Abstract
Background
Shigella is a leading cause of bacterial diarrhea morbidity and mortality affecting mainly children under five in the developing world. In Zambia, Shigella has a high prevalence of 34.7% in children with diarrhea and an attributable fraction of 6.7% in Zambian children with moderate to severe diarrhea. Zambian diarrhea management guidelines and the health ministry reporting tool Health Management Information System (HMIS) heavily rely on the WHO clinical classification of dysentery to potentially identify and estimate the burden of Shigella in children. This reliance on clinical dysentery as a proxy to shigellosis in under five children may be resulting in gross under-estimation of shigella disease burden in Zambia.
Methods
We used existing laboratory and clinical data to examine the sensitivity and predictive value of dysentery to correctly identify Shigella infection in under five children with PCR confirmed Shigella infection in Lusaka and Ndola districts, Zambia.
Results
Clinical dysentery had a sensitivity of 8.5% (34/401) in identifying under five children with Shigella by stool PCR. Dysentery was able to correctly classify Shigella in 34 of 68 bloody stool samples giving a corresponding positive predictive value of 50%. Of the 1087 with non-bloody diarrhea, 720 did not have Shigella giving a negative predictive value of 66.2%.
Conclusions
Use of clinical dysentery as a screening symptom for Shigella infection in children under five presenting with moderate to severe diarrhea has low sensitivity and low positive predictive value respectively. Clinical dysentery as a screening symptom for Shigella contributes to gross under diagnosis and reporting of Shigella infection among under five children in Zambia. Further research is required to better inform practice on more accurate methods or tools to use in support of routine diagnosis, particularly in low middle-income settings where laboratory diagnosis remains a challenge.
Introduction
Shigella causes over 250 million cases of shigellosis annually in low and middle-income countries (LMICs) and is the fourth commonest cause of moderate to severe diarrhea (MSD) causing up to 65,000 deaths in children under five years [1–4]. Shigella has four species—S sonnei, S flexneri, S boydii and S dysenteriae. These species vary in their tendency to cause dysentery, which is defined as blood in stool. S dysenteriae type 1 has traditionally been associated with bloody stools and to a lesser extent, S flexneri [5]. However, there has been changing Shigella disease epidemiology with recent studies indicating a global decline in the incidence of S dysenteriae type 1-the principal cause of epidemic or pandemic dysentery [2,6]. However, other serotypes of Shigella continue to dominate with S. flexneri becoming more prevalent in the developing world [1,2,5,6]. In Zambia, Shigella was a leading bacterial causes of MSD in under-five children in 2012 with high prevalence (34.7%) and high attributable fraction (6.7%) for MSD [7]. However, there is little published data on Shigella speciation in Zambia.
Bloody diarrhea in under five children in LMICs is largely caused by shigellosis and amoebiasis [8,9]. However, gaps remain on the sensitivity of utilizing a clinical diagnosis of dysentery to diagnose shigellosis in children. A meta-analysis by Tickell et al. demonstrated a significant and steady decline in the sensitivity of using a clinical diagnosis of dysentery to identify shigellosis in children with diarrhea over the years (1977 to 2016) [2]. Several studies from Africa and Asia have shown non-dysenteric Shigella as a more common presentation of Shigella diarrhea [2,10]. Consequently, the continued reliance on the World Health Organization (WHO) clinical dysentery to identify shigellosis may underestimate Shigella disease morbidity and mortality.
The Zambian Ministry of Health’s Health Management Information System (HMIS) and disease surveillance infrastructure currently relies on stool culture or clinical categorization of non-bloody verses bloody diarrhea to inform disease intelligence efforts, Shigella disease burden estimates and subsequently the diarrhea management protocols for children with MSD [9]. This paper examined the clinical presentation of confirmed cases of Shigella using PCR molecular techniques and determined the sensitivity and predictive value of dysentery in identifying shigellosis in children under five with diarrhea using the WHO Integrated Management of Childhood Illnesses(IMCI) [11] and the Zambia Standard Treatment Guidelines [12].
Methods
Study design and participants
We used existing clinical and laboratory data collected from a project that was aimed at evaluating the impact of a comprehensive diarrhea prevention and control program which was targeted at reducing post-neonatal, all-cause under-five mortality in Zambia. Consent was obtained from parents or guardians of the minors included in the study. Interventions included, (i) introduction of the rotavirus vaccine to the national immunization program, (ii) improved clinical case management of diarrhea, and (iii) a comprehensive community prevention and advocacy campaign on hand washing with soap, exclusive breastfeeding up to 6 months of age, and the use of ORS and Zinc. The study is described in detail elsewhere [7,13,14]. The data was collected between July 2012 and October 2013.
Briefly, children were eligible for the study if they were aged below five years; presented to a health care facility with caregiver confirmation of child having diarrhea (defined as the passing of ≥3 abnormally loose stools in the past 24 hrs). The criteria for MSD, as per IMCI guidelines was based on the following clinical features:
Sunken eyes, loss of normal skin turgor, IV fluid administration, blood in stool, hospitalization for diarrhea or dysentery or classified by clinician as MSD.
We obtained data from the Zambian Ministry of Health HMIS of all children under five reported to have had diarrhea in 2018–2020. We applied the Shigella prevalence in children under five as determined by PCR to estimate the extent of under reporting and potential missed Shigella in children under five with diarrhea.
Sample collection
At each site, the child’s care giver was asked by study staff to notify them when the child indicated a need to use the toilet or after the child had produced stool in a diaper. About 10–15 mL of bulk stool was collected from each child at enrollment in sterile specimen containers, immediately after collection, samples were transported to the laboratory at 2–8°C, then samples were stored at -80°C until testing.
Laboratory procedures
The extraction of nucleic acid was done using bead beating in SK38 soil grinding tubes (Cat KT03961-1-006.2, Bertin Corporation, USA), incubated in NucliSENS® easyMAG® lysis buffer (BioMérieux, France) containing bacteriophage MS2 as an internal positive control for the assay and centrifuged. After centrifugation, the supernatant was then used for nucleic acid extraction using the commercially available QIAamp MinElute Virus Spin Kit (Qiagen, Germany) with the nucleic acid eluted at -20°C until testing the next day.
We used the PCR based multiplex qualitative Luminex x-TAG® gastrointestinal pathogen panel, according to the manufacturer’s instructions for pathogen identification in stool samples collected. The kit detects 15 enteric pathogens (i.e. bacteria, viruses, and protozoa) simultaneously [3].
Statistics
For this effort reported here, the following statistical methods were employed: The sample size was calculated based on confidence interval for sample sensitivity and specificity [15–17]. Assuming a prevalence of 34.7% and a sensitivity of 8%, the sample size needed for a two-sided 95% sensitivity confidence interval with a width at the most extreme at 0.100, is 329. With the assumed prevalence of 34.7% and a sample specificity of 0.950, the sample size needed for a two-sided 95% specificity confidence interval with a width of at most 0.100, is 112. The whole sample size required so that both confidence intervals have a width of less than 0.100, is 329, considering the largest of the two sample sizes.
We summarized categorical data using proportions and determined association between baseline patient characteristics and Shigella positivity using chi square test. We computed the sensitivity, specificity, NPV and PPV and associated 95% confidence interval for blood in stool as a diagnostic measure for Shigella against qPCR as the gold standard. Based on the estimated prevalence NPV and PPV we estimated the number and proportion of Shigella cases misdiagnosed, using the national reported data and assuming the prevalence of Shigella remains the same over time. This assumption holds because there has not been any major intervention which might result in a reduction in the prevalence of Shigella. All statistical analyses were performed in Stata SE 16 (StataCorp, College Station, TX, USA).
Ethics statement
This study was approved by the University of Zambia Biomedical Research Ethics Committee, University of North Carolina at Chapel Hill Institutional Review Board, and the Zambian Ministry of Health under Reference No. 014-09-11. All study participants provided written informed consent.
Results
Study profile
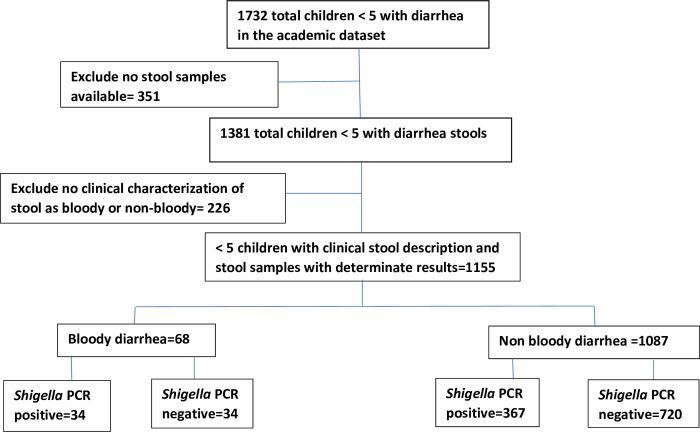
1732 children under five were recorded as having diarrhea in the study database and 1381 had stool available. Only stools that met the criteria for MSD were considered. A total of 226 stool samples were excluded from the analysis because they did not have accompanying clinical characterization of “blood” or “no blood” in stool. Up to 1155 children with clinical characterization of stools by blood and PCR lab results were considered in the analysis. Fig 1 is a flow chart showing the number of children with Shigella.
Fig 1. Shows the flow chart of children with Shigella.
Baseline characteristics
The baseline characteristics of children included age, gender and clinical symptoms at presentation to the health facility. The clinical symptoms included abdominal pain, fever and blood in stool as reported by the caregiver or observed by the health worker. Age was significantly associated with Shigella infection with a p-value 0.001 as shown in Table 1 below.
Table 1. Baseline characteristics of the children in the ACADEMIC data set.
| Characteristic | n (%) of Total | Shigella negative, n(%) | Shigella positive n(%) | p value |
|---|---|---|---|---|
| Age (n = 971) | ||||
| <12 months | 474(50.4) | 337(71.1) | 137(28.9) | 0.001 |
| 12–23 months | 275(29.2) | 162(58.9) | 113(41.1) | |
| 24–60 months | 192(20.4) | 109(56.8) | 83(43.2) | |
| Gender (n = 1115) | ||||
| Male | 544(48.8) | 366(67.3) | 178(32.7) | 0.116 |
| Female | 571(51.2) | 358(62.7) | 213(37.3) | |
| Abdominal pain (n = 1115) | ||||
| No | 349(30.2) | 223(63.9) | 126(36.1) | 0.144 |
| Yes | 686(59.4) | 443(64.6) | 243(35.4) | |
| Not reported | 120(10.4) | 88(73.3) | 32(26.7) | |
| Fever (n = 1115) | ||||
| No | 585(50.6) | 386(66) | 199(34) | 0.407 |
| Yes | 554(48) | 360(65) | 194(35) | |
| Not reported | 16(1.4) | 8(50) | 8(50) | |
| Reported blood in stool (n = 1115) | ||||
| No | 1087(94.1) | 720(66.2) | 367(33.8) | 0.006 |
| Yes | 68(5.9) | 34(50) | 34(50) |
Prevalence, sensitivity, specificity, and predictive values results
A total of 401 were diagnosed with Shigella by PCR method out of the 1155 stool samples and giving a prevalence of 34.7%. Of the 401 Shigella positive patients, 34 had reported a history of bloody stools translating into a sensitivity of 8.5%. Using the PCR technique, a total of 754 of children with both bloody and non-diarrhea did not have shigellosis. Of these, 720 had non-bloody diarrhea out of 754 with diarrhea and no Shigella giving a specificity of 95.5%. A clinical diagnosis of blood in stool was able to correctly classify Shigella in 34 of 68 stool samples giving a corresponding positive predictive value of 50%. Further, of the 1087 with non-bloody diarrhea, 720 did not have Shigella giving a negative predictive value of 66.2% as shown in Table 2 below.
Table 2. Performance of dysentery against lab confirmed Shigella.
| Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | |
|---|---|---|---|---|
| Any blood in stool | 8.5(5.9–11.7) | 95.5(93.8–96.9) | 50(37.6–62.4) | 66.2(63.3–69.1) |
Estimated under-reporting of Shigella disease burden by the HMIS data reporting tool
Utilizing Ministry of Health HMIS data reports for diarrhoea in under five children for the period 2018 to 2020, there were a total of 5,857,526, 5,730,470 and 5,478,925 cases of diarrhea in 2018, 2019 and 2020 respectively. The total dysentery cases in under five children reported for the periods 2018, 2019 and 2020 were 14, 675, 13,100 and 11,332 respectively. Assuming all reported cases of dysentery were caused by shigella (over-estimation), the estimated Shigella cases for the reporting period was less than 1%. Applying the 34.7% prevalence of Shigella as detected by PCR in Chisenga et. al, the possible Shigella cases missed by the HMIS reporting tool are estimated at above 99% from the total diarrhoea for the 2018–2020 reporting periods. Table 3 shows the estimated under reporting of Shigella prevalence by HMIS if the PCR testing was utilized in the 2018–2020 HMIS data reporting period.
Table 3. Estimated underreporting of Shigella by prevalence in under five children with diarrhea in Zambia.
| Reported dysentery cases by MOH HMIS data | Expected Shigella cases if prevalence was 34.7% | |||||
|---|---|---|---|---|---|---|
| Year | Diarrhea (non-bloody and dysentery) OPD | Dysentery cases | Dysentery (%) | Shigella cases | Missed Shigella cases | Shigella cases missed (%) |
| 2020 | 5,478,925 | 11,332 | 0.21% | 1,901,187 | 1,889,855 | 99.40% |
| 2019 | 5,730,470 | 13,100 | 0.23% | 1,988,473 | 1,975,373 | 99.34% |
| 2018 | 5,857,526 | 14,675 | 0.25% | 2,032,562 | 2,017,887 | 99.28% |
Discussion
Our study examines the utility of clinical dysentery as a clinical proxy to diagnose Shigella disease in children under five with diarrhea. Clinical dysentery has been traditionally used as a clinical proxy for Shigella disease by the WHO and the Zambian diarrhea management guidelines. Our results show three main findings.
Firstly, our results show that although the prevalence of Shigella in stools of under five children presenting with diarrhea in our study was 34.7%, relaying on the clinical symptom of dysentery may be leading to significant under reporting. Our study finding that only 8.48% (34/401) of all laboratory confirmed cases of Shigella by stool PCR presented with dysentery, is consistent with recent literature on Shigella disease epidemiology [2,18,19]. This finding shows that 91.52% of under five children in Zambia who have Shigella infection may not present with dysentery. Recent studies including the GEMS Study have shown a changing Shigella disease epidemiology with a global decline in the incidence of S dysenteriae type 1 (traditionally the principal cause of epidemic or pandemic dysentery) and S. flexneri emerging as the predominant Shigella species in the developing world [1,2,5,6]. Unfortunately, there is currently little data on the seroprevalence of different species of Shigella in Zambia [9]. If what has been documented in other parts of the developing world is true for Zambia, the change in species prevalence and disease presentation might result in fewer children with Shigella infection presenting with blood in stool leading to under-reporting and ultimately suboptimal treatment of shigellosis in under-five children presenting with diarrhea.
Secondly, the sensitivity of clinical dysentery in diagnosing Shigella infection was very low at 8.5% while having a very high specificity of 95.5%. This important finding of low sensitivity of 8.5% speaks to the use of dysentery to estimate burden of Shigella disease in many countries. The presence of dysentery has been the traditional teaching about the main presentation of shigellosis [20]. The WHO diarrhea management protocols recommend the use of antibiotics in diarrhea when there is presence of blood in stool with the assumption that Shigella infection is the leading cause of dysentery. This continued heavy reliance on the WHO diarrhea management clinical guidelines mainly emphasizing the clinical characteristic of bloody diarrhea to identify shigellosis may be contributing to the reported under treatment of shigellosis without dysentery and the likely increased mortality in the shigellosis group without dysentery [2,10,20].
The Zambian Ministry of Health (MOH) uses the HMIS classification of diarrhea as non-bloody and bloody diarrhea [21]. Only 11,332 (0.21%) out of the total 5,478,925 diarrhea cases under five were classified as bloody diarrhea (dysentery) in 2020. It is likely that Shigella disease estimates in Zambia as reported by the HMIS reporting system may grossly underestimate the true burden of Shigella disease among children. According to Chisenga et al 2018, the prevalence of Shigella in under five children with diarrhea in Lusaka province was found to be 34.7% and attributable fraction for causing MSD to be 6.7% using stool PCR [7]. Applying these proportion to the MOH HMIS data in which there were 5,467,593 OPD diarrhea non bloody and 11,332 of bloody diarrhea cases in under five children in 2020 in Zambia (total diarrhea = 5,478,925) [21], the estimated number of Shigella cases if PCR techniques were used would have probably been about 1,901,187. However, only a paltry 11,332 dysentery cases were reported with no specific breakdown of shigellosis. Assuming all 11,332 dysentery cases are caused by Shigella in 2020 (gross over estimation as there are other causes such as amoebic dysentery), we can therefore extrapolate that up to 1,889,855(99%) of Shigella cases may not be reported in the HMIS data tool which places heavy reliance on dysentery as a symptom/proxy for Shigella infection (see Table 3). This agrees with our findings of low sensitivity and low predictive value of dysentery to identify Shigella infection in Zambian children under five who have diarrhea. This resonates well with findings from a Kenyan study in which the use of clinical symptoms to identify shigellosis was found to have low sensitivity [10]. With WHO and Zambia diarrhea management guidelines recommending antibiotic use when there is dysentery or cholera, it is likely that the majority of Shigella cases may be under treated based on these guidelines and may be contributing to the high mortality arising from diarrhea [22]. This study has therefore shown that over reliance on the clinical parameter of dysentery as a clinical proxy for Shigella infection in Zambian under five children may be leading to gross under reporting and underestimating the true burden of Shigella disease burden.
Thirdly, this study shows that use of clinical dysentery as a diagnostic screening symptom for Shigella infection has low positive and negative predictive values of 50% and 66.2% respectively. Our study results show that half of all the bloody diarrhea did not have Shigella. These low predictive values mean that clinicians and health care workers may not be able to place much reliance on the symptom of blood in stool to both identify or exclude under five children with Shigella infection. Unfortunately, Zambia like most developing countries continues to use the WHO’s clinical dysentery as a proxy to estimate the burden of shigellosis [9,10]. The clinical importance of this low positive predictive value for identifying Shigella disease is that without appropriate laboratory confirmation, many children with Shigella infection may not receive appropriate treatment.
Although stool culture is the gold standard for diagnosis of shigellosis, it is not widely available in LMICs and has poor yield compared to PCR [3]. Limitations to laboratory diagnosis of Shigella include limited laboratory infrastructure and consumables, inadequate microbiology culture skills/competencies, poor clinical and laboratory staff attitudes, and sample courier logistical challenges [20,22–24]. There is therefore urgent need for Shigella diagnostics that have low cost, high sensitivity and high specificity which can be widely available like the malaria rapid diagnostic tests [25–27]. The current reality however is that these are not yet widely available and mainly under development.
The current pragmatic approach to rational antibiotic use in diarrhea case management in which the WHO recommends antibiotic use for dysentery and cholera needs to be discussed in the light of emerging evidence [2,18,19]. Where there are diagnostic challenges and the screening symptoms which have low sensitivity and low predictive values, there is need for an appropriate balance between maintaining antibiotic stewardship and addressing mortality and morbidity due to untreated shigellosis in children. Further, there is need for point of care screening tests, diarrhea scoring systems or models with better predictive values for screening against Shigella infections in children under five. In the absence of in an effective Shigella vaccine or improved diagnostics, health care workers in LMIC will need to have a high index of suspicion for shigellosis in children with non-bloody diarrhea. The findings of this paper may refine the empiric management of diarrhea management protocols for Zambia.
Study limitation
Although the strength of this study is its use of stool PCR data to challenge the WHO clinical dysentery symptoms as a diagnostic proxy for shigellosis in children under five children (and subsequent antibiotic use) where there is no laboratory culture, the major limitation of this study is that it was done on retrospective data limited by inconsistent collection of associated clinical parameters/symptoms. Factor analysis of the nature of diarrhea, associated fever, abdominal pain, and vomiting would have been very informative in predicting a model that can utilize clinical symptoms to predict shigellosis in under five children with diarrhea.
Conclusion
The use of clinical dysentery as a screening symptom for Shigella infection in children under five presenting with moderate to severe diarrhea has low sensitivity and low positive predictive value. This contributes to under diagnosing and reporting of Shigella infection among under five children in Zambia. More accurate burden of disease data will be required to strengthen the case for accelerated vaccine development which holds the possibility of effective prevention of shigellosis.
Recommendation
Well-designed studies be done that can evaluate symptoms associated with Shigella diarrhea to come up with shigella diarrhea predictive models or scoring systems for use in clinical settings.
Stool handling and microbiology laboratory processes be improved to increase diagnosis of shigellosis in LMICs. Additionally, point of care Rapid Diagnostic Testing (RDT) for Shigella are urgently needed to improve diarrhea management in LMICs.
Acknowledgments
We wish to express gratitude to the Center for Infectious Diseases Research Zambia and the Zambian Ministry of Health for supporting and facilitating the use of data from ACADEMIC dataset and the HMIS database.
Data Availability
The underlying data set cannot be made publicly available because it contains human research participant data; however, it can be made available to any interested researchers upon request. The Centre for Infectious Disease Research in Zambia (CIDRZ) Ethics and Compliance Committee is responsible for approving such requests. To request data access, one must write to the Secretary to the Committee/Head of Research Operations through this email address: info@cidrz.org, mentioning the intended use for the data, contact information, a research project title, and a description of the analysis being proposed as well as the format it is expected. The requested data should only be used for the purposes related to the original research or study. The CIDRZ Ethics and Compliance Committee will normally review all data requests within 48–72 hours (Monday-Friday), and provide notification if access has been granted or additional project information is needed.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.McArthur MA, Maciel M, Pasetti MF. Human immune responses against Shigella and enterotoxigenic E. coli: Current advances and the path forward. Vaccine. 2017;35(49, Part A):6803–6. doi: 10.1016/j.vaccine.2017.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tickell KD, Brander RL, Atlas HE, Pernica JM, Walson JL, Pavlinac PB. Identification and management of Shigella infection in children with diarrhoea: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1235–e48. doi: 10.1016/S2214-109X(17)30392-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388(10051):1291–301. doi: 10.1016/S0140-6736(16)31529-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016. Lancet Infect Dis. 2018;18(11):1229–40. Epub 2018/09/30. doi: 10.1016/S1473-3099(18)30475-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, Marohn ME, et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis. 2014;59(7):933–41. Epub 2014/06/23. doi: 10.1093/cid/ciu468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewnard JA, Rogawski McQuade ET, Platts-Mills JA, Kotloff KL, Laxminarayan R. Incidence and etiology of clinically-attended, antibiotic-treated diarrhea among children under five years of age in low- and middle-income countries: Evidence from the Global Enteric Multicenter Study. PLoS Negl Trop Dis. 2020;14(8):e0008520. Epub 2020/08/11. doi: 10.1371/journal.pntd.0008520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chisenga CC BS, Laban NM, Mwila-Kazimbaya K, Mwaba J, Simuyandi M and Chilengi R. Aetiology of Diarrhoea in Children under Five in Zambia Detected Using Luminex xTAG Gastrointestinal Pathogen Panel. Pediatric Infectious Diseases 2018;3:8. doi: 10.21767/2573-0282.100064 [DOI] [Google Scholar]
- 8.Murphy MS. Management of bloody diarrhoea in children in primary care. BMJ. 2008;336(7651):1010–5. doi: 10.1136/bmj.39542.440417.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katemba BM KN, Sakubita P, Ngomah A, Gianetti B. Trend and Disease Burden of Dysentery in Zambia 2016–2018. Health Press Zambia Bulletin 2019; 3(7);. 2019;3(7):5–9. Epub 29 September 2019. [Google Scholar]
- 10.Pavlinac PB, Denno DM, John-Stewart GC, Onchiri FM, Naulikha JM, Odundo EA, et al. Failure of Syndrome-Based Diarrhea Management Guidelines to Detect Shigella Infections in Kenyan Children. J Pediatric Infect Dis Soc. 2016;5(4):366–74. Epub 2015/09/26. doi: 10.1093/jpids/piv037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health O. Handbook: IMCI integrated management of childhood illness. Geneva: World Health Organization; 2005. [Google Scholar]
- 12.Ministry of Health ZNFC. Standard Treatment Guidelines, Essential Medicines List, Essential Laboratory Supplies for Zambia. 3rd Edition ed2013.
- 13.Beres LK, Tate JE, Njobvu L, Chibwe B, Rudd C, Guffey MB, et al. A Preliminary Assessment of Rotavirus Vaccine Effectiveness in Zambia. Clinical Infectious Diseases. 2016;62(suppl_2):S175–S82. doi: 10.1093/cid/civ1206 [DOI] [PubMed] [Google Scholar]
- 14.Bosomprah S, Beach LB, Beres LK, Newman J, Kapasa K, Rudd C, et al. Findings from a comprehensive diarrhoea prevention and treatment programme in Lusaka, Zambia. BMC Public Health. 2016;16(1):475. doi: 10.1186/s12889-016-3089-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics Journal of Biomedical Informatics. 2014;48:,193–204. doi: 10.1016/j.jbi.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 16.Newcombe RG. Two-Sided Confidence Intervals for the Single Proportion: Comparison of Seven Methods.’ Statistics in Medicine; 17 ed1998. p. 857–72. [DOI] [PubMed] [Google Scholar]
- 17.Fleiss JL, Levin B., Paik M.C. Statistical Methods for Rates and Proportions. Third Edition. ed: John Wiley & Sons. New York.; 2003. [Google Scholar]
- 18.Chang Z, Zhang J, Ran L, Sun J, Liu F, Luo L, et al. The changing epidemiology of bacillary dysentery and characteristics of antimicrobial resistance of Shigella isolated in China from 2004–2014. BMC Infectious Diseases. 2016;16(1):685. doi: 10.1186/s12879-016-1977-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taneja N. Changing Epidemiology of Shigellosis and Emergence of Ciprofloxacin-Resistant Shigellae in India. Journal of clinical microbiology. 2007;45:678–9. doi: 10.1128/JCM.02247-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO WHO. The treatment of diarrhoea: a manual for physicians and other senior health workers.2005.
- 21.Ministry of Health Z. HMIS 2020. 2021.
- 22.WHO WHO. Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1. 2005.
- 23.Cathy A. Petti CRP, Thomas C. Quinn, Allan R. Ronald, Merle A. Sande,. Laboratory Medicine in Africa: A Barrier to Effective Health Care. Clinical Infectious Diseases. 2006(42):377–82. [DOI] [PubMed] [Google Scholar]
- 24.Hegde S, Benoit SR, Arvelo W, Lindblade K, López B, McCracken JP, et al. Burden of laboratory-confirmed shigellosis infections in Guatemala 2007–2012: results from a population-based surveillance system. BMC Public Health. 2019;19(3):474. doi: 10.1186/s12889-019-6780-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham J, Jones S, Gatton ML, Barnwell JW, Cheng Q, Chiodini PL, et al. A review of the WHO malaria rapid diagnostic test product testing programme (2008–2018): performance, procurement and policy. Malaria Journal. 2019;18(1):387. doi: 10.1186/s12936-019-3028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyce MR, Menya D, Turner EL, Laktabai J, Prudhomme-O’Meara W. Evaluation of malaria rapid diagnostic test (RDT) use by community health workers: a longitudinal study in western Kenya. Malaria Journal. 2018;17(1):206. doi: 10.1186/s12936-018-2358-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yukich JO, Bennett A, Albertini A, Incardona S, Moonga H, Chisha Z, et al. Reductions in Artemisinin-Based Combination Therapy Consumption after the Nationwide Scale up of Routine Malaria Rapid Diagnostic Testing in Zambia. The American Society of Tropical Medicine and Hygiene. 2012;87(3):437–46. doi: 10.4269/ajtmh.2012.12-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The underlying data set cannot be made publicly available because it contains human research participant data; however, it can be made available to any interested researchers upon request. The Centre for Infectious Disease Research in Zambia (CIDRZ) Ethics and Compliance Committee is responsible for approving such requests. To request data access, one must write to the Secretary to the Committee/Head of Research Operations through this email address: info@cidrz.org, mentioning the intended use for the data, contact information, a research project title, and a description of the analysis being proposed as well as the format it is expected. The requested data should only be used for the purposes related to the original research or study. The CIDRZ Ethics and Compliance Committee will normally review all data requests within 48–72 hours (Monday-Friday), and provide notification if access has been granted or additional project information is needed.