Keese P K, Duncan E L, Reddel R R, Jefferson R A. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum Mol Genet. 1997;6:2011–2019. doi: 10.1093/hmg/6.12.2011. [DOI] [PubMed] [Google Scholar]
- 19.Licht J D, Collins K. Telomerase RNA function in recombinant Tetrahymena telomerase. Genes Dev. 1999;13:1116–1125. doi: 10.1101/gad.13.9.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lingner J, Hendrick L L, Cech T R. Telomerase RNAs of different ciliates have a common secondary structure and a permuted template. Genes Dev. 1994;8:1984–1998. doi: 10.1101/gad.8.16.1984. [DOI] [PubMed] [Google Scholar]
- 21.Lingner J, Hughes T R, Shevchenko A, Mann M, Lundblad V, Cech T R. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 22.McCormick-Graham M, Romero D P. Ciliate telomerase RNA structural features. Nucleic Acids Res. 1995;23:1091–1097. doi: 10.1093/nar/23.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormick-Graham M, Romero D P. A single telomerase RNA is sufficient for the synthesis of variable telomeric DNA repeats in ciliates of the genus Paramecium. Mol Cell Biol. 1996;16:1871–1879. doi: 10.1128/mcb.16.4.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller M C, Liu J K, Collins K. Template definition by Tetrahymena telomerase reverse transcriptase. EMBO J. 2000;19:4412–4422. doi: 10.1093/emboj/19.16.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell J R, Cheng J, Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol Cell Biol. 1999;19:567–576. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell J R, Collins K. Human telomerase activation requires two independent interactions between telomerase RNA and telomerase reverse transcriptase in vivo and in vitro. Mol Cell. 2000;6:361–371. doi: 10.1016/s1097-2765(00)00036-8. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell J R, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T M, Morin G B, Chapman K B, Weinrich S L, Andrews W H, Lingner J, Harley C B, Cech T R. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 29.Romero D P, Blackburn E H. A conserved secondary structure for telomerase RNA. Cell. 1991;67:343–353. doi: 10.1016/0092-8674(91)90186-3. [DOI] [PubMed] [Google Scholar]
- 30.ten Dam E, van Belkum A, Pleij K. A conserved psuedoknot in telomerase RNA. Nucleic Acids Res. 1991;19:6951. doi: 10.1093/nar/19.24.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tesmer V M, Ford L P, Holt S E, Frank B C, Yi X, Aisner D L, Ouellette M, Shay J W, Wright W E. Two inactive fragments of the integral RNA cooperate to assemble active telomerase with the human protein catalytic subunit (hTERT) in vitro. Mol Cell Biol. 1999;19:6207–6216. doi: 10.1128/mcb.19.9.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulaner G A, Hu J, Vu T H, Giudice L C, Hoffman A R. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58:4168–4172. [PubMed] [Google Scholar]
- 33.Weinrich S L, Pruzan R, Ma L, Ouellette M, Tesmer V M, Holt S E, Bodnar A G, Lichsteiner S, Kim N W, Trager J B, Taylor R D, Carlos R, Andrews W H, Wright W E, Shay J W, Harley C B, Morin G B. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet. 1997;17:498–502. doi: 10.1038/ng1297-498. [DOI] [PubMed] [Google Scholar]
- 34.Xia J, Peng Y, Mian I S, Lue N F. Identification of functionally important domains in the N-terminal region of telomerase reverse transcriptase. Mol Cell Biol. 2000;20:5196–5207. doi: 10.1128/mcb.20.14.5196-5207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zakian V A. Telomeres: beginning to understand the end. Science. 1995;270:1601–1607. doi: 10.1126/science.270.5242.1601. [DOI] [PubMed] [Google Scholar]
- 36.Zaug A J, Cech T R. Analysis of the structure of Tetrahymena nuclear RNAs in vivo: telomerase RNA, the self-splicing rRNA intron, and U2 snRNA. RNA. 1995;1:363–374. [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Mar V, Zhou W, Harrington L, Robinson M O. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 1999;13:2388–2399. doi: 10.1101/gad.13.18.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]