Abstract
Background and objective: Some variants in defensin beta 1 (DEFB1) and mannose-binding lectin 2 (MBL2) genes can be associated with oral diseases. Herein, we designed a systematic review and meta-analysis to evaluate the association of DEFB1 (rs11362, rs1799946, and rs1800972) and MBL2 (rs7096206 and rs1800450) polymorphisms with the susceptibility to dental caries (DC) in children. Materials and methods: A systematic literature search was conducted in the PubMed/Medline, Web of Science, Scopus, and Cochrane Library databases until 3 December 2022, without any restrictions. The odds ratio (OR), along with a 95% confidence interval (CI) of the effect sizes, are reported. Analyses including a subgroup analysis, a sensitivity analysis, and funnel plot analyses were conducted. Results: A total of 416 records were identified among the databases, and nine articles were entered into the meta-analysis. A significant relationship was found between the T allele of DEFB1 rs11362 polymorphism and DC susceptibility, and the T allele was related to an elevated risk of DC in children (OR = 1.225; 95%CI: 1.022, 1.469; p = 0.028; I2 = 0%). No other polymorphisms were associated with DC. All articles were of moderate quality. Egger’s test in homozygous and dominant models demonstrated a significant publication bias for the association of DEFB1 rs1799946 polymorphism with DC risk. Conclusions: The results demonstrated that the T allele of DEFB1 rs11362 polymorphism had an elevated risk for DC in children. However, there were only few studies that evaluated this association.
Keywords: dental caries, DEFB1 protein, MBL2 protein, polymorphism, meta-analysis
1. Introduction
Oral health is important for a child’s overall health and development [1]. Dental caries (DC) or tooth decay involves damage to tooth enamel [2] and is a chronic disease that is widely prevalent [2,3]. DC is the most prevalent chronic disease among children [4,5,6], with 0.5 billion prevalent cases of caries in deciduous teeth among 0–14-year-old children [7].
Oral bacteria [8,9], dietary habits (sugar intake) [10], oral health behavior (e.g., toothbrushing and using fluoridated toothpaste) [11], feeding practices (e.g., breastfeeding practice and night bottle-feeding) [11,12], geographic area [13], and socioeconomic status (e.g., income, education, and social class) are factors that could serve as determinants of DC in children. In addition to these factors, genetic variations in the formation of enamel and immune response genes could be associated with a greater predisposition to DC [14]. Recent meta-analyses [15,16,17] reported a relationship between polymorphisms and DC risk.
The human beta-defensin 1 (DEFB1) is a 36-amino-acid antimicrobial peptide depending on the defensin family [18]. This peptide is encoded by the DEFB1 gene and is the main molecule for protection from DC [19], and it has been detected in mucosal surface of airways, the gastrointestinal tract (esophagus, intestines, and stomach), urogenital tissue [20], salivary glands [21], and gingival and oral tissues [22]. The DEFB1 gene is associated with immune response, and researchers have shown that it acts as a host defense protein by influencing the non-specific immune system, as well as in adaptive immunity, thereby influencing DC progression [23,24].
Mannose-binding lectin (MBL) is a protein molecule inherent to the immune system, in which the activation of lectin (ubiquitous carbohydrate-binding protein) domains are found in relation to collagenous structures [25,26]. MBL insufficiency is one of the most common human immunodeficiencies and increases first from three single-point mutations in exon 1 of the MBL2 gene [26]. Variations in this gene can be associated with DC [27].
Two meta-analyses [19,28] reported a relationship between polymorphisms and DC risk. One meta-analysis checked the relationship of DEFB1 (rs11362, rs1799946, and rs1800972) and MBL2 (rs7096206, rs11003125, and rs1800450) polymorphisms with DC risk [28] while the other just explored the role of DEFB1 rs11362 polymorphism [19]. Both meta-analyses [19,28] analyzed the results, including individuals of all age groups. It is evident that age is a significant predictor of DC [29,30,31,32].
Although some researchers have reported selected polymorphisms of the DEFB1 and MBL2 genes to have an influence on the progression of DC, the results are still unconfirmed and inconsistent. We designed a systematic review and meta-analysis to evaluate the association of DEFB1 (rs11362, rs1799946, and rs1800972) and MBL2 (rs7096206 and rs1800450) polymorphisms with the risk of DC in children with more studies for the first time in the English literature based on our knowledge.
2. Materials and Methods
To design the study, the PRISMA guidelines provided in the Supplementary file were followed [33]. The PECO (Population, Exposure, Comparator, and Outcome) question [34,35] was as follows: Is there an association between DEFB1 and MBL2 polymorphisms and susceptibility to DC? (P: Children with DC (CDC), E: DEFB1 and MBL2 polymorphisms, C: Children free of DC (CFC); O: DC).
2.1. Search Strategy and Study Selection
The Scopus, PubMed/Medline, Cochrane Library, and Web of Science databases were searched by one author (M.S.) to retrieve records published until December 3, 2022, without any restrictions (e.g., language). The keywords or search terms were (“beta defensin*” or “β-defensin*” or “beta-defensin 1” or “β-defensin-1” or “beta-defensin-1” or “defensin beta 1” or “DEFB1” or “human beta-defensin-1” or “HBD-1” or “mannose-binding lectin” or “MBL” or “mannose binding lectin 2” or “MBL2” or “mannose binding lectin-2” or “MBL-2” or “mannan-binding lectin” or “mannan-binding protein” or “MBP”) and (“tooth decay” or “dental caries” or “caries”). Moreover, the citations of the retrieved original articles/reviews/meta-analyses linked to the subject were searched to ensure that no study was missed. A second reviewer (G.H.) evaluated the titles/abstracts of the articles linked to the subject; afterwards, the full texts of the articles that met the inclusion criteria were downloaded and screened. Any study that was excluded was tagged with the reason for exclusion. In the event of a lack of agreement among the authors, a third reviewer (M.M.I) was involved.
2.2. Quality Assessment
The quality of studies was evaluated based on the modified Newcastle–Ottawa scale (NOS) [19] by two reviewers independently (G.H. and M.S.). The scores ranged from 0 to 10 points, with >7 points being considered as “high quality”, 4 to 7 points denoting “moderate quality”, and less than 4 points being “low quality”. Disagreement between the authors was resolved by a third reviewer (M.M.I.).
2.3. Eligibility Criteria
The inclusion criteria were as follows: (I) any type of articles including two independent groups (CDC and CFCs); (II) studies with any defined DMFT/dmft score for CDC and CFCs; (III) studies including polymorphisms of DEFB1 or MBL2 genes including minimum two studies for the analysis with any amount of the Hardy–Weinberg equilibrium (HWE); and (IV) CDC and CFCs had no chronic illnesses, genetic diseases, or other disorders. Irrelevant studies, meta-analyses, studies without a control group, studies with insufficient data for analysis, case reports, and conference papers were excluded.
2.4. Data Extraction
Two authors (S.B. and R.S.) independently extracted the data of the studies. Disagreement between the authors was resolved by a third author (P.C.). The extracted data were the name of first author, publication year of the study, country of origin of the study, number of CDC and CFCs, ethnicity, age range of individuals, investigated dentition, DMFT/dmft score of the CDC and CFCs, type of reported polymorphism(s), the quality score of each study, and effect sizes (odds ratio (OR) and 95% confidence interval (CI)) for DC occurrence of each polymorphism according to five genetic models.
2.5. Statistical Analysis
To compute the effect sizes (ORs and 95% CIs) and the rest of the analyses, two authors (G.H. and M.S.) independently used comprehensive meta-analysis version 2.0 (CMA 2.0) software. Disagreement between the authors was resolved by a third author (M.M.I.). A p-value (two-sided) less than 0.05 was considered significant. The I2 statistic was used to estimate heterogeneity, with I2 > 50% (Pheterogeneity < 0.1) recommending a significant heterogeneity, and we used the fixed-effects model [36]. The publication bias across or among the studies was evaluated using Egger’s and Begg’s tests [37,38]; if p-value (two-sided) was less than 0.10 (two-sided) for one and both tests, a significant publication bias was considered to be present. With regard to the stability of the results, two sensitivity analyses, including ‘one-study-removed’ and ‘cumulative analyses’ were carried out. A subgroup analysis based on ethnicity, type of dentition, and sample size was carried out for DEFB1 rs11362 polymorphism while such analysis wasn’t possible for MBL2 polymorphisms due to an insufficient number of studies.
3. Results
3.1. Study Selection
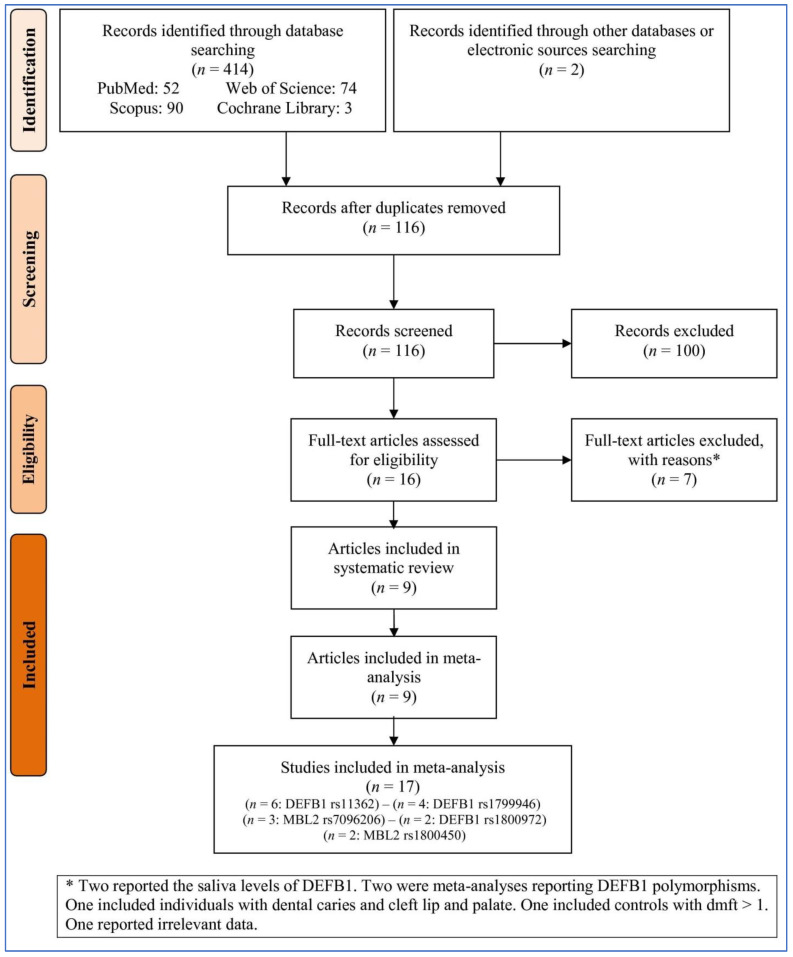
By searching the databases and electronic resources, 416 records were identified. After removing duplicates and irrelevant records, 16 full-text articles were obtained and, after that, assessed. Among the evaluated full-text articles, seven were excluded for different reasons (Figure 1). Finally, nine articles [14,27,39,40,41,42,43,44,45] involving analyses of 17 studies (several articles included more than one polymorphism, and each polymorphism was considered one independent study) were involved in the meta-analysis.
Figure 1.
Flowchart of study selection. DEFB1: beta-defensin 1. MBL2: mannose-binding lectin 2.
3.2. Characteristics of the Studies
The articles were published from 2005 to 2020 (Table 1). Four articles [14,27,42,43] included Caucasians, three [40,44,45] Asians, and two [39,41] mixed ethnicities. Four articles [14,42,43,45] investigated deciduous dentitions, two [27,40] investigated permanent dentitions, two [41,44] investigated mixed dentitions, and one [39] investigated all three dentitions. Five and four articles evaluated the association of MBL2 [27,40,43,44,45] and DEFB1 [14,39,41,42] polymorphisms, respectively with DC risk.
Table 1.
Features of articles included in the analysis.
| First Author, Publication Year | Country | Ethnicity | No. of Cases | No. of Controls | Age Range, Years | Investigated Dentition |
Caries Index (Control; Case) | Polymorphisms |
|---|---|---|---|---|---|---|---|---|
| Pehlivan, 2005 [43] | Turkey | Caucasian | 42 | 40 | < 18 | Deciduous | dmft (0; NR) | MBL2 rs1800450 |
| Mubayrik, 2014 [42] | Turkey | Caucasian | 87 | 74 | 2 to 6 | Deciduous | dmft (0; ≥1) |
DEFB1 rs11362
DEFB1 rs1800972 |
| Yang, 2013 [45] | China | Asian | 70 | 70 | 1 to 5 | Deciduous | dmft (0; ≥1) | MBL2 rs1800450 |
| Abbasoğlu, 2015 [14] | Turkey | Caucasian | 136 | 123 | 2 to 5 | Deciduous | dmft (0; ≥1) |
DEFB1 rs11362
DEFB1 rs1800972 |
| Alyousef, 2017 [27] | Saudi Arabia | Caucasian | 204 | 200 | 5 to 13 | Permanent | DMFT (0; NR) | MBL2 rs7096206 |
| Lips, 2017 [41] | Brazil | Mixed | 87 | 81 | 2 to 12 | Mixed | DMFT/dmft (0; ≥4) |
DEFB1 rs11362
DEFB1 rs1799946 |
| de Oliveira, 2018 [39] | Brazil | Mixed | 117 | 78 | 10 to 12 | Deciduous | DMFT/dmft (0; ≥1) | DEFB1 rs11362 |
| 118 | 78 | Permanent | ||||||
| 265 | 49 | Mixed | ||||||
| 117 | 78 | 6 to 12 | Deciduous | DEFB1 rs1799946 | ||||
| 118 | 78 | Permanent | ||||||
| 265 | 49 | Mixed | ||||||
| Shimomura-Kuroki, 2018 [44] | Japan | Asian | 53 | 28 | 3 to 11 | Mixed | DMFT/dmft (0; ≥1) | MBL2 rs7096206 |
| Hu, 2020 [40] | China | Asian | 198 | 162 | 12 to 15 | Permanent | DMFT (0; ≥1) | MBL2 rs7096206 |
NR: not reported. DEFB1: beta-defensin 1. MBL2: mannose-binding lectin 2. dmft: decayed, missing and filled primary teeth. DMFT: decayed, missing, and filled permanent teeth.
3.3. Quality Assessment
Table 2 shows the quality evaluation of the articles by modified NOS. All articles [14,27,39,40,41,42,43,44,45] were of moderate quality.
Table 2.
Quality evaluation of the articles by modified Newcastle–Ottawa scale.
| First Author, Publication Year | Representativeness of Cases | Source of Controls |
Hardy–Weinberg Equilibrium in Controls |
Genotyping Examination |
Association Assessment |
Total Score |
|---|---|---|---|---|---|---|
| Pehlivan, 2005 [43] | * | * * | ** | - | * * | 7 |
| Mubayrik, 2014 [42] | * | * | * * | - | * * | 6 |
| Yang, 2013 [45] | * | * | * | - | * * | 5 |
| Abbasoğlu, 2015 [14] | * | * * | * | - | * * | 6 |
| Alyousef, 2017 [27] | * | * | ** | - | * * | 6 |
| Lips, 2017 [41] | * | * * | * | - | * * | 6 |
| de Oliveira, 2018 [39] | * | * * | * | - | * * | 6 |
| Shimomura-Kuroki, 2018 [44] | * | * | ** | - | * * | 6 |
| Hu, 2020 [40] | * | * | ** | - | * * | 6 |
Each asterisk denotes 1 score.
3.4. Meta-Analysis
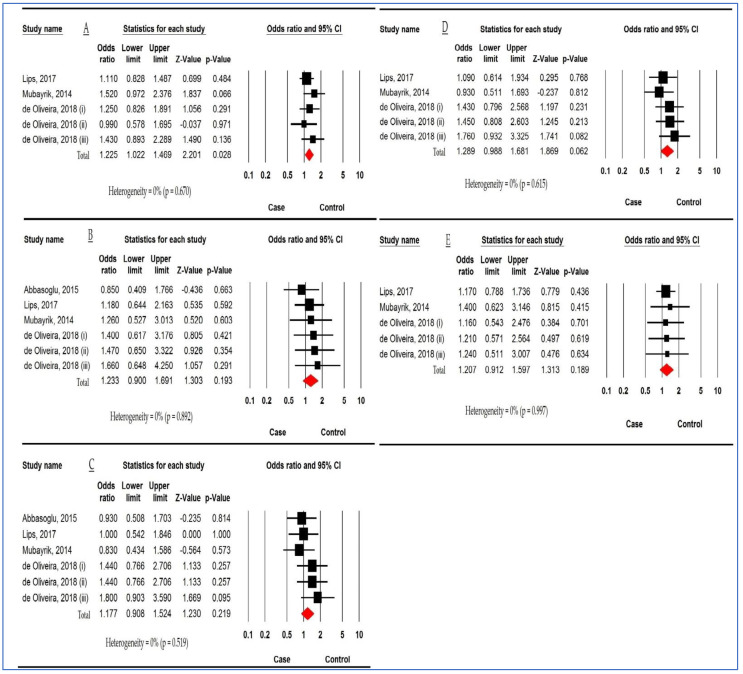
Figure 2 displays the relationship between DEFB1 rs11362 polymorphism and DC risk based on six studies for homozygous and heterozygous models and five for other models. The pooled ORs were 1.225 (95%CI: 1.022, 1.469; p = 0.028; I2 = 0%), 1.233 (95%CI: 0.900, 1.691; p = 0.193; I2 = 0%), 1.177 (95%CI: 0.908, 1.524; p = 0.219; I2 = 0%), 1.289 (95%CI: 0.988, 1.681; p = 0.062; I2 = 0%), and 1.207 (95%CI: 0.912, 1.597; p = 0.189; I2 = 0%) in the allelic model (AM), the homozygous model (HoM), the heterozygous model (HeM), the dominant model (DM), and the recessive model (RM), respectively. There was only a significant relationship between the T allele of DEFB1 rs11362 polymorphism and DC risk.
Figure 2.
Forest plot showing the association between defensin beta 1 (DEFB1) rs11362 polymorphism and dental caries risk. (A) Allelic model. (B) Homozygous model. (C) Heterozygous model. (D) Dominant model. (E) Recessive model. ((i) Deciduous dentition. (ii) Permanent dentition. (iii) Mixed dentition in the study of de Oliveira et al. [14,39,41,42]).
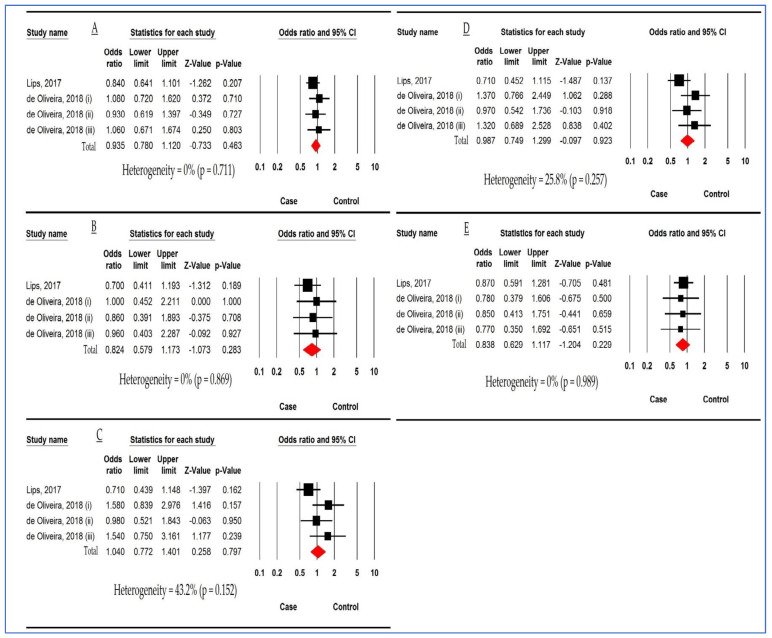
Figure 3 reports the relationship between DEFB1 rs1799946 polymorphism and DC risk based on four studies. The pooled ORs were 0.935 (95%CI: 0.780, 1.120; p = 0.463; I2 = 0%), 0.824 (95%CI: 0.579, 1.173; p = 0.283; I2 = 0%), 1.040 (95%CI: 0.772, 1.401; p = 0.797; I2 = 43.2%), 0.987 (95%CI: 0.749, 1.299; p = 0.923; I2 = 25.8%), and 0.838 (95%CI: 0.629, 1.117; p = 0.229; I2 = 0%) in AM, HoM, HeM, DM, and RM, respectively. There was no significant relationship between DEFB1 rs1799946 polymorphism and the DC susceptibility in any of the five genetic models.
Figure 3.
Forest plot analyses demonstrating the relationship between defensin beta 1 (DEFB1) rs1799946 polymorphism and dental caries risk. (A) Allelic model. (B) Homozygous model. (C) Heterozygous model. (D) Dominant model. (E) Recessive model. ((i) Deciduous dentition. (ii) Permanent dentition. (iii) Mixed dentition in the study of de Oliveira et al. [39,41]).
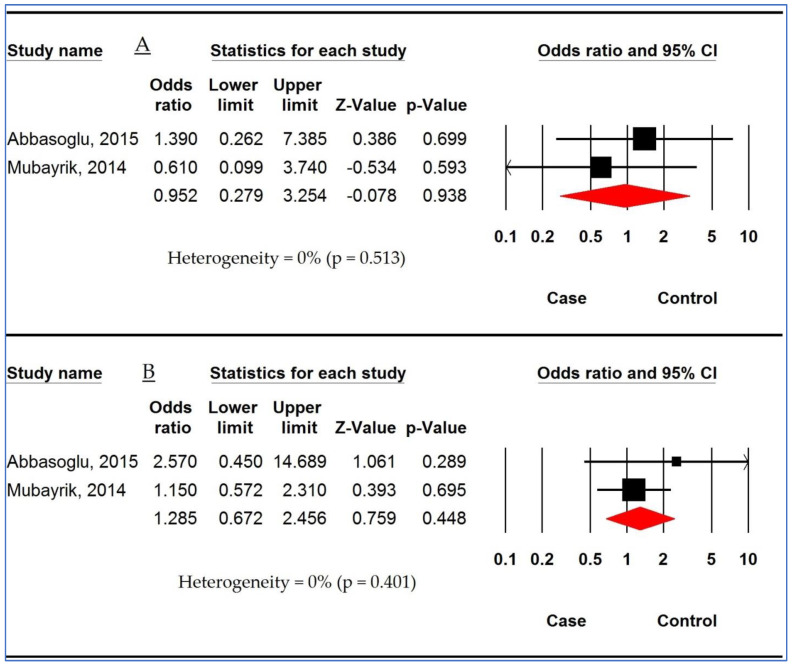
Figure 4 demonstrates the relationship between DEFB1 rs1800972 polymorphism and DC susceptibility based on three studies in two models. The pooled ORs were 0.952 (95%CI: 0.279, 3.254; p = 0.938; I2 = 0%) and 1.285 (95%CI: 0.672, 2.456; p = 0.448; I2 = 0%) in HoM and HeM, respectively. There was no significant association between DEFB1 rs1800972 polymorphism and susceptibility to DC.
Figure 4.
Forest plot for association between defensin beta 1 (DEFB1) rs1800972 polymorphism and dental caries susceptibility. (A) Homozygous model. (B) Heterozygous model [14,42].
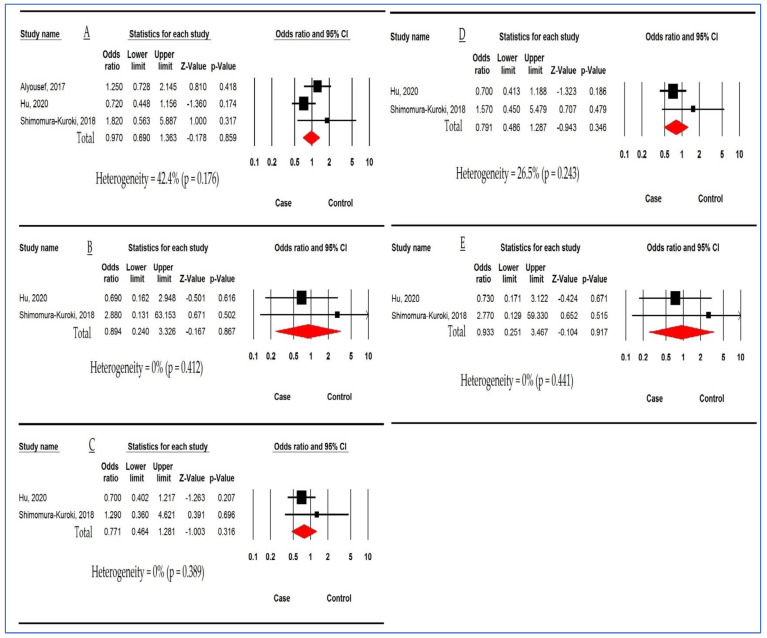
Forest plot in Figure 5 demonstrates the association between MBL2 rs7096206 polymorphism and DC susceptibility based on three studies in allelic models and two studies for other models. The pooled ORs were 0.970 (95%CI: 0.690, 1.363; p = 0.859; I2 = 42.4%), 0.894 (95%CI: 0.240, 3.326; p = 0.867; I2 = 0%), 0.771 (95%CI: 0.464, 1.281; p = 0.316; I2 = 0%), 0.791 (95%CI: 0.486, 1.287; p = 0.346; I2 = 26.5%), and 0.933 (95%CI: 0.251, 3.467; p = 0.917; I2 = 0%) in AM, HoM, HeM, DM, and RM, respectively. There was no significant association between MBL2 rs7096206 polymorphism and susceptibility to DC.
Figure 5.
Forest plot for association between mannose-binding lectin 2 (MBL2) rs7096206 polymorphism and dental caries risk. (A) Allelic model. (B) Homozygous model. (C) Heterozygous model. (D) Dominant model. (E) Recessive model [27,40,44].
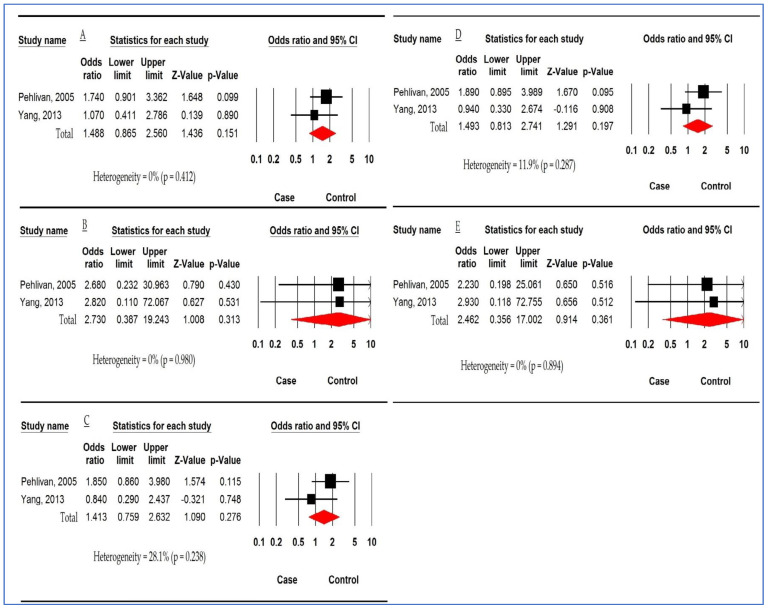
Forest plot in Figure 6 displays the association between MBL2 rs1800450 polymorphism and DC susceptibility based on two studies. The pooled ORs were 1.488 (95%CI: 1.865, 2.560; p = 0.151; I2 = 0%), 2.730 (95%CI: 0.387, 19.243; p = 0.313; I2 = 0%), 1.413 (95%CI: 759, 2.632; p = 0.276; I2 = 0%), 1.493 (95%CI: 0.813, 2.741; p = 0.197; I2 = 0%), and 2.462 (95%CI: 0.356, 17.002; p = 0.361; I2 = 0%) in AM, HoM, HeM, DM, and RM, respectively. There was no significant association between MBL2 rs1800450 polymorphism and susceptibility to DC.
Figure 6.
Forest plot for association between mannose-binding lectin 2 (MBL2) rs1800450 polymorphism and dental caries susceptibility. (A) Allelic model. (B) Homozygous model. (C) Heterozygous model. (D) Dominant model. (E) Recessive model [43,45].
3.5. Subgroup Analysis
Table 3 presents the subgroup analysis for the association between DEFB1 rs11362 polymorphism and susceptibility to DC. Among ethnicity, type of dentition, and sample size in the five genetic models, the TT + CT genotype in mixed ethnicity and T allele in deciduous dentition had elevated risks of DC. Therefore, ethnicity and type of dentition were significant factors for the association between DEFB1 rs11362 polymorphism and DC risk.
Table 3.
Subgroup analysis for beta-defensin 1 (DEFB1) rs11362 polymorphism.
| Variable | Model, N | OR | 95%CI | p-Value | I2, % |
|---|---|---|---|---|---|
| Ethnicity | |||||
| Caucasian | Allelic (1) | 1.520 | 0.972, 2.376 | 0.066 | - |
| Homozygous (2) | 1.000 | 0.571, 1.751 | 1.000 | 0 | |
| Heterozygous (2) | 0.882 | 0.567, 1.372 | 0.578 | 0 | |
| Dominant (1) | 0.930 | 0.511, 1.693 | 0.812 | - | |
| Recessive (1) | 1.400 | 0.623, 3.146 | 0.415 | - | |
| Mixed | Allelic (4) | 1.157 | 0.964, 1.432 | 0.111 | 0 |
| Homozygous (4) | 1.360 | 0.928, 1.993 | 0.115 | 0 | |
| Heterozygous (4) | 1.368 | 0.994, 1.883 | 0.055 | 0 | |
| Dominant (4) | 1.396 | 1.038, 1.878 | 0.028 | 0 | |
| Recessive (4) | 1.182 | 0.877, 1.594 | 0.272 | 0 | |
| Dentition | |||||
| Deciduous | Allelic (2) | 1.368 | 1.010, 1.854 | 0.043 | 0 |
| Homozygous (3) | 1.113 | 0.701, 1.768 | 0.649 | 0 | |
| Heterozygous (3) | 1.036 | 0.722, 1.489 | 0.846 | 0 | |
| Dominant (2) | 1.159 | 0.762, 1.762 | 0.490 | 1.3 | |
| Recessive (2) | 1.267 | 0.728, 2.203 | 0.403 | 0 | |
| Permanent | Allelic (1) | 0.990 | 0.578, 1.695 | 0.971 | - |
| Homozygous (1) | 1.470 | 0.650, 3.322 | 0.354 | - | |
| Heterozygous (1) | 1.440 | 0.766, 2.706 | 0.257 | - | |
| Dominant (1) | 1.450 | 0.808, 2.603 | 0.213 | - | |
| Recessive (1) | 1.210 | 0.571, 2.564 | 0.619 | - | |
| Mixed | Allelic (2) | 1.191 | 0.929, 1.527 | 0.167 | 0 |
| Homozygous (2) | 1.304 | 0.784, 2.170 | 0.307 | 0 | |
| Heterozygous (2) | 1.296 | 0.819, 2.049 | 0.268 | 35.8 | |
| Dominant (2) | 1.351 | 0.882, 2.068 | 0.166 | 16.8 | |
| Recessive (2) | 1.181 | 0.824, 1.694 | 0.365 | 0 | |
| Sample size | |||||
| ≥200 | Allelic (1) | 1.430 | 0.893, 2.289 | 0.136 | - |
| Homozygous (2) | 1.094 | 0.614, 1.948 | 0.760 | 17.6 | |
| Heterozygous (2) | 1.239 | 0.786, 1.953 | 0.356 | 49.7 | |
| Dominant (1) | 1.760 | 0.932, 3.325 | 0.082 | - | |
| Recessive (1) | 1.240 | 0.511, 3.007 | 0.634 | - | |
| <200 | Allelic (4) | 1.193 | 0.980, 1.451 | 0.078 | 0 |
| Homozygous (4) | 1.298 | 0.890, 1.893 | 0.175 | 0 | |
| Heterozygous (4) | 1.148 | 0.837, 1.573 | 0.392 | 0 | |
| Dominant (4) | 1.206 | 0.900, 1.617 | 0.209 | 0 | |
| Recessive (4) | 1.203 | 0.895, 1.617 | 0.220 | 0 |
OR: odds ratio. CI: confidence interval. N: number of studies. Bolded numbers mean statistically significant.
3.6. Sensitivity Analysis
The sensitivity analyses demonstrated stability of the results for all explorations where there were a minimum of three studies (results are not presented).
3.7. Publication Bias
The results of Egger’s and Begg’s tests were checked to evaluate the publication bias across the studies. The funnel plots are illustrated in the Supplementary file. The findings reported that just Egger’s test in homozygous and dominant models for association between DEFB1 rs1799946 polymorphism and DC risk showed a significant publication bias.
4. Discussion
A meta-analysis [28] reported that among DEFB1 (rs11362, rs1799946, and rs1800972) and MBL2 (rs7096206, rs11003125, and rs1800450) polymorphisms, just MBL2 rs11003125 had an association with the risk of DC, while another meta-analysis [19] found DEFB1 rs11362 polymorphism to be associated with the risk of DC in permanent dentition, not deciduous or mixed dentitions. This systematic review evaluated the association of DEFB1 (rs11362, rs1799946, and rs1800972) and MBL2 (rs7096206 and rs1800450) polymorphisms with DC risk; the findings suggest that DEFB1 rs11362 polymorphism in T allele is related to an increased likelihood of DC occurrence. In addition, ethnicity and type of dentition were significant factors in the subgroup analysis checking the relationship of DEFB1 rs11362 polymorphism with DC risk.
DC is a chronic disease that is usually affected by environmental and host agents and even genetic factors [2,46,47,48,49]. Therefore, early detection, early diagnosis, and early treatment are the main considerations for the prevention and treatment of DC [50,51].
There are many genetic agents that probably contribute to DC susceptibility and resistance, such as salivary agents, taste preference, tooth morphology, immune system, enamel structure and composition, organic and inorganic substances, and behavior [16,17,46,52,53,54]. In addition, the likelihood of DC occurrence is high in the first months after the tooth eruption but is much lower in adulthood and later stages of life, and at different ages, the DC intensity may be different [32].
Two studies [27,40] reported that the differences between the results of studies reporting the relationship between polymorphisms and susceptibility to DC can be a result of the variation in the sample sizes, experimental methods, and ethnicities. Another study [55] that included 53 genes reported as being involved in DC susceptibility showed that cytokine network relevant genes, the transforming growth factor-beta family, and the matrix metalloproteinases family had important roles in tooth development and carious lesions. It is believed that the flow of saliva, pH, and chemical composition of saliva are among other important factors in the occurrence and progress of DC [56,57,58]. The present meta-analysis reported that ethnicity and type of dentition were important factors for the relationship of DEFB1 rs11362 polymorphism with susceptibility to DC in children. Other reports showed that Africans had 32% and Mixed ethnicities had 69% more DC experience than Whites [59] and that DC prevalence was 30.4%, 39.0%, and 51.7% for White, Black, and Hispanic students, respectively [60]. For DEFB1 (rs1799946 and rs1800972) and MBL2 (rs7096206 and rs1800450) polymorphisms, we could not perform a subgroup analysis due to the limited number of reported studies. Therefore, a large number of studies are needed to prove the role of ethnicity in the prevalence of tooth decay. This can be due to the difference in ethnical factors (geographical conditions, bone structure, nutrition, etc.) that have affected dental genetics over time. To find these relationships, further studies and further emphasis on ethnicity and the risk of DC in the future can be discussed in further possible mechanisms. With regard to the type of dentition, the etiology of dental anomalies is partly environmental and partly genetic [61] and the DC phenotypes in the deciduous dentition were highly heritable [62]. Therefore, the role of type of dentition can be affected by genetics, but more studies are needed to find the possible mechanisms between type of dentition and risk of DC.
DEFB1 as an oral antimicrobial peptide gives the first line of defense against an extensive range of pathogens [63,64]. The high variability of defensin levels in oral tissues can be attributed to genetic changes in the host [65,66]. The present meta-analysis reported that DEFB1 rs1799946 polymorphism was related to the elevated risk of DC in children, and therefore, this can cause a reduction in DEFB1 and then oral infections. DC is caused by bacteria that destroy the enamel and dentin [67,68,69]. Therefore, the role of DEFB1 polymorphisms could be considered in future studies for reaching better and more accurate results.
The MBL2 plays an important role in the innate immune system and few polymorphisms in this gene can be responsible for increased susceptibility to some infectious diseases [70,71,72]; therefore, MBL2 insufficiency is related to bacterial infection [73]. The present meta-analysis could not find any association between MBL2 (rs7096206 and rs1800450) polymorphisms, perhaps due to a limited number of included studies. Therefore, more studies are needed to support or reject the present meta-analysis results.
The role of oral peptides as therapeutic agents and for clinical assessment of an individual’s susceptibility to DC can be promising in the future [74,75,76]. Oral antimicrobial peptides give the first line of defense against an extensive range of pathogens [75,77]. Their expression in saliva and all over the oral cavity denotes their role in preserving the tooth structure from DC, as well as preserving the oral mucosa, in spite of the fact that the amount of antimicrobial peptides expressed in saliva varies among people [66,76,78,79]. Therefore, paying attention to the metabolism pathways of peptides, their genetic mutations, the values of these peptides in blood and saliva, and their expression can greatly help future research in finding factors associated with susceptibility to DC.
This meta-analysis has three limitations: (1) There were a limited number of published studies, therefore, an inability to conduct subgroup analyses for most polymorphisms; therefore, more studies with more cases are needed to confirm the association of these polymorphisms with DC risk. (2) There was possibly a publication bias for some analyses, which could also be due to fewer studies being included in the analyses. (3) None of the studies were of high quality. In contrast, the stability of the results and low/lack of heterogeneity across the studies were the strengths of the meta-analysis.
5. Conclusions
The findings suggest that the T allele of DEFB1 rs11362 polymorphism is associated with an increased likelihood of DC. Therefore, this polymorphism could have a significant role in the pathogenesis of DC. The limited number of studies and the moderate quality of the included studies demonstrate that well-designed studies with more cases are needed to confirm or reject the results.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/children10020232/s1.
Author Contributions
Conceptualization, G.H. and M.S.; methodology, M.S.; software, M.S.; validation, M.M.I. and R.S.; formal analysis, M.S.; investigation, P.C. and M.S.; resources, G.H.; data curation, M.S.; writing—original draft preparation, M.S., and S.K.T.; writing—review and editing, M.S., M.M.I., F.I., R.S., and S.K.T.; visualization, M.M.I.; supervision, G.H.; project administration, M.S.; funding acquisition, S.K.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zhou N., Zhu H., Chen Y., Jiang W., Lin X., Tu Y., Chen D., Chen H. Dental caries and associated factors in 3 to 5-year-old children in Zhejiang Province, China: An epidemiological survey. BMC Oral Health. 2019;19:9. doi: 10.1186/s12903-018-0698-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selwitz R.H., Ismail A.I., Pitts N.B. Dental caries. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 3.Pitts N.B., Zero D.T., Marsh P.D., Ekstrand K., Weintraub J.A., Ramos-Gomez F., Tagami J., Twetman S., Tsakos G., Ismail A. Dental caries. Nat. Rev. Dis. Prim. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 4.Escoffié-Ramirez M., Ávila-Burgos L., Baena-Santillan E.S., Aguilar-Ayala F., Lara-Carrillo E., Minaya-Sánchez M., Mendoza-Rodríguez M., Márquez-Corona M.d.L., Medina-Solís C.E. Factors associated with dental pain in Mexican schoolchildren aged 6 to 12 years. BioMed Res. Int. 2017;2017:7431301. doi: 10.1155/2017/7431301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nomura Y., Maung K., Kay Khine E.M., Sint K.M., Lin M.P., Win Myint M.K., Aung T., Sogabe K., Otsuka R., Okada A. Prevalence of dental caries in 5-and 6-year-old Myanmar children. Int. J. Dent. 2019;2019:5948379. doi: 10.1155/2019/5948379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S., Kumar A., Badiyani B., Kumar A., Basak D., Ismail M.B. Oral health impact, dental caries experience, and associated factors in 12–15-year-old school children in India. Int. J. Adolesc. Med. Health. 2017;29:20150041. doi: 10.1515/ijamh-2015-0041. [DOI] [PubMed] [Google Scholar]
- 7.Wen P.Y.F., Chen M.X., Zhong Y.J., Dong Q.Q., Wong H.M. Global Burden and Inequality of Dental Caries, 1990 to 2019. J Dent. Res. 2022;101:392–399. doi: 10.1177/00220345211056247. [DOI] [PubMed] [Google Scholar]
- 8.Kirthiga M., Murugan M., Saikia A., Kirubakaran R. Risk factors for early childhood caries: A systematic review and meta-analysis of case control and cohort studies. Pediatr. Dent. 2019;41:95–112. [PMC free article] [PubMed] [Google Scholar]
- 9.Ledder R.G., Kampoo K., Teanpaisan R., McBain A.J. Oral microbiota in severe early childhood caries in Thai children and their families: A pilot study. Front. Microbiol. 2018;9:2420. doi: 10.3389/fmicb.2018.02420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kesim S., Çiçek B., Aral C.A., Öztürk A., Mazicioğlu M.M., Kurtoğlu S. Oral health, obesity status and nutritional habits in Turkish children and adolescents: An epidemiological study. Balk. Med. J. 2016;33:364–372. doi: 10.5152/balkanmedj.2016.16699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leong P.M., Gussy M.G., Barrow S.Y.L., de Silva-Sanigorski A., Waters E. A systematic review of risk factors during first year of life for early childhood caries. Int. J. Paediatr. Dent. 2013;23:235–250. doi: 10.1111/j.1365-263X.2012.01260.x. [DOI] [PubMed] [Google Scholar]
- 12.Tham R., Bowatte G., Dharmage S.C., Tan D.J., Lau M.X., Dai X., Allen K.J., Lodge C.J. Breastfeeding and the risk of dental caries: A systematic review and meta-analysis. Acta Paediatr. 2015;104:62–84. doi: 10.1111/apa.13118. [DOI] [PubMed] [Google Scholar]
- 13.Jain M., Namdev R., Bodh M., Dutta S., Singhal P., Kumar A. Social and behavioral determinants for early childhood caries among preschool children in India. J. Dent. Res. Dent. Clin. Dent. Prospect. 2015;9:115. doi: 10.15171/joddd.2014.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbasoğlu Z., Tanboğa İ., Küchler E.C., Deeley K., Weber M., Kaspar C., Korachi M., Vieira A.R. Early childhood caries is associated with genetic variants in enamel formation and immune response genes. Caries Res. 2015;49:70–77. doi: 10.1159/000362825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharifi R., Jahedi S., Mozaffari H.R., Imani M.M., Sadeghi M., Golshah A., Moradpoor H., Safaei M. Association of LTF, ENAM, and AMELX polymorphisms with dental caries susceptibility: A meta-analysis. BMC Oral Health. 2020;20:132. doi: 10.1186/s12903-020-01121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadeghi M., Golshah A., Godiny M., Sharifi R., Khavid A., Nikkerdar N., Tadakamadla S.K. The Most Common Vitamin D Receptor Polymorphisms (ApaI, FokI, TaqI, BsmI, and BglI) in Children with Dental Caries: A Systematic Review and Meta-Analysis. Children. 2021;8:302. doi: 10.3390/children8040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chisini L.A., Cademartori M.G., Conde M.C.M., Costa F.D.S., Salvi L.C., Tovo-Rodrigues L., Correa M.B. Single nucleotide polymorphisms of taste genes and caries: A systematic review and meta-analysis. Acta Odontol. Scand. 2021;79:147–155. doi: 10.1080/00016357.2020.1832253. [DOI] [PubMed] [Google Scholar]
- 18.Valore E.V., Park C.H., Quayle A.J., Wiles K.R., McCray P.B., Jr., Ganz T. Human beta-defensin-1: An antimicrobial peptide of urogenital tissues. J. Clin. Investig. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatipoğlu Ö., Saydam F. Association between rs11362 polymorphism in the beta-defensin 1 (DEFB1) gene and dental caries: A meta-analysis. J. Oral Biosci. 2020;62:272–279. doi: 10.1016/j.job.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Huttner K.M., Bevins C.L. Antimicrobial peptides as mediators of epithelial host defense. Pediatr. Res. 1999;45:785–794. doi: 10.1203/00006450-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Sahasrabudhe K., Kimball J., Morton T., Weinberg A., Dale B. Expression of the antimicrobial peptide, human β-defensin 1, in duct cells of minor salivary glands and detection in saliva. J. Dent. Res. 2000;79:1669–1674. doi: 10.1177/00220345000790090601. [DOI] [PubMed] [Google Scholar]
- 22.Mathews M., Jia H.P., Guthmiller J.M., Losh G., Graham S., Johnson G.K., Tack B.F., McCray P.B., Jr. Production of β-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect. Immun. 1999;67:2740–2745. doi: 10.1128/IAI.67.6.2740-2745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vieira A.R., Modesto A., Marazita M.L. Caries: Review of human genetics research. Caries Res. 2014;48:491–506. doi: 10.1159/000358333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piekoszewska-Ziętek P., Turska-Szybka A., Olczak-Kowalczyk D. Single nucleotide polymorphism in the aetiology of caries: Systematic literature review. Caries Res. 2017;51:425–435. doi: 10.1159/000476075. [DOI] [PubMed] [Google Scholar]
- 25.Fujita T. Evolution of the lectin–complement pathway and its role in innate immunity. Nat. Rev. Immunol. 2002;2:346–353. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- 26.Turner M.W. The role of mannose-binding lectin in health and disease. Mol. Immunol. 2003;40:423–429. doi: 10.1016/S0161-5890(03)00155-X. [DOI] [PubMed] [Google Scholar]
- 27.Alyousef Y.M., Borgio J.F., AbdulAzeez S., Al-Masoud N., Al-Ali A.A., Al-Shwaimi E., Al-Ali A.K. Association of MBL2 gene polymorphism with dental caries in Saudi children. Caries Res. 2017;51:12–16. doi: 10.1159/000450963. [DOI] [PubMed] [Google Scholar]
- 28.Chisini L.A., Cademartori M.G., Conde M.C.M., Costa F.D.S., Tovo-Rodrigues L., Carvalho R.V.d., Demarco F.F., Correa M.B. Genes and SNPs in the pathway of immune response and caries risk: A systematic review and meta-analysis. Biofouling. 2020;36:1100–1116. doi: 10.1080/08927014.2020.1856821. [DOI] [PubMed] [Google Scholar]
- 29.Hu J., Jiang W., Lin X., Zhu H., Zhou N., Chen Y., Wu W., Zhang D., Chen H. Dental Caries Status and Caries Risk Factors in Students Ages 12-14 Years in Zhejiang, China. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018;24:3670–3678. doi: 10.12659/MSM.907325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mwakayoka H., Masalu J.R., Namakuka Kikwilu E. Dental Caries and Associated Factors in Children Aged 2–4 Years Old in Mbeya City, Tanzania. J. Dent. 2017;18:104–111. [PMC free article] [PubMed] [Google Scholar]
- 31.Krustrup U., Petersen P.E. Dental caries prevalence among adults in Denmark--the impact of socio-demographic factors and use of oral health services. Community Dent. Health. 2007;24:225–232. [PubMed] [Google Scholar]
- 32.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan R.L., Thayer K.A., Bero L., Bruce N., Falck-Ytter Y., Ghersi D., Guyatt G., Hooijmans C., Langendam M., Mandrioli D. GRADE: Assessing the quality of evidence in environmental and occupational health. Environ. Int. 2016;92:611–616. doi: 10.1016/j.envint.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan R.L., Thayer K.A., Santesso N., Holloway A.C., Blain R., Eftim S.E., Goldstone A.E., Ross P., Guyatt G., Schünemann H.J. Evaluation of the risk of bias in non-randomized studies of interventions (ROBINS-I) and the ‘target experiment’ concept in studies of exposures: Rationale and preliminary instrument development. Environ. Int. 2018;120:382–387. doi: 10.1016/j.envint.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 36.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 38.De Oliveira D.S.B., Segato R.A.B., Oliveira S., Dutra A.L.T., Dos Santos A.S., Praxedes A.D.N., Belém L.C., Antunes L.A., Lips A., Nelson-Filho P. Association between genetic polymorphisms in DEFB1 and microRNA202 with caries in two groups of Brazilian children. Arch. Oral Biol. 2018;92:1–7. doi: 10.1016/j.archoralbio.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Hu X.-P., Zhou H.-j., Li Z.-Q., Song T.-Z., Zhu Y.-Y. Lack of associations between lactoferrin (LTF) and mannose-binding lectin 2 (MBL2) gene polymorphism and dental caries susceptibility. J. Int. Med. Res. 2020;48:0300060520943428. doi: 10.1177/0300060520943428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lips A., Antunes L.S., Antunes L.A., de Abreu J.G.B., Barreiros D., de Oliveira D.S.B., Batista A.C., Nelson-Filho P., da Silva L.A.B., da Silva R.A.B. Genetic polymorphisms in DEFB1 and miRNA202 are involved in salivary human β-defensin 1 levels and caries experience in children. Caries Res. 2017;51:209–215. doi: 10.1159/000458537. [DOI] [PubMed] [Google Scholar]
- 41.Mubayrik A.F.B., Deeley K., Patir A., Koruyucu M., Seymen F., Vieira A. Polymorphisms in the antimicrobial peptide DEFB1 are not associated with caries in primary dentition. JPDA. 2014;23:66. [Google Scholar]
- 42.Pehlivan S., Sipahi M., Ozkinay F., Pehlivan M., Koturoglu G., Alpoz A.R. Might there be a link between mannose-binding lectin polymorphism and dental caries? Mol. Immunol. 2005;42:1125–1127. doi: 10.1016/j.molimm.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Shimomura-Kuroki J., Nashida T., Miyagawa Y., Sekimoto T. The role of genetic factors in the outbreak mechanism of dental caries. J. Clin. Pediatr. Dent. 2018;42:32–36. doi: 10.17796/1053-4628-42.1.6. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y., Wang W., Qin M. Mannose-binding lectin gene polymorphisms are not associated with susceptibility to severe early childhood caries. Hum. Immunol. 2013;74:110–113. doi: 10.1016/j.humimm.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Opal S., Garg S., Jain J., Walia I. Genetic factors affecting dental caries risk. Aust. Dent. J. 2015;60:2–11. doi: 10.1111/adj.12262. [DOI] [PubMed] [Google Scholar]
- 46.Werneck R., Mira M., Trevilatto P. A critical review: An overview of genetic influence on dental caries. Oral Dis. 2010;16:613–623. doi: 10.1111/j.1601-0825.2010.01675.x. [DOI] [PubMed] [Google Scholar]
- 47.Strużycka I. The oral microbiome in dental caries. Pol. J. Microbiol. 2014;63:127. doi: 10.33073/pjm-2014-018. [DOI] [PubMed] [Google Scholar]
- 48.Cavallari T., Arima L.Y., Ferrasa A., Moysés S.J., Moysés S.T., Herai R.H., Werneck R.I. Dental caries: Genetic and protein interactions. Arch. Oral Biol. 2019;108:104522. doi: 10.1016/j.archoralbio.2019.104522. [DOI] [PubMed] [Google Scholar]
- 49.Petersen P.E. The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century–the approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2003;31:3–24. doi: 10.1046/j..2003.com122.x. [DOI] [PubMed] [Google Scholar]
- 50.Santosh A.B.R., Boyd D., Laxminarayana K.K. Clinical outline of oral diseases. Dent. Clin. 2020;64:1–10. doi: 10.1016/j.cden.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Wright J.T. The role of Genetics in Caries Risk and Resistance. Dimensions of Dental Hygiene. 2019. [(accessed on 3 December 2022)]. Available online: https://dimensionsofdentalhygiene.com/article/geneticscaries-risk.
- 52.Wang X., Willing M.C., Marazita M.L., Wendell S., Warren J.J., Broffitt B., Smith B., Busch T., Lidral A.C., Levy S.M. Genetic and environmental factors associated with dental caries in children: The Iowa Fluoride Study. Caries Res. 2012;46:177–184. doi: 10.1159/000337282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olszowski T., Adler G., Janiszewska-Olszowska J., Safranow K., Kaczmarczyk M. MBL2, MASP2, AMELX, and ENAM gene polymorphisms and dental caries in Polish children. Oral Dis. 2012;18:389–395. doi: 10.1111/j.1601-0825.2011.01887.x. [DOI] [PubMed] [Google Scholar]
- 54.Kunin A.A., Evdokimova A.Y., Moiseeva N.S. Age-related differences of tooth enamel morphochemistry in health and dental caries. EPMA J. 2015;6:3. doi: 10.1186/s13167-014-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Q., Jia P., Cuenco K.T., Feingold E., Marazita M.L., Wang L., Zhao Z. Multi-dimensional prioritization of dental caries candidate genes and its enriched dense network modules. PloS ONE. 2013;8:e76666. doi: 10.1371/journal.pone.0076666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leone C.W., Oppenheim F.G. Physical and chemical aspects of saliva as indicators of risk for dental caries in humans. J. Dent. Educ. 2001;65:1054–1062. doi: 10.1002/j.0022-0337.2001.65.10.tb03449.x. [DOI] [PubMed] [Google Scholar]
- 57.Lenander-Lumikari M., Loimaranta V. Saliva and dental caries. Adv. Dent. Res. 2000;14:40–47. doi: 10.1177/08959374000140010601. [DOI] [PubMed] [Google Scholar]
- 58.Sharifi R., Tabarzadi M.F., Choubsaz P., Sadeghi M., Tadakamadla J., Brand S., Sadeghi-Bahmani D. Evaluation of Serum and Salivary Iron and Ferritin Levels in Children with Dental Caries: A Meta-Analysis and Trial Sequential Analysis. Children. 2021;8:1034. doi: 10.3390/children8111034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drummond A.M., Ferreira E.F., Gomes V.E., Marcenes W. Inequality of Experience of Dental Caries between Different Ethnic Groups of Brazilians Aged 15 to 19 Years. PLoS ONE. 2015;10:e0145553. doi: 10.1371/journal.pone.0145553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuo G., Rozier R.G., Kranz A.M. Dental Caries: Racial and Ethnic Disparities Among North Carolina Kindergarten Students. Am. J. Public Health. 2015;105:2503–2509. doi: 10.2105/AJPH.2015.302884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cakan D.G., Ulkur F., Taner T. The genetic basis of dental anomalies and its relation to orthodontics. Eur. J. Dent. 2013;7:S143–S147. doi: 10.4103/1305-7456.119092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X., Shaffer J.R., Weyant R.J., Cuenco K.T., DeSensi R.S., Crout R., McNeil D.W., Marazita M.L. Genes and their effects on dental caries may differ between primary and permanent dentitions. Caries Res. 2010;44:277–284. doi: 10.1159/000314676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dunsche A., Açil Y., Siebert R., Harder J., Schröder J.M., Jepsen S. Expression profile of human defensins and antimicrobial proteins in oral tissues. J. Oral Pathol. Med. 2001;30:154–158. doi: 10.1034/j.1600-0714.2001.300305.x. [DOI] [PubMed] [Google Scholar]
- 64.Gursoy U.K., Könönen E. Understanding the roles of gingival beta-defensins. J. Oral Microbiol. 2012;4:15127. doi: 10.3402/jom.v4i0.15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rivas-Santiago B., Serrano C.J., Enciso-Moreno J.A. Susceptibility to infectious diseases based on antimicrobial peptide production. Infect. Immun. 2009;77:4690–4695. doi: 10.1128/IAI.01515-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dale B.A., Tao R., Kimball J.R., Jurevic R.J. Oral antimicrobial peptides and biological control of caries. BMC Oral Health. 2006;6:S13. doi: 10.1186/1472-6831-6-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen D.H., Martin J.T. Common dental infections in the primary care setting. Am. Fam. Physician. 2008;77:797–802. [PubMed] [Google Scholar]
- 68.Caufield P.W., Griffen A.L. Dental caries: An infectious and transmissible disease. Pediatr. Clin. N. Am. 2000;47:1001–1019. doi: 10.1016/S0031-3955(05)70255-8. [DOI] [PubMed] [Google Scholar]
- 69.Islam B., Khan S.N., Khan A.U. Dental caries: From infection to prevention. Med. Sci. Monit. 2007;13:RA196. [PubMed] [Google Scholar]
- 70.Ezekowitz R.A. Role of the mannose-binding lectin in innate immunity. J. Infect. Dis. 2003;187:S335–S339. doi: 10.1086/374746. [DOI] [PubMed] [Google Scholar]
- 71.Garred P. Mannose-binding lectin genetics: From A to Z. Biochem. Soc. Trans. 2008;36:1461–1466. doi: 10.1042/BST0361461. [DOI] [PubMed] [Google Scholar]
- 72.Holanda K., Lucena-Araujo A.R., Quintas A., Mendonca T., Lima A., Vasconcelos L.R., Moura P., Cavalcanti M., Machado C., Araujo A.S. Mannose-binding lectin 2 (MBL2) gene polymorphisms do not influence frequency of infections in chronic lymphocytic leukemia patients. Rev. Bras. Hematol. Hemoter. 2014;36:29–34. doi: 10.5581/1516-8484.20140010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eisen D.P., Minchinton R.M. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin. Infect. Dis. 2003;37:1496–1505. doi: 10.1086/379324. [DOI] [PubMed] [Google Scholar]
- 74.Niu J.Y., Yin I.X., Wu W.K.K., Li Q.-L., Mei M.L., Chu C.H. Antimicrobial peptides for the prevention and treatment of dental caries: A concise review. Arch. Oral Biol. 2021;122:105022. doi: 10.1016/j.archoralbio.2020.105022. [DOI] [PubMed] [Google Scholar]
- 75.Chen L., Jia L., Zhang Q., Zhou X., Liu Z., Li B., Zhu Z., Wang F., Yu C., Zhang Q. A novel antimicrobial peptide against dental-caries-associated bacteria. Anaerobe. 2017;47:165–172. doi: 10.1016/j.anaerobe.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 76.Pepperney A., Chikindas M.L. Antibacterial peptides: Opportunities for the prevention and treatment of dental caries. Probiotics Antimicrob. Proteins. 2011;3:68–96. doi: 10.1007/s12602-011-9076-5. [DOI] [PubMed] [Google Scholar]
- 77.Goeke J.E., Kist S., Schubert S., Hickel R., Huth K.C., Kollmuss M. Sensitivity of caries pathogens to antimicrobial peptides related to caries risk. Clin. Oral Investig. 2018;22:2519–2525. doi: 10.1007/s00784-018-2348-7. [DOI] [PubMed] [Google Scholar]
- 78.Tao R., Jurevic R.J., Coulton K.K., Tsutsui M.T., Roberts M.C., Kimball J.R., Wells N., Berndt J., Dale B.A. Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob. Agents Chemother. 2005;49:3883–3888. doi: 10.1128/AAC.49.9.3883-3888.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vitorino R., Lobo M.J.C., Duarte J.R., Ferrer-Correia A.J., Domingues P.M., Amado F.M. The role of salivary peptides in dental caries. Biomed. Chromatogr. 2005;19:214–222. doi: 10.1002/bmc.438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.