Abstract
Background
People living with HIV (PLWH) have increased risks of non-communicable diseases, especially cardiovascular diseases. Current HIV clinical management guidelines recommend regular cardiovascular risk screening, but the risk equation models are not specific for PLWH. Better tools are needed to assess cardiovascular risk among PLWH accurately.
Methods
We performed a prospective study to determine the performance of automatic retinal image analysis in assessing coronary artery disease (CAD) in PLWH. We enrolled PLWH with ≥1 cardiovascular risk factor. All participants had computerized tomography (CT) coronary angiogram and digital fundus photographs. The primary outcome was coronary atherosclerosis; secondary outcomes included obstructive CAD. In addition, we compared the performances of three models (traditional cardiovascular risk factors alone; retinal characteristics alone; and both traditional and retinal characteristics) by comparing the area under the curve (AUC) of receiver operating characteristic curves.
Results
Among the 115 participants included in the analyses, with a mean age of 54 years, 89% were male, 95% had undetectable HIV RNA, 45% had hypertension, 40% had diabetes, 45% had dyslipidemia, and 55% had obesity, 71 (61.7%) had coronary atherosclerosis, and 23 (20.0%) had obstructive CAD. The machine-learning models, including retinal characteristics with and without traditional cardiovascular risk factors, had AUC of 0.987 and 0.979, respectively and had significantly better performance than the model including traditional cardiovascular risk factors alone (AUC 0.746) in assessing coronary artery disease atherosclerosis. The sensitivity and specificity for risk of coronary atherosclerosis in the combined model were 93.0% and 93.2%, respectively. For the assessment of obstructive CAD, models using retinal characteristics alone (AUC 0.986) or in combination with traditional risk factors (AUC 0.991) performed significantly better than traditional risk factors alone (AUC 0.777). The sensitivity and specificity for risk of obstructive CAD in the combined model were 95.7% and 97.8%, respectively.
Conclusion
In this cohort of Asian PLWH at risk of cardiovascular diseases, retinal characteristics, either alone or combined with traditional risk factors, had superior performance in assessing coronary atherosclerosis and obstructive CAD.
Summary
People living with HIV in an Asian cohort with risk factors for cardiovascular disease had a high prevalence of coronary artery disease (CAD). A machine-learning-based retinal image analysis could increase the accuracy in assessing the risk of coronary atherosclerosis and obstructive CAD.
Background
Effective and durable anti-retroviral therapy allows us to witness tremendous improvement in life expectancy in people living with HIV (PLWH). However, substantial morbidity and mortality in PLWH are due to non-communicable diseases, such as cardiovascular diseases.
PLWH had a two-fold increased risk of cardiovascular diseases [1]. The global burden of cardiovascular diseases attributable to HIV has tripled since the 1990s, with sub-Saharan Africa and Asia-Pacific regions being the most affected areas [1]. Despite significant improvements in the management of HIV and its comorbidities over the last two decades. Recent population-based studies consistently showed that PLWH had higher cardiovascular and cerebrovascular disease risks [2, 3]. PLWH also carries a higher prevalence of risk factors for cardiovascular diseases, such as hypertension and diabetes [2]. Studies performed in Asia also showed that PLWH had high risks of coronary artery disease (CAD), partly attributable to the high prevalence of cardiovascular disease risk factors, suboptimal screening, and suboptimal management of these risk factors, especially in general low- and middle-income settings [4].
Current HIV clinical management guidelines recommend regular cardiovascular risk screening in PLWH; however, the best risk prediction model for PLWH is uncertain [5, 6]. Although various cardiovascular disease risk prediction functions are currently available. These algorithms have primarily been developed in non-HIV-infected populations. Therefore, they could not accurately predict risk in PLWH, possibly due to differences in pathogenesis underlying cardiovascular disease [7]. Moreover, many of these functions, including HIV-specific prediction models, have not been adequately validated in Asian populations of PLWH [4].
Retinal vascular characteristics have been recognized to encompass features associated with systemic diseases, such as diabetes and hypertension. Recent research has demonstrated a broader breadth of the application of retinal biomarkers for diagnostic, monitoring, and prognostic purposes in a wide range of chronic diseases [8]. Recently, retinal image characteristics have been shown to be closely linked with multiple cardiovascular risk factors and major cardiovascular events [9]. In particular, several retinal vascular characteristics, including arteriolar and venular calibre, curvature tortuosity, and branching complexity, were shown to have associations with CAD [10, 11].
Traditionally, manual interpretation of retinal images was heavily operator-dependent and time-consuming and was subjected to measurement error. Recently, the availability of automated models has shown superior accuracy and has transformed the practicality of adopting the assessment of retinal characteristics into routine clinical use [8, 12, 13]. However, most studies often involved a limited number of vascular characteristics [14]. In contrast, contemporary computing methods allow efficient and accurate measurements of a broad spectrum of retinal microvasculature characteristics. The retinal characteristics include vascular calibre, tortuosity, density, and branching complexity [15]. Retinal image analysis also has the advantages of being a simple, non-invasive procedure, requiring minimal operator training.
The application of retinal image analysis in assessing the risk of cardiovascular diseases has not yet been evaluated in PLWH. Also, HIV infection per se and associated opportunistic diseases cause various retinal abnormalities [16]. Therefore, studies showing a correlation between retinal vascular characteristics and cardiovascular diseases performed in non-HIV-infected populations might not be generalizable to PLWH. This study aimed to determine the prevalence of CAD among high-risk PLWH in a predominantly Asian population and determine the performance of machine-learning-based retinal image analysis in assessing the risk of CAD in PLWH compared to traditional risk prediction tools.
Methods
We performed a prospective study on CAD in an Asian population of PLWH with atherosclerotic risk factors at the Prince of Wales Hospital Infectious Diseases clinic in Hong Kong from February 2019 to February 2021. The primary outcome is coronary atherosclerosis; secondary outcomes include the presence of any coronary artery calcium (CAC), significant CAC, and obstructive CAD.
We enrolled PLWH aged 30 years or above with either chest pain or the presence of one or more risk factors for cardiovascular disease. Cardiovascular disease risk factors included hypertension, diabetes mellitus, dyslipidemia (defined by total cholesterol ≥6.2 mmol/L, HDL cholesterol ≤0.9 mmol/L, triglyceride ≥2.3 mmol/L, or use of the lipid-lowering drug) [17], current smoker, obesity (defined by body mass index ≥27 kg/m2) [18], and family history of CAD (defined by a first-degree relative with myocardial infarction before age 50 years) [17]. Patients with previously diagnosed CAD, creatinine clearance < 60mL/min, allergy to intravenous contrast, and pregnancy were excluded. All participants provided written informed consent. The study was approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee.
We collected demographic and clinical information, including HIV-related clinical data and comorbidities. We measured body weight, height, waist circumference, and blood pressure. After at least 8 hours of fasting, we collected blood samples for glucose, HbA1c, insulin, cholesterol, triglyceride, creatinine, C reactive protein, fibrinogen, D-dimer, sCD14, sCD163, and adiponectin.
We calculated cardiovascular risk using four cardiovascular risk prediction functions for each participant. They included Framingham risk score for 10-year coronary heart disease risk [19], QRISK3 [20], Pooled Cohort ASCVD risk equations [21], and 10-year cardiovascular disease risk using the Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study (DAD) cohort risk prediction [22]. A prediction of <10% was considered a low risk of cardiovascular disease.
All participants underwent a coronary CT angiogram, which included a non-contrast CT for calcium scoring and subsequently contrast-enhanced CT with cardiac gating for coronary angiography. We obtained a CAC score from the non-contrast CT and quantified it using the Agatston method [23]. The presence of any CAC was defined as Agatston score > 0, and significant CAC was defined as Agatston score ≥ 100. We assessed the coronary plaque burden, including the presence, the site, composition (calcified, non-calcified, or mixed), and degree of stenosis of the plaques following the Society of Cardiovascular Computed Tomography guidelines [24]. In brief, stenosis was categorized as normal or minimal (0–25%), mild (26% - 50%), moderate (51% - 75%), severe non-subtotal occlusion (76% - 90%), and severe subtotal occlusion (91–99%). Coronary atherosclerosis was defined as the presence of any plaques in one or more coronary artery segments, while moderate to severe stenosis of the lumen was considered as obstructive CAD.
A trained research nurse acquired digital fundus photographs using a Canon CR2-AF non-mydriatic retinal camera from both eyes of each participant. We then assessed retinal characteristics, for example, retinal vessel measurements, arteriole-venous nicking, arteriole occlusion, haemorrhages, exudates, tortuosity, bifurcation coefficients, asymmetry of branches, and bifurcation angles. The definitions of the retinal parameters were previously presented in detail [11, 25].
Statistical methods
We performed these measurements using R (University of Auckland, Auckland) and Matlab (MathWorks, Massachusetts, USA) computer software. The methods included fractal analysis, high-order spectra analysis, and statistical texture analysis [25].
We presented descriptive statistics for the baseline characteristics. We compared demographic, clinical, and retinal characteristics between those with and without primary and secondary outcomes using an independent two-sample Student t-test and Mann Whitney U test for continuous variables and a chi-square test for categorical variables. Using stepwise logistic regression analyses, we determined the associations of traditional cardiovascular risk factors and retinal characteristics with the primary and secondary outcomes. Three different models were evaluated: Model 1 included clinical characteristics regarded as traditional cardiovascular risk factors in PLWH; model 2 included retinal characteristics only; model 3 included both the traditional cardiovascular risk factors and retinal characteristics. All covariates with a p-value less than 0.1 were kept in the final model. We compared the performances of different models by comparing the area under the curve (AUC) of receiver operating characteristic (ROC) curves using the Delong method [26] The sensitivity and specificity of the models will also be calculated.
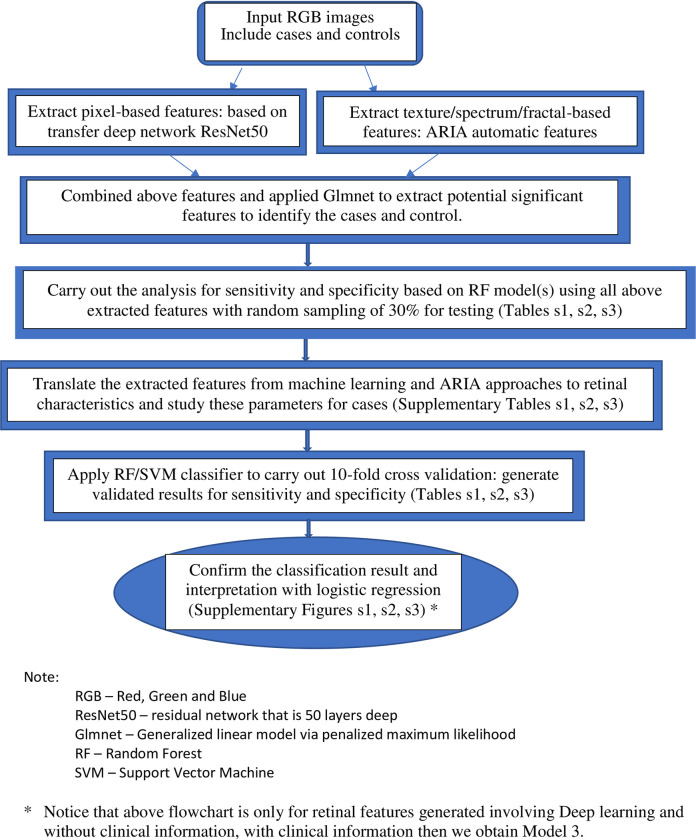
For the classification analysis, we used machine learning and deep learning techniques. Using Matlab, we first applied transfer deep network ResNet50 convolutional neural network with retinal images as input, and the outputs were features generated at the layer of ‘’fc1000_softmax’’, based on pixels associated with the specific outcome status [27]. We also extracted the texture/spectrum/fractal-based features that are associated with the specific outcome by using the automatic retinal image analysis (ARIA) algorithm written in Matlab [28]. We then used the Glmnet approach to select significant features based on the penalised maximum likelihood by using R and Matlab [29, 30]. These refined features are highly associated with the specific outcome. Finally, we translated the features extracted from the aforementioned machine learning approaches to commonly used retinal characteristics measured from the images using ImageJ. This part of the analysis helped enhance our understanding of retinal characteristics that contribute to the classification and identification of the specific outcome and was performed with SPSS. For the validation, we applied a 10-fold cross-validation method by using a support vector machine (SVM) algorithm for testing datasets that were not used in the training of the model [30, 31]. This was performed by partitioning the dataset and using a subset to train the algorithm, and the remaining subset of data for testing. Each time we ran the cross-validation analysis, we used 10% of the data for testing that were not used at all in the training data. The advantage of this method is that the data used for testing in each run were excluded from the specific training models for the purpose of validation to reduce the problem of overfitting and overestimation of the sensitivity and specificity. Because cross-validation does not use all of the data to build a model, it is a commonly used method to prevent overfitting during training. Fig 1 shows the flowchart of the described methodology.
Fig 1. Flowchart of the method for the development of the classification models.
Results
This study enrolled 120 participants during the recruitment period. Five participants were excluded from the analyses. Among them, 3 participants were excluded due to the unavailability of good quality retinal images, 1 participant was deceased, and 1 participant was lost to follow-up after recruitment without completing all study procedures. Among the 115 participants included in the analyses, the mean (±standard deviation) age was 54±10 years, 89% were male, the median (interquartile range/IQR) duration of HIV diagnosis was 12 (7–17) years, 95% had undetectable serum HIV RNA, and the median (IQR) CD4 count was 632 (451–840) cells/mm3. Among this cohort, 45% had hypertension, 40% had diabetes, 45% had dyslipidemia, and 55% had obesity. In addition, chest pain and dyspnea were present in 17% and 18% of participants. Their detailed demographic and clinical characteristics are shown in Tables 1 and 2.
Table 1. Demographic and clinical characteristics in participants with and without coronary atherosclerosis1.
| Characteristics | All participants (N = 115) | No coronary atherosclerosis (N = 44) | Coronary atherosclerosis (N = 71) | P-value |
|---|---|---|---|---|
| Age (years) | 53.7±9.5 | 49.9±9.7 | 56.1±8.7 | 0.001 |
| Male | 102 (88.7%) | 35 (79.6%) | 67 (94.4%) | 0.030 |
| Current smoker | 27 (23.7%) | 14(31.82) | 13(18.31) | 0.105 |
| Family history of premature myocardial infarction | 3 (2.6%) | 2(4.55) | 1(1.41) | 0.557 |
| Diabetes mellitus | 46 (40.0%) | 16 (36.4%) | 30(42.3%) | 0.530 |
| Hypertension | 52 (45.2%) | 15 (34.1%) | 37 (52.1%) | 0.059 |
| Dyslipidemia | 52 (45.2%) | 12 (27.3%) | 40 (56.3%) | 0.002 |
| Obesity | 63 (54.8%) | 24 (54.6%) | 39 (54.9%) | 0.968 |
| Hepatitis B | 9 (7.8%) | 5 (11.4%) | 4 (5.6%) | 0.450 |
| Hepatitis C | 3 (2.6%) | 2 (4.6%) | 1 (1.4%) | 0.557 |
| Chest pain | 20 (17.4%) | 6 (13.6%) | 14 (19.7%) | 0.403 |
| Dyspnoea | 21 (18.3%) | 8 (18.2%) | 13 (18.3%) | 0.986 |
| Duration of HIV diagnosis (years) | 12 (7–17) | 12 (8–5) | 13 (7–17) | 0.928 |
| History of AIDS | 24 (20.9%) | 11 (25.0%) | 13 (18.3%) | 0.391 |
| Duration of antiretroviral therapy (years) | 9 (5–13) | 9 (6–13) | 9 (5–13) | 0.714 |
| Current anti-retroviral drugs | ||||
| Tenofovir | 78 (67.8%) | 29 (65.9%) | 49 (69.0%) | 0.729 |
| Abacavir | 31 (27.0%) | 13 (29.5%) | 18 (25.4%) | 0.622 |
| NNRTI | 27 (23.5%) | 10 (22.7%) | 17 (23.9%) | 0.881 |
| Protease inhibitor | 19 (16.5%) | 6 (13.6%) | 13 (18.3%) | 0.512 |
| Integrase strand transfer inhibitor | 74 (64.3%) | 29 (65.9%) | 45 (63.4%) | 0.783 |
| Body weight (kg) | 74.0±13.9 | 75.4±15.1 | 73.1±13.2 | 0.381 |
| Body mass index (kg/m2) | 26.5±7.2 | 27.9±10.3 | 25.6±4.3 | 0.366 |
| Waist circumference (cm) | 91.6±11.6 | 91.9±12.8 | 91.4±10.8 | 0.805 |
| Systolic blood pressure (mmHg) | 133±18 | 130±16 | 136±19 | 0.247 |
| Diastolic blood pressure (mmHg) | 86±11 | 86±12 | 87±11 | 0.67 |
| CD4 count (cells/mm3) | 632 (451–840) | 666 (496–840) | 615 (419–865) | 0.295 |
| CD4:CD8 ratio | 0.83±0.36 | 0.95±0.36 | 0.76±0.35 | 0.003 |
| HIV RNA <50 copies/mL | 109 (94.8%) | 42(95.45) | 67(94.37) | 1.000 |
| Total cholesterol (mmol/L) | 4.70±1.08 | 4.77±1.03 | 4.65±1.12 | 0.559 |
| HDL (mmol/L) | 1.19±0.38 | 1.25±0.37 | 1.15±0.39 | 0.063 |
| LDL (mmol/L) | 2.55±0.89 | 2.71±0.87 | 2.44±0.89 | 0.120 |
| Triglycerides (mmol/L) | 1.9 (1.4–2.8) | 1.8 (1.2–2.4) | 2.2 (1.5–3.0) | 0.057 |
| Glucose (mmol/L) | 5.9 (5.2–6.8) | 5.6 (5.1–6.5) | 6.1 (5.3–7.1) | 0.124 |
| HbA1C (mmol/L) | 5.9 (5.6–6.7) | 5.9 (5.6–6.5) | 6.0 (5.6–6.7) | 0.274 |
| HOMA-IR | 2.3 (1.2–4.4) | 2.2 (1.1–3.1) | 2.3 (1.3–4.8) | 0.156 |
| Creatinine (μmol/L) | 88.2±16.5 | 85.6±16.2 | 89.7±16.6 | 0.195 |
| D-dimer (ng/mL) | 259 (177–353) | 224 (164–328) | 263 (177–351) | 0.405 |
| Fibrinogen (g/L) | 2.99±0.58 | 3.02±0.56 | 2.96±0.59 | 0.591 |
| C reactive protein (mg/L) | 1.4 (0.6–3.0) | 1.7 (0.6–3.6) | 1.4 (0.6–2.3) | 0.224 |
| sCD163 (ng/mL) | 626±144 | 622±150 | 629±141 | 0.943 |
| sCD14 (pg/mL) | 2228 (2131–2371) | 2232 (2077–2402) | 2226 (2139–2344) | 0.820 |
| Adiponectin (ng/mL) | 1305 (674–3277) | 1591 (744–3529) | 1233 (587–3115) | 0.299 |
1Data are presented as number (percentage), mean ± standard deviation, or median (interquartile range), as appropriate
Table 2. Demographic and clinical characteristics in participants with and without obstructive coronary artery disease (CAD)#.
| Characteristics | All participants (N = 115) | No obstructive CAD (N = 92) | Obstructive CAD (N = 23) | P-value |
|---|---|---|---|---|
| Age (years) | 53.7±9.5 | 52.09±9.13 | 60.22±8.40 | <0.001 |
| Male | 102 (88.7%) | 81(88.04) | 21(91.3) | 0.941 |
| Current smoker | 27 (23.7%) | 23 (25.3%) | 4 (17.4%) | 0.427 |
| Family history of premature myocardial infarction | 3 (2.6%) | 2 (2.2%) | 1 (4.4%) | 0.491 |
| Diabetes mellitus | 46 (40.0%) | 36 (39.1%) | 10 (43.5%) | 0.703 |
| Hypertension | 52 (45.2%) | 39 (42.4%) | 13 (56.5%) | 0.223 |
| Dyslipidemia | 52 (45.2%) | 39 (42.4%) | 13 (56.5%) | 0.223 |
| Obesity | 63 (54.8%) | 53 (57.6%) | 10 (43.5%) | 0.223 |
| Hepatitis B | 9 (7.8%) | 9 (9.8%) | 0 (0%) | 0.259 |
| Hepatitis C | 3 (2.6%) | 3 (3.3%) | 0 (0%) | 1.000 |
| Chest pain | 20 (17.4%) | 15 (16.3%) | 5 (21.7%) | 0.758 |
| Dyspnoea | 21 (18.3%) | 12 (13.0%) | 9 (39.1%) | 0.010 |
| Duration of HIV diagnosis (years) | 12 (7–17) | 12 (7–16) | 13(6–19) | 0.661 |
| History of AIDS | 24 (20.9%) | 20 (21.7%) | 4 (17.4%) | 0.863 |
| Duration of antiretroviral therapy (years) | 9 (5–13) | 9 (6–13) | 9 (3–14) | 0.875 |
| Current anti-retroviral drugs | ||||
| Tenofovir | 78 (67.8%) | 61 (66.3%) | 17 (73.9%) | 0.485 |
| Abacavir | 31 (27.0%) | 27 (29.3%) | 4 (17.4%) | 0.248 |
| NNRTI | 27 (23.5%) | 23 (25.0%) | 4 (17.4%) | 0.441 |
| Protease inhibitor | 19 (16.5%) | 15 (16.3%) | 4 (17.4%) | 1.000 |
| Integrase strand transfer inhibitor | 74 (64.3%) | 59 (64.1%) | 15 (62.5%) | 0.922 |
| Body weight (kg) | 74.0±13.9 | 75.1±13.9 | 69.3±13.4 | 0.119 |
| Body mass index (kg/m2) | 26.5±7.2 | 26.9±7.7 | 24.8±4.4 | 0.231 |
| Waist circumference (cm) | 91.6±11.6 | 91.9±11.4 | 90.4±12.4 | 0.605 |
| Systolic blood pressure (mmHg) | 133±18 | 133±16 | 136±23 | 0.534 |
| Diastolic blood pressure (mmHg) | 86±11 | 86±12 | 86±9 | 0.952 |
| CD4 count (cells/mm3) | 632 (451–840) | 623(455–839.5) | 686(427–865) | 0.729 |
| CD4:CD8 ratio | 0.83±0.36 | 0.85±0.38 | 0.78±0.3 | 0.418 |
| HIV RNA <50 copies/mL | 109 (94.8%) | 87 (94.6%) | 22 (95.7%) | 1.000 |
| Total cholesterol (mmol/L) | 4.70±1.08 | 4.81±1.04 | 4.25±1.14 | 0.027 |
| HDL (mmol/L) | 1.19±0.38 | 1.21±0.4 | 1.12±0.32 | 0.317 |
| LDL (mmol/L) | 2.55±0.89 | 2.64±0.86 | 2.16±0.93 | 0.021 |
| Triglycerides (mmol/L) | 1.9 (1.4–2.8) | 1.9 (1.4–2.9) | 2.2 (1.1–2.8) | 0.997 |
| Glucose (mmol/L) | 5.9 (5.2–6.8) | 5.9 (5.2–6.9) | 5.9 (5.4–6.3) | 0.761 |
| HbA1C (mmol/L) | 5.9 (5.6–6.7) | 5.9 (5.6–6.7) | 5.9 (5.7–6.6) | 0.718 |
| HOMA-IR | 2.3 (1.2–4.4) | 2.2 (1.2–3.9) | 2.9 (1.1–5.3) | 0.246 |
| Creatinine (μmol/L) | 88.2±16.5 | 88.61±16.94 | 86.3±14.65 | 0.551 |
| D-dimer (ng/mL) | 259 (177–353) | 240.0(164.0–333.5) | 267.5 (190.0–448.0) | 0.146 |
| Fibrinogen (g/L) | 2.99±0.58 | 2.99±0.54 | 2.98±0.71 | 0.965 |
| C reactive protein (mg/L) | 1.4 (0.6–3.0) | 1.4(0.6–3.0) | 1.4(0.6–3.1) | 0.960 |
| sCD163 (ng/mL) | 626±144 | 611±146 | 686±119 | 0.024 |
| sCD14 (pg/mL) | 2228 (2131–2371) | 2232 (2127–2392) | 2212 (2139–2321) | 0.756 |
| Adiponectin (ng/mL) | 1305 (674–3277) | 1378 (696–3444) | 1094 (369–2799) | 0.277 |
#Data are presented as number (percentage), mean ± standard deviation, or median (interquartile range), as appropriate
Seventy-one participants (61.7%) had coronary atherosclerosis; also, these 71 participants were found to have a presence of any CAC. Thirty-five participants (30.4%) had significant CAC, and 23 (20.0%) had obstructive CAD. Coronary atherosclerosis was associated with male gender, older age, dyslipidemia, hypertension, lower CD4:CD8 ratio, lower HDL cholesterol, and higher triglyceride (Table 1). Obstructive CAD was associated with older age, dyspnea, lower total cholesterol, lower LDL cholesterol, and higher sCD163 (Table 2). Significant CAC was associated with older age, dyslipidemia, and lower LDL cholesterol (S1 Table).
The retinal characteristics associated with coronary atherosclerosis, obstructive CAD, and significant CAC are shown in S2 and S3 Tables. Several retinal vascular characteristics were associated with coronary atherosclerosis. They include narrower arterioles, wider venules, and a lower degree of arteriolar branching,
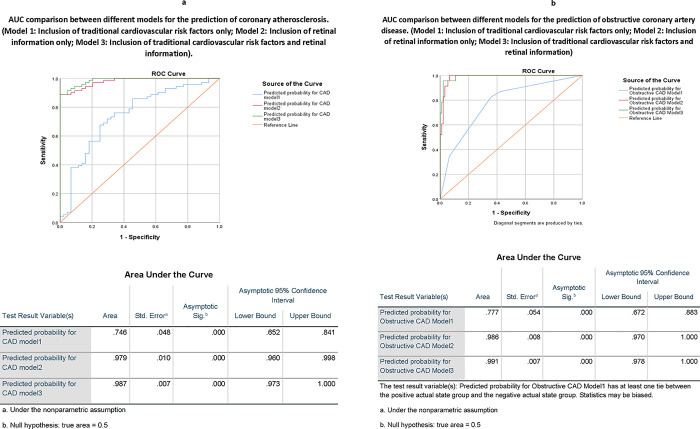
The associations between traditional cardiovascular risk factors and retinal characteristics with coronary atherosclerosis are shown in Table 3. We further improve the classification using a machine-learning approach. The model, including traditional cardiovascular risk factors and retinal characteristics, had 93.0% sensitivity and 93.2% specificity (area under the ROC curve (AUC) was 0.987, 95% CI 0.973–1.00). The performance is similar to the model with retinal characteristics alone, with 91.5% sensitivity and 88.6% specificity (AUC 0.979, 95% CI 0.960–0.998). However, both models with retinal characteristics were better than the model with traditional cardiovascular risk factors alone, with 81.7% sensitivity and 54.5% specificity (AUC 0.746, 95% CI 0.652–0.841) in assessing the risk of coronary atherosclerosis (Fig 2A).
Table 3. Stepwise logistic regression analysis showing factors associated with coronary atherosclerosis in different models.
| Odds Ratio (OR) | 95% CI for OR | P-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Model 1: Inclusion of traditional cardiovascular risk factors only | ||||
| Dyslipidemia | 3.30 | 1.41 | 7.77 | 0.006 |
| CD4:CD8 ratio | 0.21 | 0.07 | 0.68 | 0.009 |
| Hypertension | 2.56 | 1.09 | 6.04 | 0.031 |
| AUC: 0.7465 (95%CI: 0.6514–0.8416) | ||||
| Model 2: Inclusion of retinal characteristics only | ||||
| Left MBCA | 0.69 | 0.46 | 1.04 | 0.079 |
| Right CRVE | 2.16 | 1.10 | 4.24 | 0.025 |
| Right adjusted CRAE | 0.32 | 0.15 | 0.65 | 0.002 |
| Right MV asymmetry | 0.58 | 0.36 | 0.92 | 0.020 |
| AUC: 0.7298 (95%CI: 0.6356–0.8241) | ||||
| Model 3: Inclusion of traditional cardiovascular risk factors and retinal characteristics retinal characteristics | ||||
| Dyslipidemia | 3.27 | 1.31 | 8.17 | 0.011 |
| CD4:CD8 ratio | 0.23 | 0.07 | 0.79 | 0.019 |
| Hypertension | 2.23 | 0.88 | 5.62 | 0.089 |
| Left MBCA | 0.66 | 0.42 | 1.04 | 0.075 |
| Right CRVE | 2.04 | 0.98 | 4.24 | 0.057 |
| Right adjusted CRAE | 0.34 | 0.16 | 0.74 | 0.007 |
| Right MV asymmetry | 0.61 | 0.38 | 1.00 | 0.049 |
| AUC: 0.8073 (95%CI: 0.7216–0.893) | ||||
Abbreviations: CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; MBCA, bifurcation coefficient of artery; MV asymmetry, venous asymmetry index.
Fig 2.
a. AUC comparison between different models for the prediction of coronary atherosclerosis. (Model 1: Inclusion of traditional cardiovascular risk factors only; Model 2: Inclusion of retinal information only; Model 3: Inclusion of traditional cardiovascular risk factors and retinal information). b. AUC comparison between different models for the prediction of obstructive coronary artery disease. (Model 1: Inclusion of traditional cardiovascular risk factors only; Model 2: Inclusion of retinal information only; Model 3: Inclusion of traditional cardiovascular risk factors and retinal information).
The models showing the associations between traditional cardiovascular risk factors plus retinal characteristics for obstructive CAD are shown in Table 4. For assessing the risk of obstructive CAD, the model including retinal variables combined with traditional risk factors had a sensitivity and specificity of 95.7% and 97.8%, respectively (AUC 0.991, 95% CI 0.978–1.000). The model with retinal characteristics alone had sensitivity and specificity of 87.0% and 96.7%, respectively (AUC 0.986, 95% CI 0.970–1.000). Both models, including retinal characteristics, performed significantly better than the model with traditional risk factors alone and had sensitivity and specificity of 34.3% and 93.5%, respectively (AUC 0.777, 95% CI 0.672–0.883) (Fig 2B).
Table 4. Stepwise logistic regression analysis showing factors associated with obstructive coronary artery disease in different models.
| OR | 95% CI for OR | P-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Model 1: Inclusion of traditional cardiovascular risk factors only | ||||
| Age ≥55 years | 7.39 | 2.28 | 24.01 | 0.001 |
| Dyspnea | 3.20 | 1.04 | 9.79 | 0.042 |
| AUC: 0.7774 (95%CI: 0.6777–0.8771) | ||||
| Model 2: Inclusion of retinal characteristics only | ||||
| Left MBCV | 2.60 | 1.30 | 5.20 | 0.007 |
| Left AVR | 1.11 | 1.03 | 1.20 | 0.006 |
| Right MBCA | 0.14 | 0.05 | 0.42 | < .001 |
| Right AF | 2.97 | 1.03 | 8.54 | 0.044 |
| Right Tortuosity | 0.39 | 0.17 | 0.92 | 0.030 |
| Right A occlusion | 2.91 | 1.17 | 7.27 | 0.022 |
| AUC: 0.9022 (95%CI: 0.8098–0.9946) | ||||
| Model 3: Inclusion of traditional cardiovascular risk factors and retinal characteristics retinal characteristics | ||||
| Dyspnoea | 5.82 | 1.03 | 32.95 | 0.047 |
| Left MBCV | 2.36 | 1.14 | 4.87 | 0.020 |
| Left AVR | 1.11 | 1.03 | 1.21 | 0.011 |
| Right MBCA | 0.16 | 0.05 | 0.50 | 0.001 |
| Right AF | 3.27 | 1.05 | 10.17 | 0.041 |
| Right Tortuosity | 0.34 | 0.13 | 0.86 | 0.023 |
| Right A occlusion | 2.96 | 1.17 | 7.49 | 0.022 |
| AUC: 0.9168 (95%CI: 0.8420–0.9917) | ||||
Abbreviations: MBCA, bifurcation coefficient of artery; MBCV, bifurcation coefficient of venule; AF, atrial fibrillation; Tortuosity, Tortuosity; A occlusion, Arteriole occlusion; AVR, Arteriole-venule ratio.
Likewise, models including retinal characteristics alone or those combined with traditional cardiovascular risk factors performed significantly better than those with traditional risk factors alone in assessing the risk of significant CAC (S4 Table and S1 Fig). An example of retinal vascular characteristics and their related measurements is shown in Fig 3.
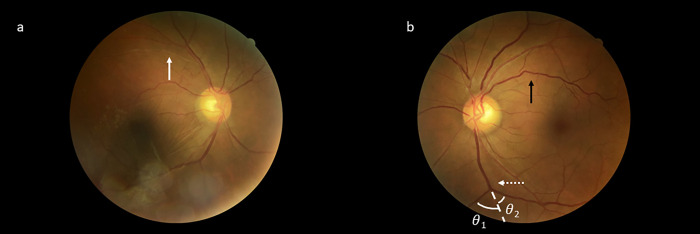
Fig 3.
a & b show the retinal images of two participants with coronary artery disease. White arrow indicates arteriole occlusion. Black arrow indicates a venous vessel with significant vascular tortuosity. The dashed white arrow indicates a venous bifurcation; θ1 and θ2 specify the bifurcation coefficients of the two branching venules (MBCV).
All conventional cardiovascular disease risk prediction functions had limited performance in assessing the risk of coronary atherosclerosis and obstructive CAD. Among those participants with coronary atherosclerosis, 45.1%, 57.7%, 52.1% and 42.3% were categorized as having low risk using Framingham risk score, QRISK3, Pooled Cohort ASCVD risk equations, and 10-year DAD cohort risk prediction, respectively. The corresponding figures for obstructive CAD among these groups were 30.4%, 52.2%, 34.8% and 34.8%, respectively. Moreover, ROC curve analyses showed that all of the prediction functions had AUC <0.7, with QRISK3 having the highest AUC for assessing both coronary atherosclerosis (AUC 0.667, 95% CI 0.566–0.767) and obstructive CAD (AUC 0.663, 95% CI 0.545–0.780) (S5 Table). For fairness in comparison, we used only the logistic regression model as a method for the comparison without using the machine-learning classification method. The models adopting the retinal characteristics alone (AUC 0.902, 95% CI 0.810–0.995) or in combination with the QRISK3 score (AUC 0.912, 95% CI 0.829–0.994) had a significantly better performance than QRISK3 score alone in assessing the risk of obstructive CAD (S6 and S7 Tables and S2 and S3 Figs).
Discussion
In this study, we evaluated machine-learning-based retinal imaging analysis to assess the risk of CAD in PLWH. In this cohort of Asian PLWH at risk of developing cardiovascular diseases, retinal characteristics, either alone or combined with traditional risk factors, enhanced the performance in assessing the risk of coronary atherosclerosis and obstructive CAD. In contrast, the performance of traditional cardiovascular risk prediction functions has a lot of room to improve.
This study showed that 62% of at-risk PLWH had coronary atherosclerosis, and 20% had obstructive CAD. The most extensive cohort study involving PLWH in Asia had recently demonstrated that traditional cardiovascular risk factors, including older age, hypertension, dyslipidemia, and high body mass index, were the major contributing factors for the development of cardiovascular disease diseases among PLWH in the region [32]. Our study further confirmed the high prevalence of CAD and its contributing risk factors among PLWH, highlighting the importance of prevention and treatment of these risk factors among PLWH. Moreover, an accurate tool to predict cardiovascular risk in these at-risk individuals is highly recommended.
We demonstrated that all of the evaluated cardiovascular risk prediction functions had limited accuracy in assessing the risks of coronary atherosclerosis and obstructive CAD in Asian PLWH. Evidence from current literature suggests that currently available cardiovascular risk prediction functions have suboptimal performance among PLWH. For example, Framingham risk scores tended to underestimate the prevalence of CAD and other atherosclerotic cardiovascular diseases among PLWH in the United States across all risk groups [33] while overestimating cardiovascular risk in European [34] and Asian [35] populations of PLWH. The available HIV-specific DAD cohort risk-prediction model was developed in a primarily European cohort [6]. It predicted a lower proportion having a high risk of cardiovascular disease among Asian PLWH than other prediction functions [35–37]. However, to the best of our knowledge, it had not yet been validated in any Asian populations of PLWH. Better tools to accurately assess the risk of CAD and other cardiovascular diseases among Asian PLWH are preferred.
Currently available studies performed in general HIV-uninfected populations have demonstrated the association of retinal vascular characteristics with multiple cardiovascular risk factors, including body mass index, smoking, hypertension, diabetes, and dyslipidemia [12, 13, 38, 39]. To supplement, these studies included multi-ethnic populations, including China [12], Britain [13], and the Middle East [38, 39]. In particular, smaller arteriolar widths, larger venular widths, and increased arteriolar and venular tortuosity were associated with cardiovascular risk factors [13]. Furthermore, using deep learning on retinal images, it was able to predict the presence of CAC and stratify cardiovascular disease risk in multi-ethnic populations [40]. In addition, the retinal vascular density and vessel branching complexity were able to predict higher mortality incidents in populations with high rates of hypertension and diabetes [15].
Regarding the prediction models of CAD, longitudinal studies showed that arteriolar narrowing, venular widening and fractal dimension accurately predicted incident CAD and CAD mortality in population-based studies [14, 41]. Patients hospitalized with acute coronary syndrome had lower retinal inner vessel length density and perfusion density than controls with lower cardiovascular risk [42]. In another study from China, patients with stable CAD had lower vessel density in superficial capillary plexus and deep capillary plexus; vessel density was also associated with CAD severity [43]. To further understand the pathogenesis of retinal vessel disease, recent genome-wide association analyses identified loci associated with retinal microvascular architecture, including genes associated with inflammatory, chemokine and angiogenesis pathways [15]. Such associations suggested that the retina may provide a window for identifying pathogenic processes underlying cardiovascular diseases in both HIV-uninfected populations and PLWH.
Our study further showed that a machine-learning-based retinal image analysis could improve the accuracy of assessing CAD risk among PLWH. In addition, it enhanced the performance of several widely adopted cardiovascular risk prediction functions in PLWH. This observation further supports that the retinal vessels provide an in-vivo examination of vascular sequelae secondary to CAD risk factors, including hypertension and diabetes. In studies performed in HIV-uninfected populations, retinal image analysis, when coupled with risk prediction functions, had better performance than risk prediction models alone in cardiovascular risk stratification [40] and prediction of cardiovascular mortality [44]. Retinal image analysis can potentially enhance the current risk prediction functions in cardiovascular disease risk stratification among PLWH.
The performance of retinal image analysis was best in assessing the risk of obstructive CAD in our cohort. While retinal characteristics can act as systemic biomarkers [8], the results from our study supported that retinal characteristics would be most helpful in identifying at-risk PLWH with obstructive CAD. This group of patients may benefit from more stringent cardiovascular risk factor control and coronary interventions.
Our study has several limitations. First, we had a relatively small sample size and involved only PLWH with cardiovascular risk factors from Asia. The cross-sectional study design precluded the evaluation of prediction of incident cardiovascular events by retinal image analysis. Future studies should evaluate the performance of retinal image analysis in predicting CAD in a more diverse population of PLWH with different ethnicities and levels of cardiovascular risk. Previous studies have shown differences in vascular calibre and fractal dimension among Asian ethnic groups [45]. Finally, this study has not involved the use of optical coherence tomography angiography (OCTA), which can provide more detailed retinal and choroidal characteristics analyses, which have also been shown to be associated with CAD [46].
In conclusion, we have demonstrated that machine-learning-based retinal image analysis accurately assesses the risk of coronary atherosclerosis and obstructive CAD among PLWH with risk factors for cardiovascular diseases. This tool should be further validated in more diverse populations of PLWH for the adoption in clinical practice for CAD risk stratification.
Supporting information
(TIF)
(TIF)
(TIF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(Model 1: Inclusion of cardiovascular risk prediction function with the highest AUC only; Model 2: Inclusion of retinal information only; Model 3: Inclusion of cardiovascular risk prediction score and retinal characteristics).
(TIFF)
(Model 1: Inclusion of cardiovascular risk prediction score with the highest AUC only; Model 2: Inclusion of retinal information only; Model 3: Inclusion of cardiovascular risk prediction score and retinal characteristics).
(TIFF)
Data Availability
Data are available from the following repository: https://doi.org/10.48668/0JVQ7V.
Funding Statement
This study is supported by the AIDS Trust Fund, HKSAR Government (Reference number MSS 318R) for Grace Lui and Benny Zee. However, the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living with the Human Immunodeficiency Virus: A Systematic Review and Meta-Analysis. Circulation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gooden TE, Gardner M, Wang J, Jolly K, Lane DA, Benjamin LA, et al. Incidence of cardiometabolic diseases in people living with and without HIV in the UK: a population-based matched cohort study. J Infect Dis. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, Marconi V. HIV Infection and Incidence of Cardiovascular Diseases: An Analysis of a Large Healthcare Database. Journal of the American Heart Association. 2019;8(14):e012241. doi: 10.1161/JAHA.119.012241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi JY, Lui GCY, Liao CT, Yang CJ. Managing cardiovascular risk in people living with HIV in Asia—where are we now? HIV Med. 2021. [DOI] [PubMed] [Google Scholar]
- 5.Ryom L, Cotter A, De Miguel R, Béguelin C, Podlekareva D, Arribas J, et al. 2019. update of the European AIDS Clinical Society Guidelines for treatment of people living with HIV version 10.0. HIV Medicine.n/a(n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation. 2019;140(2):e98–e124. doi: 10.1161/CIR.0000000000000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Agostino RB, Sr. Cardiovascular risk estimation in 2012: lessons learned and applicability to the HIV population. J Infect Dis. 2012;205 Suppl 3(Suppl 3):S362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranchod TM. Systemic retinal biomarkers. Current opinion in ophthalmology. 2021;32(5):439–44. doi: 10.1097/ICU.0000000000000784 [DOI] [PubMed] [Google Scholar]
- 9.Poplin R, Varadarajan AV, Blumer K, Liu Y, McConnell MV, Corrado GS, et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nature biomedical engineering. 2018;2(3):158–64. doi: 10.1038/s41551-018-0195-0 [DOI] [PubMed] [Google Scholar]
- 10.Wang SB, Mitchell P, Liew G, Wong TY, Phan K, Thiagalingam A, et al. A spectrum of retinal vasculature measures and coronary artery disease. Atherosclerosis. 2018;268:215–24. doi: 10.1016/j.atherosclerosis.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 11.Guo VY, Chan JC, Chung H, Ozaki R, So W, Luk A, et al. Retinal Information is Independently Associated with Cardiovascular Disease in Patients with Type 2 diabetes. Sci Rep. 2016;6:19053. doi: 10.1038/srep19053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Yuan M, An Z, Zhao X, Wu H, Li H, et al. Prediction of hypertension, hyperglycemia and dyslipidemia from retinal fundus photographs via deep learning: A cross-sectional study of chronic diseases in central China. PLoS One. 2020;15(5):e0233166. doi: 10.1371/journal.pone.0233166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen CG, Rudnicka AR, Welikala RA, Fraz MM, Barman SA, Luben R, et al. Retinal Vasculometry Associations with Cardiometabolic Risk Factors in the European Prospective Investigation of Cancer-Norfolk Study. Ophthalmology. 2019;126(1):96–106. doi: 10.1016/j.ophtha.2018.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seidelmann SB, Claggett B, Bravo PE, Gupta A, Farhad H, Klein BE, et al. Retinal Vessel Calibers in Predicting Long-Term Cardiovascular Outcomes: The Atherosclerosis Risk in Communities Study. Circulation. 2016;134(18):1328–38. doi: 10.1161/CIRCULATIONAHA.116.023425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zekavat SM, Raghu VK, Trinder M, Ye Y, Koyama S, Honigberg MC, et al. Deep Learning of the Retina Enables Phenome- and Genome-wide Analyses of the Microvasculature. Circulation. 2021. doi: 10.1161/CIRCULATIONAHA.121.057709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart MW. Human immunodeficiency virus and its effects on the visual system. Infect Dis Rep. 2012;4(1):e25. doi: 10.4081/idr.2012.e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friis-Moller N, Weber R, Reiss P, Thiebaut R, Kirk O, d’Arminio Monforte A, et al. Cardiovascular disease risk factors in HIV patients—association with anti-retroviral therapy. Results from the DAD study. AIDS. 2003;17(8):1179–93. doi: 10.1097/01.aids.0000060358.78202.c1 [DOI] [PubMed] [Google Scholar]
- 18.Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 19.Wilson PW D ’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 20.Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. doi: 10.1136/bmj.j2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goff DC jr , Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr., Gibbons R, et al. , American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friis-Moller N, Ryom L, Smith C, Weber R, Reiss P, Dabis F, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23(2):214–23. doi: 10.1177/2047487315579291 [DOI] [PubMed] [Google Scholar]
- 23.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. [DOI] [PubMed] [Google Scholar]
- 24.Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8(5):342–58. doi: 10.1016/j.jcct.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 25.Zhuo Y, Yu H, Yang Z, Zee B, Lee J, Kuang L. Prediction Factors of Recurrent Stroke among Chinese Adults Using Retinal Vasculature Characteristics. J Stroke Cerebrovasc Dis. 2017;26(4):679–85. doi: 10.1016/j.jstrokecerebrovasdis.2017.01.020 [DOI] [PubMed] [Google Scholar]
- 26.DeLong ER, DeLong DM, DL C-P. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44:837–45. [PubMed] [Google Scholar]
- 27.He, Kaiming, Xiangyu Zhang, Shaoqing Ren, and Jian Sun. "Deep residual learning for image recognition." In Proceedings of the IEEE conference on computer vision and pattern recognition, pp. 770–778. 2016. https://arxiv.org/pdf/1512.03385v1.pdf
- 28.Zee B, Lee J, Li Q, “Method and device for retinal image analysis”, Patent No. US8787638 B2, granted on 22 July 2014. https://patents.google.com/patent/US20120257164A1/en [Google Scholar]
- 29.Fan R.-E., Chen P.-H., and Lin C.-J. “Working set selection using second order information for training support vector machines.” Journal of Machine Learning Research, Vol. 6, 2005, pp. 1889–1918. https://dl.acm.org/doi/pdf/10.5555/1046920.1194907. [Google Scholar]
- 30.Hastie T., Tibshirani R., and Friedman J. The Elements of Statistical Learning, Second Edition. NY: Springer, 2008. https://web.stanford.edu/~hastie/Papers/ESLII.pdf [Google Scholar]
- 31.Scholkopf B., and Smola A. Learning with Kernels: Support Vector Machines, Regularization, Optimization and Beyond, Adaptive Computation and Machine Learning. Cambridge, MA: The MIT Press, 2002. https://dl.acm.org/doi/book/10.5555/559923 [Google Scholar]
- 32.Bijker R, Jiamsakul A, Uy E, Kumarasamy N, Ditango R, Chaiwarith R, et al. Cardiovascular disease-related mortality and factors associated with cardiovascular events in the TREAT Asia HIV Observational Database (TAHOD). HIV Med. 2019;20(3):183–91. doi: 10.1111/hiv.12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Triant VA, Perez J, Regan S, Massaro JM, Meigs JB, Grinspoon SK, et al. Cardiovascular Risk Prediction Functions Underestimate Risk in HIV Infection. Circulation. 2018;137(21):2203–14. doi: 10.1161/CIRCULATIONAHA.117.028975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krikke M, Hoogeveen RC, Hoepelman AI, Visseren FL, Arends JE. Cardiovascular risk prediction in HIV-infected patients: comparing the Framingham, atherosclerotic cardiovascular disease risk score (ASCVD), Systematic Coronary Risk Evaluation for the Netherlands (SCORE-NL) and Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) risk prediction models. HIV Med. 2016;17(4):289–97. doi: 10.1111/hiv.12300 [DOI] [PubMed] [Google Scholar]
- 35.Edwards-Jackson N, Kerr S, Tieu H, Ananworanich J, Hammer S, Ruxrungtham K, et al. Cardiovascular risk assessment in persons with HIV infection in the developing world: comparing three risk equations in a cohort of HIV-infected Thais. ?2011;12(8):510–5. [DOI] [PubMed] [Google Scholar]
- 36.Wu PY, Chen MY, Sheng WH, Hsieh SM, Chuang YC, Cheng A, et al. Estimated risk of cardiovascular disease among the HIV-positive patients aged 40 years or older in Taiwan. J Microbiol Immunol Infect. 2019;52(4):549–55. doi: 10.1016/j.jmii.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 37.Marbaniang IP, Kadam D, Suman R, Gupte N, Salvi S, Patil S, et al. Cardiovascular risk in an HIV-infected population in India. Heart Asia. 2017;9(2):e010893. doi: 10.1136/heartasia-2017-010893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Craenendonck T, Gerrits N, Buelens B, Petropoulos IN, Shuaib A, Standaert A, et al. Retinal microvascular complexity comparing mono- and multifractal dimensions in relation to cardiometabolic risk factors in a Middle Eastern population. Acta ophthalmologica. 2021;99(3):e368–e77. doi: 10.1111/aos.14598 [DOI] [PubMed] [Google Scholar]
- 39.Gerrits N, Elen B, Craenendonck TV, Triantafyllidou D, Petropoulos IN, Malik RA, et al. Age and sex affect deep learning prediction of cardiometabolic risk factors from retinal images. Sci Rep. 2020;10(1):9432. doi: 10.1038/s41598-020-65794-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rim TH, Lee CJ, Tham YC, Cheung N, Yu M, Lee G, et al. Deep-learning-based cardiovascular risk stratification using coronary artery calcium scores predicted from retinal photographs. The Lancet Digital health. 2021;3(5):e306–e16. doi: 10.1016/S2589-7500(21)00043-1 [DOI] [PubMed] [Google Scholar]
- 41.Liew G, Mitchell P, Rochtchina E, Wong TY, Hsu W, Lee ML, et al. Fractal analysis of retinal microvasculature and coronary heart disease mortality. Eur Heart J. 2011;32(4):422–9. doi: 10.1093/eurheartj/ehq431 [DOI] [PubMed] [Google Scholar]
- 42.Hannappe MA, Arnould L, Méloux A, Mouhat B, Bichat F, Zeller M, et al. Vascular density with optical coherence tomography angiography and systemic biomarkers in low and high cardiovascular risk patients. Sci Rep. 2020;10(1):16718. doi: 10.1038/s41598-020-73861-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong P, Li Z, Lin Y, Peng Q, Huang M, Jiang L, et al. Retinal microvasculature impairments in patients with coronary artery disease: An optical coherence tomography angiography study. Acta ophthalmologica. 2021. doi: 10.1111/aos.14806 [DOI] [PubMed] [Google Scholar]
- 44.Chang J, Ko A, Park SM, Choi S, Kim K, Kim SM, et al. Association of Cardiovascular Mortality and Deep Learning-Funduscopic Atherosclerosis Score derived from Retinal Fundus Images. American journal of ophthalmology. 2020;217:121–30. doi: 10.1016/j.ajo.2020.03.027 [DOI] [PubMed] [Google Scholar]
- 45.Li X, Wong WL, Cheung CY, Cheng CY, Ikram MK, Li J, et al. Racial differences in retinal vessel geometric characteristics: a multi-ethnic study in healthy Asians. Invest Ophthalmol Vis Sci. 2013;54(5):3650–6. doi: 10.1167/iovs.12-11126 [DOI] [PubMed] [Google Scholar]
- 46.Monteiro-Henriques I, Rocha-Sousa A, Barbosa-Breda J. Optical coherence tomography angiography changes in cardiovascular systemic diseases and risk factors: A Review. Acta ophthalmologica. 2021. doi: 10.1111/aos.14851 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(Model 1: Inclusion of cardiovascular risk prediction function with the highest AUC only; Model 2: Inclusion of retinal information only; Model 3: Inclusion of cardiovascular risk prediction score and retinal characteristics).
(TIFF)
(Model 1: Inclusion of cardiovascular risk prediction score with the highest AUC only; Model 2: Inclusion of retinal information only; Model 3: Inclusion of cardiovascular risk prediction score and retinal characteristics).
(TIFF)
Data Availability Statement
Data are available from the following repository: https://doi.org/10.48668/0JVQ7V.