Abstract
Dermatofibroma (DF) or fibrous histiocytoma is one of the most frequent benign cutaneous soft-tissue lesions, characterized by a post-inflammatory tissue reaction associated with fibrosis of the dermis. Clinically DFs have a polymorphous clinical aspect from the solitary, firm, single nodules to multiple papules with a relatively smooth surface. However, multiple atypical clinicopathological variants of DFs have been reported and, therefore, clinical recognition may become challenging, leading to a more burdensome identification and sometimes to misdiagnosis. Dermoscopy is considered an important tool in DFs diagnosis, as it improves diagnostic accuracy for clinically amelanotic nodules. Although typical dermoscopic patterns are most frequently seen in clinical practice, there have also been some atypical variants described, mimicking some underlying recurrent and sometimes harmful skin afflictions. Usually, no treatment is required, although an appropriate work-up may be necessary in specific cases, such as in the presence of atypical variants or a history of recent changes. This narrative review’s aim is to summarize current evidence regarding clinical presentation, positive and differential diagnosis of atypical dermatofibromas and also to raise awareness about the importance of specific characteristics of atypical variants to better differentiate them from malignant conditions.
Keywords: dermatofibroma, atypical fibrous histiocytoma, dermoscopy, benign cutaneous tumours
1. Introduction
Dermatofibroma (DF), also known as fibrous histiocytoma, is a relatively common benign cutaneous tumour characterized by a post-inflammatory tissue reaction associated with fibrosis of the dermis [1,2,3,4,5]. It mostly occurs in young or middle-aged (20 to 40 years old) adults, generally in female patients, although there are histologic variants frequently encountered in males [1,3,6]. DFs with classical morphology have also been described in children aged less than 5 years old [7]. Although various locations have been noticed (head, face, auricle, neck, trunk, shoulder, pelvic girdles, and digits), DFs usually appear on the lower extremities [1,6,7,8,9,10,11,12,13]. DFs are generally asymptomatic but sometimes can become pruritic and tender [4,5]. On palpation, upon lateral compression of the skin, DFs characteristically sink below the level of the skin, a feature also known as the dimple sign [4,5,8,9,14].
The pathogenesis of DFs is unknown, although they usually arise as consequence of local trauma (tuberculin skin testing, skin tattooing, traumatism caused by razor, thorns or wood splinters etc), insect bites or an underlying condition (folliculitis) [2,4,15,16,17,18]. Even though local recurrence and rarely distant metastases have been mentioned in the scientific literature, DFs are considered benign lesions [19].
Yamamoto et al. Addressed the role of mast cells in the development of DFs, as they were found in solitary and multiple variants [20]. Mast cells could induce histopathologic changes, such as basal melanosis, acanthosis of the epidermis, and mononuclear cell recruitment [20].
Immunohistochemical testing identified the presence of factor XIIIa, which marks dermal dendritic cells [21,22,23,24]. MAC 387, which was labeled histiocytes, did not show relevant results, and the presence of CD68-positive histiocytes was not consistent [21,22,23,24]. One study analyzing 28 cases of dermatofibromas, showed that the majority of spindle-shaped cells, independently of the histological variant, stained positively for HSP47, a marker for skin fibroblasts [21]. Transforming growth factor-beta may also stimulate the fibrosis found in dermatofibromas [25,26].
Other studies suggested that the cell surface proteoglycan, fibroblast growth factor receptor 2, which plays a role in the epithelial–mesenchymal cross-talk, and syndecan-1, may also be involved in the pathogenesis of dermatofibromas [27,28]. Furthermore, CD14+ monocytes have been proposed as the original cells of dermatofibromas [22].
Regarding gene fusion, ALKgene rearrangement and overexpression has been found in both epithelioid and atypical dermatofibromas [29,30,31,32]. As such there have been reported rare autosomal dominant familial cases [2,4].
Reactive tissue alterations and neoplastic proliferation clinical clonality have been suggested as mechanisms involved in the pathogenesis of DFs [33,34]. Spontaneous development, lack of regression and the presence of clonal markers during the analysis of X-chromosome inactivation, may also support the clonal or neoplastic mechanism [19]. Mentzel et al. Investigated 7 cases of clinically aggressive dermatofibromas and underlined the malignant transformation of a cellular dermatofibroma into a spindle cell sarcoma [35]. Chromosomal aberrations by array-comparative genomic hybridization have been proposed as possible diagnostic tools for potentially metastatic dermatofibromas [36].
DFs usually have an excellent prognosis and do not require treatment unless the lesion is changing, bleeding, becomes symptomatic or suspicious, another diagnosis is more probable or the patient demands it clinical cosmetic reasons [4,19]. Complete surgical excision with clear margins for histopathologic examination is the most common therapy [4,19,37]. Atypical variants are more prone to recur and as a result, re-excision might be necessary [37]. Another alternative is liquid nitrogen cryotherapy [37].
2. Materials and Methods
A systematic literature search was done in the PubMed, Web of Science Core Collection, and Google Scholar databases, using the terms “atypical dermatofibroma”, and “atypical fibrous histiocytoma”. A total number of 1092 articles, 135 reviews, and 571 case reports were found. All the articles, reviews, and case reports included in the study were limited to English full text in humans. Finally, 134 studies were included in the review. The pictures are from the patients admitted in “Elias” University Emergency Hospital in the period 2021–2022. Written informed consent was obtained from all subjects involved in the study (Figure 1).
Figure 1.
PRISMA flowchart [38].
3. Results
This narrative review aimed to reevaluate the clinical and land dermoscopic patterns of atypical dermatofibromas compared to the typical ones. Although the clinical diagnosis of DFs may be simple in daily practice, in the presence of various patterns, diagnosis of DFs can become challenging. Therefore, specific characterization of these atypical variants is essential in differentiating them from malignant conditions and assessing the risk of local recurrence.
3.1. Clinical Presentation
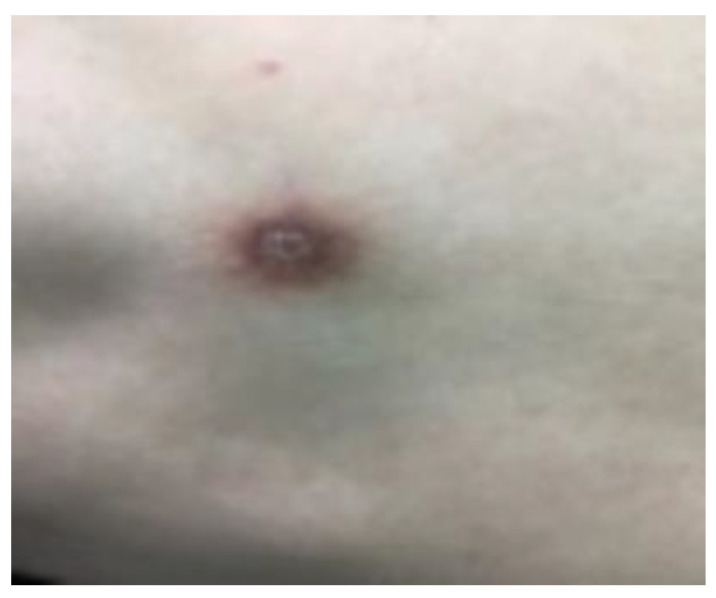
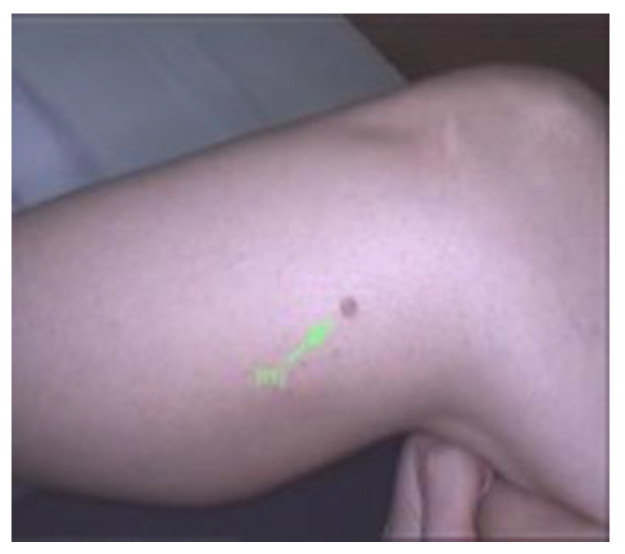
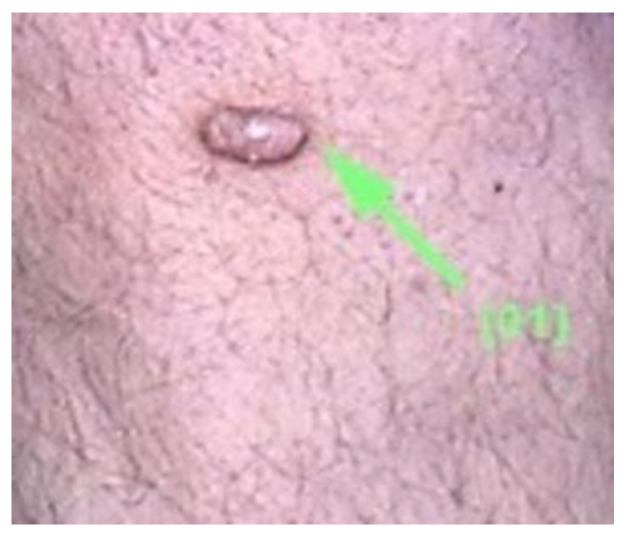
Clinically, DF usually presents as solitary, hyperkeratotic, small (0.3 to 1 cm) and slow-growing nodule with a red-brown surface [2,4,5,19] (Figure 2). The rate of recurrence seems to be higher in lesions initially greater than 1 cm [6]. Other clinical patterns include firm, flat, sometimes atrophic, single or multiple papules, plaques, with a variety of colors (light brown, dark brown, purple, red or yellow) [1,39] (Figure 3).
Figure 2.
Clinical appearance of a DF in a young woman: a solitary, well-defined, hyperkeratotic nodule with a diametre of about 1 cm with a yellow-brown surface.
Figure 3.
Another clinical pattern of a DF located on the leg: a flat, light brown, single papule.
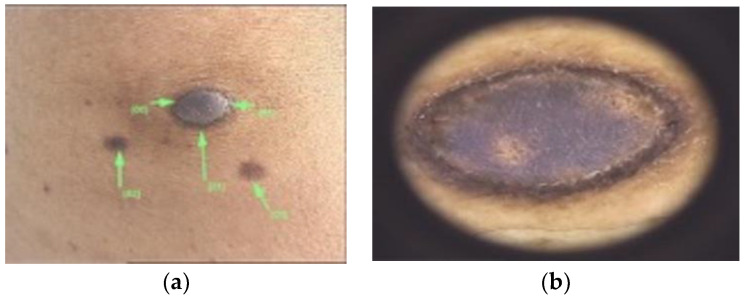
Colour may also vary depending on the Fitzpatrick fototype (Figure 4). The overlying skin can be pink, red, purple, gray, yellow, orange, blue, brown or black [40]. On palpation DFs have the consistency of a nodule, that moves freely over the subcutis [40]. The dimple sign is valuable in the diagnosis of DFs, although it may not always assure it [41].
Figure 4.
(a) DF with a nodular, blue appearance in a 4th Fitzpatrick phototype patient. Differential diagnoses may include a blue nevus. (b) Dermoscopic image of a nodular, blue DF, with well-defined borders and some scales.
Besides the classical clinical presentation, there have also been described some unusual atypical variants. Rare variants may include metastasizing benign DFs, which are usually larger than typical variants (more than 3 cm) [4,42,43]. Morphological features can be those of cellular, aneurysmal or atypical DFs and a greater number of mitosis has been noticed in this cases [4,42,43]. Extension into the subcutaneous layer and local recurrence has also been described [4,42,43]. Regarding metastatic sites, lymph nodes and lungs are the most frequent ones [4,42,43].
Giant lesions (larger than 5 cm) have also been described in the scientific literature [4,40,43] (Figure 5). The largest tumor reported measured 17 × 9 × 4 cm [44].
Figure 5.
Giant DF in a young patient, with a diameter of about 5.5 cm.
Multiple clustered DFs (more than 15) appear like a plaque with various single hyperpigmented papules [45,46]. They may also occur in children and can be either congenital or eruptive [45,46]. Atypical fibroxanthoma and dermatofibrosarcoma protuberans are differential dignosis that should be taken into consideration [45,46].
Multiple eruptive, diffuse, and persistent DFs appear in less than 1% of cases, the majority of patients suffering from an underlying affliction, such as human immunodeficiency virus infection, autoimmune diseases (systemic lupus erythematosus, dermatomyositis, myasthenia gravis, pemphigus vulgaris), Graves disease, Hashimoto thyroiditis, chromosomal alterations (Down syndrome), hematologic malignancies (leukemia, cutaneous T-cell lymphoma, myelodysplastic syndrome, multiple myeloma) atopic dermatitis, metabolic disorders (hypercholesterolemia), glycosuria, hydronephrosis, diabetes mellitus, breast cancer, ulcerative colitis, Crohn’s disease and sarcoidosis [47,48,49,50,51,52,53,54,55,56,57,58,59,60,61]. Moreover, multiple eruptive DFs have been linked to antiretroviral therapy (efalizumab and brentuximab vedotin), tyrosine kinase inhibitors (imatinib), and antitumor necrosis factor-alpha agents [62,63,64,65]. Some cases have also been described in pregnant women [4,66].
Other atypical presentations may include polypoid, atrophic, and DF with spreading satelitosis [67,68,69].
A Meyerson phenomenon adjacent to the DF has been seen [70] (Figure 6).
Figure 6.
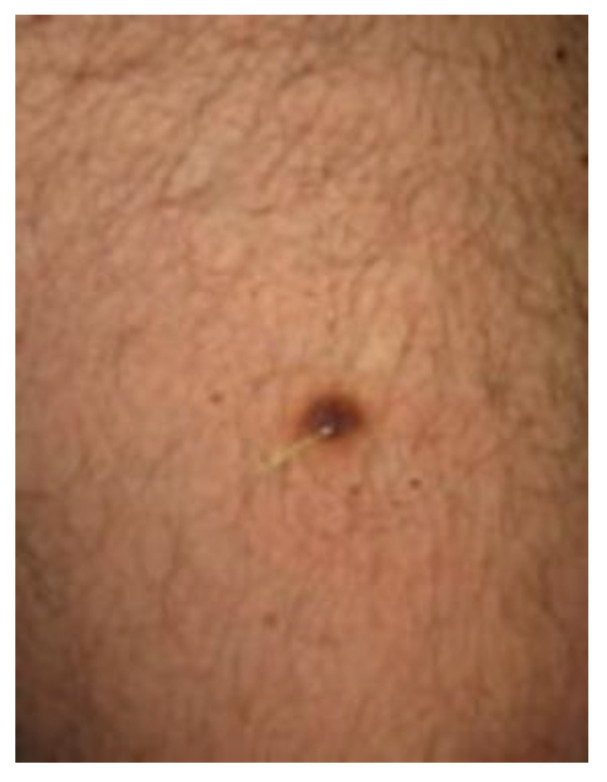
A Meyerson phenomenon is adjacent to the DF.
3.2. Diagnosis and Assessment
As multiple atypical variants of DF have been reported in the literature, clinical recognition may become challenging, leading to a more burdensome identification and sometimes to misdiagnosis [2,9]. Although diagnosis is commonly based on clinical presentation and history, further diagnostic tools such as dermoscopy, variable–frequency ultrasonography, fluorodeoxyglucose positron–emission tomography (FDG-PET) scans, and confocal laser scanning microscopy are necessary.
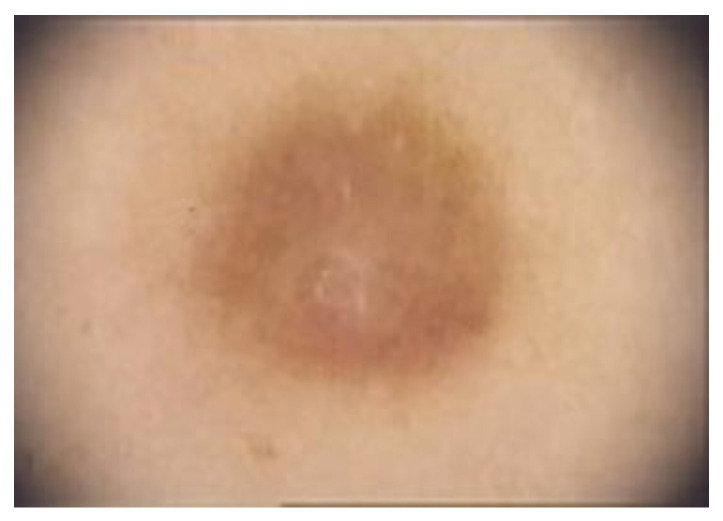
Dermoscopy is a non-invasive procedure useful for the diagnosis and management of pigmented tumours of the skin [9,71]. Among the scientific literature, various dermoscopic structures have been mentioned. DFs typical dermoscopic appearance includes the presence of a delicate, peripheral light-to-medium brown pigment network with a sharply demarcated central white scar-like area, white network and homogeneous pigmentation [1,8,9] (Figure 7).
Figure 7.
Typical dermoscopic appearance of a DF: delicate, peripheral light-to-medium brown pigment network with a sharply demarcated central white scar-like area, white network and homogeneous pigmentation.
For atypical variants, high definition optical coherence tomography can be useful as it correlates with histopathological types of DFs [72]. As DFs proved to have intense F-18 fluorodeoxyglucose uptake on positron emission tomography-computed tomography scan, the letter coud be a possible diagnostic alternative [73]. Although confocal laser scanning microsocopy is mainly used to evaluate melanocytic lesions, it is helpful in diagnosing DFs as their features (bright rings at the periphery, collagen structures at the center, central keratin) correlate with both dermoscopy and histopathology [74].
Although typical dermoscopic patterns are most frequently seen in clinical practice, some authors described atypical types of DFs (Table 1).
Table 1.
Dermoscopic features of atypical dermatofibromas.
| Study, Authors | Article Type | Year of Publication | Atypical Features with High Risk | Cases (n/%) |
|---|---|---|---|---|
| Aytekin S et al. [1] | Original article | 2021 | Irregular delicate/asymmetric pigment network Peripheral proeminent pigment network Irregular proeminent pigment network Atypical pigment network Irregular white network Irregular brown areas Dotted vessels Glomerular vessels Polymorphous/atypical vessels Ulceration White radial streaks |
31 (21.8) 3 (2.1) 2 (1.4) 2 (1.4) 3 (2.1) 9 (6.3) 34 (23.9) 2 (1.4) 6 (4.2) 5 (3.5) 8 (5.6) |
| Genc Y et al. [75] | Report | 2020 | Melanoma-like BCC-like Keratoachantoma-like Spitzoid-like |
11 (19.4) 3 (4.9) 2 (2.6) 26 (19.4) |
| Llambrich A et al. [3] | Research letter | 2019 | Dotted vessels Arborizing vessels Polymorphous/atypical vessels Shiny white streaks |
18 (50) 7 (19.4) 18 (50) 16 (44.4) |
| Lin MJ et al. [76] | Original research | 2018 | Dotted/pinpoint vessels Sharply focused arborizing vessels Linear irregular vessels Glomerular vessels Polymorphous vascular pattern White lines Ulceration Blue/grey veil |
2 (22) 0 1 (11) 1 (11) 1 (11) 0 0 0 |
| Won KY et al. [77] | Original research | 2017 | Irregular shape Spiculated margins |
8 (44) 12 (67) |
| Kelati A et al. [78] | Research article | 2017 | White streaks Ulceration Brown streks Negative-network-like appearance Dotted vessels Multicomponent melanoma-like Vascular tumor-like BCC-like Collision tumor-like Peripheral diffuse pink to red to reddish violet halo White ring around an ulceration Pink bluish pigmentation with vascularization Pigment network with a ring around follicular opening |
18 (18) 6 (6) 6 (6) 3 (3) 23.3% 20 (20%) 0 0 0 7 (7%) 6 (6%) 7 (7%) 2 (2%) |
| Marinescu SA et al. [71] | Case report | 2016 | Pinky-milk areas Peripheral pigment network Polymorphous atypical vessels |
|
| Roldán-Marín R et al. [79] | Case report | 2014 | Grey-green colour | |
| Ferrari A et al. [9] | Original article | 2013 | Melanoma-like Vascular tumour-like BCC-like Collision tumour-like |
21 (16.2) 6 (4.6) 5 (3.8) 3 (2.3) |
| Zaballos et al. [8] | Prospective study | 2008 | Proeminent atypical pigment network Irregular pigment network Irregular white network Irregular brown areas Dotted vessels Glomerular vessels Polymorphous/atypical vessels Ulceration |
13 (3.1) 8 (1.9) 3 (0.7) 125 (30.6) 3 (0.7) 10 (2.4) 18 (4.4) |
Additional clinical features may include ring-like or donut-shaped globular structures, vascular structures and sometimes ulceration, comedo-like openings, scale, crusts, or peripheral collarette fissures [8,9].
The pigment network may vary from peripheral/total/irregular delicate to peripheral/total/irregular prominent and atypical appearance [1,3,9]. Aytekin et al. evaluated dermoscopically 142 DFs of 72 patients and concluded that pigment network was found in 57% of cases, the most common subtype being irregular delicate or asymmetric pigment network [1]. Delicate pigment network seen in DFs is commonly thin, varying from light to medium brown and it is considered that it results as hyperpigmentation of rete ridges rather than the proliferation melanocytes [1]. According to the study performed by Arpaia et al., the pigment network was darker in the center, becoming gradually pale towards the periphery with brownish thin streaks [80]. In 2000, Ferrari et al. noticed that the peripheral pigment network and central white scar-like patches are more prevalent in women and among the classical histopathologic type of DFs [36]. The results may alternate due to the quality of the dermoscopy (contact/non-contact, polarized/nonpolarized light) and the difference among genders [1]. Interestingly, Zaballos et al. also identified in some DFs a significant and/or atypical pigment network [8].
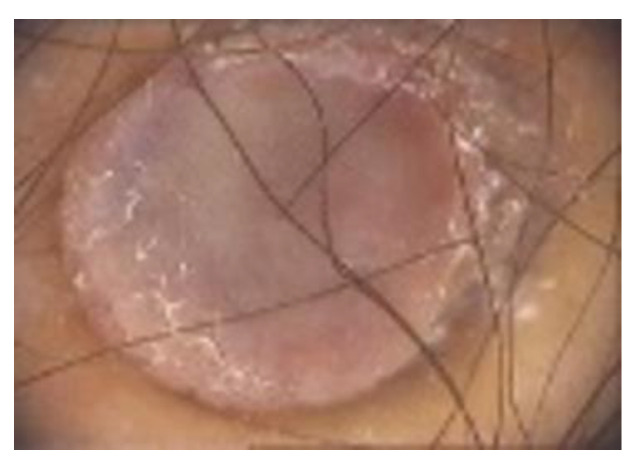
Central white scar-like patches are sharply demarcated with irregular white regions, histopathologically characterized by various grades of fibrosis in the dermis [1,9] (Figure 8).
Figure 8.
The dermoscopic appearance of central white scar-like patches sharply demarcated with irregular white regions.
Aytekin et al. have detected white scar-like patches in 37.3% of cases, the most common subtype being the central ones [1]. This structure is considered the most widespread feature of diffuse fibrous DF with a peripheral delicate pigment network [1]. It has also been noticed that the scar structure occasionally got a white radial streaks appearance, which gave the aspect of a spitzoid pattern [1]. Zaballos et al. evaluated 412 DFs and observed that white scar-like patches are mainly localized in the center part of the lesion [8]. Moreover, Arpaia et al. concluded that the central white patch was the most frequent dermoscopic feature, observed in 91.6% of cases [80].
The white network may be central, total, irregular, or crystalline-like [1,3,9]. Zaballos et al. identified a network of white lines and brown holes, which was later considered a variation of the white scar-like patch [8]. There has been raised awareness of the importance of distinguishing this structure from dysplastic nevi, Spitz nevi and the negative pigment network encountered in melanomas [1,8].
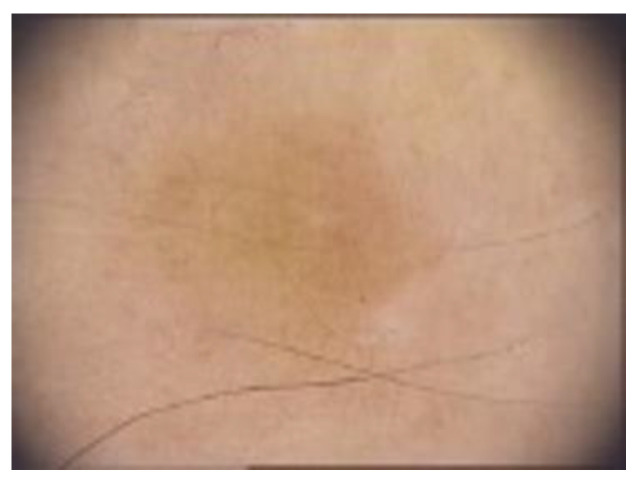
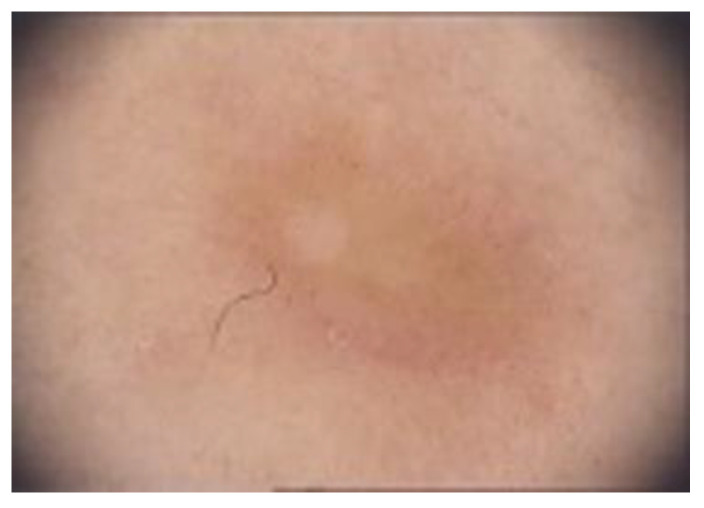
The homogenous pigmentation may include multiple colors (brown, yellow) or it may appear as hypopigmentation [1,3,9] (Figure 9).
Figure 9.
The dermoscopic appearance of a homogenous yellow-brown pigmentation of a DF.
Ferrari et al. noticed that homogeneous pigmentation was most frequently in females and DFs with sebaceous hyperplasia, whereas peripheral homogeneous pigmentation was mostly encountered in men [36]. Karaarslan et al. observed a homogeneous blueish pigmentation that was associated with the hemosiderotic type of the DF [81]. Usually, hemosiderotic variants are indicated by the green color [79].
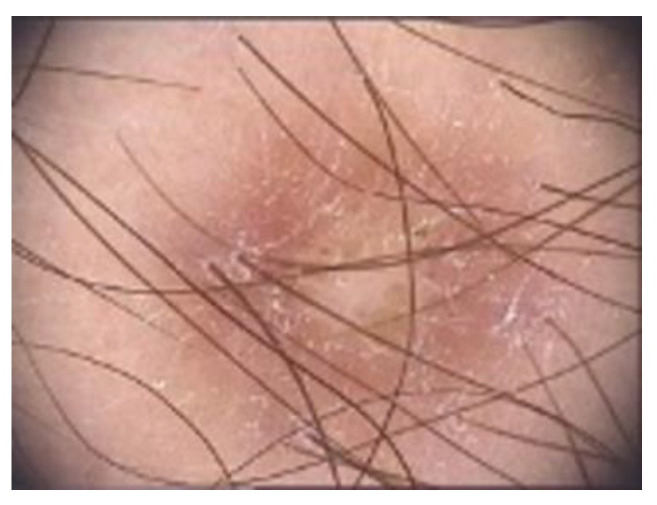
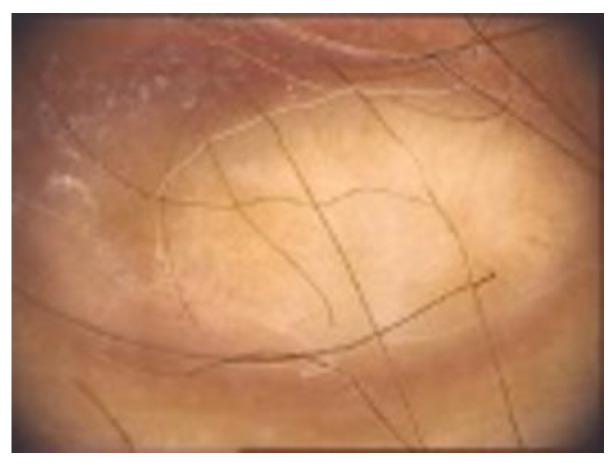
The vascular pattern has been widely discussed. Vascular structures are used in dermoscopy to diagnose melanoma and other pigmented or vascular tumors, which mimic melanoma [1,3]. Nevertheless, DFs may have peripheral, central, or total erythema, dotted, hairpin, glomerular, comma, or linear vessels, but also polymorphic and atypical ones [1,3,9] (Figure 10).
Figure 10.
The dermoscopic appearance of a DF with peripheral erythema and dotted vessels.
Contrary to other studies, Genc et al. found vascular structures to be the most frequent dermatoscopic feature and described a red to brown halo phenomenon in 4.9% of DFs [75]. Another study performed by Agero et al. concluded that blood vessels were seen in 44% of DFs when using polarized light [82]. Ferrari et al. described 2 DFs with dotted vessel patterns, whereas Aytekin et al. stated that the most frequent vascular structures in their study were erythema and dotted vessels [1,9,36].
Other not so common dermatoscopic changes may involve ring-like structures, ulceration, scales, fissures, milia-like cysts, hemorrhage, crusts or white radial streaks [1,3,9,82]. Genc et al. have conducted a study which classified DFs depending on the dermatoscopic similarities to other lesions [75]:
Melanoma-like: various colors and patterns, white structureless areas, polarizing-specific white lines, pink-red or blue-gray structureless areas, dark brown thick reticular lines, peripheral black clods and eccentric distribution of straight, curved, dotted and branched vessels [75].
Basal cell carcinoma-like: arterial structures specifically in the papillary dermis mostly at the periphery of the lesion [75] (Figure 11).
Keratoacanthoma-like: central keratin area with a surrounding radial arrangement of polymorphic vessels (curved, branched and dotted) [75].
Seborrheic keratosis-like: thick curved lines, orange, brown or white clods, brown-black crusted structures, blue-gray structureless areas and loop, dotted or coiled vessels [75].
Nevus-like: various hypopigmented structureless areas, having in between multifocal thin brown reticular lines [75] (Figure 4 and Figure 12).
Nevus sebaceous-like: white lines (associated with dermal fibrosis), peripheral thin brown reticular lines and central large yellow clods (associated with sebaceous hyperplasia) [75] (Figure 13).
Xanthogranuloma-like: yellow structureless areas, coiled vessels with a peripheral reddish halo [75].
Pyogenic granuloma-like: polymorphic vessels (curved, dotted, straight and branched) with irregular distribution, white lines and pink-red structureless areas [75].
Spitzoid-like: pink-red structureless areas, shiny white lines, white structureless areas, light brown clods, halo phenomenon, dotted vessels [75].
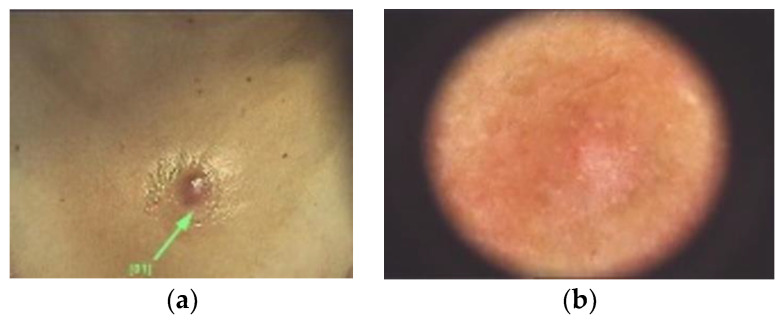
Figure 11.
(a) Clinical image of a DF with a solitary, well-defined, nodular, pink appearance in a female patient. Differential diagnoses may include basal cell carcinoma. (b) Dermoscopic image of a DF with arborizing vessels, along with the central white scar-like patch and fine delicate pigment network.
Figure 12.
Dermoscopic image of a nevus-like DF, but also with coiled vessels and some scales.
Figure 13.
Dermoscopic image of a DF with a nevus sebaceous-like appearance: white lines (associated with dermal fibrosis), peripheral thin brown reticular lines and central yellow structures.
Llambrich et al. performed a retrospective review, analyzing clinical and dermoscopic features of 36 pink nodular DFs [3]. They underlined the importance of a correct differential diagnosis as pink nodular lesions with erythema, vascular structures, shiny white streaks and a central white patch may suggest malignancy, mainly amelanotic/hypomelanotic melanoma [3]. Moreover, regarding the dermopathological types, non-fibrocollagenous variants of DFs were proned to have atypical patterns [36,75].
Melanoma-like and pyogenic granuloma-like atypical patterns were seen mostly in the case of aneurysmal DFs [75]. Furthermore DFs may have a pinkish-red pigmentation, dotted vessels and superficial white scales resembling psoriasis [9]. A “collision tumour-like” pattern was also described having a white area with focal pigment network [9]. Particularly, collision-like patterns along with melanoma-like and vascular tumour-like patterns were most commonly noticed in men [9]. Aditionally there have been described palisading, granular cell, myxoid, lichenoid, balloon cell and signet-ring cell variants [2,6,9].
As such, dermoscopy may be beneficial in increasing diagnosis and management accuracy, but since dermoscopic features may vary as well, it is certainly important to take into consideration other differential diagnoses [4,9,71,75].
3.3. Histologic Variants
Histologically, DFs contain uniform spindle cells organized in elongated fascicles [4]. Classical histopathological features of typical DFs include an overlying achantotic, hyperkeratotic and sometimes hyperpigmented epidermis [19,83,84,85,86,87,88] (Figure 14).
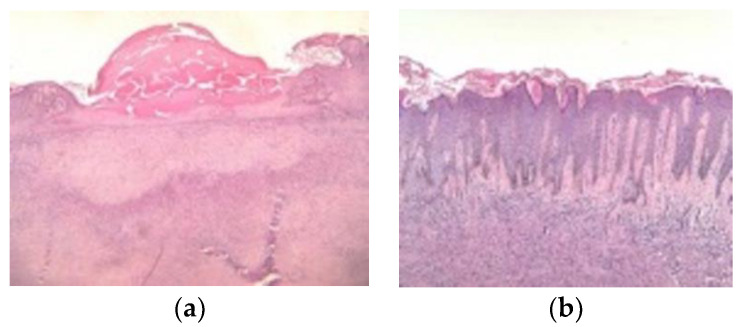
Figure 14.
(a) Histopathologic examination (hematoxylin and eosin, ×10): tumour proliferation localized in the papillary dermis and extending to the deep dermis, with interspersed collagen bundles, separated from the epidermis by a grenz zone. The overlying epidermis presents erosions centrally and collections in the keratin layer. (b) Histopathologic examination (hematoxylin and eosin, ×10): tumour proliferation composed of elongated and spindle-shape cells with elongated nuclei, in a fascicular-storiform configuration localized in the papillary dermis and extending to the deep dermis. The overlying epidermis has a hyperplastic appearance with hyperorthokeratosis, acanthosis, and elongation of the rate ridges. There is also follicular induction at the epidermis level.
Moreover, the epidermis usually exhibits elongated rete ridges containing hyperpigmented basal keratinocytes, aspect known as “dirty feet” sign [2]. There is also a proliferation of spindle-shaped fibrous cells mixed with histiocytoid cells at the level of the dermis [22,89,90]. Collagen bundles are commonly seen between the spindled fibrous cells along with the unaffected layeser, known as the “Grenz zone” [2,89,90,91].
The scientific literature has classified various types of DFs, regarding their histopathological characteristics [2]. Histological features may coexist in the same lesion [87]. As such there have been mentioned a lot of histopathological types: fibrocollagenous, cellular, keloidal, atrophic, aneurysmal, storiform, fibrocollagenous with sebaceous induction, lipidized, hemosiderotic, epithelioid, lichenoid, baloon cell, signet-ring myofibroblastic, clear cell, palisading, granular cell, myxoid and also the atypical type [2,6,71,83,84,85,87,88].
A histologic review performed by Alves et al. on 192 dermatofibromas stated that common fibrous hystiocitoma was the most frequent type, observed in 80% of cases [2]. Individual collagen bundles encompassed by lesional cells (fibroblasts, macrophages and blood vessels) and a predominantly lymphocytic inflammatory infiltrate may be seen [2].
Atypical DFs also known as DFs with monster cells, are poorly documented variants of typical fibrous histiocytomas [88]. Besides typical findings, atypical DF are comprised of pleomorphic spindle-like, hystiocite-like cells and multinucleate giant cells. [89].
LeBoit and Barr firstly described dermatofibroma with granular cells in 1991 [87,88]. This rare histologic variant can be confused with other malignant or benign cutaneous neoplasms such as: benign granular cell tumor, malignant granular cell tumor, primitive polypoid granular cell tumor, granular cell ameloblastoma, granular cell fibrous papule of the nose, granular cell basal cell carcinoma, granular cell schwannoma, granular cell leiomyoma, granular cell leiomyosarcoma or angiosarcoma and granular cell dermatofibrosarcoma protuberans [87]. Morfology of the lesion along with immunohistochemical evaluation might sometimes be decisive for the corect diagnosis [86]. For instance, benign granular cell tumors are positive for S-100 protein, CD63, CD68 and neuron-specific enolase whereas atypical fibroxanthoma stains negatively for S100 protein, Melan-A, human melanoma black (HMB)-45 pan-cytokeratin (CK) and actin and positively for CD68 and vimentin [87].
Immunohistochemistry can be useful to differentiate DFs from schwannomas, leiomyomas and leiomyosarcomas [87].
3.4. Differential Diagnosis
It is extremely important to recognize atypical DFs, as cutaneous melanoma is a vital clinical differential diagnosis and may display similar characteristics [9,19]. Nevertheless, other afflictions as well may be taken into accounts, such as intradermal nevi, basal cell carcinomas, keratoacanthomas, and dermatofibrosarcomas protuberans [4]. Differential diagnoses may also include angiokeratomas, Spitz-nevi, melanocytic nevi, blue nevi, granuloma annulare, supernumerary nipple, acrochordon, atypical fibroxanthoma, cutaneous metastasis, cutaneous T-cell lymphoma, cylindroma, pilomatrixoma or targetoid hemosiderotic hemangiomas [14,92,93].
Dermatofibrosarcoma protuberans (DFSP) appears as painless, slow-growing skin-colored nodule, with a finger-like projections pattern and should be distinguished from benign DFs as it is locally aggressive [4,19,94]. A delayed accurate diagnosis leads to clinical pitfalls [94]. A more cellular appearance and a “honeycomb” display of the subcutaneous fat is often seen in DFSP [19]. Immunohistochemical staining is also very useful, as there are various markers to differentiate the two entities [19,95,96,97,98,99,100,101,102,103]. Although DFSP stains positive for CD-34, nestin and collagen triple helix repeat containing-1 (Cthrc1) and negative for factor XIIIa, there has also been noticed an elevated expression of thrombospondin-1 (TSP-1) [19,98,100,101,103]. DFSP characteristically has a genomic reciprocal translocation in t (17;22) (q22;q13) that causes the fusion of the platelet-derived growth factor B-chain (PDGFB) and the promoter of the collagen type Iα1 (COL1A1) genes and might be detected by fluorescent in situ hybridisation or real-time PCR [94].
In comparison, DF stains positive for factor XIIIa, D2-40, insulin-like growth factor–binding protein 7 (IGFBP7), cathepsin K, CD99, leukocyte-specific protein 1 (LSP1) and 5-hydroxymethylcytosine (5-hmC) and negative for CD-34 [19,26,37,99,102,104,105,106,107]. Occasionally, the cellular type of DF may stain positive for CD34 [95,96]. Stromelysin-3 (ST-3) expression of DF shall also help to differentiate it from DFSP [97]. FGFR3/FOXN1 and FGF2/FGFR4 expression in the pathogenesis of DF is practical [104]. Fluorescence in situ hybridization (FISH) analysis is a valuable tool as well [108]. B-cell lymphoma 2 (Bcl-2) expression, autophagy marker Atg5, and phosphohistone-H3 can help to differentiate between DF and DFSP [109,110]. Moreover, Ki-67 staining shows a higher proliferation index in the case of DFSP [19].
Hemosiderotic dermatofibromas, dermatoscopically characterized by a blue/red center with white lines and maybe network and vessels at the periphery may lead to a dermoscopically differential diagnosis with Kaposi sarcoma due to the intense vascularity [28,29]. Nevertheless, Kaposi sarcoma stains are positive for CD31, CD34 and D2-40, and patients are also positive for HHV-8 [19].
CK20 positive Merkel cells, present in the follicular induction, crowding, no peripheral palisading, clear cell hyperplasia, and the absence of nuclear atypia are helpful pathologic features in differentiating DFs with follicular induction from basal cell carcinomas [19].
4. Discussion
This narrative review aimed to reevaluate the clinical and dermoscopic patterns of atypical dermatofibromas compared to the typical ones. Moreover, there have been mentioned some not-so-common etiopathogenic factors. Dermatofibromas are prevalent cutaneous benign tumours that most frequently affect young or middle-aged adults. Clinically, dermatofibromas appear as single or multiple firm papules or nodules with a smooth surface anywhere on the body, mainly on the lower extremities. They can vary in size and colour from light brown to dark brown, yellow, purple or red. Although the clinical diagnosis of DFs may be simple in daily practice, in the presence of various patterns, diagnosis of DFs can become challenging.
Therefore, it is essential to consider the possible links between dermoscopy and histology and complete surgical excision, especially in the presence of atypical variants or a history of recent changes. Thus, the precise definition of dermoscopic patterns for this frequent benign tumour is of major interest.
In a reverse manner, the possibility of a misdiagnosis of malignant skin disorders, inlcuding non-melanoma skin cancer, is a main challenge in terms of worldwide public health management. A lot of clinicians may face it and thus malignancy-related misdiagnosis remains one of the main issues in the dermatologic field. Similar to the case of melanomas or basal cell carcinomas being misdiagnosed as diabetic foot ulcer, they can be more easily considered, even by experts, as benign lesion, such as dermatofibromas [111]. As malignant tumors may sometimes mimic benign conditions, the main focus has been on finding a non-invasive, reliable, sensible and highly specific diagnostic methods to identify specific features which suggest malignancy.
Promising Differential Diagnostic Methods
Apart from dermoscopy and digital dermoscopy, not so common diagnostic tools may include variable–frequency ultrasonography, in which DFs appear as hypoechoic solid nodules and high-definition optical coherent tomography, in which DFs can resemble malignant conditions on FDG-PET scans [73,74]. Among other studied techniques there have been mentioned reflectance confocal microscopy (RCM), multiphoton microscopy, fluorescence evaluation, Raman spectroscopy and diffuse reflectance [112,113,114,115]. High-frequency ultrasonography has been a valuable diagnostic and prognostic tool in early detecting other types of malignancies, such as hepatocellular carcinoma, for a long time, but it recently has begun to represent a promising opportunity in dermatology by using deep learning-based algorithms to analyse automated images [77,116,117]. Complementary techniques, such as dermoscopy in conjunction with RCM, may also enhance diagnostic accuracy of melanocytic conditions [118,119,120]. Confocal laser scanning microscopy is also considered an alternative tool [118,119,120].
The use of fractal parameters and fractal analysis method in dermatology is promising in the evaluation of image parameters, independently of the adopted scale [121]. There are currently studies being carried regarding the usefulness of fractal parameters in building classes of disease units based upon pictures of cutaneous pigmented lesions [121,122]. It might provide fully automatic diagnostic systems able to determine the type of pigmented tumor and inform us regarding the most adequate management [121].
Taking into consideration that dermatology is a largely visual speciality, the high cost of travel expenses to urban centres, the long wait times to see a dermatologist and the shortage of dermatology services mainly in rural areas, several research studies analizying the role of an artificial intelligence (AI) system as a diagnostic tool for the management of skin conditions have been conducted, focusing mainly on the malignant ones [123,124,125,126,127,128,129]. Several studies proved that telehealth platforms, easily reachable through smartphone apps, could increase patients access to dermatological care, especially during COVID-19 pandemic [127,130,131,132]. Some of the apps can use AI to provide various differential diagnoses depending on the information provided: patient demographics, lesion type, location, symptoms and progression [127,129]. Artificial neural networks, such as convolutional neural networks (CNNs), can be used to analyse visual imagery, being very effective in recognizing automated images and equal or superior than dermatologist in recognizing skin cancers [123]. Implementing AI as a diagnostic aid in the clinical practice may be safe, useful and feasible for skin lesions accurate detection and for better differentiating malignant from benign ones [123,124,125,126,127].
In order to diminish the high degree of subjectivity and variability regarding specificity, sensitivity, and diagnostic accuracy when performing RCM, a lot of artificial intelligence algorithms were created to ensure alternatives, assistance and support od dermatologists on a daily basis [118]. AI in RCM has been used so far to point out the dermal–epidermal junction, evaluate the the quality of RCM mosaics and distinguish between different skin tumors [118].
A lot of other studies have analyzed the correlation between spectrophotometric parameters of skin color and behavioral/environmental factors to predict the risk of cutaneous malignancies [133]. They concluded that the measurment of skin melanin index measured on the arm or buttock is the simplest predictor and should be added in predictive models. Regarding the environmental/behavioral factors, the total number of sunburns appear to be the most important one. As such, spectrophotometric measurements may be considered a quick screening examination method of the skin [133].
In histopathology, AI is efficient in classifying and characterizing tissues, in detecting mitosis and segment histologic primitives as epithelium, nuclei and tubules [128].
As a consequence of complexity and intransparency of deep neural networks in classifying skin cancer, explainable artificial intelligence (XAI) has also been suggested as an alternative although further research studies are needed to evaluate the influence of XAI in detecting cutaneous cancer [134].
There are lot of opportunities that lie ahead, from automated classification of cutaneous cancer through convolutional neural networks, sequential digital dermoscopy and automated total body photography to AI and automated teledermoscopy [135]. However, the potential use of AI in clinical practice remains to be addressed due to their limitations and further studies need to be conducted in order to implement it every day medical practice [135].
5. Conclusions
Clinical diagnosis of typical dermatofibromas is easy, with a classic dermoscopic pattern of pigmented network and central white patch. However, in current clinical situations, dermatofibromas display a wide range of presentations and histological variants that make the differentiation from other tumours, such as malignant melanoma, very difficult. Specific characterization of these atypical variants is essential in differentiating them from possibly more aggressive lesions and assessing the risk of local recurrence. As a matter of fact, the definitive diagnosis of a skin condition, especially in a doubtful clinical diagnostic scenario, demands complete surgical resection and histopathological analysis. As artificial intelligence technologies had reached an impressive precision in identifying various skin lesions, along with other inovative diagnostic methods, we can emphasise that in the future it will lead to improved safety and patient care and maybe enhance dermatologists’ productivity.
Author Contributions
Conceptualization, A.M.D. and O.A.O.; methodology, A.M.D. and O.A.O.; software, A.M.D., O.A.O., C.D.G., B.B., S.A., M.M.M., L.G.P., I.T. and C.G.; validation, A.M.D., O.A.O. and C.G.; formal analysis, A.M.D., O.A.O., C.D.G., B.B., S.A., M.M.M., L.G.P., I.T. and C.G; investigation, A.M.D., O.A.O., B.B., C.D.G. and I.T.; resources, A.M.D., O.A.O., C.D.G., B.B. and S.A.; M.M.M., L.G.P., I.T. and C.G.; data curation, A.M.D., O.A.O., B.B., C.D.G. and M.M.M.; writing—original draft preparation, A.M.D., O.A.O., B.B. and C.G.; writing—review and editing, A.M.D., O.A.O., C.D.G. and B.B.; visualization, A.M.D. and B.B.; supervision, A.M.D., O.A.O., B.B. and C.G.; project administration, A.M.D., O.A.O., B.B. and L.G.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the local Ethical Committee of the “Elias” University Emergency Hospital. The research was conducted according to the Helsinki Declaration. The pictures are part of the authors’ own archive and are from the patients admitted in “Elias” University Emergency Hospital in the last 2 years. They do not contain personal data.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
This review summarizes data reported in the literature and it does not report primary data.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Aytekin S., Kaynak E., Ayhan E. Dermoscopy of dermatofibromas: A new perspective. Int. J. Clin. Pract. 2021;75:e14547. doi: 10.1111/ijcp.14547. [DOI] [PubMed] [Google Scholar]
- 2.Alves J.V.P., Matos D.M., Barreiros H.F., Bártolo E.A.F.L.F. Variants of dermatofibroma-a histopathological study. An. Bras. Dermatol. 2014;89:472–477. doi: 10.1590/abd1806-4841.20142629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llambrich A., Vila A., Terrasa F., Bañuls J., Nadal C., Zaballos P. Dermoscopy of pink nodular dermatofibromas: A study of 36 cases. Australas. J. Dermatol. 2019;60:e357–e360. doi: 10.1111/ajd.13031. [DOI] [PubMed] [Google Scholar]
- 4.uptodate.com. [(accessed on 2 June 2022)]. Available online: https://www.uptodate.com/contents/overview-of-benign-lesions-of-the-skin?search=dermatofibroma&source=search_result&selectedTitle=1~22&usage_type=default&display_rank=1#H1101419831.
- 5.Kittler H., Rosendahl C., Cameron A., Tschandl P. Dermatoscopy: Pattern Analysis of Pigmented and Non-Pigmented Lesions. 2nd ed. Facultas Verlags und Buchhandels AG; Vienna, Austria: 2016. pp. 9–52. [Google Scholar]
- 6.Gaufin M., Michaelis T., Duffy K. Cellular Dermatofibroma: Clinicopathologic Review of 218 Cases of Cellular Dermatofibroma to Determine the Clinical Recurrence Rate. Dermatol. Surg. 2019;45:1359–1364. doi: 10.1097/DSS.0000000000001833. [DOI] [PubMed] [Google Scholar]
- 7.Berklite L., Ranganathan S., John I., Picarsic J., Santoro L., Alaggio R. Fibrous histiocytoma/dermatofibroma in children: The same as adults? Hum. Pathol. 2020;99:107–115. doi: 10.1016/j.humpath.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Zaballos P., Puig S., Llambrich A., Malvehy J. Dermoscopy of Dermatofibromas: A prospective morphological study of 412 cases. Arch. Dermatol. 2008;144:75–83. doi: 10.1001/archdermatol.2007.8. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari A., Argenziano G., Buccini P., Cota C., Sperduti I., De Simone P., Eibenschutz L., Silipo V., Zalaudek I., Catricalà C. Typical and atypical dermoscopic presentations of dermatofibroma. J. Eur. Acad. Dermatol. Venereol. 2013;27:1375–1380. doi: 10.1111/jdv.12019. [DOI] [PubMed] [Google Scholar]
- 10.Bowling J. The Illustrated Guide. 1st ed. Wiley-Blackell; Oxford, UK: 2012. Diagnostic dermatology; p. 77. [Google Scholar]
- 11.Lehmer L.M., Ragsdale B.D. Digital dermatofibromas-Common lesion, uncommon location: A series of 26 cases and review of the literature. Dermatol. Online J. 2011;17 doi: 10.5070/D32HG6Q36K. [DOI] [PubMed] [Google Scholar]
- 12.Kadakia S., Chernobilsky B., Iacob C. Dermatofibroma of the Auricle. J. Drugs Dermatol. 2016;15:1270–1272. [PubMed] [Google Scholar]
- 13.Kim J.M., Cho H.J., Moon S.-H. Rare experience of keloidal dermatofibroma of forehead. Arch. Craniofacial Surg. 2018;19:72–74. doi: 10.7181/acfs.2018.19.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.emedicine.emedscape.com. [(accessed on 20 July 2022)]. Available online: https://emedicine.medscape.com/article/1056742-overview.
- 15.Evans J., Mattacks C., Clarke T., Pond C.M. Dermatofibromas and Arthropod Bites: Is There Any Evidence to Link the Two? Lancet. 1989;334:36–37. doi: 10.1016/S0140-6736(89)90267-5. [DOI] [PubMed] [Google Scholar]
- 16.Lobato-Berezo A., Churruca-Grijelmo M., Martínez-Pérez M., Imbernón-Moya A., Vargas-Laguna M.E., Fernández-Cogolludo E., Aguilar-Martínez A., Gallego-Valdés M. Dermatofibroma Arising within a Black Tattoo. Case Rep. Dermatol. Med. 2014;2014:745304. doi: 10.1155/2014/745304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe K., Fukuda H., Niiyama S., Oharasaki T., Mukai H. Multiple dermatofibromas subsequent to folliculitis. Eur. J. Dermatol. 2013;23:890–891. doi: 10.1684/ejd.2013.2164. [DOI] [PubMed] [Google Scholar]
- 18.Nomura E., Yamamoto T. Photoletter to the editor: Fibrous histiocytoma developing at the site of tuberculin skin test. J. Dermatol. Case Rep. 2012;6:130–131. doi: 10.3315/jdcr.2012.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers D.J., Fillman E.P. StatPearls [Internet] StatPearls Publishing LLC; Treasure Island, FL, USA: 2022. Dermatofibroma. [Google Scholar]
- 20.Yamamoto T., Katayama I., Nishioka K. Role of mast cells in dermatofibroma: Recent viewpoints into the pathogenesis. Eur. J. Dermatol. 2003;13:419–423. [PubMed] [Google Scholar]
- 21.Kuroda K., Tajima S. Proliferation of HSP47-positive skin fibroblasts in dermatofibroma. J. Cutan. Pathol. 2008;35:21–26. doi: 10.1111/j.1600-0560.2007.00768.x. [DOI] [PubMed] [Google Scholar]
- 22.Jin S.Y., Choi J.S., La Choi Y., Kim D.H., Lee S.H. Identification of Leukocyte-Specific Protein 1-Positive Cells: A Clue to the Cell of Origin and a Marker for the Diagnosis of Dermatofibroma. Ann. Dermatol. 2015;27:157–162. doi: 10.5021/ad.2015.27.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Törőcsik D., Bárdos H., Nagy L., Ádány R. Identification of factor XIII-A as a marker of alternative macrophage activation. Cell Mol. Life Sci. 2005;62:2132–2139. doi: 10.1007/s00018-005-5242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerio R., Spaull J., Jones E.W. Histiocytoma cutis: A tumour of dermal dendrocytes (dermal dendrocytoma) Br. J. Dermatol. 2006;120:197–206. doi: 10.1111/j.1365-2133.1989.tb07783.x. [DOI] [PubMed] [Google Scholar]
- 25.Kubo M., Ihn H., Yamane K., Tamaki K. The expression levels and the differential expression of transforming growth factor-β receptors in dermatofibroma and dermatofibrosarcoma protuberans. Br. J. Dermatol. 2006;154:919–925. doi: 10.1111/j.1365-2133.2005.06904.x. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T. Dermatofibroma: A possible model of local fibrosis with epithelial/mesenchymal cell interaction. J. Eur. Acad. Dermatol. Venereol. 2009;23:371–375. doi: 10.1111/j.1468-3083.2009.03089.x. [DOI] [PubMed] [Google Scholar]
- 27.Sellheyer K., Smoller B.R. Dermatofibroma: Upregulation of Syndecan-1 Expression in Mesenchymal Tissue. Am. J. Dermatopathol. 2003;25:392–398. doi: 10.1097/00000372-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Skroza N., Rotolo S., Ceccarelli S., Romano F., Innocenzi D., Frati L., Angeloni A., Marchese C. Modulation of the expression of the FGFR2-IIIb and FGFR2-IIIc molecules in dermatofibroma. J. Dermatol. Sci. 2008;51:53–57. doi: 10.1016/j.jdermsci.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Walther C., Hofvander J., Nilsson J., Magnusson L., Domanski H.A., Gisselsson D., Tayebwa J., Doyle L.A., Fletcher C.D.M., Mertens F. Gene fusion detection in formalin-fixed paraffin-embedded benign fibrous histiocytomas using fluorescence in situ hybridization and RNA sequencing. Lab. Investig. 2015;95:1071–1076. doi: 10.1038/labinvest.2015.83. [DOI] [PubMed] [Google Scholar]
- 30.Panagopoulos I., Gorunova L., Bjerkehagen B., Lobmaier I., Heim S. LAMTOR1-PRKCD and NUMA1-SFMBT1 fusion genes identified by RNA sequencing in aneurysmal benign fibrous histiocytoma with t(3;11)(p21;q13) Cancer Genet. 2015;208:545–551. doi: 10.1016/j.cancergen.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Doyle L.A., Mariño-Enriquez A., Fletcher C.D.M., Hornick J.L. ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod. Pathol. 2015;28:904–912. doi: 10.1038/modpathol.2015.49. [DOI] [PubMed] [Google Scholar]
- 32.Szablewski V., Laurent-Roussel S., Rethers L., Rommel A., Vaneechout P., Camboni A., Willocz P., Copie-Bergman C., Ortonne N. Atypical fibrous histiocytoma of the skin with CD30 and p80/ALK1 positivity and ALK gene rearrangement. J. Cutan. Pathol. 2014;41:715–719. doi: 10.1111/cup.12352. [DOI] [PubMed] [Google Scholar]
- 33.Chen T.-C., Kuo T.-T., Chan H.-L. Dermatofibroma is a clonal proliferative disease. J. Cutan. Pathol. 2000;27:36–39. doi: 10.1034/j.1600-0560.2000.027001036.x. [DOI] [PubMed] [Google Scholar]
- 34.Cerio R., Spaull J., Oliver G.F., Jones E.W. A Study of Factor XHIa and MAC 387 Immunolabeling in Normal and Pathological Skin. Am. J. Dermatopathol. 1990;12:221–233. doi: 10.1097/00000372-199006000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Mentzel T., Wiesner T., Cerroni L., Hantschke M., Kutzner H., Rütten A., Häberle M., Bisceglia M., Chibon F., Coindre J.-M. Malignant dermatofibroma: Clinicopathological, immunohistochemical, and molecular analysis of seven cases. Mod. Pathol. 2013;26:256–267. doi: 10.1038/modpathol.2012.157. [DOI] [PubMed] [Google Scholar]
- 36.Ferrari A., Soyer H.P., Peris K., Argenziano G., Mazzocchetti G., Piccolo D., De Giorgi V., Chimenti S. Central white scarlike patch: A dermatoscopic clue for the diagnosis of dermatofibroma. J. Am. Acad. Dermatol. 2000;43:1123–1125. doi: 10.1067/mjd.2000.109842. [DOI] [PubMed] [Google Scholar]
- 37.Lamgan S.M., Robinson T.W.E. Cryotherapy for dermatofibromas. Clin. Exp. Dermatol. 1987;12:121–123. doi: 10.1111/j.1365-2230.1987.tb01878.x. [DOI] [PubMed] [Google Scholar]
- 38.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mentzel T. Cutaneous mesenchymal tumours: An update. Pathology. 2014;46:149–159. doi: 10.1097/PAT.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 40.Fitzpatrick T.B., Gilchrest B.A. Dimple Sign to Differentiate Benign from Malignant Pigmented Cutaneous Lesions. N. Engl. J. Med. 1977;296:1518. doi: 10.1056/NEJM197706302962610. [DOI] [PubMed] [Google Scholar]
- 41.Lookingbill D.P. A Malignant Dimple. N. Engl. J. Med. 1977;297:841–842. doi: 10.1056/nejm197710132971520. [DOI] [PubMed] [Google Scholar]
- 42.Doyle L.A., Fletcher C.D. Metastasizing “Benign” Cutaneous Fibrous Histiocytoma: A clinicopathologic analysis of 16 cases. Am. J. Surg. Pathol. 2013;37:484–495. doi: 10.1097/PAS.0b013e31827070d4. [DOI] [PubMed] [Google Scholar]
- 43.Gershtenson P.C., Krunic A.L., Chen H.M. Multiple clustered dermatofibroma: Case report and review of the literature. J. Cutan. Pathol. 2010;37:e42–e45. doi: 10.1111/j.1600-0560.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- 44.Kalsi H., Rahman A., Harbol T., Sidhu J. Giant Hemosiderotic Dermatofibroma: The Largest Giant Dermatofibroma Reported to Date. Am. J. Dermatopathol. 2015;37:778–782. doi: 10.1097/DAD.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 45.Finch J., Berke A., McCusker M., Chang M.W. Congenital Multiple Clustered Dermatofibroma in a 12-Year-Old Girl. Pediatr. Dermatol. 2014;31:105–106. doi: 10.1111/j.1525-1470.2011.01681.x. [DOI] [PubMed] [Google Scholar]
- 46.Massone C., Parodi A., Virno G., Rebora A. Multiple eruptive dermatofibromas in patients with systemic lupus erythematosus treated with prednisone. Int. J. Dermatol. 2002;41:279–281. doi: 10.1046/j.1365-4362.2002.01493.x. [DOI] [PubMed] [Google Scholar]
- 47.García-Millán C., Aldanondo I., Fernández-Lorente M., Carrillo R., Jaén P. Multiple Eruptive Dermatofibromas in 2 Patients Infected with the Human Immunodeficiency Virus. Actas Dermosifiliogr. 2007;98:702–706. doi: 10.1016/S0001-7310(07)70163-1. [DOI] [PubMed] [Google Scholar]
- 48.Marque M., Pallure V., Huet P., Bessis D., Guillot B. Multiple familial “eruptive” dermatofibromas. Ann. Dermatol. Venereol. 2013;140:452–454. doi: 10.1016/j.annder.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 49.Huang P.-Y., Chu C.-Y., Hsiao C.-H. Multiple eruptive dermatofibromas in a patient with dermatomyositis taking prednisolone and methotrexate. J. Am. Acad. Dermatol. 2007;57:S81–S84. doi: 10.1016/j.jaad.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 50.López N., Fernández A., Bosch R.J., Herrera E. Multiple eruptive dermatofibromas in a patient with Graves-Basedow disease. J. Eur. Acad. Dermatol. Venereol. 2008;22:402–403. doi: 10.1111/j.1468-3083.2007.02355.x. [DOI] [PubMed] [Google Scholar]
- 51.Kimura Y., Kaneko T., Akasaka E., Nakajima K., Aizu T., Nakano H., Sawamura D. Multiple eruptive dermatofibromas associated with Hashimoto’s thyroiditis and myasthenia gravis. Eur. J. Dermatol. 2010;20:538–539. doi: 10.1684/ejd.2010.0981. [DOI] [PubMed] [Google Scholar]
- 52.Monteagudo B., Suarez-Amor O., Cabanillas M., Leon-Mateos A., Perez-Valcarcel J., de las Heras C. Down syndrome: Another cause of immunosuppression associated with multiple eruptive dermatofibromas? Dermatol. Online J. 2009;15:15. doi: 10.5070/D36VG9X727. [DOI] [PubMed] [Google Scholar]
- 53.Alexandrescu D.T., Wiernik P.H. Multiple Eruptive Dermatofibromas Occurring in a Patient with Chronic Myelogenous Leukemia. Arch. Dermatol. 2005;141:397–398. doi: 10.1001/archderm.141.3.397. [DOI] [PubMed] [Google Scholar]
- 54.Bhattacharjee P., Umar S.A., Fatteh S.M. Multiple Eruptive Dermatofibromas Occurring in a Patient with Myelodysplastic Syndrome. Acta Derm. Venereol. 2005;85:270–271. doi: 10.1080/00015550410024517. [DOI] [PubMed] [Google Scholar]
- 55.Zaccaria E., Rebora A., Rongioletti F. Multiple eruptive dermatofibromas and immunosuppression: Report of two cases and review of the literature. Int. J. Dermatol. 2008;47:723–727. doi: 10.1111/j.1365-4632.2008.03575.x. [DOI] [PubMed] [Google Scholar]
- 56.Yazici A.C., Baz K., Ikizoglu G., Koca A., Kokturk A., Apa D.D. Familial eruptive dermatofibromas in atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2006;20:90–92. doi: 10.1111/j.1468-3083.2005.01357.x. [DOI] [PubMed] [Google Scholar]
- 57.Beyazit Y., Caner S., Kurt M., Kekilli M., Aydog G., Ibis M. Dermatofibroma in a patient with Crohn’s disease: A novel clinical manifestation. J. Crohn’s Colitis. 2010;4:490–491. doi: 10.1016/j.crohns.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Hiraiwa T., Hanami Y., Yamamoto T. Hidradenitis suppurativa and multiple dermatofibromas in a patient with ulcerative colitis. J. Dermatol. 2013;40:1071–1072. doi: 10.1111/1346-8138.12316. [DOI] [PubMed] [Google Scholar]
- 59.Goldbach H., Wanat K., Rosenbach M. Multiple eruptive dermatofibromas in a patient with sarcoidosis. Cutis. 2016;98:E15–E97. [PubMed] [Google Scholar]
- 60.Bachmeyer C., Cordier F., Blum L., Cazier A., Vérola O., Aractingi S. Multiple eruptive dermatofibromas after highly active antiretroviral therapy. Br. J. Dermatol. 2000;143:1336–1337. doi: 10.1046/j.1365-2133.2000.03924.x. [DOI] [PubMed] [Google Scholar]
- 61.Queirós C., Uva L., de Almeida L.S., Filipe P. Multiple eruptive dermatofibromas associated with pregnancy—A case and literature review. Dermatol. Online J. 2019;25:12. doi: 10.5070/D3255044074. [DOI] [PubMed] [Google Scholar]
- 62.Santos-Juanes J., Coto-Segura P., Mallo S., Galache C., Soto J. Multiple Eruptive Dermatofibromas in a Patient Receiving Efalizumab. Dermatology. 2008;216:363. doi: 10.1159/000117708. [DOI] [PubMed] [Google Scholar]
- 63.Giavedoni P., Combalia A., Pigem R., Mascaró J.M. Multiple Eruptive Dermatofibromas in a Patient Treated with Brentuximab Vedotin. Actas. Dermosifiliogr. 2019;110:419–420. doi: 10.1016/j.ad.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Caldarola G., Bisceglia M., Pellicano R. Multiple eruptive plaque-like dermatofibromas during anti-TNFα treatment. Int. J. Dermatol. 2013;52:638–641. doi: 10.1111/j.1365-4632.2011.05047.x. [DOI] [PubMed] [Google Scholar]
- 65.Llamas-Velasco M., Fraga J., Solano-López G.E., Steegmann J.L., Diez A.G., Requena L. Multiple eruptive dermatofibromas related to imatinib treatment. J. Eur. Acad. Dermatol. Venereol. 2014;28:979–981. doi: 10.1111/jdv.12328. [DOI] [PubMed] [Google Scholar]
- 66.Beatrous S.V., Riahi R.R., Grisoli S.B., Cohen P.R. Associated conditions in patients with multiple dermatofibromas: Case reports and literature review. Dermatol. Online J. 2017;23 doi: 10.5070/D3239036479. [DOI] [PubMed] [Google Scholar]
- 67.Ohnishi T., Sasaki M., Nakai K., Watanabe S. Atrophic dermatofibroma. J. Eur. Acad. Dermatol. Venereol. 2004;18:580–583. doi: 10.1111/j.1468-3083.2004.00975.x. [DOI] [PubMed] [Google Scholar]
- 68.Kai H., Fujita H., Yamamoto M., Asahina A. Polypoid dermatofibroma with a slim pedicle: A case report. Dermatol. Online J. 2012;18 doi: 10.5070/D30G5926DV. [DOI] [PubMed] [Google Scholar]
- 69.Curcó N., Jucgla A., Bordas X., Moreno A. Dermatofibroma with spreading satellitosis. J. Am. Acad. Dermatol. 1992;27:1017–1019. doi: 10.1016/S0190-9622(08)80272-1. [DOI] [PubMed] [Google Scholar]
- 70.Schofield C., Weedon D., Kumar S. Dermatofibroma and halo dermatitis. Australas. J. Dermatol. 2012;53:145–147. doi: 10.1111/j.1440-0960.2010.00726.x. [DOI] [PubMed] [Google Scholar]
- 71.Marinescu S.A., Tatu A.L., Mihai I.R., Giuglea C. Correlations between clinics, dermoscopy and histopathology in a female with two dermatofibromas-a case report. Rom. J. Morphol. Embryol. 2016;57:323–326. [PubMed] [Google Scholar]
- 72.Picard A., Long-Mira E., Chuah S.Y., Passeron T., Lacour J.-P., Bahadoran P. Interest of high-definition optical coherent tomography (HD-OCT) for non-invasive imaging of dermatofibroma: A pilot study. J. Eur. Acad. Dermatol. Venereol. 2016;30:485–487. doi: 10.1111/jdv.12868. [DOI] [PubMed] [Google Scholar]
- 73.Demir M.K., Ozdemir H., Gençhallaç H., Altaner S., Kartal O. Dermatofibroma mimicking malignancy on integrated F-18 fluorodeoxyglucose PET-CT. Diagn. Interv. Radiol. 2009;15:61–63. [PubMed] [Google Scholar]
- 74.Guedes R.V.D.A.P., De Menezes N.M.N., Leite I.B., Baptista M.A. Benign fibrous histiocytoma: Particular aspects on confocal laser scanning microscopy. Eur. J. Dermatol. 2012;22:288–289. doi: 10.1684/ejd.2012.1672. [DOI] [PubMed] [Google Scholar]
- 75.Genc Y., Akay B.N., Heper A.O., Rosendahl C., Erdem C. Dermatopathological characteristics of dermatofibromas from dermatoscopic clues. Int. J. Dermatol. 2020;59:66–75. doi: 10.1111/ijd.14559. [DOI] [PubMed] [Google Scholar]
- 76.Lin M.J., Xie C., Pan Y., Jalilian C., Kelly J.W. Dermoscopy improves diagnostic accuracy for clinically amelanotic nodules. Australas. J. Dermatol. 2019;60:45–49. doi: 10.1111/ajd.12902. [DOI] [PubMed] [Google Scholar]
- 77.Won K.Y., Park S.Y., Jin W., Lew B.-L. Dermatofibroma: Sonographic findings and pathologic correlation. Acta Radiol. 2018;59:454–459. doi: 10.1177/0284185117721263. [DOI] [PubMed] [Google Scholar]
- 78.Kelati A., Aqil N., Baybay H., Gallouj S., Mernissi F.Z. Beyond classic dermoscopic patterns of dermatofibromas: A prospective research study. J. Med. Case Rep. 2017;11:266. doi: 10.1186/s13256-017-1429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roldán-Marín R., Barreiro-Capurro A., García-Herrera A., Puig S., Alarcón-Salazar I., Carrera C., Malvehy J. Green colour as a novel dermoscopic finding in the diagnosis of haemosiderotic dermatofibroma. Australas. J. Dermatol. 2014;55:196–197. doi: 10.1111/ajd.12076. [DOI] [PubMed] [Google Scholar]
- 80.Arpaia N., Cassano N., Vena G.A. Dermoscopic Patterns of Dermatofibroma. Dermatol. Surg. 2005;31:1336–1339. doi: 10.1097/00042728-200510000-00015. [DOI] [PubMed] [Google Scholar]
- 81.Karaarslan I.K., Gencoglan G., Akalin T., Ozdemir F. Different dermoscopic faces of dermatofibromas. J. Am. Acad. Dermatol. 2007;57:401–406. doi: 10.1016/j.jaad.2006.10.984. [DOI] [PubMed] [Google Scholar]
- 82.Agero A.L.C., Taliercio S., Dusza S.W., Salaro C., Chu P., Marghoob A.A. Conventional and Polarized Dermoscopy Features of Dermatofibroma. Arch. Dermatol. 2006;142:1431–1437. doi: 10.1001/archderm.142.11.1431. [DOI] [PubMed] [Google Scholar]
- 83.Han T.Y., Chang H.S., Lee J.H.K., Lee W.-M., Son S.-J. A Clinical and Histopathological Study of 122 Cases of Dermatofibroma (Benign Fibrous Histiocytoma) Ann. Dermatol. 2011;23:185–192. doi: 10.5021/ad.2011.23.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zelger B.G., Sidoroff A. Combined dermatofibroma: Co-existence of two or more variant patterns in a single lesion. Histopathology. 2000;36:529–539. doi: 10.1046/j.1365-2559.2000.00901.x. [DOI] [PubMed] [Google Scholar]
- 85.Calonje E., Brenn T., Lazar A., Mckee P. McKee’s Pathology of the Skin with Clinical Correlations. 4th ed. Elsevier Saunders; Edinburgh, UK: 2012. pp. 1643–1662. [Google Scholar]
- 86.Zeidi M., North J.P. Sebaceous induction in dermatofibroma: A common feature of dermatofibromas on the shoulder. J. Cutan. Pathol. 2015;42:400–405. doi: 10.1111/cup.12474. [DOI] [PubMed] [Google Scholar]
- 87.Cazzato G., Colagrande A., Cimmino A., Marrone M., Stellacci A., Arezzo F., Lettini T., Resta L., Ingravallo G. Granular Cell Dermatofibroma: When Morphology Still Matters. Dermatopathology. 2021;8:371–375. doi: 10.3390/dermatopathology8030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.LeBoit P.E., Barr R.J., Burall S., Metcalf J.S., Yen T.S.B., Wick M.R. Primitive Polypoid Granular-Cell Tumor and Other Cutaneous Granular-Cell Neoplasms of Apparent Nonneural Origin. Am. J. Surg. Pathol. 1991;15:48–58. doi: 10.1097/00000478-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 89.Lee M., Lee W.J., Jung J.M., Won C.H., Chang S.E., Choi J.H., Moon K.C. Clinical and histological patterns of dermatofibroma without gross skin surface change: A comparative study with conventional dermatofibroma. Indian J. Dermatol. Venereol. Leprol. 2015;81:263–269. doi: 10.4103/0378-6323.154795. [DOI] [PubMed] [Google Scholar]
- 90.Victor T.A. In: Neoplasms with Follicular Differentiation. 2nd ed. Ackerman B., Reddy V.B., Soyer H.P., editors. Blackwell Publishing Inc.; Hoboken, NJ, USA: 2002. [Google Scholar]
- 91.Kaddu S., McMenamin M.E., Fletcher C.D.M. Atypical Fibrous Histiocytoma of the Skin: Clinicopathologic analysis of 59 cases with evidence of infrequent metastasis. Am. J. Surg. Pathol. 2002;26:35–46. doi: 10.1097/00000478-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 92.Tatu A.L. Black Nodule on the Forearm. J. Cutan. Med. Surg. 2017;21:157. doi: 10.1177/1203475416679826. [DOI] [PubMed] [Google Scholar]
- 93.Agarwal A., Gopinath A., Tetzlaff M.T., Prieto V.G. Phosphohistone-H3 and Ki67: Useful Markers in Differentiating Dermatofibroma from Dermatofibrosarcoma Protuberans and Atypical Fibrohistiocytic Lesions. Am. J. Dermatopathol. 2017;39:504–507. doi: 10.1097/DAD.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 94.Trøstrup H., Bigdeli A.K., Krogerus C., Kneser U., Schmidt G., Schmidt V.J. A Multidisciplinary Approach to Complex Dermal Sarcomas Ensures an Optimal Clinical Outcome. Cancers. 2022;14:1693. doi: 10.3390/cancers14071693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Volpicelli E.R., Fletcher C.D.M. Desmin and CD34 positivity in cellular fibrous histiocytoma: An immunohistochemical analysis of 100 cases. J. Cutan. Pathol. 2012;39:747–752. doi: 10.1111/j.1600-0560.2012.01944.x. [DOI] [PubMed] [Google Scholar]
- 96.John A.M., Holahan H.H., Singh P., Handler M.Z., Lambert W.C. When Immunohistochemistry Deceives Us: The Pitfalls of CD34 and Factor XIIIa Stains in Dermatofibroma and Dermatofibrosarcoma Protuberans. Skinmed. 2017;15:53–55. [PubMed] [Google Scholar]
- 97.Kim H.J., Lee J.Y., Kim S.H., Seo Y.J., Lee J.H., Park J.K., Kim M.H., Cinn Y.W., Cho K.H., Yoon T.Y. Stromelysin-3 expression in the differential diagnosis of dermatofibroma and dermatofibrosarcoma protuberans: Comparison with factor XIIIa and CD34. Br. J. Dermatol. 2007;157:319–324. doi: 10.1111/j.1365-2133.2007.08033.x. [DOI] [PubMed] [Google Scholar]
- 98.Maekawa T., Jinnin M., Ihn H. The expression levels of thrombospondin-1 in dermatofibroma and dermatofibrosarcoma protuberans. Eur. J. Dermatol. 2011;21:534–538. doi: 10.1684/ejd.2011.1392. [DOI] [PubMed] [Google Scholar]
- 99.Li J., Yu Y., Yang Y., Wang L., Cao J., Liang X., Xiao X., Tu Y., Chen H. IGFBP7, a novel immunohistochemical marker in differentiating dermatofibroma from dermatofibrosarcoma protuberans. J. Eur. Acad. Dermatol. Venereol. 2012;26:382–385. doi: 10.1111/j.1468-3083.2011.04072.x. [DOI] [PubMed] [Google Scholar]
- 100.Mori T., Misago N., Yamamoto O., Toda S., Narisawa Y. Expression of nestin in dermatofibrosarcoma protuberans in comparison to dermatofibroma. J. Dermatol. 2008;35:419–425. doi: 10.1111/j.1346-8138.2008.00496.x. [DOI] [PubMed] [Google Scholar]
- 101.Wang L., Xiang Y.N., Zhang Y.H., Tu Y.T., Chen H.X. Collagen triple helix repeat containing-1 in the differential diagnosis of dermatofibrosarcoma protuberans and dermatofibroma. Br. J. Dermatol. 2011;164:135–140. doi: 10.1111/j.1365-2133.2010.10050.x. [DOI] [PubMed] [Google Scholar]
- 102.Yan X., Takahara M., Xie L., Tu Y., Furue M. Cathepsin K expression: A useful marker for the differential diagnosis of dermatofibroma and dermatofibrosarcoma protuberans. Histopathology. 2010;57:486–488. doi: 10.1111/j.1365-2559.2010.03628.x. [DOI] [PubMed] [Google Scholar]
- 103.Toyozawa S., Yamamoto Y., Ishida Y., Kondo T., Nakamura Y., Furukawa F. Immunohistochemical Analysis of CXCR4 Expression in Fibrohistiocytic Tumors. Acta Histochem. Cytochem. 2010;43:45–50. doi: 10.1267/ahc.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ishigami T., Hida Y., Matsudate Y., Murao K., Kubo Y. The involvement of fibroblast growth factor receptor signaling pathways in dermatofibroma and dermatofibrosarcoma protuberans. J. Med. Investig. 2013;60:106–113. doi: 10.2152/jmi.60.106. [DOI] [PubMed] [Google Scholar]
- 105.Kazlouskaya V., Malhotra S., Kabigting F.D., Lal K., Elston D.M. CD99 Expression in Dermatofibrosarcoma Protuberans and Dermatofibroma. Am. J. Dermatopathol. 2014;36:392–396. doi: 10.1097/DAD.0b013e3182a15f3e. [DOI] [PubMed] [Google Scholar]
- 106.West K.L., Cardona D.M., Su Z., Puri P.K. Immunohistochemical Markers in Fibrohistiocytic Lesions: Factor XIIIa, CD34, S-100 and p75. Am. J. Dermatopathol. 2014;36:414–419. doi: 10.1097/DAD.0b013e3182a70396. [DOI] [PubMed] [Google Scholar]
- 107.Mikoshiba Y., Ogawa E., Uchiyama R., Uchiyama A., Uhara H., Okuyama R. 5-Hydroxymethylcytosine is a useful marker to differentiate between dermatofibrosarcoma protuberans and dermatofibroma. J. Eur. Acad. Dermatol. Venereol. 2016;30:130–131. doi: 10.1111/jdv.12614. [DOI] [PubMed] [Google Scholar]
- 108.Karanian M., Pérot G., Coindre J.-M., Chibon F., Pedeutour F., Neuville A. Fluorescence in situ hybridization analysis is a helpful test for the diagnosis of dermatofibrosarcoma protuberans. Mod. Pathol. 2015;28:230–237. doi: 10.1038/modpathol.2014.97. [DOI] [PubMed] [Google Scholar]
- 109.Kaur H., Kaur J., Gill K.S., Mannan R., Arora S. Subcutaneous Dermatofibroma: A Rare Case Report with Review of Literature. J. Clin. Diagn. Res. 2014;8:FD01–FD02. doi: 10.7860/JCDR/2014/6586.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Motegi S.-I., Fujiwara C., Sekiguchi A., Yamazaki S., Yokoyama Y., Yasuda M., Ishikawa O. Possible contribution of PDGF-BB-induced autophagy in dermatofibrosarcoma protuberans: Autophagy marker Atg5 could be a differential marker between dermatofibrosarcoma protuberans and dermatofibroma. J. Dermatol. Sci. 2019;93:139–141. doi: 10.1016/j.jdermsci.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 111.Lyundup A.V., Balyasin M.V., Maksimova N.V., Kovina M.V., Krasheninnikov M.E., Dyuzheva T.G., Yakovenko S.A., Appolonova S.A., Schiöth H.B., Klabukov I.D. Misdiagnosis of diabetic foot ulcer in patients with undiagnosed skin malignancies. Int. Wound J. 2022;19:871–887. doi: 10.1111/iwj.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giovannacci I., Meleti M., Garbarino F., Cesinaro A.M., Mataca E., Pedrazzi G., Reggiani C., Paganelli A., Truzzi A., Elia F., et al. Correlation between Autofluorescence Intensity and Histopathological Features in Non-Melanoma Skin Cancer: An Ex Vivo Study. Cancers. 2021;13:3974. doi: 10.3390/cancers13163974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dessinioti C., Antoniou C., Stratigos A.J. New targeted approaches for the treatment and prevention of nonmelanoma skin cancer. Expert Rev. Dermatol. 2011;6:625–634. doi: 10.1586/edm.11.70. [DOI] [Google Scholar]
- 114.Drakaki E., Vergou T., Dessinioti C., Stratigos A.J., Salavastru C., Antoniou C. Spectroscopic methods for the photodiagnosis of nonmelanoma skin cancer. J. Biomed. Opt. 2012;18:061221. doi: 10.1117/1.JBO.18.6.061221. [DOI] [PubMed] [Google Scholar]
- 115.Seidenari S., Arginelli F., Bassoli S., Cautela J., French P.M.W., Guanti M., Guardoli D., König K., Talbot C., Dunsby C. Multiphoton Laser Microscopy and Fluorescence Lifetime Imaging for the Evaluation of the Skin. Dermatol. Res. Pract. 2011;2012:810749. doi: 10.1155/2012/810749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Balaceanu L.A. Biomarkers vs. imaging in the early detection of hepatocellular carcinoma and prognosis. World J. Clin. Cases. 2019;7:1367–1382. doi: 10.12998/wjcc.v7.i12.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Czajkowska J., Juszczyk J., Piejko L., Glenc-Ambroży M. High-Frequency Ultrasound Dataset for Deep Learning-Based Image Quality Assessment. Sensors. 2022;22:1478. doi: 10.3390/s22041478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Malciu A.M., Lupu M., Voiculescu V.M. Artificial Intelligence-Based Approaches to Reflectance Confocal Microscopy Image Analysis in Dermatology. J. Clin. Med. 2022;11:429. doi: 10.3390/jcm11020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maron R.C., Utikal J.S., Hekler A., Hauschild A., Sattler E., Sondermann W., Haferkamp S., Schilling B., Heppt M.V., Jansen P., et al. Artificial Intelligence and Its Effect on Dermatologists’ Accuracy in Dermoscopic Melanoma Image Classification: Web-Based Survey Study. J. Med. Internet Res. 2020;22:e18091. doi: 10.2196/18091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tschandl P., Wiesner T. Advances in the diagnosis of pigmented skin lesions. Br. J. Dermatol. 2018;178:9–11. doi: 10.1111/bjd.16109. [DOI] [PubMed] [Google Scholar]
- 121.Styła M., Giżewski T. The Study of Usefulness of a Set of Fractal Parameters to Build Classes of Disease Units Based on Images of Pigmented Skin Lesions. Diagnostics. 2021;11:1773. doi: 10.3390/diagnostics11101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tschandl P., Rosendahl C., Kittler H. The HAM10000 dataset, a large collection of multi-source dermatoscopic images of common pigmented skin lesions. Sci. Data. 2018;5:180161. doi: 10.1038/sdata.2018.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Felmingham C., MacNamara S., Cranwell W., Williams N., Wada M., Adler N.R., Ge Z., Sharfe A., Bowling A., Haskett M., et al. Improving Skin Cancer Management with Artificial Intelligence (SMARTI): Protocol for a preintervention/postintervention trial of an artificial intelligence system used as a diagnostic aid for skin cancer management in a specialist dermatology setting. BMJ Open. 2022;12:e050203. doi: 10.1136/bmjopen-2021-050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tschandl P., Rinner C., Apalla Z., Argenziano G., Codella N., Halpern A., Janda M., Lallas A., Longo C., Malvehy J., et al. Human–computer collaboration for skin cancer recognition. Nat. Med. 2020;26:1229–1234. doi: 10.1038/s41591-020-0942-0. [DOI] [PubMed] [Google Scholar]
- 125.Esteva A., Kuprel B., Novoa R.A., Ko J., Swetter S.M., Blau H.M., Thrun S. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115–118. doi: 10.1038/nature21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nagendran M., Chen Y., Lovejoy C.A., Gordon A.C., Komorowski M., Harvey H., Topol E.J., Ioannidis J.P.A., Collins G.S., Maruthappu M. Artificial intelligence versus clinicians: Systematic review of design, reporting standards, and claims of deep learning studies. BMJ. 2020;368:m689. doi: 10.1136/bmj.m689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ouellette S., Rao B.K. Usefulness of Smartphones in Dermatology: A US-Based Review. Int. J. Environ. Res. Public Health. 2022;19:3553. doi: 10.3390/ijerph19063553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Patrzyk S., Bielecki W., Woźniacka A. A study of attitudes among Polish dermatologists and dermatology trainees regarding modern technologies in medicine. Adv. Dermatol. Allergol. 2022;39:531–537. doi: 10.5114/ada.2022.117738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Breslavets M., Shear N.H., Lapa T., Breslavets D., Breslavets A. Validation of artificial intelligence application in clinical dermatology. J. Am. Acad. Dermatol. 2022;86:201–203. doi: 10.1016/j.jaad.2021.01.064. [DOI] [PubMed] [Google Scholar]
- 130.Koh U., Horsham C., Soyer H.P., Loescher L.J., Gillespie N., Vagenas D., Janda M. Consumer Acceptance and Expectations of a Mobile Health Application to Photograph Skin Lesions for Early Detection of Melanoma. Dermatology. 2019;235:4–10. doi: 10.1159/000493728. [DOI] [PubMed] [Google Scholar]
- 131.Fluhr J.W., Gueguen A., Legoupil D., Brenaut E., Abasq C., Araújo H., Misery L. Teledermatology in Times of COVID-19 Confinement: Comparing Patients’ and Physicians’ Satisfaction by the Standardized Brest Teledermatology Questionnaire. Dermatology. 2021;237:191–196. doi: 10.1159/000514029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Asabor E.N., Bunick C.G., Cohen J.M., Perkins S.H. Patient and physician perspectives on teledermatology at an academic dermatology department amid the COVID-19 pandemic. J. Am. Acad. Dermatol. 2021;84:158–161. doi: 10.1016/j.jaad.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fijałkowska M., Koziej M., Żądzińska E., Antoszewski B., Sitek A. Assessment of the Predictive Value of Spectrophotometric Skin Color Parameters and Environmental and Behavioral Factors in Estimating the Risk of Skin Cancer: A Case–Control Study. J. Clin. Med. 2022;11:2969. doi: 10.3390/jcm11112969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hauser K., Kurz A., Haggenmüller S., Maron R.C., von Kalle C., Utikal J.S., Meier F., Hobelsberger S., Gellrich F.F., Sergon M., et al. Explainable artificial intelligence in skin cancer recognition: A systematic review. Eur. J. Cancer. 2022;167:54–69. doi: 10.1016/j.ejca.2022.02.025. [DOI] [PubMed] [Google Scholar]
- 135.Liopyris K., Gregoriou S., Dias J., Stratigos A.J. Artificial Intelligence in Dermatology: Challenges and Perspectives. Dermatol. Ther. 2022;12:2637–2651. doi: 10.1007/s13555-022-00833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review summarizes data reported in the literature and it does not report primary data.